Vital dye analysis of cranial neural crest cell migration in the mouse embryo
|
|
|
- Alexis Roberts
- 6 years ago
- Views:
Transcription
1 Development 116, (1992) Printed in Great Britain The Company of Biologists Limited Vital dye analysis of cranial neural crest cell migration in the mouse embryo GEORGE N. SERBEDZIJA 1,*, MARIANNE BRONNER-FRASER 2 AND SCOTT E. FRASER 1 1 Department of Biology, California Institute of Technology, Pasadena, CA 91125, USA 2 Department of Developmental and Cell Biology, University of California, Irvine, Irvine, CA 92717, USA *Author for correspondence Summary The spatial and temporal aspects of cranial neural crest cell migration in the mouse are poorly understood because of technical limitations. No reliable cell markers are available and vital staining of embryos in culture has had limited success because they develop normally for only 24 hours. Here, we circumvent these problems by combining vital dye labelling with exo utero embryological techniques. To define better the nature of cranial neural crest cell migration in the mouse embryo, premigratory cranial neural crest cells were labelled by injecting DiI into the amniotic cavity on embryonic day 8. Embryos, allowed to develop an additional 1 to 5 days exo utero in the mother before analysis, showed distinct and characteristic patterns of cranial neural crest cell migration at the different axial levels. Neural crest cells arising at the level of the forebrain migrated ventrally in a contiguous stream through the mesenchyme between the eye and the diencephalon. In the region of the midbrain, the cells migrated ventrolaterally as dispersed cells through the mesenchyme bordered by the lateral surface of the mesencephalon and the ectoderm. At the level of the hindbrain, neural crest cells migrated ventrolaterally in three subectodermal streams that were segmentally distributed. Each stream extended from the dorsal portion of the neural tube into the distal portion of the adjacent branchial arch. The order in which cranial neural crest cells populate their derivatives was determined by labelling embryos at different stages of development. Cranial neural crest cells populated their derivatives in a ventral-to-dorsal order, similar to the pattern observed at trunk levels. In order to confirm and extend the findings obtained with exo utero embryos, DiI (1,1-dioctadecyl-3,3,3,3 -tetramethylindocarbocyanine perchlorate) was applied focally to the neural folds of embryos, which were then cultured for 24 hours. Because the culture technique permitted increased control of the timing and location of the DiI injection, it was possible to determine the duration of cranial neural crest cell emigration from the neural tube. Cranial neural crest cell emigration from the neural folds was completed by the 11-somite stage in the region of the rostral hindbrain, the 14-somite stage in the regions of the midbrain and caudal hindbrain and not until the 16-somite stage in the region of the forebrain. At each level, the time between the earliest and latest neural crest cells to emigrate from the neural tube appeared to be 9 hours. Some aspects of cranial neural crest cell migration were similar to those observed in avian embryos; however, the exact pathways, the timing and the axial levels that contribute to the neural crest cells were distinctly different. Key words: cell migration, cranial, DiI, mouse, neural crest. Introduction In vertebrate embryos, cranial neural crest cells arise from the lateral edges of the neural folds, at the juncture of the neuroectoderm and the ectoderm (Horstadius, 1950; Vermeij-keers and Poelmann, 1980; Nichols, 1981, 1986, 1987; Tosney, 1982). In chicken embryos, ultrastructural studies suggest that emigration of cranial neural crest cells from the neural tube begins shortly after fusion of the neural folds (Tosney, 1982). The pathways of cranial neural crest cell migration have been studied most thoroughly in avian embryos using surgically created chimeric embryos (Johnston, 1966; Le Douarin, 1982; Noden, 1988). These studies indicate that cranial neural crest cells migrate along a subectodermal pathway that extends ventrolaterally from the dorsal portion of the neural tube between the ectoderm and the underlying mesodermal mesenchyme. After migration, cranial neural crest cells give rise to a variety of craniofacial tissues (Johnston, 1966; Noden, 1975, 1983; Le Douarin, 1982; Tosney, 1982), including the sensory and parasympathetic ganglia, cartilage, bone, muscles and connective tissue of the face (Le Douarin, 1982; Noden, 1983). In the mouse, a variety of genetic mutations exist that appear to affect neural crest cell migration and differentiation (Gruneberg and Truslove, 1960; Auerbach, 1954; see review by Morrison-Graham and Weston, 1989). By com-
2 298 G. N. Serbedzija, M. Bronner-Fraser and S. E. Fraser paring neural crest cell migration and differentiation in these mutant embryos with that in normal embryos, it may be possible to learn much about the mechanisms involved in both processes. Unfortunately, we have only a limited understanding of cranial neural crest cell migration in the mouse, owing to the relative inaccessibility of the embryos to microsurgical and cell marking techniques. Our present knowledge of the pathways of migration derives from ultrastructural studies using either cellular morphology (Verwoerd and van Oostrom, 1979) or toluidine blue staining (Nichols, 1981, 1986) to identify neural crest cells. These studies suggest that cranial neural crest cells complete their emigration from the neural folds prior to neural tube closure in the head. Cranial neural crest cells are thought to migrate ventrolaterally along a subectodermal pathway, similar to the pathways observed in chick embryos (Johnston, 1966; Noden, 1975; Le Douarin, 1982). As migration continues, neural crest cells become indistinguishable, both morphologically and histologically, from cells of mesodermal origin; therefore, some method for marking neural crest cells is required in order to study the subsequent stages of cranial neural crest cell migration. Cell marking techniques have been used to follow some aspects of cranial neural crest cell migration. The microinjection of gold-conjugated wheat germ agglutinin into the amniotic cavity of mouse embryos in culture has permitted the analysis of the early stages of migration (Smits-van Prooijie et al., 1986; Chan and Tam, 1988). Wheat germ agglutinin adheres to cell surface glycoconjugates (Gesink et al., 1983), thus labelling neural crest cells while they are contiguous with the ectoderm prior to neural tube fusion (Smits-van Prooijie et al., 1986). Current culturing techniques permitted approximately 24 hours of normal development; therefore, this technique was useful for studying only the first day of cranial neural crest cell migration. Because of the nature of the label, one cannot rule out the possibility that it passed unequally to daughter cells or passed to unlabelled cells (Smits-van Prooije et al., 1986: Chan and Tam, 1988). Despite these potential limitations, these experiments clearly suggested that in the mouse, as in the chicken, cranial neural crest cells from the rostral hindbrain region migrate ventrolaterally along a subectodermal pathway and populate the trigeminal ganglia and the first branchial arch (Chan and Tam, 1988). Previously, we labelled premigratory trunk neural crest cells within the neural tube of the mouse embryo by intralumenal injection of the fluorescent carbocyanine dye, DiI (1,1-dioctadecyl-3,3,3,3 -tetramethylindocarbocyanine perchlorate; Serbedzija et al., 1990, 1991). Because DiI is lipid-soluble and hydrophobic, it incorporates nearly irreversibly into the plasma membranes of all cells that it contacts (Sims et al., 1974). This dye does not spread from labelled to unlabelled cells, nor does it appear to have any adverse effects on neuronal or neural crest cells (Honig and Hume, 1986; Serbedzija et al., 1989). However, mouse cranial neural crest cells begin migrating prior to the fusion of the neural folds (Verwoerd and van Oostrom, 1979; Nichols, 1981, 1986). This makes injection of DiI into the neural tube inappropriate for labelling this cell population. Here, we report experiments performed using a modification of the DiI-labelling technique that permits identification of premigratory cranial neural crest cells. DiI was injected into the amniotic cavity of a mouse embryo from which it labelled all cells in contact with the amniotic fluid, including the entire ectoderm, the neural plate and the neural folds. With further development, only the cells of neural crest and placodal origin become internalized. The embryos, maintained within the mother, remained attached to the uterus throughout the experimental period, utilizing the exo utero technique first developed for work on the limb (Muneoka et al., 1986). This avoided the limited developmental times over which whole embryo culture gives normal development and permits the analysis of cranial neural crest cell migration over extended periods. To confirm and refine some aspects of the migration observed in exo utero embryos, premigratory neural crest cells were labelled by injecting small amounts of DiI directly into the forming neural crest of embryos maintained in culture. By using both the exo utero and the in vitro labelling techniques, it was possible to determine the pathways of neural crest cell migration, the temporal pattern of neural crest cell emigration from the neural folds and the order in which neural crest cells populate their derivatives. While the general pattern of cranial neural crest cell migration was similar to that observed in avians, important differences were observed in the origin and the trajectories of the migrating neural crest cells. Materials and methods Animal preparation Embryos were obtained by mating CD-1 females with BDF-1 males (Charles Rivers). In the evening, male and female mice were placed in the same cage, but kept separate by a gated partition. The gate was opened at 6:00 a.m. the following morning and the male and female mice were allowed to mate for three hours. The presence of a vaginal plug at the end of the three hour period was taken to indicate pregnancy, and the time and date that the gate was opened was designated embryonic day 0 (E0). This mating scheme reduced the normal variability in the actual time of mating as well as the range of developmental stages of the embryos within a litter. On E8, a pregnant female was anesthetized with avertin, prepared by mixing 0.5 g of 2,2,2-tribromoethanol (Chemical Dynamics Corporation), 0.31 ml of 2-methyl-2-butanol (Aldrich) and 39.5 ml of distilled water. The dosage in milliliters was determined by the formula: dosage = (0.1 + weight of the mother in grams/50). After deep anesthesia was achieved, the abdomen was swabbed with 70% ethanol and the uterus was surgically exposed. An incision was made in the wall of the uterus on the side opposite the uterine arteries. After the uterus was opened, the mothers were placed in a 37 o C hood, where all subsequent manipulations took place. Those embryos to be used for experiments in culture were removed and placed in dissecting medium consisting of 20% fetal bovine serum (Hyclone), 79% Dulbecco s modified Eagle s medium (DMEM, Whittaker s Bioproducts) and 1% penicillinstreptomyocin L-glutamine (GPS, Whittaker s Bioproducts) at 37 o C. These embryos were dissected to detach partially their extraembryonic membranes, but both the embryo and the extraembryonic membranes were left attached to the placenta for the entire culture period. Embryos that were allowed to develop in the mother (exo utero embryos) were left attached to the uterus with their extraembryonic membranes intact. After experimental manipulation of the
3 Cranial neural crest cell migration 299 embryo, the mother s abdominal cavity was flushed with lactated Ringer s solution (Travenol) to prevent the exo utero embryos from drying out or from adhering to other tissues (Muneoka et al., 1986). The mother then was surgically closed with 5-0 silk (Ethicon) and allowed to recover. After 1 to 5 days of additional development, the mother was again anesthetized, killed, and the embryos removed for analysis. Preparation of DiI for microinjection All injections were made with an 0.05% solution (w/v) of 1,1- dioctadecyl-3,3,3,3 -tetramethylindocarbocyanine perchlorate (DiI, Molecular Probes), made from an 0.5% stock solution in 100% ethanol, diluted immediately before injection 1:10 in 0.3 M sucrose solution. Micropipets were backfilled with DiI solution and attached to a picospritzer (General Valve). Both the dye and the micropipets were maintained at 37 o C during the injections to avoid cold shock to the embryo. Whole ectoderm labelling The ectoderm of exo utero embryos was labelled by inserting a micropipet through the decidua capsularis, yolk sac and the amnion into the amniotic cavity and expelling a small amount of dye (Fig. 1). To determine if either the injection or the exo utero technique had any adverse affects on embryonic development, the experimental embryos were compared with embryos that were allowed to develop in utero. On the basis of both gross morphology and somite number, embryos that developed exo utero were indistinguishable from embryos that developed in utero. Because the extraembryonic membranes were left intact throughout the injection procedure, it was not possible to visualize the embryos and assess their developmental stage at the time of the injection. The stage at which an embryo was injected was deduced by extrapolating back from the elapsed time and the stage at which the embryo was fixed. Because somite development in the mouse takes approximately hours per somite pair (Theiler, 1972), 12 to 18 additional somite pairs would be expected to form after an additional 24 hours of development. This estimate of the stage of labelling appeared accurate to within a range of six somites. Because this injection technique labels the neuroectoderm only in the regions of the neural tube that have not undergone neural tube fusion, it also was possible to deduce the stage of injection by observing which regions of the neural tube contained dye. Embryos labelled prior to the formation of the 7th somite (before the fusion of the neural tube begins) contained DiI label all along the neural tube. In embryos that were labelled after the formation of the 7th somite, DiI label was not present in the neural tube at the level of the 4th and 5th somites, where neural tube fusion begins. Embryos labelled after the formation of the 14th somite contained little or no DiI in the neural tube at the cranial levels. To determine the length of time after injection that the dye remained in the amniotic fluid, several embryos were labelled by injection of the dye into the amniotic cavity in vitro. In all cases, the dye was visible at the time of the injection. However, when the amniotic sac was torn open 4 to 5 minutes after the injection, the dye was no longer visible in the amniotic fluid. This suggests that labelling is rapid, occurring transiently only at the time of injection, rather than continuously throughout the experiment. Even if dye were to remain in the amniotic cavity, no artifactual labelling would result, as only the cells of the ectoderm and neuroectoderm are in contact with the amniotic fluid during the stages examined here. Although the mechanism responsible for the disappearance of the dye is unknown, two possible explanations are: (1) that the dye becomes sequestered into the cell membranes of the embryo and the extraembryonic structures and/or (2) that dye precipitates due to the salt content of the amniotic fluid. Fig. 1. Schematic diagram of the amniotic labelling technique. An incision is made in the wall of the uterus (U), which is deflected to allow access to the embryos. The micropipet is inserted through the decidua capsularis (DC), yolk sac (Y) and the amnion (A) into the amniotic cavity and a small amount of dye is expelled. The embryo (E) is labelled for orientation. Regional labelling of premigratory neural crest cells To label neural crest cells at specific cranial levels, a micropipet was inserted into the developing cranial neural folds of embryos in culture (using a micromanipulator; Marzhauser). When the pipet was positioned at the juncture of the neuroectoderm and the ectoderm, a small amount of the DiI solution was expelled. The fidelitiy of these injections was determined by examining the embryos both at the time of the injection and at the time of analysis. At the time of the injection, the labelled site was visible through the dissecting scope as a small red spot of dye in the tissue. At the time of fixation, the rostrocaudal extent of the dye within the neural tube was observed using an epifluorescence microscope. Only those embryos in which the DiI was contained within a single region (e.g., the midbrain) both at the time of injection and at the time of fixation were analyzed. Injections in the hindbrain were divided into two groups: (1) large injections, in which the dye spanned two or more rhombomeres; and (2) small injections, in which the dye was contained within a single rhombomere. Neural tube labelling In embryos that had undergone partial or complete neural fold fusion in the cranial region, the neural tube was labelled by inserting the micropipet into the lumen of the neural tube at the level of the midbrain using a micromanipulator. In most cases, enough dye was expelled to fill the neural tube. Embryo culture Embryos, with their extraembryonic membranes and placenta attached, were cultured for a total of 24 hours in tissue culturetreated cell wells (35 mm diameter; Corning) containing 4 ml of culture medium consisting of 25% rat serum, 25% fetal bovine serum, 49% DMEM and 1% GPS. The embryos were kept in an atmosphere of 5% CO 2 in air at 37 o C. After 12 hours, an additional 4 ml of culture medium were added to each well. Cultured embryos were compared with embryos allowed to develop to similar stages in utero to ascertain if the culture period itself affected embryonic maturation. On the basis of gross morphology and somite number, embryos cultured for 24 hours appeared similar to embryos developing in utero. Our results agree with previous studies that have shown that mouse embryos develop normally in culture for 24 hours after removal from the mother (New, 1973, 1977; Sadler, 1979; Sadler and New, 1981). Histology Embryos were fixed by immersion in 4% paraformaldehyde/0.25%
4 300 G. N. Serbedzija, M. Bronner-Fraser and S. E. Fraser glutaraldehyde in 0.1 M phosphate buffer (PB) for 3 to 6 hours at 4 o C. They were viewed in whole mount through a filter set designed for rhodamine using a laser scanning confocal microscope (Biorad MRC 600 on a Zeiss Axiovert microscope). This allowed the visualization of single focal planes without the interference of scattered light from outside the plane of focus (Fine et al., 1988). Embryos were prepared for cryostat sectioning by washing in 0.1 M PB for 1 hour, followed by soaking in a 15% sucrose solution for 8 to 12 hours at 4 o C. They were embedded in 15% sucrose and 7.5% gelatin (Oxiod), rapidly frozen in liquid nitrogen and serially sectioned on a cryostat at 30 microns (HM 500 M, Microm). Sections were coverslipped in gel/mount (Biomedia Corp.) and viewed on an epifluorescence microscope equipped with a light-intensifying camera (RCA-SIT) and an image processing system (Imaging Technologies Series 151), using the VidIm software package (S. E. Fraser, G. Belford and J. Stollberg, unpublished data). Results Amniotic injection of DiI Premigratory neural crest cells were labelled by injecting a solution of DiI into the amniotic cavity of exo utero embryos on E8. Because DiI partitions into the membranes of the cells it contacts, injections of DiI into the amniotic cavity at this stage labelled both the ectodermal and the neuroectodermal cells. During neurulation, the neural tube and the neural crest were internalized, leaving the ectoderm (including the placodes) on the surface of the embryo, until approximately E9, when the first placodes invaginate. As a result of this segregation, the only labelled cells present in the mesenchyme were of neural crest or placodal origin. Embryos were maintained in the mother for 1 to 5 days. At the end of this period, the exo utero embryos were removed from the mother for analysis. Table 1 summarizes the numbers of embryos analyzed and the distribution of the DiIlabelled cells at the level of the first branchial arch; Fig. 9A schematizes the distribution of labelled cells in embryos for each of the stages examined. The findings are presented below according to the stages at which the embryos were labelled with DiI. Table 1. Summary of embryos analyzed after whole ectoderm labelling Distribution of labelled cells at the level of the first Hours of Numbers of branchial arch Stage of additional embryos injection development analyzed DBA PBA TG 0 to 6 somite to 13 somite somite / DBA, distal portion of the branchial arch; PBA, proximal portion of the branchial arch; TG, trigeminal ganglia. Embryos injected during the 0- to 6-somite stage At the 6-somite stage, the neural folds have not fused, permitting unhampered access of the DiI to the entire neuroectoderm. Embryos labelled prior to the end of the 6- somite stage and allowed to develop for 4 hours contained DiI-labelled cells in the ectoderm and neuroectoderm exclusively (Fig. 2). This confirmed that the technique labels only those cells in contact with the amniotic fluid. In embryos that developed for 24 hours after the injection, DiI-labelled cells were observed in the cranial mesenchyme and neuroectoderm in regionally distinct patterns (Fig. 3A). At the level of the forebrain, the DiI-labelled cells formed a sheet that extended ventrally from the dorsal portion of the neural tube over the region of the prosencephalon, including the optic vesicle. Transverse sections through the level of the optic vesicle revealed that these DiI-labelled cells were located in the mesoderm between the developing eye and the diencephalon (Fig. 3C). In the midbrain region, the DiIlabelled cells were dispersed individually throughout the mesoderm adjacent to both the ectoderm and the mesencephalon (similar to the pattern shown in Fig. 8B). At the level of the hindbrain, DiI-labelled cells were observed in three sub-ectodermal streams that extended from the fusion point of the neural tube to the distal end of the developing first, second and third branchial arches (Fig. 3B). Embryos allowed to develop for 48 hours after injection contained DiI-labelled cells throughout the cranial mesenchyme at the levels of the forebrain, midbrain and hindbrain (Fig. 4A), in a pattern similar to that observed 24 hours after injection of DiI. In the forebrain region, DiIlabelled cells were located between the nasal pit and the eye. At the level of the midbrain, DiI-labelled cells were distributed individually throughout the mesenchyme adjacent to the mesencephalon. At the rostral hindbrain level, DiI-labelled cells were located in the region of the developing trigeminal ganglia and in the first branchial arch (Fig. 4B). At the level of the caudal hindbrain, DiI-labelled cells were observed in the second and third branchial arches. In transverse sections taken at the level of the otic vesicle, DiI-labelled cells were present in the developing nodose and petrosal ganglia. Embryos injected during the 7- to 13-somite stage After the formation of somite 7, neural tube fusion begins at the level of somites 4 and 5 from where it progresses both rostrally and caudally. Embryos analyzed 24 hours after labelling contained DiI-labelled cells at fore- and midbrain levels in many of the same locations described for embryos injected at earlier stages (Fig. 5A). The level of the caudal hindbrain contained few, if any, DiI-labelled cells, probably because the neural tube had fused there prior to the injection. At the level of the rostral hindbrain, the streams of DiI-labelled cells extended to, but not into, the first branchial arch. Only a few DiI-labelled cells were observed in the branchial arches in any of the embryos injected at this stage. In cross-section, DiI-labelled cells formed a subectodermal stream extending from the dorsal portion of the neural tube but stopping short at the level of the forming trigeminal ganglia (Fig. 5B). Forty-eight hours after the injection of DiI, the distribution of DiI-labelled cells was similar to that observed in embryos analyzed after 24 hours. Individual DiI-labelled cells were located in the forebrain and the midbrain regions,
5
6 Cranial neural crest cell migration 301 Fig. 4. Embryos labelled by amniotic injection of DiI at the 0- to 6-somite stage and maintained exo utero for 48 hours. (A) In this fluorescent confocal image, DiI-labelled cells were located between the nasal pit (N) and the eye (E). At the level of the midbrain, individual DiI-labelled cells were observed throughout the mesenchyme adjacent to the mesencephalon. At the level of the rostral hindbrain, DiI-labelled cells (arrow) were observed in the region of the developing trigeminal ganglia (T) and the first branchial arch (1), as well as in the first and second branchial arches (1 and 2). (B) In transverse sections through the level of myelencephalon (MY), DiIlabelled cells were observed within the developing trigeminal ganglia and in the first branchial arch (arrow). Bars: A, 375 μm; B, 50 μm. including the mesenchyme adjacent to the nasal pit, around the eye and overlying the mesencephalon. At the level of the rostral hindbrain, DiI-labelled cells were located at the level of the trigeminal ganglia but not in the branchial arches (Fig. 5C). Embryos labelled at 14- or more somites Neural tube fusion in the cranial region is completed at approximately the 16-somite stage. Embryos labelled after the formation of somite 14 and allowed to develop for 24 or 48 hours contained only a few DiI-labelled cells in the cranial mesenchyme. These few cells were adjacent to the dorsal portion of the neural tube. One embryo, labelled after the fusion of the neural tube was complete, contained a few DiI-labelled cells in the trigeminal ganglia. These DiIlabelled cells probably were of placodal origin since the neural crest cells are not labelled by amniotic injection after the fusion of the neural tube. The labelled processes of these trigeminal ganglia cells could be seen extending from the ganglia into the first branchial arch (Fig. 5D). Long-term survival of embryos injected at E8 In order to identify the final destinations of the cranial neural crest cells, embryos were labelled by amniotic injection of DiI on E8 after which they were allowed to develop for 3 to 5 days before analysis. All of the somites are formed by embryonic day 11; therefore, it was not possible to deduce the exact stage at which these embryos were labelled on the basis of the exact number of somites present at the time of fixation. In addition, due to the size of the embryos and the increased opacity of their tissues, it was not possible to analyze the embryos in whole mount after embryonic day 11. Therefore, all observation were made on sectioned material. In all of the 22 embryos that were allowed to develop for 3 or more days after labelling, large numbers of DiIlabelled cells were observed in the trigeminal, the superior, the jugular, the nodose and the petrosal ganglia (Fig. 6 ). DiI-labelled cells also were observed in several neural tubederived structures, including the olfactory bulb and the optic stalk. Unlike the neural derivatives, the mesenchymal derivatives of the neural crest contained only a few detectable DiI-labelled cells in embryos examined at E11 and no detectable DiI-labelled cells at later stages. This probably was due to dilution of the dye by the extensive cell division in the mesenchymal derivatives. Regional labelling In order to confirm the above results and to examine axial differences in the migration of cranial neural crest cells, subsets of the neural crest were labelled by focally injecting small amounts of DiI into the juncture of the neuroectoderm and the ectoderm. To permit adequate visualization and control of the injection site, these injections were performed in culture. In addition, this allowed the exact stage of the embryo at the time of injection to be determined. Two types of injections were made: (1) larger injections, which labelled most or all of a particular region (e.g. the entire midbrain or forebrain) and (2) smaller injections, which labelled relatively few cells and were contained within a specific region (e.g. in the hindbrain injection sites were contained within a single rhombomere). By virtue of their position, these injections labelled both the neural crest cell precursors within the neural fold and the newly emerg-
7 302 G. N. Serbedzija, M. Bronner-Fraser and S. E. Fraser Table 2. Summary of embryos analyzed 24 hours after regional or neural tube labelling Number of Migrating Stage at time embryos cells of injection analyzed observed Forebrain labelling 0 to 5 somite to 10 somite to 15 somite somite 8 +/ Midbrain labelling 0 to 5 somite to 10 somite to 15 somite 9 +/ 16+ somite 3 Rostral hindbrain 0 to 5 somite 7 + labelling 6 to 10 somite to 15 somite somite 4 Caudal hindbrain 0 to 5 somite 5 + labelling 6 to 10 somite to 15 somite 10 +/ 16+ somite 6 Fig. 5. Embryos labelled by amniotic injection of DiI at the 7- to 13-somite stage and maintained exo utero for 24 and 48 hours. (A) After 24 hours, embryos contained a sheet of DiI-labelled cells extending temporally from the dorsal portion of the neural tube in the forebrain, over the prosencephalon (P) toward the developing eye (E). In the midbrain region, DiI-labelled cells were dispersed throughout the mesenchyme overlying the mesencephalon. In the hindbrain, DiI-labelled cells were present in the dorsal portion of the branchial arches (1 and 2), in the region of the presumptive trigeminal ganglion (arrow), and in the region overlying the mesencephalon. (B) A transverse section through the level of the first branchial arch and the myelencephalon (MY) contains DiIlabelled cells in the developing trigeminal ganglia (arrows). (C) Embryos that developed for 48 hours after the injection contained DiI-labelled cells in the mesenchyme adjacent to the nasal pit (N), around the eye (E) and overlying the mesencephalon. At the level of the rostral hindbrain, DiI-labelled cells were located in the trigeminal ganglia (T) and the dorsal portion of the branchial arches (1 and 2). (D) An embryo labelled by amniotic injection of DiI at the 14+ somite stage after fusion of the neural tube was completed up to the level of the midbrain, contained DiI-labelled cells in the trigeminal ganglia and DiI-labelled processes (arrow) extending from the trigeminal ganglion (T) into the first branchial arch. The eye (E), the trigeminal ganglion (T), the nasal pit (N), the prosencephalon (P), the myelencephalon (MY) and the first and second branchial arches (1 and 2) are indicated for orientation. Bars: A, 100 μm; B, 50 μm; C, D, 200 μm. ing neural crest cells. Following injection, embryos were cultured for 24 hours before analysis. Table 2 summarizes the number of embryos analyzed and the presence of labelled cranial neural crest cells outside the neural tube; from these data, the stages when cells emigrate from the different axial levels of the neural crest were deduced (Fig. 9B). Table 3 summarizes the number of embryos that recieved injections into individual rhombomeres. Embryos injected at the 0- to 5-somite stage Before the 5-somite stage, focal injections of DiI at any cranial level resulted in labelled cells in the cranial mesenchyme. With injections at the level of the forebrain, DiIlabelled cells were found a short distance from the neural tube toward the developing eye. Injections into the midbrain gave rise to dispersed labelled cells in the mesenchyme overlying the mesencephalon (Fig. 7A). Injections into the rostral hindbrain produced a stream of cells extending from the neural tube toward the developing first branchial arch (Fig. 7B). At the level of the caudal hindbrain, injections gave rise to a stream of labelled cells that extended into the second or the third branchial arch. For example, injections slightly caudal to the level of the otic vesicle gave rise to a stream of labelled cells extending into the third branchial arch (Fig. 7C). The distribution of labelled cells in each region was similar to that observed in embryos labelled by amniotic injection at comparable stages. Embryos injected at the 6- to 10-somite stage All focal injections at this stage produced DiI-labelled cells that migrated away from the site of the injection. Injections at the level of the forebrain gave rise to DiI-labelled cells in the region of the developing eye. Embryos labelled at Table 3. Summary of embryos analyzed after individual rhombomere labelling Stage of injection r1 r2 r3 r4 r5 r6 r7 r8 6 to NA somite 11 to 15 NA somite r, rhombomere.
8 Fig. 6. Fig. 7. Fig. 8.
9 Cranial neural crest cell migration 303 Fig. 9. (A) Schematic representation of the location of DiI-labelled cells after amniotic injection of DiI into progressively older embryos. Light gray represents the distribution of DiI-labelled cells in embryos injected at early stages of migration (0- to 6-somite stage). At the level of the forebrain, a sheet of DiI-labelled cells is observed that extends ventrally from the dorsal portion of the neural tube to encompass the developing eye (E). At the level of the midbrain, individual DiI-labelled cells are dispersed over the mesencephalon (M). At the level of the rostral hindbrain, DiI-labelled cells are observed in a stream, which extended from the fusion point of the neural tube to the distal end of the developing first branchial arch (1). In the caudal region of the hindbrain, a stream of DiI-labelled cells is observed extending into the second arch (2). Embryos injected at slightly later stages (7- to 10-somite stage) contain DiI-labelled cells in similar locations; however, the cells do not extend as far ventrally (medium gray). Embryos labelled at late stages of migration (11- to 16-somite stage) contain DiI-labelled cells only along the dorsal portion of the head (dark gray). Embryos labelled after cranial neural crest cell emigration is complete contain DiI-labelled cells only in the neural tube (black). (B) Schematic representation of the pattern in which neural crest cells stop emigrating from the neural tube. By the 11-somite stage, DiI-labelled cells no longer emigrate from the region of the rostral midbrain (black). DiI-labelled cells in the midbrain and caudal hindbrain stop emigrating from the neuroectoderm by the 14- somite stage (dark gray). In the forebrain, emigration of DiI-labelled cells ends at the 16-somite stage (light gray). The prosencephalon (P), mesencephalon (M), eye (E), otic vesicle (OV), trigeminal ganglion (T), first (1) and second (2) branchial arches and heart (H) are labelled for orientation. the level of the midbrain contained dye-labelled cells in the mesenchyme between the mesencephalon and the ectoderm (Fig. 8A and B). Focal injections into the region of the neural tube which will develop into the 1st or 2nd rhombomeres (r1 or r2) produced a stream of DiI-labelled cells that extended from the site of the injection to the level of the first branchial arch. Injections into r3, r4 or r5 gave rise to labelled cells extending from the dorsal portion of the neural tube into the second branchial arch (Fig. 8C). Injections into r6, r7 or r8 gave rise to a stream of cells extending from the injection site toward the third, and possibly the fourth branchial arch. Embryos injected at the 11- to 15-somite stage Before the 14-somite stage, injections at the level of the forebrain, midbrain or r2-8 gave similar patterns of DiIlabelled cells to those described above. In contrast, embryos injected at the level of rhombomere 1 contained no DiIlabelled cells outside the injection site (Fig. 8D), suggesting that all of the neural crest cells at the level of the first rhombomere had completed emigration by the 10-somite stage. After the 14-somite stage, DiI injections at the level of the midbrain, or r2-8 no longer gave rise to labelled cells outside the injection site, suggesting that emigration from these axial levels was completed by the 14-somite stage. In contrast, at the level of the forebrain, emigration continued through the 14- and 15-somite stage. Embryos injected at the 16+ somite stage Embryos that were labelled at the levels of the forebrain, midbrain or hindbrain after the formation of somite 16 contained no DiI-labelled cells outside the site of injection. Neural tube labelling At approximately the 16-somite stage, neural tube fusion is complete, or nearly complete, in the head of the mouse. Therefore, it was possible to label the cells of the neural tube, including any premigratory neural crest cells, by injecting DiI into the lumen of the neural tube of embryos in culture (Serbedzija et al., 1990). Embryos were labelled by intraluminal injection of DiI and then cultured for 24 hours before analysis. The neural tube at the level of the forebrain, midbrain and hindbrain contained DiI-labelled cells. However, only those embryos that were labelled prior to the formation of somite 16 contained DiI-labelled cells outside the neural tube and these were found only at the level of the forebrain. Discussion DiI labelling of premigratory and migratory neural crest cells in exo utero and cultured mouse embryos provided a direct assay of the pattern and timing of cranial neural crest cell migration. Three distinct patterns of cranial neural crest
10 304 G. N. Serbedzija, M. Bronner-Fraser and S. E. Fraser cell migration were observed. Neural crest cells from the level of the forebrain migrated ventrally through the mesenchyme in a sheet extending from the dorsal portion of the neural tube to the level of the optic vesicle and populated the mesenchyme around the eye. In contrast, neural crest cells at the level of the midbrain appeared to migrate ventrolaterally as dispersed cells through the mesenchyme between the lateral surface of the mesencephalon and the ectoderm. These cells populated the region overlying the mesencephalon. At the level of the hindbrain, neural crest cells migrated ventrolaterally along three segmentally distributed subectodermal streams, from the dorsal portion of the neural tube to the distal portion of the first, second and third branchial arches. In addition to the branchial arches, these cells populated the trigeminal, superior, jugular, petrosal and nodose ganglia. The order in which neural crest cells populated their derivatives was determined by labelling embryos at different stages of development. Injection of DiI into the amniotic cavity labelled only cells that are continuous with the
11 Cranial neural crest cell migration 305 Fig. 10. Schematic representations showing the pathways of neural crest cell migration in the mouse, chick and rat embryos. Because the labelling and the data analysis techniques varied for each of the species represented here, a small amount of artistic license was used for the sake of comparison. Light gray represents neural crest cells that migrate through the mesenchyme. Dark gray represents neural crest cells that migrate along subectodermal pathways. (A-C) Whole embryo view showing the origins and overall pathways of neural crest cell migration. (A) In the mouse embryo there are three regionally distinct pathways of neural crest cell migration. Neural crest cells from the level of the forebrain migrate ventrally in a sheet extending from the dorsal portion of the neural tube to the level of the eye (E). Neural crest cells from the level of the midbrain migrate ventrolaterally toward the maxillary process and the eye (E). At the level of the hindbrain, neural crest cells migrate ventrolaterally in three streams, from the dorsal portion of the neural tube to the distal portion of the first (1), second (2) or third (3) branchial arches. (B) In the chick embryo, only the midbrain and hindbrain give rise to cranial neural crest cells. Neural crest cells from the level of the midbrain migrate ventrolaterally toward the maxillary process and the eye (E) and rostrally toward the nasal process (N). At the level of the hindbrain, neural crest cells migrate ventrolaterally in three streams, from the dorsal portion of the neural tube to the distal portion of the first (1), second (2) or third (3) branchial arches. (C) In the rat, migratory neural crest cells arise at the level of the midbrain and hindbrain. However, it is not clear whether or not migratory neural crest cells arise from the level of the forebrain. At the level of the midbrain, neural crest cells migrate ventrolaterally toward the maxillary process and the eye (E). Neural crest cells from the hindbrain migrate ventrolaterally in three streams extending from the dorsal portion of the neural tube to the distal portion of the first (1), second (2) or third (3) branchial arches. (D-F) A cross-sectional view through the level of the eye. (D) In the mouse, neural crest cells from both the midbrain and the forebrain migrate through the mesoderm. In addition, neural crest cells from the forebrain migrate between the eye (E) and the forebrain (F). (E) In the chick, neural crest cells migrate along a subectodermal pathway from the dorsal portion of the midbrain neural tube (M) to the eye (E). (F) In the rat, neural crest cells migrate through the mesenchyme from the dorsal portion of the midbrain neural tube (M) toward the eye (E). (G-I) A cross-sectional view through the level of the first branchial arch. (G and H) In the mouse and the chick, neural crest cells migrate along a subectodermal pathway that extends from the neural tube into the first branchial arch. (I) in the rat, neural crest cells migrate through the mesenchyme as dispersed cells from the neural tube into the first branchial arch. The forebrain (F), midbrain (M), rostral hindbrain (RH), eye (E), notocord (N), otic vesicle (OV), trigeminal ganglion (T), first (1), second (2), third (3) branchial arches and heart (H) are labelled for orientation. neuroectoderm and migratory neural crest cells are left unlabelled; making it possible to mark temporally distinct subsets of the neural crest cells. Because of the dramatic changes in the anatomy of the head during these stages of development, the order in which cranial neural crest cells populated their derivatives was best illustrated at the level of the rostral hindbrain. Embryos labelled during the earliest stages of neural crest cell migration contained DiIlabelled cells throughout the first branchial arch, as well as in the region of the trigeminal ganglia (Fig. 9B, light gray). In contrast, embryos labelled at slightly later stages contained DiI-labelled cells at the level of the trigeminal ganglia, but not in the first branchial arch (Fig. 9B, medium gray). Embryos labelled at still later stages contained DiIlabelled cells adjacent to the dorsal portion of the neural tube, but not at the level of the trigeminal ganglia or in the first branchial arch (Fig. 9B, dark gray). Therefore, cranial neural crest cells populated their derivatives in a ventralto-dorsal order, similar to that observed for neural crest cells in the trunk (Serbedzija et al., 1990). Previous studies, using either ultrastructural characteristics (Nichols, 1981, 1986) or topical wheat germ agglutinin labelling (Chan and Tam, 1988) to identify neural crest cells, indicated that neural crest cell migration begins at approximately the 5-somite stage in the rostral hindbrain (Nichols, 1981, 1986; Chan and Tam, 1988). Emigration follows in the midbrain, the caudal hindbrain and finally the forebrain (Nichols, 1981). These previous studies also suggested that neural crest cell emigration at the rostral hindbrain level is complete by the 10-somite stage. By labelling premigratory neural crest cells with DiI at progressively later stages of development, we were able to make a direct determination of the stages at which neural crest cells cease emigrating from the neural folds not only at the level of the rostral hindbrain but also at each of the other axial levels (Fig. 9B). Focal injections of DiI into the juncture of the neuroectoderm and the ectoderm labelled both the neural crest cell precursors within the neural fold and the newly emerging neural crest cells. In the most rostral hindbrain (r1), neural crest cells ceased to emigrate from the neural folds by the 11-somite stage. In the midbrain and caudal hindbrain regions, neural crest cell emigration from the neural folds ended by the 14-somite stage. In the forebrain, however, neural crest cell emigration from the neural folds continued until the 16-somite stage. Because the DiI-labelling technique marks both emigrating and soon-to-emigrate neural crest cells, it is difficult to determine exactly the time of initiation of neural crest emigration by this method. However, the previous ultrastructural studies (Nichols, 1981, 1986) were well-suited to determining the stage of neural crest emigration, and generated results consistent with our own. By combining the data from these various approaches, it was possible to deduce the duration of neural crest cell emigration at each axial level. Neural crest cell emigration from the rostral hindbrain begins at the 5-somite stage and ends by the 10- somite stage, approximately 9 hours later. At other axial levels, the timing of initiation of migration and cessation of emigration paralleled one another, suggesting that the duration of cranial neural crest cell emigration at all axial levels was approximately 9 to 12 hours. The final destinations of the DiI-labelled cranial neural crest cells were examined by allowing some of the embryos labelled by amniotic injection on E8 to develop for an additional 3 to 5 days. DiI-labelled cells were found in all of the neuronal derivatives of the cranial neural crest, including the trigeminal, the superior, the jugular, the nodose and the petrosal ganglia. Surprisingly, no DiI-labelled cells were detected in any of the mesenchymal derivatives after E11, even though DiI-labelled cells were observed in the mesenchymal derivatives of embryos examined 1 to 2 days after the injection. Because a fixed amount of DiI was injected, the dye could have been diluted by extensive cell division.
12 306 G. N. Serbedzija, M. Bronner-Fraser and S. E. Fraser Consistent with this possibility, cranial ganglion cells in avians do not undergo extensive division (see review by Le Douarin, 1982), while the cells that contribute to the mesenchymal derivatives of the cranial neural crest proliferate extensively (Noden, 1988). Cranial neural crest cells in the region of the hindbrain migrate in a segmented pattern that corresponds to the location of the branchial arches. This pattern consists of three separate streams of cells, lateral to r2, r4 and r6, each of which extends from the neural tube into the adjacent branchial arch. The high degree of segmentation in the hindbrain that becomes apparent at later stages might suggest that the formation of the neural crest also may be segmented, directly contributing to this pattern. For example, the streams at r2, r4 and r6 might be created by the failure of other axial levels of the neural tube (e.g. r3 and r5) to give rise to migrating neural crest cells, similar to the pattern recently reported to occur in avians (Lumsden et al., 1991). However, injections of DiI into the mouse neural crest at each level of the hindbrain, from rhombomere 1 through 8, gives rise to labelled cells outside the neural tube. In addition, recent experiments suggest that there may be a small, but significant contribution of neural crest cells from r3 and a large contribution from r5 in the avian embryo as well (J. Sechrist, G. N. Serbedzija, S. E. Fraser, T. Scherson and T. Bonner-Fraser, unpublished data). This casts doubt on the simple hypothesis that segmented migration reflects segmental origin and suggests that the segmented pattern of mouse neural crest cell migration at the level of the hindbrain may be imposed by factors extrinsic to the neural tube, as is the case for the relationship between trunk neural crest cells and the somitic mesenchyme (Bronner-Fraser and Stern, 1991). Although the DiI-labelling technique permits defined groups of cells to be marked, it does have some limitations. First, because the intra-amniotic injection labels the whole ectoderm, the ectodermal placodes also are labelled. Because the placodes contribute to the cranial ganglia, the possibility exists that some of the labelled cells found in the mesenchyme are of placodal rather than neural crest origin (see Fig. 5D). Second, this technique does not permit the analysis of cranial neural crest cell emigration from regions where the neural tube has fused. To minimize these potential pitfalls and to confirm the results, embryos in culture were labelled by small injections of DiI into specific axial levels of the neural tube. The results from this regional labelling are consistent with the results obtained with the whole ectoderm labelling, suggesting that the whole ectoderm labelling approach gives a realistic view of neural crest cell migration in the mouse embryo. The pattern described here for mouse cranial neural crest cell migration shares some, but not all, characteristics with the patterns described for both chick and rat neural crest cell migration. At the level of the hindbrain, mouse and chicken cranial neural crest cells migrate ventrolaterally along three subectodermal streams that extend from the neural tube into each branchial arch (Fig.10A, B, G and H; Chan and Tam, 1988; Lumsden et al., 1991). In the rat, neural crest cells from the level of the hindbrain appear to migrate through the mesenchyme as dispersed cells (Fig. 10C and I; Tan and Morriss-Kay, 1986). At the midbrain level, neural crest cells of the mouse and rat migrate through the mesenchyme (Fig. 10A, C, D and F), while chick neural crest cells migrate along more superficial subectodermal pathways (Fig. 10B and E; Le Douarin, 1982; Tan and Morriss-Kay, 1986; Noden, 1988). At the level of the forebrain, we find that mouse embryos do have emigrating neural crest cells in support of previous ultrastructural results (Fig. 10 A and D; Nichols, 1986); in contrast, neither chick nor rat embryos appear to have a contribution from the forbrain to the cranial neural crest cells (Fig. 10B, C, E and F; Johnston, 1966; Noden, 1975; Tan and Morriss-Kay, 1985). Some of these apparent differences may be due to the different labelling techniques and analytical approaches used to study neural crest cell migration in each of the three species. Further comparative analyses of apparent similarities and differences of neural crest cell behavior in these three species may provide insights into the mechanisms that control the development and migration of cranial neural crest cells. In summary, by labelling premigratory cranial neural crest cells with DiI, the patterns and timing of cranial neural crest cell migration in the mouse were determined. Our observations confirm and extend the observations of both ultrastructural (Nichols, 1981, 1986) and topical labelling studies (Chan and Tam, 1988). Because our approach permitted the labelling of embryos in vivo rather than in culture, it circumvents many of the potential difficulties encountered in previous studies (Nichols, 1981, 1986; Chan and Tam, 1988) and allows the analysis of neural crest cell migration over much more extended periods of development than previously possible. The use of DiI-labelling in intact embryos has permitted, for the first time, direct analyses of the migration pathways, the duration of emigration and the order of contribution to derivatives of the mouse cranial neural crest. By defining the normal pattern of cranial neural crest cell migration, the results presented here provide a solid foundation for further experimental analysis of the factors affecting both neural crest cell migration and differentiation. This approach does not require specific mouse strains; furthermore, it can be applied to a variety of species. Future experiments using DiI-labelling to define the spatial and temporal aspects of neural crest migration in genetically manipulated embryos should permit decisive test experiments and provide new insights about the mechanisms of neural crest cell migration and differentiation in higher vertebrates. References Auerbach, R. (1954). Analysis of the developmental effects of a lethal mutation in the house mouse. J. Exp. Zool. 127, Bronner-Fraser, M. and Stern, C. (1991). Effects of mesodermal tissue on avian neural crest cell migration. Devl. Biol. 143, Chan, W. Y. and Tam, P. P. L. (1988). A morphological and experimental study of the mesencephalic neural crest cells in the mouse embryo using wheat germ agglutinin-gold conjugates as the cell marker. Development 102, Fine, A., Amos, W. B., Durbin, R. M. and McNaughton, P. A. (1988).
In ovo time-lapse analysis after dorsal neural tube ablation shows rerouting of chick hindbrain neural crest
 In ovo time-lapse analysis after dorsal neural tube ablation shows rerouting of chick hindbrain neural crest Paul Kulesa, Marianne Bronner-Fraser and Scott Fraser (2000) Presented by Diandra Lucia Background
In ovo time-lapse analysis after dorsal neural tube ablation shows rerouting of chick hindbrain neural crest Paul Kulesa, Marianne Bronner-Fraser and Scott Fraser (2000) Presented by Diandra Lucia Background
Developmental potential of trunk neural crest cells in the mouse
 Development 120, 1709-1718 (1994) Printed in Great Britain The Company of Biologists Limited 1994 1709 Developmental potential of trunk neural crest cells in the mouse George N. Serbedzija 1,, Marianne
Development 120, 1709-1718 (1994) Printed in Great Britain The Company of Biologists Limited 1994 1709 Developmental potential of trunk neural crest cells in the mouse George N. Serbedzija 1,, Marianne
Relationship between spatially restricted Krox-20 gene expression in branchial neural crest and
 The EMBO Journal vol.14 no.8 pp.1697-1710, 1995 Relationship between spatially restricted Krox-20 gene expression in branchial neural crest and segmentation in the chick embryo hindbrain M.Angela Nieto1
The EMBO Journal vol.14 no.8 pp.1697-1710, 1995 Relationship between spatially restricted Krox-20 gene expression in branchial neural crest and segmentation in the chick embryo hindbrain M.Angela Nieto1
Rhombomeric origin and rostrocaudal reassortment of neural crest cells revealed by intravital microscopy
 Development 121, 935-945 (1995) Printed in Great Britain The Company of Biologists Limited 1995 935 Rhombomeric origin and rostrocaudal reassortment of neural crest cells revealed by intravital microscopy
Development 121, 935-945 (1995) Printed in Great Britain The Company of Biologists Limited 1995 935 Rhombomeric origin and rostrocaudal reassortment of neural crest cells revealed by intravital microscopy
Developmental Zoology. Ectodermal derivatives (ZOO ) Developmental Stages. Developmental Stages
 Developmental Zoology (ZOO 228.1.0) Ectodermal derivatives 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis
Developmental Zoology (ZOO 228.1.0) Ectodermal derivatives 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis
Vital dye labelling demonstrates a sacral neural crest contribution to the enteric nervous system of chick and mouse embryos
 Development 111, 857-866 (1991) Printed in Great Britain The Company of Biologists Limited 1991 857 Vital dye labelling demonstrates a sacral neural crest contribution to the enteric nervous system of
Development 111, 857-866 (1991) Printed in Great Britain The Company of Biologists Limited 1991 857 Vital dye labelling demonstrates a sacral neural crest contribution to the enteric nervous system of
Analysis of cranial neural crest migratory pathways in axolotl using cell markers and transplantation
 Development 127, 2751-2761 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV4304 2751 Analysis of cranial neural crest migratory pathways in axolotl using cell markers and transplantation
Development 127, 2751-2761 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV4304 2751 Analysis of cranial neural crest migratory pathways in axolotl using cell markers and transplantation
Paul A. Trainor and Patrick P. L. Tam* SUMMARY
 Development 121, 2569-2582 (1995) Printed in Great Britain The Company of Biologists Limited 1995 2569 Cranial paraxial mesoderm and neural crest cells of the mouse embryo: co-distribution in the craniofacial
Development 121, 2569-2582 (1995) Printed in Great Britain The Company of Biologists Limited 1995 2569 Cranial paraxial mesoderm and neural crest cells of the mouse embryo: co-distribution in the craniofacial
Is there a ventral neural ridge in chick embryos? Implications for the origin of adenohypophyseal and other APUD cells
 /. Embryol. exp. Morph. Vol. 57, pp. 71-78, 1980 7 \ Printed in Great Britain Company of Biologists Limited 1980 Is there a ventral neural ridge in chick embryos? Implications for the origin of adenohypophyseal
/. Embryol. exp. Morph. Vol. 57, pp. 71-78, 1980 7 \ Printed in Great Britain Company of Biologists Limited 1980 Is there a ventral neural ridge in chick embryos? Implications for the origin of adenohypophyseal
A distinct development programme for the cranial paraxial mesoderm
 A distinct development programme for the cranial paraxial mesoderm Article (Published Version) Hacker, A and Guthrie, S (1998) A distinct development programme for the cranial paraxial mesoderm. Development
A distinct development programme for the cranial paraxial mesoderm Article (Published Version) Hacker, A and Guthrie, S (1998) A distinct development programme for the cranial paraxial mesoderm. Development
Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis
 Research article 1979 Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis Peter Y. Lwigale 1, Gary W. Conrad 2 and Marianne Bronner-Fraser 1, * 1
Research article 1979 Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis Peter Y. Lwigale 1, Gary W. Conrad 2 and Marianne Bronner-Fraser 1, * 1
Introduction to Embryology. He who sees things grow from the beginning will have the finest view of them.
 He who sees things grow from the beginning will have the finest view of them. Aristotle 384 322 B.C. Introduction to Embryology This lecture will introduce you to the science of developmental biology or
He who sees things grow from the beginning will have the finest view of them. Aristotle 384 322 B.C. Introduction to Embryology This lecture will introduce you to the science of developmental biology or
Expression Patterns of Engrailed-Like Proteins in the Chick Embryo
 DEVELOPMENTAL DYNAMICS 193370-388 (1992) Expression Patterns of Engrailed-Like Proteins in the Chick Embryo CHARLES A. GARDNER AND KATE F. BARALD Department of Anatomy and Cell Biology, University of Michigan
DEVELOPMENTAL DYNAMICS 193370-388 (1992) Expression Patterns of Engrailed-Like Proteins in the Chick Embryo CHARLES A. GARDNER AND KATE F. BARALD Department of Anatomy and Cell Biology, University of Michigan
Questions in developmental biology. Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration
 Questions in developmental biology Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration Representative cell types of a vertebrate zygote => embryo => adult differentiation
Questions in developmental biology Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration Representative cell types of a vertebrate zygote => embryo => adult differentiation
Biology 224 Human Anatomy and Physiology - II Week 1; Lecture 1; Monday Dr. Stuart S. Sumida. Review of Early Development of Humans.
 Biology 224 Human Anatomy and Physiology - II Week 1; Lecture 1; Monday Dr. Stuart S. Sumida Review of Early Development of Humans Special Senses Review: Historical and Developmental Perspectives Ontogeny
Biology 224 Human Anatomy and Physiology - II Week 1; Lecture 1; Monday Dr. Stuart S. Sumida Review of Early Development of Humans Special Senses Review: Historical and Developmental Perspectives Ontogeny
Conclusions. The experimental studies presented in this thesis provide the first molecular insights
 C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
Inhibition of cranial neural crest cell development by vitamin A in the cultured chick embryo
 /. Embryol. exp. Morph. Vol. 39, pp. 267-27J, 1977 267 Printed in Great Britain Inhibition of cranial neural crest cell development by vitamin A in the cultured chick embryo JOHN R. HASSELL, 1 JUDITH H.
/. Embryol. exp. Morph. Vol. 39, pp. 267-27J, 1977 267 Printed in Great Britain Inhibition of cranial neural crest cell development by vitamin A in the cultured chick embryo JOHN R. HASSELL, 1 JUDITH H.
The growth rate of sensory nerve fibres in the mammalian embryo
 Development 00, 307-3 (987) Printed in Great Britain The Company of Biologists Limited 987 307 The growth rate of sensory nerve fibres in the mammalian embryo ALUN M. DAVIES Department of Anatomy, Si George's
Development 00, 307-3 (987) Printed in Great Britain The Company of Biologists Limited 987 307 The growth rate of sensory nerve fibres in the mammalian embryo ALUN M. DAVIES Department of Anatomy, Si George's
Head and Face Development
 Head and Face Development Resources: http://php.med.unsw.edu.au/embryology/ Larsen s Human Embryology The Developing Human: Clinically Oriented Embryology Dr Annemiek Beverdam School of Medical Sciences,
Head and Face Development Resources: http://php.med.unsw.edu.au/embryology/ Larsen s Human Embryology The Developing Human: Clinically Oriented Embryology Dr Annemiek Beverdam School of Medical Sciences,
HHS Public Access Author manuscript Differentiation. Author manuscript; available in PMC 2016 July 22.
 Stage-dependent plasticity of the anterior neural folds to form olfactory placode versus neural crest Maxellende Ezin, Meyer Barembaum, and Marianne E. Bronner Division of Biology and Biological Engineering,
Stage-dependent plasticity of the anterior neural folds to form olfactory placode versus neural crest Maxellende Ezin, Meyer Barembaum, and Marianne E. Bronner Division of Biology and Biological Engineering,
Notochord grafts do not suppress formation of neural crest cells or commissural neurons
 Development 116, 877-886 (1992) Printed in Great Britain The Company of Biologists Limited 1992 877 Notochord grafts do not suppress formation of neural crest cells or commissural neurons KRISTIN B. ARTINGER
Development 116, 877-886 (1992) Printed in Great Britain The Company of Biologists Limited 1992 877 Notochord grafts do not suppress formation of neural crest cells or commissural neurons KRISTIN B. ARTINGER
A study on the pattern of prospective somites in the presomitic mesoderm of mouse embryos
 /. Embryol. exp. Morph. 9, 9-8 (98) 9 Printed in Great Britain The Company of Biologists Limited 98 A study on the pattern of prospective somites in the presomitic mesoderm of mouse embryos p. P. L. TAM
/. Embryol. exp. Morph. 9, 9-8 (98) 9 Printed in Great Britain The Company of Biologists Limited 98 A study on the pattern of prospective somites in the presomitic mesoderm of mouse embryos p. P. L. TAM
Cranial paraxial mesoderm: regionalisation of cell fate and impact on craniofacial development in mouse embryos
 Development 120, 2397-2408 (1994) Printed in Great Britain The Company of Biologists Limited 1994 2397 Cranial paraxial mesoderm: regionalisation of cell fate and impact on craniofacial development in
Development 120, 2397-2408 (1994) Printed in Great Britain The Company of Biologists Limited 1994 2397 Cranial paraxial mesoderm: regionalisation of cell fate and impact on craniofacial development in
Role of Organizer Chages in Late Frog Embryos
 Ectoderm Germ Layer Frog Fate Map Frog Fate Map Role of Organizer Chages in Late Frog Embryos Organizer forms three distinct regions Notochord formation in chick Beta-catenin localization How does beta-catenin
Ectoderm Germ Layer Frog Fate Map Frog Fate Map Role of Organizer Chages in Late Frog Embryos Organizer forms three distinct regions Notochord formation in chick Beta-catenin localization How does beta-catenin
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Overexpression of YFP::GPR-1 in the germline.
 Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
The role of FGF2 in craniofacial skeletogenesis
 The role of FGF2 in craniofacial skeletogenesis P. Ferretti, S. Sarkar, R. Moore, A. Petiot, C. J. Chan and A. Copp Summary E vidence that the major craniosynostosis syndromes are caused by mutations in
The role of FGF2 in craniofacial skeletogenesis P. Ferretti, S. Sarkar, R. Moore, A. Petiot, C. J. Chan and A. Copp Summary E vidence that the major craniosynostosis syndromes are caused by mutations in
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8
 Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Life Sciences For NET & SLET Exams Of UGC-CSIR. Section B and C. Volume-08. Contents A. BASIC CONCEPT OF DEVELOPMENT 1
 Section B and C Volume-08 Contents 5. DEVELOPMENTAL BIOLOGY A. BASIC CONCEPT OF DEVELOPMENT 1 B. GAMETOGENESIS, FERTILIZATION AND EARLY DEVELOPMENT 23 C. MORPHOGENESIS AND ORGANOGENESIS IN ANIMALS 91 0
Section B and C Volume-08 Contents 5. DEVELOPMENTAL BIOLOGY A. BASIC CONCEPT OF DEVELOPMENT 1 B. GAMETOGENESIS, FERTILIZATION AND EARLY DEVELOPMENT 23 C. MORPHOGENESIS AND ORGANOGENESIS IN ANIMALS 91 0
Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome
 Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome Marcos S. Simões-Costa 1., Sonja J. McKeown 1., Joanne Tan-Cabugao 1, Tatjana Sauka-Spengler
Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome Marcos S. Simões-Costa 1., Sonja J. McKeown 1., Joanne Tan-Cabugao 1, Tatjana Sauka-Spengler
Developmental Biology Biology Ectodermal Organs. November 22, 2005
 Developmental Biology Biology 4361 Ectodermal Organs November 22, 2005 Germinal neuroepithelium external limiting membrane neural tube neuroepithelium (stem cells) Figure 13.3 Figure 13.4 Neuroepithelial
Developmental Biology Biology 4361 Ectodermal Organs November 22, 2005 Germinal neuroepithelium external limiting membrane neural tube neuroepithelium (stem cells) Figure 13.3 Figure 13.4 Neuroepithelial
Bio Section III Organogenesis. The Neural Crest and Axonal Specification. Student Learning Objectives. Student Learning Objectives
 Bio 127 - Section III Organogenesis The Neural Crest and Axonal Specification Gilbert 9e Chapter 10 Student Learning Objectives 1. You should understand that the neural crest is an evolutionary advancement
Bio 127 - Section III Organogenesis The Neural Crest and Axonal Specification Gilbert 9e Chapter 10 Student Learning Objectives 1. You should understand that the neural crest is an evolutionary advancement
Early- and late-migrating cranial neural crest cell populations have equivalent developmental potential in vivo
 Development 124, 3077-3087 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV3724 3077 Early- and late-migrating cranial neural crest cell populations have equivalent developmental
Development 124, 3077-3087 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV3724 3077 Early- and late-migrating cranial neural crest cell populations have equivalent developmental
1. What are the three general areas of the developing vertebrate limb? 2. What embryonic regions contribute to the developing limb bud?
 Study Questions - Lecture 17 & 18 1. What are the three general areas of the developing vertebrate limb? The three general areas of the developing vertebrate limb are the proximal stylopod, zeugopod, and
Study Questions - Lecture 17 & 18 1. What are the three general areas of the developing vertebrate limb? The three general areas of the developing vertebrate limb are the proximal stylopod, zeugopod, and
Sonic hedgehog (Shh) signalling in the rabbit embryo
 Sonic hedgehog (Shh) signalling in the rabbit embryo In the first part of this thesis work the physical properties of cilia-driven leftward flow were characterised in the rabbit embryo. Since its discovery
Sonic hedgehog (Shh) signalling in the rabbit embryo In the first part of this thesis work the physical properties of cilia-driven leftward flow were characterised in the rabbit embryo. Since its discovery
Exam 3 (Final Exam) December 20, 2007
 Biology 4361 Exam 3 (Final Exam) December 20, 2007 Name: ID: Multiple choice (1 point each. Indicate the best answer.) 1. During Drosophila gastrulation, mesoderm moves in through the a. primitives streak.
Biology 4361 Exam 3 (Final Exam) December 20, 2007 Name: ID: Multiple choice (1 point each. Indicate the best answer.) 1. During Drosophila gastrulation, mesoderm moves in through the a. primitives streak.
Folding of the embryo.. the embryo is becoming a tube like structure
 The embryo is a Folding of the embryo.. the embryo is becoming a tube like structure WEEK 4 EMBRYO General features Primordia of the brain Somites Primordia of the heart Branchial arches Primordia
The embryo is a Folding of the embryo.. the embryo is becoming a tube like structure WEEK 4 EMBRYO General features Primordia of the brain Somites Primordia of the heart Branchial arches Primordia
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning
 MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
NIH Public Access Author Manuscript Dev Biol. Author manuscript; available in PMC 2012 January 15.
 NIH Public Access Author Manuscript Published in final edited form as: Dev Biol. 2011 January 15; 349(2): 238 249. doi:10.1016/j.ydbio.2010.10.032. Early regulative ability of the neuroepithelium to form
NIH Public Access Author Manuscript Published in final edited form as: Dev Biol. 2011 January 15; 349(2): 238 249. doi:10.1016/j.ydbio.2010.10.032. Early regulative ability of the neuroepithelium to form
Mesoderm Development
 Quiz rules: Spread out across available tables No phones, text books, or (lecture) notes on your desks No consultation with your colleagues No websites open other than the Quiz page No screen snap shots
Quiz rules: Spread out across available tables No phones, text books, or (lecture) notes on your desks No consultation with your colleagues No websites open other than the Quiz page No screen snap shots
INSPIRATION. The Neuron Doctrine...Henriech Wilhelm. The neuron is the anatomic, genetic, system.
 Denver Ncube 2010 INSPIRATION The Neuron Doctrine...Henriech Wilhelm Waldeyer (1891). The neuron is the anatomic, genetic, trophic and functional unit of the nervous system. BACKGROUND The differences
Denver Ncube 2010 INSPIRATION The Neuron Doctrine...Henriech Wilhelm Waldeyer (1891). The neuron is the anatomic, genetic, trophic and functional unit of the nervous system. BACKGROUND The differences
Bi 117 Final (60 pts) DUE by 11:00 am on March 15, 2012 Box by Beckman Institute B9 or to a TA
 Bi 117 Final (60 pts) DUE by 11:00 am on March 15, 2012 Box by Beckman Institute B9 or to a TA Instructor: Marianne Bronner Exam Length: 6 hours plus one 30-minute break at your discretion. It should take
Bi 117 Final (60 pts) DUE by 11:00 am on March 15, 2012 Box by Beckman Institute B9 or to a TA Instructor: Marianne Bronner Exam Length: 6 hours plus one 30-minute break at your discretion. It should take
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
Homeobox genes and the vertebrate head
 Development 103 Supplement, 17-24 (1988) Printed in Great Britain The Company of Biologists Limited 1988 17 Homeobox genes and the vertebrate head PETER W. H. HOLLAND Department of Zoology, University
Development 103 Supplement, 17-24 (1988) Printed in Great Britain The Company of Biologists Limited 1988 17 Homeobox genes and the vertebrate head PETER W. H. HOLLAND Department of Zoology, University
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1178343/dc1 Supporting Online Material for Starvation Protects Germline Stem Cells and Extends Reproductive Longevity in C. elegans This PDF file includes: Giana Angelo
www.sciencemag.org/cgi/content/full/1178343/dc1 Supporting Online Material for Starvation Protects Germline Stem Cells and Extends Reproductive Longevity in C. elegans This PDF file includes: Giana Angelo
Expression of DLX3 in chick embryos
 Mechanisms of Development 89 (1999) 189±193 Gene expression pattern Expression of DLX3 in chick embryos www.elsevier.com/locate/modo Edgar Pera 1, Michael Kessel* Max-Planck-Institut fuèr biophysikalische
Mechanisms of Development 89 (1999) 189±193 Gene expression pattern Expression of DLX3 in chick embryos www.elsevier.com/locate/modo Edgar Pera 1, Michael Kessel* Max-Planck-Institut fuèr biophysikalische
Late effects of retinoic acid on neural crest and aspects of rhombomere identity
 Development 122, 783-793 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV2020 783 Late effects of retinoic acid on neural crest and aspects of rhombomere identity Emily Gale 1,
Development 122, 783-793 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV2020 783 Late effects of retinoic acid on neural crest and aspects of rhombomere identity Emily Gale 1,
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic)
 Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons
Developmental Biology
 Developmental Biology 317 (2008) 389 400 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Review Review of fate-mapping studies of
Developmental Biology 317 (2008) 389 400 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Review Review of fate-mapping studies of
JEFFERSON COLLEGE VERTEBRATE ANATOMY
 JEFFERSON COLLEGE COURSE SYLLABUS BIO207 VERTEBRATE ANATOMY 4 Credit Hours Prepared by: Mr. Jim McCain Revised Date: November 2005 by Dr. Ken Balak Division of Arts & Science Education Dr. Mindy Selsor,
JEFFERSON COLLEGE COURSE SYLLABUS BIO207 VERTEBRATE ANATOMY 4 Credit Hours Prepared by: Mr. Jim McCain Revised Date: November 2005 by Dr. Ken Balak Division of Arts & Science Education Dr. Mindy Selsor,
The Radiata-Bilateria split. Second branching in the evolutionary tree
 The Radiata-Bilateria split Second branching in the evolutionary tree Two very important characteristics are used to distinguish between the second bifurcation of metazoans Body symmetry Germinal layers
The Radiata-Bilateria split Second branching in the evolutionary tree Two very important characteristics are used to distinguish between the second bifurcation of metazoans Body symmetry Germinal layers
Significance of the Cranial Neural Crest
 DEVELOPMENTAL DYNAMICS 229:5 13, 2004 REVIEWS A PEER REVIEWED FORUM Significance of the Cranial Neural Crest Anthony Graham,* Jo Begbie, and Imelda McGonnell The cranial neural crest has long been viewed
DEVELOPMENTAL DYNAMICS 229:5 13, 2004 REVIEWS A PEER REVIEWED FORUM Significance of the Cranial Neural Crest Anthony Graham,* Jo Begbie, and Imelda McGonnell The cranial neural crest has long been viewed
DBHR, a Gene with Homology to Dopamine -Hydroxylase, Is Expressed in the Neural Crest throughout Early Development
 Developmental Biology 234, 365 375 (2001) doi:10.1006/dbio.2001.0275, available online at http://www.idealibrary.com on DBHR, a Gene with Homology to Dopamine -Hydroxylase, Is Expressed in the Neural Crest
Developmental Biology 234, 365 375 (2001) doi:10.1006/dbio.2001.0275, available online at http://www.idealibrary.com on DBHR, a Gene with Homology to Dopamine -Hydroxylase, Is Expressed in the Neural Crest
ORDINARY section techniques have certain disadvantages when a threedimensional
 6 7 A Simultaneous Coupling Azo Dye Technique Suitable for Whole Mounts By P. R. LEWIS (From the Anatomy School, Cambridge) With one plate (fig. 1) SUMMARY A method based on the familiar coupling azo dye
6 7 A Simultaneous Coupling Azo Dye Technique Suitable for Whole Mounts By P. R. LEWIS (From the Anatomy School, Cambridge) With one plate (fig. 1) SUMMARY A method based on the familiar coupling azo dye
The allocation of cells in the presomitic mesoderm during somite segmentation in the mouse embryo
 Development 103. 379-390 (1988) Printed in Great Britain The Company of Biologists Limited 1988 379 The allocation of cells in the presomitic mesoderm during somite segmentation in the mouse embryo PATRICK
Development 103. 379-390 (1988) Printed in Great Britain The Company of Biologists Limited 1988 379 The allocation of cells in the presomitic mesoderm during somite segmentation in the mouse embryo PATRICK
Gut Tube Development. For it s rare that a man thinks of anything so seriously as his dinner!
 For it s rare that a man thinks of anything so seriously as his dinner! Ben Johnson Gut Tube Development This chapter will follow the development of the embryonic gut caudal to the developing pharynx,
For it s rare that a man thinks of anything so seriously as his dinner! Ben Johnson Gut Tube Development This chapter will follow the development of the embryonic gut caudal to the developing pharynx,
Determination of Spatiotemporal Interactions Regulating Head/Trunk Axial Pattern in the Chick Embryo
 Eukaryon, Vol. 2, January 2006, Lake Forest ollege Senior Thesis Determination of Spatiotemporal Interactions Regulating Head/Trunk Axial Pattern in the hick Embryo David Mann * Department of Biology,
Eukaryon, Vol. 2, January 2006, Lake Forest ollege Senior Thesis Determination of Spatiotemporal Interactions Regulating Head/Trunk Axial Pattern in the hick Embryo David Mann * Department of Biology,
Human Anatomy & Physiology
 Human Anatomy & Physiology Mr. Danilo Villar Rogayan Jr. Faculty, Department of Natural Sciences College of Education, Arts and Sciences Ramon Magsaysay Technological University Human Anatomy & Physiology
Human Anatomy & Physiology Mr. Danilo Villar Rogayan Jr. Faculty, Department of Natural Sciences College of Education, Arts and Sciences Ramon Magsaysay Technological University Human Anatomy & Physiology
4. Neural tube cells are specified by opposing dorsal-ventral gradients of a. Wnts and Nodal. b. FGF and Shh. c. BMPs and Wnts. d. BMPs and Shh.
 Biology 4361 Name: KEY Exam 4 ID#: August 1, 2008 Multiple choice (one point each; indicate the best answer) 1. Neural tube closure is accomplished by movement of the a. medial hinge point cells. b. medial
Biology 4361 Name: KEY Exam 4 ID#: August 1, 2008 Multiple choice (one point each; indicate the best answer) 1. Neural tube closure is accomplished by movement of the a. medial hinge point cells. b. medial
Migrating neural crest cells in the trunk of the avian embryo are multipotent
 Development 112, 913-920 (1991) Printed in Great Britain The Company of Biologists Limited 1991 913 Migrating neural crest cells in the trunk of the avian embryo are multipotent SCOTT E. FRASER 1 and MARIANNE
Development 112, 913-920 (1991) Printed in Great Britain The Company of Biologists Limited 1991 913 Migrating neural crest cells in the trunk of the avian embryo are multipotent SCOTT E. FRASER 1 and MARIANNE
Making Headway: The Roles of Hox Genes and Neural Crest Cells in Craniofacial Development
 Mini-Review TheScientificWorldJOURNAL (2003) 3, 240 264 ISSN 1537-744X; DOI 10.1100/tsw.2003.11 Making Headway: The Roles of Hox Genes and Neural Crest Cells in Craniofacial Development Paul A Trainor
Mini-Review TheScientificWorldJOURNAL (2003) 3, 240 264 ISSN 1537-744X; DOI 10.1100/tsw.2003.11 Making Headway: The Roles of Hox Genes and Neural Crest Cells in Craniofacial Development Paul A Trainor
BIOLOGY 340 Exam Study Guide All Exams Comparative Embryology Dr. Stuart S. Sumida California State University San Bernardino; Department of Biology
 BIOLOGY 340 Exam Study Guide All Exams Comparative Embryology Dr. Stuart S. Sumida California State University San Bernardino; Department of Biology Midterm and final exams may include materials studied
BIOLOGY 340 Exam Study Guide All Exams Comparative Embryology Dr. Stuart S. Sumida California State University San Bernardino; Department of Biology Midterm and final exams may include materials studied
Question Set # 4 Answer Key 7.22 Nov. 2002
 Question Set # 4 Answer Key 7.22 Nov. 2002 1) A variety of reagents and approaches are frequently used by developmental biologists to understand the tissue interactions and molecular signaling pathways
Question Set # 4 Answer Key 7.22 Nov. 2002 1) A variety of reagents and approaches are frequently used by developmental biologists to understand the tissue interactions and molecular signaling pathways
SIGNIFICANCE OF EMBRYOLOGY
 This lecture will discuss the following topics : Definition of Embryology Significance of Embryology Old and New Frontiers Introduction to Molecular Regulation and Signaling Descriptive terms in Embryology
This lecture will discuss the following topics : Definition of Embryology Significance of Embryology Old and New Frontiers Introduction to Molecular Regulation and Signaling Descriptive terms in Embryology
Comparison of the expression patterns of several sox genes between Oryzias latipes and Danio rerio
 Urun 1 Comparison of the expression patterns of several sox genes between Oryzias latipes and Danio rerio Fatma Rabia URUN ilkent University, nkara - TURKEY High mobility group domain containing transcription
Urun 1 Comparison of the expression patterns of several sox genes between Oryzias latipes and Danio rerio Fatma Rabia URUN ilkent University, nkara - TURKEY High mobility group domain containing transcription
Predator escape: an ecologically realistic scenario for the evolutionary origins of multicellularity. Student handout
 Predator escape: an ecologically realistic scenario for the evolutionary origins of multicellularity Student handout William C. Ratcliff, Nicholas Beerman and Tami Limberg Introduction. The evolution of
Predator escape: an ecologically realistic scenario for the evolutionary origins of multicellularity Student handout William C. Ratcliff, Nicholas Beerman and Tami Limberg Introduction. The evolution of
Developmental Biology 3230 Midterm Exam 1 March 2006
 Name Developmental Biology 3230 Midterm Exam 1 March 2006 1. (20pts) Regeneration occurs to some degree to most metazoans. When you remove the head of a hydra a new one regenerates. Graph the inhibitor
Name Developmental Biology 3230 Midterm Exam 1 March 2006 1. (20pts) Regeneration occurs to some degree to most metazoans. When you remove the head of a hydra a new one regenerates. Graph the inhibitor
Paraxial and Intermediate Mesoderm
 Biology 4361 Paraxial and Intermediate Mesoderm December 6, 2007 Mesoderm Formation Chick Major Mesoderm Lineages Mesodermal subdivisions are specified along a mediolateral axis by increasing amounts of
Biology 4361 Paraxial and Intermediate Mesoderm December 6, 2007 Mesoderm Formation Chick Major Mesoderm Lineages Mesodermal subdivisions are specified along a mediolateral axis by increasing amounts of
Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse
 Development 127, 933-944 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV2515 933 Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse Jeffery R. Barrow, H.
Development 127, 933-944 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV2515 933 Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse Jeffery R. Barrow, H.
9/4/2015 INDUCTION CHAPTER 1. Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology. Fig 1.
 INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
Unicellular: Cells change function in response to a temporal plan, such as the cell cycle.
 Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
Segmental lineage restrictions in the chick embryo spinal cord depend on the adjacent somites
 Development 113, 239-244 (1991) Printed in Great Britain The Company of Biologists Limited 1991 239 Segmental lineage restrictions in the chick embryo spinal cord depend on the adjacent somites 1, KAREN
Development 113, 239-244 (1991) Printed in Great Britain The Company of Biologists Limited 1991 239 Segmental lineage restrictions in the chick embryo spinal cord depend on the adjacent somites 1, KAREN
Characterization of the early development of specific hypaxial muscles from the ventrolateral myotome
 Development 126, 4305-4315 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV1451 4305 Characterization of the early development of specific hypaxial muscles from the ventrolateral
Development 126, 4305-4315 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV1451 4305 Characterization of the early development of specific hypaxial muscles from the ventrolateral
Development of neural tube basal lamina during neurulation and neural crest cell emigration in the trunk of the mouse embryo
 /. Embryol. exp. Morph. 98, 219-236 (1986) 219 Printed in Great Britain (E) The Company of Biologists Limited 1986 Development of neural tube basal lamina during neurulation and neural crest cell emigration
/. Embryol. exp. Morph. 98, 219-236 (1986) 219 Printed in Great Britain (E) The Company of Biologists Limited 1986 Development of neural tube basal lamina during neurulation and neural crest cell emigration
Paraxial and Intermediate Mesoderm
 Biology 4361 Paraxial and Intermediate Mesoderm December 7, 2006 Major Mesoderm Lineages Mesodermal subdivisions are specified along a mediolateral axis by increasing amounts of BMPs more lateral mesoderm
Biology 4361 Paraxial and Intermediate Mesoderm December 7, 2006 Major Mesoderm Lineages Mesodermal subdivisions are specified along a mediolateral axis by increasing amounts of BMPs more lateral mesoderm
2. Examine the external anatomy of the squid and identify the following structures: tentacles, arms, fins, siphon, mantle, eyes and collar.
 Cephalopod Anatomy As their name implies, members of the class Cephalopoda have modified head-foot which bears an array of prehensile tentacles and arms at the cranial end of the body. The visceral mass
Cephalopod Anatomy As their name implies, members of the class Cephalopoda have modified head-foot which bears an array of prehensile tentacles and arms at the cranial end of the body. The visceral mass
Apoptosis and proliferation in developing, mature, and regressing epibranchial placodes
 Developmental Biology 278 (2005) 86 102 www.elsevier.com/locate/ydbio Apoptosis and proliferation in developing, mature, and regressing epibranchial placodes Stefan Washausen a, Bastian Obermayer a, Guido
Developmental Biology 278 (2005) 86 102 www.elsevier.com/locate/ydbio Apoptosis and proliferation in developing, mature, and regressing epibranchial placodes Stefan Washausen a, Bastian Obermayer a, Guido
Unified School District of De Pere Advanced Biology B Benchmarks
 Content A. Students will understand that among the science disciplines, there are Standards: unifying themes: systems, order, organization, and interactions; evidence, models, and explanations; constancy,
Content A. Students will understand that among the science disciplines, there are Standards: unifying themes: systems, order, organization, and interactions; evidence, models, and explanations; constancy,
Early Development in Invertebrates
 Developmental Biology Biology 4361 Early Development in Invertebrates October 25, 2006 Early Development Overview Cleavage rapid cell divisions divisions of fertilized egg into many cells Gastrulation
Developmental Biology Biology 4361 Early Development in Invertebrates October 25, 2006 Early Development Overview Cleavage rapid cell divisions divisions of fertilized egg into many cells Gastrulation
Neural development its all connected
 Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Chapter 1- An Orientation to the Human Body NOTES
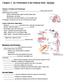 Chapter 1- An Orientation to the Human Body NOTES Overview of Anatomy and Physiology: -Anatomy- of body parts and their relationships to one another. -Gross or Macroscopic= large and easily observable
Chapter 1- An Orientation to the Human Body NOTES Overview of Anatomy and Physiology: -Anatomy- of body parts and their relationships to one another. -Gross or Macroscopic= large and easily observable
TEST BANK FOR PRESCOTTS MICROBIOLOGY 9TH EDITION BY WILLEY SHERWOOD WOOLVERTON
 TEST BANK FOR PRESCOTTS MICROBIOLOGY 9TH EDITION BY WILLEY SHERWOOD WOOLVERTON Link download full: https://testbankservice.com/download/test-bank-for-prescottsmicrobiology-9th-edition-by-willey-sherwood-woolverton/
TEST BANK FOR PRESCOTTS MICROBIOLOGY 9TH EDITION BY WILLEY SHERWOOD WOOLVERTON Link download full: https://testbankservice.com/download/test-bank-for-prescottsmicrobiology-9th-edition-by-willey-sherwood-woolverton/
Tissue Origins and Interactions in the Mammalian Skull Vault
 Developmental Biology 241, 106 116 (2002) doi:10.1006/dbio.2001.0487, available online at http://www.idealibrary.com on Tissue Origins and Interactions in the Mammalian Skull Vault Xiaobing Jiang,* Sachiko
Developmental Biology 241, 106 116 (2002) doi:10.1006/dbio.2001.0487, available online at http://www.idealibrary.com on Tissue Origins and Interactions in the Mammalian Skull Vault Xiaobing Jiang,* Sachiko
craniofacial development and WNT signaling. Shachi Bhatt
 MED23: a Mediator subunit's role in global gene transcription, regulation of craniofacial development and WNT signaling. BY 2013 Shachi Bhatt Submitted to the graduate degree program in Anatomy and Cell
MED23: a Mediator subunit's role in global gene transcription, regulation of craniofacial development and WNT signaling. BY 2013 Shachi Bhatt Submitted to the graduate degree program in Anatomy and Cell
ANATOMY AND PHYSIOLOGY Revised 11/2010
 ANATOMY AND PHYSIOLOGY Revised 11/2010 DESCRIPTION OF COURSE: Covers the basics of human anatomy and physiology including anatomical terminology, basic biochemistry, cells and tissues, and the integumentary,
ANATOMY AND PHYSIOLOGY Revised 11/2010 DESCRIPTION OF COURSE: Covers the basics of human anatomy and physiology including anatomical terminology, basic biochemistry, cells and tissues, and the integumentary,
Neural crest placode interactions mediate trigeminal ganglion formation and require Slit1 Robo2 signaling
 C h a p t e r 2 Neural crest placode interactions mediate trigeminal ganglion formation and require Slit1 Robo2 signaling 2.1 Abstract 1 The cellular and molecular interactions allowing generation of complex
C h a p t e r 2 Neural crest placode interactions mediate trigeminal ganglion formation and require Slit1 Robo2 signaling 2.1 Abstract 1 The cellular and molecular interactions allowing generation of complex
Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice
 Development 129, 2271-2282 (2002) Printed in Great Britain The Company of Biologists Limited 2002 DEV9819 2271 Novel retinoic acid generating activities in the neural tube and heart identified by conditional
Development 129, 2271-2282 (2002) Printed in Great Britain The Company of Biologists Limited 2002 DEV9819 2271 Novel retinoic acid generating activities in the neural tube and heart identified by conditional
NucView TM 488 Caspase-3 Assay Kit for Live Cells
 NucView TM 488 Caspase-3 Assay Kit for Live Cells Catalog Number: 30029 (100-500 assays) Contact Information Address: Biotium, Inc. 3423 Investment Blvd. Suite 8 Hayward, CA 94545 USA Telephone: (510)
NucView TM 488 Caspase-3 Assay Kit for Live Cells Catalog Number: 30029 (100-500 assays) Contact Information Address: Biotium, Inc. 3423 Investment Blvd. Suite 8 Hayward, CA 94545 USA Telephone: (510)
Role of Cadherin-7 in cranial neural crest development and its potential heterotypic interaction with N-cadherin in trigeminal ganglion formation
 C h a p t e r 4 Role of Cadherin-7 in cranial neural crest development and its potential heterotypic interaction with N-cadherin in trigeminal ganglion formation 4.1 Abstract Cadherins are transmembrane
C h a p t e r 4 Role of Cadherin-7 in cranial neural crest development and its potential heterotypic interaction with N-cadherin in trigeminal ganglion formation 4.1 Abstract Cadherins are transmembrane
Cell-Cell Communication in Development
 Biology 4361 - Developmental Biology Cell-Cell Communication in Development October 2, 2007 Cell-Cell Communication - Topics Induction and competence Paracrine factors inducer molecules Signal transduction
Biology 4361 - Developmental Biology Cell-Cell Communication in Development October 2, 2007 Cell-Cell Communication - Topics Induction and competence Paracrine factors inducer molecules Signal transduction
Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor nerves
 Development 122, 2367-2374 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV9463 2367 Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor
Development 122, 2367-2374 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV9463 2367 Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor
PRACTICE EXAM. 20 pts: 1. With the aid of a diagram, indicate how initial dorsal-ventral polarity is created in fruit fly and frog embryos.
 PRACTICE EXAM 20 pts: 1. With the aid of a diagram, indicate how initial dorsal-ventral polarity is created in fruit fly and frog embryos. No Low [] Fly Embryo Embryo Non-neural Genes Neuroectoderm Genes
PRACTICE EXAM 20 pts: 1. With the aid of a diagram, indicate how initial dorsal-ventral polarity is created in fruit fly and frog embryos. No Low [] Fly Embryo Embryo Non-neural Genes Neuroectoderm Genes
Developmental processes Differential gene expression Introduction to determination The model organisms used to study developmental processes
 Date Title Topic(s) Learning Outcomes: Sept 28 Oct 3 1. What is developmental biology and why should we care? 2. What is so special about stem cells and gametes? Developmental processes Differential gene
Date Title Topic(s) Learning Outcomes: Sept 28 Oct 3 1. What is developmental biology and why should we care? 2. What is so special about stem cells and gametes? Developmental processes Differential gene
Unit 4 Evaluation Question 1:
 Name: Unit 4 Evaluation Question 1: /7 points A naturally occurring dominant mutant in mice is the Doublefoot (Dbf) mutant. Below is an image of the bones from a wildtype (wt) and Doublefoot mutant mouse.
Name: Unit 4 Evaluation Question 1: /7 points A naturally occurring dominant mutant in mice is the Doublefoot (Dbf) mutant. Below is an image of the bones from a wildtype (wt) and Doublefoot mutant mouse.
Early Deletion of Neuromeres in Wnt-l-/- Mutant Mice: Evaluation by Morphological and Molecular Markers
 THE JOURNAL OF COMPARATIVE NEUROLOGY 374:246-258 (1996) Early Deletion of Neuromeres in Wnt-l-/- Mutant Mice: Evaluation by Morphological and Molecular Markers GRANT S. MASTICK, CHEN-MING FAN, MARC TESSIER-LAVIGNE,
THE JOURNAL OF COMPARATIVE NEUROLOGY 374:246-258 (1996) Early Deletion of Neuromeres in Wnt-l-/- Mutant Mice: Evaluation by Morphological and Molecular Markers GRANT S. MASTICK, CHEN-MING FAN, MARC TESSIER-LAVIGNE,
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Neuroectodermal origin of avian hypothalamo-hypophyseal complex: the role of the ventral neural ridge
 /. Embryol. exp. Morph. Vol. 34, 2,pp. 311-325, 1975 1>\\ Printed in Great Britain Neuroectodermal origin of avian hypothalamo-hypophyseal complex: the role of the ventral neural ridge By T. TAKOR TAKOR
/. Embryol. exp. Morph. Vol. 34, 2,pp. 311-325, 1975 1>\\ Printed in Great Britain Neuroectodermal origin of avian hypothalamo-hypophyseal complex: the role of the ventral neural ridge By T. TAKOR TAKOR
Kingdom Animalia. Zoology the study of animals
 Kingdom Animalia Zoology the study of animals Summary Animals are multicellular and eukaryotic. consume and digest organic materials thereby being heterotrophs. Most are motile at some time in their lives.
Kingdom Animalia Zoology the study of animals Summary Animals are multicellular and eukaryotic. consume and digest organic materials thereby being heterotrophs. Most are motile at some time in their lives.
camp Direct Immunoassay Kit
 camp Direct Immunoassay Kit Catalog Number KA0886 100 assays Version: 05 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
camp Direct Immunoassay Kit Catalog Number KA0886 100 assays Version: 05 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
Embryogenesis of an insect nervous system II: A second class of neuron precursor cells and the origin of the intersegmental connectives
 J. Embryol. exp. Morph. Vol. 61,pp. 317-330, 1981 3J7 Printed in Great Britain @ Company of Biologists Limited 1981 Embryogenesis of an insect nervous system II: A second class of neuron precursor cells
J. Embryol. exp. Morph. Vol. 61,pp. 317-330, 1981 3J7 Printed in Great Britain @ Company of Biologists Limited 1981 Embryogenesis of an insect nervous system II: A second class of neuron precursor cells
Introduction to Animals
 Introduction to Animals Characteristics of Animals multicellular Except for sponges, animal cells are arranged into tissues. Tissues are necessary to produce organs and organ systems. Tissues, organs,
Introduction to Animals Characteristics of Animals multicellular Except for sponges, animal cells are arranged into tissues. Tissues are necessary to produce organs and organ systems. Tissues, organs,
