Movements of Individual BK Ca Channels in Live Cell Membrane Monitored by Site-Specific Labeling Using Quantum Dots
|
|
|
- Scott Garrison
- 6 years ago
- Views:
Transcription
1 Biophysical Journal Volume 99 November Movements of Individual BK Ca Channels in Live Cell Membrane Monitored by Site-Specific Labeling Using Quantum Dots Sehoon Won, 6 Hae-Deun Kim, 6 Ji-Yeon Kim, 6 Byoung-Cheol Lee, Sunghoe Chang, { and Chul-Seung Park * School of Life Sciences, Bio-Imaging Research Center and Cell Dynamics Research Center, Gwangju Institute of Science Technology, Gwangju, Korea; and { Department of Biomedical Sciences, Seoul National University, Seoul, Korea ABSTRACT The movements of BK Ca channels were investigated in live cells using quantum dots (QDs). The extracellular N-terminus was metabolically tagged with biotin, labeled with streptavidin-conjugated QDs and then monitored using real-time time-lapse imaging in COS-7 cells and cultured neurons. By tracking hundreds of channels, we were able to determine the characteristics of channel movements quantitatively. Channels in COS-7 cells exhibited a confined diffusion in an area of mm 2, with an initial diffusion coefficient of mm 2 /s. In neurons, the channel movements were more heterogeneous and highly dependent on subcellular location. While the channels in soma diffused slowly without clear confinement, axodendritic channels showed more rapid and pseudo-one-dimensional movements. Intriguingly, the channel movement in somata was drastically increased by the neuronal b4 subunit, in contrast to the channels in the axodendritic area where the mobility were significantly decreased. Thus, our results demonstrate that the membrane mobility of BK Ca channels can be greatly influenced by the expression system used, subunit composition, and subcellular location. This QD-based, single-molecule tracking technique can be utilized to investigate the cellular mechanisms that determine the mobility as well as the localization of various membrane proteins in live cells. INTRODUCTION Large-conductance Ca 2þ -activated K þ channels (BK Ca channels, also known as BKor Maxi-K channels) are a family of potassium-selective ion channels that are activated synergistically by membrane depolarization and increased intracellular Ca 2þ (1 3). These channels are expressed in many tissues and involved in various physiological processes, including neuronal excitability, neurotransmitter release, contraction of smooth muscles, frequency tuning of hair cells, and immunity (4 7). Functional BK Ca channels are assembled as homotetramers of the pore-forming a-subunit, called Slowpoke (Slo, K Ca 1.1, or KCNMA1), or heterooctamers of a- and auxiliary b-subunits (8,9). The a-subunit contains seven transmembrane domains in its amino terminus responsible for voltage sensing and the selective permeation of K þ ions (10). The calcium-dependent activation of the channel is mediated by the large cytoplasmic domain at its carboxyl terminus (11). Four different types of b-subunits, b1 4 (or KCNMB1 4), are expressed differentially in various tissues (12) and they are known to regulate channel sensitivity to Ca 2þ and voltage by coassembling with the a-subunit (13). BK Ca channels are widely expressed throughout the nervous system. The a-subunit, or Slo protein, was detected immunohistochemically in various regions and subregions of the murine brains. In the mouse hippocampal formation, Submitted June 27, 2010, and accepted for publication August 25, Sehoon Won, Hae-Deun Kim, and Ji-Yeon Kim contributed equally to this work. *Correspondence: cspark@gist.ac.kr Editor: Francisco Bezanilla. for instance, BK Ca channels were observed in several different substructures including the hippocampus proper, dentate gyrus, and subiculum (14). These channels are found in both axons and somatodendritic regions of various central neurons including hippocampal pyramidal neurons (15 17). BK Ca channels accumulate in presynaptic regions and are involved in spike repolarization and the regulation of transmitter release (18). At the somatodendritic level, postsynaptic BK Ca channels have been implicated in the modulation of action potential shape and frequency (16,19). Although BK Ca channel are involved in diverse physiological and pathophysiological processes that may in part be dictated by their cellular and subcellular distribution (20), no reports to date have examined the movement of the channels in specific neurons either in tissue or in cultured conditions. It has been extremely difficult to label the channels specifically and to trace the protein molecules individually. The fluorescent proteins, for instance, were not suited for long-term imaging of single BK Ca channels due to the weak emission and photoinstability. However, recent progress in quantum dot technology has allowed individual proteins to be labeled within cells and their motion followed in real time (21). Quantum dots (QDs), also known as nanocrystals, are a special class of semiconductors ranging from 2 to 10 nanometers in diameter and composed of periodic groups of II VI, III V, or IV VI materials. The small size, long-term photostability, and narrow emission spectra of QDs have many advantages in fluorescence imaging. Their specific colors, which vary by size, and remarkable resistance to photobleaching make QDs well suited for tracking single Ó 2010 by the Biophysical Society /10/11/2853/10 $2.00 doi: /j.bpj
2 2854 Won et al. molecules in live-cell imaging and dynamics studies (21,22). Moreover, many different bioconjugated QDs, in which nanocrystals are modified with various functional biomolecules such as streptavidin, are now available for direct binding of the QDs to targets of interest (23). In this study, we visualized single BK Ca channels in live neurons by specific labeling with QDs and monitored their movements. By quantitatively analyzing the time-lapse images of QD-labeled channels, we were able to determine the factors influencing the dynamics of BK Ca channels expressed in cultured neurons. MATERIALS AND METHODS Expression of BK Ca channels and labeling with quantum dots The a-subunit of the rat BK Ca channel, or rslo (GenBank accession No. AF135265), was tagged with the acceptor peptide at its N-terminus for biotin labeling (24) and with or without a red fluorescent protein (RFP) at its C-terminus for fluorescence imaging. A truncation mutant of rslo (rslo-dc) was constructed to delete 102 amino acids from Phe 1109 to the C-terminus based on a previous report (25). These modified rslo cdnas were subcloned into a mammalian expression vector, pcdna3.1 (þ) (Invitrogen, Carlsbad, CA). The rat b4 subunit, or rb4 (GenBank accession No. AY028605), was tagged with enhanced green fluorescent protein at its C-terminus. All experiments were performed either on COS-7, African green monkey kidney fibroblast cells, or cultured rat primary hippocampal neurons. For transient expression, cells were plated onto 18-mm coverslips (Marienfeld, Lauda-Königshofen, Germany) coated with 0.05 mg/ml poly-d-lysine (Sigma-Aldrich, St. Louis, MO). The plasmid harboring rslo cdna was cotransfected with a plasmid harboring pdisplay BirA-ER, the gene for Escherichia coli biotin ligase modified to be retained in the ER, at a 2:1 ratio into COS-7 cells using a commercial reagent, Polyfect (Qiagen, Valencia, CA), and at a 3:1 ratio into primary cultured hippocampal neurons at DIV5 using calcium-phosphate precipitation. The rslo and rb4 genes were cotransfected at a 1:5 molar ratio according to a previously published method (26). Seventy-two hours after transfection, cells on coverslips were washed carefully with phosphate-buffered saline (PBS) and incubated with 0.5 nm streptavidin-conjugated QD605 (Invitrogen) in PBS for 10 min at room temperature. Cells were then carefully washed with PBS three times. Electrophysiological recordings and data analysis All macroscopic current recordings were performed using the gigaohmseal patch-clamp method in an inside-out configuration. Patch pipettes were fabricated from borosilicate glass (WPI, Sarasota, FL) and fire-polished to a resistance of 4 5 MU. The channel currents were amplified using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA), low-pass filtered at 1 or 2 khz using a four-pole Bessel filter, and digitized using a Digidata 1200A apparatus (Axon Instruments). The macroscopic currents of expressed channels were activated by voltage-clamp pulses delivered from a holding potential of 100 mv to reach membrane potentials ranging from 80 to þ200 mv in 10-mV increments. The pipette and extracellular solutions contained the following components, unless otherwise specified: 116 mm KOH, 4 mm KCl, 10 mm HEPES, and 5 mm EGTA at ph 7.2. To determine the precise free concentration of intracellular Ca 2þ ([Ca 2þ ] i ), the appropriate amount of total Ca 2þ to be added to the intracellular solution was calculated using MaxChelator software (27) ( The ph was adjusted to 7.2 with 2-(n-morpholino)ethanesulfonic acid. In order to compare channel characteristics accurately, an identical set of intracellular solutions was used throughout the experiments. Commercial software packages, which included Clampex 8.0 (Axon Instruments) and Origin 6.1 (OriginLab, Northampton, MA), were used for acquisition and analysis of macroscopic recording data. All data are presented as mean 5 SE, where n indicates the number of independent experiments. Fluorescence microscopy and live-cell imaging COS-7 cells and rat primary hippocampal neurons were kept in PBS throughout imaging. Cells were observed with an inverted microscope (Olympus IX-51; Olympus, Tokyo, Japan) connected to a charge-coupled device camera (Andor, Belfast, Northern Island) using a 60 objective oil lens and QD605 filters (SemRock, Rochester, NY). The images were captured using commercial software, MetaMorph (Universal Imaging, Downingtown, PA). The time-lapse images were collected at room temperature at 300-ms time intervals over for 15 s. For photoblinking experiments, images were acquired at room temperature at 50-ms time intervals over 50 s using the stream recording mode. The seven movies in the Supporting Material were made with stack files using MetaMorph and edited with Sony Vegas Pro 9.0 (Sony Creative Software, Madison, WI). Analysis of channel dynamics All images were analyzed using MetaMorph software. Images of BK Ca channels labeled with QD605 were converted into stack movie files, and changes in the X-Y coordinates of dots were tracked over time using imaging software. Mean square displacement (MSD) values were obtained over 50 sequential frames, or every 300 ms, and plotted against time. MSD values of individual dots are defined by the equation MSDðnDtÞ ¼ 1 N n X N-n i ¼ 1 ðxi þ n X 1 Þ 2 þðy i þ n X 1 Þ 2 ; where x i and y i are positions on frame i, x 1 and y 1 are positions on the first frame, N is the total number of frames, Dt is the time between frames and distance between steps in time t, and ndt is the time interval over which the MSD is calculated. The initial diffusion coefficient, D init, was obtained by fitting the initial slope of each MSD curve in the first 1.0 s of the plot (28) using the equation lim t/0 MSDðnDtÞ ¼ 4D initdt: When change in MSD is fitted versus time, a linear plot of MSD indicates random Brownian movement. A nonlinear plot of change in MSD over time reflects confined diffusion or directed diffusion. A decreasing slope indicates confined diffusion, while an increasing slope indicates directed diffusion. For vector plots, the relative angle of movement and the distance between one position and the next were measured along the axodendritic axis. The origin of each vector in the plot was defined as the final position of the moving dot in the previous frame. The orientation of each vector was normalized at the origin to the direction of the axodendritic process in which the dot was moving and the direction toward the soma was set at 0. RESULTS Specific labeling of BK Ca channels using quantum dots in live cells To visualize individual BK Ca channels in live cells, the a-subunit of the rat BK Ca channel (rslo) was modified in two ways. First, the extracellular N-terminus of rslo was
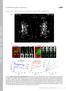
3 Membrane Mobility of BK Channels 2855 FIGURE 1 Specific labeling of functional BK Ca channels expressed in COS-7 cells using QDs. (A) Schematic illustration of the rat BK Ca channel a-subunit (or rslo) used in this experiment. An acceptor peptide (AP) was tagged at the N-terminus of rslo, and red fluorescent protein (RFP) was tagged at the C-terminus. (B) Specific labeling of AP-rSlo-RFP channels using QDs. COS-7 cells were transfected with BirA-ER and RFP (first row), AP-rSlo-RFP (second row), or AP-rSlo-RFP with BirA-ER (third row). In the fourth row, magnified views (3) of the highlighted regions in the third row are shown. In the case of BirA-ER shown in the first row, a plasmid harboring RFP (ptag-rfp-c1; Evrogen, Moscow, Russia) was cotransfected in order to visualize the transfectants. Cells were labeled with streptavidinconjugated QD605 (Invitrogen). Cells expressing either RFP or AP-rSlo-RFP are red (left column) and QD605 is blue (center column). Merged images are also shown (right column). Scale bar, 10 mm. (C) Electrophysiological characterization of rslo, AP-rSlo-RFP, and QD-labeled AP-rSlo- RFP channels (QD:AP-rSlo-RFP). Representative current traces of rslo, AP-rSlo-RFP, and QD-labeled AP-rSlo-RFP channels (QD:AP-rSlo-RFP) are shown. Ionic currents were evoked with 100-ms voltage steps from the holding potential of 100 mv to test potentials of 20, 50, and 80 mv. Intracellular Ca 2þ concentration was buffered at 10 mm. The response of untransfected HEK293 cells to voltage pulses is also shown as a control. tagged with a 15-amino-acid sequence (GLNDIFEAQ KIEWHE), called acceptor peptide (AP) (Fig. 1 A). Originating from the biotin carboxyl carrier protein subunit of bacterial acetyl-coa carboxylase, the lysine residue (K, underlined) in this peptide can be effectively biotinylated by E. coli biotin ligase, BirA (29). Second, the C-terminal end of rslo was fused with the red fluorescent protein (RFP) of a marine anemone, Discosoma striata, allowing transfected cells expressing the modified rslo channels, AP-rSlo-RFP, to be visually discerned under a fluorescent microscope. To biotinylate the channel proteins metabolically within cells, AP-rSlo-RFP was coexpressed with a modified E. coli biotin ligase, BirA-ER (24). This biotin ligase contains KDEL, an ER retention sequence, at its C-terminal end and resides within the ER lumen (30). Thus, the lysine residue of N-terminal AP can be biotinylated within the ER lumen by BirA-ER. Upon localization to the plasma membrane, these biotin-conjugated BK Ca channels expose their N-terminal biotins extracellularly and can be labeled with streptavidin-conjugated QDs (24). The specificity of QD labeling of the modified BK Ca channel expressed in COS-7 cells was initially evaluated. As shown in Fig. 1 B, COS-7 cells cotransfected with AP-rSlo-RFP and BirA-ER were heavily labeled by streptavidin-conjugated QDs (Fig. 1 B, third row). Moreover, individual fluorescent spots of QD-labeled rslo channel were clearly discernable for tracking in live cells (Fig. 1 B, third row and enlarged picture shown in fourth row). In contrast, no significant labeling of QDs was observed in the cells transfected with BirA-ER together with RFP (first row) or AP-rSlo-RFP alone (second row), showing that the biotin ligases targeted into the ER lumen recognized the AP tag of AP-rSlo-RFP and specifically biotinylated the channel proteins. The C-terminal fusion of RFP did not seem to affect the surface expression of rslo channels or their labeling by QDs significantly, because the level of QD labeling observed in cells cotransfected triply with AP-rSlo, free RFP, and BirA-ER was comparable to that of the cells cotransfected with AP-rSlo-RFP and BirA-ER (first and second rows in Fig. S1 in the Supporting Material). However, no specific QD labeling of cells cotransfected with an rslo deletion mutant lacking the last 102 amino acids of the C-terminus (AP-rSlo-DC), free RFP, or BirA-ER was observed (third row in Fig. S1), consistent with a previous report showing that this region, which contains a putative actin-binding domain, was critical for the surface expression of Slo channels (25). The functional activity of AP-rSlo-RFP channels was then examined before and after labeling with QDs (Fig. 1 C). The AP-rSlo-RFP channels were expressed robustly in COS-7 cells and activated by membrane voltages and intracellular Ca 2þ. It was evident, however, that the modifications at the N- and C-termini significantly altered the gating characteristics of the channel (Fig. S2). Compared with wild-type Slo, the half-activation voltage (V 1/2 ) of AP-rSlo-RFP channels was shifted 30 mv toward the negative direction and the deactivation rate was decreased by 55% at 80 mv in the presence of 10 mm intracellular Ca 2þ. QD labeling was also found to affect the function of AP-rSlo-RFP channels. Upon addition of QDs, the V 1/2 value of AP-rSlo-RFP channels shifted 44 mv toward the positive direction. Moreover, the QD-labeled AP-rSlo-RFP (QD:AP-rSlo-RFP) exhibited rapid deactivation, e.g., a 4.6-fold increase at 80 mv compared to AP-rSlo-RFP, and the deactivation rates in the
4 2856 Won et al. FIGURE 2 Stoichiometry of QDs on individual BK Ca channels revealed by photoblinking. (A) Photoblinking of single QD-labeled AP-rSlo-RFP channels expressed in COS-7 cells. Time-lapse images of three different fluorescence spots are shown over 0.05-s intervals. Each symbol indicates identical spots blinking intermittently in all frames. Note that the fluorescence intensity of certain spots (open arrowheads) was significantly higher than that of others (solid arrowhead). (B) Representative traces for the fluorescence intensity of QD-labeled AP-rSlo-RFP channels (red and blue) and free QD (black). Fluorescence intensity is represented in arbitrary units (A.U.). positive voltage range were not significantly voltage-dependent. Thus, these results indicated that the modified BK Ca channel a-subunit, the AP-rSlo-RFP channel, was robustly expressed in COS-7 cells and could be labeled specifically with QDs as a Ca 2þ and voltage-activated channel. Stoichiometry of quantum-dot labeled BK Ca channels We wondered how many QDs actually contributed to the individual fluorescence spots observed in the images shown in Fig. 1. To estimate the stoichiometry of QDs, time-dependent changes in fluorescence intensity were monitored. The individual fluorescence signals turned on and off intermittently, a phenomenon known as photoblinking, and some spots emitted much brighter fluorescence then others (Fig. 2 A). Photoblinking is a property of QDs in which the light emission of QDs has on and off periods due to photoionization (31). By monitoring photoblinking behavior, the number of QDs contributing individual fluorescence spots could be quantified (32). In the case of free QD particles, the fluorescence intensities were measured at ~40 arbitrary units (A.U.) and occasionally fell to basal levels (Fig. 2 B, black trace). When the intensities of 35 different fluorescence spots on COS-7 cells were monitored, two different types of fluorescence traces were obtained. Seventeen of them showed intensity levels of ~40 A.U., which intermittently dropped to near 0 A.U. (Fig. 2 B, blue trace), similar to that of free QD. The intensities of the other 18 fluorescence spots fluctuated at ~80 A.U., rapidly stepped down to 40 A.U., and then, sometimes, further down to the basal level (Fig. 2 B, red trace). By comparing the levels of photoblinking, the number of QDs contributing to individual fluorescence spots could be counted and the stoichiometry of QDs attached to single AP-rSlo-RFP channels inferred. Our results indicated that the individual fluorescence spots derived from the contribution of either one or two QDs, suggesting that each BK Ca channel had been labeled by the aforementioned numbers of QDs. On average, 1.51 QDs per channel molecule (n ¼ 35) were estimated. It is noteworthy that a fluorescence level corresponding to more than two QDs was never observed in our experiments. Membrane mobility of BK Ca channels expressed in COS-7 cells The movement of QD-labeled BK Ca channels in COS-7 cells was then investigated using time-lapse imaging. Individual fluorescent particles were tracked at 300-ms time intervals over 15 s (Movie S1). The trajectories of five different QD-labeled AP-rSlo-RFP channels are shown in Fig. 3 A. Qualitatively, the movement of the channels expressed in COS-7 cells was homogeneous and rather restricted. The dynamics of individual channels were analyzed for two different criteria: a histogram of displacement at a fixed time-interval, and time-dependent displacement. In Fig. 3 B, the mean-squared displacements (MSDs) at 15 s are plotted for a total of 300 QD-labeled rslo channels (open bars) and 100 QDs nonspecifically attached to the coverslip (hatched bar). We observed a single peak with a maximum value at (R 2 ¼ mm 2 ) from the Gaussian curve fit, suggesting a single population of channel movement (Fig. 3 B). The diffusion characteristics of individual channels were also evaluated by plotting MSD as a function of time. In the plot, the QD-labeled rslo channels expressed in COS-7 cells showed a sublinear relationship (Fig. 3 C). The initial-slope and the asymptote of the curve were estimated as mm 2 /s and mm 2, respectively. In two-dimensional diffusion, these two values are considered to represent the initial diffusion coefficient, D init, of mm 2 /s and the confinement area for diffusion of mm 2 (or a circular area of 0.76 mm in radius), respectively. Tracking of QD-labeled BK Ca channels in cultured hippocampal neurons In order to study the dynamics of BK Ca channel in live neurons, AP-rSlo-RFP and BirA-ER were expressed in cultured rat hippocampal pyramidal cells. The specific labeling of QDs was also verified in the neurons (Fig. S3), and the movement of individual QD-labeled BK Ca channels
5 Membrane Mobility of BK Channels 2857 FIGURE 3 Dynamics of individual QD-labeled BK Ca channels in live COS-7 cells. (A) Trajectories of five different QD-labeled AP-rSlo-RFP channels. QD-labeled channels were selected in the central (a c) and peripheral regions (d and e) of cells. Scale bar, 10 mm. (Lower panel) Magnified view (3) of individual trajectories. (B) Histogram of mean-squared displacement (MSD) of QDlabeled AP-rSlo-RFP at 15 s. The distribution of MSD values at 15 s is shown for QD-labeled APrSlo-RFP channels (open bars, n ¼ 300). As the control, free QDs attached nonspecifically to the coverslip are shown (hatched bar, n ¼ 100). (C) MSD versus time plot of QD-labeled AP-rSlo-RFP. Time-dependent changes in average MSD values are shown for QD-labeled AP-rSlo-RFP channels (open circles) and free QDs (diamonds). The time interval is 300 ms, and error bar represents mean 5 SE. was monitored. The trajectories of five different QD-labeled channels tracked at 300-ms time intervals over 15 s are shown in Fig. 4 A. Unlike in COS-7 cells, the movement of AP-rSlo-RFP channels in neurons was significantly heterogeneous (Movie S2). While the channels located in the neuronal cell bodies (or somata) were rather static (Fig. 4 A, a and b), those in axodendritic areas were highly mobile (Fig. 4 A, c e). Heterogeneous movements were also evident in the MSD histogram (Fig. 4 B). Whereas many channels showed slow movement peaking at 0.5 mm 2 /s, faster-moving channels were scattered at higher MSD values with discrete peaks at 2.0 and 5.5 mm 2 /s. From the trajectories of individual particles, we noticed that the QD-labeled AP-rSlo-RFP channels in axodendrites tended to move along the direction of each process. In order to appreciate this behavior of the channels, 46 consecutive movement vectors were illustrated for a single QD-labeled channel as an example (Fig. 4 C). The orientation of each vector was normalized to the direction of the dendrite at the vector origin. It was evident that most of the individual steps were along the direction of the neuronal process. Because the dynamics of QD-labeled BK Ca channels clearly differed between somata (Movie S3) and axodendrites (Movie S4), the distribution of MSD in these two areas FIGURE 4 Dynamics of individual QD-labeled BK Ca channels in cultured hippocampal neurons. (A) Trajectories of five different QD-labeled BK Ca channels. QD-labeled AP-rSlo-RFP channels were selected in the soma (a and b) and axodendritic area (c e). Scale bar, 10 mm. Magnified view (3) of individual trajectories (lower panel). (B) MSD histogram at 15 s. The distribution of displacement at 15 s is shown for QD-labeled APrSlo-RFP channels (open channel, n ¼ 1000) and free QDs (hatched dark-shaded channel, n ¼ 100). (C) Vector plot of QD-labeled AP-rSlo- RFP channels in axodendritic areas. Direction and distance are shown for 47 frames of a single representative QD-labeled AP-rSlo-RFP channel moving along a neuronal axodendrite. The orientation of each vector was normalized at the origin to the direction of the axodendritic process. The direction toward the soma was set at 0 and that toward the terminal region of the axodendrite was at 180.
6 2858 Won et al. was replotted separately (Fig. 5, A and B, upper panels). The QD-labeled rslo channels in somata showed much slower mobility, with the MSD at 15 s rapidly decaying from the origin of the plot. In contrast, the channels located in axodendritic areas exhibited much faster and broader ranges of movement, with several different peaks between 0 and 15 mm 2 /s. From the plot of MSD versus time, the initial slopes of QD-labeled rslo channels were estimated as mm 2 /s for somata and mm 2 /s in axodendritic areas. In the soma area, QD-labeled channels may experience two different types of diffusion environments, fast diffusion within 0.05 mm 2, and much slower diffusion beyond 0.05 mm 2 (Fig. 5 A, lower panel). Moreover, the MSDversus-time relationship in the slower diffusion regime was fairly linear, at least up to 15 s, suggesting a free diffusion in this region of the neuron. For the channels in axodendritic areas, a partial confinement of diffusion was evident, as indicated by a clear sublinear relationship in the initial 2.5 s of MSD versus time (Fig. 5 B, lower panel). The asymptote of MSD values observed in the initial 2.5 s was estimated as mm 2. These channels also showed a near-linear relationship between 2.5 s and 11 s, or the area between 0.7 mm 2 /s to 4 mm 2 /s, and then reached another plateau beyond 11 s. Due to the dominant movement along the direction of neuronal processes, two-dimensional D init values from the initial slope of the MSD-versus-time plot were not assigned to QD-labeled channels in axodendritic areas. Although the QD-labeled AP-rSlo-RFP channels exhibited complex and heterogeneous behavior in both neuronal somata and axodendrites, we were able to conclude that movement occurred in axodendrites more heterogeneously and much more rapidly than in the neuronal cell bodies. FIGURE 5 MSD histogram and time-dependent MSD plot of QD-labeled BK Ca channels in neuronal somata and axodendrites. (A) MSD histogram at 15 s (upper panel) and time-dependent MSD plot (lower panel) ofbk Ca channels in somata. The distribution of MSD at 15 s is shown for QD-labeled AP-rSlo-RFP channels (open dark yellow, n ¼ 500) and free QDs (hatched dark gray, n ¼ 100) in soma areas. Time-dependent average MSD values are shown for QD-labeled AP-rSlo-RFP channels (open dark-yellow circles) and free QDs (dark gray diamonds). The time-interval between adjacent circles or diamonds is 300 ms. Error bars represent mean 5 SE. (B) MSD histogram at 15 s (upper panel) and time-dependent MSD plot (lower panel) of BK Ca channels in axodendrites. The distribution of MSD at 15 s is shown for QD-labeled AP-rSlo-RFP channels (open red, n ¼ 500) and free QD (hatched dark-gray, n ¼ 100) in axodendritic areas. Time-dependent average MSD values are shown for QD-labeled rslo channels (open red circles) and free QD (dark-gray diamonds). The time-interval between adjacent circles or diamonds is 300 ms. Error bars represent mean 5 SE. Differential effects of b4 subunits on the mobility of channels expressed in somata and dendrites Composed of two different subunits, BK Ca channels can be assembled in vivo as either homotetramers comprised of only a-subunits or heterooctamers made up of a- and b-subunits. BK Ca channels in central neurons can contain the b4 subunit, a neuron-specific auxiliary b-subunit. Thus, the effects of the b4 subunit on the membrane mobility of QD-labeled BK Ca channels were investigated in both COS-7 cells and cultured hippocampal neurons. To ensure the coexpression of a- and b4 subunits in identical cells, the C-terminus of rat b4, rb4, was tagged with green fluorescent protein (GFP) (Fig. S4). In addition, a fivefold molar excess of rb4-gfp gene was used in transfection to provide enough b4 subunit for complex formation (26). The coassembly between AP-rSlo-RFP and rb4-gfp in COS-7 cells were verified by confocal imaging and coimmunoprecipitation (Fig. S5). The movement of individual QD-labeled BK Ca channels coexpressed with b4 subunit was visualized in COS-7 cells (Movie S5) and hippocampal neurons (Movie S6 and Movie S7). The trajectories of five representative QD-labeled AP-rSlo-RFP/rb4-GFP channels are shown in Fig. 6, A and B. FIGURE 6 Dynamics of QD-labeled BK Ca channels coassembled with a- and b-subunits in COS-7 cells and hippocampal neurons. (A) Trajectories of five different QD-labeled rslo/rb4 channels in COS-7 cells. QD-labeled AP-rSlo-RFP/rb4-GFP channels were selected in the central (a c) and peripheral regions (d and e) of cells. Scale bar, 10 mm. (Lower panel) Magnified view (3) of individual trajectories. (B) Trajectories of five different QD-labeled rslo/rb4 channels. QD-labeled AP-rSlo-RFP/rb4-GFP channels were selected in soma (a and b) and axodendritic areas (c e). Scale bar, 10 mm. (Lower panel) Magnified view (3) of individual trajectories.
7 Membrane Mobility of BK Channels 2859 FIGURE 7 Effects of b4-subunits on the dynamics of QD-labeled BK Ca channels in COS-7 cells and hippocampal neurons. (A) MSD histogram at 15 s (upper panel) and time-dependent MSD plot (lower panel)ofbk Ca channels in COS-7 cells. The distribution of MSD at 15 s is shown for QD-labeled AP-rSlo-RFP/ rb4-gfp channels (solid black, n ¼ 300) and AP-rSlo-RFP channels (open black, n ¼ 300). Time-dependent average MSD values are shown for QD-labeled AP-rSlo-RFP/rb4-GFP channels (solid circle, n ¼ 300) and AP-rSlo-RFP channels (open circle, n ¼ 300). (B) MSD histogram at 15 s (upper panel) and timedependent MSD plot (lower panel)ofbk Ca channels in somata of hippocampal neurons. The distribution of MSD at 15 s is shown for QD-labeled AP-rSlo-RFP/ rb4-gfp channels (solid dark yellow, n ¼ 500) and AP-rSlo-RFP channels (open dark yellow, n ¼ 500). Time-dependent average MSD values are shown for QD-labeled AP-rSlo-RFP/rb4-GFP channels (solid circle, n ¼ 500) and AP-rSlo-RFP channels (open circle, n ¼ 500). (C) MSD histogram at 15 s (upper panel) and time-dependent MSD plot (lower panel) of BK Ca channels in axodendrites of hippocampal neurons. The distribution of MSD at 15 s is shown for QD-labeled AP-rSlo-RFP/rb4-GFP channels (solid red, n ¼ 500) and AP-rSlo-RFP channels (open red, n ¼ 500). Time-dependent average MSD values are shown for QD-labeled AP-rSlo-RFP/rb4-GFP channels (solid circle, n ¼ 500) and AP-rSlo-RFP channels (open circle, n ¼ 500). The time-interval between two adjacent symbols is 300 ms. Error bars represent mean 5 SE. Compared with homomeric a-channels, the mobility of QD-labeled a/b4 heteromeric BK Ca channels was quite distinct both in COS-7 cells and neurons. In Fig. 7, the effects of the b4 subunit were quantitatively analyzed by comparing the distribution of displacement at 15 s (upper panels) and time-dependent displacement (lower panels). In COS-7 cells, b4 coexpression significantly decreased channel mobility, as evident in the shifts of the distribution to smaller MSD values (Fig. 7 A, upper panel). The MSD-versus-time plot indicated an initial slope of mm 2 /s and asymptote of mm 2, corresponding to a 16.5-fold decrease in D init and a 5.1-fold reduction in the confinement area by the coassembly of the b4 subunit. The effects of b4 were even more dramatic and intriguing in neurons. While the movement of QD-labeled BK Ca channels was decreased in axodendritic areas, the channels in somata became much more mobile with b4 coexpression (Fig. 7, B and C). The initial slopes of MSD-versus-time plots were estimated at mm 2 /s in somata and mm 2 /s in axodendrites. Thus, the mobility of BK Ca channels was decreased 5.8-fold in axodendrites but increased 6.7-fold in somata by the coexpression of the b4 subunit. Because the mobility of the channels was oppositely affected in somata and axodendrites, the diffusion coefficients of a/b heteromeric BK Ca channel became similar in these two areas. DISCUSSION As sensors for membrane voltage and intracellular Ca 2þ that link cell excitability, signaling, and metabolism, BK Ca channels are expressed widely in various tissues and involved in diverse physiological processes. Although their functional roles have been related to their specific localization to certain cellular and subcellular regions (20), the visualization of the individual BK Ca channels and tracing of their movement in cells has not previously been possible. In this study, single BK Ca channels were visualized in live cells and their movement was monitored in real time. Utilizing the acceptor peptide for biotin tagged at the extracellular N-terminus of the channel and the ER-targeted bacterial biotin ligase, we were able to express biotin-conjugated BK Ca channels on the cell surface and to achieve efficient and specific labeling of the channel with streptavidin-conjugated QDs. Compared to the metabolic labeling utilized in this study (24), the previously described method employing direct conjugation of exogenous biotin using the
8 2860 Won et al. purified biotin ligase (23) tends to result in significantly higher levels of nonspecific QD-labeling in the cells tested (S. Won and CS. Park, unpublished observation). Using the photoblinking property of QDs, the stoichiometry of QDs labeling each BK Ca channel was determined. Although up to four QDs can theoretically bind each channel, the channel was labeled by either one or two QDs. Considering the size of streptavidin-conjugated QDs used in this study, estimated at ~20 nm in diameter, the BK Ca channel may not provide sufficient space to accommodate more than two QDs. This idea is supported by a recent structural study using electron microscopy, in which the diameter of the homotetrameric BK Ca channel was determined to be ~20 nm (33), similar to the size of streptavidin-conjugated QD. These results also indicate that most, if not all, BK Ca channels expressed in COS-7 cells are not clustered in groups but separated individually on the membrane surface. In COS-7 cells, the movement of homomeric BK Ca channel can be described as a confined diffusion with the diffusion coefficient (D) of mm 2 /s and the confined area of mm 2 (Fig. 3 C). While this diffusion is substantially faster than that of highly confined membrane proteins, e.g., cystic fibrosis transmembrane conductance regulator expressed in COS-7 cells, with D ¼ mm 2 /s (34), it is comparable to that of QD-labeled aquaporin1 in COS-7 cells showing partially restricted diffusion with D ¼ mm 2 /s (35). A similar diffusion coefficient, mm 2 /s, was also reported for another type of Ca 2þ -activated K þ channel, the IK Ca channel, expressed in MDCK cells and labeled with antibody-conjugated QDs (36). We then applied the specific labeling of QDs and singleparticle tracking of the labeled BK Ca channels in cultured neurons. The behavior of homomeric BK Ca channels in cultured neurons was more complex than that in COS-7 cells. First of all, channel mobility differed markedly between somata and axodendrites (Fig. 4 and Movie S2). The initial slope of MSD versus time was 36-fold greater in axodendritic areas than in somata (Fig. 5). In fact, the diffusion coefficient measured in somata was almost 11-fold lower than that in COS-7 cells; thus, the channels moved extremely slowly. It will be intriguing to find the cellular mechanism underlying the drastic increase in BK Ca channel mobility upon entering neuronal processes from the cell body. We also wonder whether the large difference in mobility is specific for BK Ca channels or a general phenomenon among different ion channels, because there is no report describing the movement of a population of channel proteins in an entire neuron. Secondly, the trajectory of QD-labeled channels revealed that most of their movements followed the direction of axodendritic processes (Fig. 4 C). It is not clear whether this type of movement is due to the geometric constraint of neuronal processes or the role of cytoskeletal components in axodendritic transports. Nonetheless, the preferential movement along the process seems to render diffusion within this area pseudo-one-dimensional. Thirdly, the movement of individual channels within the axodendritic area was highly heterogeneous. As shown in Fig. 5 B, the position of QD-labeled BK Ca channels was fairly dispersed, with several peaks in the MSD histogram and at least three different diffusion regimes evident in the plot of MSD versus time. Considering the structural and functional heterogeneity of axodendritic arborization, individual BK Ca channels may conceivably experience quite distinct and diverse microenvironments during diffusional movement in the area. Moreover, the QD-labeled BK Ca channels in axons, expected to be a small population in our analysis, may behave significantly differently from those in dendrites. Despite the clear sublinear relationship observed in the MSD versus time plot, the heterogeneity and the pseudo-one-dimensional nature of movement preclude us from analyzing data from the neuronal axodendritic area based on the confined diffusion regime. In unraveling the behavioral complexity of the channels in neuronal cells, we may have to limit our observation to defined regions of the neuron, such as neuronal synapses, using a higher spatiotemporal resolution. In the mammalian central nervous system, the majority of BK Ca channels are assembled with two different subunits, a- and b4. Thus, we also investigated the effects of the neuronal b4 subunit on channel mobility. The molecular mass of the homotetrameric BK Ca channel is estimated to be ~515 kda. Considering the molecular mass of b4, 24 kda, the coassembly of four b4 would increase the molecular mass of channel complexes by 96 kda without taking into account any glycosylation. Coexpression of b4 significantly reduced the mobility of QD-labeled BK Ca channels in COS-7 cells and the axodendritic area of hippocampal neurons (Fig. 7, A and C). To our surprise, however, b4 coexpression dramatically increased the mobility of BK Ca channels in the soma area. The initial diffusion coefficient was increased by 7.3-fold (Fig. 7 B). Although it is still unclear whether the b4 subunit can induce similar effects on the endogenous channels, our results suggest potential and unexplored roles of this auxiliary subunit in the transport and targeting of BK Ca channels in neuronal cells. Moreover, the drastic influence of the b4 subunit on channel mobility provides us with an assay method to determine the domain (s) responsible for and the molecular mechanism underlying the effects of b4. It still remains to be examined whether there is any physiological relevance to the differential influence of b4 on channel mobility in different neuronal regions in vivo. Although only limited information is currently available regarding the dynamics of BK Ca channels in neuronal cells, a recent study demonstrated that certain neurons may contain two different pools of BK Ca channels: scattered and clustered (37). While scattered BK Ca channels were distributed throughout the somatodendritic regions of cerebellar Purkinje cells, the clustered channels were restricted to the
9 Membrane Mobility of BK Channels 2861 somata and the very proximal portion of dendrites. Na þ and Ca 2þ spikes were also found to modulate differentially the two different channel pools. Thus, it would be intriguing to determine whether the distribution, dynamics, and physiological function of BK Ca channels in specific neurons can be modulated by auxiliary subunits. In summary, we were able to probe individual BK Ca channels with QDs and monitored their movement in live cells. Our results demonstrate that the mobility of the BK Ca channel can vary significantly depending on cell type, subcellular location, and subunit composition. The experimental technique introduced in this study can be utilized to unravel the cellular mechanisms that determine the mobility and localization of BK Ca channels and other membrane proteins. SUPPORTING MATERIAL Five figures and seven movies are available at biophysj/supplemental/s (10) The authors are grateful to Dr. Alice Y. Ting (Massachusetts Institute of Technology, Cambridge, MA) for providing pdisplay BirA-ER, the members of the Laboratory of Molecular Neurobiology at Gwangju Institute of Science and Technology (GIST), Gwangju, South Korea for their valuable comments, and the Bio-Imaging Research Center at GIST for providing various imaging equipment. This research was supported in part by the grants from the Bio-Imaging Research Center and the Cell Dynamics Research Center of GIST, and the National Research Foundation of Korea (grant No. E00025) to C.-S.P. REFERENCES 1. Vergara, C., R. Latorre,., J. P. Adelman Calcium-activated potassium channels. Curr. Opin. Neurobiol. 8: Latorre, R., and S. Brauchi Large conductance Ca 2þ -activated K þ (BK) channel: activation by Ca 2þ and voltage. Biol. Res. 39: Salkoff, L., A. Butler,., A. Wei High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 7: Nelson, M. T., H. Cheng,., W. J. Lederer Relaxation of arterial smooth muscle by calcium sparks. Science. 270: Yazejian, B., D. A. DiGregorio,., A. D. Grinnell Direct measurements of presynaptic calcium and calcium-activated potassium currents regulating neurotransmitter release at cultured Xenopus nervemuscle synapses. J. Neurosci. 17: Fettiplace, R., and P. A. Fuchs Mechanisms of hair cell tuning. Annu. Rev. Physiol. 61: Brenner, R., G. J. Peréz,., R. W. Aldrich Vasoregulation by the b1 subunit of the calcium-activated potassium channel. Nature. 407: Knaus, H. G., K. Folander,., R. Swanson Primary sequence and immunological characterization of b-subunit of high conductance Ca 2þ -activated K þ channel from smooth muscle. J. Biol. Chem. 269: McManus, O. B., L. M. Helms,., R. J. Leonard Functional role of the b-subunit of high conductance calcium-activated potassium channels. Neuron. 14: Catterall, W. A Structure and function of voltage-gated ion channels. Annu. Rev. Biochem. 64: Jiang, Y., A. Pico,., R. MacKinnon Structure of the RCK domain from the E. coli K þ channel and demonstration of its presence in the human BK channel. Neuron. 29: Wallner, M., P. Meera,., L. Toro Characterization of and modulation by a b-subunit of a human maxi K Ca channel cloned from myometrium. Receptors Channels. 3: Meera, P., M. Wallner, and L. Toro A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca 2þ -activated K þ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl. Acad. Sci. USA. 97: Sausbier, U., M. Sausbier,., P. Ruth Ca 2þ -activated K þ channels of the BK-type in the mouse brain. Histochem. Cell Biol. 125: Edgerton, J. R., and P. H. Reinhart Distinct contributions of small and large conductance Ca 2þ -activated K þ channels to rat Purkinje neuron function. J. Physiol. 548: Benhassine, N., and T. Berger Homogeneous distribution of large-conductance calcium-dependent potassium channels on soma and apical dendrite of rat neocortical layer 5 pyramidal neurons. Eur. J. Neurosci. 21: Sailer, C. A., W. A. Kaufmann,., H. G. Knaus Immunolocalization of BK channels in hippocampal pyramidal neurons. Eur. J. Neurosci. 24: Hu, H., L. R. Shao,., J. F. Storm Presynaptic Ca 2þ -activated K þ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J. Neurosci. 21: Shao, L. R., R. Halvorsrud,., J. F. Storm The role of BK-type Ca 2þ -dependent K þ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J. Physiol. 521: Trimmer, J. S., and K. J. Rhodes Localization of voltage-gated ion channels in mammalian brain. Annu. Rev. Physiol. 66: Bruchez, Jr., M., M. Moronne,., A. P. Alivisatos Semiconductor nanocrystals as fluorescent biological labels. Science. 281: Han, M., X. Gao,., S. Nie Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 19: Howarth, M., K. Takao,., A. Y. Ting Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl. Acad. Sci. USA. 102: Howarth, M., and A. Y. Ting Imaging proteins in live mammalian cells with biotin ligase and monovalent streptavidin. Nat. Protoc. 3: Zou, S., S. Jha,., S. E. Dryer A novel actin-binding domain on Slo1 calcium-activated potassium channels is necessary for their expression in the plasma membrane. Mol. Pharmacol. 73: Ha, T. S., M. S. Heo, and C. S. Park Functional effects of auxiliary b4-subunit on rat large-conductance Ca 2þ -activated K þ channel. Biophys. J. 86: Patton, C., S. Thompson, and D. Epel Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 35: Kusumi, A., Y. Sako, and M. Yamamoto Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys. J. 65: Beckett, D., E. Kovaleva, and P. J. Schatz A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 8: Barat, B., and A. M. Wu Metabolic biotinylation of recombinant antibody by biotin ligase retained in the endoplasmic reticulum. Biomol. Eng. 24: Nirmal, M., B. O. Dabbousi,., L. E. Brus Fluorescence intermittency in single cadmium selenide nanocrystals. Nature. 383:
10 2862 Won et al. 32. Månsson, A., M. Sundberg,., L. Montelius In vitro sliding of actin filaments labeled with single quantum dots. Biochem. Biophys. Res. Commun. 314: Wang, L., and F. J. Sigworth Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 461: Jin, S., P. M. Haggie, and A. S. Verkman Single-particle tracking of membrane protein diffusion in a potential: simulation, detection, and application to confined diffusion of CFTR Cl channels. Biophys. J. 93: Crane, J. M., and A. S. Verkman Long-range nonanomalous diffusion of quantum dot-labeled aquaporin-1 water channels in the cell plasma membrane. Biophys. J. 94: Nechyporuk-Zloy, V., P. Dieterich,., A. Schwab Dynamics of single potassium channel proteins in the plasma membrane of migrating cells. Am. J. Physiol. Cell Physiol. 294:C1096 C Kaufmann, W. A., F. Ferraguti,., O. P. Ottersen Large-conductance calcium-activated potassium channels in purkinje cell plasma membranes are clustered at sites of hypolemmal microdomains. J. Comp. Neurol. 515:
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Changes in microtubule overlap length regulate kinesin-14-driven microtubule sliding
 Supplementary information Changes in microtubule overlap length regulate kinesin-14-driven microtubule sliding Marcus Braun* 1,2,3, Zdenek Lansky* 1,2,3, Agata Szuba 1,2, Friedrich W. Schwarz 1,2, Aniruddha
Supplementary information Changes in microtubule overlap length regulate kinesin-14-driven microtubule sliding Marcus Braun* 1,2,3, Zdenek Lansky* 1,2,3, Agata Szuba 1,2, Friedrich W. Schwarz 1,2, Aniruddha
CELL BIOLOGY - CLUTCH CH. 9 - TRANSPORT ACROSS MEMBRANES.
 !! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
!! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
Ion Channel Structure and Function (part 1)
 Ion Channel Structure and Function (part 1) The most important properties of an ion channel Intrinsic properties of the channel (Selectivity and Mode of Gating) + Location Physiological Function Types
Ion Channel Structure and Function (part 1) The most important properties of an ion channel Intrinsic properties of the channel (Selectivity and Mode of Gating) + Location Physiological Function Types
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Inhibition of S532C by MTSET at intracellular ph 6.8 indicates accessibility in the closed
 Supplementary Text Inhibition of S532C by MTSET at intracellular ph 6.8 indicates accessibility in the closed state It is difficult to examine accessibility of cysteine-substituted mutants in the fully
Supplementary Text Inhibition of S532C by MTSET at intracellular ph 6.8 indicates accessibility in the closed state It is difficult to examine accessibility of cysteine-substituted mutants in the fully
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES In Physiology Today Ohm s Law I = V/R Ohm s law: the current through a conductor between two points is directly proportional to the voltage across the
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES In Physiology Today Ohm s Law I = V/R Ohm s law: the current through a conductor between two points is directly proportional to the voltage across the
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature09450 Supplementary Table 1 Summary of kinetic parameters. Kinetic parameters were V = V / 1 K / ATP and obtained using the relationships max ( + m [ ]) V d s /( 1/ k [ ATP] + 1 k ) =,
doi:10.1038/nature09450 Supplementary Table 1 Summary of kinetic parameters. Kinetic parameters were V = V / 1 K / ATP and obtained using the relationships max ( + m [ ]) V d s /( 1/ k [ ATP] + 1 k ) =,
Neurons. The Molecular Basis of their Electrical Excitability
 Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Name: TF: Section Time: LS1a ICE 5. Practice ICE Version B
 Name: TF: Section Time: LS1a ICE 5 Practice ICE Version B 1. (8 points) In addition to ion channels, certain small molecules can modulate membrane potential. a. (4 points) DNP ( 2,4-dinitrophenol ), as
Name: TF: Section Time: LS1a ICE 5 Practice ICE Version B 1. (8 points) In addition to ion channels, certain small molecules can modulate membrane potential. a. (4 points) DNP ( 2,4-dinitrophenol ), as
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Membrane Protein Channels
 Membrane Protein Channels Potassium ions queuing up in the potassium channel Pumps: 1000 s -1 Channels: 1000000 s -1 Pumps & Channels The lipid bilayer of biological membranes is intrinsically impermeable
Membrane Protein Channels Potassium ions queuing up in the potassium channel Pumps: 1000 s -1 Channels: 1000000 s -1 Pumps & Channels The lipid bilayer of biological membranes is intrinsically impermeable
لجنة الطب البشري رؤية تنير دروب تميزكم
 1) Hyperpolarization phase of the action potential: a. is due to the opening of voltage-gated Cl channels. b. is due to prolonged opening of voltage-gated K + channels. c. is due to closure of the Na +
1) Hyperpolarization phase of the action potential: a. is due to the opening of voltage-gated Cl channels. b. is due to prolonged opening of voltage-gated K + channels. c. is due to closure of the Na +
Nature Methods: doi: /nmeth Supplementary Figure 1. In vitro screening of recombinant R-CaMP2 variants.
 Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
7.06 Cell Biology EXAM #3 April 21, 2005
 7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
me239 mechanics of the cell - syllabus me239 mechanics of the cell me239 mechanics of the cell - grading me239 mechanics of the cell - overview
 6 mechanotransduction wong, goktepe, kuhl [2010] me239 mechanics of the cell add l information http://biomechanics.stanford.edu and coursework 1 me239 mechanics of the cell - syllabus favorite topics in
6 mechanotransduction wong, goktepe, kuhl [2010] me239 mechanics of the cell add l information http://biomechanics.stanford.edu and coursework 1 me239 mechanics of the cell - syllabus favorite topics in
Membrane Physiology. Dr. Hiwa Shafiq Oct-18 1
 Membrane Physiology Dr. Hiwa Shafiq 22-10-2018 29-Oct-18 1 Chemical compositions of extracellular and intracellular fluids. 29-Oct-18 2 Transport through the cell membrane occurs by one of two basic processes:
Membrane Physiology Dr. Hiwa Shafiq 22-10-2018 29-Oct-18 1 Chemical compositions of extracellular and intracellular fluids. 29-Oct-18 2 Transport through the cell membrane occurs by one of two basic processes:
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34
 NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Cardiac cell-cell Communication Part 1 Alonso P. Moreno D.Sc. CVRTI, Cardiology
 Bioengineering 6003 Cellular Electrophysiology and Biophysics Cardiac cell-cell Communication Part 1 Alonso P. Moreno D.Sc. CVRTI, Cardiology moreno@cvrti.utah.edu November 2010 poster Physiological Relevance
Bioengineering 6003 Cellular Electrophysiology and Biophysics Cardiac cell-cell Communication Part 1 Alonso P. Moreno D.Sc. CVRTI, Cardiology moreno@cvrti.utah.edu November 2010 poster Physiological Relevance
R7.3 Receptor Kinetics
 Chapter 7 9/30/04 R7.3 Receptor Kinetics Professional Reference Shelf Just as enzymes are fundamental to life, so is the living cell s ability to receive and process signals from beyond the cell membrane.
Chapter 7 9/30/04 R7.3 Receptor Kinetics Professional Reference Shelf Just as enzymes are fundamental to life, so is the living cell s ability to receive and process signals from beyond the cell membrane.
Measuring Colocalization within Fluorescence Microscopy Images
 from photonics.com: 03/01/2007 http://www.photonics.com/article.aspx?aid=39341 Measuring Colocalization within Fluorescence Microscopy Images Two-color fluorescence-based methods are uncovering molecular
from photonics.com: 03/01/2007 http://www.photonics.com/article.aspx?aid=39341 Measuring Colocalization within Fluorescence Microscopy Images Two-color fluorescence-based methods are uncovering molecular
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016
 PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Eisner et al., http://www.jcb.org/cgi/content/full/jcb.201312066/dc1 Figure S1. Mitochondrial continuity in adult skeletal muscle. (A)
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Eisner et al., http://www.jcb.org/cgi/content/full/jcb.201312066/dc1 Figure S1. Mitochondrial continuity in adult skeletal muscle. (A)
Introduction to electrophysiology. Dr. Tóth András
 Introduction to electrophysiology Dr. Tóth András Topics Transmembran transport Donnan equilibrium Resting potential Ion channels Local and action potentials Intra- and extracellular propagation of the
Introduction to electrophysiology Dr. Tóth András Topics Transmembran transport Donnan equilibrium Resting potential Ion channels Local and action potentials Intra- and extracellular propagation of the
Fluorescence Enhancement on Silver Nanoplate at the. Single- and Sub-Nanoparticle Level
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry Supporting 2015 Information Fluorescence Enhancement on Silver Nanoplate at the Single- and Sub-Nanoparticle
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry Supporting 2015 Information Fluorescence Enhancement on Silver Nanoplate at the Single- and Sub-Nanoparticle
Waithe et al Supplementary Figures
 Waithe et al Supplementary Figures Supplementary Figure 1 Expression and properties of WT and W391A mutant YFP- Ca V 2.2. A Immunoblot using Ca V 2.2 Ab for untransfected cells (UT, lane 1), YFP-Ca V 2.2
Waithe et al Supplementary Figures Supplementary Figure 1 Expression and properties of WT and W391A mutant YFP- Ca V 2.2. A Immunoblot using Ca V 2.2 Ab for untransfected cells (UT, lane 1), YFP-Ca V 2.2
Lecture 2. Excitability and ionic transport
 Lecture 2 Excitability and ionic transport Selective membrane permeability: The lipid barrier of the cell membrane and cell membrane transport proteins Chemical compositions of extracellular and intracellular
Lecture 2 Excitability and ionic transport Selective membrane permeability: The lipid barrier of the cell membrane and cell membrane transport proteins Chemical compositions of extracellular and intracellular
Potassium channel gating and structure!
 Reading: Potassium channel gating and structure Hille (3rd ed.) chapts 10, 13, 17 Doyle et al. The Structure of the Potassium Channel: Molecular Basis of K1 Conduction and Selectivity. Science 280:70-77
Reading: Potassium channel gating and structure Hille (3rd ed.) chapts 10, 13, 17 Doyle et al. The Structure of the Potassium Channel: Molecular Basis of K1 Conduction and Selectivity. Science 280:70-77
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #1 Tuesday, September 18, 2012 1. INTRODUCTION TO EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in 1780s
ECE 795: Quantitative Electrophysiology Notes for Lecture #1 Tuesday, September 18, 2012 1. INTRODUCTION TO EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in 1780s
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #1 Wednesday, September 13, 2006 1. INTRODUCTION TO EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in
ECE 795: Quantitative Electrophysiology Notes for Lecture #1 Wednesday, September 13, 2006 1. INTRODUCTION TO EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals?
 LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
STUDENT PAPER. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters Education Program 736 S. Lombard Oak Park IL, 60304
 STUDENT PAPER Differences between Stochastic and Deterministic Modeling in Real World Systems using the Action Potential of Nerves. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters
STUDENT PAPER Differences between Stochastic and Deterministic Modeling in Real World Systems using the Action Potential of Nerves. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters
80% of all excitatory synapses - at the dendritic spines.
 Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Cells. Steven McLoon Department of Neuroscience University of Minnesota
 Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
MEMBRANE STRUCTURE. Lecture 9. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
Biomedical Instrumentation
 ELEC ENG 4BD4: Biomedical Instrumentation Lecture 5 Bioelectricity 1. INTRODUCTION TO BIOELECTRICITY AND EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in 1780s
ELEC ENG 4BD4: Biomedical Instrumentation Lecture 5 Bioelectricity 1. INTRODUCTION TO BIOELECTRICITY AND EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in 1780s
Universality of sensory-response systems
 excite.org(anism): Electrical Signaling Universality of sensory-response systems Three step process: sensation-integration-response Bacterial chemotaxis Madigan et al. Fig. 8.24 Rick Stewart (CBMG) Human
excite.org(anism): Electrical Signaling Universality of sensory-response systems Three step process: sensation-integration-response Bacterial chemotaxis Madigan et al. Fig. 8.24 Rick Stewart (CBMG) Human
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Novel Nanoparticles for Ultrasensitive Detection and Spectroscopy
 Final Technical Report (DOE-FG02-98ER14873) Project Officer: Dr. Richard Gordon / Dr. John Miller Novel Nanoparticles for Ultrasensitive Detection and Spectroscopy Shuming Nie Indiana University P. 0.
Final Technical Report (DOE-FG02-98ER14873) Project Officer: Dr. Richard Gordon / Dr. John Miller Novel Nanoparticles for Ultrasensitive Detection and Spectroscopy Shuming Nie Indiana University P. 0.
MEMBRANE POTENTIALS AND ACTION POTENTIALS:
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
BME 5742 Biosystems Modeling and Control
 BME 5742 Biosystems Modeling and Control Hodgkin-Huxley Model for Nerve Cell Action Potential Part 1 Dr. Zvi Roth (FAU) 1 References Hoppensteadt-Peskin Ch. 3 for all the mathematics. Cooper s The Cell
BME 5742 Biosystems Modeling and Control Hodgkin-Huxley Model for Nerve Cell Action Potential Part 1 Dr. Zvi Roth (FAU) 1 References Hoppensteadt-Peskin Ch. 3 for all the mathematics. Cooper s The Cell
Overview Organization: Central Nervous System (CNS) Peripheral Nervous System (PNS) innervate Divisions: a. Afferent
 Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Biology September 2015 Exam One FORM G KEY
 Biology 251 17 September 2015 Exam One FORM G KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Biology 251 17 September 2015 Exam One FORM G KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Biology September 2015 Exam One FORM W KEY
 Biology 251 17 September 2015 Exam One FORM W KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Biology 251 17 September 2015 Exam One FORM W KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
Overview of ion channel proteins. What do ion channels do? Three important points:
 Overview of ion channel proteins Protein Structure Membrane proteins & channels Specific channels Several hundred distinct types Organization Evolution We need to consider 1. Structure 2. Functions 3.
Overview of ion channel proteins Protein Structure Membrane proteins & channels Specific channels Several hundred distinct types Organization Evolution We need to consider 1. Structure 2. Functions 3.
Voltage-dependent gating of ion channels Prof. Keith S. Elmslie
 Voltage-Dependent Gating of Ion Channels Department of Pharmacology A.T. Still University Kirksville College of Osteopathic Medicine 1 The action potential (AP) E Na Threshold The N eur onal AP Sensory
Voltage-Dependent Gating of Ion Channels Department of Pharmacology A.T. Still University Kirksville College of Osteopathic Medicine 1 The action potential (AP) E Na Threshold The N eur onal AP Sensory
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
Module Membrane Biogenesis and Transport Lecture 15 Ion Channels Dale Sanders
 Module 0220502 Membrane Biogenesis and Transport Lecture 15 Ion Channels Dale Sanders 9 March 2009 Aims: By the end of the lecture you should understand The principles behind the patch clamp technique;
Module 0220502 Membrane Biogenesis and Transport Lecture 15 Ion Channels Dale Sanders 9 March 2009 Aims: By the end of the lecture you should understand The principles behind the patch clamp technique;
The neuron as a secretory cell
 The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
Deconstructing Actual Neurons
 1 Deconstructing Actual Neurons Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference: The many ionic
1 Deconstructing Actual Neurons Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference: The many ionic
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
Muscle regulation and Actin Topics: Tropomyosin and Troponin, Actin Assembly, Actin-dependent Movement
 1 Muscle regulation and Actin Topics: Tropomyosin and Troponin, Actin Assembly, Actin-dependent Movement In the last lecture, we saw that a repeating alternation between chemical (ATP hydrolysis) and vectorial
1 Muscle regulation and Actin Topics: Tropomyosin and Troponin, Actin Assembly, Actin-dependent Movement In the last lecture, we saw that a repeating alternation between chemical (ATP hydrolysis) and vectorial
Microsystems for Neuroscience and Medicine. Lecture 9
 1 Microsystems for Neuroscience and Medicine Lecture 9 2 Neural Microsystems Neurons - Structure and behaviour Measuring neural activity Interfacing with neurons Medical applications - DBS, Retinal Implants
1 Microsystems for Neuroscience and Medicine Lecture 9 2 Neural Microsystems Neurons - Structure and behaviour Measuring neural activity Interfacing with neurons Medical applications - DBS, Retinal Implants
Rahaf Nasser mohammad khatatbeh
 7 7... Hiba Abu Hayyeh... Rahaf Nasser mohammad khatatbeh Mohammad khatatbeh Brief introduction about membrane potential The term membrane potential refers to a separation of opposite charges across the
7 7... Hiba Abu Hayyeh... Rahaf Nasser mohammad khatatbeh Mohammad khatatbeh Brief introduction about membrane potential The term membrane potential refers to a separation of opposite charges across the
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary Figure S1. Pulses >3mJ reduce membrane resistance in HEK cells. Reversal potentials in a representative cell for IR-induced currents with laser pulses of 0.74 to
SUPPLEMENTARY INFORMATION Supplementary Figure S1. Pulses >3mJ reduce membrane resistance in HEK cells. Reversal potentials in a representative cell for IR-induced currents with laser pulses of 0.74 to
Electrophysiology of the neuron
 School of Mathematical Sciences G4TNS Theoretical Neuroscience Electrophysiology of the neuron Electrophysiology is the study of ionic currents and electrical activity in cells and tissues. The work of
School of Mathematical Sciences G4TNS Theoretical Neuroscience Electrophysiology of the neuron Electrophysiology is the study of ionic currents and electrical activity in cells and tissues. The work of
Neuroscience 201A Exam Key, October 7, 2014
 Neuroscience 201A Exam Key, October 7, 2014 Question #1 7.5 pts Consider a spherical neuron with a diameter of 20 µm and a resting potential of -70 mv. If the net negativity on the inside of the cell (all
Neuroscience 201A Exam Key, October 7, 2014 Question #1 7.5 pts Consider a spherical neuron with a diameter of 20 µm and a resting potential of -70 mv. If the net negativity on the inside of the cell (all
Biosciences in the 21st century
 Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Introduction to electrophysiology 1. Dr. Tóth András
 Introduction to electrophysiology 1. Dr. Tóth András Topics Transmembran transport Donnan equilibrium Resting potential Ion channels Local and action potentials Intra- and extracellular propagation of
Introduction to electrophysiology 1. Dr. Tóth András Topics Transmembran transport Donnan equilibrium Resting potential Ion channels Local and action potentials Intra- and extracellular propagation of
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing
 Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Neurons: Cellular and Network Properties HUMAN PHYSIOLOGY POWERPOINT
 POWERPOINT LECTURE SLIDE PRESENTATION by LYNN CIALDELLA, MA, MBA, The University of Texas at Austin Additional text by J Padilla exclusively for physiology at ECC UNIT 2 8 Neurons: PART A Cellular and
POWERPOINT LECTURE SLIDE PRESENTATION by LYNN CIALDELLA, MA, MBA, The University of Texas at Austin Additional text by J Padilla exclusively for physiology at ECC UNIT 2 8 Neurons: PART A Cellular and
Membrane transport 1. Summary
 Membrane transport 1. Summary A. Simple diffusion 1) Diffusion by electrochemical gradient no energy required 2) No channel or carrier (or transporter protein) is needed B. Passive transport (= Facilitated
Membrane transport 1. Summary A. Simple diffusion 1) Diffusion by electrochemical gradient no energy required 2) No channel or carrier (or transporter protein) is needed B. Passive transport (= Facilitated
Neurons and the membrane potential. N500 John Beggs 23 Aug, 2016
 Neurons and the membrane potential N500 John Beggs 23 Aug, 2016 My background, briefly Neurons Structural elements of a typical neuron Figure 1.2 Some nerve cell morphologies found in the human
Neurons and the membrane potential N500 John Beggs 23 Aug, 2016 My background, briefly Neurons Structural elements of a typical neuron Figure 1.2 Some nerve cell morphologies found in the human
Control and Integration. Nervous System Organization: Bilateral Symmetric Animals. Nervous System Organization: Radial Symmetric Animals
 Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Enhancement of Exciton Transport in Porphyrin. Aggregate Nanostructures by Controlling. Hierarchical Self-Assembly
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supporting Information for Enhancement of Exciton Transport in Porphyrin Aggregate Nanostructures
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supporting Information for Enhancement of Exciton Transport in Porphyrin Aggregate Nanostructures
Dendrites - receives information from other neuron cells - input receivers.
 The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
Membranes 2: Transportation
 Membranes 2: Transportation Steven E. Massey, Ph.D. Associate Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail:
Membranes 2: Transportation Steven E. Massey, Ph.D. Associate Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail:
Neuron. Detector Model. Understanding Neural Components in Detector Model. Detector vs. Computer. Detector. Neuron. output. axon
 Neuron Detector Model 1 The detector model. 2 Biological properties of the neuron. 3 The computational unit. Each neuron is detecting some set of conditions (e.g., smoke detector). Representation is what
Neuron Detector Model 1 The detector model. 2 Biological properties of the neuron. 3 The computational unit. Each neuron is detecting some set of conditions (e.g., smoke detector). Representation is what
Manipulating and Probing Enzymatic Conformational Fluctuations and Enzyme-Substrate Interactions by Single-Molecule FRET- Magnetic Tweezers Microscopy
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information (SI) Manipulating and Probing Enzymatic Conformational Fluctuations
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information (SI) Manipulating and Probing Enzymatic Conformational Fluctuations
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Lecture 3 13/11/2018
 Lecture 3 13/11/2018 1 Plasma membrane ALL cells have a cell membrane made of proteins and lipids. protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump Lipid bilayer allows water, carbon
Lecture 3 13/11/2018 1 Plasma membrane ALL cells have a cell membrane made of proteins and lipids. protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump Lipid bilayer allows water, carbon
The NH 2 Terminus of RCK1 Domain Regulates Ca 2 -dependent BK Ca Channel Gating
 ARTICLE The NH 2 Terminus of RCK1 Domain Regulates Ca 2 -dependent BK Ca Channel Gating Gayathri Krishnamoorthy, 1 Jingyi Shi, 2,3 David Sept, 2,4 and Jianmin Cui 2,3 1 Department of Biomedical Engineering,
ARTICLE The NH 2 Terminus of RCK1 Domain Regulates Ca 2 -dependent BK Ca Channel Gating Gayathri Krishnamoorthy, 1 Jingyi Shi, 2,3 David Sept, 2,4 and Jianmin Cui 2,3 1 Department of Biomedical Engineering,
The Potassium Ion Channel: Rahmat Muhammad
 The Potassium Ion Channel: 1952-1998 1998 Rahmat Muhammad Ions: Cell volume regulation Electrical impulse formation (e.g. sodium, potassium) Lipid membrane: the dielectric barrier Pro: compartmentalization
The Potassium Ion Channel: 1952-1998 1998 Rahmat Muhammad Ions: Cell volume regulation Electrical impulse formation (e.g. sodium, potassium) Lipid membrane: the dielectric barrier Pro: compartmentalization
Digitized single scattering nanoparticles for probing molecular binding
 Electronic Supplementary Information (ESI) Digitized single scattering nanoparticles for probing molecular binding Yue Liu a, Cheng Zhi Huang a,b* a Education Ministry Key Laboratory on Luminescence and
Electronic Supplementary Information (ESI) Digitized single scattering nanoparticles for probing molecular binding Yue Liu a, Cheng Zhi Huang a,b* a Education Ministry Key Laboratory on Luminescence and
Membrane Potentials, Action Potentials, and Synaptic Transmission. Membrane Potential
 Cl Cl - - + K + K+ K + K Cl - 2/2/15 Membrane Potentials, Action Potentials, and Synaptic Transmission Core Curriculum II Spring 2015 Membrane Potential Example 1: K +, Cl - equally permeant no charge
Cl Cl - - + K + K+ K + K Cl - 2/2/15 Membrane Potentials, Action Potentials, and Synaptic Transmission Core Curriculum II Spring 2015 Membrane Potential Example 1: K +, Cl - equally permeant no charge
Propagation& Integration: Passive electrical properties
 Fundamentals of Neuroscience (NSCS 730, Spring 2010) Instructor: Art Riegel; email: Riegel@musc.edu; Room EL 113; time: 9 11 am Office: 416C BSB (792.5444) Propagation& Integration: Passive electrical
Fundamentals of Neuroscience (NSCS 730, Spring 2010) Instructor: Art Riegel; email: Riegel@musc.edu; Room EL 113; time: 9 11 am Office: 416C BSB (792.5444) Propagation& Integration: Passive electrical
Nervous System Organization
 The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
BA, BSc, and MSc Degree Examinations
 Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Membrane transport Time Allowed: 2 hours Marking Scheme: Total marks available
Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Membrane transport Time Allowed: 2 hours Marking Scheme: Total marks available
PROPERTY OF ELSEVIER SAMPLE CONTENT - NOT FINAL. The Nervous System and Muscle
 The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
Lecture 04, 04 Sept 2003 Chapters 4 and 5. Vertebrate Physiology ECOL 437 University of Arizona Fall instr: Kevin Bonine t.a.
 Lecture 04, 04 Sept 2003 Chapters 4 and 5 Vertebrate Physiology ECOL 437 University of Arizona Fall 2003 instr: Kevin Bonine t.a.: Bret Pasch Vertebrate Physiology 437 1. Membranes (CH4) 2. Nervous System
Lecture 04, 04 Sept 2003 Chapters 4 and 5 Vertebrate Physiology ECOL 437 University of Arizona Fall 2003 instr: Kevin Bonine t.a.: Bret Pasch Vertebrate Physiology 437 1. Membranes (CH4) 2. Nervous System
7.06 Cell Biology EXAM #3 KEY
 7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
Introduction and the Hodgkin-Huxley Model
 1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
Advanced Higher Biology. Unit 1- Cells and Proteins 2c) Membrane Proteins
 Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Stable Encapsulation of Quantum Dot Barcodes with Silica Shells
 Stable Encapsulation of Quantum Dot Barcodes with Silica Shells Shang-Hsiu Hu and Xiaohu Gao Department of Bioengineering, University of Washington Seattle, WA 98195 (USA) Adv. Funct. Mater. 2010. ASAP
Stable Encapsulation of Quantum Dot Barcodes with Silica Shells Shang-Hsiu Hu and Xiaohu Gao Department of Bioengineering, University of Washington Seattle, WA 98195 (USA) Adv. Funct. Mater. 2010. ASAP
Supporting Information
 Supporting Information Mullins et al. 10.1073/pnas.0906781106 SI Text Detection of Calcium Binding by 45 Ca 2 Overlay. The 45 CaCl 2 (1 mci, 37 MBq) was obtained from NEN. The general method of 45 Ca 2
Supporting Information Mullins et al. 10.1073/pnas.0906781106 SI Text Detection of Calcium Binding by 45 Ca 2 Overlay. The 45 CaCl 2 (1 mci, 37 MBq) was obtained from NEN. The general method of 45 Ca 2
This script will produce a series of pulses of amplitude 40 na, duration 1ms, recurring every 50 ms.
 9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/1/9/e1500511/dc1 Supplementary Materials for Contractility parameters of human -cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of
advances.sciencemag.org/cgi/content/full/1/9/e1500511/dc1 Supplementary Materials for Contractility parameters of human -cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of
Control of Single Channel Conductance in the Outer Vestibule of the Kv2.1 Potassium Channel
 ARTICLE Control of Single Channel Conductance in the Outer Vestibule of the Kv2.1 Potassium Channel Josef G. Trapani, Payam Andalib, Joseph F. Consiglio, and Stephen J. Korn Department of Physiology and
ARTICLE Control of Single Channel Conductance in the Outer Vestibule of the Kv2.1 Potassium Channel Josef G. Trapani, Payam Andalib, Joseph F. Consiglio, and Stephen J. Korn Department of Physiology and
Nerve Signal Conduction. Resting Potential Action Potential Conduction of Action Potentials
 Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
SUMMARY OF THE EVENTS WHICH TRIGGER AN ELECTRICAL IMPUSLE IN NERVE CELLS (see figures on the following page)
 Anatomy and Physiology/AP Biology ACTION POTENTIAL SIMULATION BACKGROUND: The plasma membrane of cells is a selectively permeable barrier, which separates the internal contents of the cell from the surrounding
Anatomy and Physiology/AP Biology ACTION POTENTIAL SIMULATION BACKGROUND: The plasma membrane of cells is a selectively permeable barrier, which separates the internal contents of the cell from the surrounding
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Applications of Quantum Dots to Biosensing
 Applications of Quantum Dots to Biosensing CHEM 681 Student Seminar Series March 17 th, 2003 Heechang Ye Advisor : Dr. Richard M. Crooks Introduction Quantum dots (QDs) are semiconductor particles that
Applications of Quantum Dots to Biosensing CHEM 681 Student Seminar Series March 17 th, 2003 Heechang Ye Advisor : Dr. Richard M. Crooks Introduction Quantum dots (QDs) are semiconductor particles that
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell
 Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Modeling of Retinal Ganglion Cell Responses to Electrical Stimulation with Multiple Electrodes L.A. Hruby Salk Institute for Biological Studies
 Modeling of Retinal Ganglion Cell Responses to Electrical Stimulation with Multiple Electrodes L.A. Hruby Salk Institute for Biological Studies Introduction Since work on epiretinal electrical stimulation
Modeling of Retinal Ganglion Cell Responses to Electrical Stimulation with Multiple Electrodes L.A. Hruby Salk Institute for Biological Studies Introduction Since work on epiretinal electrical stimulation
