Reduced myofibrillar connectivity and increased Z-disk width in nebulin-deficient skeletal muscle
|
|
|
- Alban Gardner
- 6 years ago
- Views:
Transcription
1 384 Research Article Reduced myofibrillar connectivity and increased Z-disk width in nebulin-deficient skeletal muscle Paola Tonino 1, *, Christopher T. Pappas 2, *, Bryan D. Hudson 1, Siegfried Labeit 3, Carol C. Gregorio 2 and Henk Granzier 1, 1 Department of Physiology and 2 Department of Cell Biology and Sarver Molecular Cardiovascular Research Program, University of Arizona, Tucson, Arizona , USA 3 Institute for Integrative Pathophysiology, Universitätsmedizin Mannheim 68167, University of Heidelberg, Germany *These authors contributed equally to this work Author for correspondence (granzier@ .arizona.edu) Accepted 23 November , Published by The Company of Biologists 2010 doi: /jcs Summary A prominent feature of striated muscle is the regular lateral alignment of adjacent sarcomeres. An important intermyofibrillar linking protein is the intermediate filament protein desmin, and based on biochemical and structural studies in primary cultures of myocytes it has been proposed that desmin interacts with the sarcomeric protein nebulin. Here we tested whether nebulin is part of a novel biomechanical linker complex, by using a recently developed nebulin knockout (KO) mouse model and measuring Z-disk displacement in adjacent myofibrils of both extensor digitorum longus (EDL) and soleus muscle. Z-disk displacement increased as sarcomere length (SL) was increased and the increase was significantly larger in KO fibers than in wild-type (WT) fibers; results in 3-day-old and 10- day-old mice were similar. Immunoelectron microscopy revealed reduced levels of desmin in intermyofibrillar spaces adjacent to Z- disks in KO fibers compared with WT fibers. We also performed sirna knockdown of nebulin and expressed modules within the Z- disk portion of nebulin (M160-M170) in quail myotubes and found that this prevented the mature Z-disk localization of desmin filaments. Combined, these data suggest a model in which desmin attaches to the Z-disk through an interaction with nebulin. Finally, because nebulin has been proposed to play a role in specifying Z-disk width, we also measured Z-disk width in nebulin KO mice. Results show that most Z-disks of KO mice were modestly increased in width (~80 nm in soleus and ~40 nm in EDL fibers) whereas a small subset had severely increased widths (up to ~1 m) and resembled nemaline rod bodies. In summary, structural studies on a nebulin KO mouse show that in the absence of nebulin, Z-disks are significantly wider and that myofibrils are misaligned. Thus the functional roles of nebulin extend beyond thin filament length regulation and include roles in maintaining physiological Z-disk widths and myofibrillar connectivity. Key words: Muscle, Cytoskeleton, Contractility, Sarcomeric structure, Nemaline myopathy Introduction The sarcomere is the basic contractile unit of striated muscle, and is composed of highly ordered thin (actin) filaments and thick (myosin) filaments (Squire, 1997). The cytoskeleton surrounding this elegant contractile molecular machinery is required as a scaffold to ensure efficiency of contraction. Longitudinally, sarcomeres are connected by Z-disk lattices that anchor thin filaments and transmit force along the myofibril. The strikingly regular lateral alignment of Z-disks between adjacent myofibrils ensures that during contraction myofibrils change length in unison, thus preventing damage to membrane systems that span between myofibrils and that are responsible for activating and relaxing the sarcomeres (T-tubules, sarcoplasmic reticulum). The mechanisms and proteins responsible for maintaining lateral myofibrillar alignment are incompletely understood. An important intermyofibrillar linking protein is the intermediate filament protein desmin, but the mechanisms that regulate its attachment to the myofibril itself have not been established (Capetanaki et al., 2007; Costa et al., 2004; Wang and Ramirez-Mitchell, 1983). Here we tested whether nebulin is part of the mechanical linkage by using a recently developed nebulin knockout (KO) mouse model (Witt et al., 2006). In the absence of nebulin, Z-disks assemble (Bang et al., 2006; Witt et al., 2006) and, thus, the nebulin KO makes it possible to test the role of nebulin in Z-disk alignment. Although misalignment has been noted in previous work on a nebulin KO (Bang et al., 2006), it has not been studied in detail. These studies were motivated by in vitro studies that suggested that the Z-disk portion of nebulin interacts with desmin (Bang et al., 2002) and more recently, nebulin modules M were reported to be involved in this interaction (Conover et al., 2009). Nebulin is a giant filamentous protein that plays a role in controlling the thin filament length in skeletal muscle (Bang et al., 2006; Castillo et al., 2009; Littlefield and Fowler, 2008; McElhinny et al., 2003; Witt et al., 2006). The N-terminal region of nebulin is located near the pointed end of the thin filament, and the C-terminal region (~50 kda) is anchored in the Z-disk and contains multiple phosphorylation motifs involved in signaling events (McElhinny et al., 2003). Research into the functions of nebulin has been stimulated by the identification of mutations in the nebulin gene (including in its Z-disk region) that cause nemaline myopathy (NM) in humans. NM is a member of a class of muscle disorders that phenotypically have in common a severe muscle weakness and structural abnormalities of Z-disks (Gurgel-Giannetti et al., 2001; Pelin et al., 1999). Yeast two-hybrid screens have shown that the C-terminus of nebulin interacts with desmin (Bang et al., 2002), and that the nebulin modules M are involved in this interaction (Conover et al., 2009). The functional significance of this requires further
2 Myofibrillar connectivity and Z-disks 385 study. In the present study we investigated whether nebulin-desmin interaction is important for myofibrillar connectivity, by measuring longitudinal Z-disk displacement between adjacent myofibrils in muscle fibers from wild-type and nebulin knockout mice. We also performed immunoelectron microscopy with anti-desmin antibodies, studied the expression level of desmin, and expressed the C-terminal nebulin repeats M160-M170 in myotubes and studied the effect on desmin localization. Finally, because nebulin has been suggested to play a role in specifying Z-disk width (Millevoi et al., 1998), a role that is likely to be independent of the role of nebulin in laterally linking Z-disks, we also measured Z-disk width and determined the expression level of -actinin, a major Z-disk actin-linking protein (Sjoblom et al., 2008). Our findings suggest that nebulin plays a role in specifying Z-disk width and in lateral registration of Z-disks in adjacent myofibrils. Results The role of nebulin in myofibrillar connectivity was studied using a nebulin KO mouse model (for details, see Witt et al., 2006). We focused on EDL and soleus muscles because they contain predominately fast and a large number of slow twitch fibers (Danieli-Betto et al., 2005), respectively, allowing us to determine whether there are fiber-type-specific changes in myofibrillar connectivity in the nebulin KO mouse. The ultrastructural sarcomeric organization of WT and nebulin KO muscle is shown in Fig. 1. WT myofibrils of both EDL (Fig. 1A,C) and soleus (Fig. 1E,G) muscle had well-aligned sarcomeres (minimal Z-disk displacement in adjacent myofibrils), uniform Z-disk width and an A-band with a visible H-zone (light zone devoid of thin filaments) in the center of the sarcomere. By contrast, a number of abnormalities were observed in the KO fibers. As previously reported for the tibialis cranialis muscle (Witt et al., 2006), sarcomeres of both EDL (Fig. 1B,D) and soleus KO fibers (Fig. 1F,H) did not have H-zones and their Z-disk widths were sometimes irregular with the presence of nemaline rod bodies (rod-shaped inclusion body consisting of Z-disk material, see also below). A difference that we noted, especially in stretched fibers, was that Z- disks of adjacent myofibrils were not well in register but instead were longitudinally displaced. Thus, in addition to the absence of H-zones, which is due to variable thin filament lengths in nebulin KO mice (Witt et al., 2006), Z-disk abnormalities were present as well, including Z-disk misalignment. Z-disk misalignment is further highlighted in the sample micrographs shown in Fig. 1D,H. This figure also shows how longitudinal Z-disk displacement ( X) of adjacent myofibrils was quantified. We stretched EDL and soleus muscle fibers from nebulin KO and WT mice to different sarcomere lengths and measured X. Results are shown in Fig. 2A,B. We used linear regression analysis to determine the relationship between Z-disk displacement and sarcomere length (SL) and compared the slopes by analysis of covariance. Findings in WT fibers indicate that Z- disk displacement is relatively small at SLs of m, and that displacement increases slightly as sarcomeres are stretched, with similar findings in soleus and EDL fibers. However, slopes were not significantly different from zero. The increase with SL was significantly steeper in KO fibers than in WT fibers [EDL slopes: 0.03 and 0.11 (P 0.002); soleus slopes 0.01 and 0.26 (P 0.003)]. For soleus and EDL KO fibers the slopes were not significantly different from each other, indicating that no major differences exist between myofibrillar connectivity of these muscle types. To address the concern that differences between WT and KO muscles might be due to muscle degeneration, we also studied muscle from WT and KO mice that were only 3 days old. Supplementary material Fig. S1 shows typical electron micrographs, and analyzed results are shown in Fig. 2C,D. Results are similar to those of the 10-day old mice: displacement increased significantly more steeply with SL (P in soleus and P 0.01 in EDL) in KO fibers than in WT fibers. We determined (Fig. 3) the mean Z-disk displacement at SLs >2.9 m (to make our findings comparable to those obtained on desmin KO fibers [see Discussion by Shah et al. (Shah et al. 2002)]. In 10-day-old WT mice the mean displacement of EDL fibers was 0.08±0.05 m (n 25) and of soleus fibers, 0.07±0.03 m (n 8). For age-matched KO fibers the values were 0.21±0.09 m (n 23) and 0.31±0.03 m (n 14) for EDL and soleus fibers, respectively. The values obtained in 3-day-old mice are also shown in Fig. 3 (gray bars). The mean displacements were again significantly larger in the KO muscles. The effect of postnatal Fig. 1. Sarcomeric structure in EDL and soleus skinned skeletal muscle fibers of WT and nebulin KO mice. (A-D) EDL muscle; (E-H) soleus muscle. WT fibers have a regular structure with well-aligned sarcomeres. In KO fibers, sarcomeres are misaligned and the Z-disk structure is non-uniform, with nemaline bodies (arrows). (D,H) Measurement of longitudinal Z- disk displacement ( X, defined as longitudinal displacement of nearby Z-disk in adjacent myofibrils) in skeletal muscles. Note large Z-disk displacement in both EDL and soleus KO fibers. Scale bars: 0.5 m (A,B,E,F); 2 m (C,D,G,H). (Each result is from a different mouse. Fibers were stretched 80%.)
3 (3) Fig. 2. Z-disk displacement in relation to SL of EDL and soleus muscle from nebulin KO and WT muscles. (A,C) EDL muscle; (B,D) soleus muscle. 10-day-old mice were used for A and B and 3-day-old mice for C and D. Results from KO fibers (black circles) are significantly different from WT fibers (white circles) (P<0.001). Each value represents the mean of ~50 measurements made on a single electron micrograph; ~20 micrographs were obtained from randomly selected fibers from five different mice. day and genotype was assessed by ANOVA with Tukey s HSD post-hoc comparisons. There were statistically significant differences between all KO and WT group but not within any of the WT and KO groups. Thus, in our data, Z-disks in EDL and soleus fibers from nebulin KO mice were, at SLs >2.9 m, approximately three- to four-fold more displaced than in their WT littermates, and this was true for both age groups. Misalignment was not more severe in the 10-dayold than in 3-day-old mice suggesting that the myofibrillar misalignment is not due to a progressive pathology. Taken together these results demonstrate that nebulin is involved in the lateral registration of both fast and slow skeletal muscle myofibrils. Because it is known that the intermediate filament protein desmin is involved in laterally linking myofibrils (Shah et al., 2004; Shah et al., 2002), we performed immunoelectron microscopy with polyclonal anti-desmin antibodies and gold-conjugated secondary antibodies and studied the localization of desmin in WT and nebulin KO fibers. EDL fibers from WT mice showed an accumulation of gold particles around the Z-disks (Fig. 4A), as well as near the myonuclei (results not shown). In muscle fibers from nebulin KO mice the intermyofibrilar spaces at the level of Z-disks had fewer gold particles (Fig. 4B), whereas near nuclei and costameres enhanced staining was often observed. We quantified the number of gold particles in the Z-disk to Z-disk space (using ImageJ analysis software; Fig. 3C top). The number of gold particle per unit area was significantly reduced in the nebulin KO mice (t-test, P<0.05). Immunogold labeling was also observed throughout the sarcomere, suggesting non-specific labeling. To characterize non-specific labeling, measurements were made in randomly selected areas of the A-band region of the sarcomere. This revealed no difference between WT and KO mice (Fig. 4C, bottom). To determine whether desmin was mislocalized in nebulin KO muscle or, alternatively, had a much reduced expression level, we performed western blot Fig. 3. The mean Z-disk displacement at SLs >2.9 m. (A) Soleus muscle, (B) EDL muscle. Values are means ± s.d. for 3-day-old and 10-day-old mice. ANOVA with Tukey s HSD post-hoc tests show statistical significance (S) between all KO and WT groups but not within any of the WT and KO groups, with identical results for 3-day-old and 10-day-old mice. No significant differences exist between EDL and soleus fibers. Results are from ~50 measurements made on a single electron micrograph with ~20 micrographs obtained from randomly selected fibers from five different mice. experiments. Analysis revealed an increased desmin expression level in nebulin KO fibers when compared with WT (supplementary material Fig. S1A). Thus, reduced desmin labeling at the Z-disks of KO fibers is not due to a reduced expression level of desmin. To further study whether nebulin is involved in anchoring desmin to the Z-disk region of the sarcomere we performed cell culture experiments. Our goal was to do this work on cultured mouse fibers (from WT and KO mice) but we were unable to successfully culture these cells and instead performed experiments on quail myoblasts that turned out to be well suited for this work. In order to better understand the assembly of desmin during the differentiation of quail skeletal myotubes in culture, cells were fixed and stained for desmin and -actinin at various timepoints from 17 hours to 8 days after plating. Based on the staining for Fig. 4. Immunoelectron microscopy of EDL muscle. (A) WT muscle had gold particles (indicating the presence of desmin) near the Z-disks (black arrowheads), whereas the nebulin KO (B) muscle was largely devoid of gold particles (white arrowheads) in the Z-disk regions between myofibrils. Bar, 500 nm. (C) Particle counts in the Z-disk to Z-disk space (intermyofibrillar area enclosed by drawing lines from edge to edge of adjacent Z-disks; see examples on micrograph). Results are from three WT and three KO muscle (from three WT and three KO mice) with 15 micrographs per muscle and approx. five Z-disk regions per micrograph. We also counted particles in randomly selected areas in the A-band region of the sarcomere. Significant differences exist between WT and KO fibers for Z-disk to Z-disk measurements only. Note that no differences were found in the size of the gold particles: 14.1±3.6 nm (WT) and 16.8±4.7 nm (KO).
4 Myofibrillar connectivity and Z-disks 387 the well-characterized Z-disk component, -actinin (e.g. Rhee et al., 1994; Rudy et al., 2001) and the morphological characteristics of the cells, distinct stages of assembly were identified (supplementary material Fig. S2). At early stages of assembly (~17 hours), both -actinin and desmin were punctate in appearance. Later, (1-2 days) the dots of -actinin staining had organized into linear arrays, whereas desmin had formed a peri-sarcomeric mesh-like network that was especially evident around the nuclei. At intermediate stages of assembly (3-4 days) -actinin was assembled into discrete bands, indicating the maturation of the Z- disk, whereas desmin remained as a network. Finally, in mature stages of assembly (4-8 days) desmin also localized to the Z-disk in distinct striations. To ascertain the role of nebulin in the assembly of desmin, small interfering RNA (sirna) was used to reduce the levels of nebulin in quail skeletal myotubes (supplementary material Fig. S3). Quail myotubes were then co-stained with anti-desmin and anti- -actinin antibodies 4-8 days after sirna treatment. The reduction of nebulin levels resulted in a marked perturbation of desmin assembly at the Z-disk. The majority of cells lacked any observable desmin Z-disk assembly (Fig. 5, top two rows), whereas in the cells that did have discernible localization of desmin at the Z-disk, the staining was often broader and less well defined in comparison with the controls (Fig. 5, bottom two rows, inset). Note that the lack of desmin staining at the Z-disk is unlikely to be due to a delay in the development of the myotubes, as the phenotype persisted for days following the organization of -actinin into its mature pattern of assembly. Fig. 5. Knockdown of nebulin in primary cultures of quail myotubes perturbs the association of desmin at the Z-disk. Myotubes were double stained with antibodies generated against -actinin, as a marker of the Z-disk, and desmin, 4-8 days after sirna treatment. Treatment with nebulin-specific sirna results in a significant decrease in assembled nebulin (see supplementary material Fig. S3) with a concomitant decrease in assembled Z- disk-associated desmin. In the sirna-treated cells, desmin staining at the Z-disk was either undetectable (top two rows) or if detectable, was significantly broader and less intense, than in the controls (insets, bottom two rows). The distribution of -actinin was not disrupted in the identical myofibrils. Scale bar: 10 m. (Top two rows: example of the most typical cell type in which desmin Z-disk assembly was absent; bottom two rows: example of less typical cell type in which broad and faint Z-disk labeling of desmin was seen.) Desmin assembly at the Z-disk was also reduced following the expression of nebulin C-terminal modules M , which contain binding sites for desmin (Bang et al., 2002). Nebulin M fused to mcherry localized to the Z-disk and along the periphery of the myofibrils, whereas mcherry alone associated with the myofibrils but lacked the distinctive Z-disk distribution (Fig. 6). A significant decrease in the Z-disk localization of desmin was observed in myocytes expressing nebulin M , in comparison to cells expressing mcherry alone (Fig. 6, desmin panels). In three independent experiments we counted the number of cells that were devoid of distinct Z-disk desmin labeling (as in Fig. 6, bottom row). Of all cells transfected with mcherry alone 19.0±1.1% of the cells had diffuse and non-striated desmin labeling; this value was significantly (P<0.001) increased in cultures transfected with nebulin M fused to mcherry. Thus, both knockdown of nebulin with sirna and overexpression of M160-M170 did not interfere with the formation of normal striation patterns of -actinin but did prevent the mature Z-disk localization of desmin. Our studies also provided an opportunity to study the Z-disk structure. Previous work has suggested that nebulin plays a role in specifying Z-disk width (Millevoi et al., 1998) and the Z-disk width is thus expected to be dysregulated in nebulin-deficient muscle. (Note that the role of nebulin in Z-disk width specification is likely to be independent of its role in anchoring desmin.) As noted above, the Z-disk morphology of nebulin KO fibers was also altered, and this phenomenon is further highlighted in Fig. 7. Some Z-disks were unusually wide with an irregular or rounded shape (Fig. 7B,D), similar to the typical structure of nemaline rod bodies (Gurgel-
5 (3) Fig. 6. Introduction of a recombinant nebulin fragment (M ), which contains the desmin binding site, perturbs the Z-disk distribution of desmin in primary cultures of quail myotubes. Myotubes were double stained for desmin and -actinin, 6 days after transfection of mcherry alone or nebulin M fused to mcherry. Scale bar: 10 m. Giannetti et al., 2001). These nemaline rod bodies were found throughout the fibers but had a tendency to be more prominent in areas close to the myonuclei. Consistent with the work of Wallgren- Pettersson and colleagues (Wallgren-Pettersson et al., 1995) we were unable to detect immunogold particles in the IEM experiments described above, indicating that the presence of desmin in rod bodies is unlikely. Previous work by others has shown that the rods contain -actinin (Wallgren-Pettersson et al., 1995) and we consider it probably that this is the case in the nebulin KO as well, consistent with the increased levels of -actinin that we found in our western blot experiments (see below). In other cases, the Z-disk exhibited interruptions and a zigzag shape that appeared to be more pronounced at long sarcomere lengths, whereas in severely damaged myofibrils a disintegration of the Z-disk was also observed (which was independent of stretch). Histograms of Z-disk widths are shown in Fig. 8. The average width of the Z-disk in EDL muscle from WT mice was 127±21 nm (range nm; Fig. 8A) and in nebulin KO muscle 271±165 nm (range nm; Fig. 8B). The average width was significantly larger in soleus muscle from WT mice: 145±20 nm (range nm; Fig. 8C), and nebulin KO mice: 465±422 nm (range nm; Fig. 8D). Consistent with wider Z-disks, we found that -actinin, a major constituent of Z-disks, was upregulated several-fold in KO fibers (supplementary material Fig. S4). Discussion Linkage of myofibrils allows for lateral force transmission and limits the degree to which adjacent myofibrils translocate relative to each other during active contraction or passive stretch, thereby preventing damage to intermyofibrillar membrane systems. An important protein involved in linking adjacent Z-disks is the intermediate filament protein desmin which forms a network of filaments that surrounds myofibrils at the level of the Z-disk (Capetanaki et al., 2007; Costa et al., 2004; Lazarides and Granger, 1978; Wang and Fig. 7. Representative electron micrographs of EDL (A,B) and soleus (C,D) muscle from WT (A,C) and nebulin KO 10-day old mice (B,D). In nebulin KO fibers (B and D) wide and irregular Z-disk inclusions that resembled nemaline bodies (arrows) were commonly present. Scale bars: 2 m (A,B), and 1 m (C,D). Fibers were stretched 80% in A and D and 60% in B and C. Fig. 8. Analysis of Z-disk widths. Histograms of Z-disk widths in EDL (A,B) and soleus (C,D) fibers. Primary peaks were fit by Gaussians. Gaussian peak fit of WT fibers (broken lines) is superimposed on results of KO fibers. Results are from 82 micrographs from five muscles from five different mice. See text for details.
6 Myofibrillar connectivity and Z-disks 389 Ramirez-Mitchell, 1983). The subunit proteins of desmin filaments are elongated coiled-coils with extensive intermolecular ionic and hydrophobic interactions between individual subunits, giving rise to filaments with high tensile strength as well as plasticity (Costa et al., 2004). That desmin tethers adjacent Z-disks is supported by work on a desmin KO mouse in which Z-disk misalignment was shown to occur in stretched muscle (Shah et al., 2002). Previous in vitro work suggested that desmin binds to the sarcomeric protein nebulin (Bang et al., 2002), and in the present study using a nebulin KO mouse model we examined this possibility. We found that myofibrils translocate in muscle devoid of nebulin to a much higher degree than in WT muscle (for definition of translocation, X, see Fig. 1D,H; for results, see Fig. 2). Our work also shows that desmin is present in muscle devoid of nebulin but that it is reduced in the intermyofibrillar spaces that surround the Z-disks (Fig. 4C, top). Consistent with this, we found that both knockdown of nebulin with sirna and overexpression of M160-M170 did not interfere with the formation of normal striation patterns but did prevent desmin localization at the mature Z-disk (Figs 5 and 6). It is unclear why two distinct phenotypes were observed; that is, lack of detectable Z-disk staining and broader and less defined Z-disk staining. We predict that the broad Z-disk staining might reflect displaced Z- disk desmin. Combined, our knockdown and overexpression studies suggest a model in which desmin attaches to the Z-disk through an interaction with nebulin. This model is consistent with a previous yeast two-hybrid study that found that nebulin modules M163-M183 interact with a 19-kDa central coiled-coil domain (known as coil 1B) of desmin (Bang et al., 2002) and a more recent study in which M160-M164 was found to interact with desmin (Conover et al., 2009). In the absence of nebulin, desmin is unable to bind to these sites on the myofibril and as a result myofibrils differentially translocate when passively stretched. In our work we studied both soleus and EDL muscles, examples of predominately slow- and fastfiber-containing muscles (Danieli-Betto et al., 2005; Girgenrath et al., 2005), respectively. Essentially the same findings were obtained (Fig. 2) suggesting that the model (desmin binding to nebulin) applies equally to different muscle types. In summary our work on a nebulin KO mouse suggests that myofibrils are laterally linked at the level of the Z-disk by relatively stiff desmin filaments that bind to nebulin. It is currently unknown whether in addition to Z-disks other linkage sites exist between adjacent myofibrils that are sufficiently stiff to prevent translocation of myofibrils. A comparison of results obtained with the nebulin KO and desmin KO models might shed light on this issue. If the Z-disk is the only site of importance, largely identical Z-disk displacements are expected in the two models. Shah et al. (Shah et al., 2002) reported that in desmin KO fibers with SLs >2.90 m there was a Z-disk displacement of 0.49±0.10 m, larger that the values that we found in nebulin KO fibers [~0.2 m (EDL) and ~0.3 m (soleus)]. However, Z-disk displacement of WT fibers in the Shah et al. study was, at 0.25 m, also much larger than in our WT fibers (~0.08 m). This might indicate that forces were much higher in the Shah et al. study than in ours (we stretched the tendons very slowly and in small increment to keep passive force relatively low). It has been shown in single molecule studies (Kreplak et al., 2008) that at high force, desmin filaments can be elongated several-fold, presumably because the -helical coiledcoil dimer can be converted into -sheet-type structures upon stretching. The finding that Z-disk displacement in WT fibers is approximately constant at ~0.08 m (Fig. 2) suggests that in our study forces were insufficient to cause filament elongation. Because the increase in Z-disk displacement above that of WT fibers is similar in our study to that of Shah et al. (Shah et al., 2002) we consider it likely that the desmin-nebulin linkage site is most important for myofibrillar connectivity. Thus although it is possible that other desmin-z-disk linkages exist that do not involve nebulin [as suggested by the remaining Z-disk labeling of desmin in nebulin KO mice (see Bang et al., 2006)] the desmin-nebulin linkage is likely to be very important for establishing myofibrillar connectivity. Our similar findings in soleus and EDL muscles, representatives of slow and fast muscle types, respectively, suggest that nebulin-based myofibrillar connectivity is important in different muscle types, irrespective of their activity patterns. The importance of this linkage is also suggested by the upregulation of desmin, which might be viewed as a response to correct compromised myofibrillar connectivity. Finally, it is possible that in addition to desmin and nebulin other proteins also play a role in linking myofibrils. A good candidate is plectin, an ~500 kda linking protein with isoforms 1d and 1f binding to desmin and Z-disks (Konieczny et al., 2008). It is unknown to which Z-disk protein(s) plectin binds and it is of importance to determine whether plectin is arranged in series or in parallel with the desmin-nebulin linkage. We found that both soleus and EDL muscles of nebulin KO mice contain electron-dense Z-disk-like bodies that are similar to nemaline rod bodies that are a hallmark of nemaline myopathy in humans (Wallgren-Pettersson et al., 2004). Furthermore, histograms of Z-disk width (Fig. 6) show that the majority of all Z-disks in both WT and nebulin KO sarcomeres can be fit with a Gaussian curve that is shifted to higher values in KO than in WT fibers (by an average of ~80 nm in soleus and ~40 nm in EDL fibers). We conclude that absence of nebulin affects most Z-disks by widening them by a modest amount and that it affects a small subset of sarcomeres more severely (nemaline rod bodies). Previously titin has been suggested to play a role in Z-disk assembly (Gautel et al., 1996) and our present work indicates that nebulin also contributes to establishing and/or maintaining the Z-disk structure. The Z-disk region of titin contains a family of differentially expressed repeats, the titin Z-repeats (Gautel et al., 1996). These Z-repeats are a family of -actinin-binding motifs, which are differentially expressed in a tissue- and developmental-stage-specific fashion (Gautel et al., 1996). As previously pointed out, it is unlikely that the differential expression of the titin Z-repeats alone can determine the Z-disk width, because too few isoforms exist to account for the wide range of different Z-disk widths (Millevoi et al., 1998). Our present work supports a model in which titin and nebulin together specify Z-disk width, with titin constructing the central region of the Z-disk, including the number and positions where -actinin cross-links the thin and the titin filaments. Nebulin determines the ending of the Z-disk structure and its transition to the I-band, i.e. nebulin functions as a Z-disk terminator. The mechanism by which nebulin terminates the Z-disk might involve interaction between nebulin and Z-disk-localized proteins such as, titin and CapZ. CapZ is a barbed-end actin capping protein that binds near the C-terminus of nebulin (Pappas et al., 2008). In muscle fibers devoid of nebulin [nebulin KO (Witt et al., 2006)] or sirna knockdown of nebulin in myotubes (Pappas et al., 2008), CapZ does not localize properly, allowing the barbed ends of Z- disks to continue to grow beyond the Z-disk resulting in widened Z-disks. It is unclear why most Z-disks only widen by a modest amount and others develop into nemaline rod bodies. Because similar results were obtained in soleus and EDL, a mainly slow twitch posture muscle and fast twitch intermittently used muscle,
7 (3) respectively, differences in mechanical load might not be the main explanation. Instead we speculate that gradients in protein levels in the muscle fiber play an important role. Clearly further research is required to resolve this question. In summary, structural studies on a nebulin KO mouse show that in the absence of nebulin, Z-disks are significantly wider and that myofibrils are misaligned. Thus the functional roles of nebulin extend beyond thin filament length regulation and include maintaining physiological Z-disk widths and myofibrillar connectivity. It is interesting to note that the Z-disk functions of nebulin might be performed in cardiac muscle (where nebulin levels are very low or absent) by nebulette, a ~100 kda nebulin-like protein that shares extensive similarity with the C-terminal region of nebulin (Millevoi et al., 1998). Thus thin filament length regulation might not be the main function of nebulin (because cardiac muscle can do without) but instead the Z-disk functions of nebulin might be most crucial. Materials and Methods Muscle specimens The nebulin KO mouse model (BL6) including its genotyping has been described previously (for details, see Witt et al., 2006). Mice die at a relatively early age as a result of muscle weakness and respiratory failure and, therefore, animals in this study were used at 3 days and 10 days of age. At day 10, the KO mice weighed 2.3±0.4 g (n 8) and the WT mice 7.2±0.8 g (n 6). The values at day 3 were 2.0±0.3 g (KO, n 6) and 2.4±0.3 g (WT, n 5). Mice were killed and the skin from both hindlimbs was rapidly removed before immersing the hindlimbs in relaxing solution (40 mm BES, 10 mm EGTA, 6.56 mm MgCl 2, 5.88 mm Na-ATP, 1 mm DTT, mm potassium propionate, 15 mm creatine phosphate) containing protease inhibitors [0.005 mm leupeptin, 0.1 mm E-64, 0.2 mm phenylmethylsulphonyl fluoride (PMSF) from a stock in 100% ethanol, ph 7.0] and 1% Triton X-100 (w/v). After skinning overnight, the hindlimbs were washed with relaxing solution; EDL and soleus muscles were quickly removed and one end of their tendons was pinned down with a minuten pin (Fine Science Tools) to a Sylgard elastomer base (Dow Corning, Midland, MI) in a small Petri dish filled with relaxing solution. The muscles were then passively stretched in small increments (in ~30 seconds) to a final length that exceeded the slack length by ~20%, 40%, 60% and 80%, followed by pinning the other end of the muscle tendon to the elastomere dissection base. The muscles were kept in this stretched state for 10 minutes at 4 C, and were then processed for electron microscopy (see below). We typically studied ~20 fibers per muscle. All experiments were conducted and approved by the University of Arizona Institutional Animal Care and Use Committee, following the guidelines Using Animals in Intramural Research of the National Institutes of Health. Transmission electron microscopy For electron microscopy, stretched EDL and soleus muscle from WT and nebulin KO mice were fixed with 3% paraformaldehyde in PBS for 30 minutes. The muscles were then rinsed for 15 minutes in PBS containing protease inhibitors (Granzier et al., 1997). A secondary fixation was performed in 3% glutaraldehyde containing 2% tannic acid in PBS for 1 hour. After another PBS rinse, the muscles were postfixed in 1% OsO 4 in PBS for 30 minutes, and were then dehydrated in an ethanol series of increasing concentrations. Following dehydration, the muscles were first infiltrated with 100% propylene oxide then a mixture of 1:1 propylene oxide:araldite, and finally embedded in a pure Araldite resin. Ultrathin sections (70 nm) were obtained with a Reichert-Jung ultramicrotome and contrasted with potassium permanganate and lead citrate. During sectioning we ensured that the fiber direction was aligned with the edge of the diamond knife. We typically studied 20 fibers per muscle. Samples were observed using a JEOL 1200 EX or a Philips CM12 electron microscope, operated at 100 kv. Measurement of Z-disk displacement, sarcomere length (SL) and Z-disk width A set of digitized electron micrographs from both EDL and soleus muscles were randomly selected for analysis with Adobe Photoshop 8.0. The magnification of the microscope was used for calibration. Displacement of Z-disks ( X) in adjacent myofibrils was measured as shown in Fig. 2. Sarcomere length was measured from mid Z-disk to mid Z-disk. The Z-disk width was measured along a single pixel perpendicular line using the density as a gauge for the edge of the Z-disk (density midway between that of the center of the Z-disk and the I-band). Typically ~50 measurements were made per electron micrograph with nine micrographs per experiment (with each experiment representing a different degree of stretch: 0, 20, 40, 60 and 80%). We determined the mean and variance of all displacement values per micrograph, using KaleidaGraph 3.6 version software and plotted results as a function of SL (as in Fig. 2). The Z-disk width values were binned in 25 nm bins and plotted in histograms (as in Fig. 8). Immunoelectron microscopy (IEM) Immunogold labeling was performed as previously described (Trombitas and Granzier, 1997). Briefly, skinned muscle fiber bundles from EDL and soleus muscle (n 3) were fixed in 3% paraformaldehyde in PBS, for 30 minutes at 4 C, and washed with PBS, and PBS containing protease inhibitors. Blocking was performed with 1% BSA in PBS containing protease inhibitors for 1 hour at 4 C, followed by incubation with polyclonal anti-desmin antibody (rabbit whole serum, Sigma-Aldrich, D8281) diluted 1:10 in PBS containing protease inhibitors for 48 hours at 4 C. (Note that long labeling durations are commonly used in IEM on skinned muscle.) After rinsing in PBS, the fiber bundles were incubated for 48 hours at 4 C with secondary antirabbit Nanogold goat antibody (1.4 nm, Nanoprobes) at a dilution of 1:10 in PBS containing protease inhibitors. The bundles were rinsed with PBS and then fixed in 3% glutaraldehyde containing 0.01% tannic acid in PBS for 30 minutes at 4 C. After rinsing in PBS, aldehydes were quenched with 50 mm glycine in PBS for 15 minutes at 4 C, followed by gold enhancement (Nanoprobes; as per the manufacturer s instructions) for 3-5 minutes depending on fiber bundle thickness. Bundles were washed with PBS and postfixed with 1% OsO 4 in PBS for 15 minutes at 4 C. Fiber bundles were washed with distilled water and dehydrated in a graded series of ethanol (50%, 75%, 95%, 100%). Then the bundles were infiltrated with propylene oxide for 15 minutes, then in a mixture of 1:1 propylene oxide:araldite followed by Araldite alone. Bundles were cut into small fragments and then prepared for mounting, embedding and polymerization for 48 hours at 60 C. Sections were only lightly contrasted in lead citrate, to ensure that gold particles stood out well from the sarcomeric structures, and the sections were examined using either a Jeol 1200EX or a Philips CM12 microscope operated at 100 kv. Gel electrophoresis and western blotting analysis Gastrocnemius muscle from 10-day-old WT and nebulin KO mice (n 3) were quickfrozen in liquid nitrogen, pulverized and rapidly solubilized as previously described (Granzier and Irving, 1995). Samples were run on 12% SDS-PAGE gels, and transferred to polyvinylidene difluoride membranes. Blots were probed with antidesmin polyclonal (Sigma-Aldrich) and sarcomeric anti- -actinin monoclonal (clone EA-53; Sigma-Aldrich) primary antibodies diluted 1:500. Fluorescently labeled secondary antibodies, Alexa Fluor 680 and Rockland IR Dye 800 conjugate (Molecular Probes) were used as appropriate. Anti-myosin heavy chain (MHC) antibody was used to normalize for protein loading. Protein immunoblots were scanned and quantified by densitometry using the two-color detection Odyssey infrared imaging system (LI-COR Biosciences). MHC was used to normalize for differences in protein loading. Cell culture, transfections and sirna treatment Primary cultures of quail skeletal myotubes were prepared as described (Almenar- Queralt et al., 1999). A nebulin-specific sirna was previously designed, validated and purchased from Ambion (Austin, Texas; target cdna sequence: 5 - GTAGCTGACTCTCCAATTA-3 ); western blot analysis demonstrated that sirna treatment reduced endogenous nebulin levels by >90% (Pappas et al., 2008). As a control, a random sirna was also generated (target cdna sequence: 5 - CTCGACTAGAGTCTGTCTA-3 ). Nebulin modules , which were previously cloned from mouse heart cdna (Conover et al., 2002) were inserted into a modified pegfp-c2 vector in which GFP was replaced with mcherry. Quail myotubes were transfected with 50 nm sirna of expression plasmid DNA using the lipid-based reagent Effectene (Qiagen, Valencia, CA) according to the manufacturer s instructions, hours after plating. Four to eight days after transfection, the cells were incubated in relaxing buffer (150 mm KCl, 5 mm MgCl 2, 10 mm MOPS, ph 7.4, 1 mm EGTA, 4 mm ATP) for 15 minutes and fixed with 2% paraformaldehyde in relaxing buffer for 15 minutes. Immunofluorescence microscopy To observe sarcomeric components, cells were stained as described previously (Pappas et al., 2008). The fixed cells were permeabilized in 0.2% Triton X-100 in PBS, blocked with 2% BSA plus 1% normal donkey serum in PBS, and incubated for 1 hour with primary antibodies diluted in PBS. The primary antibodies consisted of a polyclonal anti-n-terminal nebulin antibody [3.5 g/ml (McElhinny et al., 2001)], a monoclonal anti- -actinin antibody (1:15,000; Sigma, A7811) and a polyclonal anti-desmin antibody (1:2; Biomeda, Foster City, CA; 213M). The cells were then washed with PBS for 15 minutes, and incubated with secondary antibodies in PBS for 30 minutes. The secondary antibodies, obtained from Invitrogen and Jackson ImmunoResearch Laboratories, included: Alexa-Fluor-488-conjugated goat anti-mouse and goat antirabbit IgG (1:1000), Alexa-Fluor-350-conjugated goat anti-mouse IgG (1:300) and Texas-Red-conjugated donkey anti-rabbit IgG (1:600). Alexa-Fluor-488-conjugated phalloidin was used to stain F-actin (Invitrogen). Coverslips were mounted onto slides with Aqua Poly/Mount (Polysciences, Warrington, PA). The cells were analyzed and images captured using a Deltavision deconvolution microscope (Applied Precision, Issaquah, WA) with a 100 objective (1.3 NA) and a CoolSnap HQ charge-coupled device camera (Photometrics, Tucson, AZ).
8 Myofibrillar connectivity and Z-disks 391 Statistical analysis Data were expressed as mean ± s.d. Unpaired t-tests were used to determine whether Z-disk displacements of EDL and soleus muscles were significantly different, with P<0.05 regarded as statistically significance. The slopes for displacement versus SL data from muscles of wild-type and KO mice were compared by analysis of covariance (ANCOVA) using StatView software (v. 5.01, SAS Institute). We are grateful to Germain Wright, Chi Fong, Ellen Taylor, Tiffany Pecor and Gina Zhang for excellent technical assistance. We are grateful to Christian Witt for providing the nebulin KO model, Gloria Conover for generating the M construct and Marcus DiMarco for generating the primary myocyte cultures. Thanks to Christine Davis (Washington State University, Pullman, WA) and David Elliot (University of Arizona, Tucson, AZ) for kind cooperation at the EM facility core. This work was supported by the European Union (ERARE NEMMYOP to S.L.), and by the US National Institutes of Health (HL to C.C.G. and grants AR and HL to H.G.). Deposited in PMC for release after 12 months. Supplementary material available online at References Almenar-Queralt, A., Gregorio, C. C. and Fowler, V. M. (1999). Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils. J. Cell Sci. 112, Bang, M. L., Gregorio, C. and Labeit, S. (2002). Molecular dissection of the interaction of desmin with the C-terminal region of nebulin. J. Struct. Biol. 137, Bang, M. L., Li, X., Littlefield, R., Bremner, S., Thor, A., Knowlton, K. U., Lieber, R. L. and Chen, J. (2006). Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J. Cell Biol. 173, Capetanaki, Y., Bloch, R. J., Kouloumenta, A., Mavroidis, M. and Psarras, S. (2007). Muscle intermediate filaments and their links to membranes and membranous organelles. Exp. Cell Res. 313, Castillo, A., Nowak, R., Littlefield, K. P., Fowler, V. M. and Littlefield, R. S. (2009). A nebulin ruler does not dictate thin filament lengths. Biophys. J. 96, Conover, G. M., Henderson, S. N. and Gregorio, C. C. (2009). A myopathy-linked desmin mutation perturbs striated muscle actin filament architecture. Mol. Biol. Cell 20, Costa, M. L., Escaleira, R., Cataldo, A., Oliveira, F. and Mermelstein, C. S. (2004). Desmin: molecular interactions and putative functions of the muscle intermediate filament protein. Braz. J. Med. Biol. Res. 37, Danieli-Betto, D., Esposito, A., Germinario, E., Sandona, D., Martinello, T., Jakubiec- Puka, A., Biral, D. and Betto, R. (2005). Deficiency of alpha-sarcoglycan differently affects fast- and slow-twitch skeletal muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1328-R1337. Gautel, M., Goulding, D., Bullard, B., Weber, K. and Furst, D. O. (1996). The central Z-disk region of titin is assembled from a novel repeat in variable copy numbers. J. Cell Sci. 109, Girgenrath, S., Song, K. and Whittemore, L. A. (2005). Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slowand fast-type skeletal muscle. Muscle Nerve 31, Granzier, H., Kellermayer, M., Helmes, M. and Trombitas, K. (1997). Titin elasticity and mechanism of passive force development in rat cardiac myocytes probed by thinfilament extraction. Biophys. J. 73, Granzier, H. L. and Irving, T. C. (1995). Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys. J. 68, Gurgel-Giannetti, J., Reed, U., Bang, M. L., Pelin, K., Donner, K., Marie, S. K., Carvalho, M., Fireman, M. A., Zanoteli, E., Oliveira, A. S. et al. (2001). Nebulin expression in patients with nemaline myopathy. Neuromuscul. Disord. 11, Konieczny, P., Fuchs, P., Reipert, S., Kunz, W. S., Zeold, A., Fischer, I., Paulin, D., Schroder, R. and Wiche, G. (2008). Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J. Cell Biol. 181, Kreplak, L., Herrmann, H. and Aebi, U. (2008). Tensile properties of single desmin intermediate filaments. Biophys. J. 94, Lazarides, E. and Granger, B. L. (1978). Fluorescent localization of membrane sites in glycerinated chicken skeletal muscle fibers and the relationship of these sites to the protein composition of the Z disc. Proc. Natl. Acad. Sci. USA 75, Littlefield, R. S. and Fowler, V. M. (2008). Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler. Semin. Cell Dev. Biol. 19, McElhinny, A. S., Kolmerer, B., Fowler, V. M., Labeit, S. and Gregorio, C. C. (2001). The N-terminal end of nebulin interacts with tropomodulin at the pointed ends of the thin filaments. J. Biol. Chem. 276, McElhinny, A. S., Kazmierski, S. T., Labeit, S. and Gregorio, C. C. (2003). Nebulin: the nebulous, multifunctional giant of striated muscle. Trends Cardiovasc. Med. 13, Millevoi, S., Trombitas, K., Kolmerer, B., Kostin, S., Schaper, J., Pelin, K., Granzier, H. and Labeit, S. (1998). Characterization of nebulette and nebulin and emerging concepts of their roles for vertebrate Z-discs. J. Mol. Biol. 282, Pappas, C. T., Bhattacharya, N., Cooper, J. A. and Gregorio, C. C. (2008). Nebulin interacts with CapZ and regulates thin filament architecture within the Z-disc. Mol. Biol. Cell 19, Pelin, K., Hilpela, P., Donner, K., Sewry, C., Akkari, P. A., Wilton, S. D., Wattanasirichaigoon, D., Bang, M. L., Centner, T., Hanefeld, F. et al. (1999). Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc. Natl. Acad. Sci. USA 96, Rhee, D., Sanger, J. M. and Sanger, J. W. (1994). The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil. Cytoskeleton 28, Rudy, D. E., Yatskievych, T. A., Antin, P. B. and Gregorio, C. C. (2001). Assembly of thick, thin, and titin filaments in chick precardiac explants. Dev. Dyn. 221, Shah, S. B., Su, F. C., Jordan, K., Milner, D. J., Friden, J., Capetanaki, Y. and Lieber, R. L. (2002). Evidence for increased myofibrillar mobility in desmin-null mouse skeletal muscle. J. Exp. Biol. 205, Shah, S. B., Davis, J., Weisleder, N., Kostavassili, I., McCulloch, A. D., Ralston, E., Capetanaki, Y. and Lieber, R. L. (2004). Structural and functional roles of desmin in mouse skeletal muscle during passive deformation. Biophys. J. 86, Sjoblom, B., Salmazo, A. and Djinovic-Carugo, K. (2008). Alpha-actinin structure and regulation. Cell Mol. Life Sci. 65, Squire, J. M. (1997). Architecture and function in the muscle sarcomere. Curr. Opin. Struct. Biol. 7, Trombitas, K. and Granzier, H. (1997). Actin removal from cardiac myocytes shows that near Z line titin attaches to actin while under tension. Am. J. Physiol. 273, C662-C670. Wallgren-Pettersson, C., Jasani, B., Newman, G. R., Morris, G. E., Jones, S., Singhrao, S., Clarke, A., Virtanen, I., Holmberg, C. and Rapola, J. (1995). Alpha-actinin in nemaline bodies in congenital nemaline myopathy: immunological confirmation by light and electron microscopy. Neuromuscul. Disord. 5, Wallgren-Pettersson, C., Pelin, K., Nowak, K. J., Muntoni, F., Romero, N. B., Goebel, H. H., North, K. N., Beggs, A. H. and Laing, N. G. (2004). Genotype-phenotype correlations in nemaline myopathy caused by mutations in the genes for nebulin and skeletal muscle alpha-actin. Neuromuscul. Disord. 14, Wang, K. and Ramirez-Mitchell, R. (1983). A network of transverse and longitudinal intermediate filaments is associated with sarcomeres of adult vertebrate skeletal muscle. J. Cell Biol. 96, Witt, C. C., Burkart, C., Labeit, D., McNabb, M., Wu, Y., Granzier, H. and Labeit, S. (2006). Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 25,
Review Article New Insights into the Structural Roles of Nebulin in Skeletal Muscle
 Journal of Biomedicine and Biotechnology Volume 2010, Article ID 968139, 6 pages doi:10.1155/2010/968139 Review Article New Insights into the Structural Roles of Nebulin in Skeletal Muscle Coen A. C. Ottenheijm
Journal of Biomedicine and Biotechnology Volume 2010, Article ID 968139, 6 pages doi:10.1155/2010/968139 Review Article New Insights into the Structural Roles of Nebulin in Skeletal Muscle Coen A. C. Ottenheijm
MUSCLE BIOLOGY OVERVIEW OF THE SKELETAL MUSCLE CYTOSKELETON
 MUSCLE BIOLOGY OVERVIEW OF THE SKELETAL MUSCLE CYTOSKELETON Ted Huiatt, Ph.D. Associate Professor, Department of Animal Science and Roy J. Carver Department of Biochemistry, Biophysics and Molecular Biology,
MUSCLE BIOLOGY OVERVIEW OF THE SKELETAL MUSCLE CYTOSKELETON Ted Huiatt, Ph.D. Associate Professor, Department of Animal Science and Roy J. Carver Department of Biochemistry, Biophysics and Molecular Biology,
CONTRACTION BANDS AT SHORT SARCOMERE LENGTH IN CHICK MUSCLE
 CONTRACTION BANDS AT SHORT SARCOMERE LENGTH IN CHICK MUSCLE MARTIN HAGOPIAN. From the Department of Pathology, New York Medical College, New York 10029 INTRODUCTION The sliding filament model for contraction
CONTRACTION BANDS AT SHORT SARCOMERE LENGTH IN CHICK MUSCLE MARTIN HAGOPIAN. From the Department of Pathology, New York Medical College, New York 10029 INTRODUCTION The sliding filament model for contraction
Evidence for increased myofibrillar mobility in desmin-null mouse skeletal muscle
 The Journal of Experimental Biology 205, 321 325 (2002) Printed in Great Britain The Company of Biologists Limited 2002 JEB3848 321 Evidence for increased myofibrillar mobility in desmin-null mouse skeletal
The Journal of Experimental Biology 205, 321 325 (2002) Printed in Great Britain The Company of Biologists Limited 2002 JEB3848 321 Evidence for increased myofibrillar mobility in desmin-null mouse skeletal
Titin Extensibility In Situ: Entropic Elasticity of Permanently Folded and Permanently Unfolded Molecular Segments
 Published Online: 23 February, 1998 Supp Info: http://doi.org/10.1083/jcb.140.4.853 Downloaded from jcb.rupress.org on December 27, 2018 Titin Extensibility In Situ: Entropic Elasticity of Permanently
Published Online: 23 February, 1998 Supp Info: http://doi.org/10.1083/jcb.140.4.853 Downloaded from jcb.rupress.org on December 27, 2018 Titin Extensibility In Situ: Entropic Elasticity of Permanently
Chapter 16. Cellular Movement: Motility and Contractility. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 16 Cellular Movement: Motility and Contractility Lectures by Kathleen Fitzpatrick Simon Fraser University Two eukaryotic motility systems 1. Interactions between motor proteins and microtubules
Chapter 16 Cellular Movement: Motility and Contractility Lectures by Kathleen Fitzpatrick Simon Fraser University Two eukaryotic motility systems 1. Interactions between motor proteins and microtubules
Titin is a structural protein in muscle that
 8 Journal of Undergraduate Research in Alberta Volume 2 212 The Mechanical Properties of Titin within a Sarcomere? Jens A. Herzog, Tim R. Leonard, Azim Jinha, Walter Herzog University of Calgary Titin
8 Journal of Undergraduate Research in Alberta Volume 2 212 The Mechanical Properties of Titin within a Sarcomere? Jens A. Herzog, Tim R. Leonard, Azim Jinha, Walter Herzog University of Calgary Titin
According to the diagram, which of the following is NOT true?
 Instructions: Review Chapter 44 on muscular-skeletal systems and locomotion, and then complete the following Blackboard activity. This activity will introduce topics that will be covered in the next few
Instructions: Review Chapter 44 on muscular-skeletal systems and locomotion, and then complete the following Blackboard activity. This activity will introduce topics that will be covered in the next few
138 Physiology Biochemistry and Pharmacology
 Reviews of 138 Physiology Biochemistry and Pharmacology Special Issue on The Third Filament System Edited by D. Pette and D. Fiirst (Guest Editor) Editors M.E Blaustein, Baltimore R. Greger, Freiburg H.
Reviews of 138 Physiology Biochemistry and Pharmacology Special Issue on The Third Filament System Edited by D. Pette and D. Fiirst (Guest Editor) Editors M.E Blaustein, Baltimore R. Greger, Freiburg H.
Myoblast structure affects subsequent skeletal myotube morphology and sarcomere assembly
 Available online at www.sciencedirect.com R Experimental Cell Research 291 (2003) 435 450 www.elsevier.com/locate/yexcr Myoblast structure affects subsequent skeletal myotube morphology and sarcomere assembly
Available online at www.sciencedirect.com R Experimental Cell Research 291 (2003) 435 450 www.elsevier.com/locate/yexcr Myoblast structure affects subsequent skeletal myotube morphology and sarcomere assembly
TISSUE. A) Types. (i)
 MUSCLES & MUSCLE TISSUE I. OVERVIEW - Muscle ( little mouse ) - tissue designed to cause movementt thru contraction ( shortening ). A) Types - There are some SIMILARITIES between muscle types: (i) All
MUSCLES & MUSCLE TISSUE I. OVERVIEW - Muscle ( little mouse ) - tissue designed to cause movementt thru contraction ( shortening ). A) Types - There are some SIMILARITIES between muscle types: (i) All
Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization
 Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
THE ULTRASTRUCTURE OF THE M LINE IN SKELETAL MUSCLE
 Published Online: 1 July, 1968 Supp Info: http://doi.org/10.1083/jcb.38.1.202 Downloaded from jcb.rupress.org on October 1, 2018 THE ULTRASTRUCTURE OF THE M LINE IN SKELETAL MUSCLE GUSTAV G. KNAPPEIS and
Published Online: 1 July, 1968 Supp Info: http://doi.org/10.1083/jcb.38.1.202 Downloaded from jcb.rupress.org on October 1, 2018 THE ULTRASTRUCTURE OF THE M LINE IN SKELETAL MUSCLE GUSTAV G. KNAPPEIS and
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
Lecture 13, 05 October 2004 Chapter 10, Muscle. Vertebrate Physiology ECOL 437 University of Arizona Fall instr: Kevin Bonine t.a.
 Lecture 13, 05 October 2004 Chapter 10, Muscle Vertebrate Physiology ECOL 437 University of Arizona Fall 2004 instr: Kevin Bonine t.a.: Nate Swenson Vertebrate Physiology 437 18 1. Muscle A. Sarcomere
Lecture 13, 05 October 2004 Chapter 10, Muscle Vertebrate Physiology ECOL 437 University of Arizona Fall 2004 instr: Kevin Bonine t.a.: Nate Swenson Vertebrate Physiology 437 18 1. Muscle A. Sarcomere
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016
 PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
UNIT 6 THE MUSCULAR SYSTEM
 UNIT 6 THE MUSCULAR SYSTEM I. Functions of Muscular System A. Produces Movement Internal vs. External «locomotion & manipulation «circulate blood & maintain blood pressure «move fluids, food, baby B. Maintaining
UNIT 6 THE MUSCULAR SYSTEM I. Functions of Muscular System A. Produces Movement Internal vs. External «locomotion & manipulation «circulate blood & maintain blood pressure «move fluids, food, baby B. Maintaining
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Eisner et al., http://www.jcb.org/cgi/content/full/jcb.201312066/dc1 Figure S1. Mitochondrial continuity in adult skeletal muscle. (A)
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Eisner et al., http://www.jcb.org/cgi/content/full/jcb.201312066/dc1 Figure S1. Mitochondrial continuity in adult skeletal muscle. (A)
SUPPLEMENTARY INFORMATION
 GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
Distribution of sarcomere length and intracellular calcium in mouse skeletal muscle following stretch-induced injury
 Keywords: Stretch, Skeletal muscle fibre, Calcium imaging 6441 Journal of Physiology (1997), 502.3, pp. 649 659 649 Distribution of sarcomere length and intracellular calcium in mouse skeletal muscle following
Keywords: Stretch, Skeletal muscle fibre, Calcium imaging 6441 Journal of Physiology (1997), 502.3, pp. 649 659 649 Distribution of sarcomere length and intracellular calcium in mouse skeletal muscle following
Aberrant Mitochondria with Longitudinal Cristae Observed in the Normal Rat Hepatic Parenchymal Cell. Takuma Saito and Kazuo Ozawa
 Okajimas Fol. anat. jap., 44 : 357-363, 1968 Aberrant Mitochondria with Longitudinal Cristae Observed in the Normal Rat Hepatic Parenchymal Cell By Takuma Saito and Kazuo Ozawa Department of Anatomy, Kansai
Okajimas Fol. anat. jap., 44 : 357-363, 1968 Aberrant Mitochondria with Longitudinal Cristae Observed in the Normal Rat Hepatic Parenchymal Cell By Takuma Saito and Kazuo Ozawa Department of Anatomy, Kansai
Muscle tissue. Types. Functions. Cardiac, Smooth, and Skeletal
 Types Cardiac, Smooth, and Skeletal Functions movements posture and body position Support soft tissues Guard openings body temperature nutrient reserves Muscle tissue Special Characteristics of Muscle
Types Cardiac, Smooth, and Skeletal Functions movements posture and body position Support soft tissues Guard openings body temperature nutrient reserves Muscle tissue Special Characteristics of Muscle
Intravital Imaging Reveals Ghost Fibers as Architectural Units Guiding Myogenic Progenitors during Regeneration
 Cell Stem Cell Supplemental Information Intravital Imaging Reveals Ghost Fibers as Architectural Units Guiding Myogenic Progenitors during Regeneration Micah T. Webster, Uri Manor, Jennifer Lippincott-Schwartz,
Cell Stem Cell Supplemental Information Intravital Imaging Reveals Ghost Fibers as Architectural Units Guiding Myogenic Progenitors during Regeneration Micah T. Webster, Uri Manor, Jennifer Lippincott-Schwartz,
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice Supplementary Figure 2.
 Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1
 Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
7.06 Cell Biology EXAM #3 April 21, 2005
 7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY METHODS Mef2 primary screen. RNAi hairpins from the VDRC collection were crossed to Mef2-GAL4 at 27 C. After 2 weeks lethality rate and stage was scored, and if possible 20-30 males containing
SUPPLEMENTARY METHODS Mef2 primary screen. RNAi hairpins from the VDRC collection were crossed to Mef2-GAL4 at 27 C. After 2 weeks lethality rate and stage was scored, and if possible 20-30 males containing
Transversal Stiffness and Young s Modulus of Single Fibers from Rat Soleus Muscle Probed by Atomic Force Microscopy
 418 Biophysical Journal Volume 98 February 2010 418 424 Transversal Stiffness and Young s Modulus of Single Fibers from Rat Soleus Muscle Probed by Atomic Force Microscopy Irina V. Ogneva, * Dmitry V.
418 Biophysical Journal Volume 98 February 2010 418 424 Transversal Stiffness and Young s Modulus of Single Fibers from Rat Soleus Muscle Probed by Atomic Force Microscopy Irina V. Ogneva, * Dmitry V.
Our patient for the day...
 Muscles Ch.12 Our patient for the day... Name: Eddy Age: Newborn Whole-body muscle contractions No relaxation Severe difficulty breathing due to inadequate relaxation of breathing muscles Diagnosed with
Muscles Ch.12 Our patient for the day... Name: Eddy Age: Newborn Whole-body muscle contractions No relaxation Severe difficulty breathing due to inadequate relaxation of breathing muscles Diagnosed with
SALS, a WH2-Domain-Containing Protein, Promotes Sarcomeric Actin Filament Elongation from Pointed Ends during Drosophila Muscle Growth
 Article SALS, a WH2-Domain-Containing Protein, Promotes Sarcomeric Actin Filament Elongation from Pointed Ends during Drosophila Muscle Growth Jianwu Bai, 1, * John H. Hartwig, 3 and Norbert Perrimon 1,2,
Article SALS, a WH2-Domain-Containing Protein, Promotes Sarcomeric Actin Filament Elongation from Pointed Ends during Drosophila Muscle Growth Jianwu Bai, 1, * John H. Hartwig, 3 and Norbert Perrimon 1,2,
Identifying cells using Immunofluorscence Rachel Scalzo Bio-300, 001 Professor Bentley 9/24/2014
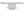 Identifying cells using Immunofluorscence Rachel Scalzo Bio-300, 001 Professor Bentley 9/24/2014 2 Abstract In this experiment, we used immunofluorescence to isolate certain organelles and see where they
Identifying cells using Immunofluorscence Rachel Scalzo Bio-300, 001 Professor Bentley 9/24/2014 2 Abstract In this experiment, we used immunofluorescence to isolate certain organelles and see where they
SUPPLEMENTARY INFORMATION
 med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
Muscle regulation and Actin Topics: Tropomyosin and Troponin, Actin Assembly, Actin-dependent Movement
 1 Muscle regulation and Actin Topics: Tropomyosin and Troponin, Actin Assembly, Actin-dependent Movement In the last lecture, we saw that a repeating alternation between chemical (ATP hydrolysis) and vectorial
1 Muscle regulation and Actin Topics: Tropomyosin and Troponin, Actin Assembly, Actin-dependent Movement In the last lecture, we saw that a repeating alternation between chemical (ATP hydrolysis) and vectorial
Slide 1. Slide 2. Slide 3. Muscles general information. Muscles - introduction. Microtubule Function
 Slide 1 Muscles general information Vertebrates and many invertebrates have three main classes of muscle Skeletal muscle connect bones are are used for complex coordianted activities. Smooth muscles surround
Slide 1 Muscles general information Vertebrates and many invertebrates have three main classes of muscle Skeletal muscle connect bones are are used for complex coordianted activities. Smooth muscles surround
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
Structural and Functional Roles of Desmin in Mouse Skeletal Muscle during Passive Deformation
 Biophysical Journal Volume 86 May 2004 2993 3008 2993 Structural and Functional Roles of Desmin in Mouse Skeletal Muscle during Passive Deformation Sameer B. Shah,* Jennifer Davis,* Noah Weisleder, y Ioanna
Biophysical Journal Volume 86 May 2004 2993 3008 2993 Structural and Functional Roles of Desmin in Mouse Skeletal Muscle during Passive Deformation Sameer B. Shah,* Jennifer Davis,* Noah Weisleder, y Ioanna
 DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr
 The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr Caroline Kemp, Ron Bardsley,, Peter Buttery Division of Nutritional Sciences, School of Biosciences, University of
The Caspase System: a potential role in muscle proteolysis and meat quality? Tim Parr Caroline Kemp, Ron Bardsley,, Peter Buttery Division of Nutritional Sciences, School of Biosciences, University of
(Be sure to clearly state the principles addressed in your discussion.)
 CELL QUESTION 1992: AP BIOLOGY A laboratory assistant prepared solutions of 0.8 M, 0.6 M, 0.4 M, and 0.2 M sucrose, but forgot to label them. After realizing the error, the assistant randomly labeled the
CELL QUESTION 1992: AP BIOLOGY A laboratory assistant prepared solutions of 0.8 M, 0.6 M, 0.4 M, and 0.2 M sucrose, but forgot to label them. After realizing the error, the assistant randomly labeled the
horseradish peroxidase-labeled anti-mouse secondary antibody were procured from
 SUPPORTING INFORMATION Characterization of anti-platelet properties of silver nanoparticles Siddhartha Shrivastava, Tanmay Bera, Sunil K. Singh, Gajendra Singh, P. Ramachandrarao and Debabrata Dash 1.
SUPPORTING INFORMATION Characterization of anti-platelet properties of silver nanoparticles Siddhartha Shrivastava, Tanmay Bera, Sunil K. Singh, Gajendra Singh, P. Ramachandrarao and Debabrata Dash 1.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature09450 Supplementary Table 1 Summary of kinetic parameters. Kinetic parameters were V = V / 1 K / ATP and obtained using the relationships max ( + m [ ]) V d s /( 1/ k [ ATP] + 1 k ) =,
doi:10.1038/nature09450 Supplementary Table 1 Summary of kinetic parameters. Kinetic parameters were V = V / 1 K / ATP and obtained using the relationships max ( + m [ ]) V d s /( 1/ k [ ATP] + 1 k ) =,
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Amanda Rawson Independent Research Project Report Bio219 Cell Biology. December 3, 2008
 Evidence That Treatment with Cytochalasin B Increases the Average Velocity of Cytoplasmic Streaming in Amoebae Proteus Amanda Rawson Independent Research Project Report Bio219 Cell Biology December 3,
Evidence That Treatment with Cytochalasin B Increases the Average Velocity of Cytoplasmic Streaming in Amoebae Proteus Amanda Rawson Independent Research Project Report Bio219 Cell Biology December 3,
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
Module 2: Foundations in biology
 alevelbiology.co.uk Module 2: Foundations in biology SPECIFICATION 2.1.1 Cell structure Learners should be able to demonstrate and apply their knowledge and understanding of: (a) The use of microscopy
alevelbiology.co.uk Module 2: Foundations in biology SPECIFICATION 2.1.1 Cell structure Learners should be able to demonstrate and apply their knowledge and understanding of: (a) The use of microscopy
Supporting Online Material. On-Chip Dielectrophoretic Co-Assembly of Live Cells and. Particles into Responsive Biomaterials
 Supporting Online Material On-Chip Dielectrophoretic Co-Assembly of Live Cells and Particles into esponsive Biomaterials Shalini Gupta, ossitza G. Alargova, Peter K. Kilpatrick and Orlin D. Velev* Description
Supporting Online Material On-Chip Dielectrophoretic Co-Assembly of Live Cells and Particles into esponsive Biomaterials Shalini Gupta, ossitza G. Alargova, Peter K. Kilpatrick and Orlin D. Velev* Description
4) Please cite Dagda et al J Biol Chem 284: , for any publications or presentations resulting from use or modification of the macro.
 Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Modeling. EC-Coupling and Contraction
 Bioeng 6460 Electrophysiology and Bioelectricity Modeling of EC-Coupling and Contraction Frank B. Sachse fs@cvrti.utah.edu Overview Quiz Excitation-Contraction Coupling Anatomy Cross Bridge Binding Coupling
Bioeng 6460 Electrophysiology and Bioelectricity Modeling of EC-Coupling and Contraction Frank B. Sachse fs@cvrti.utah.edu Overview Quiz Excitation-Contraction Coupling Anatomy Cross Bridge Binding Coupling
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Preparing Colloidal Gold for Electron Microscopy
 Corporate Headquarters 400 Valley Road Warrington, PA 18976 1-800-523-2575 FAX 1-800-343-3291 Email: info@polysciences.com www.polysciences.com Europe - Germany Polysciences Europe GmbH Handelsstr. 3 D-69214
Corporate Headquarters 400 Valley Road Warrington, PA 18976 1-800-523-2575 FAX 1-800-343-3291 Email: info@polysciences.com www.polysciences.com Europe - Germany Polysciences Europe GmbH Handelsstr. 3 D-69214
Neurite formation & neuronal polarization
 Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Supplemental material
 Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Acta Medica Okayama. An electron microscopic study on the red, white and inter-mediate muscle fibers of mouse. Takuro Ogata OCTOBER 1964
 Acta Medica Okayama Volume 18, Issue 5 1964 Article 3 OCTOBER 1964 An electron microscopic study on the red, white and inter-mediate muscle fibers of mouse Takuro Ogata Okayama University, Copyright c
Acta Medica Okayama Volume 18, Issue 5 1964 Article 3 OCTOBER 1964 An electron microscopic study on the red, white and inter-mediate muscle fibers of mouse Takuro Ogata Okayama University, Copyright c
Protocol for Minichip hybridization. Equipment and Reagents needed: BLOCKING SOLUTION (2% [w/v]) CY-3 STREPTAVIDIN
![Protocol for Minichip hybridization. Equipment and Reagents needed: BLOCKING SOLUTION (2% [w/v]) CY-3 STREPTAVIDIN Protocol for Minichip hybridization. Equipment and Reagents needed: BLOCKING SOLUTION (2% [w/v]) CY-3 STREPTAVIDIN](/thumbs/75/71532736.jpg) Protocol for Minichip hybridization Equipment and Reagents needed: 1,000mL or 250mL Filter Units (PES Membrane) (VWR, 73520-986) 1,000mL or 250mL Receiver Units (VWR, 28199-346/28199-225) Weighing Plate
Protocol for Minichip hybridization Equipment and Reagents needed: 1,000mL or 250mL Filter Units (PES Membrane) (VWR, 73520-986) 1,000mL or 250mL Receiver Units (VWR, 28199-346/28199-225) Weighing Plate
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
BIOMECHANICS 3 Origins and consequences of forces in biological systems
 BIOMECHANICS 3 Origins and consequences of forces in biological systems MOLECULAR MECHANISMS OF BIOLOGICAL MOVEMENT AT THE LEVELOF ORGANISMS MOLECULAR BASIS OF MUSCLE CONTRACTION DR. BEÁTA BUGYI - BIOPHYSICS
BIOMECHANICS 3 Origins and consequences of forces in biological systems MOLECULAR MECHANISMS OF BIOLOGICAL MOVEMENT AT THE LEVELOF ORGANISMS MOLECULAR BASIS OF MUSCLE CONTRACTION DR. BEÁTA BUGYI - BIOPHYSICS
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Tokuhiro JSHIHARA, Chotatsu TSUKAYAMA, Fumiya UCHINO
 (39) JOURNAL OF ELECTRON MICROSCOPY 39 Vol. 22, No. I, 39-44, 1973 Intramitochondrial Filamentous Structures in Human Reticulum Cells in the Bone Marrow Tokuhiro JSHIHARA, Chotatsu TSUKAYAMA, Fumiya UCHINO
(39) JOURNAL OF ELECTRON MICROSCOPY 39 Vol. 22, No. I, 39-44, 1973 Intramitochondrial Filamentous Structures in Human Reticulum Cells in the Bone Marrow Tokuhiro JSHIHARA, Chotatsu TSUKAYAMA, Fumiya UCHINO
Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc
 OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
Supplementary Information
 Supplementary Information Mechanical unzipping and rezipping of a single SNARE complex reveals hysteresis as a force-generating mechanism Duyoung Min, Kipom Kim, Changbong Hyeon, Yong Hoon Cho, Yeon-Kyun
Supplementary Information Mechanical unzipping and rezipping of a single SNARE complex reveals hysteresis as a force-generating mechanism Duyoung Min, Kipom Kim, Changbong Hyeon, Yong Hoon Cho, Yeon-Kyun
Chapter 6: A Tour of the Cell
 Chapter 6: A Tour of the Cell 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when Robert Hooke first looked at dead cells from an oak tree.
Chapter 6: A Tour of the Cell 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when Robert Hooke first looked at dead cells from an oak tree.
Supplementary Figure 1.
 Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/1/9/e1500511/dc1 Supplementary Materials for Contractility parameters of human -cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of
advances.sciencemag.org/cgi/content/full/1/9/e1500511/dc1 Supplementary Materials for Contractility parameters of human -cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of
Electron Microscopy (TEM and SEM)
 7 Electron Microscopy (TEM and SEM) Paul Verkade Wolfson Bioimaging Facility, Physiology & Pharmacology and Biochemistry, University of Bristol, UK 7.1 Basic how-to-do and why-do section 7.1.1 Electron
7 Electron Microscopy (TEM and SEM) Paul Verkade Wolfson Bioimaging Facility, Physiology & Pharmacology and Biochemistry, University of Bristol, UK 7.1 Basic how-to-do and why-do section 7.1.1 Electron
Neurite formation & neuronal polarization. The cytoskeletal components of neurons have characteristic distributions and associations
 Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Modelling Muscle Contraction a multiscale approach
 Porto Ercole, M&MKT 2016 Multiscale Systems from Particles to Continuum: Modelling and Computation Modelling Muscle Contraction a multiscale approach Giovanni Naldi Dipartimento di Matematica ``F. Enriques
Porto Ercole, M&MKT 2016 Multiscale Systems from Particles to Continuum: Modelling and Computation Modelling Muscle Contraction a multiscale approach Giovanni Naldi Dipartimento di Matematica ``F. Enriques
Unfolding of Titin Domains Explains the Viscoelastic Behavior of Skeletal Myofibrils
 1442 Biophysical Journal Volume 80 March 2001 1442 1451 Unfolding of Titin Domains Explains the Viscoelastic Behavior of Skeletal Myofibrils Ave Minajeva,* Michael Kulke,* Julio M. Fernandez, and Wolfgang
1442 Biophysical Journal Volume 80 March 2001 1442 1451 Unfolding of Titin Domains Explains the Viscoelastic Behavior of Skeletal Myofibrils Ave Minajeva,* Michael Kulke,* Julio M. Fernandez, and Wolfgang
Microtubule-dependent transport and organization of sarcomeric myosin during skeletal muscle differentiation
 The EMBO Journal (2005) 24, 3781 3792 & 2005 European Molecular Biology Organization All Rights Reserved 0261-4189/05 www.embojournal.org Microtubule-dependent transport and organization of sarcomeric
The EMBO Journal (2005) 24, 3781 3792 & 2005 European Molecular Biology Organization All Rights Reserved 0261-4189/05 www.embojournal.org Microtubule-dependent transport and organization of sarcomeric
Mitochondrial dynamics in ischemia and reperfusion
 Mitochondrial dynamics in ischemia and reperfusion Derek J Hausenloy Reader in Cardiovascular Medicine, British Heart Foundation Senior Clinical Research Fellow, The Hatter Cardiovascular Institute, University
Mitochondrial dynamics in ischemia and reperfusion Derek J Hausenloy Reader in Cardiovascular Medicine, British Heart Foundation Senior Clinical Research Fellow, The Hatter Cardiovascular Institute, University
T H E J O U R N A L O F C E L L B I O L O G Y
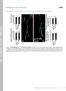 Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Mechanical Proteins. Stretching imunoglobulin and fibronectin. domains of the muscle protein titin. Adhesion Proteins of the Immune System
 Mechanical Proteins F C D B A domains of the muscle protein titin E Stretching imunoglobulin and fibronectin G NIH Resource for Macromolecular Modeling and Bioinformatics Theoretical Biophysics Group,
Mechanical Proteins F C D B A domains of the muscle protein titin E Stretching imunoglobulin and fibronectin G NIH Resource for Macromolecular Modeling and Bioinformatics Theoretical Biophysics Group,
Biochemical and Structural Properties of Titin, Nebulin and Intermediate Filaments in Muscle
 MUSCLE BIOCHEMISTRY Biochemical and Structural Properties of Titin, Nebulin and Intermediate Filaments in Muscle Richard M. Robson* Ted W. Huiatt Frederick C. Parrish, Jr. Introduction In a generic vertebrate
MUSCLE BIOCHEMISTRY Biochemical and Structural Properties of Titin, Nebulin and Intermediate Filaments in Muscle Richard M. Robson* Ted W. Huiatt Frederick C. Parrish, Jr. Introduction In a generic vertebrate
UNCORRECTED PROOF ARTICLE IN PRESS
 YEXCR-06202; No. of pages: 15; 4C: 5, 6 Experimental Cell Research xx (2004) xxx xxx www.elsevier.com/locate/yexcr 1 A novel role for non-muscle g-actin in skeletal 2 muscle sarcomere assembly 3 C.M. Lloyd,
YEXCR-06202; No. of pages: 15; 4C: 5, 6 Experimental Cell Research xx (2004) xxx xxx www.elsevier.com/locate/yexcr 1 A novel role for non-muscle g-actin in skeletal 2 muscle sarcomere assembly 3 C.M. Lloyd,
Structure of Biological Materials
 ELEC ENG 3BA3: Structure of Biological Materials Notes for Lecture #7 Monday, September 24, 2012 3.2 Muscle biomechanics Organization: skeletal muscle is made up of muscle fibers each fiber is a single
ELEC ENG 3BA3: Structure of Biological Materials Notes for Lecture #7 Monday, September 24, 2012 3.2 Muscle biomechanics Organization: skeletal muscle is made up of muscle fibers each fiber is a single
camp Direct Immunoassay Kit
 camp Direct Immunoassay Kit Catalog Number KA0886 100 assays Version: 05 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
camp Direct Immunoassay Kit Catalog Number KA0886 100 assays Version: 05 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing
 Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Desmin Cytoskeleton Linked to Muscle Mitochondrial Distribution and Respiratory Function
 Desmin Cytoskeleton Linked to Muscle Mitochondrial Distribution and Respiratory Function Derek J. Milner, Manolis Mavroidis, Noah Weisleder, and Yassemi Capetanaki Department of Molecular and Cell Biology,
Desmin Cytoskeleton Linked to Muscle Mitochondrial Distribution and Respiratory Function Derek J. Milner, Manolis Mavroidis, Noah Weisleder, and Yassemi Capetanaki Department of Molecular and Cell Biology,
CELL PRACTICE TEST
 Name: Date: 1. As a human red blood cell matures, it loses its nucleus. As a result of this loss, a mature red blood cell lacks the ability to (1) take in material from the blood (2) release hormones to
Name: Date: 1. As a human red blood cell matures, it loses its nucleus. As a result of this loss, a mature red blood cell lacks the ability to (1) take in material from the blood (2) release hormones to
T he tv~-filament cross-bridge model of muscle contraction
 The Positional Stability of Thick Filaments in Activated Skeletal Muscle Depends on Sarcomere Length: Evidence for the Role of Titin Filaments Robert Horowits and Richard J. Podolsky National nstitute
The Positional Stability of Thick Filaments in Activated Skeletal Muscle Depends on Sarcomere Length: Evidence for the Role of Titin Filaments Robert Horowits and Richard J. Podolsky National nstitute
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis):
 SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis): Aim: SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis) is one of the common methods used in the molecular biology
SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis): Aim: SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis) is one of the common methods used in the molecular biology
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 215 Electronic Supplementary Information Redox cycling-amplified enzymatic Ag deposition
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 215 Electronic Supplementary Information Redox cycling-amplified enzymatic Ag deposition
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
The Influence of the Rate of Rigor State Development on Its Tension in Single Muscle Fibre
 Gen Physiol. Biophys. (1990), 9, 245 250 M5 The Influence of the Rate of Rigor State Development on Its Tension in Single Muscle Fibre A. S. KHROMOV, L. K. SREBNITSKAYA and V. V. LFDNEV Institute of Biological
Gen Physiol. Biophys. (1990), 9, 245 250 M5 The Influence of the Rate of Rigor State Development on Its Tension in Single Muscle Fibre A. S. KHROMOV, L. K. SREBNITSKAYA and V. V. LFDNEV Institute of Biological
A Model Based Analysis of Steady-State versus Dynamic Elements in the Relationship between Calcium and Force
 A Model Based Analysis of Steady-State versus Dynamic Elements in the Relationship between Calcium and Force Casey L. Overby, Sanjeev G. Shroff BACKGROUND Cardiac contraction and calcium. Intracellular
A Model Based Analysis of Steady-State versus Dynamic Elements in the Relationship between Calcium and Force Casey L. Overby, Sanjeev G. Shroff BACKGROUND Cardiac contraction and calcium. Intracellular
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
DOI: 10.1038/ncb2362 Figure S1 CYLD and CASPASE 8 genes are co-regulated. Analysis of gene expression across 79 tissues was carried out as described previously [Ref: PMID: 18636086]. Briefly, microarray
Cytokinesis in fission yeast: Modeling the assembly of the contractile ring
 Cytokinesis in fission yeast: Modeling the assembly of the contractile ring Nikola Ojkic, Dimitrios Vavylonis Department of Physics, Lehigh University Damien Laporte, Jian-Qiu Wu Department of Molecular
Cytokinesis in fission yeast: Modeling the assembly of the contractile ring Nikola Ojkic, Dimitrios Vavylonis Department of Physics, Lehigh University Damien Laporte, Jian-Qiu Wu Department of Molecular
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R)
 Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
targets. clustering show that different complex pathway
 Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Supplementary Methods
 Supplementary Methods Modeling of magnetic field In this study, the magnetic field was generated with N52 grade nickel-plated neodymium block magnets (K&J Magnetics). The residual flux density of the magnets
Supplementary Methods Modeling of magnetic field In this study, the magnetic field was generated with N52 grade nickel-plated neodymium block magnets (K&J Magnetics). The residual flux density of the magnets
Targeted homozygous deletion of M-band titin in cardiomyocytes prevents sarcomere formation
 4322 Research Article Targeted homozygous deletion of M-band titin in cardiomyocytes prevents sarcomere formation Hanny Musa 1, Stephen Meek 2, Mathias Gautel 3, Dianna Peddie 2, Andrew J. H. Smith 2 and
4322 Research Article Targeted homozygous deletion of M-band titin in cardiomyocytes prevents sarcomere formation Hanny Musa 1, Stephen Meek 2, Mathias Gautel 3, Dianna Peddie 2, Andrew J. H. Smith 2 and
Nuclear import of DNA: genetic modification of plants
 Nuclear import of DNA: genetic modification of plants gene delivery by Agrobacterium tumefaciens T. Tzfira & V. Citovsky. 2001. Trends in Cell Biol. 12: 121-129 VirE2 binds ssdna in vitro forms helical
Nuclear import of DNA: genetic modification of plants gene delivery by Agrobacterium tumefaciens T. Tzfira & V. Citovsky. 2001. Trends in Cell Biol. 12: 121-129 VirE2 binds ssdna in vitro forms helical
Multimedia : Fibronectin and Titin unfolding simulation movies.
 I LECTURE 21: SINGLE CHAIN ELASTICITY OF BIOMACROMOLECULES: THE GIANT PROTEIN TITIN AND DNA Outline : REVIEW LECTURE #2 : EXTENSIBLE FJC AND WLC... 2 STRUCTURE OF MUSCLE AND TITIN... 3 SINGLE MOLECULE
I LECTURE 21: SINGLE CHAIN ELASTICITY OF BIOMACROMOLECULES: THE GIANT PROTEIN TITIN AND DNA Outline : REVIEW LECTURE #2 : EXTENSIBLE FJC AND WLC... 2 STRUCTURE OF MUSCLE AND TITIN... 3 SINGLE MOLECULE
6 Mechanotransduction
 6.1 Motivation The process of converting physical forces into biochemical signals and integrating these signals into the cellular response is referred to as mechnotransduction [11, 20]. To fully understand
6.1 Motivation The process of converting physical forces into biochemical signals and integrating these signals into the cellular response is referred to as mechnotransduction [11, 20]. To fully understand
Sarcomere Formation Occurs by the Assembly of Multiple Latent Protein Complexes
 Sarcomere Formation Occurs by the Assembly of Multiple Latent Protein Complexes Yanning Rui 1. *, Jianwu Bai 1., Norbert Perrimon 1,2 * 1 Department of Genetics, Harvard Medical School, Boston, Massachusetts,
Sarcomere Formation Occurs by the Assembly of Multiple Latent Protein Complexes Yanning Rui 1. *, Jianwu Bai 1., Norbert Perrimon 1,2 * 1 Department of Genetics, Harvard Medical School, Boston, Massachusetts,
me239 mechanics of the cell - syllabus me239 mechanics of the cell me239 mechanics of the cell - grading me239 mechanics of the cell - overview
 6 mechanotransduction wong, goktepe, kuhl [2010] me239 mechanics of the cell add l information http://biomechanics.stanford.edu and coursework 1 me239 mechanics of the cell - syllabus favorite topics in
6 mechanotransduction wong, goktepe, kuhl [2010] me239 mechanics of the cell add l information http://biomechanics.stanford.edu and coursework 1 me239 mechanics of the cell - syllabus favorite topics in
Chapter 6: A Tour of the Cell
 AP Biology Reading Guide Fred and Theresa Holtzclaw Chapter 6: A Tour of the Cell Name Period Chapter 6: A Tour of the Cell Concept 6.1 To study cells, biologists use microscopes and the tools of biochemistry
AP Biology Reading Guide Fred and Theresa Holtzclaw Chapter 6: A Tour of the Cell Name Period Chapter 6: A Tour of the Cell Concept 6.1 To study cells, biologists use microscopes and the tools of biochemistry
TUBULAR ELEMENTS-A NEW STRUCTURE IN BLUE-GREEN ALGAL CELLS
 J. Cell Sci. 38, 303-308 (i977) 303 Printed in Great Britain Company of Biologists Limited TUBULAR ELEMENTS-A NEW STRUCTURE IN BLUE-GREEN ALGAL CELLS Z. N. TAHMIDA KHAN AND M. B. E. GODWARD Department
J. Cell Sci. 38, 303-308 (i977) 303 Printed in Great Britain Company of Biologists Limited TUBULAR ELEMENTS-A NEW STRUCTURE IN BLUE-GREEN ALGAL CELLS Z. N. TAHMIDA KHAN AND M. B. E. GODWARD Department
