Supplementary Information
|
|
|
- Gervais Dorsey
- 5 years ago
- Views:
Transcription
1 Supplementary Information Mechanical unzipping and rezipping of a single SNARE complex reveals hysteresis as a force-generating mechanism Duyoung Min, Kipom Kim, Changbong Hyeon, Yong Hoon Cho, Yeon-Kyun Shin and Tae- Young Yoon Supplementary Figures S1 to S10 Supplementary Tables S1 to S5 Supplementary Methods Supplementary References 1
2 Supplementary Figures and Tables Supplementary Figure S1 Single molecule magnetic tweezers and force calibration. (a) Singlemolecule magnetic tweezers consists of a LED light source, a pair of magnet, a custom-built inverted microscope, a nano-positioner for objective lens, and a CCD camera. (b) Sample schematic for force calibration. The SNARE-DNA hybrid is also shown for comparison. The forces were calibrated by 2
3 analyzing lateral fluctuations of magnetic beads, which were tethered on the surface through a μm-long -DNA. It is calculated as equation [2], F k Tl x 2 B /, where B kt is the thermal energy, l the extension of the DNA tether, and 2 x the lateral fluctuations of the bead. This - DNA tether is much longer than the 350-nm DNA-handles used for the main experiments but the force differences should be less than 0.16 pn (= 1 pn( m)/100 m). (c) The calibrated forces applied to magnetic beads as a function of the magnet height from sample surface (h). The black and red histograms respectively represent the mean forces and the standard deviations (n = molecules for each data points). To increase 1 pn, the magnet should approach the sample by m in the force range between 5 and 34 pn (see Supplementary Methods for details). 3
4 Supplementary Figure S2 Electrophoresis analyses of SNARE proteins, SNARE complex and SNARE-DNA hybrid. (a) 12% SDS-PAGE showing the protein bands corresponding to syntaxin 1A, synaptobrevin 2, SNAP-25 and SNARE complex. (b) 1.3% TAE agarose gel shows the fluorescence bands corresponding to Protein-DNA and DNA-Protein-DNA constructs, indicating that the desired SNARE-DNA hybrid with two DNA handles were formed. 4
5 Supplementary Figure S3 Sequences of the SNARE proteins used for C-to-N unzipping experiment. The residues in the -7 layer and adjacent to the +7 layer of syntaxin 1A and synaptobrevin 2 were mutated to cysteins, for knotting the N-terminal covalently and crosslinking DNA handles to the C-terminus of the SNARE complex, respectively. 5
6 Supplementary Figure S4 Distribution of unzipping forces. The unzipping forces for single SNARE complexes were measured while gradually increasing the tension at a loading rate of 1 pn/s, as in Figure 1c and 1d. The average force is 33.4 pn and the standard deviation is 2.8 pn (N = 18 events from n = 12 SNARE complexes). 6
7 7
8 Supplementary Figure S5 (a-i) Additional real-time traces showing the C-to-N unzipping events of single SNARE complexes. The force was increased from 1-11 pn to 34 pn with a loading rate of 58 pn/s. The traces show one-step unzipping events with either approximately 10-nm step size (a-c) or approximately 20-nm step size (d-f), or two-step unzipping events (g-i). 8
9 Supplementary Figure S6 Error of step-size measurement. (a) Representative traces showing the determination of step sizes for the unzipping and rezipping events. (b) The error in step-size measurement ( step ) is calculated as equation [S6],, where (, ) are the i f step Ni N f i f 9
10 standard deviations before and after the unzipping event at 34 pn (or the rezipping event at 11 pn) and ( N, N ) are the number of data points used for assessing the standard deviations. (c) Errors in i f step-size measurement. The unzipping events from Figure 2d (N = 119 events) were used for the 34- pn case, and the rezipping events from Figure 3e (N = 63 events) were used for the 11-pN case. The error in our step-size measurement is smaller than 1 nm for both unzipping and rezipping events (see Supplementary Methods for details). 10
11 Supplementary Figure S7 Experiment design of N-to-C unzipping experiment. (a) Sequences of the SNARE proteins used for the N-to-C unzipping experiment. The residues in the +8 layer and adjacent to the -7 layer of syntaxin 1A and synaptobrevin 2 were mutated to cysteins, for knotting the C-terminal covalently and crosslinking DNA handles to the N-terminus of the SNARE complex, respectively. (b) Experimental scheme for the N-to-C unzipping experiment (c) Helical wheel diagram of the SNARE complex. The f and d positions of the heptad repeats are used to attach DNA handles and knot the C-terminal. 11
12 Supplementary Figure S8 (a-f) Additional real-time traces while applying cycles of the constant-force application. Fully-assembled SNARE complexes at 11 pn (green traces) were unzipped by application of the 34-pN force (red traces). When the force was lowered back to 11 pn (blue traces), the extensions were higher than those observed before 34-pN unzipping. 12
13 Supplementary Figure S9 (a and b) Energy landscape diagrams for the SNARE-complex formation at 0-pN and 11-pN force (a), at 34-pN force (b). (a) If we use the zippering of the -5 layer as a criterion for partial zippering, which corresponds to the extension difference (D) of approximately 15 nm, 86% are partially zipped and 14% still remain in the unzipped state. 14% of the rezipping processes failed to cross the first barrier (blue arrow), trapped in the unzipped state. The other 86% of the rezipping processes were all blocked by the second energy barrier and stalled in the partially-assembled state (note that no population at D=0 in Fig. 3e). (b) The first barrier (blue arrow) halts the mechanical unzipping process (at 34 pn) at the middle layers, producing partial-unzipping events observed in Figure 2. 13
14 Supplementary Figure S10 (a and b) Schematics for pulling a parallel SNARE complex (a) and an anti-parallel SNARE complex (b). 14
15 Supplementary Table S1 Estimated SNARE-complex extensions for C-to-N unzipping process at 34 pn with the t-snare precomplex structure preserved. When a SNARE complex is unzipped from the C-terminal DNA anchor position up to a specific layer, the total extension is estimated as the sum of the extensions caused by synaptobrevin 2 and precomplex, which is then subtracted by nm, the distance between the C-terminal DNA anchor residues. The extension of synaptobrevin 2 is calculated using equation [S1] of the WLC model and the precomplex extension is estimated as the layer-to-layer distance determined from the crystal structure of the SNARE complex. The extension values for the zeroth layer (ionic layer) and knotted layer are shown in red. 15
16 Supplementary Table S2 Estimated SNARE-complex extensions for N-to-C unzipping process at 34 pn with the t-snare precomplex structure preserved. When a SNARE complex is unzipped from the N-terminal DNA anchor position up to a specific layer, the total extension is estimated as the sum of the extensions caused by synaptobrevin 2 and precomplex, which is then subtracted by nm, the distance between the N-terminal DNA anchor residues. The extension of synaptobrevin 2 is calculated using equation [S1] of the WLC model and the precomplex extension is estimated as the layer-to-layer distance determined from the crystal structure of the SNARE complex. The extension values for the zeroth layer (ionic layer) and knotted layer are shown in red. 16
17 Supplementary Table S3 Estimated SNARE-complex extensions for C-to-N unzipping process at 34 pn with unstructured t-snare proteins. When a SNARE complex is unzipped from the C- terminal DNA anchor position up to a specific layer, the total extension is estimated as the sum of the extensions caused by synaptobrevin 2 and now unstructured syntaxin 1A, which is then subtracted by nm, the distance between the C-terminal DNA anchor residues. The extension of the unstructured syntaxin 1A is calculated in the same way as that used for synaptobrevin 2. The extension values for the zeroth layer (ionic layer) and knotted layer are shown in red. 17
18 Supplementary Table S4 Estimated SNARE-complex extensions for N-to-C unzipping process at 34 pn with unstructured t-snare proteins. When a SNARE complex is unzipped from the N- terminal DNA anchor position up to a specific layer, the total extension is estimated as the sum of the extensions caused by synaptobrevin 2 and now unstructured syntaxin 1A, which is then subtracted by nm, the distance between the N-terminal DNA anchor residues. The extension of the unstructured syntaxin 1A is calculated in the same way as that used for synaptobrevin 2. The extension values for the zeroth layer (ionic layer) and knotted layer are shown in red. 18
19 Supplementary Table S5 Estimated SNARE-complex extensions for C-to-N unzipping process at 11 pn with the t-snare precomplex structure preserved. The extension values are estimated using the same approach described in Supplementary Table S1. The extension values for the zeroth layer (ionic layer) and knotted layer are shown in red. 19
20 Supplementary Methods Estimation on SNARE-complex extension To map the Gaussian peaks to the corresponding structures of SNARE complex, we analyzed the extension values by using the worm-like chain (WLC) model. We first assume that synaptobrevin 2 becomes unstructured after its mechanical extraction from a single SNARE complex. This assumption seems to be reasonable because synaptobrevin has been reported many times to be intrinsically disordered in its solitary form Moreover, we are applying tens of pn forces (11 to 34 pn), which are large enough to disrupt any remaining alpha-helical contents. Therefore, when a SNARE complex is unzipped, extension x caused by stretching of an unstructured synaptobrevin 2 is estimated by the following WLC model in response to force f, 2 fl p x x, [S1] kt B 4 lc 4 lc where l c is the contour length calculated as the number of unzipped residues times 0.38 nm (Ref. 29) and l the persistence length of 0.7 nm for the p protein28,55. On the other hand, the structural status of syntaxin and SNAP-25 is a wholly open question, particularly after extraction of synaptobrevin 2. Therefore, we estimated the extension caused by the t- SNARE precomplex for the two extreme cases where the precomplex fully preserves its -helical structure or the precomplex dissembles and syntaxin 1A is completely unstructured. For the first case, the extension is estimated as the layer-to-layer distance extracted from crystal structure of a SNARE complex 37,38, assuming layer-distances of SNARE complex and precomplex are almost identical. For the second case, it is estimated as stretching of an unstructured syntaxin 1A using the WLC model given in equation [S1], as in the case of synaptobrevin 2. Consequently, when a SNARE complex is unzipped from the C-terminal DNA anchor position up to the each layer at 34-pN force, the total predicted extensions are calculated as the sum of the extensions caused by synaptobrevin 2 and precomplex, which is subtracted by nm (Ref. 37,38), the distance between the C-terminal DNA anchor residues 29,56 (Supplementary Tables S1 and S3 for both structural cases). To gain an insight toward the structural status of syntaxin and SNAP-25, the t-snare precomplex, we repeated the unzipping experiment in the reverse direction, N- to C-terminal direction. For this N-to-C unzipping experiment, we attached the DNA handles next to the -7 layer and knotted the +8 layer using a disulfide bond (Supplementary Fig. S7). We carefully chose the positions of these DNA handles and disulfide bonds so that we could see an extension very similar to that of the previous C-to-N unzipping experiment when a single SNARE complex was fully unzipped. In the N- 20
21 to-c unzipping process, the total predicted extensions from the N-terminal DNA anchor position up to the each layer are also calculated as the sum of the extensions caused by synaptobrevin 2 and precomplex, minus nm (Ref. 37,38), the distance between the N-terminal DNA anchor residues 29,56 (Supplementary Tables S2 and S4 for both structural cases). Our model estimates that when a SNARE complex is fully unzipped, an extension of 21.1 nm is predicted for the original C-to-N unzipping experiment, which is very similar to 22.3 nm of the N-to- C unzipping experiment (Supplementary Tables S1 and S2). This estimate is based on an important assumption that the t-snare precomplex fully preserves its -helical structure even after dissociation of synaptobrevin. If the t-snare precomplex disassembles, syntaxin would become unstructured and further stretched. The estimated extensions consequently increase to values close to 30 nm for both C-to-N and N-to-C unzipping experiments (28.7 nm and 29.6 nm, respectively) (Supplementary Tables S3 and S4). If the t-snare precomplex is partly unfolded, the extension would be an intermediate value between these two estimates. The experimental data of the N-to-C unzipping experiment also show two Gaussian peaks for the first unzipping steps. We note that the higher peak is at 21.4 nm, an extension very close to 19.4 nm observed from the C-to-N unzipping experiment. Thus, for both C-to-N and N-to-C unzipping experiment, the fully-unzipped SNARE complexes consistently show the extensions at approximately 20 nm, the expected values when the t-snare precomplex retains its -helicity. In the light of above discussions, we have assumed in our model that the t-snare precomplex and its -helical structure is largely maintained. With these assumptions, we also estimated extensions at 11 pn for C-to-N unzipping process (Supplementary Table S5). Extracting energy barrier parameters from the single-molecule force spectroscopy data The survival probability St ( ) in Figure 4e is defined as the fraction of SNARE complexes that have remained in the partially-assembled state until time t (the force is quenched to a specific level at t = 0) (Fig. 4a-d). St ( ) is obtained from the first-order rate equation t which can be transformed to St () exp ktdt () 0 the fully-assembled state, depends only on the force, ds() t ktst () (), [S2] dt. Since kt (), the kinetic constant for transition to fx kt () kk0 exp. [S3] kt B 21
22 Thus, the survival probability observed at time t is given by St () exptke 0 fx kt B. [S4] The survival probability after an observation time of 20 s is given as 20s 0 fx / kbt S(20) S exp 20k e and finally converted to equation [1]. We fitted the experimental data in Figure 4e and 4f with these equations to obtain x and k 0, where x is the position of the energy barrier from the partially-assembled state confining the SNARE complex and k 0 is the kinetic rate for the zippering of the C-terminal half at zero force. The free energy of activation in the absence of external force ( G ) is extracted from the Kramers equation k G kbt 0 kwe, [S5] with the pre-exponential factor presumed to be k w s 4 1 (Ref. 28). Force calibration in our magnetic tweezers apparatus One technical advantage of the magnetic tweezers is that the force delivered to a magnetic bead, and thus to a single SNARE-DNA hybrid, can be easily controlled by changing the vertical distance of the magnets from the sample. In our apparatus, when we changed the magnet height from 8 to 2 mm, the force applied to a magnetic bead increased from 3 to 34 pn (Supplementary Fig. S1). We can presume two sources of error in these force values. Firstly, even when we precisely attain the same magnet height, the force experienced by each magnetic bead can have variations mainly due to different magnetic moments of different magnetic beads. The second source of force error is that even when we achieve the precisely the same force levels, the force levels can fluctuate in time because the magnetic beads show Brownian motion, which continuously changes the position of the magnetic beads relative to the magnet. When we consider the physical dimensions of our magnetic tweezers apparatus, it becomes evident that we can safely rule out the second source of force error. To make 1 pn increase, the magnet should typically approach the sample by m (Supplementary Fig. S1c). On the other hand, the Brownian motion of the magnetic beads tethered to 1,000 base-pair DNA handles (total extension of approximately 350 nm) is typically less than 20 nm. This Brownian motion would consequently make a force fluctuation less than (20 nm/200 m)1 pn10-4 pn, a totally negligible value when we are dealing with the force range between 5 and 34 pn. 22
23 However, we need to do careful calibration for the first source of force error, variations of magnetic force from bead to bead. To this end, we performed an independent experiment, in which we analyzed lateral Brownian motions of different magnetic beads. The lateral Brownian motion of a magnetic bead and its variance 2 x gives the direct information on force experienced by each magnetic bead through equation [2] (Ref. 21,53). To amplify the Brownian motion, we used a μm-long -DNA as the DNA tether (Supplementary Fig. S1b). We note this -DNA tether is much longer than the 350-nm DNA handles used for the main experiments, but the force difference should be less than 0.16 pn (=1 pn( m)/100 m). This force calibration experiment reveals that the force values experienced by different magnetic beads are indeed different. This force variation is, however, much smaller than the mean force value in the entire force range we studied (Supplementary Fig. S1c, black versus red bars). This is also consistent with the force calibration data from previous reports 53,57,58. Error in the step-size determination Our step-size measurement first determines the positions of the extension trace before and after the unzipping (or rezipping) events, and subsequently compares these positions to finally determine the step size of each event (the difference between arithmetic mean values of records over the appropriate intervals) (Supplementary Fig. S6a). This determination of the trace positions essentially corresponds to finding the peak positions of Gaussian distributions (Supplementary Fig. S6b). The relevant error is thus the standard error of the mean, which is much smaller than the standard deviation of the distribution. To quantitatively show this, we assess the error in our step-size measurement ( equation (Ref. 59), i f step Ni N f step ) using the. [S6] This expression well illustrate that step is a kind of standard error of the mean because the standard deviations of extension trace (, ) are divided by the number of data points ( N, N ) used to obtain the standard deviations (e.g., see Supplementary Fig. S6a). i f i f This means that as a larger number of data points ( N, N ) are included for our step-size measurement, the uncertainty can be decreased to an arbitrarily smaller level. Since the fluctuation of extension traces ( i, f) is typically 5 nm and we include more than 300 data points for each i f 23
24 measurement, step 5 nm / 300 ~ 0.3 nm, indicating that we can estimate the step size with an accuracy down to a few Å level. Supplementary Figure S6c shows the distribution of step showing that the error in our step-size measurement is indeed smaller than 1 nm for both unzipping and rezipping events. On the possibility of pulling the anti-parallel SNARE complexes The SNARE complex can be in the anti-parallel configuration 60 and moreover two DNA handles can be attached to such an anti-parallel SNARE complex as shown in Supplementary Figure S10, meaning that an extension curve can also be obtained in our magnetic tweezers experiment. However, there are two reasons why we believe all the data are from single SNARE complexes in the parallel configuration. Firstly, if we pull an anti-parallel SNARE complex in our SNARE-DNA hybrid, we apply shear force which is applied to the SNARE complex in the longitudinal direction (Supplementary Fig. S10). In order to disassemble with such a shear force, we have to break all the molecular interactions simultaneously, as opposed to the sequential unzipping of interacting residues in a parallel SNARE complex. Thus, the unzipping force for the antiparallel SNARE complex would be much higher than that for the parallel SNARE complex. But, the distribution of unzipping forces shows rather a narrow distribution around 34 pn with a standard deviation of 2.8 pn (Supplementary Fig. S4) and therefore the experiment sample is thought to be quite homogeneous. In addition, it would be difficult to expect intermediates that we observed with such a shear-force unzipping. More importantly, in this anti-parallel configuration, there would be no S-S bridge inside the SNARE complex (Supplementary Fig. S10b). Therefore, if we pull and unzip such a SNARE complex, the SNARE-DNA hybrid will be completely separated into two pieces. Obviously, we cannot observe repetitive cycles of unzipping and rezipping for the anti-parallel SNARE complex. Since we have included only the data sets that show repetitive unzipping and rezipping cycles, we assure that all the data presented in this work are obtained from the parallel SNARE complex. 24
25 Supplementary References 55 Bornschlogl, T. & Rief, M. Single molecule unzipping of coiled coils: Sequence resolved stability profiles. Phys Rev Lett 96, (2006). 56 Woodside, M. T. et al. Nanomechanical measurements of the sequence-dependent folding landscapes of single nucleic acid hairpins. P Natl Acad Sci USA 103, , (2006). 57 Lipfert, J., Hao, X. M. & Dekker, N. H. Quantitative Modeling and Optimization of Magnetic Tweezers. Biophys. J. 96, , (2009). 58 Velthuis, A. J. W. T., Kerssemakers, J. W. J., Lipfert, J. & Dekkert, N. H. Quantitative Guidelines for Force Calibration through Spectral Analysis of Magnetic Tweezers Data. Biophys. J. 99, , (2010). 59 Yildiz, A. et al. Myosin V walks hand-over-hand: Single fluorophore imaging with 1.5-nm localization. Science 300, , (2003). 60 Weninger, K., Bowen, M. E., Chu, S. & Brunger, A. T. Single-molecule studies of SNARE complex assembly reveal parallel and antiparallel configurations. P Natl Acad Sci USA 100, , (2003). 25
Supplementary Information
 Supplementary Information Switching of myosin-v motion between the lever-arm swing and Brownian search-and-catch Keisuke Fujita 1*, Mitsuhiro Iwaki 2,3*, Atsuko H. Iwane 1, Lorenzo Marcucci 1 & Toshio
Supplementary Information Switching of myosin-v motion between the lever-arm swing and Brownian search-and-catch Keisuke Fujita 1*, Mitsuhiro Iwaki 2,3*, Atsuko H. Iwane 1, Lorenzo Marcucci 1 & Toshio
Multimedia : Fibronectin and Titin unfolding simulation movies.
 I LECTURE 21: SINGLE CHAIN ELASTICITY OF BIOMACROMOLECULES: THE GIANT PROTEIN TITIN AND DNA Outline : REVIEW LECTURE #2 : EXTENSIBLE FJC AND WLC... 2 STRUCTURE OF MUSCLE AND TITIN... 3 SINGLE MOLECULE
I LECTURE 21: SINGLE CHAIN ELASTICITY OF BIOMACROMOLECULES: THE GIANT PROTEIN TITIN AND DNA Outline : REVIEW LECTURE #2 : EXTENSIBLE FJC AND WLC... 2 STRUCTURE OF MUSCLE AND TITIN... 3 SINGLE MOLECULE
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/314/5801/1001/dc1 Supporting Online Material for Direct Measurement of the Full, Sequence-Dependent Folding Landscape of a Nucleic Acid Michael T. Woodside, Peter C.
www.sciencemag.org/cgi/content/full/314/5801/1001/dc1 Supporting Online Material for Direct Measurement of the Full, Sequence-Dependent Folding Landscape of a Nucleic Acid Michael T. Woodside, Peter C.
Single molecule force spectroscopy reveals a highly compliant helical
 Supplementary Information Single molecule force spectroscopy reveals a highly compliant helical folding for the 30 nm chromatin fiber Maarten Kruithof, Fan-Tso Chien, Andrew Routh, Colin Logie, Daniela
Supplementary Information Single molecule force spectroscopy reveals a highly compliant helical folding for the 30 nm chromatin fiber Maarten Kruithof, Fan-Tso Chien, Andrew Routh, Colin Logie, Daniela
Magnetic tweezers and its application to DNA mechanics
 Biotechnological Center Research group DNA motors (Seidel group) Handout for Practical Course Magnetic tweezers and its application to DNA mechanics When: 9.00 am Where: Biotec, 3 rd Level, Room 317 Tutors:
Biotechnological Center Research group DNA motors (Seidel group) Handout for Practical Course Magnetic tweezers and its application to DNA mechanics When: 9.00 am Where: Biotec, 3 rd Level, Room 317 Tutors:
Free energy recovery in single molecule experiments
 Supplementary Material Free energy recovery in single molecule experiments Single molecule force measurements (experimental setup shown in Fig. S1) can be used to determine free-energy differences between
Supplementary Material Free energy recovery in single molecule experiments Single molecule force measurements (experimental setup shown in Fig. S1) can be used to determine free-energy differences between
SUPPLEMENTAL TEXT. k = k B
 B SUPPLEMENTAL TEXT Relation between Trap Stiffness and Voltage Applied to Magnetic Tweezers At low magnetic field as used in this study (less than 100 Gauss) magnetization of magnetic beads, M can be
B SUPPLEMENTAL TEXT Relation between Trap Stiffness and Voltage Applied to Magnetic Tweezers At low magnetic field as used in this study (less than 100 Gauss) magnetization of magnetic beads, M can be
Structural investigation of single biomolecules
 Structural investigation of single biomolecules NMR spectroscopy and X-ray crystallography are currently the most common techniques capable of determining the structures of biological macromolecules like
Structural investigation of single biomolecules NMR spectroscopy and X-ray crystallography are currently the most common techniques capable of determining the structures of biological macromolecules like
Models for Single Molecule Experiments
 Models for Single Molecule Experiments Binding Unbinding Folding - Unfolding -> Kramers Bell theory 1 Polymer elasticity: - Hooke s law (?) - FJC (Freely joined chain) - WLC (Worm-like chain) 3 Molecular
Models for Single Molecule Experiments Binding Unbinding Folding - Unfolding -> Kramers Bell theory 1 Polymer elasticity: - Hooke s law (?) - FJC (Freely joined chain) - WLC (Worm-like chain) 3 Molecular
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi: 10.1038/nphys00 Peter Gross, Niels Laurens, Lene B. Oddershede, Ulrich Bockelmann 3, Erwin J. G. Peterman*, and Gijs J. L. Wuite* LaserLaB and Department of Physics and Astronomy,
SUPPLEMENTARY INFORMATION doi: 10.1038/nphys00 Peter Gross, Niels Laurens, Lene B. Oddershede, Ulrich Bockelmann 3, Erwin J. G. Peterman*, and Gijs J. L. Wuite* LaserLaB and Department of Physics and Astronomy,
BMB Class 17, November 30, Single Molecule Biophysics (II)
 BMB 178 2018 Class 17, November 30, 2018 15. Single Molecule Biophysics (II) New Advances in Single Molecule Techniques Atomic Force Microscopy Single Molecule Manipulation - optical traps and tweezers
BMB 178 2018 Class 17, November 30, 2018 15. Single Molecule Biophysics (II) New Advances in Single Molecule Techniques Atomic Force Microscopy Single Molecule Manipulation - optical traps and tweezers
Multimedia : Podcast : Sacrificial Bonds in Biological Materials; Fantner, et al. Biophys. J , 1411
 3.52 Nanomechanics o Materials and Biomaterials Tuesday 5/1/7 Pro. C. Ortiz, MIT-DMSE I LECTURE 2: THEORETICAL ASPECTS O SINGLE MOLECULE ORCE SPECTROSCOPY 2 : EXTENSIBILITY AND THE WORM LIKE CHAIN (WLC)
3.52 Nanomechanics o Materials and Biomaterials Tuesday 5/1/7 Pro. C. Ortiz, MIT-DMSE I LECTURE 2: THEORETICAL ASPECTS O SINGLE MOLECULE ORCE SPECTROSCOPY 2 : EXTENSIBILITY AND THE WORM LIKE CHAIN (WLC)
Measurements of interaction forces in (biological) model systems
 Measurements of interaction forces in (biological) model systems Marina Ruths Department of Chemistry, UMass Lowell What can force measurements tell us about a system? Depending on the technique, we might
Measurements of interaction forces in (biological) model systems Marina Ruths Department of Chemistry, UMass Lowell What can force measurements tell us about a system? Depending on the technique, we might
Magnetic Torque Tweezers: measuring torsional stiffness in DNA and RecA DNA filaments
 Magnetic Torque Tweezers: measuring torsional stiffness in DNA and RecA DNA filaments Lipfert, J., Kerssemakers, J. W., Jager, T. & Dekker, N. H. Magnetic torque tweezers: measuring torsional stiffness
Magnetic Torque Tweezers: measuring torsional stiffness in DNA and RecA DNA filaments Lipfert, J., Kerssemakers, J. W., Jager, T. & Dekker, N. H. Magnetic torque tweezers: measuring torsional stiffness
Announcements. Homework 3 (Klaus Schulten s Lecture): Due Wednesday at noon. Next homework assigned. Due Wednesday March 1.
 Announcements Homework 3 (Klaus Schulten s Lecture): Due Wednesday at noon. Next homework assigned. Due Wednesday March 1. No lecture next Monday, Feb. 27 th! (Homework is a bit longer.) Marco will have
Announcements Homework 3 (Klaus Schulten s Lecture): Due Wednesday at noon. Next homework assigned. Due Wednesday March 1. No lecture next Monday, Feb. 27 th! (Homework is a bit longer.) Marco will have
Supplemental Materials and Methods
 Supplemental Materials and Methods Time-resolved FRET (trfret) to probe for changes in the Box A/A stem upon complex assembly U3 MINI was folded and the decay of Fl fluorescence was measured at 20 ºC (see
Supplemental Materials and Methods Time-resolved FRET (trfret) to probe for changes in the Box A/A stem upon complex assembly U3 MINI was folded and the decay of Fl fluorescence was measured at 20 ºC (see
Quiz 2 Morphology of Complex Materials
 071003 Quiz 2 Morphology of Complex Materials 1) Explain the following terms: (for states comment on biological activity and relative size of the structure) a) Native State b) Unfolded State c) Denatured
071003 Quiz 2 Morphology of Complex Materials 1) Explain the following terms: (for states comment on biological activity and relative size of the structure) a) Native State b) Unfolded State c) Denatured
Biochemistry in Singulo:
 Biochemistry in Singulo: When Less Means More Carlos Bustamante University of California, Berkeley The Ensemble Approach The study of chemical transforma9ons has been dominated by the ensemble method:
Biochemistry in Singulo: When Less Means More Carlos Bustamante University of California, Berkeley The Ensemble Approach The study of chemical transforma9ons has been dominated by the ensemble method:
THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION
 THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION AND CALIBRATION Calculation of turn and beta intrinsic propensities. A statistical analysis of a protein structure
THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION AND CALIBRATION Calculation of turn and beta intrinsic propensities. A statistical analysis of a protein structure
SUPPLEMENTARY FIGURE 1. Force dependence of the unbinding rate: (a) Force-dependence
 (b) BSA-coated beads double exponential low force exponential high force exponential 1 unbinding time tb [sec] (a) unbinding time tb [sec] SUPPLEMENTARY FIGURES BSA-coated beads without BSA.2.1 5 1 load
(b) BSA-coated beads double exponential low force exponential high force exponential 1 unbinding time tb [sec] (a) unbinding time tb [sec] SUPPLEMENTARY FIGURES BSA-coated beads without BSA.2.1 5 1 load
(Entropic) Stochastic Resonance in Biological Systems at Mesoscale
 (Entropic) Stochastic Resonance in Biological Systems at Mesoscale Wokyung Sung Department of Physics, POSTECH, IBS center for Self-assembly and Complexity, Pohang, 790-784, South Korea As interconnected,
(Entropic) Stochastic Resonance in Biological Systems at Mesoscale Wokyung Sung Department of Physics, POSTECH, IBS center for Self-assembly and Complexity, Pohang, 790-784, South Korea As interconnected,
Measurement of the Phase Diagram of DNA Unzipping in the Temperature- Force Plane.
 Measurement of the Phase Diagram - Danilowicz et al. 1 Measurement of the Phase Diagram of DNA Unzipping in the Temperature- Force Plane. C.Danilowicz, Y. Kafri, R.S. Conroy, V. W. Coljee, J. Weeks, M.
Measurement of the Phase Diagram - Danilowicz et al. 1 Measurement of the Phase Diagram of DNA Unzipping in the Temperature- Force Plane. C.Danilowicz, Y. Kafri, R.S. Conroy, V. W. Coljee, J. Weeks, M.
SECOND PUBLIC EXAMINATION. Honour School of Physics Part C: 4 Year Course. Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS
 2757 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS TRINITY TERM 2013 Monday, 17 June, 2.30 pm 5.45 pm 15
2757 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS TRINITY TERM 2013 Monday, 17 June, 2.30 pm 5.45 pm 15
Many proteins spontaneously refold into native form in vitro with high fidelity and high speed.
 Macromolecular Processes 20. Protein Folding Composed of 50 500 amino acids linked in 1D sequence by the polypeptide backbone The amino acid physical and chemical properties of the 20 amino acids dictate
Macromolecular Processes 20. Protein Folding Composed of 50 500 amino acids linked in 1D sequence by the polypeptide backbone The amino acid physical and chemical properties of the 20 amino acids dictate
arxiv:cond-mat/ v2 [cond-mat.soft] 29 Nov 2004
![arxiv:cond-mat/ v2 [cond-mat.soft] 29 Nov 2004 arxiv:cond-mat/ v2 [cond-mat.soft] 29 Nov 2004](/thumbs/80/82235863.jpg) arxiv:cond-mat/411654v2 [cond-mat.soft] 29 Nov 24 ork probability distribution in single molecule experiments Alberto Imparato ( ) and Luca Peliti ( ) Dipartimento di Scienze Fisiche and Unità INFM, Università
arxiv:cond-mat/411654v2 [cond-mat.soft] 29 Nov 24 ork probability distribution in single molecule experiments Alberto Imparato ( ) and Luca Peliti ( ) Dipartimento di Scienze Fisiche and Unità INFM, Università
Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6
 Supplementary Information for: Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6 Yawei Dai 1, 2, Markus Seeger 3, Jingwei Weng 4, Song Song 1, 2, Wenning Wang 4, Yan-Wen 1, 2,
Supplementary Information for: Lipid Regulated Intramolecular Conformational Dynamics of SNARE-Protein Ykt6 Yawei Dai 1, 2, Markus Seeger 3, Jingwei Weng 4, Song Song 1, 2, Wenning Wang 4, Yan-Wen 1, 2,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature09450 Supplementary Table 1 Summary of kinetic parameters. Kinetic parameters were V = V / 1 K / ATP and obtained using the relationships max ( + m [ ]) V d s /( 1/ k [ ATP] + 1 k ) =,
doi:10.1038/nature09450 Supplementary Table 1 Summary of kinetic parameters. Kinetic parameters were V = V / 1 K / ATP and obtained using the relationships max ( + m [ ]) V d s /( 1/ k [ ATP] + 1 k ) =,
Quantitative Analysis of Forces in Cells
 Quantitative Analysis of Forces in Cells Anders Carlsson Washington University in St Louis Basic properties of forces in cells Measurement methods and magnitudes of particular types of forces: Polymerization
Quantitative Analysis of Forces in Cells Anders Carlsson Washington University in St Louis Basic properties of forces in cells Measurement methods and magnitudes of particular types of forces: Polymerization
3 Biopolymers Uncorrelated chains - Freely jointed chain model
 3.4 Entropy When we talk about biopolymers, it is important to realize that the free energy of a biopolymer in thermal equilibrium is not constant. Unlike solids, biopolymers are characterized through
3.4 Entropy When we talk about biopolymers, it is important to realize that the free energy of a biopolymer in thermal equilibrium is not constant. Unlike solids, biopolymers are characterized through
Introduction to Polymer Physics
 Introduction to Polymer Physics Enrico Carlon, KU Leuven, Belgium February-May, 2016 Enrico Carlon, KU Leuven, Belgium Introduction to Polymer Physics February-May, 2016 1 / 28 Polymers in Chemistry and
Introduction to Polymer Physics Enrico Carlon, KU Leuven, Belgium February-May, 2016 Enrico Carlon, KU Leuven, Belgium Introduction to Polymer Physics February-May, 2016 1 / 28 Polymers in Chemistry and
Denaturation and renaturation of proteins
 Denaturation and renaturation of proteins Higher levels of protein structure are formed without covalent bonds. Therefore, they are not as stable as peptide covalent bonds which make protein primary structure
Denaturation and renaturation of proteins Higher levels of protein structure are formed without covalent bonds. Therefore, they are not as stable as peptide covalent bonds which make protein primary structure
The Diffusion Coefficient for PGK Folding in Eukaryotic Cells
 Biophysical Journal, Volume 99 Supporting Material The Diffusion Coefficient for PGK Folding in Eukaryotic Cells Apratim Dhar, Simon Ebbinghaus, Zhen Shen, Tripta Mishra, and Martin Gruebele 1 Supplementary
Biophysical Journal, Volume 99 Supporting Material The Diffusion Coefficient for PGK Folding in Eukaryotic Cells Apratim Dhar, Simon Ebbinghaus, Zhen Shen, Tripta Mishra, and Martin Gruebele 1 Supplementary
Experimental biophysics: Optical tweezer lab Supervisor: Stefan Holm,
 Experimental biophysics: Optical tweezer lab :, stefan.holm@ftf.lth.se Written by Jason Beech & Henrik Persson, March 2009. Modified 2014 Karl Adolfsson, 2016 Experimental Biophysics: FAF010F, FYST23,
Experimental biophysics: Optical tweezer lab :, stefan.holm@ftf.lth.se Written by Jason Beech & Henrik Persson, March 2009. Modified 2014 Karl Adolfsson, 2016 Experimental Biophysics: FAF010F, FYST23,
Supplemental Information - Glassy Dynamics in Composite Biopolymer Networks
 Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2018 Supplemental Information - Glassy Dynamics in Composite Biopolymer Networks Tom Golde, 1 Constantin
Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2018 Supplemental Information - Glassy Dynamics in Composite Biopolymer Networks Tom Golde, 1 Constantin
Advances in nanofabrication have made it possible to
 pubs.acs.org/macroletters Conformation Model of Back-Folding and Looping of a Single DNA Molecule Confined Inside a Nanochannel Liang Dai,, Siow Yee Ng,, Patrick S. Doyle,, and Johan R. C. van der Maarel*,,
pubs.acs.org/macroletters Conformation Model of Back-Folding and Looping of a Single DNA Molecule Confined Inside a Nanochannel Liang Dai,, Siow Yee Ng,, Patrick S. Doyle,, and Johan R. C. van der Maarel*,,
GEM4 Summer School OpenCourseWare
 GEM4 Summer School OpenCourseWare http://gem4.educommons.net/ http://www.gem4.org/ Lecture: Microrheology of a Complex Fluid by Dr. Peter So. Given August 10, 2006 during the GEM4 session at MIT in Cambridge,
GEM4 Summer School OpenCourseWare http://gem4.educommons.net/ http://www.gem4.org/ Lecture: Microrheology of a Complex Fluid by Dr. Peter So. Given August 10, 2006 during the GEM4 session at MIT in Cambridge,
Anatoly B. Kolomeisky. Department of Chemistry CAN WE UNDERSTAND THE COMPLEX DYNAMICS OF MOTOR PROTEINS USING SIMPLE STOCHASTIC MODELS?
 Anatoly B. Kolomeisky Department of Chemistry CAN WE UNDERSTAND THE COMPLEX DYNAMICS OF MOTOR PROTEINS USING SIMPLE STOCHASTIC MODELS? Motor Proteins Enzymes that convert the chemical energy into mechanical
Anatoly B. Kolomeisky Department of Chemistry CAN WE UNDERSTAND THE COMPLEX DYNAMICS OF MOTOR PROTEINS USING SIMPLE STOCHASTIC MODELS? Motor Proteins Enzymes that convert the chemical energy into mechanical
SINGLE-MOLECULE PHYSIOLOGY
 SINGLE-MOLECULE PHYSIOLOGY Kazuhiko Kinosita, Jr. Center for Integrative Bioscience, Okazaki National Research Institutes Higashiyama 5-1, Myodaiji, Okazaki 444-8585, Japan Single-Molecule Physiology under
SINGLE-MOLECULE PHYSIOLOGY Kazuhiko Kinosita, Jr. Center for Integrative Bioscience, Okazaki National Research Institutes Higashiyama 5-1, Myodaiji, Okazaki 444-8585, Japan Single-Molecule Physiology under
Introduction The gramicidin A (ga) channel forms by head-to-head association of two monomers at their amino termini, one from each bilayer leaflet. Th
 Abstract When conductive, gramicidin monomers are linked by six hydrogen bonds. To understand the details of dissociation and how the channel transits from a state with 6H bonds to ones with 4H bonds or
Abstract When conductive, gramicidin monomers are linked by six hydrogen bonds. To understand the details of dissociation and how the channel transits from a state with 6H bonds to ones with 4H bonds or
Coordinated conformational and compositional dynamics drive. ribosome translocation
 Coordinated conformational and compositional dynamics drive ribosome translocation Supplementary Information Jin Chen,2, Alexey Petrov 2, Albert Tsai,2, Seán E. O Leary 2, & Joseph D. Puglisi 2,3 Department
Coordinated conformational and compositional dynamics drive ribosome translocation Supplementary Information Jin Chen,2, Alexey Petrov 2, Albert Tsai,2, Seán E. O Leary 2, & Joseph D. Puglisi 2,3 Department
Faculty of math and natural science. Universty of Split. Kratky-Porod model. Nataša Vučemilovid-Alagid. February, 2013.
 Faculty of math and natural science Universty of Split Kratky-Porod model Nataša Vučemilovid-Alagid February, 2013. Table of contents 1.Introduction:... 3 1. DNA:... 4 2. Models of polymer elasticity:...
Faculty of math and natural science Universty of Split Kratky-Porod model Nataša Vučemilovid-Alagid February, 2013. Table of contents 1.Introduction:... 3 1. DNA:... 4 2. Models of polymer elasticity:...
λmax = k d Supplementary Figures
 Supplementary Figures a b HQ CCD Transmission Grating Beam splitting lens Color CCD Objective Sample Dark-field Condenser Raw data Gaussian fit c λmax = k d k = 1.733 nm/pixel 53 nm 307 pixels d Supplementary
Supplementary Figures a b HQ CCD Transmission Grating Beam splitting lens Color CCD Objective Sample Dark-field Condenser Raw data Gaussian fit c λmax = k d k = 1.733 nm/pixel 53 nm 307 pixels d Supplementary
type GroEL-GroES complex. Crystals were grown in buffer D (100 mm HEPES, ph 7.5,
 Supplementary Material Supplementary Materials and Methods Structure Determination of SR1-GroES-ADP AlF x SR1-GroES-ADP AlF x was purified as described in Materials and Methods for the wild type GroEL-GroES
Supplementary Material Supplementary Materials and Methods Structure Determination of SR1-GroES-ADP AlF x SR1-GroES-ADP AlF x was purified as described in Materials and Methods for the wild type GroEL-GroES
Acto-myosin: from muscles to single molecules. Justin Molloy MRC National Institute for Medical Research LONDON
 Acto-myosin: from muscles to single molecules. Justin Molloy MRC National Institute for Medical Research LONDON Energy in Biological systems: 1 Photon = 400 pn.nm 1 ATP = 100 pn.nm 1 Ion moving across
Acto-myosin: from muscles to single molecules. Justin Molloy MRC National Institute for Medical Research LONDON Energy in Biological systems: 1 Photon = 400 pn.nm 1 ATP = 100 pn.nm 1 Ion moving across
Supplementary Figures:
 Supplementary Figures: Supplementary Figure 1: The two strings converge to two qualitatively different pathways. A) Models of active (red) and inactive (blue) states used as end points for the string calculations
Supplementary Figures: Supplementary Figure 1: The two strings converge to two qualitatively different pathways. A) Models of active (red) and inactive (blue) states used as end points for the string calculations
In Situ Gelation-Induced Death of Cancer Cells Based on Proteinosomes
 Supporting information for In Situ Gelation-Induced Death of Cancer Cells Based on Proteinosomes Yuting Zhou, Jianmin Song, Lei Wang*, Xuting Xue, Xiaoman Liu, Hui Xie*, and Xin Huang* MIIT Key Laboratory
Supporting information for In Situ Gelation-Induced Death of Cancer Cells Based on Proteinosomes Yuting Zhou, Jianmin Song, Lei Wang*, Xuting Xue, Xiaoman Liu, Hui Xie*, and Xin Huang* MIIT Key Laboratory
Lecture 10: Brownian Motion, Random Walk & Diffusion Side Chains of Amino Acids
 Lecture 10: Brownian Motion, Random Walk & Diffusion Side Chains of Amino Acids Lecturer: Prof. Brigita Urbanc (brigita@drexel.edu) PHYS 461 & 561, Fall 2009-2010 1 Stochastic Processes: Brownian Motion
Lecture 10: Brownian Motion, Random Walk & Diffusion Side Chains of Amino Acids Lecturer: Prof. Brigita Urbanc (brigita@drexel.edu) PHYS 461 & 561, Fall 2009-2010 1 Stochastic Processes: Brownian Motion
Proteins. Division Ave. High School Ms. Foglia AP Biology. Proteins. Proteins. Multipurpose molecules
 Proteins Proteins Multipurpose molecules 2008-2009 Proteins Most structurally & functionally diverse group Function: involved in almost everything u enzymes (pepsin, DNA polymerase) u structure (keratin,
Proteins Proteins Multipurpose molecules 2008-2009 Proteins Most structurally & functionally diverse group Function: involved in almost everything u enzymes (pepsin, DNA polymerase) u structure (keratin,
arxiv: v1 [cond-mat.soft] 4 Aug 2008
![arxiv: v1 [cond-mat.soft] 4 Aug 2008 arxiv: v1 [cond-mat.soft] 4 Aug 2008](/thumbs/74/69998376.jpg) Force Dependent Hopping Rates of RNA Hairpins can be Estimated from Accurate Measurement of the Folding Landscapes arxiv:0808.080v [cond-mat.soft] Aug 008 Changbong Hyeon, Greg Morrison,3 and D. Thirumalai,
Force Dependent Hopping Rates of RNA Hairpins can be Estimated from Accurate Measurement of the Folding Landscapes arxiv:0808.080v [cond-mat.soft] Aug 008 Changbong Hyeon, Greg Morrison,3 and D. Thirumalai,
Stability, folding dynamics, and long-range conformational transition of the synaptic t-snare complex
 Stability, folding dynamics, and long-range conformational transition of the synaptic t-snare complex Xinming Zhang a, Aleksander A. Rebane a,b,c,d,luma a, Feng Li ( 李峰 ) a,d, Junyi Jiao a,b, Hong Qu a,
Stability, folding dynamics, and long-range conformational transition of the synaptic t-snare complex Xinming Zhang a, Aleksander A. Rebane a,b,c,d,luma a, Feng Li ( 李峰 ) a,d, Junyi Jiao a,b, Hong Qu a,
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1.
 Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Dana Alsulaibi. Jaleel G.Sweis. Mamoon Ahram
 15 Dana Alsulaibi Jaleel G.Sweis Mamoon Ahram Revision of last lectures: Proteins have four levels of structures. Primary,secondary, tertiary and quaternary. Primary structure is the order of amino acids
15 Dana Alsulaibi Jaleel G.Sweis Mamoon Ahram Revision of last lectures: Proteins have four levels of structures. Primary,secondary, tertiary and quaternary. Primary structure is the order of amino acids
Supplemental Information for. Quaternary dynamics of B crystallin as a direct consequence of localised tertiary fluctuations in the C terminus
 Supplemental Information for Quaternary dynamics of B crystallin as a direct consequence of localised tertiary fluctuations in the C terminus Andrew J. Baldwin 1, Gillian R. Hilton 2, Hadi Lioe 2, Claire
Supplemental Information for Quaternary dynamics of B crystallin as a direct consequence of localised tertiary fluctuations in the C terminus Andrew J. Baldwin 1, Gillian R. Hilton 2, Hadi Lioe 2, Claire
Supplementary Figures
 Supplementary Figures Supplementary Figure. X-ray diffraction pattern of CH 3 NH 3 PbI 3 film. Strong reflections of the () family of planes is characteristics of the preferred orientation of the perovskite
Supplementary Figures Supplementary Figure. X-ray diffraction pattern of CH 3 NH 3 PbI 3 film. Strong reflections of the () family of planes is characteristics of the preferred orientation of the perovskite
Final Morphology of Complex Materials
 120314 Final Morphology of Complex Materials 1) Proteins are the prototypical model for hierarchy. a) Give the generic chemical structure for an amino acid and a protein molecule (a tripeptide). b) Label
120314 Final Morphology of Complex Materials 1) Proteins are the prototypical model for hierarchy. a) Give the generic chemical structure for an amino acid and a protein molecule (a tripeptide). b) Label
Nature Methods: doi: /nmeth Supplementary Figure 1
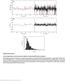 Supplementary Figure 1 Control experiments on the fluorescence stability of single Atto647N-biotin complexes. a, Exemplary signal trace (black line) in counts per second (cps) of a single Atto647N molecule
Supplementary Figure 1 Control experiments on the fluorescence stability of single Atto647N-biotin complexes. a, Exemplary signal trace (black line) in counts per second (cps) of a single Atto647N molecule
Switching of magnetic domains reveals spatially inhomogeneous superconductivity
 Switching of magnetic domains reveals spatially inhomogeneous superconductivity Simon Gerber, Marek Bartkowiak, Jorge L. Gavilano, Eric Ressouche, Nikola Egetenmeyer, Christof Niedermayer, Andrea D. Bianchi,
Switching of magnetic domains reveals spatially inhomogeneous superconductivity Simon Gerber, Marek Bartkowiak, Jorge L. Gavilano, Eric Ressouche, Nikola Egetenmeyer, Christof Niedermayer, Andrea D. Bianchi,
1. What is an ångstrom unit, and why is it used to describe molecular structures?
 1. What is an ångstrom unit, and why is it used to describe molecular structures? The ångstrom unit is a unit of distance suitable for measuring atomic scale objects. 1 ångstrom (Å) = 1 10-10 m. The diameter
1. What is an ångstrom unit, and why is it used to describe molecular structures? The ångstrom unit is a unit of distance suitable for measuring atomic scale objects. 1 ångstrom (Å) = 1 10-10 m. The diameter
Nanopores: Solid-state nanopores for these experiments were produced by using the
 Materials and Methods Nanopores: Solid-state nanopores for these experiments were produced by using the highly focused electron beam of a transmission electron microscope (TEM) to drill a single pore in
Materials and Methods Nanopores: Solid-state nanopores for these experiments were produced by using the highly focused electron beam of a transmission electron microscope (TEM) to drill a single pore in
STRAIN GAUGES YEDITEPE UNIVERSITY DEPARTMENT OF MECHANICAL ENGINEERING
 STRAIN GAUGES YEDITEPE UNIVERSITY DEPARTMENT OF MECHANICAL ENGINEERING 1 YEDITEPE UNIVERSITY ENGINEERING FACULTY MECHANICAL ENGINEERING LABORATORY 1. Objective: Strain Gauges Know how the change in resistance
STRAIN GAUGES YEDITEPE UNIVERSITY DEPARTMENT OF MECHANICAL ENGINEERING 1 YEDITEPE UNIVERSITY ENGINEERING FACULTY MECHANICAL ENGINEERING LABORATORY 1. Objective: Strain Gauges Know how the change in resistance
Single-Molecule Methods I - in vitro
 Single-Molecule Methods I - in vitro Bo Huang Macromolecules 2014.03.10 F 1 -ATPase: a case study Membrane ADP ATP Rotation of the axle when hydrolyzing ATP Kinosita group, 1997-2005 Single Molecule Methods
Single-Molecule Methods I - in vitro Bo Huang Macromolecules 2014.03.10 F 1 -ATPase: a case study Membrane ADP ATP Rotation of the axle when hydrolyzing ATP Kinosita group, 1997-2005 Single Molecule Methods
AP Biology. Proteins. AP Biology. Proteins. Multipurpose molecules
 Proteins Proteins Multipurpose molecules 2008-2009 1 Proteins Most structurally & functionally diverse group Function: involved in almost everything u enzymes (pepsin, DNA polymerase) u structure (keratin,
Proteins Proteins Multipurpose molecules 2008-2009 1 Proteins Most structurally & functionally diverse group Function: involved in almost everything u enzymes (pepsin, DNA polymerase) u structure (keratin,
Mechanical Proteins. Stretching imunoglobulin and fibronectin. domains of the muscle protein titin. Adhesion Proteins of the Immune System
 Mechanical Proteins F C D B A domains of the muscle protein titin E Stretching imunoglobulin and fibronectin G NIH Resource for Macromolecular Modeling and Bioinformatics Theoretical Biophysics Group,
Mechanical Proteins F C D B A domains of the muscle protein titin E Stretching imunoglobulin and fibronectin G NIH Resource for Macromolecular Modeling and Bioinformatics Theoretical Biophysics Group,
Supplementary Materials
 Supplementary Materials Sample characterization The presence of Si-QDs is established by Transmission Electron Microscopy (TEM), by which the average QD diameter of d QD 2.2 ± 0.5 nm has been determined
Supplementary Materials Sample characterization The presence of Si-QDs is established by Transmission Electron Microscopy (TEM), by which the average QD diameter of d QD 2.2 ± 0.5 nm has been determined
Protein Dynamics. The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron.
 Protein Dynamics The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron. Below is myoglobin hydrated with 350 water molecules. Only a small
Protein Dynamics The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron. Below is myoglobin hydrated with 350 water molecules. Only a small
Supporting Information for: Mechanism of Reversible Peptide-Bilayer. Attachment: Combined Simulation and Experimental Single-Molecule Study
 Supporting Information for: Mechanism of Reversible Peptide-Bilayer Attachment: Combined Simulation and Experimental Single-Molecule Study Nadine Schwierz a,, Stefanie Krysiak c, Thorsten Hugel c,d, Martin
Supporting Information for: Mechanism of Reversible Peptide-Bilayer Attachment: Combined Simulation and Experimental Single-Molecule Study Nadine Schwierz a,, Stefanie Krysiak c, Thorsten Hugel c,d, Martin
Supplementary information
 Supplementary information Supplementary Figure S1STM images of four GNBs and their corresponding STS spectra. a-d, STM images of four GNBs are shown in the left side. The experimental STS data with respective
Supplementary information Supplementary Figure S1STM images of four GNBs and their corresponding STS spectra. a-d, STM images of four GNBs are shown in the left side. The experimental STS data with respective
SUPPLEMENTARY INFORMATION
 In the format provided by the authors and unedited. DOI:.38/NMAT4855 A magnetic heterostructure of topological insulators as a candidate for axion insulator M. Mogi, M. Kawamura, R. Yoshimi, A. Tsukazaki,
In the format provided by the authors and unedited. DOI:.38/NMAT4855 A magnetic heterostructure of topological insulators as a candidate for axion insulator M. Mogi, M. Kawamura, R. Yoshimi, A. Tsukazaki,
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2005
 Lecture /4/ University of Washington Department of Chemistry Chemistry 4 Winter Quarter A. Polymer Properties of DNA When a linear polymer like DNA becomes long enough it can no longer be treated as a
Lecture /4/ University of Washington Department of Chemistry Chemistry 4 Winter Quarter A. Polymer Properties of DNA When a linear polymer like DNA becomes long enough it can no longer be treated as a
What is the central dogma of biology?
 Bellringer What is the central dogma of biology? A. RNA DNA Protein B. DNA Protein Gene C. DNA Gene RNA D. DNA RNA Protein Review of DNA processes Replication (7.1) Transcription(7.2) Translation(7.3)
Bellringer What is the central dogma of biology? A. RNA DNA Protein B. DNA Protein Gene C. DNA Gene RNA D. DNA RNA Protein Review of DNA processes Replication (7.1) Transcription(7.2) Translation(7.3)
What is all this fuss about Tus? Comparison of recent findings from biophysical and biochemical experiments
 Supplementary Information for: What is all this fuss about Tus? Comparison of recent findings from biophysical and biochemical experiments Bojk A. Berghuis 1,#, Vlad-Stefan Raducanu 2, Mohamed M. Elshenawy
Supplementary Information for: What is all this fuss about Tus? Comparison of recent findings from biophysical and biochemical experiments Bojk A. Berghuis 1,#, Vlad-Stefan Raducanu 2, Mohamed M. Elshenawy
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12682 Supplementary Information 1 Theoretical models of flagellum growth on the outside of a bacterial cell A classic problem in polymer physics concerns the
SUPPLEMENTARY INFORMATION doi:10.1038/nature12682 Supplementary Information 1 Theoretical models of flagellum growth on the outside of a bacterial cell A classic problem in polymer physics concerns the
Supporting Information. Diffusion Limited Cargo Loading of an Engineered Protein Container Reinhard Zschoche and Donald Hilvert
 Supporting Information Diffusion Limited Cargo Loading of an Engineered Protein Container Reinhard Zschoche and Donald Hilvert Laboratory of Organic Chemistry, ETH Zürich, 8093 Zürich, Switzerland Supporting
Supporting Information Diffusion Limited Cargo Loading of an Engineered Protein Container Reinhard Zschoche and Donald Hilvert Laboratory of Organic Chemistry, ETH Zürich, 8093 Zürich, Switzerland Supporting
Supplementary Information
 Supplementary Information Supplementary Figure 1 Raman spectroscopy of CVD graphene on SiO 2 /Si substrate. Integrated Raman intensity maps of D, G, 2D peaks, scanned across the same graphene area. Scale
Supplementary Information Supplementary Figure 1 Raman spectroscopy of CVD graphene on SiO 2 /Si substrate. Integrated Raman intensity maps of D, G, 2D peaks, scanned across the same graphene area. Scale
Protein Structure. W. M. Grogan, Ph.D. OBJECTIVES
 Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Visualizing folding of proteins (1 3) and RNA (2) in terms of
 Can energy landscape roughness of proteins and RNA be measured by using mechanical unfolding experiments? Changbong Hyeon and D. Thirumalai Chemical Physics Program, Institute for Physical Science and
Can energy landscape roughness of proteins and RNA be measured by using mechanical unfolding experiments? Changbong Hyeon and D. Thirumalai Chemical Physics Program, Institute for Physical Science and
The protein folding problem consists of two parts:
 Energetics and kinetics of protein folding The protein folding problem consists of two parts: 1)Creating a stable, well-defined structure that is significantly more stable than all other possible structures.
Energetics and kinetics of protein folding The protein folding problem consists of two parts: 1)Creating a stable, well-defined structure that is significantly more stable than all other possible structures.
From Amino Acids to Proteins - in 4 Easy Steps
 From Amino Acids to Proteins - in 4 Easy Steps Although protein structure appears to be overwhelmingly complex, you can provide your students with a basic understanding of how proteins fold by focusing
From Amino Acids to Proteins - in 4 Easy Steps Although protein structure appears to be overwhelmingly complex, you can provide your students with a basic understanding of how proteins fold by focusing
arxiv:q-bio/ v2 [q-bio.bm] 31 Jan 2006
![arxiv:q-bio/ v2 [q-bio.bm] 31 Jan 2006 arxiv:q-bio/ v2 [q-bio.bm] 31 Jan 2006](/thumbs/90/101401483.jpg) Forced-unfolding and force-quench refolding of RNA hairpins arxiv:q-bio/0601043v2 [q-bio.bm] 31 Jan 2006 Changbong Hyeon 1 and D. Thirumalai 1,2 1 Biophysics Program Institute for Physical Science and
Forced-unfolding and force-quench refolding of RNA hairpins arxiv:q-bio/0601043v2 [q-bio.bm] 31 Jan 2006 Changbong Hyeon 1 and D. Thirumalai 1,2 1 Biophysics Program Institute for Physical Science and
The Riboswitch is functionally separated into the ligand binding APTAMER and the decision-making EXPRESSION PLATFORM
 The Riboswitch is functionally separated into the ligand binding APTAMER and the decision-making EXPRESSION PLATFORM Purine riboswitch TPP riboswitch SAM riboswitch glms ribozyme In-line probing is used
The Riboswitch is functionally separated into the ligand binding APTAMER and the decision-making EXPRESSION PLATFORM Purine riboswitch TPP riboswitch SAM riboswitch glms ribozyme In-line probing is used
SUPPLEMENTARY MATERIAL FOR
 SUPPLEMENTARY MATERIAL FOR THE LIPID-BINDING DOMAIN OF WILD TYPE AND MUTANT ALPHA- SYNUCLEIN: COMPACTNESS AND INTERCONVERSION BETWEEN THE BROKEN- AND EXTENDED-HELIX FORMS. Elka R. Georgieva 1, Trudy F.
SUPPLEMENTARY MATERIAL FOR THE LIPID-BINDING DOMAIN OF WILD TYPE AND MUTANT ALPHA- SYNUCLEIN: COMPACTNESS AND INTERCONVERSION BETWEEN THE BROKEN- AND EXTENDED-HELIX FORMS. Elka R. Georgieva 1, Trudy F.
Cytoskeleton dynamics simulation of the red blood cell
 1 Cytoskeleton dynamics simulation of the red blood cell Ju Li Collaborators: Subra Suresh, Ming Dao, George Lykotrafitis, Chwee-Teck Lim Optical tweezers stretching of healthy human red blood cell 2 Malaria
1 Cytoskeleton dynamics simulation of the red blood cell Ju Li Collaborators: Subra Suresh, Ming Dao, George Lykotrafitis, Chwee-Teck Lim Optical tweezers stretching of healthy human red blood cell 2 Malaria
Ultrafast single photon emitting quantum photonic structures. based on a nano-obelisk
 Ultrafast single photon emitting quantum photonic structures based on a nano-obelisk Je-Hyung Kim, Young-Ho Ko, Su-Hyun Gong, Suk-Min Ko, Yong-Hoon Cho Department of Physics, Graduate School of Nanoscience
Ultrafast single photon emitting quantum photonic structures based on a nano-obelisk Je-Hyung Kim, Young-Ho Ko, Su-Hyun Gong, Suk-Min Ko, Yong-Hoon Cho Department of Physics, Graduate School of Nanoscience
Examples of Protein Modeling. Protein Modeling. Primary Structure. Protein Structure Description. Protein Sequence Sources. Importing Sequences to MOE
 Examples of Protein Modeling Protein Modeling Visualization Examination of an experimental structure to gain insight about a research question Dynamics To examine the dynamics of protein structures To
Examples of Protein Modeling Protein Modeling Visualization Examination of an experimental structure to gain insight about a research question Dynamics To examine the dynamics of protein structures To
Structure of the α-helix
 Structure of the α-helix Structure of the β Sheet Protein Dynamics Basics of Quenching HDX Hydrogen exchange of amide protons is catalyzed by H 2 O, OH -, and H 3 O +, but it s most dominated by base
Structure of the α-helix Structure of the β Sheet Protein Dynamics Basics of Quenching HDX Hydrogen exchange of amide protons is catalyzed by H 2 O, OH -, and H 3 O +, but it s most dominated by base
The Fic protein Doc uses an inverted substrate to phosphorylate and. inactivate EF-Tu
 The Fic protein Doc uses an inverted substrate to phosphorylate and inactivate EF-Tu Daniel Castro-Roa 1, Abel Garcia-Pino 2,3 *, Steven De Gieter 2,3, Nico A.J. van Nuland 2,3, Remy Loris 2,3, Nikolay
The Fic protein Doc uses an inverted substrate to phosphorylate and inactivate EF-Tu Daniel Castro-Roa 1, Abel Garcia-Pino 2,3 *, Steven De Gieter 2,3, Nico A.J. van Nuland 2,3, Remy Loris 2,3, Nikolay
Physics 218: Waves and Thermodynamics Fall 2002, James P. Sethna Homework 12, due Wednesday Dec. 4 Latest revision: November 26, 2002, 10:45 am
 Physics 218: Waves and Thermodynamics Fall 2002, James P. Sethna Homework 12, due Wednesday Dec. 4 Latest revision: November 26, 2002, 10:45 am Reading Feynman, I.44 Laws of Thermodynamics, I.45 Illustrations
Physics 218: Waves and Thermodynamics Fall 2002, James P. Sethna Homework 12, due Wednesday Dec. 4 Latest revision: November 26, 2002, 10:45 am Reading Feynman, I.44 Laws of Thermodynamics, I.45 Illustrations
Nucleation rate (m -3 s -1 ) Radius of water nano droplet (Å) 1e+00 1e-64 1e-128 1e-192 1e-256
 Supplementary Figures Nucleation rate (m -3 s -1 ) 1e+00 1e-64 1e-128 1e-192 1e-256 Calculated R in bulk water Calculated R in droplet Modified CNT 20 30 40 50 60 70 Radius of water nano droplet (Å) Supplementary
Supplementary Figures Nucleation rate (m -3 s -1 ) 1e+00 1e-64 1e-128 1e-192 1e-256 Calculated R in bulk water Calculated R in droplet Modified CNT 20 30 40 50 60 70 Radius of water nano droplet (Å) Supplementary
Secondary structure stability, beta-sheet formation & stability
 Protein Physics 2016 Lecture 6, February 5 Secondary structure stability, beta-sheet formation & stability Magnus Andersson magnus.andersson@scilifelab.se Theoretical & Computational Biophysics Recap of
Protein Physics 2016 Lecture 6, February 5 Secondary structure stability, beta-sheet formation & stability Magnus Andersson magnus.andersson@scilifelab.se Theoretical & Computational Biophysics Recap of
Label-Free Single-Molecule Thermoscopy Using a Laser-Heated Nanopore
 Supporting information for Label-Free Single-Molecule Thermoscopy Using a Laser-Heated Nanopore By Hirohito Yamazaki, Rui Hu,, Robert Y. Henley, Justin Halman, Kirill A. Afonin, Dapeng Yu, Qing Zhao, and
Supporting information for Label-Free Single-Molecule Thermoscopy Using a Laser-Heated Nanopore By Hirohito Yamazaki, Rui Hu,, Robert Y. Henley, Justin Halman, Kirill A. Afonin, Dapeng Yu, Qing Zhao, and
Contents. xiii. Preface v
 Contents Preface Chapter 1 Biological Macromolecules 1.1 General PrincipIes 1.1.1 Macrornolecules 1.2 1.1.2 Configuration and Conformation Molecular lnteractions in Macromolecular Structures 1.2.1 Weak
Contents Preface Chapter 1 Biological Macromolecules 1.1 General PrincipIes 1.1.1 Macrornolecules 1.2 1.1.2 Configuration and Conformation Molecular lnteractions in Macromolecular Structures 1.2.1 Weak
Nature Protocols: doi: /nprot Supplementary Figure 1
 Supplementary Figure 1 Photographs of the 3D-MTC device and the confocal fluorescence microscopy. I: The system consists of a Leica SP8-Confocal microscope (with an option of STED), a confocal PC, a 3D-MTC
Supplementary Figure 1 Photographs of the 3D-MTC device and the confocal fluorescence microscopy. I: The system consists of a Leica SP8-Confocal microscope (with an option of STED), a confocal PC, a 3D-MTC
Homework #4 Physics 498Bio Spring 2012 Prof. Paul Selvin
 Assigned Wednesday Feb. 22, 2012: Due Wednesday February 29, 10:30am. Hand in at start of class. Late homework is not accepted. (Solution sets will be posted shortly after deadline.) Note: Marco will give
Assigned Wednesday Feb. 22, 2012: Due Wednesday February 29, 10:30am. Hand in at start of class. Late homework is not accepted. (Solution sets will be posted shortly after deadline.) Note: Marco will give
Lecture 5: Macromolecules, polymers and DNA
 1, polymers and DNA Introduction In this lecture, we focus on a subfield of soft matter: macromolecules and more particularly on polymers. As for the previous chapter about surfactants and electro kinetics,
1, polymers and DNA Introduction In this lecture, we focus on a subfield of soft matter: macromolecules and more particularly on polymers. As for the previous chapter about surfactants and electro kinetics,
Phys 450 Spring 2011 Solution set 6. A bimolecular reaction in which A and B combine to form the product P may be written as:
 Problem Phys 45 Spring Solution set 6 A bimolecular reaction in which A and combine to form the product P may be written as: k d A + A P k d k a where k d is a diffusion-limited, bimolecular rate constant
Problem Phys 45 Spring Solution set 6 A bimolecular reaction in which A and combine to form the product P may be written as: k d A + A P k d k a where k d is a diffusion-limited, bimolecular rate constant
A Quantum Gas Microscope for Detecting Single Atoms in a Hubbard regime Optical Lattice
 A Quantum Gas Microscope for Detecting Single Atoms in a Hubbard regime Optical Lattice Nature 462, 74 77 (5 November 2009) Team 6 Hyuneil Kim Zhidong Leong Yulia Maximenko Jason Merritt 1 Outline Background
A Quantum Gas Microscope for Detecting Single Atoms in a Hubbard regime Optical Lattice Nature 462, 74 77 (5 November 2009) Team 6 Hyuneil Kim Zhidong Leong Yulia Maximenko Jason Merritt 1 Outline Background
Introduction to" Protein Structure
 Introduction to" Protein Structure Function, evolution & experimental methods Thomas Blicher, Center for Biological Sequence Analysis Learning Objectives Outline the basic levels of protein structure.
Introduction to" Protein Structure Function, evolution & experimental methods Thomas Blicher, Center for Biological Sequence Analysis Learning Objectives Outline the basic levels of protein structure.
Supplementary Information to. Longitudinal domain wall formation in elongated assemblies of ferromagnetic nanoparticles.
 Supplementary Information to Longitudinal domain wall formation in elongated assemblies of ferromagnetic nanoparticles authored by Miriam Varón, Marco Beleggia, Jelena Jordanovic, Jakob Schiøtz, Takeshi
Supplementary Information to Longitudinal domain wall formation in elongated assemblies of ferromagnetic nanoparticles authored by Miriam Varón, Marco Beleggia, Jelena Jordanovic, Jakob Schiøtz, Takeshi
Protein dynamics. Folding/unfolding dynamics. Fluctuations near the folded state
 Protein dynamics Folding/unfolding dynamics Passage over one or more energy barriers Transitions between infinitely many conformations B. Ozkan, K.A. Dill & I. Bahar, Protein Sci. 11, 1958-1970, 2002 Fluctuations
Protein dynamics Folding/unfolding dynamics Passage over one or more energy barriers Transitions between infinitely many conformations B. Ozkan, K.A. Dill & I. Bahar, Protein Sci. 11, 1958-1970, 2002 Fluctuations
Supplementary Figure 1 Change of the Tunnelling Transmission Coefficient from the Bulk to the Surface as a result of dopant ionization Colour-map of
 Supplementary Figure 1 Change of the Tunnelling Transmission Coefficient from the Bulk to the Surface as a result of dopant ionization Colour-map of change of the tunnelling transmission coefficient through
Supplementary Figure 1 Change of the Tunnelling Transmission Coefficient from the Bulk to the Surface as a result of dopant ionization Colour-map of change of the tunnelling transmission coefficient through
