CERAMIC MATERIALS I. Asst. Prof. Dr. Ayşe KALEMTAŞ
|
|
|
- Cuthbert Willis Taylor
- 6 years ago
- Views:
Transcription
1 CERAMIC MATERIALS I akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: Metallurgical and Materials Engineering Department
2 CLASSIFICATION OF CERAMICS Ceramic Materials Advanced Ceramics Traditional Ceramics Made from artificial or chemically modified raw materials. Mainly made from natural raw materials such as kaolinite (clay mineral), quartz and feldspar.
3 Advanced versus Traditional Ceramics Advanced Ceramics Traditional Ceramics Chemically prepared powders Precipitation, spray drying, freeze drying, vapor phase, sol-gel Raw Materials Preparation Raw minerals - Clay - Silica Slip casting, injection molding, sol-gel, hotpressing, HIPing, rapid prototyping, Forming Potters wheel, slip casting, pressing Electric furnace, hot press, reaction sintering, vapor deposition, plasma spraying, microwave furnace High temperature processing Flame kiln Erosion, laser machining, plasma spraying, ion implantation, coating Finishing Erosion, glazing Light microscopy, X-ray diffraction, electron microscopy, scanned probe microscopy, neutron diffraction, surface analytical methods Characterization Visible examination, light microscopy
4 Raw Material Selection Criterias Raw material cost Market factors Technical process parameters Performance of the desired product Market price of the product
5 CERAMIC RAW MATERIALS Ceramic Materials Naturally occurring minerals Synthetic materials their origin locations in which they can be found their relative abundance Naturally occurring minerals require extraction, which is often a regional industry located close to abundant quantities of the natural deposit. Most minerals need to go through some form of physical or chemical processing before use. The collective term for these processes is beneficiation. When you understand how oxides are manufactured, it will be clear why they are often impure and why Si, Na, Ca are the major impurities. borides (TiB 2, BN, etc.) carbides (SiC, B 4 C, TiC, etc.) nitrides (AlN, Si 3 N 4, TiN, etc.) oxides (TiO 2, Al 2 O 3, etc.) These ceramics are becoming more common, but are generally expensive and desire special processing environments. For many nonoxides the main impurities are often components of the starting material which was not reacted, e.g., Al in AlN or Si in Si 3 N 4.
6 CERAMIC RAW MATERIALS Category Purity, % Materials Crude materials Industrial minerals Industrial inorganic chemicals Special inorganic chemicals Variable Shales, stoneware clay, tile clay, crude bauxite, crude kyanite, natural ball clay, bentonite Ball clay, kaolin, refined bentonite, pyrophyllite, talc, feldspar, nepheline syenite, wollastonite, spodumene, glass sand, potter s flint (quartz), kyanite, bauxite, zircon, rutile, chrome ore, calcined kaolin, dolomite Calcined alumina (Bayer process), calcined magnesia (from brines, seawater), fused alumina, fused magnesia, aluminum nitride, silicon carbide, silicon nitride, barium carbonate, titania, calcined titanates, iron oxide, calcined ferrites, zirconia, stabilized zirconia, calcined zirconates 99.9 Various materials (Si 3 N 4, TiB 2, SiC, BN, etc)
7 Synthetic materials OXIDES The raw materials used for oxide ceramics are almost entirely produced by chemical processes to achieve a high chemical purity and to obtain the most suitable powders for component fabrication. NON-OXIDES Most of the important non-oxide ceramics do not occur naturally and therefore must be synthesized. The synthesis route is usually one of the following: Combine the metal directly with the nonmetal at high temperatures. Reduce the oxide with carbon at high temperature (carbothermal reduction) and subsequently react it with the nonmetal.
8 Natural Raw Naterials
9 Minerals Minerals are defined as (1) naturally occurring, (2) inorganic substances with a narrow range of (3) chemical composition and (4) characteristic physical properties. An example: The naturally occurring form of the compound sodium chloride is the mineral halite.
10 Minerals Mohs Hardness Scale Mohs' scale of mineral hardness quantifies the scratch resistance of minerals by comparing the ability of a harder material to scratch a softer material. The Mohs scale was invented in 1812, by the German mineralogist Friedrich Mohs. Mohs based his scale on ten minerals. Mineral Hardness Talc 1 Gypsum 2 Calcite 3 Fluorite 4 Apatite 5 Feldspar 6 Quartz 7 Topaz 8 Corundum 9 Diamond 10
11 Minerals Mohs Hardness Scale 1. Talc Talc is the world's softest mineral and the lowest mineral on the Mohs scale. Talc is a hydrated magnesium sheet silicate which is highly insoluble in water. Talc is translucent to opaque with a iridescent or pearly luster. Talc is used in cosmetics such as talcum powder, as a lubricant, and in paper manufacturing. Absolute Hardness: 1 Chemical Composition: Mg 3 Si 4 O 10 (OH) 2 2. Gypsum Gypsum is a soft mineral composed of calcium sulfate dihydrate. Gypsum occurs in nature as flattened or twinned crystals and transparent cleavable masses called selenite. When Gypsum has a silky and fibrous texture it is called Satin Spar. Absolute Hardness: 2 Chemical Composition: CaSO 4 á2h 2 O 3. Calcite Calcite is an anhydrous carbonate, and one of the most widely distributed minerals on the Earth's surface. It is a common constituent of sedimentary rocks. In crystallized form, Calcite has a vitreous luster. Absolute Hardness: 9 Chemical Composition: CaCO 3
12 Minerals Mohs Hardness Scale 4. Fluorite Fluorite (fluor-spar) is a mineral composed of calcium fluoride. It is an isometric mineral with a cubic crystal habit. Fluorite is named for its property of fluorescence, or its ability to fluoresce under ultraviolet light. Absolute Hardness: 21 Chemical Composition: CaF 2 5. Apatite Apatite (hydroxylapatite, fluorapatite, chlorapatite) is a group of phosphate minerals and is one of few minerals that are produced by biological organisms. Hydroxylapatite is the major component of tooth enamel. Absolute Hardness: 48 Chemical Composition: Ca 5 (PO 4 ) 3 (OH-,Cl-,F-) 6. Orthoclase Orthoclase (aka feldspar) in an igneous rock forming tectosilicate (silicate) mineral and is a key component in granite. Orthoclase derives its name form the Greek word for "straight fracture" because of its two cleavages at right angles to each other. Orthoclase crystallizes in the monoclinic crystal system. Absolute Hardness: 72 Chemical Composition: KAlSi 3 O 8
13 Minerals Mohs Hardness Scale 7. Quartz Quartz is one of the most common minerals found in the Earth's crust. It has a hexagonal crystal structure made of trigonal crystallized silica (silicon dioxide). The typical shape of a Quartz crystal is a six-sided prism that ends in six-sided pyramids. Absolute Hardness: 100 Chemical Composition: SiO 2 8. Topaz Topaz is a silicate or "nesosilicate" mineral created from a combination of aluminium and fluorine. It crystallizes in the orthorhombic system and it's crystals are prismatic in form. Absolute Hardness: 200 Chemical Composition: Al 2 SiO 4 (OH-,F-) 2
14 Minerals Mohs Hardness Scale 9. Corundum Corundum is the crystalline form of aluminium oxide and one of the basic rock-forming minerals. Corundum is naturally clear or colored by impurities. Due to its hardness, Corundum is used as an abrasive in sandpaper. Emery is an impure and less abrasive variety of Corundum. Absolute Hardness: 400 Chemical Composition: Al 2 O Diamond Diamond is the hardest natural occurring material. Diamond is a natural allotrope of carbon. The crystal bond structure of diamonds give the stone its hardness and differentiates it from graphite, which is the main allotrope of carbon. Absolute Hardness: 1500 Chemical Composition: C
15 WEATHERING Mechanical Weathering Physical disintegration of rock (with no chemical alteration) Chemical Weathering Chemical alteration of minerals within the rock Usually softening or dissolving the minerals Forming clays, oxides and solutes
16 WEATHERING The result of mechanical weathering Rock falls and slides Crushing and abrasion Rocks physically broken apart into sediment (but composition does NOT change) Increases surface area (lots of crushed broken pieces)
17 WEATHERING MECHANICAL WEATHERING ABRASION WATER WIND
18 WEATHERING ANIMALS Animals that burrow in ground can cause weathering
19 WEATHERING Sediments from Mechanical Weathering Sediments of: Parent rock Mineral particles Angular fragments
20 WEATHERING What is Chemical Weathering? Breakdown of rocks by chemical reactions that change the composition of rocks Hydrolysis Feldspar + Water = Clay OCCURS WHEN Water combines with minerals most often in granite (mica and feldspars) to form CLAY Feldspar A Clay Cliff
21 WEATHERING What is Chemical Weathering? Breakdown of rocks by chemical reactions that change the composition of rocks CARBONATION (causes dissolving) Rainwater containing carbon dioxide dissolves minerals (all rain water is slightly acidic) Most strongly affected are calcite minerals: Limestone and marble
22 WEATHERING What is Chemical Weathering? Breakdown of rocks by chemical reactions that change the composition of rocks RUSTING-OXIDATION OXIDATION OCCURS when oxygen combines chemically with iron to form iron oxide
23 WEATHERING Breakdown of rocks by chemical reactions that change the composition of rocks What is Chemical Weathering? Dissolving-ACID RAIN sulfuric acid-pollution in air dissolves in rainwater and eats away at buildings & rocks
24 WEATHERING Chemical Weathering Dissolving Dissolved ions Oxidation Iron in Ferromag. Minerals Iron Oxides (e.g., Hematite) Formation of Clays from silicates (e.g., Feldspar)
25 WEATHERING Chemical Weathering Dissolving Dissolved ions Oxidation Iron in Ferromag. Minerals Iron Oxides (e.g., Hematite) Formation of Clays from silicates (e.g., Feldspar) Oxidation: 4FeSiO 3 + O 2 + H 2 O FeO(OH) + 4SiO 2 Hydration: CaSO 4 + 2H 2 O CaSO 4 2H 2 O
26 WEATHERING CLIMATE CONTROLS WEATHERING PHYSICAL WEATHERING: COLD AND MOIST ALTERNATE FREEZE / THAW CHEMICAL WEATHERING: WARM AND MOIST (just like a chemical reaction) IN BOTH CASES WATER IS THE PRIMARY INGREDIENT THAT PROMOTES WEATHERING
27 WEATHERING Soil Formation and Weathering Related to Climate
28 WEATHERING Weathering Products of Common Rock-Forming Minerals
29 Minerals Minerals may be subdivided into two majors groups: Silicates (most abundant) Non-silicates (~8% of Earth s crust): Oxides O 2- Carbonates (CO 3 ) 2- Sulfides S 2- Sulfates (SO 4 ) 2- Halides Cl -, F -, Br - Native elements (single elements; e.g., Au)
30 Minerals Non-ferromagnesian Silicates (K, Na, Ca, Al) Ferromagnesian Silicates (Fe, Mg) Oxides Carbonates Sulfides/sulfates Native elements
31 Minerals Silicates Tetrahedron fundamental building block 4 oxygen ions surrounding a much smaller silicon ion Silicates are by far the most abundant mineral group accounting for more than 90% of the Earth's crust. Silicates are the major rock-forming minerals. It follows that oxygen and silicon are the most abundant elements in the crust.
32 Non-Silicates Usually form at low temperatures Carbonates Calcite - Ca CO 3 Dolomite - CaMg(CO 3 ) 2 Evaporite Minerals Gypsum - CaSO 4-2H 2 O Halite - NaCl Oxides Hematite
33 Non-Silicates
34 NATURAL RAW MATERIALS Clays Silica Feldspar Talc Wollastonite Aluminum Minerals Lithium Minerals Flourine Minerals
35 NATURAL RAW MATERIALS Non-uniform, crude materials from natural deposits clays. (Montmorillonite, illite, etc.)
36 NATURAL RAW MATERIALS The preparation, particularly of clay, by exposure to the weather for a long period. This helps to oxidize any pyrite present, rendering it soluble, so that this and other soluble impurities are to some extent leached out; the water content also becomes more uniform and agglomerates of clay are broken down with a consequent increase in plasticity. Weathering is simply the chemical and/or physical breakdown of a rock material. Weathering involves specific processes acting on rock materials at or near the surface of the Earth. Weathering products of common rock-forming minerals
37 Element Abundances SILICATES Common cations that bond with silica anions All others: 1.5%
38 Abundance of Minerals Abundance of Minerals in the Earth s Crust * * : Ceramic Materials: Science and Engineering, by C. Barry Carter and M. Grant Norton, Springer, 2007, 348.
39 THE END Thanks for your kind attention
40 Any Questions
ENVI.2030L - Minerals
 ENVI.2030L - Minerals Name I. Minerals Minerals are crystalline solids - the particles (atoms) that make-up the solid have a regular arrangement. In glasses, on the other hand, the atoms are not arranged
ENVI.2030L - Minerals Name I. Minerals Minerals are crystalline solids - the particles (atoms) that make-up the solid have a regular arrangement. In glasses, on the other hand, the atoms are not arranged
This is how we classify minerals! Silicates and Non-Silicates
 Why are some minerals harder than others? Their atomic structure and chemical formula. This is how we classify minerals! Silicates and Non-Silicates Part #1 - Silicates: Silicon and Oxygen make up 70%
Why are some minerals harder than others? Their atomic structure and chemical formula. This is how we classify minerals! Silicates and Non-Silicates Part #1 - Silicates: Silicon and Oxygen make up 70%
Periods on the Periodic Table
 Minerals Chapter 2 Matter Matter includes anything that has mass and takes up space (volume). It exists in 3 main states on Earth solid, liquid, and gas. Matter can be classified based on its physical
Minerals Chapter 2 Matter Matter includes anything that has mass and takes up space (volume). It exists in 3 main states on Earth solid, liquid, and gas. Matter can be classified based on its physical
WEATHERING. Turning Rock to Sediment and Solutions 10/22/2012
 WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
CHAPTER 2 MINERALS. Group Presentation Notes
 CHAPTER 2 MINERALS Group Presentation Notes DEFINITION OF A MINERAL A mineral is naturally occurring, inorganic solid with an orderly crystalline structure and a definite chemical composition. CHARACTERISTICS
CHAPTER 2 MINERALS Group Presentation Notes DEFINITION OF A MINERAL A mineral is naturally occurring, inorganic solid with an orderly crystalline structure and a definite chemical composition. CHARACTERISTICS
Matter and Minerals. Earth 9 th edition Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more).
 1 2 Matter and Minerals Earth 9 th edition Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more). continued... 3 4 5 6 7 8 9 10 11 12 13 14 Also crystalline,
1 2 Matter and Minerals Earth 9 th edition Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more). continued... 3 4 5 6 7 8 9 10 11 12 13 14 Also crystalline,
1 st shell holds 2 electrons. 2 nd shell holds 8 electrons
 ATOM INDIVISIBLE ELEMENTS - Nucleus = protons (+ charge) & neutrons (no charge ) - Electrons (- charge) orbit the nucleus in shells of 2, 8, 8 electrons (inner orbit outward) - Atomic number = number of
ATOM INDIVISIBLE ELEMENTS - Nucleus = protons (+ charge) & neutrons (no charge ) - Electrons (- charge) orbit the nucleus in shells of 2, 8, 8 electrons (inner orbit outward) - Atomic number = number of
Atoms>>>Elements>>>Minerals>>>Rocks>>>Continents>>>Planet
 Introduction to Minerals It s all about scale: Atoms>>>Elements>>>Minerals>>>Rocks>>>Continents>>>Planet Basic Chem: Atomic Structure Atom: smallest unit of an element that possesses the properties of
Introduction to Minerals It s all about scale: Atoms>>>Elements>>>Minerals>>>Rocks>>>Continents>>>Planet Basic Chem: Atomic Structure Atom: smallest unit of an element that possesses the properties of
Matter and Minerals Earth: Chapter Pearson Education, Inc.
 Matter and Minerals Earth: Chapter 3 Minerals: Building Blocks of Rocks By definition a mineral is: Naturally occurring An inorganic solid Ordered internal molecular structure Definite chemical composition
Matter and Minerals Earth: Chapter 3 Minerals: Building Blocks of Rocks By definition a mineral is: Naturally occurring An inorganic solid Ordered internal molecular structure Definite chemical composition
10/8/15. Earth Materials Minerals and Rocks. I) Minerals. Minerals. (A) Definition: Topics: -- naturally occurring What are minerals?
 minerals Earth Materials Minerals and Rocks I) Minerals Minerals Topics: What are minerals? Basic Chemistry Amethysts in geode: minerals Characteristics of Minerals Types of Minerals -- orderly arrangement
minerals Earth Materials Minerals and Rocks I) Minerals Minerals Topics: What are minerals? Basic Chemistry Amethysts in geode: minerals Characteristics of Minerals Types of Minerals -- orderly arrangement
Minerals Please do not write on this test packet.
 Please do not write on this test packet. 1. The diagram below shows the index minerals of Mohs hardness scale compared with the hardness of some common objects. 2. Base your answer to the following question
Please do not write on this test packet. 1. The diagram below shows the index minerals of Mohs hardness scale compared with the hardness of some common objects. 2. Base your answer to the following question
ESS Minerals. Lee. 1. The table below shows some properties of four different minerals.
 Name: ESS Minerals Pd. 1. The table below shows some properties of four different minerals. The minerals listed in the table are varieties of which mineral? (A) garnet (B) magnetite (C) olivine (D) quartz
Name: ESS Minerals Pd. 1. The table below shows some properties of four different minerals. The minerals listed in the table are varieties of which mineral? (A) garnet (B) magnetite (C) olivine (D) quartz
Earth and Space Science. Semester 2 Review, Part 2
 Earth and Space Science Semester 2 Review, Part 2 2015 Chemical Weathering -The process that breaks down rock through chemical changes. Examples that cause chemical weathering include the action of water
Earth and Space Science Semester 2 Review, Part 2 2015 Chemical Weathering -The process that breaks down rock through chemical changes. Examples that cause chemical weathering include the action of water
Chapter 4. Rocks and Minerals: Documents that Record Earth's History
 Chapter 4 Rocks and Minerals: Documents that Record Earth's History What can Minerals Tell Us? 1. Minerals may contain radioactive elements that can be used for radiometric age dating. 2. Minerals that
Chapter 4 Rocks and Minerals: Documents that Record Earth's History What can Minerals Tell Us? 1. Minerals may contain radioactive elements that can be used for radiometric age dating. 2. Minerals that
Minerals. Atoms, Elements, and Chemical Bonding. Definition of a Mineral 2-1
 Minerals In order to define a what we mean by a mineral we must first make some definitions: 2-1 Most of the Earth s surface is composed of rocky material. An element is a substance which cannot be broken
Minerals In order to define a what we mean by a mineral we must first make some definitions: 2-1 Most of the Earth s surface is composed of rocky material. An element is a substance which cannot be broken
Rocks and Minerals. Tillery, Chapter 19. Solid Earth Materials
 Rocks and Minerals Tillery, Chapter 19 Science 330 Summer 2007 No other planet in the solar system has the unique combination of fluids of Earth. Earth has a surface that is mostly covered with liquid
Rocks and Minerals Tillery, Chapter 19 Science 330 Summer 2007 No other planet in the solar system has the unique combination of fluids of Earth. Earth has a surface that is mostly covered with liquid
About Earth Materials
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Minerals: Building Blocks of Rocks Chapter 2. Based on: Earth Science, 10e
 Minerals: Building Blocks of Rocks Chapter 2 Based on: Earth Science, 10e Minerals: the building blocks of rocks Definition of a mineral Solid Inorganic Natural Crystalline Structure - Possess an orderly
Minerals: Building Blocks of Rocks Chapter 2 Based on: Earth Science, 10e Minerals: the building blocks of rocks Definition of a mineral Solid Inorganic Natural Crystalline Structure - Possess an orderly
Atoms: Building Blocks of Minerals. Why Atoms Bond. Why Atoms Bond. Halite (NaCl) An Example of Ionic Bonding. Composition of Minerals.
 Matter and Minerals Earth Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more). continued... Also crystalline, chemically specific. There! I fit it in!
Matter and Minerals Earth Chapter 3 Minerals: summary in haiku form "Mineral" defined: natural, inorganic, solid (and two more). continued... Also crystalline, chemically specific. There! I fit it in!
Minerals. What are minerals and how do we classify them?
 Minerals What are minerals and how do we classify them? 1 Minerals! Minerals are the ingredients needed to form the different types of rocks! Rock - is any naturally formed solid that is part of Earth
Minerals What are minerals and how do we classify them? 1 Minerals! Minerals are the ingredients needed to form the different types of rocks! Rock - is any naturally formed solid that is part of Earth
Sedimentary Rocks and Processes
 Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Chapter 1 Lecture Outline. Matter and Minerals
 Chapter 1 Lecture Outline Matter and Minerals Minerals: Building Blocks of Rocks Minerals are the building blocks of rocks Minerals important in human history Flint and chert for weapons and tools Gold,
Chapter 1 Lecture Outline Matter and Minerals Minerals: Building Blocks of Rocks Minerals are the building blocks of rocks Minerals important in human history Flint and chert for weapons and tools Gold,
Minerals: Minerals: Building blocks of rocks. Atomic Structure of Matter. Building Blocks of Rocks Chapter 3 Outline
 Minerals: Building Blocks of Rocks Chapter 3 Outline Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. Minerals: Building blocks of rocks Definition
Minerals: Building Blocks of Rocks Chapter 3 Outline Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. Minerals: Building blocks of rocks Definition
5/24/2018. Matter and Minerals
 1 2 3 4 5 6 7 8 9 10 11 Matter and Minerals Earth Chapter 3 Chapter 3 Matter & Minerals Figure 3.1 Minerals: Building Blocks of Rocks Geologic Definition of a Mineral: Naturally occurring Generally inorganic
1 2 3 4 5 6 7 8 9 10 11 Matter and Minerals Earth Chapter 3 Chapter 3 Matter & Minerals Figure 3.1 Minerals: Building Blocks of Rocks Geologic Definition of a Mineral: Naturally occurring Generally inorganic
Minerals II: Physical Properties and Crystal Forms. From:
 Minerals II: Physical Properties and Crystal Forms From: http://webmineral.com/data/rhodochrosite.shtml The Physical Properties of Minerals Color Streak Luster Hardness External Crystal Form Cleavage The
Minerals II: Physical Properties and Crystal Forms From: http://webmineral.com/data/rhodochrosite.shtml The Physical Properties of Minerals Color Streak Luster Hardness External Crystal Form Cleavage The
Lab #4: Minerals: Building Blocks of Rocks
 Lab #4: Minerals: Building Blocks of Rocks Minerals: Building Blocks of Rocks By definition a mineral is/has Naturally occurring Inorganic solid Ordered internal molecular structure Definite chemical composition
Lab #4: Minerals: Building Blocks of Rocks Minerals: Building Blocks of Rocks By definition a mineral is/has Naturally occurring Inorganic solid Ordered internal molecular structure Definite chemical composition
Field Trips. Field Trips
 Field Trips Saturday field trips have been scheduled October 9, October 23 and December 4 Last all day (9:00 AM to 4:00 PM) Bus transportation provided from campus Joint with GG101 laboratory, GG101 Section
Field Trips Saturday field trips have been scheduled October 9, October 23 and December 4 Last all day (9:00 AM to 4:00 PM) Bus transportation provided from campus Joint with GG101 laboratory, GG101 Section
305 ATOMS, ELEMENTS, AND MINERALS
 DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 305 ATOMS, ELEMENTS, AND MINERALS Instructions: Read each question carefully before selecting the BEST answer. Use GEOLOGIC VOCABULARY where APPLICABLE!
DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 305 ATOMS, ELEMENTS, AND MINERALS Instructions: Read each question carefully before selecting the BEST answer. Use GEOLOGIC VOCABULARY where APPLICABLE!
Introduction to Geology
 Introduction to Geology Why the heck would you want to take a geology class? 1) Geology is responsible for supplying many of the things we need. 2) Geology is closely related to the environment, which
Introduction to Geology Why the heck would you want to take a geology class? 1) Geology is responsible for supplying many of the things we need. 2) Geology is closely related to the environment, which
Review - Unit 2 - Rocks and Minerals
 Review - Unit 2 - Rocks and Minerals Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral.
Review - Unit 2 - Rocks and Minerals Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral.
PHILADELPHIA UNIVERSITY
 PHILADELPHIA UNIVERSITY FACULTY OF ENGINEERING AND TECHNOLOGY DEPARTMENT OF CIVIL ENGINEERING. Engineering Geology Part one 1 2nd semester 2018/2019 Eng. Amany Assouli 1 INTRODUCTION: What is the engineering
PHILADELPHIA UNIVERSITY FACULTY OF ENGINEERING AND TECHNOLOGY DEPARTMENT OF CIVIL ENGINEERING. Engineering Geology Part one 1 2nd semester 2018/2019 Eng. Amany Assouli 1 INTRODUCTION: What is the engineering
CH 4- MINERALS OBJECTIVES: Identify characteristics and formations of minerals. Differentiate Minerals by their groups and uses STANDARDS:
 OBJECTIVES: CH 4- MINERALS Identify characteristics and formations of minerals Differentiate Minerals by their groups and uses STANDARDS: MINERAL: WHAT IS A MINERAL? A naturally occurring Inorganic Solid
OBJECTIVES: CH 4- MINERALS Identify characteristics and formations of minerals Differentiate Minerals by their groups and uses STANDARDS: MINERAL: WHAT IS A MINERAL? A naturally occurring Inorganic Solid
305 ATOMS, ELEMENTS, AND MINERALS
 DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 305 ATOMS, ELEMENTS, AND MINERALS Instructions: Read each question carefully before selecting the BEST answer. Use GEOLOGIC VOCABULARY where APPLICABLE!
DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 305 ATOMS, ELEMENTS, AND MINERALS Instructions: Read each question carefully before selecting the BEST answer. Use GEOLOGIC VOCABULARY where APPLICABLE!
Weathering: the disintegration, or breakdown of rock material
 Weathering: the disintegration, or breakdown of rock material Mechanical Weathering: no change in chemical composition--just disintegration into smaller pieces Chemical Weathering: breakdown as a result
Weathering: the disintegration, or breakdown of rock material Mechanical Weathering: no change in chemical composition--just disintegration into smaller pieces Chemical Weathering: breakdown as a result
300 ATOMS, ELEMENTS, AND MINERALS
 DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 300 ATOMS, ELEMENTS, AND MINERALS Instructions: Read each question carefully before selecting the BEST answer. Use GEOLOGIC VOCABULARY where APPLICABLE!
DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 300 ATOMS, ELEMENTS, AND MINERALS Instructions: Read each question carefully before selecting the BEST answer. Use GEOLOGIC VOCABULARY where APPLICABLE!
What do these products have in common?
 What is a mineral? 4000 minerals on Earth!!!! A naturally occurring, inorganic solid crystalline substance with a definite chemical composition. A mineral must have these 5 major aspects: Naturally occurring
What is a mineral? 4000 minerals on Earth!!!! A naturally occurring, inorganic solid crystalline substance with a definite chemical composition. A mineral must have these 5 major aspects: Naturally occurring
Mineral Identification
 Mineral Identification! Mineral identification is a skill. " Requires learning diagnostic properties #Some properties are easily seen. $Color $Crystal shape #Some properties require handling or testing.
Mineral Identification! Mineral identification is a skill. " Requires learning diagnostic properties #Some properties are easily seen. $Color $Crystal shape #Some properties require handling or testing.
305 ATOMS, ELEMENTS, AND MINERALS
 DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 305 ATOMS, ELEMENTS, AND MINERALS Instructions: Read each question carefully before selecting the BEST answer. Use GEOLOGIC VOCABULARY where APPLICABLE!
DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 305 ATOMS, ELEMENTS, AND MINERALS Instructions: Read each question carefully before selecting the BEST answer. Use GEOLOGIC VOCABULARY where APPLICABLE!
Crust Elements. Elements of Earth. Minerals. Crystals. Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air
 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Minerals and Rocks Chapter 20
 Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
305 ATOMS, ELEMENTS, AND MINERALS
 DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 305 ATOMS, ELEMENTS, AND MINERALS Instructions: Read each question carefully before selecting the BEST answer. Use GEOLOGIC VOCABULARY where APPLICABLE!
DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 305 ATOMS, ELEMENTS, AND MINERALS Instructions: Read each question carefully before selecting the BEST answer. Use GEOLOGIC VOCABULARY where APPLICABLE!
Name: Minerals and more minerals
 1. The diagram below shows how a sample of the mineral mica breaks when hit with a rock hammer. 6. The diagrams below show the crystal shapes of two minerals. This mineral breaks in smooth, flat surfaces
1. The diagram below shows how a sample of the mineral mica breaks when hit with a rock hammer. 6. The diagrams below show the crystal shapes of two minerals. This mineral breaks in smooth, flat surfaces
Practice Test Rocks and Minerals. Name. Page 1
 Name Practice Test Rocks and Minerals 1. Which rock would be the best source of the mineral garnet? A) basalt B) limestone C) schist D) slate 2. Which mineral is mined for its iron content? A) hematite
Name Practice Test Rocks and Minerals 1. Which rock would be the best source of the mineral garnet? A) basalt B) limestone C) schist D) slate 2. Which mineral is mined for its iron content? A) hematite
Wednesday, October 10 th
 Wednesday, October 10 th Page 13a (left side) / Place Lab on table Objective: We will describe the different types of weathering and erosion and identify evidence of each type. Warm-up: 1. What is weathering?
Wednesday, October 10 th Page 13a (left side) / Place Lab on table Objective: We will describe the different types of weathering and erosion and identify evidence of each type. Warm-up: 1. What is weathering?
Emily and Megan. Earth System Science. Elements of Earth by weight. Crust Elements, by weight. Minerals. Made of atoms Earth is mostly iron, by weight
 Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Sedimentology & Stratigraphy. Thanks to Rob Viens for slides
 Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Earth Science Minerals. Moh s Scale of Hardness In which New York State landscape region was most of the garnet mined?
 Name: ate: 1. Which mineral is white or colorless, has a hardness of 2.5, and splits with cubic cleavage? A. calcite. halite C. pyrite. mica 2. ase your answer(s) to the following question(s) on the map
Name: ate: 1. Which mineral is white or colorless, has a hardness of 2.5, and splits with cubic cleavage? A. calcite. halite C. pyrite. mica 2. ase your answer(s) to the following question(s) on the map
Minerals and Rocks. Environmental Learning Community CORC 1332 Sept 21, 2010
 Minerals and Rocks Environmental Learning Community CORC 1332 Sept 21, 2010 Outline Quiz More on minerals Twinkies Rocks How can you identify one mineral from another? Distinguishing One Mineral from Another
Minerals and Rocks Environmental Learning Community CORC 1332 Sept 21, 2010 Outline Quiz More on minerals Twinkies Rocks How can you identify one mineral from another? Distinguishing One Mineral from Another
CERAMIC MATERIALS I. Asst. Prof. Dr. Ayşe KALEMTAŞ
 CERAMIC MATERIALS I akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: 211 19 17 Metallurgical and Materials Engineering Department Traditional Ceramics Clay products Main Components Clay Feldspar Silica
CERAMIC MATERIALS I akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: 211 19 17 Metallurgical and Materials Engineering Department Traditional Ceramics Clay products Main Components Clay Feldspar Silica
Geology 103 Planet Earth (QR II), Laboratory Exercises 1. Minerals
 Geology 103 Planet Earth (QR II), Laboratory Exercises 1 Student Name: Section: Minerals Minerals are naturally occurring, inorganic, crystalline solids with a characteristic chemical composition. Most
Geology 103 Planet Earth (QR II), Laboratory Exercises 1 Student Name: Section: Minerals Minerals are naturally occurring, inorganic, crystalline solids with a characteristic chemical composition. Most
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
6/20/2018. Lesson 1 (Properties of Minerals) 6 th Grade. Earth s Structure Chapter 2: Minerals and Rocks. density =
 6 th Grade Earth s Structure Chapter 2: Minerals and Rocks Mineral Lesson 1 (Properties of Minerals) a mineral must meet all four of the following requirements: 1. must be naturally-occurring (formed by
6 th Grade Earth s Structure Chapter 2: Minerals and Rocks Mineral Lesson 1 (Properties of Minerals) a mineral must meet all four of the following requirements: 1. must be naturally-occurring (formed by
EESC 4701: Igneous and Metamorphic Petrology IGNEOUS MINERALS LAB 1 HANDOUT
 EESC 4701: Igneous and Metamorphic Petrology IGNEOUS MINERALS LAB 1 HANDOUT Sources: Cornell EAS302 lab, UMass Lowell 89.301 Mineralogy, LHRIC.org The Petrographic Microscope As you know, light is an electromagnetic
EESC 4701: Igneous and Metamorphic Petrology IGNEOUS MINERALS LAB 1 HANDOUT Sources: Cornell EAS302 lab, UMass Lowell 89.301 Mineralogy, LHRIC.org The Petrographic Microscope As you know, light is an electromagnetic
Unit 2: Minerals and Rocks Practice Questions
 Name: Date: 1. Which mineral is white or colorless, has a hardness of 2.5, and splits with cubic cleavage? 6. Base your answer(s) to the following question(s) on the photograph of a sample of gneiss below.
Name: Date: 1. Which mineral is white or colorless, has a hardness of 2.5, and splits with cubic cleavage? 6. Base your answer(s) to the following question(s) on the photograph of a sample of gneiss below.
1 What Is a Mineral? Critical Thinking 2. Apply Concepts Glass is made up of silicon and oxygen atoms in a 1:2 ratio. The SiO 2
 CHAPTER 5 1 What Is a Mineral? SECTION Minerals of Earth s Crust KEY IDEAS As you read this section, keep these questions in mind: What is a mineral? What are the two main groups of minerals? What are
CHAPTER 5 1 What Is a Mineral? SECTION Minerals of Earth s Crust KEY IDEAS As you read this section, keep these questions in mind: What is a mineral? What are the two main groups of minerals? What are
Atoms, Molecules and Minerals
 Atoms, Molecules and Minerals Atoms Matter The smallest unit of an element that retain its properties Molecules - a small orderly group of atoms that possess specific properties - H 2 O Small nucleus surrounded
Atoms, Molecules and Minerals Atoms Matter The smallest unit of an element that retain its properties Molecules - a small orderly group of atoms that possess specific properties - H 2 O Small nucleus surrounded
Chapter 2 Minerals Section 1 Matter Elements and the Periodic Table
 Chapter 2 Minerals Section 1 Matter Key Concepts What is an element? What particles make up atoms? What are isotopes? What are compounds and why do they form? How do chemical bonds differ? Vocabulary element
Chapter 2 Minerals Section 1 Matter Key Concepts What is an element? What particles make up atoms? What are isotopes? What are compounds and why do they form? How do chemical bonds differ? Vocabulary element
LECTURE #2: Elements & Minerals. I. Recitations start next week! please make sure you attend the class and talk with your TA about what is expected
 GEOL 0820 Ramsey Natural Disasters Spring, 2018 LECTURE #2: Elements & Minerals Date: 11 January 2018 I. Recitations start next week! please make sure you attend the class and talk with your TA about what
GEOL 0820 Ramsey Natural Disasters Spring, 2018 LECTURE #2: Elements & Minerals Date: 11 January 2018 I. Recitations start next week! please make sure you attend the class and talk with your TA about what
Atoms Elements Minerals
 Atoms Elements Minerals Atoms The building blocks of all matter. Atoms The building blocks of all matter. Atoms The building blocks of all matter. 1 Atoms consist of a positively charged nucleus of protons
Atoms Elements Minerals Atoms The building blocks of all matter. Atoms The building blocks of all matter. Atoms The building blocks of all matter. 1 Atoms consist of a positively charged nucleus of protons
The most common elements that make up minerals are oxygen, silicon, aluminum, iron, calcium, potassium, and magnesium
 Mineralogy: The Study of Minerals and their Properties A Mineral! Occurs! Is a! Is a substance (element or compound)! Has atoms arrange in an orderly pattern ( )! Is (not formed by any process involving
Mineralogy: The Study of Minerals and their Properties A Mineral! Occurs! Is a! Is a substance (element or compound)! Has atoms arrange in an orderly pattern ( )! Is (not formed by any process involving
Chapter 6 9/25/2012. Weathering, Erosion and Soils. Introduction. How Are Earth Materials Altered? Introduction. How Are Earth Materials Altered?
 Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Minerals. Elements and Minerals
 Minerals Gypsum Crystals (actual size) Elements and Minerals 87 naturally occurring elements 12 are found in the earth s crust in amounts >1% These twelve make up 99% of the mass of the crust. 70% of the
Minerals Gypsum Crystals (actual size) Elements and Minerals 87 naturally occurring elements 12 are found in the earth s crust in amounts >1% These twelve make up 99% of the mass of the crust. 70% of the
Earth Materials: Minerals and Rocks Chapter 4
 Earth Materials: Minerals and Rocks Chapter 4 The French are bred to die for love They delight in fighting duels But I prefer a man who lives And gives expensive jewls A kill on the hand may be quite continental
Earth Materials: Minerals and Rocks Chapter 4 The French are bred to die for love They delight in fighting duels But I prefer a man who lives And gives expensive jewls A kill on the hand may be quite continental
Solid Earth materials:
 Solid Earth materials: Elements minerals rocks Nonuniform distribution of matter Molten core Contains most heavy elements Iron, nickel Thin surface crust Mostly lighter elements 8 elements make up 98.6%
Solid Earth materials: Elements minerals rocks Nonuniform distribution of matter Molten core Contains most heavy elements Iron, nickel Thin surface crust Mostly lighter elements 8 elements make up 98.6%
Minerals. Gypsum Crystals - Mexico
 Minerals Gypsum Crystals - Mexico Rocks Rocks are Earth materials made from minerals. Most rocks have more than one kind of mineral. Example: Granite Potassium feldspar. Plagioclase Feldspar. Quartz. Hornblende.
Minerals Gypsum Crystals - Mexico Rocks Rocks are Earth materials made from minerals. Most rocks have more than one kind of mineral. Example: Granite Potassium feldspar. Plagioclase Feldspar. Quartz. Hornblende.
The Use of Minerals. Chapter 3
 Section 3 The Formation, Mining, and Use of Minerals The Use of Minerals Metallic Minerals are good conductors of heat and electricity. They can be processed for various uses, including building aircraft,
Section 3 The Formation, Mining, and Use of Minerals The Use of Minerals Metallic Minerals are good conductors of heat and electricity. They can be processed for various uses, including building aircraft,
The earth is composed of various kinds
 UNIT III LANDFORMS This unit deals with Rocks and minerals major types of rocks and their characteristics Landforms and their evolution Geomorphic processes weathering, mass wasting, erosion and deposition;
UNIT III LANDFORMS This unit deals with Rocks and minerals major types of rocks and their characteristics Landforms and their evolution Geomorphic processes weathering, mass wasting, erosion and deposition;
4. The diagram of Bowen's Reaction Series below indicates the relative temperatures at which specific minerals crystallize as magma cools.
 Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral. 1. The luster of this mineral could
Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral. 1. The luster of this mineral could
OFFICIAL MID-HUDSON VALLEY GEM & MINERAL SOCIETY (MHVG&MS) 2017 EARTH SCIENCE SCAVENGER HUNT QUESTIONNAIRE. New York s Gemstone
 OFFICIAL MID-HUDSON VALLEY GEM & MINERAL SOCIETY (MHVG&MS) 2017 EARTH SCIENCE SCAVENGER HUNT QUESTIONNAIRE 2017 SHOW THEME Garnet -variety: ALMANDINE New York s Gemstone Please sign in at the Earth Science
OFFICIAL MID-HUDSON VALLEY GEM & MINERAL SOCIETY (MHVG&MS) 2017 EARTH SCIENCE SCAVENGER HUNT QUESTIONNAIRE 2017 SHOW THEME Garnet -variety: ALMANDINE New York s Gemstone Please sign in at the Earth Science
Processed Food Production. Consistent product Long shelf life Low cost
 How is the production of wheat connected to Earth and environmental sciences? Processed Food Production Consistent product Long shelf life Low cost Twinkie Ingredients Enriched bleached wheat flour [flour,
How is the production of wheat connected to Earth and environmental sciences? Processed Food Production Consistent product Long shelf life Low cost Twinkie Ingredients Enriched bleached wheat flour [flour,
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Chapter 6. Weathering, Erosion, and Soil
 Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Mechanisms
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
Physical Geography Lab Activity #07
 Physical Geography Lab Activity #07 Due date Name Rocks & Minerals COR Objective 8 7.1. Introduction One part of being a physical geographer is having a basic knowledge of the rocks around us. In this
Physical Geography Lab Activity #07 Due date Name Rocks & Minerals COR Objective 8 7.1. Introduction One part of being a physical geographer is having a basic knowledge of the rocks around us. In this
Lecture Outlines PowerPoint. Chapter 2 Earth Science 11e Tarbuck/Lutgens
 Lecture Outlines PowerPoint Chapter 2 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
Lecture Outlines PowerPoint Chapter 2 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
INTRODUCTION. From the earliest time, man has found important uses of minerals.
 CHAPTER 2: MINERALS INTRODUCTION From the earliest time, man has found important uses of minerals. E.g. clay for bricks and pottery; quartz and jade for weapons, garnet, amethyst and other coloured stones
CHAPTER 2: MINERALS INTRODUCTION From the earliest time, man has found important uses of minerals. E.g. clay for bricks and pottery; quartz and jade for weapons, garnet, amethyst and other coloured stones
Lab 4: Mineral Identification April 14, 2009
 Name: Lab 4: Mineral Identification April 14, 2009 While about 3000 minerals have been recognized as valid species, very few of these are commonly seen. Comprehensive mineralogy texts typically deal with
Name: Lab 4: Mineral Identification April 14, 2009 While about 3000 minerals have been recognized as valid species, very few of these are commonly seen. Comprehensive mineralogy texts typically deal with
it must be it must be it must have been formed by it must have it must have
 6. Minerals II (p. 78-87) What is a mineral? The five characteristics required in order for a compound to be a mineral are: it must be it must be it must have been formed by it must have it must have Characteristics
6. Minerals II (p. 78-87) What is a mineral? The five characteristics required in order for a compound to be a mineral are: it must be it must be it must have been formed by it must have it must have Characteristics
Chapter: Earth Materials
 Table of Contents Chapter: Earth Materials Section 1: Minerals Section 2: Igneous Rocks Section 3: Sedimentary Rocks Section 4: Metamorphic Rocks and the Rock Cycle 1 Minerals Common Elements Composition
Table of Contents Chapter: Earth Materials Section 1: Minerals Section 2: Igneous Rocks Section 3: Sedimentary Rocks Section 4: Metamorphic Rocks and the Rock Cycle 1 Minerals Common Elements Composition
Minerals and Rocks. Minerals
 Minerals and Rocks Minerals What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement or a D if
Minerals and Rocks Minerals What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement or a D if
ROCKS & MINERALS UNIT. 8 th Grade Earth & Space Science
 ROCKS & MINERALS UNIT 8 th Grade Earth & Space Science Characteristics of Minerals 8 th Grade Earth & Space Science Class Notes Mineral Characteristics Naturally occurring formed by natural processes Inorganic
ROCKS & MINERALS UNIT 8 th Grade Earth & Space Science Characteristics of Minerals 8 th Grade Earth & Space Science Class Notes Mineral Characteristics Naturally occurring formed by natural processes Inorganic
Weathering Cycle Teacher Notes
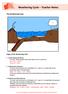 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
Prentice Hall EARTH SCIENCE
 Prentice Hall EARTH SCIENCE Tarbuck Lutgens Chapter 2 Minerals 2.1 Matter Elements and the Periodic Table Elements are the basic building blocks of minerals. Over 100 elements are known. 2.1 Matter Atoms
Prentice Hall EARTH SCIENCE Tarbuck Lutgens Chapter 2 Minerals 2.1 Matter Elements and the Periodic Table Elements are the basic building blocks of minerals. Over 100 elements are known. 2.1 Matter Atoms
CHAPTER 1: MINERALS: DEFINITION, PROPERTIES AND OCCURRENCES. Sarah Lambart
 CHAPTER 1: MINERALS: DEFINITION, PROPERTIES AND OCCURRENCES Sarah Lambart CONTENT OF CHAPTER 1 Goal: learn how to describe and classify minerals 3 elements of classification: chemistry, structure and environment
CHAPTER 1: MINERALS: DEFINITION, PROPERTIES AND OCCURRENCES Sarah Lambart CONTENT OF CHAPTER 1 Goal: learn how to describe and classify minerals 3 elements of classification: chemistry, structure and environment
And the study of mineral the branch in geology is termed as mineralogy. (Refer Slide Time: 0:29)
 Earth Sciences for Civil Engineering Professor Javed N Malik Department of Earth Sciences Indian Institute of Technology Kanpur Module 2 Lecture No 6 Rock-Forming Minerals and their Properties (Part-2)
Earth Sciences for Civil Engineering Professor Javed N Malik Department of Earth Sciences Indian Institute of Technology Kanpur Module 2 Lecture No 6 Rock-Forming Minerals and their Properties (Part-2)
Lecture 6. Physical Properties. Solid Phase. Particle Composition
 Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
1. Which mineral is mined for its iron content? A) hematite B) fluorite C) galena D) talc
 1. Which mineral is mined for its iron content? A) hematite B) fluorite C) galena D) talc 2. Which material is made mostly of the mineral quartz? A) sulfuric acid B) pencil lead C) plaster of paris D)
1. Which mineral is mined for its iron content? A) hematite B) fluorite C) galena D) talc 2. Which material is made mostly of the mineral quartz? A) sulfuric acid B) pencil lead C) plaster of paris D)
Chapter 12: Structures & Properties of Ceramics
 Chapter 12: Structures & Properties of Ceramics ISSUES TO ADDRESS... Bonding and structure of ceramic materials as compared with metals Chapter 12-1 Atomic Bonding in Ceramics Bonding: -- Can be ionic
Chapter 12: Structures & Properties of Ceramics ISSUES TO ADDRESS... Bonding and structure of ceramic materials as compared with metals Chapter 12-1 Atomic Bonding in Ceramics Bonding: -- Can be ionic
Lecture 3: Earth Materials and their Properties I: Minerals. Introduction to the Earth System EAS 2200
 Lecture 3: Earth Materials and their Properties I: Minerals Introduction to the Earth System EAS 2200 Earth Materials Plan of the Why it matters Nature of the Earth/Composition The Solid Earth Mineral
Lecture 3: Earth Materials and their Properties I: Minerals Introduction to the Earth System EAS 2200 Earth Materials Plan of the Why it matters Nature of the Earth/Composition The Solid Earth Mineral
Introduction to Prospecting. Session Three Minerals
 Introduction to Prospecting Session Three Minerals Mineral: Solid inorganic substance of natural occurrence with a specific elemental composition and crystal structure. Rock: An aggregate of minerals.
Introduction to Prospecting Session Three Minerals Mineral: Solid inorganic substance of natural occurrence with a specific elemental composition and crystal structure. Rock: An aggregate of minerals.
Chapter 23 Rocks and Minerals
 This lecture will help you understand: Chapter 23 Rocks and Minerals What Minerals and Rocks Are Mineral Properties How Minerals Form How Minerals Are Classified The Silicate Tetrahedron Igneous Rocks
This lecture will help you understand: Chapter 23 Rocks and Minerals What Minerals and Rocks Are Mineral Properties How Minerals Form How Minerals Are Classified The Silicate Tetrahedron Igneous Rocks
Element atoms join together to form an element it can not be broken down into simpler substances 118 known elements 1 92 except elements 43 and 61
 Chapter 2 What is an Element? What particles make up an atom? Element atoms join together to form an element it can not be broken down into simpler substances 118 known elements 1 92 except elements 43
Chapter 2 What is an Element? What particles make up an atom? Element atoms join together to form an element it can not be broken down into simpler substances 118 known elements 1 92 except elements 43
MINERALS Smith and Pun Chapter 2 ATOMIC STRUCTURE
 MINERALS Smith and Pun Chapter 2 1 ATOMIC STRUCTURE 2 1 ATOMIC STRUCTURE (2) (See Smith and Pun, pages 29-35) ELEMENT: Substance that cannot be broken down into other substances by ordinary chemical methods
MINERALS Smith and Pun Chapter 2 1 ATOMIC STRUCTURE 2 1 ATOMIC STRUCTURE (2) (See Smith and Pun, pages 29-35) ELEMENT: Substance that cannot be broken down into other substances by ordinary chemical methods
Rocks & Minerals. Lesson 1 Properties of Minerals. What is a mineral? What is a mineral?
 Rocks & Minerals What is a mineral? A mineral must have 5 specific characteristics to be considered a mineral a. b. c. d. e. Naturally occurring - formed by natural processes. Solid - must have a definite
Rocks & Minerals What is a mineral? A mineral must have 5 specific characteristics to be considered a mineral a. b. c. d. e. Naturally occurring - formed by natural processes. Solid - must have a definite
Weathering and Erosion
 Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Full file at
 Minerals: The Building Blocks of Rocks 2 Learning Objectives After reading, studying, and discussing the chapter, students should be able to: List the definitive characteristics that qualify certain Earth
Minerals: The Building Blocks of Rocks 2 Learning Objectives After reading, studying, and discussing the chapter, students should be able to: List the definitive characteristics that qualify certain Earth
Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Rocks Environmental Significance. Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3. Rocks Definition of a rock
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
MINERALS TAKE HOME QUIZ
 NAME 1. Which is an accurate statement about rocks? A) Rocks are located only in continental areas of the Earth. B) Rocks seldom undergo change. C) Most rocks contain fossils. D) Most rocks have several
NAME 1. Which is an accurate statement about rocks? A) Rocks are located only in continental areas of the Earth. B) Rocks seldom undergo change. C) Most rocks contain fossils. D) Most rocks have several
Layers of Earth - 3 distinct layers
 Clicker Question What is the source of the energy that drives most earthquakes and volcanoes? A. Sunlight B. Radioactive decay inside the earth C. Meteorite impacts D. Ocean tides E. None of the above
Clicker Question What is the source of the energy that drives most earthquakes and volcanoes? A. Sunlight B. Radioactive decay inside the earth C. Meteorite impacts D. Ocean tides E. None of the above
