Embedded-Polar-Group Bonded Phases for High Performance Liquid Chromatography
|
|
|
- Mervin Wilkerson
- 6 years ago
- Views:
Transcription
1 LCGC VLUME 9 NUMBE JUNE 00 Embedded-Polar-Group Bonded Phases for High Performance Liquid Chromatography In the past 0 years, embedded-polar-group bonded phases have become more popular with chromatographers. The characteristics of these phases are significantly different from traditional alkyl bonded phases, both with respect to chemical composition and chromatographic attributes. In this article, the authors chromatographically compare embedded-polar-group bonded phases with their purely alkyl counterparts. The authors describe first-generation, two-step and second-generation, one-step synthesis methodologies, and they report comparative retention mechanism studies for polar C8 and alkyl C8 phases. Embedded-polar-group phases differentiate themselves from their purely alkyl counterparts in three ways: improved peak shape for basic analytes, different selectivity, and stable retention in highly aqueous mobile phases. John E. Gara, Daniel P. Walsh, Charles H. Phoebe Jr., Bonnie A. Alden, Edouard S.P. Bouvier, Pamela C. Iraneta, Mark Capparella, and Thomas H. Walter Waters Corp., Maple Street, Milford, Massachusetts, 077 Address correspondence to J.E. Gara. The properties of reversed-phase high performance liquid chromatography (HPLC) column stationary phases have changed significantly since the inception of this analytical technique more than 0 years ago. The chemical and physical properties of early column packings bear little resemblance to those found in current state-ofthe-art materials. The primarily silica-based packings have gone from irregular to spherical in shape, from 0 m to as small as m in diameter, and from low to high purity with respect to metallic contaminants. These material changes have led to dramatic improvements in column efficiency, stability, reproducibility, and basic analyte peak shape. At the same time, bonded-phase chemistries have remained virtually unchanged. Although column developers have achieved improved surface densities, octadecyl (C8) and octyl (C8) phases generally are made from the same silanes used 0 years ago. In the early 990s, conventional C8 and C8 bonded phases continued to yield large tailing factors for basic analytes (). At that time, Nomura and co-workers () developed a novel type of bonded-phase methodology in which aminopropyl bonded phases were acylated to form an embedded polar amide functional group in the ligand s alkyl chain (see Figure ). For example, Buszewski and colleagues () reported the reaction of aminopropyl silica with acid chlorides, phenylsulfonyl chloride, and alkyl isocyanates. These and subsequent bonded phases had reduced silanol interactions with (c) Si Si Si Polar group Figure : General structures of monofunctional C8, trifunctional C8, and (c) embedded-polar-group bonded phases. alkyl.
2 LCGC VLUME 9 NUMBE JUNE 00 HPLC system was the same as described above. The mobile phase was : (v/v) 0 mm monobasic potassium phosphate (adjusted to ph 7.0 with dibasic potassium phosphate) methanol. The flow rate was.0 ml/min, and the temperature was. C. The detection wavelength was nm. We separated suprofen, ketoprofen, naproxen, tolmetin (sodium salt), fenoprofen (calcium salt), diclofenac (sodium salt), indomethacin, and ibuprofen (all from Sigma-Aldrich) on 0 mm. mm, - m d p Symmetry C 8 and Symmetry- Shield P 8 columns (Waters) as respective examples of conventional and embeddedpolar-group bonded phases. The HPLC system comprised a model LC separations module with column heater, a model 8 tunable absorbance detector, a model 7 UltraWisp autosampler, and a Millennium version. data-management system (all from Waters). The mobile phase was : (v/v) 0.% acetic acid methanol. The flow rate was.0 ml/min, and the temperature was C. The detection wavelength was nm. Adsorption thermodynamic study: We separated benzamide, aniline, m-toluidine, benzoic acid, salicylic acid, phenol, and benzyl alcohol (all from Aldrich); benzonitrile, o-toluamide, and benzophenone (all from Sigma-Aldrich); and acetophenone (J.T. Baker, Phillipsburg, New Jersey) on 0 mm.9 mm, - m d p SymmetryShield P 8 and Symmetry C 8 columns (Waters) as examples of embedded-polar-group and conventional C8 bonded phases, respectively. The HPLC system included an Alliance 90 XE separations module, a model 99 photodiode-array detector, and a Millennium version..0 datamanagement system (Waters). The column temperature was controlled using a model 90 XE column heater (Waters). The mobile phase was 80:0 (v/v) 0 mm monobasic potassium phosphate (adjusted to ph 7.0 with dibasic potassium phosphate) methanol. The flow rate was.0 ml/min, and the temperature was 0 C. The detection wavelength was nm. etention factor stability study in highly aqueous mobile phases: We separated sulfanilamide (Sigma-Aldrich) on 0 mm.9 mm, - m d p SymmetryShield P 8, SymmetryShield P 8, Symmetry C 8, and Symmetry C 8 columns (Waters) as examples of embedded-polar-group and conventional bonded phases. The HPLC system comprised a model 00E solvent-delivery system, a model 90E programmable multiwavelength detector, a model 7 Ultra- Wisp autosampler, and an ExpertEase databasic analytes (,). ther embedded polar functional groups such as ureas and carbamates were found to be similarly effective, and all three types of carbonyl phases now are commercially available. In this article, we will compare the chromatographic properties of embeddedcarbamate-group bonded phases with those of their purely alkyl counterparts. We expect that related amide, urea, and other embedded polar phases will behave in a similar manner, as long as analysts differentiate between the one- and two-step synthesis approaches (see below). Nonetheless, the chromatographic features described in this article are derived from a limited data set (column chemistry and test conditions) and should not be construed as universal for all column packings that contain embedded polar groups. As with any stationary phase, the utility of an embedded-polar-group bonded phase will depend upon the specifics of the separation problem. Experimental HPLC separations: We separated maleic acid (Aldrich Chemical Co., Milwaukee, Wisconsin) and toluamide and chlorpheniramine (maleate salt) (both from Sigma- Aldrich, St. Louis, Missouri) on a 0 mm. mm, - m d p SymmetryShield P 8 reversed-phase HPLC column (Waters Corp, Milford, Massachusetts) and on a 0 mm. mm, - m d p Supelcosil ABZ Plus reversed-phase HPLC column (Supelco, Bellefonte, Pennsylvania) as respective examples of second-generation, one-step and first-generation, two-step synthesis embedded-polar-group bonded phases. The HPLC system comprised an Alliance 90 XE separations module, a model 99 photodiode-array detector, and a Millennium version..0 datamanagement system (all from Waters) and a Neslab TE- circulating water bath (Neslab Instruments, Inc., Portsmouth, New Hampshire) for column temperature control. The mobile phase was 80:0 (v/v) 0 mm monobasic potassium phosphate (adjusted to ph.0 with phosphoric acid) and acetonitrile. The flow rate was.0 ml/min, and the temperature was. C. The detection wavelength was 0 nm. We separated uracil, propranolol (hydrochloride salt), and amitriptyline (hydrochloride salt) (all from Aldrich) and naphthalene, acenaphthene, and butyl paraben (all from Sigma-Aldrich) on 0 mm.9 mm, - m d p SymmetryShield P 8 and Symmetry C 8 columns (Waters) as respective examples of embedded-polargroup and conventional bonded phases. The management system (all from Waters). Column temperature was controlled using a Euramark Mistral column thermostat (Spark Holland, Mt. Prospect, Illinois). The mobile phases were 0 mm dibasic potassium phosphate (ph.0); :9 (v/v) methanol 0 mm dibasic potassium phosphate (ph.0); and 0:90 (v/v) methanol 0 mm dibasic potassium phosphate (ph.0). The flow rate was.0 ml/min, and the temperature was C. The detection wavelength was nm. Protocol: We equilibrated the columns for 0 min in methanol and then in 0:0 (v/v) methanol water. Later, we equilibrated the columns for 0 min in 0 0% organic mobile phase and obtained the sulfanilamide retention factor (k) before stopping the flow. We stopped the flow for h, and later resumed the flow in 0 0% organic mobile phase. We obtained the sulfanilamide retention factor after stopping the flow. The percentage decrease in retention factor was calculated using the following equation: Decrease in k 00 k before k after k before [] esults and Discussion Chromatographic differentiation of firstand second-generation phases: The first reported embedded-polar-group bonded phases were prepared by a two-step surface modification. In the first step, the native silica was bonded with an aminopropyl silane. In the second step, the amine groups were reacted with an acid chloride or isocyanate to form an amide or urea ( ). This firstgeneration methodology suffers from lessthan-complete conversion of the amine groups in the second surface reaction. As Figure shows, the resulting surface can have a mixture of derivatized and underivatized amine groups. These first-generation phases have the potential to retain analytes by both reversed-phase and anion-exchange mechanisms ( 8). The second-generation materials were prepared by a one-step surface modification in which the functional group was built into the silane (9). A single-surface reaction with the silane yields only one possible ligand structure with no possibility of anionexchange functionality. Gara and coworkers (0) showed that this approach provided excellent batch-to-batch reproducibility for a set of polar, basic, and nonpolar analytes. The absence of anion-exchange groups is an important advance with the secondgeneration materials. Figure compares a
3 LCGC VLUME 9 NUMBE JUNE 00 first- and a second-generation phase using a ph buffered mobile phase for the separation of maleic acid, toluamide, and the base chlorpheniramine. Under these conditions, the analytes were negatively charged, neutral, and positively charged, respectively. For the one-step phase, all three analytes were eluted with short retention times and good peak shape. For the two-step material, the neutral and positively charged analytes were eluted in a similar fashion, but negatively charged maleic acid was strongly retained with poor peak shape. We attributed the strong retention of maleic acid to ionexchange retention caused by residual amine groups on the surface. Thus, we recommend one-step materials over the older two-step materials for separations of negatively charged analytes. Improved peak shape for basic compounds: The most sensitive measurement of silanol interactions is achieved using highly basic probes with a ph 7 mobile phase (). At this ph, many of the residual silanols are in their ionized form (Si ), and the basic probes are completely protonated (BH ). The protonated bases interact with the ionized silanols by an ion-exchange mechanism, and the degree of tailing is a direct measure of silanol activity. As Figure illustrates, the U.S. Pharmacopeia (USP) tailing factor for amitriptyline was reduced almost 0% by substituting an embedded-polar-group C8 bonded phase for a conventional C8 bonded phase on silica (). This carbamate-bonded-phase substitution reduced silanol interactions with basic analytes on hybrid organic inorganic particles as well, in the form of a % decrease for amitriptyline s tailing factor. The diminished impact on the hybrid particles can be attributed to the inherently lower silanol concentrations generated by the methyl silicon groups on the surface (). Gara and colleagues () recently reported additional evidence to support reduced silanol interactions for embeddedpolar-group bonded phases in an experiment that measured the tailing factors of two strong bases propranolol and amitriptyline (both pk a s 9) as a function of ligand surface concentration. They found that the carbamate group reduced the interaction between bases and surface silanols in a ph 7 mobile phase, as indicated by a weak dependence of tailing factors on surface concentration. They observed a much stronger dependence for a conventional C8 bonded phase. Step : Aminopropylsilane bonding H Si NH H CH H CH Si NH H Si NH H H Step : Acylation Si CH H Si NH Cl NH Si CH H Si NH Mixture of amide and amine groups NH H H Step : Alkylcarbamate silane bonding H H Cl Si NH H H Si CH H Si H NH Single ligand on surface NH Figure : Examples of first- and second-generation approaches to the synthesis of embedded-polar-group bonded phases. alkyl.
4 8 LCGC VLUME 9 NUMBE JUNE 00 (c) H () Maleic acid pk a.8,. () Toluamide Time (min) Selectivity: Stationary phases bonded with embedded polar groups exhibit both retention and selectivity differences compared with their alkyl counterparts. Polar and basic analyte retention factors generally are reduced on embedded-polar-group phases, whereas nonpolar analytes are relatively unaffected (0). This effect is exemplified in Figure between the nonpolar analyte acenaphthene and the base amitriptyline. Figure illustrates a more complex selectivity change for the separation of a set of carboxylic-acid-substituted pharmaceuticals. The elution order changes for naproxen and talmetin and leads to better resolution on the embedded-polar-group bonded phase. Coeluted peaks for diclofenac and indomethacin were resolved by changing from the conventional C8 to the embedded-polar-group C8 column. Ibuprofen is eluted before diclofenac and indomethacin on the embedded phase. We have used adsorption thermodynamic studies to compare retention mechanisms on alkyl and embedded-polar-group phases (). As Figure shows, the log of the retention factor (k) for a series of substituted benzenes was plotted comparing C8 alkyl with C8 carbamate phases (). The slope of the plot is close to unity. Those eluites that fall on the line indicate similar retention energetics on both phases; however, a few eluites deviate from the regression line, which indicates the energetics behind selectivity are 0 8 Figure : Separation of an acid, neutral, and base mixture at low ph using embedded-polargroup bonded phases prepared using one- and two-step synthesis methods. Shown are structures of the analytes and chromatograms obtained using the one- and (c) two-step phases. NH Cl NH NH () Chlorpheniramine pk a.0, 9. different. Anything above the regression line indicates that the energetics of retention are greater on the alkylcarbamate phase; anything below the line indicates that retention selectivity is greater on the alkyl phase. The three compounds that are more strongly retained on the C8 carbamate phase are the hydrogen bond donors benzamide, aniline, and phenol. Note that addition of a methyl substituent lowers the corresponding compound s location; for example, benzamide toluamide, aniline toluidine, and benzophenone acetophenone. Benzoic acid falls below the line, but salicylic acid, with the additional hydroxy group, exhibits increased relative retention on the embedded-polar-group phase. Thus, selectivity differences between alkyl and embedded-polar-group bonded phases can be useful in method development. In many cases, when peaks are resolved unsatisfactorily on an alkyl phase, the embedded-polar-group counterpart might be substituted to achieve the desired separation with no mobile-phase adjustments. Why embedded-polar-group bonded phases have different chromatographic properties than their alkyl counterparts remains the subject of ongoing investigation. Hydrogen bond donors may interact through hydrogen bonding with the embedded polar groups, which would explain the increased retention of this class of compounds on embedded-polar-group phases compared with conventional phases (7). Accounting for the reduced retention and tailing of basic compounds on embedded-polar phases is more difficult. Buszewski and colleagues (8) showed that the presence of an embedded polar group does not reduce the acidity of residual silanols. An alternative explanation is that the presence of the embedded polar group may affect the composition of the mobile phase adsorbed in the surface layer. For conventional alkyl-bonded phases, the surface layer contains a higher concentration of the organic component than the bulk mobile phase (8). Felitsyn and Cantwell (9) recently reported data showing that when organic modifier is sorbed into a C8 bonded phase, the sorbent properties are altered considerably. The surface layer of an embedded-polar-group bonded phase should have a higher concentration of the aqueous component because of the embedded functional group s hydrogen bonding ability. Jaroniec (0) demonstrated this effect for amide bonded phases. The embedded-polar-group bonded phase sur-
5 0 LCGC VLUME 9 NUMBE JUNE 00 face layer should have a higher dielectric constant. As a result, the strength of ionic interactions between surface silanols and polar or basic compounds should be reduced and result in different selectivity and reduced tailing factors compared with the corresponding pure-alkyl-bonded phase (). We expect a better mechanistic understanding of these novel phases with additional investigations. Stable retention in highly aqueous mobile phases: Purely alkyl bonded phases can suffer to varying degrees from retention-time instability in 00% aqueous mobile phases (). ne cause of this instability is the poor wettability of alkyl surfaces by water (,). When the contact angle between the surface and the mobile phase is greater than 90, pressure must be applied to force the mobile phase into pores that contain most of the surface area (,). Several factors can contribute to this wettability problem, depending upon the particular column packing material. Factors can include the pore size of the base silica, the bonding density, and the type of ligand on the particle s surface (). For example, many of the newer alkyl C8 and C8 chromatographic packings have high bonding densities designed to improve peak shape for basic compounds and stability at high ph. However, the improvement in bonding density often can lead to retention-time instability in 00% aqueous mobile phases because of the highly hydrophobic nature of these phases. Purely alkyl phases with low bonding densities are less hydrophobic and can provide good retention-time stability in 00% aqueous mobile phases, albeit often with poor peak shape for bases. Embedded-polar-group bonded phases can provide a solution to this dilemma. ecent reports have shown that these phases provide stable and reproducible analyte retention times in 00% aqueous mobile phases (,7). Embedded-polar-group bonded phases may wet more easily for various reasons; certainly the hydrogenbonding ability of an embedded functional group with water could drop the contact angle between the surface and water to less than 90, at which water could penetrate the porous surface freely (,). To probe the difference between alkyl and embedded-polar-group bonded phases, Walter and co-workers () measured retention factor stability in 90, 9, and 00% aqueous mobile phase using a stop flow test. In this test, when the flow was stopped and the pressure was released from the column, they found that the mobile phase extruded from the pores of the purely alkyl phases because of their poor wettability by the mostly aqueous mobile phases. After restarting the flow, they observed that the mobile phase remained outside of the pores, and the particle s accessible surface area was greatly diminished. As a result, the retention factors decreased. As Figure 7 shows, the percentage decrease in retention factor for sulfanilamide approached 00% for the alkyl phases in changing from 90:0 (v/v) water methanol to 00% water. Embedded-polar-group phases were affected little across the three mobile phases most likely because the aqueous mobile phase stayed within the pores, held by hydrogen bonding even without pressure on the column., Time (min) Figure : Chromatographic separation of polar, basic, and nonpolar analytes using an embedded-polar-group C8 phase (upper chromatogram, USP tailing factor. for amitriptyline) and a conventional C8 stationary phase (lower chromatogram, USP tailing factor.9 for amitriptyline). Peaks: uracil, propranolol, butylparaben, naphthalene, amitriptyline, acenaphthene Time (min) Figure : Chromatographic separation of acidic analytes using conventional C8 and embedded-polar-group C8 stationary phases. Peaks: suprofen, ketoprofen, naproxen, tolmetin, fenoprofen, diclofenac, 7 indomethacin, 8 ibuprofen.
6 LCGC VLUME 9 NUMBE JUNE 00 Conclusion Embedded-polar-group bonded phases for HPLC were introduced during the past 0 years. These phases represent a major departure from traditional silane and bonding technology. In comparison to their purely alkyl counterparts, embedded-polar-group phases may provide utility in three areas: improved peak shape for basic analytes, different selectivity, and stable retention in highly aqueous mobile phases. esearchers have made progress in understanding the mechanism of this class of reversed-phase materials. We expect more insight as these materials are used increasingly to solve difficult separation problems. In k C8 carbamate 0 C()NH H NH H H H C H C()NH CH H H 7 NH 8 C()H H 9 CN H 0 C H C() H Acknowledgments We thank M. Dion,. Brennick, and M. Savaria of Waters Corp. for column packing. We would like to specially acknowledge U.D. Neue of Waters Corp., J.S. Petersen of GelTex Pharmaceuticals (Waltham, Massachusetts), and C.L. Niederländer of Sandoz AQ (Basel, Switzerland) for their pioneering work in the area of embedded-polar-group bonded phases. In k C8 eferences () D.V. McCalley, J. Chromatogr., 0 (99). () A. Nomura, J. Yamada, and K. Tsunoda, Anal. Sci., 09 (987). y.0x 0.9 r Figure : Log k/log k plot for substituted benzenes using embedded-polar-group C8 and conventional C8 stationary phases. Decrease in k after stopping flow (%) Alkyl Figure 7: Comparison of the decrease in retention factor for sulfanilamide under stop-flow conditions on conventional alkyl and embedded-polar-group bonded phases. 7 Mobile phases: % Aqueous 9% Aqueous 90% Aqueous C8 C8 C8 C8 Embedded polar group () B. Buszewski, J. Schmid, K. Albert, and E. Bayer, J. Chromatogr., 7 (99). () T.L. Ascah and B. Feibush, J. Chromatogr. 0, 7 9 (990). () J. Schmid, K. Albert, and E. Bayer, J. Chromatogr. 9, (99). () T. Czajkowska and M. Jaroniec, J. Liq. Chromatogr. el. Technol. 9, 89 8 (99). (7) T. Czajkowska, I. Hrabovsky, B. Buszewski,.K. Gilpin, and M. Jaroniec, J. Chromatogr. A 9, 7 (99). (8) B. Buszewski,.M. Gadzala-Kopciuch, M. Markuszewski, and. Kaliszan, Anal. Chem. 9, 77 8 (997). (9) U.D. Neue, C.L. Niederländer, and J. Petersen, U.S. patent number,7,7, 0 December 99. (0) J.E. Gara, B.A. Alden, T.H. Walter, J.S. Petersen, C.L. Niederländer, and U.D. Neue, Anal. Chem. 7, (99). () D.V. McCalley, J. Chromatogr. A 78, 9 79 (99). () J.E. Gara, D.P. Walsh, A.. Pelissey, B.A. Alden, C.A. Gendreau, P.C. Iraneta, and T.H. Walter, Investigation of C8 Bonding on Novel Hybrid rganic/inorganic Particles, paper presented at HPLC 99, Granada, Spain, 0 May June 999. () Y.-F. Cheng, T.W. Walter, Z. Lu, P. Iraneta, B.A. Alden, C. Gendreau, U.D. Neue, J.M. Grassi, J.L. Carmody, J.E. Gara, and.p. Fisk, LCGC 8(), 7 (000). () J.E. Gara, D.P. Walsh, B.A. Alden, P. Casellini, and T.H. Walter, Anal. Chem. 7, (999). () W. Melander, J. Stoveken, and Cs. Horváth, J. Chromatogr. 99, (980). () E.S.P. Bouvier, B.A. Alden, T.H. Walter, J.E. Gara, and U.D. Neue, Effect of Embedded Polar Groups on eversed-phase Chromatographic Behavior, paper presented at HPLC 98, St. Louis, Missouri, 8 May 998. (7) A. Sándi and L. Szepesy, J. Chromatogr. A 88, 7 (998). (8) B. Buszewski, M. Jaroniec, and.k. Gilpin, J. Chromatogr. A 8, 9 99 (99). (9) N. Felitsyn and F.F. Cantwell, Anal. Chem. 7, 0 08 (000). (0) M. Jaroniec, J. Chromatogr. A, 7 0 (99). () T.S. eid and.a. Henry, Am. Lab. (), 8 (999). () E.S.P. Bouvier,.M. Meirowitz, and U.D. Neue, The Effect of Contact Angle and Wetting on Performance in Solid Phase Extraction, paper presented at HPLC 97, Birmingham, United Kingdom, 7 June 997. () B. Janczuk, T. Bialopiotrowicz, and W. Wojcik, Colloids Surf. A, 9 0 (989). () U.D. Neue, HPLC Columns, Theory, Technology, and Practice (Wiley-VCH, New York, 997), pp () E.W. Washburn, Proc. Natl. Acad. Sci. 7,, (9). () T. Walter, P. Iraneta, and M. Capparella, bservations on the Wetting of eversed- Phase HPLC Packings, paper presented at HPLC 97, Birmingham, United Kingdom, 7 June 997. (7).D. Morrison and J.W. Dolan, LCGC 8(9), 9 99 (000).
Hybrid Organic Inorganic Particle Technology: Breaking Through Traditional Barriers of HPLC Separations
 6 LCGC VLUME 8 NUMBER NVEMBER 000 www.chromatographyonline.com Hybrid rganic Inorganic Particle Technology: Breaking Through Traditional Barriers of HPLC Separations Hybrid organic inorganic particles
6 LCGC VLUME 8 NUMBER NVEMBER 000 www.chromatographyonline.com Hybrid rganic Inorganic Particle Technology: Breaking Through Traditional Barriers of HPLC Separations Hybrid organic inorganic particles
OBSERVATIONS ON THE WETTING OF REVERSED-PHASE HPLC PACKINGS
 1997 Waters Corporation p. 1 980947 OBSERVATIONS ON THE WETTING OF REVERSED-PHASE HPLC PACKINGS T. Walter, P. Iraneta, and M. Capparella Waters Corporation, 34 Maple Street, Milford, MA 01757 USA Presented
1997 Waters Corporation p. 1 980947 OBSERVATIONS ON THE WETTING OF REVERSED-PHASE HPLC PACKINGS T. Walter, P. Iraneta, and M. Capparella Waters Corporation, 34 Maple Street, Milford, MA 01757 USA Presented
Preparative Chromatography with Improved Loadability
 05 LCGC RTH AMERICA VLUME 0 UMBER VEMBER 00 www.chromatographyonline.com Preparative Chromatography with Improved Loadability In combinatorial chemistry and drug development, as well as in the full-scale
05 LCGC RTH AMERICA VLUME 0 UMBER VEMBER 00 www.chromatographyonline.com Preparative Chromatography with Improved Loadability In combinatorial chemistry and drug development, as well as in the full-scale
The Retention Behavior of Reversed-Phase HPLC Columns with 100% Aqueous Mobile Phase
 9 LCGC NORTH AMERICA VOLUME NUMBER OCTOBER www.chromatographyonline.com The Retention Behavior of Reversed-Phase HPLC Columns with % Aqueous Mobile Phase The authors studied the retention behavior of reversed-phase
9 LCGC NORTH AMERICA VOLUME NUMBER OCTOBER www.chromatographyonline.com The Retention Behavior of Reversed-Phase HPLC Columns with % Aqueous Mobile Phase The authors studied the retention behavior of reversed-phase
The Effect of Contact Angle and Wetting on Performance in Solid Phase Extraction
 The Effect of Contact Angle and Wetting on Performance in Solid Phase Extraction Edouard S. P. Bouvier, Randy E. Meirowitz and Uwe D. Neue Waters Corporation, 34 Maple Street, Milford, MA 01757 USA Presented
The Effect of Contact Angle and Wetting on Performance in Solid Phase Extraction Edouard S. P. Bouvier, Randy E. Meirowitz and Uwe D. Neue Waters Corporation, 34 Maple Street, Milford, MA 01757 USA Presented
columns Acclaim Mixed-Mode WCX-1 for Separating Basic Molecules
 columns Acclaim Mixed-Mode WCX- for Separating Basic Molecules The Acclaim Mixed-Mode WCX- is a novel, high-efficiency, silica-based column specially designed for separating various basic analytes. This
columns Acclaim Mixed-Mode WCX- for Separating Basic Molecules The Acclaim Mixed-Mode WCX- is a novel, high-efficiency, silica-based column specially designed for separating various basic analytes. This
Hypersil BDS Columns TG 01-05
 TG 0-0 Hypersil BDS Columns Introduction Hypersil BDS columns have gained a reputation over the years as one of the most robust, reproducible and reliable HPLC column brands available. This Technical Guide
TG 0-0 Hypersil BDS Columns Introduction Hypersil BDS columns have gained a reputation over the years as one of the most robust, reproducible and reliable HPLC column brands available. This Technical Guide
ProntoSIL C18-EPS Reversed-Phase HPLC Columns
 ProntoSIL C8-EPS Reversed-Phase HPLC Columns Provides excellent separation of polar compounds Better peak shape for acids and bases Stabilized bonded phase for rugged, robust HPLC methods More retentive
ProntoSIL C8-EPS Reversed-Phase HPLC Columns Provides excellent separation of polar compounds Better peak shape for acids and bases Stabilized bonded phase for rugged, robust HPLC methods More retentive
Packings for HPLC. Packings for HPLC
 Summary of packings for HPLC In analytical HPLC, packings with particle sizes of 3 to 10 µm are preferred. For preparative separation tasks, also particles with diameters larger than 10 µm are applied.
Summary of packings for HPLC In analytical HPLC, packings with particle sizes of 3 to 10 µm are preferred. For preparative separation tasks, also particles with diameters larger than 10 µm are applied.
Comparison of different aqueous mobile phase HPLC techniques
 June 009 ewsletter Pharmaceutical Analysis: What is Your Problem? SIELC Technologies, Inc., Prospect Heights, IL 0070 Pharmaceutical analysis involves liquid chromatography of various compounds, from active
June 009 ewsletter Pharmaceutical Analysis: What is Your Problem? SIELC Technologies, Inc., Prospect Heights, IL 0070 Pharmaceutical analysis involves liquid chromatography of various compounds, from active
penta-hilic UHPLC COLUMNS
 penta-hilic UHPLC COLUMNS penta-hilic Highly retentive, proprietary penta-hydroxy-ligand Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
penta-hilic UHPLC COLUMNS penta-hilic Highly retentive, proprietary penta-hydroxy-ligand Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
Reversed Phase Solvents
 Part 1. General Chromatographic Theory Part 2. verview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use Reversed Phase Solvents 2 Solvents for RP Chromatography
Part 1. General Chromatographic Theory Part 2. verview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use Reversed Phase Solvents 2 Solvents for RP Chromatography
A Highly Stable Alkyl Amide Silica-Based Column Packing for Reversed-Phase HPLC of Polar and Ionizable Compounds
 A Highly Stable Alkyl Amide Silica-Based Column Packing for Reversed-Phase HPLC of Polar and Ionizable Compounds Researchers have designed a new polar-linked, reversed-phase column for separating polar,
A Highly Stable Alkyl Amide Silica-Based Column Packing for Reversed-Phase HPLC of Polar and Ionizable Compounds Researchers have designed a new polar-linked, reversed-phase column for separating polar,
penta-hilic UHPLC COLUMNS
 penta-hilic UHPLC COLUMNS Highly retentive, proprietary penta-hydroxy-ligand penta-hilic Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
penta-hilic UHPLC COLUMNS Highly retentive, proprietary penta-hydroxy-ligand penta-hilic Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
Agilent s New Weak Anion Exchange (WAX) Solid Phase Extraction Cartridges: SampliQ WAX
 Agilent s New Weak Anion Exchange (WAX) Solid Phase Extraction Cartridges: SampliQ WAX Technical Note Agilent s SampliQ WAX provides Applications for strongly acidic, acidic and neutral compounds Excellent
Agilent s New Weak Anion Exchange (WAX) Solid Phase Extraction Cartridges: SampliQ WAX Technical Note Agilent s SampliQ WAX provides Applications for strongly acidic, acidic and neutral compounds Excellent
Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity
 Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20544 Key Words Hydrophilic, HILIC,
Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20544 Key Words Hydrophilic, HILIC,
State-of-the-art C18 HPLC Columns
 An HPLC GL Sciences Newest and Most Advanced ODS Phase-New For 00 State-of-the-art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with 00%
An HPLC GL Sciences Newest and Most Advanced ODS Phase-New For 00 State-of-the-art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with 00%
LC and LC/MS Column Selection Flow Chart
 LC and LC/MS Column Selection Flow Chart To use the column selection diagram below, simply follow the path for your analyte and mobile phase. At the far right, follow your final column selection to the
LC and LC/MS Column Selection Flow Chart To use the column selection diagram below, simply follow the path for your analyte and mobile phase. At the far right, follow your final column selection to the
Chemistry Instrumental Analysis Lecture 28. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
Chromatographic Analysis
 Chromatographic Analysis Distribution of Analytes between Phases An analyte is in equilibrium between the two phases [S 1 ] [S 2 ] (in phase 1) (in phase 2) AS [S2 ] K 2 A S [S1 ] 1 AS, A 1 S Activity
Chromatographic Analysis Distribution of Analytes between Phases An analyte is in equilibrium between the two phases [S 1 ] [S 2 ] (in phase 1) (in phase 2) AS [S2 ] K 2 A S [S1 ] 1 AS, A 1 S Activity
P R O D U C T B U L L E T I N. Fused-Core particle technology for hyper-fast and super-rugged HPLC columns
 P R O D U C T B U L L E T I N Fused-Core particle technology for hyper-fast and super-rugged HPLC columns HALO column packings are not made the typical way. Instead, the particles packed into HALO columns
P R O D U C T B U L L E T I N Fused-Core particle technology for hyper-fast and super-rugged HPLC columns HALO column packings are not made the typical way. Instead, the particles packed into HALO columns
Alltech Alltima HP Introduction
 Alltech Introduction High-Stability, High-Purity, High-Performance, Low-Bleed Columns for Demanding Applications Better Peak Symmetry high-purity silica eliminates peak tailing problems Long Column Life
Alltech Introduction High-Stability, High-Purity, High-Performance, Low-Bleed Columns for Demanding Applications Better Peak Symmetry high-purity silica eliminates peak tailing problems Long Column Life
Inertsil ODS-EP Technical Information
 Technical Information Advantages of The selectivity is completely different from those of conventional columns such as ODS column due to its specific polar group in the stationary phase. Durability can
Technical Information Advantages of The selectivity is completely different from those of conventional columns such as ODS column due to its specific polar group in the stationary phase. Durability can
STUDY OF LOADABILITY AND SELECTIVITY OF PHARMACEUTICAL COMPOUNDS ON RPLC COLUMNS
 STUDY OF LOADABILITY AND SELECTIVITY OF PHARMACEUTICAL COMPOUNDS ON RPLC COLUMNS Fang Xia, Jie Y. Cavanaugh, Uwe Neue, Jeffrey R. Mazzeo, Diane M. Diehl Waters Corporation, Milford, MA USA OVERVIEW- INTRODUCTION-
STUDY OF LOADABILITY AND SELECTIVITY OF PHARMACEUTICAL COMPOUNDS ON RPLC COLUMNS Fang Xia, Jie Y. Cavanaugh, Uwe Neue, Jeffrey R. Mazzeo, Diane M. Diehl Waters Corporation, Milford, MA USA OVERVIEW- INTRODUCTION-
ANALYSIS OF BASIC COMPOUNDS
 COMPARISON OF Fully AND Superficially POROUS PARTICLE COLUMNS FOR THE ANALYSIS OF BASIC COMPOUNDS Kenneth J. Fountain, Jane Xu, Zhe Yin, Pamela C. Iraneta, Diane M. Diehl Waters Corporation GOAL Compare
COMPARISON OF Fully AND Superficially POROUS PARTICLE COLUMNS FOR THE ANALYSIS OF BASIC COMPOUNDS Kenneth J. Fountain, Jane Xu, Zhe Yin, Pamela C. Iraneta, Diane M. Diehl Waters Corporation GOAL Compare
Acclaim Mixed-Mode WCX-1
 Acclaim Mixed-Mode WCX-1 Product Manual for the Acclaim Mixed-Mode WCX-1 Column Page 1 of 28 PRODUCT MANUAL for the Acclaim Mixed-Mode WCX-1 Columns 4.6 x 150 mm, P/N (068353) 4.6 x 250 mm, P/N (068352)
Acclaim Mixed-Mode WCX-1 Product Manual for the Acclaim Mixed-Mode WCX-1 Column Page 1 of 28 PRODUCT MANUAL for the Acclaim Mixed-Mode WCX-1 Columns 4.6 x 150 mm, P/N (068353) 4.6 x 250 mm, P/N (068352)
New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns
 New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns have: the highest plate number versus any other 5-micron
New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns have: the highest plate number versus any other 5-micron
and water enriched layer at the stationary phase particle-eluent interface [9,11].
![and water enriched layer at the stationary phase particle-eluent interface [9,11]. and water enriched layer at the stationary phase particle-eluent interface [9,11].](/thumbs/87/96223751.jpg) 8 A Simple, Generally Applicable HILIC Method Development Platform Based Upon Selectivity by Alan P McKeown, Advanced Chromatography Technologies Ltd, 1 Berry Street, Aberdeen, Scotland AB25 1HF, UK amckeown@ace-hplc.com
8 A Simple, Generally Applicable HILIC Method Development Platform Based Upon Selectivity by Alan P McKeown, Advanced Chromatography Technologies Ltd, 1 Berry Street, Aberdeen, Scotland AB25 1HF, UK amckeown@ace-hplc.com
A C18 Silica Column With Exceptional Temperature and ph Stability
 A C18 lica Column With Exceptional Temperature and ph Stability Brian A. Jones 1, Stephanie J. Marin 1, Jody Clark 1, Nathan L. Porter 1, J. Andreas Lippert 2, and Todd M. Johnson 2 1. Selerity Technologies,
A C18 lica Column With Exceptional Temperature and ph Stability Brian A. Jones 1, Stephanie J. Marin 1, Jody Clark 1, Nathan L. Porter 1, J. Andreas Lippert 2, and Todd M. Johnson 2 1. Selerity Technologies,
8. Methods in Developing Mobile Phase Condition for C18 Column
 I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
Analysis - HPLC A.136. Primesep 5 µm columns B.136
 Primesep 5 µm columns Primesep columns feature double functionality of the bonding i.e : alkyl chain with anionic or cationic group, chelating group. This feature creates unique selectivities when using
Primesep 5 µm columns Primesep columns feature double functionality of the bonding i.e : alkyl chain with anionic or cationic group, chelating group. This feature creates unique selectivities when using
Basic Principles for Purification Using Supercritical Fluid Chromatography
 Basic Principles for Purification Using Supercritical Fluid Chromatography Jo-Ann M. Jablonski, Christopher J. Hudalla, Kenneth J. Fountain, Steven M. Collier, and Damian Morrison Waters Corporation, Milford,
Basic Principles for Purification Using Supercritical Fluid Chromatography Jo-Ann M. Jablonski, Christopher J. Hudalla, Kenneth J. Fountain, Steven M. Collier, and Damian Morrison Waters Corporation, Milford,
Method Development in Solid Phase Extraction using Non-Polar ISOLUTE SPE Columns for the Extraction of Aqueous Samples
 Technical Note 101 Method Development in Solid Phase Extraction using Non-Polar ISOLUTE SPE Columns for the Extraction of Aqueous Samples This technical note includes by specific information on the extraction
Technical Note 101 Method Development in Solid Phase Extraction using Non-Polar ISOLUTE SPE Columns for the Extraction of Aqueous Samples This technical note includes by specific information on the extraction
HPLC Column Classification
 HPLC Column Classification Introduction 1978 USP XIX Fourth supplement : L1 designation for C18 column 1980 USP XX : 7 columns were classified -Classified HPLC column to 56 descriptions -More than 220
HPLC Column Classification Introduction 1978 USP XIX Fourth supplement : L1 designation for C18 column 1980 USP XX : 7 columns were classified -Classified HPLC column to 56 descriptions -More than 220
Kromasil. for your analytical HPLC. Kromasil. The way to peak performance in liquid chromatography
 Kromasil for your analytical HPLC Kromasil The way to peak performance in liquid chromatography Kromasil is known, worldwide, for its high performance and excellent total economy in preparative and industrial
Kromasil for your analytical HPLC Kromasil The way to peak performance in liquid chromatography Kromasil is known, worldwide, for its high performance and excellent total economy in preparative and industrial
William E. Barber, Ph.D. Applications Chemist October
 William E. Barber, Ph.D. Applications Chemist ctober 0 00 Secrets of Good Peak Shape in HPLC Time: :00 p.m. CET Telephone Number: + 44 0 76 088 Chairperson: John Vis The Secrets of Good Peak Shape in HPLC
William E. Barber, Ph.D. Applications Chemist ctober 0 00 Secrets of Good Peak Shape in HPLC Time: :00 p.m. CET Telephone Number: + 44 0 76 088 Chairperson: John Vis The Secrets of Good Peak Shape in HPLC
Improve Peak Shape and Productivity in HPLC Analysis of Pharmaceutical Compounds with Eclipse Plus C8 Columns Application
 Improve Peak Shape and Productivity in HPLC Analysis of Pharmaceutical Compounds with Eclipse Plus C8 Columns Application Pharmaceuticals Authors John W. Henderson Jr., Nona Martone, and Cliff Woodward
Improve Peak Shape and Productivity in HPLC Analysis of Pharmaceutical Compounds with Eclipse Plus C8 Columns Application Pharmaceuticals Authors John W. Henderson Jr., Nona Martone, and Cliff Woodward
for Acclaim Trinity TM P1
 for Acclaim Trinity TM P Product Manual for Acclaim TRINITY P Columns Page of 4 Product Manual for Acclaim Trinity TM P Columns 07556 Acclaim Trinity P, 3µm, 4.6 x 00mm 07556 Acclaim Trinity P, 3µm, 4.6
for Acclaim Trinity TM P Product Manual for Acclaim TRINITY P Columns Page of 4 Product Manual for Acclaim Trinity TM P Columns 07556 Acclaim Trinity P, 3µm, 4.6 x 00mm 07556 Acclaim Trinity P, 3µm, 4.6
Ch.28 HPLC. Basic types of Liquid Chromatography Partition (LLC) Adsorption (LSC) Ion Exchange (IC) Size Exclusion (SEC or Gel Chromatography)
 Ch.28 HPLC 28.1 Basic types of Liquid Chromatography Partition (LLC) Adsorption (LSC) Ion Exchange (IC) Size Exclusion (SEC or Gel Chromatography) High Performance (Pressure) LC Glass column st.steel (high
Ch.28 HPLC 28.1 Basic types of Liquid Chromatography Partition (LLC) Adsorption (LSC) Ion Exchange (IC) Size Exclusion (SEC or Gel Chromatography) High Performance (Pressure) LC Glass column st.steel (high
Important Guidelines for Optimizing Speed and Sensitivity in Small-Molecule LC UV and LC MS
 Página 1 de 6 Important Guidelines for Optimizing Speed and Sensitivity in Small-Molecule LC UV and LC MS May 1, 2005 By: Richard A. Henry, David S. Bell LCGC North America The task of developing a new
Página 1 de 6 Important Guidelines for Optimizing Speed and Sensitivity in Small-Molecule LC UV and LC MS May 1, 2005 By: Richard A. Henry, David S. Bell LCGC North America The task of developing a new
Shodex TM ODP2 HP series columns
 HPLC Columns Shodex TM ODP2 HP series columns Better retention of highly polar substances Technical notebook No. 6 Contents 1. Introduction 1-1. Specifications 1-2. Eluent Compatibility of ODP2 HP Series
HPLC Columns Shodex TM ODP2 HP series columns Better retention of highly polar substances Technical notebook No. 6 Contents 1. Introduction 1-1. Specifications 1-2. Eluent Compatibility of ODP2 HP Series
for Acclaim Mixed-Mode WCX-1
 for Acclaim Mixed-Mode WCX- Product Manual for the Acclaim Mixed-Mode WCX- Column Page of 8 Product Manual for Acclaim Mixed-Mode WCX- Columns Acclaim Mixed-Mode WCX-, 3µm, Analytical column, 3.0 x 50mm
for Acclaim Mixed-Mode WCX- Product Manual for the Acclaim Mixed-Mode WCX- Column Page of 8 Product Manual for Acclaim Mixed-Mode WCX- Columns Acclaim Mixed-Mode WCX-, 3µm, Analytical column, 3.0 x 50mm
Polymer Coated Titania for Analytical and Preparative Reversed-Phase Chromatography
 Polymer Coated tania for Analytical and Preparative Reversed-Phase Chromatography EAS 200 BIGWE YA 1, CLAYT V. MCEFF 1, JCHE WIKLER 2 1 ZirChrom Separations, Inc., 617 Pierce Street, Anoka, M 303 2 Sachtleben,
Polymer Coated tania for Analytical and Preparative Reversed-Phase Chromatography EAS 200 BIGWE YA 1, CLAYT V. MCEFF 1, JCHE WIKLER 2 1 ZirChrom Separations, Inc., 617 Pierce Street, Anoka, M 303 2 Sachtleben,
Tracer Excel TRACER EXCEL ODS-A. Total deactivation. Maximum Stability
 Tracer Excel TRACER EXCEL is a range of totally new packings that employ the most advanced procedures of synthesis and chemical functionalization, resulting in some column packings that completely surpass
Tracer Excel TRACER EXCEL is a range of totally new packings that employ the most advanced procedures of synthesis and chemical functionalization, resulting in some column packings that completely surpass
Choosing Columns and Conditions for the Best Peak Shape
 Choosing Columns and Conditions for the Best Peak Shape What is Good Peak Shape and Why is it Important? Good peak shape can be defined as a symmetrical or gaussian peak and poor peak shape can include
Choosing Columns and Conditions for the Best Peak Shape What is Good Peak Shape and Why is it Important? Good peak shape can be defined as a symmetrical or gaussian peak and poor peak shape can include
Reversed Phase Chromatography: Mobile phase Considerations
 Reversed Phase: Mobile Phase Considerations Dr. www.forumsci.co.il/hplc Example of a Reversed Phase Method with UV Detection 1 Chromatographic Process B+A Mobile phase Elution through the Column-movie
Reversed Phase: Mobile Phase Considerations Dr. www.forumsci.co.il/hplc Example of a Reversed Phase Method with UV Detection 1 Chromatographic Process B+A Mobile phase Elution through the Column-movie
Overview Applications of NUCLEODUR HPLC Phases
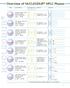 verview Applications of HPLC Phases Phase Specification Characteristics* Stability Structure A B C octadecyl phase, high density coating multi-endcapping 8% C USP L ph stability -, suitable for LC/MS (Si-
verview Applications of HPLC Phases Phase Specification Characteristics* Stability Structure A B C octadecyl phase, high density coating multi-endcapping 8% C USP L ph stability -, suitable for LC/MS (Si-
Silanol. Groups Liquid Chromatography
 Abstract umber: 0-8P Study of Secondary Interaction Based on Residual lanol Groups for Reversed-Phase Liquid Chromatography II orikazu agae Ph.D, Kouji Yamamoto, Chiaki Kadota Chromaik Technologies Inc.
Abstract umber: 0-8P Study of Secondary Interaction Based on Residual lanol Groups for Reversed-Phase Liquid Chromatography II orikazu agae Ph.D, Kouji Yamamoto, Chiaki Kadota Chromaik Technologies Inc.
Cation Exchange HPLC Columns
 Cation Exchange HPLC Columns Hamilton offers seven polymeric packing materials for cation exchange separations. Type Recommended Application(s) PRP-X00 PRP-X00 PRP-X800 HC-0 HC-7 Ca + HC-7 H + HC-7 Pb
Cation Exchange HPLC Columns Hamilton offers seven polymeric packing materials for cation exchange separations. Type Recommended Application(s) PRP-X00 PRP-X00 PRP-X800 HC-0 HC-7 Ca + HC-7 H + HC-7 Pb
Fast Separation of Vastly Different Compounds by Isocratic HPLC
 Fast Separation of Vastly Different Compounds by Isocratic HPLC Aimee N. Heyrman and Yury Zelechonok Resolution Systems, Inc. 590 E. 32nd St. Holland. MI, 49423 SIELC Technologies, 15 E. Palatine Rd, Suite
Fast Separation of Vastly Different Compounds by Isocratic HPLC Aimee N. Heyrman and Yury Zelechonok Resolution Systems, Inc. 590 E. 32nd St. Holland. MI, 49423 SIELC Technologies, 15 E. Palatine Rd, Suite
Polymer Coated Titania for Analytical and Preparative Reversed-Phase Chromatography
 Polymer Coated tania for Analytical and Preparative Reversed-Phase Chromatography Pittcon 2005 CLAYT V. MCEFF 1, BIGWE YA 1, JCHE WIKLER 2 1 ZirChrom Separations, Inc., 617 Pierce Street, Anoka, M 55303
Polymer Coated tania for Analytical and Preparative Reversed-Phase Chromatography Pittcon 2005 CLAYT V. MCEFF 1, BIGWE YA 1, JCHE WIKLER 2 1 ZirChrom Separations, Inc., 617 Pierce Street, Anoka, M 55303
Sunniest C18 Sunniest RP-AQUA Sunniest C8 Patent pending
 Patent pending Website : www.chromtech.net.au E-mail : info@chromtech.net.au telno : 0 97 0... in AUSTRALIA A Novel Bonding Technique (patent pending) An unique trifunctional silyl-reagent was developed
Patent pending Website : www.chromtech.net.au E-mail : info@chromtech.net.au telno : 0 97 0... in AUSTRALIA A Novel Bonding Technique (patent pending) An unique trifunctional silyl-reagent was developed
Technical Guide Thermo Scientific Syncronis HPLC Columns
 Technical Guide Thermo Scientific Syncronis HPLC Columns Consistent, predictable separations, Column after column, time after time Thermo Scientific Syncronis Columns When developing a new method, one
Technical Guide Thermo Scientific Syncronis HPLC Columns Consistent, predictable separations, Column after column, time after time Thermo Scientific Syncronis Columns When developing a new method, one
RP C18 column with feature of a silanol group
 RP C8 column with feature of a silanol group lanol Activity Controlled C8 Column Sunrise ctacosyl C8) Sunrise ctadecyl C8) Sunrise ctadecyl-sac C8-SAC has an interaction of silanol groups ChromaNik Sunrise
RP C8 column with feature of a silanol group lanol Activity Controlled C8 Column Sunrise ctacosyl C8) Sunrise ctadecyl C8) Sunrise ctadecyl-sac C8-SAC has an interaction of silanol groups ChromaNik Sunrise
HICHROM. Chromatography Columns and Supplies. LC COLUMNS HALO and HALO-5. Catalogue 9. Hichrom Limited
 HICHROM Chromatography Columns and Supplies LC COLUMNS HALO and HALO- Catalogue 9 The Markham Centre, Station Road Theale, Reading, Berks, RG PE, UK Tel: + (0)8 90 0 Fax: + (0)8 9 8 Email: sales@hichrom.co.uk
HICHROM Chromatography Columns and Supplies LC COLUMNS HALO and HALO- Catalogue 9 The Markham Centre, Station Road Theale, Reading, Berks, RG PE, UK Tel: + (0)8 90 0 Fax: + (0)8 9 8 Email: sales@hichrom.co.uk
viridis Columns Bringing a New Dimension to SFC Combining state-of-the-art media manufacturing with industry leading column technology, Viridis
 [ viridis columns ] viridis Columns Bringing a New Dimension to SFC Combining state-of-the-art media manufacturing with industry leading column technology, Viridis Supercritical Fluid Chromatography (SFC)
[ viridis columns ] viridis Columns Bringing a New Dimension to SFC Combining state-of-the-art media manufacturing with industry leading column technology, Viridis Supercritical Fluid Chromatography (SFC)
Orosil HPLC Columns. OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph.
 Orosil OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph. Excellent organic base selectivity with very low asymmetry values Compatible
Orosil OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph. Excellent organic base selectivity with very low asymmetry values Compatible
Zirconia: the Ideal Substrate for Ion-Exchange LC and LC-MS
 Zirconia: the Ideal Substrate for Ion-Exchange LC and LC-MS EAS 2005 Bingwen Yan 1, Clayton V. McNeff 1, Richard A. Henry 2, and David S. Bell 2 1 ZirChrom Separations, Inc., 617 Pierce Street, Anoka,
Zirconia: the Ideal Substrate for Ion-Exchange LC and LC-MS EAS 2005 Bingwen Yan 1, Clayton V. McNeff 1, Richard A. Henry 2, and David S. Bell 2 1 ZirChrom Separations, Inc., 617 Pierce Street, Anoka,
Zirconia-Based Phases as a Powerful Complement to Silica-Based Phases for LC and LC-MS under Non-extreme Mobile Phase Conditions
 Zirconia-Based Phases as a Powerful Complement to Silica-Based Phases for LC and LC-MS under on-extreme Mobile Phase Conditions Richard A. Henry, Shawn R. Wyatt, Carmen T. Santasania and David S. Bell
Zirconia-Based Phases as a Powerful Complement to Silica-Based Phases for LC and LC-MS under on-extreme Mobile Phase Conditions Richard A. Henry, Shawn R. Wyatt, Carmen T. Santasania and David S. Bell
LC Columns with Liquid Separation Cell Technology
 Obelisc LC Columns with Liquid Separation Cell Technology Obelisc HPLC Columns - Liquid Separation Cell Technology Introduction Obelisc HPLC columns are the latest, innovative columns from SIELC Technologies,
Obelisc LC Columns with Liquid Separation Cell Technology Obelisc HPLC Columns - Liquid Separation Cell Technology Introduction Obelisc HPLC columns are the latest, innovative columns from SIELC Technologies,
column If you would like a subscription, or more information, please contact your nearest Waters Office.
 column Waters A Reprint from Spring 99 If you would like a subscription, or more information, please contact your nearest Waters Office. Check the Waters website for up-to-date information: http://www.waters.com
column Waters A Reprint from Spring 99 If you would like a subscription, or more information, please contact your nearest Waters Office. Check the Waters website for up-to-date information: http://www.waters.com
Performance evaluation of the Agilent 1290 Infinity 2D-LC Solution for comprehensive two-dimensional liquid chromatography
 Performance evaluation of the Agilent 1290 Infinity 2D-LC Solution for comprehensive two-dimensional liquid chromatography Technical Overview 2D-LC Conventional 1D-LC Abstract This Technical Overview presents
Performance evaluation of the Agilent 1290 Infinity 2D-LC Solution for comprehensive two-dimensional liquid chromatography Technical Overview 2D-LC Conventional 1D-LC Abstract This Technical Overview presents
UPLC Method Development and Validation
 UPLC Method Development and Validation Rev. 2 2008 Waters Corporation Challenges of Method Development Methods are developed throughout the drug development process Samples vary in complexity Redundancy
UPLC Method Development and Validation Rev. 2 2008 Waters Corporation Challenges of Method Development Methods are developed throughout the drug development process Samples vary in complexity Redundancy
Certificate of Analysis
 National Institute of Standards & Technology Certificate of Analysis Standard Reference Material 870 Column Performance Test Mixture for Liquid Chromatography This Standard Reference Material (SRM) is
National Institute of Standards & Technology Certificate of Analysis Standard Reference Material 870 Column Performance Test Mixture for Liquid Chromatography This Standard Reference Material (SRM) is
Epic Aromatic Selectivity
 Epic Aromatic Selectivity HPLC Columns p h a r m a c e u t i c a l e n v i r o n m e n t a l c h e m i c a l b i o c h e m i c a l s e p a r a t i o n & p u r i f i c a t i o n ES Industries 701 S. Route
Epic Aromatic Selectivity HPLC Columns p h a r m a c e u t i c a l e n v i r o n m e n t a l c h e m i c a l b i o c h e m i c a l s e p a r a t i o n & p u r i f i c a t i o n ES Industries 701 S. Route
Synthesis and Use of a New Covalently Bonded C18 Modified Carbon Clad Microporous Zirconia for Fast High Temperature Separations
 Synthesis and Use of a New Covalently Bonded C18 Modified Carbon Clad Microporous Zirconia for Fast High Temperature Separations by Peter W. Carr, Clayton V. McNeff, Dwight R. Stoll, Danielle R. Hawker,
Synthesis and Use of a New Covalently Bonded C18 Modified Carbon Clad Microporous Zirconia for Fast High Temperature Separations by Peter W. Carr, Clayton V. McNeff, Dwight R. Stoll, Danielle R. Hawker,
VALIDATION OF A UPLC METHOD FOR A BENZOCAINE, BUTAMBEN, AND TETRACAINE HYDROCHLORIDE TOPICAL SOLUTION
 VALIDATION OF A UPLC METHOD FOR A BENZOCAINE, BUTAMBEN, AND TETRACAINE HYDROCHLORIDE TOPICAL SOLUTION Andrew J. Aubin and Tanya L. Jenkins Waters Corporation, Milford, MA, USA INTRODUCTION Benzocaine (4-Aminobenzoic
VALIDATION OF A UPLC METHOD FOR A BENZOCAINE, BUTAMBEN, AND TETRACAINE HYDROCHLORIDE TOPICAL SOLUTION Andrew J. Aubin and Tanya L. Jenkins Waters Corporation, Milford, MA, USA INTRODUCTION Benzocaine (4-Aminobenzoic
Novel LC/MS-Compatible Stationary Phase with Polar Selectivity
 Novel LC/MS-Compatible Stationary Phase with Polar Selectivity Carmen T. Santasania and David S. Bell Supelco 595 N. Harrison Rd., Bellefonte, PA 16823 T403168 GJU Introduction Stationary phases based
Novel LC/MS-Compatible Stationary Phase with Polar Selectivity Carmen T. Santasania and David S. Bell Supelco 595 N. Harrison Rd., Bellefonte, PA 16823 T403168 GJU Introduction Stationary phases based
Trends in Method Development for HPLC and UHPLC... the movement toward using solid-core particles
 Trends in Method Development for HPLC and UHPLC... the movement toward using solid-core particles Richard A. Henry, Separation Technology Consultant On behalf of: Supelco/Sigma-Aldrich, Bellefonte, USA
Trends in Method Development for HPLC and UHPLC... the movement toward using solid-core particles Richard A. Henry, Separation Technology Consultant On behalf of: Supelco/Sigma-Aldrich, Bellefonte, USA
HILIC Method Development in a Few Simple Steps
 HILIC Method Development in a Few Simple Steps Monica Dolci, Luisa Pereira, Dafydd Milton and Tony Edge Thermo Fisher Scientific, Runcorn, Cheshire, UK Overview This poster presents a systematic approach
HILIC Method Development in a Few Simple Steps Monica Dolci, Luisa Pereira, Dafydd Milton and Tony Edge Thermo Fisher Scientific, Runcorn, Cheshire, UK Overview This poster presents a systematic approach
Mechanisms of retention in HPLC
 Mechanisms of retention in HPLC María Celia García-Álvarez-Coque Department of Analytical Chemistry University of Valencia Valencia, Spain https://sites.google.com/site/fuschrom/ 1 Part 3 Mechanisms of
Mechanisms of retention in HPLC María Celia García-Álvarez-Coque Department of Analytical Chemistry University of Valencia Valencia, Spain https://sites.google.com/site/fuschrom/ 1 Part 3 Mechanisms of
Fractionation of Acidic, Basic, and Neutral Drugs from Plasma with an SPE Mixed Mode Strong Cation Exchange Polymeric Resin (Agilent SampliQ SCX)
 Fractionation of Acidic, Basic, and Neutral Drugs from Plasma with an SPE Mixed Mode Strong Cation Exchange Polymeric Resin (Agilent SampliQ SCX) Application Note Forensic Toxicology Authors Bellah. Pule,
Fractionation of Acidic, Basic, and Neutral Drugs from Plasma with an SPE Mixed Mode Strong Cation Exchange Polymeric Resin (Agilent SampliQ SCX) Application Note Forensic Toxicology Authors Bellah. Pule,
ACE. For increased polar retention and alternative selectivity. Alternative selectivity for method development
 ACE C8-Amide For increased polar retention and alternative selectivity Alternative selectivity for method development Improved separations with polar, acidic, basic and phenolic compounds High efficiency
ACE C8-Amide For increased polar retention and alternative selectivity Alternative selectivity for method development Improved separations with polar, acidic, basic and phenolic compounds High efficiency
HPLC. High Performance Liquid Chromatography (HPLC) Harris Chapter 25
 High Performance Liquid Chromatography (HPLC) Harris Chapter 25 12/1/2005 Chem 253 - Chapter 25 1 HPLC Separation of nonvolatile or thermally unstable compounds. If the analyte/sample can be found to be
High Performance Liquid Chromatography (HPLC) Harris Chapter 25 12/1/2005 Chem 253 - Chapter 25 1 HPLC Separation of nonvolatile or thermally unstable compounds. If the analyte/sample can be found to be
Analysis of Metals, Halides, and Inorganic Ions Using Hydrophilic Interaction Chromatography
 Application Note Inorganic Ions, Water Testing, Minerals, Metals, Basic Chemicals Analysis of Metals, Halides, and Inorganic Ions Using Hydrophilic Interaction Chromatography Authors Anne Mack, Adam Bivens
Application Note Inorganic Ions, Water Testing, Minerals, Metals, Basic Chemicals Analysis of Metals, Halides, and Inorganic Ions Using Hydrophilic Interaction Chromatography Authors Anne Mack, Adam Bivens
Thermo Scientific Accucore XL HPLC Columns. Technical Manual
 Thermo Scientific Accucore XL HPLC Columns Technical Manual Thermo Scientific Accucore XL HPLC Columns Based on Core Enhanced Technology using µm solid core particles, Accucore XL HPLC columns allow users
Thermo Scientific Accucore XL HPLC Columns Technical Manual Thermo Scientific Accucore XL HPLC Columns Based on Core Enhanced Technology using µm solid core particles, Accucore XL HPLC columns allow users
4. Ion chromatography (IC) 4.1. Educational aims and objectives
 4. Ion chromatography (IC) 4.1. Educational aims and objectives Principles of ion chromatography method and instrumentation. Methods for ionic compounds analysis including ion chromatography and its subtypes.
4. Ion chromatography (IC) 4.1. Educational aims and objectives Principles of ion chromatography method and instrumentation. Methods for ionic compounds analysis including ion chromatography and its subtypes.
Product Bulletin. ACE LC/MS and Rapid Analysis HPLC Columns. HPLC Columns
 Product Bulletin HPLC Columns ACE LC/MS and Rapid Analysis HPLC Columns 0 mm, 30 mm, 35 mm and 50 mm column lengths.0,., 3.0, 4.0 and 4.6 mm column diameters Configured for High Sample Throughput Specially
Product Bulletin HPLC Columns ACE LC/MS and Rapid Analysis HPLC Columns 0 mm, 30 mm, 35 mm and 50 mm column lengths.0,., 3.0, 4.0 and 4.6 mm column diameters Configured for High Sample Throughput Specially
for Acclaim Mixed-Mode HILIC-1 Column
 for Acclaim Mixed-Mode HILIC-1 Column Product Manual for ACCLAIM Mixed-Mode HILIC-1 Page 1 of 17 Product Manual for ACCLAIM Mixed-Mode HILIC-1 Column 5µm, 4.6 x 250mm, P/N 066844 5µm, 4.6 x 150mm, P/N
for Acclaim Mixed-Mode HILIC-1 Column Product Manual for ACCLAIM Mixed-Mode HILIC-1 Page 1 of 17 Product Manual for ACCLAIM Mixed-Mode HILIC-1 Column 5µm, 4.6 x 250mm, P/N 066844 5µm, 4.6 x 150mm, P/N
Thermo Scientific Syncronis HPLC Columns. Remarkable separations guaranteed time after time
 Thermo Scientific Syncronis HPLC Columns Remarkable separations guaranteed time after time Syncronis HPLC and UHPLC Columns Remarkable separations guaranteed time after time When developing a new method,
Thermo Scientific Syncronis HPLC Columns Remarkable separations guaranteed time after time Syncronis HPLC and UHPLC Columns Remarkable separations guaranteed time after time When developing a new method,
Solid Phase Extraction Method Development Tips and Tricks
 Solid Phase Extraction Method Development Tips and Tricks Carol Haney Ball, Ph.D. Application Scientist Agilent Technologies, Inc. February 3, 2009 Sources of Error Generated and Time Spent During a Typical
Solid Phase Extraction Method Development Tips and Tricks Carol Haney Ball, Ph.D. Application Scientist Agilent Technologies, Inc. February 3, 2009 Sources of Error Generated and Time Spent During a Typical
Value of Eclipse Plus C18 and ph Selectivity to Analyze Amine-Containing Drugs
 Value of Eclipse Plus C18 and ph Selectivity to Analyze Ae-Containing Drugs Application Pharmaceuticals Authors John W. Henderson Jr., William J. Long, and Cliff Woodward Agilent Technologies 285 Centerville
Value of Eclipse Plus C18 and ph Selectivity to Analyze Ae-Containing Drugs Application Pharmaceuticals Authors John W. Henderson Jr., William J. Long, and Cliff Woodward Agilent Technologies 285 Centerville
InertSustainSwift C8
 Physical Properties Silica Particle Size Surface Area Pore Size Pore Volume Bonded Phase End-capping Carbon Loading ph Range USP Code :ES (Evolved Surface) Silica Gel :.9 μm, μm, μm :00 m /g :00 Å (0 nm)
Physical Properties Silica Particle Size Surface Area Pore Size Pore Volume Bonded Phase End-capping Carbon Loading ph Range USP Code :ES (Evolved Surface) Silica Gel :.9 μm, μm, μm :00 m /g :00 Å (0 nm)
Chapter content. Reference
 Chapter 7 HPLC Instrumental Analysis Rezaul Karim Environmental Science and Technology Jessore University of Science and Technology Chapter content Liquid Chromatography (LC); Scope; Principles Instrumentation;
Chapter 7 HPLC Instrumental Analysis Rezaul Karim Environmental Science and Technology Jessore University of Science and Technology Chapter content Liquid Chromatography (LC); Scope; Principles Instrumentation;
Too Polar for Reversed Phase What Do You Do?
 Too Polar for Reversed Phase What Do You Do? June 20, 2013 Mark Powell Columns and Consumables Technical Support Page 1 C8 or C18 Doesn t Always Do the Job Typical reversed phase conditions involve water/buffer
Too Polar for Reversed Phase What Do You Do? June 20, 2013 Mark Powell Columns and Consumables Technical Support Page 1 C8 or C18 Doesn t Always Do the Job Typical reversed phase conditions involve water/buffer
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Intelligent RP LC Column Selection & New Technologies Peru October 2016 Ricardo Martínez
 Intelligent RP LC Column Selection & New Technologies Peru ctober 2016 Ricardo Martínez 2016 Waters Corporation 1 Agenda LC Fundamentals Columns Quality Particles & Ligands Column Efficiency ph in LC New
Intelligent RP LC Column Selection & New Technologies Peru ctober 2016 Ricardo Martínez 2016 Waters Corporation 1 Agenda LC Fundamentals Columns Quality Particles & Ligands Column Efficiency ph in LC New
Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS
 Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS 1. List two advantages of temperature programming in GC. a) Allows separation of solutes with widely varying retention factors in a reasonable
Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS 1. List two advantages of temperature programming in GC. a) Allows separation of solutes with widely varying retention factors in a reasonable
Dilution(*) Chromatography
 WA20264 Poster # 184, HPLC 2002, Montreal, 4-5 June 2002 At-Column Column-Dilution for Preparative Dilution(*) Chromatography Cecilia Mazza, Jie Cavanaugh, Ziling Lu,Tom Sirard,Tom Wheat and Uwe Neue Waters
WA20264 Poster # 184, HPLC 2002, Montreal, 4-5 June 2002 At-Column Column-Dilution for Preparative Dilution(*) Chromatography Cecilia Mazza, Jie Cavanaugh, Ziling Lu,Tom Sirard,Tom Wheat and Uwe Neue Waters
High Performance Liquid Chromatography
 High Performance Liquid Chromatography What is HPLC? It is a separation technique that involves: Injection of small volume of liquid sample Into a tube packed with a tiny particles (stationary phase).
High Performance Liquid Chromatography What is HPLC? It is a separation technique that involves: Injection of small volume of liquid sample Into a tube packed with a tiny particles (stationary phase).
A Protocol for High-Throughput Drug Mixture Quantitation: Fast LC MS or Flow Injection Analysis MS?
 60 LCGC VOLUME 19 NUMBER 1 JANUARY 2001 www.chromatographyonline.com A Protocol for High-Throughput Drug Mixture Quantitation: Fast LC MS or Flow Injection Analysis MS? The authors demonstrate the pros
60 LCGC VOLUME 19 NUMBER 1 JANUARY 2001 www.chromatographyonline.com A Protocol for High-Throughput Drug Mixture Quantitation: Fast LC MS or Flow Injection Analysis MS? The authors demonstrate the pros
Fast Screening Methods for Analgesics and Non-Steroidal Anti-Inflammatory Drugs by HPLC
 Fast Screening Methods for Analgesics and on-steroidal Anti-Inflammatory Drugs by HPLC Application ote Small Molecule Pharmaceuticals and Generics Author William Long Agilent Technologies, Inc. Introduction
Fast Screening Methods for Analgesics and on-steroidal Anti-Inflammatory Drugs by HPLC Application ote Small Molecule Pharmaceuticals and Generics Author William Long Agilent Technologies, Inc. Introduction
Open Column Chromatography, GC, TLC, and HPLC
 Open Column Chromatography, GC, TLC, and HPLC Murphy, B. (2017). Introduction to Chromatography: Lecture 1. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy, Chicago. USES OF CHROMATOGRAPHY
Open Column Chromatography, GC, TLC, and HPLC Murphy, B. (2017). Introduction to Chromatography: Lecture 1. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy, Chicago. USES OF CHROMATOGRAPHY
Introduction to Chromatography
 Introduction to Chromatography Dr. Sana Mustafa Assistant Professor Department of Chemistry, Federal Urdu University of Arts, Science & Technology, Karachi. What is Chromatography? Derived from the Greek
Introduction to Chromatography Dr. Sana Mustafa Assistant Professor Department of Chemistry, Federal Urdu University of Arts, Science & Technology, Karachi. What is Chromatography? Derived from the Greek
State - of - the -art C18 HPLC Columns
 An HPLC GL Sciences Newest and Most Advanced ODS Phase -New For State - of - the -art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with
An HPLC GL Sciences Newest and Most Advanced ODS Phase -New For State - of - the -art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with
Inertsil Technical Library
 HPLC COLUMN INERTSIL TECHNICAL LIBRARY H P L C C o l u m n Inertsil Technical Library Inertsil ODS-4, C8-4 Comparison of Performance List of Compared Columns Experimental Explanation, Analytical Conditions
HPLC COLUMN INERTSIL TECHNICAL LIBRARY H P L C C o l u m n Inertsil Technical Library Inertsil ODS-4, C8-4 Comparison of Performance List of Compared Columns Experimental Explanation, Analytical Conditions
Changing the way you think about HPLC
 3521 Silverside Road Suite 1-K Quillen Building Wilmington, DE 19810 USA info@advanced-materials-tech.com Changing the way you think about HPLC Changing the way you think about HPLC New 5-micron HALO-5
3521 Silverside Road Suite 1-K Quillen Building Wilmington, DE 19810 USA info@advanced-materials-tech.com Changing the way you think about HPLC Changing the way you think about HPLC New 5-micron HALO-5
ImpSilHPLC Columns. The solution for your chromatography IMPTECH SCIENTIFIC
 ImpSilHPLC Columns The solution for your chromatography IMPTECH SCIENTIFIC 7-1-60,203-Veena Dharam Karam Road,Ameerpet,Hyderabad-500 016 Tel:040-23740443/483 Ph:9848051071 imptechscientific@gmail.com www.impscience.com
ImpSilHPLC Columns The solution for your chromatography IMPTECH SCIENTIFIC 7-1-60,203-Veena Dharam Karam Road,Ameerpet,Hyderabad-500 016 Tel:040-23740443/483 Ph:9848051071 imptechscientific@gmail.com www.impscience.com
Fast Screening Methods for Steroids by HPLC with Agilent Poroshell 120 Columns
 Fast Screening Methods for Steroids by HPLC with Agilent Poroshell 2 Columns Application Note Pharma, BioPharma, and Clinical Research Author William Long Agilent Technologies, Inc. Introduction Steroids
Fast Screening Methods for Steroids by HPLC with Agilent Poroshell 2 Columns Application Note Pharma, BioPharma, and Clinical Research Author William Long Agilent Technologies, Inc. Introduction Steroids
