The Retention Behavior of Reversed-Phase HPLC Columns with 100% Aqueous Mobile Phase
|
|
|
- Brianna Poole
- 5 years ago
- Views:
Transcription
1 9 LCGC NORTH AMERICA VOLUME NUMBER OCTOBER The Retention Behavior of Reversed-Phase HPLC Columns with % Aqueous Mobile Phase The authors studied the retention behavior of reversed-phase high performance liquid chromatography (HPLC) columns under % aqueous mobile-phase conditions. They report about the behaviors of reversedphase HPLC columns such as C and C columns and discuss why they exhibit decreased retention times. They also suggest criteria for achieving reproducible results from reversed-phased columns under highly aqueous mobile-phase conditions. Norikazu Nagae, Toshiyuki Enami, and Sid Doshi* Nomura Chemical Co., Ltd., Anada-cho, Seto, 9-, Japan * Phenomenex, West th Street, Torrance, California 9- Address correspondence to N. Nagae. Octadecyl (C) and octyl (C) are the most widely used bonded phases in reversedphase high performance liquid chromatography (HPLC). However, C and C reversed phases also exhibit decreased and poorly reproducible retention under more than 9% aqueous conditions. This problem traditionally has been explained as being the result of ligand collapse or a matting effect. Wolcott and Dolan () reported that when a C column was washed with water, the bonded phase collapsed. Subsequent flushing with mobile phase removed the wash solvent, but the stationary phase remained in the collapsed configuration, which caused a change in retention and selectivity. They advised that washing a reversed-phase HPLC column with water should be avoided. Furthermore, if the organic solvent content of a mobile phase was too low, the stationary phase would tend to collapse onto itself in a lowenergy conformation. This collapse could lead to abnormal chromatographic behavior and generally undesirable results. To avoid this phenomenon, scientists recently have used embedded polar phases, including amide or carbamate groups ( ), which supposedly do not undergo phase collapse in % water. These stationary phases higher polarity limits their applicability in HPLC because they show shorter retention and sta- bility in reversed-phase liquid chromatography (LC). In our work, we studied retention behavior under % aqueous mobile-phase conditions for several reversed phases such as trimethylsilyl silica, C, C, and triacontylsilyl silica (C). When the pore size of the silica supports was larger, some phases showed the decreased retention times, but others had higher reproducibility in retention. Through this process, we discovered new information about the real mechanism causing the decrease in retention under % aqueous mobile-phase conditions (,). We found that standard C reversedphase columns could provide reproducible results under % aqueous mobile-phase conditions as long as they met certain criteria. Experimental Apparatus: The LC system comprised a model PU-9 pump, a model UV-9 UV detector, and a model -RI refractive index detector (all from Jasco, Tokyo, Japan); a model injector (Rheodyne, Rohnert Park, California); and a model SIC integrator (System Instrument, Tokyo, Japan). We controlled the column temperature between and C using a water bath and a heater with a model EC temperature controller (Omron, Tokyo, Japan) and between and C using a heater and
2 9 LCGC NORTH AMERICA VOLUME NUMBER OCTOBER Table I: Characteristics of the stationary phases examined in this work* Specific Surface Area Pore Volume Mean Pore Diameter Carbon Content Ligand Density Stationary Phase (m /g) (ml/g) (nm) (%) ( mol/m ) Trimethylsilyl silica Trimethylsilyl silica Trimethylsilyl silica.... C.9... C C C C..9.. C..9.. C C.... C.... C C.... * All measurements were performed on bonded phases. Table II: Retention time and weight of a C column under % aqueous mobile-phase conditions (a).9 min. min. min t t -propanol t -propanol t Mass of Column (min or ml) (min or ml) (min or ml) % (g) (b). min.. min min Initial.9... After stopping..... Initial after Time (min) Figure : Retention behavior of a C column under % aqueous mobile-phase conditions. Shown are (a) initial results and (b) results after stopping the pump for h and then starting it again. Column: mm. mm, - m d p C, -nm pore size; mobile phase: water; flow rate:. ml/min; column pressure:. MPa; temperature: C; detection: refractive index; added back pressure:. MPa after the column. Peaks: sodium nitrate (t ), -propanol. a cooler (Orion Kikai, Tokyo, Japan). The particle size, pore size, and surface area measurements were obtained using Coulter Multisizer and Coulter SA- instruments (both from Beckman Coulter, Fullerton, California). The carbon load was determined using a CN-corder MT- instrument (Yanaco, Kyoto, Japan). Reagents and chemicals: We prepared distilled water in the laboratory. Other solvents and buffer constituents were purchased from Wako (Osaka, Japan). The tested solutes also were from Wako. Table I lists the stationary phases used in our study. Trimethylsilyl silica, C, C, and C phases were generated using trimethylchlorosilane, dimethyloctylchlorosilane, dimethyloctadecylchlorosilane (all from Shin-etsu Silicon Chemicals, Tokyo, Japan), and dimethyltriacontylchlorosilane (Gelest, Tullytown, Pennsylvania) by the conventional refluxing method in toluene for h with pyridine and excess reagent. All phases, except for trimethylsilyl silica, were bonded with trimethylchlorosilane twice by the same manner as the above as an endcapping. The hydrogen-bonding capacity, which was the separation factor of caffeine and phenol obtained using : (v/v) methanol water as a mobile phase, was less than. on all phases. The silica gel used in bonding was from Nomura Chemical (Seto, Japan). Table I also lists the characteristics of the stationary phases. The bonded phases were slurry packed with chloroform into a mm. mm column blank (Sugiyama Shoji, Yokohama, Japan). Results and Discussion It is well known that the retention time of a C phase is reduced under % aqueous mobile-phase conditions. Figure shows the change of retention time of sodium nitrite and -propanol on a -nm pore size C phase at C. We used the sodium nitrite to measure the unretained time (t ). We added back pressure to the column when the mobile phase was changed to water we will explain the reason for this change below. Stopping the flow for h caused shorter retention times for both sodium nitrite and -propanol. The difference of retention time between sodium nitrite and -propanol became the essential retention of -propanol in this case. After no flow for h, not only did the t decrease from.9 min to. min, the essential retention of -propanol was only.% compared with its initial retention. Generally, a decrease of t is unexpected because t is the unretained time. We measured the column weight at the same time. The column was sealed at both ends with plugs as soon as the column pressure was reduced to MPa. The decreases in t and the column weight were.9 ml and. g, respectively, as Table II lists. The values were almost the same because the specific gravity of water is. g/ml. Furthermore, we observed that. ml of water went out of the outlet of the column during the h when no mobile phase flowed through the column.
3 9 LCGC NORTH AMERICA VOLUME NUMBER OCTOBER Figure shows the effect of the pore size. We evaluated the retention behavior of - and -nm pore size C silica. The -nm C silica showed decreased retention times of thymine and sodium nitrite as t ; however, the retention times of both sodium nitrite and thymine were unchanged on the -nm C silica. The only difference between these two C silicas is pore size. The larger pore size provided higher reproducibility in retention time under % aqueous conditions. From these results we ascertained that the mobile phase was being expelled from the pores of the packing material under % aqueous mobilephase conditions, as Figure shows. Figure illustrates the relationship between the pore size and the relative retention time. The relative retention time was the ratio of the retention time after no flow for h to the initial retention time at C. When comparing packing materials of the same pore size at nm and nm, for example a C phase showed the greatest decrease in retention, and the C phase showed the least decrease in retention. The longer the length of ligand, the smaller the decrease in retention, except for the trimethylsilyl ligand. The trimethylsilyl ligand is so small that it influences the polarity of the silica surface. In other words, trimethylsilyl silica is more polar than the other phases compared in our study, and it is this polarity of the trimethylsilyl silica that likely influences the pore size shift to the small area. As we will discuss below, residual silanol groups (a) Silica Ligand such as ODS (C) showed a similar effect on the results. Furthermore, the trimethylsilyl phase did not appear to suffer phase collapse because it was a very short ligand. Trimethylsilyl phase bonded to a -nm pore size silica, however, did show a decrease in retention time. On the other hand, the C ligand can collapse because of its long alkyl chain. We expect that the ligand of the - and -nm pore size C silica could collapse similarly under % aqueous conditions. Although the -nm C silica showed an % decrease in retention time, the -nm C silica exhibited less than a % decrease in retention time, as shown in Figure. Other phases also showed similar results, although we evaluated many pore sizes. We can assume from these findings that phase collapse has nothing to do with the decrease in retention time on reversed-phase HPLC columns when used under % aqueous mobile-phase conditions. Figures and show the effect of temperature on -nm pore size C and C phases. The C phase showed an % decrease in retention time at C; however, it showed an % decrease in retention time at C. The lower the temperature, the smaller the decrease in retention time. Even when the pump was running and pressure existed in the column, the retention time was reduced at more than C. On the other hand, the C phase showed a % decrease in retention time at C and % at C. The retention behavior of the C phase at C and at C is very similar to that of the C phase at C and C. The melting points of octadecane (C H ) and triacontane (C H ) are 9 C and 9 C, respectively. It seems that retention behavior of the C and C phases is related to their respective melting points. Recently, Doyle and coworkers () reported the characterization of C-bonded stationary phase by Raman spectroscopy. They concluded that bonded ligands did not exhibit the same phasetransition behavior as neat octadecane. The octadecane melted at a discrete temperature ( C); the spectral features of the C ligands, which more closely resemble those of liquid than solid octadecane, gradually and continually varied with changes in temperature throughout the C range investigated. The change of retention behavior in Figure was gradual and continual not a sudden phase transition. An end of the C ligand was bonded on the surface of the silica gel, so that the behavior of C ligands naturally was different from that of neat octadecane. The temperature range with changes of C ligands was higher than that Figure : Effect of the pore size on retention under % aqueous mobile-phase conditions. Column: mm. mm, - m d p C, -nm pore size; mobile phase: mm phosphate buffer (ph.); flow rate:. ml/min; column pressure:. MPa; temperature: C; detection: UV at nm. Sample: C (-nm pore size) sodium nitrite, C (-nm pore size) sodium nitrite, C (-nm pore size) thymine, C (-nm pore size) thymine. (b) Pore Silica Pore Figure : Behavior of (a) an organic solvent water mobile phase, which fills the silica gel pore, and (b) water mobile phase, which is expelled from the pore. 9 Pore diameter (nm) Figure : Relationship between pore size and relative retention time with % aqueous mobile phase. Column: C ( ), C ( ), C ( ), and trimethylsilyl silica ( ); column dimensions: mm. mm; sample: thymine. Other conditions were the same as in Figure. Relative retention time was calculated as the ratio of the decrease in retention versus initial retention time.
4 9 LCGC NORTH AMERICA VOLUME NUMBER OCTOBER Figure : Effect of temperature on C column (-nm pore size) under % aqueous mobile-phase conditions. Column dimensions: mm. mm; temperature: C, C, C, C, C. Other conditions were the same as in Figure. Figure : Effect of temperature on C (- nm pore size) under % aqueous mobilephase conditions. Column dimensions: mm. mm; temperature: C, C, C. Other conditions were the same as in Figure. Figure : Effect of stopping flow. Column: mm. mm C, -nm pore size. Sample: sodium nitrite, thymine. Other conditions were the same as in Figure. of C ligands by C. We assumed that when the temperature was higher than the melting point of the ligand, the degree of freedom for movement for each ligand became high, so we see that the mobile phase is easily expelled from the pore. This change explains why the C phase showed the greatest decrease in retention time in Figure because the melting point of octane (C H ) is lower than that of octadecane. Figures and show the effect of stopping flow and back pressure after the column. The decrease in retention time was finished in several minutes at C, as shown in Figure. This result shows that the mobile phase immediately went out of a column at C when no pressure was in the column. The particle size of packing materials tested in Figure was m, and the column pressure was. MPa. When adding back pressure after the column, the pressure on the inlet of the column was. MPa in addition to its back pressure. When the pressure was greater than. MPa around the packing materials, the retention time was. min, as shown in Figure. However, when less than. MPa of pressure existed around the packing materials, the retention time was min. In other words, - m d p, -nm pore size octadecylsilyl silica showed a decrease in retention time from the beginning without the back pressure because the pressure around the packing materials was insufficient for the mobile phase to remain in the pores of the packing materials. Even if the column was packed with - m d p packing materials and the column pressure was high, we found that the mobile phase was expelled from the pore of the packing materials near the outlet of the column, as shown in Figure 9, because of very low pressure near the outlet. A.-MPa postcolumn back pressure caused the retention time of -propanol to increase from. min to. min. This back pressure also is considered to be a factor responsible for lowering reproducibility in retention. Therefore, the back pressure after the column makes reproducibility in retention high. Table III documents the effect of endcapping for three types of C columns (Develosil by Nomura Chemical). One column was not endcapped, and the others were single- and double-endcapped with trimethylchlorosilane. All were used to evalu-. MPa Pressure MPa. MPa Pressure. MPa Inlet Column Mobile phase exists in the pores 9 Mobile phase moves out of the pores Back pressure (MPa) Figure : Effect of back pressure. Column: mm. mm - m d p C, -nm pore size; column pressure:. MPa; sample: thymine. Other conditions were the same as in Figure. Resistance was added after the column. Outlet Add the back pressure after the column (. MPa) Retention time of -propanol. min. min Figure 9: State of the packing material in the column. Conditions were the same as in Figure.
5 9 LCGC NORTH AMERICA VOLUME NUMBER OCTOBER Table III: Effect of endcapping* Hydrogen- Relative Amount Final Bonding of Residual Retention Time Column Endcapping Capacity Silanol Groups Ratio (%) Develosil ODS-A- No. Much 9. Develosil ODS-T- Single. Little. Develosil ODS-HG- Double. Very little 9.9 * Conditions as shown in Figure. Hydrogen-bonding capacity: separation factor of caffeine and phenol. The final retention time ratio is the final retention when the retention time decreased to the initial retention time. Relative retention (%) ate the effect of the residual silanol groups. We measured the amount of residual silanol groups on the bonded phase by evaluating their hydrogen-bonding capacity. The higher the hydrogen-bonding capacity, the greater the number of residual silanol groups. More extensive endcapping reduced the amount of residual silanol groups and thereby caused poor reproducibility in retention time, as shown in Table III. The residual silanol groups work as polar groups on the stationary phase. The polarity of stationary phases with high residual silanol group content is high, so these phases show high reproducibility in retention much as do embedded polar phases such as alkylamide and alkylcarbamate phases. Similarly, trimethylsilyl silica has high hydrogen-bonding capacity and is more polar than a C phase, hence a trimethylsilyl silica phase with a 9-nm pore size displays high reproducibility in retention, as shown in Figure. A C phase without endcapping is very unstable especially under neutral ph and highly aqueous conditions because of the existence of these silanol groups. Therefore, this type of C column cannot be used. Figures and illustrate the effect of salt and ion-pair reagents. The relative retention largely was influenced by the concentration of salt and ion-pairing reagent, as these figures show. Only mm of ionpairing reagent showed very high reproducibility in retention. We think that the salt and ion-pairing reagent decreased the surface tension of the mobile phase while simultaneously making the stationary phase more hydrophilic. With this low surface tension, the highly hydrophilic stationary phase easily could keep the mobile phase in the pore. After the aqueous mobile phase was expelled from the pore of the packing materials, we eluted the mobile phase with a greater than % concentration of organic solvent to return the mobile phase into the pore. We flushed the column with : (v/v) acetonitrile water at the end of each evaluation, and the column always returned to its initial state. We repeated the experiment several times using the same column, and we obtained the same results each time. Conclusion We have confirmed that reversed-phase HPLC columns show a decrease in retention under % aqueous conditions because under these conditions the mobile phase is expelled from the pore of the packing materials, which results in decreased contact between the stationary and mobile phases. Some critical parameters such as pore size, the length of the alkyl group bonded to the stationary phase, temperature, pressure, endcapping, and the concentration of salt and the ion-pairing reagent influence the decrease in retention. When the pore size of packing materials is greater than nm and the operating temperature is lower than C or mm of ion-pairing reagent is added to the mobile phase, even a common C HPLC column shows high retention reproducibility under % aqueous conditions. We also found that a -nm pore size C phase used at temperatures lower than C showed high retention reproducibility without any of the above conditions, thereby making it an ideal phase to use for highly aqueous mobile-phase applications (9). References () R.G. Wolcott and J.W. Dolan, LCGC (), (999). () J.E. O Gara, B.A. Alden, T.H. Walter, J.S. Petersen, C.L. Niederlander, and U.D. Neue, Anal. Chem., 9 (99). () T. Czajkowaka, I. Hrabovsky, B. Buszewski, R.K. Gilpin, and M. Jaronieic, J. Chromatogr. A 9, (99). () T.L. Ascah, K.M.R. Kallury, C.A. Szafravski, S.D. Corman, and F. Liu, J. Liq. Chromatogr. Rel. Technol. 9, 9 (99). Figure : Effect of concentration of salt in the mobile phase. Column: mm. mm C, -nm pore size; mobile phase: water, mm ammonium phosphate (ph.), mm ammonium phosphate (ph.). Other conditions were the same as in Figure. Relative retention (%) Figure : Effect of the concentration of the ion-pairing reagent. Column: mm. mm C, -nm pore size; mobile phase: mm sodium phosphate (ph.), mm sodium phosphate mm sodium octanesulfonate (ph.), mm sodium phosphate mm sodium octanesulfonate (ph.). Other conditions were the same as in Figure. () T. Czajkowaka and M. Jaronieic, J. Chromatogr. A, (99). () N. Nagae and T. Enami, Bunseki Kagaku 9, 9 (). () T. Enami and N. Nagae, Chromatography, 9 (). () C.A. Doyle, T.J. Vickers, C.K. Mann, and J.G. Dorsey, J. Chromatogr. A, 9 (). (9) N. Nagae, Nomura Chemical Co., Ltd., U.S. patent number,,9, June.
Sunniest C18 Sunniest RP-AQUA Sunniest C8 Patent pending
 Patent pending Website : www.chromtech.net.au E-mail : info@chromtech.net.au telno : 0 97 0... in AUSTRALIA A Novel Bonding Technique (patent pending) An unique trifunctional silyl-reagent was developed
Patent pending Website : www.chromtech.net.au E-mail : info@chromtech.net.au telno : 0 97 0... in AUSTRALIA A Novel Bonding Technique (patent pending) An unique trifunctional silyl-reagent was developed
A Highly Stable Alkyl Amide Silica-Based Column Packing for Reversed-Phase HPLC of Polar and Ionizable Compounds
 A Highly Stable Alkyl Amide Silica-Based Column Packing for Reversed-Phase HPLC of Polar and Ionizable Compounds Researchers have designed a new polar-linked, reversed-phase column for separating polar,
A Highly Stable Alkyl Amide Silica-Based Column Packing for Reversed-Phase HPLC of Polar and Ionizable Compounds Researchers have designed a new polar-linked, reversed-phase column for separating polar,
8. Methods in Developing Mobile Phase Condition for C18 Column
 I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
Retention Behavior of p-alkylphenols in Reversed-Phase Liquid Chromatography Using Ethanol/Water Mixtures
 1998 The Japan Society for Analytical Chemistry 355 Retention Behavior of p-alkylphenols in Reversed-Phase Liquid Chromatography Using Ethanol/Water Mixtures Kanji MIYABE, Noboru NOMURA, Fumie MORISHITA,
1998 The Japan Society for Analytical Chemistry 355 Retention Behavior of p-alkylphenols in Reversed-Phase Liquid Chromatography Using Ethanol/Water Mixtures Kanji MIYABE, Noboru NOMURA, Fumie MORISHITA,
penta-hilic UHPLC COLUMNS
 penta-hilic UHPLC COLUMNS penta-hilic Highly retentive, proprietary penta-hydroxy-ligand Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
penta-hilic UHPLC COLUMNS penta-hilic Highly retentive, proprietary penta-hydroxy-ligand Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
Packings for HPLC. Packings for HPLC
 Summary of packings for HPLC In analytical HPLC, packings with particle sizes of 3 to 10 µm are preferred. For preparative separation tasks, also particles with diameters larger than 10 µm are applied.
Summary of packings for HPLC In analytical HPLC, packings with particle sizes of 3 to 10 µm are preferred. For preparative separation tasks, also particles with diameters larger than 10 µm are applied.
Inertsil ODS-EP Technical Information
 Technical Information Advantages of The selectivity is completely different from those of conventional columns such as ODS column due to its specific polar group in the stationary phase. Durability can
Technical Information Advantages of The selectivity is completely different from those of conventional columns such as ODS column due to its specific polar group in the stationary phase. Durability can
RP C18 column with feature of a silanol group
 RP C8 column with feature of a silanol group lanol Activity Controlled C8 Column Sunrise ctacosyl C8) Sunrise ctadecyl C8) Sunrise ctadecyl-sac C8-SAC has an interaction of silanol groups ChromaNik Sunrise
RP C8 column with feature of a silanol group lanol Activity Controlled C8 Column Sunrise ctacosyl C8) Sunrise ctadecyl C8) Sunrise ctadecyl-sac C8-SAC has an interaction of silanol groups ChromaNik Sunrise
for Acclaim Mixed-Mode HILIC-1 Column
 for Acclaim Mixed-Mode HILIC-1 Column Product Manual for ACCLAIM Mixed-Mode HILIC-1 Page 1 of 17 Product Manual for ACCLAIM Mixed-Mode HILIC-1 Column 5µm, 4.6 x 250mm, P/N 066844 5µm, 4.6 x 150mm, P/N
for Acclaim Mixed-Mode HILIC-1 Column Product Manual for ACCLAIM Mixed-Mode HILIC-1 Page 1 of 17 Product Manual for ACCLAIM Mixed-Mode HILIC-1 Column 5µm, 4.6 x 250mm, P/N 066844 5µm, 4.6 x 150mm, P/N
penta-hilic UHPLC COLUMNS
 penta-hilic UHPLC COLUMNS Highly retentive, proprietary penta-hydroxy-ligand penta-hilic Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
penta-hilic UHPLC COLUMNS Highly retentive, proprietary penta-hydroxy-ligand penta-hilic Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
The Effect of Contact Angle and Wetting on Performance in Solid Phase Extraction
 The Effect of Contact Angle and Wetting on Performance in Solid Phase Extraction Edouard S. P. Bouvier, Randy E. Meirowitz and Uwe D. Neue Waters Corporation, 34 Maple Street, Milford, MA 01757 USA Presented
The Effect of Contact Angle and Wetting on Performance in Solid Phase Extraction Edouard S. P. Bouvier, Randy E. Meirowitz and Uwe D. Neue Waters Corporation, 34 Maple Street, Milford, MA 01757 USA Presented
LC and LC/MS Column Selection Flow Chart
 LC and LC/MS Column Selection Flow Chart To use the column selection diagram below, simply follow the path for your analyte and mobile phase. At the far right, follow your final column selection to the
LC and LC/MS Column Selection Flow Chart To use the column selection diagram below, simply follow the path for your analyte and mobile phase. At the far right, follow your final column selection to the
InertSustainSwift C8
 Physical Properties Silica Particle Size Surface Area Pore Size Pore Volume Bonded Phase End-capping Carbon Loading ph Range USP Code :ES (Evolved Surface) Silica Gel :.9 μm, μm, μm :00 m /g :00 Å (0 nm)
Physical Properties Silica Particle Size Surface Area Pore Size Pore Volume Bonded Phase End-capping Carbon Loading ph Range USP Code :ES (Evolved Surface) Silica Gel :.9 μm, μm, μm :00 m /g :00 Å (0 nm)
ProntoSIL C18-EPS Reversed-Phase HPLC Columns
 ProntoSIL C8-EPS Reversed-Phase HPLC Columns Provides excellent separation of polar compounds Better peak shape for acids and bases Stabilized bonded phase for rugged, robust HPLC methods More retentive
ProntoSIL C8-EPS Reversed-Phase HPLC Columns Provides excellent separation of polar compounds Better peak shape for acids and bases Stabilized bonded phase for rugged, robust HPLC methods More retentive
Biochemistry. Biochemical Techniques HPLC
 Description of Module Subject Name Paper Name 12 Module Name/Title 13 1. Objectives 1.1. To understand the basic concept and principle of 1.2. To understand the components and techniques of 1.3. To know
Description of Module Subject Name Paper Name 12 Module Name/Title 13 1. Objectives 1.1. To understand the basic concept and principle of 1.2. To understand the components and techniques of 1.3. To know
Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS. Table of Contents
 No. 089 SEPARATION REPORT Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS Table of Contents 1. Introduction 1 2. Column Specification 1 3. Features of Packing Materials
No. 089 SEPARATION REPORT Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS Table of Contents 1. Introduction 1 2. Column Specification 1 3. Features of Packing Materials
Inertsil Technical Library
 HPLC COLUMN INERTSIL TECHNICAL LIBRARY H P L C C o l u m n Inertsil Technical Library Inertsil ODS-4, C8-4 Comparison of Performance List of Compared Columns Experimental Explanation, Analytical Conditions
HPLC COLUMN INERTSIL TECHNICAL LIBRARY H P L C C o l u m n Inertsil Technical Library Inertsil ODS-4, C8-4 Comparison of Performance List of Compared Columns Experimental Explanation, Analytical Conditions
Luna 2.5 µm C18(2)-HST. Advantages of 2.5 µm for increasing the speed of analysis while maintaining high efficiency
 Luna 2.5 µm C18(2)-HST Advantages of 2.5 µm for increasing the speed of analysis while maintaining high efficiency Table of Contents Part 1 Theory 1.1 Abstract...3 1.2 Introduction...3 Part 2 Set Up 2.1
Luna 2.5 µm C18(2)-HST Advantages of 2.5 µm for increasing the speed of analysis while maintaining high efficiency Table of Contents Part 1 Theory 1.1 Abstract...3 1.2 Introduction...3 Part 2 Set Up 2.1
Stability of Silica-Based, Monofunctional C 18 Bonded- Phase Column Packing for HPLC at High ph
 Stability of Silica-Based, Monofunctional C 18 Bonded- Phase Column Packing for HPLC at High ph J.J. Kirkland Rockland Technologies, Inc., 538 First State Blvd., Newport, Delaware 19804 Abstract Previous
Stability of Silica-Based, Monofunctional C 18 Bonded- Phase Column Packing for HPLC at High ph J.J. Kirkland Rockland Technologies, Inc., 538 First State Blvd., Newport, Delaware 19804 Abstract Previous
Too Polar for Reversed Phase What Do You Do?
 Too Polar for Reversed Phase What Do You Do? June 20, 2013 Mark Powell Columns and Consumables Technical Support Page 1 C8 or C18 Doesn t Always Do the Job Typical reversed phase conditions involve water/buffer
Too Polar for Reversed Phase What Do You Do? June 20, 2013 Mark Powell Columns and Consumables Technical Support Page 1 C8 or C18 Doesn t Always Do the Job Typical reversed phase conditions involve water/buffer
An HPLC column offering moderate retentivity with superb peak shape Inertsil ODS Sprint
 An HPLC column offering moderate retentivity with superb peak shape Inertsil ODS Sprint An important new column in the Inertsil series Bonded-Phase Structure Perfect balance of retention for both polar
An HPLC column offering moderate retentivity with superb peak shape Inertsil ODS Sprint An important new column in the Inertsil series Bonded-Phase Structure Perfect balance of retention for both polar
Embedded-Polar-Group Bonded Phases for High Performance Liquid Chromatography
 LCGC VLUME 9 NUMBE JUNE 00 www.chromatographyonline.com Embedded-Polar-Group Bonded Phases for High Performance Liquid Chromatography In the past 0 years, embedded-polar-group bonded phases have become
LCGC VLUME 9 NUMBE JUNE 00 www.chromatographyonline.com Embedded-Polar-Group Bonded Phases for High Performance Liquid Chromatography In the past 0 years, embedded-polar-group bonded phases have become
ODS-UG,HG,MG,SR columns
 Column for reversed phase ODS-UG,HG,MG,SR columns Develosil ODS-HG Develosil ODS-UG Develosil ODS-MG Develosil ODS-SR The 2nd generation of Develosil series, very standard column This series is the column
Column for reversed phase ODS-UG,HG,MG,SR columns Develosil ODS-HG Develosil ODS-UG Develosil ODS-MG Develosil ODS-SR The 2nd generation of Develosil series, very standard column This series is the column
Voltage-Induced Sample Release from Anion Exchange Supports in Capillary Electrochromatography
 1998 The Japan Society for Analytical Chemistry 571 Voltage-Induced Sample Release from Anion Exchange Supports in Capillary Electrochromatography Shinya KITAGAWA and Takao TSUDA Department of Applied
1998 The Japan Society for Analytical Chemistry 571 Voltage-Induced Sample Release from Anion Exchange Supports in Capillary Electrochromatography Shinya KITAGAWA and Takao TSUDA Department of Applied
LIQUID CHROMATOGRAPHY
 LIQUID CHROMATOGRAPHY RECENT TECHNIQUES HPLC High Performance Liquid Chromatography RRLC Rapid Resolution Liquid Chromatography UPLC Ultra Performance Liquid Chromatography UHPLC Ultra High Pressure Liquid
LIQUID CHROMATOGRAPHY RECENT TECHNIQUES HPLC High Performance Liquid Chromatography RRLC Rapid Resolution Liquid Chromatography UPLC Ultra Performance Liquid Chromatography UHPLC Ultra High Pressure Liquid
Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity
 Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20544 Key Words Hydrophilic, HILIC,
Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20544 Key Words Hydrophilic, HILIC,
Retention mechanism of some solutes using ionic liquids as mobile phase modifier in RP-high-performance liquid chromatography (HPLC)
 Korean J. Chem. Eng., 28(2), 357-363 (2011) DOI: 10.1007/s11814-010-0273-9 INVITED REVIEW PAPER Retention mechanism of some solutes using ionic liquids as mobile phase modifier in RP-high-performance liquid
Korean J. Chem. Eng., 28(2), 357-363 (2011) DOI: 10.1007/s11814-010-0273-9 INVITED REVIEW PAPER Retention mechanism of some solutes using ionic liquids as mobile phase modifier in RP-high-performance liquid
Fast Separation of Vastly Different Compounds by Isocratic HPLC
 Fast Separation of Vastly Different Compounds by Isocratic HPLC Aimee N. Heyrman and Yury Zelechonok Resolution Systems, Inc. 590 E. 32nd St. Holland. MI, 49423 SIELC Technologies, 15 E. Palatine Rd, Suite
Fast Separation of Vastly Different Compounds by Isocratic HPLC Aimee N. Heyrman and Yury Zelechonok Resolution Systems, Inc. 590 E. 32nd St. Holland. MI, 49423 SIELC Technologies, 15 E. Palatine Rd, Suite
DEVELOPMENT OF HPLC METHOD FOR ANALYSIS OF NITRITE AND NITRATE IN VEGETABLE
 Journal of Agricultural, Food and Environmental Sciences UDC 635.546.173/.175]:543.544.5.068.7 Original scientific paper DEVELOPMENT OF HPLC METHOD FOR ANALYSIS OF NITRITE AND NITRATE IN VEGETABLE A. Najdenkoska*
Journal of Agricultural, Food and Environmental Sciences UDC 635.546.173/.175]:543.544.5.068.7 Original scientific paper DEVELOPMENT OF HPLC METHOD FOR ANALYSIS OF NITRITE AND NITRATE IN VEGETABLE A. Najdenkoska*
Looking into the HPLC Column while Running CIP
 Looking into the HPLC Column while Running CIP Imre SALLAY, PhD OSAKA SODA CO., LTD., Osaka, Japan imre@osaka-soda.co.jp The big application - By far the biggest RP silica application is insulin purification.
Looking into the HPLC Column while Running CIP Imre SALLAY, PhD OSAKA SODA CO., LTD., Osaka, Japan imre@osaka-soda.co.jp The big application - By far the biggest RP silica application is insulin purification.
Advantages of polymerbased. an alternative for ODS
 Advantages of polymerbased HPLC columns an alternative for ODS Yukiko Higai Shodex, 1 Content (1) Comparison between Silica and Polymer-based RP columns (2) Characteristics of Polymer RP columns - an example:
Advantages of polymerbased HPLC columns an alternative for ODS Yukiko Higai Shodex, 1 Content (1) Comparison between Silica and Polymer-based RP columns (2) Characteristics of Polymer RP columns - an example:
LC Technical Information
 LC Technical Information Method Transfer to Accucore.6 μm Columns Containing solid core particles, which are engineered to a diameter of.6μm and a very narrow particle size distribution; Accucore HPLC
LC Technical Information Method Transfer to Accucore.6 μm Columns Containing solid core particles, which are engineered to a diameter of.6μm and a very narrow particle size distribution; Accucore HPLC
InertSustainSwift C8
 HPLC, LC/MS Columns InertSustainSwift TM C8 New! InertSustainSwift C8 is an octyl group (C8) bonded column offering the same extreme inertness to any type of compounds just like InertSustainSwift C8, which
HPLC, LC/MS Columns InertSustainSwift TM C8 New! InertSustainSwift C8 is an octyl group (C8) bonded column offering the same extreme inertness to any type of compounds just like InertSustainSwift C8, which
Silanol. Groups Liquid Chromatography
 Abstract umber: 0-8P Study of Secondary Interaction Based on Residual lanol Groups for Reversed-Phase Liquid Chromatography II orikazu agae Ph.D, Kouji Yamamoto, Chiaki Kadota Chromaik Technologies Inc.
Abstract umber: 0-8P Study of Secondary Interaction Based on Residual lanol Groups for Reversed-Phase Liquid Chromatography II orikazu agae Ph.D, Kouji Yamamoto, Chiaki Kadota Chromaik Technologies Inc.
Shodex TM ODP2 HP series columns
 HPLC Columns Shodex TM ODP2 HP series columns Better retention of highly polar substances Technical notebook No. 6 Contents 1. Introduction 1-1. Specifications 1-2. Eluent Compatibility of ODP2 HP Series
HPLC Columns Shodex TM ODP2 HP series columns Better retention of highly polar substances Technical notebook No. 6 Contents 1. Introduction 1-1. Specifications 1-2. Eluent Compatibility of ODP2 HP Series
Alltech Alltima HP Introduction
 Alltech Introduction High-Stability, High-Purity, High-Performance, Low-Bleed Columns for Demanding Applications Better Peak Symmetry high-purity silica eliminates peak tailing problems Long Column Life
Alltech Introduction High-Stability, High-Purity, High-Performance, Low-Bleed Columns for Demanding Applications Better Peak Symmetry high-purity silica eliminates peak tailing problems Long Column Life
Preparative Chromatography with Improved Loadability
 05 LCGC RTH AMERICA VLUME 0 UMBER VEMBER 00 www.chromatographyonline.com Preparative Chromatography with Improved Loadability In combinatorial chemistry and drug development, as well as in the full-scale
05 LCGC RTH AMERICA VLUME 0 UMBER VEMBER 00 www.chromatographyonline.com Preparative Chromatography with Improved Loadability In combinatorial chemistry and drug development, as well as in the full-scale
Improve Peak Shape and Productivity in HPLC Analysis of Pharmaceutical Compounds with Eclipse Plus C8 Columns Application
 Improve Peak Shape and Productivity in HPLC Analysis of Pharmaceutical Compounds with Eclipse Plus C8 Columns Application Pharmaceuticals Authors John W. Henderson Jr., Nona Martone, and Cliff Woodward
Improve Peak Shape and Productivity in HPLC Analysis of Pharmaceutical Compounds with Eclipse Plus C8 Columns Application Pharmaceuticals Authors John W. Henderson Jr., Nona Martone, and Cliff Woodward
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
C18 Column. Care & Use Sheet
 C18 Column Care & Use Sheet HALO Description HALO C18 is a high-speed, high-performance liquid chromatography column based on a new Fused-CoreTM particle design. The Fused-Core particle provides a thin
C18 Column Care & Use Sheet HALO Description HALO C18 is a high-speed, high-performance liquid chromatography column based on a new Fused-CoreTM particle design. The Fused-Core particle provides a thin
Chemistry Instrumental Analysis Lecture 28. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
P R O D U C T B U L L E T I N. Fused-Core particle technology for hyper-fast and super-rugged HPLC columns
 P R O D U C T B U L L E T I N Fused-Core particle technology for hyper-fast and super-rugged HPLC columns HALO column packings are not made the typical way. Instead, the particles packed into HALO columns
P R O D U C T B U L L E T I N Fused-Core particle technology for hyper-fast and super-rugged HPLC columns HALO column packings are not made the typical way. Instead, the particles packed into HALO columns
Overview Applications of NUCLEODUR HPLC Phases
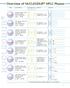 verview Applications of HPLC Phases Phase Specification Characteristics* Stability Structure A B C octadecyl phase, high density coating multi-endcapping 8% C USP L ph stability -, suitable for LC/MS (Si-
verview Applications of HPLC Phases Phase Specification Characteristics* Stability Structure A B C octadecyl phase, high density coating multi-endcapping 8% C USP L ph stability -, suitable for LC/MS (Si-
Chemistry 3200 High Performance Liquid Chromatography: Quantitative Determination of Headache Tablets
 Chemistry 3200 High Performance Liquid Chromatography: Quantitative Determination of Headache Tablets Liquid chromatography was developed by Tswett in early 1900 s and was shown to be a powerful separation
Chemistry 3200 High Performance Liquid Chromatography: Quantitative Determination of Headache Tablets Liquid chromatography was developed by Tswett in early 1900 s and was shown to be a powerful separation
Barry E. Boyes, Ph.D. Consumables and Accessories Business Unit May 10, 2000
 Barry E. Boyes, Ph.D. Consumables and Accessories Business Unit May 0, 000 HPLC Column Troubleshooting What Every HPLC User Should Know :00 a.m. EST Telephone Number: 86-650-06 Chair Person: Tim Spaeder
Barry E. Boyes, Ph.D. Consumables and Accessories Business Unit May 0, 000 HPLC Column Troubleshooting What Every HPLC User Should Know :00 a.m. EST Telephone Number: 86-650-06 Chair Person: Tim Spaeder
New L-lysine derived high molecular-shape selective organic phase with ordered functional groups for reversed-phase liquid chromatography
 Electronic Supplementary Material (ESI) for Analytical Methods. This journal is The Royal Society of Chemistry 2014 Supporting Information New L-lysine derived high molecular-shape selective organic phase
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is The Royal Society of Chemistry 2014 Supporting Information New L-lysine derived high molecular-shape selective organic phase
Chromatography. Gas Chromatography
 Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Basic principles of HPLC
 Introduction to the theory of HPLC HPLC (High Performance Liquid Chromatography) depends on interaction of sample analytes with the stationary phase (packing) and the mobile phase to effect a separation.
Introduction to the theory of HPLC HPLC (High Performance Liquid Chromatography) depends on interaction of sample analytes with the stationary phase (packing) and the mobile phase to effect a separation.
Optimisil HPLC & UPLC Column catalogue
 Optimisil HPLC & UPLC Column catalogue Analytical Hub Plot No: 37, P & T Colony, Vikrampuri, Secunderabad 500009 Tel: 040-64621313, Mobile: +91 7799931313 Website: www.analyticalhub.com email: contact@analyticalhub.com
Optimisil HPLC & UPLC Column catalogue Analytical Hub Plot No: 37, P & T Colony, Vikrampuri, Secunderabad 500009 Tel: 040-64621313, Mobile: +91 7799931313 Website: www.analyticalhub.com email: contact@analyticalhub.com
State-of-the-art C18 HPLC Columns
 An HPLC GL Sciences Newest and Most Advanced ODS Phase-New For 00 State-of-the-art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with 00%
An HPLC GL Sciences Newest and Most Advanced ODS Phase-New For 00 State-of-the-art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with 00%
and water enriched layer at the stationary phase particle-eluent interface [9,11].
![and water enriched layer at the stationary phase particle-eluent interface [9,11]. and water enriched layer at the stationary phase particle-eluent interface [9,11].](/thumbs/87/96223751.jpg) 8 A Simple, Generally Applicable HILIC Method Development Platform Based Upon Selectivity by Alan P McKeown, Advanced Chromatography Technologies Ltd, 1 Berry Street, Aberdeen, Scotland AB25 1HF, UK amckeown@ace-hplc.com
8 A Simple, Generally Applicable HILIC Method Development Platform Based Upon Selectivity by Alan P McKeown, Advanced Chromatography Technologies Ltd, 1 Berry Street, Aberdeen, Scotland AB25 1HF, UK amckeown@ace-hplc.com
A C18 Silica Column With Exceptional Temperature and ph Stability
 A C18 lica Column With Exceptional Temperature and ph Stability Brian A. Jones 1, Stephanie J. Marin 1, Jody Clark 1, Nathan L. Porter 1, J. Andreas Lippert 2, and Todd M. Johnson 2 1. Selerity Technologies,
A C18 lica Column With Exceptional Temperature and ph Stability Brian A. Jones 1, Stephanie J. Marin 1, Jody Clark 1, Nathan L. Porter 1, J. Andreas Lippert 2, and Todd M. Johnson 2 1. Selerity Technologies,
Orosil HPLC Columns. OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph.
 Orosil OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph. Excellent organic base selectivity with very low asymmetry values Compatible
Orosil OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph. Excellent organic base selectivity with very low asymmetry values Compatible
viridis Columns Bringing a New Dimension to SFC Combining state-of-the-art media manufacturing with industry leading column technology, Viridis
 [ viridis columns ] viridis Columns Bringing a New Dimension to SFC Combining state-of-the-art media manufacturing with industry leading column technology, Viridis Supercritical Fluid Chromatography (SFC)
[ viridis columns ] viridis Columns Bringing a New Dimension to SFC Combining state-of-the-art media manufacturing with industry leading column technology, Viridis Supercritical Fluid Chromatography (SFC)
COSMOSIL Applications
 1.FAQ and Troubleshooting COSMOSIL Applications COSMOSIL Application has more than 7,600 applications using COSMOSIL columns. Setting optimal HPLC experimental parameters is an important process that requires
1.FAQ and Troubleshooting COSMOSIL Applications COSMOSIL Application has more than 7,600 applications using COSMOSIL columns. Setting optimal HPLC experimental parameters is an important process that requires
Solid-Supported DNA for Asymmetric Synthesis: a Stepping Stone toward Practical Applications
 Solid-Supported DA for Asymmetric Synthesis: a Stepping Stone toward Practical Applications Soyoung Park, * a Keiichi Ikehata, a and iroshi Sugiyama*,a,b,c a Department of Chemistry, Graduate School of
Solid-Supported DA for Asymmetric Synthesis: a Stepping Stone toward Practical Applications Soyoung Park, * a Keiichi Ikehata, a and iroshi Sugiyama*,a,b,c a Department of Chemistry, Graduate School of
HPLC Praktikum Skript
 HPLC Praktikum Skript Assistants: Gianluca Bartolomeo HCI D330, 3 46 68, bartolomeo@org.chem.ethz.ch Sahar Ghiasikhou HCI E330, 2 29 29, ghiasikhou@org.chem.ethz.ch 1. Introduction In chromatographic techniques,
HPLC Praktikum Skript Assistants: Gianluca Bartolomeo HCI D330, 3 46 68, bartolomeo@org.chem.ethz.ch Sahar Ghiasikhou HCI E330, 2 29 29, ghiasikhou@org.chem.ethz.ch 1. Introduction In chromatographic techniques,
Hybrid Organic Inorganic Particle Technology: Breaking Through Traditional Barriers of HPLC Separations
 6 LCGC VLUME 8 NUMBER NVEMBER 000 www.chromatographyonline.com Hybrid rganic Inorganic Particle Technology: Breaking Through Traditional Barriers of HPLC Separations Hybrid organic inorganic particles
6 LCGC VLUME 8 NUMBER NVEMBER 000 www.chromatographyonline.com Hybrid rganic Inorganic Particle Technology: Breaking Through Traditional Barriers of HPLC Separations Hybrid organic inorganic particles
Reversed Phase Solvents
 Part 1. General Chromatographic Theory Part 2. verview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use Reversed Phase Solvents 2 Solvents for RP Chromatography
Part 1. General Chromatographic Theory Part 2. verview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use Reversed Phase Solvents 2 Solvents for RP Chromatography
Ondansetron Hydrochloride Tablets
 Ondansetron Hydrochloride Tablets Dissolution Perform the test with 1 tablet of Ondansetron Hydrochloride Tablets at 50 revolutions per minute according to the Paddle method, using 900 ml of water
Ondansetron Hydrochloride Tablets Dissolution Perform the test with 1 tablet of Ondansetron Hydrochloride Tablets at 50 revolutions per minute according to the Paddle method, using 900 ml of water
High Performance Liquid Chromatography
 High Performance Liquid Chromatography What is HPLC? It is a separation technique that involves: Injection of small volume of liquid sample Into a tube packed with a tiny particles (stationary phase).
High Performance Liquid Chromatography What is HPLC? It is a separation technique that involves: Injection of small volume of liquid sample Into a tube packed with a tiny particles (stationary phase).
Determination of Caffeine by HPLC
 Determination of Caffeine by HPLC Introduction It was a long history before real high performance liquid chromatography (HPLC) had evolved. The very first indication of a chromatographic separation was
Determination of Caffeine by HPLC Introduction It was a long history before real high performance liquid chromatography (HPLC) had evolved. The very first indication of a chromatographic separation was
Comparison of different aqueous mobile phase HPLC techniques
 June 009 ewsletter Pharmaceutical Analysis: What is Your Problem? SIELC Technologies, Inc., Prospect Heights, IL 0070 Pharmaceutical analysis involves liquid chromatography of various compounds, from active
June 009 ewsletter Pharmaceutical Analysis: What is Your Problem? SIELC Technologies, Inc., Prospect Heights, IL 0070 Pharmaceutical analysis involves liquid chromatography of various compounds, from active
Chemistry Instrumental Analysis Lecture 31. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 31 High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) Solvent Delivery
Chemistry 4631 Instrumental Analysis Lecture 31 High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) Solvent Delivery
OBSERVATIONS ON THE WETTING OF REVERSED-PHASE HPLC PACKINGS
 1997 Waters Corporation p. 1 980947 OBSERVATIONS ON THE WETTING OF REVERSED-PHASE HPLC PACKINGS T. Walter, P. Iraneta, and M. Capparella Waters Corporation, 34 Maple Street, Milford, MA 01757 USA Presented
1997 Waters Corporation p. 1 980947 OBSERVATIONS ON THE WETTING OF REVERSED-PHASE HPLC PACKINGS T. Walter, P. Iraneta, and M. Capparella Waters Corporation, 34 Maple Street, Milford, MA 01757 USA Presented
Ch.28 HPLC. Basic types of Liquid Chromatography Partition (LLC) Adsorption (LSC) Ion Exchange (IC) Size Exclusion (SEC or Gel Chromatography)
 Ch.28 HPLC 28.1 Basic types of Liquid Chromatography Partition (LLC) Adsorption (LSC) Ion Exchange (IC) Size Exclusion (SEC or Gel Chromatography) High Performance (Pressure) LC Glass column st.steel (high
Ch.28 HPLC 28.1 Basic types of Liquid Chromatography Partition (LLC) Adsorption (LSC) Ion Exchange (IC) Size Exclusion (SEC or Gel Chromatography) High Performance (Pressure) LC Glass column st.steel (high
Chromatographic Methods: Basics, Advanced HPLC Methods
 Chromatographic Methods: Basics, Advanced HPLC Methods Hendrik Küpper, Advanced Course on Bioinorganic Chemistry & Biophysics of Plants, summer semester 2018 Chromatography: Basics Chromatography a physical
Chromatographic Methods: Basics, Advanced HPLC Methods Hendrik Küpper, Advanced Course on Bioinorganic Chemistry & Biophysics of Plants, summer semester 2018 Chromatography: Basics Chromatography a physical
Luminescence transitions. Fluorescence spectroscopy
 Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
CYCLOSERINE Final text for addition to The International Pharmacopoeia. (November 2008) CYCLOSERINUM CYCLOSERINE
 December 2008 CYCLOSERINE Final text for addition to The International Pharmacopoeia (November 2008) This monograph was adopted at the Forty-third WHO Expert Committee on Specifications for Pharmaceutical
December 2008 CYCLOSERINE Final text for addition to The International Pharmacopoeia (November 2008) This monograph was adopted at the Forty-third WHO Expert Committee on Specifications for Pharmaceutical
Determination of Trace Cations in Power Plant Waters Containing Morpholine
 Application Note 8 Determination of Trace Cations in Power Plant Waters Containing Morpholine INTRODUCTION Morpholine and ammonium are used as additives in power plant waters. Morpholine acts as a corrosion
Application Note 8 Determination of Trace Cations in Power Plant Waters Containing Morpholine INTRODUCTION Morpholine and ammonium are used as additives in power plant waters. Morpholine acts as a corrosion
Determination of trace anions in concentrated hydrofluoric acid
 APPLICATION NOTE 78 Determination of trace anions in concentrated hydrofluoric acid Authors Archava Siriraks Thermo Fisher Scientific, Sunnyvale, CA Keywords HF, ICS-5000 +, IonPac AS10, IonPac AC10, ion
APPLICATION NOTE 78 Determination of trace anions in concentrated hydrofluoric acid Authors Archava Siriraks Thermo Fisher Scientific, Sunnyvale, CA Keywords HF, ICS-5000 +, IonPac AS10, IonPac AC10, ion
LUMEFANTRINUM LUMEFANTRINE
 July 2008 LUMEFANTRINE: Final text for addition to The International Pharmacopoeia (July 2008) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications for Pharmaceutical Preparations
July 2008 LUMEFANTRINE: Final text for addition to The International Pharmacopoeia (July 2008) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications for Pharmaceutical Preparations
High Pressure/Performance Liquid Chromatography (HPLC)
 High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
LC Columns with Liquid Separation Cell Technology
 Obelisc LC Columns with Liquid Separation Cell Technology Obelisc HPLC Columns - Liquid Separation Cell Technology Introduction Obelisc HPLC columns are the latest, innovative columns from SIELC Technologies,
Obelisc LC Columns with Liquid Separation Cell Technology Obelisc HPLC Columns - Liquid Separation Cell Technology Introduction Obelisc HPLC columns are the latest, innovative columns from SIELC Technologies,
HPLC. High Performance Liquid Chromatography (HPLC) Harris Chapter 25
 High Performance Liquid Chromatography (HPLC) Harris Chapter 25 12/1/2005 Chem 253 - Chapter 25 1 HPLC Separation of nonvolatile or thermally unstable compounds. If the analyte/sample can be found to be
High Performance Liquid Chromatography (HPLC) Harris Chapter 25 12/1/2005 Chem 253 - Chapter 25 1 HPLC Separation of nonvolatile or thermally unstable compounds. If the analyte/sample can be found to be
Hypersil BDS Columns TG 01-05
 TG 0-0 Hypersil BDS Columns Introduction Hypersil BDS columns have gained a reputation over the years as one of the most robust, reproducible and reliable HPLC column brands available. This Technical Guide
TG 0-0 Hypersil BDS Columns Introduction Hypersil BDS columns have gained a reputation over the years as one of the most robust, reproducible and reliable HPLC column brands available. This Technical Guide
The Role of Pore Size and Stationary Phase Composition in Preventing Aqueous-Induced Retention Time Loss in Reversed-Phase HPLC
 The Role of Pore Size and Stationary Phase Composition in Preventing Aqueous-Induced Retention Time Loss in Reversed-Phase HPLC Brian A. Bidlingmeyer and Alan D. Broske Agilent Technologies, Inc., 2850
The Role of Pore Size and Stationary Phase Composition in Preventing Aqueous-Induced Retention Time Loss in Reversed-Phase HPLC Brian A. Bidlingmeyer and Alan D. Broske Agilent Technologies, Inc., 2850
Open Column Chromatography, GC, TLC, and HPLC
 Open Column Chromatography, GC, TLC, and HPLC Murphy, B. (2017). Introduction to Chromatography: Lecture 1. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy, Chicago. USES OF CHROMATOGRAPHY
Open Column Chromatography, GC, TLC, and HPLC Murphy, B. (2017). Introduction to Chromatography: Lecture 1. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy, Chicago. USES OF CHROMATOGRAPHY
NEVIRAPINE ORAL SUSPENSION Final text for addition to The International Pharmacopoeia (February 2009)
 February 2009. NEVIRAPINE ORAL SUSPENSION Final text for addition to The International Pharmacopoeia (February 2009) This monograph was adopted at the Forty-third WHO Expert Committee on Specifications
February 2009. NEVIRAPINE ORAL SUSPENSION Final text for addition to The International Pharmacopoeia (February 2009) This monograph was adopted at the Forty-third WHO Expert Committee on Specifications
CHAPTER V ANALYTICAL METHODS ESTIMATION OF DICLOFENAC. Diclofenac (gift sample from M/s Micro Labs Ltd., Pondicherry)
 CHAPTER V ANALYTICAL METHODS ESTIMATION OF DICLOFENAC A UV spectrophotometric method based on the measurement of absorbance at 276nm in phosphate buffer of p H 7.4 was used in the present study of the
CHAPTER V ANALYTICAL METHODS ESTIMATION OF DICLOFENAC A UV spectrophotometric method based on the measurement of absorbance at 276nm in phosphate buffer of p H 7.4 was used in the present study of the
High Performance Liquid Chromatography
 STANDARDBASE techniques: High Performance Liquid Chromatography Drenthe College, The Netherlands 1. Introduction HPLC. High Performance Liquid Chromatography High Performance Liquid Chromatography (HPLC)
STANDARDBASE techniques: High Performance Liquid Chromatography Drenthe College, The Netherlands 1. Introduction HPLC. High Performance Liquid Chromatography High Performance Liquid Chromatography (HPLC)
How to Troubleshoot a Failed System Suitability Test
 Volume 34 Number 12, 881 932 Volume 34 Number 12 December 216 www.chromatographyonline.com LCGC NORTH AMERICA How to Troubleshoot a Failed System Suitability Test December 216 Preventing Carryover in GC
Volume 34 Number 12, 881 932 Volume 34 Number 12 December 216 www.chromatographyonline.com LCGC NORTH AMERICA How to Troubleshoot a Failed System Suitability Test December 216 Preventing Carryover in GC
Open Access. Yoichi Shibusawa *,1, Go Morikawa 1, Akio Yanagida 1 and Yoichiro Ito 2. Science, Horinouchi, Hachioji, Tokyo , Japan
 The Open Analytical Chemistry Journal, 2012, 6, 9-14 9 Open Access Counter-Current Chromatographic Separation of Nucleic Acid Constituents with a Polar Volatile Organic-Aqueous Two-Phase Solvent Systems
The Open Analytical Chemistry Journal, 2012, 6, 9-14 9 Open Access Counter-Current Chromatographic Separation of Nucleic Acid Constituents with a Polar Volatile Organic-Aqueous Two-Phase Solvent Systems
EFFECTS OF MOBILE PHASE COMPOSITION ON THE SEPARATION OF CATECHOLAMINES BY LIQUID CHROMATOGRAPHY WITH ELECTROCHEMICAL DETECTION
 Pharmacology EFFECTS OF MOBILE PHASE COMPOSITION ON THE SEPARATION OF CATECHOLAMINES BY LIQUID CHROMATOGRAPHY WITH ELECTROCHEMICAL DETECTION A. ISIMER N. E. BASCI A. BOZKURT S. O. KAYAALP SUMMARY: In this
Pharmacology EFFECTS OF MOBILE PHASE COMPOSITION ON THE SEPARATION OF CATECHOLAMINES BY LIQUID CHROMATOGRAPHY WITH ELECTROCHEMICAL DETECTION A. ISIMER N. E. BASCI A. BOZKURT S. O. KAYAALP SUMMARY: In this
2] The plate height in chromatography is best described as 2
![2] The plate height in chromatography is best described as 2 2] The plate height in chromatography is best described as 2](/thumbs/76/73153760.jpg) 9 Chromatography. General Topics 1] Explain the three major components of the van Deemter equation. Sketch a clearly labeled diagram describing each effect. What is the salient point of the van Deemter
9 Chromatography. General Topics 1] Explain the three major components of the van Deemter equation. Sketch a clearly labeled diagram describing each effect. What is the salient point of the van Deemter
Chapter content. Reference
 Chapter 7 HPLC Instrumental Analysis Rezaul Karim Environmental Science and Technology Jessore University of Science and Technology Chapter content Liquid Chromatography (LC); Scope; Principles Instrumentation;
Chapter 7 HPLC Instrumental Analysis Rezaul Karim Environmental Science and Technology Jessore University of Science and Technology Chapter content Liquid Chromatography (LC); Scope; Principles Instrumentation;
Abstract: An minimalist overview of chromatography for the person who would conduct chromatographic experiments, but not design experiments.
 Chromatography Primer Abstract: An minimalist overview of chromatography for the person who would conduct chromatographic experiments, but not design experiments. At its heart, chromatography is a technique
Chromatography Primer Abstract: An minimalist overview of chromatography for the person who would conduct chromatographic experiments, but not design experiments. At its heart, chromatography is a technique
 CAPCELL PAK C18 IF2 Type http://hplc.shiseido.co.jp/e/column/html/if2_index.htm Page 1 of 2 List of Sales Representatives Contact Shiseido Technical Materials Catalog List HPLC Columns HPLC Instruments
CAPCELL PAK C18 IF2 Type http://hplc.shiseido.co.jp/e/column/html/if2_index.htm Page 1 of 2 List of Sales Representatives Contact Shiseido Technical Materials Catalog List HPLC Columns HPLC Instruments
Fall 2012 Due In Class Friday, Oct. 19. Complete the following on separate paper. Show your work and clearly identify your answers.
 CHEM 322 Name Fall 2012 Due In Class Friday, Oct. 19 Complete the following on separate paper. Show your work and clearly identify your answers. General Separations 1. Describe the relative contributions
CHEM 322 Name Fall 2012 Due In Class Friday, Oct. 19 Complete the following on separate paper. Show your work and clearly identify your answers. General Separations 1. Describe the relative contributions
ACE SuperC18. ACE UHPLC and HPLC Columns. Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability
 ACE SuperC8 TM Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS compatible buffers between ph.. High efficiency µm, µm,
ACE SuperC8 TM Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS compatible buffers between ph.. High efficiency µm, µm,
Supporting Information
 Supporting Information Photo-controlled reversible guest uptake, storage and release by azobenzene-modified microporous multi-layer films of pillar[5]arenes Tomoki Ogoshi,,,,* Shu Takashima and Tada-aki
Supporting Information Photo-controlled reversible guest uptake, storage and release by azobenzene-modified microporous multi-layer films of pillar[5]arenes Tomoki Ogoshi,,,,* Shu Takashima and Tada-aki
IDENTIFICATION AND DETERMINATION OF HYDROQUINONE IN COSMETIC PRODUCTS 2 14/11/17 ACM 003 BY TLC AND HPLC
 A. IDENTIFICATION BY TLC 1. SCOPE AND FIELD OF APPLICATION The method describes the identification of hydroquinone in cosmetic products. 2. PRINCIPLE Hydroquinone is identified by thin layer chromatography
A. IDENTIFICATION BY TLC 1. SCOPE AND FIELD OF APPLICATION The method describes the identification of hydroquinone in cosmetic products. 2. PRINCIPLE Hydroquinone is identified by thin layer chromatography
637. Thiamethoxam. HPLC method
 637. Thiamethoxam HPLC method CIPAC Collaborative Trial according to CIPAC Information Sheet N o 293 Dr. Sven Adolph Syngenta Crop Protection Münchwilen AG CH-4333 Münchwilen Switzerland May 212 page 1
637. Thiamethoxam HPLC method CIPAC Collaborative Trial according to CIPAC Information Sheet N o 293 Dr. Sven Adolph Syngenta Crop Protection Münchwilen AG CH-4333 Münchwilen Switzerland May 212 page 1
Reversed-phase chromatography is by far the most widely used technique in high performance liquid chromatography
 8 LCGC ASIA PACIFIC VOLUME 6 NUMBER 1 MARCH 2003 www.chromatographyonline.com Column Watch This month s Column Watch looks at practical ways to return a contaminated column to or close to its original
8 LCGC ASIA PACIFIC VOLUME 6 NUMBER 1 MARCH 2003 www.chromatographyonline.com Column Watch This month s Column Watch looks at practical ways to return a contaminated column to or close to its original
The Effect of Flow Rate on High Performance Liquid Chromatography Column Re-equilibration after Gradient Elution
 The Effect of Flow Rate on High Performance Liquid Chromatography Column Re-equilibration after Gradient Elution A Thesis Submitted to the Faculty of Drexel University by Michael Robert Fletcher in partial
The Effect of Flow Rate on High Performance Liquid Chromatography Column Re-equilibration after Gradient Elution A Thesis Submitted to the Faculty of Drexel University by Michael Robert Fletcher in partial
Acclaim Mixed-Mode WCX-1
 Acclaim Mixed-Mode WCX-1 Product Manual for the Acclaim Mixed-Mode WCX-1 Column Page 1 of 28 PRODUCT MANUAL for the Acclaim Mixed-Mode WCX-1 Columns 4.6 x 150 mm, P/N (068353) 4.6 x 250 mm, P/N (068352)
Acclaim Mixed-Mode WCX-1 Product Manual for the Acclaim Mixed-Mode WCX-1 Column Page 1 of 28 PRODUCT MANUAL for the Acclaim Mixed-Mode WCX-1 Columns 4.6 x 150 mm, P/N (068353) 4.6 x 250 mm, P/N (068352)
HPLC Background Chem 250 F 2008 Page 1 of 24
 HPLC Background Chem 250 F 2008 Page 1 of 24 Outline: General and descriptive aspects of chromatographic retention and separation: phenomenological k, efficiency, selectivity. Quantitative description
HPLC Background Chem 250 F 2008 Page 1 of 24 Outline: General and descriptive aspects of chromatographic retention and separation: phenomenological k, efficiency, selectivity. Quantitative description
Tracer Excel TRACER EXCEL ODS-A. Total deactivation. Maximum Stability
 Tracer Excel TRACER EXCEL is a range of totally new packings that employ the most advanced procedures of synthesis and chemical functionalization, resulting in some column packings that completely surpass
Tracer Excel TRACER EXCEL is a range of totally new packings that employ the most advanced procedures of synthesis and chemical functionalization, resulting in some column packings that completely surpass
ANALYSIS OF BASIC COMPOUNDS
 COMPARISON OF Fully AND Superficially POROUS PARTICLE COLUMNS FOR THE ANALYSIS OF BASIC COMPOUNDS Kenneth J. Fountain, Jane Xu, Zhe Yin, Pamela C. Iraneta, Diane M. Diehl Waters Corporation GOAL Compare
COMPARISON OF Fully AND Superficially POROUS PARTICLE COLUMNS FOR THE ANALYSIS OF BASIC COMPOUNDS Kenneth J. Fountain, Jane Xu, Zhe Yin, Pamela C. Iraneta, Diane M. Diehl Waters Corporation GOAL Compare
