Unit 0 Measurements and Calculations in Chemistry
|
|
|
- Gloria Tate
- 5 years ago
- Views:
Transcription
1 Unit 0 Measurements and Calculations in Chemistry Question Difficulty: * Normal ** Difficult *** Very difficult A. Measurements and Units (O-0.2 O-0.5) 1. SI units and prefixes a. A measured quantity/property characterized by a number requires the use of the appropriate unit. 1
2 1. SI units and prefixes b. The SI unit system is a set of consistent units for physical properties. Base quantity (SI) Unit Symbol mass kilogram kg length meter m time second s temperature kelvin K amount of substance mole mol 1. SI units and prefixes c. There are a number of prefixes (to the unit symbol) used in SI. Prefix Symbol Meaning Tera- T Giga- G 10 9 Mega- M 10 6 Kilo- k 10 3 Deci- d 10 1 Centi- c 10 2 Milli- m 10 3 Micro Nano- n 10 9 Pico- p
3 1. SI units and prefixes d. There are few other units that are useful to chemists. For atomic mases, use atomic mass unit: 1 amu = kg For atomic distances, use angstrom: 1 A = m 2. Temperature scales a. There are three scales of (or units for) temperature. Kelvin scale using kelvin denoted K this is the SI unit Celsius scale using Celsius (or centigrade) denoted C Fahrenheit scale using Fahrenheit denoted F 3
4 2. Temperature scales b. There are formulas that can be used to transfer a temperature from one scale to another: K C or C K F 9 C or C F Temperature scales c. Formulas can de deduced based on the relationships between the three temperature scales. C 100 H 2 O boiling K 373 F 212 A a b C F B A B a 0 H 2 O melting 273 b 32 C F
5 2. Temperature scales d. One degree difference on Celsius scale is the same as one degree difference on Kelvin scale but is not the same as one degree difference on Fahrenheit scale. O-0.2 ** 1) Below is a list of common prefixes used in the SI and metric systems. Included with each is an abbreviation and meaning. Which set contains an error? a.deci- d 10-1 b.kilo- k 10 3 c.centi- c 10-2 d.mega- M 10 6 e.micro- m
6 O-0.3 2) The normal boiling point of radon is F. What is this temperature in kelvins? a. 190 K b. 202 K c. 294 K d. 224 K e. 249 K ** 3. Derived units a. Units for all other quantities can be determined from the base SI units. Area = (length) 2 so it has SI unit of m 2. Volume = (length) 3 so it has SI unit of m 3. b. Although the SI unit for volume is m 3, because 1 m 3 is a big volume, other units are typically used. 1 dm 3 = 10 3 m 3 = 1 L 1 cm 3 = 10 6 m 3 = 10 3 L = 1 ml = 1 cc 6
7 4. Density a. An important quantity is density (denoted d or D) defined as mass (denoted m or mass) over (unit of) volume (V). m m d or V or m d V V d b. Although the SI unit for density is kg/m 3, the typical unit is g/cm 3 or g/ml. Typical values of density are in the g/cm 3 range. Density of water is around 1 g/cm 3 and varies with temperature. 4. Density c. Specific gravity is the numerical value (i.e., the value with no unit) of density when expressed in g/cm 3. d. Density is an intensive property. e. Properties can be classified as: extensive when they are dependent on the amount intensive when they are independent on the amount 7
8 O-0.4 * 3) Which of the following statements about density is incorrect? a. If oil and water are placed in a container, they form two layers with oil as the top layer because it has the greater density. b. The densities of liquids are usually expressed in units of g/ml (or g/cm 3 ). c. The intensive property density can be calculated from the two extensive properties: mass and volume. d. Densities of gases change greatly with changes in temperature and pressure. e. The densities of gases are usually expressed in units of g/l. O-0.4 4) What is the specific gravity of nickel if 2.35 cm 3 of nickel has the same mass as 20.9 ml of water at room temperature? a b c d e ** 8
9 5. Energy and energy changes a. Energy can be defined as the capacity to do work or transfer heat. b. In general, there are two forms of energy: Kinetic energy (K or KE or E k = mv 2 /2) is the energy associated with the motion of particles or objects, Potential energy (V or PE or E p ) is the energy associated with the position of particles in a field of forces. c. Potential energy can sometimes be seen as stored energy like the energy stored in chemical bonds. 5. Energy and energy changes d. Both of these forms of energy are relative meaning that they should be defined with respect to a zero of energy. e. Thermal energy is the kinetic energy associated to random motion of atoms and molecules. The change in thermal energy is measured by measuring T. 9
10 5. Energy and energy changes f. The SI unit of energy is joule (J), and another accepted (and highly used) unit of energy is calorie (cal). energy = force distance; J N m kg m s 2 m kg m s cal = J exactly 5. Energy and energy changes g. The first law of thermodynamics states that, during a process, energy is not created or destroyed but just transferred. It is known as the law of conservation of energy. h. The transfer of energy is seen from the point of view of the system under observation, and processes can be classified as: exothermic when the system loses thermal energy endothermic when the system gains thermal energy 10
11 6. Accuracy and precision in measurements a. Accuracy is a measure of how close measurements are to the real value. b. Precision is a measure of how close measurements are to each other. B. Significant Figures (O-0.6 & O-0.7) 1. Identifying the number of significant figures a. Carrying out measurements of various properties are associated with uncertainty so the reported number should contain only the number of digits that are significant. b. Significant figures are the meaningful digits in a reported number. 11
12 1. Identifying the number of significant figures c. In general, numbers can be classified as: exact when there is an infinite number of significant figures inexact when there is a finite number of significant figures d. The exact numbers are: integers examples: 1, 10, 25, 100 exact numbers by definition: 1 in = 2.54 cm and 1 cal = J 1. Identifying the number of significant figures e. The number of significant figures in inexact numbers can be determined based on the following rules: digits other than 0 are always significant digit 0 between other digits is always significant leading digit 0 (i.e., before other digits) is not significant digit 0 to the right of a non-zero digit is significant if after or before the decimal point (i.e., if the decimal point is written) digit 0 to the right of a non-zero digit is ambiguous if it is before the decimal point and the decimal point is not shown 12
13 1. Identifying the number of significant figures f. Scientific notation might need to be used to write a number with the proper number of significant figures. g. For example 1200 can have: 2 significant figures and it should be written as significant figures and it should be written as significant figures and it should be written as O-0.6 5) How many significant figures are in ? a. 3 b. 4 c. 5 d. 6 e. 7 * 13
14 2. Significant figures in calculations a. For multiplications and divisions of two or more numbers, the final result should have the same number of significant figures as the smallest number of significant figures in the starting numbers. b. For additions and subtractions of two or more numbers, the final result should have the last digit as the highest power of 10 among the last digit of the starting numbers. Equivalently, the final result should not have more decimal digits (i.e, the digits to the right of the decimal point) than any of the starting numbers. c. One should carry out the calculation and round off the final result to the right number of significant figures. O-0.7 6) Perform the indicated mathematical operations and express the answer in scientific notation rounded off to the proper number of significant figures: ( ) ( ) ( ) = a b c d e * 14
15 O-0.7 7) The sum expressed to the proper number of significant figures is: a b c d. 439 e * O-0.7 8) Do the arithmetic and give the answer to the correct number of significant figures: a b c d e. 1.0 ** 15
16 O-0.7 9) Do the arithmetic and give the answer to the correct number of significant figures: ( ) a b c d e ** C. Unit and Quantity Conversion (O-0.8) 1. Unit-factor method a. The unit-factor method is also called factor-label method. b. The method can be used to transform a quantity from one unit to another using conversion factors (or unit ratios). Conversion factors are fractions (equal to 1) in which the same quantity is expressed one way in the numerator and another way in the denominator. 16
17 1. Unit-factor method c. The method can also be used to transform a quantity to another equivalent (or stoichiometric) quantity. Example: transform mass into volume using the density. d. The fraction should have: the unit to be transformed from in the denominator the unit to be transform into in the numerator 2. Applications a. To transform between in and cm, use the equality: 1 in = 2.54 cm 2.54 cm Use to transform from in into cm. 1in 1in Use to transform from cm into in cm b. To transform between m and cm, use the equality: 1 m = 100 cm. 17
18 O-0.5 & O ) What is the kinetic energy of 2.0 grams of H 2 moving at 2000 m/s? a J b J c J d J e J ** O-0.5 & O-0.8 ** 11) What is the kinetic energy of a 1800-lb car traveling at 59 miles per hour? (1 lb = kg, 1 mi = km) a J b J c J d J e J 18
19 O ) Convert 1,285 cm 2 to m 2? ** a m 2 b m 2 c m 2 d m 2 e m 2 O-0.4 & O ) The density of mercury is 13.6 g/cm 3. What is the mass of in 3 of mercury? a g b g c g d g e g ** 19
20 O ) If neon atoms (spherical) were laid in a line, each touching the next, the line would measure 2.54 miles. What is the diameter of a neon atom in Å? a Å b Å c Å d Å e Å 2.54 mile km mile 103 m 1 km 1010 Å 1 m *** = 0.71 Å *** O ) Assuming that a lithium atom is spherical, calculate its volume in cm 3. The volume of a sphere is given by V = (4/3) r 3. The radius of a lithium atom is 1.52 Å. 1 Å = 10-8 cm and = a cm 3 b cm 3 V = (4/3) 1.52 Å) 3 c cm 3 d cm 3 V = 14.7 Å 3 e cm 3 V = 14.7 Å cm 1 Å 10-8 cm 1 Å 10-8 cm 1 Å 20
21 Unit I Atoms Question Difficulty: * Normal ** Difficult *** Very difficult A. Matter and Properties (O-I.2 & O-I.3) 1. Matter a. Matter is anything that occupied space and has mass. b. Matter can be classified as: a substance matter that has a definite (i.e., constant) composition and properties a mixture matter that is a combination of two or more substances 1
22 1. Matter c. Substances can be: an element a substance that cannot be broken down into simpler substances by any means a compound a substance composed of two or more elements combined in a specific ration that cannot be separated into simpler substances using physical methods 1. Matter d. Mixtures can be: homogeneous when the composition is uniform throughout heterogeneous when the composition is not uniform throughout e. The physical states of matter are solid, liquid and gas. 2
23 2. Properties a. There are two types of properties (or changes) of matter: chemical when the chemical nature of matter changes physical when the chemical nature of matter does not change b. Chemical changes are chemical reactions. 2. Properties c. The properties can be: qualitative when there is no number associated with the property quantitative when there is a number associated with the property 3
24 3. Early experimental laws and early atomic theory a. Experiments led to some early laws: the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. 3. Early experimental laws and early atomic theory b. Dalton proposed an early atomic theory in Each element is composed of tiny, indestructible particles called atoms. All atoms of the same element have the same mass and properties that distinguish them from the atoms of other elements. Atoms combined in simple, whole-number ratios to form compounds. Atoms of one element cannot change into atoms of another element; a chemical reaction involves changing the way that atoms are bound with each other. 4
25 O-I.2 1) Which of the following is not a mixture? * a. seawater b. steel c. gasoline d. oxygen e. air O-I.2 2) Which statement is false? a. A compound is a substance that can be decomposed by chemical means into simpler substances. b. An example of a homogeneous mixture is one prepared by mixing two liquids, ethyl alcohol (grain alcohol) and water. c. All samples of a particular pure substance have the same composition and properties. d. Different mixtures of the same two substances can have different compositions. e. An example of a heterogeneous mixture is one prepared by dissolving the solid, sodium chloride (table salt), in the liquid, water. * 5
26 O-I.3 * 3) All of the following are properties of antimony. Which one is not a physical property? a. It burns in an atmosphere of chlorine. b. It has both yellow and gray forms (allotropes) in the solid state. c. It is one of the few substances that expands upon freezing. d. It is a solid at room temperature. e. The gray form melts at 631 C B. Atoms and Elements (O-I.2 O-I.6) 1. Atomic particles and structure a. An atom is the smallest quantity of matter that still retains the properties of matter. Atom means indivisible in Greek. b. Atoms contain three types of subatomic particles. electron negative charge, mass of amu proton positive charge, mass of amu neutron neutral charge, mass of amu 6
27 1. Atomic particles and structure c. Protons and neutron are heavier particles and are located in the atomic nucleus, which contains the large majority of the atom weight. Protons and neutrons are called nucleons. d. The size of the nucleus is about m while the size of atoms (or electrons in atoms) is about m. 1. Atomic particles and structure e. The historic discoveries of subatomic particles and atomic structure can be summarized in the table below. Scientist Experiment Discovery J. J. Thomson cathode rays charge/mass ratio of electron Millikan oil-drop experiment charge and mass of electron Becquerel radioactivity,, and rays Rutherford gold foil experiment Chadwick nuclear model of atom existence of neutron 7
28 2. Elements and element symbols a. An element is a substance that cannot be broken down into simpler substances by chemical means. Elements contain the same type of atoms. b. Each element has a name and a symbol. c. An element symbol has one or two letters in which the first one is a capital letter while the second one is not. Example: Ca not CA or ca. d. Element symbols are very important and need to be known for elements 136, elements from principal groups and selected transitional metals. O-I.4 4) Atoms consist principally of what three fundamental particles? a. elements, protons, and neutrons b. electrons, protons, and neutrons c. electrons, protons, and molecules d. electrons, positrons, and neutrons e. elements, positrons, and neutrons * 8
29 O-I.4 5) Which statement is false? a. The charge on a proton is positive, the charge on a neutron is negative. b. Electrons are smaller particles than protons. c. The nucleus of an atom is very small and massive. d. Molecules are the smallest unit of a compound. e. Compounds consist of more than one type of element. * O-I.4 6) The Rutherford "gold foil experiment" suggested. ** a. that electrons have negative charges b. the ratio of the mass of an electron to the charge of the electron c. the existence of canal rays d. that atoms have a tiny, positively charged, massive center e. that protons have charges equal in magnitude but opposite in sign to those of electrons 9
30 ** O-I.4 7) The cathode rays experiment of J. J. Thomson suggested. a. that electrons have negative charges b. the ratio of the mass of an electron to the charge of the electron c. the existence of canal rays d. that atoms have a tiny, positively charged, massive center e. that protons have charges equal in magnitude but opposite in sign to those of electrons O-I.4 ** 8) Which of the following statements is incorrect? a. The heavy particles constituting an atom are present in the nucleus. b. Negative particles are present in the nucleus. c. The chemical symbol of sodium is Na. d. Both negative and positive particles are present in atoms. e. The charge on an electron is negative, and the charge on a proton is positive. 10
31 O-I.5 9) What is the symbol for the element selenium? a. S b. Sn c. Si d. Se e. Sl * 3. Atomic and mass numbers a. Atomic number (denoted Z) is equal to the number of protons in an atom. It is an integer. b. Mass number (denoted A) is equal to the number of protons and neutrons in an atom (i.e., the number of heavy particles in an atom). Atomic number is also equal to the number of electrons in an atom but not equal in an ion. It is an integer. Mass number is equal to the number of nucleons. 11
32 3. Atomic and mass numbers c. Isotopes are different forms of an atom having same Z but different A (i.e., same number of protons but different number of neutrons). Example: hydrogen is a mixture of three isotopes: protium, deuterium, and tritium. d. Nuclide refers to a particular isotope (or a particular nucleus) namely an atom/element with a particular mass number. 3. Atomic and mass numbers e. A nuclide or an isotope is denoted by the chemical symbol with the mass number on the top left corner (and sometimes with the atomic number on the bottom left corner) or by name-a or by symbol-a Example: Cu or Cu or copper - 64 or Cu f. Ions are charged particles in which the number of electrons is different than the number of protons. A positive ion is called a cation, and it has less electrons than protons. A negative ion is called an anion, and it has more electrons than protons. 12
33 O-I.6 10) Which of the following nuclides have 18 neutrons? * a. 18 O b. 32 S c. 34 S d. 31 P e. 32 P O-I.6 11) Which of the following nuclides have 18 nucleons? * a. 18 O b. 32 S c. 34 S d. 31 P e. 32 P 13
34 O-I.6 12) What is the symbol for a species composed of 19 protons, 20 neutrons, and 18 electrons? a. 40 Ar b. 40 Ca c. 34 S 2- d. 39 K + e. 40 Ca 2+ ** O-I.6 13) Which of the following statements is false? a. 17 O nuclide has 8 electrons. b. 32 S 2- nuclide has 32 nucleons. c. 32 S 2- ion has 16 electrons. d. 18 O nuclide has 10 neutrons. e. 2 H 2 16 O molecule has 10 protons. ** 14
35 O-I.6 14) Give the number of protons, neutrons, and electrons in the 34 S nuclide. * a. 16 p, 18 n, 15 e b. 16 p, 18 n, 16 e c. 16 p, 16 n, 16 e d. 16 p, 18 n, 18 e e. 34 p, 16 n, 18 e O-I.6 15) Give the number of protons, neutrons, and electrons in the ion. a. 16 p, 18 n, 15 e b. 16 p, 18 n, 16 e c. 16 p, 16 n, 19 e d. 16 p, 18 n, 18 e e. 34 p, 16 n, 18 e S ** 15
36 4. Atomic masses a. The atomic mass of an element, in the periodic table, is the average mass (in amu or g/mol) of an element based on the natural abundance of element s isotopes and the isotope masses. It is a weighted average. It is not the same as the mass number A. 4. Atomic and mass numbers b. For example, consider an atom with three isotopes with masses a, b, and c and percentage natural abundances x, y, and z, respectively. The sum of all abundances should be 100: x + y + z = 100 ax by cz ax by cz Atomic mass x y z 100 Generalizing, Atomic mass = (abundance isotope mass)
37 O-I.7 ** 16) If an element consisted of three isotopes in the following relative abundance, what would the atomic weight of the element be? This is a hypothetical example % amu 50.00% amu 20.00% amu a amu b amu c amu d amu e amu C. Mole and Molar Mass (O-I.8 & O-I.9) 1. The mole and molar mass a. One mole is the amount of substance having Avogadro number of particles (i.e., atoms, ions, molecules, etc.). Avogadro number: N A = Avogadro number is the number of 12 C atoms in 12 g of 12 C. b. Molar mass (denoted M M or ) is the mass of 1 mole of substance. 17
38 1. The mole and molar mass c. Transformations between mass, number of moles, and number of particles can be done based on the following equalities: N A particles = 1 mole = M M g (or M M amu) 2. The mass of an atom a. The mass of an atom in grams is the molar mass divided by N A. b. The molar mass in grams has the same numerical value of (atomic) mass in amu. 1 amu = 1 g / N A 18
39 O-I.8 17) How many grams are in a mole of carbon? * a b. 16 c. 40 d. 52 e. 12 ** O-I.8 18) Calculate the number of moles of nitrogen atoms in 35 grams of nitrogen. a. 1.3 moles b. 1.7 moles 1 mol N = 14 g c moles d. 2.5 moles 1 mol 14 g = 1 = 1 e. 5.0 moles 14 g 1 mol 35 g = 2.5 mol 1 mol 14 g 19
40 O-I.8 19) How many grams of Mo equal 2.50 moles of Mo? a g 1 mol Mo = 95.9 g b. 238 g 95.9 g c. 178 g = 1 d. 150 g 1 mol e g 2.5 mol 95.9 g = 239 g 1 mol? g = 95.9 g 1 mol 2.5 mol * O-I.8 20) Suppose you have a 10-gram sample of each of the following elements. Which sample contains the smallest number of moles? a. Cr b. Na c. P d. N e. Pb *** 20
41 O-I.9 21) The mass of one oxygen atom is *** a g b g c g d g e g 1 mol O = 16 g = atoms 1 atom = g g atoms 21
42 Unit II Quantum Theory Question Difficulty: * Normal ** Difficult *** Very difficult A. Energy and Light (O-II.2 O-II.4) 1. Electromagnetic radiation a. Electromagnetic radiation (or wave) has an oscillating electric field perpendicular to a magnetic field. Light (or visible light) is part of electromagnetic spectrum. Waves have the property of interference. 1
43 1. Electromagnetic radiation b. Electromagnetic radiation (or wave) is characterized by: Wavelength () the distance between two maximum in amplitude Frequency () number of oscillations per unit time Energy (E) Wavenumbers ( ~ ) c. There are relations between the properties of waves: = c where c is the speed of light (c = m/s) E = h = hc/ where h is Planck s constant (h = Js) ~ 1 1. Electromagnetic radiation d. The SI unit of wavelength is m, the SI unit of frequency is s 1 = Hz, and the SI unit of wavenumber is m 1. 2
44 2. Regions of electromagnetic spectrum a. Electromagnetic spectrum includes all wavelengths of radiation. b. Electromagnetic spectrum contains the following regions: gamma-rays (-rays) X-rays UV visible IR microwave radio O-II.2 1) Which of the following regions of electromagnetic spectrum has the largest wavelength? * a. visible b. infrared c. ultraviolet d. microwave e. X-rays 3
45 O-II.3 2) Which statement about electromagnetic radiation is false? * a. As frequency increases, wavelength decreases. b. As wavelength increases, energy increases. c. As wavelength increases, frequency decreases. d. Wavelength and frequency are inversely proportional. e. Wavelength and energy are inversely proportional. O-II.3 3) A police officer is measuring traffic speed with radar operating at Hz. What is the wavelength of this electromagnetic energy? a m b nm c Å d m e m * 4
46 O-II.3 4) What is the frequency of light having a wavelength of cm? * a s 1 b s 1 c s 1 d s 1 e s 1 O-II.3 5) A tanning booth uses ultraviolet light at a wavelength of 1000 Å. What is the frequency of this light? a s -1 b s -1 c s -1 d s -1 e s -1 * 5
47 O-II.3 6) What is the wavelength in Ångstroms of radiation used by an x-ray technician, with a frequency of s 1? a Å b Å c Å d Å e Å ** B. Development of Quantum Theory (O-II.5 O-II.7) 1. Quantum theory discoveries a. A series of discoveries at the beginning of the 20 th century led to the development of quantum theory. Scientist Property Development Plank blackbody radiation quantized energy Einstein photoelectric effect light behaving as particle Rutherford gold foil experiment Bohr nuclear model of atom line spectra of H atom quantized energy of electron de Broglie wave nature of particle Heisenberg location of particles de Broglie wavelength uncertainty principle Schrödinger describing particles wave function for electron 6
48 1. Quantum theory discoveries b. Plank introduced the idea of quantized energy for oscillating particles given by E = h, where h is Planck s constant. c. To explain photoelectric effect, Einstein introduced the idea of light behaving like particle called photon. Energy of photon is given by E photon = h d. De Broglie introduced the idea of moving particles behaving like wave and having a de Broglie wavelength associated with it. h m u where is de Broglie wavelength, m is the mass, and u is the speed. 1. Quantum theory discoveries e. Heisenberg realized that the position and the momentum of a particle cannot be determined simultaneously with any desired precision. h x p 4 where x is uncertainty in position and p is uncertainty in momentum. f. Schrödinger proposed that particles should be described by wave functions () that can be obtained from the Schrodinger equation. 7
49 O-II.4 7) All of the following are true statements about electromagnetic radiation (light) except? * a. As wavelength increases frequency decreases. b. As energy increases frequency decreases. c. As wavelength increases energy decreases. d. The product of wavelength and frequency is constant. e. Amplitude is independent of frequency. O-II.4 8) Radio waves are very low energy forms of electromagnetic radiation. What is the energy of a photon of radio waves with a wavelength of 150 m? a J b J c J d J e J * 8
50 O-II.4 9) The energy of a photon is J. What is the wavelength of the corresponding light? a. 485 nm b m c m d. 485 pm e m * O-II.4 ** 10) The photoelectric work function of a metal is the minimum energy needed to eject an electron by irradiating the metal with light. For calcium, this work function equals J. What is the minimum frequency of light for the photoelectric effect in calcium? a s 1 b s 1 c s 1 d s 1 e s 1 9
51 O-II.4 11) The emission spectrum of mercury shows a line of wavelength 579 nm. How much energy is emitted as the excited electron falls to a lower energy level? a J/atom b J/atom c J/atom d J/atom e J/atom ** O-II.4 12) What is the energy, in J/photon, of ultraviolet light with a frequency of Hz? a J/photon b J/photon c J/photon d J/photon e J/photon ** 10
52 O-II.5 13) Who developed an explanation for the photoelectric effect? * a. Einstein b. Planck c. Rutherford d. Millikan e. Bohr O-II.5 ** 14) Which statement regarding the photoelectric effect is false? a. Electrons can be ejected only if the light is of sufficiently short wavelength. b. The current increases with increasing intensity of the light. c. Electrons can be ejected only if the light is of sufficiently high energy. d. The current does not depend on the color of the light as long as the wavelength is short enough. e. The wavelength limit sufficient for the ejection of electrons is the same for all metals. 11
53 O-II.6 15) Which of the following has the lowest de Broglie wavelength? ** a. A proton moving with a speed of 500 ms 1. b. A proton moving with a speed of 200 ms 1. c. An electron moving with a speed of 1000 ms 1. d. An electron moving with a speed of 500 ms 1. e. An electron moving with a speed of 200 ms 1. O-II.6 16) An electron of mass g is traveling at m/s. Calculate its de Broglie wavelength (in Å). a Å b Å c. 345 Å d Å e Å *** 12
54 2. Bohr model of H atom a. Bohr extended the nuclear model of atom proposed by Rutherford to explain the line spectrum of H atom. Line spectrum means that only certain wavelength (or frequency) are emitted or absorbed by H atom. b. The experimentally determined wavelengths were described by Rydberg equation R where n 2 > n 1. 2 n1 n2 2 R = cm 1 is the Rydberg constant. 2. Bohr model of H atom c. Bohr proposed that only certain energy levels for the electron in H atom are possible. constant E n = E = 0 n n = 3 where constant = n = 2 J. n is called quantum number, and possible values are n = 1, 2, 3, n = 1 13
55 2. Bohr model of H atom c. Bohr proposed that only certain energy levels for the electron in H atom are possible. The lowestenergy state (obtained when n = 1) is called ground state. n = 5 4 E = 0 n = 3 n = 2 n = 1 2. Bohr model of H atom c. Bohr proposed that only certain energy levels for the electron in H atom are possible. All other states (obtained when n = 2,3, ) are higher in energy and are called excited states. n = 5 4 E = 0 n = 3 n = 2 n = 1 14
56 2. Bohr model of H atom d. Bohr proposed that radiation is emitted (or absorbed) when the electron moves from one energy state (or level) to another. c E E E 1 1 n n1 constant h h n n constant R hc n1 n2 n1 n2 2 A photon is emitted when n initial > n final and energy is lost by the H atom. A photon is absorbed when n initial < n final and energy is gained by the H atom. 2. Bohr model of H atom e. Bohr model/theory of H atom explained the spectrum of H atom that contains a series of lines. Lyman series in UV is due to n = 2, 3, 4, to n = 1 transitions. Balmer series in visible is due to n = 3, 4, 5, to n = 2 transitions. Pashen series in IR is due to n = 4, 5, 6, to n = 3 transitions. Bracket series in IR is due to n = 5, 6, 7, to n = 4 transitions. 15
57 O-II.7 ** 17) When an electron of an excited hydrogen atom falls from level n = 2 to level n = 1, what wavelength of light is emitted? R = m -1 a Å b Å c Å d. 970 Å e Å ** O-II.7 18) What is the frequency of the first line in the Paschen series? a Hz b Hz c Hz d Hz e Hz 16
58 17 C. Quantum Mechanical Treatment of H Atom (O-II.8 & O-II.9) 1. Wave functions and orbitals a. Schrodinger equation provides the wave function for electron in hydrogen atom. b. The wave function contains all possible information about the electron in hydrogen atom. c. The location of the electron is not completely known so one need to discuss the probability of finding the electron or electron density, which is determined by 2. Wave function () Obtained by solving Schrödinger equation Schrödinger equation for H atom in spherical coordinates ),, ( ),, ( ),, ( z y x E z y x z y x V z y x m E r e r m r m r r r r m r e r e e 0 2, , 2 2, sin 1 2 sin sin
59 R Wave function () for H atom r) 2 m nlm ( r,, ) Rnl ( r) Y l (, ) n n l n l 1!! 1/ 2 l 3 / 2 2 l r / na r e 3 na0 ( 0 nl m Y l (2l 1) ( l m )! (, ) 4 ( l m )! 1/ 2 m P l im cos e 2l 1 2r L nl na0 1 L 1 ( x) 1 1 L 2 ( x) 2!(2 x) 3 L 3 ( x) 3! L 3 ( x) 3!(3 3x x ) 2 3 L 4 ( x) 4!(4 x) 5 L 5 ( x) 5! L 4 ( x) 4!(4 6x 2x x ) L 5 ( x) 5!(10 5x x ) 2 5 L 6 ( x) 6!(6 x) 7 L 7 ( x) 7! m m m 2 ( ) (1 ) 2 d P l x x Pl ( x) m dx 1. Wave functions and orbitals d. Schrodinger equation introduces the concept of orbital, which very generally indicate a description of an electron, but is typically used to indicate one of the following: the wave function depending on three quantum numbers the wave function square 2 probability of finding the electron or electron density boundary surface a 3-dimensional representation of the probability of finding the electron or electron density 18
60 2. Shells, subshells and orbitals in H atom a. The electron wave function (or the electron properties/behavior) in an atom is dependent on four quantum numbers that are described below. Quantum Name Possible Determines number values (or describes) n principal 1, 2, 3, 4, E or size of orbital l angular momentum 0, 1,, (n -1) shape of orbital m l or m magnetic -l,, 0,, l orientation m s spin +1/2 or -1/2 electron spin 2. Shells, subshells and orbitals in H atom a. The electron wave function (or the electron properties/behavior) in an atom is dependent on four quantum numbers that are described below. Angular momentum quantum number l is also called azimuthal or secondary quantum number. The value of angular momentum quantum number l is given by a letter as follows: l value orbital designation s p d f g h Various m l values are sometimes described by the Cartesian coordinates or combinations of Cartesian coordinates: x, y, and z for p orbitals, xy, xz, yz, x 2 -y 2, and z 2 for d orbitals. 19
61 2. Shells, subshells and orbitals in H atom b. One can construct a diagram showing all possibilities for the electron based on possible quantum numbers. l n = 4 m l n = 3 3s 3p 3d n = 2 2s 2p n = 1 1s 2. Shells, subshells and orbitals in H atom b. One can construct a diagram showing all possibilities for the electron based on possible quantum numbers. All boxes with same energy (or n) represent a shell. Each set of boxes represents a subshell. Each box represents an orbital. Each box can contain up to two electrons (with different m s ). 20
62 2. Shells, subshells and orbitals in H atom c. The quantum numbers describe various quantities. Quantum number n describes a shell, level, or period. Quantum numbers n and l describe a subshell or sublevel. Quantum numbers n, l and m l describe an orbital. Quantum numbers n, l, m l, and m s describe an electron. d. For H atom, the energy of subshells (or types of orbitals) is determined only by n and increases in the order: 1s < 2s = 2p < 3s = 3p =3d < 4s = 4p = 4d = 4f <5s=.. 2. Shells, subshells and orbitals in H atom e. The number of orbitals into a subshell is (2l + 1) and the number of orbitals into a shell is n 2. f. The maximum number of electrons that can be accommodated or fit into an orbital is 2. g. The maximum number of electrons that can be accommodated or fit into a subshell is 2(2l + 1). 2 in s subshell, 6 in p subshell, etc. h. The maximum number of electrons that can be accommodated or fit into a shell is 2n 2. 2 in shell 1, 8 in shell 2, 18 in shell 3, etc. 21
63 3. Representing orbitals a. Orbitals are typically represented by the boundary surface, which is the surface (of equal electron density) that contains 95% of probability to finding the electron. They are not surfaces on which the electron moves! 3. Representing orbitals b. (The boundary surfaces of) orbitals have different sizes (determined by n), different shapes (determined by l), and different orientations (determined by m l ). s orbitals have spherical shape 1s 2s p orbitals have bilobal/dumbbell shape 2p x 2p y 2p z 22
64 3. Representing orbitals b. (The boundary surfaces of) orbitals have different sizes (determined by n), different shapes (determined by l), and different orientations (determined by m l ). d orbitals have tetralobal shape f orbitals have octalobal shape O-II.8 19) Which of the following quantum numbers determine the orientation of an orbital? * a. n b. m l c. l d. j e. m s 23
65 O-II.8 20) Which of the following quantum numbers determine the energy of an orbital? * a. n b. m l c. l d. j e. m s O-II.8 21) Which of the following quantum numbers determine the shape of an orbital? * a. n b. m l c. l d. j e. m s 24
66 O-II.8 22) Which of the following sets of quantum numbers are permissible for an electron? * a. n = 1, l = 1, m l = 0, m s = 1/2 b. n = 2, l = 1, m l = 2, m s = 1/2 c. n = 3, l = 1, m l = 1, m s = +1 d. n = 2, l = 1, m l = 1, m s = 1/2 e. n = 3, l = 2, m l = 1, m s = +1/2 O-II.8 23) Which of the following sets of quantum numbers are NOT permissible for an electron? * a. n = 1, l = 0, m l = 0, m s = 1/2 b. n = 2, l = 1, m l = 1, m s = 1/2 c. n = 3, l = 1, m l = 1, m s = +1/2 d. n = 2, l = 1, m l = 1, m s = 1/2 e. n = 3, l = 1, m l = 0, m s = +1/2 25
67 O-II.8 24) Which of the following sets of quantum numbers (n, l, m l, m s ) is permissible for an electron in an atom? a. (4, 2, 0, +1/2) b. (2, 0, 1, 1/2) c. (3, 2, 0, +1/2) d. (3, 2, 3, 1/2) e. (2, 2, 1, 1/2) f. (2, 1, 0, 0) ** O-II.8 & O-II.9 *** 25) Which of the following statements is false? a. The spin quantum number has values of either +1/2 or 1/2. b. An f set of orbitals is filled with 10 electrons. c. The third energy level has 5 d orbitals. d. A set of p orbitals in a given energy level are equal in energy. e. The magnetic quantum number has its values restricted by the l quantum number. 26
68 O-II.9 26) What is the value of the azimuthal quantum number, l, for orbital? * a. 0 b. 1/2 c. -1 d. 1 e. 2 O-II.9 27) What is the value of l for the orbital aside? * a. 1/2 b. 1 c. 0 d. 3 e. 2 f. 1 27
69 O-II.9 ** 28) Which of the following sets of quantum numbers (n, l, m l ) are associated with the 4p subshell? a. (4, 0, 0) b. (4, 2, 1) c. (4, 1, 1) d. (3, 2, 0) e. (3, 1, 1) f. (3, 1, 1) O-II.9 29) The third energy level or shell of an atom can hold a maximum of electrons. a. 25 b. 18 c. 8 d. 16 e. 2 ** 28
70 O-II.4 *** 30) One of the spectral lines in the emission spectrum of mercury has a wavelength of m. How much energy is emitted if 1.00 mole of mercury atoms emits light of m? Express your answer in kj/mol. a. 127 kj/mol b. 485 kj/mol c. 192 kj/mol d kj/mol e kj/mol O-II.5 31) An electron is confined to a linear region with a length of the same order as the diameter of an atom (about 100 pm). What is the minimum uncertainty in its momentum? a kgms 1 b kgms 1 c kgms 1 d kgms 1 e kgms 1 *** 29
71 O-II.6 32) A moving electron has a de Broglie wavelength of 727 nm. What is its speed? *** a. 1 ms 1 b. 10 ms 1 c. 100 ms 1 d ms 1 e ms 1 O-II.7 33) A ground-state hydrogen atom absorbs a photon of light that has a wavelength of nm. What is the principal quantum number of the final state of the hydrogen atom? a. 2 b. 3 c. 4 d. 5 e. 6 *** 30
Multiple Choice Identify the choice that best completes the statement or answers the question.
 Chemi1100pretest1 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Accordingly to the periodic law the properties of elements repeat at regular intervals
Chemi1100pretest1 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Accordingly to the periodic law the properties of elements repeat at regular intervals
Ch 7 Quantum Theory of the Atom (light and atomic structure)
 Ch 7 Quantum Theory of the Atom (light and atomic structure) Electromagnetic Radiation - Electromagnetic radiation consists of oscillations in electric and magnetic fields. The oscillations can be described
Ch 7 Quantum Theory of the Atom (light and atomic structure) Electromagnetic Radiation - Electromagnetic radiation consists of oscillations in electric and magnetic fields. The oscillations can be described
Atomic Structure. Standing Waves x10 8 m/s. (or Hz or 1/s) λ Node
 Atomic Structure Topics: 7.1 Electromagnetic Radiation 7.2 Planck, Einstein, Energy, and Photons 7.3 Atomic Line Spectra and Niels Bohr 7.4 The Wave Properties of the Electron 7.5 Quantum Mechanical View
Atomic Structure Topics: 7.1 Electromagnetic Radiation 7.2 Planck, Einstein, Energy, and Photons 7.3 Atomic Line Spectra and Niels Bohr 7.4 The Wave Properties of the Electron 7.5 Quantum Mechanical View
Q1 and Q2 Review large CHEMISTRY
 Q1 and Q2 Review large CHEMISTRY Multiple Choice Identify the choice that best completes the statement or answers the question. 1. E = hv relates the following a. Energy to Planck s constant & wavelength
Q1 and Q2 Review large CHEMISTRY Multiple Choice Identify the choice that best completes the statement or answers the question. 1. E = hv relates the following a. Energy to Planck s constant & wavelength
Atomic Structure 11/21/2011
 Atomic Structure Topics: 7.1 Electromagnetic Radiation 7.2 Planck, Einstein, Energy, and Photons 7.3 Atomic Line Spectra and Niels Bohr 7.4 The Wave Properties of the Electron 7.5 Quantum Mechanical View
Atomic Structure Topics: 7.1 Electromagnetic Radiation 7.2 Planck, Einstein, Energy, and Photons 7.3 Atomic Line Spectra and Niels Bohr 7.4 The Wave Properties of the Electron 7.5 Quantum Mechanical View
CP Chemistry Semester 1 Final Review KEY. Unit 1
 CP Chemistry Semester 1 Final Review KEY Unit 1 Practice Problems 1. What tool do you use to measure volume of water? Describe how to make a proper measurement of a volume of water using a 50 ml graduated
CP Chemistry Semester 1 Final Review KEY Unit 1 Practice Problems 1. What tool do you use to measure volume of water? Describe how to make a proper measurement of a volume of water using a 50 ml graduated
Chemistry (
 Question 2.1: (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. Answer 2.1: (i) Mass of one electron = 9.10939 10 31
Question 2.1: (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. Answer 2.1: (i) Mass of one electron = 9.10939 10 31
I. Multiple Choice Questions (Type-I)
 I. Multiple Choice Questions (Type-I) 1. Which of the following conclusions could not be derived from Rutherford s α -particle scattering experiement? (i) Most of the space in the atom is empty. (ii) The
I. Multiple Choice Questions (Type-I) 1. Which of the following conclusions could not be derived from Rutherford s α -particle scattering experiement? (i) Most of the space in the atom is empty. (ii) The
Chemistry 111 Dr. Kevin Moore
 Chemistry 111 Dr. Kevin Moore Black Body Radiation Heated objects emit radiation based on its temperature Higher temperatures produce higher frequencies PhotoElectric Effect Light on a clean metal surface
Chemistry 111 Dr. Kevin Moore Black Body Radiation Heated objects emit radiation based on its temperature Higher temperatures produce higher frequencies PhotoElectric Effect Light on a clean metal surface
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
Observations. Qualitative: descriptive observation that is not numerical. Quantitative: Numerical observation.
 Mid-Term Topics Observations Qualitative: descriptive observation that is not numerical. Example: This apple is red. Quantitative: Numerical observation. Example: The temperature of this room is 23 C.
Mid-Term Topics Observations Qualitative: descriptive observation that is not numerical. Example: This apple is red. Quantitative: Numerical observation. Example: The temperature of this room is 23 C.
Ch 1 Chemistry and Measurement
 Ch 1 Chemistry and Measurement Matter - Matter is anything that has substance and occupies space. Matter also has mass and volume. - A material is any particular type of matter. - An atom is the smallest
Ch 1 Chemistry and Measurement Matter - Matter is anything that has substance and occupies space. Matter also has mass and volume. - A material is any particular type of matter. - An atom is the smallest
Chapter 9: Electrons and the Periodic Table
 C h e m i s t r y 1 2 C h 9 : E l e c t r o n s a n d P e r i o d i c T a b l e P a g e 1 Chapter 9: Electrons and the Periodic Table Work on MasteringChemistry assignments What we have learned: Dalton
C h e m i s t r y 1 2 C h 9 : E l e c t r o n s a n d P e r i o d i c T a b l e P a g e 1 Chapter 9: Electrons and the Periodic Table Work on MasteringChemistry assignments What we have learned: Dalton
10. What word is used to describe properties of a substance that depend on the quantity of substance? Give two examples of such properties.
 1. In which state does matter have a definite shape and volume? 2. In which state of matter are forces between particles least dominant? 3. What kind of change does not alter the composition or identity
1. In which state does matter have a definite shape and volume? 2. In which state of matter are forces between particles least dominant? 3. What kind of change does not alter the composition or identity
UNIT TWO TEST HISTORY OF ATOM, STRUCTURE OF ATOM, ATOMIC MASS CARBON-12
 KEY Review Sheet: UNIT TWO TEST HISTORY OF ATOM, STRUCTURE OF ATOM, ATOMIC MASS 1. Know which isotope is the standard for the atomic mass unit. CARBON-12 2. Know what the difference in masses of isotopes
KEY Review Sheet: UNIT TWO TEST HISTORY OF ATOM, STRUCTURE OF ATOM, ATOMIC MASS 1. Know which isotope is the standard for the atomic mass unit. CARBON-12 2. Know what the difference in masses of isotopes
Example 3: 4000: 1 significant digit Example 4: : 4 significant digits
 Notes: Measurement and Math 1 Accuracy and Precision Precision depends on the precision of the measuring device o For example a device that can measure to the ten thousands place (1.6829 grams) is a more
Notes: Measurement and Math 1 Accuracy and Precision Precision depends on the precision of the measuring device o For example a device that can measure to the ten thousands place (1.6829 grams) is a more
Observation information obtained through the senses; observation in science often involves measurement
 Review Sheet Unit 1: The Atom Chemistry the study of the composition of matter and the changes matter undergoes Scientific Method Scientific method a logical, systematic approach to the solution of a scientific
Review Sheet Unit 1: The Atom Chemistry the study of the composition of matter and the changes matter undergoes Scientific Method Scientific method a logical, systematic approach to the solution of a scientific
1. What is the difference between a qualitative and quantitative observation? Give at least one example of each.
 1 st 9wks Exam Review Name Per. Chemistry 1H Scientific Skills Unit - Lab Safety & Equipment: KEY EQUIPMENT TO KNOW: +beaker +Erlenmeyer flask +beaker tongs +balance +graduated cylinder +test tube +test
1 st 9wks Exam Review Name Per. Chemistry 1H Scientific Skills Unit - Lab Safety & Equipment: KEY EQUIPMENT TO KNOW: +beaker +Erlenmeyer flask +beaker tongs +balance +graduated cylinder +test tube +test
Chapter Test B. Chapter: Arrangement of Electrons in Atoms. possible angular momentum quantum numbers? energy level? a. 4 b. 8 c. 16 d.
 Assessment Chapter Test B Chapter: Arrangement of Electrons in Atoms PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question
Assessment Chapter Test B Chapter: Arrangement of Electrons in Atoms PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question
Chapter 5. The Electromagnetic Spectrum. What is visible light? What is visible light? Which of the following would you consider dangerous?
 Which of the following would you consider dangerous? X-rays Radio waves Gamma rays UV radiation Visible light Microwaves Infrared radiation Chapter 5 Periodicity and Atomic Structure 2 The Electromagnetic
Which of the following would you consider dangerous? X-rays Radio waves Gamma rays UV radiation Visible light Microwaves Infrared radiation Chapter 5 Periodicity and Atomic Structure 2 The Electromagnetic
Chem 1075 Chapter 5 Models of the Atom Lecture Outline
 Chem 1075 Chapter 5 Models of the Atom Lecture Outline Slide 2 Dalton Model of the Atom John Dalton proposed that is made up of The particles are or can be broken down into by chemical processes. cannot
Chem 1075 Chapter 5 Models of the Atom Lecture Outline Slide 2 Dalton Model of the Atom John Dalton proposed that is made up of The particles are or can be broken down into by chemical processes. cannot
8. Which of the following could be an isotope of chlorine? (A) 37 Cl 17 (B) 17 Cl 17 (C) 37 Cl 17 (D) 17 Cl 37.5 (E) 17 Cl 37
 Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
Light. Light (con t.) 2/28/11. Examples
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Chapter 1 Matter and Energy. Classifying Matter An Exercise. Chemical Classifications of Matter
 Chapter 1 Matter and Energy Matter and its Classification Physical and Chemical Changes and Properties of Matter Energy and Energy Changes Scientific Inquiry 1-1 Copyright The McGraw-Hill Companies, Inc.
Chapter 1 Matter and Energy Matter and its Classification Physical and Chemical Changes and Properties of Matter Energy and Energy Changes Scientific Inquiry 1-1 Copyright The McGraw-Hill Companies, Inc.
AP Chapter 6 Study Questions
 Class: Date: AP Chapter 6 Study Questions True/False Indicate whether the statement is true or false. 1. The wavelength of radio waves can be longer than a football field. 2. Black body radiation is the
Class: Date: AP Chapter 6 Study Questions True/False Indicate whether the statement is true or false. 1. The wavelength of radio waves can be longer than a football field. 2. Black body radiation is the
UNIT 2 - ATOMIC THEORY
 UNIT 2 - ATOMIC THEORY VOCABULARY: Allotrope Electron Configuration Nuclear Charge Anion Element Nucleons Atom Excited state Nucleus Atomic Mass Ground state Orbital Atomic Mass unit (a.m.u.) Ion Proton
UNIT 2 - ATOMIC THEORY VOCABULARY: Allotrope Electron Configuration Nuclear Charge Anion Element Nucleons Atom Excited state Nucleus Atomic Mass Ground state Orbital Atomic Mass unit (a.m.u.) Ion Proton
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Explain the term subshell. 5. Explain de-broglie equation (relationship). 6. Discuss the dual nature of electrons
 MODEL COLLEGE ASSIGNMENT SHEET FOR STRUCTURE OF ATOM. What are the main postulates of Bohr s theory of an atom?. Explain how the angular momentum of an electron in an atom is quantized? 3. What are the
MODEL COLLEGE ASSIGNMENT SHEET FOR STRUCTURE OF ATOM. What are the main postulates of Bohr s theory of an atom?. Explain how the angular momentum of an electron in an atom is quantized? 3. What are the
Honors Ch3 and Ch4. Atomic History and the Atom
 Honors Ch3 and Ch4 Atomic History and the Atom Ch. 3.1 The Atom is Defined 400 B.C. the Greek philosopher Democritus said that the world was made of two things: Empty space and tiny particles called atoms
Honors Ch3 and Ch4 Atomic History and the Atom Ch. 3.1 The Atom is Defined 400 B.C. the Greek philosopher Democritus said that the world was made of two things: Empty space and tiny particles called atoms
UNIT 2 - ATOMIC THEORY
 UNIT 2 - ATOMIC THEORY VOCABULARY: Allotrope Electron Configuration Nuclear Charge Anion Element Nucleons Atom Excited state Nucleus Atomic Mass Ground state Orbital Atomic Mass unit (a.m.u.) Ion Proton
UNIT 2 - ATOMIC THEORY VOCABULARY: Allotrope Electron Configuration Nuclear Charge Anion Element Nucleons Atom Excited state Nucleus Atomic Mass Ground state Orbital Atomic Mass unit (a.m.u.) Ion Proton
UNIT 2 - ATOMIC THEORY
 *KEY* *KEY* UNIT 2 - ATOMIC THEORY *KEY* *KEY* VOCABULARY: Allotrope Anion Atom Atomic Mass Atomic Mass unit (a.m.u.) Atomic number Bohr model Cation Compound Electron Electron Configuration Element Excited
*KEY* *KEY* UNIT 2 - ATOMIC THEORY *KEY* *KEY* VOCABULARY: Allotrope Anion Atom Atomic Mass Atomic Mass unit (a.m.u.) Atomic number Bohr model Cation Compound Electron Electron Configuration Element Excited
CHEMISTRY I - HONORS MIDTERM REVIEW* *Test may cover other topics not included on this review, yet have been covered throughout the semester.
 Name Period CHEMISTRY I - HONORS MIDTERM REVIEW* *Test may cover other topics not included on this review, yet have been covered throughout the semester. Chapter 2 Measurement & Calculations Describe the
Name Period CHEMISTRY I - HONORS MIDTERM REVIEW* *Test may cover other topics not included on this review, yet have been covered throughout the semester. Chapter 2 Measurement & Calculations Describe the
Chemistry Mid-Term Practice Exam
 Chemistry Mid-Term Practice Exam Multiple Choice. Identify the choice that best completes the statement or answers the question. 1. A measure of the 3-D space matter occupies is a. density. c. volume.
Chemistry Mid-Term Practice Exam Multiple Choice. Identify the choice that best completes the statement or answers the question. 1. A measure of the 3-D space matter occupies is a. density. c. volume.
Chapter 4 The Structure of the Atom
 Chapter 4 The Structure of the Atom Read pg. 86-97 4.1 Early Theories of Matter The Philosophers Democritus Artistotle - Artistotle s influence so great and the science so primitive (lacking!) his denial
Chapter 4 The Structure of the Atom Read pg. 86-97 4.1 Early Theories of Matter The Philosophers Democritus Artistotle - Artistotle s influence so great and the science so primitive (lacking!) his denial
Atomic Theory. Early models
 Atomic Theory Early models Ancient Greece Late 18 th century 4 elements Earth, Water, Wind, Fire: Matter is made up in different combinations of these 4 elements. First atom proposed by Democritus (Greek)
Atomic Theory Early models Ancient Greece Late 18 th century 4 elements Earth, Water, Wind, Fire: Matter is made up in different combinations of these 4 elements. First atom proposed by Democritus (Greek)
Atomic Structure Discovered. Dalton s Atomic Theory. Discovery of the Electron 10/30/2012
 Atomic Structure Discovered Ancient Greeks Democritus (460-362 BC) - indivisible particles called atoms Prevailing argument (Plato and Aristotle) - matter is continuously and infinitely divisible John
Atomic Structure Discovered Ancient Greeks Democritus (460-362 BC) - indivisible particles called atoms Prevailing argument (Plato and Aristotle) - matter is continuously and infinitely divisible John
What are the isotopes of hydrogen? H, 2 H, and 3 H
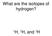 What are the isotopes of hydrogen? 1 H, 2 H, and 3 H The average mass of a boron atom is 10.81. Assuming you were able to isolate only one boron atom, the chance that you would randomly get one with a
What are the isotopes of hydrogen? 1 H, 2 H, and 3 H The average mass of a boron atom is 10.81. Assuming you were able to isolate only one boron atom, the chance that you would randomly get one with a
Chapter 7: The Quantum-Mechanical Model of the Atom
 C h e m i s t r y 1 A : C h a p t e r 7 P a g e 1 Chapter 7: The Quantum-Mechanical Model of the Atom Homework: Read Chapter 7. Work out sample/practice exercises Check for the MasteringChemistry.com assignment
C h e m i s t r y 1 A : C h a p t e r 7 P a g e 1 Chapter 7: The Quantum-Mechanical Model of the Atom Homework: Read Chapter 7. Work out sample/practice exercises Check for the MasteringChemistry.com assignment
2) The energy of a photon of light is proportional to its frequency and proportional to its wavelength.
 Advanced Chemistry Chapter 13 Review Name Per Show all work Wave Properties 1) Which one of the following is correct? A) ν + λ = c B) ν λ = c C) ν = cλ D) λ = c ν E) νλ = c 2) The energy of a photon of
Advanced Chemistry Chapter 13 Review Name Per Show all work Wave Properties 1) Which one of the following is correct? A) ν + λ = c B) ν λ = c C) ν = cλ D) λ = c ν E) νλ = c 2) The energy of a photon of
Chapter 27 Early Quantum Theory and Models of the Atom Discovery and Properties of the electron
 Chapter 27 Early Quantum Theory and Models of the Atom 27-1 Discovery and Properties of the electron Measure charge to mass ratio e/m (J. J. Thomson, 1897) When apply magnetic field only, the rays are
Chapter 27 Early Quantum Theory and Models of the Atom 27-1 Discovery and Properties of the electron Measure charge to mass ratio e/m (J. J. Thomson, 1897) When apply magnetic field only, the rays are
Light. October 16, Chapter 5: Electrons in Atoms Honors Chemistry. Bohr Model
 Chapter 5: Electrons in Atoms Honors Chemistry Bohr Model Niels Bohr, a young Danish physicist and a student of Rutherford improved Rutherford's model. Bohr proposed that an electron is found only in specific
Chapter 5: Electrons in Atoms Honors Chemistry Bohr Model Niels Bohr, a young Danish physicist and a student of Rutherford improved Rutherford's model. Bohr proposed that an electron is found only in specific
What is a theory? An organized system of accepted knowledge that applies in a variety of circumstances to explain a specific set of phenomena
 Atomic Structure What is a theory? An organized system of accepted knowledge that applies in a variety of circumstances to explain a specific set of phenomena Early Theories Democritus: 4 B.C.: atom He
Atomic Structure What is a theory? An organized system of accepted knowledge that applies in a variety of circumstances to explain a specific set of phenomena Early Theories Democritus: 4 B.C.: atom He
EOC review questions I
 Name: Class: _ Date: _ EOC review questions I Matching Match each item with the correct statement below. a. mixture d. reactant b. product e. heterogeneous mixture c. phase f. vapor 1. gaseous state of
Name: Class: _ Date: _ EOC review questions I Matching Match each item with the correct statement below. a. mixture d. reactant b. product e. heterogeneous mixture c. phase f. vapor 1. gaseous state of
Democritus & Leucippus (~400 BC) Greek philosophers: first to propose that matter is made up of particles called atomos, the Greek word for atoms
 Chemistry Ms. Ye Name Date Block The Evolution of the Atomic Model Since atoms are too small to see even with a very powerful microscope, scientists rely upon indirect evidence and models to help them
Chemistry Ms. Ye Name Date Block The Evolution of the Atomic Model Since atoms are too small to see even with a very powerful microscope, scientists rely upon indirect evidence and models to help them
Section 3.1 Substances Are Made of Atoms
 Section 3.1 Substances Are Made of Atoms Objectives: 1. State the three laws that support the existence of atoms. 2. List the five principles of John Dalton s atomic theory. Vocabulary: law of definite
Section 3.1 Substances Are Made of Atoms Objectives: 1. State the three laws that support the existence of atoms. 2. List the five principles of John Dalton s atomic theory. Vocabulary: law of definite
10/4/2011. Tells you the number of protons
 Atomic Structure The arrangement of the subatomic particles within the atom determines the chemical properties of the elements How they interact with one another The types of ions and structures that they
Atomic Structure The arrangement of the subatomic particles within the atom determines the chemical properties of the elements How they interact with one another The types of ions and structures that they
Chapter 2: The Structure of the Atom and the Periodic Table
 Chapter 2: The Structure of the Atom and the Periodic Table 1. What are the three primary particles found in an atom? A) neutron, positron, and electron B) electron, neutron, and proton C) electron, proton,
Chapter 2: The Structure of the Atom and the Periodic Table 1. What are the three primary particles found in an atom? A) neutron, positron, and electron B) electron, neutron, and proton C) electron, proton,
Atoms, Electrons and Light MS. MOORE CHEMISTRY
 Atoms, Electrons and Light MS. MOORE CHEMISTRY Atoms Remember Rutherford??? What did he discover with his gold foil experiment. A: Atoms contain a dense nucleus where the protons and neutrons reside. ATOMS
Atoms, Electrons and Light MS. MOORE CHEMISTRY Atoms Remember Rutherford??? What did he discover with his gold foil experiment. A: Atoms contain a dense nucleus where the protons and neutrons reside. ATOMS
CHE 105 Exam 1 Spring 2016
 CHE 105 Exam 1 Spring 2016 Your Name: Your ID: Question #: 1 Which one of the following states of matter does not take on the shape of its container? A. solid B. liquid C. gas Question #: 2 Which statement
CHE 105 Exam 1 Spring 2016 Your Name: Your ID: Question #: 1 Which one of the following states of matter does not take on the shape of its container? A. solid B. liquid C. gas Question #: 2 Which statement
Chapter 3. Table of Contents. Section 1 The Atom: From Philosophical Idea to Scientific Theory. Section 2 The Structure of the Atom
 Atoms: The Building Blocks of Matter Table of Contents Section 1 The Atom: From Philosophical Idea to Scientific Theory Section 2 The Structure of the Atom Section 1 The Atom: From Philosophical Idea to
Atoms: The Building Blocks of Matter Table of Contents Section 1 The Atom: From Philosophical Idea to Scientific Theory Section 2 The Structure of the Atom Section 1 The Atom: From Philosophical Idea to
Ch. 7 The Quantum Mechanical Atom. Brady & Senese, 5th Ed.
 Ch. 7 The Quantum Mechanical Atom Brady & Senese, 5th Ed. Index 7.1. Electromagnetic radiation provides the clue to the electronic structures of atoms 7.2. Atomic line spectra are evidence that electrons
Ch. 7 The Quantum Mechanical Atom Brady & Senese, 5th Ed. Index 7.1. Electromagnetic radiation provides the clue to the electronic structures of atoms 7.2. Atomic line spectra are evidence that electrons
Mid-Term Review Multiple Choice: Ch. 3 Identify the letter of the choice that best completes the statement or answers the question.
 HONORS CHEMISTRY MID-TERM PRACTICE January 2013 Mrs. Allen from HS North put together a collection of multiple choice questions for you to use as a study tool. Not all of the midterm topics are covered
HONORS CHEMISTRY MID-TERM PRACTICE January 2013 Mrs. Allen from HS North put together a collection of multiple choice questions for you to use as a study tool. Not all of the midterm topics are covered
Name Date Class MODELS OF THE ATOM
 5.1 MODELS OF THE ATOM Section Review Objectives Identify inadequacies in the Rutherford atomic model Identify the new assumption in the Bohr model of the atom Describe the energies and positions of electrons
5.1 MODELS OF THE ATOM Section Review Objectives Identify inadequacies in the Rutherford atomic model Identify the new assumption in the Bohr model of the atom Describe the energies and positions of electrons
2. For the following two compounds between oxygen and hydrogen: 3. Tell what discoveries were made by each of the following scientists:
 EXTRA HOMEWORK 1A 1. When Dalton proposed that matter was composed of atoms, why was his Atomic Theory accepted? 2. For the following two compounds between oxygen and hydrogen: Mass of O Mass of H Compound
EXTRA HOMEWORK 1A 1. When Dalton proposed that matter was composed of atoms, why was his Atomic Theory accepted? 2. For the following two compounds between oxygen and hydrogen: Mass of O Mass of H Compound
Semester 1 Final Whiteboard Review!
 Semester 1 Final Whiteboard Review! Identify the following data as being qualitative or quantitative: a. Blue and fuzzy qualitative b. 1.045 m quantitative c. warm and dry qualitative d. 101 F quantitative
Semester 1 Final Whiteboard Review! Identify the following data as being qualitative or quantitative: a. Blue and fuzzy qualitative b. 1.045 m quantitative c. warm and dry qualitative d. 101 F quantitative
CHAPTER 5. The Structure of Atoms
 CHAPTER 5 The Structure of Atoms Chapter Outline Subatomic Particles Fundamental Particles The Discovery of Electrons Canal Rays and Protons Rutherford and the Nuclear Atom Atomic Number Neutrons Mass
CHAPTER 5 The Structure of Atoms Chapter Outline Subatomic Particles Fundamental Particles The Discovery of Electrons Canal Rays and Protons Rutherford and the Nuclear Atom Atomic Number Neutrons Mass
Chapter 6. Electronic. Electronic Structure of Atoms Pearson Education
 Chapter 6 Laser: step-like energy transition 6.1 The Wave Nature of Light 6.2 Quantized Energy and Photons 6.3 Line Spectra and the Bohr Model 6.4 The Wave Behavior of Matter 6.5 Quantum Mechanics and
Chapter 6 Laser: step-like energy transition 6.1 The Wave Nature of Light 6.2 Quantized Energy and Photons 6.3 Line Spectra and the Bohr Model 6.4 The Wave Behavior of Matter 6.5 Quantum Mechanics and
1. The most important aspects of the quantum theory.
 Lecture 5. Radiation and energy. Objectives: 1. The most important aspects of the quantum theory: atom, subatomic particles, atomic number, mass number, atomic mass, isotopes, simplified atomic diagrams,
Lecture 5. Radiation and energy. Objectives: 1. The most important aspects of the quantum theory: atom, subatomic particles, atomic number, mass number, atomic mass, isotopes, simplified atomic diagrams,
Chapter 7 QUANTUM THEORY & ATOMIC STRUCTURE Brooks/Cole - Thomson
 Chapter 7 QUANTUM THEORY & ATOMIC STRUCTURE 1 7.1 The Nature of Light 2 Most subatomic particles behave as PARTICLES and obey the physics of waves. Light is a type of electromagnetic radiation Light consists
Chapter 7 QUANTUM THEORY & ATOMIC STRUCTURE 1 7.1 The Nature of Light 2 Most subatomic particles behave as PARTICLES and obey the physics of waves. Light is a type of electromagnetic radiation Light consists
Page 1 of 5. Chapter 1: Matter & Change 1) Define the following: and Give Examples A. an atom smallest piece of an element that
 NAME: CHEMISTRY I HONORS MID-COURSE REVIEW BLOCK: Chapter 1: Matter & Change 1) Define the following: and Give Examples A. an atom smallest piece of an element that retains all properties B. an element
NAME: CHEMISTRY I HONORS MID-COURSE REVIEW BLOCK: Chapter 1: Matter & Change 1) Define the following: and Give Examples A. an atom smallest piece of an element that retains all properties B. an element
Chapter 7. The Quantum- Mechanical Model of the Atom. Chapter 7 Lecture Lecture Presentation. Sherril Soman Grand Valley State University
 Chapter 7 Lecture Lecture Presentation Chapter 7 The Quantum- Mechanical Model of the Atom Sherril Soman Grand Valley State University The Beginnings of Quantum Mechanics Until the beginning of the twentieth
Chapter 7 Lecture Lecture Presentation Chapter 7 The Quantum- Mechanical Model of the Atom Sherril Soman Grand Valley State University The Beginnings of Quantum Mechanics Until the beginning of the twentieth
Which choice lists the states of matter in order from least compressible to most compressible?
 DRAFT Do Not Use Until Posted. Course Name: - Question #: 1 Which choice lists the states of matter in order from least compressible to most compressible? A. solid liquid
DRAFT Do Not Use Until Posted. Course Name: - Question #: 1 Which choice lists the states of matter in order from least compressible to most compressible? A. solid liquid
Chemistry CRT Study Guide First Quarter
 Number AL COS # 1. #1.0 Classify sodium chloride as an element, mixture, compound, or colloid. Compound 2. #1.0 Classify air as an element, mixture, compound, or colloid. Mixture 3. #1.0 Classify a blueberry
Number AL COS # 1. #1.0 Classify sodium chloride as an element, mixture, compound, or colloid. Compound 2. #1.0 Classify air as an element, mixture, compound, or colloid. Mixture 3. #1.0 Classify a blueberry
Glossary. acid A substance that releases H + ions when dissolved in water.
 Glossary acid A substance that releases H + ions when dissolved in water. acid-base titration A procedure in which a solution of a base (or an acid) is carefully added to another solution containing an
Glossary acid A substance that releases H + ions when dissolved in water. acid-base titration A procedure in which a solution of a base (or an acid) is carefully added to another solution containing an
Georgia Institute of Technology CHEM 1310 revised 10/8/09 Spring The Development of Quantum Mechanics. ν (nu) = frequency (in s -1 or hertz)
 The Development of Quantum Mechanics Early physicists used the properties of electromagnetic radiation to develop fundamental ideas about the structure of the atom. A fundamental assumption for their work
The Development of Quantum Mechanics Early physicists used the properties of electromagnetic radiation to develop fundamental ideas about the structure of the atom. A fundamental assumption for their work
Teacher: Mr. gerraputa. Name: Base your answer to the question on the information below. Given the electron dot diagram:
 Teacher: Mr. gerraputa Print Close Name: 1. Given the electron dot diagram: The valence electrons represented by the electron dot diagram could be those of atoms in Group 1. 13 3. 3 2. 15 4. 16 2. Which
Teacher: Mr. gerraputa Print Close Name: 1. Given the electron dot diagram: The valence electrons represented by the electron dot diagram could be those of atoms in Group 1. 13 3. 3 2. 15 4. 16 2. Which
Absorber Alpha emission Alpha particle Atom. Atomic line spectra Atomic mass unit Atomic number Atomic structure. Background radiation
 Material that prevent radioactive emission from passing through it Release of alpha particle from unstable nucleus(a 2+ helium ion or a helium nucleus) The nucleus of a helium atom (two protons and two
Material that prevent radioactive emission from passing through it Release of alpha particle from unstable nucleus(a 2+ helium ion or a helium nucleus) The nucleus of a helium atom (two protons and two
The History of the Atom. How did we learn about the atom?
 The History of the Atom How did we learn about the atom? The Atomic Theory of Matter All matter is made up of fundamental particles. What does fundamental mean? The Greek Philosophers, 400 B.C. Democritus
The History of the Atom How did we learn about the atom? The Atomic Theory of Matter All matter is made up of fundamental particles. What does fundamental mean? The Greek Philosophers, 400 B.C. Democritus
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin
 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Models of the Atom by Christopher G. Hamaker Illinois State University Dalton Model of the Atom John Dalton
Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Models of the Atom by Christopher G. Hamaker Illinois State University Dalton Model of the Atom John Dalton
Chapter 6. Quantum Theory and the Electronic Structure of Atoms Part 1
 Chapter 6 Quantum Theory and the Electronic Structure of Atoms Part 1 The nature of light Quantum theory Topics Bohr s theory of the hydrogen atom Wave properties of matter Quantum mechanics Quantum numbers
Chapter 6 Quantum Theory and the Electronic Structure of Atoms Part 1 The nature of light Quantum theory Topics Bohr s theory of the hydrogen atom Wave properties of matter Quantum mechanics Quantum numbers
structure, properties changes energy ELEMENTS COMPOUNDS PHYSICAL CHEMICAL change MATTER: ATOMS WEIGHT: versus MASS: ELEMENT COMPOUND force amount
 Unit 1a Matter and Energy Chemistry is 1. The study of matter (structure, properties) 2. The changes that matter undergoes and 3. The energy involved in those changes. 1. Classify substances as either
Unit 1a Matter and Energy Chemistry is 1. The study of matter (structure, properties) 2. The changes that matter undergoes and 3. The energy involved in those changes. 1. Classify substances as either
Professor K. Atomic structure
 Professor K Atomic structure Review Reaction- the formation and breaking of chemical bonds Bond- a transfer or sharing of electrons Electrons Abbreviated e - What are they? How were they discovered? Early
Professor K Atomic structure Review Reaction- the formation and breaking of chemical bonds Bond- a transfer or sharing of electrons Electrons Abbreviated e - What are they? How were they discovered? Early
Matter What is Chemistry? Chemistry is the study of matter and the changes it undergoes.
 Matter What is Chemistry? Chemistry is the study of matter and the changes it undergoes. What is matter? Matter is anything that has mass and occupies space. Chemists use a scientific method to study matter.
Matter What is Chemistry? Chemistry is the study of matter and the changes it undergoes. What is matter? Matter is anything that has mass and occupies space. Chemists use a scientific method to study matter.
CHAPTER 4 10/11/2016. Properties of Light. Anatomy of a Wave. Components of a Wave. Components of a Wave
 Properties of Light CHAPTER 4 Light is a form of Electromagnetic Radiation Electromagnetic Radiation (EMR) Form of energy that exhibits wavelike behavior and travels at the speed of light. Together, all
Properties of Light CHAPTER 4 Light is a form of Electromagnetic Radiation Electromagnetic Radiation (EMR) Form of energy that exhibits wavelike behavior and travels at the speed of light. Together, all
INTRODUCTORY CHEMISTRY Concepts & Connections
 INTRODUCTORY CHEMISTRY Concepts & Connections Sixth Edition by Charles H. Corwin Chapter 5 Models of the Atom by Christopher Hamaker Chapter 5 1 Dalton Model of the Atom John Dalton proposed that all matter
INTRODUCTORY CHEMISTRY Concepts & Connections Sixth Edition by Charles H. Corwin Chapter 5 Models of the Atom by Christopher Hamaker Chapter 5 1 Dalton Model of the Atom John Dalton proposed that all matter
CHEMISTRY. CHM201 Class #2 CHEMISTRY. Chapter 1 Continued. Chapter 1 Outline for Class #2. Particles of Matter: Measurements and the Tools of Science
 CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies CHM201 Class #2 Chemistry, 5 th Edition Copyright 2017, W. W. Norton & Company CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies Chapter
CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies CHM201 Class #2 Chemistry, 5 th Edition Copyright 2017, W. W. Norton & Company CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies Chapter
Chapter 3. Chapter 3. Objectives. Table of Contents. Chapter 3. Chapter 3. Foundations of Atomic Theory, continued. Foundations of Atomic Theory
 Atoms: The Building Blocks of Matter Table of Contents Philosophical Idea to Scientific Theory Objectives Explain the law of conservation of mass, the law of definite proportions, and the law of multiple
Atoms: The Building Blocks of Matter Table of Contents Philosophical Idea to Scientific Theory Objectives Explain the law of conservation of mass, the law of definite proportions, and the law of multiple
Honors Chemistry - 1st Semester Final Practice Exam
 Honors Chemistry - 1st Semester Final Practice Exam Mr. Matthew Totaro MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which statement about the
Honors Chemistry - 1st Semester Final Practice Exam Mr. Matthew Totaro MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which statement about the
CHAPTER 4. Atomic Structure. 4.1 Atoms. Dalton s Atomic Theory
 CHAPTER 4 Atomic Structure 4.1 Atoms Democritus first suggested the idea of atoms Indivisible & Indestructible 460 B.C. 370 B.C. Dalton s Atomic Theory 1. All elements are composed of submicroscopic indivisible
CHAPTER 4 Atomic Structure 4.1 Atoms Democritus first suggested the idea of atoms Indivisible & Indestructible 460 B.C. 370 B.C. Dalton s Atomic Theory 1. All elements are composed of submicroscopic indivisible
Accounts for certain objects being colored. Used in medicine (examples?) Allows us to learn about structure of the atom
 1.1 Interaction of Light and Matter Accounts for certain objects being colored Used in medicine (examples?) 1.2 Wavelike Properties of Light Wavelength, : peak to peak distance Amplitude: height of the
1.1 Interaction of Light and Matter Accounts for certain objects being colored Used in medicine (examples?) 1.2 Wavelike Properties of Light Wavelength, : peak to peak distance Amplitude: height of the
Chapter 5 Electrons In Atoms
 Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
THE NATURE OF THE ATOM. alpha particle source
 chapter THE NATURE OF THE ATOM www.tutor-homework.com (for tutoring, homework help, or help with online classes) Section 30.1 Rutherford Scattering and the Nuclear Atom 1. Which model of atomic structure
chapter THE NATURE OF THE ATOM www.tutor-homework.com (for tutoring, homework help, or help with online classes) Section 30.1 Rutherford Scattering and the Nuclear Atom 1. Which model of atomic structure
Name: Electrons in Atoms Chemical Periodicity Chapters 13 and 14
 Name: Electrons in Atoms Chemical Periodicity Chapters 13 and 14 1 Chapter 13 Electrons in Atoms We need to further develop our understanding of atomic structure to help us understand how atoms bond to
Name: Electrons in Atoms Chemical Periodicity Chapters 13 and 14 1 Chapter 13 Electrons in Atoms We need to further develop our understanding of atomic structure to help us understand how atoms bond to
CHEM Chapter 6. Basic Quantum Chemistry (Homework). WL36
 CHEM 1411. Chapter 6. Basic Quantum Chemistry (Homework). WL36 1. The Bohr model of the hydrogen atom found its greatest support in experimental work on the photoelectric effect. A) True B) False 2. A
CHEM 1411. Chapter 6. Basic Quantum Chemistry (Homework). WL36 1. The Bohr model of the hydrogen atom found its greatest support in experimental work on the photoelectric effect. A) True B) False 2. A
Unit 4. Electrons in Atoms
 Unit 4 Electrons in Atoms When were most of the subatomic particles discovered? Who discovered densely packed nucleus surrounded by fast moving electrons? Rutherford s Model Major development Lacked detail
Unit 4 Electrons in Atoms When were most of the subatomic particles discovered? Who discovered densely packed nucleus surrounded by fast moving electrons? Rutherford s Model Major development Lacked detail
Atomic Theory. Contribution to Modern Atomic Theory
 Alief High School Chemistry STAAR Review Reporting Category 2: Atomic Structure and Nuclear Chemistry C.6.A Understand the experimental design and conclusions used in the development of modern atomic theory,
Alief High School Chemistry STAAR Review Reporting Category 2: Atomic Structure and Nuclear Chemistry C.6.A Understand the experimental design and conclusions used in the development of modern atomic theory,
Worksheet 2.1. Chapter 2: Atomic structure glossary
 Worksheet 2.1 Chapter 2: Atomic structure glossary Acceleration (in a mass spectrometer) The stage where the positive ions are attracted to negatively charged plates. Alpha decay The emission of an alpha
Worksheet 2.1 Chapter 2: Atomic structure glossary Acceleration (in a mass spectrometer) The stage where the positive ions are attracted to negatively charged plates. Alpha decay The emission of an alpha
Part One: Light Waves, Photons, and Bohr Theory. 2. Beyond that, nothing was known of arrangement of the electrons.
 CHAPTER SEVEN: QUANTUM THEORY AND THE ATOM Part One: Light Waves, Photons, and Bohr Theory A. The Wave Nature of Light (Section 7.1) 1. Structure of atom had been established as cloud of electrons around
CHAPTER SEVEN: QUANTUM THEORY AND THE ATOM Part One: Light Waves, Photons, and Bohr Theory A. The Wave Nature of Light (Section 7.1) 1. Structure of atom had been established as cloud of electrons around
Notes:&&Unit&4:&Atomics& & & & & & & & & & & & & & & & &
 Name: RegentsChemistry:Mr.Palermo Notes:Unit4:Atomics! www.mrpalermo.com Name: $ Key$Ideas$ Themodernmodeloftheatomhasevolvedoveralongperiodoftimethroughtheworkofmany scientists.(3.1a) Eachatomhasanucleus,withanoverallpositivecharge,surroundedbyoneormorenegatively
Name: RegentsChemistry:Mr.Palermo Notes:Unit4:Atomics! www.mrpalermo.com Name: $ Key$Ideas$ Themodernmodeloftheatomhasevolvedoveralongperiodoftimethroughtheworkofmany scientists.(3.1a) Eachatomhasanucleus,withanoverallpositivecharge,surroundedbyoneormorenegatively
Chapter 6 Electronic Structure of Atoms. 許富銀 ( Hsu Fu-Yin)
 Chapter 6 Electronic Structure of Atoms 許富銀 ( Hsu Fu-Yin) 1 The Wave Nature of Light The light we see with our eyes, visible light, is one type of electromagnetic radiation. electromagnetic radiation carries
Chapter 6 Electronic Structure of Atoms 許富銀 ( Hsu Fu-Yin) 1 The Wave Nature of Light The light we see with our eyes, visible light, is one type of electromagnetic radiation. electromagnetic radiation carries
CHEMISTRY - TRO 4E CH.7 - THE QUANTUM-MECHANICAL MODEL OF THE ATOM
 !! www.clutchprep.com CONCEPT: THE NATURE OF LIGHT Visible light represents a small portion of the continuum of radiant energy known as. The visible light spectrum ranges from to. Its wave properties of
!! www.clutchprep.com CONCEPT: THE NATURE OF LIGHT Visible light represents a small portion of the continuum of radiant energy known as. The visible light spectrum ranges from to. Its wave properties of
CHAPTER ONE. The Foundations of Chemistry
 CHAPTER ONE The Foundations of Chemistry Why is Chemistry Important? Materials for our homes Components for computers and other electronic devices Cooking Fuel Body functions 2 Some definitions / Vocabulary
CHAPTER ONE The Foundations of Chemistry Why is Chemistry Important? Materials for our homes Components for computers and other electronic devices Cooking Fuel Body functions 2 Some definitions / Vocabulary
Electrons in Atoms. Section 5.1 Light and Quantized Energy
 Name Date Class 5 Electrons in Atoms Section 5.1 Light and Quantized Energy In your textbook, read about the wave nature of light. Use each of the terms below just once to complete the passage. amplitude
Name Date Class 5 Electrons in Atoms Section 5.1 Light and Quantized Energy In your textbook, read about the wave nature of light. Use each of the terms below just once to complete the passage. amplitude
SUMMARY (p. 44) The atom is % empty space and is composed of three particles. a. b. c.
 2.1 Atomic Structure and Subatomic Particles (p. 40) There are two types of charge. 1. _ 2. Electrons In 1897 J. J. Thomson, using a cathode ray tube, measured the ratio of the charge-to-mass of the electron:
2.1 Atomic Structure and Subatomic Particles (p. 40) There are two types of charge. 1. _ 2. Electrons In 1897 J. J. Thomson, using a cathode ray tube, measured the ratio of the charge-to-mass of the electron:
3. SI Base Units used in Chemistry: Quantity Unit Name Abbreviation Tool to Measure Length Mass Time Temperature Amount of Substance
 Honors Chemistry Unit 1 Intro and Atomic Theory Notes Intro Scientific Measurements: 1. Chemistry is the study of. 2. Why do scientists worldwide use the SI system of measurement? 3. SI Base Units used
Honors Chemistry Unit 1 Intro and Atomic Theory Notes Intro Scientific Measurements: 1. Chemistry is the study of. 2. Why do scientists worldwide use the SI system of measurement? 3. SI Base Units used
CHAPTER ONE. The Foundations of Chemistry
 CHAPTER ONE The Foundations of Chemistry Red pigment CHAPTER 1 The Foundations of Chemistry The rose on the right is in an atmosphere of sulfur dioxide, SO 2. Gaseous SO 2 and aqueous solutions of HSO
CHAPTER ONE The Foundations of Chemistry Red pigment CHAPTER 1 The Foundations of Chemistry The rose on the right is in an atmosphere of sulfur dioxide, SO 2. Gaseous SO 2 and aqueous solutions of HSO
Calendar. October 23, Chapter 5 Notes Waves.notebook Waves vocab waves ws. quiz PSAT. Blank. elements test. demo day
 Calendar Sunday Monday Tuesday Wednesday Thursday Friday Saturday 13 14 Waves vocab waves ws 20 PSAT make notecards 7th 15 21 22 quiz 16 23 17 24 27 28 29 30 31 elements test demo day Blank 1 The Nature
Calendar Sunday Monday Tuesday Wednesday Thursday Friday Saturday 13 14 Waves vocab waves ws 20 PSAT make notecards 7th 15 21 22 quiz 16 23 17 24 27 28 29 30 31 elements test demo day Blank 1 The Nature
b. Na. d. So. 1 A basketball has more mass than a golf ball because:
 Chem I Semester Review All of the following are general characteristics of a substance in the liquid state except a. definite volume. c. not easily compressed. b. able to flow. d. definite shape. In the
Chem I Semester Review All of the following are general characteristics of a substance in the liquid state except a. definite volume. c. not easily compressed. b. able to flow. d. definite shape. In the
Properties of Light and Atomic Structure. Chapter 7. So Where are the Electrons? Electronic Structure of Atoms. The Wave Nature of Light!
 Properties of Light and Atomic Structure Chapter 7 So Where are the Electrons? We know where the protons and neutrons are Nuclear structure of atoms (Chapter 2) The interaction of light and matter helps
Properties of Light and Atomic Structure Chapter 7 So Where are the Electrons? We know where the protons and neutrons are Nuclear structure of atoms (Chapter 2) The interaction of light and matter helps
