Chapter 2 Energy and Matter 2.1 Energy
|
|
|
- Julia Beasley
- 5 years ago
- Views:
Transcription
1 Chapter 2 Energy and Matter 2.1 Energy 1
2 Energy Energy makes objects move. makes things stop. is needed to do work. 2
3 Work Work is done when you climb. you lift a bag of groceries. you ride a bicycle. you breathe. your heart pumps blood. water goes over a dam. Copyright 2009 by Pearson Education, Inc. 3
4 Potential Energy Potential energy is stored energy. Examples are water behind a dam. a compressed spring. chemical bonds in gasoline, coal, or food. Copyright 2009 by Pearson Education, Inc. 4
5 Kinetic Energy Kinetic energy is the energy of motion. Examples are swimming. water flowing over a dam. working out. burning gasoline. Copyright 2009 by Pearson Education, Inc. 5
6 Learning Check Identify the energy as potential or kinetic. A. Rollerblading B. a peanut butter and jelly sandwich C. mowing the lawn D. gasoline in the gas tank 6
7 Solution Identify the energy as potential or kinetic. A. Rollerblading (kinetic) B. a peanut butter and jelly sandwich (potential) C. mowing the lawn (kinetic) D. gasoline in the gas tank (potential) 7
8 Units for Measuring Energy or Heat Heat is measured in joules or calories Joules (J) = 1 calorie (cal) 1 kj = 1000 J 1 kilocalorie (kcal) = 1000 calories (cal) 8
9 Examples of Energy In Joules 9
10 Learning Check How many cal are obtained from a pat of butter if it provides 150 J of energy when metabolized? 1) 0.36 cal 2) 36 cal 3) 630 cal 10
11 Solution How many cal are obtained from a pat of butter if it provides 150 J of energy when metabolized? Answer 2) Solution: Given: Need: Plan: Equality: Setup: 150 J calories J -> cal 1 cal = J 150 J x 1 cal = 36 cal J 11
12 Chapter 2 Energy and Matter 2.2 Energy and Nutrition Copyright 2009 by Pearson Education, Inc. 12
13 Calorimeters A calorimeter is used to measure heat transfer. can be made with a coffee cup and a thermometer. indicates the heat lost by a sample indicates the heat Copyright 2009 by Pearson Education, Inc. 13
14 Energy and Nutrition On food labels, energy is shown as the nutritional Calorie, written with a capital C. In countries other than the U.S., energy is shown in kilojoules (kj). 1 Cal = 1000 calories 1 Cal = 1 kcal 1 Cal = 1000 cal 14
15 Caloric Food Values The caloric or energy values for foods indicate the number of kcal (Cal) provided by 1 g of each type of food. Carbohydrate: Fat (lipid): Protein: 4 kcal 1 g 9 kcal 1 g 4 kcal 1 g 15
16 Energy Values for Some Foods 16
17 Energy Requirements The amount of energy needed each day depends on age, sex, and physical activity. 17
18 Learning Check A cup of whole milk contains 12 g of carbohydrate, 9 g of fat, and 5 g of protein. How many kcal (Cal) does a cup of milk contain (round answer to the tens place)? 1) 50 kcal (or Cal) 2) 80 kcal (or Cal) 3) 150 kcal (or Cal) 18
19 Solution A cup of whole milk contains 12 g of carbohydrate, 9 g of fat, and 5 g of protein. How many kcal (Cal) does a cup of milk contain? 3) 150 kcal (or Cal) 12 g carbohydrates x 4 kcal/g = 50 kcal 9 g fat x 9 kcal/g = 80 kcal 5 g protein x 4 kcal/g = 20 kcal 150 kcal 19
20 Chapter 2 Energy and Matter 2.3 Temperature Conversions Copyright 2009 by Pearson Education, Inc. 20
21 Temperature Temperature is a measure of how hot or cold an object is compared to another object. indicates that heat flows from the object with a higher temperature to the object with a lower temperature. is measured using a thermometer. Copyright 2009 by Pearson Education, Inc. 21
22 Temperature Scales Temperature Scales are Fahrenheit, Celsius, and Kelvin. have reference points for the boiling and freezing points of water. Copyright 2009 by Pearson Education, Inc. 22
23 Learning Check A. What is the temperature of freezing water? 1) 0 F 2) 0 C 3) 0 K B. What is the temperature of boiling water? 1) 100 F 2) 32 F 3) 373 K C. How many Celsius units are between the boiling and freezing points of water? 1) 100 2) 180 3)
24 Solution A. What is the temperature of freezing water? 2) 0 C B. What is the temperature of boiling water? 3) 373 K C. How many Celsius units are between the boiling and freezing points of water? 1)
25 Fahrenheit Formula On the Fahrenheit scale, there are 180 F between the freezing and boiling points; on the Celsius scale there are 100 C. 180 F = 9 F = 1.8 F 100 C 5 C 1 C In the formula for the Fahrenheit temperature, adding 32 adjusts the zero point of water from 0 C to 32 F. T F = 9/5 T C + 32 or T F = 1.8 T C
26 Celsius Formula T C is obtained by rearranging the equation for T F. T F = 1.8T C + 32 Subtract 32 from both sides. T F - 32 = 1.8 T C ( ) T F - 32 = 1.8 T C Divide by 1.8 = F - 32 = 1.8 T C T F -32 = T C
27 Solving A Temperature Problem A person with hypothermia has a body temperature of 34.8 C. What is that temperature in F? T F = 1.8 T C + 32 T F = 1.8 (34.8 C) + 32 exact 3 SFs exact = (addition) = 94.6 F tenth s Copyright 2009 by Pearson Education, Inc. 27
28 Learning Check The normal body temperature of a chickadee is F. What is that temperature on the Celsius scale? 1) 73.8 C 2) 58.8 C 3) 41.0 C 28
29 Solution 3) 41.0 C T C = (T F 32 ) 1.8 = ( exact) 1.8 = 73.8 F (3 SFs) = 41.0 C 1.8 (exact) (division, 3SFs) 29
30 Learning Check A pepperoni pizza is baked at 455 F. What temperature is needed on the Celsius scale? 1) 423 C 2) 235 C 3) 221 C 30
31 Solution A pepperoni pizza is baked at 455 F. What temperature is needed on the Celsius scale? 2) 235 C T F -32 = T C 1.8 ( ) = 235 C
32 Learning Check On a cold winter day, the temperature is 15 C. What is that temperature in F? 1) 19 F 2) 59 F 3) 5 F 32
33 Solution Answer 3) 5 F Solution: T F = 1.8T C + 32 T F = 1.8 ( 15 C) + 32 = 27 F + 32 = 5 F Note: Be sure to use the change sign key on your calculator to enter the minus ( ) sign. 1.8 x 15 +/ = 27 33
34 Study Tip: Temperature Math The temperature equation involves the exact numbers 1.8 and 32. Only the temperature is measured. To convert C to F, a multiplication rule is followed by an addition rule. multiplication step 1.8 ( 15 C) = 27 F (2 SF) addition step 27 F (ones place) + 32 (exact) = 5 F (ones place) 34
35 Kelvin Temperature Scale The Kelvin temperature scale has 100 units between the freezing and boiling points of water. 100 K = 100 C or 1 K = 1 C is obtained by adding 273 to the Celsius temperature. T K = T C contains the lowest possible temperature, absolute zero (0 K). 0 K = 273 C 35
36 Temperatures 36
37 Learning Check What is normal body temperature of 37 C in kelvins? 1) 236 K 2) 310 K 3) 342 K 37
38 Solution What is normal body temperature of 37 C in kelvins? 2) 310 K T K = T C = 37 C = 310. K (to ones place) 38
39 Chapter 2 Energy and Matter 2.4 Specific Heat Copyright 2009 by Pearson Education, Inc. 39
40 Specific Heat Specific heat is different for different substances. is the amount of heat that raises the temperature of 1 g of a substance by 1 C. in the SI system has units of J/g C. in the metric system has units of cal/g C. 40
41 Examples of Specific Heats 41
42 Learning Check A. When ocean water cools, the surrounding air 1) cools. 2) warms. 3) stays the same. B. Sand in the desert is hot in the day, and cool at night. Sand must have a 1) high specific heat. 2) low specific heat. 42
43 Solution A. When ocean water cools, the surrounding air 2) warms. B. Sand in the desert is hot in the day, and cool at night. Sand must have a 2) low specific heat. 43
44 Learning Check What is the specific heat if 24.8 g of a metal absorbs 275 J of energy and the temperature rises from 20.2 C to 24.5 C? 44
45 Solution Given: 24.8 g metal, 275 J of energy, 20.2 C to 24.5 C Need: Specific heat J/g C Plan: specific heat (SH) = Heat (J) g C T = 24.5 C 20.2 C = 4.3 C Setup: 275 J = 2.6 J/g C (24.8 g)(4.3 C) 45
46 Heat Equation The amount of heat lost or gained by a substance is calculated from the mass of substance (g). temperature change ( T). specific heat of the substance (J/g C). This is expressed as the heat equation. Heat = g x C x J = J g C 46
47 Learning Check How many kj are needed to raise the temperature of 325 g of water from 15.0 C to 77.0 C? 1) 20.4 kj 2) 77.7 kj 3) 84.3 kj 47
48 Study Tip: Using Specific Heat 48
49 Solution Answer: 3) 84.3 kj 77.0 C 15.0 C = 62.0 C 325 g x 62.0 C x J x 1 kj g C 1000 J = 84.3 kj 49
50 Coral Bleaching Coral contains algae that produce sugars (food) and the bright red and orange pigments of coral. expels the algae when water temperatures increase as little as 1 C. bleaches as it loses its food supply and color. dies if the stress of higher temperatures continues. reefs in Australia and the Indian Ocean have been badly damaged by increases in ocean temperatures. 50
51 Learning Check How many kcal are absorbed by ocean water if 3 x L of water in the Caribbean has an increase of 1 C. Assume the specific heat of ocean water is the same as water. Assume the density of ocean water is 1.0 g/ml. 1) 3 x kcal 2) 3 x kcal 3) 3 x kcal 51
52 Solution ml 3 10 L 1 L 21 = 3 10 g of sea seawater water seawater 1 g 1 ml g 1 C 1 cal 1 kcal g C 1000 cal 18 = 3 10 kcal of heat absorbed (Answer 2) 52
53 Chapter 2 Energy and Matter 2.5 States of Matter Copyright 2009 by Pearson Education, Inc. 53
54 Solids Solids have a definite shape. a definite volume. particles that are close together in a fixed arrangement. particles that move very slowly. 54
55 Liquids Liquids have an indefinite shape, but a definite volume. the same shape as their container. particles that are close together, but mobile. particles that move slowly. 55
56 Gases Gases have an indefinite shape. an indefinite volume. the same shape and volume as their container. particles that are far apart. particles that move very fast. 56
57 Three States of Matter for Water Copyright 2009 by Pearson Education, Inc. 57
58 Summary of the States of Matter 58
59 Learning Check Identify each as: 1) solid, 2) liquid, or 3) gas. A. It has a definite volume, but takes the shape of the container. B. Its particles are moving rapidly. C. It fills the volume of a container. D. It has particles in a fixed arrangement. E. It has particles close together that are mobile. 59
60 Solution Identify each as: 1) solid, 2) liquid, or 3) gas. 2 A. It has a definite volume, but takes the shape of the container. 3 B. Its particles are moving rapidly. 3 C. It fills the volume of a container. 1 D. It has particles in a fixed arrangement. 2 E. It has particles close together that are mobile. 60
General, Organic, and Biological Chemistry. Fourth Edition Karen Timberlake. Chapter 2. Energy and Matter Pearson Education, Inc.
 General, Organic, and Biological Chemistry Fourth Edition Karen Timberlake Chapter 2 Energy and Matter 2013 Pearson Education, Inc. Section 2.1 Energy Energy is the ability to do work or transfer heat.
General, Organic, and Biological Chemistry Fourth Edition Karen Timberlake Chapter 2 Energy and Matter 2013 Pearson Education, Inc. Section 2.1 Energy Energy is the ability to do work or transfer heat.
Slide 1. Slide 2. Slide 3. Chapter 2 Matter and Energy. 2.1 Classification of Matter. Matter. Pure Substances
 Slide 1 Chapter 2 Matter and Energy 1 2.1 Classification of Matter Slide 2 2 Matter Matter is the material that makes up all things is anything that has mass and occupies space Slide 3 Pure Substances
Slide 1 Chapter 2 Matter and Energy 1 2.1 Classification of Matter Slide 2 2 Matter Matter is the material that makes up all things is anything that has mass and occupies space Slide 3 Pure Substances
Chapter 2 Energy and Matter
 Chapter 2 Energy and Matter Energy makes objects move makes things stop is needed to do work Kinetic Energy Work is done when you climb you lift a bag of groceries you ride a bicycle you breathe your heart
Chapter 2 Energy and Matter Energy makes objects move makes things stop is needed to do work Kinetic Energy Work is done when you climb you lift a bag of groceries you ride a bicycle you breathe your heart
Chapter States and Properties of Matter. The Periodic Table of the Elements Classification of Matter. 3.5 Energy and Nutrition
 Chapter 3 Atoms are the building blocks from which all other things are built. The Periodic Table of the Elements 3.1 - Classification of Matter 3.2 States and Properties of Matter 3.3 Temperature 3.4
Chapter 3 Atoms are the building blocks from which all other things are built. The Periodic Table of the Elements 3.1 - Classification of Matter 3.2 States and Properties of Matter 3.3 Temperature 3.4
Thermodynamics. Internal Energy. Study of energy and its transformations Thermochemistry
 Internal Energy 5.1- Thermodynamics Study of energy and its transformations Thermochemistry Study of energy changes that accompany chemical and physical changes. Energy the capacity to do work or transfer
Internal Energy 5.1- Thermodynamics Study of energy and its transformations Thermochemistry Study of energy changes that accompany chemical and physical changes. Energy the capacity to do work or transfer
Thermochemistry. The study of energy changes that occur during chemical reactions and changes in state.
 Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Ch 100: Fundamentals for Chemistry
 Ch 100: Fundamentals for Chemistry Chapter 4: Properties of Matter Lecture Notes Physical & Chemical Properties Physical Properties are the characteristics of matter that can be changed without changing
Ch 100: Fundamentals for Chemistry Chapter 4: Properties of Matter Lecture Notes Physical & Chemical Properties Physical Properties are the characteristics of matter that can be changed without changing
Start Part 2. Tro's "Introductory Chemistry", Chapter 3
 Start Part 2 1 Separation of Mixtures Separate mixtures based on different physical properties of the components. Physical change. Different Physical Property Boiling point State of matter (solid/liquid/gas)
Start Part 2 1 Separation of Mixtures Separate mixtures based on different physical properties of the components. Physical change. Different Physical Property Boiling point State of matter (solid/liquid/gas)
ENERGY. Unit 12: IPC
 ENERGY Unit 12: IPC WHAT IS ENERGY? Energy- is the ability to do work. Energy is the ability to cause a change. Energy can change an object s: motion shape temperature color THERMAL internal motion of
ENERGY Unit 12: IPC WHAT IS ENERGY? Energy- is the ability to do work. Energy is the ability to cause a change. Energy can change an object s: motion shape temperature color THERMAL internal motion of
CHEMISTRY: Chapter 10 Prep-Test
 CHEMISTRY: Chapter 10 Prep-Test Matching Match each item with the correct statement below. a. calorimeter d. temperature b. calorie e. specific heat c. joule f. heat 1. quantity of heat needed to raise
CHEMISTRY: Chapter 10 Prep-Test Matching Match each item with the correct statement below. a. calorimeter d. temperature b. calorie e. specific heat c. joule f. heat 1. quantity of heat needed to raise
Temp vs. Heat. Absolute Temperature Scales. Common Temperature Scales. Thermal Energy. Heat and Temperature are not the same!!
 Thermal Energy Heat and Temperature are not the same!! Cold is the absence of heat, not an energy Same concept as light/dark Cold can t come in, heat flows out Heat flows from High Temp Low Temp Temp vs.
Thermal Energy Heat and Temperature are not the same!! Cold is the absence of heat, not an energy Same concept as light/dark Cold can t come in, heat flows out Heat flows from High Temp Low Temp Temp vs.
Zeroth Law of Thermodynamics
 Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
Thermal Equilibrium. Zeroth Law of Thermodynamics 2/4/2019. Temperature
 Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
3.2 Units of Measurement > Chapter 3 Scientific Measurement. 3.2 Units of Measurement. 3.1 Using and Expressing Measurements
 Chapter 3 Scientific Measurement 3.1 Using and Expressing Measurements 3.2 Units of Measurement 3.3 Solving Conversion Problems 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
Chapter 3 Scientific Measurement 3.1 Using and Expressing Measurements 3.2 Units of Measurement 3.3 Solving Conversion Problems 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
the energy of motion!
 What are the molecules of matter doing all the time?! Heat and Temperature! Notes! All matter is composed of continually jiggling atoms or molecules! The jiggling is! If something is vibrating, what kind
What are the molecules of matter doing all the time?! Heat and Temperature! Notes! All matter is composed of continually jiggling atoms or molecules! The jiggling is! If something is vibrating, what kind
Ch06. Energy. Thermochemistry, understanding energy, heat & work. version 1.5
 Ch06 Energy Thermochemistry, understanding energy, heat & work. version 1.5 Nick DeMello, PhD. 2007-2016 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal
Ch06 Energy Thermochemistry, understanding energy, heat & work. version 1.5 Nick DeMello, PhD. 2007-2016 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal
2 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
 CHEMISTRY & YOU Chapter 17 Thermochemistry 17.1 The Flow of Energy 17. Measuring and Expressing Enthalpy Changes 17.3 in Changes of State 17.4 Calculating s of Reaction Why does lava cool faster in water
CHEMISTRY & YOU Chapter 17 Thermochemistry 17.1 The Flow of Energy 17. Measuring and Expressing Enthalpy Changes 17.3 in Changes of State 17.4 Calculating s of Reaction Why does lava cool faster in water
Chapter 4. Properties of Matter
 Chapter 4 Properties of Matter A burning log undergoes chemical change resulting in the release of energy in the form of heat and light. The physical properties of the log change during the Introduction
Chapter 4 Properties of Matter A burning log undergoes chemical change resulting in the release of energy in the form of heat and light. The physical properties of the log change during the Introduction
Chemistry 104 Chapter Two PowerPoint Notes
 Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
Measurement Chapter 1.6-7
 Unit 1 Essential Skills Measurement Chapter 1.6-7 The Unit 1 Test will cover material from the following Chapters and Sections: 1.all 2.5-8 3.all 2 Two types of Data: When we make observations of matter,
Unit 1 Essential Skills Measurement Chapter 1.6-7 The Unit 1 Test will cover material from the following Chapters and Sections: 1.all 2.5-8 3.all 2 Two types of Data: When we make observations of matter,
Name Date Class THERMOCHEMISTRY
 Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
The Kinetic Theory of Matter. Temperature. Temperature. Temperature. Temperature. Chapter 6 HEAT
 The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
SUMMARY OF PROPERTIES OF MATTER State Shape Volume Particles Compressibility Solid Definite Definite Densely packed Very slight
 MATTER & ITS FORMS Matter is defined as anything that has mass and occupies space. Matter can be classified by its states: solid, liquid, and gas. Solid: Densely packed matter with definite shape and volume.
MATTER & ITS FORMS Matter is defined as anything that has mass and occupies space. Matter can be classified by its states: solid, liquid, and gas. Solid: Densely packed matter with definite shape and volume.
Lecture PowerPoints. Chapter 14 Physics: Principles with Applications, 6 th edition Giancoli
 Lecture PowerPoints Chapter 14 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for
Lecture PowerPoints Chapter 14 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for
Chapter 14 Heat. Lecture PowerPoints. Chapter 14 Physics: Principles with Applications, 7 th edition Giancoli
 Lecture PowerPoints Chapter 14 Physics: Principles with Applications, 7 th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
Lecture PowerPoints Chapter 14 Physics: Principles with Applications, 7 th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
What are the states of Matter?
 What are the states of Matter? Solid Lowest energy/heat Molecules barely moving Definite, uniform shape Example: ice States of Matter Liquid Medium energy/heat Molecules slowly moving Shape of container
What are the states of Matter? Solid Lowest energy/heat Molecules barely moving Definite, uniform shape Example: ice States of Matter Liquid Medium energy/heat Molecules slowly moving Shape of container
Physics 111. Lecture 35 (Walker: ) Thermal Physics I: Temperature Thermal Expansion. April 29, Temperature (T)
 Physics 111 Lecture 35 (Walker: 16.1-3) Thermal Physics I: Temperature Thermal Expansion April 29, 2009 Lecture 35 1/26 Temperature (T) Temperature (T) is a measure of how hot or cold something is Temperature
Physics 111 Lecture 35 (Walker: 16.1-3) Thermal Physics I: Temperature Thermal Expansion April 29, 2009 Lecture 35 1/26 Temperature (T) Temperature (T) is a measure of how hot or cold something is Temperature
Chapter 12. Temperature and Heat
 Chapter 12 Temperature and Heat 12.1 Common Temperature Scales Temperatures are reported in degrees Celsius or degrees Fahrenheit. Kelvin Scale 100 o C or 212 o F T K = T + 273.15 Temperature changes,
Chapter 12 Temperature and Heat 12.1 Common Temperature Scales Temperatures are reported in degrees Celsius or degrees Fahrenheit. Kelvin Scale 100 o C or 212 o F T K = T + 273.15 Temperature changes,
Page 1 SPH3U. Heat. What is Heat? Thermal Physics. Waterloo Collegiate Institute. Some Definitions. Still More Heat
 SPH3U Thermal Physics electrons and holes in semiconductors An Introductory ourse in Thermodynamics converting energy into work magnetism thin films and surface chemistry thermal radiation (global warming)
SPH3U Thermal Physics electrons and holes in semiconductors An Introductory ourse in Thermodynamics converting energy into work magnetism thin films and surface chemistry thermal radiation (global warming)
Honors Chemistry Energy and Specific Heat Lab
 Honors Chemistry Energy and Lab Name Date Objectives: Calculate the amount of heat absorbed or released by a substance as its temperature changes. Describe how a calorimeter is used to measure energy that
Honors Chemistry Energy and Lab Name Date Objectives: Calculate the amount of heat absorbed or released by a substance as its temperature changes. Describe how a calorimeter is used to measure energy that
3.4. Classify each of the following pure substances as either an element or a compound.
 CH 112 Chapter 3 Homework Key (Due 4/17/2018) 3.4. Classify each of the followin pure substances as either an element or a compound. a. Helium as (He) element b. Mercury (H) in a thermometer element c.
CH 112 Chapter 3 Homework Key (Due 4/17/2018) 3.4. Classify each of the followin pure substances as either an element or a compound. a. Helium as (He) element b. Mercury (H) in a thermometer element c.
Chapter 14 Temperature and Heat
 Chapter 14 Temperature and Heat To understand temperature and temperature scales. To describe thermal expansion and its applications. To explore and solve problems involving heat, phase changes and calorimetry.
Chapter 14 Temperature and Heat To understand temperature and temperature scales. To describe thermal expansion and its applications. To explore and solve problems involving heat, phase changes and calorimetry.
Temperature and Its Measurement
 Temperature and Its Measurement When the physical properties are no longer changing, the objects are said to be in thermal equilibrium. Two or more objects in thermal equilibrium have the same temperature.
Temperature and Its Measurement When the physical properties are no longer changing, the objects are said to be in thermal equilibrium. Two or more objects in thermal equilibrium have the same temperature.
0 o K is called absolute zero. Water Freezes: 273 o K Water Boils: 373 o K
 Part I Notes Temperature and Heat The terms at the right all mean the same thing. The heat energy of a substance is the sum of the kinetic and potential energies of all of the atoms and molecules in the
Part I Notes Temperature and Heat The terms at the right all mean the same thing. The heat energy of a substance is the sum of the kinetic and potential energies of all of the atoms and molecules in the
Physical Science Chapter 5 Cont2. Temperature & Heat
 Physical Science Chapter 5 Cont2 Temperature & Heat What are we going to study? Temperature Heat Specific Heat and Latent Heat Heat Transfer Phases of Matter The Kinetic Theory of Gases Thermodynamics
Physical Science Chapter 5 Cont2 Temperature & Heat What are we going to study? Temperature Heat Specific Heat and Latent Heat Heat Transfer Phases of Matter The Kinetic Theory of Gases Thermodynamics
BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7
 BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7 Chemistry - the study of matter, its behavior and interactions. matter - anything that takes up space and has mass mass - the substance which makes up the
BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7 Chemistry - the study of matter, its behavior and interactions. matter - anything that takes up space and has mass mass - the substance which makes up the
Physics Mechanics
 1 Physics 170 - Mechanics Lecture 35 Heat 2 Definition and Units of Heat Heat is a form of energy, and therefore is measured in joules. There are other units of heat, the most common one is the kilocalorie:
1 Physics 170 - Mechanics Lecture 35 Heat 2 Definition and Units of Heat Heat is a form of energy, and therefore is measured in joules. There are other units of heat, the most common one is the kilocalorie:
CP Chapter 17 Thermochemistry
 CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
A). Yes. B). No. Q15 Is it possible for a solid metal ball to float in mercury?
 Q15 Is it possible for a solid metal ball to float in mercury? A). Yes. B). No. The upward force is the weight of liquid displaced and the downward force is the weight of the ball. If the density of the
Q15 Is it possible for a solid metal ball to float in mercury? A). Yes. B). No. The upward force is the weight of liquid displaced and the downward force is the weight of the ball. If the density of the
Thermal Energy. Thermal Energy is the TRANSFER of kinetic energy between two objects that are at different temperatures.
 Thermal Energy Thermal Energy is the TRANSFER of kinetic energy between two objects that are at different temperatures. And remember: heat will always transfer from a warm object to a cold object. HEAT
Thermal Energy Thermal Energy is the TRANSFER of kinetic energy between two objects that are at different temperatures. And remember: heat will always transfer from a warm object to a cold object. HEAT
* Defining Temperature * Temperature is proportional to the kinetic energy of atoms and molecules. * Temperature * Internal energy
 * Defining Temperature * We associate temperature with how hot or cold an object feels. * Our sense of touch serves as a qualitative indicator of temperature. * Energy must be either added or removed from
* Defining Temperature * We associate temperature with how hot or cold an object feels. * Our sense of touch serves as a qualitative indicator of temperature. * Energy must be either added or removed from
CHAPTER 17 Thermochemistry
 CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
Chapter 5: Thermochemistry. Problems: , , 5.100, 5.106, 5.108, , 5.121, 5.126
 Chapter 5: Thermochemistry Problems: 5.1-5.95, 5.97-98, 5.100, 5.106, 5.108, 5.118-5.119, 5.121, 5.126 Energy: Basic Concepts and Definitions energy: capacity to do work or to produce heat thermodynamics:
Chapter 5: Thermochemistry Problems: 5.1-5.95, 5.97-98, 5.100, 5.106, 5.108, 5.118-5.119, 5.121, 5.126 Energy: Basic Concepts and Definitions energy: capacity to do work or to produce heat thermodynamics:
Temperature Energy and Heat
 CHAPTER 3 Temperature Energy and Heat 3.1 Temperature What is temperature? Why is temperature important in chemistry? How is energy related to temperature? 2 3.1 Temperature Milk fat particles are being
CHAPTER 3 Temperature Energy and Heat 3.1 Temperature What is temperature? Why is temperature important in chemistry? How is energy related to temperature? 2 3.1 Temperature Milk fat particles are being
Phase Change Diagram. Rank Solids, liquids and gases from weakest attractive forces to strongest:
 Unit 11 Kinetic molecular theory packet Page 1 of 13 Chemistry Unit 11 Kinetic Theory Unit Quiz: Test Objectives Be able to define pressure and memorize the basic pressure units. Be able to convert to/from:
Unit 11 Kinetic molecular theory packet Page 1 of 13 Chemistry Unit 11 Kinetic Theory Unit Quiz: Test Objectives Be able to define pressure and memorize the basic pressure units. Be able to convert to/from:
HW #1: 1.42, 1.52, 1.54, 1.64, 1.66, 1.70, 1.76, 1.78, 1.80, 1.82, 1.84, 1.86, 1.92, 1.94, 1.98, 1.106, 1.110, 1.116
 Chemistry 121 Lecture 5: Measuring Temperature; Energy and Heat; Specific Heat and the Heating Curve for Water Sections 1.13, 8.15 in McMurry, Ballantine, et. al. 7 th edition HW #1: 1.42, 1.52, 1.54,
Chemistry 121 Lecture 5: Measuring Temperature; Energy and Heat; Specific Heat and the Heating Curve for Water Sections 1.13, 8.15 in McMurry, Ballantine, et. al. 7 th edition HW #1: 1.42, 1.52, 1.54,
2. If the volume of a container holding a gas is reduced, what will happen to the presure within the container?
 1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
kinetic molecular theory thermal energy.
 Thermal Physics 1 Thermal Energy The kinetic molecular theory is based on the assumption that matter is made up of tiny particles that are always in motion. In a hot object the particles are moving faster
Thermal Physics 1 Thermal Energy The kinetic molecular theory is based on the assumption that matter is made up of tiny particles that are always in motion. In a hot object the particles are moving faster
matter/index.html
 http://www.colorado.edu/physics/2000/index.pl http://www.harcourtschool.com/activity/states_of_ matter/index.html Thermal Energy Ch 6-1 Temperature and Heat Objectives Explain the kinetic theory of matter
http://www.colorado.edu/physics/2000/index.pl http://www.harcourtschool.com/activity/states_of_ matter/index.html Thermal Energy Ch 6-1 Temperature and Heat Objectives Explain the kinetic theory of matter
Exercises Temperature (pages ) 1. Define temperature. 2. Explain how a common liquid thermometer works.
 Exercises 21.1 Temperature (pages 407 408) 1. Define temperature. 2. Explain how a common liquid thermometer works. Match each number with the corresponding description. Temperature Description 3. 273
Exercises 21.1 Temperature (pages 407 408) 1. Define temperature. 2. Explain how a common liquid thermometer works. Match each number with the corresponding description. Temperature Description 3. 273
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Thermochemistry AP Chemistry Lecture Outline
 Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermal energy. Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules.
 Thermal energy Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules. Heat is the transfer of thermal energy between substances. Until the
Thermal energy Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules. Heat is the transfer of thermal energy between substances. Until the
Measurement and Calculations
 Measurement and Calculations Quantitative Observation How much? Need Measurement Measurement is the comparison of a physical quantity to be measured with a unit of measurement-that is a fixed standard
Measurement and Calculations Quantitative Observation How much? Need Measurement Measurement is the comparison of a physical quantity to be measured with a unit of measurement-that is a fixed standard
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
Thermochemistry. Using Heats of Reaction - Hess s Law - Standard Enthalpies of Formation - Fuels Foods, Commercial Fuels, and Rocket Fuels
 Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law
 Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law Units of Chapter 17 & 19 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work
Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law Units of Chapter 17 & 19 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work
Chapter 21: Temperature, Heat and Expansion
 Chapter 21: Temperature, Heat and Expansion All matter solid, liquid and gas is made of atoms or molecules, which are continually jiggling. As this jiggling is a movement, all these particles must have
Chapter 21: Temperature, Heat and Expansion All matter solid, liquid and gas is made of atoms or molecules, which are continually jiggling. As this jiggling is a movement, all these particles must have
Conceptual Physics Fundamentals
 Conceptual Physics Fundamentals Chapter 8: TEMPERATURE, HEAT, AND THERMODYNAMICS This lecture will help you understand: Temperature Absolute Zero Internal Energy Heat Quantity of Heat The Laws of Thermodynamics
Conceptual Physics Fundamentals Chapter 8: TEMPERATURE, HEAT, AND THERMODYNAMICS This lecture will help you understand: Temperature Absolute Zero Internal Energy Heat Quantity of Heat The Laws of Thermodynamics
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
EDULABZ INTERNATIONAL. Heat ASSIGNMENT
 Heat ASSIGNMENT 1. Fill in the blank spaces by choosing the correct words from the list given below : List : substance, thermal capacity, mass, latent, heat, cold, constant, water, J C 1, fusion, hot.
Heat ASSIGNMENT 1. Fill in the blank spaces by choosing the correct words from the list given below : List : substance, thermal capacity, mass, latent, heat, cold, constant, water, J C 1, fusion, hot.
ORGANIC CHEMISTRY. Serkan SAYINER, DVM PhD, Assist. Prof.
 ORGANIC CHEMISTRY Serkan SAYINER, DVM PhD, Assist. Prof. serkan.sayiner@neu.edu.tr ENERGY AND MATTER The Units of Energy, Energy and Nutrition, The Three States of Matter, Classification of Matter, Intermolecular
ORGANIC CHEMISTRY Serkan SAYINER, DVM PhD, Assist. Prof. serkan.sayiner@neu.edu.tr ENERGY AND MATTER The Units of Energy, Energy and Nutrition, The Three States of Matter, Classification of Matter, Intermolecular
Chapter 3. Matter, Changes and Energy
 Chapter 3 Matter, Changes and Energy Formulating some questions What are the most basic forms of matter? What are the criteria that allow us to distinguish one substance from another? How do we describe
Chapter 3 Matter, Changes and Energy Formulating some questions What are the most basic forms of matter? What are the criteria that allow us to distinguish one substance from another? How do we describe
Exam 2--PHYS 151--S16
 Name: Exam 2--PHYS 5--S6 Multiple Choice Identify the choice that best completes the statement or answers the question.. Which of these are characteristics of ideal fluid flow? I. steady II. laminar III.
Name: Exam 2--PHYS 5--S6 Multiple Choice Identify the choice that best completes the statement or answers the question.. Which of these are characteristics of ideal fluid flow? I. steady II. laminar III.
Conceptual Physics Fundamentals
 Conceptual Physics Fundamentals Chapter 8: TEMPERATURE, HEAT, AND THERMODYNAMICS This lecture will help you understand: Temperature Absolute Zero Internal Energy Heat Quantity of Heat The Laws of Thermodynamics
Conceptual Physics Fundamentals Chapter 8: TEMPERATURE, HEAT, AND THERMODYNAMICS This lecture will help you understand: Temperature Absolute Zero Internal Energy Heat Quantity of Heat The Laws of Thermodynamics
October 5 A. Bell Work B. Have 4.2 Notes I Out for a stamp C. Today we will work on 4.2 Notes II D. Homework: 4.2 Notes II (due tomorrow end of
 October 5 A. Bell Work B. Have 4.2 Notes I Out for a stamp C. Today we will work on 4.2 Notes II D. Homework: 4.2 Notes II (due tomorrow end of class) Read 4.3 and take notes (due Monday) 4.2 Key Concept:
October 5 A. Bell Work B. Have 4.2 Notes I Out for a stamp C. Today we will work on 4.2 Notes II D. Homework: 4.2 Notes II (due tomorrow end of class) Read 4.3 and take notes (due Monday) 4.2 Key Concept:
Chapter 10 Temperature and Heat
 Chapter 10 Temperature and Heat Thermodynamics deals with 1. Temperature. 2. The transfer and transformation of energy. 3. The relationship between macroscopic properties and microscopic dynamics. Temperature
Chapter 10 Temperature and Heat Thermodynamics deals with 1. Temperature. 2. The transfer and transformation of energy. 3. The relationship between macroscopic properties and microscopic dynamics. Temperature
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
Scientific Measurement
 Scientific Measurement A quantity is anything having a measurable size or amount For Example: 5 But 5 what? A unit assigns value to a measured quantity For Example: 5 ft, 5 gal, 5 sec, 5 m, 5 g. Base Units
Scientific Measurement A quantity is anything having a measurable size or amount For Example: 5 But 5 what? A unit assigns value to a measured quantity For Example: 5 ft, 5 gal, 5 sec, 5 m, 5 g. Base Units
SPECIFIC HEAT CAPACITY AND HEAT OF FUSION
 SPECIFIC HEAT CAPACITY AND HEAT OF FUSION Apparatus on each table: Thermometer, metal cube, complete calorimeter, outer calorimeter can (aluminum only), balance, 4 styrofoam cups, graduated container,
SPECIFIC HEAT CAPACITY AND HEAT OF FUSION Apparatus on each table: Thermometer, metal cube, complete calorimeter, outer calorimeter can (aluminum only), balance, 4 styrofoam cups, graduated container,
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
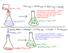 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Bell Ringer. What are the formulas to obtain the force, acceleration, and mass? And corresponding units. F= ma M= f/a A= f/m
 Bell Ringer What are the formulas to obtain the force, acceleration, and mass? And corresponding units. F= ma M= f/a A= f/m F= N M= kg A= m/s^2 What did we learn about the acceleration rate and gravitational
Bell Ringer What are the formulas to obtain the force, acceleration, and mass? And corresponding units. F= ma M= f/a A= f/m F= N M= kg A= m/s^2 What did we learn about the acceleration rate and gravitational
Chapter 2 Heat, Temperature and the First Law of Thermodynamics
 Chapter 2 Heat, Temperature and the First Law of Thermodynamics 2.1. Temperature and the Zeroth Law of Thermodynamics 2.2. Thermal Expansion 2.3. Heat and the Absorption of Heat by Solids and Liquids 2.4.
Chapter 2 Heat, Temperature and the First Law of Thermodynamics 2.1. Temperature and the Zeroth Law of Thermodynamics 2.2. Thermal Expansion 2.3. Heat and the Absorption of Heat by Solids and Liquids 2.4.
Practice Packet: Energy. Regents Chemistry: Dr. Shanzer. Practice Packet. Chapter 4: Energy.
 Regents Chemistry: Dr. Shanzer Practice Packet Chapter 4: Energy http:/drshanzerchemistry.weebly.com Energy Objectives Define energy. Demonstrate the difference between endothermic and exothermic reactions
Regents Chemistry: Dr. Shanzer Practice Packet Chapter 4: Energy http:/drshanzerchemistry.weebly.com Energy Objectives Define energy. Demonstrate the difference between endothermic and exothermic reactions
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
2,000-gram mass of water compared to a 1,000-gram mass.
 11.2 Heat To change the temperature, you usually need to add or subtract energy. For example, when it s cold outside, you turn up the heat in your house or apartment and the temperature goes up. You know
11.2 Heat To change the temperature, you usually need to add or subtract energy. For example, when it s cold outside, you turn up the heat in your house or apartment and the temperature goes up. You know
2012 Thermodynamics Division C
 Team: Team Number: Team Member Names: 1. 2. Instructions: Answer all questions on the test paper. If you need more room, you may attach extra paper. The test is worth a total of 50 points. Show all work
Team: Team Number: Team Member Names: 1. 2. Instructions: Answer all questions on the test paper. If you need more room, you may attach extra paper. The test is worth a total of 50 points. Show all work
Core Concepts. PowerPoint Lectures to accompany Physical Science, 8e. Chapter 4 Heat and Temperature. New Symbols for this Chapter 2/14/2011
 PowerPoint Lectures to accompany Physical Science, 8e Chapter 4 Heat and Temperature Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. New Symbols for this Chapter
PowerPoint Lectures to accompany Physical Science, 8e Chapter 4 Heat and Temperature Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. New Symbols for this Chapter
EXPERIMENT 14 SPECIFIC HEAT OF WATER. q = m s T
 EXPERIMENT 14 SPECIFIC HEAT OF WATER INTRODUCTION: Heat is a form of energy which can pass from an object of relatively high temperature to an object of relatively low temperature. One physical property
EXPERIMENT 14 SPECIFIC HEAT OF WATER INTRODUCTION: Heat is a form of energy which can pass from an object of relatively high temperature to an object of relatively low temperature. One physical property
Heat and Temperature
 Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
Welcome to Chemistry 121
 General, Organic, and Biological Chemistry Fourth Edition Karen Timberlake Welcome to Chemistry 121 2013 Pearson Education, Inc. General, Organic, and Biological Chemistry Fourth Edition Karen Timberlake
General, Organic, and Biological Chemistry Fourth Edition Karen Timberlake Welcome to Chemistry 121 2013 Pearson Education, Inc. General, Organic, and Biological Chemistry Fourth Edition Karen Timberlake
Lecture 23. Specific Heat and Phase Changes
 Lecture 23 Specific Heat and Phase Changes Today s Topics: Heat and Temperature Change Specific heat Heat and Phase Change Latent heat Heat and Temperature Change Heat is energy that flows from a higher-temperature
Lecture 23 Specific Heat and Phase Changes Today s Topics: Heat and Temperature Change Specific heat Heat and Phase Change Latent heat Heat and Temperature Change Heat is energy that flows from a higher-temperature
2.1 Units of Measurement. Copyright 2011 Pearson Education, Inc.
 Chapter 2 Measurements 2.1 Units of Measurement 1 Measurement You make a measurement every time you measure your height read your watch take your temperature weigh a cantaloupe 2 Measurement in Chemistry
Chapter 2 Measurements 2.1 Units of Measurement 1 Measurement You make a measurement every time you measure your height read your watch take your temperature weigh a cantaloupe 2 Measurement in Chemistry
CHAPTER 17: THERMOCHEMISTRY. Mrs. Brayfield
 CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
Introduction to Thermochemistry. Thermochemistry Unit. Definition. Terminology. Terminology. Terminology 07/04/2016. Chemistry 30
 Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
What kinds of energy do you see?
 Energy Transfer ICP FTF Day 2 April 16, 2012 HW: none Topic: Light (last day) Questions: 1. When light passes through a prism, which colors of light are bent the most? 2. Which two colors of paint could
Energy Transfer ICP FTF Day 2 April 16, 2012 HW: none Topic: Light (last day) Questions: 1. When light passes through a prism, which colors of light are bent the most? 2. Which two colors of paint could
Thermochemistry. Section The flow of energy
 Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
THERMOCHEMISTRY. This section explains the relationship between energy and heat, and distinguishes between heat capacity and specific heat.
 I Name _ Date _ Class _ THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY-HEAT AND WORK (pages 505-510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
I Name _ Date _ Class _ THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY-HEAT AND WORK (pages 505-510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Chapter Notes: Temperature, Energy and Thermal Properties of Materials Mr. Kiledjian
 Chapter 10-11 Notes: Temperature, Energy and Thermal Properties of Materials Mr. Kiledjian 1) Temperature 2) Expansion of Matter 3) Ideal Gas Law 4) Kinetic Theory of Gases 5) Energy, Heat transfer and
Chapter 10-11 Notes: Temperature, Energy and Thermal Properties of Materials Mr. Kiledjian 1) Temperature 2) Expansion of Matter 3) Ideal Gas Law 4) Kinetic Theory of Gases 5) Energy, Heat transfer and
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy. Copyright 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings
 Energy Copyright 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings 1 Energy is a substance like quantity that can cause change. Makes objects move. Makes things stop. Is needed to do work.
Energy Copyright 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings 1 Energy is a substance like quantity that can cause change. Makes objects move. Makes things stop. Is needed to do work.
Problem: Convert 25kg to ug. Problem: Convert 482mL to cl. Problem: How many micrograms are in a kilogram? Problem: How many ml are in 1 ounce?
 Problem: Convert 25kg to ug. Problem: Convert 482mL to cl. Problem: How many micrograms are in a kilogram? Problem: How many ml are in 1 ounce? Problem: How many ml are in 1 tsp? Problem: A nerve impulse
Problem: Convert 25kg to ug. Problem: Convert 482mL to cl. Problem: How many micrograms are in a kilogram? Problem: How many ml are in 1 ounce? Problem: How many ml are in 1 tsp? Problem: A nerve impulse
Practice Packet Unit 7: Heat
 Regents Chemistry: Mr. Palermo Practice Packet Unit 7: Heat Review (Things you need to know in order to understand the new stuff ) Particle Diagrams Draw a particle diagram of a compound of CaCl2, using
Regents Chemistry: Mr. Palermo Practice Packet Unit 7: Heat Review (Things you need to know in order to understand the new stuff ) Particle Diagrams Draw a particle diagram of a compound of CaCl2, using
Chapter 10 Test Form B
 Chapter 10 Test Form A 1. B 2. A 3. A 4. B 5. D 6. B 7. B 8. A 9. A 10. A 11. B 12. D 13. A 14. C 15. No, heat and cold do not flow between objects. Energy transferred between objects changes the temperature
Chapter 10 Test Form A 1. B 2. A 3. A 4. B 5. D 6. B 7. B 8. A 9. A 10. A 11. B 12. D 13. A 14. C 15. No, heat and cold do not flow between objects. Energy transferred between objects changes the temperature
Chapter 1. Matter, Measurement, and Problem Solving Copyright 2011 Pearson Education, Inc. 28/11/1435
 Chapter 1 Matter, Measurement, and Problem Solving Chemistry: A Molecular Approach, Second Edition Nivaldo J. Tro CRS Clicker Questions Jason A. Kautz University of Nebraska-Lincoln Which of the following
Chapter 1 Matter, Measurement, and Problem Solving Chemistry: A Molecular Approach, Second Edition Nivaldo J. Tro CRS Clicker Questions Jason A. Kautz University of Nebraska-Lincoln Which of the following
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
