Osnove meteorologije z nalogami za študente 2. letnika programa Fizika Del 2: termodinamika vlažnega zraka in bilanca energije
|
|
|
- Dylan Morgan
- 6 years ago
- Views:
Transcription
1 Osnove meteorologije z nalogami za študente 2. letnika programa Fizika Del 2: termodinamika vlažnega zraka in bilanca energije izr.prof.dr. Nedjeljka Žagar Fakulteta za matemaako in fiziko Univerza v Ljubljani Ljubljana, 2014
2 Tematski sklop 1: Osnovne spremenljivke za opis vlage Stabilnost in dviganje vlažnega zraka Termodinamični diagrami
3 Spremenljivke za opis vlažnega zraka e = ρ v R v T p = p d + e q = e p r = e p e R d R v R d R v = ε e p e = ε p e ε e p R = e e s 100 r r s 100 q q s 100 p = ρr d T v de s dt = Le s R v T 2 " T v = T e % $ ' # p & T d = " $ # 1 1 T o R v L ln e s e so = T ( r) % ' & 1
4 Enačba Clausius- Clapeyrona opisuje spremembe nasičenega tlaka vodne pare v odvisnosa od temperature de s dt = Le s R v T 2 Različne oblike C- C enačbe v uporabi v meteorološki praksi: npr. Tetenova formula, formula WMO Več na hsp:// /~voemel/vp.html Arhiv sondaž dostopen na spletu (University of Wyoming) hsp://weather.uwyo.edu/upperair/sounding.html
5 SaturaAon vapor pressure Količina vodne pare potrebna za nasičenje 1 kg suhega zraka na različnih temperaturah. Približno se podvoji vsakih 10 C 11/8/10
6 Naloga S pomočjo osnovnih plinskih zakonov pokaži, da je vlažen zrak lažji od suhega ρ v <ρ d
7 Merjenje vlažnosa zraka Določanje relaavne vlage v F s pomočjo psihrometra Izmerjene vrednosa: p = 980 hpa (dostopno s postaje pred FMF) T= 23.0 o C temperatura suhega termometra Tm = 18.0 o C temperatura mokrega termometra Naloga: Izračunaj relaavno vlago iz izmerjenih podatkov Postopek: Uporabimo 1. stavek termodinamike pri p=konst LΔq = C p ΔT ( ) = C p ( T T m ) L q s q q s določimo iz enačbe Clausius- Clapeyrona (ki da e s ) in izraza Iz zgornje enačbe (q s, T, T m ) določimo količino vlage v F4 RelaAvna vlaga R = q q s 100% q s = ε e s p
8 Naloga: izračun relaavne vlage Izračunaj relaavno vlago v učilnici F4 na Jadranski 19 iz naslednjih izmerjenih podatkov: Temperatura suhega termometra T=23 o C Temperatura mokrega termometra T m =18 o C Tlak p=980 hpa RH=? Rešitev: 86%
9 Nenasičeni adiabatni procesi
10 Odvisno je o količini vlage v zraku Dviganje vlažnega zraka Mokro- adiabatni dvig: ~5 C/km Suho- adiabatani dvig: 9.8 C/km
11 Ekvivaletna potencialna temperatura Lq s Cp T θ e θ Maksimalna temperature, ki jo delec zraka lahko doseže v primeru, ko se vsa vlaga kondenzira, sproščena latentna toplota preda okolici in delec adiabatno spusa nazaj na 1000 hpa. Ohranjena za mokro- adiabatne procese (nasičen zrak). Mokra adiabata=krivulja θ e = konst.
12 Termodinamični diagrami Različne vrste diagramov so v uporabi od konca 19. stoletja. Vsi so zgrajeni na istem principu in upoštevajo osnovne termodinamične zakone in povezave med T,p,q Osnovni princip konstrukcije diagrama: enake površine predstavljajo enako energijo v vsaki točki diagrama Vsaki diagram vsebuje pet vrst izolinij 5 izolinij: T, p, r s, Θ, Θe Diagrami omogočajo enostavno določanje nivoja kondenzacije, nivoja in temperature proste konvekcije, energije dostopne za konvekcijo itn.
13 the Emagram the Tephigram Vrste termodinamičnih diagramov the SkewT/Log P diagram (modified emagram) the PsuedoadiabaAc (or Stüve) diagram ** The emagram was devised in 1884 by H. Hertz. In this plot, the dry adiabaac lines have an angle of about 45degrees with the isobars; isopleths of saturaaon mixing raao are almost straight and veracal. In 1947, N. Herlofson proposed a modificaaon to the emagram which allows straight, horizontal isobars, and provides for a large angle between isotherms and dry adiabats, similar to that in the tephigram. ** The Tephigram takes its name from the rectangular Cartesian coordinates : temperature and entropy. The Greek leser 'phi' was used for entropy, hence Te- phi- gram (or T- F- gram). The diagram was developed by Sir William Napier Shaw, a BriAsh meteorologist about 1922 or 1923, and was officially adopted by the InternaAonal Commission for the ExploraAon of the Upper Air in 1925.
14 Vrste termodinamičnih diagramov (2) ** The Stüve diagram was developed circa 1927 by G. Stüve and gained widespread acceptance in the United States: it uses straight lines for the three primary variables, pressure, temperature and poten<al temperature. In doing so we sacrifices the equal- area requirements (from the original Clapeyron diagram) that are saasfied in the other two diagrams. ** The SkewT/Log(- P) diagram is also in widespread use in North America, and in many services with which the United States (various) weather services have had connecaons. This is in fact a variaaon on the original Emagram, which was first devised in 1884 by H. Hertz.
15 y = - RlnP x = T + klnp Termodinamični diagrami: SKEW- T/LOG(P) Parameter k se določi tako, da je kot med izotermami in suhimi adiabatmi priližno 90 o.
16 Termodinamični diagrami 5 vrst izolinij: T, p, r s, Θ, Θe SKEW- T/LOG(P) T p r s Θ Θe
17 Termodinamični diagrami T izoterme p izobare r s izograme Θ suha (nenasičena) adiabata: Θe mokra (nasičena) adiabata: Γ d Γ m
18 Stabilnost vlažnega zraka Γ = Γ d Nenasičeno nevtralno ozračje Γ = Γ m (B) Nasičeno nevtralno ozračje Γ > Γ d (D) Absolutno labilno ozračje Γ < Γ m (A) Absolutno stabilno ozračje Γ < Γ d in Γ > Γ m (C) Pogojno stabilno ozračje: labilno glede na nasičeno in stabilno glede na nenasičeno (suho) adiabato
19 Stabilnost vlažnega zraka pogojno stabilno labilno stabilno Θ suha (nenasičena) adiabata: Θe mokra (nasičena) adiabata: Γ d Γ m
20 Parametri, ki jih določamo s pomočjo termodinamičnega diagrama LCL kondenzacijski nivo CCL nivo konvekavne kondenzacije (baza oblakov Cu) LFC nivo proste konvekcije (točka poziavnega vzgona) EL ravnovesni nivo (točka ničlega vzgona, ~vrh konv. oblaka) CAPE energija, dostopna za konvekavni razvoj CIN energija, potrebna za dvig delca na LFC
21 Struktura ozračja nad Ljubljano, , ob 6
22 11/8/10 Struktura ozračja nad Ljubljano,
23 Udine: struktura ozračja , ob 12 UTC
24 Konvekcija
25 Energija, dostopna za konvekcijo CAPE = convecave available potenaal energy CAPE z LNB z LFC g T v,delec T v,okolica T v,delec dz CAPE = w 2 max 2 J/kg CIN = convecave inhibiaon CIN=energija, potrebna za dvig delca na nivo proste konvekcije
26 Razvoj konvekcije: Apična sondaža
27 Naloga: dviganje vlažnega delca na Jadranski 19 Nadaljevanje naloge za izračun relaavne vlage v F4 P=980 hpa T= 23.0 o C Tm = 18.0 o C 1) izračunaj naslednje spremenljivke: qs, q, in RH Rešitev: 86% 2) Določi kondenzacijski nivo delca F4: LCL (p,t)=? 3) Če delec nadaljuje z dviganjem za dodatnih 200 hpa, določi p,t na novem polozaju in količino vodne pare kondenzirane med dvigom
28 Tematski sklop 2: Globalni hidrološki cikel Klimatologija padavin
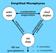
29 Globalni hidrološki cikel Ozračje oceani kopno Kon<uniran cikel!
30 Globalni hidrološki cikel E- evaporaaon, Q- atmospheric water vapour advecaon, P- precipitaaon, Ro- river runoff, Ru- undergraound runoff Q Q Q E C E P P Ro Ru Pet komponent sistema: Oceani, ledeniki in sneg, vode na kopnu, ozračje in biosfera Vodna bilanca sistema: S=P- E- Ro- Ru totalna količina vode v sistemu je ohranjena Vir: Peixoto&Oort
31 Globalni hidrološki cikel <0.1% ~97.5% ~2.4% Če kondenziramo vso vodo v ozračju višina stolpca vode znaša približno 2.5 cm Vir: Peixoto&Oort
32 Globalni hidrološki cikel
33 Globalni hidrološki cikel Procesi v oceanih so dominanten vir in ponor vode v ozračju 350,000 Volume of Water (km 3 ) 300, , , , ,000 50,000 0 Ocean Evap Ocean Precip Land Precip Evap/trans Runoff process Ocean Evap Ocean Precip Land Precip Evap/trans Runoff
34 Padavine: globalna klimatologija letno povprečje (mm/dan) Variabilnost na skali > leta Vir: reanalize ERA40 (
35 Izhlapevanje- Padavine (letno povprečje) Vir: reanalize ERA40 (
36 Padavine Globalno letno povprečje padavin: ~1 m vode Povprečna količina vode v zraku: ~2.5 cm Povprečna življenska doba vodne kaplje: 0.025/1 na leto kar znaša okoli 9 dni Sledi, da se voda v ozračju v povprečju izmeni vsakih 40 dni
37 Padavine v Sloveniji: (30- letno povprečje, mm/leto) Vir: ARSO
38 Klimatologija padavin na območju Alp Vir: hsp:// doc/rr_clim.htm Letno povprečje (mm/dan) Postaje Povprečje za november Povprečje za julij
39 Tematski sklop 3: Sevanje in njegove spremembe na poa do tal Energijska bilanca ozračja
40 Oblike energijske v atmosferi E=E i +E p +E k +E lh notranja energija potencialna energija kineačna energija energija zaradi sproščanja latentne toplote Skupna energija klimatskega sistema (atmosfera, oceani, tla) je ohranjena
41 Prenosi energije v klimatskem sistemu Totalna energija sistema je ohranjena sevanje, kondukcija, konvekcija
42 Prvi zakon termodinamike dq=mc v T+pdα opravljeno delo izmenjena toplota notranja energija dq=mc p T- Vdp 1 m dq dt = C p dt dt dp α dt dt dt dp = α C dt p 1 mc p dq dt
43 Sevanje Osnovne spremenljivke za opis prenosa energije sevanjem: valovna dolžina - λ (m) frekvenca - ν (s - 1 oz. Hz) c = λ ν c - svetlobna hitrost ( m/s) P = 1 A ΔE Δt P A = ΔE Δt gostota energijskega toka sevanja (v enotah J/sm 2 = W/m 2 ) energijski tok (v enotah W = J/s )
44 Spekter elektromagnetnega sevanja
45 Osnovni fizikalni zakoni, ki se uporabljajo za opis sevanja 1) Planckov zakon: porazdelitev gostote energijskega toka v spektru valovnih dolžin P λ 2hc ( T) dλ = / 2 d [ ] λ 5 ch kλt e 1 λ Intenziteta monokrom. sevanja (energija po enoa površine v enoa časa po enoa kota) c - svetlobna hitrost ( m/s) h - Planckova konstanta (6, Js) k - Boltzmannova konstanta (1, J/K)
46 Osnovni fizikalni zakoni, ki se uporabljajo za opis sevanja 2) Wienov zakon: spekter sevanja črnega telesa ima maksimum pri valovni dolžini λ T = c max W c W - Wien- ova konstanta (c W =2898 Kμm) λ max - valovna dolžina pri kateri telo seva največ (m) T - temperatura telesa (K) Torej, toplejša telesa sevajo več pri manjših valovnih dolžinah, kot hladnejša.
47 Osnovni fizikalni zakoni, ki se uporabljajo za opis sevanja 3) Stefan- Boltzmannov zakon (črno telo s T višjo od absolutne ničle, oddaja energijo s sevanjem): 0 P( T) = P ( T) dλ ~ T λ P( T)cosθ dωda = σt 4 4 Stefan- Boltzmannova konstanta σ σ= Wm - 2 K - 4 Za sivo telo: P = ε σ T 4 ε emisivnost ali sposobnost oddajanja
48 Gostota energijskega toka na vrhu ozračja na povprečni oddaljenosa od Sonca Sonce seva pri ~5800 K, Vidna (λ med 0,4 in 0,75 µm), IR (0,2-0,4 µm) in UV (0,4-24 µm) svetloba S o = Q =1367 Wm 2 2 4πr Q intenziteta sončnega sevanja (3, W) r povprečna razdalja Sonce- Zemlja ( m)
49
50 Sončno in terestrično sevanje λ max = 2898 T Kµm Sončno sevanje ima max v področju vidne svetlobe (~0.6 µm), teresačno v infrardečem delu (~14 µm)
51 Gostota energijskega toka na vrhu ozračja na povprečni oddaljenosa od Sonca: S o =1367 Wm - 2 Sonce seva pri ~6000 K, Na vrhu ozračja: ~46% energije je med 0.4 in 0.75 µm (vidno sevanje), ~46% energije je med 0.75 in 24 µm (IR, infrardece sevanje), ~7% energije je med 0.2 in 0.4 µm (UV, ultravijolicno sevanje).
52 Kaj se zgodi s S o na poa do tal? Vhodno sončno sevanje se delno - absorbira (upija) - sipa - odbija (reflekara) - prepušča (transmisivnost) Sposobnost absorpcije: Koeficient absorpavnosa Sposobnost oddajanja: Koeficient emisivnosa ε P = εσt 4 Odbita energija Upadla energija = α koeficient refleksivnosa (odboja), ALBEDO
53 Albedo
54 Albedo pri tleh Albedo is majhen za površino oceanov, (2-10)%, Večji za kopno, posebej za puščave, (35-45)%, Največji pa za območja ledu in pod snegom (80% in večji)
55 Albedo za različne površine
56 Emisijska temperatura Zemlje
57 Bilanca energije na vrhu ozračja Letno povprečje Vhodno sončno sevanje 340 W/m 2 Absorbirano sončno sevanje 240 W/m 2 Planetary albedo 0.30 Oddano sevanje Zemlje 240 W/m 2 Emisijska temperatura Zemlje 255 K
58 Bilanca energije na vrhu ozračja Sončno sevanje Albedo Sevanje Zemlje Bilanca Vir: Barkstrom et al., 1989
59 Bilanca energije in globalni tokovi
60 Vloga ozračja: transport Skupni transport energije skozi atmosfero in oceane proa poloma Petawatt=10 15 W
61 Vloga spodnjih robnih pogojev: T površine Temperatura površine morja januarja Temperatura površine morja julija
62 Vloga spodnjih robnih pogojev: vlaga Odvisnost e s od temperature Globalna porazdelitev e s Okoli 70% površine zemlje je mokro
63 Vloga spodnjih robnih pogojev: ostali faktorji Nadmorska višina (orografija) Toplotna kapaciteta Hrapavost podlage Vegetacija Morski led Kopenski led
64 Profil ravnovesne temperature
65 Kaj se zgodi s S o na poa do tal?
66 Ocena energijske bilance Zemljina klimatskega sistema (v W/m 2 ) Vir: AMS
67 Ocena energijske bilance Zemljina klimatskega sistema (v %)
68 Porazdelitev Sončnega sevanja pri tleh
69 Lokalna bilanca energije Bilanca = +SW (sončno vhodno) SW (odbito) +LW (IR) LW (IR)
70 Energijska bilanca Zemlje P = P SW + P Povprečni energijski tok (ang. LW energy flux) P SW ( ) 1 α P = PSW PSW = SW α povprečni albedo P LW = P LW PLW Vse skupaj: P ( ) 4 1 α P εσt + P = SW Z LW SW: kratkovalovno LW: dolgovalovno
71 Energijska bilanca Zemlje: vrh ozračja (1) P TA ( ) 1 α P ds P ds 0 = SW top top LW Energijska bilanca na vrhu ozračja (TOA=top of the atmosphere) α povprečni albedo πr Z ( ) α zemlja+ozračje, v povprečju α S = 238 4πR 2 2 Z o Wm - 2 Za Zemljo kot črno telo, ravnovesna temperatura za (1): σt 4 e = 238 Te = 255 K Wm - 2 emisivnost ε=1
72 Energijska bilanca pri tleh P T s T e ( ) 4 1 A P εσt + P = SW e LW = T e ΔT ΔT = 33 K K emisivnost ε=1 P Tok zaznavne toplote P SH P LH Tok latentne toplote P G P M Toplotni tok v globlje sloje = 0 Energija, porabljena za taljenje snega, leda ali zmrzovanje vode
73 Bilanca energije na površini Zemlje Letno povprečje Absorbirano sončno sevanje (SW) 176 W/m 2 Dolgovalovno sevanje proa tlom (LW ) 312 W/m 2 Dolgovalovno sevanje navzven (LW ) W/m 2 Totalno dolgovalovno sevanje (LW) - 73 W/m 2 Bilanca sevanja na površini (SW+LW) 103 W/m 2 Latentna toplota (LH) - 79 W/m 2 Zaznavna toplota (SH) - 24 W/m 2 Poznamo jo le približno (napaka znaša kakšnih 20%)
74 Meddelovanje med oblaki in sevanjem
75 Naloga: enostavni modeli toplogrednega učinka Predpostavi, da je atmosfera opisana z N slojev kot je predstavljeno na sliki: Vir: Naloga 2.5 iz učbenika Marshall in Plumb: Atmosphere, Ocean and Climate Dynamics: an introductory text. InternaAonal Geophysics Series.
76 Naloga: enostavni modeli toplogrednega učinka (2) Predpostavi, da je atmosfera popolnoma prosojna za kratkovalovno (sončno, SW) sevanje in da delno propušča dolgovalovno (zemljino, LW) sevanje. Vsaki sloj popolnoma absorbira sevanje LW. a) S pomočjo energijske bilance pri tleh pokaži, da temperatura pri tleh mora bia večja od temperature najnižjega sloja ozračja. T s > T N b) S pomočjo energijske bilance za n- A sloj pokaži, da v ravnovesju velja 2T n 4 = T n T n 1 T n je tempertura sloja n, za n>1. Iz tega lahko sledi, da je ravnovesna temperatura pri tleh T T n je emisijska T planeta. s = N +1 ( ) 1/4 T e [Navodilo: Za reševanje naloge b) uporabi rezultat naloge a), določi T 1 in uporabi (*) za določanje razlike T sosednih slojev.] (*)
Attempt to prepare seasonal weather outlook for Slovenia
 Attempt to prepare seasonal weather outlook for Slovenia Main available sources (ECMWF, EUROSIP, IRI, CPC.NCEP.NOAA,..) Two parameters (T and RR anomally) Textual information ( Met Office like ) Issued
Attempt to prepare seasonal weather outlook for Slovenia Main available sources (ECMWF, EUROSIP, IRI, CPC.NCEP.NOAA,..) Two parameters (T and RR anomally) Textual information ( Met Office like ) Issued
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
ENAČBA STANJA VODE IN VODNE PARE
 ENAČBA STANJA VODE IN VODNE PARE SEMINARSKA NALOGA PRI PREDMETU JEDRSKA TEHNIKA IN ENERGETIKA TAMARA STOJANOV MENTOR: IZRED. PROF. DR. IZTOK TISELJ NOVEMBER 2011 Enačba stanja idealni plin: pv = RT p tlak,
ENAČBA STANJA VODE IN VODNE PARE SEMINARSKA NALOGA PRI PREDMETU JEDRSKA TEHNIKA IN ENERGETIKA TAMARA STOJANOV MENTOR: IZRED. PROF. DR. IZTOK TISELJ NOVEMBER 2011 Enačba stanja idealni plin: pv = RT p tlak,
Lecture 3a: Surface Energy Balance
 Lecture 3a: Surface Energy Balance Instructor: Prof. Johnny Luo http://www.sci.ccny.cuny.edu/~luo Total: 50 pts Absorption of IR radiation O 3 band ~ 9.6 µm Vibration-rotation interaction of CO 2 ~
Lecture 3a: Surface Energy Balance Instructor: Prof. Johnny Luo http://www.sci.ccny.cuny.edu/~luo Total: 50 pts Absorption of IR radiation O 3 band ~ 9.6 µm Vibration-rotation interaction of CO 2 ~
KLIMATSKI DEJAVNIKI (2) 5 skupin
 KLIMATSKI DEJAVNIKI (2) 5 skupin Sončno obsevanje Transmisijske lastnosti atmosfere za prenos različnih sevanj (aerosoli in plini tople grede) Cirkulacija atmosfere in oceanov Lastnosti površja Relief
KLIMATSKI DEJAVNIKI (2) 5 skupin Sončno obsevanje Transmisijske lastnosti atmosfere za prenos različnih sevanj (aerosoli in plini tople grede) Cirkulacija atmosfere in oceanov Lastnosti površja Relief
Lecture 3a: Surface Energy Balance
 Lecture 3a: Surface Energy Balance Instructor: Prof. Johnny Luo http://www.sci.ccny.cuny.edu/~luo Surface Energy Balance 1. Factors affecting surface energy balance 2. Surface heat storage 3. Surface
Lecture 3a: Surface Energy Balance Instructor: Prof. Johnny Luo http://www.sci.ccny.cuny.edu/~luo Surface Energy Balance 1. Factors affecting surface energy balance 2. Surface heat storage 3. Surface
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
IZRAČUN MEMBRANSKE RAZTEZNE POSODE - "MRP" za HLADNOVODNE SISTEME (DIN 4807/2)
 IZPIS IZRAČUN MEMBRANSKE RAZTEZNE POSODE - "MRP" za HLADNOVODNE SISTEME Izhodiščni podatki: Objkt : Vrtc Kamnitnik Projkt : PZI Uporaba MRP : Črpalna vrtina Datum : 30.8.2017 Obdlal : Zupan Skupna hladilna
IZPIS IZRAČUN MEMBRANSKE RAZTEZNE POSODE - "MRP" za HLADNOVODNE SISTEME Izhodiščni podatki: Objkt : Vrtc Kamnitnik Projkt : PZI Uporaba MRP : Črpalna vrtina Datum : 30.8.2017 Obdlal : Zupan Skupna hladilna
The Tropical Atmosphere: Hurricane Incubator
 The Tropical Atmosphere: Hurricane Incubator Images from journals published by the American Meteorological Society are copyright AMS and used with permission. A One-Dimensional Description of the Tropical
The Tropical Atmosphere: Hurricane Incubator Images from journals published by the American Meteorological Society are copyright AMS and used with permission. A One-Dimensional Description of the Tropical
General Concepts of Atmospheric Thermodynamic Atmospheric Thermodynamic Theory
 General Concepts of Atmospheric Thermodynamic Atmospheric Thermodynamic Theory The theory of thermodynamics is one of the important cornerstones of classical physics. It has applications not only in physics,
General Concepts of Atmospheric Thermodynamic Atmospheric Thermodynamic Theory The theory of thermodynamics is one of the important cornerstones of classical physics. It has applications not only in physics,
Clouds and turbulent moist convection
 Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
GEF2200 Atmosfærefysikk 2012
 GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
TERMODINAMIKA, BIOENERGETIKA
 TERMODINAMIKA, BIOENERGETIKA Osnovni termodinamski koncepti Fizikalni pomen termodinamskih količin ph in standardni pogoji Sklopljeni procesi Energijsko bogate biomolekule Osnovni termodinamski koncepti
TERMODINAMIKA, BIOENERGETIKA Osnovni termodinamski koncepti Fizikalni pomen termodinamskih količin ph in standardni pogoji Sklopljeni procesi Energijsko bogate biomolekule Osnovni termodinamski koncepti
Exam 1 (Chaps. 1-6 of the notes)
 10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
Chapter 4 Water Vapor
 Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
EAS270, The Atmosphere 2 nd Mid-term Exam 2 Nov. 2016
 EAS270, The Atmosphere 2 nd Mid-term Exam 2 Nov. 2016 Professor: J.D. Wilson Time available: 50 mins Value: 25% No formula sheets; no use of tablet computers etc. or cell phones. Formulae/data at back.
EAS270, The Atmosphere 2 nd Mid-term Exam 2 Nov. 2016 Professor: J.D. Wilson Time available: 50 mins Value: 25% No formula sheets; no use of tablet computers etc. or cell phones. Formulae/data at back.
UPORABA TERMOGRAFIJE V ELEKTRIČNIH NAPRAVAH
 I UNIVERZA V MARIBORU FAKULTETA ZA ELEKTROTEHNIKO, RAČUNALNIŠTVO IN INFORMATIKO 2000 Maribor, Smetanova ul. 17 Diplomska naloga visokošolskega strokovnega študijskega programa UPORABA TERMOGRAFIJE V ELEKTRIČNIH
I UNIVERZA V MARIBORU FAKULTETA ZA ELEKTROTEHNIKO, RAČUNALNIŠTVO IN INFORMATIKO 2000 Maribor, Smetanova ul. 17 Diplomska naloga visokošolskega strokovnega študijskega programa UPORABA TERMOGRAFIJE V ELEKTRIČNIH
2. Illustration of Atmospheric Greenhouse Effect with Simple Models
 2. Illustration of Atmospheric Greenhouse Effect with Simple Models In the first lecture, I introduced the concept of global energy balance and talked about the greenhouse effect. Today we will address
2. Illustration of Atmospheric Greenhouse Effect with Simple Models In the first lecture, I introduced the concept of global energy balance and talked about the greenhouse effect. Today we will address
Clouds and atmospheric convection
 Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
1) The energy balance at the TOA is: 4 (1 α) = σt (1 0.3) = ( ) 4. (1 α) 4σ = ( S 0 = 255 T 1
 EAS488/B8800 Climate & Climate Change Homework 2: Atmospheric Radiation and Climate, surface energy balance, and atmospheric general circulation Posted: 3/12/18; due: 3/26/18 Answer keys 1. (10 points)
EAS488/B8800 Climate & Climate Change Homework 2: Atmospheric Radiation and Climate, surface energy balance, and atmospheric general circulation Posted: 3/12/18; due: 3/26/18 Answer keys 1. (10 points)
Earth Systems Science Chapter 3
 Earth Systems Science Chapter 3 ELECTROMAGNETIC RADIATION: WAVES I. Global Energy Balance and the Greenhouse Effect: The Physics of the Radiation Balance of the Earth 1. Electromagnetic Radiation: waves,
Earth Systems Science Chapter 3 ELECTROMAGNETIC RADIATION: WAVES I. Global Energy Balance and the Greenhouse Effect: The Physics of the Radiation Balance of the Earth 1. Electromagnetic Radiation: waves,
Baroklina nestabilnost
 Baroklina nestabilnost Navodila za projektno nalogo iz dinamične meteorologije 2012/2013 Januar 2013 Nedjeljka Zagar in Rahela Zabkar Naloga je zasnovana na dvoslojnem modelu baroklinega razvoja, napisana
Baroklina nestabilnost Navodila za projektno nalogo iz dinamične meteorologije 2012/2013 Januar 2013 Nedjeljka Zagar in Rahela Zabkar Naloga je zasnovana na dvoslojnem modelu baroklinega razvoja, napisana
Radiative Transfer Chapter 3, Hartmann
 Radiative Transfer Chapter 3, Hartmann Shortwave Absorption: Clouds, H 2 0, O 3, some CO 2 Shortwave Reflection: Clouds, surface, atmosphere Longwave Absorption: Clouds, H 2 0, CO 2, CH 4, N 2 O Planck
Radiative Transfer Chapter 3, Hartmann Shortwave Absorption: Clouds, H 2 0, O 3, some CO 2 Shortwave Reflection: Clouds, surface, atmosphere Longwave Absorption: Clouds, H 2 0, CO 2, CH 4, N 2 O Planck
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere
![Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere](/thumbs/72/66535634.jpg) Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Monday 9 September, :30-11:30 Class#03
 Monday 9 September, 2013 10:30-11:30 Class#03 Topics for the hour Solar zenith angle & relationship to albedo Blackbody spectra Stefan-Boltzman Relationship Layer model of atmosphere OLR, Outgoing longwave
Monday 9 September, 2013 10:30-11:30 Class#03 Topics for the hour Solar zenith angle & relationship to albedo Blackbody spectra Stefan-Boltzman Relationship Layer model of atmosphere OLR, Outgoing longwave
Governing Equations and Scaling in the Tropics
 Governing Equations and Scaling in the Tropics M 1 ( ) e R ε er Tropical v Midlatitude Meteorology Why is the general circulation and synoptic weather systems in the tropics different to the those in the
Governing Equations and Scaling in the Tropics M 1 ( ) e R ε er Tropical v Midlatitude Meteorology Why is the general circulation and synoptic weather systems in the tropics different to the those in the
Earth: the Goldilocks Planet
 Earth: the Goldilocks Planet Not too hot (460 C) Fig. 3-1 Not too cold (-55 C) Wave properties: Wavelength, velocity, and? Fig. 3-2 Reviewing units: Wavelength = distance (meters or nanometers, etc.) Velocity
Earth: the Goldilocks Planet Not too hot (460 C) Fig. 3-1 Not too cold (-55 C) Wave properties: Wavelength, velocity, and? Fig. 3-2 Reviewing units: Wavelength = distance (meters or nanometers, etc.) Velocity
Outline. Aim. Gas law. Pressure. Scale height Mixing Column density. Temperature Lapse rate Stability. Condensation Humidity.
 Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
CONDENSATION CORROSION: A THEORETICAL APPROACH
 ACTA CARSOLOGICA 34/2 2 317-348 LJUBLJANA 2005 COBISS: 1.01 CONDENSATION CORROSION: A THEORETICAL APPROACH KONDENZACIJSKA KOROZIJA: TEORETIČNI PRISTOP WOLFGANG DREYBRODT 1,2, FRANCI GABROVŠEK 2 & MATIJA
ACTA CARSOLOGICA 34/2 2 317-348 LJUBLJANA 2005 COBISS: 1.01 CONDENSATION CORROSION: A THEORETICAL APPROACH KONDENZACIJSKA KOROZIJA: TEORETIČNI PRISTOP WOLFGANG DREYBRODT 1,2, FRANCI GABROVŠEK 2 & MATIJA
Physical Fundamentals of Global Change Processes
 University of Applied Sciences Eberswalde Master Study Program Global Change Management Manfred Stock Potsdam Institute for Climate Impact Research Module: Physical Fundamentals of Global Change Processes
University of Applied Sciences Eberswalde Master Study Program Global Change Management Manfred Stock Potsdam Institute for Climate Impact Research Module: Physical Fundamentals of Global Change Processes
OA07 ANNEX 4: SCOPE OF ACCREDITATION IN CALIBRATION
 OA07 ANNEX 4: SCOPE OF ACCREDITATION IN CALIBRATION Table of contents 1 TECHNICAL FIELDS... 2 2 PRESENTING THE SCOPE OF A CALIBRATION LABOORATORY... 2 3 CONSIDERING CHANGES TO SCOPES... 6 4 CHANGES WITH
OA07 ANNEX 4: SCOPE OF ACCREDITATION IN CALIBRATION Table of contents 1 TECHNICAL FIELDS... 2 2 PRESENTING THE SCOPE OF A CALIBRATION LABOORATORY... 2 3 CONSIDERING CHANGES TO SCOPES... 6 4 CHANGES WITH
Overview of fundamental atmospheric concepts
 Fundamentals of Earth s Atmosphere AOSC 433/633 & CHEM 433/633 Tim Canty Class Web Site: http://www.atmos.umd.edu/~rjs/class/spr2013 Notes: Ross, Tim, & Allison co-teach this class; please include all
Fundamentals of Earth s Atmosphere AOSC 433/633 & CHEM 433/633 Tim Canty Class Web Site: http://www.atmos.umd.edu/~rjs/class/spr2013 Notes: Ross, Tim, & Allison co-teach this class; please include all
Planetary Atmospheres
 Planetary Atmospheres Structure Composition Clouds Meteorology Photochemistry Atmospheric Escape EAS 4803/8803 - CP 11:1 Structure Generalized Hydrostatic Equilibrium P( z) = P( 0)e z # ( ) " dr / H r
Planetary Atmospheres Structure Composition Clouds Meteorology Photochemistry Atmospheric Escape EAS 4803/8803 - CP 11:1 Structure Generalized Hydrostatic Equilibrium P( z) = P( 0)e z # ( ) " dr / H r
( ) = 1005 J kg 1 K 1 ;
 Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
The atmosphere: A general introduction Niels Woetmann Nielsen Danish Meteorological Institute
 The atmosphere: A general introduction Niels Woetmann Nielsen Danish Meteorological Institute Facts about the atmosphere The atmosphere is kept in place on Earth by gravity The Earth-Atmosphere system
The atmosphere: A general introduction Niels Woetmann Nielsen Danish Meteorological Institute Facts about the atmosphere The atmosphere is kept in place on Earth by gravity The Earth-Atmosphere system
Boundary layer equilibrium [2005] over tropical oceans
![Boundary layer equilibrium [2005] over tropical oceans Boundary layer equilibrium [2005] over tropical oceans](/thumbs/96/128963638.jpg) Boundary layer equilibrium [2005] over tropical oceans Alan K. Betts [akbetts@aol.com] Based on: Betts, A.K., 1997: Trade Cumulus: Observations and Modeling. Chapter 4 (pp 99-126) in The Physics and Parameterization
Boundary layer equilibrium [2005] over tropical oceans Alan K. Betts [akbetts@aol.com] Based on: Betts, A.K., 1997: Trade Cumulus: Observations and Modeling. Chapter 4 (pp 99-126) in The Physics and Parameterization
Hand in Question sheets with answer booklets Calculators allowed Mobile telephones or other devices not allowed
 York University Department of Earth and Space Science and Engineering ESSE 3030 Department of Physics and Astronomy PHYS 3080 Atmospheric Radiation and Thermodynamics Final Examination 2:00 PM 11 December
York University Department of Earth and Space Science and Engineering ESSE 3030 Department of Physics and Astronomy PHYS 3080 Atmospheric Radiation and Thermodynamics Final Examination 2:00 PM 11 December
2. Energy Balance. 1. All substances radiate unless their temperature is at absolute zero (0 K). Gases radiate at specific frequencies, while solids
 I. Radiation 2. Energy Balance 1. All substances radiate unless their temperature is at absolute zero (0 K). Gases radiate at specific frequencies, while solids radiate at many Click frequencies, to edit
I. Radiation 2. Energy Balance 1. All substances radiate unless their temperature is at absolute zero (0 K). Gases radiate at specific frequencies, while solids radiate at many Click frequencies, to edit
Fundamentals of Earth s Atmosphere. AOSC 434/658R & CHEM 434/678A Tim Canty
 Fundamentals of Earth s Atmosphere AOSC 434/658R & CHEM 434/678A Tim Canty Class Web Site: http://www.atmos.umd.edu/~rjs/class/spr2011 Notes: Please include both Ross and Tim on any class related email
Fundamentals of Earth s Atmosphere AOSC 434/658R & CHEM 434/678A Tim Canty Class Web Site: http://www.atmos.umd.edu/~rjs/class/spr2011 Notes: Please include both Ross and Tim on any class related email
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
THERMODYNAMICS 1 /43
 THERMODYNAMICS 1 Atmosphere A multi-component Multi-Phase System The gas phase atmospheric cons1tuents; major gases; fixed propor1ons by volume (dry air) Nitrogen (N 2 ) 78,08 % Oxygen (O 2 ) 29,05 % Argon
THERMODYNAMICS 1 Atmosphere A multi-component Multi-Phase System The gas phase atmospheric cons1tuents; major gases; fixed propor1ons by volume (dry air) Nitrogen (N 2 ) 78,08 % Oxygen (O 2 ) 29,05 % Argon
SEGREVANJE VODNIKOV V USTALJENEM STANJU. Žiga VORŠIČ, Vitodrag Kumperščak, Jože PIHLER
 . osvetovanje "KOMUNALNA ENERGETIKA / POWER ENGINEERING", Maribor, 015 1 SEGREVANJE VODNIKOV V USTALJENEM STANJU Žiga VORŠIČ, Vitodrag Kumerščak, Jože PIHLER POVZETEK V načrtovanju razdeljevalnih in renosnih
. osvetovanje "KOMUNALNA ENERGETIKA / POWER ENGINEERING", Maribor, 015 1 SEGREVANJE VODNIKOV V USTALJENEM STANJU Žiga VORŠIČ, Vitodrag Kumerščak, Jože PIHLER POVZETEK V načrtovanju razdeljevalnih in renosnih
ENERGY AND MASS SPECTROSCOPY OF IONS AND NEUTRALS IN COLD PLASMA
 UDK621.3:(53+54+621 +66), ISSN0352-9045 Informaclje MIDEM 3~(~UU8)4, Ljubljana ENERGY AND MASS SPECTROSCOPY OF IONS AND NEUTRALS IN COLD PLASMA Marijan Macek 1,2* Miha Cekada 2 1 University of Ljubljana,
UDK621.3:(53+54+621 +66), ISSN0352-9045 Informaclje MIDEM 3~(~UU8)4, Ljubljana ENERGY AND MASS SPECTROSCOPY OF IONS AND NEUTRALS IN COLD PLASMA Marijan Macek 1,2* Miha Cekada 2 1 University of Ljubljana,
Radiation in climate models.
 Lecture. Radiation in climate models. Objectives:. A hierarchy of the climate models.. Radiative and radiative-convective equilibrium.. Examples of simple energy balance models.. Radiation in the atmospheric
Lecture. Radiation in climate models. Objectives:. A hierarchy of the climate models.. Radiative and radiative-convective equilibrium.. Examples of simple energy balance models.. Radiation in the atmospheric
Atmospheric Dynamics: lecture 3
 Atmospheric Dynamics: lecture 3 Moist convection Dew point temperature/lapse rate/lcl Equivalent potential temperature Conditional and potential instability Thermodynamic diagram CAPE Introduction to Python
Atmospheric Dynamics: lecture 3 Moist convection Dew point temperature/lapse rate/lcl Equivalent potential temperature Conditional and potential instability Thermodynamic diagram CAPE Introduction to Python
where p oo is a reference level constant pressure (often 10 5 Pa). Since θ is conserved for adiabatic motions, a prognostic temperature equation is:
 1 Appendix C Useful Equations Purposes: Provide foundation equations and sketch some derivations. These equations are used as starting places for discussions in various parts of the book. C.1. Thermodynamic
1 Appendix C Useful Equations Purposes: Provide foundation equations and sketch some derivations. These equations are used as starting places for discussions in various parts of the book. C.1. Thermodynamic
Atsc final Equations: page 1/6
 Atsc. 405 2012 final Equations: page 1/6 Answer each of these 7 questions (note weight). Show all your work on all questions (needed for partial credit). Be sure to put your name on any detached pages.
Atsc. 405 2012 final Equations: page 1/6 Answer each of these 7 questions (note weight). Show all your work on all questions (needed for partial credit). Be sure to put your name on any detached pages.
Planetary Atmospheres
 Planetary Atmospheres Structure Composition Clouds Meteorology Photochemistry Atmospheric Escape EAS 4803/8803 - CP 17:1 Structure Generalized Hydrostatic Equilibrium P( z) = P( 0)e z # ( ) " dr / H r
Planetary Atmospheres Structure Composition Clouds Meteorology Photochemistry Atmospheric Escape EAS 4803/8803 - CP 17:1 Structure Generalized Hydrostatic Equilibrium P( z) = P( 0)e z # ( ) " dr / H r
Radiation, Sensible Heat Flux and Evapotranspiration
 Radiation, Sensible Heat Flux and Evapotranspiration Climatological and hydrological field work Figure 1: Estimate of the Earth s annual and global mean energy balance. Over the long term, the incoming
Radiation, Sensible Heat Flux and Evapotranspiration Climatological and hydrological field work Figure 1: Estimate of the Earth s annual and global mean energy balance. Over the long term, the incoming
Solar Radiation and Environmental Biophysics Geo 827, MSU Jiquan Chen Oct. 6, 2015
 Solar Radiation and Environmental Biophysics Geo 827, MSU Jiquan Chen Oct. 6, 2015 1) Solar radiation basics 2) Energy balance 3) Other relevant biophysics 4) A few selected applications of RS in ecosystem
Solar Radiation and Environmental Biophysics Geo 827, MSU Jiquan Chen Oct. 6, 2015 1) Solar radiation basics 2) Energy balance 3) Other relevant biophysics 4) A few selected applications of RS in ecosystem
Reševanje problemov in algoritmi
 Reševanje problemov in algoritmi Vhod Algoritem Izhod Kaj bomo spoznali Zgodovina algoritmov. Primeri algoritmov. Algoritmi in programi. Kaj je algoritem? Algoritem je postopek, kako korak za korakom rešimo
Reševanje problemov in algoritmi Vhod Algoritem Izhod Kaj bomo spoznali Zgodovina algoritmov. Primeri algoritmov. Algoritmi in programi. Kaj je algoritem? Algoritem je postopek, kako korak za korakom rešimo
1. Heterogeneous Systems and Chemical Equilibrium
 1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
Atmospheric science research for numerical weather prediction and climate modeling in Slovenia: contribution of the PECS program
 Atmospheric science research for numerical weather prediction and climate modeling in Slovenia: contribution of the PECS program Matic Šavli, Žiga Zaplotnik, Veronika Hladnik, Gregor Skok and Nedjeljka
Atmospheric science research for numerical weather prediction and climate modeling in Slovenia: contribution of the PECS program Matic Šavli, Žiga Zaplotnik, Veronika Hladnik, Gregor Skok and Nedjeljka
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics
 Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
MECHANICAL EFFICIENCY, WORK AND HEAT OUTPUT IN RUNNING UPHILL OR DOWNHILL
 original scientific article UDC: 796.4 received: 2011-05-03 MECHANICAL EFFICIENCY, WORK AND HEAT OUTPUT IN RUNNING UPHILL OR DOWNHILL Pietro Enrico DI PRAMPERO University of Udine, Department of Biomedical
original scientific article UDC: 796.4 received: 2011-05-03 MECHANICAL EFFICIENCY, WORK AND HEAT OUTPUT IN RUNNING UPHILL OR DOWNHILL Pietro Enrico DI PRAMPERO University of Udine, Department of Biomedical
A Case Study on Diurnal Boundary Layer Evolution
 UNIVERSITY OF OKLAHOMA A Case Study on Diurnal Boundary Layer Evolution Meteorological Measurement Systems Fall 2010 Jason Godwin 12/9/2010 Lab partners: Sam Irons, Charles Kuster, Nathan New, and Stefan
UNIVERSITY OF OKLAHOMA A Case Study on Diurnal Boundary Layer Evolution Meteorological Measurement Systems Fall 2010 Jason Godwin 12/9/2010 Lab partners: Sam Irons, Charles Kuster, Nathan New, and Stefan
Chapter 3 Energy Balance and Temperature. Astro 9601
 Chapter 3 Energy Balance and Temperature Astro 9601 1 Topics to be covered Energy Balance and Temperature (3.1) - All Conduction (3..1), Radiation (3.. and 3...1) Convection (3..3), Hydrostatic Equilibrium
Chapter 3 Energy Balance and Temperature Astro 9601 1 Topics to be covered Energy Balance and Temperature (3.1) - All Conduction (3..1), Radiation (3.. and 3...1) Convection (3..3), Hydrostatic Equilibrium
Glaciology HEAT BUDGET AND RADIATION
 HEAT BUDGET AND RADIATION A Heat Budget 1 Black body radiation Definition. A perfect black body is defined as a body that absorbs all radiation that falls on it. The intensity of radiation emitted by a
HEAT BUDGET AND RADIATION A Heat Budget 1 Black body radiation Definition. A perfect black body is defined as a body that absorbs all radiation that falls on it. The intensity of radiation emitted by a
1. Basic state values of matter
 1. Basic state values of matter Example 1.1 The pressure inside a boiler is p p = 115.10 5 Pa and p v = 9.44.10 4 Pa inside a condenser. Calculate the absolute pressure inside the boiler and condenser
1. Basic state values of matter Example 1.1 The pressure inside a boiler is p p = 115.10 5 Pa and p v = 9.44.10 4 Pa inside a condenser. Calculate the absolute pressure inside the boiler and condenser
Termodinamika. FIZIKA PSS-GRAD 29. studenog Copyright 2015 John Wiley & Sons, Inc. All rights reserved.
 Termodinamika FIZIKA PSS-GRAD 29. studenog 2017. 15.1 Thermodynamic Systems and Their Surroundings Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey.
Termodinamika FIZIKA PSS-GRAD 29. studenog 2017. 15.1 Thermodynamic Systems and Their Surroundings Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey.
Friday 8 September, :00-4:00 Class#05
 Friday 8 September, 2017 3:00-4:00 Class#05 Topics for the hour Global Energy Budget, schematic view Solar Radiation Blackbody Radiation http://www2.gi.alaska.edu/~bhatt/teaching/atm694.fall2017/ notes.html
Friday 8 September, 2017 3:00-4:00 Class#05 Topics for the hour Global Energy Budget, schematic view Solar Radiation Blackbody Radiation http://www2.gi.alaska.edu/~bhatt/teaching/atm694.fall2017/ notes.html
Buoyancy and Coriolis forces
 Chapter 2 Buoyancy and Coriolis forces In this chapter we address several topics that we need to understand before starting on our study of geophysical uid dynamics. 2.1 Hydrostatic approximation Consider
Chapter 2 Buoyancy and Coriolis forces In this chapter we address several topics that we need to understand before starting on our study of geophysical uid dynamics. 2.1 Hydrostatic approximation Consider
Chapter 3 Energy Balance and Temperature. Topics to be covered
 Chapter 3 Energy Balance and Temperature Astro 9601 1 Topics to be covered Energy Balance and Temperature (3.1) - All Conduction (3..1), Radiation (3.. and31) 3...1) Convection (3..3), Hydrostatic Equilibrium
Chapter 3 Energy Balance and Temperature Astro 9601 1 Topics to be covered Energy Balance and Temperature (3.1) - All Conduction (3..1), Radiation (3.. and31) 3...1) Convection (3..3), Hydrostatic Equilibrium
Določanje stopnje oblačnosti z metodo merjenja temperature neba
 Univerza v Ljubljani Fakulteta za elektrotehniko Štefan Mikuž Določanje stopnje oblačnosti z metodo merjenja temperature neba Diplomsko delo univerzitetnega študija Mentor: doc. dr. Marko Jankovec Ljubljana,
Univerza v Ljubljani Fakulteta za elektrotehniko Štefan Mikuž Določanje stopnje oblačnosti z metodo merjenja temperature neba Diplomsko delo univerzitetnega študija Mentor: doc. dr. Marko Jankovec Ljubljana,
SEMESTER I EXAMINATION 2009/2010. MAPH Physical Meteorology
 SEMESTER I EXAMINATION 2009/2010 MAPH 40240 Physical Meteorology Extern examiner: Professor Keith Shine Head of School: Professor Micheal O Searcoid Examiner: Dr. Rodrigo Caballero Time Allowed: 2 hours
SEMESTER I EXAMINATION 2009/2010 MAPH 40240 Physical Meteorology Extern examiner: Professor Keith Shine Head of School: Professor Micheal O Searcoid Examiner: Dr. Rodrigo Caballero Time Allowed: 2 hours
Climate Dynamics Simple Climate Models
 Climate Dynamics Simple Climate Models John Shepherd School of Ocean & Earth Science Southampton Oceanography Centre 1) Basic facts and findings Overview : 4 Lectures The global energy balance Zero-dimensional
Climate Dynamics Simple Climate Models John Shepherd School of Ocean & Earth Science Southampton Oceanography Centre 1) Basic facts and findings Overview : 4 Lectures The global energy balance Zero-dimensional
GEF2200 atmospheric physics 2018
 GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
Data and formulas at the end. Real exam is Wednesday May 8, 2002
 ATMS 31: Physical Climatology Practice Mid Term Exam - Spring 001 page 1 Atmospheric Sciences 31 Physical Climatology Practice Mid-Term Examination: Would be Closed Book Data and formulas at the end. Real
ATMS 31: Physical Climatology Practice Mid Term Exam - Spring 001 page 1 Atmospheric Sciences 31 Physical Climatology Practice Mid-Term Examination: Would be Closed Book Data and formulas at the end. Real
Let s make a simple climate model for Earth.
 Let s make a simple climate model for Earth. What is the energy balance of the Earth? How is it controlled? ó How is it affected by humans? Energy balance (radiant energy) Greenhouse Effect (absorption
Let s make a simple climate model for Earth. What is the energy balance of the Earth? How is it controlled? ó How is it affected by humans? Energy balance (radiant energy) Greenhouse Effect (absorption
WaVaCS summerschool Autumn 2009 Cargese, Corsica
 Introduction Part I WaVaCS summerschool Autumn 2009 Cargese, Corsica Holger Tost Max Planck Institute for Chemistry, Mainz, Germany Introduction Overview What is a parameterisation and why using it? Fundamentals
Introduction Part I WaVaCS summerschool Autumn 2009 Cargese, Corsica Holger Tost Max Planck Institute for Chemistry, Mainz, Germany Introduction Overview What is a parameterisation and why using it? Fundamentals
P607 Climate and Energy (Dr. H. Coe)
 P607 Climate and Energy (Dr. H. Coe) Syllabus: The composition of the atmosphere and the atmospheric energy balance; Radiative balance in the atmosphere; Energy flow in the biosphere, atmosphere and ocean;
P607 Climate and Energy (Dr. H. Coe) Syllabus: The composition of the atmosphere and the atmospheric energy balance; Radiative balance in the atmosphere; Energy flow in the biosphere, atmosphere and ocean;
The Behaviour of the Atmosphere
 3 The Behaviour of the Atmosphere Learning Goals After studying this chapter, students should be able to: apply the ideal gas law and the concept of hydrostatic balance to the atmosphere (pp. 49 54); apply
3 The Behaviour of the Atmosphere Learning Goals After studying this chapter, students should be able to: apply the ideal gas law and the concept of hydrostatic balance to the atmosphere (pp. 49 54); apply
dt T du T = C V = Nk ln = Nk ln 1 + V ]
![dt T du T = C V = Nk ln = Nk ln 1 + V ] dt T du T = C V = Nk ln = Nk ln 1 + V ]](/thumbs/80/81150773.jpg) PHYSICS 218 SOLUION O HW 10 Created: November 25, 2004 23:26pm Last updated: December 9, 2004 1:01am 1. Schroeder 3.32 (a) In moving the piston through a distance of x = 1 mm, the work done on the system
PHYSICS 218 SOLUION O HW 10 Created: November 25, 2004 23:26pm Last updated: December 9, 2004 1:01am 1. Schroeder 3.32 (a) In moving the piston through a distance of x = 1 mm, the work done on the system
Determining the Leakage Flow through Water Turbines and Inlet- Water Gate in the Doblar 2 Hydro Power Plant
 Elektrotehniški vestnik 77(4): 39-44, 010 Electrotechnical Review: Ljubljana, Slovenija Določanje puščanja vodnih turbin in predturbinskih zapornic v hidroelektrarni Doblar Miha Leban 1, Rajko Volk 1,
Elektrotehniški vestnik 77(4): 39-44, 010 Electrotechnical Review: Ljubljana, Slovenija Določanje puščanja vodnih turbin in predturbinskih zapornic v hidroelektrarni Doblar Miha Leban 1, Rajko Volk 1,
G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1. Time allowed 50 mins. Total possible points: 40 number of pages: 5
 G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1 Time allowed 50 mins. Total possible points: 40 number of pages: 5 Part A: Short Answer & Problems (12), Fill in the Blanks (6).
G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1 Time allowed 50 mins. Total possible points: 40 number of pages: 5 Part A: Short Answer & Problems (12), Fill in the Blanks (6).
What did we learn in Ch. 1? Final Exam 2014: 6 Questions. Energy Transfers Link Ch Reversible-Adiabatic-Work. What did we learn in Ch. 3?
 Final Exam 0: 6 Questions. First/Second Laws Question. Definitions Question 3. Clausius-Clapeyron with q/h/e) Question. Köhler, Clouds, and Stability Question 5. Climate Model and Sensitivity Question
Final Exam 0: 6 Questions. First/Second Laws Question. Definitions Question 3. Clausius-Clapeyron with q/h/e) Question. Köhler, Clouds, and Stability Question 5. Climate Model and Sensitivity Question
Calculation of stress-strain dependence from tensile tests at high temperatures using final shapes of specimen s contours
 RMZ Materials and Geoenvironment, Vol. 59, No. 4, pp. 331 346, 2012 331 Calculation of stress-strain dependence from tensile tests at high temperatures using final shapes of specimen s contours Določitev
RMZ Materials and Geoenvironment, Vol. 59, No. 4, pp. 331 346, 2012 331 Calculation of stress-strain dependence from tensile tests at high temperatures using final shapes of specimen s contours Določitev
First Law of Thermodynamics
 First Law of Thermodynamics September 11, 2013 The first law of thermodynamics is the conservation of energy applied to thermal systems. Here, we develop the principles of thermodynamics for a discrete
First Law of Thermodynamics September 11, 2013 The first law of thermodynamics is the conservation of energy applied to thermal systems. Here, we develop the principles of thermodynamics for a discrete
UNIVERSITY OF SOUTHAMPTON VERY IMPORTANT NOTE. Section A answers MUST BE in a separate blue answer book. If any blue
 UNIVERSITY OF SOUTHAMPTON PHYS1013W1 SEMESTER 2 EXAMINATION 2011/12 ENERGY AND MATTER SOLUTIONS Duration: 120 MINS VERY IMPORTANT NOTE Section A answers MUST BE in a separate blue answer book. If any blue
UNIVERSITY OF SOUTHAMPTON PHYS1013W1 SEMESTER 2 EXAMINATION 2011/12 ENERGY AND MATTER SOLUTIONS Duration: 120 MINS VERY IMPORTANT NOTE Section A answers MUST BE in a separate blue answer book. If any blue
TOPLJENEC ASOCIIRA LE V VODNI FAZI
 TOPLJENEC ASOCIIRA LE V VODNI FAZI V primeru asociacij molekul topljenca v vodni ali organski fazi eksperimentalno določeni navidezni porazdelitveni koeficient (P n ) v odvisnosti od koncentracije ni konstanten.
TOPLJENEC ASOCIIRA LE V VODNI FAZI V primeru asociacij molekul topljenca v vodni ali organski fazi eksperimentalno določeni navidezni porazdelitveni koeficient (P n ) v odvisnosti od koncentracije ni konstanten.
Chemistry 431 Practice Final Exam Fall Hours
 Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
ρ x + fv f 'w + F x ρ y fu + F y Fundamental Equation in z coordinate p = ρrt or pα = RT Du uv tanφ Dv Dt + u2 tanφ + vw a a = 1 p Dw Dt u2 + v 2
 Fundamental Equation in z coordinate p = ρrt or pα = RT Du uv tanφ + uw Dt a a = 1 p ρ x + fv f 'w + F x Dv Dt + u2 tanφ + vw a a = 1 p ρ y fu + F y Dw Dt u2 + v 2 = 1 p a ρ z g + f 'u + F z Dρ Dt + ρ
Fundamental Equation in z coordinate p = ρrt or pα = RT Du uv tanφ + uw Dt a a = 1 p ρ x + fv f 'w + F x Dv Dt + u2 tanφ + vw a a = 1 p ρ y fu + F y Dw Dt u2 + v 2 = 1 p a ρ z g + f 'u + F z Dρ Dt + ρ
Chapter 7: Thermodynamics
 Chapter 7: Thermodynamics 7.1 Sea surface heat budget In Chapter 5, we have introduced the oceanic planetary boundary layer-the Ekman layer. The observed T and S in this layer are almost uniform vertically,
Chapter 7: Thermodynamics 7.1 Sea surface heat budget In Chapter 5, we have introduced the oceanic planetary boundary layer-the Ekman layer. The observed T and S in this layer are almost uniform vertically,
Simplified Microphysics. condensation evaporation. evaporation
 Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Chemistry 795T. Lecture 7. Electromagnetic Spectrum Black body Radiation. NC State University
 Chemistry 795T Lecture 7 Electromagnetic Spectrum Black body Radiation NC State University Black body Radiation An ideal emitter of radiation is called a black body. Observation: that peak of the energy
Chemistry 795T Lecture 7 Electromagnetic Spectrum Black body Radiation NC State University Black body Radiation An ideal emitter of radiation is called a black body. Observation: that peak of the energy
Chemistry 795T. Black body Radiation. The wavelength and the frequency. The electromagnetic spectrum. Lecture 7
 Chemistry 795T Lecture 7 Electromagnetic Spectrum Black body Radiation NC State University Black body Radiation An ideal emitter of radiation is called a black body. Observation: that peak of the energy
Chemistry 795T Lecture 7 Electromagnetic Spectrum Black body Radiation NC State University Black body Radiation An ideal emitter of radiation is called a black body. Observation: that peak of the energy
Equation for Global Warming
 Equation for Global Warming Derivation and Application Contents 1. Amazing carbon dioxide How can a small change in carbon dioxide (CO 2 ) content make a critical difference to the actual global surface
Equation for Global Warming Derivation and Application Contents 1. Amazing carbon dioxide How can a small change in carbon dioxide (CO 2 ) content make a critical difference to the actual global surface
Chemistry 431. Lecture 1. Introduction Statistical Averaging Electromagnetic Spectrum Black body Radiation. NC State University
 Chemistry 431 Lecture 1 Introduction Statistical Averaging Electromagnetic Spectrum Black body Radiation NC State University Overview Quantum Mechanics Failure of classical physics Wave equation Rotational,
Chemistry 431 Lecture 1 Introduction Statistical Averaging Electromagnetic Spectrum Black body Radiation NC State University Overview Quantum Mechanics Failure of classical physics Wave equation Rotational,
The Atmospheric Boundary Layer. The Surface Energy Balance (9.2)
 The Atmospheric Boundary Layer Turbulence (9.1) The Surface Energy Balance (9.2) Vertical Structure (9.3) Evolution (9.4) Special Effects (9.5) The Boundary Layer in Context (9.6) What processes control
The Atmospheric Boundary Layer Turbulence (9.1) The Surface Energy Balance (9.2) Vertical Structure (9.3) Evolution (9.4) Special Effects (9.5) The Boundary Layer in Context (9.6) What processes control
Clouds and atmospheric convection
 Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 Clouds and atmospheric convection 2 Clouds and atmospheric
Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 Clouds and atmospheric convection 2 Clouds and atmospheric
The Clausius-Clapeyron and the Kelvin Equations
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
THE EXOSPHERIC HEAT BUDGET
 E&ES 359, 2008, p.1 THE EXOSPHERIC HEAT BUDGET What determines the temperature on earth? In this course we are interested in quantitative aspects of the fundamental processes that drive the earth machine.
E&ES 359, 2008, p.1 THE EXOSPHERIC HEAT BUDGET What determines the temperature on earth? In this course we are interested in quantitative aspects of the fundamental processes that drive the earth machine.
4.1 LAWS OF MECHANICS - Review
 4.1 LAWS OF MECHANICS - Review Ch4 9 SYSTEM System: Moving Fluid Definitions: System is defined as an arbitrary quantity of mass of fixed identity. Surrounding is everything external to this system. Boundary
4.1 LAWS OF MECHANICS - Review Ch4 9 SYSTEM System: Moving Fluid Definitions: System is defined as an arbitrary quantity of mass of fixed identity. Surrounding is everything external to this system. Boundary
Monitoring Climate Change from Space
 Monitoring Climate Change from Space Richard Allan (email: r.p.allan@reading.ac.uk twitter: @rpallanuk) Department of Meteorology, University of Reading Why Monitor Earth s Climate from Space? Global Spectrum
Monitoring Climate Change from Space Richard Allan (email: r.p.allan@reading.ac.uk twitter: @rpallanuk) Department of Meteorology, University of Reading Why Monitor Earth s Climate from Space? Global Spectrum
Temperature. Vertical Thermal Structure. Earth s Climate System. Lecture 1: Introduction to the Climate System
 Lecture 1: Introduction to the Climate System T mass (& radiation) T & mass relation in vertical mass (& energy, weather..) Energy T vertical stability vertical motion thunderstorm What are included in
Lecture 1: Introduction to the Climate System T mass (& radiation) T & mass relation in vertical mass (& energy, weather..) Energy T vertical stability vertical motion thunderstorm What are included in
ATMOS 5130 Lecture 9. Enthalpy Conservation Property The Second Law and Its Consequences Entropy
 ATMOS 5130 Lecture 9 Enthalpy Conservation Property The Second Law and Its Consequences Entropy CLASS Presentation Form group of 2 students Present ~20 minute presentation (~ 10 minute each person) Focus
ATMOS 5130 Lecture 9 Enthalpy Conservation Property The Second Law and Its Consequences Entropy CLASS Presentation Form group of 2 students Present ~20 minute presentation (~ 10 minute each person) Focus
df dz = dp dt Essentially, this is just a statement of the first law in one of the forms we derived earlier (expressed here in W m 3 ) dq p dt dp
 A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
Atmospheric Thermodynamics
 Atmospheric Thermodynamics R. Wordsworth February 12, 2015 1 Objectives Derive hydrostatic equation Derive dry and moist adiabats Understand how the theory relates to observed properties of real atmospheres
Atmospheric Thermodynamics R. Wordsworth February 12, 2015 1 Objectives Derive hydrostatic equation Derive dry and moist adiabats Understand how the theory relates to observed properties of real atmospheres
Atmospheric Thermodynamics
 Atmospheric Thermodynamics Atmospheric Composition What is the composition of the Earth s atmosphere? Gaseous Constituents of the Earth s atmosphere (dry air) Constituent Molecular Weight Fractional Concentration
Atmospheric Thermodynamics Atmospheric Composition What is the composition of the Earth s atmosphere? Gaseous Constituents of the Earth s atmosphere (dry air) Constituent Molecular Weight Fractional Concentration
Department of Earth & Climate Sciences Spring 2016 Meteorology 260
 Department of Earth & Climate Sciences Spring 2016 Meteorology 260 Name Laboratory #9 Key: Joplin Tornado Day Subsynoptic, Thermodynamic, and Wind Shear Setting Part A: 1600 UTC Surface Chart Subsynoptic
Department of Earth & Climate Sciences Spring 2016 Meteorology 260 Name Laboratory #9 Key: Joplin Tornado Day Subsynoptic, Thermodynamic, and Wind Shear Setting Part A: 1600 UTC Surface Chart Subsynoptic
