Exam 1 (Chaps. 1-6 of the notes)
|
|
|
- Evan Cross
- 6 years ago
- Views:
Transcription
1 10/12/06 ATS Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10 pts] a) Check the following quantities that are conserved when unsaturated air experiences an adiabatic expansion (e.g., by vertical lifting with no mixing)? dewpoint temperature (T d ) enthalpy (h) entropy (s) equivalent potential temperature (θ e ) mixing ratio (r v ) potential temperature (θ) relative humidity (f) water vapor pressure (e) wet-bulb potential temperature (θ w ) wet-bulb temperature (T w ) a) Check the following quantities that are conserved when saturated air is lifted adiabatically (no mixing, and all condensed water stays with the parcel)? dewpoint temperature (T d ) enthalpy (h) entropy (s) equivalent potential temperature (θ e ) mixing ratio (r v ) potential temperature (θ) relative humidity (f) water vapor pressure (e) wet-bulb potential temperature (adiabatic, θ w ) wet-bulb temperature (T w )
2 10/12/06 ATS Atmospheric Thermodynamics and Cloud Physics 2 2. [30 pts] Consider a parcel with the following thermodynamic values: p = 900 hpa T = 25 C r v = 10 g kg -1 Using the skew-t diagram on the following pages, find the following parameters graphically. Also write the equation that would be used to determine the most precise values. a) saturation point T and p values (T sp and p sp ; give equation for T sp only) b) saturation mixing ratio c) potential temperature d) equivalent potential temperature f) wet-bulb potential temperature g) dew-point temperature h) relative humidity i) vapor pressure j) the temperature of a parcel that ascends adiabatically to 600 mb k) the adiabatic mixing ratio of the parcel at 600 mb Show all work on the skew-t diagrams on the next two pages.
3 10/12/06 ATS Atmospheric Thermodynamics and Cloud Physics 3
4 10/12/06 ATS Atmospheric Thermodynamics and Cloud Physics 4
5 10/12/06 ATS Atmospheric Thermodynamics and Cloud Physics 5 3. [15 pts] What is the volume, in m 3, of 5 kg of dry air at 300 mb and -40 C?
6 10/12/06 ATS Atmospheric Thermodynamics and Cloud Physics 6 4. [10 pts] A parcel of moist air has a total pressure of 975 hpa and a temperature of 15 C. If the mixing ratio is 1.8 g kg -1, what are the water vapor pressure and the virtual temperature?
7 10/12/06 ATS Atmospheric Thermodynamics and Cloud Physics 7 5. [15 pts] An isolated rain drop that is evaporating into air with a temperature of 18 C has a water surface temperature of 12 C. a) Find (using a skew-t) the mixing ratio of the air. b) Indicate what equation you would use to solve this problem precisely.
8 10/12/06 ATS Atmospheric Thermodynamics and Cloud Physics 8 6. [20 pts] a) Convert the First Law of Thermodynamics from the T, α form (dq = c v dt + pdα) to the T, p form. Show all steps. b) Derive potential temperature (θ) from this form (dq = c p dt - αdp) of the First Law c) Explain why a correction factor in the exponent is used when water vapor is present.
9 10/12/06 ATS Atmospheric Thermodynamics and Cloud Physics 9 Equations and constants pα = (R*/M)T = RT p = ρ m R d T v. e = ρ v R v T dq = c v dt + pdα. dq = c p dt - αdp. dq = Tds = du + pdα. Tα η-1 = const (T c vα R d = const) Tp -κ = const (T c pp -R d = const κ = R d /c p and η = c p /c v. pα η = const (p c vα c p = const) c pm = c pd ( r v ) R m = R d ( r v ). θe Lvlr vs = θexp cptsp θ e 2675r = θ exp Tsp vs ds dq T rev θ = p0 T p R c d p ( r )) v f u Ts g = u - Ts + pα dp/dt = Δs/Δα = -ΔH fusion /(TΔα) dlne s /dt = L vl /(R v T 2 ) e s (T) = Ae -B/T A = 2.53x10 8 kpa, B = 5.42x10 3 K r v = m v /m d = εe / [p-(1-e)e] εe/p q v = εe / [p-(1-e)e] ε = f = r v / r vs (T,p) = r vs (T d,p)/r vs (T,p) e/e s (T) T v T(1+0.61r v ) T d = T d (r vs,p) = B Aε ln rp v = f(r v,p) T iw = T - (L lv /c p )[(ε/p)ae -B/T w - rv ], T ie = T + L lv r v /c p. T sp 2840 = lnT ln e χ = ρ m [r vs (T sp,p sp ) - r vs (T sat,p) θe=const ]
10 10/12/06 ATS Atmospheric Thermodynamics and Cloud Physics 10 θ = T e K 1000 p (1 0.28r ) v exp r Tsp v ( r ) v c v = 717 J K -1 kg -1 c p = J K -1 kg -1 c wv = 1463 J K -1 kg -1 c wp = 1952 J K -1 kg -1 R d = 287 J kg -1 K -1 R v = J kg -1 K -1 L vl = 2.50 x 10 6 J kg -1 (0 o C) latent heat of condensation (function of T) L vl = 2.25 x 10 6 J kg -1 (100 o C) L il = 3.34 x 10 5 J kg -1 latent heat of melting L vi = 2.83 x 10 6 J kg -1 (0 o C) latent heat of deposition L vl = ( at c ) x 10 6 J kg -1, where a = C -1 and T c is the dry bulb temperature in C. Table 5.1. Saturation vapor pressures over water and ice, and latent heats of condensation and deposition. T ( C) e s (Pa) e i (Pa) L vl (J kg -1 ) x 10 6 L vi (J kg -1 ) x
Chapter 5. On-line resource
 Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
Chapter 4 Water Vapor
 Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Temperature and Thermodynamics, Part II. Topics to be Covered
 Teperature and Therodynaics, Part II Topics to be Covered Profiles of Teperature in the Boundary Layer Potential teperature Adiabatic Lapse Rate Theral Stratification 1/8/17 Why are We Interested in Theral
Teperature and Therodynaics, Part II Topics to be Covered Profiles of Teperature in the Boundary Layer Potential teperature Adiabatic Lapse Rate Theral Stratification 1/8/17 Why are We Interested in Theral
Sec Water vapour variables each has its own usefulness 2/11 The ideal gas law inter-relates vapour pressure (e) & absolute humidity ( ρv) 1 e
 Ch7. Water vapour: the most variable gas & most important GHG Absolute humidity Specific humidity ρv ρv = q q= mass of water vapour volume of sample EAS270_Ch7_WaterVapour_A.odp JDW, EAS Ualberta, last
Ch7. Water vapour: the most variable gas & most important GHG Absolute humidity Specific humidity ρv ρv = q q= mass of water vapour volume of sample EAS270_Ch7_WaterVapour_A.odp JDW, EAS Ualberta, last
GEF2200 Atmosfærefysikk 2012
 GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
Measuring State Parameters of the Atmosphere
 Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Chapter 5 - Atmospheric Moisture
 Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Name 28-MAY-08. FA RP 1 Mr. Chase. 1. Which weather-station model shows an air pressure of millibars?
 FA RP 1 Mr. Chase Name 28-MAY-08 1. Which weather-station model shows an air pressure of 993.4 millibars? 2. Which station model shows the correct form for indicating a northwest wind at 25 knots and an
FA RP 1 Mr. Chase Name 28-MAY-08 1. Which weather-station model shows an air pressure of 993.4 millibars? 2. Which station model shows the correct form for indicating a northwest wind at 25 knots and an
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
First Law of Thermodynamics
 First Law of Thermodynamics September 11, 2013 The first law of thermodynamics is the conservation of energy applied to thermal systems. Here, we develop the principles of thermodynamics for a discrete
First Law of Thermodynamics September 11, 2013 The first law of thermodynamics is the conservation of energy applied to thermal systems. Here, we develop the principles of thermodynamics for a discrete
ATMO 551a Fall 08. Equivalent Potential Temperature
 Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
ATMO 551a Moist Adiabat Fall Change in internal energy: ΔU
 Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere
![Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere](/thumbs/72/66535634.jpg) Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]
![Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]](/thumbs/81/83694823.jpg) Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a
Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a
7. The weather instrument below can be used to determine relative humidity.
 1. What is the dewpoint temperature when the dry-bulb temperature is 12 C and the wet-bulb temperature is 7 C? A) 1 C B) -2 C C) -5 C D) 4 C 2. A parcel of air has a dry-bulb temperature reading of 16
1. What is the dewpoint temperature when the dry-bulb temperature is 12 C and the wet-bulb temperature is 7 C? A) 1 C B) -2 C C) -5 C D) 4 C 2. A parcel of air has a dry-bulb temperature reading of 16
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics
 Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Atmospheric Composition הרכב האטמוספירה
 Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Meteorology 6150 Cloud System Modeling
 Meteorology 6150 Cloud System Modeling Steve Krueger Spring 2009 1 Fundamental Equations 1.1 The Basic Equations 1.1.1 Equation of motion The movement of air in the atmosphere is governed by Newton s Second
Meteorology 6150 Cloud System Modeling Steve Krueger Spring 2009 1 Fundamental Equations 1.1 The Basic Equations 1.1.1 Equation of motion The movement of air in the atmosphere is governed by Newton s Second
Outline. Aim. Gas law. Pressure. Scale height Mixing Column density. Temperature Lapse rate Stability. Condensation Humidity.
 Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Kelvin Effect. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
CAE 331/513 Building Science Fall 2017
 CAE 331/513 Building Science Fall 2017 October 5, 2017 Psychrometrics (equations) Advancing energy, environmental, and sustainability research within the built environment www.built-envi.com Twitter: @built_envi
CAE 331/513 Building Science Fall 2017 October 5, 2017 Psychrometrics (equations) Advancing energy, environmental, and sustainability research within the built environment www.built-envi.com Twitter: @built_envi
CAE 331/513 Building Science Fall 2015
 CAE 331/513 Building Science Fall 2015 Week 5: September 24, 2015 Psychrometrics (equations) Advancing energy, environmental, and sustainability research within the built environment www.built-envi.com
CAE 331/513 Building Science Fall 2015 Week 5: September 24, 2015 Psychrometrics (equations) Advancing energy, environmental, and sustainability research within the built environment www.built-envi.com
Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties
 Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties For Further Reading Most information contained within these lecture notes is drawn from Chapters 1, 2, 4, and 6 of The Use of the Skew
Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties For Further Reading Most information contained within these lecture notes is drawn from Chapters 1, 2, 4, and 6 of The Use of the Skew
1. Heterogeneous Systems and Chemical Equilibrium
 1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
ATMOS 5130 Lecture 9. Enthalpy Conservation Property The Second Law and Its Consequences Entropy
 ATMOS 5130 Lecture 9 Enthalpy Conservation Property The Second Law and Its Consequences Entropy CLASS Presentation Form group of 2 students Present ~20 minute presentation (~ 10 minute each person) Focus
ATMOS 5130 Lecture 9 Enthalpy Conservation Property The Second Law and Its Consequences Entropy CLASS Presentation Form group of 2 students Present ~20 minute presentation (~ 10 minute each person) Focus
Parcel Model. Meteorology September 3, 2008
 Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
4 WATER VAPOR. Contents 4.1. VAPOR PRESSURE AT SATURATION
 Copyright 2017 by Roland Stull. Practical Meteorology: An Algebra-based Survey of Atmospheric Science. v1.02b 4 WATER VAPOR Contents 4.1. Vapor Pressure at Saturation 87 HIGHER MATH Clausius-Clapeyron
Copyright 2017 by Roland Stull. Practical Meteorology: An Algebra-based Survey of Atmospheric Science. v1.02b 4 WATER VAPOR Contents 4.1. Vapor Pressure at Saturation 87 HIGHER MATH Clausius-Clapeyron
THERMODYNAMICS 1 /43
 THERMODYNAMICS 1 Atmosphere A multi-component Multi-Phase System The gas phase atmospheric cons1tuents; major gases; fixed propor1ons by volume (dry air) Nitrogen (N 2 ) 78,08 % Oxygen (O 2 ) 29,05 % Argon
THERMODYNAMICS 1 Atmosphere A multi-component Multi-Phase System The gas phase atmospheric cons1tuents; major gases; fixed propor1ons by volume (dry air) Nitrogen (N 2 ) 78,08 % Oxygen (O 2 ) 29,05 % Argon
Numerical Example An air parcel with mass of 1 kg rises adiabatically from sea level to an altitude of 3 km. What is its temperature change?
 Numerical Example An air parcel with mass of 1 kg rises adiabatically from sea level to an altitude of 3 km. What is its temperature change? From the 1 st law, T = -g/c p z + Q/m air /c p Here, Q = 0,
Numerical Example An air parcel with mass of 1 kg rises adiabatically from sea level to an altitude of 3 km. What is its temperature change? From the 1 st law, T = -g/c p z + Q/m air /c p Here, Q = 0,
Clouds and turbulent moist convection
 Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Atsc final Equations: page 1/6
 Atsc. 405 2012 final Equations: page 1/6 Answer each of these 7 questions (note weight). Show all your work on all questions (needed for partial credit). Be sure to put your name on any detached pages.
Atsc. 405 2012 final Equations: page 1/6 Answer each of these 7 questions (note weight). Show all your work on all questions (needed for partial credit). Be sure to put your name on any detached pages.
Simplified Microphysics. condensation evaporation. evaporation
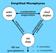 Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents
 CHAPTER 6 HUMIDITY, SATURATION, AND STABILITY MULTIPLE CHOICE QUESTIONS 1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents 2., annual precipitation
CHAPTER 6 HUMIDITY, SATURATION, AND STABILITY MULTIPLE CHOICE QUESTIONS 1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents 2., annual precipitation
Naraine Persaud, Entry Code ME-11 1
 Naraine Persaud, Entry Code ME-11 1 Persaud, N. 2005. Adiabatic cooling. In: Water Encyclopedia Volume 4: Oceanography; Meteorology; Physics and Chemistry; Water Law; and Water History, Art, and Culture.
Naraine Persaud, Entry Code ME-11 1 Persaud, N. 2005. Adiabatic cooling. In: Water Encyclopedia Volume 4: Oceanography; Meteorology; Physics and Chemistry; Water Law; and Water History, Art, and Culture.
The Clausius-Clapeyron and the Kelvin Equations
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
Atmospheric Dynamics: lecture 2
 Atmospheric Dynamics: lecture 2 Topics Some aspects of advection and the Coriolis-effect (1.7) Composition of the atmosphere (figure 1.6) Equation of state (1.8&1.9) Water vapour in the atmosphere (1.10)
Atmospheric Dynamics: lecture 2 Topics Some aspects of advection and the Coriolis-effect (1.7) Composition of the atmosphere (figure 1.6) Equation of state (1.8&1.9) Water vapour in the atmosphere (1.10)
On Formulas for Equivalent Potential Temperature
 SEPTEMBER 2009 N O T E S A N D C O R R E S P O N D E N C E 3137 On Formulas for Equivalent Potential Temperature ROBERT DAVIES-JONES NOAA/National Severe Storms aboratory, Norman, Oklahoma (Manuscript
SEPTEMBER 2009 N O T E S A N D C O R R E S P O N D E N C E 3137 On Formulas for Equivalent Potential Temperature ROBERT DAVIES-JONES NOAA/National Severe Storms aboratory, Norman, Oklahoma (Manuscript
 Physical Chemistry Physical chemistry is the branch of chemistry that establishes and develops the principles of Chemistry in terms of the underlying concepts of Physics Physical Chemistry Main book: Atkins
Physical Chemistry Physical chemistry is the branch of chemistry that establishes and develops the principles of Chemistry in terms of the underlying concepts of Physics Physical Chemistry Main book: Atkins
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Introduction. Lecture 6: Water in Atmosphere. How Much Heat Is Brought Upward By Water Vapor?
 Lecture 6: Water in Atmosphere Introduction Over 70% of the planet is covered by water Water is unique in that it can simultaneously exist in all three states (solid, liquid, gas) at the same temperature
Lecture 6: Water in Atmosphere Introduction Over 70% of the planet is covered by water Water is unique in that it can simultaneously exist in all three states (solid, liquid, gas) at the same temperature
First Law of Thermodyamics U = Q + W. We can rewrite this by introducing two physical. Enthalpy, H, is the quantity U + pv, as this
 First Law of Thermodyamics U = Q + W where U is the increase in internal energy of the system, Q is the heat supplied to the system and W is the work done on the system. We can rewrite this by introducing
First Law of Thermodyamics U = Q + W where U is the increase in internal energy of the system, Q is the heat supplied to the system and W is the work done on the system. We can rewrite this by introducing
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1
 Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
Outline. Property diagrams involving entropy. Heat transfer for internally reversible process
 Outline roperty diagrams involving entropy What is entropy? T-ds relations Entropy change of substances ure substances (near wet dome) Solids and liquids Ideal gases roperty diagrams involving entropy
Outline roperty diagrams involving entropy What is entropy? T-ds relations Entropy change of substances ure substances (near wet dome) Solids and liquids Ideal gases roperty diagrams involving entropy
Phase Changes and Latent Heat
 Review Questions Why can a person remove a piece of dry aluminum foil from a hot oven with bare fingers without getting burned, yet will be burned doing so if the foil is wet. Equal quantities of alcohol
Review Questions Why can a person remove a piece of dry aluminum foil from a hot oven with bare fingers without getting burned, yet will be burned doing so if the foil is wet. Equal quantities of alcohol
ME 201 Thermodynamics
 Spring 01 ME 01 Thermodynamics Property Evaluation Practice Problems II Solutions 1. Air at 100 K and 1 MPa goes to MPa isenthapically. Determine the entropy change. Substance Type: Ideal Gas (air) Process:
Spring 01 ME 01 Thermodynamics Property Evaluation Practice Problems II Solutions 1. Air at 100 K and 1 MPa goes to MPa isenthapically. Determine the entropy change. Substance Type: Ideal Gas (air) Process:
Atmospheric Dynamics: lecture 3
 Atmospheric Dynamics: lecture 3 Moist convection Dew point temperature/lapse rate/lcl Equivalent potential temperature Conditional and potential instability Thermodynamic diagram CAPE Introduction to Python
Atmospheric Dynamics: lecture 3 Moist convection Dew point temperature/lapse rate/lcl Equivalent potential temperature Conditional and potential instability Thermodynamic diagram CAPE Introduction to Python
Physical Fundamentals of Global Change Processes
 University of Applied Sciences Eberswalde Master Study Program Global Change Management Manfred Stock Potsdam Institute for Climate Impact Research Module: Physical Fundamentals of Global Change Processes
University of Applied Sciences Eberswalde Master Study Program Global Change Management Manfred Stock Potsdam Institute for Climate Impact Research Module: Physical Fundamentals of Global Change Processes
Measuring State Parameters of the Atmosphere
 Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
1. Basic state values of matter
 1. Basic state values of matter Example 1.1 The pressure inside a boiler is p p = 115.10 5 Pa and p v = 9.44.10 4 Pa inside a condenser. Calculate the absolute pressure inside the boiler and condenser
1. Basic state values of matter Example 1.1 The pressure inside a boiler is p p = 115.10 5 Pa and p v = 9.44.10 4 Pa inside a condenser. Calculate the absolute pressure inside the boiler and condenser
Chapter 5. Atmospheric Moisture
 Chapter 5 Atmospheric Moisture hydrologic cycle--movement of water in all forms between earth & atmosphere Humidity: amount of water vapor in air vapor pressure saturation vapor pressure absolute humidity
Chapter 5 Atmospheric Moisture hydrologic cycle--movement of water in all forms between earth & atmosphere Humidity: amount of water vapor in air vapor pressure saturation vapor pressure absolute humidity
1. Second Law of Thermodynamics
 1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. However, the first law cannot explain certain facts about thermal
1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. However, the first law cannot explain certain facts about thermal
Final Examination. Part A Answer ONLY TWELVE QUESTIONS in Part A. (Each question is 3 points)
 ATS 210 Spring Term 2001 NAME: Final Examination This is a 2 hour, closed-book examination. Calculators may be used. All answers should be written on the examination paper. Use the final sheet for any
ATS 210 Spring Term 2001 NAME: Final Examination This is a 2 hour, closed-book examination. Calculators may be used. All answers should be written on the examination paper. Use the final sheet for any
The Tropical Atmosphere: Hurricane Incubator
 The Tropical Atmosphere: Hurricane Incubator Images from journals published by the American Meteorological Society are copyright AMS and used with permission. A One-Dimensional Description of the Tropical
The Tropical Atmosphere: Hurricane Incubator Images from journals published by the American Meteorological Society are copyright AMS and used with permission. A One-Dimensional Description of the Tropical
Phase Diagrams. NC State University
 Chemistry 433 Lecture 18 Phase Diagrams NC State University Definition of a phase diagram A phase diagram is a representation of the states of matter, solid, liquid, or gas as a function of temperature
Chemistry 433 Lecture 18 Phase Diagrams NC State University Definition of a phase diagram A phase diagram is a representation of the states of matter, solid, liquid, or gas as a function of temperature
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
Moist Convection. Chapter 6
 Moist Convection Chapter 6 1 2 Trade Cumuli Afternoon cumulus over land 3 Cumuls congestus Convectively-driven weather systems Deep convection plays an important role in the dynamics of tropical weather
Moist Convection Chapter 6 1 2 Trade Cumuli Afternoon cumulus over land 3 Cumuls congestus Convectively-driven weather systems Deep convection plays an important role in the dynamics of tropical weather
The Water Cycle. Water in the Atmosphere AOSC 200 Tim Canty. Class Web Site:
 Water in the Atmosphere AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Latent Heat Evaporation & Saturation Relative Humidity Dew Point Lecture 11 Oct 2 2018
Water in the Atmosphere AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Latent Heat Evaporation & Saturation Relative Humidity Dew Point Lecture 11 Oct 2 2018
Project 3 Convection and Atmospheric Thermodynamics
 12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
Theory. Humidity h of an air-vapor mixture is defined as the mass ratio of water vapor and dry air,
 Theory Background In a cooling tower with open water circulation, heat is removed from water because of the material and heat exchange between the water and the ambient air. The cooling tower is a special
Theory Background In a cooling tower with open water circulation, heat is removed from water because of the material and heat exchange between the water and the ambient air. The cooling tower is a special
Weather, Atmosphere and Meteorology
 S c i e n c e s Weather, Atmosphere and Meteorology Key words: Atmosphere, Ozone, Water vapor, solar radiation, Condensation, Evaporation, Humidity, Dew-Point Temperature, Cirrus Clouds, Stratus Clouds,
S c i e n c e s Weather, Atmosphere and Meteorology Key words: Atmosphere, Ozone, Water vapor, solar radiation, Condensation, Evaporation, Humidity, Dew-Point Temperature, Cirrus Clouds, Stratus Clouds,
Name Class Date. 3. In what part of the water cycle do clouds form? a. precipitation b. evaporation c. condensation d. runoff
 Skills Worksheet Directed Reading B Section: Water in the Air 1. What do we call the condition of the atmosphere at a certain time and place? a. the water cycle b. weather c. climate d. precipitation THE
Skills Worksheet Directed Reading B Section: Water in the Air 1. What do we call the condition of the atmosphere at a certain time and place? a. the water cycle b. weather c. climate d. precipitation THE
Clouds and atmospheric convection
 Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
METEO 431: Atmospheric Thermodynamics
 METEO 431: Atmospheric Thermodynamics Final Exam (100 points) INSTRUCTIONS: Please write as legibly as you can and make sure your thoughts are organized. I am looking for your understanding of the key
METEO 431: Atmospheric Thermodynamics Final Exam (100 points) INSTRUCTIONS: Please write as legibly as you can and make sure your thoughts are organized. I am looking for your understanding of the key
Thermodynamics Introduction and Basic Concepts
 Thermodynamics Introduction and Basic Concepts by Asst. Prof. Channarong Asavatesanupap Mechanical Engineering Department Faculty of Engineering Thammasat University 2 What is Thermodynamics? Thermodynamics
Thermodynamics Introduction and Basic Concepts by Asst. Prof. Channarong Asavatesanupap Mechanical Engineering Department Faculty of Engineering Thammasat University 2 What is Thermodynamics? Thermodynamics
Thermodynamics We Can See! Adapted from ATS 541 notes (Dr. Susan van den Heever)
 Thermodynamics We Can See! Adapted from ATS 541 notes (Dr. Susan van den Heever) What we have learned so far ~ Moist Adiabatic Latent Heating ρ ρ Condensation ~ Dry Adiabatic Cloud Types Skew- T log- P
Thermodynamics We Can See! Adapted from ATS 541 notes (Dr. Susan van den Heever) What we have learned so far ~ Moist Adiabatic Latent Heating ρ ρ Condensation ~ Dry Adiabatic Cloud Types Skew- T log- P
Radiation, Sensible Heat Flux and Evapotranspiration
 Radiation, Sensible Heat Flux and Evapotranspiration Climatological and hydrological field work Figure 1: Estimate of the Earth s annual and global mean energy balance. Over the long term, the incoming
Radiation, Sensible Heat Flux and Evapotranspiration Climatological and hydrological field work Figure 1: Estimate of the Earth s annual and global mean energy balance. Over the long term, the incoming
Parcel Model. Atmospheric Sciences September 30, 2012
 Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
GEF2200 atmospheric physics 2018
 GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
1. Static Stability. (ρ V ) d2 z (1) d 2 z. = g (2) = g (3) T T = g T (4)
 NCAR (National Center for Atmospheric Research) has an excellent resource for education called COMET-MetEd. There you can find some really great tutorials on SkewT-LogP plots: visit http://www.meted.ucar.edu/mesoprim/skewt/index.htm.
NCAR (National Center for Atmospheric Research) has an excellent resource for education called COMET-MetEd. There you can find some really great tutorials on SkewT-LogP plots: visit http://www.meted.ucar.edu/mesoprim/skewt/index.htm.
UNIVERSITY OF SOUTHAMPTON
 UNIVERSITY OF SOUTHAMPTON PHYS1013W1 SEMESTER 2 EXAMINATION 2014-2015 ENERGY AND MATTER Duration: 120 MINS (2 hours) This paper contains 8 questions. Answers to Section A and Section B must be in separate
UNIVERSITY OF SOUTHAMPTON PHYS1013W1 SEMESTER 2 EXAMINATION 2014-2015 ENERGY AND MATTER Duration: 120 MINS (2 hours) This paper contains 8 questions. Answers to Section A and Section B must be in separate
1. Base your answer to the following question on the weather map below, which shows a weather system that is affecting part of the United States.
 1. Base your answer to the following question on the weather map below, which shows a weather system that is affecting part of the United States. Which sequence of events forms the clouds associated with
1. Base your answer to the following question on the weather map below, which shows a weather system that is affecting part of the United States. Which sequence of events forms the clouds associated with
1. (10) True or False: A material with an ideal thermal equation of state must have a constant c v.
 AME 54531 Intermediate hermodynamics Examination : Prof. J. M. Powers 7 November 018 1. 10) rue or False: A material with an ideal thermal equation of state must have a constant c v. False. Forsuchamaterialc
AME 54531 Intermediate hermodynamics Examination : Prof. J. M. Powers 7 November 018 1. 10) rue or False: A material with an ideal thermal equation of state must have a constant c v. False. Forsuchamaterialc
Monday 7 October 2013, Class #15
 Monday 7 October 2013, Class #15 Concepts for Today (Basics for Thermodynamics) Weather versus climate Lapse Rate (Adiabatic Lapse Rate) Ideal Gas Law Adiabatic Processes Potential Temperature Hydrostatic
Monday 7 October 2013, Class #15 Concepts for Today (Basics for Thermodynamics) Weather versus climate Lapse Rate (Adiabatic Lapse Rate) Ideal Gas Law Adiabatic Processes Potential Temperature Hydrostatic
Water-Temperature-Dependent Wet Bulb Temperature Calculation
 Water-Temperature-Dependent Wet Bulb Temperature Calculation Oxycom Fresh Air BV December 7th, 2012 Abstract A numerical solution for the exact calculation of the wet bulb temperature of air has been derived,
Water-Temperature-Dependent Wet Bulb Temperature Calculation Oxycom Fresh Air BV December 7th, 2012 Abstract A numerical solution for the exact calculation of the wet bulb temperature of air has been derived,
latent heat/humidity
 1. Base your answer(s) to the following question(s) on the Earth Science Reference Tables, the graph below, and your knowledge of Earth science. The graph shows variations in air temperature and relative
1. Base your answer(s) to the following question(s) on the Earth Science Reference Tables, the graph below, and your knowledge of Earth science. The graph shows variations in air temperature and relative
UNIVERSITY OF TORONTO FACULTY OF APPLIED SCIENCE AND ENGINEERING TERM TEST 2 17 MARCH First Year APS 104S
 UNIERSIY OF ORONO Please mark X to indicate your tutorial section. Failure to do so will result in a deduction of 3 marks. U 0 U 0 FACULY OF APPLIED SCIENCE AND ENGINEERING ERM ES 7 MARCH 05 U 03 U 04
UNIERSIY OF ORONO Please mark X to indicate your tutorial section. Failure to do so will result in a deduction of 3 marks. U 0 U 0 FACULY OF APPLIED SCIENCE AND ENGINEERING ERM ES 7 MARCH 05 U 03 U 04
R13. II B. Tech I Semester Regular Examinations, Jan THERMODYNAMICS (Com. to ME, AE, AME) PART- A
 SET - 1 II B. Tech I Semester Regular Examinations, Jan - 2015 THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max. Marks: 70 Note 1. Question Paper consists of two parts (Part-A and Part-B) 2. Answer
SET - 1 II B. Tech I Semester Regular Examinations, Jan - 2015 THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max. Marks: 70 Note 1. Question Paper consists of two parts (Part-A and Part-B) 2. Answer
Tropical Cyclones: Steady State Physics
 Tropical Cyclones: Steady State Physics Energy Production Carnot Theorem: Maximum efficiency results from a particular energy cycle: Isothermal expansion Adiabatic expansion Isothermal compression Adiabatic
Tropical Cyclones: Steady State Physics Energy Production Carnot Theorem: Maximum efficiency results from a particular energy cycle: Isothermal expansion Adiabatic expansion Isothermal compression Adiabatic
1 Thermodynamics: some Preliminaries
 1 Thermodynamics: some Preliminaries Beforewebegintoconsider thetransfer ofradiation through an atmosphere, let s consider the structure of an atmosphere with a little thermodynamics. This material hopefully
1 Thermodynamics: some Preliminaries Beforewebegintoconsider thetransfer ofradiation through an atmosphere, let s consider the structure of an atmosphere with a little thermodynamics. This material hopefully
Application of Thermodynamics in Phase Diagrams. Today s Topics
 Lecture 23 Application of Thermodynamics in Phase Diagrams The Clausius Clapeyron Equation A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics The Clapeyron equation Integration
Lecture 23 Application of Thermodynamics in Phase Diagrams The Clausius Clapeyron Equation A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics The Clapeyron equation Integration
1 in = ft (round answer to nearest integer)
 Hydrology LAB 1: DRAINAGE-BASIN PROPERTIES AND SIMPLE METEOROLOGIC CALCULATIONS OBJECTIVES: a. to develop your ability to extract basic drainage-basin data from topographic maps b. to learn how to make
Hydrology LAB 1: DRAINAGE-BASIN PROPERTIES AND SIMPLE METEOROLOGIC CALCULATIONS OBJECTIVES: a. to develop your ability to extract basic drainage-basin data from topographic maps b. to learn how to make
1. Second Law of Thermodynamics
 1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. he second law deals with direction of thermodynamic processes
1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. he second law deals with direction of thermodynamic processes
Hurricanes are intense vortical (rotational) storms that develop over the tropical oceans in regions of very warm surface water.
 Hurricanes: Observations and Dynamics Houze Section 10.1. Holton Section 9.7. Emanuel, K. A., 1988: Toward a general theory of hurricanes. American Scientist, 76, 371-379 (web link). http://ww2010.atmos.uiuc.edu/(gh)/guides/mtr/hurr/home.rxml
Hurricanes: Observations and Dynamics Houze Section 10.1. Holton Section 9.7. Emanuel, K. A., 1988: Toward a general theory of hurricanes. American Scientist, 76, 371-379 (web link). http://ww2010.atmos.uiuc.edu/(gh)/guides/mtr/hurr/home.rxml
Last Name or Student ID
 10/06/08, Chem433 Exam # 1 Last Name or Student ID 1. (3 pts) 2. (3 pts) 3. (3 pts) 4. (2 pts) 5. (2 pts) 6. (2 pts) 7. (2 pts) 8. (2 pts) 9. (6 pts) 10. (5 pts) 11. (6 pts) 12. (12 pts) 13. (22 pts) 14.
10/06/08, Chem433 Exam # 1 Last Name or Student ID 1. (3 pts) 2. (3 pts) 3. (3 pts) 4. (2 pts) 5. (2 pts) 6. (2 pts) 7. (2 pts) 8. (2 pts) 9. (6 pts) 10. (5 pts) 11. (6 pts) 12. (12 pts) 13. (22 pts) 14.
ln P s T and P s T where R 22,105, D A 27, E B 97.
 ASAE D271.2 DEC94 Psychrometric Data Reviewed by ASAE s Structures and Environment Division and the Food Engineering Division Standards Committees; approved by the Electric Power and Processing Division
ASAE D271.2 DEC94 Psychrometric Data Reviewed by ASAE s Structures and Environment Division and the Food Engineering Division Standards Committees; approved by the Electric Power and Processing Division
Entropy and the Second Law of Thermodynamics
 Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Liquid water static energy page 1/8
 Liquid water static energy age 1/8 1) Thermodynamics It s a good idea to work with thermodynamic variables that are conserved under a known set of conditions, since they can act as assive tracers and rovide
Liquid water static energy age 1/8 1) Thermodynamics It s a good idea to work with thermodynamic variables that are conserved under a known set of conditions, since they can act as assive tracers and rovide
III A-PROPATH: Moist Air
 III A-PROPATH: Moist Air Use is made of two different formulations. One is that of ideal gas mixture of dry air and steam. Another is that of real fluid. 466 A-PROPATH: Moist Air 1. General Features 1.1
III A-PROPATH: Moist Air Use is made of two different formulations. One is that of ideal gas mixture of dry air and steam. Another is that of real fluid. 466 A-PROPATH: Moist Air 1. General Features 1.1
( ) = 1005 J kg 1 K 1 ;
 Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
Moisture, Clouds, and Precipitation Earth Science, 13e Chapter 17
 Moisture, Clouds, and Precipitation Earth Science, 13e Chapter 17 Stanley C. Hatfield Southwestern Illinois College Changes of state of water, H 2 O Water is the only substance in atmosphere that exists
Moisture, Clouds, and Precipitation Earth Science, 13e Chapter 17 Stanley C. Hatfield Southwestern Illinois College Changes of state of water, H 2 O Water is the only substance in atmosphere that exists
Chapter The transition from water vapor to liquid water is called. a. condensation b. evaporation c. sublimation d.
 Chapter-6 Multiple Choice Questions 1. The transition from water vapor to liquid water is called. a. condensation b. evaporation c. sublimation d. deposition 2. The movement of water among the great global
Chapter-6 Multiple Choice Questions 1. The transition from water vapor to liquid water is called. a. condensation b. evaporation c. sublimation d. deposition 2. The movement of water among the great global
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Atmospheric Thermodynamics
 Atmospheric Thermodynamics Atmospheric Composition What is the composition of the Earth s atmosphere? Gaseous Constituents of the Earth s atmosphere (dry air) Constituent Molecular Weight Fractional Concentration
Atmospheric Thermodynamics Atmospheric Composition What is the composition of the Earth s atmosphere? Gaseous Constituents of the Earth s atmosphere (dry air) Constituent Molecular Weight Fractional Concentration
MAHALAKSHMI ENGINEERING COLLEGE
 MAHALAKSHMI ENGINEERING COLLEGE TIRUCHIRAPALLI 621 213. Department: Mechanical Subject Code: ME2202 Semester: III Subject Name: ENGG. THERMODYNAMICS UNIT-I Basic Concept and First Law 1. What do you understand
MAHALAKSHMI ENGINEERING COLLEGE TIRUCHIRAPALLI 621 213. Department: Mechanical Subject Code: ME2202 Semester: III Subject Name: ENGG. THERMODYNAMICS UNIT-I Basic Concept and First Law 1. What do you understand
P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.
![P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C. P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.](/thumbs/79/79184286.jpg) Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur
 Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 21 Psychometric Processes Good afternoon, yesterday we
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 21 Psychometric Processes Good afternoon, yesterday we
where p oo is a reference level constant pressure (often 10 5 Pa). Since θ is conserved for adiabatic motions, a prognostic temperature equation is:
 1 Appendix C Useful Equations Purposes: Provide foundation equations and sketch some derivations. These equations are used as starting places for discussions in various parts of the book. C.1. Thermodynamic
1 Appendix C Useful Equations Purposes: Provide foundation equations and sketch some derivations. These equations are used as starting places for discussions in various parts of the book. C.1. Thermodynamic
Orographic Precipitation II: Effects of Phase Change on Orographic Flow. Richard Rotunno. National Center for Atmospheric Research, USA
 Orographic Precipitation II: Effects of Phase Change on Orographic Flow Richard Rotunno National Center for Atmospheric Research, USA Condensation D Dt vs w( x, ) vs U Large-Scale Flow 0 H L Dynamics w
Orographic Precipitation II: Effects of Phase Change on Orographic Flow Richard Rotunno National Center for Atmospheric Research, USA Condensation D Dt vs w( x, ) vs U Large-Scale Flow 0 H L Dynamics w
