GEF2200 atmospheric physics 2018
|
|
|
- Drusilla Pitts
- 5 years ago
- Views:
Transcription
1 GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities are conserved: Potential temperature and mixing ratio. For the second pair, only equvivalent potential temperature is conserved, but saturation mixing ratio changes. WH06 3.8s Saturated air is lifted (pseudoadiabatically): Only equvivalent potential temperature is conserved. Potential temperature, mixing ratio and saturation mixing ratio change and are not conserved. WH06 3.8t The frost point is the temperature at which water vapor becomes saturated with respect to ice. Considering Figure 3.9 in WH, we see that the ice saturation pressure is lower than the water saturation pressure, for a given temperature. But since the saturation pressures increase with temperature, this means that for a given ambient vapor pressure, we will reach saturation w.r.t. ice at a higher temperature than saturation w.r.t. water, which means that the frost point temperature is higher than the dew point temperature. This is why we get frost and not dew on the ground when the temperature drops to below 0 C. WH06 3.8v The refridgerator is driven by a reversed heat engine transferring heat from the cold reservoir (within it) to the warm reservoir (the kitchen). As we have learned in chapter 3.7, such an installation needs external work to be transferred into heat. This means that the total system of the kitchen plus refridgerator is heated. As the door is left open, the heat engine works harder
2 Obs p(hpa) T(C) T d (C) θ(k) dθ/dz w s θ e (K) dθ e /dz A < 0 < 0 B < 0 C (> 0) 0 (or > 0) D > 0 < 0 E > 0 < 0 F > 0 < 0 G Table : Souning of WH to maintain the chill of the refridgerator, so that more work is transferred into heat. If the heat engine were to cool the kitchen, we would have to use something other than the kitchen as the warm reservoir, for example the outdoor air. This is what is done in air-condition systems. WH a We calculate the potential temperature and equlivalent potential temperature and see how they change with height (given in Table ). We know the following about the static stability of dry/unsaturated air: dθ/dz < 0: the layer is unstable. dθ/dz = 0 the layer is neutral. dθ/dz > 0 the layer is stable. Potential temperature may be calculated from the well known equation ( ) 000hPa R/cp θ = T () p or you can read the values off the sonde diagram. Thus, for dry air, layer AB is unstable, BC is neutral and the rest are stable. 2
3 However, if the layer is saturated we have to consider the moist adiabat, not the dry adiabat! This is done by looking at the equivalent potential temperature. There are two ways to find θ e, but we need the sonde diagram anyway:. We can read it off the sonde diagram, by lifting the air in each point (along the dry adiabat) to saturation, and then upwards along the pseudo adiabat, until all moisture has been removed. We then follow the air downwards along the dry adiabat and read off the temperature at 000hPa. 2. We calculate it from Equation (3.7) ( ) Lv w s θ e = θ exp c p T (2) To do this, we read off the saturation mixing ratio of water vapor (w s ) for the temperature T at each point (w s (T ), not w(t ) = w s (T d ), you should know the difference). L v = Jkg, and c p = 004Jkg K. The θ e -values are given in Table. Both point C and D are saturated (T = T d ), but the equivalent potential temperature increases with height (or is almost unchanged). Therefore, this layer is stable (or neutral). Alternative solution by use of skew-t ln p chart Alternatively, just consider the lapse rate compared to the dry and moist adiabates in Figure (). b Considering convective instability, the criterion for instability is dθ e /dz < 0, so we see that all layers are convetively unstable, except layer CD which we have already noted is stable (or neutral). 3
4 Figure : Plot of the radiosonde values of Exercise WH From (3.83): Q Q2 = T T2 (3) T2 T 273.5K = 20J = 4.6J K Q2 = Q (4) Q2 (5) and the work done in one cycle is: W = Q Q2 (6) So the work done in 0 cycles is: W0 = 0 W = 0 (Q Q2 ) = 0 ( )J = 54J (7) The engine thus rejects 4.64J 0 = 46.4J. The efficiency of the maschine is the work done divided by the heat absorbed: η= Q Q2 54J = = 0.27 Q 0 20J 4 (8)
5 WH We use that at the boiling temperature, T B, the saturation vapor pressure of water is equal to the atmospheric pressure: e s (T B ) = p atmos. (9) We now use the Clausius Clapeyron equation (eq 3.92) pluss the expression above to get the expression: or dp atmos dt B = L v T B (α 2 α ) (0) dt B = T B(α 2 α ) () dp atmos L v Now, notice that the spesific volume of liquid water α is very much smaller than that of gas α 2, so we can ignore this term. Also, we use the ideal gas law to express α 2 = T BR v p atmos. This gives us: dt B = T B( TBRv dp atmos L v (2) dt B R v = dp atmos L v p atmos (3) dt B = R v dp atmos L v p atmos (4) T 2 B TB (600hPa) T 2 B T B (000hPa) TB 2 T B + dt B = p atmos ) 600hPa 000hPa R v dp atmos (5) L v p atmos 373.5K = R v ln 600hPa (6) L v 000hPa ( T B (600hPa) = 373.5K R v ln 600hPa ) (7) L v 000hPa T B (600hPa) = 360K = 87.5 C (8) So the boiling point is reduced to 87.5 C at 600hPa. A.20.T A.20.T a) Potential temperature (θ): The temperature an air parcel will have if it is compressed (lowered) or expanded (lifted) from its pressure to the standard pressure p 0. We use p 0 = 000hPa. ( ) R/cp p0 θ = T (9) p 5
6 Static stability: The layer is stable when the lapse rate of the surroundings (Γ) is less than the dry adiabatic lapse rate (Γ d ), where air lifted will be colder than its surroundings. In other words: Γ < Γ d (20) dt g < T dz c p T (2) d ln T g < dz c p T (22) (23) Now we can express ln T in terms of potential temperature θ. ( p0 ) R/cp θ = T (24) p ( p0 ) R/cp T = θ (25) p ln T = ln θ + R ( p ) ln (26) c p p 0 Putting this into the expression above: ( d ln θ dz d ln T g < dz c p T + R dp c p p dz ) < g c p T (27) (28) (29) (30) Now we can use the ideal gas law and substitute p (p = ρrt ), and the hydrostatic equation, dp dz = ρg: ( d ln θ dz + R c p (ρrt ) ( ρg)) < g c p T d ln θ dz (3) + g c p T < g c p T (32) d ln θ dz > 0 (33) (34) In other words: if potential temperature increases with height, the layer is stable. If potential height decreases with height, the layer is unstable. b) Since θ increase with height, the layer is stable. We can also see that Γ < Γ d. 6
7 Figure 2: Exercise A.20.T d) c) The frequency is called the Brunt-Väisälä frequency [ g N = T (Γ d Γ)] (35) d) To find the mixing ratio at A and B, we use the definition of the relative humidity: RH = w w s 00% (36) which gives: w A = RH w s = 7.5g/kg 0.95 = 7.g/kg 00% (37) w B = RH w s = 5.g/kg 0.80 = 4.0g/kg 00% (38) We can now mark these saturation vapor pressure lines the skew-t lnp chart, as in figure 2. We noe raise a theoretical air parcel from point A: First we follow the dry adiabat because the parcel is not yet saturated, but when we reach the level/temperature where 7.g/kg is the saturation mixing ratio, we know that we have reached saturation and LCL. From this level we follow the pseudoadiabat. We reach the level of free convection when the temperature of the theoretical air parcel is warmer than its surroundings. In this case, this is approximately at 420hPa. When we do the same for point B, we get that as soon as the air parcel reaches saturation it becomes warmer than its surroundings, i.e. LCL is equal to LFC. Seeing as the height air at A has to be lifted in order to become buoyant is so high compared to B, and that the negative buoyancy it has to 7
8 Figure 3: Temperatur T (heltrukken linje) og duggpunkt T d (stiplet) for en sonde. overcome is so large, it seems more likely that air at B will release the potential instability. A.74.T Eksamen GEF See figure 4. Part of a skew-t log p chart is shown in figure 3, in which the temperature T and the dew point temperature T d are given for a layer A-B. a. How is the stability of layer A-B? Layer A to B is stable. We can see this because the environmental sonding crosses the adiabats such that potential temperature increses with height. b. The layer A-B is now lifted by 00hPa. Is the layer more or less stable now? We lift the end points as an approximation. We know that the air at point A is saturated because the temperature is equal to the dew point temperature. We thus follow the pseudoadiabat. B is not saturated so we follow the dry adiabat to B. The layer is less stable. c. The layer is lifted another 00hPa. What is the stability now? the airparcel A is still saturated so we keep following the pseudoadiabat. For 8
9 Figure 4: Temperatur T (heltrukken linje) og duggpunkt T d (stiplet) for en sonde. the air parcel at B we are now approaching saturation and follow the pseudoadiabat from the LCL. The layer is now conditionally unstable! 9
GEF2200 Atmosfærefysikk 2012
 GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
Project 3 Convection and Atmospheric Thermodynamics
 12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]
![Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]](/thumbs/81/83694823.jpg) Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a
Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a
1. Static Stability. (ρ V ) d2 z (1) d 2 z. = g (2) = g (3) T T = g T (4)
 NCAR (National Center for Atmospheric Research) has an excellent resource for education called COMET-MetEd. There you can find some really great tutorials on SkewT-LogP plots: visit http://www.meted.ucar.edu/mesoprim/skewt/index.htm.
NCAR (National Center for Atmospheric Research) has an excellent resource for education called COMET-MetEd. There you can find some really great tutorials on SkewT-LogP plots: visit http://www.meted.ucar.edu/mesoprim/skewt/index.htm.
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
ATMO 551a Fall 08. Equivalent Potential Temperature
 Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Clouds and turbulent moist convection
 Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Introduction. Lecture 6: Water in Atmosphere. How Much Heat Is Brought Upward By Water Vapor?
 Lecture 6: Water in Atmosphere Introduction Over 70% of the planet is covered by water Water is unique in that it can simultaneously exist in all three states (solid, liquid, gas) at the same temperature
Lecture 6: Water in Atmosphere Introduction Over 70% of the planet is covered by water Water is unique in that it can simultaneously exist in all three states (solid, liquid, gas) at the same temperature
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere
![Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere](/thumbs/72/66535634.jpg) Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
References: Parcel Theory. Vertical Force Balance. ESCI Cloud Physics and Precipitation Processes Lesson 3 - Stability and Buoyancy Dr.
 References: ESCI 340 - Cloud Physics and Precipitation Processes Lesson 3 - Stability and Buoyancy Dr. DeCaria Glossary of Meteorology, 2nd ed., American Meteorological Society A Short Course in Cloud
References: ESCI 340 - Cloud Physics and Precipitation Processes Lesson 3 - Stability and Buoyancy Dr. DeCaria Glossary of Meteorology, 2nd ed., American Meteorological Society A Short Course in Cloud
Atmospheric Dynamics: lecture 3
 Atmospheric Dynamics: lecture 3 Moist convection Dew point temperature/lapse rate/lcl Equivalent potential temperature Conditional and potential instability Thermodynamic diagram CAPE Introduction to Python
Atmospheric Dynamics: lecture 3 Moist convection Dew point temperature/lapse rate/lcl Equivalent potential temperature Conditional and potential instability Thermodynamic diagram CAPE Introduction to Python
Convection and buoyancy oscillation
 Convection and buoyancy oscillation Recap: We analyzed the static stability of a vertical profile by the "parcel method"; For a given environmental profile (of T 0, p 0, θ 0, etc.), if the density of an
Convection and buoyancy oscillation Recap: We analyzed the static stability of a vertical profile by the "parcel method"; For a given environmental profile (of T 0, p 0, θ 0, etc.), if the density of an
Chapter 4 Water Vapor
 Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 5. Atmospheric Moisture
 Chapter 5 Atmospheric Moisture hydrologic cycle--movement of water in all forms between earth & atmosphere Humidity: amount of water vapor in air vapor pressure saturation vapor pressure absolute humidity
Chapter 5 Atmospheric Moisture hydrologic cycle--movement of water in all forms between earth & atmosphere Humidity: amount of water vapor in air vapor pressure saturation vapor pressure absolute humidity
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Chapter 5 - Atmospheric Moisture
 Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Thermodynamic Energy Equation
 Thermodynamic Energy Equation The temperature tendency is = u T x v T y w T z + dt dt (1) where dt/dt is the individual derivative of temperature. This temperature change experienced by the air parcel
Thermodynamic Energy Equation The temperature tendency is = u T x v T y w T z + dt dt (1) where dt/dt is the individual derivative of temperature. This temperature change experienced by the air parcel
Clouds and atmospheric convection
 Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Dynamic Meteorology: lecture 2
 Dynamic Meteorology: lecture 2 Sections 1.3-1.5 and Box 1.5 Potential temperature Radiatively determined temperature (boxes 1.1-1.4) Buoyancy (-oscillations) and static instability, Brunt-Vaisala frequency
Dynamic Meteorology: lecture 2 Sections 1.3-1.5 and Box 1.5 Potential temperature Radiatively determined temperature (boxes 1.1-1.4) Buoyancy (-oscillations) and static instability, Brunt-Vaisala frequency
P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.
![P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C. P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.](/thumbs/79/79184286.jpg) Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Atmospheric Composition הרכב האטמוספירה
 Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Outline. Aim. Gas law. Pressure. Scale height Mixing Column density. Temperature Lapse rate Stability. Condensation Humidity.
 Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Moist Convection. Chapter 6
 Moist Convection Chapter 6 1 2 Trade Cumuli Afternoon cumulus over land 3 Cumuls congestus Convectively-driven weather systems Deep convection plays an important role in the dynamics of tropical weather
Moist Convection Chapter 6 1 2 Trade Cumuli Afternoon cumulus over land 3 Cumuls congestus Convectively-driven weather systems Deep convection plays an important role in the dynamics of tropical weather
5 Atmospheric Stability
 5 Atmospheric Stability Introduction In Chapter 4, we examined the concept of horizontal and vertical atmospheric motion, and how pressure differences create the winds that form on a variety of different
5 Atmospheric Stability Introduction In Chapter 4, we examined the concept of horizontal and vertical atmospheric motion, and how pressure differences create the winds that form on a variety of different
Orographic Precipitation II: Effects of Phase Change on Orographic Flow. Richard Rotunno. National Center for Atmospheric Research, USA
 Orographic Precipitation II: Effects of Phase Change on Orographic Flow Richard Rotunno National Center for Atmospheric Research, USA Condensation D Dt vs w( x, ) vs U Large-Scale Flow 0 H L Dynamics w
Orographic Precipitation II: Effects of Phase Change on Orographic Flow Richard Rotunno National Center for Atmospheric Research, USA Condensation D Dt vs w( x, ) vs U Large-Scale Flow 0 H L Dynamics w
Atmospheric Dynamics: lecture 2
 Atmospheric Dynamics: lecture 2 Topics Some aspects of advection and the Coriolis-effect (1.7) Composition of the atmosphere (figure 1.6) Equation of state (1.8&1.9) Water vapour in the atmosphere (1.10)
Atmospheric Dynamics: lecture 2 Topics Some aspects of advection and the Coriolis-effect (1.7) Composition of the atmosphere (figure 1.6) Equation of state (1.8&1.9) Water vapour in the atmosphere (1.10)
Parcel Model. Atmospheric Sciences September 30, 2012
 Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle,
 Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle, like a mountain) or by buoyancy forces. Air that is
Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle, like a mountain) or by buoyancy forces. Air that is
The Behaviour of the Atmosphere
 3 The Behaviour of the Atmosphere Learning Goals After studying this chapter, students should be able to: apply the ideal gas law and the concept of hydrostatic balance to the atmosphere (pp. 49 54); apply
3 The Behaviour of the Atmosphere Learning Goals After studying this chapter, students should be able to: apply the ideal gas law and the concept of hydrostatic balance to the atmosphere (pp. 49 54); apply
Thermodynamics We Can See! Adapted from ATS 541 notes (Dr. Susan van den Heever)
 Thermodynamics We Can See! Adapted from ATS 541 notes (Dr. Susan van den Heever) What we have learned so far ~ Moist Adiabatic Latent Heating ρ ρ Condensation ~ Dry Adiabatic Cloud Types Skew- T log- P
Thermodynamics We Can See! Adapted from ATS 541 notes (Dr. Susan van den Heever) What we have learned so far ~ Moist Adiabatic Latent Heating ρ ρ Condensation ~ Dry Adiabatic Cloud Types Skew- T log- P
Monteverdi Metr 201 Quiz #4 100 pts.
 DEPARTMENT OF GEOSCIENCES Name San Francisco State University April 27, 2012 Monteverdi Metr 201 Quiz #4 100 pts. A. Definitions. (5 points each for a total of 25 points in this section). (a) Convective
DEPARTMENT OF GEOSCIENCES Name San Francisco State University April 27, 2012 Monteverdi Metr 201 Quiz #4 100 pts. A. Definitions. (5 points each for a total of 25 points in this section). (a) Convective
Parcel Model. Meteorology September 3, 2008
 Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties
 Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties For Further Reading Most information contained within these lecture notes is drawn from Chapters 1, 2, 4, and 6 of The Use of the Skew
Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties For Further Reading Most information contained within these lecture notes is drawn from Chapters 1, 2, 4, and 6 of The Use of the Skew
Temperature and Thermodynamics, Part II. Topics to be Covered
 Teperature and Therodynaics, Part II Topics to be Covered Profiles of Teperature in the Boundary Layer Potential teperature Adiabatic Lapse Rate Theral Stratification 1/8/17 Why are We Interested in Theral
Teperature and Therodynaics, Part II Topics to be Covered Profiles of Teperature in the Boundary Layer Potential teperature Adiabatic Lapse Rate Theral Stratification 1/8/17 Why are We Interested in Theral
Chapter 4. Atmospheric Temperature and Stability
 Chapter 4. Atmospheric Temperature and Stability 4.1 The temperature structure of the atmosphere Most people are familiar with the fact that the temperature of the atmosphere decreases with altitude. The
Chapter 4. Atmospheric Temperature and Stability 4.1 The temperature structure of the atmosphere Most people are familiar with the fact that the temperature of the atmosphere decreases with altitude. The
Measuring State Parameters of the Atmosphere
 Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
( ) = 1005 J kg 1 K 1 ;
 Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
ATMO 551a Moist Adiabat Fall Change in internal energy: ΔU
 Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
A Case Study on Diurnal Boundary Layer Evolution
 UNIVERSITY OF OKLAHOMA A Case Study on Diurnal Boundary Layer Evolution Meteorological Measurement Systems Fall 2010 Jason Godwin 12/9/2010 Lab partners: Sam Irons, Charles Kuster, Nathan New, and Stefan
UNIVERSITY OF OKLAHOMA A Case Study on Diurnal Boundary Layer Evolution Meteorological Measurement Systems Fall 2010 Jason Godwin 12/9/2010 Lab partners: Sam Irons, Charles Kuster, Nathan New, and Stefan
Lecture 3: Convection
 EESC V2100 The Climate System spring 2004 Lecture 3: Convection Yochanan Kushnir Lamont Doherty Earth Observatory of Columbia University Palisades, NY 10964, USA kushnir@ldeo.columbia.edu Layers of the
EESC V2100 The Climate System spring 2004 Lecture 3: Convection Yochanan Kushnir Lamont Doherty Earth Observatory of Columbia University Palisades, NY 10964, USA kushnir@ldeo.columbia.edu Layers of the
Buoyancy and Coriolis forces
 Chapter 2 Buoyancy and Coriolis forces In this chapter we address several topics that we need to understand before starting on our study of geophysical uid dynamics. 2.1 Hydrostatic approximation Consider
Chapter 2 Buoyancy and Coriolis forces In this chapter we address several topics that we need to understand before starting on our study of geophysical uid dynamics. 2.1 Hydrostatic approximation Consider
EAS270, The Atmosphere Mid-term Exam 28 Oct. 2011
 EAS270, The Atmosphere Mid-term Exam 28 Oct. 2011 Professor: J.D. Wilson Time available: 50 mins Value: 20% Instructions: For each of the 30 multi-choice questions, choose the most logical option. Use
EAS270, The Atmosphere Mid-term Exam 28 Oct. 2011 Professor: J.D. Wilson Time available: 50 mins Value: 20% Instructions: For each of the 30 multi-choice questions, choose the most logical option. Use
Lecture 3: Convective Heat Transfer I
 Lecture 3: Convective Heat Transfer I Kerry Emanuel; notes by Paige Martin and Daniel Mukiibi June 18 1 Introduction In the first lecture, we discussed radiative transfer in the climate system. Here, we
Lecture 3: Convective Heat Transfer I Kerry Emanuel; notes by Paige Martin and Daniel Mukiibi June 18 1 Introduction In the first lecture, we discussed radiative transfer in the climate system. Here, we
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics
 Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents
 CHAPTER 6 HUMIDITY, SATURATION, AND STABILITY MULTIPLE CHOICE QUESTIONS 1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents 2., annual precipitation
CHAPTER 6 HUMIDITY, SATURATION, AND STABILITY MULTIPLE CHOICE QUESTIONS 1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents 2., annual precipitation
Correction for Dry Bias in Vaisala Radiosonde RH Data
 Correction for Dry Bias in Vaisala Radiosonde RH Data E. R. Miller, J. Wang, and H. L. Cole National Center for Atmospheric Research Atmospheric Technology Division Boulder, Colorado Abstract Extensive
Correction for Dry Bias in Vaisala Radiosonde RH Data E. R. Miller, J. Wang, and H. L. Cole National Center for Atmospheric Research Atmospheric Technology Division Boulder, Colorado Abstract Extensive
39th International Physics Olympiad - Hanoi - Vietnam Theoretical Problem No. 3 / Solution. Solution
 Solution. For an altitude change dz, the atmospheric pressure change is : dp = ρgdz () where g is the acceleration of gravity, considered constant, ρ is the specific mass of air, which is considered as
Solution. For an altitude change dz, the atmospheric pressure change is : dp = ρgdz () where g is the acceleration of gravity, considered constant, ρ is the specific mass of air, which is considered as
Quasi-equilibrium transitions
 Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Boundary layer equilibrium [2005] over tropical oceans
![Boundary layer equilibrium [2005] over tropical oceans Boundary layer equilibrium [2005] over tropical oceans](/thumbs/96/128963638.jpg) Boundary layer equilibrium [2005] over tropical oceans Alan K. Betts [akbetts@aol.com] Based on: Betts, A.K., 1997: Trade Cumulus: Observations and Modeling. Chapter 4 (pp 99-126) in The Physics and Parameterization
Boundary layer equilibrium [2005] over tropical oceans Alan K. Betts [akbetts@aol.com] Based on: Betts, A.K., 1997: Trade Cumulus: Observations and Modeling. Chapter 4 (pp 99-126) in The Physics and Parameterization
Chapter The transition from water vapor to liquid water is called. a. condensation b. evaporation c. sublimation d.
 Chapter-6 Multiple Choice Questions 1. The transition from water vapor to liquid water is called. a. condensation b. evaporation c. sublimation d. deposition 2. The movement of water among the great global
Chapter-6 Multiple Choice Questions 1. The transition from water vapor to liquid water is called. a. condensation b. evaporation c. sublimation d. deposition 2. The movement of water among the great global
Introduction to Skew-T Diagrams
 Introduction to Skew-T Diagrams Have a think about a few things I m going to throw to you it will hopefully make you think a little outside the square! - LIs of -15 can give you clear skies - LIs of 0
Introduction to Skew-T Diagrams Have a think about a few things I m going to throw to you it will hopefully make you think a little outside the square! - LIs of -15 can give you clear skies - LIs of 0
Chapter 3 Convective Dynamics
 Chapter 3 Convective Dynamics Photographs Todd Lindley 3.2 Ordinary or "air-mass storm 3.2.1. Main Characteristics Consists of a single cell (updraft/downdraft pair) Forms in environment characterized
Chapter 3 Convective Dynamics Photographs Todd Lindley 3.2 Ordinary or "air-mass storm 3.2.1. Main Characteristics Consists of a single cell (updraft/downdraft pair) Forms in environment characterized
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1
 Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
Temperature. Vertical Thermal Structure. Earth s Climate System. Lecture 1: Introduction to the Climate System
 Lecture 1: Introduction to the Climate System T mass (& radiation) T & mass relation in vertical mass (& energy, weather..) Energy T vertical stability vertical motion thunderstorm What are included in
Lecture 1: Introduction to the Climate System T mass (& radiation) T & mass relation in vertical mass (& energy, weather..) Energy T vertical stability vertical motion thunderstorm What are included in
SEVERE AND UNUSUAL WEATHER
 SEVERE AND UNUSUAL WEATHER Basic Meteorological Terminology Adiabatic - Referring to a process without the addition or removal of heat. A temperature change may come about as a result of a change in the
SEVERE AND UNUSUAL WEATHER Basic Meteorological Terminology Adiabatic - Referring to a process without the addition or removal of heat. A temperature change may come about as a result of a change in the
Water in the Atmosphere Understanding Weather and Climate
 Water in the Atmosphere Understanding Weather and Climate Climate 2 1 Cloud Development and Forms Understanding Weather and Climate Climate 2 2 Learning Objectives 1. The various atmospheric lifting mechanisms
Water in the Atmosphere Understanding Weather and Climate Climate 2 1 Cloud Development and Forms Understanding Weather and Climate Climate 2 2 Learning Objectives 1. The various atmospheric lifting mechanisms
The atmosphere s water
 The atmosphere s water Atmospheric Moisture and Precipitation Properties of Water The Hydrosphere and the Hydrologic Cycle Humidity The Adiabatic Process Clouds Precipitation Air Quality Main points for
The atmosphere s water Atmospheric Moisture and Precipitation Properties of Water The Hydrosphere and the Hydrologic Cycle Humidity The Adiabatic Process Clouds Precipitation Air Quality Main points for
Chapter 6. Cloud Development and Forms
 Cloud Formation Chapter 6 Cloud Development and Forms Condensation (i.e. clouds,fog) results from: Diabatic cooling (important for fog) Adiabatic cooling (important for clouds) Clouds form due to adiabatic
Cloud Formation Chapter 6 Cloud Development and Forms Condensation (i.e. clouds,fog) results from: Diabatic cooling (important for fog) Adiabatic cooling (important for clouds) Clouds form due to adiabatic
ATMO 551a Fall The Carnot Cycle
 What is a arnot ycle and Why do we care The arnot ycle arnot was a French engineer who was trying to understand how to extract usable mechanical work from a heat engine, that is an engine where a gas or
What is a arnot ycle and Why do we care The arnot ycle arnot was a French engineer who was trying to understand how to extract usable mechanical work from a heat engine, that is an engine where a gas or
Moisture, Clouds, and Precipitation Earth Science, 13e Chapter 17
 Moisture, Clouds, and Precipitation Earth Science, 13e Chapter 17 Stanley C. Hatfield Southwestern Illinois College Changes of state of water, H 2 O Water is the only substance in atmosphere that exists
Moisture, Clouds, and Precipitation Earth Science, 13e Chapter 17 Stanley C. Hatfield Southwestern Illinois College Changes of state of water, H 2 O Water is the only substance in atmosphere that exists
Hurricanes are intense vortical (rotational) storms that develop over the tropical oceans in regions of very warm surface water.
 Hurricanes: Observations and Dynamics Houze Section 10.1. Holton Section 9.7. Emanuel, K. A., 1988: Toward a general theory of hurricanes. American Scientist, 76, 371-379 (web link). http://ww2010.atmos.uiuc.edu/(gh)/guides/mtr/hurr/home.rxml
Hurricanes: Observations and Dynamics Houze Section 10.1. Holton Section 9.7. Emanuel, K. A., 1988: Toward a general theory of hurricanes. American Scientist, 76, 371-379 (web link). http://ww2010.atmos.uiuc.edu/(gh)/guides/mtr/hurr/home.rxml
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Aviation Hazards: Thunderstorms and Deep Convection
 Aviation Hazards: Thunderstorms and Deep Convection TREND Empirical thunderstorm forecasting techniques Contents Necessary conditions for convection: Instability Low-level moisture Trigger mechanism Forecasting
Aviation Hazards: Thunderstorms and Deep Convection TREND Empirical thunderstorm forecasting techniques Contents Necessary conditions for convection: Instability Low-level moisture Trigger mechanism Forecasting
Exam 1 (Chaps. 1-6 of the notes)
 10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
An alternative, less empirical approach (though still full of brazen assumptions) is the following:
 ERTH 500: More Notes on Final Project: Dr. Dave Dempsey Earth Systems II Modeling the Dept. of (Spring 2016) Cenozoic Icehouse Earth Earth & Climate Sciences More notes on Upslope/Monsoon Precipitation
ERTH 500: More Notes on Final Project: Dr. Dave Dempsey Earth Systems II Modeling the Dept. of (Spring 2016) Cenozoic Icehouse Earth Earth & Climate Sciences More notes on Upslope/Monsoon Precipitation
The atmospheric boundary layer: Where the atmosphere meets the surface. The atmospheric boundary layer:
 The atmospheric boundary layer: Utrecht Summer School on Physics of the Climate System Carleen Tijm-Reijmer IMAU The atmospheric boundary layer: Where the atmosphere meets the surface Photo: Mark Wolvenne:
The atmospheric boundary layer: Utrecht Summer School on Physics of the Climate System Carleen Tijm-Reijmer IMAU The atmospheric boundary layer: Where the atmosphere meets the surface Photo: Mark Wolvenne:
PHYSICAL GEOGRAPHY. By Brett Lucas
 PHYSICAL GEOGRAPHY By Brett Lucas INTRODUCTION TO ATMOSPHERIC MOISTURE Atmospheric Moisture The Nature of Water The Hydrologic Cycle Evaporation Measures of Humidity Condensation The Buoyancy of Air Precipitation
PHYSICAL GEOGRAPHY By Brett Lucas INTRODUCTION TO ATMOSPHERIC MOISTURE Atmospheric Moisture The Nature of Water The Hydrologic Cycle Evaporation Measures of Humidity Condensation The Buoyancy of Air Precipitation
Answers to Clicker Questions
 Answers to Clicker Questions Chapter 1 What component of the atmosphere is most important to weather? A. Nitrogen B. Oxygen C. Carbon dioxide D. Ozone E. Water What location would have the lowest surface
Answers to Clicker Questions Chapter 1 What component of the atmosphere is most important to weather? A. Nitrogen B. Oxygen C. Carbon dioxide D. Ozone E. Water What location would have the lowest surface
Instabilities and Basic Convection
 Instabilities and Basic Convection Buoyant Instability (gravity is restoring force) Assume a stationary incompressible fluid (like water), so that ρ = ρ 0 + ρ/ z z and also it is in hydrostatic equilibrium
Instabilities and Basic Convection Buoyant Instability (gravity is restoring force) Assume a stationary incompressible fluid (like water), so that ρ = ρ 0 + ρ/ z z and also it is in hydrostatic equilibrium
Thermodynamics of Cloud Formation
 Thermodynamics of Cloud Formation Energy Partitioning! Griffith et al. 2008! Orographic Clouds Above the PBL Titan s boundary layer Tokano et al. 2006 Stratiform Clouds! Convective clouds! Cumulonimbus!
Thermodynamics of Cloud Formation Energy Partitioning! Griffith et al. 2008! Orographic Clouds Above the PBL Titan s boundary layer Tokano et al. 2006 Stratiform Clouds! Convective clouds! Cumulonimbus!
Lecture Outlines PowerPoint. Chapter 17 Earth Science 11e Tarbuck/Lutgens
 Lecture Outlines PowerPoint Chapter 17 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
Lecture Outlines PowerPoint Chapter 17 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
Weather, Atmosphere and Meteorology
 S c i e n c e s Weather, Atmosphere and Meteorology Key words: Atmosphere, Ozone, Water vapor, solar radiation, Condensation, Evaporation, Humidity, Dew-Point Temperature, Cirrus Clouds, Stratus Clouds,
S c i e n c e s Weather, Atmosphere and Meteorology Key words: Atmosphere, Ozone, Water vapor, solar radiation, Condensation, Evaporation, Humidity, Dew-Point Temperature, Cirrus Clouds, Stratus Clouds,
Lecture 10: Climate Sensitivity and Feedback
 Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Monday 7 October 2013, Class #15
 Monday 7 October 2013, Class #15 Concepts for Today (Basics for Thermodynamics) Weather versus climate Lapse Rate (Adiabatic Lapse Rate) Ideal Gas Law Adiabatic Processes Potential Temperature Hydrostatic
Monday 7 October 2013, Class #15 Concepts for Today (Basics for Thermodynamics) Weather versus climate Lapse Rate (Adiabatic Lapse Rate) Ideal Gas Law Adiabatic Processes Potential Temperature Hydrostatic
4 WATER VAPOR. Contents 4.1. VAPOR PRESSURE AT SATURATION
 Copyright 2017 by Roland Stull. Practical Meteorology: An Algebra-based Survey of Atmospheric Science. v1.02b 4 WATER VAPOR Contents 4.1. Vapor Pressure at Saturation 87 HIGHER MATH Clausius-Clapeyron
Copyright 2017 by Roland Stull. Practical Meteorology: An Algebra-based Survey of Atmospheric Science. v1.02b 4 WATER VAPOR Contents 4.1. Vapor Pressure at Saturation 87 HIGHER MATH Clausius-Clapeyron
Atmospheric Moisture and Precipitation
 Atmospheric Water Atmospheric Moisture and Precipitation Properties of Water The Hydrosphere and the Hydrologic Cycle Humidity The Adiabatic Processes Clouds Precipitation Air Quality Main topics for today
Atmospheric Water Atmospheric Moisture and Precipitation Properties of Water The Hydrosphere and the Hydrologic Cycle Humidity The Adiabatic Processes Clouds Precipitation Air Quality Main topics for today
(Assignment continues on next page) 1
 MEA712: Mesoscale Modeling Class mesoscale model (CMM) project, assignment 1 Due at start of class on Thursday, 5 October. In class supervised work period on Tuesday, 3 October. We will be following a
MEA712: Mesoscale Modeling Class mesoscale model (CMM) project, assignment 1 Due at start of class on Thursday, 5 October. In class supervised work period on Tuesday, 3 October. We will be following a
Theory. Humidity h of an air-vapor mixture is defined as the mass ratio of water vapor and dry air,
 Theory Background In a cooling tower with open water circulation, heat is removed from water because of the material and heat exchange between the water and the ambient air. The cooling tower is a special
Theory Background In a cooling tower with open water circulation, heat is removed from water because of the material and heat exchange between the water and the ambient air. The cooling tower is a special
What did we learn in Ch. 1? Final Exam 2014: 6 Questions. Energy Transfers Link Ch Reversible-Adiabatic-Work. What did we learn in Ch. 3?
 Final Exam 0: 6 Questions. First/Second Laws Question. Definitions Question 3. Clausius-Clapeyron with q/h/e) Question. Köhler, Clouds, and Stability Question 5. Climate Model and Sensitivity Question
Final Exam 0: 6 Questions. First/Second Laws Question. Definitions Question 3. Clausius-Clapeyron with q/h/e) Question. Köhler, Clouds, and Stability Question 5. Climate Model and Sensitivity Question
WaVaCS summerschool Autumn 2009 Cargese, Corsica
 Introduction Part I WaVaCS summerschool Autumn 2009 Cargese, Corsica Holger Tost Max Planck Institute for Chemistry, Mainz, Germany Introduction Overview What is a parameterisation and why using it? Fundamentals
Introduction Part I WaVaCS summerschool Autumn 2009 Cargese, Corsica Holger Tost Max Planck Institute for Chemistry, Mainz, Germany Introduction Overview What is a parameterisation and why using it? Fundamentals
EAS270, The Atmosphere 2 nd Mid-term Exam 2 Nov. 2016
 EAS270, The Atmosphere 2 nd Mid-term Exam 2 Nov. 2016 Professor: J.D. Wilson Time available: 50 mins Value: 25% No formula sheets; no use of tablet computers etc. or cell phones. Formulae/data at back.
EAS270, The Atmosphere 2 nd Mid-term Exam 2 Nov. 2016 Professor: J.D. Wilson Time available: 50 mins Value: 25% No formula sheets; no use of tablet computers etc. or cell phones. Formulae/data at back.
ATS 351, Spring 2010 Lab #9 Weather Radar - 55 points
 ATS 351, Spring 2010 Lab #9 Weather Radar - 55 points 1. (5 points) If a radar has a maximum unambiguous range of 300km, what is its PRF? (The speed of light, c, is equal to 3x10 8 m/s) 2. (5 points) Explain
ATS 351, Spring 2010 Lab #9 Weather Radar - 55 points 1. (5 points) If a radar has a maximum unambiguous range of 300km, what is its PRF? (The speed of light, c, is equal to 3x10 8 m/s) 2. (5 points) Explain
Air stability. About. Precipitation. air in unstable equilibrium will move--up/down Fig. 5-1, p.112. Adiabatic = w/ no exchange of heat from outside!
 Air stability About clouds Precipitation A mass of moist, stable air gliding up and over these mountains condenses into lenticular clouds. Fig. 5-CO, p.110 air in unstable equilibrium will move--up/down
Air stability About clouds Precipitation A mass of moist, stable air gliding up and over these mountains condenses into lenticular clouds. Fig. 5-CO, p.110 air in unstable equilibrium will move--up/down
Isentropic Analysis. Much of this presentation is due to Jim Moore, SLU
 Isentropic Analysis Much of this presentation is due to Jim Moore, SLU Utility of Isentropic Analysis Diagnose and visualize vertical motion - through advection of pressure and system-relative flow Depict
Isentropic Analysis Much of this presentation is due to Jim Moore, SLU Utility of Isentropic Analysis Diagnose and visualize vertical motion - through advection of pressure and system-relative flow Depict
I. Convective Available Potential Energy (CAPE)
 Calculating CAPE, Lifted Index and Strength of Maximum Convective Updraft CAPE Calculation Lifted Index Calculation Maximum Updraft Strength Calculation I. Convective Available Potential Energy (CAPE)
Calculating CAPE, Lifted Index and Strength of Maximum Convective Updraft CAPE Calculation Lifted Index Calculation Maximum Updraft Strength Calculation I. Convective Available Potential Energy (CAPE)
Clouds and atmospheric convection
 Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 Clouds and atmospheric convection 2 Clouds and atmospheric
Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 Clouds and atmospheric convection 2 Clouds and atmospheric
ESS55: EARTH S ATMOSPHERE / Homework #6 / (due 5/24/2018)
 ESS55: EARTH S ATMOSPHERE / Homework #6 / (due 5/24/2018) Name Student ID: 1) 21) 41) 2) 22) 42) 3) 23) 43) 4) 24) 44) 5) 25) 45) 6) 26) 46) 7) 27) 47) 8) 28) 48) 9) 29) 49) 10) 30) 50) 11) 31) 51) 12)
ESS55: EARTH S ATMOSPHERE / Homework #6 / (due 5/24/2018) Name Student ID: 1) 21) 41) 2) 22) 42) 3) 23) 43) 4) 24) 44) 5) 25) 45) 6) 26) 46) 7) 27) 47) 8) 28) 48) 9) 29) 49) 10) 30) 50) 11) 31) 51) 12)
The State of Atmosphere Stability and Instability Effects on Air Quality
 The International Journal of Engineering and Science (IJES) Volume 6 Issue 4 Pages PP 74-79 2017 ISSN (e): 2319 1813 ISSN (p): 2319 1805 The State of Atmosphere Stability and Instability Effects on Air
The International Journal of Engineering and Science (IJES) Volume 6 Issue 4 Pages PP 74-79 2017 ISSN (e): 2319 1813 ISSN (p): 2319 1805 The State of Atmosphere Stability and Instability Effects on Air
Meteorology Lecture 4. Equation of State of Moist Air Ideal Gas Law for water vapor Mixing ratio and specific humidity Virtual Temperature
 Meteorology 3510 Lecture 4 Equation of State of Moist Air Ideal Gas Law for water vapor Mixing ratio and specific humidity Virtual Temperature CLASS Presentation Form group of 2 students Present ~20 minute
Meteorology 3510 Lecture 4 Equation of State of Moist Air Ideal Gas Law for water vapor Mixing ratio and specific humidity Virtual Temperature CLASS Presentation Form group of 2 students Present ~20 minute
VIII. CLOUDS AND STABILITY:
 VIII. CLOUDS AND STABILITY: A. Cloud Classification: 1) Clouds are classified with respect to the height of the cloud base --- low, middle, or high. a. Strato is the first prefix used to indicate cloud
VIII. CLOUDS AND STABILITY: A. Cloud Classification: 1) Clouds are classified with respect to the height of the cloud base --- low, middle, or high. a. Strato is the first prefix used to indicate cloud
Measuring State Parameters of the Atmosphere
 Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Simplified Microphysics. condensation evaporation. evaporation
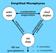 Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Løsningsforslag: oppgavesett kap. 9 (2 av 3) GEF2200
 Løsningsforslag: oppgavesett kap. 9 (2 av 3) GEF2200 s.m.blichner@geo.uio.no Oppgave 1 a) The turbulent vertical flux of sensible heat (Q H ) in the atmospheric boundary layer often takes place through
Løsningsforslag: oppgavesett kap. 9 (2 av 3) GEF2200 s.m.blichner@geo.uio.no Oppgave 1 a) The turbulent vertical flux of sensible heat (Q H ) in the atmospheric boundary layer often takes place through
AT351 Lab Seven Skew-T Stability Analysis
 AT351 Lab Seven Skew-T Stability Analysis Twice a day, all around the planet, small instrument packages called radiosondes are launched into the atmosphere using balloons. These instruments record the
AT351 Lab Seven Skew-T Stability Analysis Twice a day, all around the planet, small instrument packages called radiosondes are launched into the atmosphere using balloons. These instruments record the
MEA 716 Exercise, BMJ CP Scheme With acknowledgements to B. Rozumalski, M. Baldwin, and J. Kain Optional Review Assignment, distributed Th 2/18/2016
 MEA 716 Exercise, BMJ CP Scheme With acknowledgements to B. Rozumalski, M. Baldwin, and J. Kain Optional Review Assignment, distributed Th 2/18/2016 We have reviewed the reasons why NWP models need to
MEA 716 Exercise, BMJ CP Scheme With acknowledgements to B. Rozumalski, M. Baldwin, and J. Kain Optional Review Assignment, distributed Th 2/18/2016 We have reviewed the reasons why NWP models need to
The Earth s Radiation Balance
 The Earth s Radiation Balance Incoming Energy = Outgoing Energy (absorbed sunshine)(area) = (thermal loss)(area) S(1-a)pr 2 = s T 4 (4 pr 2 ) Solve for T T = -18 C; (0 F) The radiative equilibrium temperature
The Earth s Radiation Balance Incoming Energy = Outgoing Energy (absorbed sunshine)(area) = (thermal loss)(area) S(1-a)pr 2 = s T 4 (4 pr 2 ) Solve for T T = -18 C; (0 F) The radiative equilibrium temperature
ATMO/OPTI 656b Spring 09. Physical properties of the atmosphere
 The vertical structure of the atmosphere. Physical properties of the atmosphere To first order, the gas pressure at the bottom of an atmospheric column balances the downward force of gravity on the column.
The vertical structure of the atmosphere. Physical properties of the atmosphere To first order, the gas pressure at the bottom of an atmospheric column balances the downward force of gravity on the column.
EARTH SCIENCE. Prentice Hall Water in the Atmosphere Water in the Atmosphere Water in the Atmosphere.
 Prentice Hall EARTH SCIENCE Tarbuck Lutgens Water s Changes of State 1. Precipitation is any form of water that falls from a cloud. a. Examples: Snow, rain, hail, sleet 3 States of matter of water: 1.
Prentice Hall EARTH SCIENCE Tarbuck Lutgens Water s Changes of State 1. Precipitation is any form of water that falls from a cloud. a. Examples: Snow, rain, hail, sleet 3 States of matter of water: 1.
Temperature Pressure Wind Moisture
 Chapter 1: Properties of Atmosphere Temperature Pressure Wind Moisture Thickness of the Atmosphere (from Meteorology Today) 90% 70% The thickness of the atmosphere is only about 2% of Earth s thickness
Chapter 1: Properties of Atmosphere Temperature Pressure Wind Moisture Thickness of the Atmosphere (from Meteorology Today) 90% 70% The thickness of the atmosphere is only about 2% of Earth s thickness
