THERMODYNAMICS 1 /43
|
|
|
- Tobias Weaver
- 5 years ago
- Views:
Transcription
1 THERMODYNAMICS 1
2 Atmosphere A multi-component Multi-Phase System The gas phase atmospheric cons1tuents; major gases; fixed propor1ons by volume (dry air) Nitrogen (N 2 ) 78,08 % Oxygen (O 2 ) 29,05 % Argon (Ar) 0,934 % Variable vapors and minor gases: Carbon dioxide (CO 2 ) Neon, Helium, Nitrous Oxide, Ozon, Methan, Sulfur compounds, organics Water vapor (0 4 % by volume) Because of its proclivity to change phase and the manner in which these phase changes affect the local temperature on the one hand, and foster diverse interac1ons with radiant energy on the other, water indelibly marks mo1ons in the lower atmosphere on all 1me and spa1al scales. 2
3 Notation The basic theromdynamic proper1es of the atmosphere depend on its component parts. These are defined in terms of their mass, such that for an equilibrium system four cons1tuents of the moist atmosphere can be defined: d dry air v water vapor l liquid water i ice Total mass of the system: m=m d +m v +m l +m i To describe the amount of ma[er we use: Specific mass: q x =m x /m It is useful in a thermodynamic descrip1on for an equilibrium system (the normalizing mass is invariant as long as the basic flow describes the mo1on of the dry air) Mixing ra1o: r x =m x /m d It is helpful when considering the possibility that different components of a mass element have their own velocity 3
4 We dis1nguish between: equilibrium condensed phases associated with clouds, which evolve with the thermodynamic state in a more or less reversible way, and larger hydrometeors which evolve in irreversible way Larger hydrometeors, like rain-drops and most forms of ice, develop through irreversible microphysical processes such as the collision and coalescence of water droplets. These are more difficult to approximate as an equilibrium phase. Because they are larger, non-equilibrium phases of water in the atmosphere are also more dilute and shortlived The present lecture (Thermodynamics) focuses on reversible thermodynamic processes. 4
5 Thermodynamic systems in local equilibrium are iden1fied with mass elements, some1mes referred to as air- or fluid-parcels. Formally the concept gains validity for mass elements small enough that the volume they occupy encompasses a scale much smaller than the scale over which thermodynamic proper1es vary, but much larger than the mean-free path. Diffusion rapidly homogenizes the atmosphere on scales smaller than the Kolmogorov lenght scale, η=(ν 3 /ε) 1/4 ν - viscosity of the atmosphere, ε - turbulent disipaaon rate In vigorous cumulus clouds: ε=0.05 m 2 /s 3, ν= m 2 /s η=0.5 mm; this is several thousand 1mes larger than the mean free path of an air molecule, making the concept of an air parcel a useful one. 5
6 6 Typically the specific condensate mass within a cloud is less than 1 g kg -1 ; much less than the specific mass of water vapor What is the volume frac1on of the water condensate? The dilutness of condensate can come into conflict with the concept of an equilibrium thermodynamic system, as on the Kolmogorov scale, the condensate is not con1nuously distributed d l d l 3 l 3 d d d l l d l 10 m cm 1 V V kg g 1 m m ; cm g 1 ; m kg 1 m m V V = = = = = = ρ ρ ρ ρ
7 In presence of the condensate (droplets, ice) that is not con1nously distributed the concept of air parcel should be enlarged. One ofen imagines an ir parcel as being on the scale of arround 1 m 3. Strictly speeking volumes of air this large cannot be thought of in terms of a single temperature, but the error of this approxima1on is typically much less than those associated with other approxima1ons invoked in the descrip1on of such systems. 7
8 Subscripts notation d dry air v water vapor l liquid water i solid water (ice) c condensate t total water (irrespec1ve of phase) s saturated state, or process e equivalent (all condensate) reference state l! liquid-free (all vapor) reference state 8
9 Equation of State Taking an air-parcel to be comprised of an ideal mixture of ideal gases, perhaps in the presence of condensate, the equa1on of state is that for an ideal gas of variable composi1on, such that: p = p d + p v = m R d d V + m v R v V T R d = 287JK 1 kg 1 R v = 461JK 1 kg 1 Defining the density of the gaseous/vapor mixture as ρ=(m d +m v )/V allows one to formulate the equa1on of state as: d v l i c t s e dry air water vapor liquid water solid water (ice) condensate total water (irrespec1ve of phase) saturated state, or process equivalent (all condensate) reference state p = ρrt R = q d R d + q v R v = 1 q v q c = R d 1+ R v 1 q v q c R d ( ) R d + q v R v To avoid dealing with a state dependent gas constant it is customary to define a density temperature, such that: p = ρ R d T ρ T ρ = T ( 1+ εʹq v q c ) m = m d + m v + m c 1= q d + q v + q c q d =1 q v q c ε ʹ = R v R d l! liquid-free (all vapor) reference state / m ε = R d R v
10 Density temperature, virtual temperature T ρ = T ( 1+ εʹq v q c ) T v = T ( 1+ εʹq v ) = T ( q v ) In the absence of water the density temperature is the air temperature, otherwise can be interpreted as the temperature of a dry air parcel having the same density and pressure as the given air parcel. In the literature the density temperature is ofen called the virtual temperature. 10
11 Buoyancy Given the pressure, the density temperature determines the density and thus is important to the concept of the buoyancy. The buoyancy (b) of a fluid parcel can be measured by the extent to which its density differs from a background or reference density. Assume that locally the density is given in terms of a devia1on from such a reference state density: ρ = ρ 0 + ρʹ b g ρʹ Tʹ g ρ 0 T 0 Rʹ = g R 0 ʹ T ρ T ρ 0 p = ρrt The approxima1on of the buoyancy in terms of the density temperature follows from the assump1on that the rela1ve change in pressure is small compared to the rela1ve change in density. 11
12 Enthalpy Entropy Gibbs free energy Thermodynamic functions of state 12
13 Enthalpy For an atmospheric system it proves useful to use temperature and pressure to describe the state of the system. The First Law becomes (h specific enthalpy): q = dh υdp h = q d h d + q v h v + q l h l + q i h i ( ) p c p = h T h = h 0 + ( q d c p,d + q v c p,v + q l c l + q i c i )T Limi1ng our considera1ons to ideal gases implies that enthalpy depends ONLY on temperature. For water and ice the subscript p for specific heat is omi[ed because water and ice are non-compressible. Physically only enthalpy differences are relevant. 13
14 Enthalpy Vaporisa1on enthalpy (latent heat): L υ = h υ h l It proves useful to rewrite the expression for the enthalpy in terms of the phase-change enthalpies, so that it only refers to the reference enthalpy of one of the phases : using L v to subs1tute h v in expressiion: h = q d h d + q υ h υ + q l h l h e = c e T + q v L v c e = c d + q t ( c l c d ) = q d c d + q t c l using L v to subs1tute h l h l = c l T q l L v c l = c d + q t + ( c υ c d ) = q d c d + q t c υ The subscripts e and l serves as a reminder of which reference state has been adopted. Both expressions of enthalpy are simillar if: ( c l c e )T = q t L υ = q t ( c υ c l )T 14
15 Entropy The Second Law postulates the existence of an entropy state func1on, S, defined by the property that in equilibrium the state of the system is that which maximizes the entropy func1on. Such a func1on has the property that q Tds Tds = dh υdp As an extensive state func1on the entropy, like the enthalpy, can be decomposed into its cons1tuent parts:: s = q d s d + q υ s υ + q l s l + q i s i 15
16 Dry air and water vapor entropy Dry air (ideal gas): dh = Tds +υdp dh = c d dt, υ = R d T p d ds = c d d lnt R d d ln p d s d = s d,0 + c d ln T T 0 ( ) R d ln( p d p 0 ) s d,0 is the reference entropy of dry air at the temperature T 0 and pressure p 0. Water vapor (ideal gas): s υ = s υ,0 + c υ ln( T T 0 ) R υ ln( p υ p 0 ) It is assumed that the specific heats are constant between T and T 0. 16
17 Entropy for condensed phases The condensate is assumed to be ideal so that changes in pressure do not contribute to entropy. s l = s l,0 + c l ln( T T 0 ) 17
18 The general expression for the composite entropy with respect to the equivalent reference state s e = q d s d + q υ s υ + q l s l ( ) = q d s d + q t s l + q υ s υ s l = q d s d,0 + q t s l,0 + q d c d ln T q d R d ln p d + q t c l ln T + q υ ( s υ s l ) T 0 p 0 T 0 q l = q t q υ s e = s e,0 + c e ln T R e ln p d + q υ ( s υ s l ) T 0 p 0 ( ) ( c l c d ) s e,0 = q d s d,0 + q t s l,0 = s d,0 + q t s υ,0 s d,0 c e = q d c d + q t c l = c d + q t R e = q d R d s e,0 is determined by the amount of water in the system and the reference state temperature and pressure denoted by T 0 and p 0 respec1vely 18
19 Entropy for the liquid-free reference state s l = q d s d + q υ s υ + q l s l ( ) = q d s d + q t s υ q l s υ s l = q d s d,0 + q t s υ,0 + q d c d ln T T 0 q d R d ln p d p 0 + q t c υ ln T T 0 qr υt ln p υ p 0 q l ( s υ s l ) q υ = q t q l s l = s l,0 + c e ln T T 0 q d R d ln p d p 0 q t R υ ln p υ p 0 q l s l,0 = s d,0 + q t s υ,0 s d,0 ( ) ( c υ c d ) c l = q d c d + q t c υ = c d + q t ( s υ s l ) 19
20 Gibbs free energy For a closed isobaric and isothermal system it follows from equa1on reversible system 0 = d ( H TS) Which introduces the Gibbs free energy, or Gibbs poten1al as G = H TS i.e. the energy available to do work in an isothermal and isobaric system. TdS = dh Vdp that for a From this defini1on it follows that the difference in the Gibbs energy of two cons1tuents is related to the differencies in their enthalpies and entropies: g υ g l = h υ h l T(s υ s l ) From the postulates of thermodynamics, whereby in equilibrium H and T adopt values that maximize S, it follows that the Gibbs free energy of a system in equilibrium is a minimum. In equilibrium the specific Gibbs energy of each phase must be equal, otherwise a redistribu1on of the mass between the phases could lower the total Gibbs energy. 20
21 The Clausius-Clapeyron Equation g = h Ts dg = dh sdt Tds = υdp sdt Tds = dh υdp dg υ = υ υ dp s υ dt dg l = υ l dp s l dt dg υ = dg l dp = s υ s l υ υ υ l dt Clapeyron equa1on υ υ = R υ T p υ υ >> υ l L υ T = s υ s l Saturated water vapor pressure is ofen denoted by e s d(lne s ) = L v R υ T 2 dt Clausius-Clapeyron equa1on 21
22 Satura:on vapor pressure over liquid (solid line) and ice (dashed line). Colored circles and lines show vapor pressure in the atmosphere, binned according to temperature for different pressure levels (900 hpa, black; 700 hpa, blue, 500 hpa, orange, 300 hpa, red). At T = 0C the satura1on vapor pressure is Pa. At T = 30C the satura1on vapor pressure over liquid water is 50.8 Pa as compared to 38.0 Pa over ice at the same temperature. Satura1on with respect to liquid for T < 0C is relevant because supercooled water is ofen present in the atmosphere, with homogeneous nuclea1on of ice par1cles first occurring at about T = -38C. 22
23 The Clausius-Clapeyron equation The Clausius-Clapeyron equa1on very effec1vely delimits the distribu1on of water throughout the atmosphere because: The atmosphere sits atop a reservoir of water, which endeavors to bring the air above it into satura1on, but how much moisture can be maintained in an air parcel is strongly constrained by the satura1on value. If the amount of moisture exceeds the satura1on value it condenses, and condensate is effec1vely removed by precipita1on by the system. Hence the satura1on specific humidity limits the amount of water in the atmosphere. Because the Clausius-Clapeyron equa1on so strongly controls the distribu1on of water in the atmosphere, if one had to single out a par1cular equa1on as being the most important for the func1oning of Earth s climate, it would be this equa1on. 23
24 Potential temperatures 24
25 Potential temperatures The first and second laws dictate how temperature changes between a given state, and a reference state defined by its pressure p ϑ, and phase distribu1on, given by the triplet {p ϑ,q v,q l }. The temperature in this reference state is called the poten1al temperature (denoted by θ) as it measures the temperature the system would have to have in the reference state for the entropy of this state to be iden1cal to that of the given state. Poten1al temperatures are invariant under an isentropic process, but their proper1es and absolute values depend on the choice of the reference. The poten1al temperature, rather than the sensible temperature, is ofen preferred as a state variable because it is invariant for reversible transforma1ons of the air parcel. The poten1al temperature provides a convenient way to compare air parcels in different parts of the atmosphere, where for instance the pressure or humidity may vary. 25
26 Equivalent potential temperature θ e Reference state {p ϑ,q v,q l }= {10 5 Pa,0,q t } All the vapor is condensed into liquid at p ϑ =1000 hpa. For historical reasons this par1cular reference state is called an equivalent state and denoted by subscript e. For the equivalent reference state we equate the equa1on for s e with the value of T 0 chosen so that s e,0 =s e. s e = s e,0 + c e ln T R e ln p d + q υ ( s υ s l ) θ e p θ c e lnθ e = c e lnt R e ln p d + q υ ( s υ s l ) p θ 26
27 Equivalent potential temperature.. We express the pressure of the dry air in terms of the total pressure and the specific humidity p d = R d Tρ d p = RTρ R = q d R d + q υ R υ p d = p q R d d = p R e R R We express the entropy difference (s v -s l ) rela1ve to the vapor entropy in satura1on s υ s l = s υ s s + s s s l = s υ s s + L υ T s υ s s = c υ ln T θ e R υ ln p υ p θ c υ ln T θ e + R υ ln p s p θ = R υ ln p υ p s = R υ lnϕ φ rela1ve humidity 27
28 Equivalent potential temperature.. c e lnθ e = c e lnt R e ln p p θ R e R q υ R υ lnϕ + q L υ υ T θ e = T p θ p R e ce Ωe exp q L υ υ c e T Ω = R e R e R e ce ϕ q υr υ c e R e = q d R d c e = c d + q t ( c l c d ) R = R d + q t ( R υ R d ) For q t =0 the equivalent poten1al temperature gets a simpler form Ω e is a factor that is very near unity, and (because q t <<1) depends only very weakly on the thermodynamic state. π θ = T p R d c d 28
29 Liquid water potential temperature The counterpart to the equivalent poten1al temperature is the liquid-water poten1al temperature, which is the temperature an air parcel would have if were reversibly brought to the {p ϑ,q v,0} reference state. For the liquid-free reference state we equate the equa1on for s l with the value of T 0 chosen so that s l,0 =s l. θ l = T p θ p R l cl Ωl exp q L l υ R Ω l = c l T q d R d q d R d cl R q υ R υ q t R υ cl R l = R d + q t ( R υ R d ) c l = c d + q t ( c υ c d ) R = R d + q t ( R υ R d ) 29
30 Approximate forms: θ e, θ l Many of the nuances that moisture brings to thermodynamic descrip1ons can be neglected when considering small perturba1ons about a given Enthalpies h e and h l both describe the enthalpy of moist liquid-vapor system, hence in general h e =h l. However if, as is common, one assumes that: c e c d = c p c l c d = c p Large differences between h l and h e become apparent. These differences are however differences only in the absolute sense. In saturated case qʹv = qʹs = qlʹ perturba1ons in general are similar. It is customary to define many of the moist thermodynamic variables in an approximate form apprropriate to the considera1on of small perturba1ons. L θe = θ exp c v p L θl = θ exp c q T v p v ql T where θ π = T p R d c p 30
31 Saturated equivalent potential temperature L θ s θ exp c θ s is only a func1on of temperature and pressure, so it measures the thermal structure of the atmosphere θ s is constant following a saturated pseudo-adiabat. The word pseudo arises because formally the process corresponding to constant θ s is similar to a reversible adiabat, but the removal of condensate upon condensa1on, as implied by the use of qs instead of qt impies a loss of condensate enthalpy by the system, hence it is not truly adiaba1c. The difference between θ s and θ e measures the subsatura1on, as θ e < θ s. v p q T s 31
32 Moist adiabatic lapse rate Γ s dt dz θe = γγ d γ c R 1+ q s β υ T d R c p L 1+ q s β υ T c p T β T lnq s lnt = L υ R υ T lnq s ln p 5400K T c p = q d c d + q υ c υ Γ d = g c d 32
33 Non-dimensional lapse rate γ pressure: hpa the air ini1ally saturated at 300 K solid curve pseudo-adiabat dashed curve adiaba1c lapse rate Γ s dt dz θe = γγ d γ c R 1+ q s β υ T d R c p L 1+ q s β υ T c p T β T 5400K T 33
34 Non-dimensional lapse rate γ pressure: 800 hpa the air ini1ally saturated at 300 K solid curve pseudo-adiabat dashed curve adiaba1c lapse rate Γ s dt dz θe = γγ d γ c R 1+ q s β υ T d R c p L 1+ q s β υ T c p T β T 5400K T 34
35 Water condensated in pseudoadiabatic process Equa1on for enthalpy of a composite system for adiaba1c process. dh = q +υdp q = 0, dh l = c l dt L υ dq l q l dl υ c p = q d c d + q υ c υ + q l c l 0 = c p dt L υ dq l υdp c p dt L υ dq l υdp = 0 L υ dq l = c p dt + gdz dq l = c p L υ dt dz + g c p υdp = gdz dz Γ = g d, Γ s = dt c p dz dq l = c p L υ ( Γ d Γ s )dz LWC = q d l ρ c p ( LWC) = ( Γ Γ )dz L v ρ d s 35
36 Rate of condensation d ( LWC) = c w ( T, p)dz c w (T, p) = c p L υ ρ Γ d ( 1 γ ) c p L υ ρ Γ d kg / m 4 = g / m 4 c w = g / m 4 36
37 p=1000 hpa 37
38 p=800 hpa 38
39 EUCREX Pawlowska et al., Atmos. Res
40 Pawlowska et al., Atmos. Res
41 Pawlowska et al., Atmos. Res
42 ACE-2 Brenguier, Pawlowska, Schueller, JGR
43 EUCAARI - IMPACT Jarecka et al.,, ACP.,
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics
 Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Atmospheric Thermodynamics
 Atmospheric Thermodynamics Atmospheric Composition What is the composition of the Earth s atmosphere? Gaseous Constituents of the Earth s atmosphere (dry air) Constituent Molecular Weight Fractional Concentration
Atmospheric Thermodynamics Atmospheric Composition What is the composition of the Earth s atmosphere? Gaseous Constituents of the Earth s atmosphere (dry air) Constituent Molecular Weight Fractional Concentration
Exam 1 (Chaps. 1-6 of the notes)
 10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
The Clausius-Clapeyron and the Kelvin Equations
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Reversible vs. irreversible processes
 Reversible vs. irreversible processes A reversible process is one for which the final states of the universe (system and environment) are iden
Reversible vs. irreversible processes A reversible process is one for which the final states of the universe (system and environment) are iden
Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]
![Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]](/thumbs/81/83694823.jpg) Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a
Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a
ATMO 551a Moist Adiabat Fall Change in internal energy: ΔU
 Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
ATMOSPHERIC THERMODYNAMICS
 ATMOSPHERIC THERMODYNAMICS 1. Introduction 1.1 The field of thermodynamics Classical thermodynamics deals with energy and the transformations of the nature of energy. To a certain extent, it classifies
ATMOSPHERIC THERMODYNAMICS 1. Introduction 1.1 The field of thermodynamics Classical thermodynamics deals with energy and the transformations of the nature of energy. To a certain extent, it classifies
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
df dz = dp dt Essentially, this is just a statement of the first law in one of the forms we derived earlier (expressed here in W m 3 ) dq p dt dp
 A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION
 1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
1. Heterogeneous Systems and Chemical Equilibrium
 1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
Atmospheric Composition הרכב האטמוספירה
 Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
AT 620 Notes. These notes were prepared by Prof. Steven A. Rutledge. (and adapted slightly for the Fall 2009 course, and again slightly for this year)
 AT 620 Notes These notes were prepared by Prof. Steven A. Rutledge (and adapted slightly for the Fall 2009 course, and again slightly for this year) You may access Prof. Cotton s notes, password cloud9
AT 620 Notes These notes were prepared by Prof. Steven A. Rutledge (and adapted slightly for the Fall 2009 course, and again slightly for this year) You may access Prof. Cotton s notes, password cloud9
First Law of Thermodynamics
 First Law of Thermodynamics September 11, 2013 The first law of thermodynamics is the conservation of energy applied to thermal systems. Here, we develop the principles of thermodynamics for a discrete
First Law of Thermodynamics September 11, 2013 The first law of thermodynamics is the conservation of energy applied to thermal systems. Here, we develop the principles of thermodynamics for a discrete
The Atmosphere EVPP 110 Lecture Fall 2003 Dr. Largen
 1 Physical Environment: EVPP 110 Lecture Fall 2003 Dr. Largen 2 Physical Environment: Atmosphere Composition Heat transfer Atmospheric moisture Atmospheric circulation Weather and climate 3 Physical Environment:
1 Physical Environment: EVPP 110 Lecture Fall 2003 Dr. Largen 2 Physical Environment: Atmosphere Composition Heat transfer Atmospheric moisture Atmospheric circulation Weather and climate 3 Physical Environment:
Some properties of the Helmholtz free energy
 Some properties of the Helmholtz free energy Energy slope is T U(S, ) From the properties of U vs S, it is clear that the Helmholtz free energy is always algebraically less than the internal energy U.
Some properties of the Helmholtz free energy Energy slope is T U(S, ) From the properties of U vs S, it is clear that the Helmholtz free energy is always algebraically less than the internal energy U.
The Atmosphere. 1 Global Environments: 2 Global Environments:
 1 Global Environments: 2 Global Environments: Composition Vertical structure Heat transfer Atmospheric moisture Atmospheric circulation Weather and climate 3 Global Environments: The earth s atmosphere
1 Global Environments: 2 Global Environments: Composition Vertical structure Heat transfer Atmospheric moisture Atmospheric circulation Weather and climate 3 Global Environments: The earth s atmosphere
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Chapter 5. On-line resource
 Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
UNRESOLVED ISSUES. 1. Spectral broadening through different growth histories 2. Entrainment and mixing 3. In-cloud activation
 URESOLVED ISSUES. Spectral broadening through different growth histories 2. Entrainment and mixing. In-cloud activation /4 dr dt ξ ( S ) r, ξ F D + F K 2 dr dt 2ξ ( S ) For a given thermodynamic conditions
URESOLVED ISSUES. Spectral broadening through different growth histories 2. Entrainment and mixing. In-cloud activation /4 dr dt ξ ( S ) r, ξ F D + F K 2 dr dt 2ξ ( S ) For a given thermodynamic conditions
1. Second Law of Thermodynamics
 1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. However, the first law cannot explain certain facts about thermal
1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. However, the first law cannot explain certain facts about thermal
MME 2010 METALLURGICAL THERMODYNAMICS II. Fundamentals of Thermodynamics for Systems of Constant Composition
 MME 2010 METALLURGICAL THERMODYNAMICS II Fundamentals of Thermodynamics for Systems of Constant Composition Thermodynamics addresses two types of problems: 1- Computation of energy difference between two
MME 2010 METALLURGICAL THERMODYNAMICS II Fundamentals of Thermodynamics for Systems of Constant Composition Thermodynamics addresses two types of problems: 1- Computation of energy difference between two
arxiv: v1 [physics.ao-ph] 12 Oct 2015
![arxiv: v1 [physics.ao-ph] 12 Oct 2015 arxiv: v1 [physics.ao-ph] 12 Oct 2015](/thumbs/77/74746419.jpg) arxiv:1510.03239v1 [physics.ao-ph] 12 Oct 2015 Parameterization of Atmospheric Convection (COST-ES0905 book) in R. S. Plant and J.-I. Yano, editors. Vol. 2: Current issues and new theories. Chapter 22:
arxiv:1510.03239v1 [physics.ao-ph] 12 Oct 2015 Parameterization of Atmospheric Convection (COST-ES0905 book) in R. S. Plant and J.-I. Yano, editors. Vol. 2: Current issues and new theories. Chapter 22:
Outline. Aim. Gas law. Pressure. Scale height Mixing Column density. Temperature Lapse rate Stability. Condensation Humidity.
 Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
1. Second Law of Thermodynamics
 1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. he second law deals with direction of thermodynamic processes
1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. he second law deals with direction of thermodynamic processes
Simplified Microphysics. condensation evaporation. evaporation
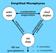 Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Physical Fundamentals of Global Change Processes
 University of Applied Sciences Eberswalde Master Study Program Global Change Management Manfred Stock Potsdam Institute for Climate Impact Research Module: Physical Fundamentals of Global Change Processes
University of Applied Sciences Eberswalde Master Study Program Global Change Management Manfred Stock Potsdam Institute for Climate Impact Research Module: Physical Fundamentals of Global Change Processes
Sec Water vapour variables each has its own usefulness 2/11 The ideal gas law inter-relates vapour pressure (e) & absolute humidity ( ρv) 1 e
 Ch7. Water vapour: the most variable gas & most important GHG Absolute humidity Specific humidity ρv ρv = q q= mass of water vapour volume of sample EAS270_Ch7_WaterVapour_A.odp JDW, EAS Ualberta, last
Ch7. Water vapour: the most variable gas & most important GHG Absolute humidity Specific humidity ρv ρv = q q= mass of water vapour volume of sample EAS270_Ch7_WaterVapour_A.odp JDW, EAS Ualberta, last
Gibb s free energy change with temperature in a single component system
 Gibb s free energy change with temperature in a single component system An isolated system always tries to maximize the entropy. That means the system is stable when it has maximum possible entropy. Instead
Gibb s free energy change with temperature in a single component system An isolated system always tries to maximize the entropy. That means the system is stable when it has maximum possible entropy. Instead
Parcel Model. Atmospheric Sciences September 30, 2012
 Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere
![Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere](/thumbs/72/66535634.jpg) Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Name: Discussion Section:
 CBE 141: Chemical Engineering Thermodynamics, Spring 2017, UC Berkeley Midterm 2 FORM B March 23, 2017 Time: 80 minutes, closed-book and closed-notes, one-sided 8 ½ x 11 equation sheet allowed lease show
CBE 141: Chemical Engineering Thermodynamics, Spring 2017, UC Berkeley Midterm 2 FORM B March 23, 2017 Time: 80 minutes, closed-book and closed-notes, one-sided 8 ½ x 11 equation sheet allowed lease show
Quasi-equilibrium transitions
 Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Parcel Model. Meteorology September 3, 2008
 Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
ESCI 341 Atmospheric Thermodynamics Lesson 12 The Energy Minimum Principle
 ESCI 341 Atmospheric Thermodynamics Lesson 12 The Energy Minimum Principle References: Thermodynamics and an Introduction to Thermostatistics, Callen Physical Chemistry, Levine THE ENTROPY MAXIMUM PRINCIPLE
ESCI 341 Atmospheric Thermodynamics Lesson 12 The Energy Minimum Principle References: Thermodynamics and an Introduction to Thermostatistics, Callen Physical Chemistry, Levine THE ENTROPY MAXIMUM PRINCIPLE
GEF2200 atmospheric physics 2018
 GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
Kelvin Effect. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Rain rate. If the drop size distribu0on is n(d), and fall speeds v(d), net ver0cal flux of drops (m - 2 s - 1 )
 Rain rate If the drop size distribu0on is n(d), and fall speeds v(d), net ver0cal flux of drops (m - 2 s - 1 ) Φ = 0 (w v(d))n(d)dd The threshold diameter has v(d th ) = w. Smaller drops move up, larger
Rain rate If the drop size distribu0on is n(d), and fall speeds v(d), net ver0cal flux of drops (m - 2 s - 1 ) Φ = 0 (w v(d))n(d)dd The threshold diameter has v(d th ) = w. Smaller drops move up, larger
II/IV B.Tech (Regular) DEGREE EXAMINATION. (1X12 = 12 Marks) Answer ONE question from each unit.
 Page 1 of 8 Hall Ticket Number: 14CH 404 II/IV B.Tech (Regular) DEGREE EXAMINATION June, 2016 Chemical Engineering Fourth Semester Engineering Thermodynamics Time: Three Hours Maximum : 60 Marks Answer
Page 1 of 8 Hall Ticket Number: 14CH 404 II/IV B.Tech (Regular) DEGREE EXAMINATION June, 2016 Chemical Engineering Fourth Semester Engineering Thermodynamics Time: Three Hours Maximum : 60 Marks Answer
Outline Review Example Problem 1. Thermodynamics. Review and Example Problems: Part-2. X Bai. SDSMT, Physics. Fall 2014
 Review and Example Problems: Part- SDSMT, Physics Fall 014 1 Review Example Problem 1 Exponents of phase transformation : contents 1 Basic Concepts: Temperature, Work, Energy, Thermal systems, Ideal Gas,
Review and Example Problems: Part- SDSMT, Physics Fall 014 1 Review Example Problem 1 Exponents of phase transformation : contents 1 Basic Concepts: Temperature, Work, Energy, Thermal systems, Ideal Gas,
Engineering Thermodynamics. Chapter 6. Entropy: a measure of Disorder 6.1 Introduction
 Engineering hermodynamics AAi Chapter 6 Entropy: a measure of Disorder 6. Introduction he second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of
Engineering hermodynamics AAi Chapter 6 Entropy: a measure of Disorder 6. Introduction he second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of
Introduction Statistical Thermodynamics. Monday, January 6, 14
 Introduction Statistical Thermodynamics 1 Molecular Simulations Molecular dynamics: solve equations of motion Monte Carlo: importance sampling r 1 r 2 r n MD MC r 1 r 2 2 r n 2 3 3 4 4 Questions How can
Introduction Statistical Thermodynamics 1 Molecular Simulations Molecular dynamics: solve equations of motion Monte Carlo: importance sampling r 1 r 2 r n MD MC r 1 r 2 2 r n 2 3 3 4 4 Questions How can
ATMO 551a Fall 08. Equivalent Potential Temperature
 Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Chapter 4 Water Vapor
 Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Practice Examinations Chem 393 Fall 2005 Time 1 hr 15 min for each set.
 Practice Examinations Chem 393 Fall 2005 Time 1 hr 15 min for each set. The symbols used here are as discussed in the class. Use scratch paper as needed. Do not give more than one answer for any question.
Practice Examinations Chem 393 Fall 2005 Time 1 hr 15 min for each set. The symbols used here are as discussed in the class. Use scratch paper as needed. Do not give more than one answer for any question.
Lecture 20. The Chemical Potential
 MIT 3.00 Fall 2002 c W.C Carter 135 Last Time Internal Degrees of Freedom Lecture 20 The Chemical Potential At constant P, T : G, which represents the internal degrees of freedom, is minimized. The Chemical
MIT 3.00 Fall 2002 c W.C Carter 135 Last Time Internal Degrees of Freedom Lecture 20 The Chemical Potential At constant P, T : G, which represents the internal degrees of freedom, is minimized. The Chemical
CHAPTER 7 ENTROPY. Copyright Hany A. Al-Ansary and S. I. Abdel-Khalik (2014) 1
 CHAPTER 7 ENTROPY S. I. Abdel-Khalik (2014) 1 ENTROPY The Clausius Inequality The Clausius inequality states that for for all cycles, reversible or irreversible, engines or refrigerators: For internally-reversible
CHAPTER 7 ENTROPY S. I. Abdel-Khalik (2014) 1 ENTROPY The Clausius Inequality The Clausius inequality states that for for all cycles, reversible or irreversible, engines or refrigerators: For internally-reversible
Last Name or Student ID
 10/06/08, Chem433 Exam # 1 Last Name or Student ID 1. (3 pts) 2. (3 pts) 3. (3 pts) 4. (2 pts) 5. (2 pts) 6. (2 pts) 7. (2 pts) 8. (2 pts) 9. (6 pts) 10. (5 pts) 11. (6 pts) 12. (12 pts) 13. (22 pts) 14.
10/06/08, Chem433 Exam # 1 Last Name or Student ID 1. (3 pts) 2. (3 pts) 3. (3 pts) 4. (2 pts) 5. (2 pts) 6. (2 pts) 7. (2 pts) 8. (2 pts) 9. (6 pts) 10. (5 pts) 11. (6 pts) 12. (12 pts) 13. (22 pts) 14.
Clouds and turbulent moist convection
 Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
1 mol ideal gas, PV=RT, show the entropy can be written as! S = C v. lnt + RlnV + cons tant
 1 mol ideal gas, PV=RT, show the entropy can be written as! S = C v lnt + RlnV + cons tant (1) p, V, T change Reversible isothermal process (const. T) TdS=du-!W"!S = # "Q r = Q r T T Q r = $W = # pdv =
1 mol ideal gas, PV=RT, show the entropy can be written as! S = C v lnt + RlnV + cons tant (1) p, V, T change Reversible isothermal process (const. T) TdS=du-!W"!S = # "Q r = Q r T T Q r = $W = # pdv =
Thermodynamics of phase transitions
 Thermodynamics of phase transitions Katarzyna Sznajd-Weron Department of Theoretical of Physics Wroc law University of Science and Technology, Poland March 12, 2017 Katarzyna Sznajd-Weron (WUST) Thermodynamics
Thermodynamics of phase transitions Katarzyna Sznajd-Weron Department of Theoretical of Physics Wroc law University of Science and Technology, Poland March 12, 2017 Katarzyna Sznajd-Weron (WUST) Thermodynamics
 Physical Chemistry Physical chemistry is the branch of chemistry that establishes and develops the principles of Chemistry in terms of the underlying concepts of Physics Physical Chemistry Main book: Atkins
Physical Chemistry Physical chemistry is the branch of chemistry that establishes and develops the principles of Chemistry in terms of the underlying concepts of Physics Physical Chemistry Main book: Atkins
Atsc final Equations: page 1/6
 Atsc. 405 2012 final Equations: page 1/6 Answer each of these 7 questions (note weight). Show all your work on all questions (needed for partial credit). Be sure to put your name on any detached pages.
Atsc. 405 2012 final Equations: page 1/6 Answer each of these 7 questions (note weight). Show all your work on all questions (needed for partial credit). Be sure to put your name on any detached pages.
Liquids and Solids. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Liquids and Solids Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Gases, Liquids and Solids Gases are compressible fluids. They have no proper volume and proper
Liquids and Solids Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Gases, Liquids and Solids Gases are compressible fluids. They have no proper volume and proper
Thermodynamic condition for equilibrium between two phases a and b is G a = G b, so that during an equilibrium phase change, G ab = G a G b = 0.
 CHAPTER 5 LECTURE NOTES Phases and Solutions Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between
CHAPTER 5 LECTURE NOTES Phases and Solutions Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between
5/6/ :41 PM. Chapter 6. Using Entropy. Dr. Mohammad Abuhaiba, PE
 Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
ME 2202 ENGINEERING THERMODYNAMICS TWO MARKS QUESTIONS AND ANSWERS UNIT I BASIC CONCEPTS AND FIRST LAW
 ME 2202 ENGINEERING THERMODYNAMICS TWO MARKS QUESTIONS AND ANSWERS UNIT I BASIC CONCEPTS AND FIRST LAW 1. What is thermodynamics? It is a basic science that deals with energy and its transformations. The
ME 2202 ENGINEERING THERMODYNAMICS TWO MARKS QUESTIONS AND ANSWERS UNIT I BASIC CONCEPTS AND FIRST LAW 1. What is thermodynamics? It is a basic science that deals with energy and its transformations. The
GEF2200 Atmosfærefysikk 2012
 GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
Thermochemistry X.S. Bai Thermochemistry
 Lecture 2 Thermochemistry Design a power plant X.S. Bai Thermochemistry When we study a combustion device, what do we want to know? heat generated power production combustion efficiency combustion control
Lecture 2 Thermochemistry Design a power plant X.S. Bai Thermochemistry When we study a combustion device, what do we want to know? heat generated power production combustion efficiency combustion control
Outline Review Example Problem 1 Example Problem 2. Thermodynamics. Review and Example Problems. X Bai. SDSMT, Physics. Fall 2013
 Review and Example Problems SDSMT, Physics Fall 013 1 Review Example Problem 1 Exponents of phase transformation 3 Example Problem Application of Thermodynamic Identity : contents 1 Basic Concepts: Temperature,
Review and Example Problems SDSMT, Physics Fall 013 1 Review Example Problem 1 Exponents of phase transformation 3 Example Problem Application of Thermodynamic Identity : contents 1 Basic Concepts: Temperature,
CHEMICAL ENGINEERING THERMODYNAMICS. Andrew S. Rosen
 CHEMICAL ENGINEERING THERMODYNAMICS Andrew S. Rosen SYMBOL DICTIONARY 1 TABLE OF CONTENTS Symbol Dictionary... 3 1. Measured Thermodynamic Properties and Other Basic Concepts... 5 1.1 Preliminary Concepts
CHEMICAL ENGINEERING THERMODYNAMICS Andrew S. Rosen SYMBOL DICTIONARY 1 TABLE OF CONTENTS Symbol Dictionary... 3 1. Measured Thermodynamic Properties and Other Basic Concepts... 5 1.1 Preliminary Concepts
MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7
 2017 Spring Semester MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7 Byungha Shin ( 신병하 ) Dept. of MSE, KAIST Largely based on lecture notes of Prof. Hyuck-Mo Lee and Prof. WooChul
2017 Spring Semester MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7 Byungha Shin ( 신병하 ) Dept. of MSE, KAIST Largely based on lecture notes of Prof. Hyuck-Mo Lee and Prof. WooChul
ATMO/OPTI 656b Spring 08. Physical Properties of the Atmosphere
 Physical Properties of the Atmosphere Thin as a piece of paper The atmosphere is a very thin layer above the solid Earth and its oceans. This is true of the atmospheres of all of the terrestrial planets.
Physical Properties of the Atmosphere Thin as a piece of paper The atmosphere is a very thin layer above the solid Earth and its oceans. This is true of the atmospheres of all of the terrestrial planets.
Chemistry. Lecture 10 Maxwell Relations. NC State University
 Chemistry Lecture 10 Maxwell Relations NC State University Thermodynamic state functions expressed in differential form We have seen that the internal energy is conserved and depends on mechanical (dw)
Chemistry Lecture 10 Maxwell Relations NC State University Thermodynamic state functions expressed in differential form We have seen that the internal energy is conserved and depends on mechanical (dw)
THERMODYNAMICS NOTES. These notes give a brief overview of engineering thermodynamics. They are based on the thermodynamics text by Black & Hartley.
 THERMODYNAMICS NOTES These notes give a brief overview of engineering thermodynamics. They are based on the thermodynamics text by Black & Hartley. Topics covered include: concepts; properties; conservation
THERMODYNAMICS NOTES These notes give a brief overview of engineering thermodynamics. They are based on the thermodynamics text by Black & Hartley. Topics covered include: concepts; properties; conservation
Project 3 Convection and Atmospheric Thermodynamics
 12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
On Free Energy and Internal Combustion Engine Cycles. William D Harris rd Street Apt A Oakland, CA
 On Free Energy and Internal Combustion Engine Cycles. William D arris 548 43 rd Street Apt A Oakland, CA 94609 wdharris31416@gmail.com Abstract: The performance of one type of Internal Combustion Engine
On Free Energy and Internal Combustion Engine Cycles. William D arris 548 43 rd Street Apt A Oakland, CA 94609 wdharris31416@gmail.com Abstract: The performance of one type of Internal Combustion Engine
CHAPTER 3 LECTURE NOTES 3.1. The Carnot Cycle Consider the following reversible cyclic process involving one mole of an ideal gas:
 CHATER 3 LECTURE NOTES 3.1. The Carnot Cycle Consider the following reversible cyclic process involving one mole of an ideal gas: Fig. 3. (a) Isothermal expansion from ( 1, 1,T h ) to (,,T h ), (b) Adiabatic
CHATER 3 LECTURE NOTES 3.1. The Carnot Cycle Consider the following reversible cyclic process involving one mole of an ideal gas: Fig. 3. (a) Isothermal expansion from ( 1, 1,T h ) to (,,T h ), (b) Adiabatic
Chapter 3. The Second Law Fall Semester Physical Chemistry 1 (CHM2201)
 Chapter 3. The Second Law 2011 Fall Semester Physical Chemistry 1 (CHM2201) Contents The direction of spontaneous change 3.1 The dispersal of energy 3.2 The entropy 3.3 Entropy changes accompanying specific
Chapter 3. The Second Law 2011 Fall Semester Physical Chemistry 1 (CHM2201) Contents The direction of spontaneous change 3.1 The dispersal of energy 3.2 The entropy 3.3 Entropy changes accompanying specific
Generating cloud drops from CCN. Wallace & Hobbs (1977)
 Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Lecture Phase transformations. Fys2160,
 Lecture 12 01.10.2018 Phase transformations Fys2160, 2018 1 A phase transformation Discontinuous change in the properties of substance when the environent is changed infinitesimaly. Change between phases
Lecture 12 01.10.2018 Phase transformations Fys2160, 2018 1 A phase transformation Discontinuous change in the properties of substance when the environent is changed infinitesimaly. Change between phases
The Gibbs Phase Rule F = 2 + C - P
 The Gibbs Phase Rule The phase rule allows one to determine the number of degrees of freedom (F) or variance of a chemical system. This is useful for interpreting phase diagrams. F = 2 + C - P Where F
The Gibbs Phase Rule The phase rule allows one to determine the number of degrees of freedom (F) or variance of a chemical system. This is useful for interpreting phase diagrams. F = 2 + C - P Where F
Chapter 7. Entropy. by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn
 Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
Diabatic Processes. Diabatic processes are non-adiabatic processes such as. entrainment and mixing. radiative heating or cooling
 Diabatic Processes Diabatic processes are non-adiabatic processes such as precipitation fall-out entrainment and mixing radiative heating or cooling Parcel Model dθ dt dw dt dl dt dr dt = L c p π (C E
Diabatic Processes Diabatic processes are non-adiabatic processes such as precipitation fall-out entrainment and mixing radiative heating or cooling Parcel Model dθ dt dw dt dl dt dr dt = L c p π (C E
Measuring State Parameters of the Atmosphere
 Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Phase Diagrams. Department of Mechanical Engineering Indian Institute of Technology Kanpur Kanpur India
 Phase Diagrams 1 Increasing the temperature isobarically T-v diagram of constant-pressure phase-change processes of a pure substance at various pressures numerical values are for water. 2 Temperature -
Phase Diagrams 1 Increasing the temperature isobarically T-v diagram of constant-pressure phase-change processes of a pure substance at various pressures numerical values are for water. 2 Temperature -
Chapter 1 Introduction and Basic Concepts
 Chapter 1 Introduction and Basic Concepts 1-1 Thermodynamics and Energy Application Areas of Thermodynamics 1-2 Importance of Dimensions and Units Some SI and English Units Dimensional Homogeneity Unity
Chapter 1 Introduction and Basic Concepts 1-1 Thermodynamics and Energy Application Areas of Thermodynamics 1-2 Importance of Dimensions and Units Some SI and English Units Dimensional Homogeneity Unity
Atmospheric Basics Atmospheric Composition
 Atmospheric Basics Atmospheric Composition Air is a combination of many gases, each with its own unique characteristics. About 99 percent of the atmosphere is composed of nitrogen and oxygen, with the
Atmospheric Basics Atmospheric Composition Air is a combination of many gases, each with its own unique characteristics. About 99 percent of the atmosphere is composed of nitrogen and oxygen, with the
Chemical Potential. Combining the First and Second Laws for a closed system, Considering (extensive properties)
 Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Naraine Persaud, Entry Code ME-11 1
 Naraine Persaud, Entry Code ME-11 1 Persaud, N. 2005. Adiabatic cooling. In: Water Encyclopedia Volume 4: Oceanography; Meteorology; Physics and Chemistry; Water Law; and Water History, Art, and Culture.
Naraine Persaud, Entry Code ME-11 1 Persaud, N. 2005. Adiabatic cooling. In: Water Encyclopedia Volume 4: Oceanography; Meteorology; Physics and Chemistry; Water Law; and Water History, Art, and Culture.
Temperature. Vertical Thermal Structure. Earth s Climate System. Lecture 1: Introduction to the Climate System
 Lecture 1: Introduction to the Climate System T mass (& radiation) T & mass relation in vertical mass (& energy, weather..) Energy T vertical stability vertical motion thunderstorm What are included in
Lecture 1: Introduction to the Climate System T mass (& radiation) T & mass relation in vertical mass (& energy, weather..) Energy T vertical stability vertical motion thunderstorm What are included in
Multi-Scale Modeling of Turbulence and Microphysics in Clouds. Steven K. Krueger University of Utah
 Multi-Scale Modeling of Turbulence and Microphysics in Clouds Steven K. Krueger University of Utah 10,000 km Scales of Atmospheric Motion 1000 km 100 km 10 km 1 km 100 m 10 m 1 m 100 mm 10 mm 1 mm Planetary
Multi-Scale Modeling of Turbulence and Microphysics in Clouds Steven K. Krueger University of Utah 10,000 km Scales of Atmospheric Motion 1000 km 100 km 10 km 1 km 100 m 10 m 1 m 100 mm 10 mm 1 mm Planetary
Thermodynamics Free E and Phase D. J.D. Price
 Thermodynamics Free E and Phase D J.D. Price Force - the acceleration of matter (N, kg m/s 2 ) Pressure (P)( ) - a force applied over an area (N/m 2 ) Work (W) - force multiplied by distance (kg( m 2 /s
Thermodynamics Free E and Phase D J.D. Price Force - the acceleration of matter (N, kg m/s 2 ) Pressure (P)( ) - a force applied over an area (N/m 2 ) Work (W) - force multiplied by distance (kg( m 2 /s
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A
 ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A 1. What is meant by thermodynamics system? (A/M 2006) Thermodynamics system is defined as any space or matter or group of matter
ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A 1. What is meant by thermodynamics system? (A/M 2006) Thermodynamics system is defined as any space or matter or group of matter
Thermodynamics! for Environmentology!
 1 Thermodynamics! for Environmentology! Thermodynamics and kinetics of natural systems Susumu Fukatsu! Applied Quantum Physics Group! The University of Tokyo, Komaba Graduate School of Arts and Sciences
1 Thermodynamics! for Environmentology! Thermodynamics and kinetics of natural systems Susumu Fukatsu! Applied Quantum Physics Group! The University of Tokyo, Komaba Graduate School of Arts and Sciences
UBMCC11 - THERMODYNAMICS. B.E (Marine Engineering) B 16 BASIC CONCEPTS AND FIRST LAW PART- A
 UBMCC11 - THERMODYNAMICS B.E (Marine Engineering) B 16 UNIT I BASIC CONCEPTS AND FIRST LAW PART- A 1. What do you understand by pure substance? 2. Define thermodynamic system. 3. Name the different types
UBMCC11 - THERMODYNAMICS B.E (Marine Engineering) B 16 UNIT I BASIC CONCEPTS AND FIRST LAW PART- A 1. What do you understand by pure substance? 2. Define thermodynamic system. 3. Name the different types
water Plays dominant role in radiation All three phases emit and absorb in longwave radiation
 4.,4. water Plays dominant role in radiation All three phases emit and absorb in longwave radiation Some shortwave (solar) radiation is absorbed by all phases of water Principal role in the shortwave radiation
4.,4. water Plays dominant role in radiation All three phases emit and absorb in longwave radiation Some shortwave (solar) radiation is absorbed by all phases of water Principal role in the shortwave radiation
Clouds and atmospheric convection
 Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Chapter 5 - Atmospheric Moisture
 Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Chapter 3. Property Relations The essence of macroscopic thermodynamics Dependence of U, H, S, G, and F on T, P, V, etc.
 Chapter 3 Property Relations The essence of macroscopic thermodynamics Dependence of U, H, S, G, and F on T, P, V, etc. Concepts Energy functions F and G Chemical potential, µ Partial Molar properties
Chapter 3 Property Relations The essence of macroscopic thermodynamics Dependence of U, H, S, G, and F on T, P, V, etc. Concepts Energy functions F and G Chemical potential, µ Partial Molar properties
Lecture Notes 2014March 13 on Thermodynamics A. First Law: based upon conservation of energy
 Dr. W. Pezzaglia Physics 8C, Spring 2014 Page 1 Lecture Notes 2014March 13 on Thermodynamics A. First Law: based upon conservation of energy 1. Work 1 Dr. W. Pezzaglia Physics 8C, Spring 2014 Page 2 (c)
Dr. W. Pezzaglia Physics 8C, Spring 2014 Page 1 Lecture Notes 2014March 13 on Thermodynamics A. First Law: based upon conservation of energy 1. Work 1 Dr. W. Pezzaglia Physics 8C, Spring 2014 Page 2 (c)
Thermodynamics of solids 5. Unary systems. Kwangheon Park Kyung Hee University Department of Nuclear Engineering
 Thermodynamics of solids 5. Unary systems Kwangheon ark Kyung Hee University Department of Nuclear Engineering 5.1. Unary heterogeneous system definition Unary system: one component system. Unary heterogeneous
Thermodynamics of solids 5. Unary systems Kwangheon ark Kyung Hee University Department of Nuclear Engineering 5.1. Unary heterogeneous system definition Unary system: one component system. Unary heterogeneous
Marine stratocumulus clouds
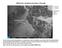 Marine stratocumulus clouds E. Pacific E. Atlantic Subtropical high-p regions Typical cold sea surface temperatures, in conjunc6on with the subsidence associated with the subtropical high, are responsible
Marine stratocumulus clouds E. Pacific E. Atlantic Subtropical high-p regions Typical cold sea surface temperatures, in conjunc6on with the subsidence associated with the subtropical high, are responsible
Lecture 4 Clausius Inequality
 Lecture 4 Clausius Inequality Entropy distinguishes between irreversible and reversible processes. irrev S > 0 rev In a spontaneous process, there should be a net increase in the entropy of the system
Lecture 4 Clausius Inequality Entropy distinguishes between irreversible and reversible processes. irrev S > 0 rev In a spontaneous process, there should be a net increase in the entropy of the system
Lecture 10: Climate Sensitivity and Feedback
 Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
