Measuring State Parameters of the Atmosphere
|
|
|
- Herbert Burns
- 5 years ago
- Views:
Transcription
1 Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial
2 Introduction Goals of This Presentation Present two complementary aspects related to atmospheric thermodynamics: 1 Discuss some basics regarding how measurements of thermodynamic state variables are measured by a research aircraft 2 Show some useful applications of atmospheric thermodynamics to how those measurements are made
3 STATE VARIABLES What Are State Variables? Those variables needed to specify the thermodynamic state of the system, in this case the atmosphere. If we consider a moist atmosphere, in general we need three variables to specify the state. They may be taken, for example, to be temperature, pressure, and water vapor pressure. Other variables can then be determined from these, for example: density from the perfect gas law relative humidity from knowledge of the equilibrium vapor pressure vs T for water dew point also from knowledge of the equilibrium vapor pressure vs T for water institution-log
4 TEMPERATURE SENSORS Temperature Humidity Pressure Types of Temperature Sensors 1 Resistive-element sensors: 1 often, platinum wire 2 resistance varies with temperature 2 radiometric: 1 CO 2 absorbs and re-emits radiation in short distances at some specic wavelengths 2 The intensity of such radiation varies with the temperature 3 Others sensors are also sometimes used, including thermocouple junctions and thermistors
5 THE STANDARD SENSOR Temperature Humidity Pressure
6 Temperature Humidity Pressure EFFECT OF AIRSPEED ON IN SITU SENSORS Airow Approaching a Stagnation Point At boundaries, airspeed tends to zero relative to the boundary. The result is compressional adiabatic heating of the air The sensing wire therefore is in contact with warmed air, not ambient air institution-log
7 WHAT IS TTX? Temperature Humidity Pressure Denition TTX is the measured temperature determined from the resistance of the wire. It is named total temperature because it is approximately the total temperature of air brought to a stagnation point. It is actually closer to the recovery temperature, dened below
8 Temperature Humidity Pressure HOW IS TTX RELATED TO ATX? Conservation of Energy First Law: du = δ Q δ W, and for a perfect gas du = c v dt On a streamline starting with varying speed V, δ Q = 0and δw = pδv To U, must add kinetic energy 1 2 ρv 2 a, with air density ρ a: 1 2 V 2 + c v T + p ρ a = Constant Because p ρ a = R d T and c v + R d = c p, 1 2 V 2 + c p T = Constant institution-log
9 Temperature Humidity Pressure MAGNITUDE OF THE HEATING Heating is about 5 C at 100 m/s, increasing to about 30 C at airspeeds reached by the GV. Accurate correction for this airspeed thus is quite important, especially at high airspeed.
10 THE RECOVERY FACTOR Temperature Humidity Pressure A Further Correction That Depends On Probe Geometry The air does not reach complete stagnation at a distance in thermal contact with the sensing wire. One might expect that the temperature that aects the wire is that present at a distance from the sensor of about a mean free path for air molecules. This is usually dealt with via a recovery factor that varies with sensor but may be as high as 0.98 (where 1.0 would apply for a stagnation point). Often this is determined from ight maneuvers where the aircraft varies airspeed while ying through a region of uniform temperature so the eect of airspeed on the measurement can be detected. institution-log
11 WHAT HAPPENS IN CLOUD? Temperature Humidity Pressure If the sensor becomes wet, the measurement will be wrong Suppose the relative humidity is 100% in the cloud and the sensor becomes covered with water As the air is heated on approach to the sensor, the humidity falls below 100% because the cloud droplets cannot evaporate fast enough to raise the humidity The water on the sensor will evaporate into the subsaturated air, cooling the sensor At the extreme, the sensor will approach the wet-bulb temperature appropriate for the near-stagnation air, potentially introducing errors of a few degrees Celsius
12 Temperature Humidity Pressure THE WET-BULB TEMPERATURE Basic Formula rl V + c p T = Constant r is the water-vapor mixing ratio, L v the latent heat of vaporization, c p the specic heat at constant pressure, and T the temperature. As the air evaporates from the sensing wire and cloud drops, T decreases and r increases. At saturation, r = r s (T WB) ). The wet-bulb temperature is the temperature for which this condition is satised. Conceptually, one could plot the quantity r s (T )L v + c p T vs T and nd the point at which that curve intersects the value specied by the formula above for ambient conditions {r,t }. In practice, the equation is usually solved iteratively. institution-log
13 HUMIDITY SENSORS Temperature Humidity Pressure Basic Sensor Types: Dew point hygrometers: Devices that detect the presence of condensate on a chilled mirror Light-absorption hygrometers: Devices that measure the absorption of radiation at a wavelength where there is strong water absorption Wet-bulb thermometers: Devices that measure the cooling of a wetted sensor Capacitance measurements or hygristor (resistance) devices: Common in radiosondes, seldom used in research aircraft
14 Temperature Humidity Pressure A CHILLED-MIRROR HYGROMETER The sensor housing
15 Temperature Humidity Pressure A CHILLED-MIRROR HYGROMETER Photograph as mounted on the GV institution-log
16 Temperature Humidity Pressure A CHILLED-MIRROR HYGROMETER The control process Reected light from the mirror is measured If the reected light decreases, the mirror is heated, and v.v. The control loop is adjusted to keep just threshold condensation on the mirror
17 Temperature Humidity Pressure USING CHILLED-MIRROR MEASUREMENTS Finding the Water Vapor Pressure Denition The dew point is the temperature at which the water vapor pressure would be in equilibrium with a plane water surface.
18 Temperature Humidity Pressure USING CHILLED-MIRROR MEASUREMENTS Finding the Water Vapor Pressure The functional dependence is usually expressed as e = e s (T DP ) where e s is the equilibrium vapor pressure function, e is the vapor pressure, and T DP is the dew point.
19 Temperature Humidity Pressure USING CHILLED-MIRROR MEASUREMENTS Finding the Water Vapor Pressure Formulas exist to express the function e s, including the Clausius-Clapeyron equation, the Go-Gratch formula, or the Murphy-Koop formula. We now use the latter.
20 FURTHER CONSIDERATIONS Temperature Humidity Pressure Three additional considerations when using these formulas: 1 The condensate on the mirror may be frost, not dew. 2 The pressure in the sensing chamber may dier from the ambient. 3 In the presence of dry air, the equilibrium vapor pressure over a plane surface is slightly higher than the equilibrium value in the absence of air. All these require corrections. The rst two are often substantial.
21 Temperature Humidity Pressure HUMIDITY SENSORS THAT MEASURE ABSORPTION Beer's Law di I = σn dl [I is the intensity of radiation, l distance, σ the molecular crosssection for absorption, and n the number density of molecules.] I = I 0 e σln The quantity measured by instruments using absorption is then n or ρ w = m w n, the mass density of water vapor. Two types of radiometric hygrometer are in common use: 1 Lyman-alpha hygrometers based on absorption of the Lyman-alpha line of hydrogen, which lies in the UV 2 Tunable diode laser (TDL) hygrometers that work in the near IR institution-log
22 THE HUMIDITY VARIABLES Temperature Humidity Pressure Original Measurements: DPB, DPT (mirror temperatures) Corrected for frost-dew dierence, etc: DPBC, DPTC, DPXC Derived: EDPC water vapor pressure MR water vapor mixing ratio RHUM relative humidity RHODT water vapor density Experimental: MIRRORT_CR2 (cryogenic hygrometer) and some measurements from a TDL hygrometer. These are not yet processed to nal engineering-unit form.
23 Temperature Humidity Pressure CALCULATING THE DERIVED VARIABLES Preferred dew-point sensor One of the dew point measurements is designated as the preferred measurement; e.g., DPXC=DPTC. Derived measurements are determined from this basic measurement. EDPC: determined from e=e s (T DP ) after corrections as discussed earlier RHUM: e/e s (T ) where T is the ambient temperature MR: r = εe p e where ε = M W /M a is the ratio of the molecular weight of water to that of air and p is the total pressure RHODT: ρ w = e R w T where R w is the gas constant for water vapor. This equation is also used to obtain e from measurements of ρ w or n, such as provided by the radiometric hygrometers. institution-log
24 PRESSURE MEASUREMENT Temperature Humidity Pressure The Sensors Many dierent transducers are available to measure absolute or dierential pressure. Among the most accurate are digital quartz crystal sensors that change oscillation frequency with pressure. Others are capacitative, piezoelectric, piezoresistive,... On aircraft, these attach to static ports designed to provide pressure close to the ight-level pressure
25 Temperature Humidity Pressure HOW DO STATIC PORTS WORK? The Key Problem Airow around the surfaces of the aircraft creates a varying pressure eld that makes accurate measurement dicult. An example was encountered earlier in connection with temperature measurement: V 2 p = ρ a 2 which can produce an error of about 70 hpa under the following conditions: p = 200 hpa, T = 40 C, V =220 m/s. Pressure ports are located at special locations where this eect is minimized. Corrections are still necessary. Calibration: trailing cone and ight maneuvers to test the eects of angle of attack and sideslip institution-log
26 MAPPING PRESSURE FIELDS Temperature Humidity Pressure Heights on a constant-pressure surface show pressure gradients GPS measurements give the height of the aircraft to few-cm accuracy. (Synoptic maps of pressure elds often use contour increments of 50 m or more.) It is possible to map mesoscale pressure elds by ying on constant-pressure surfaces. Accuracy considerations: If uncertainty in p is 0.5 mb, then the corresponding uncertainty in height can be estimated from the hydrostatic equation: dp p = g RT dz For δ p = 0.5 mb, p = 500 mb, T = 263 K, gives δ Z = 7.5 m. institution-log
27 Pseudo-adiabatic EQUIVALENT POTENTIAL TEMPERATURE (advanced topic) Call attention to the new equation of Davies-Jones (2009)
28 What Is? Rossby Form L v and c pd are kept constant. Θ [Rossby] p { } Lv r = Θ d exp c pd T
29 What Is? Rossby Form L v and c pd are kept constant. Basis for thermodynamic diagrams Θ [Rossby] p { } Lv r = Θ d exp c pd T
30 What Is? Rossby Form L v and c pd are kept constant. Basis for thermodynamic diagrams Θ [Rossby] p { } Lv r = Θ d exp c pd T Revised Forms Like Bolton Take into account the temperature dependence of L v and c pd
31 What Is? Rossby Form L v and c pd are kept constant. Basis for thermodynamic diagrams Θ [Rossby] p { } Lv r = Θ d exp c pd T Revised Forms Like Bolton Take into account the temperature dependence of L v and c pd Often adjust L v to minimize errors
32 What Is? Rossby Form L v and c pd are kept constant. Basis for thermodynamic diagrams Revised Forms Like Bolton Take into account the temperature dependence of L v and c pd Often adjust L v to minimize errors New: Davies-Jones, 2009 Θ [Rossby] p Davies-Jones (2009): { } Lv r = Θ d exp c pd T { [L } Θ 0 L 1 (T L T 0 )]r+k 2 r2 E = Θe c pd T L where L 0, L 1, and K 2 are coecients that are adjusted to minimize errors. institution-log
33 Terminology Wet vs Dry
34 Terminology Wet vs Dry wet-equivalent: carry all water with parcel (Θ q )
35 Terminology Wet vs Dry wet-equivalent: carry all water with parcel (Θ q ) AMS Glossary: Equivalent Potential Temperature A thermodynamic quantity, with its natural logarithm proportional to the entropy of moist air, that is conserved in a reversible moist adiabatic process.
36 Terminology Wet vs Dry wet-equivalent: carry all water with parcel (Θ q ) pseudo-adiabatic equivalent: all water is removed as it condenses AMS Glossary: Equivalent Potential Temperature A thermodynamic quantity, with its natural logarithm proportional to the entropy of moist air, that is conserved in a reversible moist adiabatic process.
37 Terminology Wet vs Dry wet-equivalent: carry all water with parcel (Θ q ) pseudo-adiabatic equivalent: all water is removed as it condenses Pseudo-adiabatic Preferred terminology for all water removed when condensed Equivalent to neglecting the specic heat of liquid water AMS Glossary: Equivalent Potential Temperature A thermodynamic quantity, with its natural logarithm proportional to the entropy of moist air, that is conserved in a reversible moist adiabatic process. institution-log
38 Terminology Wet vs Dry wet-equivalent: carry all water with parcel (Θ q ) pseudo-adiabatic equivalent: all water is removed as it condenses Pseudo-adiabatic Preferred terminology for all water removed when condensed Equivalent to neglecting the specic heat of liquid water AMS Glossary: Equivalent Potential Temperature A thermodynamic quantity, with its natural logarithm proportional to the entropy of moist air, that is conserved in a reversible moist adiabatic process. institution-log
39 Terminology Wet vs Dry wet-equivalent: carry all water with parcel (Θ q ) pseudo-adiabatic equivalent: all water is removed as it condenses Pseudo-adiabatic Preferred terminology for all water removed when condensed Equivalent to neglecting the specic heat of liquid water AMS Glossary: Equivalent Potential Temperature A thermodynamic quantity, with its natural logarithm proportional to the entropy of moist air, that is conserved in a reversible moist adiabatic process. Usage Here: pseudo-adiabatic equivalent Θ p instead of Θ e Θ q for wet-equivalent institution-log
40 Terminology Wet vs Dry wet-equivalent: carry all water with parcel (Θ q ) pseudo-adiabatic equivalent: all water is removed as it condenses Pseudo-adiabatic Preferred terminology for all water removed when condensed Equivalent to neglecting the specic heat of liquid water AMS Glossary: Equivalent Potential Temperature A thermodynamic quantity, with its natural logarithm proportional to the entropy of moist air, that is conserved in a reversible moist adiabatic process. Usage Here: pseudo-adiabatic equivalent Θ p instead of Θ e Θ q for wet-equivalent institution-log
41 Terminology Wet vs Dry wet-equivalent: carry all water with parcel (Θ q ) pseudo-adiabatic equivalent: all water is removed as it condenses Pseudo-adiabatic Preferred terminology for all water removed when condensed Equivalent to neglecting the specic heat of liquid water AMS Glossary: Equivalent Potential Temperature A thermodynamic quantity, with its natural logarithm proportional to the entropy of moist air, that is conserved in a reversible moist adiabatic process. Usage Here: pseudo-adiabatic equivalent Θ p instead of Θ e Θ q for wet-equivalent institution-log
42 Terminology Wet vs Dry wet-equivalent: carry all water with parcel (Θ q ) pseudo-adiabatic equivalent: all water is removed as it condenses Pseudo-adiabatic Preferred terminology for all water removed when condensed Equivalent to neglecting the specic heat of liquid water AMS Glossary: Equivalent Potential Temperature A thermodynamic quantity, with its natural logarithm proportional to the entropy of moist air, that is conserved in a reversible moist adiabatic process. Usage Here: pseudo-adiabatic equivalent Θ p instead of Θ e Θ q for wet-equivalent institution-log
43 Equations Θ q = T ( p0 p d ) Rd /c pt ( ) Lv r exp c pt T (1) Quantities in red vary with temperature. Equation (1) is a straightforward denition if L v and c pd (entering c pt = c pd + r t c w ) are taken at the level of the LCL
44 Equations Θ q = T ( p0 p d ) Rd /c pt ( ) Lv r exp c pt T (1) Quantities in red vary with temperature. Equation (1) is a straightforward denition if L v and c pd (entering c pt = c pd + r t c w ) are taken at the level of the LCL
45 Equations Θ q = T ( p0 p d ) Rd /c pt ( ) Lv r exp c pt T (1) Bolton: If Θ D is the dry-air potential temperature at the LCL, e the vapor pressure in mb, T K the air temperature in kelvin, T L the temperature at the LCL in kelvin and r the mixing ratio T L = lnT K lne Θ Bolton p = Θ D exp {( ) } r( r) T L institution-log
46 Equations Θ q = T ( p0 p d ) Rd /c pt ( ) Lv r exp c pt T (1) dt = TR d + L v r dp d p d (c pd + r t c w ) + T ε p d ( Lv e s (T ) T T ) p d 1 (2)
47 Three Associated Analyses 1 Do revised vapor-pressure equations matter?
48 Three Associated Analyses 1 Do revised vapor-pressure equations matter? Davies-Jones (2009) still uses an approximate formula instead of a more accurate representation of e s (T ).
49 Three Associated Analyses 1 Do revised vapor-pressure equations matter? Davies-Jones (2009) still uses an approximate formula instead of a more accurate representation of e s (T ). Expect small eect because dierences are at low temperature where vapor pressure is low.
50 Three Associated Analyses 1 Do revised vapor-pressure equations matter? Davies-Jones (2009) still uses an approximate formula instead of a more accurate representation of e s (T ). Expect small eect because dierences are at low temperature where vapor pressure is low. 2 What is the eect of including the temperature dependence of the specic heats, especially for supercooled water?
51 Three Associated Analyses 1 Do revised vapor-pressure equations matter? Davies-Jones (2009) still uses an approximate formula instead of a more accurate representation of e s (T ). Expect small eect because dierences are at low temperature where vapor pressure is low. 2 What is the eect of including the temperature dependence of the specic heats, especially for supercooled water? Neglected in previous studies, although Murphy and Koop show that variation in c w is particularly signicant.
52 Three Associated Analyses 1 Do revised vapor-pressure equations matter? Davies-Jones (2009) still uses an approximate formula instead of a more accurate representation of e s (T ). Expect small eect because dierences are at low temperature where vapor pressure is low. 2 What is the eect of including the temperature dependence of the specic heats, especially for supercooled water? Neglected in previous studies, although Murphy and Koop show that variation in c w is particularly signicant. Applies to both Θ p and Θ q.
53 Three Associated Analyses 1 Do revised vapor-pressure equations matter? Davies-Jones (2009) still uses an approximate formula instead of a more accurate representation of e s (T ). Expect small eect because dierences are at low temperature where vapor pressure is low. 2 What is the eect of including the temperature dependence of the specic heats, especially for supercooled water? Neglected in previous studies, although Murphy and Koop show that variation in c w is particularly signicant. Applies to both Θ p and Θ q. 3 How accurate is the Bolton formula for the temperature at the LCL?
54 Three Associated Analyses 1 Do revised vapor-pressure equations matter? Davies-Jones (2009) still uses an approximate formula instead of a more accurate representation of e s (T ). Expect small eect because dierences are at low temperature where vapor pressure is low. 2 What is the eect of including the temperature dependence of the specic heats, especially for supercooled water? Neglected in previous studies, although Murphy and Koop show that variation in c w is particularly signicant. Applies to both Θ p and Θ q. 3 How accurate is the Bolton formula for the temperature at the LCL? How much error is introduced?
55 Three Associated Analyses 1 Do revised vapor-pressure equations matter? Davies-Jones (2009) still uses an approximate formula instead of a more accurate representation of e s (T ). Expect small eect because dierences are at low temperature where vapor pressure is low. 2 What is the eect of including the temperature dependence of the specic heats, especially for supercooled water? Neglected in previous studies, although Murphy and Koop show that variation in c w is particularly signicant. Applies to both Θ p and Θ q. 3 How accurate is the Bolton formula for the temperature at the LCL? How much error is introduced? Do we need to use a numerical solution to obtain better accuracy? institution-log
56 The Approaches Used For all three questions, the approach was to compare solutions from equations to numerical solutions
57 The Approaches Used For all three questions, the approach was to compare solutions from equations to numerical solutions Include new vapor pressure formulas
58 The Approaches Used For all three questions, the approach was to compare solutions from equations to numerical solutions Include new vapor pressure formulas Allow specic heats and the latent heat of vaporization to vary with temperature
59 The Approaches Used For all three questions, the approach was to compare solutions from equations to numerical solutions Include new vapor pressure formulas Allow specic heats and the latent heat of vaporization to vary with temperature Example: Adiabatic motion from in initial point with (T 1,p 1 ) to a new point with (T 2, p 2 ): Given {T 1,p 1,p 2 }, nd T 2 two ways:
60 The Approaches Used For all three questions, the approach was to compare solutions from equations to numerical solutions Include new vapor pressure formulas Allow specic heats and the latent heat of vaporization to vary with temperature Example: Adiabatic motion from in initial point with (T 1,p 1 ) to a new point with (T 2, p 2 ): Given {T 1,p 1,p 2 }, nd T 2 two ways: 1 Integrate the exact equation for dt /dp d from point 1 to point 2 to nd T 2.
61 The Approaches Used For all three questions, the approach was to compare solutions from equations to numerical solutions Include new vapor pressure formulas Allow specic heats and the latent heat of vaporization to vary with temperature Example: Adiabatic motion from in initial point with (T 1,p 1 ) to a new point with (T 2, p 2 ): Given {T 1,p 1,p 2 }, nd T 2 two ways: 1 Integrate the exact equation for dt /dp d from point 1 to point 2 to nd T 2. 2 Evaluate the equation for potential temperature at point 1, then invert it at point 2 to nd T 2. institution-log
62 NEW VARIABLES 1. Change to (6.5) of Davies-Jones (2009), and change the variable name to pseudo-adiabatic equivalent potential temperature. Continue to use (21) of Bolton (1980) to determine the saturation temperature T L. Θ [DJ} p { (L 0 = Θ DL exp L (T } 1 L T 0 ) + K 2 r)r c pd T L ( ) ( ) r TK Θ DL = T k p d T L T L = lnT K lne institution-log
63 NEW VARIABLES 2. Add a new variable wet-equivalent potential temperature and use the standard equation for its evaluation.. Θ q = T ( p0 p d ) Rd /c pt ( ) Lv r exp c pt T where c pt = c pd + r t c w and r tot is the total water mixing ratio, r tot = r + r w where r w = χ/ρ d with χ the liquid water content and ρ d the density of dry air: ρ d = (p e)/(r d T ).
64 MIXING DIAGRAMS (Advanced Topic) [to be added later]
65 More Information: Contact Information: phone:
Measuring State Parameters of the Atmosphere
 Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Exam 1 (Chaps. 1-6 of the notes)
 10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
Introduction. Lecture 6: Water in Atmosphere. How Much Heat Is Brought Upward By Water Vapor?
 Lecture 6: Water in Atmosphere Introduction Over 70% of the planet is covered by water Water is unique in that it can simultaneously exist in all three states (solid, liquid, gas) at the same temperature
Lecture 6: Water in Atmosphere Introduction Over 70% of the planet is covered by water Water is unique in that it can simultaneously exist in all three states (solid, liquid, gas) at the same temperature
Chapter 4 Water Vapor
 Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.
![P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C. P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.](/thumbs/79/79184286.jpg) Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
ATMO 551a Moist Adiabat Fall Change in internal energy: ΔU
 Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Buoyancy and Coriolis forces
 Chapter 2 Buoyancy and Coriolis forces In this chapter we address several topics that we need to understand before starting on our study of geophysical uid dynamics. 2.1 Hydrostatic approximation Consider
Chapter 2 Buoyancy and Coriolis forces In this chapter we address several topics that we need to understand before starting on our study of geophysical uid dynamics. 2.1 Hydrostatic approximation Consider
Parcel Model. Meteorology September 3, 2008
 Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
GEF2200 atmospheric physics 2018
 GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
Chapter 5 - Atmospheric Moisture
 Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
First Law of Thermodyamics U = Q + W. We can rewrite this by introducing two physical. Enthalpy, H, is the quantity U + pv, as this
 First Law of Thermodyamics U = Q + W where U is the increase in internal energy of the system, Q is the heat supplied to the system and W is the work done on the system. We can rewrite this by introducing
First Law of Thermodyamics U = Q + W where U is the increase in internal energy of the system, Q is the heat supplied to the system and W is the work done on the system. We can rewrite this by introducing
Simplified Microphysics. condensation evaporation. evaporation
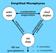 Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Parcel Model. Atmospheric Sciences September 30, 2012
 Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Module 2. Measurement Systems. Version 2 EE IIT, Kharagpur 1
 Module 2 Measurement Systems Version 2 EE IIT, Kharagpur 1 Lesson 8 Measurement of Level, Humidity and ph Version 2 EE IIT, Kharagpur 2 Instructional Objectives At the end of this lesson, the student will
Module 2 Measurement Systems Version 2 EE IIT, Kharagpur 1 Lesson 8 Measurement of Level, Humidity and ph Version 2 EE IIT, Kharagpur 2 Instructional Objectives At the end of this lesson, the student will
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere
![Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere](/thumbs/72/66535634.jpg) Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]
![Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]](/thumbs/81/83694823.jpg) Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a
Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a
Outline. Aim. Gas law. Pressure. Scale height Mixing Column density. Temperature Lapse rate Stability. Condensation Humidity.
 Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Weather is the state or condition of the atmosphere at a given location for a brief time period.
 Topic 8: WEATHER Workbook chapter 7 Weather is the state or condition of the atmosphere at a given location for a brief time period. Differences in how Earth s surfaces absorb and reradiate energy from
Topic 8: WEATHER Workbook chapter 7 Weather is the state or condition of the atmosphere at a given location for a brief time period. Differences in how Earth s surfaces absorb and reradiate energy from
Project 3 Convection and Atmospheric Thermodynamics
 12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
The Water Cycle. Water in the Atmosphere AOSC 200 Tim Canty. Class Web Site:
 Water in the Atmosphere AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Latent Heat Evaporation & Saturation Relative Humidity Dew Point Lecture 11 Oct 2 2018
Water in the Atmosphere AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Latent Heat Evaporation & Saturation Relative Humidity Dew Point Lecture 11 Oct 2 2018
P 1 P * 1 T P * 1 T 1 T * 1. s 1 P 1
 ME 131B Fluid Mechanics Solutions to Week Three Problem Session: Isentropic Flow II (1/26/98) 1. From an energy view point, (a) a nozzle is a device that converts static enthalpy into kinetic energy. (b)
ME 131B Fluid Mechanics Solutions to Week Three Problem Session: Isentropic Flow II (1/26/98) 1. From an energy view point, (a) a nozzle is a device that converts static enthalpy into kinetic energy. (b)
Atmospheric Composition הרכב האטמוספירה
 Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Melting of ice particles:
 Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Clouds and turbulent moist convection
 Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Correcting Dew Point Measurements for Pressure
 Background Correcting Dew Point Measurements for Pressure RAF Algorithm Review 10/12/2011 Background WHY MEASURE HOUSING PRESSURE? The Housing Chilled mirror inside Note small hole Accelerated air ow around
Background Correcting Dew Point Measurements for Pressure RAF Algorithm Review 10/12/2011 Background WHY MEASURE HOUSING PRESSURE? The Housing Chilled mirror inside Note small hole Accelerated air ow around
Final Examination. Part A Answer ONLY TWELVE QUESTIONS in Part A. (Each question is 3 points)
 ATS 210 Spring Term 2001 NAME: Final Examination This is a 2 hour, closed-book examination. Calculators may be used. All answers should be written on the examination paper. Use the final sheet for any
ATS 210 Spring Term 2001 NAME: Final Examination This is a 2 hour, closed-book examination. Calculators may be used. All answers should be written on the examination paper. Use the final sheet for any
4 WATER VAPOR. Contents 4.1. VAPOR PRESSURE AT SATURATION
 Copyright 2017 by Roland Stull. Practical Meteorology: An Algebra-based Survey of Atmospheric Science. v1.02b 4 WATER VAPOR Contents 4.1. Vapor Pressure at Saturation 87 HIGHER MATH Clausius-Clapeyron
Copyright 2017 by Roland Stull. Practical Meteorology: An Algebra-based Survey of Atmospheric Science. v1.02b 4 WATER VAPOR Contents 4.1. Vapor Pressure at Saturation 87 HIGHER MATH Clausius-Clapeyron
On Formulas for Equivalent Potential Temperature
 SEPTEMBER 2009 N O T E S A N D C O R R E S P O N D E N C E 3137 On Formulas for Equivalent Potential Temperature ROBERT DAVIES-JONES NOAA/National Severe Storms aboratory, Norman, Oklahoma (Manuscript
SEPTEMBER 2009 N O T E S A N D C O R R E S P O N D E N C E 3137 On Formulas for Equivalent Potential Temperature ROBERT DAVIES-JONES NOAA/National Severe Storms aboratory, Norman, Oklahoma (Manuscript
The Clausius-Clapeyron and the Kelvin Equations
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
Review: Saturation vapor pressure
 Hygrometry Dr. Christopher M. Gofrey University of North Carolina at Asheville Photo: C. Gofrey Hygrometry refers to the measurement of atmospheric humiity Several variables escribe the quantity of ater
Hygrometry Dr. Christopher M. Gofrey University of North Carolina at Asheville Photo: C. Gofrey Hygrometry refers to the measurement of atmospheric humiity Several variables escribe the quantity of ater
Understanding the uncertainties associated with using the 5128A RHapid Cal portable humidity generator
 Understanding the uncertainties associated with using the 5128A RHapid Cal portable humidity generator Introduction Humidity generators are mechanical devices that provide stable temperature and humidity
Understanding the uncertainties associated with using the 5128A RHapid Cal portable humidity generator Introduction Humidity generators are mechanical devices that provide stable temperature and humidity
Thermodynamics Introduction and Basic Concepts
 Thermodynamics Introduction and Basic Concepts by Asst. Prof. Channarong Asavatesanupap Mechanical Engineering Department Faculty of Engineering Thammasat University 2 What is Thermodynamics? Thermodynamics
Thermodynamics Introduction and Basic Concepts by Asst. Prof. Channarong Asavatesanupap Mechanical Engineering Department Faculty of Engineering Thammasat University 2 What is Thermodynamics? Thermodynamics
SHEBA GLASS SOUNDING DATA
 SHEBA GLASS SOUNDING DATA Erik R. Miller and Kathryn Beierle - 12 June 2000 NCAR ATD Considerable speculation has been brought to bear as to whether there is a dry bias in the SHEBA radiosonde data. Two
SHEBA GLASS SOUNDING DATA Erik R. Miller and Kathryn Beierle - 12 June 2000 NCAR ATD Considerable speculation has been brought to bear as to whether there is a dry bias in the SHEBA radiosonde data. Two
Chapter 7. Water and Atmospheric Moisture. Water on Earth Unique Properties of Water Humidity Atmospheric Stability Clouds and Fog
 Chapter 7 Water and Atmospheric Moisture Robert W. Christopherson Charlie Thomsen Water kept both the terrestrial and marine ecosystems closely linked with the atmosphere. (1) Air carries water vapor and
Chapter 7 Water and Atmospheric Moisture Robert W. Christopherson Charlie Thomsen Water kept both the terrestrial and marine ecosystems closely linked with the atmosphere. (1) Air carries water vapor and
Planetary Atmospheres
 Planetary Atmospheres Structure Composition Meteorology Clouds Photochemistry Atmospheric Escape EAS 4803/8803 - CP 20:1 Cloud formation Saturated Vapor Pressure: Maximum amount of water vapor partial
Planetary Atmospheres Structure Composition Meteorology Clouds Photochemistry Atmospheric Escape EAS 4803/8803 - CP 20:1 Cloud formation Saturated Vapor Pressure: Maximum amount of water vapor partial
df dz = dp dt Essentially, this is just a statement of the first law in one of the forms we derived earlier (expressed here in W m 3 ) dq p dt dp
 A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
Atmosphere Properties and Instruments. Outline. AT351 Lab 2 January 30th, 2008
 Atmosphere Properties and Instruments AT351 Lab 2 January 30th, 2008 Outline 1. Atmospheric Variables and How We Measure Them 2. Composition of the Atmosphere 3. How to Represent Weather Data Visually
Atmosphere Properties and Instruments AT351 Lab 2 January 30th, 2008 Outline 1. Atmospheric Variables and How We Measure Them 2. Composition of the Atmosphere 3. How to Represent Weather Data Visually
Atsc final Equations: page 1/6
 Atsc. 405 2012 final Equations: page 1/6 Answer each of these 7 questions (note weight). Show all your work on all questions (needed for partial credit). Be sure to put your name on any detached pages.
Atsc. 405 2012 final Equations: page 1/6 Answer each of these 7 questions (note weight). Show all your work on all questions (needed for partial credit). Be sure to put your name on any detached pages.
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics
 Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1
 Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
CAE 331/513 Building Science Fall 2017
 CAE 331/513 Building Science Fall 2017 October 5, 2017 Psychrometrics (equations) Advancing energy, environmental, and sustainability research within the built environment www.built-envi.com Twitter: @built_envi
CAE 331/513 Building Science Fall 2017 October 5, 2017 Psychrometrics (equations) Advancing energy, environmental, and sustainability research within the built environment www.built-envi.com Twitter: @built_envi
Understanding uncertainties associated with the 5128A RHapid-Cal Humidity Generator
 Understanding uncertainties associated with the 5128A RHapid-Cal Humidity Generator Technical Note The Fluke Calibration 5128A RHapid-Cal Humidity Generator provides a portable, stable test environment
Understanding uncertainties associated with the 5128A RHapid-Cal Humidity Generator Technical Note The Fluke Calibration 5128A RHapid-Cal Humidity Generator provides a portable, stable test environment
Topic 1 The Atmosphere and Atmospheric Variables
 Name Notes: Topic 1 The Atmosphere Regents Earth Science Topic 1 The Atmosphere and Atmospheric Variables What is the atmosphere? Meteorology is the study of A. Structure of the Atmosphere: What two gases
Name Notes: Topic 1 The Atmosphere Regents Earth Science Topic 1 The Atmosphere and Atmospheric Variables What is the atmosphere? Meteorology is the study of A. Structure of the Atmosphere: What two gases
Diffusional Growth of Liquid Phase Hydrometeros.
 Diffusional Growth of Liquid Phase Hydrometeros. I. Diffusional Growth of Liquid Phase Hydrometeors A. Basic concepts of diffusional growth. 1. To understand the diffusional growth of a droplet, we must
Diffusional Growth of Liquid Phase Hydrometeros. I. Diffusional Growth of Liquid Phase Hydrometeors A. Basic concepts of diffusional growth. 1. To understand the diffusional growth of a droplet, we must
Temperature * OpenStax
 OpenStax-CNX module: m42214 1 Temperature * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Dene temperature. Convert temperatures
OpenStax-CNX module: m42214 1 Temperature * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Dene temperature. Convert temperatures
Governing Equations and Scaling in the Tropics
 Governing Equations and Scaling in the Tropics M 1 ( ) e R ε er Tropical v Midlatitude Meteorology Why is the general circulation and synoptic weather systems in the tropics different to the those in the
Governing Equations and Scaling in the Tropics M 1 ( ) e R ε er Tropical v Midlatitude Meteorology Why is the general circulation and synoptic weather systems in the tropics different to the those in the
Weather, Atmosphere and Meteorology
 S c i e n c e s Weather, Atmosphere and Meteorology Key words: Atmosphere, Ozone, Water vapor, solar radiation, Condensation, Evaporation, Humidity, Dew-Point Temperature, Cirrus Clouds, Stratus Clouds,
S c i e n c e s Weather, Atmosphere and Meteorology Key words: Atmosphere, Ozone, Water vapor, solar radiation, Condensation, Evaporation, Humidity, Dew-Point Temperature, Cirrus Clouds, Stratus Clouds,
Quasi-equilibrium transitions
 Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION
 1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
Department of Mechanical Engineering ME 96. Free and Forced Convection Experiment. Revised: 25 April Introduction
 CALIFORNIA INSTITUTE OF TECHNOLOGY Department of Mechanical Engineering ME 96 Free and Forced Convection Experiment Revised: 25 April 1994 1. Introduction The term forced convection refers to heat transport
CALIFORNIA INSTITUTE OF TECHNOLOGY Department of Mechanical Engineering ME 96 Free and Forced Convection Experiment Revised: 25 April 1994 1. Introduction The term forced convection refers to heat transport
D501 and D501-RS. Microscanner D-Series IR Thermometers. no effect. no effect. no effect. no effect no effect
 Microscanner D-Series The Only Certified Accurate Surface Instruments in the World A MUST FOR ISO 9001 ISO 9002 ISO 9003 TRACEABILITY PROGRAMS Common Surface Measurement Errors D501 and D501-RS Microscanner
Microscanner D-Series The Only Certified Accurate Surface Instruments in the World A MUST FOR ISO 9001 ISO 9002 ISO 9003 TRACEABILITY PROGRAMS Common Surface Measurement Errors D501 and D501-RS Microscanner
LAB 3: Atmospheric Pressure & Moisture
 Name School LAB 3: Atmospheric Pressure & Moisture Our atmosphere is a very dynamic area especially when we see what type of interactions it has with the surrounding environment. This lab will begin discussing
Name School LAB 3: Atmospheric Pressure & Moisture Our atmosphere is a very dynamic area especially when we see what type of interactions it has with the surrounding environment. This lab will begin discussing
Correction for Dry Bias in Vaisala Radiosonde RH Data
 Correction for Dry Bias in Vaisala Radiosonde RH Data E. R. Miller, J. Wang, and H. L. Cole National Center for Atmospheric Research Atmospheric Technology Division Boulder, Colorado Abstract Extensive
Correction for Dry Bias in Vaisala Radiosonde RH Data E. R. Miller, J. Wang, and H. L. Cole National Center for Atmospheric Research Atmospheric Technology Division Boulder, Colorado Abstract Extensive
GEF2200 Atmosfærefysikk 2012
 GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
MICHELL TECHNOLOGIES FOR MOISTURE. Calibration. General Principles & Theory, Equipment Considerations
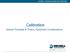 Calibration General Principles & Theory, Equipment Considerations Metrological Principles Accurate but not Precise Calibration A A+ Correction = Precise but not Accurate Correction B Ref Std (Radio Link
Calibration General Principles & Theory, Equipment Considerations Metrological Principles Accurate but not Precise Calibration A A+ Correction = Precise but not Accurate Correction B Ref Std (Radio Link
Aircraft Icing Icing Physics
 Aircraft Icing Icing Physics Prof. Dr. Dept. Aerospace Engineering, METU Fall 2015 Outline Formation of ice in the atmosphere Supercooled water droplets Mechanism of aircraft icing Icing variations Ice
Aircraft Icing Icing Physics Prof. Dr. Dept. Aerospace Engineering, METU Fall 2015 Outline Formation of ice in the atmosphere Supercooled water droplets Mechanism of aircraft icing Icing variations Ice
PHASE CHANGE. Freezing Sublimation
 Melting Graphic Organizer Deposition PHASE CHANGE Freezing Sublimation Boiling Evaporation Condensation PHASE CHANGE Phase change happens as the temperature changes. All matter can move from one state
Melting Graphic Organizer Deposition PHASE CHANGE Freezing Sublimation Boiling Evaporation Condensation PHASE CHANGE Phase change happens as the temperature changes. All matter can move from one state
Chapter 14 Temperature and Heat
 Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 14 Temperature and Heat Thermodynamics Starting a different area of physics called thermodynamics Thermodynamics focuses on energy rather than
Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 14 Temperature and Heat Thermodynamics Starting a different area of physics called thermodynamics Thermodynamics focuses on energy rather than
1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents
 CHAPTER 6 HUMIDITY, SATURATION, AND STABILITY MULTIPLE CHOICE QUESTIONS 1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents 2., annual precipitation
CHAPTER 6 HUMIDITY, SATURATION, AND STABILITY MULTIPLE CHOICE QUESTIONS 1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents 2., annual precipitation
Lecture 9: Climate Sensitivity and Feedback Mechanisms
 Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Topic 3 &10 Review Thermodynamics
 Name: Date: Topic 3 &10 Review Thermodynamics 1. The kelvin temperature of an object is a measure of A. the total energy of the molecules of the object. B. the total kinetic energy of the molecules of
Name: Date: Topic 3 &10 Review Thermodynamics 1. The kelvin temperature of an object is a measure of A. the total energy of the molecules of the object. B. the total kinetic energy of the molecules of
MAHALAKSHMI ENGINEERING COLLEGE
 MAHALAKSHMI ENGINEERING COLLEGE TIRUCHIRAPALLI 621 213. Department: Mechanical Subject Code: ME2202 Semester: III Subject Name: ENGG. THERMODYNAMICS UNIT-I Basic Concept and First Law 1. What do you understand
MAHALAKSHMI ENGINEERING COLLEGE TIRUCHIRAPALLI 621 213. Department: Mechanical Subject Code: ME2202 Semester: III Subject Name: ENGG. THERMODYNAMICS UNIT-I Basic Concept and First Law 1. What do you understand
Name... Class... Date... Specific heat capacity and specific latent heat
 Specific heat capacity and specific latent heat Specification references: P3.2.2 Temperature changes in a system and specific heat capacity P3.2.3 Changes of heat and specific latent heat Aims This is
Specific heat capacity and specific latent heat Specification references: P3.2.2 Temperature changes in a system and specific heat capacity P3.2.3 Changes of heat and specific latent heat Aims This is
Sec Water vapour variables each has its own usefulness 2/11 The ideal gas law inter-relates vapour pressure (e) & absolute humidity ( ρv) 1 e
 Ch7. Water vapour: the most variable gas & most important GHG Absolute humidity Specific humidity ρv ρv = q q= mass of water vapour volume of sample EAS270_Ch7_WaterVapour_A.odp JDW, EAS Ualberta, last
Ch7. Water vapour: the most variable gas & most important GHG Absolute humidity Specific humidity ρv ρv = q q= mass of water vapour volume of sample EAS270_Ch7_WaterVapour_A.odp JDW, EAS Ualberta, last
The calibration of Hygrometer (LectureandTraining )
 The calibration of Hygrometer (LectureandTraining ) HAGIYA Satoshi 8Nov.2016 Outline 1.Measu rementmethods of midity(theory) Hu 2.Traceab ili ty in JMA 3.Cali brationmethodsof Hyg rometer 4.Practice (Ca
The calibration of Hygrometer (LectureandTraining ) HAGIYA Satoshi 8Nov.2016 Outline 1.Measu rementmethods of midity(theory) Hu 2.Traceab ili ty in JMA 3.Cali brationmethodsof Hyg rometer 4.Practice (Ca
1/2/2016 WEATHER DEFINITION
 WEATHER DEFINITION Weather state or condition of the variables of the atmosphere at a given time Weather variables temperature, air pressure, wind, moisture, cloud cover, precipitation, storms Weather
WEATHER DEFINITION Weather state or condition of the variables of the atmosphere at a given time Weather variables temperature, air pressure, wind, moisture, cloud cover, precipitation, storms Weather
Thermodynamics (Lecture Notes) Heat and Thermodynamics (7 th Edition) by Mark W. Zemansky & Richard H. Dittman
 Thermodynamics (Lecture Notes Heat and Thermodynamics (7 th Edition by Mark W. Zemansky & Richard H. Dittman 2 Chapter 1 Temperature and the Zeroth Law of Thermodynamics 1.1 Macroscopic Point of View If
Thermodynamics (Lecture Notes Heat and Thermodynamics (7 th Edition by Mark W. Zemansky & Richard H. Dittman 2 Chapter 1 Temperature and the Zeroth Law of Thermodynamics 1.1 Macroscopic Point of View If
Water in the Atmosphere
 Water in the Atmosphere Characteristics of Water solid state at 0 o C or below (appearing as ice, snow, hail and ice crystals) liquid state between 0 o C and 100 o C (appearing as rain and cloud droplets)
Water in the Atmosphere Characteristics of Water solid state at 0 o C or below (appearing as ice, snow, hail and ice crystals) liquid state between 0 o C and 100 o C (appearing as rain and cloud droplets)
Response Characteristics of Dew Point Sensor with Aluminum Oxide by means of Correlation between Purging Rate and Tube Length
 Response Characteristics of Dew Point Sensor with Aluminum Oxide by means of Correlation between Purging Rate and Tube Length Yun-Kyung Bae and In-Jik Jeong Industrial & Physical Instrument Center, Korea
Response Characteristics of Dew Point Sensor with Aluminum Oxide by means of Correlation between Purging Rate and Tube Length Yun-Kyung Bae and In-Jik Jeong Industrial & Physical Instrument Center, Korea
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur
 Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 21 Psychometric Processes Good afternoon, yesterday we
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 21 Psychometric Processes Good afternoon, yesterday we
Kelvin Effect. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Dynamic Meteorology: lecture 2
 Dynamic Meteorology: lecture 2 Sections 1.3-1.5 and Box 1.5 Potential temperature Radiatively determined temperature (boxes 1.1-1.4) Buoyancy (-oscillations) and static instability, Brunt-Vaisala frequency
Dynamic Meteorology: lecture 2 Sections 1.3-1.5 and Box 1.5 Potential temperature Radiatively determined temperature (boxes 1.1-1.4) Buoyancy (-oscillations) and static instability, Brunt-Vaisala frequency
ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A
 ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A 1. What is meant by thermodynamics system? (A/M 2006) Thermodynamics system is defined as any space or matter or group of matter
ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A 1. What is meant by thermodynamics system? (A/M 2006) Thermodynamics system is defined as any space or matter or group of matter
water Plays dominant role in radiation All three phases emit and absorb in longwave radiation
 4.,4. water Plays dominant role in radiation All three phases emit and absorb in longwave radiation Some shortwave (solar) radiation is absorbed by all phases of water Principal role in the shortwave radiation
4.,4. water Plays dominant role in radiation All three phases emit and absorb in longwave radiation Some shortwave (solar) radiation is absorbed by all phases of water Principal role in the shortwave radiation
Performance of the Meteolabor Snow White chilled-mirror hygrometer. Masatomo Fujiwara Hokkaido University, Japan
 Performance of the Meteolabor Snow White chilled-mirror hygrometer Masatomo Fujiwara Hokkaido University, Japan Coauthors and Collaborators F. Hasebe, M. Shiotani, H. Voemel, S. J. Oltmans, H. Takashima,
Performance of the Meteolabor Snow White chilled-mirror hygrometer Masatomo Fujiwara Hokkaido University, Japan Coauthors and Collaborators F. Hasebe, M. Shiotani, H. Voemel, S. J. Oltmans, H. Takashima,
Clouds and atmospheric convection
 Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Temperature Scales. Temperature, and Temperature Dependent on Physical Properties. Temperature. Temperature Scale
 Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle,
 Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle, like a mountain) or by buoyancy forces. Air that is
Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle, like a mountain) or by buoyancy forces. Air that is
Earth s Energy Balance and the Atmosphere
 Earth s Energy Balance and the Atmosphere Topics we ll cover: Atmospheric composition greenhouse gases Vertical structure and radiative balance pressure, temperature Global circulation and horizontal energy
Earth s Energy Balance and the Atmosphere Topics we ll cover: Atmospheric composition greenhouse gases Vertical structure and radiative balance pressure, temperature Global circulation and horizontal energy
Radiation, Sensible Heat Flux and Evapotranspiration
 Radiation, Sensible Heat Flux and Evapotranspiration Climatological and hydrological field work Figure 1: Estimate of the Earth s annual and global mean energy balance. Over the long term, the incoming
Radiation, Sensible Heat Flux and Evapotranspiration Climatological and hydrological field work Figure 1: Estimate of the Earth s annual and global mean energy balance. Over the long term, the incoming
Naraine Persaud, Entry Code ME-11 1
 Naraine Persaud, Entry Code ME-11 1 Persaud, N. 2005. Adiabatic cooling. In: Water Encyclopedia Volume 4: Oceanography; Meteorology; Physics and Chemistry; Water Law; and Water History, Art, and Culture.
Naraine Persaud, Entry Code ME-11 1 Persaud, N. 2005. Adiabatic cooling. In: Water Encyclopedia Volume 4: Oceanography; Meteorology; Physics and Chemistry; Water Law; and Water History, Art, and Culture.
Atmospheric Dynamics: lecture 2
 Atmospheric Dynamics: lecture 2 Topics Some aspects of advection and the Coriolis-effect (1.7) Composition of the atmosphere (figure 1.6) Equation of state (1.8&1.9) Water vapour in the atmosphere (1.10)
Atmospheric Dynamics: lecture 2 Topics Some aspects of advection and the Coriolis-effect (1.7) Composition of the atmosphere (figure 1.6) Equation of state (1.8&1.9) Water vapour in the atmosphere (1.10)
ATMO 551a Fall 08. Equivalent Potential Temperature
 Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Meteorology 6150 Cloud System Modeling
 Meteorology 6150 Cloud System Modeling Steve Krueger Spring 2009 1 Fundamental Equations 1.1 The Basic Equations 1.1.1 Equation of motion The movement of air in the atmosphere is governed by Newton s Second
Meteorology 6150 Cloud System Modeling Steve Krueger Spring 2009 1 Fundamental Equations 1.1 The Basic Equations 1.1.1 Equation of motion The movement of air in the atmosphere is governed by Newton s Second
A Case Study on Diurnal Boundary Layer Evolution
 UNIVERSITY OF OKLAHOMA A Case Study on Diurnal Boundary Layer Evolution Meteorological Measurement Systems Fall 2010 Jason Godwin 12/9/2010 Lab partners: Sam Irons, Charles Kuster, Nathan New, and Stefan
UNIVERSITY OF OKLAHOMA A Case Study on Diurnal Boundary Layer Evolution Meteorological Measurement Systems Fall 2010 Jason Godwin 12/9/2010 Lab partners: Sam Irons, Charles Kuster, Nathan New, and Stefan
Chapter 6 Clouds. Cloud Development
 Chapter 6 Clouds Chapter overview Processes causing saturation o Cooling, moisturizing, mixing Cloud identification and classification Cloud Observations Fog Why do we care about clouds in the atmosphere?
Chapter 6 Clouds Chapter overview Processes causing saturation o Cooling, moisturizing, mixing Cloud identification and classification Cloud Observations Fog Why do we care about clouds in the atmosphere?
G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1. Time allowed 50 mins. Total possible points: 40 number of pages: 5
 G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1 Time allowed 50 mins. Total possible points: 40 number of pages: 5 Part A: Short Answer & Problems (12), Fill in the Blanks (6).
G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1 Time allowed 50 mins. Total possible points: 40 number of pages: 5 Part A: Short Answer & Problems (12), Fill in the Blanks (6).
Module 11: Meteorology Topic 3 Content: Weather Instruments Notes
 Introduction In order for meteorologists to accurately predict the weather, they take thousands of different weather measurements each day. Meteorologists need to use many tools in order to draw an accurate
Introduction In order for meteorologists to accurately predict the weather, they take thousands of different weather measurements each day. Meteorologists need to use many tools in order to draw an accurate
Chapter 5. Sound Waves and Vortices. 5.1 Sound waves
 Chapter 5 Sound Waves and Vortices In this chapter we explore a set of characteristic solutions to the uid equations with the goal of familiarizing the reader with typical behaviors in uid dynamics. Sound
Chapter 5 Sound Waves and Vortices In this chapter we explore a set of characteristic solutions to the uid equations with the goal of familiarizing the reader with typical behaviors in uid dynamics. Sound
Series tore word. Acknowledgements
 Series tore word p. xi Preface p. xiii Acknowledgements p. xv Disclaimer p. xvii Introduction p. 1 The instrumental age p. 2 Measurements and the climate record p. 2 Clouds and rainfall p. 3 Standardisation
Series tore word p. xi Preface p. xiii Acknowledgements p. xv Disclaimer p. xvii Introduction p. 1 The instrumental age p. 2 Measurements and the climate record p. 2 Clouds and rainfall p. 3 Standardisation
ATMOS 5130 Lecture 9. Enthalpy Conservation Property The Second Law and Its Consequences Entropy
 ATMOS 5130 Lecture 9 Enthalpy Conservation Property The Second Law and Its Consequences Entropy CLASS Presentation Form group of 2 students Present ~20 minute presentation (~ 10 minute each person) Focus
ATMOS 5130 Lecture 9 Enthalpy Conservation Property The Second Law and Its Consequences Entropy CLASS Presentation Form group of 2 students Present ~20 minute presentation (~ 10 minute each person) Focus
Water Vapour Effects in Mass Measurement
 Water Vapour Effects in Mass Measurement N.-E. Khélifa To cite this version: N.-E. Khélifa. Water Vapour Effects in Mass Measurement. Measurement. Water Vapour Effects in Mass Measurement, May 2007, Smolenice,
Water Vapour Effects in Mass Measurement N.-E. Khélifa To cite this version: N.-E. Khélifa. Water Vapour Effects in Mass Measurement. Measurement. Water Vapour Effects in Mass Measurement, May 2007, Smolenice,
Supporting Information: On Localized Vapor Pressure Gradients Governing Condensation and Frost Phenomena
 Supporting Information: On Localized Vapor Pressure Gradients Governing Condensation and Frost Phenomena Saurabh Nath and Jonathan B. Boreyko Department of Biomedical Engineering and Mechanics, Virginia
Supporting Information: On Localized Vapor Pressure Gradients Governing Condensation and Frost Phenomena Saurabh Nath and Jonathan B. Boreyko Department of Biomedical Engineering and Mechanics, Virginia
5) The amount of heat needed to raise the temperature of 1 gram of a substance by 1 C is called: Page Ref: 69
 Homework #2 Due 9/19/14 1) If the maximum temperature for a particular day is 26 C and the minimum temperature is 14 C, what would the daily mean temperature be? (Page Ref: 66) 2) How is the annual mean
Homework #2 Due 9/19/14 1) If the maximum temperature for a particular day is 26 C and the minimum temperature is 14 C, what would the daily mean temperature be? (Page Ref: 66) 2) How is the annual mean
Lecture 10: Climate Sensitivity and Feedback
 Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Temperature Pressure Wind Moisture
 Chapter 1: Properties of Atmosphere Temperature Pressure Wind Moisture Thickness of the Atmosphere (from Meteorology Today) 90% 70% The thickness of the atmosphere is only about 2% of Earth s thickness
Chapter 1: Properties of Atmosphere Temperature Pressure Wind Moisture Thickness of the Atmosphere (from Meteorology Today) 90% 70% The thickness of the atmosphere is only about 2% of Earth s thickness
