Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]
|
|
|
- Cordelia Emmeline Paul
- 5 years ago
- Views:
Transcription
1 Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]
2 Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a reversible process by an amount equal to the heat absorbed or emitted divided by the thermodynamic temperature. It is measured in joules per kelvin [J/K]. 2. (Statistical Physics) a statistical measure of the disorder of a closed system expressed by S = k B log W + c where W is the probability that a particular state of the system exists, and c is an arbitrary constant 3. (General Physics) lack of pattern or organization; disorder 4. (Electronics & Computer Science / Communications & Information) a measure of the efficiency of a system, such as a code or language, in transmitting information
3 1st Law of Thermodynamics & Thermodynamic Identity du = d 'q! d 'w The notation dʹ denotes an inexact differential, i.e., an integral over the quantity depends on the path taken. s(u,{x i })! ds =!s $ # & "!u% {x i }!!s $ # & = 1 "!u%{x i } T! du +!s $ # & "!x i % " du = Tds!T!s % $ ' #!x i &! du = Tds +T!s $ # & " d" % u u,{x n(i } u,{x n'i } dx i dx i d" = Tds ' pd" = d 'q! d 'w (1) 1st Law Energy conservation: the change in energy of a system du must equal the heat added to/removed from the system dʹ q and the work done on/by the system dʹ w. (2) Definition of temperature [from entropy s, which is written as a function of u and a set of other variables {x i } ] Expanding s as a linear function of it s first partial derivatives and rearranging, using (2) leads to (3) the thermodynamic identity. Here, pressure p is related to the partial derivative of s w.r.t. system volume.
4 Aside: Thermodynamic Potentials Potential: generalized force F i multiplied by a generalized displacement d i : Internal energy (U): " = F i d i U = U(S,V, { N i }) = TS " pv + µ i N i Helmholtz free energy (A): A = A(T,V, { N i }) = U " TS Gibbs free energy (G): G = G(T, p, { N i }) = U " TS + pv Enthalpy (H): H = H(S, p, { N i }) = U + pv Landau or Grand Potential (Ω): " = "(T,V,{ µ i }) = U # TS # µ i N i Chemical potential (µ i ): force related to particle exchange (of species i) between systems Euler s homogeneous function theorem allows U to be written in terms of TS, pv, T,p,µ i are intensive variables: they are unchanged if 2 or more identical systems are combined. S,V,N i [and, e.g., U] are extensive, scaling with system size. Legendre transforms applied to obtain other potentials Properties of potentials can be exploited for different purposes, e.g., Gibbs is useful for processes in T-p space. Maxwell relations: equivalence of mixed partial derivatives
5 Hydrostatic equilibrium & buoyancy Newton s 2nd Law of Motion in the z direction:! F = m! a z Consider first the case with equal environmental and parcel densities, i.e., " e = " b = " ("#x#y#z) d 2 #z dt 2 = $("#x#y#z)g + p(z)#x#y $ p(z + #z)#x#y In equilibrium, and applying limit δz 0, 0 = "#g " $p $z Consider now : z+δz z y δx p(z+δz) " e # " b (" b #x#y#z) d 2 #z = $(" dt 2 b #x#y#z)g + p(z)#x#y $ p(z + #z)#x#y " d 2 #z = $g + 1 &p dt 2 % b &z = $g ' 1$ % * e ), - B Buoyancy ( + % b ρ b x p(z) δy g ρ e
6 Relating buoyancy and entropy Defining the specific volume α as " = 1 #, we can rewrite the buoyancy as: $ B = "g 1" # e & % # b ' $ ) = "g 1" * b & ( % * e ' $ ) = g * b "* e ' & ) = g +* ( % ( * * e where " # " e and "# $ # b %# e Consider α=α(s,p). Then, at constant pressure: d! = "! "s p ds = "T " p s ds α is equivalent to specific enthalpy, h. The second equality here thus follows from the Maxwell relations for mixed second partial derivatives of h. Thus, B = g!!! = g ""T % $ '! #" p & s ds = ( "T "z s ds = )ds Use the hydrostatic relationship to covert derivative to a function of z.
7 Dry adiabatic lapse rate Adiabatic parcel + ideal gas law [+hydrostatic eq] Tds = 0 = du + pd! p" = R d T For an ideal gas, the internal energy is a function of T only. [R d " 287Jkg #1 K #1 ] z leads to the dry adiabatic lapse rate γ d : % " d = # $T ( ' * & $z ) ds= 0 = g c p +10K /km [c p "1004Jkg #1 K #1 ] z 0 +δz T(z 0 +δz) T e (z 0 +δz) From the 1D force equation in the vertical [again applying the ideal gas law]: dw dt = g " e # " = g T # T e " T e T(z 0 + "z) # T e (z 0 ) $ % d "z z 0 T(z 0 ) =T e (z 0 ) T e (z 0 ) dw dt = g"z(# e $ # d ) /T e T e (z 0 + "z) # T e (z 0 ) $ % e "z
8 (Dry) Static Stability w = d"z dt # d 2 "z dt 2 & $ g % e $ % d ( ' T e ) + "z = 0 * d 2 "z + N 2 (z)"z = 0 N(z) = "g # " # e d dt 2 T e (z) N is known as the Brunt- V äisälä frequency. If γ e < γ d, N 2 (z) > 0, so solutions to the perturbation equation are oscillatory (sinusoids). In this case, the equilibrium is stable. If γ e > γ d, N 2 (z) < 0, so solutions to the perturbation equation are exponential (hyperbolic sine/cosine). In this case, the equilibrium is unstable. Height Slope: -γ Stable d Slope: Unstable Temperature -γ e
9 Let s re-evaluate radiative equilibrium Emanuel, 2005 Pure radiative equilibrium is in fact unstable for conditions in the troposphere [but is reasonable in the stratosphere]. Tropospheric γ d significantly exceeds the observed lapse rate (~6.5K/km) Need to account for vertical heat transport via convection [more shortly and later]
10 Moist atmosphere Ideal gas law for dry air (denoted d ) and water vapor (denoted v ): p d " d = R d T e" v = R v T p = p d + e # % $ R d R v = m v " 18 m d 28.9 = & ( ' The total pressure of moist (dry +vapor) air [Dalton s Law of Partial Pressures]: The density of moist air is: " = " d + " v = p $ & R d T 1# e ' ) % p( Virtual temperature (T v ): temperature to which dry air must be raised to have the same density as moist the same pressure # T v = T% 1" e "1 & ( $ p' Specific humidity (q): q = " v " = 0.622e p # 0.378e $ e p Using the definitions of q and T v, the equation of state of moist air is approximately: p " #R d T( q)
11 Saturation Consider the relative humidity (rh): rh = e e s Here, the saturation vapor pressure (e s ) is the maximum vapor pressure attainable at a given temperature The Clausius-Clapeyron equation governs the temperature dependence of e s for two-phase equilibrium: de s T = L T(" 2 #" 2 ) If 1 denotes the condensed phase (solid or liquid) and 2 the gaseous phase, α 2 >>α 1. From prior definitions and after integration: $ e s (T) "exp #0.622 L ' & ) % R d T(
12 Adiabatic process in a moist atmosphere Consider first the potential temperature for dry air, θ, which is the temperature a parcel of air would have if displaced, adiabatically and reversibly, to a reference pressure p 0 [typically, 1000 mb]: # " = T p & 0 % ( $ p ' ) ;) = R d c p [To derive: apply 1st law for an adiabatic process (ds=0), use definition of internal energy and ideal gas law, and integrate.] The (dry) entropy can be expressed in terms of θ:!s d = c p ln! d!s d = 0 " d! = 0 Note: the Δ notation indicates entropy is defined up to an arbitrary constant, i.e., Δs d =s d -constant. Now, for an adiabatic upward displacement of saturated air, condensation will occur, leading to a release of latent heat in the amount -Ldq s. The entropy change associated with this release is dδs c =(-Ldq s )/T. Equating dδs d and dδs c and integrating gives the equivalent potential temperature θ e : c p dln" = # L cdq s T % $ #d L cq s ( ' * & T ) # " e = " exp Lq & s % ( $ c p T'
13 Moist Stability Starting with the vertical acceleration and using the definition of potential temperature, it can be shown that: d 2 "z dt 2 + N 2 (z)"z = 0 Thus: "# "z > 0 Stable "# "z < 0 Unstable d 2 "z dt + % g $# ( ' *"z = 0 2 &# $z ) Equivalent potential temperature can be used to evaluate stability for a moist atmosphere; by analogy, a moist adiabatic lapse γ m rate can be defined. A region of conditional instability emerges, for γ m < γ e < γ d. For unsaturated air, this region is stable; for saturated air, it s unstable. Height Conditionally Unstable m=-γ d Absolutely Unstable m=-γ m Absolutely Stable Temperature
14 Overview of convection [more later] Convection is an important source of atmospheric heating associated with H 2 O phase changes. Vertical motion field associated with convection is an important control on the space-time behavior of H 2 O vapor, other tracers, aerosols, and clouds. Global hydrologic cycle balance implies balance between global mean precipitation and evaporation rates [~3 mm day -1 ] although significant regional [sub-global] heterogeneity exists. Evaporation [aka latent heating] is an important component of the surface energy budget.
15 Convective adjustment Eliminates atmospheric column instability Environmental conditions that favor instability: Cooling aloft via cold air advection or longwave radiative cooling Warming of surface via warm air advection or solar heating Lifting of air mass through low-level convergence
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere
![Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere](/thumbs/72/66535634.jpg) Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Chapter 4 Water Vapor
 Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics
 Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Project 3 Convection and Atmospheric Thermodynamics
 12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.
![P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C. P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.](/thumbs/79/79184286.jpg) Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Outline. Aim. Gas law. Pressure. Scale height Mixing Column density. Temperature Lapse rate Stability. Condensation Humidity.
 Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
ATMO/OPTI 656b Spring 09. Physical properties of the atmosphere
 The vertical structure of the atmosphere. Physical properties of the atmosphere To first order, the gas pressure at the bottom of an atmospheric column balances the downward force of gravity on the column.
The vertical structure of the atmosphere. Physical properties of the atmosphere To first order, the gas pressure at the bottom of an atmospheric column balances the downward force of gravity on the column.
Clouds and turbulent moist convection
 Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
GEF2200 atmospheric physics 2018
 GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
Dynamic Meteorology: lecture 2
 Dynamic Meteorology: lecture 2 Sections 1.3-1.5 and Box 1.5 Potential temperature Radiatively determined temperature (boxes 1.1-1.4) Buoyancy (-oscillations) and static instability, Brunt-Vaisala frequency
Dynamic Meteorology: lecture 2 Sections 1.3-1.5 and Box 1.5 Potential temperature Radiatively determined temperature (boxes 1.1-1.4) Buoyancy (-oscillations) and static instability, Brunt-Vaisala frequency
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
ATMO 551a Fall 08. Equivalent Potential Temperature
 Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Atmospheric Dynamics: lecture 2
 Atmospheric Dynamics: lecture 2 Topics Some aspects of advection and the Coriolis-effect (1.7) Composition of the atmosphere (figure 1.6) Equation of state (1.8&1.9) Water vapour in the atmosphere (1.10)
Atmospheric Dynamics: lecture 2 Topics Some aspects of advection and the Coriolis-effect (1.7) Composition of the atmosphere (figure 1.6) Equation of state (1.8&1.9) Water vapour in the atmosphere (1.10)
Lecture 3: Convective Heat Transfer I
 Lecture 3: Convective Heat Transfer I Kerry Emanuel; notes by Paige Martin and Daniel Mukiibi June 18 1 Introduction In the first lecture, we discussed radiative transfer in the climate system. Here, we
Lecture 3: Convective Heat Transfer I Kerry Emanuel; notes by Paige Martin and Daniel Mukiibi June 18 1 Introduction In the first lecture, we discussed radiative transfer in the climate system. Here, we
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Thermodynamic Energy Equation
 Thermodynamic Energy Equation The temperature tendency is = u T x v T y w T z + dt dt (1) where dt/dt is the individual derivative of temperature. This temperature change experienced by the air parcel
Thermodynamic Energy Equation The temperature tendency is = u T x v T y w T z + dt dt (1) where dt/dt is the individual derivative of temperature. This temperature change experienced by the air parcel
The Tropical Atmosphere: Hurricane Incubator
 The Tropical Atmosphere: Hurricane Incubator Images from journals published by the American Meteorological Society are copyright AMS and used with permission. A One-Dimensional Description of the Tropical
The Tropical Atmosphere: Hurricane Incubator Images from journals published by the American Meteorological Society are copyright AMS and used with permission. A One-Dimensional Description of the Tropical
Radiative-Convective Models. The Hydrological Cycle Hadley Circulation. Manabe and Strickler (1964) Course Notes chapter 5.1
 Climate Modeling Lecture 8 Radiative-Convective Models Manabe and Strickler (1964) Course Notes chapter 5.1 The Hydrological Cycle Hadley Circulation Prepare for Mid-Term (Friday 9 am) Review Course Notes
Climate Modeling Lecture 8 Radiative-Convective Models Manabe and Strickler (1964) Course Notes chapter 5.1 The Hydrological Cycle Hadley Circulation Prepare for Mid-Term (Friday 9 am) Review Course Notes
References: Parcel Theory. Vertical Force Balance. ESCI Cloud Physics and Precipitation Processes Lesson 3 - Stability and Buoyancy Dr.
 References: ESCI 340 - Cloud Physics and Precipitation Processes Lesson 3 - Stability and Buoyancy Dr. DeCaria Glossary of Meteorology, 2nd ed., American Meteorological Society A Short Course in Cloud
References: ESCI 340 - Cloud Physics and Precipitation Processes Lesson 3 - Stability and Buoyancy Dr. DeCaria Glossary of Meteorology, 2nd ed., American Meteorological Society A Short Course in Cloud
ATMO 551a Moist Adiabat Fall Change in internal energy: ΔU
 Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Lecture 10: Climate Sensitivity and Feedback
 Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
1. Static Stability. (ρ V ) d2 z (1) d 2 z. = g (2) = g (3) T T = g T (4)
 NCAR (National Center for Atmospheric Research) has an excellent resource for education called COMET-MetEd. There you can find some really great tutorials on SkewT-LogP plots: visit http://www.meted.ucar.edu/mesoprim/skewt/index.htm.
NCAR (National Center for Atmospheric Research) has an excellent resource for education called COMET-MetEd. There you can find some really great tutorials on SkewT-LogP plots: visit http://www.meted.ucar.edu/mesoprim/skewt/index.htm.
Reversible vs. irreversible processes
 Reversible vs. irreversible processes A reversible process is one for which the final states of the universe (system and environment) are iden
Reversible vs. irreversible processes A reversible process is one for which the final states of the universe (system and environment) are iden
Quasi-equilibrium transitions
 Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
GEF2200 Atmosfærefysikk 2012
 GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
1. The vertical structure of the atmosphere. Temperature profile.
 Lecture 4. The structure of the atmosphere. Air in motion. Objectives: 1. The vertical structure of the atmosphere. Temperature profile. 2. Temperature in the lower atmosphere: dry adiabatic lapse rate.
Lecture 4. The structure of the atmosphere. Air in motion. Objectives: 1. The vertical structure of the atmosphere. Temperature profile. 2. Temperature in the lower atmosphere: dry adiabatic lapse rate.
ATMO/OPTI 656b Spring 08. Physical Properties of the Atmosphere
 Physical Properties of the Atmosphere Thin as a piece of paper The atmosphere is a very thin layer above the solid Earth and its oceans. This is true of the atmospheres of all of the terrestrial planets.
Physical Properties of the Atmosphere Thin as a piece of paper The atmosphere is a very thin layer above the solid Earth and its oceans. This is true of the atmospheres of all of the terrestrial planets.
Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle,
 Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle, like a mountain) or by buoyancy forces. Air that is
Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle, like a mountain) or by buoyancy forces. Air that is
Lecture 9: Climate Sensitivity and Feedback Mechanisms
 Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Chapter 5. Atmospheric Moisture
 Chapter 5 Atmospheric Moisture hydrologic cycle--movement of water in all forms between earth & atmosphere Humidity: amount of water vapor in air vapor pressure saturation vapor pressure absolute humidity
Chapter 5 Atmospheric Moisture hydrologic cycle--movement of water in all forms between earth & atmosphere Humidity: amount of water vapor in air vapor pressure saturation vapor pressure absolute humidity
First Law of Thermodyamics U = Q + W. We can rewrite this by introducing two physical. Enthalpy, H, is the quantity U + pv, as this
 First Law of Thermodyamics U = Q + W where U is the increase in internal energy of the system, Q is the heat supplied to the system and W is the work done on the system. We can rewrite this by introducing
First Law of Thermodyamics U = Q + W where U is the increase in internal energy of the system, Q is the heat supplied to the system and W is the work done on the system. We can rewrite this by introducing
Parcel Model. Atmospheric Sciences September 30, 2012
 Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Parcel Model. Meteorology September 3, 2008
 Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Introduction. Lecture 6: Water in Atmosphere. How Much Heat Is Brought Upward By Water Vapor?
 Lecture 6: Water in Atmosphere Introduction Over 70% of the planet is covered by water Water is unique in that it can simultaneously exist in all three states (solid, liquid, gas) at the same temperature
Lecture 6: Water in Atmosphere Introduction Over 70% of the planet is covered by water Water is unique in that it can simultaneously exist in all three states (solid, liquid, gas) at the same temperature
Naraine Persaud, Entry Code ME-11 1
 Naraine Persaud, Entry Code ME-11 1 Persaud, N. 2005. Adiabatic cooling. In: Water Encyclopedia Volume 4: Oceanography; Meteorology; Physics and Chemistry; Water Law; and Water History, Art, and Culture.
Naraine Persaud, Entry Code ME-11 1 Persaud, N. 2005. Adiabatic cooling. In: Water Encyclopedia Volume 4: Oceanography; Meteorology; Physics and Chemistry; Water Law; and Water History, Art, and Culture.
z g + F w (2.56) p(x, y, z, t) = p(z) + p (x, y, z, t) (2.120) ρ(x, y, z, t) = ρ(z) + ρ (x, y, z, t), (2.121)
 = + dw dt = 1 ρ p z g + F w (.56) Let us describe the total pressure p and density ρ as the sum of a horizontally homogeneous base state pressure and density, and a deviation from this base state, that
= + dw dt = 1 ρ p z g + F w (.56) Let us describe the total pressure p and density ρ as the sum of a horizontally homogeneous base state pressure and density, and a deviation from this base state, that
df dz = dp dt Essentially, this is just a statement of the first law in one of the forms we derived earlier (expressed here in W m 3 ) dq p dt dp
 A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
Buoyancy and Coriolis forces
 Chapter 2 Buoyancy and Coriolis forces In this chapter we address several topics that we need to understand before starting on our study of geophysical uid dynamics. 2.1 Hydrostatic approximation Consider
Chapter 2 Buoyancy and Coriolis forces In this chapter we address several topics that we need to understand before starting on our study of geophysical uid dynamics. 2.1 Hydrostatic approximation Consider
Lecture 3: Convection
 EESC V2100 The Climate System spring 2004 Lecture 3: Convection Yochanan Kushnir Lamont Doherty Earth Observatory of Columbia University Palisades, NY 10964, USA kushnir@ldeo.columbia.edu Layers of the
EESC V2100 The Climate System spring 2004 Lecture 3: Convection Yochanan Kushnir Lamont Doherty Earth Observatory of Columbia University Palisades, NY 10964, USA kushnir@ldeo.columbia.edu Layers of the
Classical Thermodynamics. Dr. Massimo Mella School of Chemistry Cardiff University
 Classical Thermodynamics Dr. Massimo Mella School of Chemistry Cardiff University E-mail:MellaM@cardiff.ac.uk The background The field of Thermodynamics emerged as a consequence of the necessity to understand
Classical Thermodynamics Dr. Massimo Mella School of Chemistry Cardiff University E-mail:MellaM@cardiff.ac.uk The background The field of Thermodynamics emerged as a consequence of the necessity to understand
Exam 1 (Chaps. 1-6 of the notes)
 10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
Isentropic Analysis. Much of this presentation is due to Jim Moore, SLU
 Isentropic Analysis Much of this presentation is due to Jim Moore, SLU Utility of Isentropic Analysis Diagnose and visualize vertical motion - through advection of pressure and system-relative flow Depict
Isentropic Analysis Much of this presentation is due to Jim Moore, SLU Utility of Isentropic Analysis Diagnose and visualize vertical motion - through advection of pressure and system-relative flow Depict
The perturbation pressure, p, can be represented as the sum of a hydrostatic pressure perturbation p h and a nonhydrostatic pressure perturbation p nh
 z = The perturbation pressure, p, can be represented as the sum of a hydrostatic pressure perturbation p h and a nonhydrostatic pressure perturbation p nh, that is, p = p h + p nh. (.1) The former arises
z = The perturbation pressure, p, can be represented as the sum of a hydrostatic pressure perturbation p h and a nonhydrostatic pressure perturbation p nh, that is, p = p h + p nh. (.1) The former arises
Atmospheric Dynamics: lecture 3
 Atmospheric Dynamics: lecture 3 Moist convection Dew point temperature/lapse rate/lcl Equivalent potential temperature Conditional and potential instability Thermodynamic diagram CAPE Introduction to Python
Atmospheric Dynamics: lecture 3 Moist convection Dew point temperature/lapse rate/lcl Equivalent potential temperature Conditional and potential instability Thermodynamic diagram CAPE Introduction to Python
ATMO551a Fall Vertical Structure of Earth s Atmosphere
 Vertical Structure of Earth s Atmosphere Thin as a piece of paper The atmosphere is a very thin layer above the solid Earth and its oceans. This is true of the atmospheres of all of the terrestrial planets.
Vertical Structure of Earth s Atmosphere Thin as a piece of paper The atmosphere is a very thin layer above the solid Earth and its oceans. This is true of the atmospheres of all of the terrestrial planets.
1. Heterogeneous Systems and Chemical Equilibrium
 1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
4. Atmospheric transport. Daniel J. Jacob, Atmospheric Chemistry, Harvard University, Spring 2017
 4. Atmospheric transport Daniel J. Jacob, Atmospheric Chemistry, Harvard University, Spring 2017 Forces in the atmosphere: Gravity g Pressure-gradient ap = ( 1/ ρ ) dp / dx for x-direction (also y, z directions)
4. Atmospheric transport Daniel J. Jacob, Atmospheric Chemistry, Harvard University, Spring 2017 Forces in the atmosphere: Gravity g Pressure-gradient ap = ( 1/ ρ ) dp / dx for x-direction (also y, z directions)
Water in the Atmosphere Understanding Weather and Climate
 Water in the Atmosphere Understanding Weather and Climate Climate 2 1 Cloud Development and Forms Understanding Weather and Climate Climate 2 2 Learning Objectives 1. The various atmospheric lifting mechanisms
Water in the Atmosphere Understanding Weather and Climate Climate 2 1 Cloud Development and Forms Understanding Weather and Climate Climate 2 2 Learning Objectives 1. The various atmospheric lifting mechanisms
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
Chapter 4. Atmospheric Temperature and Stability
 Chapter 4. Atmospheric Temperature and Stability 4.1 The temperature structure of the atmosphere Most people are familiar with the fact that the temperature of the atmosphere decreases with altitude. The
Chapter 4. Atmospheric Temperature and Stability 4.1 The temperature structure of the atmosphere Most people are familiar with the fact that the temperature of the atmosphere decreases with altitude. The
Boundary layer equilibrium [2005] over tropical oceans
![Boundary layer equilibrium [2005] over tropical oceans Boundary layer equilibrium [2005] over tropical oceans](/thumbs/96/128963638.jpg) Boundary layer equilibrium [2005] over tropical oceans Alan K. Betts [akbetts@aol.com] Based on: Betts, A.K., 1997: Trade Cumulus: Observations and Modeling. Chapter 4 (pp 99-126) in The Physics and Parameterization
Boundary layer equilibrium [2005] over tropical oceans Alan K. Betts [akbetts@aol.com] Based on: Betts, A.K., 1997: Trade Cumulus: Observations and Modeling. Chapter 4 (pp 99-126) in The Physics and Parameterization
Temperature profile of the Troposphere
 roposphere he troposphere is the lowest atmospheric layer reaching an altitude o about 20 km. Density, pressure and temperature decline with altitude. he troposphere is largely convective, which translates
roposphere he troposphere is the lowest atmospheric layer reaching an altitude o about 20 km. Density, pressure and temperature decline with altitude. he troposphere is largely convective, which translates
Crib Sheet David Randall Atmosphere, Clouds and Climate Princeton U Press Cooke
 Crib Sheet David Randall Atmosphere, Clouds and Climate Princeton U Press 2012. Cooke Units Radiation Budget Planetary Energy Balance Turbulence Feedbacks snow and Ice feedback water vapor feedback combining
Crib Sheet David Randall Atmosphere, Clouds and Climate Princeton U Press 2012. Cooke Units Radiation Budget Planetary Energy Balance Turbulence Feedbacks snow and Ice feedback water vapor feedback combining
2. Conservation laws and basic equations
 2. Conservation laws and basic equations Equatorial region is mapped well by cylindrical (Mercator) projection: eastward, northward, upward (local Cartesian) coordinates:,, velocity vector:,,,, material
2. Conservation laws and basic equations Equatorial region is mapped well by cylindrical (Mercator) projection: eastward, northward, upward (local Cartesian) coordinates:,, velocity vector:,,,, material
Convection and buoyancy oscillation
 Convection and buoyancy oscillation Recap: We analyzed the static stability of a vertical profile by the "parcel method"; For a given environmental profile (of T 0, p 0, θ 0, etc.), if the density of an
Convection and buoyancy oscillation Recap: We analyzed the static stability of a vertical profile by the "parcel method"; For a given environmental profile (of T 0, p 0, θ 0, etc.), if the density of an
AT 620 Notes. These notes were prepared by Prof. Steven A. Rutledge. (and adapted slightly for the Fall 2009 course, and again slightly for this year)
 AT 620 Notes These notes were prepared by Prof. Steven A. Rutledge (and adapted slightly for the Fall 2009 course, and again slightly for this year) You may access Prof. Cotton s notes, password cloud9
AT 620 Notes These notes were prepared by Prof. Steven A. Rutledge (and adapted slightly for the Fall 2009 course, and again slightly for this year) You may access Prof. Cotton s notes, password cloud9
1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents
 CHAPTER 6 HUMIDITY, SATURATION, AND STABILITY MULTIPLE CHOICE QUESTIONS 1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents 2., annual precipitation
CHAPTER 6 HUMIDITY, SATURATION, AND STABILITY MULTIPLE CHOICE QUESTIONS 1., annual precipitation is greater than annual evapotranspiration. a. On the ocean *b. On the continents 2., annual precipitation
Simplified Microphysics. condensation evaporation. evaporation
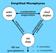 Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Radiative Transfer Chapter 3, Hartmann
 Radiative Transfer Chapter 3, Hartmann Shortwave Absorption: Clouds, H 2 0, O 3, some CO 2 Shortwave Reflection: Clouds, surface, atmosphere Longwave Absorption: Clouds, H 2 0, CO 2, CH 4, N 2 O Planck
Radiative Transfer Chapter 3, Hartmann Shortwave Absorption: Clouds, H 2 0, O 3, some CO 2 Shortwave Reflection: Clouds, surface, atmosphere Longwave Absorption: Clouds, H 2 0, CO 2, CH 4, N 2 O Planck
Atmospheric Thermodynamics
 Atmospheric Thermodynamics Atmospheric Composition What is the composition of the Earth s atmosphere? Gaseous Constituents of the Earth s atmosphere (dry air) Constituent Molecular Weight Fractional Concentration
Atmospheric Thermodynamics Atmospheric Composition What is the composition of the Earth s atmosphere? Gaseous Constituents of the Earth s atmosphere (dry air) Constituent Molecular Weight Fractional Concentration
Hurricanes are intense vortical (rotational) storms that develop over the tropical oceans in regions of very warm surface water.
 Hurricanes: Observations and Dynamics Houze Section 10.1. Holton Section 9.7. Emanuel, K. A., 1988: Toward a general theory of hurricanes. American Scientist, 76, 371-379 (web link). http://ww2010.atmos.uiuc.edu/(gh)/guides/mtr/hurr/home.rxml
Hurricanes: Observations and Dynamics Houze Section 10.1. Holton Section 9.7. Emanuel, K. A., 1988: Toward a general theory of hurricanes. American Scientist, 76, 371-379 (web link). http://ww2010.atmos.uiuc.edu/(gh)/guides/mtr/hurr/home.rxml
Kelvin Effect. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Atmospheric Composition הרכב האטמוספירה
 Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
2.1 Effects of a cumulus ensemble upon the large scale temperature and moisture fields by induced subsidence and detrainment
 Atmospheric Sciences 6150 Cloud System Modeling 2.1 Effects of a cumulus ensemble upon the large scale temperature and moisture fields by induced subsidence and detrainment Arakawa (1969, 1972), W. Gray
Atmospheric Sciences 6150 Cloud System Modeling 2.1 Effects of a cumulus ensemble upon the large scale temperature and moisture fields by induced subsidence and detrainment Arakawa (1969, 1972), W. Gray
Quasi-equilibrium Theory of Small Perturbations to Radiative- Convective Equilibrium States
 Quasi-equilibrium Theory of Small Perturbations to Radiative- Convective Equilibrium States See CalTech 2005 paper on course web site Free troposphere assumed to have moist adiabatic lapse rate (s* does
Quasi-equilibrium Theory of Small Perturbations to Radiative- Convective Equilibrium States See CalTech 2005 paper on course web site Free troposphere assumed to have moist adiabatic lapse rate (s* does
Chapter 6 Clouds. Cloud Development
 Chapter 6 Clouds Chapter overview Processes causing saturation o Cooling, moisturizing, mixing Cloud identification and classification Cloud Observations Fog Why do we care about clouds in the atmosphere?
Chapter 6 Clouds Chapter overview Processes causing saturation o Cooling, moisturizing, mixing Cloud identification and classification Cloud Observations Fog Why do we care about clouds in the atmosphere?
p = ρrt p = ρr d = T( q v ) dp dz = ρg
 Chapter 1: Properties of the Atmosphere What are the major chemical components of the atmosphere? Atmospheric Layers and their major characteristics: Troposphere, Stratosphere Mesosphere, Thermosphere
Chapter 1: Properties of the Atmosphere What are the major chemical components of the atmosphere? Atmospheric Layers and their major characteristics: Troposphere, Stratosphere Mesosphere, Thermosphere
Final Examination. Part A Answer ONLY TWELVE QUESTIONS in Part A. (Each question is 3 points)
 ATS 210 Spring Term 2001 NAME: Final Examination This is a 2 hour, closed-book examination. Calculators may be used. All answers should be written on the examination paper. Use the final sheet for any
ATS 210 Spring Term 2001 NAME: Final Examination This is a 2 hour, closed-book examination. Calculators may be used. All answers should be written on the examination paper. Use the final sheet for any
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
where p oo is a reference level constant pressure (often 10 5 Pa). Since θ is conserved for adiabatic motions, a prognostic temperature equation is:
 1 Appendix C Useful Equations Purposes: Provide foundation equations and sketch some derivations. These equations are used as starting places for discussions in various parts of the book. C.1. Thermodynamic
1 Appendix C Useful Equations Purposes: Provide foundation equations and sketch some derivations. These equations are used as starting places for discussions in various parts of the book. C.1. Thermodynamic
2 Equations of Motion
 2 Equations of Motion system. In this section, we will derive the six full equations of motion in a non-rotating, Cartesian coordinate 2.1 Six equations of motion (non-rotating, Cartesian coordinates)
2 Equations of Motion system. In this section, we will derive the six full equations of motion in a non-rotating, Cartesian coordinate 2.1 Six equations of motion (non-rotating, Cartesian coordinates)
Chapter 5 - Atmospheric Moisture
 Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Temperature. Vertical Thermal Structure. Earth s Climate System. Lecture 1: Introduction to the Climate System
 Lecture 1: Introduction to the Climate System T mass (& radiation) T & mass relation in vertical mass (& energy, weather..) Energy T vertical stability vertical motion thunderstorm What are included in
Lecture 1: Introduction to the Climate System T mass (& radiation) T & mass relation in vertical mass (& energy, weather..) Energy T vertical stability vertical motion thunderstorm What are included in
Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties
 Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties For Further Reading Most information contained within these lecture notes is drawn from Chapters 1, 2, 4, and 6 of The Use of the Skew
Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties For Further Reading Most information contained within these lecture notes is drawn from Chapters 1, 2, 4, and 6 of The Use of the Skew
Model description of AGCM5 of GFD-Dennou-Club edition. SWAMP project, GFD-Dennou-Club
 Model description of AGCM5 of GFD-Dennou-Club edition SWAMP project, GFD-Dennou-Club Mar 01, 2006 AGCM5 of the GFD-DENNOU CLUB edition is a three-dimensional primitive system on a sphere (Swamp Project,
Model description of AGCM5 of GFD-Dennou-Club edition SWAMP project, GFD-Dennou-Club Mar 01, 2006 AGCM5 of the GFD-DENNOU CLUB edition is a three-dimensional primitive system on a sphere (Swamp Project,
A new theory for moist convection in statistical equilibrium
 A new theory for moist convection in statistical equilibrium A. Parodi(1), K. Emanuel(2) (2) CIMA Research Foundation,Savona, Italy (3) EAPS, MIT, Boston, USA True dynamics: turbulent, moist, non-boussinesq,
A new theory for moist convection in statistical equilibrium A. Parodi(1), K. Emanuel(2) (2) CIMA Research Foundation,Savona, Italy (3) EAPS, MIT, Boston, USA True dynamics: turbulent, moist, non-boussinesq,
NENG 301 Week 8 Unary Heterogeneous Systems (DeHoff, Chap. 7, Chap )
 NENG 301 Week 8 Unary Heterogeneous Systems (DeHoff, Chap. 7, Chap. 5.3-5.4) Learning objectives for Chapter 7 At the end of this chapter you will be able to: Understand the general features of a unary
NENG 301 Week 8 Unary Heterogeneous Systems (DeHoff, Chap. 7, Chap. 5.3-5.4) Learning objectives for Chapter 7 At the end of this chapter you will be able to: Understand the general features of a unary
EAS270, The Atmosphere Mid-term Exam 28 Oct. 2011
 EAS270, The Atmosphere Mid-term Exam 28 Oct. 2011 Professor: J.D. Wilson Time available: 50 mins Value: 20% Instructions: For each of the 30 multi-choice questions, choose the most logical option. Use
EAS270, The Atmosphere Mid-term Exam 28 Oct. 2011 Professor: J.D. Wilson Time available: 50 mins Value: 20% Instructions: For each of the 30 multi-choice questions, choose the most logical option. Use
1 Introduction to Governing Equations 2 1a Methodology... 2
 Contents 1 Introduction to Governing Equations 2 1a Methodology............................ 2 2 Equation of State 2 2a Mean and Turbulent Parts...................... 3 2b Reynolds Averaging.........................
Contents 1 Introduction to Governing Equations 2 1a Methodology............................ 2 2 Equation of State 2 2a Mean and Turbulent Parts...................... 3 2b Reynolds Averaging.........................
Outline Review Example Problem 1. Thermodynamics. Review and Example Problems: Part-2. X Bai. SDSMT, Physics. Fall 2014
 Review and Example Problems: Part- SDSMT, Physics Fall 014 1 Review Example Problem 1 Exponents of phase transformation : contents 1 Basic Concepts: Temperature, Work, Energy, Thermal systems, Ideal Gas,
Review and Example Problems: Part- SDSMT, Physics Fall 014 1 Review Example Problem 1 Exponents of phase transformation : contents 1 Basic Concepts: Temperature, Work, Energy, Thermal systems, Ideal Gas,
What did we learn in Ch. 1? Final Exam 2014: 6 Questions. Energy Transfers Link Ch Reversible-Adiabatic-Work. What did we learn in Ch. 3?
 Final Exam 0: 6 Questions. First/Second Laws Question. Definitions Question 3. Clausius-Clapeyron with q/h/e) Question. Köhler, Clouds, and Stability Question 5. Climate Model and Sensitivity Question
Final Exam 0: 6 Questions. First/Second Laws Question. Definitions Question 3. Clausius-Clapeyron with q/h/e) Question. Köhler, Clouds, and Stability Question 5. Climate Model and Sensitivity Question
Brief Overview of the Global Atmosphere
 Brief Overview of the Global Atmosphere Images on pages 3, 5 through 9, 19, 77 through 80 and 90 are copyrighted by Academic Press, NY, 1999. The images are in a chapter entitled "Quasi-equilibrium thinking"
Brief Overview of the Global Atmosphere Images on pages 3, 5 through 9, 19, 77 through 80 and 90 are copyrighted by Academic Press, NY, 1999. The images are in a chapter entitled "Quasi-equilibrium thinking"
Atmospheric Sciences 321. Science of Climate. Lecture 13: Surface Energy Balance Chapter 4
 Atmospheric Sciences 321 Science of Climate Lecture 13: Surface Energy Balance Chapter 4 Community Business Check the assignments HW #4 due Wednesday Quiz #2 Wednesday Mid Term is Wednesday May 6 Practice
Atmospheric Sciences 321 Science of Climate Lecture 13: Surface Energy Balance Chapter 4 Community Business Check the assignments HW #4 due Wednesday Quiz #2 Wednesday Mid Term is Wednesday May 6 Practice
Clouds and atmospheric convection
 Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Orographic Precipitation II: Effects of Phase Change on Orographic Flow. Richard Rotunno. National Center for Atmospheric Research, USA
 Orographic Precipitation II: Effects of Phase Change on Orographic Flow Richard Rotunno National Center for Atmospheric Research, USA Condensation D Dt vs w( x, ) vs U Large-Scale Flow 0 H L Dynamics w
Orographic Precipitation II: Effects of Phase Change on Orographic Flow Richard Rotunno National Center for Atmospheric Research, USA Condensation D Dt vs w( x, ) vs U Large-Scale Flow 0 H L Dynamics w
MAHALAKSHMI ENGINEERING COLLEGE
 MAHALAKSHMI ENGINEERING COLLEGE TIRUCHIRAPALLI 621 213. Department: Mechanical Subject Code: ME2202 Semester: III Subject Name: ENGG. THERMODYNAMICS UNIT-I Basic Concept and First Law 1. What do you understand
MAHALAKSHMI ENGINEERING COLLEGE TIRUCHIRAPALLI 621 213. Department: Mechanical Subject Code: ME2202 Semester: III Subject Name: ENGG. THERMODYNAMICS UNIT-I Basic Concept and First Law 1. What do you understand
Governing Equations and Scaling in the Tropics
 Governing Equations and Scaling in the Tropics M 1 ( ) e R ε er Tropical v Midlatitude Meteorology Why is the general circulation and synoptic weather systems in the tropics different to the those in the
Governing Equations and Scaling in the Tropics M 1 ( ) e R ε er Tropical v Midlatitude Meteorology Why is the general circulation and synoptic weather systems in the tropics different to the those in the
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1
 Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
Answers to Clicker Questions
 Answers to Clicker Questions Chapter 1 What component of the atmosphere is most important to weather? A. Nitrogen B. Oxygen C. Carbon dioxide D. Ozone E. Water What location would have the lowest surface
Answers to Clicker Questions Chapter 1 What component of the atmosphere is most important to weather? A. Nitrogen B. Oxygen C. Carbon dioxide D. Ozone E. Water What location would have the lowest surface
A B C D PROBLEMS Dilution of power plant plumes. z z z z
 69 PROBLEMS 4. Dilution of power plant plumes Match each power plant plume (-4) to the corresponding atmospheric lapse rate (A-D, solid lines; the dashed line is the adiabatic lapse rate Γ). Briefly comment
69 PROBLEMS 4. Dilution of power plant plumes Match each power plant plume (-4) to the corresponding atmospheric lapse rate (A-D, solid lines; the dashed line is the adiabatic lapse rate Γ). Briefly comment
A History of Modifications to SUBROUTINE CONVECT by Kerry Emanuel
 A History of Modifications to SUBROUTINE CONVECT by Kerry Emanuel The following represents modifications to the convection scheme as it was originally described in Emanuel, K.A., 1991, J. Atmos. Sci.,
A History of Modifications to SUBROUTINE CONVECT by Kerry Emanuel The following represents modifications to the convection scheme as it was originally described in Emanuel, K.A., 1991, J. Atmos. Sci.,
( ) = 1005 J kg 1 K 1 ;
 Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
Global Energy Balance: Greenhouse Effect
 Global Energy Balance: Greenhouse Effect Atmospheric Composition & Structure Physical Causes of Greenhouse Effects Chapter 3: 44 48. Atmospheric Composition Why does water vapor vary so much? Saturation
Global Energy Balance: Greenhouse Effect Atmospheric Composition & Structure Physical Causes of Greenhouse Effects Chapter 3: 44 48. Atmospheric Composition Why does water vapor vary so much? Saturation
ATMO 551a Intro to Optical Depth Fall τ υ,z. dz = di υ. B[ v,t(z) ]e
![ATMO 551a Intro to Optical Depth Fall τ υ,z. dz = di υ. B[ v,t(z) ]e ATMO 551a Intro to Optical Depth Fall τ υ,z. dz = di υ. B[ v,t(z) ]e](/thumbs/89/97485998.jpg) Atmospheric Radiative Transfer We need to understand how energy is transferred via radiation within the atmosphere. We introduce the concept of optical depth. We will further show that the light moves
Atmospheric Radiative Transfer We need to understand how energy is transferred via radiation within the atmosphere. We introduce the concept of optical depth. We will further show that the light moves
ScienceDirect. Numerical modeling of atmospheric water content and probability evaluation. Part I
 Available online at www.sciencedirect.com ScienceDirect Procedia Engineering 70 ( 2014 ) 321 329 12th International Conference on Computing and Control for the Water Industry, CCWI2013 Numerical modeling
Available online at www.sciencedirect.com ScienceDirect Procedia Engineering 70 ( 2014 ) 321 329 12th International Conference on Computing and Control for the Water Industry, CCWI2013 Numerical modeling
(Assignment continues on next page) 1
 MEA712: Mesoscale Modeling Class mesoscale model (CMM) project, assignment 1 Due at start of class on Thursday, 5 October. In class supervised work period on Tuesday, 3 October. We will be following a
MEA712: Mesoscale Modeling Class mesoscale model (CMM) project, assignment 1 Due at start of class on Thursday, 5 October. In class supervised work period on Tuesday, 3 October. We will be following a
Moist Convection. Chapter 6
 Moist Convection Chapter 6 1 2 Trade Cumuli Afternoon cumulus over land 3 Cumuls congestus Convectively-driven weather systems Deep convection plays an important role in the dynamics of tropical weather
Moist Convection Chapter 6 1 2 Trade Cumuli Afternoon cumulus over land 3 Cumuls congestus Convectively-driven weather systems Deep convection plays an important role in the dynamics of tropical weather
The atmosphere s water
 The atmosphere s water Atmospheric Moisture and Precipitation Properties of Water The Hydrosphere and the Hydrologic Cycle Humidity The Adiabatic Process Clouds Precipitation Air Quality Main points for
The atmosphere s water Atmospheric Moisture and Precipitation Properties of Water The Hydrosphere and the Hydrologic Cycle Humidity The Adiabatic Process Clouds Precipitation Air Quality Main points for
WaVaCS summerschool Autumn 2009 Cargese, Corsica
 Introduction Part I WaVaCS summerschool Autumn 2009 Cargese, Corsica Holger Tost Max Planck Institute for Chemistry, Mainz, Germany Introduction Overview What is a parameterisation and why using it? Fundamentals
Introduction Part I WaVaCS summerschool Autumn 2009 Cargese, Corsica Holger Tost Max Planck Institute for Chemistry, Mainz, Germany Introduction Overview What is a parameterisation and why using it? Fundamentals
