Weaver, P.P.E., Schmincke, H.-U., Firth, J.V., and Duffield, W. (Eds.), 1998 Proceedings of the Ocean Drilling Program, Scientific Results, Vol.
|
|
|
- Elwin Scott
- 5 years ago
- Views:
Transcription
1 Weaver, P.P.E., Schmincke, H.-U., Firth, J.V., and Duffield, W. (Eds.), 1998 Proceedings of the Ocean Drilling Program, Scientific Results, Vol SULFUR, CHLORINE, AND FLUORINE IN GLASS INCLUSIONS IN OLIVINE AND CLINOPYROXENE FROM BASALTIC HYALOCLASTITES REPRESENTING THE GRAN CANARIA SHIELD STAGE AT SITES 953 AND Thor H. Hansteen 2 and Andrey A. Gurenko 2 ABSTRACT Sulfur, chlorine, and fluorine concentrations of primary silicate glass inclusions in olivine and clinopyroxene from basaltic hyaloclastites representing the Gran Canaria shield stage at Sites 953 and 956 were investigated using an electron microprobe. Inclusion compositions corrected for post-entrapment, host-mineral crystallization range from 46.3 to 54.4 wt% SiO 2, and from 5.7 to 1.3 wt% MgO. Significant spread in S and Cl values (2 239 ppm S, and ppm Cl) and the lack of correlation between S and Cl with MgO and P suggest partial degassing of the magmas prior to eruption. F was not degassed during magma evolution and thus can be used to discuss magma source compositions. The lack of correlation between SiO 2 and F in the calculated parental magmas suggests that pressure is not likely to have exerted the main control on volatile contents. A source with heterogeneous F contents is inferred. The occurrence of S 2, as the only detectable S species in the glass inclusions, suggests crystallization conditions with fo 2 levels at or below the Ni-NiO (NNO) buffer. INTRODUCTION One purpose of Leg 157 was to recover samples from the submarine and early shield stages of Gran Canaria and to tie these into the evolutionary history of the island. Because the seamount and shield stages of an oceanic island represent the highest magma production and eruption rates during its history (e.g., Schmincke et al., 1995), investigations of melt generation and evolution during these early phases are of essential importance for better understanding the origin of oceanic intraplate volcanism. During Leg 157, at Sites 953 through 956, core samples covering practically the complete time interval from the Quaternary to middle Miocene, were recovered from the volcaniclastic apron north and south of Gran Canaria (Schmincke, Weaver, Firth et al., 1995; Fig. 1). Samples recovered from Sites 953 and 956 include Miocene sequences corresponding to the shield stage of Gran Canaria. Hyaloclastites of Miocene age, recovered during Leg 157, are described in detail by Schmincke and Segschneider (Chap. 12, this volume). We have studied the same 13 samples of basaltic hyaloclastic tuffs and lapillistones from Sites 953 and 956 as Gurenko et al. (Chap. 22, this volume; Table 1). Sample descriptions, compositions of liquidus minerals and fluid inclusions, and the major and trace element compositions of glass inclusions are given in that paper. The samples are strongly to moderately altered, but contain optically fresh phenocrysts of clinopyroxene and, more rarely, optically fresh olivine cores; both minerals contain abundant primary glass, crystal, and fluid inclusions. This paper focuses on the halogen and sulfur concentrations of primary glass inclusions in these minerals. The abundance and evolution of such volatile and incompatible elements are used to make inferrences about the mantle source during the shield stage of Gran Canaria, and to describe the degassing behavior at depth in the lithosphere and during eruption. ANALYTICAL METHODS Electron Microprobe Analysis Fluorine, chlorine, sulfur, and phosphorous were analyzed as trace elements with a Cameca SX-5 electron microprobe at Research Center for Marine Geosciences, Federal Republic of Germany (GEOMAR), using an acceleration potential of 15 kv and a beam current of 2 25 na. The electron beam was rastered between 3 µm 4 µm and 1 µm 12 µm, and peak counting times were 6 s. The standards used were chalcopyrite for S, scapolite USNM R66-1 for 16 W 15 W 29 N 29 N TENERIFE 1 28 N GRAN 28 N CANARIA N DSDP 27 N FU. 16 W 15 W 1 Weaver, P.P.E., Schmincke, H.-U., Firth, J.V., and Duffield, W. (Eds.), Proc. ODP Sci. Results, 157: College Station, TX (Ocean Drilling Program). 2 GEOMAR Forschungszentrum,Wischhofstraße 1-3, D Kiel, Federal Republic of Germany. thansteen@geomar.de Figure 1. Schematic map showing locations of Site 953 through 956 in relation to the Canary Islands (after Funck, 1996). Fields are seismically defined volcaniclastic aprons of Fuerteventura, Gran Canaria, and Tenerife. 43
2 T.H. HANSTEEN, A.A. GURENKO Table 1. Volatile compositions of selected major element data of melt inclusions, and calculated volatile compositions of the trapped melts. Sample Phase SiO 2 MgO K 2 O Cl F S P Mg#, Fo Ol add Cpx add 82R-1, a gl mlt cpx ND ND ND ND ND R-1, gl mlt cpx ND ND ND ND ND R-7, a gl mlt cpx ND ND ND ND ND b gl mlt cpx ND ND ND ND ND.83 89R-1, gl mlt cpx ND ND ND ND ND.838 9R-1, gl ND mlt ND b gl ND mlt ND cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND R-6, gl mlt cpx ND ND ND ND ND gl mlt ol ND ND ND ND ND gl mlt b gl mlt ol ND ND ND ND ND gl mlt ol ND ND ND ND ND gl mlt ol ND ND ND ND ND gl mlt ol ND ND ND ND ND gl mlt ol ND ND ND ND ND gl mlt ol ND ND ND ND ND gl mlt b gl mlt ol ND ND ND ND ND gl mlt b gl mlt ol ND ND ND ND ND 8 43 gl mlt ol ND ND ND ND ND R-2, gl ND mlt ND cpx ND ND ND ND ND R-1, gl mlt cpx ND ND ND ND ND gl mlt
3 SULFUR, CHLORINE, AND FLUORINE IN GLASS INCLUSIONS Table 1 (continued). Sample Phase SiO 2 MgO K 2 O Cl F S P Mg#, Fo Ol add Cpx add cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND R-1, gl mlt cpx ND ND ND ND ND.84 16b gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND R-3, gl mlt cpx ND ND ND ND ND.785 1b gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND gl ND mlt ND cpx ND ND ND ND ND R-3, gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND b gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND R-CC, gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND gl mlt cpx ND ND ND ND ND.75 Notes: Sample = sample studied (core, section, interval [cm], and analyzed grain). Mg# = atomic ratio Mg/(Mg + Fe tot ) in glass inclusions and host clinopyroxenes. Fo = mol% forsterite in host olivine, gl = glass (i.e., glass inclusion), mlt = melt (i.e., calculated composition of trapped melt), Cpx = host clinopyroxene, Ol = host olivine, ND = not determined, and = not calculated. All values are given in weight percent. Cl, Durango fluorapatite USNM 1421 for F, and basaltic glass USNM /1 (VG-A99) for P, respectively (Jarosewich et al., 198). The monitor samples used were basaltic glass ALV981R23 for S (Metrich and Clocchiatti, 1989) and comenditic glass KN18 for F and Cl (Mosbah et al., 1991). Within each silicate glass inclusion, three to eight spots were measured. Test analyses for F, Cl, S, and P using lower beam currents (1-15 na) and shorter counting times (2 4 s) were performed on selected inclusions and compositionally similar glasses. When three or more spots per sample were measured, no systematic differences were found between results obtained using the various analytical conditions, except for significant variations in the counting statistics. 45
4 T.H. HANSTEEN, A.A. GURENKO The oxidation state of sulfur dissolved in the glass inclusions was measured through precise determinations of wavelengths for the S Kα peak relative to standards with known sulfur valence states: BaSO 4 (barite) was used for calibration of the S 6+ Kα peak, and FeS (pyrrhotite) for S 2 (e.g., Carroll and Rutherford, 1985; Wallace and Carmichael, 1992). Only reduced sulfur (S 2 ) was detected in the inclusions. Detailed results of these studies will be presented elsewhere (J. E. Gardner, unpubl. data). GLASS INCLUSIONS IN OLIVINE AND CLINOPYROXENE Occurrence of Glass Inclusions Olivine and clinopyroxene phenocrysts contain (1) inclusions of crystals (inclusions of olivine and plagioclase in clinopyroxene, inclusions of clinopyroxene in olivine, and inclusions of opaque minerals in both olivine and clinopyroxene); (2) glass inclusions (multiphase inclusions of partially crystallized glass, and naturally quenched glass inclusions); and (3) gas/liquid fluid inclusions. Following the classification of Roedder (1984), we subdivide glass, crystal, and fluid inclusions into primary, pseudosecondary, and secondary types. We studied only primary inclusions because they contain direct information on the conditions of magma evolution. Pseudosecondary and secondary inclusions were excluded from further considerations. The investigated primary glass inclusions range in size from 3 to 15 µm, and either contain glass ± gas bubble(s), or additionally contain daughter crystals. In one case, a droplet of metal sulfide was found as a primary inclusion in clinopyroxene. Within a group of presumably syngenetic inclusions, the individual inclusions may range from completely glassy to having a daughter mineral content of several tens of percent. We restricted our measurements to inclusions that are dominated by glass (>9%) compared to daughter crystals and do not contain spinel or titanomagnetite daughter crystals. Only inclusions containing optically fresh glass were analyzed. Further inclusion descriptions have been presented by Gurenko et al. (Chap. 22, this volume). Volatile Contents P F Cl All measurements were performed on naturally quenched glass inclusions in olivine and clinopyroxene. As would be expected for such small systems, variable amounts of host mineral crystallization have occurred on the outer margins of the glass inclusions because of imperfect natural quenching or pre-eruptive cooling within the magma plumbing system. This was detected through low and apparently variable Fe-Mg distribution coefficients between inclusions and host olivine or clinopyroxene (e.g., Roeder and Emslie, 197), suggesting crystallization of normally zoned mineral rims along the inclusion margins prior to final quenching (Gurenko et al., Chap. 22, this volume). Compositions of the entrapped melts were thus recalculated by adding wt% increments of the inferred momentary equilibrated host mineral composition to the glass inclusions until the Fe-Mg equilibrium was reached between the measured host mineral and the recalculated inclusion composition. This correction procedure is described in detail by Gurenko et al. (Chap. 22, this volume). Concentrations of S, F, Cl, P, and selected major elements for 47 glass inclusions are given in Table 1. (Full major element analyses are given in Gurenko et al., Chap. 22, this volume). Only small variations in S, F, Cl, and P contents were found for inclusions within single crystals. Compositions corrected for host-mineral crystallization are listed in Table 1 and presented in Figures 2, 3, and 4. F and Cl cover restricted ranges at 158 ± 52 ppm and 34 ± 17 ppm, respectively, throughout the entire range of major element compositions ( wt% SiO 2, and wt% MgO; Fig. 2). P and F show good negative correlations with MgO, suggesting that both el- S MgO Figure 2. MgO variation diagrams for the corrected compositions of glass inclusions in olivine and clinopyroxene in Table 1. All values are in weight percent. Legend: Open squares = Site 956, and solid diamonds = Site
5 SULFUR, CHLORINE, AND FLUORINE IN GLASS INCLUSIONS F Cl S Figure 3. Variation diagrams for the corrected compositions of glass inclusions in olivine and clinopyroxene in Table 1. Legend as in Figure 2. S Figure 4. Variation diagrams for the corrected compositions of glass inclusions in olivine and clinopyroxene in Table 1. Legend as in Figure 2. Cl P ements have behaved incompatibly and were enriched in the melt during crystal fractionation. S shows considerable scatter over the entire range of MgO, which can be ascribed to either deep-seated degassing or mantle source heterogeneity. DISCUSSION Degassing Upon Magma Evolution Concentrations of S, F, and Cl were plotted against the incompatible element P (Fig. 3). Some of the data scatter is probably caused by the relatively high analytical uncertainties at these low concentration levels. The fair correlation between F and P (and between F and K) suggests that F was retained in the melt during crystal fractionation at mantle depths, as was also shown for Pliocene sideromelane glasses at Sites 953, 954, and 956 by Gurenko and Schmincke (Chap. 25, this volume). On the other hand, Cl and S show almost no correlation with P, but positive correlation with each other (Figs. 3, 4). Two possible explanations could be suggested for this behavior. First, F was retained almost quantitatively in the liquid during magma ascent and fractional crystallization, and the less soluble elements such as S and Cl seem to have been weakly partitioned into the coexisting fluid phase upon early fluid oversaturation, probably at upper mantle depths. This is in partial agreement with experimental and glass inclusion data from basaltic rocks (e.g., Metrich and Clocchiatti, 1989). Because of the higher volatility of S relative to Cl, the covariation between S and Cl may be fortuitous. This is further underlined by the occurrence of sulfide as a primary inclusion in clinopyroxene, suggesting that sulfide saturation was reached upon liquidus evolution for at least some melt batches. Second, an alternative explanation is mantle source heterogeneity and possible differences in the degrees of its partial melting, which can only be traced using volatile elements if the melts were not degassed prior to entrapment in their current host minerals. Source characteristics are discussed below. The glass inclusions analyzed represent evolved compositions after 1% 35% crystallization from the parental melts (Table 2) within the Gran Canaria magma plumbing system and have been demonstrated to have evolved in crustal and uppermost mantle magma reservoirs (Gurenko et al., 1996, and Chap. 22, this volume). At these levels, degassing may be an important process in basaltic magmas. Because Cl and S are more strongly partitioned into a coexisting fluid phase than F, the occurrence of relatively low Cl and S concentrations in some of the glass inclusions is likely to reflect shallow lithospheric degassing for these particular melt portions. An important consequence is that the inclusions represent samples of different magma batches, which may have evolved at various depths within the plumbing system. These melts were mixed in reservoirs at shallow lithospheric levels before eruption. Compositional Variations in the Parental Magmas Compositions of parental magmas, which are believed to have been equilibriated with the mantle source, were calculated under the assumption that between partial melting and melt extraction in the source and entrapment of the glass inclusions in phenocrysts, fractional crystallization was the only process operating. This was shown not to be the case for S and Cl, so their concentrations are regarded as minimum values. Parental magma compositions were calculated by simulating the reverse path of fractional crystallization on the basis of major element compositions of glass inclusions, using a similar calculation routine as that applied for compositional correction of the post-entrapment crystallization in the glass inclusions (Gurenko et al., Chap. 22, this volume). The calculations were carried out in two steps. First, we modeled the reverse path of olivine-clinopyroxene cotectic crystallization until the composition of the calculated melt became equilibrated with the most magnesian clinopyroxene found 47
6 T.H. HANSTEEN, A.A. GURENKO Table 2. Calculated volatile contents of parental magmas. Sample SiO 2 MgO K 2 O Cl F S P 82R-1, R-1, R-7, -12 Min: Max: Mean: R-1, R-1, Min: Max: Mean: R-6, Min: Max: Mean: R-2, R-1, -12 Min: Max: Mean: R-1, 8-28 Min: Max: Mean: R-3, Min: Max: Mean: R-3, Min: Max: Mean: R-CC,7-17 Min: Max: Mean: Note: Min = minimum, and max = maximum. All values are in weight percent. in each particular sample. Second, we modeled reversed fractional crystallization of olivine only, using the melt compositions obtained during the first step. The calculation was ended as soon as the melt reached equilibrium with olivine Fo 9, the most magnesian olivine phenocrysts found in Miocene shield basalts of Gran Canaria (Gurenko et al., 1996). Compositions of the calculated parental magmas averaged for each sample are listed in Table 2. It should be noted that calculated parental melt compositions depend strongly on the model used, and on the clinopyroxene composition assumed. The calculated major element data therefore should be treated with care. However, this is not a problem for the incompatible volatile elements, for which the addition of clinopyroxene and olivine to the melts mainly amounts to a dilution. The average S concentration estimated for the Gran Canaria primitive magmas is ~5 ppm. This average probably represents a minimum value because of partial degassing within holding chambers. This is slightly lower than the S content of primitive mid-ocean-ridge basalt (MORB) magmas (8 1 ppm), which is consistent with their formation by 1% 2% partial melting of a mantle source containing 8 3 ppm of S (Chaussidon et al., 1989). The common presence of sulfides in peridotites of mantle origin implies that basaltic magma may form in equilibrium with small but variable amounts of sulfide (Lorand, 199). Average fractionation-corrected Cl contents are 49 ppm for normal-morb (N-MORB) and 153 ppm for enriched MORB (E-MORB; Jambon et al., 1995). Our average estimate is 23 ppm Cl in the primitive magmas, that is, similar but somewhat higher than those of E-MORB. This may point to similar S and halogen contents in the E-MORB source and the source of the Gran Canaria shield stage magmas. Aoki et al. (1981) noted that a correlation between K 2 O and F can be used to demonstrate the presence of phlogopite in the source. In Figure 5, no clear correlation exists between K 2 O and F, so phlogopite control in the source cannot be the only explanation for the variable F concentrations in the Gran Canaria shield magmas. However, Hoernle and Schmincke (1993a, 1993b) found trace element evidence for the presence of phlogopite in the mantle source. A likely scenario would be that the lithospheric mantle beneath Gran Canaria was impregnated with reaction products of deep-seated basaltic magmas prior to the shield-stage volcanism, similar to what has been inferred for several Canary Islands (on the basis of mantle xenolith studies; Neumann, 1991; Hansteen et al., 1991; Neumann et al., 1995). Partial melting of a mantle source metasomatized or enriched in basaltic components, which may contain variable amounts of phlogopite and sulfides ± amphibole, could possibly explain the scatter in F and probably, to some extent, S and Cl contents in the glass inclusions. A new generation of experimental studies on partial melting of peridotites indicates that the SiO 2 contents of the primary melts are good indicators of the pressure of melting, where low SiO 2 contents in partial melts imply higher pressures of magma generation (Hirose and Kushiro, 1993; Hirose and Kawamoto, 1995). SiO 2 does not correlate significantly with F in the calculated parental magmas (Fig. 6). Note that the calculated range in SiO 2 for the parental melts ( wt%) reflects a similar range of SiO 2 in the inclusions corrected only for post-entrapment crystallization ( wt%). When it is assumed that the source is peridotitic, it follows that there is probably little or no depth control on the parental F (and possibly also Cl and S) contents. This is in agreement with the model of Gurenko et al. (Chap. 22, this volume). The relative importance of the degrees of partial melting and source heterogeneity on the parental volatile contents cannot be resolved from the present data alone. Sulfur Speciation and Oxygen Fugacity Sulfur may occur in multiple valence states in basaltic liquids (Fincham and Richardson, 1954). Experimental data demonstrate that sulfur solubility in silicate liquids is dependent on temperature, pressure, bulk composition, and the fugacities of oxygen (fo 2 ) and sulfur (fs 2 ; e.g., Haughton et al., 1974; Luhr, 199). However, the dissolved S content in a basic magma does not change significantly as a function of pressure at lithospheric depths (Wallace and Carmichael, 1992). It has also been shown that at magmatic temperatures, S is present predominantly as sulfide at fo 2 more reduced than the fayalite-magnetite-quartz (FMQ) buffer (e.g., Nagashima and Katsura, 1973; Carrol and Rutherford, 1985). The S 6+ /S tot ratio further correlates positively with fo 2 for sulfur-undersaturated melts. For natural basaltic melts, >9% of the sulfur occurs as S 2 at fo 2 below the Ni- NiO (NNO) buffer (Wallace and Carmichael, 1994). For the Gran Canaria shield stage magmas, Gurenko et al (Chap. 22, this volume) have calculated fo 2 according to Kilinc et al. (1983), using the equilibrium Fe 2+ /Fe 3+ ratios in spinel and coexisting glass inclusions (based on experimental data on spinel-silicate melt equilibria of Maurel and Maurel, 1982), major element compositions of the melt, and estimated T of crystallization. Redox conditions of magma crystallization were calculated at below the FMQ buffer for the early stages of magma evolution, evolving to conditions of NNO ±1 during the late stages. If sulfide saturation is not reached in a basaltic system, the relative contents of S 6+ /S tot is expected to correlate with fo 2. A complication here is that an immiscible sulfide droplet has been found as a primary 48
7 SULFUR, CHLORINE, AND FLUORINE IN GLASS INCLUSIONS F evolved under different pressure conditions within the magma plumbing system. Residence of large melt volumes in crustal or upper mantle magma chambers resulted in partial S and Cl degassing of some of the magma batches before eruption. F contents in the inclusions have not been significantly affected by degassing, and thus reflect the composition of the mantle source. The occurrence of practically only reduced S species (S 2 ) in the glass inclusions suggests crystallization at fo 2 levels close to or below the NNO buffer. ACKNOWLEDGMENTS F S K 2 O Figure 5. Variation diagrams for the calculated compositions of parental melts in Table 2. Legend as in Figure SiO 2 Figure 6. Variation diagrams for the calculated compositions of parental melts in Table 2. Legend as in Figure 2. inclusion, meaning that sulfide saturation was probably reached for some magma batches during evolution at depth. Although only S 2 was found, the uncertainty of the measurements probably allows for ~1% of oxidized S species to be present in the glass. However, the occurrence of S 2, as the dominant sulfur species in the Gran Canaria shield phase inclusions, strongly supports an origin of the magmas at fo 2 values below the NNO buffer. This is in qualitative agreement with the results of Gurenko et al. (Chap. 22, this volume), which may thus be regarded as maximum values. CONCLUSIONS Silicate glass inclusions in clinopyroxene and olivine from basaltic hyaloclastic tuffs and lapillistones from the Gran Canaria shield stage (Sites 953 and 956) represent several batches of magma This work was supported by grants from the Deutsche Forschungsgemeinschaft HA 21/2-1 to THH, and an Alexander von Humboldt research fellowship to AAG. Thanks are owed to Hans-Ulrich Schmincke and James E. Gardner for fruitful discussions, and to J.F. Luhr and P.J. Michael for helpful reviews. REFERENCES Aoki, K., Ishikawa, K., and Kanisawa, S., Fluorine geochemistry of basaltic rocks from continental and oceanic regions and petrogenetic application. Contrib. Mineral. Petrol., 76: Carroll, M.R., and Rutherford, M.J., Sulfide and sulfate saturation in hydrous silicate melts. J. Geophys. Res., 9 (Suppl.):C61 C612. Chaussidon, M., Albarede, F., and Sheppard, S.M.F., Sulphur isotope variations in the mantle from ion microprobe analyses of micro-sulphide inclusions. Earth Planet. Sci. Lett., 92: Fincham, C.J.B., and Richardson, F.D., The behaviour of sulfur in silicate and aluminate melts. Proc. R. Soc. London A, 233:4 62. Funck, T., Structure of the volcanic apron north of Gran Canaria deduced from reflection seismic, bathymetric and borehole data [Ph.D. dissert.]. Univ. Kiel. Gurenko, A.A., Hansteen, T.H., and Schmincke H.-U., Evolution of parental magmas of Miocene shield basalts of Gran Canaria (Canary Islands): constraints from crystal, melt and fluid inclusions in minerals. Contrib. Mineral. Petrol., 124: Hansteen, T.H., Andersen, T., Neumann, E.-R., and Jelsma, H., Fluid and silicate glass inclusions in ultramafic and mafic xenoliths from Hierro, Canary Islands: Implications for mantle metasomatism. Contrib. Mineral. Petrol., 17: Haughton, D.R., Roeder, P.L., and Skinner, B.J., Solubility of sulfur in mafic magmas. Econ. Geol., 69: Hirose, K., Kawamoto, T., Hydrous partial melting of lherzolite at 1 GPa: the effect of H 2 O on the genesis of basaltic magmas. Earth Planet. Sci. Lett., 133: Hirose, K., and Kushiro, I., Partial melting of dry peridotites at high pressures: determination of compositions of melts segregated from peridotite using aggregates of diamond. Earth Planet. Sci. Lett., 114: Hoernle, K., and Schmincke, H.-U., 1993a. The petrology of the tholeiites through melilite nephelinites on Gran Canaria, Canary Islands: crystal fractionation, accumulation, and depth of melting. J. Petrol., 34: , 1993b. The role of partial melting in the 15-Ma geochemical evolution of Gran Canaria: a blob model for the Canary Hotspot. J. Petrol., 34: Jambon, A., Déruelle, B., Dreibus, G., and Pineau, F., Chlorine and bromine abundance in MORB: the contrasting behaviour of the Mid- Atlantic Ridge and East Pacific Rise and implications for chlorine geodynamic cycle. Chem. Geol., 126: Jarosewich, E., Nelen, J.A., and Norberg, J.A., 198. Reference samples for electron microprobe analysis. Geostand. Newsl., 4: Kilinc, A., Carmichael, I.S.E., Rivers, M.L., and Sack, R.O., The ferric ferrous ratio of natural silicate liquids equilibrated in air. Contrib. Mineral. Petrol., 83: Lorand, J.P., 199. Are spinel lherzolite xenoliths representative of the abundance of sulfur in the upper mantle? Geochim. Cosmochim. Acta, 54: Luhr, J.F., 199. Experimental phase relations of water- and sulfur-saturated arc magmas and the 1982 eruptions of El Chichón volcano. J. Petrol., 31:
8 T.H. HANSTEEN, A.A. GURENKO Maurel, C., and Maurel, P., Etude expérimentale de l équilibre Fe 2+ - Fe 3+ dans les spinelles chromifères et les liquides silicatés basiques coexistants, à 1 atm. C.R. Acad. Sci. Paris, 285: Metrich, N., and Clocchiatti, C., Melt inclusion investigation of the volatile behaviour in historic alkali basaltic magmas of Etna. Bull. Volcanol., 51: Mosbah, M., Metrich, N. and Massiot, P., PIGME fluorine determination using a nuclear microprobe with application to glass inclusions. Nucl. Instr. Meth. Phys. Res., B58: Nagashima, S., and Katsura, T., The solubility of sulfur in Na 2 O-SiO 2 melts under various oxygen partial pressures at 11, 125 and 13 C. Bull. Chem. Soc. Jpn., 46: Neumann, E.-R., Ultramafic and mafic xenoliths from Hierro, Canary Islands: evidence for melt infiltration in the upper mantle. Contrib. Mineral. Petrol., 16: Neumann, E.-R., Wulff-Pedersen, E., Johnsen, K., Andersen, T., and Krogh, E., Petrogenesis of spinel harzburgite and dunite suite xenoliths from Lanzarote, eastern Canary Islands: implications for the upper mantle. Lithos, 35: Roedder, E., Fluid inclusions. Rev. Mineral., Mineral. Soc. Am., 12. Roeder, P.L., and Emslie, R.F., 197. Olivine-liquid equilibrium. Contrib. Mineral. Petrol., 29: Schmincke, H.-U., Weaver, P.P.E., and Firth, J., The clastic apron of Gran Canaria and the Madeira Abyssal Plain. JOIDES J., 21: Schmincke, H.-U., Weaver, P.P.E., Firth, J.V., et al., Proc. ODP, Init. Repts., 157: College Station, TX (Ocean Drilling Program). Wallace, P., and Carmichael, I.S.E., Sulfur in basaltic magmas. Geochim. Cosmochim. Acta, 56: , S speciation in submarine basaltic glasses as determined by measurements of S Kα X-ray wavelength shift. Am. Mineral., 79: Date of initial receipt: 3 June 1996 Date of acceptance: 6 January 1997 Ms 157SR
Weaver, P.P.E., Schmincke, H.-U., Firth, J.V., and Duffield, W. (Eds.), 1998 Proceedings of the Ocean Drilling Program, Scientific Results, Vol.
 Weaver, P.P.E., Schmincke, H.-U., Firth, J.V., and Duffield, W. (Eds.), 998 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 57. MELT, CRYSTAL, AND FLUID INCLUSIONS IN OLIVINE AND CLINOPYROXENE
Weaver, P.P.E., Schmincke, H.-U., Firth, J.V., and Duffield, W. (Eds.), 998 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 57. MELT, CRYSTAL, AND FLUID INCLUSIONS IN OLIVINE AND CLINOPYROXENE
J. Mangas and F.J. Perez-Torrado. Departamento de Física. Universidad de Las Palmas de Gran Canaria Las Palmas de Gran Canaria.
 Magmatic processes in the oceanic lithosphere: characterization of the ultramafic and mafic materials from the Holocene volcanic centers of Bandama and La Caldera de Pinos de Gáldar (Gran Canaria, Canary
Magmatic processes in the oceanic lithosphere: characterization of the ultramafic and mafic materials from the Holocene volcanic centers of Bandama and La Caldera de Pinos de Gáldar (Gran Canaria, Canary
Worked Example of Batch Melting: Rb and Sr
 Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools -
 High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
Partial melting of mantle peridotite
 Partial melting of mantle peridotite 1100 1200 1300 1400 1500 (TºC) Depth (km) 50 100 150 Plag lherzolite (ol-opx-cpx-pl) Spinel lherzolite (Ol-opx-cpx-sp) Garnet lherzolite (Ol-opx-cpx-gar) Graphite Diamond
Partial melting of mantle peridotite 1100 1200 1300 1400 1500 (TºC) Depth (km) 50 100 150 Plag lherzolite (ol-opx-cpx-pl) Spinel lherzolite (Ol-opx-cpx-sp) Garnet lherzolite (Ol-opx-cpx-gar) Graphite Diamond
Effect of tectonic setting on chemistry of mantle-derived melts
 Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
High-T T heating stage: : application for igneous petrogenesis and mantle processes - melt inclusions as key tools -
 High-T T heating stage: : application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
High-T T heating stage: : application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
Lecture 24 Hawaii. Hawaii
 Lecture 24 Hawaii Friday, April 22 nd 2005 Hawaii The Hawaiian Islands, in the middle of the Pacific Ocean, are volcanic islands at the end of a long chain of submerged volcanoes. These volcanoes get progressively
Lecture 24 Hawaii Friday, April 22 nd 2005 Hawaii The Hawaiian Islands, in the middle of the Pacific Ocean, are volcanic islands at the end of a long chain of submerged volcanoes. These volcanoes get progressively
Origin of Basaltic Magma. Geology 346- Petrology
 Origin of Basaltic Magma Geology 346- Petrology 2 principal types of basalt in the ocean basins Tholeiitic Basalt and Alkaline Basalt Table 10-1 Common petrographic differences between tholeiitic and alkaline
Origin of Basaltic Magma Geology 346- Petrology 2 principal types of basalt in the ocean basins Tholeiitic Basalt and Alkaline Basalt Table 10-1 Common petrographic differences between tholeiitic and alkaline
Volatile solubility models and their application to magma storage and transport in the mantle and he crust. Julie Roberge ESIA-Ticoman, IPN Mexico
 Volatile solubility models and their application to magma storage and transport in the mantle and he crust Julie Roberge ESIA-Ticoman, IPN Mexico Melt Inclusions What are they? How to use them volatiles
Volatile solubility models and their application to magma storage and transport in the mantle and he crust Julie Roberge ESIA-Ticoman, IPN Mexico Melt Inclusions What are they? How to use them volatiles
Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints
 REFERENCES RELEVANT TO PERIDOTITE PARTIAL MELTING IN THE MANTLE WEDGE Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints at converging plate boundaries - I: Dehydration in
REFERENCES RELEVANT TO PERIDOTITE PARTIAL MELTING IN THE MANTLE WEDGE Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints at converging plate boundaries - I: Dehydration in
SEA-FLOOR SPREADING. In the 1950 s and early 1960 s detailed study of the oceans revealed the following surprising information:-
 SEA-FLOOR SPREADING In the 1950 s and early 1960 s detailed study of the oceans revealed the following surprising information:- Detailed bathymetric (depth) studies showed that there was an extensive submarine
SEA-FLOOR SPREADING In the 1950 s and early 1960 s detailed study of the oceans revealed the following surprising information:- Detailed bathymetric (depth) studies showed that there was an extensive submarine
Recent advances in the analysis of volatiles and fluid-mobile elements in melt inclusions by Secondary Ion Mass Spectrometry (SIMS)
 Edinburgh Research Explorer Recent advances in the analysis of volatiles and fluid-mobile elements in melt inclusions by Secondary Ion Mass Spectrometry (SIMS) Citation for published version: De Hoog,
Edinburgh Research Explorer Recent advances in the analysis of volatiles and fluid-mobile elements in melt inclusions by Secondary Ion Mass Spectrometry (SIMS) Citation for published version: De Hoog,
13. PETROLOGY OF BASALTS FROM DEEP SEA DRILLING PROJECT, LEG 38
 . PETROLOGY OF BASALTS FROM DEEP SEA DRILLING PROJECT, LEG W.I. Ridley, M.R. Perfit, and ML. Adams, LamontDoherty Geological Observatory, Columbia University, Palisades, New York INTRODUCTION We have determined
. PETROLOGY OF BASALTS FROM DEEP SEA DRILLING PROJECT, LEG W.I. Ridley, M.R. Perfit, and ML. Adams, LamontDoherty Geological Observatory, Columbia University, Palisades, New York INTRODUCTION We have determined
GEOL 2312 Igneous and Metamorphic Petrology Spring 2009 Sc ore / 40
 GEOL 2312 Igneous and Metamorphic Petrology Name Spring 2009 Sc ore / 40 QUIZ 3 1) Name two geologic features that provide physical evidence for the mineralogy of the earth s mantle (2 pts) Ophiolites,
GEOL 2312 Igneous and Metamorphic Petrology Name Spring 2009 Sc ore / 40 QUIZ 3 1) Name two geologic features that provide physical evidence for the mineralogy of the earth s mantle (2 pts) Ophiolites,
Metal saturation in the upper mantle
 Vol 449 27 September 2007 Metal saturation in the upper mantle A. Rohrbach, C. Ballhaus, U. Golla Schindler, P. Ulmer,V.S. Kamenetsky, D.V. Kuzmin NS seminar 2007.10.25 The uppermost mantle is oxidized.
Vol 449 27 September 2007 Metal saturation in the upper mantle A. Rohrbach, C. Ballhaus, U. Golla Schindler, P. Ulmer,V.S. Kamenetsky, D.V. Kuzmin NS seminar 2007.10.25 The uppermost mantle is oxidized.
Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated:
 Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated: 7-22-05 Cpx-Plag-Ol Thermobar is an Excel workbook that can be used to calculate crystallization pressures and temperatures for clinopyroxene-
Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated: 7-22-05 Cpx-Plag-Ol Thermobar is an Excel workbook that can be used to calculate crystallization pressures and temperatures for clinopyroxene-
Lecture 38. Igneous geochemistry. Read White Chapter 7 if you haven t already
 Lecture 38 Igneous geochemistry Read White Chapter 7 if you haven t already Today. Magma mixing/afc 2. Spot light on using the Rare Earth Elements (REE) to constrain mantle sources and conditions of petrogenesis
Lecture 38 Igneous geochemistry Read White Chapter 7 if you haven t already Today. Magma mixing/afc 2. Spot light on using the Rare Earth Elements (REE) to constrain mantle sources and conditions of petrogenesis
CO 2 quantification in magmatic systems
 CO 2 quantification in magmatic systems Innovative coupling of X-ray micro-tomography, in-situ microanalysis and thermodynamic modelling By Laura Créon Créon et al. (2018) Problematics 7 years work to
CO 2 quantification in magmatic systems Innovative coupling of X-ray micro-tomography, in-situ microanalysis and thermodynamic modelling By Laura Créon Créon et al. (2018) Problematics 7 years work to
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY
 WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
12 Chemistry (Mg,Fe) 2 SiO 4 Olivine is forms what is called an isomorphous solid solution series that ranges between two end members: Forsterite Mg
 11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
Trace Elements. Today s lecture
 Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
Nitrogen speciation in upper mantle fluids and the origin of Earth s nitrogen-rich atmosphere
 Supporting Online Material for Nitrogen speciation in upper mantle fluids and the origin of Earth s nitrogen-rich atmosphere Sami Mikhail & Dimitri Sverjensky S1. Supplementary discussion S1.1 The selection
Supporting Online Material for Nitrogen speciation in upper mantle fluids and the origin of Earth s nitrogen-rich atmosphere Sami Mikhail & Dimitri Sverjensky S1. Supplementary discussion S1.1 The selection
Continental Alkaline Magmatism. The East African Rift
 Announcements No lecture on Friday Lab final begins at 1 PM Today s agenda: Lecture/Demo Go through field trip pics Call for pizza Lab review Lecture Review Make poster Continental Alkaline Magmatism.
Announcements No lecture on Friday Lab final begins at 1 PM Today s agenda: Lecture/Demo Go through field trip pics Call for pizza Lab review Lecture Review Make poster Continental Alkaline Magmatism.
Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m
 Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m (b). Dashed lines represent the two successive ridge
Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m (b). Dashed lines represent the two successive ridge
Igneous Rocks. Igneous Rocks. Genetic Classification of
 Igneous Rocks Fig. 5.1 Genetic Classification of Igneous Rocks Intrusive: crystallized from slowly cooling magma intruded within the Earth s crust; e.g. granite, gabbro 1 Fig. 5.2 Genetic Classification
Igneous Rocks Fig. 5.1 Genetic Classification of Igneous Rocks Intrusive: crystallized from slowly cooling magma intruded within the Earth s crust; e.g. granite, gabbro 1 Fig. 5.2 Genetic Classification
Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere. 07/11/2017 GEO-DEEP 9300 Claire Aupart
 Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere 07/11/2017 GEO-DEEP 9300 Claire Aupart Introduction Introduction Getting samples Cores: Maximum depth reach in
Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere 07/11/2017 GEO-DEEP 9300 Claire Aupart Introduction Introduction Getting samples Cores: Maximum depth reach in
Tectonic-Igneous Associations
 Tectonic-Igneous Associations Associations on a larger scale than the petrogenetic provinces An attempt to address global patterns of igneous activity by grouping provinces based upon similarities in occurrence
Tectonic-Igneous Associations Associations on a larger scale than the petrogenetic provinces An attempt to address global patterns of igneous activity by grouping provinces based upon similarities in occurrence
GEOL 3313 Petrology of Igneous and Metamorphic Rocks Study Guide for Final Examination Glen Mattioli
 GEOL 3313 Petrology of Igneous and Metamorphic Rocks Study Guide for Final Examination Glen Mattioli Chapter 5: Crystal-Melt phase diagrams Effect of water pressure on feldspar stability Hypersolvus vs.
GEOL 3313 Petrology of Igneous and Metamorphic Rocks Study Guide for Final Examination Glen Mattioli Chapter 5: Crystal-Melt phase diagrams Effect of water pressure on feldspar stability Hypersolvus vs.
Lecture 6 - Igneous Rocks and Volcanoes
 Lecture 6 - Igneous Rocks and Volcanoes Learning objectives Understand and be able to predict where and why magma will be forming at different tectonic settings Understand the factors controlling magma
Lecture 6 - Igneous Rocks and Volcanoes Learning objectives Understand and be able to predict where and why magma will be forming at different tectonic settings Understand the factors controlling magma
Abstract. 1. Introduction
 Abstract 1. Introduction 2. Geological position and host volcanics 3. Summary of primary mineralogy of Tirich and other peridotites of Dzhilinda River Figure 1. Composition of clinopyroxenes from the Dzhilinda
Abstract 1. Introduction 2. Geological position and host volcanics 3. Summary of primary mineralogy of Tirich and other peridotites of Dzhilinda River Figure 1. Composition of clinopyroxenes from the Dzhilinda
Overview of the KAHT system. Ian E.M. Smith, School of Environment, University of Auckland
 Overview of the KAHT system Ian E.M. Smith, School of Environment, University of Auckland Tonga-Kermadec-New Zealand Arc Developed on the Pacific - Australian convergent margin Mainly intraoceanic except
Overview of the KAHT system Ian E.M. Smith, School of Environment, University of Auckland Tonga-Kermadec-New Zealand Arc Developed on the Pacific - Australian convergent margin Mainly intraoceanic except
Fluids, melts, and supercriticality in the MSH system and element transport in subduction zones
 cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
Lecture 36. Igneous geochemistry
 Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Supplementary Information
 Supplementary Information Supplementary Tables Major element compositions of host and daughter crystals: Table 1 reports composition for host-olivine. No daughter olivine has been found. The number for
Supplementary Information Supplementary Tables Major element compositions of host and daughter crystals: Table 1 reports composition for host-olivine. No daughter olivine has been found. The number for
Oxygen isotope heterogeneity of the mantle beneath the Canary Islands: insights
 1 2 3 4 5 Oxygen isotope heterogeneity of the mantle beneath the Canary Islands: insights from olivine phenocrysts 6 7 Andrey A. Gurenko a *, Ilya N. Bindeman b, Marc Chaussidon a 8 9 10 11 12 a Centre
1 2 3 4 5 Oxygen isotope heterogeneity of the mantle beneath the Canary Islands: insights from olivine phenocrysts 6 7 Andrey A. Gurenko a *, Ilya N. Bindeman b, Marc Chaussidon a 8 9 10 11 12 a Centre
Imagine the first rock and the cycles that it has been through.
 A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one type of rocky material gets transformed into another The Rock Cycle Representation
A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one type of rocky material gets transformed into another The Rock Cycle Representation
34. PETROLOGY OF BASALTS FROM SITE 487, DEEP SEA DRILLING PROJECT LEG 66, MIDDLE AMERICA TRENCH AREA OFF MEXICO 1
 34. PETRLGY F BASALTS FRM SITE 487, DEEP SEA DRILLING PRJECT LEG 66, MIDDLE AMERICA TRENCH AREA FF MEXIC 1 Shoji Arai, Institute of Geosciences, Faculty of Science, Shizuoka University, Shizuoka, 422 Japan
34. PETRLGY F BASALTS FRM SITE 487, DEEP SEA DRILLING PRJECT LEG 66, MIDDLE AMERICA TRENCH AREA FF MEXIC 1 Shoji Arai, Institute of Geosciences, Faculty of Science, Shizuoka University, Shizuoka, 422 Japan
The Nature of Igneous Rocks
 The Nature of Igneous Rocks Form from Magma Hot, partially molten mixture of solid liquid and gas Mineral crystals form in the magma making a crystal slush Gases - H 2 O, CO 2, etc. - are dissolved in
The Nature of Igneous Rocks Form from Magma Hot, partially molten mixture of solid liquid and gas Mineral crystals form in the magma making a crystal slush Gases - H 2 O, CO 2, etc. - are dissolved in
Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics
 Chemical Geology 183 (2002) 143 168 www.elsevier.com/locate/chemgeo Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics Marc D. Norman a, *, Michael
Chemical Geology 183 (2002) 143 168 www.elsevier.com/locate/chemgeo Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics Marc D. Norman a, *, Michael
Lecture 12 COMPLEX MELTING MODELS. (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007))
 Lecture 12 COMPLEX MELTING MODELS (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007)) Thus far we have considered two end-member melting models, batch melting
Lecture 12 COMPLEX MELTING MODELS (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007)) Thus far we have considered two end-member melting models, batch melting
What can isotopes tell us about mantle dynamics? Sujoy Mukhopadhyay. Harvard University
 What can isotopes tell us about mantle dynamics? Sujoy Mukhopadhyay Harvard University The mantle zoo Hofmann, 1997 187 Os/ 188 Os 0.168 0.156 0.144 0.132 EM1 Hawaii Pitcairn DMM peridotites Shield Basalts
What can isotopes tell us about mantle dynamics? Sujoy Mukhopadhyay Harvard University The mantle zoo Hofmann, 1997 187 Os/ 188 Os 0.168 0.156 0.144 0.132 EM1 Hawaii Pitcairn DMM peridotites Shield Basalts
Petrogenetic Constraints at Mount Rainier Volcano, Washington
 Petrogenetic Constraints at Mount Rainier Volcano, Washington S. C. Kuehn and P. R. Hooper, Department of Geology, Washington State University, Pullman, WA A. E. Eggers and C. Kerrick, Department of Geology,
Petrogenetic Constraints at Mount Rainier Volcano, Washington S. C. Kuehn and P. R. Hooper, Department of Geology, Washington State University, Pullman, WA A. E. Eggers and C. Kerrick, Department of Geology,
Week 7 Submarine Pyroclastic Activity (there was no lecture for week 6)
 Week 7 Submarine Pyroclastic Activity (there was no lecture for week 6) Note: some of these slides were provided by Dave Clague, MBARI Two topics: fluidal clasts and bubble wall fragments internal water
Week 7 Submarine Pyroclastic Activity (there was no lecture for week 6) Note: some of these slides were provided by Dave Clague, MBARI Two topics: fluidal clasts and bubble wall fragments internal water
AN EXPERIMENTAL STUDY
 NORDIC VOLCANOLOGICAL INSTITUTE 78 01 UNIVERSITY OF!CELANO PARTITIONING OF Cr, Mn, Co AND Ni BETWEEN OLIVINE AND BASALTIC LIQUID: AN EXPERIMENTAL STUDY by Heikki Makipaa This is a preliminary report from
NORDIC VOLCANOLOGICAL INSTITUTE 78 01 UNIVERSITY OF!CELANO PARTITIONING OF Cr, Mn, Co AND Ni BETWEEN OLIVINE AND BASALTIC LIQUID: AN EXPERIMENTAL STUDY by Heikki Makipaa This is a preliminary report from
Lecture 25 Subduction Related Magmatism
 Lecture 25 Subduction Related Magmatism Monday, May 2 nd 2005 Subduction Related Magmatism Activity along arcuate volcanic chains along subduction zones Distinctly different from the mainly basaltic provinces
Lecture 25 Subduction Related Magmatism Monday, May 2 nd 2005 Subduction Related Magmatism Activity along arcuate volcanic chains along subduction zones Distinctly different from the mainly basaltic provinces
Constitution of Magmas. Magmas. Gas Law. Composition. Atomic Structure of Magma. Structural Model. PV = nrt H 2 O + O -2 = 2(OH) -
 Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
Volatiles (H, C, N, F, S, Cl) in the lunar mantle, crust, and regolith: What questions remain and where to go next?
 Volatiles (H, C, N, F, S, Cl) in the lunar mantle, crust, and regolith: What questions remain and where to go next? Francis M. McCubbin & Charles K. Shearer Motivation behind this work 100, In Revision,
Volatiles (H, C, N, F, S, Cl) in the lunar mantle, crust, and regolith: What questions remain and where to go next? Francis M. McCubbin & Charles K. Shearer Motivation behind this work 100, In Revision,
Lecture 37. Igneous geochemistry. Crystallization Let's look at how we use differences in element distribution to understand crystallization process.
 Reading White Chapter 7 Lecture 37 Igneous geochemistry Today: Using trace elements to study 1. crystallization 2. melting Crystallization Let's look at how we use differences in element distribution to
Reading White Chapter 7 Lecture 37 Igneous geochemistry Today: Using trace elements to study 1. crystallization 2. melting Crystallization Let's look at how we use differences in element distribution to
Essentials of Geology, 11e
 Essentials of Geology, 11e Igneous Rocks and Intrusive Activity Chapter 3 Instructor Jennifer Barson Spokane Falls Community College Geology 101 Stanley Hatfield Southwestern Illinois College Characteristics
Essentials of Geology, 11e Igneous Rocks and Intrusive Activity Chapter 3 Instructor Jennifer Barson Spokane Falls Community College Geology 101 Stanley Hatfield Southwestern Illinois College Characteristics
GEOL 2312 Igneous and Metamorphic Petrology Spring 2016 Score / 58. Midterm 1 Chapters 1-10
 GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
THE MONTE MAGGIORE PERIDOTITE (CORSICA)
 MONTE MAGGIORE CAPO CORSO CORSICA Giovanni B. Piccardo THE MONTE MAGGIORE PERIDOTITE (CORSICA) FIELD RELATIONSHIPS MORB Gabbro Spinel (ex-garnet) pyroxenites L ESCURSIONE A MONTE MAGGIORE The Monte Maggiore
MONTE MAGGIORE CAPO CORSO CORSICA Giovanni B. Piccardo THE MONTE MAGGIORE PERIDOTITE (CORSICA) FIELD RELATIONSHIPS MORB Gabbro Spinel (ex-garnet) pyroxenites L ESCURSIONE A MONTE MAGGIORE The Monte Maggiore
A model of sulphur solubility for hydrous mafic melts: application to the determination of magmatic fluid compositions of Italian volcanoes
 ANNALS OF GEOPHYSICS, VOL. 48, N. 4/5, August/October 2005 A model of sulphur solubility for hydrous mafic melts: application to the determination of magmatic fluid compositions of Italian volcanoes ISTO-CNRS,
ANNALS OF GEOPHYSICS, VOL. 48, N. 4/5, August/October 2005 A model of sulphur solubility for hydrous mafic melts: application to the determination of magmatic fluid compositions of Italian volcanoes ISTO-CNRS,
Composition of the Earth and its reservoirs: Geochemical observables
 Composition of the Earth and its reservoirs: Geochemical observables Cin-Ty A. Lee Rice University MYRES-I 2004 The Earth is dynamic and heterogeneous Atmosphere Midocean Ridge Plume Ocean Crust Oceanic
Composition of the Earth and its reservoirs: Geochemical observables Cin-Ty A. Lee Rice University MYRES-I 2004 The Earth is dynamic and heterogeneous Atmosphere Midocean Ridge Plume Ocean Crust Oceanic
- low FeO*/MgO is controlled by the reaction relation oliv + liq! opx.
 Mantle melting trend to high-sio 2 - low FeO*/MgO is controlled by the reaction relation oliv + liq! opx. 4 3 2 1 Ol+Opx +Cpx+Sp +liq 79-35g 82-66 87S35 85-44 85-41c -ol +opx Mantle melting/reaction 0
Mantle melting trend to high-sio 2 - low FeO*/MgO is controlled by the reaction relation oliv + liq! opx. 4 3 2 1 Ol+Opx +Cpx+Sp +liq 79-35g 82-66 87S35 85-44 85-41c -ol +opx Mantle melting/reaction 0
Revision 1. Origin and Petrogenetic Implications of Anomalous Olivine From a Cascade Forearc Basalt
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 Revision 1 Origin and Petrogenetic Implications of Anomalous Olivine From a Cascade Forearc
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 Revision 1 Origin and Petrogenetic Implications of Anomalous Olivine From a Cascade Forearc
2009 (25) ! & "# $ % / 5 1 / 3 & ) * / 1 1 / 8 " # (, 3 4 # 1# 2.( /0# ) "! %2 : % % 1 #
 2009 (25)! " & ' "# $ % ( 2008/05/13 &) * 2009/01/18 " # (, 34# 1#2 (. /0#) "! - %2 :% % 1# 7 80 " 1#9 # - 6!5 # >4 :% 62 %2 :% (2 1# - " = #
2009 (25)! " & ' "# $ % ( 2008/05/13 &) * 2009/01/18 " # (, 34# 1#2 (. /0#) "! - %2 :% % 1# 7 80 " 1#9 # - 6!5 # >4 :% 62 %2 :% (2 1# - " = #
DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER
 Geol 2312 Igneous and Metamorphic Petrology Spring 2009 Name DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER Objective: This exercise is intended to improve understanding
Geol 2312 Igneous and Metamorphic Petrology Spring 2009 Name DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER Objective: This exercise is intended to improve understanding
Basaltic and Gabbroic Rocks
 Page 1 of 18 EENS 212 Prof. Stephen A. Nelson Basaltic and Gabbroic Rocks Petrology Tulane University This document last updated on 04-Mar-2004 Although basaltic and gabbroic rocks are found in nearly
Page 1 of 18 EENS 212 Prof. Stephen A. Nelson Basaltic and Gabbroic Rocks Petrology Tulane University This document last updated on 04-Mar-2004 Although basaltic and gabbroic rocks are found in nearly
Supplementary material to accompany the manuscript, Redox variations in HSDP2
 Supplementary material to accompany the manuscript, Redox variations in HSDP2 Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth s oxygenation by M. Brounce,
Supplementary material to accompany the manuscript, Redox variations in HSDP2 Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth s oxygenation by M. Brounce,
Larsen, H.C., Duncan, R.A., Allan, J.F., Brooks, K. (Eds.), 1999 Proceedings of the Ocean Drilling Program, Scientific Results, Vol.
 Larsen, H.C., Duncan, R.A., Allan, J.F., Brooks, K. (Eds.), 1999 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 163 9. LOW-PRESSURE MELTING STUDIES OF BASALT AND BASALTIC ANDESITE
Larsen, H.C., Duncan, R.A., Allan, J.F., Brooks, K. (Eds.), 1999 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 163 9. LOW-PRESSURE MELTING STUDIES OF BASALT AND BASALTIC ANDESITE
Description of Supplementary Files
 Description of Supplementary Files File Name: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References File Name: Peer Review File Supplementary figure
Description of Supplementary Files File Name: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References File Name: Peer Review File Supplementary figure
12. ABUNDANCES OF RARE EARTHS AND OTHER TRACE ELEMENTS LEG 46 BASALTS (DSDP)
 2. ABUNDANCES OF RARE EARTHS AND OTHER TRACE ELEMENTS LEG 6 BASALTS (DSDP) R. Emmermann and H. Puchelt, Institut für Petrographie und Geochemie der Universitàt (T.H.) Karlsruhe, Germany ABSTRACT On a total
2. ABUNDANCES OF RARE EARTHS AND OTHER TRACE ELEMENTS LEG 6 BASALTS (DSDP) R. Emmermann and H. Puchelt, Institut für Petrographie und Geochemie der Universitàt (T.H.) Karlsruhe, Germany ABSTRACT On a total
Earth Science 232 Petrography
 Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Differentiation 1: core formation OUTLINE
 Differentiation 1: core formation Reading this week: White Ch 12 OUTLINE Today 1.Finish some slides 2.Layers 3.Core formation 1 Goldschmidt Classification/Geochemical Periodic Chart Elements can be assigned
Differentiation 1: core formation Reading this week: White Ch 12 OUTLINE Today 1.Finish some slides 2.Layers 3.Core formation 1 Goldschmidt Classification/Geochemical Periodic Chart Elements can be assigned
Evidences for geochemically distinct mantle components
 Evidences for geochemically distinct mantle components 1 Mantle Array Oceanic basalts, including seamounts, oceanic islands and middle ocean ridge basalts, were used. 2 Binary All analyses fall between
Evidences for geochemically distinct mantle components 1 Mantle Array Oceanic basalts, including seamounts, oceanic islands and middle ocean ridge basalts, were used. 2 Binary All analyses fall between
Rare Earth Elements in some representative arc lavas
 Rare Earth Elements in some representative arc lavas Low-K (tholeiitic), Medium-K (calc-alkaline), and High-K basaltic andesites and andesites. A typical N-MORB pattern is included for reference Notes:
Rare Earth Elements in some representative arc lavas Low-K (tholeiitic), Medium-K (calc-alkaline), and High-K basaltic andesites and andesites. A typical N-MORB pattern is included for reference Notes:
GY303 Igneous & Metamorphic Petrology. Lecture 7: Magma Sources and Tectonic Environments
 GY303 Igneous & Metamorphic Petrology Lecture 7: Magma Sources and Tectonic Environments Factors controlling Magma production Source rock composition Amount of fluids, especially H 2 O Pressure (Depth)
GY303 Igneous & Metamorphic Petrology Lecture 7: Magma Sources and Tectonic Environments Factors controlling Magma production Source rock composition Amount of fluids, especially H 2 O Pressure (Depth)
Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2.
 Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2. Screening for olivine interference in Fe-µ-XANES spectra When collecting Fe-µ-XANES
Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2. Screening for olivine interference in Fe-µ-XANES spectra When collecting Fe-µ-XANES
Chapter 9: Trace Elements
 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8.2. Harker variation diagram for 310 analyzed volcanic rocks from Crater Lake (Mt. Mazama), Oregon Cascades. Data compiled by Rick
Chapter 9: Trace Elements Note magnitude of major element changes Figure 8.2. Harker variation diagram for 310 analyzed volcanic rocks from Crater Lake (Mt. Mazama), Oregon Cascades. Data compiled by Rick
Chapter 4 8/27/2013. Igneous Rocks. and Intrusive Igneous Activity. Introduction. The Properties and Behavior of Magma and Lava
 Introduction Chapter 4 Igneous rocks form by the cooling of magma (or lava). Large parts of the continents and all the oceanic crust are composed of. and Intrusive Igneous Activity The Properties and Behavior
Introduction Chapter 4 Igneous rocks form by the cooling of magma (or lava). Large parts of the continents and all the oceanic crust are composed of. and Intrusive Igneous Activity The Properties and Behavior
Chapter 4 Rocks & Igneous Rocks
 Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Florida Atlantic University PETROLOGY -- MIDTERM TWO KEY
 GLY4310 Name 60 points March 26, 2012 12 took exam - Numbers to the left of the question number in red are the number of incorrect responses. Instructor comments are in blue. Florida Atlantic University
GLY4310 Name 60 points March 26, 2012 12 took exam - Numbers to the left of the question number in red are the number of incorrect responses. Instructor comments are in blue. Florida Atlantic University
Igneous Rocks of the Convergent Margins
 Page 1 of 10 EENS 2120 Petrology Prof. Stephen A. Nelson Tulane University Igneous Rocks of the This document last updated on 08-Feb-2011 The convergent plate margins are the most intense areas of active
Page 1 of 10 EENS 2120 Petrology Prof. Stephen A. Nelson Tulane University Igneous Rocks of the This document last updated on 08-Feb-2011 The convergent plate margins are the most intense areas of active
Volcanism at the Edge of the Hawaiian Plume: Petrogenesis of Submarine Alkalic Lavas from the North Arch Volcanic Field
 JOURNAL OF PETROLOGY VOLUME 41 NUMBER 5 PAGES 667 691 2000 Volcanism at the Edge of the Hawaiian Plume: Petrogenesis of Submarine Alkalic Lavas from the North Arch Volcanic Field F. A. FREY 1, D. CLAGUE
JOURNAL OF PETROLOGY VOLUME 41 NUMBER 5 PAGES 667 691 2000 Volcanism at the Edge of the Hawaiian Plume: Petrogenesis of Submarine Alkalic Lavas from the North Arch Volcanic Field F. A. FREY 1, D. CLAGUE
Silicic volcanism and plutonism in the IBM arc
 NSF-IFREE MARGINS Subduction Factory Workshop Hawaii, 8-12 September 2002 MARGINS Web Site: http://www.ldeo.columbia.edu/margins Silicic volcanism and plutonism in the IBM arc Yoshihiko Tamura IFREE, JAMSTEC,
NSF-IFREE MARGINS Subduction Factory Workshop Hawaii, 8-12 September 2002 MARGINS Web Site: http://www.ldeo.columbia.edu/margins Silicic volcanism and plutonism in the IBM arc Yoshihiko Tamura IFREE, JAMSTEC,
GLY 155 Introduction to Physical Geology, W. Altermann. Grotzinger Jordan. Understanding Earth. Sixth Edition
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 4: IGNEOUS ROCKS Solids from Melts 2011 by W. H. Freeman and Company Chapter 4: Igneous Rocks: Solids from Melts 1 About Igneous Rocks Igneous
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 4: IGNEOUS ROCKS Solids from Melts 2011 by W. H. Freeman and Company Chapter 4: Igneous Rocks: Solids from Melts 1 About Igneous Rocks Igneous
GSA Data Repository
 GSA Data Repository 218145 Parolari et al., 218, A balancing act of crust creation and destruction along the western Mexican convergent margin: Geology, https://doi.org/1.113/g39972.1. 218145_Tables DR1-DR4.xls
GSA Data Repository 218145 Parolari et al., 218, A balancing act of crust creation and destruction along the western Mexican convergent margin: Geology, https://doi.org/1.113/g39972.1. 218145_Tables DR1-DR4.xls
Engineering Geology ECIV 2204
 Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Depths of Partial Crystallization of H 2 O-bearing MORB: Phase Equilibria Simulations of Basalts at the MAR near Ascension Island (7^118S)
 JOURNAL OF PETROLOGY VOLUME 49 NUMBER1 PAGES 25^45 2008 doi:10.1093/petrology/egm068 Depths of Partial Crystallization of H 2 O-bearing MORB: Phase Equilibria Simulations of Basalts at the MAR near Ascension
JOURNAL OF PETROLOGY VOLUME 49 NUMBER1 PAGES 25^45 2008 doi:10.1093/petrology/egm068 Depths of Partial Crystallization of H 2 O-bearing MORB: Phase Equilibria Simulations of Basalts at the MAR near Ascension
The application of olivine geothermometry to infer crystallization temperatures of parental liquids: Implications for the temperature of MORB magmas
 Chemical Geology 241 (2007) 207 233 www.elsevier.com/locate/chemgeo The application of olivine geothermometry to infer crystallization temperatures of parental liquids: Implications for the temperature
Chemical Geology 241 (2007) 207 233 www.elsevier.com/locate/chemgeo The application of olivine geothermometry to infer crystallization temperatures of parental liquids: Implications for the temperature
Crystallization temperatures of tholeiite parental liquids: Implications for the existence of thermally driven mantle plumes
 Crystallization temperatures of tholeiite parental liquids: Implications for the existence of thermally driven mantle plumes Trevor J. Falloon* School of Earth Sciences and Centre for Marine Science, UTAS,
Crystallization temperatures of tholeiite parental liquids: Implications for the existence of thermally driven mantle plumes Trevor J. Falloon* School of Earth Sciences and Centre for Marine Science, UTAS,
Petrology and Geochronology of Iran Tepe volcano, Eastern Rhodopes, Bulgaria: Age relationship with the Ada Tepe gold deposit. (preliminary data)
 Petrology and Geochronology of Iran Tepe volcano, Eastern Rhodopes, Bulgaria: Age relationship with the Ada Tepe gold deposit. (preliminary data) Peter Kibarov, Peter Marchev, Maria Ovtcharova, Raya Raycheva,
Petrology and Geochronology of Iran Tepe volcano, Eastern Rhodopes, Bulgaria: Age relationship with the Ada Tepe gold deposit. (preliminary data) Peter Kibarov, Peter Marchev, Maria Ovtcharova, Raya Raycheva,
C = 3: Ternary Systems: Example 1: Ternary Eutectic
 Phase Equilibrium C = 3: Ternary Systems: Example 1: Ternary Eutectic Adding components, becomes increasingly difficult to depict 1-C: P - T diagrams easy 2-C: isobaric T-X, isothermal P-X 3-C:?? Still
Phase Equilibrium C = 3: Ternary Systems: Example 1: Ternary Eutectic Adding components, becomes increasingly difficult to depict 1-C: P - T diagrams easy 2-C: isobaric T-X, isothermal P-X 3-C:?? Still
Ore deposits related to mafic igneous rocks Diamonds - GLY 361 Lecture 3
 Ore deposits related to mafic igneous rocks Diamonds - GLY 361 Lecture 3 A short history of diamonds Derived from the ancient Greek αδάμας (adámas): unbreakable Thought to have been first recognized and
Ore deposits related to mafic igneous rocks Diamonds - GLY 361 Lecture 3 A short history of diamonds Derived from the ancient Greek αδάμας (adámas): unbreakable Thought to have been first recognized and
GEOLOGY. Subject : GEOLOGY (For under graduate student.) Paper No. : Paper 02 Introduction to Geology 02
 GEOLOGY Subject : GEOLOGY (For under graduate student.) Paper No. : Paper 02 Introduction to Geology 02 Topic No. & Title : 37 Magma Bowen Series (Part 01) Academic Script What is Igneous Petrology? Igneous
GEOLOGY Subject : GEOLOGY (For under graduate student.) Paper No. : Paper 02 Introduction to Geology 02 Topic No. & Title : 37 Magma Bowen Series (Part 01) Academic Script What is Igneous Petrology? Igneous
29. CHEMICAL ZONATION OF PLAGIOCLASE PHENOCRYSTS FROM LEG 51, 52, AND 53 BASALTS
 29. CHEMICAL ZONATION OF PLAGIOCLASE PHENOCRYSTS FROM LEG 51, 52, AND BASALTS Claire Bollinger and Michel Semet, Laboratoire de Géochimie et Cosmochimie, Institut de Physique du Globe de Paris, Département
29. CHEMICAL ZONATION OF PLAGIOCLASE PHENOCRYSTS FROM LEG 51, 52, AND BASALTS Claire Bollinger and Michel Semet, Laboratoire de Géochimie et Cosmochimie, Institut de Physique du Globe de Paris, Département
Structure of the Earth and the Origin of Magmas
 Page 1 of 12 EENS 2120 Petrology Tulane University Prof. Stephen A. Nelson Structure of the Earth and the Origin of Magmas This document last updated on 23-Jan-2015 Magmas do not form everywhere beneath
Page 1 of 12 EENS 2120 Petrology Tulane University Prof. Stephen A. Nelson Structure of the Earth and the Origin of Magmas This document last updated on 23-Jan-2015 Magmas do not form everywhere beneath
LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES
 Geology 316 (Petrology) (03/26/2012) Name LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES INTRODUCTION Ultramafic rocks are igneous rocks containing less than 10% felsic minerals (quartz + feldspars
Geology 316 (Petrology) (03/26/2012) Name LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES INTRODUCTION Ultramafic rocks are igneous rocks containing less than 10% felsic minerals (quartz + feldspars
Igneous & Metamorphic Petrology I LECTURE 11
 Igneous & Metamorphic Petrology I LECTURE 11 The Earth s Mantle 1. Structure of the Earth A Reminder The velocities of seismic waves differ with the elastic properties and densities of rocks and allow
Igneous & Metamorphic Petrology I LECTURE 11 The Earth s Mantle 1. Structure of the Earth A Reminder The velocities of seismic waves differ with the elastic properties and densities of rocks and allow
Fluorine and Chlorine in Alkaline Rocks and A-type Granites
 Fluorine and Chlorine in Alkaline Rocks and A-type Granites Using the fluorine and chlorine content of Amphibole, Apatite and Biotite to monitor magma halogen content Chilwa Province, Malawi, and Carboniferous
Fluorine and Chlorine in Alkaline Rocks and A-type Granites Using the fluorine and chlorine content of Amphibole, Apatite and Biotite to monitor magma halogen content Chilwa Province, Malawi, and Carboniferous
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10326 Supplementary Discussion All known modern terrestrial mantle reservoirs evolved from a primitive precursor with superchondritic 143 Nd/ 144 Nd. What is this reservoir? The terms
doi:10.1038/nature10326 Supplementary Discussion All known modern terrestrial mantle reservoirs evolved from a primitive precursor with superchondritic 143 Nd/ 144 Nd. What is this reservoir? The terms
Igneous and Metamorphic Rock Forming Minerals. Department of Geology Mr. Victor Tibane SGM 210_2013
 Igneous and Metamorphic Rock Forming Minerals Department of Geology Mr. Victor Tibane 1 SGM 210_2013 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 4: IGNEOUS ROCKS Solids from Melts 2011
Igneous and Metamorphic Rock Forming Minerals Department of Geology Mr. Victor Tibane 1 SGM 210_2013 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 4: IGNEOUS ROCKS Solids from Melts 2011
Chapter 9: Trace Elements
 Lecture 13 Introduction to Trace Elements Wednesday, March 9, 2005 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8-2. Harker variation diagram for 310 analyzed volcanic rocks
Lecture 13 Introduction to Trace Elements Wednesday, March 9, 2005 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8-2. Harker variation diagram for 310 analyzed volcanic rocks
Mineral chemistry of submarine lavas from Hilo Ridge, Hawaii: implications for magmatic processes within Hawaiian rift zones
 Contrib Mineral Petrol (1999) 135: 355±372 Ó Springer-Verlag 1999 Huai-Jen Yang á Frederick A. Frey David A. Clague á Michael O. Garcia Mineral chemistry of submarine lavas from Hilo Ridge, Hawaii: implications
Contrib Mineral Petrol (1999) 135: 355±372 Ó Springer-Verlag 1999 Huai-Jen Yang á Frederick A. Frey David A. Clague á Michael O. Garcia Mineral chemistry of submarine lavas from Hilo Ridge, Hawaii: implications
SUPPLEMENTARY INFORMATION
 Sample Description Rock 14053 is one of the most well-studied high-al basalts in the Apollo collection 2,3. This sample (14053,241) was chipped from a large (approx. 0.5 m long) boulder at station C2,
Sample Description Rock 14053 is one of the most well-studied high-al basalts in the Apollo collection 2,3. This sample (14053,241) was chipped from a large (approx. 0.5 m long) boulder at station C2,
Geol. 656 Isotope Geochemistry. Stable Isotope Geochemistry II: High Temperature Applications
 Stable Isotope Geochemistry II: High Temperature Applications 9.1 INTRODUCTION Stable isotopes have a number of uses in high temperature geochemistry (i.e., igneous and metamorphic geochemistry). Perhaps
Stable Isotope Geochemistry II: High Temperature Applications 9.1 INTRODUCTION Stable isotopes have a number of uses in high temperature geochemistry (i.e., igneous and metamorphic geochemistry). Perhaps
doi: /nature09369
 doi:10.1038/nature09369 Supplementary Figure S1 Scanning electron microscope images of experimental charges with vapour and vapour phase quench. Experimental runs are in the order of added water concentration
doi:10.1038/nature09369 Supplementary Figure S1 Scanning electron microscope images of experimental charges with vapour and vapour phase quench. Experimental runs are in the order of added water concentration
Magmatic Processes at Subduction Zones
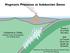 Magmatic Processes at Subduction Zones Katherine A. Kelley Graduate School of Oceanography Univ. of Rhode Island Thanks to Terry Plank Erik Hauri GVP: Liz Cottrell Simon Carn Jennifer Jay Ed Venzke Subduction
Magmatic Processes at Subduction Zones Katherine A. Kelley Graduate School of Oceanography Univ. of Rhode Island Thanks to Terry Plank Erik Hauri GVP: Liz Cottrell Simon Carn Jennifer Jay Ed Venzke Subduction
