Matter and Energy: What are atoms?
|
|
|
- Marcia Flowers
- 5 years ago
- Views:
Transcription
1 Matter and Energy: What are atoms? By Encyclopaedia Britannica, adapted by Newsela staff on Word Count 546 Level 820L An illustration of an atom. The nucleus, containing neutrons and protons, is at the center. Circling around it are electrons. Image from: Pixabay. The tiny particles called atoms are the basic building blocks of all matter. Atoms can be combined with other atoms to form molecules. However, they cannot be divided into smaller parts by ordinary means. The word atom derives from the Greek word "atomos," which means indivisible. Something that is indivisible cannot be divided. The ancient Greeks were the first to think of the atom as the basic unit of all matter. But it was not until the 1800s that scientists began to understand how atoms work. This article is available at 5 reading levels at 1
2 Atoms Made Up Of Subatomic Particles Each individual atom is made up of smaller particles called electrons, protons and neutrons. These are known as subatomic particles. At the center of each atom is the nucleus. The nucleus consists of protons and neutrons. Protons carry a positive electrical charge, while neutrons carry no electrical charge. Together, protons and neutrons are called nucleons. Surrounding the nucleus is a cloud of negatively charged electrons. Scientists believe that subatomic particles are themselves made up of even smaller substances. These substances are called quarks and leptons. This article is available at 5 reading levels at 2
3 Atomic Number Determines What Kind Of Atom It Is The most important thing to know about an atom is how many protons it has in its nucleus. This is known as its atomic number. The atomic number determines what kind of atom it is. Every atom is associated with a certain chemical element. An atom is the smallest unit of an element, and each chemical element has its own atomic number. For instance, hydrogen has an atomic number of 1. That's because every hydrogen atom has one proton in its nucleus. No other element has an atomic number of 1. Each atom also has a mass number and an atomic weight. The mass number is equal to the total number of protons and neutrons in an atom. The atomic weight is equal to the mass number divided by a certain number that scientists have come up with. The mass number and atomic weight are very similar. For example, carbon's mass number is 12. Its atomic weight is Some atoms have the same atomic number, but different mass numbers. These kinds of atoms are called isotopes. For example, Carbon-12 is the ordinary form of carbon. It has six protons and six neutrons in each atom. Carbon-14 is an isotope with six protons and eight neutrons in each atom. It still has six protons. If it did not have six protons, it would not be carbon. An ordinary atom has an equal number of protons and electrons. Therefore its positive and negative charges are balanced. But sometimes atoms lose or gain electrons. This can happen through chemical reactions or by colliding into other particles. Ordinary atoms that either gain or lose electrons are called ions. If an ordinary atom loses an electron, it becomes a positive ion. If it gains an electron, it becomes a negative ion. This article is available at 5 reading levels at 3
4 This article is available at 5 reading levels at 4
5 Quiz 1 Read the summary below. Choose the answer that BEST fits into the blank to complete the summary. Atoms are the tiny building blocks of everything in nature. The number of protons an atom has is called its atomic number, which decides what kind of atom it is. Changes in the number of neutrons make an isotope, and changes to the number of electrons make an ion. They are made up of subatomic particles called protons, electrons and neutrons. They are made up of only negative and neutral electrical charges in the nucleus. They are made up of a nucleus with a surrounding cloud of neutrons and protons. They are made up of quarks and leptons that are divided into even smaller particles. 2 Read the following paragraph from the article. An ordinary atom has an equal number of protons and electrons. Therefore, its positive and negative charges are balanced. But sometimes atoms lose or gain electrons. This can happen through chemical reactions, or by colliding into other particles. Ordinary atoms that either gain or lose electrons are called ions. If an ordinary atom loses an electron, it becomes a positive ion. If it gains an electron, it becomes a negative ion. Which answer choice BEST describes the structure of the paragraph? chronological order compare and contrast cause and effect problem and solution This article is available at 5 reading levels at 5
6 3 Which statement from the section "Atoms Made Up Of Subatomic Particles" suggests that there might be particles tinier than protons, electrons and neutrons? Together, protons and neutrons are called nucleons. Scientists believe that subatomic particles are themselves made up of even smaller substances. The tiny particles called atoms are the basic building blocks of all matter. These are known as subatomic particles. 4 Based on information in the article, which of these statements is TRUE? The atomic number is determined by how many protons are in the nucleus. The atomic weight is determined by how many electrons are in the nucleus. The atomic number is determined by dividing the protons by the neutrons. The atomic weight is determined by dividing the neutrons by the electrons. This article is available at 5 reading levels at 6
7 Answer Key 1 Read the summary below. Choose the answer that BEST fits into the blank to complete the summary. Atoms are the tiny building blocks of everything in nature. The number of protons an atom has is called its atomic number, which decides what kind of atom it is. Changes in the number of neutrons make an isotope, and changes to the number of electrons make an ion. They are made up of subatomic particles called protons, electrons and neutrons. They are made up of only negative and neutral electrical charges in the nucleus. They are made up of a nucleus with a surrounding cloud of neutrons and protons. They are made up of quarks and leptons that are divided into even smaller particles. 2 Read the following paragraph from the article. An ordinary atom has an equal number of protons and electrons. Therefore, its positive and negative charges are balanced. But sometimes atoms lose or gain electrons. This can happen through chemical reactions, or by colliding into other particles. Ordinary atoms that either gain or lose electrons are called ions. If an ordinary atom loses an electron, it becomes a positive ion. If it gains an electron, it becomes a negative ion. Which answer choice BEST describes the structure of the paragraph? chronological order compare and contrast cause and effect problem and solution This article is available at 5 reading levels at 7
8 3 Which statement from the section "Atoms Made Up Of Subatomic Particles" suggests that there might be particles tinier than protons, electrons and neutrons? Together, protons and neutrons are called nucleons. Scientists believe that subatomic particles are themselves made up of even smaller substances. The tiny particles called atoms are the basic building blocks of all matter. These are known as subatomic particles. 4 Based on information in the article, which of these statements is TRUE? The atomic number is determined by how many protons are in the nucleus. The atomic weight is determined by how many electrons are in the nucleus. The atomic number is determined by dividing the protons by the neutrons. The atomic weight is determined by dividing the neutrons by the electrons. This article is available at 5 reading levels at 8
Matter and Energy: What are atoms?
 Matter and Energy: What are atoms? By Encyclopaedia Britannica, adapted by Newsela staff on 03.31.17 Word Count 518 Level MAX An illustration of an atom. The nucleus, containing neutrons and protons, is
Matter and Energy: What are atoms? By Encyclopaedia Britannica, adapted by Newsela staff on 03.31.17 Word Count 518 Level MAX An illustration of an atom. The nucleus, containing neutrons and protons, is
An Atom Apart by Leslie Cargile
 Have you ever walked through a cloud of gnats on a hot summer day, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
Have you ever walked through a cloud of gnats on a hot summer day, only to have them follow you? No matter how you swat at them, or even if you run, they won t leave you alone. If so, then you have something
CHEMISTRY Matter and Change. Chapter 4: The Structure of the Atom
 CHEMISTRY Matter and Change Chapter 4: The Structure of the Atom CHAPTER 4 Table Of Contents Section 4.1 Section 4.2 Section 4.3 Section 4.4 Early Ideas About Matter Defining the Atom How Atoms Differ
CHEMISTRY Matter and Change Chapter 4: The Structure of the Atom CHAPTER 4 Table Of Contents Section 4.1 Section 4.2 Section 4.3 Section 4.4 Early Ideas About Matter Defining the Atom How Atoms Differ
THE ATOM atoms. electrons nucleus, protons neutrons atomic number atomic weight isotopes
 1 THE ATOM All matter is made up of atoms. An atom is made up of two major parts: First, there is a cloud of electrons (very light, negatively charged particles filling most of the volume), and second,
1 THE ATOM All matter is made up of atoms. An atom is made up of two major parts: First, there is a cloud of electrons (very light, negatively charged particles filling most of the volume), and second,
The following pages contain all the notes so far this semester
 The following pages contain all the notes so far this semester This packet is for you to check your notebook. You cannot use this packet on the quiz. Monday, January 29 Tuesday, January 30 particle part
The following pages contain all the notes so far this semester This packet is for you to check your notebook. You cannot use this packet on the quiz. Monday, January 29 Tuesday, January 30 particle part
Topic 2 Atomic Structure. IB Chemistry SL Coral Gables Senior High School Ms. Kiely
 Topic 2 Atomic Structure IB Chemistry SL Coral Gables Senior High School Ms. Kiely Prepare for Lab Quiz: Put everything away except for a pen (no pencil) and a calculator. Bell-Ringer This is an example
Topic 2 Atomic Structure IB Chemistry SL Coral Gables Senior High School Ms. Kiely Prepare for Lab Quiz: Put everything away except for a pen (no pencil) and a calculator. Bell-Ringer This is an example
Atomic Structure. For thousands of years, people had many ideas about matter Ancient Greeks believed that everything was made up of the four elements
 An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
Structure of the Atom. Atomic Components
 Chapter 19 Atoms 0 Matter is anything that takes up space and has mass. All matter is made of atoms. 0 Atoms are the basic building blocks of matter. They make up everything around us; Your desk, the board,
Chapter 19 Atoms 0 Matter is anything that takes up space and has mass. All matter is made of atoms. 0 Atoms are the basic building blocks of matter. They make up everything around us; Your desk, the board,
Atoms and Their Isotopes
 Atoms and Their Isotopes Why? Atoms and isotopes are identified by the number of protons, neutrons, and electrons that they contain. Before you can understand the properties of atoms, how atoms combine
Atoms and Their Isotopes Why? Atoms and isotopes are identified by the number of protons, neutrons, and electrons that they contain. Before you can understand the properties of atoms, how atoms combine
HIGH SCHOOL SCIENCE. Physical Science 9: Atomic Structure
 HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
Chemistry. Robert Taggart
 Chemistry Robert Taggart Table of Contents To the Student..................................................v Unit 1: Matter and Measurement Lesson 1: Chemistry and the Scientific Method...................3
Chemistry Robert Taggart Table of Contents To the Student..................................................v Unit 1: Matter and Measurement Lesson 1: Chemistry and the Scientific Method...................3
Unit 3 Lesson 1 The Atom. Copyright Houghton Mifflin Harcourt Publishing Company
 As a Matter of Fact What makes up matter? The Greek philosopher Democritus thought matter could be divided into smaller units until you obtained a particle that could not be cut. He called this particle
As a Matter of Fact What makes up matter? The Greek philosopher Democritus thought matter could be divided into smaller units until you obtained a particle that could not be cut. He called this particle
Elements and Atoms NEVER TRUST AN ATOM THEY MAKE UP EVERYTHING
 Elements and Atoms NEVER TRUST AN ATOM THEY MAKE UP EVERYTHING Atoms Matter! All matter is made up of tiny, fundamental particles called atoms Atoms are the smallest particles of an element that retain
Elements and Atoms NEVER TRUST AN ATOM THEY MAKE UP EVERYTHING Atoms Matter! All matter is made up of tiny, fundamental particles called atoms Atoms are the smallest particles of an element that retain
1 The Development of Atomic Theory
 CHAPTER 4 1 The Development of Atomic Theory SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What scientists helped to develop atomic theory? What part of atoms did Thomson
CHAPTER 4 1 The Development of Atomic Theory SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What scientists helped to develop atomic theory? What part of atoms did Thomson
Chapt. 2 The Nature of Molecules
 Most of the Universe consists of matter and energy. Energy is the capacity to do work. Matter has mass and occupies space. All matter is composed of basic elements. Elements are substances consisting of
Most of the Universe consists of matter and energy. Energy is the capacity to do work. Matter has mass and occupies space. All matter is composed of basic elements. Elements are substances consisting of
Particle Theory of Matter. By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that:
 Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
CHEMISTRY. Matter and Change. Table Of Contents. Section 4.1 Early Ideas About Matter. Unstable Nuclei and Radioactive Decay
 CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
Chapter #1 - Atomic Structure
 Chapter #1 - Atomic Structure Atomic Theories Democritus (460-340 BC) Democritus believed that all matter consisted of extremely small particles that could not be divided. He called them atoms from the
Chapter #1 - Atomic Structure Atomic Theories Democritus (460-340 BC) Democritus believed that all matter consisted of extremely small particles that could not be divided. He called them atoms from the
Early Atomic Theory. Alchemy. The atom
 Early Atomic Theory Chapter 3 Democritus 460 BC- ~ 370 BC Nothing exists except atoms and empty space; everything else is opinion. Matter is composed of small indivisible particles, atomos meaning Indivisible
Early Atomic Theory Chapter 3 Democritus 460 BC- ~ 370 BC Nothing exists except atoms and empty space; everything else is opinion. Matter is composed of small indivisible particles, atomos meaning Indivisible
The structure of the Atom. Chemistry chapter 4
 The structure of the Atom Chemistry chapter 4 Rutherford-Bohr Model Niels Bohr (1922) Proposed improvements to Rutherford Atomic Model. For this reason the planetary model of the atoms is sometimes called
The structure of the Atom Chemistry chapter 4 Rutherford-Bohr Model Niels Bohr (1922) Proposed improvements to Rutherford Atomic Model. For this reason the planetary model of the atoms is sometimes called
Learn the photosynthesis formula
 Learn the photosynthesis formula By ThoughtCo.com, adapted by Newsela staff on 10.16.17 Word Count 481 Level 800L In plants, photosynthesis occurs mainly within the leaves. Photo from the public domain
Learn the photosynthesis formula By ThoughtCo.com, adapted by Newsela staff on 10.16.17 Word Count 481 Level 800L In plants, photosynthesis occurs mainly within the leaves. Photo from the public domain
Chapter 3. Atom. Table of Contents. 1. Atom and History of Atom 2. Subatomic Particles 3. Isotopes 4. Ions 5. Atomic Terminology
 Atom Table of Contents 1. Atom and History of Atom 2. Subatomic Particles 3. Isotopes 4. Ions 5. Atomic Terminology The History of The Atom Warm up Make a list of inferences about any properties of objects
Atom Table of Contents 1. Atom and History of Atom 2. Subatomic Particles 3. Isotopes 4. Ions 5. Atomic Terminology The History of The Atom Warm up Make a list of inferences about any properties of objects
Erasmus Plus INS Ramon Berenguer IV LESSON PLAN
 LESSON PLAN Subject: Chemistry Topic: Matter matters! Age of students: 17 Language level: B1/B2 Time: 45-60 minutes Content aims: After completing the lesson, the student will be able to: Understand the
LESSON PLAN Subject: Chemistry Topic: Matter matters! Age of students: 17 Language level: B1/B2 Time: 45-60 minutes Content aims: After completing the lesson, the student will be able to: Understand the
Ancient Atomic Theories
 Atomic Theory What is an Atom? An ATOM is the smallest part of an element that has all of the element s properties. Atoms of different elements are different from each other. Atomic Theory This is the
Atomic Theory What is an Atom? An ATOM is the smallest part of an element that has all of the element s properties. Atoms of different elements are different from each other. Atomic Theory This is the
Matter: Properties and Change
 Matter: Properties and Change 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element are
Matter: Properties and Change 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element are
Name Date Class DEFINING THE ATOM
 4.1 DEFINING THE ATOM Section Review Objectives Describe Democritus s ideas about atoms Explain Dalton s atomic theory Describe the size of an atom Vocabulary atom Dalton s atomic theory Part A Completion
4.1 DEFINING THE ATOM Section Review Objectives Describe Democritus s ideas about atoms Explain Dalton s atomic theory Describe the size of an atom Vocabulary atom Dalton s atomic theory Part A Completion
Understanding the Atom
 Name Date Period 3.1 Discovering Parts of an Atom Directions: On the line before each statement, write correct if the statement is correct or not correct if the statement is not correct. If the statement
Name Date Period 3.1 Discovering Parts of an Atom Directions: On the line before each statement, write correct if the statement is correct or not correct if the statement is not correct. If the statement
Matter. Anything that has mass and takes up space. (has volume)
 Matter and more Matter Anything that has mass and takes up space. (has volume) Mass is the measure of the amount of matter something is made of. It is measured in grams Weight is the measure of gravitational
Matter and more Matter Anything that has mass and takes up space. (has volume) Mass is the measure of the amount of matter something is made of. It is measured in grams Weight is the measure of gravitational
Atomic Models. A model uses familiar ideas to explain unfamiliar facts observed in nature. A model can be changed as new information is collected.
 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. Atomic Models A model uses familiar ideas
This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. Atomic Models A model uses familiar ideas
CHAPTER 4: THE ATOM PHYSICAL SCIENCE
 CHAPTER 4: THE ATOM PHYSICAL SCIENCE By C. Goodman, Doral Academy Preparatory High School, 2011-2013 Based on a PowerPoint presentation by Mrs. S. Temple, Doral Academy Preparatory High School Essential
CHAPTER 4: THE ATOM PHYSICAL SCIENCE By C. Goodman, Doral Academy Preparatory High School, 2011-2013 Based on a PowerPoint presentation by Mrs. S. Temple, Doral Academy Preparatory High School Essential
The structure of Atom III
 The structure of Atom III Atomic Structure If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement
The structure of Atom III Atomic Structure If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement
Chemistry is taking place in your body all the time. Your body is made up of a variety of chemicals, and chemical reactions that take place within
 Basic Chemistry Chemistry is taking place in your body all the time. Your body is made up of a variety of chemicals, and chemical reactions that take place within you. There is also chemistry taking place
Basic Chemistry Chemistry is taking place in your body all the time. Your body is made up of a variety of chemicals, and chemical reactions that take place within you. There is also chemistry taking place
2-1 The Nature of Matter
 Biology 1 of 40 2 of 40 The study of chemistry begins with the basic unit of matter, the atom. The Greek philosopher Democritus called the smallest fragment of matter the atom, from the Greek word atomos.
Biology 1 of 40 2 of 40 The study of chemistry begins with the basic unit of matter, the atom. The Greek philosopher Democritus called the smallest fragment of matter the atom, from the Greek word atomos.
Matter and Energy: What is matter?
 Matter and Energy: What is matter? By Encyclopaedia Britannica, adapted by Newsela staff on 03.30.17 Word Count 544 Level 830L This photo of John Muir Glacier in Alaska shows two of the three main states
Matter and Energy: What is matter? By Encyclopaedia Britannica, adapted by Newsela staff on 03.30.17 Word Count 544 Level 830L This photo of John Muir Glacier in Alaska shows two of the three main states
Matter and Atoms. The Structure of Atoms
 CHAPTER 11 Matter and Atoms LESSON 2 The Structure of Atoms What do you think? Read the three statements below and decide whether you agree or disagree with them. Place an A in the Before column if you
CHAPTER 11 Matter and Atoms LESSON 2 The Structure of Atoms What do you think? Read the three statements below and decide whether you agree or disagree with them. Place an A in the Before column if you
Directed Reading B. Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY. is a(n). DALTON S ATOMIC THEORY BASED ON EXPERIMENTS
 Skills Worksheet Directed Reading B Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY 1. The word atom comes from the Greek word atomos, which means a. dividable. b. invisible. c.
Skills Worksheet Directed Reading B Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY 1. The word atom comes from the Greek word atomos, which means a. dividable. b. invisible. c.
Early Ideas About Matter
 Early Ideas About Matter Democritus (460 370 BC) believed that matter is made of small, solid objects called atomos, from which the English word atom is derived. Early Ideas About Matter (cont.) Aristotle
Early Ideas About Matter Democritus (460 370 BC) believed that matter is made of small, solid objects called atomos, from which the English word atom is derived. Early Ideas About Matter (cont.) Aristotle
Card 1 Chapter 18. Card 2. Chapter 18. Negative particles that surround the nucleus (like planets around the sun)
 Card 1 Card 2 Positive particles in the nucleus of the atom Negative particles that surround the nucleus (like planets around the sun) Card 3 Card 4 Neutral particles in the nucleus of the atom. They help
Card 1 Card 2 Positive particles in the nucleus of the atom Negative particles that surround the nucleus (like planets around the sun) Card 3 Card 4 Neutral particles in the nucleus of the atom. They help
Structure of the Atom
 1 Structure of the Atom Scientific Shorthand Scientists have developed their own shorthand for dealing with long, complicated names. Chemical symbols consist of one capital letter or a capital letter plus
1 Structure of the Atom Scientific Shorthand Scientists have developed their own shorthand for dealing with long, complicated names. Chemical symbols consist of one capital letter or a capital letter plus
Mastery Quiz #4: Atomic Structure
 Mastery Quiz #4: Atomic Structure Multiple Choice: 1. The literal translation to English of the Greek word atomos (ατομως) is a. Particle b. Indivisible c. Tiny d. Anti æther 2. Which of the following
Mastery Quiz #4: Atomic Structure Multiple Choice: 1. The literal translation to English of the Greek word atomos (ατομως) is a. Particle b. Indivisible c. Tiny d. Anti æther 2. Which of the following
SCIENCE 8 WORKBOOK Section 2 Atomic Theory Ms. Jamieson 2018 This workbook belongs to:
 SCIENCE 8 WORKBOOK Section 2 Atomic Theory Ms. Jamieson 2018 This workbook belongs to: Eric Hamber Secondary 5025 Willow Street Vancouver, BC Table of Contents A. Models in Science Making Sense of Models....
SCIENCE 8 WORKBOOK Section 2 Atomic Theory Ms. Jamieson 2018 This workbook belongs to: Eric Hamber Secondary 5025 Willow Street Vancouver, BC Table of Contents A. Models in Science Making Sense of Models....
Write the letter that best answers the question or completes the statement on the line provided.
 Chapter 4 Atomic Structure Chapter Test A Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. Democritus thought that matter was made of
Chapter 4 Atomic Structure Chapter Test A Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. Democritus thought that matter was made of
ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:
 ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:. READING 1 The atom Lesson 4. JOURNEY TO THE INTERIOR OF MATTER Wise men and women of the great ancient civilisations thought a long time ago about
ACTIVITY SHEETS PHYSICS AND CHEMISTRY 2 nd ESO) NAME:. READING 1 The atom Lesson 4. JOURNEY TO THE INTERIOR OF MATTER Wise men and women of the great ancient civilisations thought a long time ago about
Introduction to Pre-IB Chemistry I
 Introduction to Pre-IB Chemistry I Unit 1, Part 1 Slides Ms. Kiely Coral Gables Senior High Pre-IB Chemistry Welcome to Pre-IB Chemistry -Syllabus -Periodic Table Welcome to IB Chemistry SL Some general
Introduction to Pre-IB Chemistry I Unit 1, Part 1 Slides Ms. Kiely Coral Gables Senior High Pre-IB Chemistry Welcome to Pre-IB Chemistry -Syllabus -Periodic Table Welcome to IB Chemistry SL Some general
TEACHER. The Atom 4. Make a drawing of an atom including: Nucleus, proton, neutron, electron, shell
 Click on the SUBATOMIC roadmap button on the left. Explore the Subatomic Universe Roadmap to answer the following questions. Matter 1. What 3 atoms is a water molecule made of? Two Hydrogen atoms and one
Click on the SUBATOMIC roadmap button on the left. Explore the Subatomic Universe Roadmap to answer the following questions. Matter 1. What 3 atoms is a water molecule made of? Two Hydrogen atoms and one
Chemistry Vocabulary. These vocabulary words appear on the Chemistry CBA in addition to being tested on the Chemistry Vocabulary Test.
 Chemistry Vocabulary These vocabulary words appear on the Chemistry CBA in addition to being tested on the Chemistry Vocabulary Test. atom the smallest unit of an element that still represents that element.
Chemistry Vocabulary These vocabulary words appear on the Chemistry CBA in addition to being tested on the Chemistry Vocabulary Test. atom the smallest unit of an element that still represents that element.
Atomic Structure. A model uses familiar ideas to explain unfamiliar facts observed in nature.
 Atomic Structure 1 2 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. A model uses familiar
Atomic Structure 1 2 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. A model uses familiar
THE CHEMISTRY OF LIFE. The Nature of Matter
 THE CHEMISTRY OF LIFE The Nature of Matter What do all of These Pictures Have in Common? And last, but not least GEICO S Gecko! MATTER All matter is made up of different combinations of elements.
THE CHEMISTRY OF LIFE The Nature of Matter What do all of These Pictures Have in Common? And last, but not least GEICO S Gecko! MATTER All matter is made up of different combinations of elements.
Atomic Structure element element: Location and Charge of Subatomic Particles Neutron: Proton: Electron:
 Our world is full of diversity, found in all of the materials, substances, and living things that exist on Earth. Take a look at the picture on the right. Even in a small aquarium, there are green plants
Our world is full of diversity, found in all of the materials, substances, and living things that exist on Earth. Take a look at the picture on the right. Even in a small aquarium, there are green plants
ATOMS. reflect. what do you think?
 reflect Our world is full of diversity, found in all of the materials, substances, and living things that exist on Earth. Take a look at the picture on the right. Even in a small aquarium, there are green
reflect Our world is full of diversity, found in all of the materials, substances, and living things that exist on Earth. Take a look at the picture on the right. Even in a small aquarium, there are green
Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video
 Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video 2 CH 4- Atoms 1 Discovering the Atom In this lesson we will take a look at the scientists who explored the
Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video 2 CH 4- Atoms 1 Discovering the Atom In this lesson we will take a look at the scientists who explored the
Scientist wanted to understand how the atom looked. It was known that matter was neutral. It was known that matter had mass
 Atom Models Scientist wanted to understand how the atom looked It was known that matter was neutral It was known that matter had mass They used these ideas to come up with their models, however science
Atom Models Scientist wanted to understand how the atom looked It was known that matter was neutral It was known that matter had mass They used these ideas to come up with their models, however science
Atomic Structure. From Molecules to Quarks
 Atomic Structure From Molecules to Quarks ELECTRONS Negatively charged particles that circle the nucleus. Bohr s Atom electrons in orbits nucleus Inside the Atom The Nucleus Contains Protons & Neutrons
Atomic Structure From Molecules to Quarks ELECTRONS Negatively charged particles that circle the nucleus. Bohr s Atom electrons in orbits nucleus Inside the Atom The Nucleus Contains Protons & Neutrons
Chapter 3. Chemistry of Life
 Chapter 3 Chemistry of Life Content Objectives Write these down! I will be able to identify: The make-up of matter. Why atoms form bonds. Some important interactions between substances in living things.
Chapter 3 Chemistry of Life Content Objectives Write these down! I will be able to identify: The make-up of matter. Why atoms form bonds. Some important interactions between substances in living things.
Atomic Structure & the Periodic Table
 Atomic Structure & the Periodic Table Structure of the Atom Symbols Symbols are used to represent different elements. Example: C carbon, Al aluminum Some symbols are from the Latin words. Example: sodium
Atomic Structure & the Periodic Table Structure of the Atom Symbols Symbols are used to represent different elements. Example: C carbon, Al aluminum Some symbols are from the Latin words. Example: sodium
The Chemical Basis of Animal Life. Chapter 2
 The Chemical Basis of Animal Life Chapter 2 Chemistry The branch of science dealing with composition of substances and reactions among these substances. A knowledge of chemistry is essential for understanding
The Chemical Basis of Animal Life Chapter 2 Chemistry The branch of science dealing with composition of substances and reactions among these substances. A knowledge of chemistry is essential for understanding
Exploring The Planets: Jupiter
 Exploring The Planets: Jupiter By Encyclopaedia Britannica, adapted by Newsela staff on 08.28.17 Word Count 691 Level 800L New Horizons spacecraft took this collection of images of Jupiter and Io in 2007.
Exploring The Planets: Jupiter By Encyclopaedia Britannica, adapted by Newsela staff on 08.28.17 Word Count 691 Level 800L New Horizons spacecraft took this collection of images of Jupiter and Io in 2007.
SNC1D1 History of the Atom
 SNC1D1 History of the Atom What is the atom? Atoms are the building block for all matter: Atoms make up elements! Elements combine to make compounds!2 ATOMIC MODEL TIMELINE 400 B.C PRESENT DAY ATOMIC MODEL
SNC1D1 History of the Atom What is the atom? Atoms are the building block for all matter: Atoms make up elements! Elements combine to make compounds!2 ATOMIC MODEL TIMELINE 400 B.C PRESENT DAY ATOMIC MODEL
To remain valid, models and theories must:
 Note Taking Guide: Episode 301 Model: A idea used to explain facts in. Theory: An of facts and. To remain valid, models and theories must: all known enable to make correct Democritus: proposed the of an
Note Taking Guide: Episode 301 Model: A idea used to explain facts in. Theory: An of facts and. To remain valid, models and theories must: all known enable to make correct Democritus: proposed the of an
Name... Class... Date... In this activity you will have an opportunity to explore the nuclear model of the atom by building your own.
 Model of an atom Specification references: C1.1.4 Relative electrical charges of subatomic particles C1.1.5 Size and mass of atoms WS 1.2 Aims In this activity you will have an opportunity to explore the
Model of an atom Specification references: C1.1.4 Relative electrical charges of subatomic particles C1.1.5 Size and mass of atoms WS 1.2 Aims In this activity you will have an opportunity to explore the
Passing an electric current makes a beam appear to move from the negative to the positive end.
 Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Smallest
Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Smallest
The Story of the Atom. A history of atomic theory over many years
 The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
tomic tructure Chapter 3
 tomic tructure Chapter 3 Early Theories of Matter 460 BC Democritus Proposed the matter was not infinitely divisible. Believed matter composed of particles called atoms. Early Theories of Matter Aristotle
tomic tructure Chapter 3 Early Theories of Matter 460 BC Democritus Proposed the matter was not infinitely divisible. Believed matter composed of particles called atoms. Early Theories of Matter Aristotle
4/14/2013 ATOMIC STRUCTURE THE ATOMIC MODEL
 ATOMIC STRUCTURE R E G E N T S C H E M I S T R Y M R S. T I L A R O HISTORY OF THE ATOM O L D A N D M O D E R N A T O M THE ATOMIC MODEL Model of the atom is based on indirect experimental data. Model
ATOMIC STRUCTURE R E G E N T S C H E M I S T R Y M R S. T I L A R O HISTORY OF THE ATOM O L D A N D M O D E R N A T O M THE ATOMIC MODEL Model of the atom is based on indirect experimental data. Model
Biochemistry. The study of chemical processes in living organisms. Introduction to Chemistry Properties of Water Acids and Bases.
 Biochemistry The study of chemical processes in living organisms. Introduction to Chemistry Properties of Water Acids and Bases Chemistry Of Life Matter Everything living AND non living is made up of matter.
Biochemistry The study of chemical processes in living organisms. Introduction to Chemistry Properties of Water Acids and Bases Chemistry Of Life Matter Everything living AND non living is made up of matter.
Chap 4 Bell -Ringers
 Chap 4 Bell -Ringers The Structure of the Atom The Atom has a Structure What we ve seen so far Chapter 1 The Science of Chemistry - Chemistry is about discovering and understanding natural laws using the
Chap 4 Bell -Ringers The Structure of the Atom The Atom has a Structure What we ve seen so far Chapter 1 The Science of Chemistry - Chemistry is about discovering and understanding natural laws using the
Matter: Properties and Change
 Matter: Properties and Change Date: 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element
Matter: Properties and Change Date: 6.P.2 Understand the structure, classifications and physical properties of matter. 6.P.2.1 Recognize that all matter is made up of atoms and atoms of the same element
Name: Date: Pd: Unit 2: Atomic Structure: Isotopes Guided Notes. Positively charged (+1). Found in the nucleus.
 _ Atoms: The Building Blocks of everything? Unit 2: Atomic Structure: Isotopes Guided Notes An atom consists of a An atom is the smallest unit of an element that is possible. All the matter around us is
_ Atoms: The Building Blocks of everything? Unit 2: Atomic Structure: Isotopes Guided Notes An atom consists of a An atom is the smallest unit of an element that is possible. All the matter around us is
Date: Hybrid Chemistry Regents Prep Ms. Hart/Mr. Kuhnau. Unit 1: All About Atoms Lesson 1.1: Parts of an atom
 Unit 1: All About Atoms Lesson 1.1: Parts of an atom Do Now: By the end of today, you will have an answer to: How do we determine the number of subatomic particles in an atom? 1. A neutron has a charge
Unit 1: All About Atoms Lesson 1.1: Parts of an atom Do Now: By the end of today, you will have an answer to: How do we determine the number of subatomic particles in an atom? 1. A neutron has a charge
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS
 CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
Chapter 4. History of the atom. History of Atom Smallest possible piece? Atomos - not to be cut. Atoms and their structure
 Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Looked at
Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Looked at
Conceptual Physics Matter Atoms
 Conceptual Physics Matter Atoms Lana Sheridan De Anza College July 20, 2017 Last time projectile motion orbits escape speed heliocentric model Kepler s laws review Overview matter atoms elements atomic
Conceptual Physics Matter Atoms Lana Sheridan De Anza College July 20, 2017 Last time projectile motion orbits escape speed heliocentric model Kepler s laws review Overview matter atoms elements atomic
Test Review- Final- Physical Science- Strausser Chapters 1-5. Due May 5, 2014
 Test Review- Final- Physical Science- Strausser 2013-2014- Chapters 1-5 Due May 5, 2014 Name Date Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Technology
Test Review- Final- Physical Science- Strausser 2013-2014- Chapters 1-5 Due May 5, 2014 Name Date Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Technology
4-1 Notes. Defining the Atom
 4-1 Notes Defining the Atom Early Models of the Atom All matter is composed of atoms Atoms are the smallest particles of an element that retains their identity in a chemical reaction Greek philosopher
4-1 Notes Defining the Atom Early Models of the Atom All matter is composed of atoms Atoms are the smallest particles of an element that retains their identity in a chemical reaction Greek philosopher
Atomic Structure. Defining the Atom. Defining the Atom. Sizing up the Atom. Structure of the Atom 9/18/2012
 Atomic Structure Defining the Atom Atom smallest particle of an that retains the chemical of that element 1 2 Defining the Atom The Greek philosopher Democritus (460 B.C. 370 B.C.) was among the to suggest
Atomic Structure Defining the Atom Atom smallest particle of an that retains the chemical of that element 1 2 Defining the Atom The Greek philosopher Democritus (460 B.C. 370 B.C.) was among the to suggest
Joke of the Day. Progress of the Atom. Discovering the Atom X X. CH 4- Atoms 1. Democritus Dalton Thomson. Rutherford X X Bohr X X X.
 Joke of the Day Discovering the Atom Progress of the Atom In this lesson we will take a look at the scientists who explored the wonders of the atom. QuickTime and a decompressor are needed to see this
Joke of the Day Discovering the Atom Progress of the Atom In this lesson we will take a look at the scientists who explored the wonders of the atom. QuickTime and a decompressor are needed to see this
You can call the center of the atom, the nucleus. Most atoms in our environment have a stable nucleus.
 Build an Atom Simulation Part One Learning Objectives: Draw models that show atoms Use information about the number of protons, neutrons, and electrons to Identify an element and its position on the periodic
Build an Atom Simulation Part One Learning Objectives: Draw models that show atoms Use information about the number of protons, neutrons, and electrons to Identify an element and its position on the periodic
1.1 The Fundamental Chemistry of life
 1.1 The Fundamental Chemistry of life Matter makes up everything in the universe, including all living organisms. Matter is composed of elements, a pure substance that cannot be broken down into simpler
1.1 The Fundamental Chemistry of life Matter makes up everything in the universe, including all living organisms. Matter is composed of elements, a pure substance that cannot be broken down into simpler
Atomic Pudding Models of the Atom
 Atomic Pudding Models of the Atom Think About It The drawing depicts a very tiny sample of gold taken from a gold ring. The spheres in the cube of gold are so small that they cannot be seen. What are the
Atomic Pudding Models of the Atom Think About It The drawing depicts a very tiny sample of gold taken from a gold ring. The spheres in the cube of gold are so small that they cannot be seen. What are the
Big Idea 5. What do atoms look like? Day 1. Weekly Question
 1 Nearly everything in the universe is made of matter. This paper is made of matter. You are made of matter. Even the air we breathe is made of matter. But what is matter? Matter is anything that has both
1 Nearly everything in the universe is made of matter. This paper is made of matter. You are made of matter. Even the air we breathe is made of matter. But what is matter? Matter is anything that has both
Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na
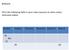 Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Science Enhanced Scope and Sequence Grade 6. Modeling the Atom
 Modeling the Atom Strand Topic Matter Investigating atoms, elements, molecules, and compounds Primary SOL 6.4 The student will investigate and understand that all matter is made up of atoms. Key concepts
Modeling the Atom Strand Topic Matter Investigating atoms, elements, molecules, and compounds Primary SOL 6.4 The student will investigate and understand that all matter is made up of atoms. Key concepts
Lesson 12: Atoms and Subatomic Particles
 NOTES Name: Date: Class: Lesson 12: Atoms and Subatomic Particles Element: fundamental substance that ; all matter consists of ~100 elements Atom: that can exist; smallest unit of an element that can enter
NOTES Name: Date: Class: Lesson 12: Atoms and Subatomic Particles Element: fundamental substance that ; all matter consists of ~100 elements Atom: that can exist; smallest unit of an element that can enter
Chapter 2 Energy, Force, and Motion Lesson 6 Describing Motion C, D; 8.2C, D; 8.4A; 8.6B
 Table of Contents Texas Essential Knowledge and Skills Correlation Chart....... 7 Chapter 1 Matter..................................... 11 Lesson 1 Atoms and Elements.......................... 12 6.5A*,
Table of Contents Texas Essential Knowledge and Skills Correlation Chart....... 7 Chapter 1 Matter..................................... 11 Lesson 1 Atoms and Elements.......................... 12 6.5A*,
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE. 4.1 Introduction to Atoms
 NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.1 Introduction to Atoms The first people to think about the nature of matter were the ancient Greeks. Around 430 B.C, Democritus, a Greek philosopher,
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.1 Introduction to Atoms The first people to think about the nature of matter were the ancient Greeks. Around 430 B.C, Democritus, a Greek philosopher,
VIII. Progression of the atomic model Democritus/Dalton --> Thomson --> Rutherford --> Bohr --> Quantum Mechanical
 HISTORY OF ATOMIC THEORY NOTES I. Ancient Greeks A. Aristotle ~ believed there were 4 elements: B. Democritus ~ believed in the "particle theory": ~ named the small particles "atoms" which means II. Dalton
HISTORY OF ATOMIC THEORY NOTES I. Ancient Greeks A. Aristotle ~ believed there were 4 elements: B. Democritus ~ believed in the "particle theory": ~ named the small particles "atoms" which means II. Dalton
What are atoms? -:-.L'::'
 What are atoms? -:-.L'::' What is the smallest particle of dust? thing you can think of? A single grain of sand? A Now try to imagine something so small that you would need millions of them to make one
What are atoms? -:-.L'::' What is the smallest particle of dust? thing you can think of? A single grain of sand? A Now try to imagine something so small that you would need millions of them to make one
The Chemistry of Life Chapter 2. Prof. J. Dodd
 The Chemistry of Life Chapter 2 Prof. J. Dodd Why should we study chemistry in C, H, O, N Atoms are composed of 3 main particles: (subatomic particles) Protons (+) Neutrons Electrons (-) Protons and Neutrons
The Chemistry of Life Chapter 2 Prof. J. Dodd Why should we study chemistry in C, H, O, N Atoms are composed of 3 main particles: (subatomic particles) Protons (+) Neutrons Electrons (-) Protons and Neutrons
1 amu 1 amu 0 amu. Chapter 2 part 1.notebook September 16, Modern Atomic Theory
 Chapter 2 The Atom Elements are the basic substances that make up all matter. An atom is the smallest particle of an element. Average atoms are 10 10 m in diameter. If you could put 6.02 x 10 23 p + and
Chapter 2 The Atom Elements are the basic substances that make up all matter. An atom is the smallest particle of an element. Average atoms are 10 10 m in diameter. If you could put 6.02 x 10 23 p + and
Chapter 2 Notes The Chemistry of Life
 Name: Chapter 2 Notes The Chemistry of Life Section 2-1 The Nature of Matter Date: Atoms (p. 35) The study of chemistry begins with the basic unit of matter, the. Comes from the Greek word atomos, meaning
Name: Chapter 2 Notes The Chemistry of Life Section 2-1 The Nature of Matter Date: Atoms (p. 35) The study of chemistry begins with the basic unit of matter, the. Comes from the Greek word atomos, meaning
The Atom. How Small Is an Atom? 318 Chapter 11 Introduction to Atoms
 Objectives Describe the size of an atom. Name the parts of an atom. Describe the relationship between numbers of protons and neutrons and atomic number. State how isotopes differ. Calculate atomic masses.
Objectives Describe the size of an atom. Name the parts of an atom. Describe the relationship between numbers of protons and neutrons and atomic number. State how isotopes differ. Calculate atomic masses.
Atoms Section 2 Section 2: The Structure of Atoms
 Section 2: The Structure of Atoms Preview Key Ideas Bellringer What Is in an Atom? Atomic Number and Mass Number Isotopes Atomic Masses Math Skills Key Ideas What is the difference between protons, neutrons,
Section 2: The Structure of Atoms Preview Key Ideas Bellringer What Is in an Atom? Atomic Number and Mass Number Isotopes Atomic Masses Math Skills Key Ideas What is the difference between protons, neutrons,
2.1 The Nature of Matter
 2.1 The Nature of Matter Lesson Objectives Identify the three subatomic particles found in atoms. Explain how all of the isotopes of an element are similar and how they are different. Explain how compounds
2.1 The Nature of Matter Lesson Objectives Identify the three subatomic particles found in atoms. Explain how all of the isotopes of an element are similar and how they are different. Explain how compounds
SCH3U1 This presentation and more can be found at
 SCH3U1 Today s Learning goals: Review history of the atomic model Practice using standard atomic notation Introduce radioisotopes This presentation and more can be found at http://lorenowicz.weebly.com
SCH3U1 Today s Learning goals: Review history of the atomic model Practice using standard atomic notation Introduce radioisotopes This presentation and more can be found at http://lorenowicz.weebly.com
Do atoms always have an equal number of protons, neutrons and electrons? 1. Yes. 2. No.
 Self Quiz Do atoms always have an equal number of protons, neutrons and electrons? 1. Yes. 2. No. Do atoms always have an equal number of protons, neutrons and electrons? 1. Yes. 2. No. A chemical bond
Self Quiz Do atoms always have an equal number of protons, neutrons and electrons? 1. Yes. 2. No. Do atoms always have an equal number of protons, neutrons and electrons? 1. Yes. 2. No. A chemical bond
CLIL Content Language Integrated Learning
 Atomos CLIL Content Language Integrated Learning PET - Preliminary English Test Teacher: Mr Pierluigi Stroppa Tutor: Mrs Angela Valentini 1 Investigating atoms You should be able to: Describe the particle
Atomos CLIL Content Language Integrated Learning PET - Preliminary English Test Teacher: Mr Pierluigi Stroppa Tutor: Mrs Angela Valentini 1 Investigating atoms You should be able to: Describe the particle
Time to develop a model
 ATOMIC THEORY ONCE UPON A TIME People have been fascinated with matter for a long time. What is matter? What is all this stuff around us made of? Can it be broken down? Are there different types of matter?
ATOMIC THEORY ONCE UPON A TIME People have been fascinated with matter for a long time. What is matter? What is all this stuff around us made of? Can it be broken down? Are there different types of matter?
Atoms to Minerals CH 5.1
 Atoms to Minerals CH 5.1 Objectives Identify the characteristics of matter Compare the particles that make up atoms of elements Describe the three types of chemical bonds Identify the characteristics of
Atoms to Minerals CH 5.1 Objectives Identify the characteristics of matter Compare the particles that make up atoms of elements Describe the three types of chemical bonds Identify the characteristics of
Introduction to Atomic Structure Multiple Choice HW
 Introduction to Atomic Structure Multiple Choice HW PSI Chemistry Name------------------------------------------- Energy and Light 1. What principle is responsible for the pattern below: 2. This principal
Introduction to Atomic Structure Multiple Choice HW PSI Chemistry Name------------------------------------------- Energy and Light 1. What principle is responsible for the pattern below: 2. This principal
Vocabulary QUIZ: 1. The total number of particles in the nucleus 2. 1 / 12
 Sep 29 11:29 AM Vocabulary QUIZ: 1. The total number of particles in the nucleus 2. 1 / 12 th of the mass of a carbon atom 3. The weighted average mass of all the isotopes of a particular element 4. A
Sep 29 11:29 AM Vocabulary QUIZ: 1. The total number of particles in the nucleus 2. 1 / 12 th of the mass of a carbon atom 3. The weighted average mass of all the isotopes of a particular element 4. A
