The Story of the Atom. A history of atomic theory over many years
|
|
|
- Nelson Lambert
- 5 years ago
- Views:
Transcription
1 The Story of the Atom A history of atomic theory over many years
2 Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up with the atomic theory, stating that "the universe is composed of two elements, the atoms and the void in which they exist and move." Democritus' theory: 1. Matter is composed of empty space through which atoms move 2. Atoms are solid, homogeneous, indestructible & indivisible. 3. Different kinds of atoms have different sizes and shapes 4. The different sizes and shapes cause the different properties of matter 5. Changes in matter are due to changes in groupings of atoms, not because the atom it self changes. Democritus thought we were all made of small solid particles that cannot be divided. He said that these were called "atomos" meaning indivisible. Without any of the scientific tools, there was no evidence to back this up, but the term "atom" and the original thought of what an atom is came from Democritus. At the same time Aristotle's theory was that all matter consists of 4 elements, fire, water, air & earth, and that the combination of these elements gives each material its qualities. This theory went on to influence the alchemists for about 2,000 years.
3 Alchemy For the next few thousand years many discoveries were made. Alchemy began around 300BC and continued until the 1700's when the science of chemistry began. The alchemists developed many of the tools we use for science today in order to pursue their dreams of finding one of two things: One goal of the alchemists was to turn "base metals" the inexpensive metals, into gold. The other goal was to find eternal life through creation of the "Philosophers stone" or the "elixir of life". Although neither of these tasks were possible, the experimentation of the alchemists lead its way into the science we now know as chemistry. Alchemists used experiment and observations to learn about the world around them. In the 18th century we began to practice modern chemistry. One main difference was that Chemistry was quantitative, meaning that everything was measured and could be studied that way. Alchemy was primarily Qualitative, looking merely at the physical outcome and physical properties, without significant measurement. Realizing that "air" was made of "gasses" was a huge turning point, because it meant that Aristotle was wrong about air being an element itself. The ability to measure everything that went into and out of an experiment was a significant change in science, leading to the Quantitative science of chemistry, where the temperature and mass of all substances was recorded during reactions. You will learn about some of the key chemists in the transition from Alchemy to Chemistry throughout the school year. Robert Boyle objected to the Aristotelian elements. Antoine Lavoisier began "the chemical revolution" with his law of conservation of Mass, stating that no matter can be created or destroyed. It is then, in 1773, that Chemistry was truly born. Robert Boyle Antoine Lavoisier
4 Dalton In 1803, John Dalton wanted to condense the information that was known about the atom. Through many quantitative experments, he was able to discover mass ratios of elements involved in reactions, leading to further development of the ideas that Democritus had two thousand years earlier. He ended up with 5 statements that make up Dalton's atomic theory. DALTON'S ATOMIC THEORY: All matter is made of extremely tiny particles called atoms 2. All atoms of a given element are identical in mass and properties, and they are different from any other element 3. Atoms cannot be created, divided into smaller particles or destroyed. 4. Atoms combine in simple whole number ratios to form compounds 5. In chemical reactions, atoms are separated, combined or rearranged. Although we have found some exceptions for the above rules in the last 200+ years (isotopes, nuclear reactions & subparticles), these are key ideas that were used as the foundation of modern chemistry. Dalton considered the atom to be a sphere, and that the atom was the smallest possible particle.
5 JJ. Thompson Scientists like to study the unknown. If you have ever been shocked when you touched a friend or a piece of metal and wondered what just happened, you are not unlike JJ Thompson and other early scientists who decided to study this phenomenon. What they did was take a glass tube and pump most of the air out of it. They then passed electricity through the tube. They found that the electricity formed rays from the cathode to the anode across the tube, giving it the name Cathode Ray Tube or CRT. By the end of the 1800's they had found that these "rays" were actually a stream of negatively charged particles. In the 1890's JJ Thompson decided to find out how much mass each particle had when compared to it's charge. The amazing thing was that JJ Thompson found out that the mass of one of these particles was smaller than the Hydrogen atom... but at the time it was thought that the Hydrogen atom was the smallest possible particle! This meant that this particle, called the electron, was smaller than an atom, and was part of what made up an atom. JJ Thompson had disproved Dalton and found that Atoms were divisible into smaller subatomic particles, because the electron was one of them! BEFORE JJ Thompson: DISCOVERY: 1890 After JJ Thompson Electrons are a sub atomic particle Atoms are the smallest particle Plumb Pudding Model: Atoms are made of negative electrons in a positive sphere.
6 Ernest Rutherford Rutherford wanted to find out what happens when you pass alpha particles (emitted from a radioactive source) were passed through an atom. Because gold could be made very very thin without breaking, he decided a very thin piece of gold foil would be the perfect way to do his experiment. If the radioactive source was in a small box with one hole, the alpha particles would make a stream. Rutherford thought the atom looked like JJ Thompson's plumb pudding model, and so he thought that most of these positive alpha particles would go right through, but some would pass close enough to electrons for them to shift their path slightly. When he began his experiment and some of the particles bounced backwards he said "it is like shooting a missile at a piece of paper and having it bounce back at you" because the actual results were so unexpected. The alpha particle was very large and the only way for it to bounce back is if there was something with greater density inside the atom. Based on these findings, the atom could not look like JJ Thompson had modeled it. Rutherford considered the data in order to come up with a new atomic model. The only way this could have happened is if there was a tiny dense region he called the nucleus in the center of the atom. He concluded it contained all of the positive charge and most of its mass. He said that the positively charged particles in the nucleus were protons. The rest of the atom, about % of its volume, was empty space! Before Rutherford: Discoveries After Rutherford: Dense nucleus contains positive protons The atom is 99.99% empty space Plumb Pudding Model: Atoms are made of negative electrons in a positive sphere. Rutherford's Model includes a central nucleus made of protons and electrons moving through empty space
7 Niels Bohr When Rutherford discovered the nucleus, it was known that a new model of the atom was needed. Bohr created his idea of the atomic model in 1915, and it can also be called the "planetary model" of the atom. The electrons orbit the nucleus like planets orbiting the sun. No actual image of this model can be "to scale" because if the atom was 2 football fields in diameter, the nucleus would be the size of a nickel. This means hat no model of the atom is actually to scale. The key to the Bohr model is that the orbits are quantized, meaning they are at specific distances from the nucleus, with no orbitals (no electrons) in between. Each orbital has an energy level. If the electrons are in the lowest available orbital they are in the ground state, but they can move up to higher levels if they are excited by energy. Each element has different distances between the nucleus and orbitals, giving that element specific properties. You will study this more in depth when you find out more about electron configuration, but the organization of electrons into these "orbitals" is the key to many properties of each type of atom. Before Bohr Discovery: 1915 After Bohr Bohr merged the ideas of Rutherford and Plank to build a model of the atom explaining the behavior of electrons Rutherford's Model includes a central nucleus made of protons and electrons moving through empty space The Bohr model shows electrons orbiting around the nucleus at quantized levels
8 James Chadwick Chadwick worked in Rutherford's lab, where they bombarded all kinds of things with alpha particles, studying the atomic nuclei. He set up an experiment where he shot alpha particles at beryllium foil, which caused an uncharged radiation to be emitted. This radiation hit the wax, which bumped the protons off of the wax. Using charges he was able to discover that the radiation from the beryllium was not charged, however it had mass. This was the discovery of a neutral particle with mass that Chadwick called the neutron. Chadwick worked with Hans Geiger (of the Geiger counter) until he was imprisoned in Germany during WWI, then moved to America and eventually went on to be part of the Manhattan project, developing the world's first atomic weapons. Before Chadwick The Bohr model shows electrons orbiting around the nucleus at quantized levels Discovery: 1932 The atom contains neutral particles with large mass in the nucleus. These are called neutrons. After Chadwick The nucleus now has protons AND neutrons.
9 Schrodinger The Bohr model continues to be a helpful way to imagine the distribution of electrons and some of the behaviors of electrons, but Schrodinger set out to include more information about the orbitals and the dual wave- particle nature of the electron. His model is based on probability, because we cannot know where an electron is, but rather where it might be. Schrodinger uses quantum numbers as coordinates to describe the size, shape and orientation in space of the orbitals. His model is known as the Quantum Mechanical Model of the atom. The probable area of finding an electron is represented by an electron cloud in the shape of the orbital. Each electron pair's probability is represented by an orbital cloud and these orbitals are all layered over one another to make the atom: Before Schrodinger The Bohr model shows electrons orbiting the nucleus Discovery: 1926 The electrons behave based on probability and can be thought of as a particle or a wave After Schrodinger The current model of the atom is known to have orbital clouds representing electrons probable locations.
10 This booklet was created by Jewyl Clarke using Glencoe's Matter and Change as well as the following references history- of- the- atom.wikispaces.com/ postulates.shtml bio.html false- false- false- in- x- none- ar.html
Get out your diagram from your research paper. Get out a sheet of paper to take some notes on.
 Bellwork: Get out your diagram from your research paper. Get out a sheet of paper to take some notes on. Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons
Bellwork: Get out your diagram from your research paper. Get out a sheet of paper to take some notes on. Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons
Dalton Thompson Rutherford Bohr Modern Model ("Wave. Models of the Atom
 Dalton Thompson Rutherford Bohr Modern Model ("Wave Models of the Atom Mechanical" Model) Aim: To discuss the scientists and their contributions to the current atomic model. Focus: Rutherford's Gold Foil
Dalton Thompson Rutherford Bohr Modern Model ("Wave Models of the Atom Mechanical" Model) Aim: To discuss the scientists and their contributions to the current atomic model. Focus: Rutherford's Gold Foil
Atomic Structure. For thousands of years, people had many ideas about matter Ancient Greeks believed that everything was made up of the four elements
 An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
SNC1D1 History of the Atom
 SNC1D1 History of the Atom What is the atom? Atoms are the building block for all matter: Atoms make up elements! Elements combine to make compounds!2 ATOMIC MODEL TIMELINE 400 B.C PRESENT DAY ATOMIC MODEL
SNC1D1 History of the Atom What is the atom? Atoms are the building block for all matter: Atoms make up elements! Elements combine to make compounds!2 ATOMIC MODEL TIMELINE 400 B.C PRESENT DAY ATOMIC MODEL
The History of Atomic Theory Chapter 3--Chemistry
 The History of Atomic Theory Chapter 3--Chemistry In this lesson, we ll learn about the men whose quests for knowledge about the fundamental nature of the universe helped define our views. The atomic model
The History of Atomic Theory Chapter 3--Chemistry In this lesson, we ll learn about the men whose quests for knowledge about the fundamental nature of the universe helped define our views. The atomic model
Atomic Models. A model uses familiar ideas to explain unfamiliar facts observed in nature. A model can be changed as new information is collected.
 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. Atomic Models A model uses familiar ideas
This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. Atomic Models A model uses familiar ideas
Atomic Theory Timeline
 Atomic Theory Timeline Democritus 450 B.C. Democritus was a Greek philosopher who came to the conclusion that everything was made up of tiny particles. He used the term atomos. Unfortunately, since Democritus
Atomic Theory Timeline Democritus 450 B.C. Democritus was a Greek philosopher who came to the conclusion that everything was made up of tiny particles. He used the term atomos. Unfortunately, since Democritus
SNC1D CHEMISTRY 2/8/2013. ATOMS, ELEMENTS, & COMPOUNDS L Atomic Theory (P ) Atomic Theory. Atomic Theory
 SNC1D CHEMISTRY ATOMS, ELEMENTS, & COMPOUNDS L Atomic Theory (P.168-175) Atomic Theory Thousands of years ago Greek philosophers were asking themselves questions like, If you take a gold bar and cut it
SNC1D CHEMISTRY ATOMS, ELEMENTS, & COMPOUNDS L Atomic Theory (P.168-175) Atomic Theory Thousands of years ago Greek philosophers were asking themselves questions like, If you take a gold bar and cut it
Atomic Theory. Democritus to the Planetary Model
 Atomic Theory Democritus to the Planetary Model Democritus Greek philosopher (460-370 BCE) Believed in the philosophy of materialism With Leucippus, they though that matter can not be divided infinitely.
Atomic Theory Democritus to the Planetary Model Democritus Greek philosopher (460-370 BCE) Believed in the philosophy of materialism With Leucippus, they though that matter can not be divided infinitely.
Atomic Structure. A model uses familiar ideas to explain unfamiliar facts observed in nature.
 Atomic Structure 1 2 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. A model uses familiar
Atomic Structure 1 2 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. A model uses familiar
Scientists to Know CHADWICK THOMSON RUTHERFORD DEMOCRITUS BOHR HEISENBERG DALTON
 Unit 1: Atoms Scientists to Know CHADWICK THOMSON RUTHERFORD DEMOCRITUS BOHR HEISENBERG DALTON The History of Discovering the Atom The Timeline of Discovery 1. Philosophical Era 2. Alchemical Era 3. Classical
Unit 1: Atoms Scientists to Know CHADWICK THOMSON RUTHERFORD DEMOCRITUS BOHR HEISENBERG DALTON The History of Discovering the Atom The Timeline of Discovery 1. Philosophical Era 2. Alchemical Era 3. Classical
Particle Theory of Matter. By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that:
 Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
Atomic Theory. The History of Atomic Theory
 Atomic Theory The History of Atomic Theory This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels.
Atomic Theory The History of Atomic Theory This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels.
Nuclear Chemistry. Atomic Structure Notes Start on Slide 20 from the second class lecture
 Nuclear Chemistry Atomic Structure Notes Start on Slide 20 from the second class lecture The Birth of an Idea Democritus, 400 B.C. coined the term atom If you divide matter into smaller and smaller pieces,
Nuclear Chemistry Atomic Structure Notes Start on Slide 20 from the second class lecture The Birth of an Idea Democritus, 400 B.C. coined the term atom If you divide matter into smaller and smaller pieces,
Investigating Atoms and Atomic Theory
 Investigating Atoms and Atomic Theory Students should be able to: Describe the particle theory of matter. PS.2a Use the Bohr model to differentiate among the three basic particles in the atom (proton,
Investigating Atoms and Atomic Theory Students should be able to: Describe the particle theory of matter. PS.2a Use the Bohr model to differentiate among the three basic particles in the atom (proton,
Chemistry. Robert Taggart
 Chemistry Robert Taggart Table of Contents To the Student..................................................v Unit 1: Matter and Measurement Lesson 1: Chemistry and the Scientific Method...................3
Chemistry Robert Taggart Table of Contents To the Student..................................................v Unit 1: Matter and Measurement Lesson 1: Chemistry and the Scientific Method...................3
The structure of Atom III
 The structure of Atom III Atomic Structure If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement
The structure of Atom III Atomic Structure If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement
Major upsetting discoveries: Today s Objectives/Agenda. Notice: New Unit: with Ms. V. after school Before Friday 9/22.
 Bellwork Monday 9 18 17 Tuesday 9 19 17 *This should be the last box on bellwork: 1. What are some major points in history where common knowledge was upset by a new discovery? 2. Draw what you think an
Bellwork Monday 9 18 17 Tuesday 9 19 17 *This should be the last box on bellwork: 1. What are some major points in history where common knowledge was upset by a new discovery? 2. Draw what you think an
An Introduction to Atomic Theory. VCE Chemistry Unit 1: The Big Ideas of Chemistry Area of Study 1 The Periodic Table
 An Introduction to Atomic Theory VCE Chemistry Unit 1: The Big Ideas of Chemistry Area of Study 1 The Periodic Table From Democritus to Dalton Two thousand years ago, Democritus proposed that matter consisted
An Introduction to Atomic Theory VCE Chemistry Unit 1: The Big Ideas of Chemistry Area of Study 1 The Periodic Table From Democritus to Dalton Two thousand years ago, Democritus proposed that matter consisted
Atomic Theory. Past and Present: pieces of a puzzle
 Atomic Theory Past and Present: pieces of a puzzle The First Atomic Hypothesis Democritus (460 370 BC): Greek philosopher Speculated that matter is composed of atoms which move through empty space Atoms
Atomic Theory Past and Present: pieces of a puzzle The First Atomic Hypothesis Democritus (460 370 BC): Greek philosopher Speculated that matter is composed of atoms which move through empty space Atoms
Chapter 4. Atomic Structure
 Chapter 4 Atomic Structure Warm Up We have not yet discussed this material, but what do you know already?? What is an atom? What are electron, neutrons, and protons? Draw a picture of an atom from what
Chapter 4 Atomic Structure Warm Up We have not yet discussed this material, but what do you know already?? What is an atom? What are electron, neutrons, and protons? Draw a picture of an atom from what
Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video
 Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video 2 CH 4- Atoms 1 Discovering the Atom In this lesson we will take a look at the scientists who explored the
Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video 2 CH 4- Atoms 1 Discovering the Atom In this lesson we will take a look at the scientists who explored the
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS
 CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
CHEMISTRY. Matter and Change. Table Of Contents. Section 4.1 Early Ideas About Matter. Unstable Nuclei and Radioactive Decay
 CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
The History of the Atom. How did we learn about the atom?
 The History of the Atom How did we learn about the atom? The Atomic Theory of Matter All matter is made up of fundamental particles. What does fundamental mean? The Greek Philosophers, 400 B.C. Democritus
The History of the Atom How did we learn about the atom? The Atomic Theory of Matter All matter is made up of fundamental particles. What does fundamental mean? The Greek Philosophers, 400 B.C. Democritus
HISTORY OF THE ATOM ATOMA
 S.MORRIS 2006 HISTORY OF THE ATOM 460 BC Democritus develops the idea of atoms he pounded up materials in his pestle and mortar until he had reduced them to smaller and smaller particles which he called
S.MORRIS 2006 HISTORY OF THE ATOM 460 BC Democritus develops the idea of atoms he pounded up materials in his pestle and mortar until he had reduced them to smaller and smaller particles which he called
Chapter 4. The structure of the atom. AL-COS Objectives 1, 2,3,4,7, 10, 15, 20, 21, 22, 27and 28
 Chapter 4 The structure of the atom AL-COS Objectives 1, 2,3,4,7, 10, 15, 20, 21, 22, 27and 28 You ll learn to Identify the experiments that led to the development of the nuclear model of atomic structure
Chapter 4 The structure of the atom AL-COS Objectives 1, 2,3,4,7, 10, 15, 20, 21, 22, 27and 28 You ll learn to Identify the experiments that led to the development of the nuclear model of atomic structure
Early Ideas About Matter
 Early Ideas About Matter Democritus (460 370 BC) believed that matter is made of small, solid objects called atomos, from which the English word atom is derived. Early Ideas About Matter (cont.) Aristotle
Early Ideas About Matter Democritus (460 370 BC) believed that matter is made of small, solid objects called atomos, from which the English word atom is derived. Early Ideas About Matter (cont.) Aristotle
Rhonda Alexander IC Science Robert E. Lee
 Rhonda Alexander IC Science Robert E. Lee Atom The smallest particle of an element that retains all of the chemical properties of the element. The Theory & Evidence for John Dalton s Atomic Theory: Around
Rhonda Alexander IC Science Robert E. Lee Atom The smallest particle of an element that retains all of the chemical properties of the element. The Theory & Evidence for John Dalton s Atomic Theory: Around
Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Early Ideas About Matter A. atom the smallest particle of an element retaining
Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Early Ideas About Matter A. atom the smallest particle of an element retaining
EARLY VIEWS: The Ancient Greeks
 Feb 7 11:59 AM EARLY VIEWS: The Ancient Greeks Empedocles (c. 450 B.C.) proposed Four Element theory he thought that matter was composed of four elements: AIR, EARTH, FIRE and WATER elements mixed together
Feb 7 11:59 AM EARLY VIEWS: The Ancient Greeks Empedocles (c. 450 B.C.) proposed Four Element theory he thought that matter was composed of four elements: AIR, EARTH, FIRE and WATER elements mixed together
Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester?
 Chemistry Ms. Ye Name Date Block Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester? Atoms Video: 1. Proper Portioned Giant Atom Model of Science: Structure
Chemistry Ms. Ye Name Date Block Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester? Atoms Video: 1. Proper Portioned Giant Atom Model of Science: Structure
Origins of the Atom. Atoms: The Building Blocks of Matter. Let s Get Ready to Rumble. Aristotle s Theory of the Atom CHAPTER 3
 Origins of the Atom CHAPTER 3 Atoms: The Building Blocks of Matter Let s Get Ready to Rumble The idea of the atom was met with great skepticism, especially among great thinkers. The most vocal critic of
Origins of the Atom CHAPTER 3 Atoms: The Building Blocks of Matter Let s Get Ready to Rumble The idea of the atom was met with great skepticism, especially among great thinkers. The most vocal critic of
DEMOCRITUS - A philosopher in the year 400 B.C. - He didn t do experiments and he wondered if atoms kept on being divided, that there would only be
 DEMOCRITUS A philosopher in the year 400 B.C. He didn t do experiments and he wondered if atoms kept on being divided, that there would only be one undividable particle left. He discovered that this was
DEMOCRITUS A philosopher in the year 400 B.C. He didn t do experiments and he wondered if atoms kept on being divided, that there would only be one undividable particle left. He discovered that this was
1. Based on Dalton s evidence, circle the drawing that demonstrates Dalton s model.
 Various models of the ATOM Dalton Model John Dalton developed the first atomic model in 1808. Before him people, mostly philosophers, had speculated about the smallest unit of matter and two theories prevailed.
Various models of the ATOM Dalton Model John Dalton developed the first atomic model in 1808. Before him people, mostly philosophers, had speculated about the smallest unit of matter and two theories prevailed.
Atoms, Elements, and the Periodic Table
 chapter 00 3 3 Atoms, Elements, and the Periodic Table section 1 Structure of Matter Before You Read Take a deep breath. What fills your lungs? Can you see it or hold it in your hand? What You ll Learn
chapter 00 3 3 Atoms, Elements, and the Periodic Table section 1 Structure of Matter Before You Read Take a deep breath. What fills your lungs? Can you see it or hold it in your hand? What You ll Learn
PROGRESSION OF THE ATOMIC MODEL
 PROGRESSION OF THE ATOMIC MODEL By 1808, it was widely accepted that matter was made up of ELEMENTS, which consisted of tiny PARTICLES called ATOMS. After 2000 years - DEMOCRITUS was right all along John
PROGRESSION OF THE ATOMIC MODEL By 1808, it was widely accepted that matter was made up of ELEMENTS, which consisted of tiny PARTICLES called ATOMS. After 2000 years - DEMOCRITUS was right all along John
H CHEM - WED, 9/7/16. Do Now Be ready for notes. Sigfig review problem. Agenda Atomic Theory. Homework. Error Analysis
 H CHEM - WED, 9/7/16 Do Now Be ready for notes. Sigfig review problem Agenda Atomic Theory Error Analysis Homework Possibly atomic theory paragraph THE ATOM DEFINITION TO START Atom smallest particle
H CHEM - WED, 9/7/16 Do Now Be ready for notes. Sigfig review problem Agenda Atomic Theory Error Analysis Homework Possibly atomic theory paragraph THE ATOM DEFINITION TO START Atom smallest particle
democritus (~440 bc) who was he? theorized: A Greek philosopher
 democritus (~440 bc) who was he? A Greek philosopher theorized: Everything in the world is made up small particles that we cannot see The shape of these particles determine the properties of a substance
democritus (~440 bc) who was he? A Greek philosopher theorized: Everything in the world is made up small particles that we cannot see The shape of these particles determine the properties of a substance
Chemistry Chapter 3. Atoms: The Building Blocks of Matter
 Chemistry Chapter 3 Atoms: The Building Blocks of Matter I. From Philosophical Idea to Scientific Theory History of the Atom The Ancient Greeks were the first to come up with the idea of the atom. Democritus
Chemistry Chapter 3 Atoms: The Building Blocks of Matter I. From Philosophical Idea to Scientific Theory History of the Atom The Ancient Greeks were the first to come up with the idea of the atom. Democritus
Glencoe: Chapter 4. The Structure of the Atom
 Glencoe: Chapter 4 The Structure of the Atom Section One: Early Ideas about Matter Atomists and Democritus : 400 B.C. From Thrace in Greece. Atoms- Uncut-Table Indivisible parts which cannot be broken
Glencoe: Chapter 4 The Structure of the Atom Section One: Early Ideas about Matter Atomists and Democritus : 400 B.C. From Thrace in Greece. Atoms- Uncut-Table Indivisible parts which cannot be broken
Bellwork: 2/6/2013. atom is the. atom below. in an atom is found in the. mostly. 2. The smallest part of an. 1. Label the parts of the
 Bellwork: 2/6/2013 1. Label the parts of the atom below. B 2. The smallest part of an atom is the. 3. The majority of the mass in an atom is found in the. A C 4. An atom is made up of mostly. Bellwork:
Bellwork: 2/6/2013 1. Label the parts of the atom below. B 2. The smallest part of an atom is the. 3. The majority of the mass in an atom is found in the. A C 4. An atom is made up of mostly. Bellwork:
CLIL Content Language Integrated Learning
 Atomos CLIL Content Language Integrated Learning PET - Preliminary English Test Teacher: Mr Pierluigi Stroppa Tutor: Mrs Angela Valentini 1 Investigating atoms You should be able to: Describe the particle
Atomos CLIL Content Language Integrated Learning PET - Preliminary English Test Teacher: Mr Pierluigi Stroppa Tutor: Mrs Angela Valentini 1 Investigating atoms You should be able to: Describe the particle
Democritus of Abdera. John Dalton. Dalton s Atom. Dalton s Atomic Theory Ancient Greece - 4th century BC. Eaglesfield, England
 Democritus of Abdera Ancient Greece - 4th century BC first suggested the existence of tiny fundamental particles that make up matter. atoms = indestructible did not agree with the current sci theory -
Democritus of Abdera Ancient Greece - 4th century BC first suggested the existence of tiny fundamental particles that make up matter. atoms = indestructible did not agree with the current sci theory -
"Brief" History of the Atom About two thousand years in under 15 minutes
 "Brief" History of the Atom About two thousand years in under 15 minutes Chemistry C2 Structure and properties of atoms Atom: Smallest particle of an element that retains its identity in a chemical reaction
"Brief" History of the Atom About two thousand years in under 15 minutes Chemistry C2 Structure and properties of atoms Atom: Smallest particle of an element that retains its identity in a chemical reaction
1 The Development of Atomic Theory
 CHAPTER 4 1 The Development of Atomic Theory SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What scientists helped to develop atomic theory? What part of atoms did Thomson
CHAPTER 4 1 The Development of Atomic Theory SECTION Atoms KEY IDEAS As you read this section, keep these questions in mind: What scientists helped to develop atomic theory? What part of atoms did Thomson
Democritus thought atoms were indivisible & indestructible Lacked experimental support 4 th century B.C.
 Chapter 5 Democritus thought atoms were indivisible & indestructible Lacked experimental support 4 th century B.C. Democritus thought atoms were indivisible & indestructible Lacked experimental support
Chapter 5 Democritus thought atoms were indivisible & indestructible Lacked experimental support 4 th century B.C. Democritus thought atoms were indivisible & indestructible Lacked experimental support
AP Atomic Structure Models
 AP Atomic Structure Models What is a Model? On a scrap piece of paper, write down your definition of a model with at least two examples. A model is a representation of an object, idea, action, or concept.
AP Atomic Structure Models What is a Model? On a scrap piece of paper, write down your definition of a model with at least two examples. A model is a representation of an object, idea, action, or concept.
Atomic Structure. History of Atomic Theory
 Atomic Structure History of Atomic Theory Democritus (460-370 BC) Was the to come up with the idea of atom Believed that all matter was composed of Which is derived from the Greek word Atomos meaning He
Atomic Structure History of Atomic Theory Democritus (460-370 BC) Was the to come up with the idea of atom Believed that all matter was composed of Which is derived from the Greek word Atomos meaning He
Atomic Theory. Introducing the Atomic Theory:
 Atomic Theory Chemistry is the science of matter. Matter is made up of things called atoms, elements, and molecules. But have you ever wondered if atoms and molecules are real? Would you be surprised to
Atomic Theory Chemistry is the science of matter. Matter is made up of things called atoms, elements, and molecules. But have you ever wondered if atoms and molecules are real? Would you be surprised to
Scientist wanted to understand how the atom looked. It was known that matter was neutral. It was known that matter had mass
 Atom Models Scientist wanted to understand how the atom looked It was known that matter was neutral It was known that matter had mass They used these ideas to come up with their models, however science
Atom Models Scientist wanted to understand how the atom looked It was known that matter was neutral It was known that matter had mass They used these ideas to come up with their models, however science
The Development of Atomic Theory
 The Development of Atomic Theory Ideas & Theories in Science Change Our theory about the atom has changed over time as new studies are done. Even though no one has ever seen an atom up close we are still
The Development of Atomic Theory Ideas & Theories in Science Change Our theory about the atom has changed over time as new studies are done. Even though no one has ever seen an atom up close we are still
The Atom. protons, neutrons, and electrons oh my!
 The Atom protons, neutrons, and electrons oh my! What s an Atom? An atom is the smallest physical particle of an element that still retains the properties of that element. How Big is an Atom? At sea level,
The Atom protons, neutrons, and electrons oh my! What s an Atom? An atom is the smallest physical particle of an element that still retains the properties of that element. How Big is an Atom? At sea level,
Unit 3 Lesson 1 The Atom. Copyright Houghton Mifflin Harcourt Publishing Company
 As a Matter of Fact What makes up matter? The Greek philosopher Democritus thought matter could be divided into smaller units until you obtained a particle that could not be cut. He called this particle
As a Matter of Fact What makes up matter? The Greek philosopher Democritus thought matter could be divided into smaller units until you obtained a particle that could not be cut. He called this particle
Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester?
 Chemistry Ms. Ye Name Date Block Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester? Atoms Video: 1. Proper Portioned Giant Atom Model of Science: Structure
Chemistry Ms. Ye Name Date Block Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester? Atoms Video: 1. Proper Portioned Giant Atom Model of Science: Structure
What is matter? Matter is anything that has mass and takes up space. Matter is made up of atoms.
 Matter What is matter? Matter is anything that has mass and takes up space. Matter is made up of atoms. Is it matter? Can you measure the object? Does it take up space? Does the object have a mass? Come
Matter What is matter? Matter is anything that has mass and takes up space. Matter is made up of atoms. Is it matter? Can you measure the object? Does it take up space? Does the object have a mass? Come
The origins of atomic theory
 Models of the atom It is important to realise that a lot of what we know about the structure of atoms has been developed over a long period of time. This is often how scientific knowledge develops, with
Models of the atom It is important to realise that a lot of what we know about the structure of atoms has been developed over a long period of time. This is often how scientific knowledge develops, with
Atomic Theory. Developing the Nuclear Model of the Atom. Saturday, January 20, 18
 Atomic Theory Developing the Nuclear Model of the Atom Democritus Theory: Atom, the indivisible particle c. 300 BC Democritus Problem: No scientific evidence c. 300 BC Dalton Theory: The solid sphere model
Atomic Theory Developing the Nuclear Model of the Atom Democritus Theory: Atom, the indivisible particle c. 300 BC Democritus Problem: No scientific evidence c. 300 BC Dalton Theory: The solid sphere model
SCH3U1 This presentation and more can be found at
 SCH3U1 Today s Learning goals: Review history of the atomic model Practice using standard atomic notation Introduce radioisotopes This presentation and more can be found at http://lorenowicz.weebly.com
SCH3U1 Today s Learning goals: Review history of the atomic model Practice using standard atomic notation Introduce radioisotopes This presentation and more can be found at http://lorenowicz.weebly.com
Atomic Structure. How do you discover and study something you can t see?
 Atomic Structure How do you discover and study something you can t see? WHAT IS A THEORY? A hypothesis is a proposed explanation made as a starting point for further investigation (It s bright outside
Atomic Structure How do you discover and study something you can t see? WHAT IS A THEORY? A hypothesis is a proposed explanation made as a starting point for further investigation (It s bright outside
History of the OBJECTIVES. ESSENTIAL QUESTION What evidence is there for the existence of atoms and their sub-atomic particles?
 History of the 09/15/2016 OBJECTIVES Understand the law of definite proportions. Define a scientific law and identify how observations become a law. Explain that a scientific theory is not established
History of the 09/15/2016 OBJECTIVES Understand the law of definite proportions. Define a scientific law and identify how observations become a law. Explain that a scientific theory is not established
Ancient Greek Models of Atoms
 Atomic Theory Ancient Greek Models of Atoms The philosopher Democritus believed that all matter consisted of extremely small particles that could not be divided. He called these particles atoms from the
Atomic Theory Ancient Greek Models of Atoms The philosopher Democritus believed that all matter consisted of extremely small particles that could not be divided. He called these particles atoms from the
What is matter? Matter is anything that has mass and takes up space. What is matter made of??
 What is matter? Matter is anything that has mass and takes up space What is matter made of?? Atoms. All matter is made of atoms. Atoms are the building blocks of Matter Remember???? The Cell theory - 3
What is matter? Matter is anything that has mass and takes up space What is matter made of?? Atoms. All matter is made of atoms. Atoms are the building blocks of Matter Remember???? The Cell theory - 3
Atomic Theory Development
 Atomic Theory Development Born as early as 400 BC, it took more than 2000 years before Science was ready to accept the idea of atomic structure of matter and another 150 years to develop a good model!
Atomic Theory Development Born as early as 400 BC, it took more than 2000 years before Science was ready to accept the idea of atomic structure of matter and another 150 years to develop a good model!
Atomic theory. Atoms: The Building Blocks of Matter
 Atomic theory Atoms: The Building Blocks of Matter First, there was Democritus Democritus was a Greek philosopher atomos He came up with the idea of the atom around 400BCE He had no evidence, he just thought
Atomic theory Atoms: The Building Blocks of Matter First, there was Democritus Democritus was a Greek philosopher atomos He came up with the idea of the atom around 400BCE He had no evidence, he just thought
Topic III Quest Study Guide
 Topic III Quest Study Guide A. Early Concepts: Democritus: Democritus: Greek Philosopher 400 B.C. Matter is composed of atoms, which move through empty space Atoms are solid, homogeneous indestructible
Topic III Quest Study Guide A. Early Concepts: Democritus: Democritus: Greek Philosopher 400 B.C. Matter is composed of atoms, which move through empty space Atoms are solid, homogeneous indestructible
7.1 Development of a Modern Atomic Theory
 7.1 Development of a Modern Atomic Theory Development of the Atomic Theory Many scientists in different countries have contributed to the understanding of matter - atoms John Dalton Credited with developing
7.1 Development of a Modern Atomic Theory Development of the Atomic Theory Many scientists in different countries have contributed to the understanding of matter - atoms John Dalton Credited with developing
Atom s Structure. Chemistry is the study of the structure, characteristics and behavior of matter. The basic
 AlMarzooqi 1 Hamad AlMarzooqi Professor Kafle ESL 015 26 February 2013 Atom s Structure Chemistry is the study of the structure, characteristics and behavior of matter. The basic unit of matter is an atom,
AlMarzooqi 1 Hamad AlMarzooqi Professor Kafle ESL 015 26 February 2013 Atom s Structure Chemistry is the study of the structure, characteristics and behavior of matter. The basic unit of matter is an atom,
Greek Philosophers (cont.)
 Greek Philosophers (cont.) Many ancient scholars believed matter was composed of such things as earth, water, air, and fire. Many believed matter could be endlessly divided into smaller and smaller pieces.
Greek Philosophers (cont.) Many ancient scholars believed matter was composed of such things as earth, water, air, and fire. Many believed matter could be endlessly divided into smaller and smaller pieces.
9/23/2012. Democritus 400 B.C. Greek philosopher Proposed that all materials are made from atoms. Coined Greek word atmos, meaning indivisible.
 Mr. Sudbury Atoms are too small to see with your eyes. Atoms are too small to see with the most powerful microscopes. Scientist use models to explain atoms. A scientific model is an representation containing
Mr. Sudbury Atoms are too small to see with your eyes. Atoms are too small to see with the most powerful microscopes. Scientist use models to explain atoms. A scientific model is an representation containing
The following is a quote by Democritus (c. 460 c. 370 bce). Paraphrase this quote in your own words in your science journal.
 Section 1 Development of the Atomic Theory Bellringer The following is a quote by Democritus (c. 460 c. 370 bce). Paraphrase this quote in your own words in your science journal. Color exists by convention,
Section 1 Development of the Atomic Theory Bellringer The following is a quote by Democritus (c. 460 c. 370 bce). Paraphrase this quote in your own words in your science journal. Color exists by convention,
Democritus & Leucippus (~400 BC) Greek philosophers: first to propose that matter is made up of particles called atomos, the Greek word for atoms
 AP Chemistry Ms. Ye Name Date Block The Evolution of the Atomic Model Since atoms are too small to see even with a very powerful microscope, scientists rely upon indirect evidence and models to help them
AP Chemistry Ms. Ye Name Date Block The Evolution of the Atomic Model Since atoms are too small to see even with a very powerful microscope, scientists rely upon indirect evidence and models to help them
Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na
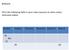 Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
1 Development of the Atomic Theory
 CHAPTER 11 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
CHAPTER 11 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
Evolution of Atomic Theory
 Evolution of Atomic Theory Mrs. Baldessari Chemistry Scientists that changed our view of the atom?greatest Chemistry Discoveries?? YouTube Aristotle What: All matter is a combo of fire, air, earth or water
Evolution of Atomic Theory Mrs. Baldessari Chemistry Scientists that changed our view of the atom?greatest Chemistry Discoveries?? YouTube Aristotle What: All matter is a combo of fire, air, earth or water
The term valent means outermost, therefore only the electrons on the outermost shell are called valent.
 The atom is the smallest basic unit of an element which possesses the properties of the element. Structure of the Atom The term valent means outermost, therefore only the electrons on the outermost shell
The atom is the smallest basic unit of an element which possesses the properties of the element. Structure of the Atom The term valent means outermost, therefore only the electrons on the outermost shell
Early Ideas about Matter
 Early Ideas about Matter atom The smallest piece of the element with all the chemical properties of the element an old and new idea Greeks Discontinuists Democritus Continuists Aristotle Discontinuists
Early Ideas about Matter atom The smallest piece of the element with all the chemical properties of the element an old and new idea Greeks Discontinuists Democritus Continuists Aristotle Discontinuists
Make sure this is handed in!
 Make sure this is handed in! Based on the 3 groups in early atomic history, pick one of the groups and explain how they progressed the current knowledge of atoms and elements at their time. OR Explain
Make sure this is handed in! Based on the 3 groups in early atomic history, pick one of the groups and explain how they progressed the current knowledge of atoms and elements at their time. OR Explain
CHAPTER 4 Atomic Structure
 CHAPTER 4 Atomic Structure 4.1 Early Theories of Matter Earth, Water, Air, Fire Matter was thought to be infinitely divisible No method was available to test theories Democritus (460 B.C. 370 B.C.) First
CHAPTER 4 Atomic Structure 4.1 Early Theories of Matter Earth, Water, Air, Fire Matter was thought to be infinitely divisible No method was available to test theories Democritus (460 B.C. 370 B.C.) First
Early Atomic Theory. Alchemy. The atom
 Early Atomic Theory Chapter 3 Democritus 460 BC- ~ 370 BC Nothing exists except atoms and empty space; everything else is opinion. Matter is composed of small indivisible particles, atomos meaning Indivisible
Early Atomic Theory Chapter 3 Democritus 460 BC- ~ 370 BC Nothing exists except atoms and empty space; everything else is opinion. Matter is composed of small indivisible particles, atomos meaning Indivisible
Name: Block Unit 3- The Atom
 Name: Block Unit 3- The Atom DEMOCRITUS 1. Was Democritus a scientist? Notes 2. In what time of history did he live? 3. Describe Democritus thoughts about gold. 4. What was Democritus word for something
Name: Block Unit 3- The Atom DEMOCRITUS 1. Was Democritus a scientist? Notes 2. In what time of history did he live? 3. Describe Democritus thoughts about gold. 4. What was Democritus word for something
atomos is a Greek word which means indivisible
 The History of Atomic Theory i.e. the history of the development of thought about what an atom is. 1st timeframe: around 5 B.C. : This was the time of the Ancient Greeks (in Athens, Greece). During the
The History of Atomic Theory i.e. the history of the development of thought about what an atom is. 1st timeframe: around 5 B.C. : This was the time of the Ancient Greeks (in Athens, Greece). During the
CHAPTER 4: Matter is Made up of Atoms
 CHAPTER 4: Matter is Made up of Atoms ATOMS & THEIR STRUCTURE Aristotle thought matter was made of air, earth, fire and water. Democritus (250 B.C.)- Said the world is made of empty space & tiny particles
CHAPTER 4: Matter is Made up of Atoms ATOMS & THEIR STRUCTURE Aristotle thought matter was made of air, earth, fire and water. Democritus (250 B.C.)- Said the world is made of empty space & tiny particles
Ancient Atomic Theories
 Atomic Theory What is an Atom? An ATOM is the smallest part of an element that has all of the element s properties. Atoms of different elements are different from each other. Atomic Theory This is the
Atomic Theory What is an Atom? An ATOM is the smallest part of an element that has all of the element s properties. Atoms of different elements are different from each other. Atomic Theory This is the
Chapter 2. Atoms and Ions
 Chapter 2 Atoms and Ions A History of Atomic Models 400 B.C.E. (Democritus, a early atomist) 1804 (Dalton) Law of Conservation of Mass Antoine Lavoisier 1743-1794 In a chemical reaction, matter is neither
Chapter 2 Atoms and Ions A History of Atomic Models 400 B.C.E. (Democritus, a early atomist) 1804 (Dalton) Law of Conservation of Mass Antoine Lavoisier 1743-1794 In a chemical reaction, matter is neither
Ancient Theories of Atoms
 Ancient Theories of Atoms The word atom was coined somewhere around 450 B.C. (it is an unfortunate side note about this period of history that it is usually quite difficult to give precise dates; one wishes
Ancient Theories of Atoms The word atom was coined somewhere around 450 B.C. (it is an unfortunate side note about this period of history that it is usually quite difficult to give precise dates; one wishes
Chapter 4: Atomic Structure Section 4.1 Defining the Atom
 Chapter 4: Atomic Structure Section 4.1 Defining the Atom Early Models of the Atom atom the smallest particle of an element that retains its identity in a chemical reaction Democritus s Atomic Philosophy
Chapter 4: Atomic Structure Section 4.1 Defining the Atom Early Models of the Atom atom the smallest particle of an element that retains its identity in a chemical reaction Democritus s Atomic Philosophy
HIGH SCHOOL SCIENCE. Physical Science 9: Atomic Structure
 HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
CHAPTER 3. Atoms: The Building Blocks of Matter
 CHAPTER 3 Atoms: The Building Blocks of Matter Origins of the Atom Democritus: Greek philosopher (460 BC - 370 BC) Coined the term atom from the Greek word atomos Democritus believes that atoms were indivisible
CHAPTER 3 Atoms: The Building Blocks of Matter Origins of the Atom Democritus: Greek philosopher (460 BC - 370 BC) Coined the term atom from the Greek word atomos Democritus believes that atoms were indivisible
Name Chemistry-PAP Per. Notes: Atomic Structure
 Name Chemistry-PAP Per. I. Historical Development of the Atomic Model Ancient Greek Model Notes: Atomic Structure Democritus (460-370 BC) was an ancient Greek philosopher credited with the first particle
Name Chemistry-PAP Per. I. Historical Development of the Atomic Model Ancient Greek Model Notes: Atomic Structure Democritus (460-370 BC) was an ancient Greek philosopher credited with the first particle
CH4 HOMEWORK : ATOMIC STRUCTURE
 Name Date Class 4 CH4 HOMEWORK : ATOMIC STRUCTURE SECTION 4.1 DEFINING THE ATOM (pages 101 103) This section describes early atomic theories of matter and provides ways to understand the tiny size of individual
Name Date Class 4 CH4 HOMEWORK : ATOMIC STRUCTURE SECTION 4.1 DEFINING THE ATOM (pages 101 103) This section describes early atomic theories of matter and provides ways to understand the tiny size of individual
The Structure of the Atom
 Main Ideas Atoms contain positive and negative particles. Atoms have small, dense, positively-charged nuclei. A nucleus contains protons and neutrons. The radii of atoms are expressed in picometers. FIGURE
Main Ideas Atoms contain positive and negative particles. Atoms have small, dense, positively-charged nuclei. A nucleus contains protons and neutrons. The radii of atoms are expressed in picometers. FIGURE
Chemistry. Chapter 14 Section 1
 Chemistry Chapter 14 Section 1 What is matter? Matter is anything that has mass and takes up space What is matter made of?? Atoms. All matter is made of atoms. Atoms are the building blocks of Matter There
Chemistry Chapter 14 Section 1 What is matter? Matter is anything that has mass and takes up space What is matter made of?? Atoms. All matter is made of atoms. Atoms are the building blocks of Matter There
Early Atomic Models. Atoms: the smallest particle of an element that retains the properties of that element.
 Chapter 5 Early Atomic Models Atoms: the smallest particle of an element that retains the properties of that element. (Greek: atomos = indivisible) Democritus (Greek teacher in the 4 th century BC) First
Chapter 5 Early Atomic Models Atoms: the smallest particle of an element that retains the properties of that element. (Greek: atomos = indivisible) Democritus (Greek teacher in the 4 th century BC) First
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE. 4.1 Introduction to Atoms
 NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.1 Introduction to Atoms The first people to think about the nature of matter were the ancient Greeks. Around 430 B.C, Democritus, a Greek philosopher,
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.1 Introduction to Atoms The first people to think about the nature of matter were the ancient Greeks. Around 430 B.C, Democritus, a Greek philosopher,
ATOMIC STRUCTURE. Name: Period: Date: 1) = a generalization of scientific observations that what happens (does explain)
 ATOMIC STRUCTURE Name: Period: Date: I. LAW vs. THEORY: 1) = a generalization of scientific observations that what happens (does explain) 2) (model) = a set of assumptions used to explain observations
ATOMIC STRUCTURE Name: Period: Date: I. LAW vs. THEORY: 1) = a generalization of scientific observations that what happens (does explain) 2) (model) = a set of assumptions used to explain observations
3.1 Early History of Atomic Theories
 Figure 1 In Dalton s atomic model, an atom is a solid sphere, similar to a billiard ball. This simple model is still used today to represent the arrangement of atoms in molecules. DID YOU KNOW? William
Figure 1 In Dalton s atomic model, an atom is a solid sphere, similar to a billiard ball. This simple model is still used today to represent the arrangement of atoms in molecules. DID YOU KNOW? William
Passing an electric current makes a beam appear to move from the negative to the positive end.
 Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Smallest
Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Smallest
The Atom and the Subatomic Particles
 The Atom and the Subatomic Particles The purpose of this handout is to familiarize the chemistry student with the history, development and structure of the atomic model. In the hope, that the student will
The Atom and the Subatomic Particles The purpose of this handout is to familiarize the chemistry student with the history, development and structure of the atomic model. In the hope, that the student will
In many ways, Dalton's ideas are still useful today. For example, they help us to understand elements, compounds, and molecules.
 History of the Atom Name: Reading excerpt from Absorb Chemistry for GCSE by Lawrie Ryan http://www.absorblearning.com/chemistry/demo/units/lr301.html Introduction Our understanding of the physical world
History of the Atom Name: Reading excerpt from Absorb Chemistry for GCSE by Lawrie Ryan http://www.absorblearning.com/chemistry/demo/units/lr301.html Introduction Our understanding of the physical world
