Vocabulary QUIZ: 1. The total number of particles in the nucleus 2. 1 / 12
|
|
|
- Naomi Nichols
- 5 years ago
- Views:
Transcription
1 Sep 29 11:29 AM Vocabulary QUIZ: 1. The total number of particles in the nucleus 2. 1 / 12 th of the mass of a carbon atom 3. The weighted average mass of all the isotopes of a particular element 4. A helium nucleus is the same as this, two protons and two neutrons 5. An extra negative electron is the same as this 6. The rays and particles emitted by uranium 7. Unstable atoms under go this 8. I'm "positive" this particle is in the nucleus 9. This particle is like Switzerland 10. Two atoms differ only in mass number but not atomic number, they must be. Sep 11 9:25 AM 1
2 Atom Models Scientists wanted to understand how the atom looked It was known that matter was neutral It was known that matter had mass They used these to ideas to come up with their models, however science changed as technology developed Video Sep 11 12:31 PM History of Atomic Theory ΕΡΛΨ ΓΡΣΣΚ ΘΟΤ Democritus and Leucippus- Greek philosophers who came up with the concept of an atom from the Greek word atomos which means indivisible. OR Aristotle - Famous philosopher who believed that all substances were made of 4 elements: Fire Hot, Air light, Earth - cool, heavy, and Water wet. Blend these in different proportions to get all substances Who Was Right? Sep 10 9:05 AM 2
3 notes 2015.notebook Medieval Times In medieval times, alchemists began experimentation. They are known for trying to change lead to gold. Out of this practice emerged the modern chemists, the first being Sir Robert Boyle in the 1600s. He was the first to do actual chemical experiments. He helped end the idea of the four elements. Sep 10 12:20 PM Late 1700 s - John Dalton, an English school teacher, summarized results of his experiments and those of other scientists. His conclusions led to the first scientific theory of the atom. What is a scientific theory? Sep 10 1:04 PM 3
4 Dalton s Atomic Theory 1. All matter is made of tiny indivisible particles called atoms. 2. Atoms of the same element are identical, those of different elements are different. 3. Atoms of different elements combine in whole number ratios to form compounds. 4. Chemical reactions involve the rearrangement of atoms. No new atoms are created nor destroyed. Sep 10 1:07 PM MODERN VIEWS of the ATOM Atom: The smallest particle of an element that retains the properties of the element. Most atoms consists of three particles: proton neutron electron Sep 10 1:10 PM 4
5 History of the Atom Plum Pudding model: (J. J. Thomson in 1897) Spherically shaped Electrons (negatively charged) positioned throughout it like chocolate chips in a cookie The sphere was a positively charged mass, like the cookie dough Idea did not last long Video Sep 5 8:04 PM History of the Atom Ernest Rutherford (1911) Shot a beam of radiation particles at a thin sheet of gold He expected the particles to go straight through if the atom was based off the Plum Pudding Model. Particles should only alter course if they hit an electron in the atom Sep 5 8:58 PM 5
6 History of the Atom Ernest Rutherford (1911) The atom was made up of mostly empty space where the electrons moved The positive charge of the atom was within the center of the atom: The nucleus a small dense positively charged space at the center of an atom Electrons are held within the atom due to the attraction of the nucleus The diameter of the atom was calculated to be 10,000 times the diameter of the nucleus (comparison: if the diameter of the nucleus was the size of my desk (1.34 m), how big would the diameter of the atom be?) in miles? 0.62 miles=1km Sep 6 9:04 AM History of the Atom James Chadwick Video Sep 6 9:11 AM 6
7 History of the Atom The Nucleus contains protons and neutrons, containing 99.97% of the atoms mass Protons are a positively charged subatomic particle Neutrons are neutrally charged subatomic particle Nucleus is surrounded by negatively charged electrons, with negligible mass Protons and electrons are attracted to each other Atoms are mostly empty space Video Sep 6 9:11 AM The Atom 6 C Carbon Atomic Number Element Symbol Element Name Atomic Mass Sep 7 8:13 AM 7
8 The Atom Atomic Number 6 C Carbon This tells you how many protons are in the atom (ALWAYS!!!) The # of protons is the DNA of the atom, it makes it that element This also tells you how many electrons there are in a neutral atom (remember protons are positive and electrons are negative Sep 7 8:13 AM The Atom 6 C Carbon Atomic Mass Measured in Atomic Mass Units (AMUs) Atomic mass number of any one atom = # Protons + # Neutrons Remember Electrons mass is Negligible 12 amu 6 protons = 6 neutrons Round the mass to a whole number Sep 7 9:11 AM 8
9 The Atom 6 C Carbon Average Atomic Mass (Why is this number not a whole number?) This is the weighted sum of all the atomic masses possible for this element Since protons do not EVER change, and electrons mass is negligible, the only thing that can change the mass is a change in Neutrons Isotopes: Atoms with the same number of protons but different number of Neutrons Sep 7 9:11 AM Oct 8 1:53 PM 9
10 Writing isotopes Uranium 238 mass number U \ atomic # Sep 13 10:21 AM Isotopes amu oxygen Li Bromine 80 p n e Ba Au Sep 13 10:25 AM 10
11 Oct 8 2:10 PM Isotopes amu Nitrogen Ca p n e - Radon Mo Ag Sep 13 10:25 AM 11
12 The Atom Atomic Mass Calculation [% of Mass 1 x (Mass 1)] + [% of Mass 2 x (Mass 2)] +... Copper has two isotopes: 69.2% of all copper has a mass of 63 amus and 30.8% of all copper has a mass of 65 amus. What is copper's atomic mass? Sep 7 9:22 AM Warm up 10/6/2015 Tin (Sn) has four different isotopes. What is the average atomic mass of a sample of Tin that has 55.0% of its isotopes having 118 amus, 19.0% having 119 amus, 20.0% having 120 amus, and 6.0% having 121 amus? Oct 2 8:45 AM 12
13 Radioactivity Fission Fusion + + Oct 13 1:39 PM Splitting the Atom Radioactive decay occurs when an atom undergoes a nuclear reaction. The nucleus breaks apart into a more stable structure. When this happens particles and energy are released. These particles and energy are called radiation. Three types of radiation: alpha beta gamma Sep 11 1:14 PM 13
14 Alpha Radiation Alpha radiation is a very weak form of radiation. It can easily be stopped by human skin or paper. In alpha radiation, alpha particles are given off. An alpha particle contains two protons and two neutrons (a helium nucleus). It carries a +2 charge Ra Rn + α Sep 12 10:14 AM Alpha Radiation Niobium 94 undergoes alpha decay Barium 138 undergoes alpha decay Lead 209 undergoes alpha decay Oct 13 2:01 PM 14
15 Beta Radiation Beta radiation is a stronger form of radiation. It can be stopped by aluminum foil or an inch of acrylic. In beta radiation, beta particles are given off. A beta particle is a particle with a -1 charge 14 6 C 14 7N + β - + neutrino How does this happen? Sep 12 9:07 AM Beta radiation (cont.) A neutron decomposes into a positive proton and releases the negative portion as a negatively charged particle and the extra energy as a neutrino 14 6 C 14 7N + β - + neutrino Sep 12 11:08 AM 15
16 Beta Radiation Carbon 14 undergoes beta decay Potassium 40 undergoes beta decay Strontium 88 undergoes beta decay Oct 13 2:10 PM Gamma radiation Gamma radiation is the strongest form of radiation. It is deadly to life. It can be stopped by an inch of lead or a meter of concrete. It will not turn you into the Hulk. Sep 12 11:13 AM 16
17 Gamma radiation In gamma radiation, a very unstable nucleus has too much energy. To become stable, the nucleus releases the energy in the form of gamma rays and also other particles (beta, alpha, and/or neutrons) Sep 13 9:28 AM Gamma radiation Rubidium 86 undergoes only gamma decay Rubidium 86 undergoes gamma decay and the releases a neutron Rubidium 86 undergoes gamma and alpha decay Rubidium 86 undergoes gamma and beta decay Oct 13 2:18 PM 17
18 Practice Mn undergoes beta decay. What is the final product? Antimony 122 goes through alpha decay and then a beta decay. What is produced? 3. What new element is created by the beta decay of polonium 210? If 53I was produced by two beta decays, what was the original element? Oct 14 12:28 PM Oct 6 9:12 AM 18
Scientist wanted to understand how the atom looked. It was known that matter was neutral. It was known that matter had mass
 Atom Models Scientist wanted to understand how the atom looked It was known that matter was neutral It was known that matter had mass They used these ideas to come up with their models, however science
Atom Models Scientist wanted to understand how the atom looked It was known that matter was neutral It was known that matter had mass They used these ideas to come up with their models, however science
CHEMISTRY. Matter and Change. Table Of Contents. Section 4.1 Early Ideas About Matter. Unstable Nuclei and Radioactive Decay
 CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
Chapter 4. History of the atom. History of Atom Smallest possible piece? Atomos - not to be cut. Atoms and their structure
 Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Looked at
Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Looked at
Atoms and their structure
 Atoms and their structure History of atomic theory Not the history of atom, but the idea of the atom Original idea Ancient Greece (400 B.C..) Democritus and Leucippus Greek philosophers Another Greek Aristotle
Atoms and their structure History of atomic theory Not the history of atom, but the idea of the atom Original idea Ancient Greece (400 B.C..) Democritus and Leucippus Greek philosophers Another Greek Aristotle
Passing an electric current makes a beam appear to move from the negative to the positive end.
 Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Smallest
Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Smallest
The structure of Atom III
 The structure of Atom III Atomic Structure If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement
The structure of Atom III Atomic Structure If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement
General Chemistry Standard : Identify the significance of the various outcomes of Thomson s and Rutherford s experiments
 Not the history of the atom, but the idea of the atom The atom was not discovered until recently Original Idea Ancient Greece (400 BC) Proposed by lesser-known scientists They looked at a beach made of
Not the history of the atom, but the idea of the atom The atom was not discovered until recently Original Idea Ancient Greece (400 BC) Proposed by lesser-known scientists They looked at a beach made of
Nuclear Chemistry. Atomic Structure Notes Start on Slide 20 from the second class lecture
 Nuclear Chemistry Atomic Structure Notes Start on Slide 20 from the second class lecture The Birth of an Idea Democritus, 400 B.C. coined the term atom If you divide matter into smaller and smaller pieces,
Nuclear Chemistry Atomic Structure Notes Start on Slide 20 from the second class lecture The Birth of an Idea Democritus, 400 B.C. coined the term atom If you divide matter into smaller and smaller pieces,
Early Atomic Models. Atoms: the smallest particle of an element that retains the properties of that element.
 Chapter 5 Early Atomic Models Atoms: the smallest particle of an element that retains the properties of that element. (Greek: atomos = indivisible) Democritus (Greek teacher in the 4 th century BC) First
Chapter 5 Early Atomic Models Atoms: the smallest particle of an element that retains the properties of that element. (Greek: atomos = indivisible) Democritus (Greek teacher in the 4 th century BC) First
Chapter 4. The structure of the atom. AL-COS Objectives 1, 2,3,4,7, 10, 15, 20, 21, 22, 27and 28
 Chapter 4 The structure of the atom AL-COS Objectives 1, 2,3,4,7, 10, 15, 20, 21, 22, 27and 28 You ll learn to Identify the experiments that led to the development of the nuclear model of atomic structure
Chapter 4 The structure of the atom AL-COS Objectives 1, 2,3,4,7, 10, 15, 20, 21, 22, 27and 28 You ll learn to Identify the experiments that led to the development of the nuclear model of atomic structure
Chemistry Chapter 3. Atoms: The Building Blocks of Matter
 Chemistry Chapter 3 Atoms: The Building Blocks of Matter I. From Philosophical Idea to Scientific Theory History of the Atom The Ancient Greeks were the first to come up with the idea of the atom. Democritus
Chemistry Chapter 3 Atoms: The Building Blocks of Matter I. From Philosophical Idea to Scientific Theory History of the Atom The Ancient Greeks were the first to come up with the idea of the atom. Democritus
2. Electrons: e - charge = negative -1 mass ~ 0
 Notes Ch. and 5: Atomic Structure and Nuclear Chemistry History and Structure the Nuclear Atom The Atom smallest particle an element that retains all properties the element I. Early Models the Atom A.
Notes Ch. and 5: Atomic Structure and Nuclear Chemistry History and Structure the Nuclear Atom The Atom smallest particle an element that retains all properties the element I. Early Models the Atom A.
Structure of the Nuclear Atom
 Structure of the Nuclear Atom I. The II. A. The is the smallest particle of an element that retains its of the element. History of the Atom A. Democritus 1. Democritus (460 B.C. 370 B.C) was the first
Structure of the Nuclear Atom I. The II. A. The is the smallest particle of an element that retains its of the element. History of the Atom A. Democritus 1. Democritus (460 B.C. 370 B.C) was the first
CHEMISTRY Matter and Change. Chapter 4: The Structure of the Atom
 CHEMISTRY Matter and Change Chapter 4: The Structure of the Atom CHAPTER 4 Table Of Contents Section 4.1 Section 4.2 Section 4.3 Section 4.4 Early Ideas About Matter Defining the Atom How Atoms Differ
CHEMISTRY Matter and Change Chapter 4: The Structure of the Atom CHAPTER 4 Table Of Contents Section 4.1 Section 4.2 Section 4.3 Section 4.4 Early Ideas About Matter Defining the Atom How Atoms Differ
CHAPTER 3. Atoms: The Building Blocks of Matter
 CHAPTER 3 Atoms: The Building Blocks of Matter Origins of the Atom Democritus: Greek philosopher (460 BC - 370 BC) Coined the term atom from the Greek word atomos Democritus believes that atoms were indivisible
CHAPTER 3 Atoms: The Building Blocks of Matter Origins of the Atom Democritus: Greek philosopher (460 BC - 370 BC) Coined the term atom from the Greek word atomos Democritus believes that atoms were indivisible
Honors Chemistry Unit 2: The Atom & Its Nucleus
 Honors Chemistry Unit 2: The Atom & Its Nucleus (2017-2018) Bunsen, I must tell you how excellent your study of chemical spectroscopy is, as is your pioneer work in photochemistry but what really impresses
Honors Chemistry Unit 2: The Atom & Its Nucleus (2017-2018) Bunsen, I must tell you how excellent your study of chemical spectroscopy is, as is your pioneer work in photochemistry but what really impresses
Dalton Thompson Rutherford Bohr Modern Model ("Wave. Models of the Atom
 Dalton Thompson Rutherford Bohr Modern Model ("Wave Models of the Atom Mechanical" Model) Aim: To discuss the scientists and their contributions to the current atomic model. Focus: Rutherford's Gold Foil
Dalton Thompson Rutherford Bohr Modern Model ("Wave Models of the Atom Mechanical" Model) Aim: To discuss the scientists and their contributions to the current atomic model. Focus: Rutherford's Gold Foil
Understanding the Atom
 Name Date Period 3.1 Discovering Parts of an Atom Directions: On the line before each statement, write correct if the statement is correct or not correct if the statement is not correct. If the statement
Name Date Period 3.1 Discovering Parts of an Atom Directions: On the line before each statement, write correct if the statement is correct or not correct if the statement is not correct. If the statement
Mass number i. Example U (uranium 235) and U (uranium 238) atomic number e. Average atomic mass weighted of the isotopes of that element i.
 CP NT Ch. 4&25 I. Atomic Theory and Structure of the Atom a. Democritus all matter consists of very small, indivisible particles, which he named i. Atom smallest particle of an element that retains all
CP NT Ch. 4&25 I. Atomic Theory and Structure of the Atom a. Democritus all matter consists of very small, indivisible particles, which he named i. Atom smallest particle of an element that retains all
CHAPTER 4 Atomic Structure
 CHAPTER 4 Atomic Structure 4.1 Early Theories of Matter Earth, Water, Air, Fire Matter was thought to be infinitely divisible No method was available to test theories Democritus (460 B.C. 370 B.C.) First
CHAPTER 4 Atomic Structure 4.1 Early Theories of Matter Earth, Water, Air, Fire Matter was thought to be infinitely divisible No method was available to test theories Democritus (460 B.C. 370 B.C.) First
Unit 2 continued-chemical Foundations Atoms, Ions, &Elements
 Unit 2 continuedchemical Foundations Atoms, Ions, &Elements The Elements Most abundant elements in/on Earth: Oxygen 49.2% Silicon25.7% Most abundant in the human body: Oxygen65.0% Carbon18.0 % Hydrogen10.0%
Unit 2 continuedchemical Foundations Atoms, Ions, &Elements The Elements Most abundant elements in/on Earth: Oxygen 49.2% Silicon25.7% Most abundant in the human body: Oxygen65.0% Carbon18.0 % Hydrogen10.0%
Unit 2 Atomic Structure and Nuclear Chemistry
 Chemistry 1 West Linn High School Unit 2 Packet and Goals Name: Period: Unit 2 Atomic Structure and Nuclear Chemistry Unit Goals: As you work through this unit, you should be able to: 1. describe Dalton
Chemistry 1 West Linn High School Unit 2 Packet and Goals Name: Period: Unit 2 Atomic Structure and Nuclear Chemistry Unit Goals: As you work through this unit, you should be able to: 1. describe Dalton
Atomic Structure. For thousands of years, people had many ideas about matter Ancient Greeks believed that everything was made up of the four elements
 An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Early Ideas About Matter A. atom the smallest particle of an element retaining
Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Early Ideas About Matter A. atom the smallest particle of an element retaining
Origins of the Atom. Atoms: The Building Blocks of Matter. Let s Get Ready to Rumble. Aristotle s Theory of the Atom CHAPTER 3
 Origins of the Atom CHAPTER 3 Atoms: The Building Blocks of Matter Let s Get Ready to Rumble The idea of the atom was met with great skepticism, especially among great thinkers. The most vocal critic of
Origins of the Atom CHAPTER 3 Atoms: The Building Blocks of Matter Let s Get Ready to Rumble The idea of the atom was met with great skepticism, especially among great thinkers. The most vocal critic of
Chemistry. Robert Taggart
 Chemistry Robert Taggart Table of Contents To the Student..................................................v Unit 1: Matter and Measurement Lesson 1: Chemistry and the Scientific Method...................3
Chemistry Robert Taggart Table of Contents To the Student..................................................v Unit 1: Matter and Measurement Lesson 1: Chemistry and the Scientific Method...................3
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS
 CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
Chapter 4: Atomic Structure Section 4.1 Defining the Atom
 Chapter 4: Atomic Structure Section 4.1 Defining the Atom Early Models of the Atom atom the smallest particle of an element that retains its identity in a chemical reaction Democritus s Atomic Philosophy
Chapter 4: Atomic Structure Section 4.1 Defining the Atom Early Models of the Atom atom the smallest particle of an element that retains its identity in a chemical reaction Democritus s Atomic Philosophy
4.1 Structure of the Atom
 4.1 Structure of the Atom How do atoms differ from each other? What are atoms composed of? What are the subatomic particles? 2-1 Structure of the Atom Atoms actually are divisible. They are composed of
4.1 Structure of the Atom How do atoms differ from each other? What are atoms composed of? What are the subatomic particles? 2-1 Structure of the Atom Atoms actually are divisible. They are composed of
Early Atomic Theory. Alchemy. The atom
 Early Atomic Theory Chapter 3 Democritus 460 BC- ~ 370 BC Nothing exists except atoms and empty space; everything else is opinion. Matter is composed of small indivisible particles, atomos meaning Indivisible
Early Atomic Theory Chapter 3 Democritus 460 BC- ~ 370 BC Nothing exists except atoms and empty space; everything else is opinion. Matter is composed of small indivisible particles, atomos meaning Indivisible
Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video
 Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video 2 CH 4- Atoms 1 Discovering the Atom In this lesson we will take a look at the scientists who explored the
Development of Atomic Theory Elements of chemistry- Atoms, the building blocks of matter Video 2 CH 4- Atoms 1 Discovering the Atom In this lesson we will take a look at the scientists who explored the
Atomic Structure & Nuclear Chemistry Unit 3 Notes
 Atomic Structure & Nuclear Chemistry Unit 3 Notes Academic Chemistry Name 52 24 Cr Mass Number Symbol Atomic Number Unit #3 Test Date You can never learn less, you can only learn more. R. Buckminster Fuller
Atomic Structure & Nuclear Chemistry Unit 3 Notes Academic Chemistry Name 52 24 Cr Mass Number Symbol Atomic Number Unit #3 Test Date You can never learn less, you can only learn more. R. Buckminster Fuller
Chapter 4. Atomic Structure
 Chapter 4 Atomic Structure Warm Up We have not discussed this material, what do you know already?? What is an atom? What are electron, neutrons, and protons? Draw a picture of an atom from what you know
Chapter 4 Atomic Structure Warm Up We have not discussed this material, what do you know already?? What is an atom? What are electron, neutrons, and protons? Draw a picture of an atom from what you know
The Structure of the Atom
 The Structure of the Atom Section 4.1 Early Theories of Matter In your textbook, read about the philosophers, John Dalton, and defining the atom. For each statement below, write true or false. 1. Ancient
The Structure of the Atom Section 4.1 Early Theories of Matter In your textbook, read about the philosophers, John Dalton, and defining the atom. For each statement below, write true or false. 1. Ancient
EARLY VIEWS: The Ancient Greeks
 Feb 7 11:59 AM EARLY VIEWS: The Ancient Greeks Empedocles (c. 450 B.C.) proposed Four Element theory he thought that matter was composed of four elements: AIR, EARTH, FIRE and WATER elements mixed together
Feb 7 11:59 AM EARLY VIEWS: The Ancient Greeks Empedocles (c. 450 B.C.) proposed Four Element theory he thought that matter was composed of four elements: AIR, EARTH, FIRE and WATER elements mixed together
Chapter 4 Jeopardy Review
 Chapter 4 Jeopardy Review Atom Models of the Atom Atomic Theory Calculating Subatomic Particles Isotopes 100 100 100 100 100 200 200 200 200 200 300 300 300 300 300 400 400 400 400 400 500 500 500 500
Chapter 4 Jeopardy Review Atom Models of the Atom Atomic Theory Calculating Subatomic Particles Isotopes 100 100 100 100 100 200 200 200 200 200 300 300 300 300 300 400 400 400 400 400 500 500 500 500
Chapter 4 The Atom. Philosophers and scientists have proposed many ideas on the structure of atoms.
 Chapter4 TheAtom 4.1 Early Models of the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. Philosophers and scientists have proposed many ideas on the
Chapter4 TheAtom 4.1 Early Models of the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. Philosophers and scientists have proposed many ideas on the
Atomic Structure. ppst.com
 Atomic Structure ppst.com Defining the Atom The Greek philosopher (460 B.C. 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word ) He believed that atoms were and His ideas
Atomic Structure ppst.com Defining the Atom The Greek philosopher (460 B.C. 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word ) He believed that atoms were and His ideas
SNC1D1 History of the Atom
 SNC1D1 History of the Atom What is the atom? Atoms are the building block for all matter: Atoms make up elements! Elements combine to make compounds!2 ATOMIC MODEL TIMELINE 400 B.C PRESENT DAY ATOMIC MODEL
SNC1D1 History of the Atom What is the atom? Atoms are the building block for all matter: Atoms make up elements! Elements combine to make compounds!2 ATOMIC MODEL TIMELINE 400 B.C PRESENT DAY ATOMIC MODEL
Atomic Theory & the Atom. it s elemental
 Atomic Theory & the Atom it s elemental Our view of the atom has changed over time the ATOM the smallest particle of an element that still retains the chemical properties of that element Here is a model
Atomic Theory & the Atom it s elemental Our view of the atom has changed over time the ATOM the smallest particle of an element that still retains the chemical properties of that element Here is a model
DescribeDemocritus s Democritus s ideas
 Atomic Structure Section 4.1 Defining the Atom DescribeDemocritus s Democritus s ideas about atoms. Section 4.1 Defining the Atom Explain Dalton s atomic theory. Section 4.1 Defining the Atom Identifywhat
Atomic Structure Section 4.1 Defining the Atom DescribeDemocritus s Democritus s ideas about atoms. Section 4.1 Defining the Atom Explain Dalton s atomic theory. Section 4.1 Defining the Atom Identifywhat
Chapter 4 Atomic Structure. Chemistry- Lookabaugh Moore High School
 Chapter 4 Atomic Structure Chemistry- Lookabaugh Moore High School Section 4.1 Defining the Atom Democritus (460 B.C 370 B.C.) first used the term atomon to describe the smallest particle of matter possible.
Chapter 4 Atomic Structure Chemistry- Lookabaugh Moore High School Section 4.1 Defining the Atom Democritus (460 B.C 370 B.C.) first used the term atomon to describe the smallest particle of matter possible.
Name Chemistry-PAP Per. Notes: Atomic Structure
 Name Chemistry-PAP Per. I. Historical Development of the Atomic Model Ancient Greek Model Notes: Atomic Structure Democritus (460-370 BC) was an ancient Greek philosopher credited with the first particle
Name Chemistry-PAP Per. I. Historical Development of the Atomic Model Ancient Greek Model Notes: Atomic Structure Democritus (460-370 BC) was an ancient Greek philosopher credited with the first particle
ATOMIC STRUCTURE. Name: Period: Date: 1) = a generalization of scientific observations that what happens (does explain)
 ATOMIC STRUCTURE Name: Period: Date: I. LAW vs. THEORY: 1) = a generalization of scientific observations that what happens (does explain) 2) (model) = a set of assumptions used to explain observations
ATOMIC STRUCTURE Name: Period: Date: I. LAW vs. THEORY: 1) = a generalization of scientific observations that what happens (does explain) 2) (model) = a set of assumptions used to explain observations
Early Models of the Atom
 Early Models of the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms,
Early Models of the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms,
Atomic Theory: Early Models of the Atom:
 Atomic Theory: Our next job in Chemistry 11 is to learn about what matter is made of. After we have done this, we can start to understand why matter behaves the way it does. Everything that has volume
Atomic Theory: Our next job in Chemistry 11 is to learn about what matter is made of. After we have done this, we can start to understand why matter behaves the way it does. Everything that has volume
Greek Philosophers (cont.)
 Greek Philosophers (cont.) Many ancient scholars believed matter was composed of such things as earth, water, air, and fire. Many believed matter could be endlessly divided into smaller and smaller pieces.
Greek Philosophers (cont.) Many ancient scholars believed matter was composed of such things as earth, water, air, and fire. Many believed matter could be endlessly divided into smaller and smaller pieces.
Particle Theory of Matter. By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that:
 Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
PreAP Chemistry. Unit 4 Atomic Structure, the Periodic Table, and Nuclear Radiation
 PreAP Chemistry Unit 4 Atomic Structure, the Periodic Table, and Nuclear Radiation Democritus A Greek who lived ~400 BC, was the first to suggest the existence of atoms. He believed atoms to be indivisible
PreAP Chemistry Unit 4 Atomic Structure, the Periodic Table, and Nuclear Radiation Democritus A Greek who lived ~400 BC, was the first to suggest the existence of atoms. He believed atoms to be indivisible
Atomic Theory. Democritus to the Planetary Model
 Atomic Theory Democritus to the Planetary Model Democritus Greek philosopher (460-370 BCE) Believed in the philosophy of materialism With Leucippus, they though that matter can not be divided infinitely.
Atomic Theory Democritus to the Planetary Model Democritus Greek philosopher (460-370 BCE) Believed in the philosophy of materialism With Leucippus, they though that matter can not be divided infinitely.
1 Development of the Atomic Theory
 CHAPTER 4 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
CHAPTER 4 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
Name Date Class DEFINING THE ATOM
 4.1 DEFINING THE ATOM Section Review Objectives Describe Democritus s ideas about atoms Explain Dalton s atomic theory Describe the size of an atom Vocabulary atom Dalton s atomic theory Part A Completion
4.1 DEFINING THE ATOM Section Review Objectives Describe Democritus s ideas about atoms Explain Dalton s atomic theory Describe the size of an atom Vocabulary atom Dalton s atomic theory Part A Completion
Chapter 3 Atoms: The Building Blocks of Matter. Honors Chemistry 412
 Chapter 3 Atoms: The Building Blocks of Matter Honors Chemistry 412 Foundations of Atomic Theory Democritus Greek Philosopher 460-370 B.C. Stated Matter could be divided into smaller & smaller particles
Chapter 3 Atoms: The Building Blocks of Matter Honors Chemistry 412 Foundations of Atomic Theory Democritus Greek Philosopher 460-370 B.C. Stated Matter could be divided into smaller & smaller particles
Chemistry Review Unit 1 Study Guide
 1. Draw and label a Bohr model of a C 14 atom. 2. Describe the following about a proton a. mass: the mass of a proton is 1 atomic mass unit (AMU) b. charge: protons have a positive charge c. location:
1. Draw and label a Bohr model of a C 14 atom. 2. Describe the following about a proton a. mass: the mass of a proton is 1 atomic mass unit (AMU) b. charge: protons have a positive charge c. location:
Friday, 05/06/16 6) HW QUIZ MONDAY Learning Target (NEW)
 Friday, 05/06/16 1) Warm-up: If you start with 100g of a radioactive substance, how much will be left after 3 half-lives? 2) Review HW & Nuclear Notes 3) Complete Modeling Energy Investigation 4) Complete:
Friday, 05/06/16 1) Warm-up: If you start with 100g of a radioactive substance, how much will be left after 3 half-lives? 2) Review HW & Nuclear Notes 3) Complete Modeling Energy Investigation 4) Complete:
1 Development of the Atomic Theory
 CHAPTER 11 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
CHAPTER 11 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
All are made of atoms. The, your and even are made of atoms. Atoms are. One atom is only one of a meter wide!
 Name: Atoms & The Periodic Table WHAT IS AN ATOM? What is an atom? All are made of atoms. The, your and even are made of atoms. Atoms are. One atom is only one of a meter wide! DEMOCRITIS The idea of an
Name: Atoms & The Periodic Table WHAT IS AN ATOM? What is an atom? All are made of atoms. The, your and even are made of atoms. Atoms are. One atom is only one of a meter wide! DEMOCRITIS The idea of an
Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester?
 Chemistry Ms. Ye Name Date Block Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester? Atoms Video: 1. Proper Portioned Giant Atom Model of Science: Structure
Chemistry Ms. Ye Name Date Block Do Now: Recall 1. What is an atom? What have you learned about the word atom so far this semester? Atoms Video: 1. Proper Portioned Giant Atom Model of Science: Structure
HIGH SCHOOL SCIENCE. Physical Science 9: Atomic Structure
 HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
What is matter? Matter is anything that has mass and takes up space. Matter is made up of atoms.
 Matter What is matter? Matter is anything that has mass and takes up space. Matter is made up of atoms. Is it matter? Can you measure the object? Does it take up space? Does the object have a mass? Come
Matter What is matter? Matter is anything that has mass and takes up space. Matter is made up of atoms. Is it matter? Can you measure the object? Does it take up space? Does the object have a mass? Come
Glencoe: Chapter 4. The Structure of the Atom
 Glencoe: Chapter 4 The Structure of the Atom Section One: Early Ideas about Matter Atomists and Democritus : 400 B.C. From Thrace in Greece. Atoms- Uncut-Table Indivisible parts which cannot be broken
Glencoe: Chapter 4 The Structure of the Atom Section One: Early Ideas about Matter Atomists and Democritus : 400 B.C. From Thrace in Greece. Atoms- Uncut-Table Indivisible parts which cannot be broken
Early Ideas about Matter
 Early Ideas about Matter atom The smallest piece of the element with all the chemical properties of the element an old and new idea Greeks Discontinuists Democritus Continuists Aristotle Discontinuists
Early Ideas about Matter atom The smallest piece of the element with all the chemical properties of the element an old and new idea Greeks Discontinuists Democritus Continuists Aristotle Discontinuists
Chapter 11 Study Questions Name: Class:
 Chapter 11 Study Questions Name: Class: Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. The discovery of which particle proved that the atom
Chapter 11 Study Questions Name: Class: Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. The discovery of which particle proved that the atom
SCH3U1 This presentation and more can be found at
 SCH3U1 Today s Learning goals: Review history of the atomic model Practice using standard atomic notation Introduce radioisotopes This presentation and more can be found at http://lorenowicz.weebly.com
SCH3U1 Today s Learning goals: Review history of the atomic model Practice using standard atomic notation Introduce radioisotopes This presentation and more can be found at http://lorenowicz.weebly.com
The Story of the Atom. A history of atomic theory over many years
 The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
Get out your diagram from your research paper. Get out a sheet of paper to take some notes on.
 Bellwork: Get out your diagram from your research paper. Get out a sheet of paper to take some notes on. Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons
Bellwork: Get out your diagram from your research paper. Get out a sheet of paper to take some notes on. Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons
Alta Chemistry CHAPTER 25. Nuclear Chemistry: Radiation, Radioactivity & its Applications
 CHAPTER 25 Nuclear Chemistry: Radiation, Radioactivity & its Applications Nuclear Chemistry Nuclear Chemistry deals with changes in the nucleus The nucleus of an atom contains Protons Positively Charged
CHAPTER 25 Nuclear Chemistry: Radiation, Radioactivity & its Applications Nuclear Chemistry Nuclear Chemistry deals with changes in the nucleus The nucleus of an atom contains Protons Positively Charged
7.1 Development of a Modern Atomic Theory
 7.1 Development of a Modern Atomic Theory Development of the Atomic Theory Many scientists in different countries have contributed to the understanding of matter - atoms John Dalton Credited with developing
7.1 Development of a Modern Atomic Theory Development of the Atomic Theory Many scientists in different countries have contributed to the understanding of matter - atoms John Dalton Credited with developing
Chapter 2. Atoms and Ions
 Chapter 2 Atoms and Ions A History of Atomic Models 400 B.C.E. (Democritus, a early atomist) 1804 (Dalton) Law of Conservation of Mass Antoine Lavoisier 1743-1794 In a chemical reaction, matter is neither
Chapter 2 Atoms and Ions A History of Atomic Models 400 B.C.E. (Democritus, a early atomist) 1804 (Dalton) Law of Conservation of Mass Antoine Lavoisier 1743-1794 In a chemical reaction, matter is neither
Chapter 3 https://youtu.be/thndxfdkzzs?list=pl8dpuualjx tphzzyuwy6fyeax9mqq8ogr
 Chapter 3 https://youtu.be/thndxfdkzzs?list=pl8dpuualjx tphzzyuwy6fyeax9mqq8ogr The smallest particle of an element that retains the chemical properties of that element. Regions: Nucleus: very small region
Chapter 3 https://youtu.be/thndxfdkzzs?list=pl8dpuualjx tphzzyuwy6fyeax9mqq8ogr The smallest particle of an element that retains the chemical properties of that element. Regions: Nucleus: very small region
Atomic Structure. A model uses familiar ideas to explain unfamiliar facts observed in nature.
 Atomic Structure 1 2 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. A model uses familiar
Atomic Structure 1 2 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. A model uses familiar
SCIENCE 10: (7.1) ATOMIC THEORY, ISOTOPES AND RADIOACTIVE DECAY Name: Date: Block: (Textbook Reference pp in BC Science 10) into an
 SCIENCE 10: (7.1) ATOMIC THEORY, ISOTOPES AND RADIOACTIVE DECAY Name: Date: Block: (Textbook Reference pp. 286-301 in BC Science 10) Natural background radiation: It has the ability to interact with an
SCIENCE 10: (7.1) ATOMIC THEORY, ISOTOPES AND RADIOACTIVE DECAY Name: Date: Block: (Textbook Reference pp. 286-301 in BC Science 10) Natural background radiation: It has the ability to interact with an
Chemistry. - Many philosophers concluded that matter was composed of things such as earth, -Democritus: -Aristotle: -Dalton:
 Chemistry Made by Saleem Abu-Tayeh Chapter 4 - Many philosophers concluded that matter was composed of things such as earth, water, air, and fire. It was also commonly accepted that matter could be endlessly
Chemistry Made by Saleem Abu-Tayeh Chapter 4 - Many philosophers concluded that matter was composed of things such as earth, water, air, and fire. It was also commonly accepted that matter could be endlessly
Early Ideas About Matter
 Early Ideas About Matter Democritus (460 370 BC) believed that matter is made of small, solid objects called atomos, from which the English word atom is derived. Early Ideas About Matter (cont.) Aristotle
Early Ideas About Matter Democritus (460 370 BC) believed that matter is made of small, solid objects called atomos, from which the English word atom is derived. Early Ideas About Matter (cont.) Aristotle
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE. 4.1 Introduction to Atoms
 NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.1 Introduction to Atoms The first people to think about the nature of matter were the ancient Greeks. Around 430 B.C, Democritus, a Greek philosopher,
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.1 Introduction to Atoms The first people to think about the nature of matter were the ancient Greeks. Around 430 B.C, Democritus, a Greek philosopher,
Nuclear Physics Questions. 1. What particles make up the nucleus? What is the general term for them? What are those particles composed of?
 Nuclear Physics Questions 1. What particles make up the nucleus? What is the general term for them? What are those particles composed of? 2. What is the definition of the atomic number? What is its symbol?
Nuclear Physics Questions 1. What particles make up the nucleus? What is the general term for them? What are those particles composed of? 2. What is the definition of the atomic number? What is its symbol?
1. Based on Dalton s evidence, circle the drawing that demonstrates Dalton s model.
 Various models of the ATOM Dalton Model John Dalton developed the first atomic model in 1808. Before him people, mostly philosophers, had speculated about the smallest unit of matter and two theories prevailed.
Various models of the ATOM Dalton Model John Dalton developed the first atomic model in 1808. Before him people, mostly philosophers, had speculated about the smallest unit of matter and two theories prevailed.
THE ATOM Pearson Education, Inc.
 THE ATOM Title and Highlight Right Side NOTES ONLY TN Ch 4.1-4.2 Topic: EQ: Date Reflect Question: Reflect on the material by asking a question (its not suppose to be answered from notes) NOTES: Write
THE ATOM Title and Highlight Right Side NOTES ONLY TN Ch 4.1-4.2 Topic: EQ: Date Reflect Question: Reflect on the material by asking a question (its not suppose to be answered from notes) NOTES: Write
 Radioactive Decay What is Radioactivity? http://explorecuriocity.org/explore/articleid/3033 http://explorecuriocity.org/explore/articleid/3035 http://explorecuriocity.org/explore/articleid/2160 Quick Review
Radioactive Decay What is Radioactivity? http://explorecuriocity.org/explore/articleid/3033 http://explorecuriocity.org/explore/articleid/3035 http://explorecuriocity.org/explore/articleid/2160 Quick Review
Directed Reading B. Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY. is a(n). DALTON S ATOMIC THEORY BASED ON EXPERIMENTS
 Skills Worksheet Directed Reading B Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY 1. The word atom comes from the Greek word atomos, which means a. dividable. b. invisible. c.
Skills Worksheet Directed Reading B Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY 1. The word atom comes from the Greek word atomos, which means a. dividable. b. invisible. c.
Atomic Structure and Nuclear Chemistry Multiple Choice Questions PSI Chemistry
 Atomic Structure and Nuclear Chemistry Multiple Choice Questions PSI Chemistry Name: 1. What was the first particle discovered inside an atom? A. Proton C. Electron 2. What characteristic of cathode rays
Atomic Structure and Nuclear Chemistry Multiple Choice Questions PSI Chemistry Name: 1. What was the first particle discovered inside an atom? A. Proton C. Electron 2. What characteristic of cathode rays
Radioactive Decay. Scientists have discovered that when atoms of one kind of element emit radiation, they can change into atoms of a NEW element.
 Radioactive Decay Radioactive Decay Scientists have discovered that when atoms of one kind of element emit radiation, they can change into atoms of a NEW element. Why would an atom emit radiation in the
Radioactive Decay Radioactive Decay Scientists have discovered that when atoms of one kind of element emit radiation, they can change into atoms of a NEW element. Why would an atom emit radiation in the
Atomic Models. A model uses familiar ideas to explain unfamiliar facts observed in nature. A model can be changed as new information is collected.
 This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. Atomic Models A model uses familiar ideas
This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. Atomic Models A model uses familiar ideas
The Atom. protons, neutrons, and electrons oh my!
 The Atom protons, neutrons, and electrons oh my! What s an Atom? An atom is the smallest physical particle of an element that still retains the properties of that element. How Big is an Atom? At sea level,
The Atom protons, neutrons, and electrons oh my! What s an Atom? An atom is the smallest physical particle of an element that still retains the properties of that element. How Big is an Atom? At sea level,
4-1 Notes. Defining the Atom
 4-1 Notes Defining the Atom Early Models of the Atom All matter is composed of atoms Atoms are the smallest particles of an element that retains their identity in a chemical reaction Greek philosopher
4-1 Notes Defining the Atom Early Models of the Atom All matter is composed of atoms Atoms are the smallest particles of an element that retains their identity in a chemical reaction Greek philosopher
1. Explain the law of conservation of mass in your own words. Matter is neither created nor destroyed in a chemical reaction.
 Chapter 3 Exam Review KEY I. Atomic Theory 1. Explain the law of conservation of mass in your own words. Matter is neither created nor destroyed in a chemical reaction. 2. What were Rutherford s two main
Chapter 3 Exam Review KEY I. Atomic Theory 1. Explain the law of conservation of mass in your own words. Matter is neither created nor destroyed in a chemical reaction. 2. What were Rutherford s two main
) The nucleus of an atom, when compared to the entire atom, is (Circle two).
 Unit 3: The Atom Review Packet Directions: Answer the following questions WITHOUT using your notes first. This will be a great way to study for your test. Then, get out your notes and go back and fill
Unit 3: The Atom Review Packet Directions: Answer the following questions WITHOUT using your notes first. This will be a great way to study for your test. Then, get out your notes and go back and fill
H CHEM - WED, 9/7/16. Do Now Be ready for notes. Sigfig review problem. Agenda Atomic Theory. Homework. Error Analysis
 H CHEM - WED, 9/7/16 Do Now Be ready for notes. Sigfig review problem Agenda Atomic Theory Error Analysis Homework Possibly atomic theory paragraph THE ATOM DEFINITION TO START Atom smallest particle
H CHEM - WED, 9/7/16 Do Now Be ready for notes. Sigfig review problem Agenda Atomic Theory Error Analysis Homework Possibly atomic theory paragraph THE ATOM DEFINITION TO START Atom smallest particle
Chapter 3. Table of Contents. Section 1 The Atom: From Philosophical Idea to Scientific Theory. Section 2 The Structure of the Atom
 Atoms: The Building Blocks of Matter Table of Contents Section 1 The Atom: From Philosophical Idea to Scientific Theory Section 2 The Structure of the Atom Section 1 The Atom: From Philosophical Idea to
Atoms: The Building Blocks of Matter Table of Contents Section 1 The Atom: From Philosophical Idea to Scientific Theory Section 2 The Structure of the Atom Section 1 The Atom: From Philosophical Idea to
CHAPTER -4 STRUCTURE OF ATOM CONCEPT DETAILS
 CHAPTER -4 STRUCTURE OF ATOM CONCEPT DETAILS KEY CONCEPTS : [ *rating as per the significance of concept] 1. Dalton s Atomic theory ** 2. J J Thomson Experiments *** 3. Rutherford s Scattering Experiments
CHAPTER -4 STRUCTURE OF ATOM CONCEPT DETAILS KEY CONCEPTS : [ *rating as per the significance of concept] 1. Dalton s Atomic theory ** 2. J J Thomson Experiments *** 3. Rutherford s Scattering Experiments
Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na
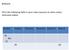 Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
NJCTL.org 2015 AP Physics 2 Nuclear Physics
 AP Physics 2 Questions 1. What particles make up the nucleus? What is the general term for them? What are those particles composed of? 2. What is the definition of the atomic number? What is its symbol?
AP Physics 2 Questions 1. What particles make up the nucleus? What is the general term for them? What are those particles composed of? 2. What is the definition of the atomic number? What is its symbol?
Chapter 4. Atomic Structure
 Chapter 4 Atomic Structure Warm Up We have not yet discussed this material, but what do you know already?? What is an atom? What are electron, neutrons, and protons? Draw a picture of an atom from what
Chapter 4 Atomic Structure Warm Up We have not yet discussed this material, but what do you know already?? What is an atom? What are electron, neutrons, and protons? Draw a picture of an atom from what
Fundamental Forces of the Universe
 Fundamental Forces of the Universe There are four fundamental forces, or interactions in nature. Strong nuclear Electromagnetic Weak nuclear Gravitational Strongest Weakest Strong nuclear force Holds the
Fundamental Forces of the Universe There are four fundamental forces, or interactions in nature. Strong nuclear Electromagnetic Weak nuclear Gravitational Strongest Weakest Strong nuclear force Holds the
Early Atomic Theories and the Origins of Quantum Theory. Chapter 3.1
 Early Atomic Theories and the Origins of Quantum Theory Chapter 3.1 What is Matter Made of? People have wondered about the answer to this question for thousands of years Philosophers Matter is composed
Early Atomic Theories and the Origins of Quantum Theory Chapter 3.1 What is Matter Made of? People have wondered about the answer to this question for thousands of years Philosophers Matter is composed
JJ Thompson (1897) Robert Millikan (1909)
 Atomic Structure Matter Consists of Particles Aristotle Democritus John Dalton 183 I am a genius! Atomic Theory of Matter based on the following postulates: 1) Each element is composed of particles called
Atomic Structure Matter Consists of Particles Aristotle Democritus John Dalton 183 I am a genius! Atomic Theory of Matter based on the following postulates: 1) Each element is composed of particles called
Practice Packet Unit 4: Atomic Structure
 Name: Regents Chemistry Practice Packet Unit 4: Atomic Structure Assess Yourself: Vocab: Lesson 1: Lesson 2: Lesson 3: Lesson 4: Lesson 5: Lesson 6: Lesson 7: 1 Vocabulary: Check your understanding. Describe
Name: Regents Chemistry Practice Packet Unit 4: Atomic Structure Assess Yourself: Vocab: Lesson 1: Lesson 2: Lesson 3: Lesson 4: Lesson 5: Lesson 6: Lesson 7: 1 Vocabulary: Check your understanding. Describe
Unit 3 Atomic Structure Chapter 3 of your book.
 Unit 3 Atomic Structure Chapter 3 of your book. Early Booklet E.C.: / 2 Unit 3 Hwk. Pts: / 24 Unit 3 Lab Pts: / 16 Late, Incomplete, No Work, No Units Fees? Y / N Learning Targets for Unit 3 1.1 I can
Unit 3 Atomic Structure Chapter 3 of your book. Early Booklet E.C.: / 2 Unit 3 Hwk. Pts: / 24 Unit 3 Lab Pts: / 16 Late, Incomplete, No Work, No Units Fees? Y / N Learning Targets for Unit 3 1.1 I can
Vocabulary Review. Atom Cathode Ray Electrons Protons Neutrons Nucleus
 2/19/16 Do Now On your half sheet of paper, identify the scientist that either discovered a part of the atom or developed a theory related to atomic structure. Vocabulary Review Atom Cathode Ray Electrons
2/19/16 Do Now On your half sheet of paper, identify the scientist that either discovered a part of the atom or developed a theory related to atomic structure. Vocabulary Review Atom Cathode Ray Electrons
Atomic Structure. Chapters 4, 8, Bravo 15,000 kilotons
 Atomic Structure Chapters 4, 8, 18.1-18.3 Bravo 15,000 kilotons What is an atom? Smallest unit of an element that retains all the properties of the element Can combine with other atoms to form compound
Atomic Structure Chapters 4, 8, 18.1-18.3 Bravo 15,000 kilotons What is an atom? Smallest unit of an element that retains all the properties of the element Can combine with other atoms to form compound
