I C(CH 3 ) 3. (c)2ch 3 OH+(CH 3 ) 3 CCl (CH 3 ) 3 COCH 3 +CH 3 OH + 2 +Cl - (d) CH 3 CH 2 CH 2 Br+NaCN CH 3 CH 2 CH 2 CN+NaBr
|
|
|
- Albert Bell
- 6 years ago
- Views:
Transcription
1 6.1 Writer the following as net ionic reactions and designate the nucleophile, substrate, and leaving group in each reaction. (a) Na Na (b) Na Na (c) 2 3 +( 3 ) 3 ( 3 ) (d) NaN N+Na (e) N N 2 +N 4 ANSWER: (a) nucleophile: substrate: 3 leaving group: - (b) nucleophile: - substrate: 3 2 leaving group: - (c)2 3 +( 3 ) 3 ( 3 ) nucleophile: 3 substrate: ( 3 ) 3 leaving group: - (d) N N+ - nucleophile: N - substrate: leaving group: - (e) N N 2 +N nucleophile: N 2 substrate: leaving group: Use chair conformational structures (Sect.4.12) and show the nucleophilic substitution reaction that would take place when trans-1-bromo-4-tert-butylcyclohexane reacts with iodide ion. (Show the most stable conformation of the reactant and the product.) ( 3 ) 3 ( 3 ) S N 2 reactions that involve breaking a bond to a stereocenter can be used to relate configurations of molecules because the stereochemistry of the reaction is known, (a) llustrate how this is true by assigning configurations to the 2-chloro-butane enantiomers based on the following date. [The configuration of (--)-2-butanol is given in Section 5.7.]
2 (+)-2-hlorobutane - S N 2 (-)-2-Butanol 25 [ α ] = [ α ] = Enantiomerically pure Enantiomerically pure (b) When optically pure (+)-2-chlorobutane is allowed to react with potassium iodide in acetone in an S N 2 reaction, the 2-iodobutane that is produced has a minus rotation. What is the configuration of (--)-2-iodobutane? f (+)-2-iodobutane? Answer, (a) Et Et + Me Me (b) An inversion of configuration R-(-)-2-iodobutane S-(+)-2-iodobutane 6.4 Keep in mind that carbocations have a trigonal planar structure, (a) write a structure for the carbocation intermediate and (b) write structures for the alcohol (or alcohols) that you would expect from the following reaction: ( 3 ) SN1 (a) Structure of the carbocation intermediate: (b) Structures of the alcohols would be:
3 3 ( 3 ) 3 ( 3 ) What product(s) would you expect from the methanolysis of the cyclohexane derivative given as the reaction in Problem 6.4? (3 ) The product is following: (3 ) 3 3, ( 3 ) the reaction is as 3 (3 ) (3 ) ( 3 ) The relative rates of ethanolysis of four primary alkyl halides are as follows: 3 2, 1.0; 3 2 2, 0.28; ( 3 ) 2 2, 0.030; ( 3 ) 3 2, (a). Are each of these reactions likely to be S N 1 or S N 2? (b) Provide an explanation for the relative reactivities that are observed. (a) All the reactions are likely to be S N 2. (b) For particles (molecules and ions) to react, their reactive centers must be able to come within bonding distance of each other. Large and bulky groups can often hinder the formation of the required transition state. So the relative rates of the above alkyl halides are from high to low. 6.7 assify the following solvents as being protic or aprotic: formic acid, ; acetone, 3 3 ;acetonitrile, 3 N ; formamide, N 2 ; sulfur
4 3 dioxide, S 2 ; ammonia, N 3 ; trimethylamine, 3 N 3 ; ethylene glycol, 2 2. Protic:, N 2, N 3, Aptotic: 3 3, 3 N, 3 N 3, S Would you expect the reaction of propyl bromide with sodium cyanide (NaN), that is, Na N + Na to occur faster in MF or in ethanol? Explain you answer. N 3 MF 3 The reaction occurs faster in MF than in ethanol. Because it don t solvate anions to any appreciable extent. These naked anions are highly reactive both as base and nucleophiles. This is the opposite of their strength as nucleophiles in alcohol or water solutions. The rates of S N 2 reactions generally are vastly increased when they are carried out in polar aprotic solvents. The increase in rate can be as large as a millionfold. 6.9 Which would you expect to be the stronger nucleophile in a protic solvent: - (a). 3 2 or 3 -? 3 - is the stronger nucleophile. (b). 2 or 2 S? 2 S is the stronger nucleophile. (c). ( 3 ) 3 P or ( 3 ) 3 N? ( 3 ) 3 P is the stronger nucleophile in a protic solvent When tert-butyl bromide undergoes solvolysis in a mixture of methanol and water, the rate of solvoysis (measured by the rate at which bromide ions from in the mixture) increases when the percentage of water in the water increased. (a) Explain this occurrence. (b) Provide an explanation for the observation that the rate of the S N 2 reaction of ethyl chloride with potassium iodide in methanol and water decreases when the percentage of water in the mixture is increased.
5 (a) Because water is the most effective solvent for promoting ionization. t made the intermediate more stable. (b) Because the charge of the transition state is more dispersed than the charge in the starting material. ncreasing the polarity of the solvent will increase the stability of the starting material. Therefore, it will decrease the rate of the reaction Starting with (S)-2-bromobutane, outline syntheses of each of the following compounds. (a) (R)- (b) 3 2 (R) (c) (R) (d) (R) S S 3 (a) (b) (c) 3 3 S + S (d) + 3 S 3 S + 3 3
6 6.13 Show how you might use a nucleophilic substitution reaction of propyl bromide to synthesize each of the following compounds. (You may use any other compounds that are necessary.) (a) (b) (c) (d) S 3 (e) (f) N 3 3 (g) 3 N (g) N (h) S Answers: (a) (b) (c) (d) 3 S S 3 + (e)
7 (f) N N 3 + (g) ( 3 ) 3 N ( 3 ) 3 N (h) N N + (i) S S Which alkyl halide would you expect to react more rapidly by an S N 2 mechanism? Explain your answer. (a) or ( 3 ) 2 (b) or (c) ( 3 ) 2 2 or (d) ( 3 ) or 3 2 ( 3 ) 2 (e) 6 5 or (a) 1-omopropane. Because it is less hindered. (b) 1-odobutane. Because - is a good leaving group. (c) 1-hlorobutane. Because it is less hindered. (d) 1-hloro-3-methylbutane. Because the carbon bearing the leaving group is less hindered than in 1-hloro-2-methylbutane. (e) 1-chlorohexane. Phenyl halides are unreactive in S N Which S N 2 reaction of each pair would you expect to take place more rapidly in a protic solvent?
8 (a) (1) (2) (b) (1) (2) S S (c) (1) ( 6 5 ) 3 N N( 6 5 ) (2) ( 6 5 ) 3 P P( 6 5 ) (d) (1) (1.0M) (1.0M) (2) (1.0M) (2.0M) each pair. (a) (1),(b) (2),(c) (2),(d) (2) are expected to take place more rapidly in a protic solvent in 6.16 Which S N 1 reaction of each pair would you expect to take place more rapidly? Explain your answer. (a) (b) (1)( 3 ) ( 3 ) 3 + (2)( 3 ) ( 3 ) 3 + (1)( 3 ) ( 3 ) 3 + (2)( 3 ) ( 3 ) 3 + () (1)( 3 ) 3 (1.0M) (1.0M) ( 3 ) (2)( 3 ) 3 (2.0M) (1.0M) ( 3 ) (d) (1)( 3 ) 3 (1.0M) (1.0M) ( 3 ) (2)( 3 ) 3 (1.0M) (2.0M) ( 3 ) (e) (1)( 3 ) ( 3 ) 3 + (2) (a) Reaction2 will be more rapidly. Because bromide ion is better leaving group than chloride ion (b) Reaction1 will be more rapidly. Because 2 and ethanol are both solvent and nucleophile, and the water is stranger polar protic solvent than ethanol. (c) Reaction2 will be more rapidly. Because the substrate has bigger concentration. (d) The two reactions have same reaction rate. Because the Nu has no effect on the reaction. (e) Reaction1 will be more rapidly. Because ( 3 ) 3 + is more stable.
9 6.17 With methyl, ethyl, or cyclopentyl halides as your organic starting materials and using any needed solvents or inorganic reagents, outline syntheses of each of the following. More than one step may be necessary and you need not repeat steps carried out in earlier parts of this problem. (a) 3 (b) 3 2 (c) 3 (d) 3 2 (e) 3 S (f) 3 2 S (g) 3 N (h) 3 2 N (i) 3 3 (j) (k) yclopentene (a) (b) (c) (d) (e) 3 S 3 + S - 3 S (f) 3 2 S S S + - (g) 3 N 3 + N N + - (h) 3 2 N N N + - (i) Na 2 3 Na o (j) (k) yclopentene 50 o Na Na + 3 2
10 6.18 Listed below are several hypothetical nucleophilic substitution reactions. None is synthetically useful because the product indicated is not formed at an appreciable rate. n each case provide an explanation for the failure of the reaction to take place as indicated. (a) (b) (c) N (d) +N (e) N N (f) N N Answer (a) Alkanide ion ( - 3 ) is very powerful base and it s virtually never acting as leaving groups. Therefore, reaction (a) is not feasible. (b) ydride ion (: - ) is very powerful base and it s virtually never acting as leaving groups. Therefore, reaction (b) is not feasible. (c) Alkanide ion (R: - ) is very powerful base and it s virtually never acting as leaving groups. Therefore, reaction (c) is not feasible. (d) Tertiary alkyl bromide can t be the substrate for the S N 2 reaction. (e) 3 - is a strongly basic ion and rarely acts as leaving group. Therefore, reaction e is not feasible. (f) only appear in a strong acid and N 3 can t exist in a strong acid You have the task of preparing styrene ( 6 5 = 2 ) by dehydrohalogenation of either 1-bromo-2-phenylethane or 1-bromo-1-phenylethane use K in ethanol. Which halide would you choose as your starting material to give the better yield of the alkene? Explain your answer. Answers: 1-omo-1-phenylethane will be used as the starting material, because a 1º alkyl bromide gives mainly S N 2 except with a hindered strong base and then gives mainly E2. A 2º alkyl bromide gives mainly E2 with strong bases Your task is to prepare isopropyl methyl ether. 3 ( 3 ) 2, by one of the following reactions. Which reaction would give the better yield? Explain your choice. (1) 3 Na + ( 3 ) 2 3 ( 3 ) 2 (2) ( 3 ) 2 Na ( 3 ) 2 The second reaction would give the better yield because the desired reaction is an SN2 reaction, and the substrate is a methyl halide. Use of reaction (1) would result in considerable elimination by an E2 pathway Which product (or products) would you expect to obtain from each of the following reactions? n each part give the mechanism (S N 1, SN2, E1, or E2) by which each product is formed and predict the relative amount of each product (i.e., would the product be the only product, the major product, a minor
11 product, etc.?) (a) S N 2 major product ( 2 3 ) minor product = 2 (b) E2 major product ( 3 ) 3-50 ( 3 ) = 2 minor product ( 3 ) 3-50 ( 3 ) ( 3 ) (c) E2 the only product ( 3 ) (d) E1 the only product ( 3 ) 3 + ( 3 ) 3-50 ( 3 )
12 (e) S N 2 the only product ( 3 ) acetone ( 3 ) 3 (f) S N 1 the only product 3 ( 3 ) ( 3 ) ( 3 ) 3 3 (g) E1 3-hloropentane = 3 3-hloropentane ( 3 ) (h) S N 1 3-hloropentane ( 2 3 ) hloropentane = 2 3 (i) E2 major product - + (R)-2-bromobutane minor product - + (R)-2-bromobutane 25
13 (j) S N 1 (S)-3-omo-3-methylhexane (k) S N (+)- (S)-2-omooctane (R)-2-odooctane Write conformational structures for the substitution products of the following deuterium-labeled compounds: (a) - 3? (b) - 3? (c) - 3? (d) 3 2 3? The answer:
14 (a) (b) (c) (d) Although ethyl bromide and isobutyl bromide are both primary halides. Ethyl bromide undergoes S N 2 reactions more than 10 times faster than isobutyl bromide does. When each compound is treated with a strong base/ nucleophile ( ), isobutyl bromide gives a greater yield of elimination products than substitution products, whereas with ethyl bromide this behavior is reversed. What factor account for these results? The reason ethyl bromide undergoes S N 2 reactions faster than isobutyl bromide is steric effect. This steric hindrance causes isopropyl bromide to react more slowly in S N 2 reactions and to give relatively more elimination (by an E2) when a strong base is used onsider the reaction of - with 3 2. (a) Would you expect the reaction to be S N 1 or S N 2?
15 The rate constant for the reaction at 60 is 5*10-5 L mol -1 s -1. What is the reaction rate if [ - ]=0.1mol L -1 and [ 3 2 ]=0.1 mol L -1? (c) f [ - ]=0.1mol L -1 and [ 3 2 ]=0.2mol L -1? (d) f [ - ]=0.2mol L -1 and [ 3 2 ]=0.1 mol L -1? (e) f [ - ]=0.2mol L -1 and [ 3 2 ]=0.2 mol L -1? Because the substrate is the primary alkyl halide, the reaction would be S N 2. V=k*[ - ][ 3 2 ] (b) v =5*10-5 *0.1*0.1=5*10-7 (c) v =5*10-5 *0.1*0.2=1*10-6 (d) v =5*10-5 *0.2*0.1=1*10-6 (e) v =5*10-5 *0.2*0.2=2* Which reagent in each pair listed here would be the more reactive nucleophile in a protic solvent? (a) 3 N - or 3 N 2 (e) 2 or 3 + (b) 3 - or 3 - (f) N 3 or N 4 (c) 3 S or 3 (g) 2 S or S - (d) ( 6 5 ) 3N or ( 6 5 ) 3P (h) 3 - or - (a) 3 N - (b) 3 - (c) 3 S (d) ( 6 5 ) 3P (e) 2 (f) N 3 (g) S - (h) write mechanisms that account for the products of the following reaction (a) 2 2 _ 2 (b) 2 N _ 2 N ANSWER: (a)
16 - (b) 2 N N - N 6.27 Many S N 2 reactions of alkyl chlorides and alkyl bromides are catalyzed by the addition of sodium or potassium iodide. For example, the hydrolysis of methyl bromide takes place much faster in the presence of sodium iodide. Explain. Because Alkyl chlorides and bromides are also easily converted to alkyl iodides by nucleophilic substitution reactions. R R + - R + - R + - The effect of the leaving group in S N 2 reaction: R > R > R > R F That is why many S N 2 reactions of alkyl chlorides and alkyl bromides are catalyzed by the addition of sodium or potassium iodide take place much faster in the presence of sodium iodide. odide ion is a good nucleophile and a good leaving group Explain the following observations: When tert-butyl bromide is treated with sodium methoxide in a mixture of methanol and water, the rate of formation of tert-butyl alcohol and tert-butyl methyl ether does not change appreciably as the concentration of sodium methoxide is increased. owever, increasing the concentration of sodium methoxide causes a marked increase in the rate at which tert-butyl bromide disappears from the mixture. tert-butyl alcohol and tert-butyl methyl ether are formed via an S N 1 mechanism. The rate of the reaction is independent of the concentration of methoxide ion. owever, A cpompetetion reaction of this reaction is the E2 elimination reaction that is associated with the concentration of the methoxide ion. This mechanism will cause the disappearance of the tert-butyl bromide. The mechanism is as follows: ( 3 ) 2 + -
17 6.29 (a) onsider the general problem of converting a tertiary alkyl halide to an alkene, for example, the conversion of tert-butyl chloride to 2-methylpropene. What experimental conditions would you choose to ensure that elimination is favored over substitution? (b) onsider the opposite problem, that of carrying out a substitution reaction on a tertiary alkyl halide. Use as your example the conversion of tert-butyl chloride to tert-butyl ethyl ether. What experimental conditions would you employ to ensure the highest possible yield of the ether? (a.) t is hard to influence the relative partition between S N 1 and E1 products, E2 reaction should be chosen to ensure that elimination is favored, because S N 2 reaction is hard to take place as the result of the steric hindrance. So high temperature, bulky strong base are required. n the conversion of tert-butyl chloride to 2-methylpropene, a base of 2 5 Na solved in 2 5 and a high temperature should be offered. (b.) Because of the steric hindrance, S N 2 reaction couldn t take place, so the only way to ensure the substitution is to ensure the S N 1 reaction. We should use a neutral nucleophile at low temperature. For example, when ( 3 ) 3 and 2 5 are mixed at a low temperature, they can produce ( 3 ) omobicyclo[2,2,1]heptane is extremely unreactive in either S N 2 or S N 1 reactions. Provide explanations for this behavior. 1-omobicyclo[2,2,1]heptane First it is one kind of bicycloalkane, so the ring strain in structure baffles the nucleophile attacking to the substrate from back. So it is unreactive in S N 2. Second, it is hard for the bridge carbon atom forming carbocation, because the sp 2 hybridization should be in one plane. So it is unreactive in S N Starting with an appropriate alkyl halide, and using any other needed reagents, outline syntheses of each of the following. When alternative possibilities exist for a synthesis you should be careful to choose the one that gives the better yield (a) Butyl sec-butyl ether (g)(s)-2-pentanol (b) 3 2 S( 3 ) (h)(r)-2-odo-4-methylpentane (c) Methyl neopentyl ether (i)( 3 ) 3 2 (d) Methyl phenyl ether (j) cis-4-sopropylcyclohexanol (e) N (k)(r)- 3 (N) 2 3 (f) N (l) trans-1-odo-4-methylcyclohexane
18 (a) + Na Butylsec-butyl ether (b) + S S (c) + (d) + (e) N N - (f) + Na (g) + Na + Na (h) (i) 3 2 Na
19 (j) cis-4-sopropylcyclohexanol (k) N + N (R)- 3 (N) 2 3 (l) trans-1-odo-4-methylcyclohexane 6.33 Give structures for the products of each of the following reactions: (a) (b) 1,4-ichlorohexane(1mol) + Na(1 mol) F acetone + Na(1mol) 5 8 F + Na acetone Na () 1,2-ibromoethane(1mol) + NaS22SNa 4 8 S 2 +2Na - 2 heat (d) 4-hloro-1-butanol +Na Et Na Et Na -N 3 (e) Propyne + NaN3 liq.n Na Na Answers: (a) 5 8 F F
20 (b) hloro-1-iodo-hexane (c) 4 8 S 2 S S (d) 4 8 Na Na + (e) 3 3 Na 3 Na When tert-butyl bromide undergoes S N 1 hydrolysis, adding a common ion (e.g.,na) to the aqueous solution has no effect on the rate. n the other hand, when ( 6 5 ) 2 undergoes S N 1 hydrolysis, adding Na retards the reaction.given that the ( 6 5 ) 2 + cation is known to be much more stable than the ( 3 ) 3 + cation (and we shall see why in section 15.12A), provide an explanation for the different behavior of the two compounds Ph Ph + - Ph Ph 2 Ph Ph
21 The rate determining step of the S N 1 reaction is the formation of the carbocation, in the hydrolysis of tert-butyl bromide, the carbocation intermediate is relative unstable comparing with the diphenyl methyl cation. Which means that diphenyl methyl cation could combine with the bromine anion, a reversible reaction takes place easier than the first reaction. Therefore, increasing the concentration of bromine anion will increase the reaction speed of the reverse one, and retard the hydrolysis reaction When the alkyl bromides ( listed here ) were subjected to hydrolysis in a mixture of ethanol and water ( 80% 2 5 / 20% 2 ) at 55, the rates of the reaction showed the following order: ( 3 ) 3 > 3 > 3 2 > ( 3 ) 2 Provide an explanation for this order of reactivity. The relative reactivity of the substrates towards SN2 is as follows: 3 X> 3 2 X> ( 3 ) 2 X The relative reactivity of the substrates towards SN1 is as follows: ( 3 ) 3 > ( 3 ) 2 > 3 2 > 3 ombine those two mechanisms, the relative reactivity of the substrates towards the nucleophilic substitution reaction is: ( 3 ) 3 > 3 > 3 2 > ( 3 ) The reaction of 1º alkyl halides with nitrite salts produces both RN 2 and RN. Account for this behavior. The nucleophilic sites for the N - 2 are: N N N - δ R δ X N δ R δ X N R X - transition state - N δ R δ X - δ N + - δ R X N+ R X -
22 6.37 What would be the effect of increasing solvent polarity on the rate of each of the following nucleophilic substitution reaction? (a) Nu: + R L R Nu + + :L - (b) R L + R + + :L δ + δ - The transition state for the reaction (a) should be: Nu R L, in which the charge is developing. The more polar the solvent, the better it can solvate the transition state, thus lowing the free energy of the activation and increasing the reaction rate. δ + δ + The transition state for the reaction (b) should be: R L, in which the charge is becoming dispersed. A polar solvent is less able to solvate this transition state than it is to solvate the reactant. The free energy of activation, therefore, will become somewhat larger as the solvent polarity increases, and the rate will be slower ompetition experiments are those in which two reactants at the same concentration (or one reactant with two reactive sites) compete for a reagent. Predict the major product resulting from each of the following competent experiment. (1) n the solvent MF, is more reactive than in water or methanol as no hydrogen bonds is formed. So it carries out a reaction S N 2. The carbon atom 1 is bearing a bulky group which has a dramatic inhibiting effect. (2) acetone is a neutral molecule, and it can only carry out a reaction S N 1. The tertiary carbocation is more stable than the primary carbocation.
23 acetone n contrast to S N 2 reactions, S N 1 reactions show relatively little nucleophile selectivity. That is, when more than one nucleophile is present in the reaction medium, S N 1 reaction show only a slight tendency to discriminate between weak nucleophiles and strong nucleophiles, whereas S N 2 reactions show a marked tendency to discriminate. (a) Provide an explanation for this behavior. (b) Show how your answer accounts for the fact that reacts with 0.01 M NaN in ethanol to yield primarily N, whereas under the same conditions ( 3 ) 3 reacts to give primarily ( 3 ) (a) Reaction of alkyl halides by an S N 1 mechanism are favored by the use of substrates that can from relatively stable carbocation, by the use of weak nucleophiles, and by the use of highly ionizing solvent. S N 1 mechanism, therefore, are important in solvolysis reactions of tertiary halides, especially when the solvent is highly polar. f we want to favor the reaction of an alkyl halide by an S N 2 mechanism, we should use a relatively unhindered alkyl halide, a strong nucleophile, a polar aprotic solvent, and a high concentration of the nucleophile. So they have different behavior. (b) and 0.01 M NaN in ethanol use S N 2 reactions, and show a marked tendency to NaN. So the product is primarily N. Whereas ( 3 ) 3 show slight tendency to NaN. Because in 0.01 M NaN in ethanol solution, ethanol is much more than NaN. So the product is primarily ( 3 ) n the gas phase, the homolytic bond dissociation energy (Section 10.2A) for the carbon-chlorine bond of tert-butyl chloride is +328KJ mol -1 ; the ionization potential for a tert-butyl radical is +715KJ mol -1 ; and the electron affinity of chlorine is 330KJ mol -1. Using these data, calculate the enthalpy change for the gas phase ionization of tert-butyl chloride to a tert-butyl cation and a chloride ion (this is the heterolytic bond dissociation energy of the carbon chlorine bond). ( 3 ) 3 ( 3 ) KJ / mol ( 3 ) 3 + ( 3 ) 3 e KJ / mol + e KJ / mol ( 3 ) 3 ( 3 ) KJ / mol
24 +328KJ +715KJ -330KJ 6.41 The reaction of chloroethane with water in the gas phase to produce ethanol and hydrogen chloride has a Δ = KJ mol -1 and a ΔS = J K -1 mol -1 at 25 (a) Which of these terms, if either, favors the reaction going to completion Solution: The entropy term is slightly favorable. (b) alculate ΔG for the reaction. What can you now say about whether the reaction will proceed to completion? Solution: ΔG =Δ -TΔS = *4.81*10-3 = > 0 So, chloroethane with water in the gas phase will produce ethanol and hydrogen chloride at 25 (c) alculate the equilibrium constant for the reaction Solution: ΔG = - RT ln K K = 3.84*10-5 (d) n aqueous solution the equilibrium constant is very much larger than the one you just calculated. ow can you account for this fact? The equilibrium is very much more favorable in aqueous solution because solvation of the products takes place and thereby stabilizes them When (S)-2-bromopropanoic acid [ (S)- 3 2 ] reacts with concentrated sodium hydroxide the product formed (after acidification) is (R)-2-hydroxypropanoic acid [(R)- 3 2, commonly known as (R)-lactic acid]. This is, of course, the normal stereochemical result for an S N 2 reaction. owever, when the same reaction is carried out with a low concentration of hydroxide ion in the presence of Ag 2 (where Ag acts as a Lewis acid), it takes place with overall retention of configuration to produce (S)-2-hydroxypropanoic acid. The mechanism of this reaction involves a phenomenon called neighboring group participation. Write a detailed mechanism for this reaction that accounts for the net retention of configuration when Ag and a low concentration of hydroxide are used. at the first condition, there exists sodium hydroxide, a strong base and a good nucleophile. So S N 2 reaction takes place. But at the second condition, there exists Ag +, the reaction takes place in the following way: 3 Ag The phenomenon of configuration inversion in a chemical reaction was discovered in1896 by Paul von Walden. Walden s proof of configuration inversion was base on the following cycle:
25 Ag (-)-hlorosuccinic acid P 5 K 2 2 () 2 (-)-Malic acid 2 2 () 2 (+)-Malic acid P 5 K (+)-hlorosuccinic acid Ag 2 2 (a) Basing your answer on the preceding problem, which reaction of the Walden cycle are likely to take place with overall inversion of configuration and which are likely to occur with overall retention of configuration? (b) Malic acid with a negative optical rotation is now known to have the (s) configuration. What are the configuration of the other compounds in the Walden cycle? (c) Walden also found that when (+)-malic acid is treated with thionyl chloride (rather P 5 ), the product of the reaction is (+)-chlorosuccinic acid. ow can you explain this result? (d) Assuming that the reaction of (-)-malic acid and thionyl chloride has the same steteochemistry, outline a Walden cycle based on the vse of thionyl chloride instead of P5. ANSWER: (a) The reaction of the Walden cycle are likely to take place with overall inversion of configuration: K 2 2 () (-)-Malic acid P (+)-hlorosuccinic acid (-)-hlorosuccinic acid K P () 2 (+)-Malic acid The reaction which are likely to occur with overall retention of configuration: Ag () 2 (-)-hlorosuccinic acid 2 (-)-Malic acid (+)-hlorosuccinic acid Ag () 2 (+)-Malic acid (b) (-)-hlorosuccinic acid is (S)
26 2 2 () 2 (+)-Malic acid is (R) (+)-hlorosuccinic acid is (R) (c)the type of reaction is the retention of the configuration. 2 2 () 2 (+)-Malic acid S (+)-hlorosuccinic acid (d) The new cycle is K (+)-hlorosuccinic acid thionyl chloride 2 2 () 2 (-)-Malic acid 2 2 () 2 (+)-Malic acid thionyl chloride K (-)-hlorosuccinic acid 6.44 (R)-(3-hloro-2-methylpropyl ) methyl ether ( A ) on reaction with azide ion (N - 3 ) in aqueous ethanol gives ( S )-( 3-azido-2-methylpropyl ) methyl ether (B). ompound A has the structure 2 ( 3 ) 2 3. ( a ) raw wedge-dashed wedge-line formulas of both A and B. ( b ) s there a change of configuration during this reaction? A B 2 2 N There is no change of configuration during this reaction cis-4-omocyclohexanol t-bu - in t-bu racemic 6 10 (compound )
27 ompound has infrared absorption in the 1620 to 1680 cm - and in the 3590 to 3650 cm - regions. raw and label the (R) and (S) enantiomers of produce Answer
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Elimination Reactions Heating an alkyl halide with a strong base causes elimination of a. molecule of HX
 Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
(b) (CH 3 ) 2 CCH 2 CH 3 D 2 O. (e) A. CH 3 CCl OSO 2 CH 3 C 6 H 5 H 3 C
 278 h a p t e r 7 Further Reactions of aloalkanes 3. arbocations are stabilized by hyperconjugation: Tertiary are the most stable, followed by secondary. Primary and methyl cations are too unstable to
278 h a p t e r 7 Further Reactions of aloalkanes 3. arbocations are stabilized by hyperconjugation: Tertiary are the most stable, followed by secondary. Primary and methyl cations are too unstable to
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
Chapter 11: Nucleophilic Substitution and Elimination Walden Inversion
 hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
CHE 275 NUCLEOPHILIC SUBSTITUTUION CHAP 8 ASSIGN. 1. Which best depicts the partial charges on methyl bromide and sodium methoxide?
 CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
 11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Halogen Compounds
 8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
Chapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution
 hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
7. Haloalkanes (text )
 2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
FOURTH EXAMINATION. (1)..20 pts... (2)..24 pts... (3)..18 pts... (4)..12 pts... (5)..12 pts... (6).. 6 pts... (7).. 8 pts... Bonus 4 pts...
 Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
Chapter 7 Substitution Reactions 7.1 Introduction to Substitution Reactions Substitution Reactions: two reactants exchange parts to give new products
 hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
The Electrophile. S N 2 and E2 least stable most stable least hindered most hindered. S N 1 and E1. > x > >
 The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 7 Substitution Reactions
 Chapter 7 Substitution Reactions Review of Concepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of Chapter 7. Each of the sentences below appears
Chapter 7 Substitution Reactions Review of Concepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of Chapter 7. Each of the sentences below appears
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Chapter 8 I. Nucleophilic Substitution (in( II. Competion with Elimination. Nucleophilic Substitution
 hapter 8 I. Nucleophilic Substitution (in( depth) II. ompetion with Elimination Nucleophilic Substitution Substrate is a sp3 hybridized carbon atom (cannot be an a vinylic halide or an aryl halide except
hapter 8 I. Nucleophilic Substitution (in( depth) II. ompetion with Elimination Nucleophilic Substitution Substrate is a sp3 hybridized carbon atom (cannot be an a vinylic halide or an aryl halide except
+ + CH 11: Substitution and Elimination Substitution reactions
 C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
CHAPTER 8 HW SOLUTIONS: ELIMINATIONS REACTIONS
 APTER 8 W SLUTNS: ELMNATNS REATNS S-TRANS SMERSM 1. Use a discussion and drawing of orbitals to help explain why it is generally easy to rotate around single bonds at room temperature, while it is difficult
APTER 8 W SLUTNS: ELMNATNS REATNS S-TRANS SMERSM 1. Use a discussion and drawing of orbitals to help explain why it is generally easy to rotate around single bonds at room temperature, while it is difficult
Chapter 5. 3-Chloro-2-methylpentane. Cl 2. 2-Chloro-2-methylpentane. 1-Chloro-2-methylpentane. Cl 2-Chloro-4-methylpentane. 1-Chloro-4-methylpentane
 hapter 5 5.1 lassify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) 3Br K 3 KBr (b) 3 2 2 2 2 (c) 2 2 2 3 3 a. substitution b. elimination c. addition
hapter 5 5.1 lassify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) 3Br K 3 KBr (b) 3 2 2 2 2 (c) 2 2 2 3 3 a. substitution b. elimination c. addition
Lecture 18 Organic Chemistry 1
 CEM 232 rganic Chemistry I at Chicago Lecture 18 rganic Chemistry 1 Professor Duncan Wardrop March 9, 2010 1 Nucleophilicity nucleophilicity: measures the strength of the nucleophile ; more nucleophilic
CEM 232 rganic Chemistry I at Chicago Lecture 18 rganic Chemistry 1 Professor Duncan Wardrop March 9, 2010 1 Nucleophilicity nucleophilicity: measures the strength of the nucleophile ; more nucleophilic
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams.
 2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
CHEM 2311 HW1. COMPLETELY FILL THE BOXES IN DARK PENCIL, i.e.:, NOT or or STRUCTURE
 EM 2311 W1 Provide answers to the following multiple choice questions on the Scantron form provided in lecture. Make sure that you put your name and bubble in your GTID # on the answer form. MPLETELY FILL
EM 2311 W1 Provide answers to the following multiple choice questions on the Scantron form provided in lecture. Make sure that you put your name and bubble in your GTID # on the answer form. MPLETELY FILL
Chapter 9. Nucleophilic Substitution and ß-Elimination
 Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
CHEM 2312 practice final. Version - II
 EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
Halo Alkanes and Halo Arenes
 alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
1) (100 pts) 5) (20 pts) 3) (35 pts) 4) (25pts. Total (200 pts) More Tutorial at
 Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
PRACTICE PROBLEMS UNIT 8
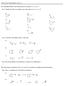 PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
PAPER No. 05: TITLE: ORGANIC CHEMISTRY-II MODULE No. 12: TITLE: S N 1 Reactions
 Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
Dr. Anand Gupta Mr Mahesh Kapil
 Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
1-What is substitution reaction? 2-What are can Nucleophilic Substitution Reaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2
 1-What is substitution reaction? 2-What are can Nucleophilic Substitution eaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2 1- SUBSTITUTION EACTIONS 1-Substitution eaction In this type
1-What is substitution reaction? 2-What are can Nucleophilic Substitution eaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2 1- SUBSTITUTION EACTIONS 1-Substitution eaction In this type
S N 1 Displacement Reactions
 S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
Nucleophilic Substitution and Elimination
 Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
CONCERTED sp 2 H. HO Et
 Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
PAPER No. : 5; Organic Chemistry-II MODULE No. : 13; Mixed S N 1 and S N 2 Reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Chapter 10. BrCH 2 CH 2 CH 2 CCH 2 Br CH 3. CH 3 CCH 2 CH 2 Cl CH 3 CHCH 2 CH 2 CHCH Give IUPAC names for the following alkyl halides:
 hapter 10 10.1 Give IUPA names for the following alkyl halides: (a), 3 2 2 2 I 1-iodobutane 3 (b), 3 2 2 l 1-chloro-3-methylbutane (c), 3 2 2 2 2 3 1,5-Dibromo-2,2-dimethylpentane 3 3 2 2 l (d), l 1,3-Dichloro-3-methylbutane
hapter 10 10.1 Give IUPA names for the following alkyl halides: (a), 3 2 2 2 I 1-iodobutane 3 (b), 3 2 2 l 1-chloro-3-methylbutane (c), 3 2 2 2 2 3 1,5-Dibromo-2,2-dimethylpentane 3 3 2 2 l (d), l 1,3-Dichloro-3-methylbutane
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution
 7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution Preview 7-4 7.1 Nucleophilic Substitution Reactions of Haloalkanes 7-5 Nucleophilic Substitution Mechanisms (7.1A) 7-5 The SN1
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution Preview 7-4 7.1 Nucleophilic Substitution Reactions of Haloalkanes 7-5 Nucleophilic Substitution Mechanisms (7.1A) 7-5 The SN1
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
3-chloro-1-propene 1-chloropropane 2-chloropropene
 ANSWERS #1. (from 50 minute exam #3, Fall 2000) 5. (6 points) For each group of 3 compounds, identify the compound that expresses the indicated property the MOST and the compound that expresses it the
ANSWERS #1. (from 50 minute exam #3, Fall 2000) 5. (6 points) For each group of 3 compounds, identify the compound that expresses the indicated property the MOST and the compound that expresses it the
Classes of Halides. Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination. Polarity and Reactivity. Classes of Alkyl Halides
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
4.15 Halogenation of Alkanes RH + X 2 RX + HX
 4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
Test Date: (Sunday) Test Time: 1:00 pm to 2:00 pm Test Venue: Lajpat Bhawan, Madhya Marg, Sector 15-B, Chandigarh
 Test ate: 1.09.015 (Sunday) Test Time: 1:00 pm to :00 pm Test Venue: Lajpat hawan, Madhya Marg, Sector 15-, handigarh r. Sangeeta Khanna Ph. 1 EMISTRY OING IRLE G:\+ Grand test-4 L-1.doc r. Sangeeta Khanna
Test ate: 1.09.015 (Sunday) Test Time: 1:00 pm to :00 pm Test Venue: Lajpat hawan, Madhya Marg, Sector 15-, handigarh r. Sangeeta Khanna Ph. 1 EMISTRY OING IRLE G:\+ Grand test-4 L-1.doc r. Sangeeta Khanna
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Text Sections (N0 1.9, 9-11) Homework: Chapter 1:
 CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
Organic Chemistry 1 CHM 2210 Exam 3 (November 9, 2001)
 Organic Chemistry 1 CM 2210 Exam 3 (November 9, 2001) Name (print): Signature: Student ID Number: There are 14 multiple choice problems (5 points each) on this exam, however, you will only be graded out
Organic Chemistry 1 CM 2210 Exam 3 (November 9, 2001) Name (print): Signature: Student ID Number: There are 14 multiple choice problems (5 points each) on this exam, however, you will only be graded out
Chapter 10 Radical Reactions
 Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
 EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
an axial "X" is necessary for a succesful E2 reaction and also works better for S N 2
 cleophilic Substitution & Elimination hemistry 1 Templates for predicting S2 and E2 reactions primary - β α α β two different perspectives secondary - β β α β β α two different perspectives tertiary -
cleophilic Substitution & Elimination hemistry 1 Templates for predicting S2 and E2 reactions primary - β α α β two different perspectives secondary - β β α β β α two different perspectives tertiary -
CHEM 2312 practice final. Version I
 CEM 2312 practice final Version The following standardized final examination covers the entire introductory year of organic chemistry (CEM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
CEM 2312 practice final Version The following standardized final examination covers the entire introductory year of organic chemistry (CEM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
20 Halogenoalkanes:substitution and elimination reactions
 20 alogenoalkanes:substitution and elimination reactions s to worked examples WE 20.1 Allylic halogenations (on p. 922 in Chemistry 3 ) 1-(Chloromethyl)benzene (benzyl chloride, PhC 2 ) is made on a large
20 alogenoalkanes:substitution and elimination reactions s to worked examples WE 20.1 Allylic halogenations (on p. 922 in Chemistry 3 ) 1-(Chloromethyl)benzene (benzyl chloride, PhC 2 ) is made on a large
much more stable then the
 8.1 Give the structure and name of the product that would be obtained from the ionic addition of I to propene. I I I 8.3 Provide mechanistic explanations for the following observations:(a)the addition
8.1 Give the structure and name of the product that would be obtained from the ionic addition of I to propene. I I I 8.3 Provide mechanistic explanations for the following observations:(a)the addition
MUNISH KAKAR s INSTITUTE OF CHEMISTRY
 REACTONS & PREPARATON OF HALOGENATED COMPDS. WS#3 Q1. Reagent for preparing a chloroalkane from an alcohol is (a) SOCl 2 (b) HCl/ZnCl 2 (c) PCl 3 (d) All of these Q2. n the addition of H to propene in
REACTONS & PREPARATON OF HALOGENATED COMPDS. WS#3 Q1. Reagent for preparing a chloroalkane from an alcohol is (a) SOCl 2 (b) HCl/ZnCl 2 (c) PCl 3 (d) All of these Q2. n the addition of H to propene in
8-3 This exercise is worked out on page 293 as "Working with Concepts".
 Copyright 2009 James K Whitesell 8-1 This exercise combines your knowledge of basic nomenclature rules and concepts of stereochemistry introduced in Chapters 4 and 5. 8-2 Keep in mind that faculty at UCSD
Copyright 2009 James K Whitesell 8-1 This exercise combines your knowledge of basic nomenclature rules and concepts of stereochemistry introduced in Chapters 4 and 5. 8-2 Keep in mind that faculty at UCSD
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl
 Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Organic Chemistry I. Summer Final Exam
 Print Name: rganic hemistry Summer 2014 Final Exam SPEAL NSTRUTNS: Please make sure to read each questions VERY carefully!! Some questions will look similar to questions on previous tests, but contain
Print Name: rganic hemistry Summer 2014 Final Exam SPEAL NSTRUTNS: Please make sure to read each questions VERY carefully!! Some questions will look similar to questions on previous tests, but contain
8.8 Unimolecular Nucleophilic Substitution S N 1
 8.8 Unimolecular Nucleophilic Substitution S N 1 A question. Tertiary alkyl halides are very unreactive in substitutions that proceed by the S N 2 mechanism. Do they undergo nucleophilic substitution at
8.8 Unimolecular Nucleophilic Substitution S N 1 A question. Tertiary alkyl halides are very unreactive in substitutions that proceed by the S N 2 mechanism. Do they undergo nucleophilic substitution at
Alkyl Halides. Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group.
 Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Chapter 11. Solution: 11.4 What product would you expect from S N 2 reaction of 1-bromobutane with each of the following?
 hapter 11 11.1 What product would you expect to obtain from a nucleophilic substitution reaction of (S)-2-bromohexane with acetate ion, 3-2? Assume that inversion of configuration occur, and show the stereochemistry
hapter 11 11.1 What product would you expect to obtain from a nucleophilic substitution reaction of (S)-2-bromohexane with acetate ion, 3-2? Assume that inversion of configuration occur, and show the stereochemistry
1. Name the following compound. Use the IUPAC system and include the stereochemical designations.
 Chemistry 51 Exam 1, Summer 2004 This is a closed book exam. The exam lasts 100 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer
Chemistry 51 Exam 1, Summer 2004 This is a closed book exam. The exam lasts 100 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer
What are radicals? H. Cl. Chapter 10 Radical Reactions. Production of radicals. Reactions of radicals. Electronic structure of methyl radical
 What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
Organic Chemistry CHM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl Halides: Substitution Reactions - Chapter 6 (Wade)
 rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
Introduction to Alkyl Halides, Alcohols, Ethers, Thiols, and Sulfides
 8 Introduction to Alkyl alides, Alcohols, Ethers, Thiols, and Sulfides Solutions to In-Text Problems 8.1 (b) exyl iodide is a primary alkyl halide. (d) Tert-butyl chloride is a tertiary alkyl halide. 8.2
8 Introduction to Alkyl alides, Alcohols, Ethers, Thiols, and Sulfides Solutions to In-Text Problems 8.1 (b) exyl iodide is a primary alkyl halide. (d) Tert-butyl chloride is a tertiary alkyl halide. 8.2
HALOALKANES AND HALOARENES
 Unit - 10 HALOALKANES AND HALOARENES 1. Write the IUPAC names of the following compounds. Br CH = CH C CH (vi) (vii) (ix) (CCl 3 ) 3 CCl 103 XII Chemistry 2. Write the structure of following halogen compounds
Unit - 10 HALOALKANES AND HALOARENES 1. Write the IUPAC names of the following compounds. Br CH = CH C CH (vi) (vii) (ix) (CCl 3 ) 3 CCl 103 XII Chemistry 2. Write the structure of following halogen compounds
OChem1 Old Exams. Chemistry 3719 Practice Exams
 OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
Hydrogen iodide is a strong acid and will drive the reverse reaction, meaning the forward reaction will not occur.
 EM 261 Oct 18, 2018 Photosynthesis and Related Reactions O 2 2 O 6 12 O 6 2 O N 3, S, Fe, u, o, other Natural Products D-Glucose R O R OR Ionic substitution S N 1 & R X X 2 hv Petroleum/ Alkanes R N 2
EM 261 Oct 18, 2018 Photosynthesis and Related Reactions O 2 2 O 6 12 O 6 2 O N 3, S, Fe, u, o, other Natural Products D-Glucose R O R OR Ionic substitution S N 1 & R X X 2 hv Petroleum/ Alkanes R N 2
Reactions SN2 and SN1
 Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
3.2.8 Haloalkanes. Elimination. 148 minutes. 145 marks. Page 1 of 22
 3.2.8 Haloalkanes Elimination 148 minutes 145 marks Page 1 of 22 Q1. Reaction of 2-bromobutane with potassium hydroxide can produce two types of product depending on the solvent used. In aqueous solution,
3.2.8 Haloalkanes Elimination 148 minutes 145 marks Page 1 of 22 Q1. Reaction of 2-bromobutane with potassium hydroxide can produce two types of product depending on the solvent used. In aqueous solution,
CHE 321 Summer 2012 Exam 2 Form Select the correct number of chirality centers in heroin, the illicit drug shown below.
 Multiple Choice Questions: 50 points 1. Select the correct number of chirality centers in heroin, the illicit drug shown below. A 4 B 5 C 6 D 7 E 8 F more than 8 2. Choose the correct IUPAC name for the
Multiple Choice Questions: 50 points 1. Select the correct number of chirality centers in heroin, the illicit drug shown below. A 4 B 5 C 6 D 7 E 8 F more than 8 2. Choose the correct IUPAC name for the
SECTION-I (SINGLE CORRECT CHOICE)
 SECTI-I (SIGLE CRRECT CICE) 1. Which of the following phrases are not correctly associated with an S1 reaction? 1. Rearrangement is possible. 2. Rate is affected by solvent polarity. 3. The strength of
SECTI-I (SIGLE CRRECT CICE) 1. Which of the following phrases are not correctly associated with an S1 reaction? 1. Rearrangement is possible. 2. Rate is affected by solvent polarity. 3. The strength of
12AL Experiment 11 (3 days): Nucleophilic Substitution Reactions
 12AL Experiment 11 (3 days): Nucleophilic Substitution Reactions Instructor note: Day 1 (half of the class); Day 2 (other half); Day 3 (everyone to finish up any separation & purification steps etc). Initial
12AL Experiment 11 (3 days): Nucleophilic Substitution Reactions Instructor note: Day 1 (half of the class); Day 2 (other half); Day 3 (everyone to finish up any separation & purification steps etc). Initial
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Walden discovered a series of reactions that could interconvert (-)-malic acid and (+)-malic acid.
 Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2
 Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Chemistry 210 Organic Chemistry I Summer Semester 1999 Dr. Somnath Sarkar
 Chemistry 210 rganic Chemistry I Summer Semester 1999 Dr. Somnath Sarkar Final Examination Name: Thursday, July 29, 1999, 6:30 8:30 Question 1 Stereochemistry(MC#1) 16 Question 2. Substitution(MC #2) 16
Chemistry 210 rganic Chemistry I Summer Semester 1999 Dr. Somnath Sarkar Final Examination Name: Thursday, July 29, 1999, 6:30 8:30 Question 1 Stereochemistry(MC#1) 16 Question 2. Substitution(MC #2) 16
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
c. Cl H Page 1 of 7 major P (E > Z and more substituted over less substituted alkene) LG must be axial are the same Cl -
 CEM 109A 1. Predict the products of the following reactions (a-c E2, d-f E1 KEY focuses only on elimination products, in most cases there will also be substitution products.) a. - LG must be axial - are
CEM 109A 1. Predict the products of the following reactions (a-c E2, d-f E1 KEY focuses only on elimination products, in most cases there will also be substitution products.) a. - LG must be axial - are
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Reactions of Haloalkanes
 Reactions of aloalkanes N Goalby chemrevise.org aloalkanes ontain a halogen atom covalently bonded to a carbon atom General formula: R-X where X is a halogen atom (F, l,, I) and R is the carbon chain.
Reactions of aloalkanes N Goalby chemrevise.org aloalkanes ontain a halogen atom covalently bonded to a carbon atom General formula: R-X where X is a halogen atom (F, l,, I) and R is the carbon chain.
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
CHEM 302 Organic Chemistry I Problem Set VII Chapter 7 Answers
 CEM 302 rganic Chemistry Problem Set V Chapter 7 Answers 1) The pk a values of acetic acid and trifluroacetic acid are 4.5 and 0.9 respectively. Based on this information, which anion, C 3 C - or CF 3
CEM 302 rganic Chemistry Problem Set V Chapter 7 Answers 1) The pk a values of acetic acid and trifluroacetic acid are 4.5 and 0.9 respectively. Based on this information, which anion, C 3 C - or CF 3
CHE 321 Summer 2010 Exam 2 Form Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III
 CE 321 Summer 2010 Exam 2 orm 2 Multiple Choice Questions: 60 points 1. Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III (A) I only (B) II only (C) I and II only II
CE 321 Summer 2010 Exam 2 orm 2 Multiple Choice Questions: 60 points 1. Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III (A) I only (B) II only (C) I and II only II
C h a p t e r S e v e n : Substitution Reactions S N 2 O H H H O H H. Br -
 C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
Columbia University C99ORG12.DOC S3443D Summer 99 Professor Grace B. Borowitz Exam No. 2 June 14, 1999
 olumbia University 99RG12.D SD Summer 99 Professor Grace B. Borowitz Exam No. 2 June 1, 1999 Name: Sterie hemistry Grade: Please use a non-red pen. Answer questions in the provided space. If you write
olumbia University 99RG12.D SD Summer 99 Professor Grace B. Borowitz Exam No. 2 June 1, 1999 Name: Sterie hemistry Grade: Please use a non-red pen. Answer questions in the provided space. If you write
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
Química Orgânica I. Organic Reactions
 Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
