Alkyl Halides. Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group.
|
|
|
- Britton Knight
- 6 years ago
- Views:
Transcription
1 Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant CFC) CF 3 CHClBr (Halothane: anesthetic) Halogen atoms are more electronegative than carbon atoms, and so the C-Hal bond is polarized. The C-Hal (often written C-X) bond is polarized in such a way that there is partial positive charge on the carbon and partial negative charge on the halogen. Ch06 Alkyl Halides (landscape).doc Page 1
2 Dipole moment Electronegativities decrease in the order of: F > Cl > Br > I Carbon-halogen bond lengths increase in the order of: C-F < C-Cl < C-Br < C-I Bond Dipole Moments decrease in the order of: C-Cl > C-F > C-Br > C-I = 1.56D 1.51D 1.48D 1.29D Typically the chemistry of alkyl halides is dominated by this effect, and usually results in the C-X bond being broken (either in a substitution or elimination process). This reactivity makes alkyl halides useful chemical reagents. Ch06 Alkyl Halides (landscape).doc Page 2
3 Nomenclature According to IUPAC, alkyl halides are treated as alkanes with a halogen (Halo-) substituent. The halogen prefixes are Fluoro-, Chloro-, Bromo- and Iodo-. Examples: Often compounds of CH 2 X 2 type are called methylene halides. (CH 2 Cl 2 is methylene chloride). CHX 3 type compounds are called haloforms. (CHI 3 is iodoform). CX 4 type compounds are called carbon tetrahalides. (CF 4 is carbon tetrafluoride). Alkyl halides can be primary (1 ), secondary (2 ) or tertiary (3 ). Other types: A geminal (gem) dihalide has two halogens on the same carbon. A vicinal dihalide has halogens on adjacent carbon atoms. Ch06 Alkyl Halides (landscape).doc Page 3
4 Preparation of Alkyl Halides Numerous ways to make alkyl halides. (1a) Free Radical Halogenation Usually this method gives mixtures of mono-, di-, tri- etc halogenated compounds, which is considered an inefficient method for the synthesis of a desired compound. Consider propane: Sometimes if there can be control over the selectivity of halogenation this is a useful route. Ch06 Alkyl Halides (landscape).doc Page 4
5 (1b) Allylic Bromination (Allylic means adjacent to a C=C double bond) The bromination of cyclohexene produces a high yield of 3-bromocyclohexene. An allylic hydrogen has been substituted for a bromine. The bromine atom abstracts an allylic hydrogen because the allylic radical is resonance stabilized. The radical then reacts with a bromine molecule to continue the chain. Ch06 Alkyl Halides (landscape).doc Page 5
6 A common reagent for these allylic brominations is N-bromosuccinamide (NBS) because it continually generates small amounts of Br 2 through reaction with HBr. Other methods for Preparation (These will be covered in detail in appropriate later chapters). From alkenes and alkynes: Ch06 Alkyl Halides (landscape).doc Page 6
7 From alcohols: From other halides: Reactions of Alkyl Halides The alkyl halides are chemically versatile. The halogen atom may leave with its bonding pair of electrons to give a halide ion which is stable a halide is called a good leaving group. If an atom replaces the halide the overall reaction is a substitution. If the halide loss is accompanied by the loss of another atom, the overall reaction is called an elimination. Very often the other atom lost is a hydrogen (as H + ). The elimination of H-X is common, and is called a dehydrohalogenation. Often substitution and elimination reactions will occur in competition with each other. Ch06 Alkyl Halides (landscape).doc Page 7
8 Nucleophilic Substitution The nucleophile Nuc: displaces the leaving group (producing X ) from the carbon atom by using its lone pair to form a new bond to the carbon atom. Elimination A new bond is formed by the elimination of halide ion and another atom (usually H + ). In a dehydrohalogenation, the base B: abstracts a proton from the alkyl halide. Most nucleophiles can also act as bases, therefore the preference for elimination or substitution depends on the reaction conditions and the alkyl halide used. Ch06 Alkyl Halides (landscape).doc Page 8
9 The S N 2 reaction S N 2 means substitution nucleophilic bimolecular. Consider the reaction of hydroxide ion with methyl iodide, to yield methanol. The hydroxide ion is a good nucleophile since the oxygen atom has a negative charge and a pair of unshared electrons. The carbon atom is electrophilic since it is bound to a (more electronegative) halogen, which pulls electron density away from the carbon, thus polarizing the bond with carbon bearing partial positive charge and the halogen bearing partial negative charge. The nucleophile is attracted to the electrophile by electrostatic charges. The nucleophile attacks the electrophilic carbon through donation of 2 electrons. Carbon can only have a maximum of 8 valence electrons, so as the carbon-nucleophile bond is forming, then the carbon-leaving group bond must be breaking. Iodide is the leaving group since it leaves with the pair of electrons that once bound it to carbon. Ch06 Alkyl Halides (landscape).doc Page 9
10 The reaction is said to be concerted, taking place in a single step with the new bond forming as the old bond is breaking. The transition state is a point of highest energy (not an intermediate). Ch06 Alkyl Halides (landscape).doc Page 10
11 Kinetic information tells us that the rate is doubled when the [CH 3 I] is doubled, and also doubled when the [HO - ] is doubled. The rate is first order w.r.t. both reactants and is therefore 2 nd order overall. Rate = k r [CH 3 I] [HO - ] The rate and mechanism are consistent since the mechanism requires a collision between the hydroxide ion and methyl iodide. Both species are present in the transition state, and the frequency of collisions is proportional to the concentrations of the reactants. S N 2 = substitution nucleophilic bimolecular. Bimolecular means that the transition state of the R.D.S. involves the collision of two molecules. (Bimolecular reactions generally have 2 nd order overall rate equations). Ch06 Alkyl Halides (landscape).doc Page 11
12 Versatility of the S N 2 mechanism The S N 2 mechanism is a common reaction mechanism and can cover a variety of functional group transformations of alkyl halides. All of the type: Ch06 Alkyl Halides (landscape).doc Page 12
13 Halogen exchange reactions are normally used to prepare either iodo- of fluoro- compounds from other alkyl halides since direct iodination is too slow and direct fluorination is too violent. Nucleophile Strength The rate of the S N 2 reaction strongly depends on the nature of the nucleophile a good nucleophile gives faster rates than a worse nucleophile. Consider methanol (CH 3 OH) and methoxide (CH 3 O ) reacting with CH 3 I. It is found that methoxide reacts about a million times faster in S N 2 reactions than methanol. Generally, negatively charged species are much better nucleophiles than analogous neutral species. The two transition states are different energetically. Ch06 Alkyl Halides (landscape).doc Page 13
14 The two transition states are different energetically. The T.S. with methoxide has the negative charge shared over the oxygen atom and the leaving halide. (Good as both are electronegative). In the methanol case, there is no negative charge. The halide has a partial negative charge and the oxygen has a partial positive charge. This is of higher energy. Ch06 Alkyl Halides (landscape).doc Page 14
15 Basicity and Nucleophilicity Basicity is defined by the equilibrium constant for abstracting a proton. Nucleophilicity is defined by the rate of attack on an electrophilic carbon atom. Trends in Nucleophilicity (there are three) 1) Species with a negative charge are stronger nucleophiles than analogous species without a negative charge. (Bases are always stronger nucleophiles than their conjugate acids). OH > H 2 O SH > H 2 S NH 2 > NH 3 2) Nucleophilicity decreases from left to right across the periodic table. (The more electronegative elements hold on more tightly to their non-bonding electrons). NH 2 > OH > F NH 3 > H 2 O (CH 3 CH 2 ) 3 P > (CH 3 CH 2 ) 2 S Ch06 Alkyl Halides (landscape).doc Page 15
16 3) Nucleophilicity increases down the periodic table. (Increase in polarizability and size). I > Br > Cl > F HSe > HS > HO (CH 3 CH 2 ) 3 P > (CH 3 CH 2 ) 3 N As the size of an atom increases, its outer electrons get further from the attractive force of the nucleus. The electrons are held less tightly and are said to be more polarizable they are more able to move toward a positive charge. More polarizable atoms can form bonds at greater distances, which gives rise to stronger bonding in the T.S. Fluoride is a hard or low polarizability nucleophile, with its electrons held close to the nucleus, and it must approach the carbon nucleus closely before orbital overlap can occur. The outer shell of the soft iodide has loosely held electrons, and these can easily shift and overlap with the carbon atom at a relatively far distance. Ch06 Alkyl Halides (landscape).doc Page 16
17 Effect of Solvents Different solvents have different effects on the nucleophilicity of a species. Solvents with acidic protons are called protic solvents (usually O-H or N-H groups). Polar, protic solvents are often used for S N 2 reactions, since the polar reactants (nucleophile and alkyl halide) generally dissolve well in them. Small anions are much more strongly solvated than larger anions, and sometimes this can have an adverse effect. Certain anions, like F -, can be solvated so well in polar protic solvents that that their nucleophilicity is reduced by the solvation. For efficient S N 2 reactions with small anions it is usual to use polar aprotic solvents. The solvents are still polar, but have no O-H or N-H bonds to form hydrogen bonds to the small anions. Ch06 Alkyl Halides (landscape).doc Page 17
18 Steric Effects In general, the steric bulk has a detrimental effect on nucleophilicity. Since nucleophilicity involves the attack of the nucleophile at a carbon center, large groups tend to hinder this process. Steric effects are not as important for basicity since this involves the abstraction of an unhindered proton. Substrate Effects -leaving group effects -steric effects Leaving group effects A good leaving group has the following features: (1) Electron withdrawing (to polarize the C-X bond, making the C electrophilic). (2) Stable once it has left (not a strong base). (3) Polarizable (to stabilize the T.S. like I previously) Common leaving groups: (ions) Cl, Br, I, ROSO 2 (alkylsulfonate), ROSO 3 (alkylsulfate), ROPO 3 (alkylphosphate). (neutral) H 2 O, R-OH, R 3 N, R 3 P. Ch06 Alkyl Halides (landscape).doc Page 18
19 Hydroxide ions are not good leaving groups (strong bases), but in acidic media, the O gets protonated, and now H 2 O can serve as a good leaving group. Neutral molecules can be good leaving groups from positively charged electrophiles. But the need to protonate the electrophile first limits the choice of nucleophiles to those that are not strong bases, since the nucleophile would simply get protonated. Ch06 Alkyl Halides (landscape).doc Page 19
20 Steric Effects of the Substrate Large groups on the electrophile hinder the approach of the nucleophile. Rel. rates for S N 2: CH 3 X > 1 > 2 > 3 alkyl halides. For an S N 2 reaction, the nucleophile must approach the small backside lobe of the C-X sp 3 orbital. Generally, one alkyl group slows the reaction, two alkyl groups make it difficult, three alkyl groups close to impossible. Ch06 Alkyl Halides (landscape).doc Page 20
21 Stereochemistry of the S N 2 Reaction A nucleophile donates its electron density into (attacks) the small back lobe of the sp 3 hybridized C-X bond, since the leaving group itself blocks attack from any other direction. This is called back side attack. The product has its stereochemistry inverted by an S N 2 reaction. The S N 2 reaction is called a stereospecific reaction since a certain stereoisomer reacts to give one specific stereoisomer as product. SN2 reactions always proceed with inversion. Ch06 Alkyl Halides (landscape).doc Page 21
22 First Order Nucleophilic Substitution There is also an S N 1 reaction. (Substitution, nucleophilic, unimolecular). Consider the reaction of t-butylbromide and methanol: (CH 3 ) 3 C-Br + CH 3 -OH (CH 3 ) 3 C-O-CH 3 + H-Br The rate was found to depend only on the concentration of t-butylbromide. Rate = k r [(CH 3 ) 3 C-Br] The rate is first order overall unimolecular. It appears that the nucleophile is not present in the R.D.S., but must react somewhere after the R.D.S. has occurred. Mechanism: Ch06 Alkyl Halides (landscape).doc Page 22
23 The S N 1 reaction is a two step process, with the first being a slow ionization reaction generating a carbocation. The second is the quick nucleophilic attack by the nucleophile on the carbocation. (In some case, like when water or alcohol is the nucleophile, a quick loss of a proton gives the final product). In general: SLOW (RDS) FAST The S N 1 reaction has two transition states, whereas the S N 2 only has one transition state. Ch06 Alkyl Halides (landscape).doc Page 23
24 Substituent Effects The ionization to form the carbocation in the R.D.S is an endothermic process (breaking bonds), therefore the Hammond postulate tells us that the T.S. for this process should resemble the carbocation. Consequently, SN1 rates depend on carbocation stability. Since alkyl groups are known to stabilize carbocations (inductive effects and hyperconjugation), S N 1 reactivities decrease in the order of: (Notice this is the opposite for S N 2 reactivity). Ch06 Alkyl Halides (landscape).doc Page 24
25 Resonance stabilized cations are also important for S N 1 reactivity, for example, allyl bromide is much more reactive than other primary halides in S N 1 reactions. Leaving Group Effects In the R.D.S. for an S N 1 reaction, the bond to the leaving group is breaking, therefore a highly polarizable leaving group helps stabilize the T.S. through partial bonding as it leaves (like for the S N 2 case). The leaving group should be stable after it has left with the bonding electrons, and also be a weak base. The leaving group starts to take on partial negative charge as the cation starts to form. Good leaving groups are essential for both S N 1 and S N 2 reactions. Ch06 Alkyl Halides (landscape).doc Page 25
26 Solvent Effects The R.D.S. of an S N 1 reaction involves the formation of 2 ions, therefore polar solvents (which stabilize ions) enhance S N 1 reactivities. Protic solvents are especially useful since the hydrogen bonding stabilizes the anionic leaving group after ionization. Dielectric Constant Dielectric constant ( ) is a measure of a solvents polarity. Solvent Rel. Rate Water Methanol Ethanol Acetone 21 1 Diethyl ether Hexane 2.0 < Ionization requires the stabilization of both positive and negative charges, solvents with higher have faster rates for S N 1 reactions. Ch06 Alkyl Halides (landscape).doc Page 26
27 Stereochemistry of the S N 1 Reaction (Recall S N 2 reaction is stereospecific, with inversion). The SN1 reaction is not stereospecific. Consider the reaction below: The carbocation produced is planar and sp 2 hybridized. The nucleophile may attack from either the top or bottom face (typically it will do both). If the nucleophile attacks from the side that the leaving group was originally attached, the product displays retention of configuration. If the nucleophile attacks from what would have been the back side of the leaving group, then the product displays an inversion of configuration. A combination of inversion and retention is called racemization. Often complete racemization is not achieved since the leaving group will partially block one face of the molecule as it ionizes, thus giving a major product of inversion. Ch06 Alkyl Halides (landscape).doc Page 27
28 Rearrangements in S N 1 Reactions Carbocations will often undergo rearrangements, producing more stable ions. For example the products of the S N 1 reaction of 2-bromo-3-methylbutane and ethanol are a mixture of structural isomers the expected product and a rearranged product. The two products arise from the same carbocation. In one case the cation is trapped by the nucleophile before it can rearrange, whereas the second product arises by quenching of the rearranged cation by the nucleophile. Mechanism Step 1: Formation of carbocation Ch06 Alkyl Halides (landscape).doc Page 28
29 Step 2: Attack of solvent before and after rearrangement. Rearrangement: The hydrogen moves with its two electrons. Sometimes called hydrogen shift, or H~, or H migration. Ch06 Alkyl Halides (landscape).doc Page 29
30 Another Rearrangement Neopentylbromide gives exclusively a rearranged product, which results from a methyl shift. This rearrangement produces a tertiary cation (stable) instead of a primary cation (unstable). In the cationic rearrangements, the moving group (H or CH 3 ) take their bonding electrons with them (H - and CH 3 - ). Rearrangements occur when a more stable cation can be produced by a hydrogen or alkyl group shift. (Rearrangements do not occur in S N 2 reactions since carbocations are not intermediates)! Ch06 Alkyl Halides (landscape).doc Page 30
31 Recap of S N 1 and S N 2 S N 1 S N 2 Nuc strength is unimportant Strong nucleophiles required 3 > 2 (1 / CH 3 X don t go) CH 3 X > 1 > 2 > 3 Good ionizing solvent required Rate = k r [R-X] Racemization (inversion/retention) Possible rearrangements May go faster in less polar solvent Rate = k r [R-X] [Nuc ] Complete inversion No rearrangements Ch06 Alkyl Halides (landscape).doc Page 31
32 Eliminations An elimination is the loss of two atoms or groups from a molecule, which will typically result in the formation of a new bond. For example the loss of H-Br to generate alkenes: Bromide can be lost first, and then a proton removed to convert the carbocation into an alkene. (First order elimination: E1). Alternatively, the bromide and the proton can be lost synchronously. (Second order elimination: E2). Ch06 Alkyl Halides (landscape).doc Page 32
33 This is elimination, unimolecular. E1 Elimination Unimolecular means the R.D.S. involves one molecule. Again it is the slow ionization to generate a carbocation. The second step is the fast removal of a proton by the solvent. The electrons that were used for the C-H bond (which is broken) go to form the new C=C bond. In general for an E1: Step 1: carbocation formation (slow, rate determining) Step 2: removal of a proton (fast) Ch06 Alkyl Halides (landscape).doc Page 33
34 Competition of E1 with S N 1 Often when the carbocation is formed, S N 1 and E1 processes compete with each other, and mixtures of elimination and substitution products occur. For example the reaction of t-butylbromide and ethanol: Energy diagram The E1 reaction has a large endothermic ionization, and this is the rate determining transition state. The second step is a fast exothermic removal of a proton. The base does not figure in this reaction until after the R.D.S. and therefore does not appear in the rate equation. Ch06 Alkyl Halides (landscape).doc Page 34
35 Cation Summary Cations can react in 4 different fashions: (1) React with the leaving group to return to the starting material (2) React with a nucleophile to give a substitution product (3) Lose a proton to give an elimination product (4) Rearrange to give a more stable cation, and then react further. Second Order Elimination It is possible to have a second order elimination. Generally this occurs with a strong base, which eliminates a proton quicker than the substrate can ionize. Normally the S N 2 reaction does not compete with E2 since there is steric crowding around the C-X bond which retards the S N 2 process. The rate is related to the concentrations of the substrate and the base, giving a second order rate equation. E2 is elimination, bimolecular. Ch06 Alkyl Halides (landscape).doc Page 35
36 The methoxide ion is acting as a base rather than a nucleophile. The reaction takes place in one concerted step, with the C-H and C-X bonds breaking as the B-H and C-C bonds are forming. For example Ch06 Alkyl Halides (landscape).doc Page 36
37 The elimination requires a hydrogen adjacent to the leaving group. If there are two or more possibilities of adjacent hydrogens, mixtures of products can result. For example: Saytzeff (Zaitsev) Rule The major product of an elimination will be the one with the most highly substituted double bond. R2C=CR2 > R2C=CRH > RHC=CHR and R2C=CH 2 > RCH=CH 2 This order is also the order of stability of alkenes. Ch06 Alkyl Halides (landscape).doc Page 37
38 Stereochemistry of the E2 Like the S N 2, the E2 follows a concerted mechanism one where bond breaking and formation occur at the same time. The partial formation of bonds in the T.S. lowers the energy of the T.S. (no free cations). Typically, concerted mechanisms require suitable geometries so that the orbitals of the atoms involved in the bond breaking and formation can overlap just right, so that electrons can smoothly flow from one bond to another. The S N 2 reaction requires back side attack. The E2 reaction requires a coplanar arrangement of orbitals. The partial bond in the T.S. requires the parallel alignment of two p orbitals. The electrons that were once the C-H bond must start to overlap with the orbital that the leaving group is vacating. The two sp 3 orbitals involved must be arranged parallel to achieve this overlap. Two different arrangements satisfy these requirements, although one is preferred over the other. When the hydrogen and leaving group are anti to each other ( = 180 ), this is called the anti-coplanar conformation. When =0, the hydrogen and leaving group eclipse each other, and this is the syn-coplanar conformation. Ch06 Alkyl Halides (landscape).doc Page 38
39 The anti-coplanar is of lower energy and is by far the most common. In the anti-coplanar conformation the base and leaving group are well separated, thus removing electron repulsions. The syn-coplanar conformation requires the base to approach much closer to the leaving group which is energetically unfavorable. Ch06 Alkyl Halides (landscape).doc Page 39
40 Certain molecules which are held in a fixed eclipsed type conformation may undergo syn-coplanar eliminations. These are exceptions rather than the rule. E2 Eliminations using Diastereomers The E2 reaction is a stereospecific reaction. A particular stereoisomer reacts to give one specific stereoisomer. It is stereospecific since it prefers the anti-coplanar T.S. for elimination. Ch06 Alkyl Halides (landscape).doc Page 40
41 Consider the following two diastereomers: The (S,R) diastereomer gives a trans alkene. Whereas the (R,R) diastereomer gives a cis alkene. The E2 is stereospecific. Ch06 Alkyl Halides (landscape).doc Page 41
42 E2 Reactions in Cyclohexane Systems Almost all cyclohexane systems are most stable in the chair conformations. In a chair, adjacent axial positions are in an anti-coplanar arrangement, ideal for E2 eliminations. Adjacent axial positions are said to be in a trans-diaxial arrangement. E2 s only proceed in chair conformations from trans-diaxial positions, and chair chair interconversions allow the hydrogen and leaving group to attain the trans-diaxial arrangement. The elimination of HBr from bromocyclohexane gives cyclohexene. The bromine must be in an axial position before it can leave. Ch06 Alkyl Halides (landscape).doc Page 42
43 Comparison of E1 and E2 E1 Base strength unimportant Good ionizing solvent required E2 Strong bases are required Solvent polarity not so important Rate = k r [R-X] Rate = k r [R-X][B - ] Saytzeff Orientation No special geometry required Rearrangements are common Saytzeff Orientation Coplanarity required (anti >> syn) No rearrangements Ch06 Alkyl Halides (landscape).doc Page 43
44 Substitution versus Elimination Guidelines (a) The strength of a base or nucleophile will dictate the order of a reaction. (Strong bases/nucleophiles will react more quickly and create 2 nd order kinetics). (b) Primary halides usually undergo S N 2 with good nucleophiles. Also watch for rearrangements to more stable cations if ionization is possible. (c) Tertiary halides usually do not undergo S N 2 reactions. More likely to undergo E2 with a good base, or E1 and S N 1 otherwise. (d) Secondary halides can react in all ways (hard to predict). (e) High temperatures favor elimination. (f) The nucleophile/base will usually favor one or the other type of reaction. (t-butoxide favors elimination, bromide and iodide favor substitution). Ch06 Alkyl Halides (landscape).doc Page 44
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Classes of Halides. Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination. Polarity and Reactivity. Classes of Alkyl Halides
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
Chapter 9. Nucleophilic Substitution and ß-Elimination
 Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
The Electrophile. S N 2 and E2 least stable most stable least hindered most hindered. S N 1 and E1. > x > >
 The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 7 Substitution Reactions 7.1 Introduction to Substitution Reactions Substitution Reactions: two reactants exchange parts to give new products
 hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Organic Halogen Compounds
 8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
10. Organohalides. Based on McMurry s Organic Chemistry, 7 th edition
 10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
7. Haloalkanes (text )
 2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Chapter 8 Alkyl Halides and Elimination Reactions
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 8 Alkyl Halides and Elimination Reactions Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 8 Alkyl Halides and Elimination Reactions Prepared by Rabi Ann Musah State University of New York at Albany Copyright
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
 11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Elimination Reactions Heating an alkyl halide with a strong base causes elimination of a. molecule of HX
 Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Dehydrohalogenation of Alkyl Halides E2 and E1 Reactions in Detail
 Dehydrohalogenation of Alkyl Halides E2 and E1 Reactions in Detail b-elimination Reactions Overview dehydration of alcohols: X = H; Y = OH dehydrohalogenation of alkyl halides: X = H; Y = Br, etc. X C
Dehydrohalogenation of Alkyl Halides E2 and E1 Reactions in Detail b-elimination Reactions Overview dehydration of alcohols: X = H; Y = OH dehydrohalogenation of alkyl halides: X = H; Y = Br, etc. X C
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
Elimination reactions
 Chapter 9 Elimination reactions E2 and E1 reactions Competition between S N and E Elimination reactions Ch 9 #2 elimination and/or substitution 2 mechanisms ~ E2 and E1 E2: bimolecular elimination rxn
Chapter 9 Elimination reactions E2 and E1 reactions Competition between S N and E Elimination reactions Ch 9 #2 elimination and/or substitution 2 mechanisms ~ E2 and E1 E2: bimolecular elimination rxn
Nucleophilic Substitution and Elimination
 Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Dr. Anand Gupta Mr Mahesh Kapil
 Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
CONCERTED sp 2 H. HO Et
 Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Chapter 11: Nucleophilic Substitution and Elimination Walden Inversion
 hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
Walden discovered a series of reactions that could interconvert (-)-malic acid and (+)-malic acid.
 Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
Chapter 7 - Alkenes and Alkynes I
 Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name
Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name
PAPER No. 5: REACTION MECHANISM MODULE No. 2: Types of Organic Reaction Mechanisms
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
1-What is substitution reaction? 2-What are can Nucleophilic Substitution Reaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2
 1-What is substitution reaction? 2-What are can Nucleophilic Substitution eaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2 1- SUBSTITUTION EACTIONS 1-Substitution eaction In this type
1-What is substitution reaction? 2-What are can Nucleophilic Substitution eaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2 1- SUBSTITUTION EACTIONS 1-Substitution eaction In this type
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
ORGANIC - EGE 5E CH. 7 - NUCLEOPHILIC SUBSTITUTION AND ELIMINATION REACTIONS
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
!! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
Chapter 7 Alkenes; Elimination Reactions
 hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Chapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution
 hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
Reactions SN2 and SN1
 Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
PAPER No. : 5; Organic Chemistry-II MODULE No. : 13; Mixed S N 1 and S N 2 Reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
1. Which statement is not true of nucleophillic substitution reactions?!
 Mock Exam 3 CH 235-2F Ch. 7, 8, 9, and 11 Multiple Choice: 1. Which statement is not true of nucleophillic substitution reactions? a. SN1 proceeds through a cation intermediate. b. SN2 occurs with inversion
Mock Exam 3 CH 235-2F Ch. 7, 8, 9, and 11 Multiple Choice: 1. Which statement is not true of nucleophillic substitution reactions? a. SN1 proceeds through a cation intermediate. b. SN2 occurs with inversion
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction
 Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction Nucleophilic substitution and base induced elimination are among most widely occurring and versatile reaction types in organic
Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction Nucleophilic substitution and base induced elimination are among most widely occurring and versatile reaction types in organic
Elimination Reactions. Chapter 6 1
 Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Química Orgânica I. Organic Reactions
 Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
What is the major product of the following reaction?
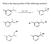 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
S N 1 Displacement Reactions
 S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Some Arrow-Pushing Guidelines (Section 1.14) 1. Arrows follow electron movement.
 Chem 350 Jasperse Ch. 1 Notes 1 Note: The headers and associated chapters don t actually jive with the textbook we are using this summer. But otherwise this highlights a lot of the chemistry from Organic
Chem 350 Jasperse Ch. 1 Notes 1 Note: The headers and associated chapters don t actually jive with the textbook we are using this summer. But otherwise this highlights a lot of the chemistry from Organic
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution
 7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution Preview 7-4 7.1 Nucleophilic Substitution Reactions of Haloalkanes 7-5 Nucleophilic Substitution Mechanisms (7.1A) 7-5 The SN1
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution Preview 7-4 7.1 Nucleophilic Substitution Reactions of Haloalkanes 7-5 Nucleophilic Substitution Mechanisms (7.1A) 7-5 The SN1
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
+ + CH 11: Substitution and Elimination Substitution reactions
 C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
What are radicals? H. Cl. Chapter 10 Radical Reactions. Production of radicals. Reactions of radicals. Electronic structure of methyl radical
 What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
Chem 3719 Example Exams. Chemistry 3719 Practice Exams
 Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
PAPER No. 05: TITLE: ORGANIC CHEMISTRY-II MODULE No. 12: TITLE: S N 1 Reactions
 Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
Organic Chemistry. Alkenes (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
10. Alkyl Halides. What Is an Alkyl Halide. An organic compound containing at least one carbonhalogen
 10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry CHM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl Halides: Substitution Reactions - Chapter 6 (Wade)
 rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides"
 Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Substitution and Elimination reactions
 PART 3 Substitution and Elimination reactions Chapter 8. Substitution reactions of RX 9. Elimination reactions of RX 10. Substit n/elimin n of other comp ds 11. Organometallic comp ds 12. Radical reactions
PART 3 Substitution and Elimination reactions Chapter 8. Substitution reactions of RX 9. Elimination reactions of RX 10. Substit n/elimin n of other comp ds 11. Organometallic comp ds 12. Radical reactions
2/26/18. Practice Questions. Practice Questions B F. How many steps are there in this reaction?
 Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2
 Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Study of Chemical Reactions
 Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
General Glossary. General Glossary
 General Glossary Absolute configuration The actual three-dimensional structure of a chiral molecule. Absolute configurations are specified verbally by the Cahn-Ingold-Prelog R,S convention and are represented
General Glossary Absolute configuration The actual three-dimensional structure of a chiral molecule. Absolute configurations are specified verbally by the Cahn-Ingold-Prelog R,S convention and are represented
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2018
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2015
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
1. Radical Substitution on Alkanes. 2. Radical Substitution with Alkenes. 3. Electrophilic Addition
 1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
c. Cl H Page 1 of 7 major P (E > Z and more substituted over less substituted alkene) LG must be axial are the same Cl -
 CEM 109A 1. Predict the products of the following reactions (a-c E2, d-f E1 KEY focuses only on elimination products, in most cases there will also be substitution products.) a. - LG must be axial - are
CEM 109A 1. Predict the products of the following reactions (a-c E2, d-f E1 KEY focuses only on elimination products, in most cases there will also be substitution products.) a. - LG must be axial - are
Chapter 7: Alkenes and Alkynes
 Chapter 7: Alkenes and Alkynes ydrocarbons Containing Double and Triple Bonds Unsaturated Compounds (Less than Maximum Atoms) Alkenes also Referred to as Olefins Properties Similar to those of Corresponding
Chapter 7: Alkenes and Alkynes ydrocarbons Containing Double and Triple Bonds Unsaturated Compounds (Less than Maximum Atoms) Alkenes also Referred to as Olefins Properties Similar to those of Corresponding
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
6-2 This exercise is worked out on page 220 as "Working with Concepts".
 Copyright 2009 James K Whitesell 6-1 Although we can approach this exercise from a chemical perspective, one can also teach a non-chemist how to derive the answer once the name of the starting material
Copyright 2009 James K Whitesell 6-1 Although we can approach this exercise from a chemical perspective, one can also teach a non-chemist how to derive the answer once the name of the starting material
C C. sp 2. π M.O. atomic. orbitals. carbon 1. σ M.O. molecular. orbitals. H C C rotate D. D H zero overlap of p orbitals: π bond broken!
 Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
ORGANIC - CLUTCH CH. 8 - ELIMINATION REACTIONS.
 !! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
!! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
1) (100 pts) 5) (20 pts) 3) (35 pts) 4) (25pts. Total (200 pts) More Tutorial at
 Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
Chapter 7 Substitution Reactions
 Chapter 7 Substitution Reactions Review of Concepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of Chapter 7. Each of the sentences below appears
Chapter 7 Substitution Reactions Review of Concepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of Chapter 7. Each of the sentences below appears
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
