1-What is substitution reaction? 2-What are can Nucleophilic Substitution Reaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2
|
|
|
- Dominic Fletcher
- 5 years ago
- Views:
Transcription
1
2
3 1-What is substitution reaction? 2-What are can Nucleophilic Substitution eaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2
4 1- SUBSTITUTION EACTIONS
5 1-Substitution eaction In this type of reaction one atom, ion, or group is substituted for another. Its two types: A. Nucleophilic Substitution eaction B. Electrophilic Substitution eaction
6 X + Y Y + X X is called leaving group, a term meaning any group that can be displaced from a carbon atom. alide ions are good leaving groups as they are very week bases. Strong bases such as O - are very poor leaving group. In substitution reaction of alkyl halides, the Iodide ion is the halide most easily displaced.
7 F Cl Br I Increase of eactivity The species that attacks an usually alkyl in substitution reaction is called a nucleophile (abbreviated Nu: - ). Generally, a nucleophile is any species that is attracted to positive center. It is a Lewis base. Most nucleophiles are anions, however, some neutral polar molecules such as 2 O, O, N 2 can also act as nucleophiles by their unshared electrons that can be used to form sigma bonds.
8 Substitutions by nucleophiles are called nucleophilic substitution or nucleophilic displacement. The electrophile (abbreviated E + ) is any species that is attracted toward a negative center. It is a Lewis acid. Some common electrophiles and nucleophiles common electrophiles (E + ) + (Cl) + (-X) -C + =O (COX) NO 2 + (NO 3 ) X + (X 2 ) AlCl 3 AlBr 3 Common nucleophiles (Nu: - ) O - ( 2 O), O - (O), COO - Na + N - 2, 3 N: X - (Cl -, Br - ) Na + CN - - Mg + X, Ar - Mg + X () 2 C=C( 1 ) 2 C 6 6
9 A. Nucleophilic Substitution eaction Q: What is nucleophilic substitution reaction? A species which has ability to donates a pair of electrons is termed as a nucleophile A reaction in which Nu is substituted by another Nu can occur by an: a. S N 1 path b. S N 2 path Most common reaction of alkyl halides (X) and alcohols (O)
10 Nucleophilic Substitution eactions The S N 1 Mechanism
11 S N 1 eaction Unimolecular Nucleophilic Substitution It is a 2 step mechanism involving: 1. Slow: (rate determining) step - ionization of the alkyl halide to form a carbocation (Carbonium ion) Slow X + X 2. Fast step : addition of the nucleophile to the carbocation (Carbonium ion) + Nu Fast Nu
12 The S N 1 Mechanism Acetone is used to dissolve everything! Water is the solvent and nucleophile (solvolysis). This sequence of reactions can be represented on an energy diagram. The formation of the carbocation (carbonium ion) is the high energy (slow) step. Addition of the nucleophile to the carbocation (carbonium ion) is very rapid. 1) 2) C : Br: C + carbocation slow + : O : _ C + + : Br : fast C + : O E C Br t.s.1? t.s. 2? Carbocation intermediate C Nu 3) C + : O fast + C + : O ( ) 3 CBr + 2 O ( ) 3 CO+ + Progress of reaction
13 S N 1 eaction: stereochemistry () Br planar carbocation sp2 50% + C - O 50% attacks top and bottom equally (S) O O + enantiomers () ACEMIZATION
14 Pr C Br 3 C Et 3 o substrate (S) enantiomer Slow -O- polar protic solvent! Pr C 3 C Et planar carbocation Pr C -O- front side attack Pr 3 C Et C O fast Pr C O Et 50% (S) 3 C Et -O- Fast back side attack 3 C O Pr Et fast 3 C O Pr Et 50% ()
15 The S N 1 Mechanism 1) The rate of SN1 reaction Is in the following order C Br > C Br > -C 2 -Br > -Br 2) 3 X undergo SN1 reaction exclusively tertiary secondary primary 3) When weak Nu such as 2 O or O is used the rate of S N 1 reaction Is in the following order: C + + C + C 2 + C 6 5 C2X > C 2 =CC 2 X > 3 X 4) When a strong Nu as CN- is used 3 X undergo SN1 reaction exclusively, tertiary carbocation (very stable) three methyl groups secondary carbocation two methyl groups primary carbocation (unstable) one methyl group very unstable carbocation no methyl groups C 6 5 C 2 X or C 2 =CC 2 X S N 1 2 O or O S N 2 CN - C 6 5 C 2 O or C 2 =CC 2 O C 6 5 C 2 CN or C 2 =CC 2 CN
16 S N 1 eaction: kinetics The 1 indicates that the reaction is unimolecular - only one reactant is involved in the slow step of the reaction. The rate depends only on the concentration of the alkyl halide, not the nucleophile. S N 1 does not involve the nucleophile in the rate determining step. Thus nucleophile has no effect on the reaction rate.
17 S N 1 eaction solvents Usually S N 1 reactions are run in polar protic solvents, compounds with O- groups, as Polar solvent stabilizes the carbocation! The polar protic solvent acts as BOT nucleophile as well as the solvent. Common solvent/nucleophiles include: water, ethanol, methanol, acetic acid, and formic acid.
18 S N 1 eaction solvents Usually S N 1 reactions are run in polar protic solvents, compounds with O- groups, as Polar solvent stabilizes the carbocation! Water O Methanol O OMe The polar protic solvent acts as BOT nucleophile as well as the solvent in S N 1 reactions - solvolysis:. Common solvent/nucleophiles include: water, ethanol, methanol, acetic acid, and formic acid. Ethanol Acetic acid Formic acid O C 2 OEt O C O O C O OAc S N 1 reactions prefer polar-protic solvents that can solvate the anion and cation formed in the rate-determining step. -X + + X - rate-determining step ions solvation of both ions speeds the ionization Carbocation
19 S N 1 eaction solvents Nonpolar Polar Aprotic Solvents Polar Protic Solvents CF 3 COO 2 O POLA S N 2 O C N O - O + S C S N 1 CF 3 C 2 O COO O C 2 O COO Water O Methanol O OMe C 2 O C 2 CCl 4 Ethanol O C 2 OEt C 2 C 2 C 2 Acetic acid O C O OAc Formic acid O C O NONPOLA
20 .W -1 Q1: List the following carbocation in order of increasing stability C 2 3. C( ) 2 Q2: Which of the following compounds is more reactive toward SN1 reaction. Explain why 1. C 6 5 C 2 Br 2. C3Br 3. C 2 =CC 2 Br
21 The S N 2 Mechanism Substitution eactions
22 S N 2 eactions Bimolecular nucleophilic substitution, one-step mechanism, which involves a transition state. Nu attacks from back-side Bimolecular reaction, because both Nu and X are involved in the transition state. Transition state
23 S N 2 eactions The S N 2 mechanism: a) is a single step process b) involves no intermediates c) involves only one transition state, which is of low polarity d) follows second order (bimolecular) kinetics. That is, rate=k[substrate][nucleophile]
24 S N 2 eactions It is second order reaction, because it is proportional to conc. Of Nu & X Increase the steric hindrance around the halogenated carbon Decreases the rate of S N 2 reaction. 3 X are too hindered to undergo S N 2 reaction. X C 2 X 2 CX increasing steric hindrance, decreasing S N 2 rate X most reactive 2 [ 2 CX ] react slowly 3 [ 3 X ].no react by S N 2 When strong Nu as CN- is used, the S N 2 rate in the following order benzylic halide > Allylic halide > Methyl halide ** X and C 2 X (1 X) undergo S N 2 exclusively, irrespective of the strength of Nu-
25 S N 2 eactions Mechanism O + O + Br Br Energy of T. S. O C Br Potential Energy (E) E act transition state for reaction one step O: C Br Average energy of reactants Average energy of products O C Br - Progress of reaction
26 Nu 1 C X Nu 1 C X 1 Nu C + X PC 211/ Dr. Ahmed M. Alafeefy
27 S N 2 eactions Mechanism nucleophilic attack O : attacks back lobe C ()-config. : Br : O : C INVESION (S)-config.
28 S N 2 eactions Mechanism ENEGY POFILE S N 2 ANIMATION C Cl: Press the slide show button to see the animation. Press ESC to finish.
29 S N 2 eactions Mechanism ENEGY POFILE S N 2 ANIMATION :Br: C Cl:
30 S N 2 eactions Mechanism ENEGY POFILE S N 2 ANIMATION :Br: C Cl:
31 S N 2 eactions Mechanism ENEGY POFILE S N 2 ANIMATION :Br: C Cl:
32 S N 2 eactions Mechanism ENEGY POFILE S N 2 ANIMATION :Br: C Cl:
33 S N 2 eactions Mechanism ENEGY POFILE S N 2 ANIMATION :Br C Cl: d- d- Transition State Activated Complex
34 S N 2 eactions Mechanism ENEGY POFILE S N 2 ANIMATION :Br C :Cl:
35 S N 2 eactions Mechanism ENEGY POFILE S N 2 ANIMATION :Br C :Cl:
36 S N 2 eactions Mechanism ENEGY POFILE S N 2 ANIMATION :Br C :Cl:
37 S N 2 eactions Mechanism ENEGY POFILE S N 2 ANIMATION :Br C
38 S N 2 eactions Mechanism The rate depends on both the concentration of the -X and the nucleophile. Stereochemistry of S N 2 eactions When the nucleophile attacks in an S N 2 it is on the opposite side to the position of the leaving group. As a result, the reaction will proceed with an inversion of configuration. O 3 C C C 6 13 Br O C Br C 6 13 O C + C 6 13 Br ()-(-)-2-Bromooctane (S)-(+)-2-Octanol () (S)
39 o Effects of - S N 2 eactions Mechanism The rate of S N 2 reaction is inversely proportional to the streic hindrance around the carbon attached to the leaving group. eactivity order - > C 2 - > ( ) 2 C- > ( ) 3 C- -Br > -C 2 -Br > C C Br Br > primary secondary tertiary eactivity order---- fastest to slowest!
40 S N 2 eactions Mechanism O : C : Br S N 2 - SUBSTATE large groups introduce steric hindrance O : C : Br easy access no steric hindrance
41 S N 2 eactions Mechanism Effects of Nucleophile Since the nucleophile is involved in the rate determining step, the nature of the nucleophile is very important in an S N 2 reaction. More reactive nucleophiles will favor an S N 2 reaction. elative Nucleophilicity O - > O - >>CO 2 - > O > 2 O O 2 O O C _ O _ O _ O _ O I S _ C N Increasing Nucleophilicty 1 ) In general, stronger bases are better nucleophiles 2) owever, iodide doesn t fit that pattern (weak base, but great nucleophile!) 3) Cyanide is an excellent nucleophile because of its linear structure 4) Sulfur is better than oxygen as a nucleophile
42 S N 2 eaction solvents Nonpolar Polar Aprotic Solvents Polar Protic Solvents CF 3 COO 2 O POLA S N 2 O C N O - O + S C S N 1 CF 3 C 2 O COO O C 2 O COO Water O Methanol O OMe C 2 O C 2 CCl 4 Ethanol O C 2 OEt C 2 C 2 C 2 Acetic acid O C O OAc Formic acid O C NONPOLA O
43 S N 2 eaction solvents S N 2 reactions prefer non-polar solvents, or polar-aprotic solvents that do not solvate the nucleophile. S N 2 reactions are accelerated in polar, aprotic solvents S N 2 reactions are retarded (slowed) in polar, protic solvents : X : SMALL, UNSOLVATED C : Br
44 Examples on S N 2 eaction C 2 Cl + O - C 2 O + Cl - C 2 Br + S - C 2 S + Br - C 2 I + O - C 2 O + I - C 2 Br + S - C 2 S + Br - C 2 Cl + 2 N C 2 N 2 + Cl - C 2 Br + CC - C 2 CC + Br - C 2 I + NC - C 2 CN + I -
45 .W -2 Q: Outline all steps in the mechansim of each of the following reaction: 1. C 6 5 C 2 Br + NaCN C 6 5 C 2 CN + NaBr 2. C 6 5 C 2 Br + 2 O C 6 5 C 2 O + Br 3. ( ) 3 CCl + O - Na + ( ) 3 CO + NaCl
46 .W -3 Which member of the following pairs of compounds will react more rapidly with Nu in an SN2 reaction? Explain a. Br or C 2 C 2 Br b. C 2 C 2 Cl or C c. Cl or I d. C 2 C 2 C 2 Cl or 3 CC Cl
47 General concepts for S N 1 & S N 2
48 Solvents Protic solvents: Aprotic solvents: those that contain -O or -N groups worst solvents for S N 2 reactions. Typical protic solvents: water, methanol, ethanol, acetic acid, formic acid have strong dipoles but don t have -O or -N groups. Best for S N 2 reactions. Typical aprotic solvents: acetone, DMF, DMSO, acetonitrile
49 Summary of S N 1 & S N 2 Mechanisms S N 2 A bimolecular reaction Back-side attack 2 ed order in rate Inversion of configuration X > 1 o X > 2 o X S N 1 A unimolecular reaction An ionization reaction 1 st order in rate No inversion of configuration 3 o X > 2 o X
50 Summary of S N 1 & S N 2 Mechanisms Benzylic and allylic undergo both type of substitution S N 1 & S N 2 Mechanisms Depending on the strength of Nu if weak Nu S N 1 if strong Nu. S N 2 Energy required for 3 o alkylhalid is very high, not obtained even with heating, but tertiary alkylhalid is very reactive and proceed via S N 1 reaction. Factors egulate S N 2 and S N 1 Mechanism 1) Nature of the nucleophile 2) Nature of the solvent 3) Nature of the halogen atom
51 Linkage with the life sciences Information Enrichment
52 Medically Speaking Pharmacology And Drug Design Pharmacology is the study of how drugs interact with biological systems, including the mechanisms that explain drug action. Pharmacology is a very important field of study because it serves as the basis for the design of new drugs.
53 Chlorambucil
Chapter 7 Substitution Reactions 7.1 Introduction to Substitution Reactions Substitution Reactions: two reactants exchange parts to give new products
 hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 11: Nucleophilic Substitution and Elimination Walden Inversion
 hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
Chapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution
 hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
8.8 Unimolecular Nucleophilic Substitution S N 1
 8.8 Unimolecular Nucleophilic Substitution S N 1 A question. Tertiary alkyl halides are very unreactive in substitutions that proceed by the S N 2 mechanism. Do they undergo nucleophilic substitution at
8.8 Unimolecular Nucleophilic Substitution S N 1 A question. Tertiary alkyl halides are very unreactive in substitutions that proceed by the S N 2 mechanism. Do they undergo nucleophilic substitution at
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
Lecture 18 Organic Chemistry 1
 CEM 232 rganic Chemistry I at Chicago Lecture 18 rganic Chemistry 1 Professor Duncan Wardrop March 9, 2010 1 Nucleophilicity nucleophilicity: measures the strength of the nucleophile ; more nucleophilic
CEM 232 rganic Chemistry I at Chicago Lecture 18 rganic Chemistry 1 Professor Duncan Wardrop March 9, 2010 1 Nucleophilicity nucleophilicity: measures the strength of the nucleophile ; more nucleophilic
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
C h a p t e r S e v e n : Substitution Reactions S N 2 O H H H O H H. Br -
 C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
Nucleophilic Substitution and Elimination
 Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
S N 1 Displacement Reactions
 S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
Substitution and Elimination reactions
 PART 3 Substitution and Elimination reactions Chapter 8. Substitution reactions of RX 9. Elimination reactions of RX 10. Substit n/elimin n of other comp ds 11. Organometallic comp ds 12. Radical reactions
PART 3 Substitution and Elimination reactions Chapter 8. Substitution reactions of RX 9. Elimination reactions of RX 10. Substit n/elimin n of other comp ds 11. Organometallic comp ds 12. Radical reactions
Chapter 9. Nucleophilic Substitution and ß-Elimination
 Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
+ + CH 11: Substitution and Elimination Substitution reactions
 C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction
 Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction Nucleophilic substitution and base induced elimination are among most widely occurring and versatile reaction types in organic
Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction Nucleophilic substitution and base induced elimination are among most widely occurring and versatile reaction types in organic
Elimination Reactions Heating an alkyl halide with a strong base causes elimination of a. molecule of HX
 Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Dr. Anand Gupta Mr Mahesh Kapil
 Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams.
 2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
PAPER No. 05: TITLE: ORGANIC CHEMISTRY-II MODULE No. 12: TITLE: S N 1 Reactions
 Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
11. Nucleophilic Substitution Reactions
 11. Nucleophilic Substitution Reactions A. Introduction It would be beneficial if you review the chapter on substitution reactions in your textbook prior to lab. This is Ch. 11 in the 9 th edition McMurry
11. Nucleophilic Substitution Reactions A. Introduction It would be beneficial if you review the chapter on substitution reactions in your textbook prior to lab. This is Ch. 11 in the 9 th edition McMurry
7. Haloalkanes (text )
 2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
Reactions SN2 and SN1
 Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Chapter 8 I. Nucleophilic Substitution (in( II. Competion with Elimination. Nucleophilic Substitution
 hapter 8 I. Nucleophilic Substitution (in( depth) II. ompetion with Elimination Nucleophilic Substitution Substrate is a sp3 hybridized carbon atom (cannot be an a vinylic halide or an aryl halide except
hapter 8 I. Nucleophilic Substitution (in( depth) II. ompetion with Elimination Nucleophilic Substitution Substrate is a sp3 hybridized carbon atom (cannot be an a vinylic halide or an aryl halide except
The Electrophile. S N 2 and E2 least stable most stable least hindered most hindered. S N 1 and E1. > x > >
 The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
Chapter 9:Nucleophiles & Substitution Reactions
 1. a. Place the following nucleophiles in order of strength (1= strongest; 3 = weakest). i. ii. b. Place the following in order of leaving group ability (1= best; 7 = worst). (A pka table may help you!)
1. a. Place the following nucleophiles in order of strength (1= strongest; 3 = weakest). i. ii. b. Place the following in order of leaving group ability (1= best; 7 = worst). (A pka table may help you!)
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Substitution Reactions
 Substitution Reactions Substitution reactions are reactions in which a nucleophile displaces an atom or group of atoms (the leaving group) from a tetrahedral carbon atom. onsider the following general
Substitution Reactions Substitution reactions are reactions in which a nucleophile displaces an atom or group of atoms (the leaving group) from a tetrahedral carbon atom. onsider the following general
Organic Chemistry CHM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl Halides: Substitution Reactions - Chapter 6 (Wade)
 rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
PAPER No. : 5; Organic Chemistry-II MODULE No. : 13; Mixed S N 1 and S N 2 Reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Walden discovered a series of reactions that could interconvert (-)-malic acid and (+)-malic acid.
 Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
 11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
Halo Alkanes and Halo Arenes
 alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
Organic Halogen Compounds
 8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
Problem Set 8: Substitution Reactions-ANSWER KEY. (b) nucleophile NH 3 H C. (d) H 3 N
 Problem Set 8: Substitution Reactions-ANSWER KEY hemistry 260 rganic hemistry 1. The answers are (1), (3) and (5). Nucleophiles generally have lone pair and/or negative charge. 2. (a) neither 4 (c) nucleophile
Problem Set 8: Substitution Reactions-ANSWER KEY hemistry 260 rganic hemistry 1. The answers are (1), (3) and (5). Nucleophiles generally have lone pair and/or negative charge. 2. (a) neither 4 (c) nucleophile
Lab 11 Guide: Nucleophilic Substitution (Nov 10 16)
 Lab 11 Guide: Nucleophilic Substitution (Nov 10 16) Nucleophilic Substitution of Alkyl Halides, Exp. 17A, B, and C, pages 187-192 in Taber This week you will be doing examining real life S N 1 and S N
Lab 11 Guide: Nucleophilic Substitution (Nov 10 16) Nucleophilic Substitution of Alkyl Halides, Exp. 17A, B, and C, pages 187-192 in Taber This week you will be doing examining real life S N 1 and S N
CHE 275 NUCLEOPHILIC SUBSTITUTUION CHAP 8 ASSIGN. 1. Which best depicts the partial charges on methyl bromide and sodium methoxide?
 CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
Alkyl Halides and Nucleophilic Subs5tu5on Reac5ons. S N 2 and S N 1 Reac,ons
 Alkyl Halides and Nucleophilic Subs5tu5on Reac5ons S N 2 and S N 1 Reac,ons 1 Alkyl Halides The electronega5ve halogen atom in alkyl halides creates a polar C X bond, making the carbon atom electron deficient.
Alkyl Halides and Nucleophilic Subs5tu5on Reac5ons S N 2 and S N 1 Reac,ons 1 Alkyl Halides The electronega5ve halogen atom in alkyl halides creates a polar C X bond, making the carbon atom electron deficient.
12AL Experiment 11 (3 days): Nucleophilic Substitution Reactions
 12AL Experiment 11 (3 days): Nucleophilic Substitution Reactions Instructor note: Day 1 (half of the class); Day 2 (other half); Day 3 (everyone to finish up any separation & purification steps etc). Initial
12AL Experiment 11 (3 days): Nucleophilic Substitution Reactions Instructor note: Day 1 (half of the class); Day 2 (other half); Day 3 (everyone to finish up any separation & purification steps etc). Initial
Alkyl Halides. Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group.
 Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Reaction mechanism 1/17/19. HCl. HCl. 3 radical can form here. Br 2. hint!
 If the reagents or reaction conditions favor radicals, then it is likely to be a radical reaction. Look for hints like peroxide, AIBN, NBS, hn (UV light). 3 radical can form here Reaction mechanism How
If the reagents or reaction conditions favor radicals, then it is likely to be a radical reaction. Look for hints like peroxide, AIBN, NBS, hn (UV light). 3 radical can form here Reaction mechanism How
Chap. 8 Substitution Reactions
 Chap. 8 Substitution Reactions Y + R X R' Y + X Nucleophilic not necessarily the same as R Electrophilic S N 1 slow (C 3 ) 3 CCl (C + Cl - 3 ) 3 C + (C 3 ) 3 C + OC 2 C 3 C 3 C 2 O C 3 C 2 O d[( C ) 3CCl]
Chap. 8 Substitution Reactions Y + R X R' Y + X Nucleophilic not necessarily the same as R Electrophilic S N 1 slow (C 3 ) 3 CCl (C + Cl - 3 ) 3 C + (C 3 ) 3 C + OC 2 C 3 C 3 C 2 O C 3 C 2 O d[( C ) 3CCl]
Sn1 or Sn2 Reactions: A Guide to Deciding Which Reaction is Occurring
 Sn1 or Sn2 Reactions: A Guide to Deciding Which Reaction is Occurring The following is a discussion of the approach you should use in order to determine if a chemical reaction occurs via a Sn1 or Sn2 mechanism.
Sn1 or Sn2 Reactions: A Guide to Deciding Which Reaction is Occurring The following is a discussion of the approach you should use in order to determine if a chemical reaction occurs via a Sn1 or Sn2 mechanism.
Elimination reactions
 Chapter 9 Elimination reactions E2 and E1 reactions Competition between S N and E Elimination reactions Ch 9 #2 elimination and/or substitution 2 mechanisms ~ E2 and E1 E2: bimolecular elimination rxn
Chapter 9 Elimination reactions E2 and E1 reactions Competition between S N and E Elimination reactions Ch 9 #2 elimination and/or substitution 2 mechanisms ~ E2 and E1 E2: bimolecular elimination rxn
Chapter 7 Substitution Reactions
 Chapter 7 Substitution Reactions Review of Concepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of Chapter 7. Each of the sentences below appears
Chapter 7 Substitution Reactions Review of Concepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of Chapter 7. Each of the sentences below appears
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
CONCERTED sp 2 H. HO Et
 Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Chemistry 35 Exam 2 Answers - April 9, 2007
 Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
10. Organohalides. Based on McMurry s Organic Chemistry, 7 th edition
 10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
PRACTICE PROBLEMS UNIT 8
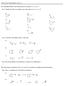 PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
Effect of nucleophile on reaction
 1 Effect of nucleophile on reaction X DS c X c c X DS c + X cleophile not involved in DS of S N 1 so does not effect the reaction (well obviously it controls the formula of the product!) cleophile has
1 Effect of nucleophile on reaction X DS c X c c X DS c + X cleophile not involved in DS of S N 1 so does not effect the reaction (well obviously it controls the formula of the product!) cleophile has
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
1. The Substrate: CH3, 1 o, 2 o, 3 o, Allyl or Benzyl
 Putting it all together: Substitution and elimination reactions are almost always in competition with each other. In order to predict the products of a reaction, it is necessary to determine which mechanisms
Putting it all together: Substitution and elimination reactions are almost always in competition with each other. In order to predict the products of a reaction, it is necessary to determine which mechanisms
ORGANIC - CLUTCH CH. 8 - ELIMINATION REACTIONS.
 !! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
!! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution
 7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution Preview 7-4 7.1 Nucleophilic Substitution Reactions of Haloalkanes 7-5 Nucleophilic Substitution Mechanisms (7.1A) 7-5 The SN1
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution Preview 7-4 7.1 Nucleophilic Substitution Reactions of Haloalkanes 7-5 Nucleophilic Substitution Mechanisms (7.1A) 7-5 The SN1
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #4 - December 9, 2002 ANSWERS
 INSTRUCTINS Department of Chemistry SUNY/neonta Chem 221 - rganic Chemistry I Examination #4 - December 9, 2002 ANSWERS This examination is in multiple choice format; the questions are in this Exam Booklet
INSTRUCTINS Department of Chemistry SUNY/neonta Chem 221 - rganic Chemistry I Examination #4 - December 9, 2002 ANSWERS This examination is in multiple choice format; the questions are in this Exam Booklet
3-chloro-1-propene 1-chloropropane 2-chloropropene
 ANSWERS #1. (from 50 minute exam #3, Fall 2000) 5. (6 points) For each group of 3 compounds, identify the compound that expresses the indicated property the MOST and the compound that expresses it the
ANSWERS #1. (from 50 minute exam #3, Fall 2000) 5. (6 points) For each group of 3 compounds, identify the compound that expresses the indicated property the MOST and the compound that expresses it the
Classes of Halides. Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination. Polarity and Reactivity. Classes of Alkyl Halides
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
Nucleophilic Substitution Synthesis of 1-Iodobutane.
 Nucleophilic Substitution Synthesis of 1-Iodobutane Nucleophilic Substitution of Alkyl alides: Synthesis of 1-Iodobutane C 4 9 Br MW 137.02 d 1.28 g/ml BP 100-104 o C Br + NaI I + NaBr 1-Bromobutane is
Nucleophilic Substitution Synthesis of 1-Iodobutane Nucleophilic Substitution of Alkyl alides: Synthesis of 1-Iodobutane C 4 9 Br MW 137.02 d 1.28 g/ml BP 100-104 o C Br + NaI I + NaBr 1-Bromobutane is
Organic Chemistry Review: Topic 10 & Topic 20
 Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
UCF - ORGANIC CHEMISTRY 1 - PROF. DAOUDI UCF PROF. DAOUDI EXAM 3 REVIEW.
 UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
EXPERIMENT 8 RELATIVE RATES OF NUCLEOPHILIC SUBSTITUTION REACTIONS
 EXPERIMENT 8 RELATIVE RATES OF NUCLEOPHILIC SUBSTITUTION REACTIONS Reading Assignment: Smith, Chapter 7 Pre-lab Questions: 1) What determines whether 2-bromobutane undergoes an S N 1 or an S N 2 reaction?
EXPERIMENT 8 RELATIVE RATES OF NUCLEOPHILIC SUBSTITUTION REACTIONS Reading Assignment: Smith, Chapter 7 Pre-lab Questions: 1) What determines whether 2-bromobutane undergoes an S N 1 or an S N 2 reaction?
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
Week 4. Even harder stuff!
 Week 4 Even harder stuff! Focus: SN1 and SN2 Two organic reactions Learn about two basic pathways for how these reactions happen Focus on stereochemistry Focus: SN1 and SN2 You need a couple of things
Week 4 Even harder stuff! Focus: SN1 and SN2 Two organic reactions Learn about two basic pathways for how these reactions happen Focus on stereochemistry Focus: SN1 and SN2 You need a couple of things
CHEM Lecture 7
 CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
I C(CH 3 ) 3. (c)2ch 3 OH+(CH 3 ) 3 CCl (CH 3 ) 3 COCH 3 +CH 3 OH + 2 +Cl - (d) CH 3 CH 2 CH 2 Br+NaCN CH 3 CH 2 CH 2 CN+NaBr
 6.1 Writer the following as net ionic reactions and designate the nucleophile, substrate, and leaving group in each reaction. (a) 3 + 3 2 Na 3 2 3 +Na (b) Na+ 3 2 3 2 +Na (c) 2 3 +( 3 ) 3 ( 3 ) 3 3 + 3
6.1 Writer the following as net ionic reactions and designate the nucleophile, substrate, and leaving group in each reaction. (a) 3 + 3 2 Na 3 2 3 +Na (b) Na+ 3 2 3 2 +Na (c) 2 3 +( 3 ) 3 ( 3 ) 3 3 + 3
Organic Chemistry HL IB CHEMISTRY HL
 Organic Chemistry HL IB CHEMISTRY HL Understandings: Nucleophilic Substitution Reactions: SN1 represents a nucleophilic unimolecular substitution reaction and SN2 represents a nucleophilic bimolecular
Organic Chemistry HL IB CHEMISTRY HL Understandings: Nucleophilic Substitution Reactions: SN1 represents a nucleophilic unimolecular substitution reaction and SN2 represents a nucleophilic bimolecular
Only five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those five.
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I
 Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
Chapter 08 Nucleophilic Substitution Part 02
 hapter 08 Nucleophilic Substitution Part 02 EM 341: Spring 2012 Prof. Greg ook 2012 Gregory R ook Solvent Effects in Nucleophilic Substitution R X Y R Y X SLVENT Polar reactions - we need polar solvents
hapter 08 Nucleophilic Substitution Part 02 EM 341: Spring 2012 Prof. Greg ook 2012 Gregory R ook Solvent Effects in Nucleophilic Substitution R X Y R Y X SLVENT Polar reactions - we need polar solvents
ORGANIC - EGE 5E CH. 7 - NUCLEOPHILIC SUBSTITUTION AND ELIMINATION REACTIONS
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
!! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
Hydrogen iodide is a strong acid and will drive the reverse reaction, meaning the forward reaction will not occur.
 EM 261 Oct 18, 2018 Photosynthesis and Related Reactions O 2 2 O 6 12 O 6 2 O N 3, S, Fe, u, o, other Natural Products D-Glucose R O R OR Ionic substitution S N 1 & R X X 2 hv Petroleum/ Alkanes R N 2
EM 261 Oct 18, 2018 Photosynthesis and Related Reactions O 2 2 O 6 12 O 6 2 O N 3, S, Fe, u, o, other Natural Products D-Glucose R O R OR Ionic substitution S N 1 & R X X 2 hv Petroleum/ Alkanes R N 2
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
6-2 This exercise is worked out on page 220 as "Working with Concepts".
 Copyright 2009 James K Whitesell 6-1 Although we can approach this exercise from a chemical perspective, one can also teach a non-chemist how to derive the answer once the name of the starting material
Copyright 2009 James K Whitesell 6-1 Although we can approach this exercise from a chemical perspective, one can also teach a non-chemist how to derive the answer once the name of the starting material
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
ORGANIC & BIOLOGICAL CHEMISTRY
 GARETH J ROWLANDS ORGANIC & BIOLOGICAL CHEMISTRY LECTURE FIVE Now we re on the homestretch......well for the first 6 lectures (section 1 if you like) 1 Now we will start looking at specific reactions...and
GARETH J ROWLANDS ORGANIC & BIOLOGICAL CHEMISTRY LECTURE FIVE Now we re on the homestretch......well for the first 6 lectures (section 1 if you like) 1 Now we will start looking at specific reactions...and
c. Cl H Page 1 of 7 major P (E > Z and more substituted over less substituted alkene) LG must be axial are the same Cl -
 CEM 109A 1. Predict the products of the following reactions (a-c E2, d-f E1 KEY focuses only on elimination products, in most cases there will also be substitution products.) a. - LG must be axial - are
CEM 109A 1. Predict the products of the following reactions (a-c E2, d-f E1 KEY focuses only on elimination products, in most cases there will also be substitution products.) a. - LG must be axial - are
CHAPTER 7 HW: SUBSTITUTIONS
 CHAPTER 7 HW: SUBSTTUTNS REACTN TYPES 1. assify each reaction as either a substitution, elimination, or addition reaction. Reaction Type NaH H + Na H + (cat.) H CH CH 2 H 2 CH H H + (cat.) H 2 + H 2 TERMNLGY
CHAPTER 7 HW: SUBSTTUTNS REACTN TYPES 1. assify each reaction as either a substitution, elimination, or addition reaction. Reaction Type NaH H + Na H + (cat.) H CH CH 2 H 2 CH H H + (cat.) H 2 + H 2 TERMNLGY
Homework - Chapter 9 Chem 2310
 Homework - Chapter 9 Chem 2310 me:. ntroduction to organic halides 1. Draw a line structure for a compound with the following description: an alkyl halide with the formula C 5 H 11 a vinyl halide with
Homework - Chapter 9 Chem 2310 me:. ntroduction to organic halides 1. Draw a line structure for a compound with the following description: an alkyl halide with the formula C 5 H 11 a vinyl halide with
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
PAPER No. 5: REACTION MECHANISM MODULE No. 2: Types of Organic Reaction Mechanisms
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
