Reaction mechanism 1/17/19. HCl. HCl. 3 radical can form here. Br 2. hint!
|
|
|
- Caitlin Simpson
- 5 years ago
- Views:
Transcription
1 If the reagents or reaction conditions favor radicals, then it is likely to be a radical reaction. Look for hints like peroxide, AIBN, NBS, hn (UV light). 3 radical can form here Reaction mechanism How to identify the most likely pathway for a reactant and it s reagents. 2 hν hint! If the starting material is π-bonded and has no leaving group, then it is most likely an ADDITIN reaction, but check for radicals, too. This is where Markovnikov s rule and carbocation mechanisms often occur. Note: addition can also happen when there is a leaving group present. 2 H hν less substituted, 1 H H H more substituted, 0 Hs 1
2 Note: addition can also happen when there is a leaving group present. H H If the starting material has a leaving group (for example, a halogen atom) then look for ACID/BASE, Nucleophilic SUBSITUTIN or ELIMINATIN as a likely pathway. If the starting material has a leaving group (for example, a halogen atom) then look for Acid/Base, Nucleophilic substitution or Elimination as a likely pathway. First, since all nucelophiles are bases, is the reaction an acid/base reaction? If the starting material has a leaving group (for example, a halogen atom) then look for Acid/Base, Nucleophilic substitution or Elimination as a likely pathway. Estimate K a First, since all nucelophiles are bases, is the reaction an acid/base reaction? 1. Identify the substrate (containing the leaving group), estimate its K a value 2. If the nucleophile is a powerful enough base to react with the substrate in an acid/base reaction then that is the outcome because acid/base reactions are always very fast. H H 2
3 Estimate K a nucleophilic elimination H H H H most acidic acid/base nucleophilic elimination H H H H H acid/base THERWISE Determine the type of substrate based on the carbon with the leaving group. It is either methyl 1, 2, 3 or vinylic (leaving group attached to sp 2 carbon. If vinylic then no reaction will occur. If vinylic then no reaction will occur. If methyl SN2 substitution will happen. If 1 then S N2 substitution is favored, except in the case of a nucleophile that is a very sterically hindered strong base like (CH3)3C ( ) in which case E2 elimination is favored. If 2, then with a strong base (hydroxide or stronger) E2 elimination is favored, following Zaisev s rule; if the base is weaker than hydroxide, then SN2 substitution is favored. Elimination is favored with higher temp and protic solvents. Lower temps and aprotic solvents favor substitution. If 3 then most likely pathway is elimination except in the case where the nucleophile is the solvent. This is called solvolysis which usually follows an S N1 pathway, especially at lower temperatures. Higher temps and stronger bases favor E2 elimination. 3
4 If methyl S N 2 substitution will happen. NR CH 3 If methyl S N 2 substitution will happen. If 1 then SN2 substitution is favored, except in the case of a nucleophile that is a very sterically hindered strong base like (CH3)3C ( ) in which case E2 elimination is favored. CH 3 CH 3 H CH 3 - If 1 then SN2 substitution is favored, except in the case of a nucleophile that is a very sterically hindered strong base like (CH3)3C ( ) in which case E2 elimination is favored. CH 3 - (CH 3 ) 3 C - CH3 4
5 If 2, then with a strong base (hydroxide or stronger) E2 elimination is favored, following Zaisev s rule; if the base is weaker than hydroxide, then SN2 substitution is favored. Elimination is favored with higher temp and protic solvents. Lower temps and aprotic solvents favor substitution. (CH 3 ) 3 C - NaCN Note that CN ( ) is a weaker base than H ( ) What temperature and solvent is best in this case? NaCN NC NaCN NC What temperature and solvent is best in this case? Hydroxide is the starting point for elimination Room temperature or lower Aprotic polar solvents such as: DMS Acetone Diethyl ether THF DMF 5
6 What temperature and solvent is best in this case? 2 Hs Higher than rt (ex. 65 C) Polar protic solvent, such as: Alcohol Water Carboxylic acid Amine 1 H If 3 then the most likely pathway is elimination except in the case where the nucleophile is the solvent. This is called solvolysis which usually follows an SN1 pathway, especially at lower temperatures. Higher temps and stronger bases favor E2 elimination. Why is it racemic? CH 3 CH 2 H CH 3 CH 2 H CH 2 CH 3 racemic CH 3 CH 3 Na CH 3 CH 3 Na 6
7 What conditions are best? What is the minor product? CH 3 CH 3 Na major 7
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
The Electrophile. S N 2 and E2 least stable most stable least hindered most hindered. S N 1 and E1. > x > >
 The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Chemistry CHM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl Halides: Substitution Reactions - Chapter 6 (Wade)
 rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
PRACTICE PROBLEMS UNIT 8
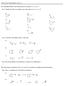 PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
Chapter 9:Nucleophiles & Substitution Reactions
 1. a. Place the following nucleophiles in order of strength (1= strongest; 3 = weakest). i. ii. b. Place the following in order of leaving group ability (1= best; 7 = worst). (A pka table may help you!)
1. a. Place the following nucleophiles in order of strength (1= strongest; 3 = weakest). i. ii. b. Place the following in order of leaving group ability (1= best; 7 = worst). (A pka table may help you!)
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
1. The Substrate: CH3, 1 o, 2 o, 3 o, Allyl or Benzyl
 Putting it all together: Substitution and elimination reactions are almost always in competition with each other. In order to predict the products of a reaction, it is necessary to determine which mechanisms
Putting it all together: Substitution and elimination reactions are almost always in competition with each other. In order to predict the products of a reaction, it is necessary to determine which mechanisms
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
Chapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution
 hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
10. Organohalides. Based on McMurry s Organic Chemistry, 7 th edition
 10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
Nucleophilic Substitution and Elimination
 Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
+ + CH 11: Substitution and Elimination Substitution reactions
 C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
Lecture 18 Organic Chemistry 1
 CEM 232 rganic Chemistry I at Chicago Lecture 18 rganic Chemistry 1 Professor Duncan Wardrop March 9, 2010 1 Nucleophilicity nucleophilicity: measures the strength of the nucleophile ; more nucleophilic
CEM 232 rganic Chemistry I at Chicago Lecture 18 rganic Chemistry 1 Professor Duncan Wardrop March 9, 2010 1 Nucleophilicity nucleophilicity: measures the strength of the nucleophile ; more nucleophilic
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Elimination reactions
 Chapter 9 Elimination reactions E2 and E1 reactions Competition between S N and E Elimination reactions Ch 9 #2 elimination and/or substitution 2 mechanisms ~ E2 and E1 E2: bimolecular elimination rxn
Chapter 9 Elimination reactions E2 and E1 reactions Competition between S N and E Elimination reactions Ch 9 #2 elimination and/or substitution 2 mechanisms ~ E2 and E1 E2: bimolecular elimination rxn
Chapter 7 Substitution Reactions 7.1 Introduction to Substitution Reactions Substitution Reactions: two reactants exchange parts to give new products
 hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Only five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those five.
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
Classes of Halides. Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination. Polarity and Reactivity. Classes of Alkyl Halides
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
1-What is substitution reaction? 2-What are can Nucleophilic Substitution Reaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2
 1-What is substitution reaction? 2-What are can Nucleophilic Substitution eaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2 1- SUBSTITUTION EACTIONS 1-Substitution eaction In this type
1-What is substitution reaction? 2-What are can Nucleophilic Substitution eaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2 1- SUBSTITUTION EACTIONS 1-Substitution eaction In this type
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Sn1 or Sn2 Reactions: A Guide to Deciding Which Reaction is Occurring
 Sn1 or Sn2 Reactions: A Guide to Deciding Which Reaction is Occurring The following is a discussion of the approach you should use in order to determine if a chemical reaction occurs via a Sn1 or Sn2 mechanism.
Sn1 or Sn2 Reactions: A Guide to Deciding Which Reaction is Occurring The following is a discussion of the approach you should use in order to determine if a chemical reaction occurs via a Sn1 or Sn2 mechanism.
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Walden discovered a series of reactions that could interconvert (-)-malic acid and (+)-malic acid.
 Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams.
 2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
Substitution and Elimination reactions
 PART 3 Substitution and Elimination reactions Chapter 8. Substitution reactions of RX 9. Elimination reactions of RX 10. Substit n/elimin n of other comp ds 11. Organometallic comp ds 12. Radical reactions
PART 3 Substitution and Elimination reactions Chapter 8. Substitution reactions of RX 9. Elimination reactions of RX 10. Substit n/elimin n of other comp ds 11. Organometallic comp ds 12. Radical reactions
Organic Chemistry CHM 224
 rganic Chemistry CHM 224 Exam I Review Questions This set of questions is a compilation of old exams and additional questions, and does not represent the typical length of an exam - this is WAY longer!
rganic Chemistry CHM 224 Exam I Review Questions This set of questions is a compilation of old exams and additional questions, and does not represent the typical length of an exam - this is WAY longer!
PAPER No. : 5; Organic Chemistry-II MODULE No. : 13; Mixed S N 1 and S N 2 Reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
CHAPTER 7 HW: SUBSTITUTIONS
 CHAPTER 7 HW: SUBSTTUTNS REACTN TYPES 1. assify each reaction as either a substitution, elimination, or addition reaction. Reaction Type NaH H + Na H + (cat.) H CH CH 2 H 2 CH H H + (cat.) H 2 + H 2 TERMNLGY
CHAPTER 7 HW: SUBSTTUTNS REACTN TYPES 1. assify each reaction as either a substitution, elimination, or addition reaction. Reaction Type NaH H + Na H + (cat.) H CH CH 2 H 2 CH H H + (cat.) H 2 + H 2 TERMNLGY
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction
 Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction Nucleophilic substitution and base induced elimination are among most widely occurring and versatile reaction types in organic
Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction Nucleophilic substitution and base induced elimination are among most widely occurring and versatile reaction types in organic
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
7. Haloalkanes (text )
 2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
Chapter 08 Nucleophilic Substitution Part 02
 hapter 08 Nucleophilic Substitution Part 02 EM 341: Spring 2012 Prof. Greg ook 2012 Gregory R ook Solvent Effects in Nucleophilic Substitution R X Y R Y X SLVENT Polar reactions - we need polar solvents
hapter 08 Nucleophilic Substitution Part 02 EM 341: Spring 2012 Prof. Greg ook 2012 Gregory R ook Solvent Effects in Nucleophilic Substitution R X Y R Y X SLVENT Polar reactions - we need polar solvents
Dr. Anand Gupta Mr Mahesh Kapil
 Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
!!!! Organic Chemistry CHM 224. Exam I Questions
 ld Exam I Questions - CHM 224 rganic Chemistry CHM 224 Exam I Questions This set of questions is a compilation of old exams, and does not represent the typical length of an exam - there are more examples,
ld Exam I Questions - CHM 224 rganic Chemistry CHM 224 Exam I Questions This set of questions is a compilation of old exams, and does not represent the typical length of an exam - there are more examples,
11. Nucleophilic Substitution Reactions
 11. Nucleophilic Substitution Reactions A. Introduction It would be beneficial if you review the chapter on substitution reactions in your textbook prior to lab. This is Ch. 11 in the 9 th edition McMurry
11. Nucleophilic Substitution Reactions A. Introduction It would be beneficial if you review the chapter on substitution reactions in your textbook prior to lab. This is Ch. 11 in the 9 th edition McMurry
S N 1 Displacement Reactions
 S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
C h a p t e r S e v e n : Substitution Reactions S N 2 O H H H O H H. Br -
 C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
Chemistry 35 Exam 2 Answers - April 9, 2007
 Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
CHE 275 NUCLEOPHILIC SUBSTITUTUION CHAP 8 ASSIGN. 1. Which best depicts the partial charges on methyl bromide and sodium methoxide?
 CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
Chem ORGANIC CHEMISTRY I
 ORGANIC CHEMISTRY I CHEM 221 /4 02 Final Examination April 20, 2005 0900-1200 Dr. Cerrie ROGERS x x periodic table & pk a data table provided non-programmable calculators allowed molecular model kits allowed
ORGANIC CHEMISTRY I CHEM 221 /4 02 Final Examination April 20, 2005 0900-1200 Dr. Cerrie ROGERS x x periodic table & pk a data table provided non-programmable calculators allowed molecular model kits allowed
Chapter 11: Nucleophilic Substitution and Elimination Walden Inversion
 hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
ORGANIC - EGE 5E CH. 7 - NUCLEOPHILIC SUBSTITUTION AND ELIMINATION REACTIONS
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
!! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
CONCERTED sp 2 H. HO Et
 Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
PAPER No. 05: TITLE: ORGANIC CHEMISTRY-II MODULE No. 12: TITLE: S N 1 Reactions
 Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
ORGANIC - CLUTCH CH. 8 - ELIMINATION REACTIONS.
 !! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
!! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
Elimination Reactions The E2 Mechanism
 Elimination Reactions The E2 Mechanism The E2 Mechanism X X- B: B- δ- B:- δ+ R 1 δ- R 2 δ+ X δ- The E2 Mechanism R 3 R 4 transition state Free energy (G) Eact B:- B R 1 R 2 X R 1 R 2 R 3 R 4 R 4 R 3 X:-
Elimination Reactions The E2 Mechanism The E2 Mechanism X X- B: B- δ- B:- δ+ R 1 δ- R 2 δ+ X δ- The E2 Mechanism R 3 R 4 transition state Free energy (G) Eact B:- B R 1 R 2 X R 1 R 2 R 3 R 4 R 4 R 3 X:-
Effect of nucleophile on reaction
 1 Effect of nucleophile on reaction X DS c X c c X DS c + X cleophile not involved in DS of S N 1 so does not effect the reaction (well obviously it controls the formula of the product!) cleophile has
1 Effect of nucleophile on reaction X DS c X c c X DS c + X cleophile not involved in DS of S N 1 so does not effect the reaction (well obviously it controls the formula of the product!) cleophile has
Ch 18 Ethers and Epoxides
 Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 8 I. Nucleophilic Substitution (in( II. Competion with Elimination. Nucleophilic Substitution
 hapter 8 I. Nucleophilic Substitution (in( depth) II. ompetion with Elimination Nucleophilic Substitution Substrate is a sp3 hybridized carbon atom (cannot be an a vinylic halide or an aryl halide except
hapter 8 I. Nucleophilic Substitution (in( depth) II. ompetion with Elimination Nucleophilic Substitution Substrate is a sp3 hybridized carbon atom (cannot be an a vinylic halide or an aryl halide except
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution
 7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution Preview 7-4 7.1 Nucleophilic Substitution Reactions of Haloalkanes 7-5 Nucleophilic Substitution Mechanisms (7.1A) 7-5 The SN1
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution Preview 7-4 7.1 Nucleophilic Substitution Reactions of Haloalkanes 7-5 Nucleophilic Substitution Mechanisms (7.1A) 7-5 The SN1
ELECTROPHILIC ADDITIONS OF ALKENES AS THE COUNTERPART OF ELIMINATIONS
 ELECTRPHILIC ADDITINS F ALKENES AS THE CUNTERPART F ELIMINATINS INTRDUCTIN - Chapter 8 is mostly about alkene reactions. That is, how one can transform alkenes into other functional groups. Most of these
ELECTRPHILIC ADDITINS F ALKENES AS THE CUNTERPART F ELIMINATINS INTRDUCTIN - Chapter 8 is mostly about alkene reactions. That is, how one can transform alkenes into other functional groups. Most of these
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
Loudon Chapter 9 Review: Reactions of Alkyl Halides Jacquie Richardson, CU Boulder Last updated 5/1/2016
 Chapter 9 covers reactions you can do with alkyl halides. For the most part, these break down into two categories: substitution and elimination. Substitution results in replacing the halogen with some
Chapter 9 covers reactions you can do with alkyl halides. For the most part, these break down into two categories: substitution and elimination. Substitution results in replacing the halogen with some
Objective 11. Apply Reactivity Principles to Substitution and Elimination Reactions: compare size and strength of nucleophile to predict major product
 Objective 11 Apply Reactivity Principles to Substitution and Elimination Reactions: compare size and strength of nucleophile to predict major product Given Reactants ----> Predict Products How to figure
Objective 11 Apply Reactivity Principles to Substitution and Elimination Reactions: compare size and strength of nucleophile to predict major product Given Reactants ----> Predict Products How to figure
Chapter 13: Alcohols and Phenols
 Chapter 13: Alcohols and Phenols [ Chapter 9 Sections: 9.10; Chapter 13 Sections: 13.1-13.3, 13.9-13.10] 1. Nomenclature of Alcohols simple alcohols C3 C3C2 Eddie Sachs 1927-1964 larger alcohols find the
Chapter 13: Alcohols and Phenols [ Chapter 9 Sections: 9.10; Chapter 13 Sections: 13.1-13.3, 13.9-13.10] 1. Nomenclature of Alcohols simple alcohols C3 C3C2 Eddie Sachs 1927-1964 larger alcohols find the
(c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
 KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
Chem 251 Fall Learning Objectives
 Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Substitution Reactions
 Substitution Reactions Substitution reactions are reactions in which a nucleophile displaces an atom or group of atoms (the leaving group) from a tetrahedral carbon atom. onsider the following general
Substitution Reactions Substitution reactions are reactions in which a nucleophile displaces an atom or group of atoms (the leaving group) from a tetrahedral carbon atom. onsider the following general
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Chapter 9. Nucleophilic Substitution and ß-Elimination
 Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Reading: Finish reading Ch. 9 if you haven't already HW - finish smartwork above and begin working on competition problems from the Ch. 9 Handout.
 Ch. 8 Smartwork available - due Tomorrow @ 10 AM - don't forget to check for carbocation rearrangements! Ch. 9 Smartwork available - due Saturday (12/9) @ 11:59 PM Final Exam (Monday 12/11 @ 8 AM here)
Ch. 8 Smartwork available - due Tomorrow @ 10 AM - don't forget to check for carbocation rearrangements! Ch. 9 Smartwork available - due Saturday (12/9) @ 11:59 PM Final Exam (Monday 12/11 @ 8 AM here)
Lecture 21 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
Loudon Chapter 17 Review: Allylic/Benzylic Reactivity
 Chapter 17 is all about reactions that happen at the position one away from an aromatic ring, or one away from a double bond. These are called the benzylic and allylic positions respectively. Benzyl and
Chapter 17 is all about reactions that happen at the position one away from an aromatic ring, or one away from a double bond. These are called the benzylic and allylic positions respectively. Benzyl and
UCF - ORGANIC CHEMISTRY 1 - PROF. DAOUDI UCF PROF. DAOUDI EXAM 3 REVIEW.
 UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
Organic Chemistry. Unit 10
 Organic Chemistry Unit 10 Halides Primary Carbons Secondary Carbons Tertiary Carbons IMPORTANCE?? REACTIONS!! Benzene C6H6 Aromatic functional group - C6H5 (IUPAC name - phenyl) Substitution Reactions
Organic Chemistry Unit 10 Halides Primary Carbons Secondary Carbons Tertiary Carbons IMPORTANCE?? REACTIONS!! Benzene C6H6 Aromatic functional group - C6H5 (IUPAC name - phenyl) Substitution Reactions
Name: Student Number: University of Manitoba - Department of Chemistry CHEM Introductory Organic Chemistry II - Term Test 1
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 11, 2016; 7-9 PM This is a 2-hour test, marked out
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 11, 2016; 7-9 PM This is a 2-hour test, marked out
Learning Guide for Chapter 10 - Alkyl Halides II
 Learning Guide for Chapter 10 - Alkyl Halides II I. Elimination Reactions of Alkyl Halides Introduction Mechanisms Beta hydrogens, constitutional isomers, and stereoisomers E2 vs E1 Strong and Weak Bases
Learning Guide for Chapter 10 - Alkyl Halides II I. Elimination Reactions of Alkyl Halides Introduction Mechanisms Beta hydrogens, constitutional isomers, and stereoisomers E2 vs E1 Strong and Weak Bases
Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron sheet.
 Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
LECTURE #22 Thurs., Nov.15, 2007
 Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
CH 334. Tuesday, December 5, Name KEY
 CH 334 Final Exam Tuesday, December 5, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Tables of possibly useful data are included on the
CH 334 Final Exam Tuesday, December 5, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Tables of possibly useful data are included on the
MUNISH KAKAR s INSTITUTE OF CHEMISTRY
 REACTONS & PREPARATON OF HALOGENATED COMPDS. WS#3 Q1. Reagent for preparing a chloroalkane from an alcohol is (a) SOCl 2 (b) HCl/ZnCl 2 (c) PCl 3 (d) All of these Q2. n the addition of H to propene in
REACTONS & PREPARATON OF HALOGENATED COMPDS. WS#3 Q1. Reagent for preparing a chloroalkane from an alcohol is (a) SOCl 2 (b) HCl/ZnCl 2 (c) PCl 3 (d) All of these Q2. n the addition of H to propene in
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
 11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
H 3 C C C H H. Hydride Shift
 Exam II SN1, E1, SN2, E2 Reactions Markovnikov Addition Ant-Markovnikov Addition Reaction Mechanisms offman and Saytzeff Eliminations Enantiomers and Diastereomers SN1 Reactions: By-Products - Whenever
Exam II SN1, E1, SN2, E2 Reactions Markovnikov Addition Ant-Markovnikov Addition Reaction Mechanisms offman and Saytzeff Eliminations Enantiomers and Diastereomers SN1 Reactions: By-Products - Whenever
CHEM 263 Oct 25, stronger base stronger acid weaker acid weaker base
 CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
use iso-, tert-, sec- prefixes in your substituent names, where necessary
 Chapter 07.2: 1 1) Name. Include E/Z stereochemistry in name, if necessary. a) 3 C b) c) d) e) f) use iso-, tert-, sec- prefixes in your substituent names, where necessary g) Do NT use iso-, tert-, sec-
Chapter 07.2: 1 1) Name. Include E/Z stereochemistry in name, if necessary. a) 3 C b) c) d) e) f) use iso-, tert-, sec- prefixes in your substituent names, where necessary g) Do NT use iso-, tert-, sec-
Writing a Correct Mechanism
 Chapter 2 1) Balancing Equations Writing a Correct Mechanism 2) Using Arrows to show Electron Movement 3) Mechanisms in Acidic and Basic Media 4) Electron rich Species: Nucleophile or Base? 5) Trimolecular
Chapter 2 1) Balancing Equations Writing a Correct Mechanism 2) Using Arrows to show Electron Movement 3) Mechanisms in Acidic and Basic Media 4) Electron rich Species: Nucleophile or Base? 5) Trimolecular
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions SN2 and SN1
 Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Alkyl Halides. Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group.
 Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
1. Radical Substitution on Alkanes. 2. Radical Substitution with Alkenes. 3. Electrophilic Addition
 1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
12AL Experiment 11 (3 days): Nucleophilic Substitution Reactions
 12AL Experiment 11 (3 days): Nucleophilic Substitution Reactions Instructor note: Day 1 (half of the class); Day 2 (other half); Day 3 (everyone to finish up any separation & purification steps etc). Initial
12AL Experiment 11 (3 days): Nucleophilic Substitution Reactions Instructor note: Day 1 (half of the class); Day 2 (other half); Day 3 (everyone to finish up any separation & purification steps etc). Initial
CHE2060 Lecture 5: Acid-base chemistry. CHE2060 Lecture 5: Acid-base chemistry
 CHE2060 Lecture 5: Acid-base chemistry 5.1 Acids & bases: overview & basics 5.2 Acid & base strength 5.3 Equilibrium acid-base reactions 5.4 The leveling effect of solvents 5.5 Estimation of acidity by
CHE2060 Lecture 5: Acid-base chemistry 5.1 Acids & bases: overview & basics 5.2 Acid & base strength 5.3 Equilibrium acid-base reactions 5.4 The leveling effect of solvents 5.5 Estimation of acidity by
