Organic Chemistry CHM 224
|
|
|
- Stuart Daniels
- 5 years ago
- Views:
Transcription
1 rganic Chemistry CHM 224 Exam I Review Questions This set of questions is a compilation of old exams and additional questions, and does not represent the typical length of an exam - this is WAY longer! First three questions are based on the following four spectra. 1H triplet (hard to see)
2 ! This compound contains C, H, and atoms. Based on the spectral data, determine the most likely molecular formula for this compound. Analysis of the IR spectrum of this molecule indicates what key functional group is/are present? Propose a structure for this compound.
3 The next three questions are based on the following four spectra.
4
5 This molecule contains C, H and - based on the spectral data, determine the most likely molecular formula for this compound. Analysis of the IR spectrum of this molecule indicates what key functional group is present? Propose a structure for this compound. How could you tell the difference between the following two compounds using 13 C NMR only? How could you tell the difference between the following two compounds using 1 H NMR only? Be specific! H 3 C CH 2 CH 3
6 The Williamson Ether synthesis you completed in the laboratory is shown below. Draw a stepwise arrow-pushing mechanism that accounts for the formation of the major organic product of the reaction. You may ignore the role of the tetrabutylammonium fluoride (Bu4NF). H NaH, Bu 4 NF I + H 2 + NaI + Bu 4 NF CH 3 CH 3 The substitution reaction shown in the above question follows an SN1 or SN2 mechanism? (this one is from the book!): (S)-2-Butanol slowly racemizes on standing in dilute sulfuric acid. Explain by drawing a mechanism that accounts for this racemization. Assume each chemical below was made using either an SN1 or SN2 reaction. Identify the BND that was most likely created in the substitution reaction that led to the formation of the products shown by either circling it or using an arrow that unambiguously points to it. For some of the chemicals below, there may more than one possible choice - identifying NE (for each of the 4 below) is sufficient to receive full credit. CN CH 3 N
7 ! Predict the major product of each of the following reactions. Make sure to indicate stereochemistry when appropriate.: NaN 3 DMF NaC(CH 3 ) 3 (CH 3 ) 3 CH NaC(CH 3 ) 3 (CH 3 ) 3 CH Na + C C CH 3 I DMS Write the complete stepwise mechanism the justifies the formation of both products. Show all electron flow with arrows and draw all reaction intermediates. Circle which of the two products is formed in higher yield. KH Acetone +
8 ! (S)-1-omo-2-cyclohexene reacts with sodium ethoxide in ethanol to produce a racemic mixture of substitution products, whereas (S)-1-bromo-3-cyclohexene produces a 20:60 mixture of substitution products (both reactions also produce minor amounts of elimination products) Propose an explanation for why these two reactions do not both produce the same ratio of substitution products. Be as specific as you can to get all the points! (10 pts) Na + Ethanol 50:50 Na + Ethanol 20:60 For the next set of three questions, draw the product(s) of the following reactions, and indicate if the reaction follows an SN1 or SN2 mechanism (circle one). S Na CH 3 CH 2 DMF SN2 or SN1 H CH 3 C CH 3 NaCN acetone SN2 or SN1
9 CH 3 CH 2 H ethanol SN2 or SN1 Consider the reactions below to answer the next four questions: NaN 3 DMS N 3 + Na NaN 3 DMS N 3 + Na The alkyl bromide starting materials in these reactions are classified as: 3º b. 2º c. 1º d. 4º The solvent in these reactions is: protic b. aprotic The nucleophile in these reactions is: a. Na + b. the alkyl group c. - d. N3 - Which reaction is should proceed at a faster rate (first or second one?) a. SN1 b. SN2
10 Consider the pair of reactions below to answer the next four questions: KI acetone I KI acetone I The alkyl bromide starting material in these reactions are classified as: 3º b. 2º c. 1º d. 4º The solvent in these reactions is: a. protic b. aprotic The nucleophile in these reactions is: a. K + b. alkyl group c. - d. I - Which reaction is faster (first or second one?) The mechanism for these reactions is: a. SN2 b. E2 c. SN1 d. E1 1H NMR is highly useful in determining products of organic chemical reactions. ne might question whether or not the following reaction proceeds through a substitution or elimination mechanism. However, studying the 1 H NMR spectrum of the product(s) reveals all signals appear between 2 and 0 ppm. Draw the major organic product(s) of this reaction. CH 3 H
11 n the graph below, draw an energy diagram for an SN1 substitution. early label the electrophile/starting material (S.M.), the product (P) and any relevant intermediates (I) or transition states (TS). Energy reaction coordinate Draw the product(s) of the following reactions. If more than one product exists because of a mixture of possible mechanisms, circle the major product. NaI HCH 2 CH 3 NaSH DMS DBU DMS DBU = N N H 2 heat I
12 K DMF K CH 3 CN I HMPA HCH 3 Select the mechanism(s) (SN1, SN2, E1, E2) [there may be more than one] that fit the description provided. - This reaction mechanism is characterized by inversion of stereochemistry at a stereogenic reaction center and exhibits second-order kinetics. - This reaction mechanism is characterized by partial or complete racemization at a stereogenic reaction center and exhibits first-order kinetics. - This reaction mechanism is characterized by a carbocation intermediate. - This reaction mechanism is characterized by the requirement that a leaving group and a hydrogen on an adjacent carbon be anti-coplanar. - This reaction mechanism is favored by 3 substrates, high temperatures, and a strong base.
13 ! The following molecule was prepared using a Diels Alder reaction. Write the structures of the starting diene and dienophile necessary. Pay special attention to the stereochemistry of the dienophile C 2 CH 3 C 2 CH 3 Consider the reaction below to answer questions H + A B C D 1. The nucleophile in the first step of this reaction is (A - D). 2. The electrophile in the first step of this reaction is (A - D). 3. The kinetically controlled product in this reaction is (A - D). 4. The product that results from 1,4-addition is (A - D). 5. Draw a stepwise arrow-pushing mechanism that accounts for the formation of both products shown (C and D). Show all intermediate structures.
14 ! When an organic molecule is irradiated with ultraviolet radiation, the energy absorbed by the molecule corresponds to: a. the amount necessary to increase molecular motions in functional groups b. the amount necessary to excite electrons from one molecular orbital to another c. the amount necessary to "flip" the spin of atomic nuclei d. the amount necessary to strip a molecule of one electron to generate a radical cation Draw the product of the following intramolecular Diels Alder reaction. heat Write the structure(s) of the product(s) for the Diels-Alder reaction below (no mechanism is needed). Be sure to indicate any relevant stereochemistry. Which of the following molecules is expected to have the longest wavelength for maximum absorbance in the UV spectrum? a) ethylene b) 1,3-butadiene c) 1,3,5-hexatriene d) 1,3,7,9-decatetraene
15 ! The following questions refer to the following chemical reaction: H A B C D The acid in the first step of this reaction mechanism is The base in the first step of this reaction mechanism is The kinetically controlled product of this reaction is The product that has the lowest overall energy is Starting from A and B, draw a STEPWISE arrow-pushing mechanism that accounts for the formation of D.
!!!! Organic Chemistry CHM 224. Exam I Questions
 ld Exam I Questions - CHM 224 rganic Chemistry CHM 224 Exam I Questions This set of questions is a compilation of old exams, and does not represent the typical length of an exam - there are more examples,
ld Exam I Questions - CHM 224 rganic Chemistry CHM 224 Exam I Questions This set of questions is a compilation of old exams, and does not represent the typical length of an exam - there are more examples,
CHAPTER 7 HW: SUBSTITUTIONS
 CHAPTER 7 HW: SUBSTTUTNS REACTN TYPES 1. assify each reaction as either a substitution, elimination, or addition reaction. Reaction Type NaH H + Na H + (cat.) H CH CH 2 H 2 CH H H + (cat.) H 2 + H 2 TERMNLGY
CHAPTER 7 HW: SUBSTTUTNS REACTN TYPES 1. assify each reaction as either a substitution, elimination, or addition reaction. Reaction Type NaH H + Na H + (cat.) H CH CH 2 H 2 CH H H + (cat.) H 2 + H 2 TERMNLGY
Only five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those five.
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
a) 1. O 3 2. (CH 3 ) 2 S
 Name: 1 Chemistry 250B Final Exam December 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) Complete the following reactions. Show the stereochemistry
Name: 1 Chemistry 250B Final Exam December 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) Complete the following reactions. Show the stereochemistry
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
UCF - ORGANIC CHEMISTRY 1 - PROF. DAOUDI UCF PROF. DAOUDI EXAM 3 REVIEW.
 UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams.
 2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
PRACTICE PROBLEMS UNIT 8
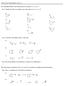 PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
Chem 232. Problem Set 9. Question 1. D. J. Wardrop
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
FOURTH EXAMINATION. (1)..20 pts... (2)..24 pts... (3)..18 pts... (4)..12 pts... (5)..12 pts... (6).. 6 pts... (7).. 8 pts... Bonus 4 pts...
 Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
CHEMISTRY MIDTERM # 3 March 31, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 Name: CEMISTRY 1-01 MIDTERM # March 1, 2009 The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (11 pts) Mark as true (T) false (F) the following statements.
Name: CEMISTRY 1-01 MIDTERM # March 1, 2009 The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (11 pts) Mark as true (T) false (F) the following statements.
Chemistry 35 Exam 2 Answers - April 9, 2007
 Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
and Ultraviolet Spectroscopy
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #4 - December 7, 1998*
 Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #4 - December 7, 1998* INSTRUTINS --- This examination is in multiple choice format; the questions are in this Exam Booklet and
Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #4 - December 7, 1998* INSTRUTINS --- This examination is in multiple choice format; the questions are in this Exam Booklet and
Chapter 9:Nucleophiles & Substitution Reactions
 1. a. Place the following nucleophiles in order of strength (1= strongest; 3 = weakest). i. ii. b. Place the following in order of leaving group ability (1= best; 7 = worst). (A pka table may help you!)
1. a. Place the following nucleophiles in order of strength (1= strongest; 3 = weakest). i. ii. b. Place the following in order of leaving group ability (1= best; 7 = worst). (A pka table may help you!)
Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems
 Some Topics to Study (very broad): Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems Reactivity stuff Classify atoms as electrophilic or nucleophilic Definitions of nucleophiles/electrophiles
Some Topics to Study (very broad): Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems Reactivity stuff Classify atoms as electrophilic or nucleophilic Definitions of nucleophiles/electrophiles
diene. If stereoisomeric mixtures form, indicate by drawing one and writing "+ enantiomer" and/or "+ diastereomer" in the box.
 I. (9 points) Page A. Consider how varying the conditions of a reaction can vastly influence the rate of a reaction as well as the product(s) formed. First draw the product(s) that form in the reference
I. (9 points) Page A. Consider how varying the conditions of a reaction can vastly influence the rate of a reaction as well as the product(s) formed. First draw the product(s) that form in the reference
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
Homework for Chapter 17 Chem 2320
 Homework for Chapter 17 Chem 2320 I. Cumulated, isolated, and conjugated dienes Name 1. Draw structures which fit the following descriptions. Use correct geometry! a conjugated diene with the formula C
Homework for Chapter 17 Chem 2320 I. Cumulated, isolated, and conjugated dienes Name 1. Draw structures which fit the following descriptions. Use correct geometry! a conjugated diene with the formula C
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Chem 232. Problem Set 4. Table. Substrate Types and the Choice of S N 1 of S N 2
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem et 4 Does the nucleophilic substitution of substrate X proceed via an N 2 or N 1 mechanism? is a perennial question in rganic Chemistry. Use the table below
Chem 232 D. J. Wardrop wardropd@uic.edu Problem et 4 Does the nucleophilic substitution of substrate X proceed via an N 2 or N 1 mechanism? is a perennial question in rganic Chemistry. Use the table below
CHE 321 Summer 2012 Exam 2 Form Select the correct number of chirality centers in heroin, the illicit drug shown below.
 Multiple Choice Questions: 50 points 1. Select the correct number of chirality centers in heroin, the illicit drug shown below. A 4 B 5 C 6 D 7 E 8 F more than 8 2. Choose the correct IUPAC name for the
Multiple Choice Questions: 50 points 1. Select the correct number of chirality centers in heroin, the illicit drug shown below. A 4 B 5 C 6 D 7 E 8 F more than 8 2. Choose the correct IUPAC name for the
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #4 - December 9, 2002 ANSWERS
 INSTRUCTINS Department of Chemistry SUNY/neonta Chem 221 - rganic Chemistry I Examination #4 - December 9, 2002 ANSWERS This examination is in multiple choice format; the questions are in this Exam Booklet
INSTRUCTINS Department of Chemistry SUNY/neonta Chem 221 - rganic Chemistry I Examination #4 - December 9, 2002 ANSWERS This examination is in multiple choice format; the questions are in this Exam Booklet
CHEMISTRY 112A FALL 2016 EXAM 2 OCTOBER 20, 2016 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE: Points (Maximum)
 CEMISTRY 112A FALL 2016 EXAM 2 CTBER 20, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN TE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure drawings
CEMISTRY 112A FALL 2016 EXAM 2 CTBER 20, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN TE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure drawings
Chemistry 250B Final Exam Answer Key December 19, 2008
 Name: NSWER KEY 1 hemistry 250 Final Exam nswer Key ecember 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) omplete the following reactions. Show
Name: NSWER KEY 1 hemistry 250 Final Exam nswer Key ecember 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) omplete the following reactions. Show
1. (6 points) Provide IUPAC accepted names for the following compounds. 2. (6 points) Provide a structure for the following compounds.
 Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Conjugated Systems. With conjugated double bonds resonance structures can be drawn
 Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Reaction mechanism 1/17/19. HCl. HCl. 3 radical can form here. Br 2. hint!
 If the reagents or reaction conditions favor radicals, then it is likely to be a radical reaction. Look for hints like peroxide, AIBN, NBS, hn (UV light). 3 radical can form here Reaction mechanism How
If the reagents or reaction conditions favor radicals, then it is likely to be a radical reaction. Look for hints like peroxide, AIBN, NBS, hn (UV light). 3 radical can form here Reaction mechanism How
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Organic Chemistry CHM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl Halides: Substitution Reactions - Chapter 6 (Wade)
 rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
PLEASE DO NOT OPEN THIS EXAM UNTIL YOU ARE INSTRUCTED TO DO SO.
 CEMISTRY 0 :5 AM Section EXAM 2 Nov 2009 Name: Note: Your exam should consist of 5 pages including the cover page and grade tabulation sheet. Skim the entire exam, and solve the easiest problems first.
CEMISTRY 0 :5 AM Section EXAM 2 Nov 2009 Name: Note: Your exam should consist of 5 pages including the cover page and grade tabulation sheet. Skim the entire exam, and solve the easiest problems first.
Partial Periodic Table
 CEM 33, Fall 00 Professor Walba Third our Exam November 8, 00 Scores: ) 0 ) 0 3) 0 4) 0 5) 0 00 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, have neither given nor received
CEM 33, Fall 00 Professor Walba Third our Exam November 8, 00 Scores: ) 0 ) 0 3) 0 4) 0 5) 0 00 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, have neither given nor received
Name: Student Number: University of Manitoba - Department of Chemistry CHEM Introductory Organic Chemistry II - Term Test 1
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 11, 2016; 7-9 PM This is a 2-hour test, marked out
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 11, 2016; 7-9 PM This is a 2-hour test, marked out
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I
 Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Lecture 18 Organic Chemistry 1
 CEM 232 rganic Chemistry I at Chicago Lecture 18 rganic Chemistry 1 Professor Duncan Wardrop March 9, 2010 1 Nucleophilicity nucleophilicity: measures the strength of the nucleophile ; more nucleophilic
CEM 232 rganic Chemistry I at Chicago Lecture 18 rganic Chemistry 1 Professor Duncan Wardrop March 9, 2010 1 Nucleophilicity nucleophilicity: measures the strength of the nucleophile ; more nucleophilic
CHEMISTRY MIDTERM # 2 ANSWER KEY July 10, 2002
 EMISTRY 314-81 MIDTERM # 2 ANSWER KEY July 10, 2002 Statistics: Average: 47 pts (67%); ighest: 69 pts (99%); Lowest: 26 pts (37%) Number of students performing at or above average: 12 (46%) 1. (7 pts)
EMISTRY 314-81 MIDTERM # 2 ANSWER KEY July 10, 2002 Statistics: Average: 47 pts (67%); ighest: 69 pts (99%); Lowest: 26 pts (37%) Number of students performing at or above average: 12 (46%) 1. (7 pts)
2. Examining the infrared spectrum of a compound allows us to:
 CHEM 204 2010 Ass. 1 Problem 1. The amount of energy in infrared light corresponds to: a. the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital b. the amount
CHEM 204 2010 Ass. 1 Problem 1. The amount of energy in infrared light corresponds to: a. the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital b. the amount
Conjugated Dienes. Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry
 Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
Cyclooctatetraene (2R)-2-phenylbutane benzyl chloride cycloocta-1,3,5,7-tetraene
 CM238-02 S05 Practice Material for xam 1 This is a compilation from relevant problems from last spring s exam 1&2. This is too long! 1. (4 pts) Draw 2 of the following 3: Cross one out or graded in order.
CM238-02 S05 Practice Material for xam 1 This is a compilation from relevant problems from last spring s exam 1&2. This is too long! 1. (4 pts) Draw 2 of the following 3: Cross one out or graded in order.
a) Write the mechanism of Friedel-Crafts alkylation of ethyl benzene to give 1,4- diethylbenzene. Show all arrow pushing.
 a) Write the mechanism of Friedel-Crafts alkylation of ethyl benzene to give 1,4- diethylbenzene. Show all arrow pushing. Br AlBr 3 b) Using resonance and inductive effects, explain why an ethyl group
a) Write the mechanism of Friedel-Crafts alkylation of ethyl benzene to give 1,4- diethylbenzene. Show all arrow pushing. Br AlBr 3 b) Using resonance and inductive effects, explain why an ethyl group
CHE 275 NUCLEOPHILIC SUBSTITUTUION CHAP 8 ASSIGN. 1. Which best depicts the partial charges on methyl bromide and sodium methoxide?
 CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
CHEM Exam 2 October 25, 2007
 CEM 3311-200 Exam 2 October 25, 2007 By printing my name below, I pledge that "On my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized assistance on
CEM 3311-200 Exam 2 October 25, 2007 By printing my name below, I pledge that "On my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized assistance on
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
Conjugated Dienes and Ultraviolet Spectroscopy
 Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
The Final Learning Experience
 hemistry 210 rganic hemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #5 Reactions of Alcohols and Related Reactions The Final Learning Experience Wednesday, December 20, 2000, 1:00-3:00 Name:
hemistry 210 rganic hemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #5 Reactions of Alcohols and Related Reactions The Final Learning Experience Wednesday, December 20, 2000, 1:00-3:00 Name:
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Partial Periodic Table
 CEM 3311, Fall 2011 Professor Walba Third our Exam November 17, 2011 scores: 1) 20 2) 20 3) 20 4) 20 5) 20 100 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither
CEM 3311, Fall 2011 Professor Walba Third our Exam November 17, 2011 scores: 1) 20 2) 20 3) 20 4) 20 5) 20 100 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither
1. In the reaction shown above the nucleophile is. (a) Na (b) NaC CH (c) HC C (d) HC CH. 2. In the reaction shown above the nucleophile is
 Chemistry 247B Hanson Sample Exam 4B In each case, read each possible answer, use a process of elimination, and circle the BEST answer. If you are having trouble deciding between two answers, briefly explain
Chemistry 247B Hanson Sample Exam 4B In each case, read each possible answer, use a process of elimination, and circle the BEST answer. If you are having trouble deciding between two answers, briefly explain
Chapter 14: Conjugated Dienes
 Chapter 14: Conjugated Dienes Coverage: 1. Conjugated vs Nonconjugated dienes and Stability 2. MO picture of 1,3-butadiene 3. Electrophilic addition to Dienes 4. Kinetic vs Thermodynamic Control 5. Diels-Alder
Chapter 14: Conjugated Dienes Coverage: 1. Conjugated vs Nonconjugated dienes and Stability 2. MO picture of 1,3-butadiene 3. Electrophilic addition to Dienes 4. Kinetic vs Thermodynamic Control 5. Diels-Alder
Chem ORGANIC CHEMISTRY I
 ORGANIC CHEMISTRY I CHEM 221 /4 02 Final Examination April 20, 2005 0900-1200 Dr. Cerrie ROGERS x x periodic table & pk a data table provided non-programmable calculators allowed molecular model kits allowed
ORGANIC CHEMISTRY I CHEM 221 /4 02 Final Examination April 20, 2005 0900-1200 Dr. Cerrie ROGERS x x periodic table & pk a data table provided non-programmable calculators allowed molecular model kits allowed
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
ORGANIC - CLUTCH CH. 8 - ELIMINATION REACTIONS.
 !! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
!! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
Columbia University C99ORG12.DOC S3443D Summer 99 Professor Grace B. Borowitz Exam No. 2 June 14, 1999
 olumbia University 99RG12.D SD Summer 99 Professor Grace B. Borowitz Exam No. 2 June 1, 1999 Name: Sterie hemistry Grade: Please use a non-red pen. Answer questions in the provided space. If you write
olumbia University 99RG12.D SD Summer 99 Professor Grace B. Borowitz Exam No. 2 June 1, 1999 Name: Sterie hemistry Grade: Please use a non-red pen. Answer questions in the provided space. If you write
ORGANIC - EGE 5E CH. 7 - NUCLEOPHILIC SUBSTITUTION AND ELIMINATION REACTIONS
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
!! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
Ethers. Chapter 14: Ethers, Epoxides, & Sulfides. General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
 Chamras Chemistry 106 Lecture Notes Examination 1 Materials Chapter 14: Ethers, Epoxides, & Sulfides Ethers General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
Chamras Chemistry 106 Lecture Notes Examination 1 Materials Chapter 14: Ethers, Epoxides, & Sulfides Ethers General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. A) I B) II C) III D) IV E) V
 Practice Questions : Chem 226 / Exam 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about benzene is correct?
Practice Questions : Chem 226 / Exam 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about benzene is correct?
The Final Learning Experience
 Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #5 Reactions of Alcohols and Related Reactions The Final Learning Experience Wednesday, December 20, 2000, 1:00-3:00 Name:
Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #5 Reactions of Alcohols and Related Reactions The Final Learning Experience Wednesday, December 20, 2000, 1:00-3:00 Name:
Conjugated Dienes. Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry
 Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
Exam (6 pts) Show which starting materials are used to produce the following Diels-Alder products:
 Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
use iso-, tert-, sec- prefixes in your substituent names, where necessary
 Chapter 07.2: 1 1) Name. Include E/Z stereochemistry in name, if necessary. a) 3 C b) c) d) e) f) use iso-, tert-, sec- prefixes in your substituent names, where necessary g) Do NT use iso-, tert-, sec-
Chapter 07.2: 1 1) Name. Include E/Z stereochemistry in name, if necessary. a) 3 C b) c) d) e) f) use iso-, tert-, sec- prefixes in your substituent names, where necessary g) Do NT use iso-, tert-, sec-
1. The Substrate: CH3, 1 o, 2 o, 3 o, Allyl or Benzyl
 Putting it all together: Substitution and elimination reactions are almost always in competition with each other. In order to predict the products of a reaction, it is necessary to determine which mechanisms
Putting it all together: Substitution and elimination reactions are almost always in competition with each other. In order to predict the products of a reaction, it is necessary to determine which mechanisms
CM 241 Organic Chemistry Fall 1999
 M 241 rganic hemistry Fall 1999 1 UR EXAM III KEY Date: 12/14/99 The marks for each question are given in italics. Write all answers in booklets provided. Question 1. (2+2+2+2) Give names for the following
M 241 rganic hemistry Fall 1999 1 UR EXAM III KEY Date: 12/14/99 The marks for each question are given in italics. Write all answers in booklets provided. Question 1. (2+2+2+2) Give names for the following
*Assignments could be reversed. *
 Name Key 5 W-Exam No. Page I. (6 points) Identify the indicated pairs of hydrogens in each of the following compounds as (i) homotopic, (ii) enantiotopic, or (iii) diastereotopic s. Write the answers as
Name Key 5 W-Exam No. Page I. (6 points) Identify the indicated pairs of hydrogens in each of the following compounds as (i) homotopic, (ii) enantiotopic, or (iii) diastereotopic s. Write the answers as
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
Exam. Name. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements is incorrect about benzene? 1) A) All of the carbon
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements is incorrect about benzene? 1) A) All of the carbon
C h a p t e r S e v e n : Substitution Reactions S N 2 O H H H O H H. Br -
 C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
FALL 2018 CEM 251: Organic Chemistry I Sections September 25, 2018
 ALL 2018 CEM 251: rganic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only what
ALL 2018 CEM 251: rganic Chemistry Sections 25-36 TUE-TU 8:00 9:20 AM September 25, 2018 Name: Section: PD: TA: This is a closed book and note examination. f boxes are provided for your answers, only what
Chemistry Fall Semester 2007; Midterm 3 Exam
 1 Chemistry 2521 Fall Semester 2007; Midterm 3 Exam December 7, Friday, 1:00 to 1:50 pm This exam has 7 problems (100 pts) on 9 pages. Make sure your copy is complete and correct. Printed Name (Last, First)
1 Chemistry 2521 Fall Semester 2007; Midterm 3 Exam December 7, Friday, 1:00 to 1:50 pm This exam has 7 problems (100 pts) on 9 pages. Make sure your copy is complete and correct. Printed Name (Last, First)
CHE 321 Summer 2010 Exam 2 Form Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III
 CE 321 Summer 2010 Exam 2 orm 2 Multiple Choice Questions: 60 points 1. Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III (A) I only (B) II only (C) I and II only II
CE 321 Summer 2010 Exam 2 orm 2 Multiple Choice Questions: 60 points 1. Choose the structure(s) that represent cis-1-sec-butyl-4-methylcyclohexane. I II III (A) I only (B) II only (C) I and II only II
CHEM 8A Summer Student ID # Organic Chemistry FINAL EXAM (400 points)
 CEM 8A Summer 2017 UCSC, Binder Name Student ID # rganic Chemistry FINAL EXAM (400 points) In each of the following problems, you will use your knowledge of organic chemistry conventions to answer the
CEM 8A Summer 2017 UCSC, Binder Name Student ID # rganic Chemistry FINAL EXAM (400 points) In each of the following problems, you will use your knowledge of organic chemistry conventions to answer the
Name: CHEM 633/634 Problem Set 1: Review Due Tues, Aug 29, 2017 (First Lecture!)
 ame: CEM 633/634 Problem Set 1: Review Due Tues, Aug 29, 2017 (First Lecture!) Please print this problem set. Answers must be in the spaces or boxes provided to receive full credit. You may work in groups,
ame: CEM 633/634 Problem Set 1: Review Due Tues, Aug 29, 2017 (First Lecture!) Please print this problem set. Answers must be in the spaces or boxes provided to receive full credit. You may work in groups,
Write your name and date on the cover page Do not open exam until instructed to do so
 Write your name and date on the cover page Do not open exam until instructed to do so Name: Date: Exam III hem. 210 Do not open exam until told to do so. Get out your pencil, eraser, and scientific nongraphing
Write your name and date on the cover page Do not open exam until instructed to do so Name: Date: Exam III hem. 210 Do not open exam until told to do so. Get out your pencil, eraser, and scientific nongraphing
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Points: 1 / 15 / Total: 100 points /
 Chemistry 6372 Fall 2002 omework #6 Answer Key Name Points: 1 / 15 / 2 / 10 / 3 / 10 / 4 / 10 / 5 / 10 / 6 / 10 / 7 / 35 / Total: 100 points / Average: 73 1) Answer the following questions from our book:
Chemistry 6372 Fall 2002 omework #6 Answer Key Name Points: 1 / 15 / 2 / 10 / 3 / 10 / 4 / 10 / 5 / 10 / 6 / 10 / 7 / 35 / Total: 100 points / Average: 73 1) Answer the following questions from our book:
CHEMISTRY 112A FALL 2015 FINAL EXAM DECEMBER 16, 2015 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE:
 CEMISTRY 112A FALL 2015 FINAL EXAM DECEMBER 16, 2015 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN TE LABRATRY CURSE: You will have 2 hours 45 minutes minutes in which to work. Problem Points
CEMISTRY 112A FALL 2015 FINAL EXAM DECEMBER 16, 2015 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN TE LABRATRY CURSE: You will have 2 hours 45 minutes minutes in which to work. Problem Points
+ + CH 11: Substitution and Elimination Substitution reactions
 C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Final Exam December 13, 2005 Professor Rebecca Hoenigman
 CEM 3311-200, all 2005 inal Exam December 13, 2005 Professor Rebecca oenigman Average core = 158 igh core = 276 Low core = 20 I pledge to uphold the CU onor Code: ignature Name (printed) Last four digits
CEM 3311-200, all 2005 inal Exam December 13, 2005 Professor Rebecca oenigman Average core = 158 igh core = 276 Low core = 20 I pledge to uphold the CU onor Code: ignature Name (printed) Last four digits
Problem Set 8: Substitution Reactions-ANSWER KEY. (b) nucleophile NH 3 H C. (d) H 3 N
 Problem Set 8: Substitution Reactions-ANSWER KEY hemistry 260 rganic hemistry 1. The answers are (1), (3) and (5). Nucleophiles generally have lone pair and/or negative charge. 2. (a) neither 4 (c) nucleophile
Problem Set 8: Substitution Reactions-ANSWER KEY hemistry 260 rganic hemistry 1. The answers are (1), (3) and (5). Nucleophiles generally have lone pair and/or negative charge. 2. (a) neither 4 (c) nucleophile
1) (100 pts) 5) (20 pts) 3) (35 pts) 4) (25pts. Total (200 pts) More Tutorial at
 Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
CHEM 341: Organic Chemistry I at North Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:!
 CHEM 341: rganic Chemistry I at Nth Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:! You may use the blank pages of your test f scratch paper. Please read through each question carefully
CHEM 341: rganic Chemistry I at Nth Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:! You may use the blank pages of your test f scratch paper. Please read through each question carefully
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Homework - Chapter 9 Chem 2310
 Homework - Chapter 9 Chem 2310 me:. ntroduction to organic halides 1. Draw a line structure for a compound with the following description: an alkyl halide with the formula C 5 H 11 a vinyl halide with
Homework - Chapter 9 Chem 2310 me:. ntroduction to organic halides 1. Draw a line structure for a compound with the following description: an alkyl halide with the formula C 5 H 11 a vinyl halide with
CONCERTED sp 2 H. HO Et
 Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
3) The delocalized π system in benzene is formed by a cyclic overlap of 6 orbitals. A) s B) p C) sp D) sp2 E) sp3
 Chapter 8 Questions 1) Which of the following statements is incorrect about benzene? A) All of the carbon atoms are sp hybridized. B) It has delocalized electrons. C) The carbon-carbon bond lengths are
Chapter 8 Questions 1) Which of the following statements is incorrect about benzene? A) All of the carbon atoms are sp hybridized. B) It has delocalized electrons. C) The carbon-carbon bond lengths are
KWANTLEN UNIVERSITY COLLEGE CHEMISTRY R10 Organic Chemistry I
 KWANTLEN UNIVERSITY LLEGE EMISTRY 2320 - R10 rganic hemistry I ecember 14, 2001 180 Total Marks Time: 3 hours (180 minutes) Budget your time and good luck!! NAME: This paper consists of 12 pages (11 questions)
KWANTLEN UNIVERSITY LLEGE EMISTRY 2320 - R10 rganic hemistry I ecember 14, 2001 180 Total Marks Time: 3 hours (180 minutes) Budget your time and good luck!! NAME: This paper consists of 12 pages (11 questions)
CHEM 302 Organic Chemistry I Problem Set VII Chapter 7 Answers
 CEM 302 rganic Chemistry Problem Set V Chapter 7 Answers 1) The pk a values of acetic acid and trifluroacetic acid are 4.5 and 0.9 respectively. Based on this information, which anion, C 3 C - or CF 3
CEM 302 rganic Chemistry Problem Set V Chapter 7 Answers 1) The pk a values of acetic acid and trifluroacetic acid are 4.5 and 0.9 respectively. Based on this information, which anion, C 3 C - or CF 3
ORGANIC - BRUICE 8E CH.8 - DELOCALIZED ELECTRONS AND THEIR EFFECT
 !! www.clutchprep.com CONCEPT: RESONANCE STRUCTURES Resonance theory is used to represent all the different ways that the same molecule can distribute its electrons. Atoms move! The only thing that moves
!! www.clutchprep.com CONCEPT: RESONANCE STRUCTURES Resonance theory is used to represent all the different ways that the same molecule can distribute its electrons. Atoms move! The only thing that moves
11/5/ Conjugated Dienes. Conjugated Dienes. Conjugated Dienes. Heats of Hydrogenation
 8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
Elimination Reactions Heating an alkyl halide with a strong base causes elimination of a. molecule of HX
 Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Chapter 9. Nucleophilic Substitution and ß-Elimination
 Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
