CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl
|
|
|
- Gabriella O’Connor’
- 6 years ago
- Views:
Transcription
1 Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of Methane 4 l 2 3 l l 3 l l 2 2 l 2 l 2 l 2 l 2 l 3 l l 3 l 2 l 4 l Free adicals 416 Structure and Stability of Free adicals contain unpaired electrons Examples: O 2 :O O: NO : N O : l : l
2 Alkyl adicals Figure 417 Structure of Methyl adical Most free radicals in which carbon bears the unpaired electron are too unstable to be isolated Alkyl radicals are classified as primary, secondary, or tertiary in the same way that carbocations are Methyl radical is planar, which suggests that carbon is sp 2 hybridized and that the unpaired electron is in a p orbital Alkyl adicals The order of stability of free radicals is the same as for carbocations Alkyl adicals Methyl radical less stable than 3 less stable than 3 Isopropyl radical (secondary) 3 Ethyl radical (primary) 3 less stable than 3 3 tert-butyl radical (tertiary) Alkyl adicals The order of stability of free radicals can be determined by measuring bond strengths By "bond strength" we mean the energy required to break a covalent bond A chemical bond can be broken in two different ways heterolytically or homolytically In a homolytic bond cleavage, the two electrons in the bond are divided equally between the two atoms One electron goes with one atom, the second with the other atom In a heterolytic cleavage, one atom retains both electrons omolytic eterolytic
3 omolytic The species formed by a homolytic bond cleavage of a neutral molecule are free radicals Therefore, measure enthalpy cost of homolytic bond cleavage to gain information about stability of free radicals The more stable the free-radical products, the weaker the bond, and the lower the bond-dissociation energy Measures of Free adical Stability Bond-dissociation enthalpy measurements tell us that isopropyl radical is 13 kj/mol more stable than propyl Measures of Free adical Stability Bond-dissociation enthalpy measurements tell us that tert-butyl radical is 30 kj/mol more stable than isobutyl ( 3 ) 2 2 ( 3 ) Mechanism of hlorination of Methane ( 3 ) 3 Mechanism of hlorination of Methane Free-radical chain mechanism Initiation step: (Light or eat is Necessary) : l : l: : l l : The initiation step "gets the reaction going" by producing free radicals chlorine chlorine atoms from chlorine molecules in this case Initiation step is followed by propagation steps Each propagation step consumes one free radical but generates another one First propagation step: 3 : Mechanism of hlorination of Methane l: 3 : l: Second propagation step: 3 : l : l : l: 3 : l:
4 First propagation step: 3 : Mechanism of hlorination of Methane l: 3 Second propagation step: 3 : l : l: 3 : l: : l: l: Almost all of the product is formed by repetitive cycles of the two propagation steps First propagation step: 3 : l: 3 Second propagation step: 3 : l : l: 3 : l: : l: l: 3 : : l : l : 3 : l: : l: 3 : : l : l : 3 : l: : l: Termination Steps Question 11 stop chain reaction by consuming free radicals 3 l: 3 : l: hardly any product is formed by termination step because concentration of free radicals at any instant is extremely low The step shown below is a step of the free-radical chlorination of chloromethane A) initiation B) propagation ) chain-terminating D) bond cleavage Question 16 For the free-radical reaction below, light is involved in which of the following reaction steps? 418 alogenation of igher Alkanes A) Initiation only B) Propagation only ) Termination only D) Initiation and propagation
5 hlorination of Alkanes can be used to prepare alkyl chlorides from alkanes in which all of the hydrogens are equivalent to one another l l l (78%) l 2 l l (73%) Major limitation: hlorination of Alkanes hlorination gives every possible monochloride derived from original carbon skeleton Not much difference in reactivity of different hydrogens in molecule Example hlorination of butane gives a mixture of 1-chlorobutane and 2-chlorobutane Percentage of Product that esults from Substitution of Indicated ydrogen if Every ollision with hlorine Atoms is Productive l 2 (28%) (72%) l l Percentage of Product that Actually esults from eplacement of Indicated ydrogen 46% 46% 46% 46% 46% 46% elative ates of ydrogen Atom Abstraction divide by = = 39 46% A secondary hydrogen is abstracted 39 times faster than a primary hydrogen by a chlorine atom
6 Similarly, chlorination of 2-methylbutane gives a mixture of isobutyl chloride and tert-butyl chloride Question l 2 (63%) l 3 ow many monochlorination products do you expect to obtain from the chlorination of 2-methylbutane? A) two B) three ) four (37%) 3 3 l D) five Answer 10 Percentage of Product that esults from eplacement of Indicated ydrogen ow many monochlorination products do you expect to obtain from the chlorination of 2-methylbutane? A) two B) three ) four D) five 70% 37% elative ates of ydrogen Atom Abstraction divide by = = 53 A tertiary hydrogen is abstracted 53 times faster than a primary hydrogen by a chlorine atom Selectivity of Free-radical alogenation 3 > 2 2 > 3 chlorination: bromination: hlorination of an alkane gives a mixture of every possible isomer having the same skeleton as the starting alkane Useful for synthesis only when all hydrogens in a molecule are equivalent Bromination is highly regioselective for substitution of tertiary hydrogens Major synthetic application is in synthesis of tertiary alkyl bromides
7 Synthetic Application of hlorination of an Alkane Question 20 l 2 (64%) l hlorination is useful for synthesis only when all of the hydrogens in a molecule are equivalent An alkane with a molecular formula of 8 18 reacts with l 2 in the presence of light and heat to give a single monochloride 8 17 l What is the most reasonable structure for the starting alkane? A) B) ( 3 2 ) ) ( 3 ) ( 3 ) 2 D) ( 3 ) ( 3 3 ) 3 Synthetic Application of Bromination of an Alkane (A) Barbamide, a cyanobacterial peptide containing a trichloromethyl group and (B) dysidenin, a barbamide-related compound isolated from a sponge-cyanobacterial association Br 2 Br (76%) Bromination is highly selective for substitution of tertiary hydrogens Major synthetic application is in synthesis of tertiary alkyl bromides Question 19 Which of the following best describes a mechanistic feature of the free-radical bromination (Br 2, light) of 2- methylpropane? A) The initiation step involves cleavage of a - bond B) The free-radical ( 3 ) 3 is produced in one propagation step and reacts with Br 2 in another ) The reaction is characterized by the homolytic cleavage of the -Br bond D) The reaction is concerted; ie, it occurs in a single step
4.15 Halogenation of Alkanes RH + X 2 RX + HX
 4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Free Radicals
 hapter 10 Free Radicals This is an example of a free radical reaction. A radical is a species that has a free unpaired electron. There are several examples of stable radicals, the most common of which
hapter 10 Free Radicals This is an example of a free radical reaction. A radical is a species that has a free unpaired electron. There are several examples of stable radicals, the most common of which
Chapter 10 Radical Reactions
 Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Organic Chemistry(I) Chapter 3
 Organic Chemistry(I) Chapter 3 1. Carbon-carbon bonds are not easily broken. Which bond in the following compound would be the least difficult to break homolytically? 2. Which of the following molecules
Organic Chemistry(I) Chapter 3 1. Carbon-carbon bonds are not easily broken. Which bond in the following compound would be the least difficult to break homolytically? 2. Which of the following molecules
What are radicals? H. Cl. Chapter 10 Radical Reactions. Production of radicals. Reactions of radicals. Electronic structure of methyl radical
 What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
CHEM 241 ALCOHOLS AND ALKYL HALIDES CHAP 5 ASSIGN
 CEM 241 ALCOOLS AND ALKYL ALIDES CAP 5 ASSIGN 1. What is the IUPAC name of the compound below? A. 3-isobutyl-2-hexanol B. 2-methyl-5-propyl-6-heptanol C. 2-methyl-5-(1-hydroxyethyl)octane D. 6-methyl-3-propyl-2-heptanol
CEM 241 ALCOOLS AND ALKYL ALIDES CAP 5 ASSIGN 1. What is the IUPAC name of the compound below? A. 3-isobutyl-2-hexanol B. 2-methyl-5-propyl-6-heptanol C. 2-methyl-5-(1-hydroxyethyl)octane D. 6-methyl-3-propyl-2-heptanol
What is the major product of the following reaction?
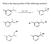 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane.
 Chemistry 51 DS Quiz 2 1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane. 2. When 2,3-dimethylbutane is monochlorinated
Chemistry 51 DS Quiz 2 1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane. 2. When 2,3-dimethylbutane is monochlorinated
CHEM Lecture 6
 EM 494 Special Topics in hemistry Illinois at hicago EM 494 - Lecture 6 Prof. Duncan Wardrop October 15, 2012 Midterm Papers Factors that ontrol ydrocarbon Acidity Factors that ontrol ydrocarbon onformation
EM 494 Special Topics in hemistry Illinois at hicago EM 494 - Lecture 6 Prof. Duncan Wardrop October 15, 2012 Midterm Papers Factors that ontrol ydrocarbon Acidity Factors that ontrol ydrocarbon onformation
Organic Chemistry. for Students of Medicine and Biology 大学化学 III 和大学化学 III(2)
 Organic Chemistry for Students of Medicine and Biology 大学化学 III 和大学化学 III(2) March 4, 2015 Refining of petroleum, a major natural source of alkanes Chapter 4 Alkanes and Cycloalkanes ( 烷烃和环烷烃 ) March 3,
Organic Chemistry for Students of Medicine and Biology 大学化学 III 和大学化学 III(2) March 4, 2015 Refining of petroleum, a major natural source of alkanes Chapter 4 Alkanes and Cycloalkanes ( 烷烃和环烷烃 ) March 3,
Chapter 5. 3-Chloro-2-methylpentane. Cl 2. 2-Chloro-2-methylpentane. 1-Chloro-2-methylpentane. Cl 2-Chloro-4-methylpentane. 1-Chloro-4-methylpentane
 hapter 5 5.1 lassify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) 3Br K 3 KBr (b) 3 2 2 2 2 (c) 2 2 2 3 3 a. substitution b. elimination c. addition
hapter 5 5.1 lassify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) 3Br K 3 KBr (b) 3 2 2 2 2 (c) 2 2 2 3 3 a. substitution b. elimination c. addition
An unknown molecule A has 4 signals in the 1 H NMR spectrum. Which of the following corresponds to molecule A
 An unknown molecule A has 4 signals in the 1 H NMR spectrum. Which of the following corresponds to molecule A How many nonequivalent protons does the following structure have? 4 Reading from left to right,
An unknown molecule A has 4 signals in the 1 H NMR spectrum. Which of the following corresponds to molecule A How many nonequivalent protons does the following structure have? 4 Reading from left to right,
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms
 Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
Reaction of alkanes with bromine / chlorine in UV light. This is the overall reaction, but a more complex mixture of products is actually formed
 8. The aloalkanes opyright N Goalby Bancroft's School Synthesis of chloroalkanes Reaction of alkanes with bromine / chlorine in UV light In the presence of UV light alkanes react with chlorine to form
8. The aloalkanes opyright N Goalby Bancroft's School Synthesis of chloroalkanes Reaction of alkanes with bromine / chlorine in UV light In the presence of UV light alkanes react with chlorine to form
ORGANIC - BROWN 8E CH.8 - HALOALKANES, HALOGENATION AND RADICALS
 !! www.clutchprep.com CONCEPT: ALKYL HALIDES Alkyl halides are named by naming them as a substituent before the root chain and indicating their location. Prefixes: -F, -Cl -Br -I Alkyl halides have NO
!! www.clutchprep.com CONCEPT: ALKYL HALIDES Alkyl halides are named by naming them as a substituent before the root chain and indicating their location. Prefixes: -F, -Cl -Br -I Alkyl halides have NO
THE CHEMISTRY OF ALKANES
 AN INTRODUCTION TO THE CHEMISTRY OF ALKANES Information taken from a presentation by: KNOCKHARDY PUBLISHING General ALKANES members of a homologous series general formula is C n H 2n+2 for non-cyclic alkanes
AN INTRODUCTION TO THE CHEMISTRY OF ALKANES Information taken from a presentation by: KNOCKHARDY PUBLISHING General ALKANES members of a homologous series general formula is C n H 2n+2 for non-cyclic alkanes
Organic Chemistry. Radical Reactions
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Radical Reactions by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Radical Reactions by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
Química Orgânica I. Organic Reactions
 Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Alkanes are aliphatic saturated hydrocarbons (no C=C double bonds, C and H atoms only). They are identified by having a ane name ending.
 Alkanes Alkanes are aliphatic saturated hydrocarbons (no = double bonds, and atoms only). They are identified by having a ane name ending. The alkanes have similar chemistry to one another because they
Alkanes Alkanes are aliphatic saturated hydrocarbons (no = double bonds, and atoms only). They are identified by having a ane name ending. The alkanes have similar chemistry to one another because they
ORGANIC CHEMISTRY 307
 ORGANIC CHEMISTRY 307 CHAPTER 3 LECTURE NOTES R. Boikess II. Principles of Organic Reactions 1. Chemical reactions are the result of bond breaking and bond making. a. Most (but not all) bond making and
ORGANIC CHEMISTRY 307 CHAPTER 3 LECTURE NOTES R. Boikess II. Principles of Organic Reactions 1. Chemical reactions are the result of bond breaking and bond making. a. Most (but not all) bond making and
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2
 Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical
 C h a p t e r E l e v e n: Radical Reactions α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical CM 321: Summary of Important Concepts YConcepts for Chapter 11: Radical
C h a p t e r E l e v e n: Radical Reactions α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical CM 321: Summary of Important Concepts YConcepts for Chapter 11: Radical
dihalogenoalkane H 2, Nickel Catalyst KOH alcoholic HBr, HCl Br Cl Elimination KOH aqueous heat under reflux Nucleophilic substitution
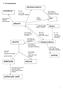 7 AS mechanisms dihalogenoalkane poly(alkene) Br 2, 2 KO aqueous room temp Electrophilic addition heat under reflux Nucleophilic substitution high pressure atalyst polymerization alkene KMnO 4 oxidation
7 AS mechanisms dihalogenoalkane poly(alkene) Br 2, 2 KO aqueous room temp Electrophilic addition heat under reflux Nucleophilic substitution high pressure atalyst polymerization alkene KMnO 4 oxidation
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
Mass Spectrometry. 2000, Paul R. Young University of Illinois at Chicago, All Rights Reserved
 Mass Spectrometry 2000, Paul R. Young University of Illinois at Chicago, All Rights Reserved Mass Spectrometry When a molecule is bombarded with high-energy electrons, one of the process that can occur
Mass Spectrometry 2000, Paul R. Young University of Illinois at Chicago, All Rights Reserved Mass Spectrometry When a molecule is bombarded with high-energy electrons, one of the process that can occur
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
3.1 Introduction to Organic Chemistry
 3.1 Introduction to Organic hemistry Organic hemistry is the study of carbon chemistry as carbon has the ability to join together in chains, rings, balls etc. arbon also joins with other elements easily
3.1 Introduction to Organic hemistry Organic hemistry is the study of carbon chemistry as carbon has the ability to join together in chains, rings, balls etc. arbon also joins with other elements easily
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Vision. Cis-trans isomerism is key to vision. How rods work H 3 C CH 3. Protein opsin. 11-cis-retinal. Opsin. Rhodopsin.
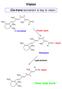 Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
dihalogenoalkane H 2, KOH alcoholic heat under reflux Elimination PCl 5, PBr 3, PI 3 Heat under reflux substitution KOH aqueous heat under reflux
 7. AS mechanisms dihalogenoalkane poly(alkene) 2, l 2 room temp Electrophilic addition KO aqueous heat under reflux Nucleophilic substitution high pressure atalyst polymerization alkene KMnO 4 oxidation
7. AS mechanisms dihalogenoalkane poly(alkene) 2, l 2 room temp Electrophilic addition KO aqueous heat under reflux Nucleophilic substitution high pressure atalyst polymerization alkene KMnO 4 oxidation
Monday /3/2017. (Lecture No. 3-4 : Alkanes )
 Babylon University / Pharmacy Collage Department of Pharmaceutical Chemistry Monday.. 13-16/3/2017 (Lecture No. 3-4 : Alkanes ) Classification by structure: the family The basis of organic chemistry, we
Babylon University / Pharmacy Collage Department of Pharmaceutical Chemistry Monday.. 13-16/3/2017 (Lecture No. 3-4 : Alkanes ) Classification by structure: the family The basis of organic chemistry, we
CH320/328 M Spring 2014
 CH320/328 M Spring 2014 HW Set #3 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your
CH320/328 M Spring 2014 HW Set #3 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your
Introduction to Alkyl Halides, Alcohols, Ethers, Thiols, and Sulfides
 8 Introduction to Alkyl alides, Alcohols, Ethers, Thiols, and Sulfides Solutions to In-Text Problems 8.1 (b) exyl iodide is a primary alkyl halide. (d) Tert-butyl chloride is a tertiary alkyl halide. 8.2
8 Introduction to Alkyl alides, Alcohols, Ethers, Thiols, and Sulfides Solutions to In-Text Problems 8.1 (b) exyl iodide is a primary alkyl halide. (d) Tert-butyl chloride is a tertiary alkyl halide. 8.2
The Simplest Alkanes. Physical Properties 2/16/2012. Butanes are still gases. bp -160 C bp -89 C bp -42 C. CH 3 CH 2 CH 2 CH 2 CH 3 n-pentane.
 The Simplest Alkanes Butanes are still gases Methane (CH 4 ) Ethane (C 2 H 6 ) Propane (C 3 H 8 ) n-butane CH 2 CH 2 Isobutane ( ) 3 CH bp -160 C bp -89 C bp -42 C bp -0.4 C bp -10.2 C Branched isomer
The Simplest Alkanes Butanes are still gases Methane (CH 4 ) Ethane (C 2 H 6 ) Propane (C 3 H 8 ) n-butane CH 2 CH 2 Isobutane ( ) 3 CH bp -160 C bp -89 C bp -42 C bp -0.4 C bp -10.2 C Branched isomer
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
The carbon-carbon double bond is the distinguishing feature of alkenes.
 Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Organic Chemistry. Why are these compounds called Organic. What is a Hydrocarbon? Questions: P167 Read
 Organic Chemistry The fact that carbon can form a wide variety of relatively stable long chain molecules results in this very important branch of Chemistry: Organics. Carbon forms strong covalent bonds
Organic Chemistry The fact that carbon can form a wide variety of relatively stable long chain molecules results in this very important branch of Chemistry: Organics. Carbon forms strong covalent bonds
Classification of Reactions by:
 lassification of Reactions by: 1) Functional group 2) Kind a) Addition: A + B > b) Elimination: A > B + c) Substitution: A-B + -D > A- + B-D d) Rearrangement: A > B, where B is a constitutional isomer
lassification of Reactions by: 1) Functional group 2) Kind a) Addition: A + B > b) Elimination: A > B + c) Substitution: A-B + -D > A- + B-D d) Rearrangement: A > B, where B is a constitutional isomer
Chapter 20. Mass Spectroscopy
 Chapter 20 Mass Spectroscopy Mass Spectrometry (MS) Mass spectrometry is a technique used for measuring the molecular weight and determining the molecular formula of an organic compound. Mass Spectrometry
Chapter 20 Mass Spectroscopy Mass Spectrometry (MS) Mass spectrometry is a technique used for measuring the molecular weight and determining the molecular formula of an organic compound. Mass Spectrometry
Study of Chemical Reactions
 Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
Part C- section 1 p-bonds as nucleophiles
 Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Lesmahagow High School CfE Advanced Higher Chemistry
 Lesmahagow High School AHChemistry Organic Chemistry& Instrumental Analysis Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Alkanes, Alkenes and
Lesmahagow High School AHChemistry Organic Chemistry& Instrumental Analysis Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Alkanes, Alkenes and
Alkanes and Alkenes. The Alkanes
 Alkanes and Alkenes The Alkanes Alkanes are hydrocarbons (i.e. compounds of carbon and hydrogen only). They are called saturated hydrocarbons because they contain no double bonds, and so cannot undergo
Alkanes and Alkenes The Alkanes Alkanes are hydrocarbons (i.e. compounds of carbon and hydrogen only). They are called saturated hydrocarbons because they contain no double bonds, and so cannot undergo
CHEMISTRY 241 Section 004 EXAMINATION I TUESDAY, October 11, :30-11:50 AM Professor William P. Dailey NAME: QUESTIONS POINTS SCORE
 CEMISTRY 241 Section 004 EXAMIATI I TUESDAY, ctober 11, 2005 10:30-11:50 AM Professor William P. Dailey AME: Student ID number : QUESTIS PITS SCRE 1. 16 2. 10 3. 12 4. 12 5. 12 6. 8 7. 9 8. 9 9. 15 10.
CEMISTRY 241 Section 004 EXAMIATI I TUESDAY, ctober 11, 2005 10:30-11:50 AM Professor William P. Dailey AME: Student ID number : QUESTIS PITS SCRE 1. 16 2. 10 3. 12 4. 12 5. 12 6. 8 7. 9 8. 9 9. 15 10.
Lab Activity 9: Introduction to Organic Chemical Reactivity, Lab 5 Prelab, Reflux
 Lab Activity 9: Introduction to Organic Chemical Reactivity, Lab 5 Prelab, Reflux Objectives 1. Identify structural features (pi bonds, bond polarity, lone pairs) of a compound 2. Determine whether a structural
Lab Activity 9: Introduction to Organic Chemical Reactivity, Lab 5 Prelab, Reflux Objectives 1. Identify structural features (pi bonds, bond polarity, lone pairs) of a compound 2. Determine whether a structural
CHEMISTRY MIDTERM # 2 October 27, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 EMISTRY 313-01 MIDTERM # 2 ctober 27, 2005 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (7 pts) Mark as true (T) or false (F) the following
EMISTRY 313-01 MIDTERM # 2 ctober 27, 2005 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (7 pts) Mark as true (T) or false (F) the following
An Overview of Organic Reactions. Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants:
 An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser
 hemistry 210 rganic hemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #2 Alkyl alides: Their Synthesis by alogenation of Alkanes and Their Nucleophilic Substitution Reactions. Friday, ctober
hemistry 210 rganic hemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #2 Alkyl alides: Their Synthesis by alogenation of Alkanes and Their Nucleophilic Substitution Reactions. Friday, ctober
Chapter 04 Alcohols and Alkyl Halides part 01
 hapter 04 Alcohols and Alkyl alides part 01 EM 341: Spring 2012 Prof. Greg ook Functional Groups A functional group is a structural feature in a molecule that has characteristic reactivity. A functional
hapter 04 Alcohols and Alkyl alides part 01 EM 341: Spring 2012 Prof. Greg ook Functional Groups A functional group is a structural feature in a molecule that has characteristic reactivity. A functional
WELCOME TO MODERN ORGANIC CHEMISTRY
 WELCOME TO MODERN ORGANIC CEMISTRY Organic Chemistry, 5 th Edition L. G. Wade, Jr. Chapter 4 The Study of Chemical Reactions WAT IS A REACTION MECANISM A DESCRIPTION OF STRUCTURES AN ENERGIES OF STARTING
WELCOME TO MODERN ORGANIC CEMISTRY Organic Chemistry, 5 th Edition L. G. Wade, Jr. Chapter 4 The Study of Chemical Reactions WAT IS A REACTION MECANISM A DESCRIPTION OF STRUCTURES AN ENERGIES OF STARTING
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #1 - September 22, 2004 ANSWERS
 ame Department of hemistry SUY/neonta hem 221 - rganic hemistry I Examination #1 - September 22, 2004 ASWERS ISTRUTIS This examination has two parts. The first part is in multiple choice format; the questions
ame Department of hemistry SUY/neonta hem 221 - rganic hemistry I Examination #1 - September 22, 2004 ASWERS ISTRUTIS This examination has two parts. The first part is in multiple choice format; the questions
Chapter 10. BrCH 2 CH 2 CH 2 CCH 2 Br CH 3. CH 3 CCH 2 CH 2 Cl CH 3 CHCH 2 CH 2 CHCH Give IUPAC names for the following alkyl halides:
 hapter 10 10.1 Give IUPA names for the following alkyl halides: (a), 3 2 2 2 I 1-iodobutane 3 (b), 3 2 2 l 1-chloro-3-methylbutane (c), 3 2 2 2 2 3 1,5-Dibromo-2,2-dimethylpentane 3 3 2 2 l (d), l 1,3-Dichloro-3-methylbutane
hapter 10 10.1 Give IUPA names for the following alkyl halides: (a), 3 2 2 2 I 1-iodobutane 3 (b), 3 2 2 l 1-chloro-3-methylbutane (c), 3 2 2 2 2 3 1,5-Dibromo-2,2-dimethylpentane 3 3 2 2 l (d), l 1,3-Dichloro-3-methylbutane
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Electronegativity Scale F > O > Cl, N > Br > C, H
 Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
An Overview of Organic Reactions
 An Overview of Organic Reactions Radical Reactions Reactions involving symmetrical bond breaking and bond forming omolytic bond breaking omogenic bond formation Radical Reaction with alkanes and uv light
An Overview of Organic Reactions Radical Reactions Reactions involving symmetrical bond breaking and bond forming omolytic bond breaking omogenic bond formation Radical Reaction with alkanes and uv light
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Organic Chemistry. Nomenclature: Alkanes
 Organic Chemistry Nomenclature: Alkanes Alkanes Hydrocarbon chains where all the bonds between carbons are SINGLE bonds Name uses the ending ane Examples: Methane, Propane, Butane, Octane, 2-methylpentane
Organic Chemistry Nomenclature: Alkanes Alkanes Hydrocarbon chains where all the bonds between carbons are SINGLE bonds Name uses the ending ane Examples: Methane, Propane, Butane, Octane, 2-methylpentane
Introduction to Organic Chemistry: Hydrocarbons
 Introduction to Organic Chemistry: Hydrocarbons Chapter 12 Chapter 12 12.1 Organic Compounds 12.2 Alkanes 12.3 Alkanes with Substituents 12.4 Properties of Alkanes 12.5 Alkenes and Alkynes 12.6 Cis-Trans
Introduction to Organic Chemistry: Hydrocarbons Chapter 12 Chapter 12 12.1 Organic Compounds 12.2 Alkanes 12.3 Alkanes with Substituents 12.4 Properties of Alkanes 12.5 Alkenes and Alkynes 12.6 Cis-Trans
Organic Compounds. Introduction to Organic Chemistry: Hydrocarbons. also contain other nonmetals such as oxygen, nitrogen,
 Introduction to Organic Chemistry: Hydrocarbons Chapter 12 12.1 Organic Compounds Identify properties characteristic of organic or inorganic compounds. Chapter 12 12.1 Organic Compounds 12.2 Alkanes 12.3
Introduction to Organic Chemistry: Hydrocarbons Chapter 12 12.1 Organic Compounds Identify properties characteristic of organic or inorganic compounds. Chapter 12 12.1 Organic Compounds 12.2 Alkanes 12.3
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
Q.1 Draw out and name the structural isomers of C 5 H 12 and C 6 H 14.
 Alkanes F22 1 ALKANES General a homologous series with general formula n 2n+2 - non-cyclic only saturated hydrocarbons - all carbon-carbon bonding is single bonds are spaced tetrahedrally about carbon
Alkanes F22 1 ALKANES General a homologous series with general formula n 2n+2 - non-cyclic only saturated hydrocarbons - all carbon-carbon bonding is single bonds are spaced tetrahedrally about carbon
Chapter 2: Alkanes MULTIPLE CHOICE
 Chapter 2: Alkanes MULTIPLE CHOICE 1. Which of the following orbitals is properly described as an antibonding orbital? a. sp + 1s d. sp 2 1s b. sp 2 + 1s e. sp 2 + sp 2 sp 3 + 1s D DIF: Easy REF: 2.2 2.
Chapter 2: Alkanes MULTIPLE CHOICE 1. Which of the following orbitals is properly described as an antibonding orbital? a. sp + 1s d. sp 2 1s b. sp 2 + 1s e. sp 2 + sp 2 sp 3 + 1s D DIF: Easy REF: 2.2 2.
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
10/6/2010. Chapter 6 An Overview of Organic Reactions. Organic Chemical Reactions. 6.1 Kinds of Organic Reactions
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 6 An Overview of Organic Reactions Richard Morrison University of Georgia, Athens Organic Chemical Reactions Organic chemical reactions
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 6 An Overview of Organic Reactions Richard Morrison University of Georgia, Athens Organic Chemical Reactions Organic chemical reactions
Chapter 15. Free Radical Reactions
 Grossman, CE 230 Chapter 15. Free Radical Reactions A free radical is a species containing one or more unpaired electrons. Free radicals are electrondeficient species, but they are usually uncharged, so
Grossman, CE 230 Chapter 15. Free Radical Reactions A free radical is a species containing one or more unpaired electrons. Free radicals are electrondeficient species, but they are usually uncharged, so
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
Chapter 2 Alkanes and Cycloalkanes: Introduction to Hydrocarbons
 Chapter 2 Alkanes and Cycloalkanes: Introduction to ydrocarbons Copyright The McGraw-ill Companies, Inc. Permission required for reproduction or display. 2.1 Classes of ydrocarbons ydrocarbons Aliphatic
Chapter 2 Alkanes and Cycloalkanes: Introduction to ydrocarbons Copyright The McGraw-ill Companies, Inc. Permission required for reproduction or display. 2.1 Classes of ydrocarbons ydrocarbons Aliphatic
Ch.10 Alkyl Halides. Organic halides are valuable as industrial solvents, inhaled anesthetics in medicine, refrigerants, and pesticides.
 Ch.10 Alkyl alides Organic halides are valuable as industrial solvents, inhaled anesthetics in medicine, refrigerants, and pesticides. F F C C F Trichloroethylene (a solvent) alothane (an inhaled anesthetic)
Ch.10 Alkyl alides Organic halides are valuable as industrial solvents, inhaled anesthetics in medicine, refrigerants, and pesticides. F F C C F Trichloroethylene (a solvent) alothane (an inhaled anesthetic)
Q.1 Draw out suitable structures which fit the molecular formula C 6 H 6
 Aromatic compounds 2814 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out suitable structures which fit the molecular formula
Aromatic compounds 2814 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out suitable structures which fit the molecular formula
Ethers & Epoxides. Chapter 5. Dr. Seham ALTERARY. Chem 340-2nd semester
 Ethers & Epoxides Chapter 5 Dr. Seham ALTERARY Chapter s out line Ethers Definition; General formula; Classification and Types Nomenclature - Common Names. - IUPAC Naming. Physical Properties General methods
Ethers & Epoxides Chapter 5 Dr. Seham ALTERARY Chapter s out line Ethers Definition; General formula; Classification and Types Nomenclature - Common Names. - IUPAC Naming. Physical Properties General methods
1. Which of the following reactions would have the smallest energy of activation?.
 Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
First Midterm Exam Monday, October 16, You may use model kits but no other material with chemical information without instructor approval.
 CH 334 Form A First Midterm Exam Monday, October 16, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Please do not use any electronic devices
CH 334 Form A First Midterm Exam Monday, October 16, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Please do not use any electronic devices
ALKANES STRUCTURE, PROPERTIES, AND SYNTHESIS A STUDENT WHO HAS MASTERED THE MATERIAL IN THIS SECTION SHOULD BE ABLE TO:
 ALKANES STRUCTURE, PROPERTIES, AND SYNTESIS A STUDENT WO AS MASTERED TE MATERIAL IN TIS SECTION SOULD BE ABLE TO: 1. Predict relative boiling points of alkanes, in comparison with other alkanes and with
ALKANES STRUCTURE, PROPERTIES, AND SYNTESIS A STUDENT WO AS MASTERED TE MATERIAL IN TIS SECTION SOULD BE ABLE TO: 1. Predict relative boiling points of alkanes, in comparison with other alkanes and with
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
CHEM Exam 2 October 25, 2007
 CEM 3311-200 Exam 2 October 25, 2007 By printing my name below, I pledge that "On my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized assistance on
CEM 3311-200 Exam 2 October 25, 2007 By printing my name below, I pledge that "On my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized assistance on
ORGANIC CHEMISTRY: SATURATED HYDROCARBONS
 19 09/16/2013 13:54:37 Page 283 APTER 19 ORGANI EMISTRY: SATURATED YDROARBONS SOLUTIONS TO REVIEW QUESTIONS 1. Two of the major reasons for the large number of organic compounds is the ability of carbon
19 09/16/2013 13:54:37 Page 283 APTER 19 ORGANI EMISTRY: SATURATED YDROARBONS SOLUTIONS TO REVIEW QUESTIONS 1. Two of the major reasons for the large number of organic compounds is the ability of carbon
Unit 2, Lesson 01: Introduction to Organic Chemistry and Hydrocarbons
 Unit 2, Lesson 01: Introduction to Organic Chemistry and Hydrocarbons Organic Chemistry: is the branch of chemistry that deals with carbon-based covalent compounds. living organisms are made up of a huge
Unit 2, Lesson 01: Introduction to Organic Chemistry and Hydrocarbons Organic Chemistry: is the branch of chemistry that deals with carbon-based covalent compounds. living organisms are made up of a huge
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems
 Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Rearrangement: a single reactant rearranges its
 Chapter 5: An overview of organic reactions 5.1 Kinds of organic reactions Even though there are hundreds of reactions to study, organic chemistry is governed by only a few key ideas that determine chemical
Chapter 5: An overview of organic reactions 5.1 Kinds of organic reactions Even though there are hundreds of reactions to study, organic chemistry is governed by only a few key ideas that determine chemical
First Midterm Exam Monday, October 16, You may use model kits but no other material with chemical information without instructor approval.
 CH 334 Form B First Midterm Exam Monday, October 16, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Please do not use any electronic devices
CH 334 Form B First Midterm Exam Monday, October 16, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Please do not use any electronic devices
Review: Atoms and Orbitals. Electrons = and charged; held
 hapter 1 Review: Atoms and Orbitals 1.1 Molecules are composed of Atoms be broken into smaller, stable units (except by physicists Elements are : Pb Au Atom = Nucleus + Electrons: Nucleus =,, charged core
hapter 1 Review: Atoms and Orbitals 1.1 Molecules are composed of Atoms be broken into smaller, stable units (except by physicists Elements are : Pb Au Atom = Nucleus + Electrons: Nucleus =,, charged core
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
10. Alkyl Halides. What Is an Alkyl Halide. An organic compound containing at least one carbonhalogen
 10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
CHEM 203. Topics Discussed on Oct. 16
 EM 203 Topics Discussed on Oct. 16 ydrogenation (= saturation) of olefins in the presence of finely divided transition metal catalysts (Ni, Pd, Pt, Rh, Ru...): generic alkene R 1 finely divided Pd (or
EM 203 Topics Discussed on Oct. 16 ydrogenation (= saturation) of olefins in the presence of finely divided transition metal catalysts (Ni, Pd, Pt, Rh, Ru...): generic alkene R 1 finely divided Pd (or
Lecture 21 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
Alkanes. The reaction is initiated by the formation of chlorine radicals from chlorine. ...
 Alkanes 1. Butane, 4 10, reacts with chlorine to produce a chloroalkane with molecular formula 4 9 l. The reaction is initiated by the formation of chlorine radicals from chlorine. What is meant by the
Alkanes 1. Butane, 4 10, reacts with chlorine to produce a chloroalkane with molecular formula 4 9 l. The reaction is initiated by the formation of chlorine radicals from chlorine. What is meant by the
Model 1 Homolysis Reactions are Highly Endothermic
 Chem 201 Activity 24: Radical chain mechanisms (What do radicals do? What does a radical chain mechanism look like) Model 1 Homolysis Reactions are Highly Endothermic Heterolysis Homolysis Y Z Y + Z Y
Chem 201 Activity 24: Radical chain mechanisms (What do radicals do? What does a radical chain mechanism look like) Model 1 Homolysis Reactions are Highly Endothermic Heterolysis Homolysis Y Z Y + Z Y
CHAPTER 12: SATURATED HYDROCARBONS
 CHAPTER 12: SATURATED HYDROCARBONS MULTIPLE CHOICE 1. Which of the following statements concerning organic compounds is correct? Organic compounds are found only in non-living systems. b. Organic compounds
CHAPTER 12: SATURATED HYDROCARBONS MULTIPLE CHOICE 1. Which of the following statements concerning organic compounds is correct? Organic compounds are found only in non-living systems. b. Organic compounds
Topic 2 Alkenes and alkynes
 Topic 2 Alkenes and alkynes lassification of rganic Reactions Four basic types of organic reaction Acid base reactions Substitution reactions Addition elimination reactions xidation reduction reactions
Topic 2 Alkenes and alkynes lassification of rganic Reactions Four basic types of organic reaction Acid base reactions Substitution reactions Addition elimination reactions xidation reduction reactions
HISTORY OF ORGANIC CHEMISTRY
 ISTORY OF ORGANI EMISTRY In the early days of chemistry, scientists classified chemical substances into 2 groups: 1. Inorganic: those that were composed of minerals, such as rocks and nonliving matter.
ISTORY OF ORGANI EMISTRY In the early days of chemistry, scientists classified chemical substances into 2 groups: 1. Inorganic: those that were composed of minerals, such as rocks and nonliving matter.
