Part C- section 1 p-bonds as nucleophiles
|
|
|
- Leslie Rich
- 5 years ago
- Views:
Transcription
1 Part C- section 1 p-bonds as nucleophiles
2 Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around the double bond) F Cl 2
3 Nomenclature 1. Select the longest chain and replace the suffix ane with ene 2. Start numbering the chain at the end nearer to the double bond 3. Designate the location of the double bond C 2 =CC 2 C 3 C 3 C=CC 2 C 2 C 3 1-butene 2-hexene (not 3-butene) (not 4-hexene) 4. Indicate the location of substituents by the number of the C atoms C 3 C 3 C 3 C 3 C 3 C 3 C=CC 3 C 3 C=CCC 3 C 3 C=CC 2 C-C methyl-2-butene C 3 2,4-dimethyl-2-pentene 2,5,5-trimethyl-2-hexene 3
4 Nomenclature 5. Number susbtituted cycloalkenes in the way that C atoms at the double bonds have 1 and 2 positions and that substituents have lower # at the first point of difference methylcyclopentene (not 2-methylcyclopentene ,5-dimethylcyclohexene not 4,6-dimethylcyclohexene) 6. If an alcohol group is present give the lowest possible # to it. C 3 C 3 C=CCC 3 4-methyl-3-penten-2-ol 4
5 7. Decide about cis and trans or E and Z Two «common» names: C 2 =C- vinyl C 2 =CC 2 - allyl 5
6 E and Z Cl F Z = heavier groups on the same side (Z)-2-bromo-1-chloro-1-fluoroethene E = heavier groups on the two opposite sides Cl F (E)-2-bromo-1-chloro-1-fluoroethene 6
7 (E)-1-bromo-1-choro-1-pentene Cl C 2 C 2 C 3 Cl (E)-2-bromo-1-chloro-1-iodo-1-butene I C 2 C 3 3 C C 2 C(C 3 ) 2 (Z)-3,5-dimethyl-2-hexene C 3 7
8
9
10 Evaluating alkene relative stability 10
11 trans more stable than cis (release of steric hindrance) By using either heat of combustion or heat of hydrogenation It is possible to demonstrate that : The greater # of groups to the double bond the greater is the alkene stability > > > > > > Electron donating ability of alkyl groups to satisfy the increased electronegativity of sp 2 C atoms 11
12 Cycloalkenes Always cis till cycloheptene cis-cyclooctene trans-cyclooctene 12
13 Additions to Alkenes -X X -S 3 S 3 - X-X X X 13
14 Additions to Alkenes + X-Y p-bond s-bond two s-bonds X Y bonds broken eactions usually hexothermic bonds formed Electron-rich region Susceptible of electrophilic attack 14 Electron-rich region
15 X- X X Electrophiles are: Lewis Acids 15
16 Markovnikov s rule bservation: C atom with the greater # of 16
17 Markovnikov s rule Explanation: 3 0 carbocation - t-butyl bromide!! 17
18 18
19 General formulation: In the ionic addition of an asymmetrical reagent to a double bond, the positive portion of the reagent bonds in a way which generates the most stable carbocation. +d -d I-Cl I 3 0 carbocation Cl I Cl These reactions are called: regioselective 19
20 Stereochemistry of the addition reaction X Et-C = C 2 -X Et C 2 - X Et (S) C 3 C3 Et () X Addition of 2 S 4 to form the alkyl hydrogen sulphate X = S 4 Addition of 2 to form the corresponding alcohol (it needs acid as a catalyst. Carbocation transpositions are possible with 1 0 and 2 0 carbocations) X = A valuable reaction 20
21 21
22 Synthesis of alcohols 1. acid-catalyzed hydration of alkenes (follows Markovnikov s rule) Et-C = C 2 -X Et C 2-2 Et C 3 Markovnikov rule: the bulkier group goes on the most crowded C atom 22
23 Carbocation rearrangements 3 C 3 C C 3 C C 3 3 C 3 C d + d+ C 3 C C 3 2 C C 3 C 3 C C 3 23
24 Synthesis of alcohols 2. xymercuration-demercuration (also follows Markovnikov s rule but no skeletal rearrangement) (1) g(ac) 2, 2 (2) NaB 4 24
25 chanism 1 st step: 2 nd step: g(ac) 2 g(ac) + + Ac - +d g(ac) +d g(ac) 3 rd step: +d 2 2 g(ac) g(ac) g(ac) +d 4 th step: NaB 4 g(ac) 25
26 The reaction can also be used for the synthesis of ethers just using instead of 2 +d g(ac) g(ac) g(ac) +d NaB 4 g(ac) 26
27 27
28 28
29 3. Addition of water to the double bond (anti-markovnikov) 1 st step: ydroboration Less crowded C atom 3 C 3 C 3 C B B syn attack!! B B 3 B 2 29
30 3. Addition of water to the double bond anti-markovnikov 2 nd step: oxidation / hydrolysis 1) Na 3 B Na 3 B 3 2) acid 3 C 3 C B -- B C configuration retained!! + B 30
31 Summary of Stereochemistry of hydroboration 1. Anti-markovnikov addition of 2 to the olefin 2. Syn addition C 3 1. hydroboration C 3 2. oxidation/hydrolysis + enantiomer 31
32 The basic principle of asymmetric synthesis C % (S) B 2 B* C 3 () + B* (S) C 3 100% (S) 100% (S) S, S,S diastereomers!!! (different stability and rate of formation) Enantiomeric enrichment 32
33 33
34 34
35 Provide the structure of the major organic product of the reaction below. Answer:! 35
36 adical Alkene Additions--Anti-Markovnikov Addition of Before 1933, the addition of to alkenes gave confusing results. In contrast to Cl, which always gave the Markovnikov addition product, the reaction sometimes gave the anti-markovnikov product. Cl + Cl isopropyl chloride (Markovnikov Product) + sometimes propyl bromide (Anti-Markovnikov Product)
37 The ole of rganic Peroxides In 1933, Morris Kharasch and Frank Mayo (U of Chicago) reported that the erratic behavior of was due to trace amounts of organic peroxides, sometimes present as a contaminant in the alkenes, that initiated a competing free radical chain addition. : : : : --- organic peroxide : : : : --- organic hydroperoxide They showed the anti-markovnikov addition product could be suppressed by adding small amounts of free radical "traps" that inhibited the propagation of the free radical chain reaction.
38 Peroxides : : : : : heat or radiation 2 -. : : alkoxyl radical Azo Compounds :heat or :N=N: radiation : 2. alkyl radicals + :N N: alogens : : X X : : : : : heat or radiation 2 : X. : : halogen radical
39 eactivity of Free adicals Most free radicals are highly reactive, short-lived species. They react with other radicals upon collision to form covalent bonds. They also react with molecules in atom abstraction or addition reactions. These reactions are driven by bond strength energetics.
40 Examples : Cl. + :C 3 methane : : heat or radiation :Cl: +. C 3 methyl radical : : Chlorine radical abstracts a hydrogen atom from methane producing hydrogen chloride and methyl radical. C. +. =... oxygen : : heat or radiation C -. methyl peroxy radical : : : : : thyl radical rapidly reacts with oxygen ( 2 ) to produce the methyl peroxy radical. C. + C 2 =CC 6 5 styrene heat or radiation. C 3 C 2 CC phenyl-1-propyl radical thyl radical adds to styrene
41 chanism of the Free adical Addition eaction The role of the peroxides is to initiate a free radical chain reaction by homolysis of the weak peroxy bond. Initiation : : : : --- heat 2 -. alkoxy radicals : : Δ o ~ 146 kj/mol : : : : : -- : : (Δ o = -366) (Δ o ~ -431) + :. a chain carrier : : Δ o ~ - 65 kj/mol
42 Propagation (1) :. + C 2 =C-C. 3 -C 2 -C-C 3 : : (D o ~ - 251) (D o ~ - 290) Δ o ~ - 39 kj/mol (2). -C 2 -C-C C 2 -C-C 3 + :. : : (D o = - 366) (D o = - 395) Δ o ~ - 29 kj/mol Note: Each propagation step is exothermic so there is no "kinetic bottleneck."
43 egioselectivity of the Addition The regioselectivity in the free radical chain addition of to propene (anti-markovnikov) is determined by the relative stabilities of the alkyl radical intermediates. :. faster. + C 2 =C-C 3 C 2 CC 3 secondary alkyl radical (more stable) : : :. + C 2 =C-C 3 slower. C 2 CC 3 primary alkyl radical (less stable) : : In the absence of peroxide initiators, or with added radical traps, the ionic addition dominates: + - C 3 C=C 2 + C 3 CC 3 C 3 CC 3 more stable carbocation
44 An Evaluation of the Addition of Cl to Propene Why does Cl only react by an ionic mechanism? The energetics of the two propagating steps in the free radical chain mechanism are: (1) : Cl. + C 2 =C-C 3 : :. ClC 2 CC 3 (D o = - 431) (D o = - 395) Δ o ~ + 36 kj/mol (2). Cl-C 2 -C-C 3 + -Cl Cl-C 2 -C-C 3 (D o = - 251) (D o = - 341) + : Cl. : : Δ o ~ - 90 kj/mol
45 Quiz Chapter 10 Section 9 In the presence of peroxides, isobutene reacts with to give 1-bromo-2- methylpropane. Provide the initiation and propagation steps for this reaction. (C 3 ) 2 C=C 2 + (peroxides) (C 3 ) 2 CC 2 Initiation heat or hν Propagation (C 3 ) 2 C = C 2 +. (C 3 ) 2 C-C 2. (C 3 ) 2 C-C 2 + (C 3 ) 2 CC chain carriers
46 Alkene xidations Syn-hydroxylation -, 2 C 2 C 2 + KMn 4 2 C C 2 Two methods: Na 2 S 3 / 2 C 2 C 2 + s 4 2 C C 2 46
47 Alkene oxidations: chanism Mn Mn Mn s s s 47
48 xidative cleavage 3 C 3 C KMn 4, - heat + C 3 C 3 KMn 4, - heat + C 2 ' ' 48
49 Alkyne xidations KMn 4 heat 49
50 50
51 zonolysis Zn ozonide 51
52 52
53 Epoxide formation Let s do the following experiment C + C Epoxide (oxyrane) 53
54 eactions of epoxides ing opening with acid 3 + with base 2 54
55 Stereochemistry of ring opening Acid catalyzed esembles a 3 0 carbocation C 3 (S) 3 + +d C 3 C 3 C 3 () Base catalyzed (S) C 3 C 3 (S) C 3 55
56 56
57 57
58 Anti hydroxylation of alkenes C() 3 + trans 58
59 Stereochemistry cis meso 2,3-2,3-butanediol 2S,3S-2,3-butanediol 59
60 racemate trans meso-2,3-butanediol 60
61 (S)-*C() (S) (S) () () ' ' ' () (S) ' () (S) ' 61
62 Addition of halogens Fast, high yield reactions forming vic-dihalides trans 62
63 chanism + anti addition: a stereospecific reaction 63
64 Stereospecificity, S,S trans-1,2-dibromocyclopentane The reaction forms only one steroisomeric form (trans) but is not enantiospecific 64
65 Stereospecificity C 3 3 C 2 2,3S (meso) CCl 4 trans-2-butene C 3 C 3 3 C C 3 C 3 2 CCl C cis-2-butene (MDELS) C 3 2,3 2S,3S C 3 65
66 Stereospecificity Et 2 Et Et 2 Et Et S 66
67 Formation of halohydrins (addition of X 2 and 2 ) minor major Use excess of water to favor halohydrin 67
68 Formation of halohydrins (addition of X 2 and 2 ) mechanism anti attack 68
69 69
70 70
71 71
72 The acidity of terminal alkynes sp sp 2 sp 3 pk a More s character, more stable anion, weaker conjugated base, better acid Acidity C 3 C 3 < C 2 =C 2 < CC Basicity C 3 C 2 > C 2 =C > CC < < CC < N 2 < C=C 2 < C 2 -C 3 72
73 Alkynes on the same line as alkenes Addition of 2 and Cl Addition of X follows the Markovnikov's rule xidative cleavage ' 3 C 2 + 'C 2 73
74 ydrogenation of alkynes Syn addition for the formation of cis-alkenes 2 Anti addition for the formation of trans-alkenes Li, EtN 2 74
75 Anti addition: the mechanism (reminder) -NEt Li Li -NEt trans 75
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 8 Alkenes and Alkynes II: Addition Reactions "
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Alkenes. Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula C n H 2n. Nomenclature
 Alkenes Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula n 2n. Nomenclature Alkenes are named in the same manner as alkanes with the following adjustments. 1. Find the longest
Alkenes Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula n 2n. Nomenclature Alkenes are named in the same manner as alkanes with the following adjustments. 1. Find the longest
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
HONORS ORGANIC CHEM. HAHS MRS. RICHARDS
 NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
CH Organic Chemistry I (Katz) Practice Exam #3- Fall 2013
 C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry
 Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Electrophilic Addition
 . Reactivity of = Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. arbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the
. Reactivity of = Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. arbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the
nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes
 240 Chem nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes 1 Chapter 3 Alkenes or Olefines Crabon-Carbon double bond C n H 2n Hybridization in Alkenes: 1.34 A 2 Nomenclature of Alkenes and Cycloalkenes
240 Chem nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes 1 Chapter 3 Alkenes or Olefines Crabon-Carbon double bond C n H 2n Hybridization in Alkenes: 1.34 A 2 Nomenclature of Alkenes and Cycloalkenes
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
2/26/18. Practice Questions. Practice Questions B F. How many steps are there in this reaction?
 Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes hapter 8 Alkenes and Alkynes II: Addition eactions Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The carbocation
Additions to Alkenes hapter 8 Alkenes and Alkynes II: Addition eactions Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The carbocation
When H and OH add to the alkyne, an enol is formed, which rearranges to form a carbonyl (C=O) group:
 Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
6.10 Acid-Catalyzed Hydration of Alkenes. Acid-Catalyzed Hydration of Alkenes
 Acid-atalyzed ydration of Alkenes 6.10 Acid-atalyzed ydration of Alkenes O O reaction is acid catalyzed; typical hydration medium is 50% 2 SO 4-50% 2 O Follows Markovnikov's Rule Follows Markovnikov's
Acid-atalyzed ydration of Alkenes 6.10 Acid-atalyzed ydration of Alkenes O O reaction is acid catalyzed; typical hydration medium is 50% 2 SO 4-50% 2 O Follows Markovnikov's Rule Follows Markovnikov's
Reactivity of C=C. Chapter 8 Reactions of Alkenes. Types of Additions. Electrophilic Addition. Addition of HX (1) Addition of HX (2)
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 8 Reactions of Alkenes Jo Blackburn Richland ollege, allas, TX allas ounty ommunity ollege istrict 2003, Prentice all Reactivity of = Electrons in pi
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 8 Reactions of Alkenes Jo Blackburn Richland ollege, allas, TX allas ounty ommunity ollege istrict 2003, Prentice all Reactivity of = Electrons in pi
Chapter 7: Alkenes: Reactions and Synthesis
 hapter 7: Alkenes: Reactions and Synthesis alcohol alkane halohydrin 1,2-diol 1,2-dihalide carbonyl halide halide Addition Y Y Elimination Electrophilic Addition Dehydrohalogenation: loss of from an alkyl
hapter 7: Alkenes: Reactions and Synthesis alcohol alkane halohydrin 1,2-diol 1,2-dihalide carbonyl halide halide Addition Y Y Elimination Electrophilic Addition Dehydrohalogenation: loss of from an alkyl
Chapter 8 Reactions of Alkenes
 Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Organic Chemistry, Second Edition. Janice Gorzynski Smith University of Hawai i. Chapter 10 Alkenes
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Chapter 8: Addition Reactions
 Chapter 8: Addition Reactions Addition Reactions to Alkenes (Section 8.1) Markovnikov s Rule (Section 8.2) Stereochemistry of Ionic Addition to Alkenes (Section 8.3) 2 S 4 Additions to Alkenes (Section
Chapter 8: Addition Reactions Addition Reactions to Alkenes (Section 8.1) Markovnikov s Rule (Section 8.2) Stereochemistry of Ionic Addition to Alkenes (Section 8.3) 2 S 4 Additions to Alkenes (Section
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
4.15 Halogenation of Alkanes RH + X 2 RX + HX
 4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
Chapter 7: Alkene reactions conversion to new functional groups
 hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
Chapter 7 Alkenes; Elimination Reactions
 hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
1. Radical Substitution on Alkanes. 2. Radical Substitution with Alkenes. 3. Electrophilic Addition
 1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
Chapter 8 - Alkenes and Alkynes II Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles
 Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Chapter 9 Alkynes. Introduction
 hapter 9 Alkynes Introduction Alkynes contain a triple bond. General formula is n 2n-2. Two elements of unsaturation for each triple bond. MST reactions are like alkenes: addition and oxidation. Some reactions
hapter 9 Alkynes Introduction Alkynes contain a triple bond. General formula is n 2n-2. Two elements of unsaturation for each triple bond. MST reactions are like alkenes: addition and oxidation. Some reactions
Chapter 4. Reactions of alkenes. Addition reactions Carbocations Selectivity of reactions
 Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
Organic Chemistry. Alkenes
 Organic Chemistry by Nurlin Abu Samah, Dr. Md. Shaheen & Dr. Nadeem Akhtar Faculty of Industrial Sciences & Technology nurlin@ump.edu.my Chapter Description Aims The students should understand the fundamental
Organic Chemistry by Nurlin Abu Samah, Dr. Md. Shaheen & Dr. Nadeem Akhtar Faculty of Industrial Sciences & Technology nurlin@ump.edu.my Chapter Description Aims The students should understand the fundamental
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
sp 2 geometry tetrahedral trigonal planar linear ΔH C-C ΔH C-H % s character pk a 464 KJ/mol 33% 44
 hapter 10: Alkynes 10.1 Introduction to Alkynes ~ 111 ~ 122 1.06 Å 180 1.1 Å ~ 116 1.08 Å 1.54 Å 1.34 Å 1.20 Å hybridization of sp 3 sp 2 sp geometry tetrahedral trigonal planar linear 368 KJ/mol 632 KJ/mol
hapter 10: Alkynes 10.1 Introduction to Alkynes ~ 111 ~ 122 1.06 Å 180 1.1 Å ~ 116 1.08 Å 1.54 Å 1.34 Å 1.20 Å hybridization of sp 3 sp 2 sp geometry tetrahedral trigonal planar linear 368 KJ/mol 632 KJ/mol
ORGANIC - CLUTCH CH ADDITION REACTIONS.
 !! www.clutchprep.com CONCEPT: GENERAL MECHANISM Addition reactions are ones in which 1 bond is broken and 2 new bonds are formed. They are the inverse of reactions EXAMPLE: Provide the mechanism for the
!! www.clutchprep.com CONCEPT: GENERAL MECHANISM Addition reactions are ones in which 1 bond is broken and 2 new bonds are formed. They are the inverse of reactions EXAMPLE: Provide the mechanism for the
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of H X 2. Addition of H OH and addition of Y X 3. Addition to allene and
 Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
Double and Triple Bonds. The addition of an electrophile and a
 Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) Can act as weak nucleophiles
 Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Lecture 11 Organic Chemistry 1
 EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
ORGANIC CHEMISTRY- 1
 ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
1. Addition of HBr to alkenes
 eactions of Alkenes I eading: Wade chapter 8, sections 8-1- 8-8 tudy Problems: 8-47, 8-48, 8-55, 8-66, 8-67, 8-70 Key Concepts and kills: Predict the products of additions to alkenes, including regiochemistry
eactions of Alkenes I eading: Wade chapter 8, sections 8-1- 8-8 tudy Problems: 8-47, 8-48, 8-55, 8-66, 8-67, 8-70 Key Concepts and kills: Predict the products of additions to alkenes, including regiochemistry
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem reaction what is added to the C=C what kind of molecule results addition of HX HX only
 I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
What is the major product of the following reaction?
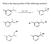 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
C h a p t e r N i n e: Addition Reactions of Alkenes
 C h a p t e r N i n e: Addition Reactions of Alkenes. H C 2 H Biosynthesis of a prostaglandin from arachidonic acid: intermediate intramolecular radical addition CHM 321: Summary of Important Concepts
C h a p t e r N i n e: Addition Reactions of Alkenes. H C 2 H Biosynthesis of a prostaglandin from arachidonic acid: intermediate intramolecular radical addition CHM 321: Summary of Important Concepts
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
Ether, NaOH. This is a two-step process, but is most commonly done as a one-pot procedure (upper right).
 CAPTE 8. ALKENES: EACTINS AND SYNTESIS ydration of Alkenes by xymercuration-eduction - concerted formation of three-membered ring intermediate - addition of water occurs through anti stereoselectivity
CAPTE 8. ALKENES: EACTINS AND SYNTESIS ydration of Alkenes by xymercuration-eduction - concerted formation of three-membered ring intermediate - addition of water occurs through anti stereoselectivity
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008
 Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections
 Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
CHEM Lecture 7
 CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
CHEM Exam 2 ANSWER KEY
 CEM 3311-200 Exam 2 ANSWER KEY ctober 23, 2012 Time: 2 ours Please copy and sign the onor Pledge on the scantron sheet in the space below the double lines. I pledge that n my honor, as a University of
CEM 3311-200 Exam 2 ANSWER KEY ctober 23, 2012 Time: 2 ours Please copy and sign the onor Pledge on the scantron sheet in the space below the double lines. I pledge that n my honor, as a University of
Organic Chemistry I: Reactions and Overview
 Organic Chemistry I: Reactions and Overview Andrew Rosen Editor: Raghav Malik January 13, 2013 Contents I Library of Synthetic Reactions 3 II Organic Trends and Essentials 4 1 The Basics: Bonding and Molecular
Organic Chemistry I: Reactions and Overview Andrew Rosen Editor: Raghav Malik January 13, 2013 Contents I Library of Synthetic Reactions 3 II Organic Trends and Essentials 4 1 The Basics: Bonding and Molecular
Chapter 8 Addition Reactions to Alkenes
 . 8 hapter 8 Addition eactions to Alkenes In this chapter will we study the addition reactions of alkenes. We will see that the π electrons of the double bond are loosely held and that their maximum electron
. 8 hapter 8 Addition eactions to Alkenes In this chapter will we study the addition reactions of alkenes. We will see that the π electrons of the double bond are loosely held and that their maximum electron
CHEM 263 Oct 25, stronger base stronger acid weaker acid weaker base
 CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
trans-cyclooctene 4-fluoro-1-butanol 2-cyclohexenol 4. (5 pts) Provide structural formula for each of the following molecules: Br OH Cl OH
 CEMISTRY 313-01 MIDTERM # 2 answer key March 12, 2009 Statistics: Average: 72 pts (72%); ighest: 98 pts (98%); Lowest: 21 pts (21%) umber of students performing at or above average: 22 (50%) umber of students
CEMISTRY 313-01 MIDTERM # 2 answer key March 12, 2009 Statistics: Average: 72 pts (72%); ighest: 98 pts (98%); Lowest: 21 pts (21%) umber of students performing at or above average: 22 (50%) umber of students
Organic Chemistry. Alkenes (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
Loudon Chapter 14 Review: Reactions of Alkynes Jacquie Richardson, CU Boulder Last updated 1/16/2018
 An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
Chapter 5. Reactions of Alkenes and Alkynes
 Chapter 5. Reactions of Alkenes and Alkynes Learning objectives: 1. Identify the followings from a reaction coordinate diagram when applicable: endothermic or exothermic reactions, activation energy, heat
Chapter 5. Reactions of Alkenes and Alkynes Learning objectives: 1. Identify the followings from a reaction coordinate diagram when applicable: endothermic or exothermic reactions, activation energy, heat
Chapter 8. Alkenes and Alkynes II: Addition Reactions. Ch. 8-1
 hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
Alkenes - Addition Reactions
 Alkenes - Addition Reactions Alkenes- reactions. Addition Ionic Free radical Reduction Oxidation Substitution Reactions, alkenes: 1. Addition of hydrogen (reduction). 2. Addition of halogens. 3. Addition
Alkenes - Addition Reactions Alkenes- reactions. Addition Ionic Free radical Reduction Oxidation Substitution Reactions, alkenes: 1. Addition of hydrogen (reduction). 2. Addition of halogens. 3. Addition
CHAPTER 3 ALKENES, ALKYNES & CONJUGATE DIENES
 CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
Alcohol Synthesis. Dr. Sapna Gupta
 Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
10.1. Electrophilic Addition of to to give. Works with HCl HBr HI. Electrophilic Addition Mechanism. H Cl Cl. 3D picture of intermediate: Rxn Coord
 10.1 Electrophilic Addition of to to give Works with I F Electrophilic Addition Mechanism 3D picture of intermediate: E xn oord Thermodynamics: + Δ (kcal/mol) + + = Alkene ydration: Electrophilic Addition
10.1 Electrophilic Addition of to to give Works with I F Electrophilic Addition Mechanism 3D picture of intermediate: E xn oord Thermodynamics: + Δ (kcal/mol) + + = Alkene ydration: Electrophilic Addition
1. Which of the following reactions would have the smallest energy of activation?.
 Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
CHEMISTRY 231 GENERAL ORGANIC CHEMISTRY I FALL 2014 List of Topics / Examination Schedule
 Page 1 of 5 CHEMISTRY 231 FALL 2014 List of Topics / Examination Schedule Unit Starts Topic of Study 20 Aug 2014 STRUCTURE AND BONDING Suggested Reading: Chapter 1 29 Aug 2014 ALKANES & CYCLOALKANES Suggested
Page 1 of 5 CHEMISTRY 231 FALL 2014 List of Topics / Examination Schedule Unit Starts Topic of Study 20 Aug 2014 STRUCTURE AND BONDING Suggested Reading: Chapter 1 29 Aug 2014 ALKANES & CYCLOALKANES Suggested
C C. sp 2. π M.O. atomic. orbitals. carbon 1. σ M.O. molecular. orbitals. H C C rotate D. D H zero overlap of p orbitals: π bond broken!
 Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
1. Which of the following compounds is the weakest base?
 I. Multiple-choice Questions Fall 2018 1. Which of the following compounds is the weakest base? a. C3C2 b. C3C2 c. N3 d. C3 e. N2 2. Which of the following functional groups is indicated by a strong and
I. Multiple-choice Questions Fall 2018 1. Which of the following compounds is the weakest base? a. C3C2 b. C3C2 c. N3 d. C3 e. N2 2. Which of the following functional groups is indicated by a strong and
1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors
 1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors I, Cl, N 3, 3 P 4 pka 10 to 5 Super strong acids 3 + pka 1.7 RC 2 pka ~ 5 acids Ph pka ~ 10 get 2, R pka ~ 16 weaker RCC (alkynes) pka ~ 26 RN 2 pka
1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors I, Cl, N 3, 3 P 4 pka 10 to 5 Super strong acids 3 + pka 1.7 RC 2 pka ~ 5 acids Ph pka ~ 10 get 2, R pka ~ 16 weaker RCC (alkynes) pka ~ 26 RN 2 pka
Introduction & Definitions Catalytic Hydrogenations Dissolving Metal Reduction Reduction by Addition of H- and H+ Oxidation of Alcohols Oxidation of
 CEM 241- UNIT 4 xidation/reduction Reactions Redox chemistry 1 utline Introduction & Definitions Catalytic ydrogenations Dissolving Metal Reduction Reduction by Addition of - and + xidation of Alcohols
CEM 241- UNIT 4 xidation/reduction Reactions Redox chemistry 1 utline Introduction & Definitions Catalytic ydrogenations Dissolving Metal Reduction Reduction by Addition of - and + xidation of Alcohols
CHEMISTRY MIDTERM # 2 October 27, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 EMISTRY 313-01 MIDTERM # 2 ctober 27, 2005 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (7 pts) Mark as true (T) or false (F) the following
EMISTRY 313-01 MIDTERM # 2 ctober 27, 2005 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (7 pts) Mark as true (T) or false (F) the following
CHEMISTRY Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay
 EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
ELECTROPHILIC ADDITIONS OF ALKENES AS THE COUNTERPART OF ELIMINATIONS
 ELECTRPHILIC ADDITINS F ALKENES AS THE CUNTERPART F ELIMINATINS INTRDUCTIN - Chapter 8 is mostly about alkene reactions. That is, how one can transform alkenes into other functional groups. Most of these
ELECTRPHILIC ADDITINS F ALKENES AS THE CUNTERPART F ELIMINATINS INTRDUCTIN - Chapter 8 is mostly about alkene reactions. That is, how one can transform alkenes into other functional groups. Most of these
10. Organohalides. Based on McMurry s Organic Chemistry, 7 th edition
 10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chem Lecture 23 Page- 1 -
 Chem 230-001 Lecture 23 Page- 1 - ydroboration/ oxidation - acemic trans-2-methylcyclohexanol may be neatly synthesized from 1-methyl- 1-cyclohexene by using a reagent called borane, 3. 0.33 equivs. 3,
Chem 230-001 Lecture 23 Page- 1 - ydroboration/ oxidation - acemic trans-2-methylcyclohexanol may be neatly synthesized from 1-methyl- 1-cyclohexene by using a reagent called borane, 3. 0.33 equivs. 3,
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Chapter 7. dehydration
 hapter 7 7.1 ne problem with elimination reactions is that mixtures of products are often formed. For example, treatment of 2-bromo-2-methylbutane with K in ethanol yields a mixture of two alkene products.
hapter 7 7.1 ne problem with elimination reactions is that mixtures of products are often formed. For example, treatment of 2-bromo-2-methylbutane with K in ethanol yields a mixture of two alkene products.
Lecture 20 Organic Chemistry 1
 CEM 232 rganic Chemistry I at Chicago Lecture 20 rganic Chemistry 1 Professor Duncan Wardrop March 18, 2010 1 Self-Test Question Capnellene (4) is a marine natural product that was isolated from coral
CEM 232 rganic Chemistry I at Chicago Lecture 20 rganic Chemistry 1 Professor Duncan Wardrop March 18, 2010 1 Self-Test Question Capnellene (4) is a marine natural product that was isolated from coral
Chapter 7. Alkenes: Reactions and Synthesis
 Chapter 7. Alkenes: Reactions and Synthesis 1 Synthesis of Alkenes: Elimination Reactions 1. Dehydrohalogenation of alkyl halides. loss of requires CH 2 CH 2 Cl Zaitsev s Rule: CH 2 C 2. Dehydration of
Chapter 7. Alkenes: Reactions and Synthesis 1 Synthesis of Alkenes: Elimination Reactions 1. Dehydrohalogenation of alkyl halides. loss of requires CH 2 CH 2 Cl Zaitsev s Rule: CH 2 C 2. Dehydration of
B. (2 pts) The regiochemistry of alcohol dehydration is determined by the: a. Zaitsev rule; b. Hammond postulate;
 CEMISTRY 313-01 MIDTERM # answer key ctober 1, 008 Statistics: Average: 73 pts (73%); ighest: 98 pts (98%); Lowest: 3 pts (3%) Number of students performing at or above average: 36 (58%) Number of students
CEMISTRY 313-01 MIDTERM # answer key ctober 1, 008 Statistics: Average: 73 pts (73%); ighest: 98 pts (98%); Lowest: 3 pts (3%) Number of students performing at or above average: 36 (58%) Number of students
Ethers. Chapter 14: Ethers, Epoxides, & Sulfides. General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
 Chamras Chemistry 106 Lecture Notes Examination 1 Materials Chapter 14: Ethers, Epoxides, & Sulfides Ethers General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
Chamras Chemistry 106 Lecture Notes Examination 1 Materials Chapter 14: Ethers, Epoxides, & Sulfides Ethers General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
Chapter 11 - Alcohols and Ethers 1
 Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
+ HBr!H = OH CH CH 2. HgOAc
 radical substitution Cl hv + Cl 2 + Cl! = Energy radical addition RR + electrophilic addition + C3 C3! = Energy Energy + 2 acetone water + 2 2 +! =! = Energy Energy acid catalyzed hydration + 2 +! = Energy
radical substitution Cl hv + Cl 2 + Cl! = Energy radical addition RR + electrophilic addition + C3 C3! = Energy Energy + 2 acetone water + 2 2 +! =! = Energy Energy acid catalyzed hydration + 2 +! = Energy
What are radicals? H. Cl. Chapter 10 Radical Reactions. Production of radicals. Reactions of radicals. Electronic structure of methyl radical
 What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
CH320/328 M Spring 2014
 CH320/328 M Spring 2014 HW Set #3 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your
CH320/328 M Spring 2014 HW Set #3 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your
Chemistry 210 Organic Chemistry I Winter Semester 2001 Dr. Rainer Glaser
 hemistry 210 rganic hemistry I Winter Semester 2001 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, April 20, 2001, 9:00-9:50 Name: Answer Key Question
hemistry 210 rganic hemistry I Winter Semester 2001 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, April 20, 2001, 9:00-9:50 Name: Answer Key Question
CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl
 Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
