An unknown molecule A has 4 signals in the 1 H NMR spectrum. Which of the following corresponds to molecule A
|
|
|
- Alexina Strickland
- 6 years ago
- Views:
Transcription
1 An unknown molecule A has 4 signals in the 1 H NMR spectrum. Which of the following corresponds to molecule A
2 How many nonequivalent protons does the following structure have? 4
3 Reading from left to right, what multiplicity would be found for the three nonequivalent sets of protons in the 1 H NMR spectrum of the following compound? d, d, s
4 Give the number of lines in the coupling pattern for each type of hydrogen. 2, 7, 7, 2
5 Introduction Homolytic bond cleavage leads to the formation of radicals(also called free radicals) Radicals are highly reactive,, short lived species Single headed arrows are used to show the movement fo single electrons. Production of Radicals Homolysis of relatively weak bonds such as O-O and X-X bonds can occur with the addition of energy in the form of heat or light.
6 Carbon radicals are categorized as primary (1 ), secondary (2 ) and tertiary (3 ) based on the number of attached R groups. RCH2R2CHR3CH A carbon radical is sp 2 hybridized with a trigonal planar geometry with the unpaired electron in the unhybridized p orbital. Bond dissociation energy is used as a measure of radical stability.
7 Two different radicals can be formed with the cleavage of a C-H bond. Basically, the more alkyl groups attached to the radical carbon the more stable it is. Also the more stable the radical, the less energy it takes to break the C-H bond.
8
9 What type of radical are each of the following? H3CH3CCH2CH3 H3CCHH3CCHCH3 H3CCHH2CCH2CH Of these three radicals, which is the most stable? H3CH3CCH2CH3
10 Radical Reactions of Alkanes Abstraction of a H from a C-H σ bond in which one electron is sued to form H-X while the other is left on the new alkyl radical. H+X+HX A radical can also add to a alkene by adding onto a double bond and leaving the other carbon that was part fo the double bond as a radical. XX
11 Radicals are highly reactive and unstable and usually react quickly with a sigma or pi bond. However sometimes they can react with another radical. X+XXX When oxygen, a diradical, is present it acts as a radical inhibitor or scavenger. Meaning it prevents the radical from attacking any alkanes or alkenes. OO+XOOX
12 In the presence of heat and light, alkanes and halogens will react to form alkyl halides. H3CH+Cl2H3CCl+HClH+Br2Br+HBrH3CH2CCH3+2Cl2H3CH2CC
13 Predict the products from the monobromination of the foloowing compound? CHCHH3CH3CCH2ClCH3CHCClH3CH3CCH3C CHCHH3CH3CCH3CH3Br2hv/ he
14 Step 1 - Initiation ClClhv/heat2Cl Step 2 Propagation H3CH2CH+ClH3CCH2+HClH3CCH2+ClClH3CH2CCl+ Step 3 - Termination ClCl+ClClH3CCH2CH3H2C+H3CH2CH2CCH3H3CCH2+ClH3CCH
15 In each step of the propagation a bond is broken and formed. And because the overall step has a -ΔH it is exothermic. Step 1 is called the rate determini g step because it is higher in energy.
16 Transition States Cl----H----CH 2 CH 3 Cl---Cl---- CH 2 CH 3
17 H3CH2CCH3+ClClH3CH2CCH2Cl+H3CHClCCH3 There are 6 Methyl H s and 2 Methylene H s. Based on this, the ratio fo the two products should be 3:1(primary to secondary). However, the ratio is 1:1. The more stable the radical being formed is, the easier it is to cleave the C-H bond.
18 Which C-H bond in each compound is most reactive? H H HH
19 Chlorination Vs. Bromination H3CH2CCH3+Cl2H3CH2CCH2Cl+H3CHClCCH3 1:1
20 H3CH2CCH3+Br2H3CH2CCH2Br+H3CHBrCCH3 99%
21 Chlorination is faster and nonselective. This is due to it s rate determining step being exothermic. Bromination is slower and chooses the most stablew radical. This due to it s rate determining step being endothermic. H3CH2CCHCH3CH3+BR2 H3CH2CCClCH3 +Br2 Br
22 Halogenation is useful in the formation of alkenes. +Cl2Cl+K+-OH An elimination in the presence of a strong base is responsible for the formation of the alkene. Cl-OHH+H2O+KBr
23 aq. H2SO4OH+ Conversion of the alkene to an alcohol via nucleophillic substitution is an extension of the utility of radical halogenation. H+++-HSO4HOHOHH-HSO4OH
24 Hg(OAc)2NaBH4OCH3 Oxymercuration-demercuration of an alkene results in the formation of an ether. +HgOAc+HgOAcMeOHHCH3HgOAcOCH3HgOAcNaBH4OCH3
25 H3CH2CH2CCl2H3CH2CH2CH3CHClCH2C+CH3CH2ClCH3 Radical halogenations give a racemic mixture of prodcuts when possible. This halogenation of an achiral compound results in 3 products. A primary and secondary alkyl halide. The secondary halide exist as a pair of enantiomers due to the creation of a stereogenic center upon halogenation.
26 H3CCH2CH3BrHClH3CBrCH2CH3Cl2Cl2H3CCH2CH3BrClH3 enantiomers H3CCH2CH3BrHClH3CCHCH3BrHCl2Cl2H3CCH3BrClHHH3CC diastereomers
27 H3CCH2CH3BrHCl2ClH2CCH2CH3BrH+H3CCH2CH2ClBrH Only achieve enantiomers or diastereomers if the halogenation takes place at a stereogenic center.
Organic Chemistry(I) Chapter 3
 Organic Chemistry(I) Chapter 3 1. Carbon-carbon bonds are not easily broken. Which bond in the following compound would be the least difficult to break homolytically? 2. Which of the following molecules
Organic Chemistry(I) Chapter 3 1. Carbon-carbon bonds are not easily broken. Which bond in the following compound would be the least difficult to break homolytically? 2. Which of the following molecules
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
What are radicals? H. Cl. Chapter 10 Radical Reactions. Production of radicals. Reactions of radicals. Electronic structure of methyl radical
 What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
Organic Chemistry. Radical Reactions
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Radical Reactions by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Radical Reactions by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
4.15 Halogenation of Alkanes RH + X 2 RX + HX
 4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
Chapter 10 Radical Reactions
 Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl
 Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
ORGANIC - BROWN 8E CH.8 - HALOALKANES, HALOGENATION AND RADICALS
 !! www.clutchprep.com CONCEPT: ALKYL HALIDES Alkyl halides are named by naming them as a substituent before the root chain and indicating their location. Prefixes: -F, -Cl -Br -I Alkyl halides have NO
!! www.clutchprep.com CONCEPT: ALKYL HALIDES Alkyl halides are named by naming them as a substituent before the root chain and indicating their location. Prefixes: -F, -Cl -Br -I Alkyl halides have NO
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2018
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2015
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Chapter 10 Free Radicals
 hapter 10 Free Radicals This is an example of a free radical reaction. A radical is a species that has a free unpaired electron. There are several examples of stable radicals, the most common of which
hapter 10 Free Radicals This is an example of a free radical reaction. A radical is a species that has a free unpaired electron. There are several examples of stable radicals, the most common of which
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
CHEM Lecture 6
 EM 494 Special Topics in hemistry Illinois at hicago EM 494 - Lecture 6 Prof. Duncan Wardrop October 15, 2012 Midterm Papers Factors that ontrol ydrocarbon Acidity Factors that ontrol ydrocarbon onformation
EM 494 Special Topics in hemistry Illinois at hicago EM 494 - Lecture 6 Prof. Duncan Wardrop October 15, 2012 Midterm Papers Factors that ontrol ydrocarbon Acidity Factors that ontrol ydrocarbon onformation
Chapter 10 Lecture Outline
 Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
CHEM 241 ALCOHOLS AND ALKYL HALIDES CHAP 5 ASSIGN
 CEM 241 ALCOOLS AND ALKYL ALIDES CAP 5 ASSIGN 1. What is the IUPAC name of the compound below? A. 3-isobutyl-2-hexanol B. 2-methyl-5-propyl-6-heptanol C. 2-methyl-5-(1-hydroxyethyl)octane D. 6-methyl-3-propyl-2-heptanol
CEM 241 ALCOOLS AND ALKYL ALIDES CAP 5 ASSIGN 1. What is the IUPAC name of the compound below? A. 3-isobutyl-2-hexanol B. 2-methyl-5-propyl-6-heptanol C. 2-methyl-5-(1-hydroxyethyl)octane D. 6-methyl-3-propyl-2-heptanol
Study of Chemical Reactions
 Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Química Orgânica I. Organic Reactions
 Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
ORGANIC CHEMISTRY 307
 ORGANIC CHEMISTRY 307 CHAPTER 3 LECTURE NOTES R. Boikess II. Principles of Organic Reactions 1. Chemical reactions are the result of bond breaking and bond making. a. Most (but not all) bond making and
ORGANIC CHEMISTRY 307 CHAPTER 3 LECTURE NOTES R. Boikess II. Principles of Organic Reactions 1. Chemical reactions are the result of bond breaking and bond making. a. Most (but not all) bond making and
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Organic Chemistry. Alkenes (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2
 Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
What is the major product of the following reaction?
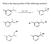 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
First Midterm Exam Monday, October 16, You may use model kits but no other material with chemical information without instructor approval.
 CH 334 Form B First Midterm Exam Monday, October 16, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Please do not use any electronic devices
CH 334 Form B First Midterm Exam Monday, October 16, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Please do not use any electronic devices
Chapter 15. Free Radical Reactions
 Grossman, CE 230 Chapter 15. Free Radical Reactions A free radical is a species containing one or more unpaired electrons. Free radicals are electrondeficient species, but they are usually uncharged, so
Grossman, CE 230 Chapter 15. Free Radical Reactions A free radical is a species containing one or more unpaired electrons. Free radicals are electrondeficient species, but they are usually uncharged, so
Chapter 8 - Alkenes and Alkynes II Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles
 Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem reaction what is added to the C=C what kind of molecule results addition of HX HX only
 I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 7-1. Alkyl Halides Based on McMurry s Organic Chemistry, 6 th edition What Is an Alkyl Halide? An
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 7-1. Alkyl Halides Based on McMurry s Organic Chemistry, 6 th edition What Is an Alkyl Halide? An
Model 1 Homolysis Reactions are Highly Endothermic
 Chem 201 Activity 24: Radical chain mechanisms (What do radicals do? What does a radical chain mechanism look like) Model 1 Homolysis Reactions are Highly Endothermic Heterolysis Homolysis Y Z Y + Z Y
Chem 201 Activity 24: Radical chain mechanisms (What do radicals do? What does a radical chain mechanism look like) Model 1 Homolysis Reactions are Highly Endothermic Heterolysis Homolysis Y Z Y + Z Y
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms
 Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
REALLY, REALLY STRONG BASES. DO NOT FORGET THIS!!!!!
 CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
CHEM 263 Oct 25, stronger base stronger acid weaker acid weaker base
 CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Lesmahagow High School CfE Advanced Higher Chemistry
 Lesmahagow High School AHChemistry Organic Chemistry& Instrumental Analysis Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Alkanes, Alkenes and
Lesmahagow High School AHChemistry Organic Chemistry& Instrumental Analysis Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Alkanes, Alkenes and
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Worksheet Chapter 10: Organic chemistry glossary
 Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
Chapter 5. 3-Chloro-2-methylpentane. Cl 2. 2-Chloro-2-methylpentane. 1-Chloro-2-methylpentane. Cl 2-Chloro-4-methylpentane. 1-Chloro-4-methylpentane
 hapter 5 5.1 lassify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) 3Br K 3 KBr (b) 3 2 2 2 2 (c) 2 2 2 3 3 a. substitution b. elimination c. addition
hapter 5 5.1 lassify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) 3Br K 3 KBr (b) 3 2 2 2 2 (c) 2 2 2 3 3 a. substitution b. elimination c. addition
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
BIOB111 - Tutorial activities for session 8
 BIOB111 - Tutorial activities for session 8 General topics for week 4 Session 8 Physical and chemical properties and examples of these functional groups (methyl, ethyl in the alkyl family, alkenes and
BIOB111 - Tutorial activities for session 8 General topics for week 4 Session 8 Physical and chemical properties and examples of these functional groups (methyl, ethyl in the alkyl family, alkenes and
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Organic Chemistry, Second Edition. Janice Gorzynski Smith University of Hawai i. Chapter 10 Alkenes
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry Review: Topic 10 & Topic 20
 Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Chapter 11 - Alcohols and Ethers 1
 Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
CH320/328 M Spring 2014
 CH320/328 M Spring 2014 HW Set #3 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your
CH320/328 M Spring 2014 HW Set #3 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your
10. Alkyl Halides. What Is an Alkyl Halide. An organic compound containing at least one carbonhalogen
 10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
Some Arrow-Pushing Guidelines (Section 1.14) 1. Arrows follow electron movement.
 Chem 350 Jasperse Ch. 1 Notes 1 Note: The headers and associated chapters don t actually jive with the textbook we are using this summer. But otherwise this highlights a lot of the chemistry from Organic
Chem 350 Jasperse Ch. 1 Notes 1 Note: The headers and associated chapters don t actually jive with the textbook we are using this summer. But otherwise this highlights a lot of the chemistry from Organic
Mechanisms. . CCl2 F + Cl.
 Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Organic Chemistry. Why are these compounds called Organic. What is a Hydrocarbon? Questions: P167 Read
 Organic Chemistry The fact that carbon can form a wide variety of relatively stable long chain molecules results in this very important branch of Chemistry: Organics. Carbon forms strong covalent bonds
Organic Chemistry The fact that carbon can form a wide variety of relatively stable long chain molecules results in this very important branch of Chemistry: Organics. Carbon forms strong covalent bonds
CHEM 203. Midterm Exam 1 October 31, 2008 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
First Midterm Exam Monday, October 16, You may use model kits but no other material with chemical information without instructor approval.
 CH 334 Form A First Midterm Exam Monday, October 16, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Please do not use any electronic devices
CH 334 Form A First Midterm Exam Monday, October 16, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Please do not use any electronic devices
The carbon-carbon double bond is the distinguishing feature of alkenes.
 Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Chapter 5. Reactions of Alkenes and Alkynes
 Chapter 5. Reactions of Alkenes and Alkynes Learning objectives: 1. Identify the followings from a reaction coordinate diagram when applicable: endothermic or exothermic reactions, activation energy, heat
Chapter 5. Reactions of Alkenes and Alkynes Learning objectives: 1. Identify the followings from a reaction coordinate diagram when applicable: endothermic or exothermic reactions, activation energy, heat
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
dihalogenoalkane H 2, Nickel Catalyst KOH alcoholic HBr, HCl Br Cl Elimination KOH aqueous heat under reflux Nucleophilic substitution
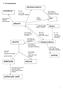 7 AS mechanisms dihalogenoalkane poly(alkene) Br 2, 2 KO aqueous room temp Electrophilic addition heat under reflux Nucleophilic substitution high pressure atalyst polymerization alkene KMnO 4 oxidation
7 AS mechanisms dihalogenoalkane poly(alkene) Br 2, 2 KO aqueous room temp Electrophilic addition heat under reflux Nucleophilic substitution high pressure atalyst polymerization alkene KMnO 4 oxidation
Organic Chemistry. Unit 10
 Organic Chemistry Unit 10 Halides Primary Carbons Secondary Carbons Tertiary Carbons IMPORTANCE?? REACTIONS!! Benzene C6H6 Aromatic functional group - C6H5 (IUPAC name - phenyl) Substitution Reactions
Organic Chemistry Unit 10 Halides Primary Carbons Secondary Carbons Tertiary Carbons IMPORTANCE?? REACTIONS!! Benzene C6H6 Aromatic functional group - C6H5 (IUPAC name - phenyl) Substitution Reactions
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
An Overview of Organic Reactions. Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants:
 An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane.
 Chemistry 51 DS Quiz 2 1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane. 2. When 2,3-dimethylbutane is monochlorinated
Chemistry 51 DS Quiz 2 1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane. 2. When 2,3-dimethylbutane is monochlorinated
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
 EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
Ethers can be symmetrical or not:
 Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22
 Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
https://en.wikipedia.org/wiki/sni
 KNOW MORE Weblinks https://en.wikipedia.org/wiki/allyl http://courses.chem.psu.edu/chem210/mol-gallery/allyl/allyl.html https://en.wikipedia.org/wiki/nucleophilic_substitution http://polymer.zju.edu.cn/attachments/2012-11/01-1352193505-
KNOW MORE Weblinks https://en.wikipedia.org/wiki/allyl http://courses.chem.psu.edu/chem210/mol-gallery/allyl/allyl.html https://en.wikipedia.org/wiki/nucleophilic_substitution http://polymer.zju.edu.cn/attachments/2012-11/01-1352193505-
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Organic Mechanisms 1
 Organic Mechanisms 1 Concepts The key ideas required to understand this section are: Concept Book page Chemical properties of alkanes 314 Chemical properties of alkenes 318 Bonding in alkenes 320 Bonding
Organic Mechanisms 1 Concepts The key ideas required to understand this section are: Concept Book page Chemical properties of alkanes 314 Chemical properties of alkenes 318 Bonding in alkenes 320 Bonding
Reaction of alkanes with bromine / chlorine in UV light. This is the overall reaction, but a more complex mixture of products is actually formed
 8. The aloalkanes opyright N Goalby Bancroft's School Synthesis of chloroalkanes Reaction of alkanes with bromine / chlorine in UV light In the presence of UV light alkanes react with chlorine to form
8. The aloalkanes opyright N Goalby Bancroft's School Synthesis of chloroalkanes Reaction of alkanes with bromine / chlorine in UV light In the presence of UV light alkanes react with chlorine to form
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Chapter 8 Alkenes and Alkynes II: Addition Reactions "
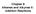 Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Organic Chemistry. for Students of Medicine and Biology 大学化学 III 和大学化学 III(2)
 Organic Chemistry for Students of Medicine and Biology 大学化学 III 和大学化学 III(2) March 4, 2015 Refining of petroleum, a major natural source of alkanes Chapter 4 Alkanes and Cycloalkanes ( 烷烃和环烷烃 ) March 3,
Organic Chemistry for Students of Medicine and Biology 大学化学 III 和大学化学 III(2) March 4, 2015 Refining of petroleum, a major natural source of alkanes Chapter 4 Alkanes and Cycloalkanes ( 烷烃和环烷烃 ) March 3,
1. Which of the following reactions would have the smallest energy of activation?.
 Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
Lab Activity 9: Introduction to Organic Chemical Reactivity, Lab 5 Prelab, Reflux
 Lab Activity 9: Introduction to Organic Chemical Reactivity, Lab 5 Prelab, Reflux Objectives 1. Identify structural features (pi bonds, bond polarity, lone pairs) of a compound 2. Determine whether a structural
Lab Activity 9: Introduction to Organic Chemical Reactivity, Lab 5 Prelab, Reflux Objectives 1. Identify structural features (pi bonds, bond polarity, lone pairs) of a compound 2. Determine whether a structural
