1: The Human Body: An Orientation
|
|
|
- Donna Cook
- 5 years ago
- Views:
Transcription
1 1: The Human Body: An Orientation I. An Overview of Anatomy and Physiology (pp. 2 3) A. Anatomy is the study of the structure of body parts and their relationships to each other, and physiology is the study of the function of body parts (p. 2). B. Topics of Anatomy (p. 2) 1. Gross (macroscopic) anatomy is the study of structures large enough to be seen with the naked eye. a. Regional anatomy is the study of all body structures in a given body region. b. Systemic anatomy is the study of all structures in a body system. c. Surface anatomy is the study of internal body structures as they relate to the overlying skin. 2. Microscopic anatomy is the study of structures that are too small to be seen with the naked eye. a. Cytology is the study of individual cells. b. Histology is the study of tissues. 3. Developmental anatomy is the study of the change in body structures over the course of a lifetime; embryology focuses on development that occurs before birth. (embryo, prior to 3 months development; fetus, from the 3 rd month until birth) II. Levels of Structural Organization (p. 3; Figs. 1.1, 1.3) A. The chemical level is the simplest level of organization (Fig. 1.1). 1. Atoms, tiny building blocks of matter, combine to form molecules. 2. Molecules combine in specific ways to form organelles, which are the basic unit of living cells. B. The cellular level is the smallest unit of life, and varies widely in size and shape according to the cells function. C. The tissue level is groups of cells having a common function. D. The organ level is made up of discrete structures that are composed of at least two groups of tissues that work together to perform a specific function in the body. E. The organ system level is a group of organs that work closely together to accomplish a specific purpose (Fig. 1.3). F. The organismal level is the total of all structures working together to promote life. III. Maintaining Life (pp. 4 8; Fig. 1.2) A. Necessary Life Functions (pp. 4 8; Fig. 1.2) 1. Maintaining boundaries allows an organism to maintain separate internal and external environments, or separate internal chemical environments. 2. Movement allows the organism to travel through the environment, and allows transport of molecules within the organism. 3. Responsiveness, or irritability, is the ability to detect changes in the internal or external environment and respond to them. 4. Digestion is the process of breaking down food into molecules that are usable by the body. 5. Metabolism includes all chemical reactions that occur in the body. 6. Excretion is the process of removing wastes. 7. Reproduction is the process of producing more cells or organisms. 8. Growth is an increase in size in body parts or the whole organism. B. Survival Needs (p. 8) 1. Nutrients are consumed chemical substances that are used for energy and cell building. 2. Oxygen is required by the chemical reactions that release energy from foods. 3. Water, the most abundant chemical substance in the body, provides an environment for chemical reactions and a fluid medium for secretions and excretions.
2 4. Normal body temperature is required for the chemical reactions of the body to occur at the proper rate. 5. Atmospheric pressure must be within an appropriate range so that proper gas exchange occurs in the lungs. IV. Homeostasis (pp. 8 11; Figs ) A. Homeostasis is the ability of the body to maintain a relatively constant internal environment, regardless of environmental changes (p. 8). B. Homeostatic Control Mechanisms (pp. 9 11; Figs ) 1. Components a. Variable: the regulated factor or event. b. Receptor: structure that monitors changes in the environment and sends information to the control center. c. Control center: structure that determines the set point for a variable, analyzes input, and coordinates an appropriate response. d. Effector: structure that carries out the response directed by the control center. 2. Negative Feedback Mechanisms a. Most homeostatic control mechanisms are negative feedback mechanisms. b. A negative feedback mechanism causes the variable to change in a way that opposes the initial change. c. Both the nervous system and the endocrine system are important to the maintenance of homeostasis. d. The goal of negative feedback mechanisms is to prevent sudden, severe changes in the body. 3. Positive Feedback Mechanisms a. A positive feedback mechanism causes the variable to change in the same direction as the original change, resulting in a greater deviation from the set point. b. Positive feedback mechanisms typically activate events that are self-perpetuating. c. Most positive feedback mechanisms are not related to the maintenance of homeostasis. 4. Homeostatic imbalance often results in disease. V. The Language of Anatomy (pp ; Figs ; Table 1.1) A. Anatomical Position and Directional Terms (pp ; Fig. 1.7; Table 1.1) 1. Anatomical position is a position in which the body is erect, palms face forward, and thumbs point away from the body. a. In anatomical position, right and left refer to the right and left sides of the person viewed. b. In anatomy, anatomical position is always assumed, regardless of the actual position of the body. 2. Directional terms are used to explain exactly where one body part is in relation to another. B. Regional Terms (p. 14; Fig. 1.7) 1. There are two fundamental divisions of the body. a. The axial region includes the head, neck, and trunk. b. The appendicular region consists of the upper and lower limbs. 2. Regional terms designate specific areas within the axial and appendicular divisions. C. Body Planes and Sections (p. 14; Fig. 1.8) 1. Body planes are flat surfaces that lie at right angles to each other. a. Sagittal plane: a vertical plane that separates the body into right and left parts. i. Median, or midsagittal plane: lies exactly along the body s midline. ii. Parasagittal plane: lies offset from the midline. b. Frontal plane: a vertical plane that separates the body into anterior and posterior parts. c. Transverse, or horizontal, plane: a plane that runs horizontally from right to left, and divides the body into superior and inferior parts.
3 D. Body Cavities and Membranes (pp ; Figs ) 1. Body cavities are spaces within the body that are closed to the outside and contain the internal organs. 2. The dorsal body cavity is the space that houses the central nervous system, and has two subdivisions: the cranial cavity and the vertebral cavity. a. The cranial cavity is within the skull, and houses the brain. b. The vertebral, or spinal, cavity is within the vertebral column, and houses the spinal cord. 3. The ventral body cavity is anterior to and larger than the dorsal cavity and has two main subdivisions: the thoracic cavity and the abdominopelvic cavity. a. The thoracic cavity is a superior division of the ventral cavity that is further subdivided into the lateral pleural cavities that surround the lungs. b. The thoracic cavity also contains the medial mediastinum, which includes the pericardial cavity surrounding the heart and the space surrounding the other thoracic structures. 4. The ventral body cavity houses the body organs, or viscera. 2: Chemistry Comes Alive PART 1: BASIC CHEMISTRY I. Definition of Concepts: Matter and Energy (pp ) A. Matter is anything that occupies space and has mass (p. 24). 1. Mass is equal to the amount of matter in the object. 2. Mass remains constant regardless of gravity. B. States of Matter (p. 24) 1. Matter exists in one of three states: solid, liquid, or gas. C. Energy (pp ) 1. Energy is the capacity to do work, and it exists in two forms. a. Kinetic energy is the energy of motion. b. Potential energy is stored energy. 2. Forms of Energy a. Chemical energy is energy stored in chemical bonds. b. Electrical energy results from the movement of charged particles. c. Mechanical energy is energy directly involved with moving matter. d. Radiant energy is energy that travels in waves. 3. Energy is easily converted from one form to another. II. Composition of Matter: Atoms and Elements (pp ; Figs ; Table 2.1) A. Basic Terms (p. 25; Table 2.1) 1. Elements are unique substances that cannot be broken down into simpler substances by ordinary chemical means. 2. Four important elements from the periodic table: carbon, hydrogen, oxygen, and nitrogen make up roughly 96% of body weight. 3. Atoms are the smallest particles of an element that retain the characteristics of that element. 4. Elements are designated by a one- or two-letter abbreviation called the atomic symbol. B. Atomic Structure (pp ; Figs ) 1. Each atom has a central nucleus with tightly packed protons and neutrons. a. Protons have a positive charge and weigh 1 atomic mass unit (amu). b. Neutrons do not have a charge and weigh 1amu.
4 2. Electrons are found moving around the nucleus, have a negative charge, and are weightless (0 amu). 3. Atoms are electrically neutral and the number of electrons is equal to the number of protons. 4. The planetary model is a simplified, two-dimensional model of atomic structure. 5. The orbital model is a more accurate three-dimensional model talking about orbital regions instead of set orbital patterns. C. Identifying Elements (pp ; Fig. 2.3) 1. Elements are identified based on their number of protons, neutrons, and electrons. 2. The atomic number of an element is equal to the number of protons of an element. a. Because the number of protons is equal to the number of electrons, the atomic number indirectly tells us the number of electrons. 3. The mass number of an element is equal to the number of protons plus the number of neutrons. a. The electron is weightless, and is ignored in calculating the mass number. 4. Isotopes are structural variations of an atom that have the same number of protons, but differ in the number of neutrons. 5. The atomic weight is an average of the relative weights of all known isotopes of an element, taking into account their relative abundance in nature. 6. Radioisotopes are heavier, unstable isotopes of an element that spontaneously decompose into more stable forms. a. The time for a radioisotope to lose one-half of its radioactivity is called the half-life. III. How Matter Is Combined: Molecules and Mixtures (pp ; Fig. 2.4) A. Molecules and Compounds (pp ) 1. A combination of two or more atoms is called a molecule. 2. If two or more atoms of the same element combine it is called a molecule of that element. 3. If two or more atoms of different elements combine it is called a molecule of a compound. B. Mixtures (pp ; Fig. 2.4) 1. Mixtures are substances made of two or more components mixed physically. 2. Solutions are homogeneous mixtures of compounds that may be gases, liquids, or solids. a. The substance present in the greatest amount is called the solvent. b. Substances present in smaller amounts are called solutes. c. Solutions may be described by their concentrations. These may be expressed as a percent or in terms of molarity. IV. Chemical Bonds (pp ; Figs ) A. A chemical bond is an energy relationship between the electrons of the reacting atoms (p. 31; Fig. 2.5). 1. The Role of Electrons in Chemical Bonding a. Electrons occupy regions of space called electron shells that surround the nucleus in layers. b. Each electron shell represents a different energy level. c. Each electron shell holds a specific number of electrons, and shells tend to fill consecutively from the closest to the nucleus to the furthest away. d. The octet rule, or rule of eights, states that except for the first energy shell (stable with two electrons), atoms are stable with eight electrons in their outermost (valence) shell. B. Types of Chemical Bonds (pp ; Figs ) 1. Ionic bonds are chemical bonds that form between two atoms that transfer one or more electrons from one atom to the other. a. Ions are charged particles. b. An anion is an electron acceptor carrying a net negative charge due to the extra electron.
5 c. A cation is an electron donor carrying a net positive charge due to the loss of an electron. d. Crystals are large structures of cations and anions held together by ionic bonds. 2. Covalent bonds form when electrons are shared between two atoms. a. Some atoms are capable of sharing two or three electrons between them, resulting in double covalent or triple covalent bonds. b. Nonpolar molecules share their electrons evenly between two atoms. c. In polar molecules, electrons spend more time around one atom thus providing that atom with a partial negative charge, while the other atom takes on a partial positive charge. d. A polar molecule is often referred to as a dipole due to the two poles of charges contained in the molecule. 3. Hydrogen bonds are weak attractions that form between partially charged atoms found in polar molecules. a. Surface tension is due to hydrogen bonds between water molecules. b. Intramolecular bonds may form between partially charged atoms in a large molecule, and are important in maintaining the shape of that molecule. V. Chemical Reactions (pp ; Fig. 2.11) A. Chemical Reactions (pp ) 1. Chemical reactions occur whenever bonds are formed, rearranged, or broken. 2. Chemical Equations a. A chemical equation describes what happens in a reaction. b. Chemical reactions denote the kinds and number of reacting substances, called reactants; the chemical composition of the products; and the relative proportion of each reactant and product, if balanced. B. Patterns of Chemical Reactions (pp ; Fig. 2.11) 1. In a synthesis (combination) reaction, larger molecules are formed from smaller molecules. 2. In a decomposition reaction a molecule is broken down into smaller molecules. 3. Exchange (displacement) reactions involve both synthesis and decomposition reactions. 4. Oxidation-reduction reactions are special exchange reactions in which electrons are exchanged between reactants. E. Factors Influencing the Rate of Chemical Reactions (pp ) 1. Chemicals react when they collide with enough force to overcome the repulsion by their electrons. 2. An increase in temperature increases the rate of a chemical reaction. 3. Smaller particle size results in a faster rate of reaction. 4. Higher concentration of reactants results in a faster rate of reaction. 5. Catalysts increase the rate of a chemical reaction without taking part in the reaction. PART 2: BIOCHEMISTRY VI. Inorganic Compounds (pp ; Figs ) A. Water (pp ) 1. Water is the most important inorganic molecule, and makes up 60 80% of the volume of most living cells. 2. Water has a high heat capacity, meaning that it absorbs and releases a great deal of heat before it changes temperature. 3. Water has a high heat of vaporization, meaning that it takes a great deal of energy (heat) to break the bonds between water molecules. 4. Water is a polar molecule and is called the universal solvent. 5. Water is an important reactant in many chemical reactions. 6. Water forms a protective cushion around organs of the body. B. Salts (p. 39; Fig. 2.12)
6 1. Salts are ionic compounds containing cations other than H + and anions other than the hydroxyl (OH ) ion; all ions are electrolytes or substances that conduct an electrical charge. 2. When salts are dissolved in water they dissociate into their component ions. C. Acids and Bases (pp ; Fig. 2.13) 1. Acids are also known as proton donors, and dissociate in water to yield hydrogen ions and anions. 2. Bases are also called proton acceptors, and absorb hydrogen ions. 3. The relative concentration of hydrogen ions is measured in concentration units called ph units. a. The greater the concentration of hydrogen ions in a solution, the more acidic the solution is. b. The greater the concentration of hydroxyl ions, the more basic, or alkaline, the solution is. c. The ph scale extends from A ph of 7 is neutral; a ph below 7 is acidic; a ph above 7 is basic or alkaline. VII. Organic Compounds (pp ; Figs ; Tables ) A. Carbohydrates, lipids, proteins, and nucleic acids are molecules unique to living systems, and all contain carbon, making them organic compounds (p. 42). B. Carbohydrates (p. 43; Figs ) 1. Carbohydrates are a group of molecules including sugars and starches. 2. Carbohydrates contain carbon, hydrogen, and oxygen. 3. The major function of carbohydrates in the body is to provide cellular fuel. 4. Monosaccharides are simple sugars that are single-chain or single-ring structures. 5. Disaccharides are formed when two monosaccharides are joined by a dehydration synthesis. 6. Polysaccharides are long chains of monosaccharides linked together by dehydration synthesis. C. Lipids (pp ; Fig. 2.16; Table 2.2) 1. Lipids are insoluble in water but dissolve readily in nonpolar solvents. 2. Triglycerides (neutral fats) are commonly known as fats when solid and oils when liquid. 3. Phospholipids are diglycerides with a phosphorus-containing group and two fatty acid chains. 4. Steroids are flat molecules made up of four interlocking hydrocarbon rings. 5. Eicosanoids are a group of diverse lipids derived from arachidonic acid. D. Proteins (pp ; Figs ; Table 2.3) 1. Proteins compose 10 30% of cell mass. a. They are the basic structural material of the body. b. They also play vital roles in cell function. 2. Proteins are long chains of amino acids connected by peptide bonds. E. Nucleic Acids (DNA and RNA) (pp ; Fig. 2.22; Table 2.4) 1. Nucleic acids composed of carbon, oxygen, hydrogen, nitrogen, and phosphorus are the largest molecules in the body. 2. Nucleotides are the structural units of nucleic acids. 3. Each nucleotide consists of three components: a pentose sugar, a phosphate group, and a nitrogen-containing base. 4. There are five nitrogenous bases used in nucleic acids: Adenine (A), Guanine (G), Cytosine (C), Uracil (U), and Thymine (T). 5. DNA, or Deoxyribonucleic Acid a. DNA is the genetic material of the cell, and is found within the nucleus. b. DNA replicates itself before cell division and provides instructions for making all of the proteins found in the body. c. The structure of DNA is a double-stranded polymer containing the nitrogenous bases A, T, G, and C, and the sugar deoxyribose.
7 d. Bonding of the nitrogenous bases in DNA is very specific; A bonds to T, and G bonds to C. e. The bases that always bind together are known as complementary bases. 6. RNA, or Ribonucleic Acid a. RNA is located outside the nucleus, and is used to make proteins using the instructions provided by the DNA. b. The structure of RNA is a single-stranded polymer containing the nitrogenous bases A, G, C, and U, and the sugar ribose. c. In RNA, G bonds with C, and A bonds with U. F. ATP, or Adenosine Triphosphate (pp ; Figs ) 1. ATP is the energy currency used by the cell. 2. ATP is an adenine-containing RNA nucleotide that has two additional phosphate groups attached. 3. The additional phosphate groups are connected by high-energy bonds. 4. Breaking the high-energy bonds releases energy the cell can use to do work. CHAPTER 3: CELLS- THE LIVING UNITS I. Overview of the Cellular Basis of Life (pp ; Figs ) B. Characteristics of Cells (p. 62; Figs ) 1. Cells vary greatly in their size, shape, and function. 2. All cells are composed primarily of carbon, hydrogen, nitrogen, and oxygen. 3. All cells have the same basic parts and some common functions. 4. A generalized human cell contains the plasma membrane, the cytoplasm, and the nucleus. II. The Plasma Membrane: Structure (pp ; Figs ) A. The Fluid Mosaic Model (pp ; Figs ) 1. The plasma membrane is composed of a double layer of phospholipids in which small amounts of cholesterol and proteins are embedded. 2. The phospolipid bilayer is composed of two layers of phospholipids lying tail to tail, with their polar heads exposed to water inside and outside the cell. III. The Plasma Membrane: Membrane Transport (pp ; Figs ; Tables ) A. Passive processes do not use energy (ATP) to move substances down their concentration gradient (pp ; Figs ; Table 3.1). 1. Diffusion is the movement of molecules down their concentration gradient. The rate of diffusion is influenced by the size of the molecule and the temperature. 2. Simple diffusion is diffusion through the plasma membrane. 3. In facilitated diffusion substances are moved through the plasma membrane by binding to protein carriers in the membrane or by moving through channels. 4. Osmosis is the diffusion of water through a selectively permeable membrane. B. Active transport processes use energy (ATP) to move substances across a membrane (pp ; Figs ; Table 3.2). 1. Active transport uses solute pumps to move substances against a concentration gradient. The two kinds of active transport are primary active transport and secondary active transport. 2. Vesicular transport is the means by which large particles, macromolecules, and fluids are transported across the plasma membrane, or within the cell. 3. Exocytosis is a process used to move substances from inside the cell to the extracellular environment.
8 4. Endocytosis, transcytosis, and vesicular trafficking are vesicular transport processes that move molecules using protein-coated vesicles. VI. The Cytoplasm (pp ; Figs ; Table 3.3) A. The cytoplasm is the cellular material between the cell membrane and the nucleus, and is the site of most cellular activity (pp ). 1. There are three major elements of the cytoplasm: cytosol, cytoplasmic organelles, and cytoplasmic inclusions. B. Cytoplasmic Organelles (pp ; Figs ; Table 3.3) 1. Mitochondria are sausage-shaped membranous organelles that are the power plants of the cell, producing most of its ATP. 2. Ribosomes are small staining granules consisting of protein and ribosomal RNA that are the site of protein synthesis. 3. The endoplasmic reticulum is an extensive system of tubes and membranes enclosing fluid-filled cavities, called cisternae, that extend throughout the cytosol. a. The rough endoplasmic reticulum has ribosomes that manufacture all proteins that are secreted from cells. b. Smooth ER is a continuation of rough ER, consisting of a looping network of tubules. Its enzymes catalyze reactions involved in several processes. 4. The Golgi apparatus is a series of stacked, flattened, membranous sacs associated with groups of membranous vesicles. a. The main function of the Golgi apparatus is to modify, concentrate, and package the proteins and lipids made at the rough ER. b. The Golgi apparatus creates vesicles containing lipids and transmembrane proteins for incorporation into the cell membrane. c. The Golgi apparatus packages digestive enzymes into lysosomes. 5. Lysosomes are spherical membranous organelles that contain digestive enzymes. a. Lysosomes function best in acidic environments, can digest almost any kind of biological molecule, and are abundant in phagocytes. b. The membrane of the lysosome functions to allow products of digestion to be released to the cytosol, yet contain the acid hydrolases used to digest molecules. 6. The endomembrane system includes the ER, Golgi apparatus, secretory vesicles, lysosomes, and nuclear membrane. a. The endomembrane system functions together to produce, store, and export biological molecules, as well as degrade potentially harmful substances. 7. Peroxisomes are membranous sacs containing enzymes, such as oxidases and catalases, which are used to detoxify harmful substances such as alcohol, formaldehyde, and free radicals. 8. The cytoskeleton is a series of rods running through the cytosol, supporting cellular structures and aiding in cell movement. a. There are three types of rods in the cytoskeleton: microtubules, microfilaments, and intermediate filaments. VII. The Nucleus (pp ; Figs ) A. Basic Characteristics (p. 91; Fig. 3.29) 1. The nucleus is the control center of the cell and contains the cellular DNA. 2. Most cells have only one nucleus, but very large cells may be multinucleate. 3. All body cells except mature red blood cells have nuclei. 4. The nucleus is larger than the cytoplasmic organelles; it has three regions and protein-containing subcompartments.
9 VIII. Cell Growth and Reproduction (pp ; Figs ) A. The Cell Life Cycle (pp ; Figs ) 1. The cell life cycle is a series of changes a cell goes through from the time it is formed to the time it reproduces. 2. Interphase and cell division are the two main periods of the cell cycle. 3. Interphase is the period from cell formation to cell division, and has three subphases. a. During the G 1, or gap 1, subphase the cell is synthesizing proteins and actively growing. b. During the S phase, DNA is replicated. c. During the G 2, or gap 2, subphase enzymes and other proteins are synthesized and distributed throughout the cell. d. DNA replication takes place when the DNA helix uncoils, and the hydrogen bonds between its base pairs are broken. Then each nucleotide strand of the DNA acts as a template for the construction of a complementary nucleotide strand. 4. Cell division is a process necessary for growth and tissue repair. There are three main events of cell division. a. Mitosis is the process of nuclear division in which cells contain all genes. b. Meiosis is the process of nuclear division found only in egg and sperm cells in which the cells have half the genes found in other body cells. c. Cytokinesis is the process of dividing the cytoplasm. d. Control of cell division depends on surface-volume relationships, chemical signaling, and contact inhibition. B. Protein Synthesis (pp ; Figs ) 1. DNA specifies the structure of protein molecules that act as structural or functional molecules. 2. Proteins are composed of polypeptide chains made up of amino acids. 3. Each gene is a segment of DNA that carries instructions for one polypetide chain, as well as exons that specify amino acid informational sequences and noncoding sequences called introns. 4. Each sequence of three nucleotide bases of DNA is called a triplet, and specifies a particular amino acid. 5: The Integumentary System I. The Skin (pp ; Figs ) A. The hypodermis, also called the superficial fascia, is subcutaneous tissue beneath the skin consisting mostly of adipose tissue that anchors the skin to underlying muscle, allows skin to slide over muscle, and acts as a shock absorber and insulator (p. 149; Fig. 5.1). B. Epidermis (pp ; Fig. 5.2) 1. The epidermis is a keratinized stratified squamous epithelium. 2. Cells of the Epidermis a. The majority of epidermal cells are keratinocytes that produce a fibrous protective protein called keratin. b. Melanocytes are epithelial cells that synthesize the pigment melanin. c. Epidermal dendritic cells or Langerhans cells, are macrophages that help activate the immune system. d. Tactile cells are associated with sensory nerve endings. 3. Layers of the Epidermis a. The stratum basale (basal layer) is the deepest epidermal layer and is the site of mitosis. b. The stratum spinosum (prickly layer) is several cell layers thick and contains keratinocytes, melanin granules, and the highest concentration of epidermal dendritic cells. c. The stratum granulosum (granular layer) contains keratinocytes that are undergoing a great deal of physical changes, turning them into the tough outer cells of the epidermis. d. The stratum lucidum (clear layer) is found only in thick skin and is composed of dead keratinocytes.
10 e. The stratum corneum (horny layer) is the outermost protective layer of the epidermis composed of a thick layer of dead keratinocytes. C. Dermis (pp ; Figs ) 1. The dermis is composed of strong, flexible connective tissue. 2. The dermis is made up of two layers: the thin, superficial papillary layer is highly vascularized areolar connective tissue containing a woven mat of collagen and elastin fibers; and the reticular layer, accounting for 80% of the thickness of the dermis, is dense irregular connective tissue. D. Skin color is determined by three pigments: melanin, hemoglobin, and carotene (pp ). II. Appendages of the Skin (pp ; Figs ) A. Sweat (Sudoriferous) Glands (pp ; Fig. 5.5) 1. Eccrine sweat glands, or merocrine sweat glands, produce true sweat, are the most numerous of the sweat glands, and are particularly abundant on the palms of the hands, soles of the feet, and forehead. 2. Apocrine sweat glands are confined to the axillary and anogenital areas and produce true sweat with the addition of fatty substances and proteins. 3. Ceruminous glands are modified sweat glands found lining the ear canal that secrete earwax, or cerumen. 4. Mammary glands are modified sweat glands found in the breasts that secrete milk. B. Sebaceous (Oil) Glands (pp ; Fig. 5.5) 1. Sebaceous glands are simple alveolar glands found all over the body except the palms of the hands and soles of the feet that secrete sebum, an oily secretion. 2. The sebaceous glands function as holocrine glands, secreting their product into a hair follicle or to a pore on the surface of the skin. 3. Secretion by sebaceous glands is stimulated by hormones. C. Hairs and Hair Follicles (pp ; Fig. 5.6) 1. Hairs, or pili, are flexible strands produced by hair follicles that consist of dead, keratinized cells. a. The main regions of a hair are the shaft and the root. b. A hair has three layers of keratinized cells: the inner core is the medulla, the middle layer is the cortex, and the outer layer is the cuticle. c. Hair pigments (melanin of different colors) are made by melanocytes at the base of the hair follicle. 2. Structure of a Hair Follicle a. Hair follicles fold down from the epidermis into the dermis and occasionally into the hypodermis. b. The deep end of a hair follicle is expanded, forming a hair bulb, which is surrounded by a knot of sensory nerve endings called a hair follicle receptor, or root hair plexus. c. The wall of a hair follicle is composed of an outer connective tissue root sheath, a thickened basement membrane called a glossy membrane, and an inner epithelial root sheath. d. Associated with each hair follicle is a bundle of smooth muscle cells called an arrector pili muscle. 3. Types and Growth of Hair a. Hairs come in various sizes and shapes, but can be classified as vellus or terminal. b. Hair growth and density are influenced by many factors, such as nutrition and hormones. c. The rate of hair growth varies from one body region to another and with sex and age. D. Nails (p. 160; Fig. 5.7) 1. A nail is a scalelike modification of the epidermis that forms a clear, protective covering. 2. Nails are made up of hard keratin and have a free edge, a body, and a proximal root. III. Functions of the Integumentary System (pp ) A. Protection (pp ) 1. Chemical barriers include skin secretions and melanin.
11 2. Physical or mechanical barriers are provided by the continuity of the skin, and the hardness of the keratinized cells. 3. Biological barriers include the epidermal dendritic cells, the macrophages of the dermis, and the DNA itself. B. The skin plays an important role in body temperature regulation by using the sweat glands of the skin to cool the body, and constriction of dermal capillaries to prevent heat loss (p. 161). IV. Homeostatic Imbalances of Skin (pp ; Figs ) A. Skin Cancer (pp ; Fig. 5.8) 1. Basal cell carcinoma is the least malignant and the most common skin cancer. 2. Squamous cell carcinoma tends to grow rapidly and metastasize if not removed. 3. Melanoma is the most dangerous of the skin cancers because it is highly metastatic and resistant to chemotherapy. B. Burns (pp ; Figs ) 1. A burn is tissue damage inflicted by intense heat, electricity, radiation, or certain chemicals, all of which denature cell proteins and cause cell death to infected areas. 2. The most immediate threat to a burn patient is dehydration and electrolyte imbalance due to fluid loss. 3. After the first 24 hours has passed, the threat to a burn patient becomes infection to the wound site. 4. Burns are classified according to their severity. a. First-degree burns involve damage only to the epidermis. b. Second-degree burns injure the epidermis and the upper region of the dermis. c. Third-degree burns involve the entire thickness of the skin. 6: Bones and Skeletal Tissues I. Skeletal Cartilages (p. 173; Fig. 6.1) A. Basic Structure, Types, and Locations (p. 173; Fig. 6.1) 1. Skeletal cartilages are made from cartilage, surrounded by a layer of dense irregular connective tissue called the perichondrium. 2. Hyaline cartilage is the most abundant skeletal cartilage, and includes the articular, costal, respiratory, and nasal cartilages. 3. Elastic cartilages are more flexible than hyaline, and are located only in the external ear and the epiglottis of the larynx. 4. Fibrocartilage is located in areas that must withstand a great deal of pressure or stretch, such as the cartilages of the knee and the intervertebral discs. II. Classification of Bones (pp ; Figs ) A. There are two main divisions of the bones of the skeleton: the axial skeleton, consisting of the skull, vertebral column, and rib cage; and the appendicular skeleton, consisting of the bones of the upper and lower limbs, and the girdles that attach them to the axial skeleton (pp ; Fig. 6.1). B. Shape (pp ; Fig. 6.2) 1. Long bones are longer than they are wide, have a definite shaft and two ends, and consist of all limb bones except patellas, carpals, and tarsals. 2. Short bones are somewhat cube shaped and include the carpals and tarsals. 3. Flat bones are thin, flattened, often curved bones that include most skull bones, the sternum, scapulae, and ribs. 4. Irregular bones have complicated shapes that do not fit in any other class, such as the vertebrae and coxae.
12 III. Functions of Bones (pp ) A. Bones support the body and cradle the soft organs, protect vital organs, allow movement, store minerals such as calcium and phosphate, and house hematopoietic tissue in specific marrow cavities (pp ). IV. Bone Structure (pp ; Figs ; Table 6.1) A. Gross Anatomy (pp ; Figs. 6.3, 6.5; Table 6.1) 1. Bone markings are projections, depressions, and openings found on the surface of bones that function as sites of muscle, ligament, and tendon attachment, as joint surfaces, and as openings for the passage of blood vessels and nerves. 2. Bone Textures: Compact and Spongy Bone a. All bone has a dense outer layer consisting of compact bone that appears smooth and solid. b. Internal to compact bone is spongy bone, which consists of honeycomb, needle-like, or flat pieces, called trabeculae. 3. Structure of a Typical Long Bone a. Long bones have a tubular bone shaft, consisting of a bone collar surrounding a hollow medullary cavity, which is filled with yellow bone marrow in adults. b. Epiphyses are at the ends of the bone, and consist of internal spongy bone covered by an outer layer of compact bone. c. The epiphyseal line is located between the epiphyses and diaphysis, and is a remnant of the epiphyseal plate. d. The external surface of the bone is covered by the periosteum. e. The internal surface of the bone is lined by a connective tissue membrane called the endosteum. 4. Structure of Short, Flat, and Irregular Bones a. Short, flat, and irregular bones consist of thin plates of periosteum-covered compact bone on the outside, and endosteum-covered spongy bone inside, which houses bone marrow between the trabeculae. 5. Location of Hematopoietic Tissue in Bones a. Hematopoietic tissue of bones, red bone marrow, is located within the trabecular cavities of the spongy bone in flat bones, and in the epiphyses of long bones. b. Red bone marrow is found in all flat bones, epiphyses, and medullary cavities of infants, but in adults, distribution is restricted to flat bones and the proximal epiphyses of the humerus and femur. B. Microscopic Anatomy of Bone (pp ; Figs ) 1. The structural unit of compact bone is the osteon, or Haversian system, which consists of concentric tubes of bone matrix (the lamellae) surrounding a central Haversian canal that serves as a passageway for blood vessels and nerves. 2. Spongy bone lacks osteons but has trabeculae that align along lines of stress, which contain irregular lamellae. C. Chemical Composition of Bone (p. 180) 1. Organic components of bone include cells (osteoblasts, osteocytes, and osteoclasts) and osteoid (ground substance and collagen fibers), which contribute to the flexibility and tensile strength of bone. 2. Inorganic components make up 65% of bone by mass, and consist of hydroxyapatite, a mineral salt that is largely calcium phosphate, which accounts for the hardness and compression resistance of bone. VI. Bone Homeostasis: Remodeling and Repair (pp ; Figs ; Table 6.2) A. Bone Remodeling (pp ; Figs ) 1. In adult skeletons, bone remodeling is balanced bone deposit and removal; bone deposit occurs at a greater rate when bone is injured; and bone resorption allows minerals of degraded bone matrix to move into the blood. B. Bone Repair (pp ; Fig. 6.15; Table 6.2)
13 1. Fractures are breaks in bones, and are classified by: the position of the bone ends after fracture, completeness of break, orientation of the break relative to the long axis of the bone, and whether the bone ends penetrate the skin. 2. Repair of fractures involves four major stages: hematoma formation, fibrocartilaginous callus formation, bony callus formation, and remodeling of the bony callus. VII. Homeostatic Imbalances of Bone (pp , 194; Fig. 6.16) 2. Rickets is inadequate mineralization of bones in children caused by insufficient calcium or vitamin D deficiency. B. Osteoporosis refers to a group of disorders in which the rate of bone resorption exceeds the rate of formation (pp , Fig. 6.16). 1. Bones have normal bone matrix, but bone mass is reduced and the bones become more porous and lighter, increasing the likelihood of fractures. 2. Older women are especially vulnerable to osteoporosis, due to the decline in estrogen after menopause. 15: The Special Senses I. The Eye and Vision (pp ; Figs ) A. Vision is our dominant sense; 70% of our body s sensory receptors are found in the eye (p. 548). B. Accessory Structures of the Eye (pp ; Figs ) 1. Eyebrows are short, coarse hairs overlying the supraorbital margins of the eye that shade the eyes and keep perspiration out. 2. Eyelids (palpebrae), eyelashes, and their associated glands help to protect the eye from physical danger as well as from drying out. 3. Conjunctiva is a transparent mucous membrane that lines the eyelids and the whites of the eyes. It produces a lubricating mucus that prevents the eye from drying out. 4. The lacrimal apparatus consists of the lacrimal gland, which secretes a dilute saline solution that cleanses and protects the eye as it moistens it, and ducts that drain excess fluid into the nasolacrimal duct. 5. The movement of each eyeball is controlled by six extrinsic eye muscles that are innervated by the abducens and trochlear nerves. C. Structure of the Eyeball (pp ; Figs ) 1. Three layers form the wall of the eyeball. a. The fibrous tunic is the outermost coat of the eye and is made of a dense avascular connective tissue with two regions: the sclera and the cornea. b. The vascular tunic (uvea) is the middle layer and has three regions: the choroid, the ciliary body, and the iris. c. The inner layer (retina) is the innermost layer made up of two layers: the outer pigmented layer absorbs light; the inner neural layer contains millions of photoreceptors (rods and cones) that transduce light energy. 2. Internal Chambers and Fluids a. The posterior segment (cavity) is filled with a clear gel called vitreous humor that transmits light, supports the posterior surface of the lens, holds the retina firmly against the pigmented layer, and contributes to intraocular pressure. b. The anterior segment (cavity) is filled with aqueous humor that supplies nutrients and oxygen to the lens and cornea while carrying away wastes. 3. The lens is an avascular, biconcave, transparent, flexible structure that can change shape to allow precise focusing of light on the retina. D. Physiology of Vision (pp ; Figs ) 1. Overview: Light and Optics
14 a. Electromagnetic radiation includes all energy waves from long waves to short waves, and includes the visible light that our eyes see as color. b. Refraction of a light ray occurs when it meets the surface of a different medium at an oblique angle rather than a right angle. 2. Focusing of Light on the Retina a. Light is bent three times: as it enters the cornea and on entering and leaving the lens. b. The far point of vision is that distance beyond which no change in lens shape is required (about 6 meters or 20 feet). c. Focusing for close vision demands that the eye make three adjustments: accommodation of the lens, constriction of the pupils, and convergence of the eyeballs. d. Myopia, or nearsightedness, occurs when objects focus in front of the retina and results in seeing close objects without a problem but distant objects are blurred. e. Hyperopia, or farsightedness, occurs when objects are focused behind the retina and results in seeing distant objects clearly but close objects are blurred. 3. Photoreception is the process by which the eye detects light energy. a. Photoreceptors are modified neurons that structurally resemble tall epithelial cells. b. Rods are highly sensitive and are best suited to night vision. Cones are less sensitive to light and are best adapted to bright light and color vision. c. Photoreceptors contain a light-absorbing molecule called retinal.
Unit 2: Basic Chemistry
 Unit 2: Basic Chemistry I. Matter and Energy A. Matter anything that occupies space and has mass (weight) B. Energy the ability to do work 1. Chemical 2. Electrical 3. Mechanical 4. Radiant C. Composition
Unit 2: Basic Chemistry I. Matter and Energy A. Matter anything that occupies space and has mass (weight) B. Energy the ability to do work 1. Chemical 2. Electrical 3. Mechanical 4. Radiant C. Composition
UNIT 2 CHEMISTRY. Atomic Structure: Ionic Bond: Covalent Bond: Hydrogen Bond:
 UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
UNIT 2 CHEMISTRY. Atomic Structure: Ionic Bond: Covalent Bond: Hydrogen Bond:
 UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
NATIONAL REVIEW COURSE. Cells, Tissues, and Membranes
 NATIONAL REVIEW COURSE Cells, Tissues, and Membranes I. Cell Types A. Prokaryote bacteria cells; a cell that does not have a nucleus in which to store its genetic material. B. Eukaryote plant or animal
NATIONAL REVIEW COURSE Cells, Tissues, and Membranes I. Cell Types A. Prokaryote bacteria cells; a cell that does not have a nucleus in which to store its genetic material. B. Eukaryote plant or animal
Chapter 2: Chemical Basis of Life
 Chapter 2: Chemical Basis of Life Chemistry is the scientific study of the composition of matter and how composition changes. In order to understand human physiological processes, it is important to understand
Chapter 2: Chemical Basis of Life Chemistry is the scientific study of the composition of matter and how composition changes. In order to understand human physiological processes, it is important to understand
Ch. 2 BASIC CHEMISTRY. Copyright 2010 Pearson Education, Inc.
 Ch. 2 BASIC CHEMISTRY Matter and Composition of Matter Definition: Anything that has mass and occupies space Matter is made up of elements An element cannot be broken down by ordinary chemical means Atoms
Ch. 2 BASIC CHEMISTRY Matter and Composition of Matter Definition: Anything that has mass and occupies space Matter is made up of elements An element cannot be broken down by ordinary chemical means Atoms
BIO 115 SP 2012 Homework 1: Introduction to A&P and Chemistry Please note that this is not a required assignment but it is recommended.
 BIO 115 SP 2012 Homework 1: Introduction to A&P and Chemistry Please note that this is not a required assignment but it is recommended. 1. When a body is in the anatomical position, it is A. Standing erect,
BIO 115 SP 2012 Homework 1: Introduction to A&P and Chemistry Please note that this is not a required assignment but it is recommended. 1. When a body is in the anatomical position, it is A. Standing erect,
1. In regard to the characteristics of connective tissue, which of the following is TRUE?
 Anatomy and Physiology Fall 2017: Exam I Form A Name: 1. In regard to the characteristics of connective tissue, which of the following is TRUE? a. Once formed the ground substance found in areolar CT is
Anatomy and Physiology Fall 2017: Exam I Form A Name: 1. In regard to the characteristics of connective tissue, which of the following is TRUE? a. Once formed the ground substance found in areolar CT is
A Brief Overview of Biochemistry. And I mean BRIEF!
 A Brief Overview of Biochemistry And I mean BRIEF! Introduction A. Chemistry deals with the composition of substances and how they change. B. A knowledge of chemistry is necessary for the understanding
A Brief Overview of Biochemistry And I mean BRIEF! Introduction A. Chemistry deals with the composition of substances and how they change. B. A knowledge of chemistry is necessary for the understanding
Basic Chemistry. Chemistry Review. Bio 250: Anatomy & Physiology
 Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Module A BODY PLAN & ORGANIZATION
 Module A BODY PLAN & ORGANIZATION Topic from Anatomical position Body planes & sections Body cavities & regions Directional terms Basic terminology Levels of organization Survey of body systems 1. Describe
Module A BODY PLAN & ORGANIZATION Topic from Anatomical position Body planes & sections Body cavities & regions Directional terms Basic terminology Levels of organization Survey of body systems 1. Describe
Chemistry Basics. Matter anything that occupies space and has mass Energy the ability to do work. Chemical Electrical Mechanical Radiant. Slide 2.
 Chemistry Basics Matter anything that occupies space and has mass Energy the ability to do work Chemical Electrical Mechanical Radiant Slide 2.1 Composition of Matter Elements Fundamental units of matter
Chemistry Basics Matter anything that occupies space and has mass Energy the ability to do work Chemical Electrical Mechanical Radiant Slide 2.1 Composition of Matter Elements Fundamental units of matter
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
 Exam Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Figure 2.1 Using Figure 2.1, match the following: 1) Lipid. 2) Functional protein. 3) Nucleotide.
Exam Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Figure 2.1 Using Figure 2.1, match the following: 1) Lipid. 2) Functional protein. 3) Nucleotide.
Figure ) Letter E represents a nucleic acid building block known as a. Answer: nucleotide Diff: 3 Page Ref: 54
 Essentials of Human Anatomy and Physiology, 10e (Marieb) Chapter 2 Basic Chemistry 2.1 Short Answer Figure 2.1 Using Figure 2.1, identify the following: 1) Which letter represents a carbohydrate polymer?
Essentials of Human Anatomy and Physiology, 10e (Marieb) Chapter 2 Basic Chemistry 2.1 Short Answer Figure 2.1 Using Figure 2.1, identify the following: 1) Which letter represents a carbohydrate polymer?
Chapter 2. Introduction: Chapter Chemical Basis of Life. Structure of Matter:
 Chapter 2.1-2.2 Read text 2.1 and describe why chemistry is important in understanding life. Read text 2.2 and discuss how atomic structure determines how atoms interact. Also describe the types of chemical
Chapter 2.1-2.2 Read text 2.1 and describe why chemistry is important in understanding life. Read text 2.2 and discuss how atomic structure determines how atoms interact. Also describe the types of chemical
Hole s Human Anatomy and Physiology Eleventh Edition. Chapter 2
 Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
BIOCHEMISTRY GUIDED NOTES - AP BIOLOGY-
 BIOCHEMISTRY GUIDED NOTES - AP BIOLOGY- ELEMENTS AND COMPOUNDS - anything that has mass and takes up space. - cannot be broken down to other substances. - substance containing two or more different elements
BIOCHEMISTRY GUIDED NOTES - AP BIOLOGY- ELEMENTS AND COMPOUNDS - anything that has mass and takes up space. - cannot be broken down to other substances. - substance containing two or more different elements
1. Know the hierarchy of organization: chemical, cellular, tissue, organ, organ system, organismal
 Anatomy and Physiology Anatomical Organization 1. Know the hierarchy of organization: chemical, cellular, tissue, organ, organ system, organismal 2. Be able to identify and describe the necessary life
Anatomy and Physiology Anatomical Organization 1. Know the hierarchy of organization: chemical, cellular, tissue, organ, organ system, organismal 2. Be able to identify and describe the necessary life
2.1 Basic Chemistry 1
 2.1 Basic Chemistry 1 A. Introduction 1. Matter anything that takes up space and has mass 2. States of matter a. Solid b. Liquid c. Gas 2 B. Elements and Atoms 1. Elements basic substances that make up
2.1 Basic Chemistry 1 A. Introduction 1. Matter anything that takes up space and has mass 2. States of matter a. Solid b. Liquid c. Gas 2 B. Elements and Atoms 1. Elements basic substances that make up
Chapter 2 Chemical Aspects of Life
 Chapter 2 Chemical Aspects of Life Multiple Choice Questions 1. Anything that has weight and occupies space can be described as A. an atom. B. matter. C. a compound. D. a molecule. #1 Learning Outcome:
Chapter 2 Chemical Aspects of Life Multiple Choice Questions 1. Anything that has weight and occupies space can be described as A. an atom. B. matter. C. a compound. D. a molecule. #1 Learning Outcome:
The Chemistry of Microbiology
 PowerPoint Lecture Presentations prepared by Mindy Miller-Kittrell, North Carolina State University C H A P T E R 2 The Chemistry of Microbiology Atoms Matter anything that takes up space and has mass
PowerPoint Lecture Presentations prepared by Mindy Miller-Kittrell, North Carolina State University C H A P T E R 2 The Chemistry of Microbiology Atoms Matter anything that takes up space and has mass
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
 ch 2 chemical basis of life Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Fill in the blank or provide a short answer: 1) When a change in matter
ch 2 chemical basis of life Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Fill in the blank or provide a short answer: 1) When a change in matter
Chemical Basis of Life
 Chemical Basis of Life Jan 30 11:42 AM In order to understand digestion and nutrition, we need some basic biochemistry Chemistry studies the composition of matter and its changes as well as the change
Chemical Basis of Life Jan 30 11:42 AM In order to understand digestion and nutrition, we need some basic biochemistry Chemistry studies the composition of matter and its changes as well as the change
The Human Body: An Orientation
 The Human Body: An Orientation Prepared by Dr. Naim Kittana Dr. Suhaib Hattab Faculty of Medicine & Health Sciences An-Najah National University 1 Declaration The content and the figures of this seminar
The Human Body: An Orientation Prepared by Dr. Naim Kittana Dr. Suhaib Hattab Faculty of Medicine & Health Sciences An-Najah National University 1 Declaration The content and the figures of this seminar
Basic Chemistry. Chapter 2 BIOL1000 Dr. Mohamad H. Termos
 Basic Chemistry Chapter 2 BIOL1000 Dr. Mohamad H. Termos Chapter 2 Objectives Following this chapter, you should be able to describe: - Atoms, molecules, and ions - Composition and properties - Types of
Basic Chemistry Chapter 2 BIOL1000 Dr. Mohamad H. Termos Chapter 2 Objectives Following this chapter, you should be able to describe: - Atoms, molecules, and ions - Composition and properties - Types of
Chapter 1. The Human Organism 1-1
 Chapter 1 The Human Organism 1-1 Overview of Anatomy and Physiology Anatomy: Scientific discipline that investigates the body s structure Physiology: Scientific investigation of the processes or functions
Chapter 1 The Human Organism 1-1 Overview of Anatomy and Physiology Anatomy: Scientific discipline that investigates the body s structure Physiology: Scientific investigation of the processes or functions
Essentials of Human Anatomy and Physiology, 12e (Marieb) Chapter 2 Basic Chemistry. 2.1 Multiple Choice Part I Questions
 Essentials of Human Anatomy and Physiology 12th Edition Marieb TEST BANK Full download at: https://testbankrealcom/download/essentialshuman-anatomy-physiology-12th-edition-mariebtest-bank/ Essentials of
Essentials of Human Anatomy and Physiology 12th Edition Marieb TEST BANK Full download at: https://testbankrealcom/download/essentialshuman-anatomy-physiology-12th-edition-mariebtest-bank/ Essentials of
Chapter 2: Chemistry. What does chemistry have to do with biology? Vocabulary BIO 105
 Chapter 2: Chemistry What does chemistry have to do with biology? BIO 105 Vocabulary 1. Matter anything that takes up space and has mass Atoms are the smallest units of matter that can participate in chemical
Chapter 2: Chemistry What does chemistry have to do with biology? BIO 105 Vocabulary 1. Matter anything that takes up space and has mass Atoms are the smallest units of matter that can participate in chemical
Essentials of Human Anatomy and Physiology, 11e (Marieb) Chapter 2 Basic Chemistry. 2.1 Multiple Choice Part I Questions
 Essentials of Human Anatomy and Physiology, 11e (Marieb) Chapter 2 Basic Chemistry 2.1 Multiple Choice Part I Questions Using Figure 2.1, identify the following: 1) Which letter represents a carbohydrate
Essentials of Human Anatomy and Physiology, 11e (Marieb) Chapter 2 Basic Chemistry 2.1 Multiple Choice Part I Questions Using Figure 2.1, identify the following: 1) Which letter represents a carbohydrate
Chapter 1- An Orientation to the Human Body NOTES
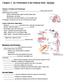 Chapter 1- An Orientation to the Human Body NOTES Overview of Anatomy and Physiology: -Anatomy- of body parts and their relationships to one another. -Gross or Macroscopic= large and easily observable
Chapter 1- An Orientation to the Human Body NOTES Overview of Anatomy and Physiology: -Anatomy- of body parts and their relationships to one another. -Gross or Macroscopic= large and easily observable
Objectives continued- Answer each of the objectives on a separate sheet of paper to demonstrate content mastery. Attach answers to back of packet.
 Anatomy and Physiology Chapter 2: Basic Chemistry Name: Objectives- By the end of this chapter I will be able to: 1. Differentiate between matter and energy. 2. Define chemical element, and list the four
Anatomy and Physiology Chapter 2: Basic Chemistry Name: Objectives- By the end of this chapter I will be able to: 1. Differentiate between matter and energy. 2. Define chemical element, and list the four
Hole s Human Anatomy and Physiology Tenth Edition. Chapter 2
 PowerPoint Lecture Outlines to accompany Hole s Human Anatomy and Physiology Tenth Edition Shier w Butler w Lewis Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
PowerPoint Lecture Outlines to accompany Hole s Human Anatomy and Physiology Tenth Edition Shier w Butler w Lewis Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Full file at Essentials of Anatomy & Physiology (Martini/ Bartholomew) Chapter 2 The Chemical Level of Organization
 Essentials of Anatomy & Physiology (Martini/ Bartholomew) Chapter 2 The Chemical Level of Organization Multiple Choice 1) An unstable isotope that emits subatomic particles spontaneously is called A) a
Essentials of Anatomy & Physiology (Martini/ Bartholomew) Chapter 2 The Chemical Level of Organization Multiple Choice 1) An unstable isotope that emits subatomic particles spontaneously is called A) a
CYTOLOGY & HISTOLOGY THE STUDY OF CELLS AND TISSUES
 NAME: DATE: PARTNER: CYTOLOGY & HISTOLOGY THE STUDY OF CELLS AND TISSUES For ease of study, multicellular animals are often examined at various levels of structural organization. Starting from the most
NAME: DATE: PARTNER: CYTOLOGY & HISTOLOGY THE STUDY OF CELLS AND TISSUES For ease of study, multicellular animals are often examined at various levels of structural organization. Starting from the most
2/25/2013. Electronic Configurations
 1 2 3 4 5 Chapter 2 Chemical Principles The Structure of Atoms Chemistry is the study of interactions between atoms and molecules The atom is the smallest unit of matter that enters into chemical reactions
1 2 3 4 5 Chapter 2 Chemical Principles The Structure of Atoms Chemistry is the study of interactions between atoms and molecules The atom is the smallest unit of matter that enters into chemical reactions
An atom is the smallest unit of an element. It has: A general understanding of chemistry is necessary for understanding human physiology.
 8/29/11 Chapter 2 I. Atoms, Ions, and Chemical Bonds Chemical Composition of the Body Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Body
8/29/11 Chapter 2 I. Atoms, Ions, and Chemical Bonds Chemical Composition of the Body Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Body
Objectives. in living cells.
 Objectives The studient will know the definition of matter, energy potential energy and kinetic energy. the student will be able to define element and be able to list the elements that make up most of
Objectives The studient will know the definition of matter, energy potential energy and kinetic energy. the student will be able to define element and be able to list the elements that make up most of
Tissues: - A group of cells similar in structure and performing a particular function forms a tissue.
 Plant Tissues Class- IX Tissues: - A group of cells similar in structure and performing a particular function forms a tissue. PLANT TISSUES ANIMAL TISSUES 1. Most of the plant tissues are Most of the tissues
Plant Tissues Class- IX Tissues: - A group of cells similar in structure and performing a particular function forms a tissue. PLANT TISSUES ANIMAL TISSUES 1. Most of the plant tissues are Most of the tissues
the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together Chemical structure Covalent bond Ionic bond
 Chemical structure the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together Covalent bond bond formed by the sharing of valence electrons between atoms Ionic bond
Chemical structure the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together Covalent bond bond formed by the sharing of valence electrons between atoms Ionic bond
CHAPTER 9 BODY ORGANIZATION. Copyright 2007 by Mosby, Inc., an affiliate of Elsevier Inc. 1
 CHAPTER 9 BODY ORGANIZATION Copyright 2007 by Mosby, Inc., an affiliate of Elsevier Inc. 1 Anatomy and Physiology Four basic properties of life: Reception The ability of the organism to control its actions
CHAPTER 9 BODY ORGANIZATION Copyright 2007 by Mosby, Inc., an affiliate of Elsevier Inc. 1 Anatomy and Physiology Four basic properties of life: Reception The ability of the organism to control its actions
Organization of Vertebrate Body. Organization of Vertebrate Body
 The Animal Body and Principles of Regulation Chapter 43 There are four levels of organization: 1. Cells 2. Tissues 3. Organs 4. Organ systems Bodies of vertebrates are composed of different cell types
The Animal Body and Principles of Regulation Chapter 43 There are four levels of organization: 1. Cells 2. Tissues 3. Organs 4. Organ systems Bodies of vertebrates are composed of different cell types
Full file at
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following is an uncharged particle found in the nucleus of 1) an atom and which has
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following is an uncharged particle found in the nucleus of 1) an atom and which has
Chemistry of Life. Chapters 2 & 3. Credit: Larry Stepanowicz. Learning Objectives
 Chemistry of Life Chapters 2 & 3 Credit: Larry Stepanowicz Learning Objectives 1. Differentiate between the definitions of an atom, element, ion, and molecule. 2. Describe why and how atoms react chemically.
Chemistry of Life Chapters 2 & 3 Credit: Larry Stepanowicz Learning Objectives 1. Differentiate between the definitions of an atom, element, ion, and molecule. 2. Describe why and how atoms react chemically.
2) Matter composed of a single type of atom is known as a(n) 2) A) element. B) mineral. C) electron. D) compound. E) molecule.
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following is a particle found in the nucleus of an atom and that has no electrical
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following is a particle found in the nucleus of an atom and that has no electrical
PRESENTATION TITLE. Chemistry. Chemistry
 PRESENTATION TITLE Chemistry Chemistry Chemistry is the study of the smallest forms of matter and their interactions. Matter is anything that has mass and takes up space. Generally, chemistry deals with
PRESENTATION TITLE Chemistry Chemistry Chemistry is the study of the smallest forms of matter and their interactions. Matter is anything that has mass and takes up space. Generally, chemistry deals with
Chapter 1. DNA is made from the building blocks adenine, guanine, cytosine, and. Answer: d
 Chapter 1 1. Matching Questions DNA is made from the building blocks adenine, guanine, cytosine, and. Answer: d 2. Matching Questions : Unbranched polymer that, when folded into its three-dimensional shape,
Chapter 1 1. Matching Questions DNA is made from the building blocks adenine, guanine, cytosine, and. Answer: d 2. Matching Questions : Unbranched polymer that, when folded into its three-dimensional shape,
Chapter 2 The Chemistry of Biology. Dr. Ramos BIO 370
 Chapter 2 The Chemistry of Biology Dr. Ramos BIO 370 2 Atoms, Bonds, and Molecules Matter - all materials that occupy space and have mass Matter is composed of atoms. Atom simplest form of matter not divisible
Chapter 2 The Chemistry of Biology Dr. Ramos BIO 370 2 Atoms, Bonds, and Molecules Matter - all materials that occupy space and have mass Matter is composed of atoms. Atom simplest form of matter not divisible
Matter and Substances Section 3-1
 Matter and Substances Section 3-1 Key Idea: All matter is made up of atoms. An atom has a positively charges core surrounded by a negatively charged region. An atom is the smallest unit of matter that
Matter and Substances Section 3-1 Key Idea: All matter is made up of atoms. An atom has a positively charges core surrounded by a negatively charged region. An atom is the smallest unit of matter that
Chapter 2. Chemical Basis of Life
 hapter 2 hemical Basis of Life opyright The McGrawill ompanies, Inc. Permission required for reproduction or display. Introduction: A. hemistry deals with the composition of matter and how it changes.
hapter 2 hemical Basis of Life opyright The McGrawill ompanies, Inc. Permission required for reproduction or display. Introduction: A. hemistry deals with the composition of matter and how it changes.
Overview of Cells. Prokaryotes vs Eukaryotes The Cell Organelles The Endosymbiotic Theory
 Overview of Cells Prokaryotes vs Eukaryotes The Cell Organelles The Endosymbiotic Theory Prokaryotic Cells Archaea Bacteria Come in many different shapes and sizes.5 µm 2 µm, up to 60 µm long Have large
Overview of Cells Prokaryotes vs Eukaryotes The Cell Organelles The Endosymbiotic Theory Prokaryotic Cells Archaea Bacteria Come in many different shapes and sizes.5 µm 2 µm, up to 60 µm long Have large
Microbiology with Diseases by Taxonomy, 5e (Bauman) Chapter 2 The Chemistry of Microbiology. 2.1 Multiple Choice Questions
 Microbiology with Diseases by Taxonomy, 5e (Bauman) Chapter 2 The Chemistry of Microbiology 2.1 Multiple Choice Questions 1) Which of the following does not contribute significantly to the mass of an atom?
Microbiology with Diseases by Taxonomy, 5e (Bauman) Chapter 2 The Chemistry of Microbiology 2.1 Multiple Choice Questions 1) Which of the following does not contribute significantly to the mass of an atom?
Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 2 Basic Chemistry. Short Answer. Figure 2.1
 Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 2 Basic Chemistry Short Answer Figure 2.1 Using Figure 2.1, identify the following: 1) The structure of the functional protein is. Diff: 3 Page
Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 2 Basic Chemistry Short Answer Figure 2.1 Using Figure 2.1, identify the following: 1) The structure of the functional protein is. Diff: 3 Page
1. Which of the cellular components listed above are part of the endomembrane system and surrounded by a double membrane?
 Anatomy and Physiology 141 Fall 2014: Exam IA Name: Use the following list to answer Question 1. 1 Golgi apparatus 2 Nuclear membrane 3 Mitochondria 4 Rough ER 5 Smooth ER 1. Which of the cellular components
Anatomy and Physiology 141 Fall 2014: Exam IA Name: Use the following list to answer Question 1. 1 Golgi apparatus 2 Nuclear membrane 3 Mitochondria 4 Rough ER 5 Smooth ER 1. Which of the cellular components
2: CHEMICAL COMPOSITION OF THE BODY
 1 2: CHEMICAL COMPOSITION OF THE BODY Although most students of human physiology have had at least some chemistry, this chapter serves very well as a review and as a glossary of chemical terms. In particular,
1 2: CHEMICAL COMPOSITION OF THE BODY Although most students of human physiology have had at least some chemistry, this chapter serves very well as a review and as a glossary of chemical terms. In particular,
Chapter 2: Fundamentals of Chemistry. Question Type: Multiple Choice. 1) Which of the following pairs is mismatched?
 Microbiology Principles and Explorations 9th Edition Black TEST BANK Full clear download at: https://testbankreal.com/download/microbiology-principles-explorations- 9th-edition-black-test-bank/ Microbiology
Microbiology Principles and Explorations 9th Edition Black TEST BANK Full clear download at: https://testbankreal.com/download/microbiology-principles-explorations- 9th-edition-black-test-bank/ Microbiology
Now starts the fun stuff Cell structure and function
 Now starts the fun stuff Cell structure and function Cell Theory The three statements of the cell theory are: All organisms are composed of one or more cells and the processes of life occur in these cells.
Now starts the fun stuff Cell structure and function Cell Theory The three statements of the cell theory are: All organisms are composed of one or more cells and the processes of life occur in these cells.
NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE. Honors Biology I
 NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
Dr. Dina A. A. Hassan Associate Professor, Pharmacology
 Cytology Dr. Dina A. A. Hassan Associate Professor, Pharmacology Email: da.hassan@psau.edu.sa Cells All living things are made up of cells Basic building blocks of life It is the smallest functional and
Cytology Dr. Dina A. A. Hassan Associate Professor, Pharmacology Email: da.hassan@psau.edu.sa Cells All living things are made up of cells Basic building blocks of life It is the smallest functional and
Chemistry Comes to Life
 BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 2 Chemistry Comes to Life Lecture Presentation Anne Gasc Hawaii Pacific University and University of Hawaii
BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 2 Chemistry Comes to Life Lecture Presentation Anne Gasc Hawaii Pacific University and University of Hawaii
Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the study of physiology because
 Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 11 th ed. Chapter 2: Chemical Basis of Life Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the
Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 11 th ed. Chapter 2: Chemical Basis of Life Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the
The Chemical Level of Organization
 Scuola di Ingegneria Industriale e dell Informazione Course 096125 (095857) Introduction to Green and Sustainable Chemistry The Chemical Level of Organization Prof. (and Ada Truscello) Dept. CMIC http://iscamap.chem.polimi.it/citterio/education/course-topics/
Scuola di Ingegneria Industriale e dell Informazione Course 096125 (095857) Introduction to Green and Sustainable Chemistry The Chemical Level of Organization Prof. (and Ada Truscello) Dept. CMIC http://iscamap.chem.polimi.it/citterio/education/course-topics/
Topic 1: The Chemical Context of Life, Holtzclaw and Holtzclaw, 2014
 Name Block Topic 1: The Chemical Context of Life, Holtzclaw and Holtzclaw, 2014 1. Complete the vocabulary on a separate piece of paper. 2. What are the elements that make up most of living matter? What
Name Block Topic 1: The Chemical Context of Life, Holtzclaw and Holtzclaw, 2014 1. Complete the vocabulary on a separate piece of paper. 2. What are the elements that make up most of living matter? What
Chapter 02 Chemistry of Life
 Chapter 02 Chemistry of Life Multiple Choice Questions 1. The smallest unit of matter is the A. molecule. B. atom. C. compound. D. isotope. HAPS Objective: C.01.03 Compare and contrast the terms atoms,
Chapter 02 Chemistry of Life Multiple Choice Questions 1. The smallest unit of matter is the A. molecule. B. atom. C. compound. D. isotope. HAPS Objective: C.01.03 Compare and contrast the terms atoms,
Anatomy and Physiology. Science Curriculum Framework
 Anatomy and Physiology Science Curriculum Framework Revised 005 Anatomy and Physiology Anatomy and Physiology should develop an understanding of the organization of the human body through studies of body
Anatomy and Physiology Science Curriculum Framework Revised 005 Anatomy and Physiology Anatomy and Physiology should develop an understanding of the organization of the human body through studies of body
BIOCHEMISTRY 10/9/17 CHEMISTRY OF LIFE. Elements: simplest form of a substance - cannot be broken down any further without changing what it is
 BIOCHEMISTRY CHEMISTRY OF LIFE Elements: simplest form of a substance - cannot be broken down any further without changing what it is THE ATOM Just like cells are the basic unit of life, the ATOM is the
BIOCHEMISTRY CHEMISTRY OF LIFE Elements: simplest form of a substance - cannot be broken down any further without changing what it is THE ATOM Just like cells are the basic unit of life, the ATOM is the
Atoms. Atoms 9/9/2015
 The Chemistry of Life The Nature of Matter, Water,Carbon Compounds, Chemical Reactions and Enzymes The Nature of Matter B.1.9 Both living and nonliving things are composed of compounds, which are themselves
The Chemistry of Life The Nature of Matter, Water,Carbon Compounds, Chemical Reactions and Enzymes The Nature of Matter B.1.9 Both living and nonliving things are composed of compounds, which are themselves
Copy into Note Packet and Return to Teacher
 Copy into Note Packet and Return to Teacher Section 1: Nature of Matter Objectives: Differentiate between atoms and elements. Analyze how compounds are formed. Distinguish between covalent bonds, hydrogen
Copy into Note Packet and Return to Teacher Section 1: Nature of Matter Objectives: Differentiate between atoms and elements. Analyze how compounds are formed. Distinguish between covalent bonds, hydrogen
Chapter 02 Chemical Composition of the Body
 Chapter 02 Chemical Composition of the Body 1. In an atom, the number of Student: A. Protons always equals the number of neutrons B. Of protons always equals the number of electrons C. Of neutrons always
Chapter 02 Chemical Composition of the Body 1. In an atom, the number of Student: A. Protons always equals the number of neutrons B. Of protons always equals the number of electrons C. Of neutrons always
The Chemistry and Energy of Life
 2 The Chemistry and Energy of Life Chapter 2 The Chemistry and Energy of Life Key Concepts 2.1 Atomic Structure Is the Basis for Life s Chemistry 2.2 Atoms Interact and Form Molecules 2.3 Carbohydrates
2 The Chemistry and Energy of Life Chapter 2 The Chemistry and Energy of Life Key Concepts 2.1 Atomic Structure Is the Basis for Life s Chemistry 2.2 Atoms Interact and Form Molecules 2.3 Carbohydrates
1/23/2012. Atoms. Atoms Atoms - Electron Shells. Chapter 2 Outline. Planetary Models of Elements Chemical Bonds
 Chapter 2 Outline Atoms Chemical Bonds Acids, Bases and the p Scale Organic Molecules Carbohydrates Lipids Proteins Nucleic Acids Are smallest units of the chemical elements Composed of protons, neutrons
Chapter 2 Outline Atoms Chemical Bonds Acids, Bases and the p Scale Organic Molecules Carbohydrates Lipids Proteins Nucleic Acids Are smallest units of the chemical elements Composed of protons, neutrons
Chapter 3: Cells and Their Functions. Copyright 2013 Wolters Kluwer Health Lippincott Williams & Wilkins
 Chapter 3: Cells and Their Functions Overview Key Terms active transport filtration mitochondria cancer gene mitosis carcinogen hemolysis mutation chromosome hypertonic nucleus cytology hypotonic organelle
Chapter 3: Cells and Their Functions Overview Key Terms active transport filtration mitochondria cancer gene mitosis carcinogen hemolysis mutation chromosome hypertonic nucleus cytology hypotonic organelle
4. Which of the following organelles digests waste using hydrolytic enzymes:
 Multichoice questions section. You must answer ALL questions. 1. A cell contains many organelles, each of which has a specific function. What is function of mitochondria? a) production of plasma membrane
Multichoice questions section. You must answer ALL questions. 1. A cell contains many organelles, each of which has a specific function. What is function of mitochondria? a) production of plasma membrane
Foundations in Microbiology Seventh Edition
 Lecture PowerPoint to accompany Foundations in Microbiology Seventh Edition Talaro Chapter 2 The Chemistry of Biology Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Lecture PowerPoint to accompany Foundations in Microbiology Seventh Edition Talaro Chapter 2 The Chemistry of Biology Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chemical Principles and Biomolecules (Chapter 2) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus
 Chemical Principles and Biomolecules (Chapter 2) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Source for figures and content: Tortora, G.J. Microbiology
Chemical Principles and Biomolecules (Chapter 2) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Source for figures and content: Tortora, G.J. Microbiology
Bio10 Cell and Molecular Lecture Notes SRJC
 Basic Chemistry Atoms Smallest particles that retain properties of an element Made up of subatomic particles: Protons (+) Electrons (-) Neutrons (no charge) Isotopes Atoms of an element with different
Basic Chemistry Atoms Smallest particles that retain properties of an element Made up of subatomic particles: Protons (+) Electrons (-) Neutrons (no charge) Isotopes Atoms of an element with different
Chemistry of Life Cells & Bioprocesses CRT Review
 Chemistry of Life Cells & Bioprocesses CRT Review Chapter 2: The Chemistry of Life macromolecules - The four types of macromolecules are carbohydrates, lipids, nucleic acids, and proteins Types of Macromolecules
Chemistry of Life Cells & Bioprocesses CRT Review Chapter 2: The Chemistry of Life macromolecules - The four types of macromolecules are carbohydrates, lipids, nucleic acids, and proteins Types of Macromolecules
CHAPTER 3. Cell Structure and Genetic Control. Chapter 3 Outline
 CHAPTER 3 Cell Structure and Genetic Control Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell Nucleus and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division
CHAPTER 3 Cell Structure and Genetic Control Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell Nucleus and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division
Chapter 02 Chemistry of Life
 Maders Understanding Human Anatomy and Physiology 9th Edition Longenbaker Test Bank Full Download: http://testbanklive.com/download/maders-understanding-human-anatomy-and-physiology-9th-edition-longenbaker
Maders Understanding Human Anatomy and Physiology 9th Edition Longenbaker Test Bank Full Download: http://testbanklive.com/download/maders-understanding-human-anatomy-and-physiology-9th-edition-longenbaker
Honors Biology-CW/HW Cell Biology 2018
 Class: Date: Honors Biology-CW/HW Cell Biology 2018 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Hooke s discovery of cells was made observing a. living
Class: Date: Honors Biology-CW/HW Cell Biology 2018 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Hooke s discovery of cells was made observing a. living
Chapter 2 Chemistry of Life
 Chapter 2 Chemistry of Life Section 2.1 Atoms, Ions and Molecules Section 2.2 Properties of water Section 2.3 Carbon-based Molecules Section 2.4 Chemical Reactions Section 2.5 - Enzymes 1 Atoms, Ions and
Chapter 2 Chemistry of Life Section 2.1 Atoms, Ions and Molecules Section 2.2 Properties of water Section 2.3 Carbon-based Molecules Section 2.4 Chemical Reactions Section 2.5 - Enzymes 1 Atoms, Ions and
Introduction to the Human Body, 10th Edition Tortora Test Bank TEST BANK for Introduction to the Human Body, 10th Edition by Gerard J.
 Introduction to the Human Body, 10th Edition Tortora Test Bank TEST BANK for Introduction to the Human Body, 10th Edition by Gerard J. Tortora Test Bank for all chapters are included. Download link: https://testbankreal.com/download/introduction-human-body-10thedition-kindle-edition-test-bank-tortora/
Introduction to the Human Body, 10th Edition Tortora Test Bank TEST BANK for Introduction to the Human Body, 10th Edition by Gerard J. Tortora Test Bank for all chapters are included. Download link: https://testbankreal.com/download/introduction-human-body-10thedition-kindle-edition-test-bank-tortora/
Honors Biology summer assignment. Review the notes and study them. There will be a test on this information the 1 st week of class
 Honors Biology summer assignment Review the notes and study them. There will be a test on this information the 1 st week of class Biomolecules Molecules that make up living things. There are 4 molecules
Honors Biology summer assignment Review the notes and study them. There will be a test on this information the 1 st week of class Biomolecules Molecules that make up living things. There are 4 molecules
Chapter 6 Chemistry in Biology. 6.1 Atoms, Elements & Compounds 6.2 Chemical Reactions 6.3 Water and Solutions 6.4 The Building Blocks of Life
 Chapter 6 Chemistry in Biology 6.1 Atoms, Elements & Compounds 6.2 Chemical Reactions 6.3 Water and Solutions 6.4 The Building Blocks of Life 6.1 Atoms, Elements, and Compounds Main idea: Matter is composed
Chapter 6 Chemistry in Biology 6.1 Atoms, Elements & Compounds 6.2 Chemical Reactions 6.3 Water and Solutions 6.4 The Building Blocks of Life 6.1 Atoms, Elements, and Compounds Main idea: Matter is composed
Chemistry of Life. Chapter 2
 Chemistry of Life Chapter 2 Elements Simplest form of matter Made of atoms of one type Cannot be chemically broken down into simpler substances About 117 known elements The Periodic Table of Elements Each
Chemistry of Life Chapter 2 Elements Simplest form of matter Made of atoms of one type Cannot be chemically broken down into simpler substances About 117 known elements The Periodic Table of Elements Each
Chapter 002 The Chemistry of Biology
 Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
Chapter 2: The Chemical Level of. Organization. Copyright 2009, John Wiley & Sons, Inc.
 Chapter 2: Organization The Chemical Level of Introduction Since chemicals compose your body and all body activities are chemical in nature, it is important to become familiar with the language and fundamental
Chapter 2: Organization The Chemical Level of Introduction Since chemicals compose your body and all body activities are chemical in nature, it is important to become familiar with the language and fundamental
Nature of matter. Chemical bond is a force that joins atoms
 Nature of matter Atom the smallest unit of matter that cannot be broken down by chemical means The subatomic particles of an atom consist of protons, neutrons and electrons Element is a pure substance
Nature of matter Atom the smallest unit of matter that cannot be broken down by chemical means The subatomic particles of an atom consist of protons, neutrons and electrons Element is a pure substance
Chapter 2: The Chemical Basis of Life
 Chapter 2: The Chemical Basis of Life I. Basic Chemistry A. Matter, Mass, and Weight 1. All living and nonliving things are composed of 2. represents the amount of matter. 3. is caused by the gravitational
Chapter 2: The Chemical Basis of Life I. Basic Chemistry A. Matter, Mass, and Weight 1. All living and nonliving things are composed of 2. represents the amount of matter. 3. is caused by the gravitational
Introduction to Science and the Chemistry of Life
 Introduction to Science and the Chemistry of Life I Nature of Science 1.Forms a) Pure Science - study for the pure gain of knowledge b) Applied Science - directed study to solve a known problem. 2. Procedures
Introduction to Science and the Chemistry of Life I Nature of Science 1.Forms a) Pure Science - study for the pure gain of knowledge b) Applied Science - directed study to solve a known problem. 2. Procedures
Cells: The Living Units
 Golgi Apparatus Cells: The Living Units Chapter 3, PPT 2 Membrane Yes, stacked and flattened Description Stacked and flattened membranous sacs Modify, concentrate, and package proteins & lipids made at
Golgi Apparatus Cells: The Living Units Chapter 3, PPT 2 Membrane Yes, stacked and flattened Description Stacked and flattened membranous sacs Modify, concentrate, and package proteins & lipids made at
Human Biology. The Chemistry of Living Things. Concepts and Current Issues. All Matter Consists of Elements Made of Atoms
 2 The Chemistry of Living Things PowerPoint Lecture Slide Presentation Robert J. Sullivan, Marist College Michael D. Johnson Human Biology Concepts and Current Issues THIRD EDITION Copyright 2006 Pearson
2 The Chemistry of Living Things PowerPoint Lecture Slide Presentation Robert J. Sullivan, Marist College Michael D. Johnson Human Biology Concepts and Current Issues THIRD EDITION Copyright 2006 Pearson
Ch. 2 Chemistry Comes to Life
 BIOL 164 Human Biology Ch 2 Chemistry Ch. 2 Chemistry Comes to Life Basic Chemistry Helps Us Understand Human Biology Chemistry Science of the composi9on and proper9es of ma:er Carbohydrates, Lipids, Proteins,
BIOL 164 Human Biology Ch 2 Chemistry Ch. 2 Chemistry Comes to Life Basic Chemistry Helps Us Understand Human Biology Chemistry Science of the composi9on and proper9es of ma:er Carbohydrates, Lipids, Proteins,
2.1 Atoms, Ions, and Molecules
 2.1 Atoms, Ions, and Molecules Living things consist of atoms of different elements. An atom is the smallest basic unit of matter. An element is one type of atom. 6 elements make up 99% of all living things
2.1 Atoms, Ions, and Molecules Living things consist of atoms of different elements. An atom is the smallest basic unit of matter. An element is one type of atom. 6 elements make up 99% of all living things
Cell Organelles. 2. Cells are the basic unit of organization in an organism Cells tissues organ organ system organism
 Cell Organelles What are some of the differences you see between these two cells? A. Cell Theory 1. All organisms are made up of one or more cells 2. Cells are the basic unit of organization in an organism
Cell Organelles What are some of the differences you see between these two cells? A. Cell Theory 1. All organisms are made up of one or more cells 2. Cells are the basic unit of organization in an organism
Chapter 02 The Chemical Basis of Life
 Chapter 02 The Chemical Basis of Life Multiple Choice Questions 1. The amount of matter in an object is its A. element. B. mass. C. ionic charge. D. atomic number. E. weight. HAPS Objective: C01.01d Distinguish
Chapter 02 The Chemical Basis of Life Multiple Choice Questions 1. The amount of matter in an object is its A. element. B. mass. C. ionic charge. D. atomic number. E. weight. HAPS Objective: C01.01d Distinguish
The Chemical Level of Organization
 PowerPoint Lecture Slides prepared by Meg Flemming Austin Community College C H A P T E R 2 The Chemical Level of Organization Chapter 2 Learning Outcomes 2-1 2-2 2-3 2-4 Describe an atom and how atomic
PowerPoint Lecture Slides prepared by Meg Flemming Austin Community College C H A P T E R 2 The Chemical Level of Organization Chapter 2 Learning Outcomes 2-1 2-2 2-3 2-4 Describe an atom and how atomic
BIOL Anatomy and Physiology I ( version L )
 BIOL 2113 - Anatomy and Physiology I ( version 213L ) Course Title Course Development Learning Support Anatomy and Physiology I Standard No Course Description Introduces the anatomy and physiology of the
BIOL 2113 - Anatomy and Physiology I ( version 213L ) Course Title Course Development Learning Support Anatomy and Physiology I Standard No Course Description Introduces the anatomy and physiology of the
Elements and Isotopes
 Section 2-1 Notes Atoms Life depends on chemistry. The basic unit of matter is the atom. Atoms are incredibly small The subatomic particles that make up atoms are protons, neutrons, and electrons. Parts
Section 2-1 Notes Atoms Life depends on chemistry. The basic unit of matter is the atom. Atoms are incredibly small The subatomic particles that make up atoms are protons, neutrons, and electrons. Parts
ANATOMY AND PHYSIOLOGY Revised 11/2010
 ANATOMY AND PHYSIOLOGY Revised 11/2010 DESCRIPTION OF COURSE: Covers the basics of human anatomy and physiology including anatomical terminology, basic biochemistry, cells and tissues, and the integumentary,
ANATOMY AND PHYSIOLOGY Revised 11/2010 DESCRIPTION OF COURSE: Covers the basics of human anatomy and physiology including anatomical terminology, basic biochemistry, cells and tissues, and the integumentary,
Cell Structure and Cell Cycle
 E X E R C I S E 4 Cell Structure and Cell Cycle Materials model or diagram of a cell compound microscopes and lens paper prepared slides of human skeletal muscle cells, pseudostratified ciliated columnar
E X E R C I S E 4 Cell Structure and Cell Cycle Materials model or diagram of a cell compound microscopes and lens paper prepared slides of human skeletal muscle cells, pseudostratified ciliated columnar
