Hydrogen bonds in the phenol-formaldehyde-ortho-naphthoquinondiazidewater
|
|
|
- Alberta Barrett
- 5 years ago
- Views:
Transcription
1 doi: /ecsoc Conference Proceedings Paper Hydrogen bonds in the phenol-formaldehyde-ortho-naphthoquinondiazidewater system Valeria Chernuha *, Dmitriy Fomichev, and Sergei Zelentsov Lobachevsky State University of Nizhni Novgorod * Correspondence: valeria.chernuxa@yandex.ru Abstract: Hydrogen bonds are formed in the phenol-formaldehyde-ortho-naphthoquinondiazide-water system. In our work quantum-chemical calculations were made using the HF/3-21G ++ theory level and it was found out that phenol-formaldehyde forms three types of hydrogen bonds: between its hydroxyl group and carbonyl group of ortho-naphthoquinondiazide, between its hydroxyl group and water and between its hydroxyl groups intramolecularly forming a "circle-like" structure, and this type of bonds prevails. Keywords: phenol-formaldehyde, ortho-naphthoquinondiazide, hydrogen bonds, photoresist 1. Introduction Naphthoquinondiazides are light-sensitive substances that are used in photolithography as a component of photoresists. The other component is a polymer, for example phenol-formaldehyde resin. These substanses form hydrogen bonds, which inhibit the dissolution of the photoresist until the light irradiation [1], [2], [3]. 2. Materials and methods All calculations described in this paper were performed in the North-West Chemistry software package [4]. The HF/3-21G ++ theory level was chosen because of the large amount of atoms, which makes the computations to take a lot of time. 3. Results In Fig.1 a model of tetramer of phenol-formaldehyde resin is shown. There are two intramolecular hydrogen bonds. Their lengths were calculated and placed in Table 1. Figure 1. The model of the phenol-formaldehyde tetramer: white atoms - H, gray atoms - C, red atoms - O, pink lines - hydrogen bonds.
2 2 of 5 Table 1. The lengths of hydrogen bonds from Fig , ,737 There is a model of pentamer phenol-formaldehyde resin shown in Fig.2. It forms a structure like a half of a circle by intramolecular hydrogen bonds. Similar bonds can be found in polyalcohols [5]. Their lengths can be found in Table 2. Figure 2. The model of the pentamer phenol-formaldehyde resin: white atoms - H, gray atoms - C, red atoms - O, pink lines - hydrogen bonds. Table 2. The lengths of hydrogen bonds from Fig In Fig.3 a model of the tetramer of phenol-formaldehyde resin accompanied by 5 water molecules is presented. Table 3 contains the lengths of hydrogen bonds formed in this system. It can be seen from the figure that hydrogen groups of tetramer tend to form hydrogen bonds with water molecules. Figure 3. The model of the phenol-formaldehyde tetramer with water: white atoms - H, gray atoms - C, red atoms - O, pink lines - hydrogen bonds.
3 3 of 5 Table 3. The lengths of hydrogen bonds from Fig In Fig.4 a model of phenol-formaldehyde pentamer with water molecules can be seen (initially there were 5 water molecules added; the ones which didn't form hydrogen bonds with phenol-formaldehyde resin during the optimization are not shown in the picture). Their lengths are shown in Table 4. Figure 4. The model of the phenol-formaldehyde pentamer with water: white atoms - H, gray atoms - C, red atoms - O, pink lines - hydrogen bonds. Table 4. The lengths of hydrogen bonds from Fig In Fig.5, there is a model of interaction of the phenol-formaldehyde pentamer and the ortho-naphthoquinondiazide molecule. The pentamer forms a "half-a-circle-like" structure. Diazide group is in its center of this structure, but no hydrogen bonds were detected with this group [1]. Although the ortho-naphthoquinondiazide carbonyl group forms a hydrogen bond with terminal hydroxyl group of the phenol-formaldehyde resin. It looks like an ortho-naphthoquinondiazide fragment closes that "circle". The lengths are placed in Table 5.
4 4 of 5 Figure 5. The model of the ortho-naphthoquinondiazide with the phenol-formaldehyde pentamer: white atoms - H, gray atoms - C, red atoms - O, blue atoms - N, pink lines - hydrogen bonds. Table 5. The lengths of hydrogen bonds from Fig Fig.6 contains a model from Fig.5, but some water molecules were added (water molecules which did not form hydrogen bonds with any non-water molecules are also not shown). It can be seen that only the two of five initially added water molecules formed hydrogen bonds in this system. The lengths of the hydrogen bonds are shown in Table 6. Figure 6. The model of ortho-naphthoquinondiazide with phenol-formaldehyde pentamer and water: white atoms - H, gray atoms - C, red atoms - O, blue atoms - N, pink lines - hydrogen bonds.
5 5 of 5 Table 6. The lengths of hydrogen bonds from Fig Discussion The results of this work let us to make a conclusion that phenol-formaldehyde resin together with ortho-naphtoquinonediazide compound is less liable to form hydrogen bonds with water than phenol-formaldehyde resin itself. It is more liable to form hydrogen bonds with ortho-naphtoquinonediazide compounds than with water molecules. In our further work we are planning to calculate energies of hydrogen bonds. We are also planning to continue quantum-chemical calculations using the higher theory level. Acknowledgments: Authors are grateful to the Volga Research and Education Center for Supercomputer Technologies for providing access to resources of the Lobachevsky supercomputer. References 1. Zelentsov, S.V.; Zelentsova, N.V. Contemporary photolithography. Educational and methodological material for the advanced training program "New materials of electronics and optoelectronics for information and telecommunication systems"; Publisher: Nizhny Novgorod, Russia, p Lebedev, V.I. and others (4). The influence of intermolecular hydrogen bonds on photoresist mask properties. The Bulletin of the Lobachevsky University of Nizhny Novgorod 2014, 4(1), Zelentsov, S.V. Photochemical reactions of organic compounds; Nizhny Novgorod, Russia, Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; Van Dam, H.J.J.; Wang, D.; Nieplocha, J.; Apra, E.; Windus, T.L.; de Jong, W.A. NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations. Computer Physics Communications 2010, 181, bibtex: valiev_nwchem:_ Rumyantsev, M.; Zelentsov, S.V.; Gushchin, A.V. Retardation effect in acetalization of poly(vinyl alcohol) with butyaldehyde. European Polymer Journal 2013, 49, p by the authors. Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (
Supporting Information for. Dynamics Study"
 Supporting Information for "CO 2 Adsorption and Reactivity on Rutile TiO 2 (110) in Water: An Ab Initio Molecular Dynamics Study" Konstantin Klyukin and Vitaly Alexandrov,, Department of Chemical and Biomolecular
Supporting Information for "CO 2 Adsorption and Reactivity on Rutile TiO 2 (110) in Water: An Ab Initio Molecular Dynamics Study" Konstantin Klyukin and Vitaly Alexandrov,, Department of Chemical and Biomolecular
Supporting information for: Mechanisms and Dynamics of Protonation and. Lithiation of Ferrocene
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 215 Supporting information for: Mechanisms and Dynamics of Protonation and Lithiation
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 215 Supporting information for: Mechanisms and Dynamics of Protonation and Lithiation
Supporting Information for. Ab Initio Metadynamics Study of VO + 2 /VO2+ Redox Reaction Mechanism at the Graphite. Edge Water Interface
 Supporting Information for Ab Initio Metadynamics Study of VO + 2 /VO2+ Redox Reaction Mechanism at the Graphite Edge Water Interface Zhen Jiang, Konstantin Klyukin, and Vitaly Alexandrov,, Department
Supporting Information for Ab Initio Metadynamics Study of VO + 2 /VO2+ Redox Reaction Mechanism at the Graphite Edge Water Interface Zhen Jiang, Konstantin Klyukin, and Vitaly Alexandrov,, Department
Supporting Information for: Dependence and Quantitative Modeling
 Supporting Information for: Tip-Enhanced Raman Voltammetry: Coverage Dependence and Quantitative Modeling Michael Mattei, Gyeongwon Kang, Guillaume Goubert, Dhabih V. Chulhai, George C. Schatz, Lasse Jensen,
Supporting Information for: Tip-Enhanced Raman Voltammetry: Coverage Dependence and Quantitative Modeling Michael Mattei, Gyeongwon Kang, Guillaume Goubert, Dhabih V. Chulhai, George C. Schatz, Lasse Jensen,
Electronic Supporting Information Topological design of porous organic molecules
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Electronic Supporting Information Topological design of porous organic molecules Valentina Santolini,
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Electronic Supporting Information Topological design of porous organic molecules Valentina Santolini,
Supporting Informations
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Supporting Informations Fabio Gabas, Giovanni Di Liberto, Riccardo Conte, and Michele Ceotto
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Supporting Informations Fabio Gabas, Giovanni Di Liberto, Riccardo Conte, and Michele Ceotto
Basic introduction of NWChem software
 Basic introduction of NWChem software Background NWChem is part of the Molecular Science Software Suite Designed and developed to be a highly efficient and portable Massively Parallel computational chemistry
Basic introduction of NWChem software Background NWChem is part of the Molecular Science Software Suite Designed and developed to be a highly efficient and portable Massively Parallel computational chemistry
The Electronic Structure Studies of Hydroxyl Substituted Benzoic Acids
 Journal of Photopolymer Science and Technology Volume 12,Number2(1999) 331-338 1999TAPJ i The Electronic Structure Studies of Hydroxyl Substituted Benzoic Acids Wang Liyuan and Yu Shangxian Department
Journal of Photopolymer Science and Technology Volume 12,Number2(1999) 331-338 1999TAPJ i The Electronic Structure Studies of Hydroxyl Substituted Benzoic Acids Wang Liyuan and Yu Shangxian Department
Infrared Spectroscopy
 Infrared Spectroscopy IR Spectroscopy Used to identify organic compounds IR spectroscopy provides a 100% identification if the spectrum is matched. If not, IR at least provides information about the types
Infrared Spectroscopy IR Spectroscopy Used to identify organic compounds IR spectroscopy provides a 100% identification if the spectrum is matched. If not, IR at least provides information about the types
THEORETICAL EXAMINATION ANSWER SHEETS
 CEMISTRY: ART, SCIENCE, FUN TEORETICAL EXAMINATION ANSWER SEETS JULY 20, 2007 MOSCOW, RUSSIA 1 Quest. 1.1 1.2 2.1 3.1 3.2 3.3 3.4 Tot Points Student code: Marks 3 3 2 4.5 2 4 6 24.5 7 1.1.1 Structures:
CEMISTRY: ART, SCIENCE, FUN TEORETICAL EXAMINATION ANSWER SEETS JULY 20, 2007 MOSCOW, RUSSIA 1 Quest. 1.1 1.2 2.1 3.1 3.2 3.3 3.4 Tot Points Student code: Marks 3 3 2 4.5 2 4 6 24.5 7 1.1.1 Structures:
Sergei D. Plechovich, Sergei V. Zelentsov, Dmitry A. Fomichev, Dmitry V. Ovsyannikov, Nikolai V. Kryukov.
 The mechanism of the photochemical oxidation of substrates of different nature by nitro compounds Sergei D. Plechovich, Sergei V. Zelentsov, Dmitry A. Fomichev, Dmitry V. Ovsyannikov, Nikolai V. Kryukov
The mechanism of the photochemical oxidation of substrates of different nature by nitro compounds Sergei D. Plechovich, Sergei V. Zelentsov, Dmitry A. Fomichev, Dmitry V. Ovsyannikov, Nikolai V. Kryukov
Deduce the following information from the structure of estradiol, a phenol contain compound.
 A2 Chemistry: F324 Rings, Polymers and Analysis The Chemistry of Phenol Learning Outcomes: All (E) State the uses of phenols Most (C) Describe the reactions of phenol with aqueous alkalis and with sodium
A2 Chemistry: F324 Rings, Polymers and Analysis The Chemistry of Phenol Learning Outcomes: All (E) State the uses of phenols Most (C) Describe the reactions of phenol with aqueous alkalis and with sodium
Molecule Dynamics Simulation of Epoxy Resin System
 Molecule Dynamics Simulation of Epoxy Resin System Wu, Peilin Department of Physics, City University of Hong Kong. Lam, Tran Department of Biology, University of Pennsylvania. (RECSEM-2017, Joint Institute
Molecule Dynamics Simulation of Epoxy Resin System Wu, Peilin Department of Physics, City University of Hong Kong. Lam, Tran Department of Biology, University of Pennsylvania. (RECSEM-2017, Joint Institute
Supporting Information for the Paper
 Supporting Information for the Paper Bent Keto Form of Curcumin, Preferential Stabilization of Enol by Piperine, and Isomers of Curcumin Cyclodextrin Complexes: Insights from Ion Mobility Mass Spectrometry
Supporting Information for the Paper Bent Keto Form of Curcumin, Preferential Stabilization of Enol by Piperine, and Isomers of Curcumin Cyclodextrin Complexes: Insights from Ion Mobility Mass Spectrometry
Composition and Photochemical Mechanisms of Photoresists
 OpenStax-CNX module: m25525 1 Composition and Photochemical Mechanisms of Photoresists Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License
OpenStax-CNX module: m25525 1 Composition and Photochemical Mechanisms of Photoresists Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License
THz-Raman Identification of labile products in the system
 THz-Raman Identification of labile products in the system «phenol-semiquinone-quinone». E.A. Iasenko 1, Dr., V.P. Chelibanov 1, Dr., A.M. Polubotko 2 1 State University of Information Technologies, Mechanics,
THz-Raman Identification of labile products in the system «phenol-semiquinone-quinone». E.A. Iasenko 1, Dr., V.P. Chelibanov 1, Dr., A.M. Polubotko 2 1 State University of Information Technologies, Mechanics,
Lecture 4 Chapter 13 - Polymers. Functional Groups Condensation Rxns Free Radical Rxns
 Lecture 4 Chapter 13 - Polymers Functional Groups Condensation Rxns Free Radical Rxns Chemistry the whole year on one page Last semester Basic atomic theory Stoichiometry, balancing reactions Thermodynamics
Lecture 4 Chapter 13 - Polymers Functional Groups Condensation Rxns Free Radical Rxns Chemistry the whole year on one page Last semester Basic atomic theory Stoichiometry, balancing reactions Thermodynamics
Intermolecular Forces I
 I How does the arrangement of atoms differ in the 3 phases of matter (solid, liquid, gas)? Why doesn t ice just evaporate into a gas? Why does liquid water exist at all? There must be some force between
I How does the arrangement of atoms differ in the 3 phases of matter (solid, liquid, gas)? Why doesn t ice just evaporate into a gas? Why does liquid water exist at all? There must be some force between
Naming Organic Halides. Properties of Organic Halides
 Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
MODELLING OF THE 3D-STRUCTURE OF CAM:OMA PHOTOPOLYMERS BY USING OF COMPUTATIONAL CHEMISTRY PROGRAM *
 MODELLING OF THE 3D-STRUCTURE OF CAM:OMA PHOTOPOLYMERS BY USING OF COMPUTATIONAL CHEMISTRY PROGRAM * VALERIU BIVOL Center of Optoelectronics of Academy of Sciences of Moldova, 1 Academiei Str., Chisinau,
MODELLING OF THE 3D-STRUCTURE OF CAM:OMA PHOTOPOLYMERS BY USING OF COMPUTATIONAL CHEMISTRY PROGRAM * VALERIU BIVOL Center of Optoelectronics of Academy of Sciences of Moldova, 1 Academiei Str., Chisinau,
Lecture 2. The framework to build materials and understand properties
 Lecture 2 The framework to build materials and understand properties 1 Trees are made into a solid materials/structures in an environment that consists of small molecules: CO 2, N 2, H 2 0, CH 4 O C 2.58Ǻ
Lecture 2 The framework to build materials and understand properties 1 Trees are made into a solid materials/structures in an environment that consists of small molecules: CO 2, N 2, H 2 0, CH 4 O C 2.58Ǻ
SCH 4U Unit Test Forces and Molecular Properties. 1. Fill in each table as done on the assignment. Including the oxidation state of the central atom:
 CH 4U Unit Test Forces and Molecular Properties Name: 1. Fill in each table as done on the assignment. Including the oxidation state of the central atom: BO 3 3- total # of e - pairs σ bonding pairs lone
CH 4U Unit Test Forces and Molecular Properties Name: 1. Fill in each table as done on the assignment. Including the oxidation state of the central atom: BO 3 3- total # of e - pairs σ bonding pairs lone
Comparing Ionic and Covalent Compounds
 Comparing Ionic and Covalent Compounds It takes energy to overcome the forces holding particles together. Thus, it takes energy to cause a substance to go from the liquid to the gaseous state. The boiling
Comparing Ionic and Covalent Compounds It takes energy to overcome the forces holding particles together. Thus, it takes energy to cause a substance to go from the liquid to the gaseous state. The boiling
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Carbonyl Group in Aldehydes and Ketones
 Lecture 4: Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Carbonyl Group in Aldehydes and Ketones A carbonyl group (C=) In an aldehyde is attached to at least one atom. In a ketone
Lecture 4: Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Carbonyl Group in Aldehydes and Ketones A carbonyl group (C=) In an aldehyde is attached to at least one atom. In a ketone
Topic 2.11 ANALYTICAL TECHNIQUES. High Resolution Mass Spectrometry Infra-red Spectroscopy
 Topic 2.11 ANALYTICAL TECHNIQUES High Resolution Mass Spectrometry Infra-red Spectroscopy HIGH RESOLUTION MASS SPECTROMETRY The technique of mass spectrometry was used in Unit 1 to: a) determine the relative
Topic 2.11 ANALYTICAL TECHNIQUES High Resolution Mass Spectrometry Infra-red Spectroscopy HIGH RESOLUTION MASS SPECTROMETRY The technique of mass spectrometry was used in Unit 1 to: a) determine the relative
Investigation of Acoustical Parameters of Polyvinyl Acetate
 Investigation of Acoustical Parameters of Polyvinyl Acetate Pankaj K.Singh (Corresponding author) Department of Physics, H.N.B.Garhwal University Srinagar (Garhwal) 246174 Uttarakhand, India Tel: 91-989-710-3871
Investigation of Acoustical Parameters of Polyvinyl Acetate Pankaj K.Singh (Corresponding author) Department of Physics, H.N.B.Garhwal University Srinagar (Garhwal) 246174 Uttarakhand, India Tel: 91-989-710-3871
Chapter 16 Aldehydes and Ketones Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 16 Aldehydes and Ketones Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 16 Aldehydes and Ketones Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Matter is made of atoms and molecules
 Name Per Talking to the Text Atoms and Molecules pt.2 Author Says (important ideas, vocabulary) Matter is made of atoms and molecules We have already used the term atom and molecule a couple of times.
Name Per Talking to the Text Atoms and Molecules pt.2 Author Says (important ideas, vocabulary) Matter is made of atoms and molecules We have already used the term atom and molecule a couple of times.
An alcohol is a compound obtained by substituting a hydoxyl group ( OH) for an H atom on a carbon atom of a hydrocarbon group.
 Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Chemistry Review: Atoms
 Chemistry Review: Atoms Atoms are made up : nucleus containing protons and neutrons orbitals containing electrons (2, 8, 8,...). Valence electrons outermost electrons Chemistry Review: Atoms All atoms
Chemistry Review: Atoms Atoms are made up : nucleus containing protons and neutrons orbitals containing electrons (2, 8, 8,...). Valence electrons outermost electrons Chemistry Review: Atoms All atoms
Ball-and-Stick Models ChemCatalyst
 I-9: New Smells, New Ideas Ball-and-Stick Models ChemCatalyst Do you think any of these molecules will smell similar? What evidence do you have to support your prediction? citronellol, C 10 H 20 O menthol,
I-9: New Smells, New Ideas Ball-and-Stick Models ChemCatalyst Do you think any of these molecules will smell similar? What evidence do you have to support your prediction? citronellol, C 10 H 20 O menthol,
Probability theory and mathematical statistics:
 N.I. Lobachevsky State University of Nizhni Novgorod Probability theory and mathematical statistics: Geometric probability Practice Associate Professor A.V. Zorine Geometric probability Practice 1 / 7
N.I. Lobachevsky State University of Nizhni Novgorod Probability theory and mathematical statistics: Geometric probability Practice Associate Professor A.V. Zorine Geometric probability Practice 1 / 7
BU Practice Quiz Which type of bond is present in copper wire? 1. An ionic bond can be formed when one or more electrons are
 BU Practice Quiz 1 Name: ate: 1. n ionic bond can be formed when one or more electrons are. equally shared by two atoms B. unequally shared by two atoms 3. Which type of bond is present in copper wire?.
BU Practice Quiz 1 Name: ate: 1. n ionic bond can be formed when one or more electrons are. equally shared by two atoms B. unequally shared by two atoms 3. Which type of bond is present in copper wire?.
CHEMISTRY 110 EXAM 2 Feb 25, 2013 FORM A
 EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
Chemistry 14CL. Worksheet for the Molecular Modeling Workshop. (Revised FULL Version 2012 J.W. Pang) (Modified A. A. Russell)
 Chemistry 14CL Worksheet for the Molecular Modeling Workshop (Revised FULL Version 2012 J.W. Pang) (Modified A. A. Russell) Structure of the Molecular Modeling Assignment The molecular modeling assignment
Chemistry 14CL Worksheet for the Molecular Modeling Workshop (Revised FULL Version 2012 J.W. Pang) (Modified A. A. Russell) Structure of the Molecular Modeling Assignment The molecular modeling assignment
Chapter 23 Phenols CH. 23. Nomenclature. The OH group takes precedence as the parent phenol.
 CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
RW Session ID = MSTCHEM1 Intermolecular Forces
 RW Session ID = MSTCHEM1 Intermolecular Forces Sections 9.4, 11.3-11.4 Intermolecular Forces Attractive forces between molecules due to charges, partial charges, and temporary charges Higher charge, stronger
RW Session ID = MSTCHEM1 Intermolecular Forces Sections 9.4, 11.3-11.4 Intermolecular Forces Attractive forces between molecules due to charges, partial charges, and temporary charges Higher charge, stronger
Lecture 2. The framework to build materials and understand properties
 Lecture 2 The framework to build materials and understand properties 1 Trees are made into a solid materials/structures in an environment that consists of small molecules: C 2, N 2, H 2 0, CH 4 C 2.58Ǻ?
Lecture 2 The framework to build materials and understand properties 1 Trees are made into a solid materials/structures in an environment that consists of small molecules: C 2, N 2, H 2 0, CH 4 C 2.58Ǻ?
Atoms, Molecules, Pure Substances, and Mixtures Activity How do atoms combine to make different types of matter?
 Atoms, Molecules, Pure Substances, and Mixtures Activity How do atoms combine to make different types of matter? Background: Everything is made of matter. Anything you touch, see, taste, or smell even
Atoms, Molecules, Pure Substances, and Mixtures Activity How do atoms combine to make different types of matter? Background: Everything is made of matter. Anything you touch, see, taste, or smell even
1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions:
 1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions: a. largest radius 2 b. stronger acid (first ionization) HN 3 H 3 P 4 H 2 S 4 c. largest radius N 3 2 F e. highest
1. (8 pts) Circle the formula (only one) that best fits each of the following descriptions: a. largest radius 2 b. stronger acid (first ionization) HN 3 H 3 P 4 H 2 S 4 c. largest radius N 3 2 F e. highest
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
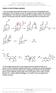 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
AN INTEGRATED SYSTEM USING TEMPERATURE BASED SAMPLING FOR POLYMER CHARACTERIZATION
 AN INTEGRATED SYSTEM USING TEMPERATURE BASED SAMPLING FOR POLYMER CHARACTERIZATION Paper # 164-8P Pittsburgh Conference 24 T. Wampler, C. Zawodny, L. Mancini CDS Analytical, Inc 465 Limestone Road, Oxford,
AN INTEGRATED SYSTEM USING TEMPERATURE BASED SAMPLING FOR POLYMER CHARACTERIZATION Paper # 164-8P Pittsburgh Conference 24 T. Wampler, C. Zawodny, L. Mancini CDS Analytical, Inc 465 Limestone Road, Oxford,
PAPER No.12 :Organic Spectroscopy MODULE No.29: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part I
 Subject Chemistry Paper No and Title Module No and Title Module Tag 12: rganic Spectroscopy 29: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part I CHE_P12_M29 TABLE F CNTENTS 1. Learning utcomes
Subject Chemistry Paper No and Title Module No and Title Module Tag 12: rganic Spectroscopy 29: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part I CHE_P12_M29 TABLE F CNTENTS 1. Learning utcomes
Kinetic Molecular Theory of Gases used to account for Ideal Gas Behavior when gases approach high temperatures and low pressures
 LIQUIDS AND SOLIDS Kinetic Molecular Theory of Gases used to account for Ideal Gas Behavior when gases approach high temperatures and low pressures GASES are very different from solids and liquids. We
LIQUIDS AND SOLIDS Kinetic Molecular Theory of Gases used to account for Ideal Gas Behavior when gases approach high temperatures and low pressures GASES are very different from solids and liquids. We
Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers
 Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Paper No and Title 14: Organic Chemistry IV (Advance Organic
 Subject Chemistry Paper o and Title 14: Organic Chemistry IV (Advance Organic Module o and 1: Fundamentals of disconnection approach Title Module Tag CHE_P14_M1 TABLE OF COTETS 1. Learning Outcomes 2.
Subject Chemistry Paper o and Title 14: Organic Chemistry IV (Advance Organic Module o and 1: Fundamentals of disconnection approach Title Module Tag CHE_P14_M1 TABLE OF COTETS 1. Learning Outcomes 2.
2) C 2 H 2 (g) + 2 H 2 (g) ---> C 2 H 6 (g) Information about the substances
 Thermochemistry 1) 2 C 4 H 10 (g) + 13 O 2 (g) ------> 8 CO 2 (g) + 10 H 2 O(l) The reaction represented above is spontaneous at 25 C. Assume that all reactants and products are in their standard states.
Thermochemistry 1) 2 C 4 H 10 (g) + 13 O 2 (g) ------> 8 CO 2 (g) + 10 H 2 O(l) The reaction represented above is spontaneous at 25 C. Assume that all reactants and products are in their standard states.
THz Oscillations in DNA Monomers, Dimers and Trimers
 Progress In Electromagnetics Research Symposium Proceedings 879 THz Oscillations in DNA Monomers, Dimers and Trimers K. Lamropoulos, K. Kaklamanis, G. Georgiadis, M. Theodorakou, M. Chatzieleftheriou,
Progress In Electromagnetics Research Symposium Proceedings 879 THz Oscillations in DNA Monomers, Dimers and Trimers K. Lamropoulos, K. Kaklamanis, G. Georgiadis, M. Theodorakou, M. Chatzieleftheriou,
DIFFERENT TYPES OF INTEMOLECULAR FORCES INTERMOLECULAR FORCES
 DIFFERENT TYPES OF INTEMOLECULAR FORCES Do all the exercises in your studyguide COMPARISON OF THE THREE PHASES OF MATTER. Matter is anything that occupy space and has mass. There are three states of matter:
DIFFERENT TYPES OF INTEMOLECULAR FORCES Do all the exercises in your studyguide COMPARISON OF THE THREE PHASES OF MATTER. Matter is anything that occupy space and has mass. There are three states of matter:
Diagnosis of Active Groups of Samples Taken (Aniline-Benzoic Acid -Acetic Anhydride and Comparing: The Results of the Laboratory)
 ; ISSN 1916-9698 E-ISSN 1916-9701 Published by Canadian Center of Science and Education Diagnosis of Active Groups of Samples Taken (Aniline-Benzoic Acid -Acetic Anhydride and Comparing: The Results of
; ISSN 1916-9698 E-ISSN 1916-9701 Published by Canadian Center of Science and Education Diagnosis of Active Groups of Samples Taken (Aniline-Benzoic Acid -Acetic Anhydride and Comparing: The Results of
Chemistry 2000 Lecture 19: Organic acids
 Chemistry 2000 Lecture 19: Organic acids Marc R. Roussel March 8, 2018 Marc R. Roussel Chemistry 2000 Lecture 19: Organic acids March 8, 2018 1 / 22 Organic acids The acid dissociation constant, K a The
Chemistry 2000 Lecture 19: Organic acids Marc R. Roussel March 8, 2018 Marc R. Roussel Chemistry 2000 Lecture 19: Organic acids March 8, 2018 1 / 22 Organic acids The acid dissociation constant, K a The
Dr. Mohamed El-Newehy
 By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Aldehydes and Ketones 1 Structure of Aldehydes and Ketones - Aldehydes and ketones
By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Aldehydes and Ketones 1 Structure of Aldehydes and Ketones - Aldehydes and ketones
Radical Initiation 2017/2/ ) Thermal Decomposition of Initiators
 adical Initiation Production of radicals (from initiator) to initiate chain polymerization. A variety of initiator systems can be used to bring about the radical polymerization. 1) Thermal Decomposition
adical Initiation Production of radicals (from initiator) to initiate chain polymerization. A variety of initiator systems can be used to bring about the radical polymerization. 1) Thermal Decomposition
EXPT. 9 DETERMINATION OF THE STRUCTURE OF AN ORGANIC COMPOUND USING UV, IR, NMR AND MASS SPECTRA
 EXPT. 9 DETERMINATION OF THE STRUCTURE OF AN ORGANIC COMPOUND USING UV, IR, NMR AND MASS SPECTRA Structure 9.1 Introduction Objectives 9.2 Principle 9.3 Requirements 9.4 Strategy for the Structure Elucidation
EXPT. 9 DETERMINATION OF THE STRUCTURE OF AN ORGANIC COMPOUND USING UV, IR, NMR AND MASS SPECTRA Structure 9.1 Introduction Objectives 9.2 Principle 9.3 Requirements 9.4 Strategy for the Structure Elucidation
CHAPTER 2: RESONANCE THEORY
 CAPTER 2: RESACE TERY The Basics. Use the Rules for writing acceptable contributing structures for this exercise (some hydrogens have been added for clarity) 1. For each example, add curved arrows to the
CAPTER 2: RESACE TERY The Basics. Use the Rules for writing acceptable contributing structures for this exercise (some hydrogens have been added for clarity) 1. For each example, add curved arrows to the
Look for absorption bands in decreasing order of importance:
 1. Match the following to their IR spectra (30 points) Look for absorption bands in decreasing order of importance: a e a 2941 1716 d f b 3333 c b 1466 1.the - absorption(s) between 3100 and 2850 cm-1.
1. Match the following to their IR spectra (30 points) Look for absorption bands in decreasing order of importance: a e a 2941 1716 d f b 3333 c b 1466 1.the - absorption(s) between 3100 and 2850 cm-1.
Honors Chemistry. Chapter 10: Forces of Attraction, Phase Change, Date / / Period and Solids Answer Key. Intermolecular and Intramolecular Forces
 Honors Chemistry Name Chapter 10: Forces of Attraction, Phase Change, Date / / Period and Solids Answer Key Complete each of the following questions directly on this worksheet. Intermolecular and Intramolecular
Honors Chemistry Name Chapter 10: Forces of Attraction, Phase Change, Date / / Period and Solids Answer Key Complete each of the following questions directly on this worksheet. Intermolecular and Intramolecular
Organic Chemistry. Introduction to Organic Molecules and Functional Groups
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
Improving the quality of coal is one of the factors in intensification of combustion processes in coal-based energy
 Improving the quality of coal is one of the factors in intensification of combustion processes in coal-based energy Yuriy Patrakov 1, Svetlana Semenova 1, and Anna Usanina 1,2* 1 The Federal Research Center
Improving the quality of coal is one of the factors in intensification of combustion processes in coal-based energy Yuriy Patrakov 1, Svetlana Semenova 1, and Anna Usanina 1,2* 1 The Federal Research Center
Supporting Information
 Supporting Information Distinct roles of the catalytic cysteine and histidine in the protease and ligase mechanisms of human legumain as revealed by DFT based QM/MM simulations *Brigitta Elsässer, Florian
Supporting Information Distinct roles of the catalytic cysteine and histidine in the protease and ligase mechanisms of human legumain as revealed by DFT based QM/MM simulations *Brigitta Elsässer, Florian
Name Date Class. aryl halides substitution reaction
 23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
Introduction to Botany
 Introduction to Botany Alexey Shipunov Minot State University Lecture 5 Shipunov (MSU) Introduction to Botany Lecture 5 1 / 14 Outline 1 Questions and answers Quiz 2 Shipunov (MSU) Introduction to Botany
Introduction to Botany Alexey Shipunov Minot State University Lecture 5 Shipunov (MSU) Introduction to Botany Lecture 5 1 / 14 Outline 1 Questions and answers Quiz 2 Shipunov (MSU) Introduction to Botany
Electonegativity, Polar Bonds, and Polar Molecules
 Electonegativity, Polar Bonds, and Polar Molecules Some Definitions Electronegativity: the ability of an atom to attract bonding electrons to itself. Intramolecular forces: the attractive force between
Electonegativity, Polar Bonds, and Polar Molecules Some Definitions Electronegativity: the ability of an atom to attract bonding electrons to itself. Intramolecular forces: the attractive force between
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
14.1 Aldehydes and Ketones Copyright 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings
 Chapter 14 Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Copyright 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings Carbonyl Group in Aldehydes A carbonyl group and
Chapter 14 Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Copyright 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings Carbonyl Group in Aldehydes A carbonyl group and
POLYSTYRENE (General purpose)(gpps)
 Eco-profiles of the European Plastics Industry POLYSTYRENE (General purpose)(gpps) A report by I Boustead for PlasticsEurope Data last calculated March 2005 gpps 1 IMPORTANT NOTE Before using the data
Eco-profiles of the European Plastics Industry POLYSTYRENE (General purpose)(gpps) A report by I Boustead for PlasticsEurope Data last calculated March 2005 gpps 1 IMPORTANT NOTE Before using the data
Basic introduction of NWChem software
 Basic introduction of NWChem software Background! NWChem is part of the Molecular Science Software Suite! Designed and developed to be a highly efficient and portable Massively Parallel computational chemistry
Basic introduction of NWChem software Background! NWChem is part of the Molecular Science Software Suite! Designed and developed to be a highly efficient and portable Massively Parallel computational chemistry
WKS Name Intermolecular Forces Period Date
 WKS Name Intermolecular orces Period Date Introduction: Substances exist in three states of matter: solids, liquids and gases. We know that molecules are... (a) far apart in gases; (b) close together,
WKS Name Intermolecular orces Period Date Introduction: Substances exist in three states of matter: solids, liquids and gases. We know that molecules are... (a) far apart in gases; (b) close together,
Part 1: Types of Chemical Bonds
 Unit 7: Bonding Review Directions: Complete as much of this packet as you can WITHOUT USING YOUR NOTES. Treat it as a practice test. Star any questions that you cannot answer on your own, and then go back
Unit 7: Bonding Review Directions: Complete as much of this packet as you can WITHOUT USING YOUR NOTES. Treat it as a practice test. Star any questions that you cannot answer on your own, and then go back
Chemistry Physical, Chemical, and Nuclear Changes
 Chemistry 1010 Physical, Chemical, and Nuclear Changes Review Which state of matter matches the following pictures? gas solid liquid What could the circles in these pictures represent? usually molecules,
Chemistry 1010 Physical, Chemical, and Nuclear Changes Review Which state of matter matches the following pictures? gas solid liquid What could the circles in these pictures represent? usually molecules,
Quiz Act # s Study Guide
 Name: Activity #15-Families of Elements 1.) Define an element Quiz Act # s 15-21 Study Guide 2.) Whether something is in the solid, liquid, or gas form is known as its state of matter. Is an elements state
Name: Activity #15-Families of Elements 1.) Define an element Quiz Act # s 15-21 Study Guide 2.) Whether something is in the solid, liquid, or gas form is known as its state of matter. Is an elements state
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
BIOB111_CHBIO - Tutorial activities for session 9
 BIOB111_CHBIO - Tutorial activities for session 9 General topics for week 5 Session 9 Physical properties and chemical reactions of organic compounds (functional groups: alcohols, phenols, ethers, aldehydes,
BIOB111_CHBIO - Tutorial activities for session 9 General topics for week 5 Session 9 Physical properties and chemical reactions of organic compounds (functional groups: alcohols, phenols, ethers, aldehydes,
STUDY OF ACYLATION REACTION OF FUNCTIONALLY SUBSTITUTED AROMATIC MONO- AND DIAMINES WITH CHLOROANHYDRIDE OF NAPHTHENIC ACID
 STUDY OF ACYLATION REACTION OF FUNCTIONALLY SUBSTITUTED AROMATIC MONO- AND DIAMINES WITH CHLOROANHYDRIDE OF NAPHTHENIC ACID M.A.Rustamov, G.A.Mirzayeva, Sh.M.Eyvazova, N. A.Veysova Azerbaijan Technical
STUDY OF ACYLATION REACTION OF FUNCTIONALLY SUBSTITUTED AROMATIC MONO- AND DIAMINES WITH CHLOROANHYDRIDE OF NAPHTHENIC ACID M.A.Rustamov, G.A.Mirzayeva, Sh.M.Eyvazova, N. A.Veysova Azerbaijan Technical
Core v Valence Electrons
 Bonding Core v Valence Electrons The core electrons (represented by the noble gas from the previous row) are those electrons held within the atom. These electrons are not involved in the bonding, but contribute
Bonding Core v Valence Electrons The core electrons (represented by the noble gas from the previous row) are those electrons held within the atom. These electrons are not involved in the bonding, but contribute
Photolithography Overview 9/29/03 Brainerd/photoclass/ECE580/Overvie w/overview
 http://www.intel.com/research/silicon/mooreslaw.htm 1 Moore s law only holds due to photolithography advancements in reducing linewidths 2 All processing to create electric components and circuits rely
http://www.intel.com/research/silicon/mooreslaw.htm 1 Moore s law only holds due to photolithography advancements in reducing linewidths 2 All processing to create electric components and circuits rely
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
To learn how to use molecular modeling software, a commonly used tool in the chemical and pharmaceutical industry.
 NAME: Lab Day/Time: Molecular Modeling BV 1/2009 Purpose The purposes of this experiment are: To learn how to use molecular modeling software, a commonly used tool in the chemical and pharmaceutical industry.
NAME: Lab Day/Time: Molecular Modeling BV 1/2009 Purpose The purposes of this experiment are: To learn how to use molecular modeling software, a commonly used tool in the chemical and pharmaceutical industry.
AS Demonstrate understanding of the properties of selected organic compounds. Collated Polymer questions
 AS 91165 Demonstrate understanding of the properties of selected organic compounds Collated Polymer questions (2017) (a) Polyvinyl chloride (polychloroethene) is often used to make artificial leather.
AS 91165 Demonstrate understanding of the properties of selected organic compounds Collated Polymer questions (2017) (a) Polyvinyl chloride (polychloroethene) is often used to make artificial leather.
Appendix: Teaching timetable and associated language focused activities.
 Electronic Supplementary Material (ESI) for Chemistry Education Research and Practice. This journal is The Royal Society of Chemistry 2018 Appendix: Teaching timetable and associated language focused activities.
Electronic Supplementary Material (ESI) for Chemistry Education Research and Practice. This journal is The Royal Society of Chemistry 2018 Appendix: Teaching timetable and associated language focused activities.
Chapter 11. Intermolecular Forces, Liquids, and Solids
 Sample Exercise 11.1 (p. 450) In which of the following substances is hydrogen bonding likely to play an important role in determining physical properties: methane (CH 4 ), hydrazine (H 2 NNH 2 ), methyl
Sample Exercise 11.1 (p. 450) In which of the following substances is hydrogen bonding likely to play an important role in determining physical properties: methane (CH 4 ), hydrazine (H 2 NNH 2 ), methyl
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
P O L Y M E R S. The Academic Support Daytona State College (Science 106, Page 1 of 25
 P O L Y M E R S The Academic Support Center @ Daytona State College (Science 106, Page 1 of 25 POLYMERS Polymers are large, long-chain molecules. found in nature, including cellulose in plants, starches
P O L Y M E R S The Academic Support Center @ Daytona State College (Science 106, Page 1 of 25 POLYMERS Polymers are large, long-chain molecules. found in nature, including cellulose in plants, starches
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
An Auxiliary-Field Quantum Monte Carlo Study of the Chromium Dimer. Abstract
 An Auxiliary-Field Quantum Monte Carlo Study of the Chromium Dimer Wirawan Purwanto, Shiwei Zhang, and Henry Krakauer Department of Physics, College of William and Mary, Williamsburg, Virginia 23187-8795,
An Auxiliary-Field Quantum Monte Carlo Study of the Chromium Dimer Wirawan Purwanto, Shiwei Zhang, and Henry Krakauer Department of Physics, College of William and Mary, Williamsburg, Virginia 23187-8795,
Properties of Solutions. Review
 Properties of Solutions Review Matter Pure substance Mixture of substances compound element homogeneous heterogeneous Solution Definitions A solution is a homogeneous mixture of two or more substances.
Properties of Solutions Review Matter Pure substance Mixture of substances compound element homogeneous heterogeneous Solution Definitions A solution is a homogeneous mixture of two or more substances.
UNIVERSITY OF CALIFORNIA College of Engineering Department of Electrical Engineering and Computer Sciences. Professor Ali Javey. Spring 2009.
 UNIVERSITY OF CALIFORNIA College of Engineering Department of Electrical Engineering and Computer Sciences EE143 Professor Ali Javey Spring 2009 Exam 1 Name: SID: Closed book. One sheet of notes is allowed.
UNIVERSITY OF CALIFORNIA College of Engineering Department of Electrical Engineering and Computer Sciences EE143 Professor Ali Javey Spring 2009 Exam 1 Name: SID: Closed book. One sheet of notes is allowed.
Solutions and solubility - Grade 11
 OpenStax-CNX module: m35865 1 Solutions and solubility - Grade 11 Rory Adams Free High School Science Texts Project Heather Williams This work is produced by OpenStax-CNX and licensed under the Creative
OpenStax-CNX module: m35865 1 Solutions and solubility - Grade 11 Rory Adams Free High School Science Texts Project Heather Williams This work is produced by OpenStax-CNX and licensed under the Creative
Chapter 7: Alcohols, Phenols and Thiols
 Chapter 7: Alcohols, Phenols and Thiols Nomenclature of Alcohols In the IUPAC system, the hydroxyl group in alcohols is indicated by the ending ol. In common names, the separate word alcohol is placed
Chapter 7: Alcohols, Phenols and Thiols Nomenclature of Alcohols In the IUPAC system, the hydroxyl group in alcohols is indicated by the ending ol. In common names, the separate word alcohol is placed
The Basics of General, Organic, and Biological Chemistry
 The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
PCCP Accepted Manuscript
 PCCP Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published
PCCP Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published
tert-butyl alcohol n-butyl alcohol methyl propyl ether (c) Explain briefly why n-butyl alcohol has a much higher bp than methyl propyl ether.
 1. (15 points) Three 4 10 isomers are shown below, along with their boiling points. ( 3 ) 3 3 2 2 2 3 2 2 3 tert-butyl alcohol n-butyl alcohol methyl propyl ether bp: 82 118 39 (a) Based on their boiling
1. (15 points) Three 4 10 isomers are shown below, along with their boiling points. ( 3 ) 3 3 2 2 2 3 2 2 3 tert-butyl alcohol n-butyl alcohol methyl propyl ether bp: 82 118 39 (a) Based on their boiling
How do electronegativity values determine the charge distribution in a polar bond?
 Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright Pearson Education, Inc., or its affiliates. All Rights
Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright Pearson Education, Inc., or its affiliates. All Rights
Sparks CH301. WHY IS EVERYTHING SO DIFFERENT? Gas, Liquid or Solid? UNIT 3 Day 7
 Sparks CH301 WHY IS EVERYTHING SO DIFFERENT? Gas, Liquid or Solid? UNIT 3 Day 7 What are we going to do today? Discuss types of intermolecular forces. Compare intermolecular forces for different molecules.
Sparks CH301 WHY IS EVERYTHING SO DIFFERENT? Gas, Liquid or Solid? UNIT 3 Day 7 What are we going to do today? Discuss types of intermolecular forces. Compare intermolecular forces for different molecules.
ORGANIC - EGE 5E CH. 2 - COVALENT BONDING AND CHEMICAL REACTIVITY
 !! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
!! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
Section 2: How Substances Dissolve. Preview Key Ideas Bellringer Water: A Common Solvent The Dissolving Process Surface Area
 : How Substances Dissolve Preview Key Ideas Bellringer Water: A Common Solvent The Dissolving Process Surface Area Key Ideas Why is water called the universal solvent? Why do substances dissolve? Bellringer
: How Substances Dissolve Preview Key Ideas Bellringer Water: A Common Solvent The Dissolving Process Surface Area Key Ideas Why is water called the universal solvent? Why do substances dissolve? Bellringer
Maya Alekseeva, Elena Fedoseeva 1, Vadim Frolov 1, Viacheslav Nistratov 1, Larisa Smirnova
 THE STRENGTH OF CHITOSAN FILMS. THE ROLE OF MOLECULAR WEIGHT, THE DEGREE OF ORDER, THE NATURE OF CONTRE-ION Maya Alekseeva, Elena Fedoseeva 1, Vadim Frolov 1, Viacheslav Nistratov 1, Larisa Smirnova Nizhny
THE STRENGTH OF CHITOSAN FILMS. THE ROLE OF MOLECULAR WEIGHT, THE DEGREE OF ORDER, THE NATURE OF CONTRE-ION Maya Alekseeva, Elena Fedoseeva 1, Vadim Frolov 1, Viacheslav Nistratov 1, Larisa Smirnova Nizhny
Luckily this intermediate has three saturated carbons between the carbonyls, which again points to a Michael reaction:
 Retrosynthesis Practice Problems Answer Key October 1, 2013 1. Draw a retrosynthesis for how to make the compound shown below from starting materials with eight or fewer carbon atoms. The first step is
Retrosynthesis Practice Problems Answer Key October 1, 2013 1. Draw a retrosynthesis for how to make the compound shown below from starting materials with eight or fewer carbon atoms. The first step is
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water.
 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
