scientific report Separate fusion of outer and inner mitochondrial membranes scientificreport
|
|
|
- Basil Tucker
- 6 years ago
- Views:
Transcription
1 scientificreport Separate fusion of outer and inner mitochondrial membranes Florence Malka 1, Olwenn Guillery 1, Carmen Cifuentes-Diaz 2, Emmanuelle Guillou 3, Pascale elenguer 3, nne Lombès 1 & Manuel Rojo 1+ 1 INSERM U582, Institut de Myologie, Université Pierre et Marie Curie, IFR14, Groupe Hospitalier Pitié-Salpêtrière, Paris, France, 2 INSERM U706/536, Paris, France, and 3 LCMCP, CNRS UMR 5088, Université Paul Sabatier, Toulouse, France Mitochondria are enveloped by two closely apposed boundary membranes with different properties and functions. It is known that they undergo fusion and fission, but it has remained unclear whether outer and inner membranes fuse simultaneously, coordinately or separately. We set up assays for the study of inner and outer membrane fusion in living human cells. Inner membrane fusion was more sensitive than outer membrane fusion to inhibition of glycolysis. Fusion of the inner membrane, but not of the outer membrane, was abolished by dissipation of the inner membrane potential with K þ (valinomycin) or H þ ionophores (cccp). In addition, outer and inner membrane fusion proceeded separately in the absence of any drug. The separate fusion of outer and inner membranes and the different requirements of these fusion reactions point to the existence of fusion machineries that can function separately. Keywords: membrane fusion; mitochondrial fusion; mitochondrial fission; membrane potential; Drp1 EMO reports (2005) 6, doi: /sj.embor INTRODUCTION Mitochondria are enveloped by two closely apposed membranes with different properties and functions. The inner membrane delimits the matrix space and contains respiratory complexes for oxidative phosphorylation. The outer membrane mediates exchange between cytosol and intermembrane space (Frey & Mannella, 2000). Signalling cascades converging to mitochondria affect outer and inner membranes differentially, and various apoptotic stimuli that induce selective permeabilization of the outer membrane leave the permeability and function of the inner membrane transiently unaffected (Waterhouse et al, 2002). 1 INSERM U582, Institut de Myologie, Université Pierre et Marie Curie, IFR14, Groupe Hospitalier Pitié-Salpêtrière, 47 blvd de l Hôpital, Paris Cedex 13, France 2 INSERM U706/536, 17 rue de Fer à Moulin, Paris, France 3 LCMCP, CNRS UMR 5088, Université Paul Sabatier, 118 Route de Narbonne 31062, Toulouse, France + Corresponding author. Tel: þ /17 (office/lab); Fax: þ ; m.rojo@myologie.chups.jussieu.fr Received 20 December 2004; revised 17 June 2005; accepted 20 June 2005; published online 29 July 2005 Mitochondria are dynamic organelles that fuse and divide. Fusion mediates molecular exchanges between mitochondria (Legros et al, 2004) and its disruption provokes severe defects (Chen et al, 2003). Three essential fusion factors are known in yeast: two transmembrane proteins of the outer membrane (Fzo1 and Ugo1) and one intermembrane space protein associated with the inner membrane (Mgm1). The interactions between yeast Ugo1, Fzo1 and Mgm1 (Wong et al, 2003; Sesaki & Jensen, 2004) and the localization of Fzo1 to contact sites between the outer and inner membranes (Fritz et al, 2001) suggest that fusion of double membranes is mediated by a single protein complex. In contrast, the recent identification of fusion intermediates in vitro (Meeusen et al, 2004) suggests that the outer and inner membranes may fuse in successive reactions. The situation is less clear in mammals. They have two homologues of Fzo1 (mitofusins Mfn1 and Mfn2; Rojo et al, 2002), one homologue of Mgm1 (OP1; Olichon et al, 2003) and no homologue of Ugo1. The role of mitofusins in fusion has been clearly established (Chen et al, 2003), but the precise function of OP1 remains unclear (Olichon et al, 2003; Cipolat et al, 2004; Griparic et al, 2004). The exchange of matrix proteins indicates fusion of mitochondrial double membranes (Legros et al, 2002), but it has remained unclear whether outer and inner membranes fuse in a single reaction or in successive steps. We set up assays for the study of outer and inner membrane fusion in living human cells and showed that the fusion reactions have different requirements and can proceed separately. RESULTS ND DISCUSSION Deoxyglucose affects mitochondrial fusion reactions To study the requirements of outer and inner membrane fusion, we fused cells containing differently labelled mitochondria in the presence of known inhibitors of energy production. Cells were first treated with deoxyglucose, an inhibitor of glycolysis, or oligomycin, an inhibitor of TP synthase. Cells survived for days with either treatment, but a combination of both drugs led to rapid cell death (data not shown). Cellular TP levels were almost unaffected by treatment with oligomycin, but decreased significantly in the presence of deoxyglucose (supplementary Table 1 &2005 EUROPEN MOLECULR IOLOGY ORGNIZTION EMO reports VOL 6 NO
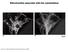
2 Separate fusion of mitochondrial membranes online), as previously described for HeLa cells (Legros et al, 2002). This shows that glycolysis alone can maintain normal cellular TP levels, but that oxidative phosphorylation cannot fully compensate the lack of cytosolic TP synthesis. To study the fusion of mitochondrial double membranes, we fused cells containing different fluorescent proteins targeted to the matrix (matrix red fluorescent protein (mtrfp) or matrix green fluorescent protein (mtgfp); Fig 1). Cells were fixed 16 h after fusion, a period that is sufficient to achieve distribution of matrix proteins throughout mitochondria (Fig 1, no drug). Mitochondrial fusion was inhibited in 70% of deoxyglucose-treated polykaryons: mixing of fluorescent proteins was restricted to small areas and large regions were devoid of one of the fluorescent proteins (Fig 1, deoxyglucose). t this stage, it was not possible to infer whether deoxyglucose treatment had affected a step preceding fusion (such as mitochondrial mobility, tethering or docking) and/or the fusion of mitochondrial membranes. We therefore fused cells containing mtrfp with cells expressing a GFP molecule anchored to the outer membrane (GFPOM; see the supplementary information online). In the presence of deoxyglucose, all mtrfp-labelled mitochondria became positive for GFPOM in all polykaryons (Fig 1), showing that outer membrane fusion and the preceding tethering/docking steps were not inhibited. In contrast, the absence of mtrfp from large regions in numerous polykaryons (Fig 1) showed that inner membrane fusion was partially inhibited by deoxyglucose. The differential sensitivity of outer and inner membrane fusion to deoxyglucose showed that the fusion reactions can proceed separately and have different requirements. The inhibition of inner membrane fusion could be due to the changes in mitochondrial structure provoked by stimulated respiration (see later) or to the decrease of cellular TP levels. In vitro studies have shown that, in the absence of cytosol, the fusion of docked yeast mitochondria requires GTP (not TP) for completion (Meeusen et al, 2004). It is thus possible that deoxyglucose lowers the concentration of GTP and/or the GTP/GDP ratio. When mitochondrial TP synthesis was inhibited with oligomycin, most of the polykaryons had fluorescent proteins distributed throughout the mitochondrial compartment (Fig 1, colocalization), suggesting that oxidative phosphorylation is dispensable for mitochondrial fusion. It confirms previous studies of mitochondrial fusion in human cells and in yeast cells devoid of mitochondrial DN (and thus of a functional respiratory chain; Okamoto et al, 1998; Legros et al, 2002, 2004). In contrast, yeast mitochondria fuse in vitro only if they have been isolated from respiring cells (Meeusen et al, 2004). The differences between in vivo and in vitro fusion may be due to the absence in vitro of yet unknown factors or to changes of mitochondrial properties accompanying cell homogenization and subcellular fractionation. Ionophores selectively inhibit inner membrane fusion We have previously shown that dissipation of the mitochondrial membrane potential (DC m ) with the protonophore cccp abolishes mitochondrial fusion (Legros et al, 2002). To investigate whether mitochondrial fusion was dependent on the proton gradient or on DC m, we compared the effects of cccp (a protonophore) and valinomycin (a potassium-specific ionophore). oth drugs mediate the influx of cations until dissipation of DC m and uncouple respiration from TP synthesis. ccordingly, each ionophore had No drug Deoxyglucose cccp Valinomycin Colocalization % polykaryons mtrfp+mgfp mtrfp+gfpom Total Partial No Total Partial No % polykaryons nd dg om cp vm nd dg om cp vm Fig 1 Outer and inner membrane fusion is differentially affected by drugs. Cells containing matrix red fluorescent protein (mtrfp) were fused with cells containing matrix green fluorescent protein (mtgfp; ) or GFPOM (a GFP molecule anchored to the outer membrane; ) and maintained under control conditions (nd) or in the presence of deoxyglucose (dg), oligomycin (om), cccp (cp) or valinomycin (vm). fter 16 h, polykaryons were fixed and analysed for fluorescent protein distribution. Scale bars, 10 mm. The quantitative analysis of colocalization indicates the percentage of polykaryons (nx100 for each condition) with total, partial or no colocalization of mtrfp and mtgfp () or mtrfp and GFPOM (). Matrix mtrfp and mtgfp as well as outer membrane GFPOM distributed throughout mitochondria under control conditions. Deoxyglucose partially prevented the diffusion of matrix mtrfp and mtgfp but not that of outer membrane GFPOM. Valinomycin and cccp abolished the exchange of mtrfp and mtgfp but not that of GFPOM. Intermixing of mtgfp, mtrfp and GFPOM was not significantly affected by oligomycin. 854 EMO reports VOL 6 NO &2005 EUROPEN MOLECULR IOLOGY ORGNIZTION
3 Separate fusion of mitochondrial membranes scientific report a minor effect on cellular TP levels, as observed with oligomycin (supplementary Table 1 online). To study the effect of ionophores on mitochondrial double membrane fusion, cells expressing different matrix fluorescent proteins (mtgfp or mtrfp) were fused in the presence of cccp or valinomycin. fter 16 h, the mitochondria of all polykaryons remained singly labelled, indicating complete inhibition of mitochondrial fusion (Fig 1). The finding that both ionophores were inhibitory indicates that DC m, rather than the proton or potassium gradients, is required for fusion, as in yeast (Meeusen et al, 2004). To investigate whether ionophores inhibited fusion of the outer membrane, inner membrane or both, we fused cells containing mtrfp with cells containing GFPOM. fter 16 h in the presence of ionophore, all mtrfp-containing mitochondria had become GFPOM positive (Fig 1), suggesting that the outer membranes had fused in the absence of DC m. The persistence of GFPOMpositive, mtrfp-negative mitochondria (Fig 1) showed that inner membrane fusion was inhibited. Selective inhibition of inner membrane fusion after dissipation of DC m confirms that outer and inner membrane fusion can proceed separately and have different requirements. These results contradict in vitro studies in which cccp, not valinomycin, abolished outer membrane fusion of yeast mitochondria (Meeusen et al, 2004). This divergence may show differences between yeast and human mitochondria or point to different requirements of in vivo and in vitro fusion. Drp1-mediated outer membrane fission In cccp-treated cells, mitochondria appeared as punctate structures that remained small and were largely scattered (Fig 1; supplementary Fig S1 online). Inhibition of fission with a dominant-negative mutant of dynamin-related protein 1 (Drp1 K38) abolished cccp-induced fragmentation (supplementary Fig S1 online), as previously described (Legros et al, 2002). This confirms that fusion, not fission, was inhibited with cccp and that Drp1-mediated fission provokes mitochondrial fragmentation. The transfer of GFPOM and the punctate morphology of mitochondria in cccp-treated polykaryons (Fig 1) suggest that outer membrane fusion was followed by outer membrane fission. To investigate this, we developed a novel fusion assay in which one cell population (donor RMGO cells) is doubly labelled with matrix mtrfp and outer membrane GFPOM (Fig 2). The second cell population (acceptor CN cells) has unlabelled mitochondria and is identified by CFPnuc, a cyan fluorescent protein targeted to the nucleus visible in the GFP channel (Fig 2). cceptor and donor cells were co-plated, fused and fixed after 16 h in the absence or presence of drugs. Polykaryons resulting from the fusion of donor and acceptor cells were identified by the coexistence of GFPOM, mtrfp and CFPnuc. Under control conditions, both mtrfp and GFPOM distributed throughout the network of filamentous mitochondria (Fig 2). In the presence of deoxyglucose, when inner membrane fusion is inhibited (Fig 1), numerous polykaryons contained GFPOM-positive, mtrfp-negative mitochondria (Fig 2C). In the presence of cccp, the coexistence of doubly labelled donor mitochondria and acceptor mitochondria labelled with GFPOM only (Fig 3) showed that only the outer membrane had undergone fusion, which was followed by outer membrane fission. To study fission, donor and acceptor cells were transfected with dominant-negative Drp1 K38 before cell fusion. The coexistence of filamentous and punctate mitochondria (Fig 3) showed that Drp1 K38 had partially inhibited fission. Residual fission probably resulted from the fusion of some untransfected cells devoid of Drp1 K38. Nevertheless, the observation of filamentous singly labelled acceptor mitochondria connected to doubly labelled donor mitochondria (Fig 3, arrowheads) indicated that Drp1 K38 had inhibited outer membrane fission in mitochondria that had undergone outer membrane fusion. This shows that, in cccp-treated cells, outer membrane fusion is followed by Drp1-mediated outer membrane fission, which leads to the punctate mitochondrial morphology. Despite its similar effect on outer and inner membrane fusion, valinomycin induced significantly different changes in mitochondrial distribution and morphology: the mitochondria of valinomycin-treated cells were significantly enlarged and coalesced perinuclearly (Fig 1; supplementary Fig S1 online). Enlargement is probably due to the entry of water that follows potassium influx and swells mitochondria (ernardi, 1999), but the mechanisms of perinuclear coalescence are unknown. Interestingly, the expression of Drp1 K38 did not hamper the appearance and coalescence of swollen mitochondria, especially after long incubations (supplementary Fig S1 online). fter fusion of donor RMGO and acceptor CN cells in the presence of valinomycin, doubly labelled donor mitochondria coexisted with acceptor mitochondria that were positive for GFPOM only (Fig 3C), confirming fusion of only the outer membrane. large number of donor and acceptor mitochondria were not scattered but remained in close proximity (Fig 3C), as observed previously (Fig 1; supplementary Fig S1 online). The transfection of dominant-negative Drp1 K38 before fusion of valinomycintreated cells had no noticeable effect on the distribution of mitochondria and on the selective transfer of GFPOM (data not shown), suggesting that outer membrane fission did not follow outer membrane fusion. Mitochondrial ultrastructure in drug-treated cells Electron microscopy showed that mitochondria have an orthodox structure (light matrix space and narrow cristae) in control cells (Fig 4). In cells treated with deoxyglucose, mitochondria were a normal size but contained an electron-dense matrix and some swollen cristae (Fig 4). These features, characteristic of the condensed conformation that mitochondria adopt after stimulation of mitochondrial respiration (Hackenbrock et al, 1971), indicate that oxidative phosphorylation is stimulated in deoxyglucose-treated cells. s predicted by fluorescence microscopy, the mitochondria of cccp-treated cells were small, whereas those of valinomycin-treated cells were significantly larger than those in control cells (Fig 4C,D). In valinomycin-treated cells, large swollen mitochondria with a clear matrix space and few cristae coexisted with fewer mitochondria having a more orthodox structure (Fig 4D). Interestingly, numerous mitochondria contained separate matrix compartments that were enveloped by a single outer membrane, but had different electron densities and differently structured cristae (Fig 4D, arrowheads; supplementary Fig S2 online). This indicates that the mitochondria enveloped by a single outer membrane contained matrices separated by an unfused inner membrane and that mitochondrial outer membrane fission is inhibited in valinomycin-treated cells. It cannot be excluded that a potassium gradient across the inner membrane is required for outer membrane fission. However, it is tempting to speculate that the &2005 EUROPEN MOLECULR IOLOGY ORGNIZTION EMO reports VOL 6 NO
4 Separate fusion of mitochondrial membranes C Overlay R+G mtrfp GFPOM/CFPnuc COX2 Fig 2 ssay for the parallel observation of outer and inner membrane fusion. Donor RMGO cells expressing two fluorescent proteins targeted to mitochondria (matrix red fluorescent protein (mtrfp) and a green fluorescent protein molecule anchored to the outer membrane (GFPOM)) and acceptor CN cells expressing CFPnuc (a cyan fluorescent protein targeted to the nucleus) were co-plated () and fused for 16 h under control conditions () or in the presence of deoxyglucose (C). fter fixation, all mitochondria were visualized with antibodies against COX2. Mitochondria of RMGO cells are doubly labelled with mtrfp and GFPOM and the nucleus of CN cells is labelled with CFPnuc (). fter fusion of RMGO and CN cells, nuclei are labelled with CFPnuc and the entire mitochondrial compartment is labelled with GFPOM and mtrfp (). In the presence of deoxyglucose, polykaryons depict mitochondria positive for GFPOM and negative for mtrfp (C, arrowheads). Scale bars, 10 mm. increase in size provoked by swelling, rather than the absence of a potassium gradient, inhibits outer membrane fission. Outer and inner membranes can fuse separately The capacity to uncouple outer and inner membrane fusion with drugs (this work) or after in vitro reconstitution (Meeusen et al, 2004) suggests that both reactions may also be uncoupled in living cells. To compare the fusion of outer and inner membranes in the absence of drugs, we used an assay based on the fusion of doubly labelled donor mitochondria with nonlabelled acceptor mitochondria (RMGO and CN cells; Fig 2). Donor and acceptor cells were co-plated, fused and fixed after 4 h, a time period that is insufficient for diffusion of matrix fluorescent proteins throughout mitochondria. Fixed cells were decorated with antibodies against mitochondrial proteins to visualize the entire mitochondrial compartment. If outer and inner membrane fusion proceeded simultaneously, acceptor mitochondria should be equally labelled with both GFPOM and mtrfp. However, if outer and inner membrane fusion proceeded separately, some mitochondria should be labelled with only GFPOM, at least transiently. t 4 h after fusion, most of the polykaryons contained mitochondria that were doubly labelled with GFPOM and mtrfp (Fig 5, quantification), indicating that fusion of outer and inner membranes had occurred simultaneously. However, the presence of a significant proportion of polykaryons (Fig 5, quantification) containing GFPOM-positive, mtrfp-negative mitochondria (Fig 5, arrowheads and boxed area) suggested that the transfer of GFPOM (by outer membrane fusion) was not accompanied by that of mtrfp (by inner membrane fusion). To investigate the possible influence of mitochondrial respiration on the coupling of outer and inner membrane fusion, we fused cells devoid of mitochondrial DN that lack a functional respiratory chain and rely on glycolysis for energy supply. The separate fusion of outer and inner membranes (Fig 5) was observed in a similar proportion of polykaryons (Fig 5, quantification), showing that separate fusion of outer and inner membranes occurs with similar frequency in respiring and nonrespiring mitochondria. We performed control experiments to confirm that the segregation of GFPOM and mtrfp 856 EMO reports VOL 6 NO &2005 EUROPEN MOLECULR IOLOGY ORGNIZTION
5 Separate fusion of mitochondrial membranes scientific report C C D Fig 3 Outer membrane fusion is followed by Drp1-mediated outer membrane fission. Donor RMGO cells expressing two fluorescent proteins targeted to mitochondria (matrix red fluorescent protein (mtrfp) and a green fluorescent protein molecule anchored to the outer membrane (GFPOM)) and acceptor CN cells expressing CFPnuc (a cyan fluorescent protein targeted to the nucleus) were fused and maintained for 16 h in the presence of cccp (,) or valinomycin (C). Cells in () were transfected with dominant-negative Drp1 K38 before fusion. The selective inhibition of inner membrane fusion leads to the coexistence of doubly labelled donor mitochondria and acceptor mitochondria that are singly labelled with GFPOM ( C). Under cccp treatment, donor and acceptor mitochondria are largely scattered, indicating that outer membrane fusion was followed by fission (). fter inhibition of fission with Drp1 K38, some cccp-treated mitochondria remain filamentous and some GFPOM-positive acceptor mitochondria (arrowheads) remain in contact with doubly labelled donor mitochondria (). Under valinomycin treatment, numerous donor and acceptor mitochondria remain in close proximity (C). Scale bars, 10 mm (,C) and 3 mm (). Fig 4 Mitochondrial ultrastructure in drug-treated cells. Cells maintained under control conditions () or treated for 16 h with deoxyglucose (), cccp (C) or valinomycin (D) were processed for electron microscopy. Mitochondria depict an orthodox conformation under control conditions () and adopt a condensed conformation (electron-dense matrix and swollen cristae) after incubation with deoxyglucose (). The mitochondria of cccp-treated cells are small (C) and those of valinomycin-treated cells are significantly enlarged (D). Valinomycintreated cells show mitochondria enveloped by a continuous outer membrane but containing matrix spaces with different electron densities and cristae morphologies, suggesting that these matrix spaces are separated by unfused inner membrane (arrowheads). Scale bars, 400 nm. &2005 EUROPEN MOLECULR IOLOGY ORGNIZTION EMO reports VOL 6 NO
6 Separate fusion of mitochondrial membranes COX2/VDC GFPOM/CFPnuc b Fig 5 Outer and inner membranes can fuse separately. RMGO cells expressing two fluorescent proteins targeted to mitochondria (matrix red fluorescent protein (mtrfp) and a green fluorescent protein molecule anchored to the outer membrane (GFPOM)) and CN cells expressing a cyan fluorescent protein targeted to the nucleus (CFPnuc) were co-plated and fused for 4 h. RMGO and CN cells were either normal () or devoid of mitochondrial DN (). fter fixation, all mitochondria were visualized with antibodies against COX2 () or VDC (). significant fraction of all polykaryons (nx100) contain mitochondria positive for GFPOM but negative for mtrfp (arrowheads in (), inset in ()). (C) Model of mitochondrial fusion and fission reactions. Under normal conditions, the fusion of outer and inner membranes mediates the successive transfer of GFPOM and mtrfp; mitochondria singly labelled with GFPOM exist only transiently. Inhibition of inner membrane fusion with cccp (1) does not affect outer membrane fusion or fission and leads to fragmentation and scattering of mitochondria. Inhibition of inner membrane fusion and outer membrane fission with valinomycin (1 þ 2) does not affect outer membrane fusion and leads to the accumulation of mitochondria with unfused inner membranes. Scale bars, 10 mm. Overlay R+G mtrfp Quantification C GFPOM = mtrfp GFPOM > mtrfp Percentage of polykaryons Percentage of polykaryons unspecific segregation of GFPOM and mtrfp by PEG treatment and/or cell fusion. fter fusion of doubly labelled donor cells expressing mtrfp and mtgfp (instead of GFPOM) with acceptor CN cells, acceptor mitochondria were all labelled with both matrix fluorescent proteins (supplementary Fig S3 online), excluding that segregation may result from the various properties (such as size, solubility, oligomeric state and fluorescence intensity) of GFP and RFP (DsRed). ltogether, these results show that, in living cells and in the absence of any drug, outer and inner membrane fusion can proceed separately. Speculation The different requirements and kinetics of outer and inner membrane fusion, as well as the possibility of uncoupling them, point to the existence of fusion machineries that can function separately (Fig 5C). This enables regulation of mitochondrial fusion at two levels. It is theoretically possible that regulated outer membrane fusion is followed by uncontrolled inner membrane fusion. However, it is also possible that inner membrane fusion is regulated separately and/or represents the main (or only?) target of regulation. Indeed, the dependence of inner membrane fusion on DC m provides the means to link the dynamics of mitochondria to its respiratory activity. Further characterization of outer and inner membrane fusion reactions and of the molecules involved in these processes is required to validate any of these hypotheses. 1 2 METHODS Reagents and experimental procedures are described in the supplementary information online. Supplementary information is available at EMO reports online ( was due to the separate fusion of outer and inner membranes. fter fusion between the RMGO cells, all mitochondria were labelled with GFPOM and mtrfp (data not shown), thus excluding CKNOWLEDGEMENTS We thank P. Frachon for valuable assistance, and F. Chevessier and P. de Camilli for expression vectors encoding CFPnuc and GFPOM, respectively. We are grateful to. Rouche and P. ozin for assistance in electron microscopy. M.R. is an investigator of CNRS. This work was supported by INSERM and by grants from FM and from Ministère Délégué à la Recherche (CI CMS). 858 EMO reports VOL 6 NO &2005 EUROPEN MOLECULR IOLOGY ORGNIZTION
7 Separate fusion of mitochondrial membranes scientific report REFERENCES ernardi P (1999) Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79: Chen H, Detmer S, Ewald J, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell iol 160: Cipolat S, de rito OM, Dal Zilio, Scorrano L (2004) OP1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl cad Sci US 101: Frey TG, Mannella C (2000) The internal structure of mitochondria. Trends iochem Sci 25: Fritz S, Rapaport D, Klanner E, Neupert W, Westermann (2001) Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organellar fusion. J Cell iol 152: Griparic L, Van Der Wel NN, Orozco IJ, Peters PJ, Van Der liek M (2004) Loss of the intermembrane space protein Mgm1/Opa1 induces swelling and localized constrictions along the lengths of mitochondria. J iol Chem 279: Hackenbrock CR, Rehn TG, Weinbach EC, Lemasters JJ (1971) Oxidative phosphorylation and ultrastructural transformation in mitochondria in the intact ascites tumor cell. J Cell iol 51: Legros F, Lombes, Frachon P, Rojo M (2002) Mitochondrial fusion in human cells is efficient, requires the inner membrane potential and is mediated by mitofusins. Mol iol Cell 13: Legros F, Malka F, Frachon P, Lombès, Rojo M (2004) Organization and dynamics of human mitochondrial DN. J Cell Sci 117: Meeusen S, McCaffery JM, Nunnari J (2004) Mitochondrial fusion intermediates revealed in vitro. Science 305: Okamoto K, Perlman PS, utow R (1998) The sorting of mitochondrial DN and mitochondrial proteins in zygotes: preferential transmission of mitochondrial DN to the medial bud. J Cell iol 142: Olichon, aricault L, Gas N, Guillou E, Valette, elenguer P, Lenaers G (2003) Loss of OP1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J iol Chem 278: Rojo M, Legros F, Chateau D, Lombes (2002) Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 115: Sesaki H, Jensen RE (2004) Ugo1p links the Fzo1p and Mgm1p GTPases for mitochondrial fusion. J iol Chem 279: Waterhouse NJ, Ricci JE, Green DR (2002) nd all of a sudden it s over: mitochondrial outer-membrane permeabilization in apoptosis. iochimie 84: Wong ED, Wagner J, Scott SV, Okreglak V, Holewinske TJ, Cassidy-Stone, Nunnari J (2003) The intramitochondrial dynamin-related GTPase, Mgm1p, is a component of a protein complex that mediates mitochondrial fusion. J Cell iol 160: &2005 EUROPEN MOLECULR IOLOGY ORGNIZTION EMO reports VOL 6 NO
Supplementary Figure 1.
 Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Lectures by Kathleen Fitzpatrick
 Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Chapter 10 Chemotrophic Energy Metabolism: Aerobic Respiration Lectures by Kathleen Fitzpatrick Simon Fraser University Figure 10-1 Figure 10-6 Conversion of pyruvate The conversion of pyruvate to acetyl
Mitochondria are crucial organelles for life and death of the
 OPA1 requires mitofusin 1 to promote mitochondrial fusion Sara Cipolat, Olga Martins de Brito, Barbara Dal Zilio, and Luca Scorrano* Dulbecco Telethon Institute, Venetian Institute of Molecular Medicine,
OPA1 requires mitofusin 1 to promote mitochondrial fusion Sara Cipolat, Olga Martins de Brito, Barbara Dal Zilio, and Luca Scorrano* Dulbecco Telethon Institute, Venetian Institute of Molecular Medicine,
Scale in the biological world
 Scale in the biological world 2 A cell seen by TEM 3 4 From living cells to atoms 5 Compartmentalisation in the cell: internal membranes and the cytosol 6 The Origin of mitochondria: The endosymbion hypothesis
Scale in the biological world 2 A cell seen by TEM 3 4 From living cells to atoms 5 Compartmentalisation in the cell: internal membranes and the cytosol 6 The Origin of mitochondria: The endosymbion hypothesis
Mitochondrial kiss-and-run : interplay between mitochondrial motility and fusion fission dynamics
 The EMBO Journal (2009) 28, 3074 3089 & 2009 European Molecular Biology Organization All Rights Reserved 0261-4189/09 www.embojournal.org : interplay between mitochondrial motility and fusion fission dynamics
The EMBO Journal (2009) 28, 3074 3089 & 2009 European Molecular Biology Organization All Rights Reserved 0261-4189/09 www.embojournal.org : interplay between mitochondrial motility and fusion fission dynamics
Organization and dynamics of human mitochondrial DNA
 Research Article 2653 Organization and dynamics of human mitochondrial DNA Frédéric Legros, Florence Malka, Paule Frachon, Anne Lombès and Manuel Rojo* INSERM U582 (IFR 14, UPMC) Institut de Myologie,
Research Article 2653 Organization and dynamics of human mitochondrial DNA Frédéric Legros, Florence Malka, Paule Frachon, Anne Lombès and Manuel Rojo* INSERM U582 (IFR 14, UPMC) Institut de Myologie,
Scale in the biological world
 Scale in the biological world From living cells to atoms A cell seen by TEM Compartmentalisation in the cell: internal membranes and the cytosol The cytosol: more than just H2O RNAs Proteins Ribosomes
Scale in the biological world From living cells to atoms A cell seen by TEM Compartmentalisation in the cell: internal membranes and the cytosol The cytosol: more than just H2O RNAs Proteins Ribosomes
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
Functions and dysfunctions of mitochondrial dynamics
 Functions and dysfunctions of mitochondrial dynamics Scott A. Detmer and David C. Chan Abstract Recent findings have sparked renewed appreciation for the remarkably dynamic nature of mitochondria. These
Functions and dysfunctions of mitochondrial dynamics Scott A. Detmer and David C. Chan Abstract Recent findings have sparked renewed appreciation for the remarkably dynamic nature of mitochondria. These
Mitochondrial Inner-Membrane Fusion and Crista Maintenance Requires the Dynamin-Related GTPase Mgm1
 Mitochondrial Inner-Membrane Fusion and Crista Maintenance Requires the Dynamin-Related GTPase Mgm1 Shelly Meeusen, 1,3 Rachel DeVay, 1 Jennifer Block, 1,4 Ann Cassidy-Stone, 1 Sarah Wayson, 1,5 J. Michael
Mitochondrial Inner-Membrane Fusion and Crista Maintenance Requires the Dynamin-Related GTPase Mgm1 Shelly Meeusen, 1,3 Rachel DeVay, 1 Jennifer Block, 1,4 Ann Cassidy-Stone, 1 Sarah Wayson, 1,5 J. Michael
Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity
 Research Article 6535 Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity Naotada Ishihara, Yuka Eura and Katsuyoshi Mihara* Department of Molecular Biology, Graduate
Research Article 6535 Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity Naotada Ishihara, Yuka Eura and Katsuyoshi Mihara* Department of Molecular Biology, Graduate
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Importance of Protein sorting. A clue from plastid development
 Importance of Protein sorting Cell organization depend on sorting proteins to their right destination. Cell functions depend on sorting proteins to their right destination. Examples: A. Energy production
Importance of Protein sorting Cell organization depend on sorting proteins to their right destination. Cell functions depend on sorting proteins to their right destination. Examples: A. Energy production
Mitochondries, Hypoxie et Cancer
 Mitochondries, Hypoxie et Cancer CNRS - UMR 6543 Institute of Signaling, Developmental Biology and Cancer Research University of Nice-Sophia Antipolis - FRANCE Colon tumor necrosis Blood vessel Colon tumor
Mitochondries, Hypoxie et Cancer CNRS - UMR 6543 Institute of Signaling, Developmental Biology and Cancer Research University of Nice-Sophia Antipolis - FRANCE Colon tumor necrosis Blood vessel Colon tumor
Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with
 Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with DsRed-mito. Right panels are time-course enlarged images
Supplementary Figure 1. CoMIC in 293T, HeLa, and HepG2 cells. (a) Mitochondrial morphology in 293T, HeLa and HepG2 cells. Cells were transfected with DsRed-mito. Right panels are time-course enlarged images
Lecture 7 Cell Biolog y ٢٢٢ ١
 Lecture 7 ١ Mitochondria ٢ Mitochondria Mitochondria are the energy factories of the cells. The energy currency for the work that animals must do is the energy-rich molecule adenosine triphosphate (ATP).
Lecture 7 ١ Mitochondria ٢ Mitochondria Mitochondria are the energy factories of the cells. The energy currency for the work that animals must do is the energy-rich molecule adenosine triphosphate (ATP).
7.06 Cell Biology EXAM #3 April 21, 2005
 7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
4) Please cite Dagda et al J Biol Chem 284: , for any publications or presentations resulting from use or modification of the macro.
 Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Oxidative Phosphorylation
 Paper : 04 Metabolism of carbohydrates Module : 15 Principal Investigator Paper Coordinator Content Reviewer Content Writer Dr.S.K.Khare,Professor IIT Delhi. Dr. Ramesh Kothari,Professor UGC-CAS Department
Paper : 04 Metabolism of carbohydrates Module : 15 Principal Investigator Paper Coordinator Content Reviewer Content Writer Dr.S.K.Khare,Professor IIT Delhi. Dr. Ramesh Kothari,Professor UGC-CAS Department
Chapter 10. Thermodynamics of Transport. Thermodynamics of Transport, con t. BCH 4053 Summer 2001 Chapter 10 Lecture Notes. Slide 1.
 BCH 4053 Summer 2001 Chapter 10 Lecture Notes 1 Chapter 10 Membrane Transport 2 3 Thermodynamics of Transport Free Energy change is given by difference in electrochemical potential and the quantity transported
BCH 4053 Summer 2001 Chapter 10 Lecture Notes 1 Chapter 10 Membrane Transport 2 3 Thermodynamics of Transport Free Energy change is given by difference in electrochemical potential and the quantity transported
ΔG o' = ηf ΔΕ o' = (#e ( V mol) ΔΕ acceptor
 Reading: Sec. 19.1 Electron-Transfer Reactions in Mitochondria (listed subsections only) 19.1.1 Electrons are Funneled to Universal Electron Acceptors p. 692/709 19.1.2 Electrons Pass through a Series
Reading: Sec. 19.1 Electron-Transfer Reactions in Mitochondria (listed subsections only) 19.1.1 Electrons are Funneled to Universal Electron Acceptors p. 692/709 19.1.2 Electrons Pass through a Series
Cellular Respiration Stage 4: Electron Transport Chain
 Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Cellular Respiration Stage 4: Electron Transport Chain 2006-2007 Cellular respiration What s the point? The point is to make ATP! ATP 2006-2007 ATP accounting so far Glycolysis 2 ATP Kreb s cycle 2 ATP
Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization
 Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
Cell Metabolism Supplemental Information Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization Prashant Mishra, Grigor Varuzhanyan,
20. Electron Transport and Oxidative Phosphorylation
 20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
20. Electron Transport and Oxidative Phosphorylation 20.1 What Role Does Electron Transport Play in Metabolism? Electron transport - Role of oxygen in metabolism as final acceptor of electrons - In inner
7.06 Problem Set
 7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
Cellular respiration ATP. Cellular Respiration Stage 4: Electron Transport Chain. AP Biology. The point is to make ATP! What s the point?
 ellular respiration ellular Respiration Stage 4: Electron Transport hain What s the point? The point is to make! accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to
ellular respiration ellular Respiration Stage 4: Electron Transport hain What s the point? The point is to make! accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of energy to run, need to
Lecture 6 - Intracellular compartments and transport I
 01.26.11 Lecture 6 - Intracellular compartments and transport I Intracellular transport and compartments 1. Protein sorting: How proteins get to their appropriate destinations within the cell 2. Vesicular
01.26.11 Lecture 6 - Intracellular compartments and transport I Intracellular transport and compartments 1. Protein sorting: How proteins get to their appropriate destinations within the cell 2. Vesicular
Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health
 I R E V I E W S E Review in Advance first posted online on August 29, 2012. (Changes may still occur before final publication online and in print.) N C A D V A N Fusion and Fission: Interlinked Processes
I R E V I E W S E Review in Advance first posted online on August 29, 2012. (Changes may still occur before final publication online and in print.) N C A D V A N Fusion and Fission: Interlinked Processes
ATP. Division Ave. High School AP Biology. Cellular Respiration Stage 4: Electron Transport Chain. Cellular respiration. The point is to make ATP!
 ellular Respiration Stage 4: Electron Transport hain 2006-2007 ellular respiration What s the point? The point is to make! 2006-2007 1 accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of
ellular Respiration Stage 4: Electron Transport hain 2006-2007 ellular respiration What s the point? The point is to make! 2006-2007 1 accounting so far Glycolysis 2 Kreb s cycle 2 Life takes a lot of
Transporters and Membrane Motors Nov 15, 2007
 BtuB OM vitamin B12 transporter F O F 1 ATP synthase Human multiple drug resistance transporter P-glycoprotein Transporters and Membrane Motors Nov 15, 2007 Transport and membrane motors Concentrations
BtuB OM vitamin B12 transporter F O F 1 ATP synthase Human multiple drug resistance transporter P-glycoprotein Transporters and Membrane Motors Nov 15, 2007 Transport and membrane motors Concentrations
Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo
 Research Article 1663 Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo Manuel Rojo, Frédéric Legros, Danielle Chateau* and Anne
Research Article 1663 Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo Manuel Rojo, Frédéric Legros, Danielle Chateau* and Anne
Mitochondria Mitochondria were first seen by kollicker in 1850 in muscles and called them sarcosomes. Flemming (1882) described these organelles as
 Mitochondria Mitochondria were first seen by kollicker in 1850 in muscles and called them sarcosomes. Flemming (1882) described these organelles as filia Altmann (1890) observed these structures and named
Mitochondria Mitochondria were first seen by kollicker in 1850 in muscles and called them sarcosomes. Flemming (1882) described these organelles as filia Altmann (1890) observed these structures and named
The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells
 Research Article 3049 The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells Daniel Tondera*, Frank Czauderna, Katharina Paulick, Rolf Schwarzer, Jörg Kaufmann and Ansgar
Research Article 3049 The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells Daniel Tondera*, Frank Czauderna, Katharina Paulick, Rolf Schwarzer, Jörg Kaufmann and Ansgar
Mitochondrial Fusion and Fission in Mammals
 Annu. Rev. Cell Dev. Biol. 2006. 22:79 99 First published online as a Review in Advance on May 11, 2006 The Annual Review of Cell and Developmental Biology is online at http://cellbio.annualreviews.org
Annu. Rev. Cell Dev. Biol. 2006. 22:79 99 First published online as a Review in Advance on May 11, 2006 The Annual Review of Cell and Developmental Biology is online at http://cellbio.annualreviews.org
Biophysics 490M Project
 Biophysics 490M Project Dan Han Department of Biochemistry Structure Exploration of aa 3 -type Cytochrome c Oxidase from Rhodobacter sphaeroides I. Introduction: All organisms need energy to live. They
Biophysics 490M Project Dan Han Department of Biochemistry Structure Exploration of aa 3 -type Cytochrome c Oxidase from Rhodobacter sphaeroides I. Introduction: All organisms need energy to live. They
Bio102 Problems Photosynthesis
 Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
Bio102 Problems Photosynthesis 1. Why is it advantageous for chloroplasts to have a very large (in surface area) thylakoid membrane contained within the inner membrane? A. This limits the amount of stroma
5 Mitochondrial Dynamics in Mammals
 5 Mitochondrial Dynamics in Mammals Hsiuchen Chen and David C. Chan Division of Biology California Institute of Technology Pasadena, California 91125 I. Introduction II. The Central Molecular Players A.
5 Mitochondrial Dynamics in Mammals Hsiuchen Chen and David C. Chan Division of Biology California Institute of Technology Pasadena, California 91125 I. Introduction II. The Central Molecular Players A.
Aerobic Cellular Respiration
 Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
Aerobic Cellular Respiration Under aerobic conditions (oxygen gas is available), cells will undergo aerobic cellular respiration. The end products of aerobic cellular respiration are carbon dioxide gas,
!"#$%&'%()*%+*,,%-&,./*%01%02%/*/3452*%3&.26%&4752*,,*1%%
 !"#$%&'%()*%+*,,%-&,./*%01%02%/*/3452*%3&.26%&4752*,,*1%% !"#$%&'(")*++*%,*'-&'./%/,*#01#%-2)#3&)/% 4'(")*++*% % %5"0)%-2)#3&) %%% %67'2#72'*%%%%%%%%%%%%%%%%%%%%%%%4'(")0/./% % 8$+&'&,+"/7 % %,$&7&/9)7$*/0/%%%%%%%%%%
!"#$%&'%()*%+*,,%-&,./*%01%02%/*/3452*%3&.26%&4752*,,*1%% !"#$%&'(")*++*%,*'-&'./%/,*#01#%-2)#3&)/% 4'(")*++*% % %5"0)%-2)#3&) %%% %67'2#72'*%%%%%%%%%%%%%%%%%%%%%%%4'(")0/./% % 8$+&'&,+"/7 % %,$&7&/9)7$*/0/%%%%%%%%%%
University of Groningen
 University of Groningen An evidence based hypothesis on the existence of two pathways of mitochondrial crista formation Harner, Max E.; Unger, Ann-Katrin; Geerts, Willie J. C.; Mari, Muriel; Izawa, Toshiaki;
University of Groningen An evidence based hypothesis on the existence of two pathways of mitochondrial crista formation Harner, Max E.; Unger, Ann-Katrin; Geerts, Willie J. C.; Mari, Muriel; Izawa, Toshiaki;
Mitochondria associate with the cytoskeleton. Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008)
 Mitochondria associate with the cytoskeleton Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008) Special arrangements of mitochondria Figure 14-6 Molecular Biology of the Cell ( Garland Science
Mitochondria associate with the cytoskeleton Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008) Special arrangements of mitochondria Figure 14-6 Molecular Biology of the Cell ( Garland Science
Mitochondrie et muscle lisse. Vladimir Veksler
 Mitochondrie et muscle lisse Vladimir Veksler Main mitochonrial functions Energy production ATP consumption rate in smooth and striated muscle smooth muscle striated (cardiac) muscle at rest 30 µmol/min/g
Mitochondrie et muscle lisse Vladimir Veksler Main mitochonrial functions Energy production ATP consumption rate in smooth and striated muscle smooth muscle striated (cardiac) muscle at rest 30 µmol/min/g
18 Efficiency of Acanthamoeba castellanii uncoupling protein in energy-dissipating processes
 18 Efficiency of Acanthamoeba castellanii uncoupling protein in energy-dissipating processes W. Jarmuszkiewicz 1,L.Hryniewiecka 1, C.M. Sluse-Goffart 2 and F.E. Sluse 2 1 Department of Bioenergetics, Adam
18 Efficiency of Acanthamoeba castellanii uncoupling protein in energy-dissipating processes W. Jarmuszkiewicz 1,L.Hryniewiecka 1, C.M. Sluse-Goffart 2 and F.E. Sluse 2 1 Department of Bioenergetics, Adam
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
Supplementary Information
 Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Photosynthesis and Cellular Respiration Practice Test Name
 Photosynthesis and Cellular Respiration Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which H+ has just passed through the
Photosynthesis and Cellular Respiration Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which H+ has just passed through the
Biochemical bases for energy transformations. Biochemical bases for energy transformations. Nutrition 202 Animal Energetics R. D.
 Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Biochemical bases for energy transformations Biochemical bases for energy transformations Nutrition 202 Animal Energetics R. D. Sainz Lecture 02 Energy originally from radiant sun energy Captured in chemical
Photosynthesis. Chapter 10. Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece
 Chapter 10 Photosynthesis Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece Edited by William Wischusen, Louisiana State University
Chapter 10 Photosynthesis Active Lecture Questions for use with Classroom Response Systems Biology, Seventh Edition Neil Campbell and Jane Reece Edited by William Wischusen, Louisiana State University
M i t o c h o n d r i a
 M i t o c h o n d r i a Dr. Diala Abu-Hassan School of Medicine dr.abuhassand@gmail.com Mitochondria Function: generation of metabolic energy in eukaryotic cells Generation of ATP from the breakdown of
M i t o c h o n d r i a Dr. Diala Abu-Hassan School of Medicine dr.abuhassand@gmail.com Mitochondria Function: generation of metabolic energy in eukaryotic cells Generation of ATP from the breakdown of
POSSIBLE ROLE OF THE MITOCHONDRIAL OUTER MEMBRANE AS AN ONCOTIC REGULATOR OF MITOCHONDRIAL VOLUME
 Volume 88. number 1 FEBS LETTERS April 1978 Hypothesis POSSIBLE ROLE OF THE MITOCHONDRIAL OUTER MEMBRANE AS AN ONCOTIC REGULATOR OF MITOCHONDRIAL VOLUME John J. LEMASTERS Laboratories for Cell Biology,
Volume 88. number 1 FEBS LETTERS April 1978 Hypothesis POSSIBLE ROLE OF THE MITOCHONDRIAL OUTER MEMBRANE AS AN ONCOTIC REGULATOR OF MITOCHONDRIAL VOLUME John J. LEMASTERS Laboratories for Cell Biology,
Energy Converion: Mitochondria and Chloroplasts. Pınar Tulay, Ph.D.
 Energy Converion: Mitochondria and Chloroplasts Pınar Tulay, Ph.D. pintulay@gmail.com Energy Conversion Prokaryotes use plasma membrane to produce adenosine triphosphate (ATP) used in the cell function
Energy Converion: Mitochondria and Chloroplasts Pınar Tulay, Ph.D. pintulay@gmail.com Energy Conversion Prokaryotes use plasma membrane to produce adenosine triphosphate (ATP) used in the cell function
Biological Process Term Enrichment
 Biological Process Term Enrichment cellular protein localization cellular macromolecule localization intracellular protein transport intracellular transport generation of precursor metabolites and energy
Biological Process Term Enrichment cellular protein localization cellular macromolecule localization intracellular protein transport intracellular transport generation of precursor metabolites and energy
Plant mitochondrial dynamics
 Plant mitochondrial dynamics Peroxisome Chloroplast Nucleus Mitochondria from Alberts et al. 1994 Living Arabidopsis leaf 10 µm Logan & Leaver (2000) Journal of Experimental Botany, 51: 865-871 5 µm S.
Plant mitochondrial dynamics Peroxisome Chloroplast Nucleus Mitochondria from Alberts et al. 1994 Living Arabidopsis leaf 10 µm Logan & Leaver (2000) Journal of Experimental Botany, 51: 865-871 5 µm S.
Lecture 10: Cyclins, cyclin kinases and cell division
 Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as
Mitochondrial Biogenesis is the process by which new mitochondria are formed in the cell.
 Mitochondrial Biogenesis is the process by which new mitochondria are formed in the cell. Purpose of Mitochondria: The mitochondria are organelles within the cell that are responsible for the biochemical
Mitochondrial Biogenesis is the process by which new mitochondria are formed in the cell. Purpose of Mitochondria: The mitochondria are organelles within the cell that are responsible for the biochemical
Life Depends on Photosynthesis
 Photosynthesis Life Depends on Photosynthesis Most energy Comes from the Sun Life Depends on Photosynthesis Most energy Comes from the Sun Life Depends on Photosynthesis Most energy Comes from the Sun
Photosynthesis Life Depends on Photosynthesis Most energy Comes from the Sun Life Depends on Photosynthesis Most energy Comes from the Sun Life Depends on Photosynthesis Most energy Comes from the Sun
OPA1 dependent cristae modulation is essential for cellular adaptation to metabolic demand
 Manuscript EMBO-2014-88349 OPA1 dependent cristae modulation is essential for cellular adaptation to metabolic demand David A. Patten, Jacob Wong, Mireille Khacho, Vincent Soubannier, Ryan J. Mailloux,
Manuscript EMBO-2014-88349 OPA1 dependent cristae modulation is essential for cellular adaptation to metabolic demand David A. Patten, Jacob Wong, Mireille Khacho, Vincent Soubannier, Ryan J. Mailloux,
Importance of Mitochondrial Dynamics During Meiosis and Sporulation
 Molecular Biology of the Cell Vol. 15, 4369 4381, October 2004 Importance of Mitochondrial Dynamics During Meiosis and Sporulation Steven W. Gorsich* and Janet M. Shaw Department of Biology, University
Molecular Biology of the Cell Vol. 15, 4369 4381, October 2004 Importance of Mitochondrial Dynamics During Meiosis and Sporulation Steven W. Gorsich* and Janet M. Shaw Department of Biology, University
Energy and Cells. Appendix 1. The two primary energy transformations in plants are photosynthesis and respiration.
 Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
The Mitochondrion. Definition Structure, ultrastructure Functions
 The Mitochondrion Definition Structure, ultrastructure Functions Organelle definition Etymology of the name Carl Benda (1903): (mitos) thread; (khondrion) granule. Light microscopy identification First
The Mitochondrion Definition Structure, ultrastructure Functions Organelle definition Etymology of the name Carl Benda (1903): (mitos) thread; (khondrion) granule. Light microscopy identification First
Spatial and temporal dynamics of budding yeast mitochondria lacking the division component Fis1p
 Research Article 2005 Spatial and temporal dynamics of budding yeast mitochondria lacking the division component Fis1p Stefan Jakobs 1, *,, Nadia Martini 1, *, Astrid C. Schauss 1, Alexander Egner 1, Benedikt
Research Article 2005 Spatial and temporal dynamics of budding yeast mitochondria lacking the division component Fis1p Stefan Jakobs 1, *,, Nadia Martini 1, *, Astrid C. Schauss 1, Alexander Egner 1, Benedikt
Cellular Respiration. Anaerobic vs Aerobic
 Cellular Respiration Anaerobic vs Aerobic What is Cellular Respiration? Process where organisms use GLUCOSE (sugar) to create ENERGY! The energy that is released from chemical bonds during Cellular Respiration
Cellular Respiration Anaerobic vs Aerobic What is Cellular Respiration? Process where organisms use GLUCOSE (sugar) to create ENERGY! The energy that is released from chemical bonds during Cellular Respiration
Membrane depolarization activates the mitochondrial protease OMA1 by stimulating self-cleavage
 Scientific Report Membrane depolarization activates the mitochondrial protease OMA1 by stimulating self-cleavage Kuan Zhang, Huihui Li & Zhiyin Song* Abstract Mitochondrial inner membrane fusion depends
Scientific Report Membrane depolarization activates the mitochondrial protease OMA1 by stimulating self-cleavage Kuan Zhang, Huihui Li & Zhiyin Song* Abstract Mitochondrial inner membrane fusion depends
Origami Organelles tm
 Mighty Mitochondria Cat no OO-001 Origami Organelles tm 折り紙オルガネラ We d love to hear any feedback, comments or questions you have! Post: Discovering DNA Ltd, PO Box 280 Hertford, SG13 9DG, UK email: info@
Mighty Mitochondria Cat no OO-001 Origami Organelles tm 折り紙オルガネラ We d love to hear any feedback, comments or questions you have! Post: Discovering DNA Ltd, PO Box 280 Hertford, SG13 9DG, UK email: info@
Role of Mitochondrial Remodeling in Programmed Cell Death in
 Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
Protein Sorting, Intracellular Trafficking, and Vesicular Transport
 Protein Sorting, Intracellular Trafficking, and Vesicular Transport Noemi Polgar, Ph.D. Department of Anatomy, Biochemistry and Physiology Email: polgar@hawaii.edu Phone: 692-1422 Outline Part 1- Trafficking
Protein Sorting, Intracellular Trafficking, and Vesicular Transport Noemi Polgar, Ph.D. Department of Anatomy, Biochemistry and Physiology Email: polgar@hawaii.edu Phone: 692-1422 Outline Part 1- Trafficking
Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts)
 1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
1 Life 21 - Aerobic respiration Raven & Johnson Chapter 9 (parts) Objectives 1: Describe the overall action of the Krebs cycle in generating ATP, NADH and FADH 2 from acetyl-coa 2: Understand the generation
Metabolism Review. A. Top 10
 A. Top 10 Metabolism Review 1. Energy production through chemiosmosis a. pumping of H+ ions onto one side of a membrane through protein pumps in an Electron Transport Chain (ETC) b. flow of H+ ions across
A. Top 10 Metabolism Review 1. Energy production through chemiosmosis a. pumping of H+ ions onto one side of a membrane through protein pumps in an Electron Transport Chain (ETC) b. flow of H+ ions across
BCH 4054 Spring 2001 Chapter 21 Lecture Notes
 BCH 4054 Spring 2001 Chapter 21 Lecture Notes 1 Chapter 21 Electron Transport and Oxidative Phosphorylation 2 Overview Oxidation of NADH and CoQH 2 produced in TCA cycle by O 2 is very exergonic. Some
BCH 4054 Spring 2001 Chapter 21 Lecture Notes 1 Chapter 21 Electron Transport and Oxidative Phosphorylation 2 Overview Oxidation of NADH and CoQH 2 produced in TCA cycle by O 2 is very exergonic. Some
Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1
 Research Article 4141 Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1 Tianzheng Yu 1, Randall J. Fox 1, Lindsay S. Burwell 2 and Yisang Yoon 1, * 1 Department
Research Article 4141 Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hfis1 Tianzheng Yu 1, Randall J. Fox 1, Lindsay S. Burwell 2 and Yisang Yoon 1, * 1 Department
The Proton Motive Force. Overview. Compartmentalization 11/6/2015. Chapter 21 Stryer Short Course. ATP synthesis Shuttles
 The Proton Motive Force Chapter 21 Stryer Short Course Redox reactions Electron transport chain Proton gradient Overview ATP synthesis Shuttles Analogy: How does burning coal put flour in the grocery store?
The Proton Motive Force Chapter 21 Stryer Short Course Redox reactions Electron transport chain Proton gradient Overview ATP synthesis Shuttles Analogy: How does burning coal put flour in the grocery store?
Supporting Information
 Supporting Information Fleissner et al. 10.1073/pnas.0907039106 Fig. S1. (A) MAK-2-GFP localized to CATs tips is not bound by membrane. his-3::pccg1 mak-2-gfp; mak-2 strain labeled with membrane dye FM4
Supporting Information Fleissner et al. 10.1073/pnas.0907039106 Fig. S1. (A) MAK-2-GFP localized to CATs tips is not bound by membrane. his-3::pccg1 mak-2-gfp; mak-2 strain labeled with membrane dye FM4
GR QUIZ WITH ANS KEY Cellular Processes. Part I: Multiple Choice. 1. In leaf cell, the synthesis of ATP occurs in which of the following?
 GR QUIZ WITH ANS KEY Cellular Processes Part I: Multiple Choice 1. In leaf cell, the synthesis of ATP occurs in which of the following? I. Ribosomes II. Mitochondria III. Chloroplasts A. I only B. II only
GR QUIZ WITH ANS KEY Cellular Processes Part I: Multiple Choice 1. In leaf cell, the synthesis of ATP occurs in which of the following? I. Ribosomes II. Mitochondria III. Chloroplasts A. I only B. II only
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Lecture 4. Protein Translocation & Nucleocytoplasmic Transport
 Lecture 4 Protein Translocation & Nucleocytoplasmic Transport Chapter 12 MBoC (5th Edition) Alberts et al. Reference paper: Tran and Wente, Cell 125, 1041-1053, 2006 2/8/2012 1 Page 713 Molecular Biology
Lecture 4 Protein Translocation & Nucleocytoplasmic Transport Chapter 12 MBoC (5th Edition) Alberts et al. Reference paper: Tran and Wente, Cell 125, 1041-1053, 2006 2/8/2012 1 Page 713 Molecular Biology
REVIEW 3: METABOLISM UNIT RESPIRATION & PHOTOSYNTHESIS. A. Top 10 If you learned anything from this unit, you should have learned:
 Period Date REVIEW 3: METABOLISM UNIT RESPIRATION & PHOTOSYNTHESIS A. Top 10 If you learned anything from this unit, you should have learned: 1. Energy production through chemiosmosis a. pumping of H+
Period Date REVIEW 3: METABOLISM UNIT RESPIRATION & PHOTOSYNTHESIS A. Top 10 If you learned anything from this unit, you should have learned: 1. Energy production through chemiosmosis a. pumping of H+
Effect of enzyme deficiencies on oxidative phosphorylation: from isolated mitochondria to intact tissues. Theoretical studies.
 Effect of enzyme deficiencies on oxidative phosphorylation: from isolated mitochondria to intact tissues. Theoretical studies. Bernard Korzeniewski Institute of Molecular Biology and Biotechnology, Jagiellonian
Effect of enzyme deficiencies on oxidative phosphorylation: from isolated mitochondria to intact tissues. Theoretical studies. Bernard Korzeniewski Institute of Molecular Biology and Biotechnology, Jagiellonian
ETC/CHEMIOSIS. By: Leslie, Kelsey, Morgan
 ETC/CHEMIOSIS By: Leslie, Kelsey, Morgan WHY THIS IS IMPORTANT House Clip SO3E7 The Son of a Coma Guy- Time: 32:00 Patient was visiting his father who was in a vegetative state for 10 years, and his only
ETC/CHEMIOSIS By: Leslie, Kelsey, Morgan WHY THIS IS IMPORTANT House Clip SO3E7 The Son of a Coma Guy- Time: 32:00 Patient was visiting his father who was in a vegetative state for 10 years, and his only
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): The manuscript by Malli and coworkers reports the successful development and characterization of the first K+ specific FRET-based sensor protein.
Reviewers' comments: Reviewer #1 (Remarks to the Author): The manuscript by Malli and coworkers reports the successful development and characterization of the first K+ specific FRET-based sensor protein.
Chapter 12: Intracellular sorting
 Chapter 12: Intracellular sorting Principles of intracellular sorting Principles of intracellular sorting Cells have many distinct compartments (What are they? What do they do?) Specific mechanisms are
Chapter 12: Intracellular sorting Principles of intracellular sorting Principles of intracellular sorting Cells have many distinct compartments (What are they? What do they do?) Specific mechanisms are
REGULATION OF MITOCHONDRIAL DIVISION BY THE DRP1 RECEPTORS. Thesis by. Oliver Calvin Losόn
 REGULATION OF MITOCHONDRIAL DIVISION BY THE DRP1 RECEPTORS Thesis by Oliver Calvin Losόn In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy CALIFORNIA INSTITUTE OF TECHNOLOGY
REGULATION OF MITOCHONDRIAL DIVISION BY THE DRP1 RECEPTORS Thesis by Oliver Calvin Losόn In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy CALIFORNIA INSTITUTE OF TECHNOLOGY
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová
 Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
Electron Transport Chain (Respiratory Chain) - exercise - Vladimíra Kvasnicová Respiratory chain (RCH) a) is found in all cells b) is located in a mitochondrion c) includes enzymes integrated in the inner
Supporting Information
 Supporting Information Development of a mitochondriotropic antioxidant based on caffeic acid: proof of concept on cellular and mitochondrial oxidative stress models José Teixeira a,b, Fernando Cagide a,
Supporting Information Development of a mitochondriotropic antioxidant based on caffeic acid: proof of concept on cellular and mitochondrial oxidative stress models José Teixeira a,b, Fernando Cagide a,
Physiologisch-Chemisches Institut der Universitdt G6ttingen, Humboldtallee 7, 3400 Gottingen, F.R.G.
 iosystems, 12 (1980) 283-287 283 Elsevier/North-Holland Scientific Publishers Ltd. ASSEMLY OF MrroCHONDRIA: SYNTHESIS AND INTRACEIJ.ULAR TRANSFER OF MITOCHONDRIAL PROTEINS MATTHEW A. HARMEY*, EVA-MARIA
iosystems, 12 (1980) 283-287 283 Elsevier/North-Holland Scientific Publishers Ltd. ASSEMLY OF MrroCHONDRIA: SYNTHESIS AND INTRACEIJ.ULAR TRANSFER OF MITOCHONDRIAL PROTEINS MATTHEW A. HARMEY*, EVA-MARIA
Cancer Letters 323 (2012) Contents lists available at SciVerse ScienceDirect. Cancer Letters. journal homepage:
 Cancer Letters 323 (212) 62 68 Contents lists available at SciVerse ScienceDirect Cancer Letters journal homepage: www.elsevier.com/locate/canlet Mitofusin 1 inhibits an apoptosis-associated amino-terminal
Cancer Letters 323 (212) 62 68 Contents lists available at SciVerse ScienceDirect Cancer Letters journal homepage: www.elsevier.com/locate/canlet Mitofusin 1 inhibits an apoptosis-associated amino-terminal
Membranes 2: Transportation
 Membranes 2: Transportation Steven E. Massey, Ph.D. Associate Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail:
Membranes 2: Transportation Steven E. Massey, Ph.D. Associate Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail:
b) What is the gradient at room temperature? Du = J/molK * 298 K * ln (1/1000) = kj/mol
 Chem350 Practice Problems Membranes 1. a) What is the chemical potential generated by the movement of glucose by passive diffusion established by a 1000 fold concentration gradient at physiological temperature?
Chem350 Practice Problems Membranes 1. a) What is the chemical potential generated by the movement of glucose by passive diffusion established by a 1000 fold concentration gradient at physiological temperature?
Signal Transduction. Dr. Chaidir, Apt
 Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Utah School of Medicine, Salt Lake City, UT Tel.: ; Fax: ;
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 281, NO. 25, pp. 17312 17320, June 23, 2006 2006 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Dimeric Dnm1-G385D Interacts
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 281, NO. 25, pp. 17312 17320, June 23, 2006 2006 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Dimeric Dnm1-G385D Interacts
The Novel Tail-anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells
 Molecular Biology of the Cell Vol. 19, 2402 2412, June 2008 The Novel Tail-anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells Shilpa Gandre-Babbe and Alexander
Molecular Biology of the Cell Vol. 19, 2402 2412, June 2008 The Novel Tail-anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells Shilpa Gandre-Babbe and Alexander
1 INTRODUCTION. 1.1 Mitochondria structure and function
 1 INTRODUCTION Mitochondria are essential mammalian organelles surrounded by two lipid bilayers (Alberts et al., 1995). This complex structural feature is mirrored by their all-round participation in life
1 INTRODUCTION Mitochondria are essential mammalian organelles surrounded by two lipid bilayers (Alberts et al., 1995). This complex structural feature is mirrored by their all-round participation in life
The Cell. C h a p t e r. PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas
 C h a p t e r 2 The Cell PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Introduction
C h a p t e r 2 The Cell PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Introduction
Supplemental material
 Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Name: TF: Section Time: LS1a ICE 5. Practice ICE Version B
 Name: TF: Section Time: LS1a ICE 5 Practice ICE Version B 1. (8 points) In addition to ion channels, certain small molecules can modulate membrane potential. a. (4 points) DNP ( 2,4-dinitrophenol ), as
Name: TF: Section Time: LS1a ICE 5 Practice ICE Version B 1. (8 points) In addition to ion channels, certain small molecules can modulate membrane potential. a. (4 points) DNP ( 2,4-dinitrophenol ), as
Mitochondrial Membrane Potential by Object Spot Counting
 A p p l i c a t i o n N o t e Mitochondrial Membrane Potential by Object Spot Counting Using Gen5 to Analyze Mitochondrial Membrane Potential Sarah Beckman, PhD, Principal Scientist, BioTek Instruments,
A p p l i c a t i o n N o t e Mitochondrial Membrane Potential by Object Spot Counting Using Gen5 to Analyze Mitochondrial Membrane Potential Sarah Beckman, PhD, Principal Scientist, BioTek Instruments,
Msp1p is an intermembrane space dynamin-related protein that mediates mitochondrial fusion in a Dnm1p-dependent manner in S. pombe
 FEBS 29249 FEBS Letters 579 (2005) 1109 1116 Msp1p is an intermembrane space dynamin-related protein that mediates mitochondrial fusion in a Dnm1p-dependent manner in S. pombe Emmanuelle Guillou, Céline
FEBS 29249 FEBS Letters 579 (2005) 1109 1116 Msp1p is an intermembrane space dynamin-related protein that mediates mitochondrial fusion in a Dnm1p-dependent manner in S. pombe Emmanuelle Guillou, Céline
2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of October
 Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
targets. clustering show that different complex pathway
 Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Tuesday 9/6/2018 Mike Mueckler
 Tuesday 9/6/2018 Mike Mueckler mmueckler@wustl.edu Intracellular Targeting of Nascent Polypeptides Mitochondria are the Sites of Oxidative ATP Production Sugars Triglycerides Figure 14-10 Molecular Biology
Tuesday 9/6/2018 Mike Mueckler mmueckler@wustl.edu Intracellular Targeting of Nascent Polypeptides Mitochondria are the Sites of Oxidative ATP Production Sugars Triglycerides Figure 14-10 Molecular Biology
