Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo
|
|
|
- Trevor Bond
- 6 years ago
- Views:
Transcription
1 Received 23 Sep 2015 Accepted 2 Nov 2015 Published 7 Dec 2015 DOI: /ncomms10098 Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo Duy T. Tran 1, Andrius Masedunskas 2,3, Roberto Weigert 2 & Kelly G. Ten Hagen 1 OPEN The actin cytoskeleton plays crucial roles in many cellular processes, including regulated secretion. However, the mechanisms controlling F-actin dynamics in this process are largely unknown. Through 3D time-lapse imaging in a secreting organ, we show that F-actin is actively disassembled along the apical plasma membrane at the site of secretory vesicle fusion and re-assembled directionally on vesicle membranes. Moreover, we show that fusion pore formation and PIP 2 redistribution precedes actin and myosin recruitment to secretory vesicle membranes. Finally, we show essential roles for the branched actin nucleators Arp2/3- and WASp in the process of secretory cargo expulsion and integration of vesicular membranes with the apical plasma membrane. Our results highlight previously unknown roles for branched actin in exocytosis and provide a genetically tractable system to image the temporal and spatial dynamics of polarized secretion in vivo. 1 Developmental Glycobiology Section, NIDCR, National Institutes of Health, Building 30, Room 426, 30 Convent Drive, MSC 4370, Bethesda, Maryland 20892, USA. 2 Intracellular Membrane Trafficking Section, NIDCR, National Institutes of Health, 30 Convent Drive, Bethesda, Maryland 20892, USA. 3 Oncology Research Unit, School of Medical Sciences, University of New South Wales, Wallace Wurth Building East (C27), Room 227, Sydney, New South Wales 2052, Australia. Correspondence and requests for materials should be addressed to K.G.T.H. ( Kelly.Tenhagen@nih.gov). NATURE COMMUNICATIONS 6:10098 DOI: /ncomms
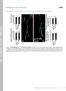
2 Regulated exocytosis is a fundamental cellular process that involves the release of stored secretory cargo from membranous vesicles into the extracellular space. This type of secretion involves the formation of secretory vesicles and their subsequent docking and fusion with the plasma membrane (PM) in response to an external stimulus. Various tissues have different modes of vesicle formation and release, likely due to the different physical and functional properties of the secreted cargo as well as the size/geometry of the secretory vesicles. Regulated secretion is used by specialized tissues to produce and secrete bioactive molecules that mediate diverse functions including neurotransmission, immunity, reproduction and digestion (reviewed in refs 1 3). Recent studies have highlighted essential, yet often disparate roles for the actin cytoskeleton in regulated exocytosis and in compensatory endocytosis. For example, actin along the cortex of cells acts as a physical barrier to prevent premature fusion of granules with the PM 4 6. In Xenopus eggs, where secretory vesicles never integrate into the PM but are maintained as shells, actin and myosin recruitment enable vesicles to be retrieved via compensatory endocytosis Other studies have demonstrated that F-actin is required for proper vesicle compression and expulsion of cargo and to regulate the expansion of the fusion pore 15. In addition, intravital imaging in rodent salivary glands (SGs) has shown that F-actin is required to regulate both the stabilization and collapse of secretory vesicles after fusion with the PM in vivo 16. However, the molecular machinery controlling the formation of specific F-actin structures and the spatial kinetics of actin assembly in the process of cargo expulsion and membrane integration has not been fully defined 3. Actin exists as monomeric units (G-actin) and in a variety of filaments that can be linear, branched or bundled (F-actin). Linear actin filaments are initiated by the formin-family of actin nucleators/elongation factors that are activated by the concerted action of small Rho-GTPases and phosphoinositides Rho and formin influence actin coat formation on lamellar bodies during surfactant secretion 12,13. Branched actin filaments, which are essential for cell polarity and migration 20,21, are formed through the coordinated action of the Actin-related protein 2/Actin-related protein 3 (Arp2/3) complex and a variety of nucleation-promoting factors (NPFs), such as WASp, Wash, Whamy and suppressor of car (SCAR) 20, Arp2/3 present on linear actin filaments is subsequently activated by NPFs, which bind Arp2/3 and induce a structural change. Branched actin is then formed at the site of Arp2/3 attachment at a 70 angle from the existing actin filament 25,26. Actin nucleators influence glucose-induced biphasic insulin secretion, but their specific effects on actin remodelling in this process are unclear 27. The role of branched actin assembly and structure is less well understood in other forms of secretion that involve large secretory vesicles containing bulky cargo 3. The factors that govern regulated secretion have been challenging to decipher, as many mammalian cells lose polarity and secretory capacity once excised and cultured ex vivo 28. Additionally, in many systems, the small size of vesicular structures and the lack of markers for cargo impede the ability to image actin dynamics, secretory vesicles and secretion with sufficient resolution. Furthermore, limited genetic tools in many systems have restricted the ability to interrogate the function of specific genes involved in actin formation in vivo. To investigate the role of actin dynamics and branched actin in secretion, we performed high-resolution, real-time imaging at single-granule resolution in Drosophila SGs, which are amenable to genetic manipulation and contain large secretory granules (3-8 mm in diameter) filled with high-molecular-weight cargo. Using Drosophila lines expressing fluorescently-labelled molecules, we are able to directly image secretory vesicle cargo, apical membrane dynamics and actin and myosin recruitment in real time to define the temporal and spatial sequence of events occurring during regulated exocytosis. By performing 4-dimensional (4D) imaging of actin dynamics during secretion, we show rearrangement of cortical actin at the point of vesicle fusion followed by coordinated recruitment of actin to the fused vesicle from the PM. We further demonstrate that fusion pore formation and redistribution of the phosphoinositide, PIP 2, occurs before actin recruitment. By imaging cargo directly, we show that fusion pore formation and cargo secretion are temporally distinct events, with the latter being dependent on actin for completion. Finally, using in vivo RNA interference (RNAi), we identify essential roles for the branched actin nucleators Arp2, Arp3 and their activator, WASp, in mediating cargo expulsion and secretory granule membrane integration with the PM. This study demonstrates a previously unknown role for branched actin nucleators in exocytosis and offers a high-resolution, genetically tractable platform for interrogating the role of any factor involved in actin dynamics and regulated secretion in a living organ in real time. Results Spatial and temporal dynamics of regulated secretion. The Drosophila larval salivary system consists of two glands comprised of secretory epithelial cells that connect to a common duct (Fig. 1a). Secretory cells are filled with large secretory granules (3 8 mm in diameter) containing high molecular weight, highly glycosylated mucins (salivary gland secretion proteins or sgs proteins) 29,30. Regulated secretion in Drosophila SGs begins with a small pulse of the steroid hormone 20-hydroxyecdysone (20E) during early third instar larval stage. This stimulus induces the expression of the sgs genes that encode the cargo that will be packaged into secretory vesicles; then, a second pulse of 20E during late third instar instructs these newly formed secretory granules to fuse with the apical PM to begin secretion To image secretion events in real time, we took advantage of Drosophila lines expressing fluorescent markers for secretory cargo (Sgs3-GFP) 32, F-actin (Lifeact-Ruby, Lifeact-RFP or Lifeact-GFP) 34,35 and the apical membrane (Myr-tdTomato) 36. SGs from Sgs3-GFP-expressing larvae were harvested during late third instar and cultured ex vivo. Cultured SGs maintain their polarity and respond to the exogenous addition of 20E, which triggers fusion of the secretory vesicles with the apical PM and release of their contents into the lumen (Fig. 1b). As secretion proceeds, the diameter of the lumen expands as it fills with the Sgs3-GFP cargo (Fig. 1b and Supplementary Movie 1), similar to what is seen in vivo (Supplementary Fig. 1a). (Real-time videos of all still images shown throughout this paper are in the Supplementary Movies section.) Imaging the dynamics of individual secretory granules using Drosophila lines expressing Sgs3-GFP cargo and the apical PM marker Myr-tdTomato revealed apical membrane deformations at the point of vesicle fusion (Fig. 1c). In addition, individual secretory vesicles undergo a slight expansion after fusion with the apical PM, likely due to hydration-related expansion of the mucin cargo. Real-time imaging of secretory cargo revealed that secretion proceeds with complete expulsion of cargo followed by integration of the vesicle membranes with the PM (Fig. 1c and Supplementary Movie 2). We next imaged the dynamics of actin recruitment to individual secretory granules using Drosophila lines expressing both Sgs3-GFP and Lifeact-Ruby. Secretory vesicles in proximity to the apical membrane are rapidly coated with actin, which precedes vesicle compression and membrane integration (Fig. 1d 2 NATURE COMMUNICATIONS 6:10098 DOI: /ncomms
3 Sgs3-GFP ARTICLE a Duct Lumen Secretory cell Secretory granules Secretory cells Larval salivary glands Apical membrane b Apical Basal * T: 0 min T: 50 min T: 100 min T: 150 min T: 200 min c Sgs3-GFP Myr-tdTomato d T: 0 s T: 25 s T: 50 s T: 75 s T: 100 s T: 125 s Sgs-GFP Lifeact-Ruby e T: 0 s T: 75 s T: 150 s T: 225 s T: 300 s T: 375 s T: 0 s T: 5 s T: 10 s T: 15 s T: 20 s T: 25 s Cortical actin T: 450 s Lifeact T: 30 s T: 35 s T: 40 s T: 45 s T: 50 s T: 55 s Figure 1 Real-time imaging of actin dynamics during regulated exocytosis. (a) Schematic diagram of third instar larval salivary glands (SGs). Inset depicts a single secretory cell. (b) Third instar larval SGs expressing Sgs3-GFP cargo (green) were imaged in real time after induction of secretion by the addition of 20E. Sgs3-GFP cargo in secretory vesicles is secreted into the lumen over time, leading to lumenal expansion. The distal tip of the gland is at the bottom of the image. Apical and basal surfaces are labelled. The SG lumen is denoted by a *. A representative time series from four independent experiments is shown. Scale bar, 50 mm. (c) Individual granules from SGs expressing Sgs3-GFP (green) and Myr-tdTomato (red; an apical PM marker) were imaged to show vesicle fusion with the PM and deformation of the PM at the site of fusion. After cargo expulsion, integration of granular membrane with the PM is seen. A representative time series from six independent experiments is shown. Scale bar, 5 mm. (d) Granule fusion in glands expressing Sgs3-GFP (green) and Lifeact-Ruby (red) shows actin recruitment to the fusing secretory granule, followed by expulsion of green secretory cargo into the lumen and integration of the granule into the PM. A representative time series from six independent experiments is shown. Scale bar, 5 mm. (e) Surface reconstruction of 4D Lifeact-GFP (white) imaging during granule secretion showing actin clearance at the apical PM followed by actin recruitment from the PM to the secretory vesicle. A representative time series from nine granule reconstructions over three independent experiments is shown. Scale bar, 3 mm. Complete movies for each time series can be found in the Supplementary Movie section. and Supplementary Movie 3). To image actin recruitment around the entirety of the vesicle, we performed three-dimensional imaging in real time (4D imaging) in Drosophila lines expressing Lifeact-GFP. Interestingly, 4D reconstruction of actin dynamics showed that cortical actin present along the apical membrane is cleared or disrupted at the site of vesicle fusion, forming a small hole that continues to expand in size (Fig. 1e and Supplementary Fig. 1b,c and Supplementary Movies 4, and 5). This actin clearance is followed by actin recruitment to the secretory vesicle, originating at the PM (Fig. 1e and Supplementary Fig. 1b, c and Supplementary Movie 4, and 5). These 4D reconstructions demonstrate dynamic actin clearance at the PM and subsequent directional actin recruitment to the secretory vesicle from the PM during SG secretion. Taken together, we show secretion of NATURE COMMUNICATIONS 6:10098 DOI: /ncomms
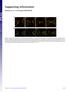
4 mucinous cargo at single granule resolution that involves dynamic rearrangement of both the cortical actin and the apical membrane at the site of secretory vesicle fusion. This process culminates in complete expulsion of cargo and integration of the granular membrane with the PM. We next addressed the temporal order of membrane fusion (fusion pore formation) and actin recruitment to the secretory vesicles. By infusing a small molecular weight (MW) fluorescent dextran (A kda dextran, stoke radius B2 nm (ref. 15)) into the lumens of SGs expressing Lifeact-GFP, we demonstrate that the fusion pore between the vesicle and the PMs forms before F-actin recruitment to the secretory vesicle (Fig. 2a, d and Supplementary Fig. 2a). Indeed, dextran diffusion from the SG lumen into the vesicle was detected before Lifeact-GFP (F-actin) on vesicle membranes (Supplementary Movie 6). The average time difference in the detection of dextran relative to actin was 57±18 s (n ¼ 11; ± refers to standard deviation). In addition, we did not see any evidence for compound exocytosis (for example, the sequential fusion of vesicles with one another at the PM), consistent with what was previously reported in mammalian SGs 16. Membrane mixing and the redistribution of phosphoinositides are known to regulate actin polymerization and membrane fusion events in other systems 37. In particular, the membrane phosphoinositide PI(4, 5)P 2 (PIP 2 ) is known to stimulate actin polymerization by regulating the activity of actin nucleators 38,39. Therefore, we next examined whether redistribution of PIP 2 occurs during SG secretion by using Drosophila lines expressing both the PIP 2 reporter PLCd-PH-EGFP 40 and Lifeact-RFP. Indeed, evidence for membrane mixing via PIP 2 movement from the apical PM to secretory vesicle membranes is seen (Fig. 2b,e and Supplementary Movie 7). Interestingly, PIP 2 recruitment precedes F-actin recruitment (by 51±19 s (n ¼ 12)), consistent with a role for PIP 2 in F-actin formation along secretory vesicle membranes (Fig. 2b,e and Supplementary Fig. 2b and Supplementary Movie 7). In rodent exocrine glands, it was shown that the actin motor non-muscle myosin II is recruited onto the secretory granules, but the temporal sequence of events is not known 16. To determine the kinetics of myosin recruitment relative to actin recruitment, we performed time-lapse imaging in flies co-expressing the myosin marker non-muscle myosin II (Zip-GFP) 41,42 and Lifeact-Ruby. As shown in Fig. 2c,f and Supplementary Fig. 3a and Supplementary Movie 8, myosin is detected on fused vesicles after F-actin. The average time difference in the detection of actin relative to myosin was 25±13 s (n ¼ 11). Altogether, our data show that secretion in Drosophila SGs begins with fusion pore formation and redistribution of PIP 2, followed by directional actin recruitment to secretory vesicle membranes and, finally, myosin recruitment. Moreover, secretion of the cargo is followed by complete membrane integration of secretory vesicles with the apical PM. Therefore, our real-time imaging system provides spatial and temporal resolution to image dynamic events occurring on the membranes of individual secretory granules in a living organ. Arp2/3 F-actin formation is required for secretion. The structural complexities associated with large, highly glycosylated cargo such as mucins 43,44, may require actomyosin contractile forces for cargo expulsion and membrane integration. We therefore examined the role of F-actin in SG secretion. We had previously shown that pharmacological disruption of the actin cytoskeleton by latrunculin A (LA) or cytochalasin D (CD) treatment impairs the gradual collapse of the secretory granules in rodent SGs in vivo 16. Likewise, treatment of Drosophila SGs with LA or CD also resulted in disrupted secretion (Fig. 3a,b, Supplementary Fig. 5a,c and Supplementary Movies 9 12). Treatment with either drug severely inhibited vesicle collapse as fused vesicles expanded dramatically in size, underwent compound fusion and failed to undergo membrane integration (Fig. 3a,b, Supplementary Fig. 5a,c and Supplementary Movies 9 12). The efficacy of each treatment on the actin cytoskeleton was assessed by examining both Lifeact-Ruby in real time and by staining fixed glands with phalloidin (Fig. 3c, Supplementary Figs 4a,b and 5b and Supplementary Movies 13 16). Taken together, these results demonstrate a requirement for F-actin in regulated secretion in this system and highlight its role in vesicle collapse and the prevention of compound fusion. Conventional light microscopy cannot resolve the organization of actin filaments on the surface of the membranes of the secretory granules in Drosophila SGs. Nonetheless, based on the fact that F-actin on the granules prevents compound exocytosis, we speculated that filaments may be tightly organized around the membranes, possibly by employing extensive branching. Branched actin is nucleated from linear filaments via the Arp2/3 complex and NPFs. To determine which of these proteins may be involved in regulated secretion within Drosophila SGs, we performed immunofluorescent staining on actively secreting glands. Arp3, WASp, Whamy and SCAR (but not Wash) each co-localized with phalloidin on granules that had fused with the apical PM, suggesting a role for these factors in secretion (Fig. 4a). We next examined the temporal order of recruitment of the Arp3 complex onto the secreting granules by creating a Drosophila line expressing both GFP-labelled Arp3 (Arp3-GFP) and Lifeact-Ruby. Real-time imaging of Lifeact-Ruby and Arp3-GFP during secretion revealed that F-actin is seen on granules before Arp3 recruitment (Fig. 4b,c, Supplementary Fig. 3b and Supplementary Movie 17). The average time difference in the detection of F-actin relative to Arp3 was 29±14 s (n ¼ 13). As the Arp2/3 complex is known to bind linear actin and is required for branched actin formation, our results suggest a model where fused vesicles are initially coated with linear actin, which is detected by Lifeact, and then subsequently bound by Arp2/3 to form a branched actin network. To investigate whether branched actin is required for regulated exocytosis, we next performed in vivo knockdown of components of the Arp2/3 complex in flies expressing Sgs3-GFP or Lifeact-Ruby. RNAi to Arp3 substantially decreased Arp3 expression and resulted in granules that fused with the apical membrane, but failed to secrete cargo and failed to undergo membrane integration (Fig. 5a, Supplementary Figs 6a,b and 7a and Supplementary Movies 18 and 19). Upon loss of Arp3, fused granules continued to enlarge, similar to what was observed in SGs treated with LA. However, unlike LA treatment, no evidence of compound exocytosis was seen, suggesting that linear actin still present on granules may function to block compound fusion. Interestingly, some F-actin (likely linear actin) was still detected on fused granules in the Arp3 RNAi background, although no longer in a homogenous distribution (Supplementary Fig. 6c, d and Supplementary Movies 20 and 21). Likewise, RNAi to Arp2 (which forms a functional complex with Arp3) phenocopied the knockdown of Arp3, adding further support for the role of Arp2/ 3-mediated branched actin in proper cargo expulsion and membrane integration (Fig. 5b and Supplementary Figs 6a, b and 7b and Supplementary Movies 22 and 23). We next examined the role of the Arp2/3 activator, WASp. RNAi to WASp, an NPF that can activate Arp2/3, also resulted in granules that grew larger in size, failed to expel cargo and failed to undergo membrane integration; these granules also maintained limited and unevenly distributed staining for F-actin (likely detecting linear actin) and did not show evidence of compound fusion (Fig. 5c and Supplementary Figs 6a,b,c,e and 7c and Supplementary 4 NATURE COMMUNICATIONS 6:10098 DOI: /ncomms
5 ARTICLE a A647- Dextran Lifeact-GFP T: 0 s T: 20 s T: 40 s T: 60 s T: 80 s T: 100 s b PLCδPH-EGFP Lifeact-RFP T: 0 s T: 25 s T: 50 s T: 75 s T: 100 s T: 125 s T: 150 s c Zip-GFP Lifeact-Ruby T: 0 s T: 10 s T: 20 s T: 30 s T: 40 s T: 50 s d e f % Fluorescent intensity Dextran 25 Lifeact Time (s) % Fluorescent intensity PLCδPH Lifeact Time (s) % Fluorescent intensity Lifeact Myosin Time (s) Figure 2 Fusion pore formation and membrane mixing occur before actin and myosin recruitment on individual secretory granules. (a) The lumen of glands expressing Lifeact-GFP (green) was infused with A647-dextran (cyan) to image fusion pore formation between the granule and the apical PM. Realtime imaging shows fusion pore formation (via diffusion of dextran from the SG lumen into the granules) before actin recruitment to the granule. A representative time series from five independent experiments is shown. Scale bar, 5 mm. (b) SGs expressing the PIP 2 reporter, PLCdPH-EGFP (green) and Lifeact-RFP (red) were imaged in real time to demonstrate that diffusion of PIP 2 from the apical membrane to the fused vesicle membrane occurs before actin recruitment. A representative time series from four independent experiments is shown. Scale bar, 5 mm. (c) Glands expressing Lifeact-Ruby (red) and Zip-GFP (green; non-muscle myosin II) were imaged in real time to show that actin recruitment to fused granules occurs before myosin recruitment. A representative time series from eight independent experiments is shown. Scale bar, 5 mm. (d) Quantification of Lifeact recruitment relative to time of dextran entry into fused granules. (e) Quantification of PLCdPH appearance relative to Lifeact recruitment to fused granules. (f) Quantification of Lifeact recruitment relative to myosin recruitment to fused granules. Representative graphs plotting percent fluorescent intensity of each marker as a function of time for individual secretory events are shown. Quantification was performed on 11 individual secretory events over two independent experiments, 12 individual secretory events over two independent experiments and 11 individual secretory events over four independent experiments, respectively. Additional graphs are shown in Supplementary Figs 2a, b and 3a. Complete movies for each time series can be found in the Supplementary Movie section. Movies 24 27). Finally, large, expanded granules could also be seen in the SGs in the intact pupae in Arp3 RNAi, Arp2 RNAi or WASp RNAi backgrounds (Supplementary Fig. 8), again indicating that our ex vivo SG system faithfully recapitulates what is occurring in vivo. Taken together, our studies demonstrate an essential role for the branched actin nucleators Arp2, Arp3 and WASp in secretory cargo expulsion and membrane integration during regulated exocytosis in vivo (Fig. 5d). Discussion Here, we show temporal and spatial dynamics of F-actin formation during regulated exocytosis and define essential roles for the branched actin nucleators Arp2, Arp3 and WASp in cargo secretion and membrane integration (Fig. 5d). By imaging actin dynamics in four dimensions at single granule resolution in a secreting organ, we demonstrate that actin along the PM is dynamically cleared at the point of vesicle fusion. This NATURE COMMUNICATIONS 6:10098 DOI: /ncomms
6 a Sgs3-GFP Latrunculin A a WASp Whamy SCAR Wash Arp3 b Sgs3-GFP c Lifeact-Ruby Zip-GFP Latrunculin A T: 0 min T: 15 min T: 0 min T: 3 min T: 6 min T: 9 min T: 12 min T: 15 min Latrunculin A T: 0 min T: 2 min T: 4 min T: 6 min T: 8 min T: 10 min Figure 3 Actin is required for secretion and inhibition of compound vesicle fusion. (a) Secreting glands expressing Sgs3-GFP (white) were treated with latrunculin A and individual granule fusion events were imaged in real time. Red boxes denote areas shown as magnified images in b. (b) Drug treatment resulted in compound fusion events between granules that continue to expand and fail to collapse once fused with the apical membrane. Dashed red lines outline secretory granules undergoing expansion and compound fusion events. A representative times series from three independent experiments is shown. (c) Secreting glands expressing Lifeact-Ruby (red) and Zip-GFP (green) treated with latrunculin A show that actin is no longer recruited to granules that have fused with the apical membrane. A representative time series from four independent experiments is shown. All scale bars, 5 mm. Complete movies for each time series can be found in the Supplementary Movie section. observation supports a role for cortical actin acting as a barrier to premature vesicle fusion, as has been proposed for other modes of secretion 4 6,45. Shortly after this cortical rearrangement, actin begins to expand over the secretory vesicle from the site of PM contact. Previous studies imaging actin (as it is involved in exocytosis) in two dimensions suggest that recruitment occurs simultaneously over all regions of the vesicle 46. However, our 4D renderings provide spatial and temporal resolution that has not been seen previously and show that actin recruitment to the secretory vesicle begins at the apical PM. These dynamic changes in actin organization suggest a highly ordered process whereby factors on the PM and vesicle act in concert to remodel actin to mediate secretory vesicle docking/fusion as well as to reform the actomyosin machinery around the fused vesicle. This mode of actin recruitment may provide the continuity with cortical actin required to generate the appropriate forces needed for cargo expulsion/release, especially in cases of large, highly glycosylated cargo, such as mucins. Actin recruitment to secretory vesicles was preceded by the formation of the fusion pore between the apical PM and the secretory vesicle membrane. In addition, membrane mixing and redistribution of the membrane lipid PIP 2 also occurred before F-actin recruitment. As PIP 2 is known to mediate actin formation in other systems 38,39, our results suggest that this membrane b Lifeact-Ruby Actin Arp3-GFP c Merge T: 0 s T: 20 s T: 40 s T: 60 s T: 80 s T: 100 s % Fluorescent intensity Lifeact Arp Time (s) Figure 4 Arp2/3 and WASp are recruited to granules undergoing secretion. (a) Secreting glands were fixed and stained for Arp3, WASp, Whamy, SCAR or Wash (green) along with phalloidin (red). Merged images are shown in yellow. Representative images from three independent experiments are shown. (b) Secreting glands expressing both Arp3-GFP (green) and Lifeact-Ruby (red) were imaged in real time to show that actin is detected on fused granules before Arp3, suggesting that linear actin first coats fused granules, followed by branched actin formation. A representative time series from 11 independent experiments is shown. (c) Quantification of actin recruitment relative to Arp3 recruitment to fused granules. Representative graph plotting percent fluorescent intensity of each marker as a function of time for an individual secretory event is shown. Quantification was performed on 13 individual secretory events from four independent experiments. Additional graphs are shown in Supplementary Fig. 3b. All scale bars, 5 mm. Complete movies for each time series can be found in the Supplementary Movie section. mixing may trigger actin polymerization on secretory granule membranes. Interestingly, actin polymerization appears to occur in two distinct steps, as the recruitment of Lifeact to secretory granules is detected before Arp3 recruitment. As the Arp2/3 complex is known to bind linear actin, this suggests that actin formation on fused secretory granules begins with linear actin formation (detected by Lifeact), which originates from the apical PM. Linear actin is then bound by the Arp2/3 complex, which catalyses the formation of branched actin. Myosin recruitment occurs after actin is present, as has been seen in cell-based systems 12. Future work will employ this system to temporally dissect the recruitment of other factors involved in secretion. The ordered recruitment of linear and branched forms of actin suggests essential roles for each in regulated secretion. However, specific roles for branched actin in regulated secretion were previously unknown. Taking advantage of genetic tools, we directly interrogated the role of branched actin in regulated 6 NATURE COMMUNICATIONS 6:10098 DOI: /ncomms
7 ARTICLE secretion by performing RNAi to the genes that regulate its formation. In vivo knockdown of Arp2 or Arp3, both of which are involved in the synthesis of branched actin, resulted in similar phenotypes, where secretory vesicles fused with the PM but were unable to secrete their cargo and unable to undergo membrane integration. These vesicles grew very large and in some instances, separated from the PM and floated back into the cytoplasm. In addition, in vivo knockdown of the NPF WASp resulted in similar phenotypes, identifying it as an additional regulator of exocytosis in this system. Taken together, our results suggest that branched actin is required to generate the force necessary to complete expulsion of the secretory cargo and integration of vesicular membranes with the PM. In the absence of branched actin, the fused secretory vesicles continue to expand in size, likely due to the hydration and expansion of the highly glycosylated mucinous cargo present within. These results have implications for the importance of branched actin formation in other systems such as the digestive tract, where secretion of large, highly glycosylated/ cross-linked cargo is essential for extracellular matrix formation, microbial interactions and protection of epithelial cell surfaces47,48. Our results also suggest unique roles for linear actin in regulated exocytosis. LA and CD, which disrupt the formation of both linear and branched actin, resulted in compound fusion of secretory vesicles with one another, which was not seen upon Arp3 RNAi Sgs3-GFP a T: 0 s T: 150 s T: 225 s T: 300 s T: 375 s Arp2 RNAi Sgs3-GFP b T: 75 s T: 0 s T: 75 s T: 150 s T: 225 s T: 300 s T: 375 s T: 75 s T: 150 s T: 225 s T: 300 s T: 375 s WASp RNAi Sgs3-GFP c T: 0 s d knockdown of Arp2, Arp3 or WASp. This suggests that the linear actin first recruited to the fused secretory vesicle may act as a barrier to prevent the fusion of other secretory vesicles. However, we cannot rule out the possibility that residual branched actin present in our knockdown animals may be able to prevent compound fusion events. Future studies combining fluorescent imaging and electron microscopy of genetically manipulated SGs will aim to identify specific actin structures present on fused secretory granules and how they influence vesicle interactions. Our study is unique in that we image secretory cargo as a direct indicator of secretion. Previous studies have used visualization of the fusion pore as evidence of cargo secretion8. However, in our system, fusion pore formation and cargo expulsion are temporally distinct events, indicating that fusion pore formation alone is not sufficient for secretion of vesicle contents. In addition to the forces mediated by branched actin, other factors may be required for the secretion of large, glycosylated cargos. This system can be used to interrogate other genes that regulate the processing, packaging and release of complex cargos. In summary, our study highlights coordinated actin clearance and directional recruitment from the PM as well as essential roles for branched actin nucleators in regulated exocytosis in a living, secreting organ. The powerful combination of Drosophila genetics with in vivo and ex vivo imaging allows one to rapidly interrogate the role of factors in many aspects of secretion, including vesicle biogenesis, vesicle movement, fusion with the PM, release of granule cargo and expansion of cargo once in the lumen. Given that many genes and mechanisms involved in secretion are conserved across species, this system can provide insight into the factors that regulate mammalian secretion. Cortical actin clearance and PI(4,5)P2 redistribution Secretory granules Secretory cargo PI(4,5)P2 F-actin Arp2/3 protein complex WASp Non-muscle myosin II Arp2/3-dependent cargo expulsion and membrane integration Figure 5 Arp2/3 and WASp are required for cargo secretion and membrane integration. (a) In vivo RNAi to Arp3 was performed in Drosophila lines that also express Sgs3-GFP (white) and secretion was imaged in real time. Knockdown of Arp3 resulted in granules that fused with the apical membrane but failed to collapse and secrete their contents. A representative time series from three independent experiments is shown. (b) In vivo RNAi to Arp2 was performed in Drosophila lines that also express Sgs3-GFP (white) and secretion was imaged in real time. Loss of Arp2 mimicked the secretion defects seen upon loss of Arp3. A representative time series from three independent experiments is shown. (c) In vivo RNAi to WASp was performed in Drosophila lines that also express Sgs3-GFP (white) and secretion was imaged in real time. Loss of WASp mimicked the secretion defects see upon loss of either Arp2 or Arp3. A representative time series from four independent experiments is shown. All scale bars, 5 mm. Complete movies for each time series can be found in the Supplementary Movie section. (d) Model of temporal and spatial events occurring during regulated exocytosis in Drosophila SGs. Fully formed secretory granules reside in the secretory cells of third instar larval SGs until secretion is triggered by a pulse of 20E. The early events of secretion involve cortical actin clearance along with fusion pore formation and redistribution of apical PIP2 to the vesicle membranes. The exact order of these events is not known. Following these early events, components of the actin cytoskeleton are assembled on fused granules. First, linear actin is recruited followed by the recruitment of the Arp2/3 protein complex, which forms branched actin structures from existing linear filaments. Actin recruitment occurs in a directional manner originating from the PM. Actin recruitment is followed by recruitment of non-muscle myosin II. Finally, expulsion of cargo from secretory granules and integration of granular membrane with the apical PM is dependent on Arp2-, Arp3- and WASpmediated actin formation. NATURE COMMUNICATIONS 6:10098 DOI: /ncomms
8 Methods Fly strains and genetics. The following Drosophila lines were used: Bloomington #5884 (w*; P{Sgs3-GFP}2) 32, #5885 (w*; P{Sgs3-GFP}3), #35544 (y 1 w*; P{UAS-Lifeact-GFP}VIE-260B), #35545 (y 1 w*; P{UAS-Lifeact-Ruby} VIE-19A), #7118 (w 1118 ; P{UAS-myr-mRFP}1), #32221 (w*; P{10XUAS-IVSmyr::tdTomato}attP2), #39723 (w*; P{UASp-Arp3.GFP}2) 49, #39693 (y 1 w*; P{UAS-PLCd-PH-EGFP}3), #58362 (w*; P{UASt-Lifeact-RFP}3). RNAi lines containing upstream activating sequence (UAS)-inducible inverted repeats (IR) were from the Vienna Drosophila RNAi Center (VDRC) 50 and were the following stocks: VDRC #29944 (P{UAS-Arp2IR), VDRC # (P{UAS-Arp3IR), VDRC # (P{UAS-WaspIR), VDRC #60008 (w 1118 ; P{UAS-dicer2 w þ }) and VDRC (w 1118 ; P{UAS-dicer2 w þ }). The wild-type stocks used were either Oregon R or w 1118 (VDRC #60000). The Gal4 driver line used in these studies was Bloomington stock #6978 (w 1118 ; P{GawB}c135), which drives expression in the Drosophila SG beginning during embryonic stage 15 and continuing through the third instar larval stage. The c135-gal4 driver stock was recombined with Sgs3-GFP to generate c135-gal44sgs3-gfp. VDRC #60008, which expresses dicer2 under the control of the UAS promoter, was crossed to VDRC # and VDRC # to enhance RNAi-mediated knockdown. VDRC #60009, which also expresses dicer2, was crossed to VDRC #29944 to enhance gene knockdown. Sgs3-GFP was recombined with Bloomington #3554 to generate Sgs3-GFP, UAS-Lifeact-Ruby. Sgs3-GFP was recombined with Bloomington #3222 1to generate Sgs3-GFP, UAS- Myr-tdTomato. Bloomington #3554 was recombined with Flytrap line #CC01626 to generate Zip-GFP, UAS-Lifeact-Ruby. The c135-gal4 driver was recombined with Zip-GFP, UAS-Lifeact-Ruby to generate c135-gal44zip-gfp, UAS-Lifeact- Ruby. Bloomington #3554 was recombined with Bloomington #39723 to generate UAS-Arp3-GFP, UAS-Lifeact-Ruby. The c135-gal4 driver was recombined with Bloomington #58362 to generate c135-gal44 UAS-Lifeact-RFP. All Drosophila crosses were kept on MM media (KD Medical, Inc.) at 25 C unless specified otherwise. Crosses to generate expression of dsrna were performed using flies from a UAS-IR transgenic line and the c135-gal44sgs3-gfp or c135-gal44zip- GFP, UAS-Lifeact-Ruby stocks. Ex vivo SG culture and real-time imaging. SGs from third instar wandering larvae were dissected in Schneider s Drosophila medium (Gibco) and transferred to glass bottom culture dishes (MatTek) containing 50 ml media. To immediately image glands that were actively secreting, the majority of the media were then removed from the dish and a 13-mm, 0.05 mm, polycarbonate membrane filter (Sterlitech) was gently placed on top of the gland. The media that were removed from the dish were then placed on top of the membrane filter and the sample was subsequently imaged. For imaging sessions longer than 2 h, 0.5% low melt agarose in Schneider s Drosophila medium was layered on top of glands and the sample was subsequently imaged. To induce secretion in non-secreting glands, 20-hydroxyecdysone (Sigma) was added to glands in glass bottom dishes to a final concentration of 700 mm and dishes were placed in a humidified chamber at room temperature for 90 min. The media from these dishes were then removed, a membrane was gently placed on top of the glands and media were added back to the top of the membrane. For experiments involving fluorescent dextran, SGs from non-secreting third instar wandering larvae were dissected and transferred to glass bottom culture dishes containing 50 ml media. Alexa Fluor 647, Alexa Fluor 680 or Rhodamine B Dextran (Invitrogen) was subsequently added to the media to a final concentration of 1 mm and then nutated at room temperature for 45 min. 20-Hydroxyecdysone was then added to a final concentration of 700 mm and placed in a humidified chamber at room temperature for 90 min. The media from these dishes were then removed, a membrane was gently placed on top of the glands and media were added back to the top of the membrane. For inhibitor experiments, LA (final concentration 3 mm) or CD (final concentration 10 mm) was added to the media resting on top of the polycarbonate membrane immediately before imaging. All glands were imaged on either a Zeiss LSM 510, Zeiss LSM 700 with a C-Apochromat 63/1.2 W Corr UV-VIS-IR objective or a Nikon A1R þ confocal microscope with both CFI Lambda S Apo LWD 40/1.15 numerical aperture (NA) water immersion (WI) and CFI Plan Apo IR 60/1.27 NA WI objectives. In vivo SG imaging. Wandering third instar larvae were immobilized between two mm 2 coverslips in sufficient Schneider s media such that the liquid spread evenly across the entire area of the coverslip. Imaging was performed on a Nikon A1R þ confocal microscope, with 10 Plan Apo 0.45 NA or 20 Plan Apo VC 0.75 NA objective, using the keep object in view function of the advanced tracking module to compensate for X Y movement. Single confocal sections were acquired every 5 s to keep glands in the imaging window and a Z-stack was imaged every minute to accommodate movement in the Z plane. sample such that SGs were closest to the cover glass. Z-stacks were acquired on a Nikon A1R þ confocal microscope with a 20 Plan Apo VC 0.75 NA objective. SG fixation and staining. SGs from third instar wandering larvae were dissected in Schneider s Drosophila Medium (Gibco) and immediately transferred to 4% paraformaldehyde in PBS. Glands were fixed for 20 min at 4 C. Samples were then washed three times in PBST (PBS, 0.1% Triton X-100) and transferred to blocking buffer (2% Goat serum/pbs, 0.1% Triton X-100) for 1 h at room temperature on a rocker. Samples were then incubated with primary antibodies overnight at 4 C. Primary antibodies used were mouse anti-wasp (P5E1-Developmental Studies Hybridoma Bank, 1:10), mouse anti-wash (P3H3-Developmental Studies Hybridoma Bank, 1:20), mouse anti-scar (P1C1-Developmental Studies Hybridoma Bank, 1:50), mouse anti-whamy (P4A8-Developmental Studies Hybridoma Bank, 1:20) 23 and rabbit anti-arp3 (the kind gift of Dr W. Theurkauf, 1:500) (ref. 51). Samples were then washed three times for 15 min each in PBST and incubated with secondary antibodies for 3 h at room temperature on a rocker. Alexa Fluor 488- or Alexa Fluor 647-conjugated secondary antibodies (Invitrogen, 1:50) and TRITC-conjugated phalloidin (Sigma, 1:100) were used. Samples were then washed three times for 15 min each in PBST, mounted in aqueous mounting medium (Electron Microscopy Sciences) and imaged using a Zeiss LSM 700 confocal microscope. Quantitative RT PCR. Quantitative RT PCR was used to determine efficiency of gene silencing when RNAi was induced. cdna prepared from SGs from third instar wandering larvae of c135-gal4,sgs3-gfp4ir crosses or controls (c135- Gal4,Sgs3-GFP 4VDRC#60000 or OregonR) was used in qpcr reactions. Briefly, RNA was isolated using the RNAqueous-Micro Total RNA Isolation Kit (Ambion). cdna synthesis was performed using iscript cdna Synthesis Kit (Bio-Rad). PCR primers (Supplementary Fig. 7d) were designed using Beacon Designer software (Bio-Rad). QRT-PCR was performed on a CFX96 real-time PCR thermocycler (Bio-Rad) using the SYBR-Green PCR Master Mix (Bio-Rad). RNA levels were normalized to 18S rrna. Values represent mean value±s.e.m. Significance values were calculated using two-tailed Student s t-test. Statistical analyses. Number of replicates used for each analysis is specified in figure legends. To measure percent fluorescent intensity for Lifeact/Dextran, Lifeact/PLCd-PH, Lifeact/Zip or Lifeact/Arp3 experiments, Nikon Elements software was used. A rectangular or oval region of interest (ROI) was drawn around individual secreting granules and the Time Measurement analysis tool was used to measure average fluorescent intensity within the ROI for each channel. Values were exported to Microsoft Excel and percent fluorescent intensity was calculated. The percent fluorescent intensity of each channel at each time point was calculated as: % fluorescent intensity ¼ (Fluorescent intensity Fluorescent intensity of T 0 )/(Fluorescent intensity of T max Fluorescent intensity of T 0 ) 100. T 0 is the time frame at which one of the two channels being measured begins to continually increase in fluorescent intensity. T max is the maximum fluorescent intensity value reached for each respective channel being measured. Each graph was plotted from T 0 until at least two time point beyond T max for each channel. Percent fluorescent intensity values that were negative due to fluctuations in intensity values as a result of granule movement were set to 0. Quantification was only performed on granules that did not move within or through the imaging plane during the entire process of secretion. To determine the average time difference in the detection of each component in the pairwise comparisons, we calculated the time between T 0 and when fluorescent intensity of the second component began to continually increase and was at least 1% higher than the previous time point. Measurements were performed on 11 granules for Lifeact/Dextran, 12 granules for Lifeact/PLCd-PH, 11 granules for Lifeact/Zip and 13 granules for Lifeact/Arp3. Averages were calculated for each pair and are expressed as mean values±s.d. To measure fold change in size of secreting Sgs3-GFP granules, Fiji ( was used to outline and measure the area of individual secreting granules as a function of time after fusion. Granule fusion with the apical membrane correlates with a change in fluorescent intensity of the fusing granule. Time 0 s represents the frame immediately before a change in fluorescent intensity of a given granule is detected. Values were exported to Microsoft Excel and fold change in granule size was calculated. Graphs represent mean values of all granules measured±s.d. Quantification was only performed on granules where fusion with the apical membrane could be detected and could be clearly visualized throughout the time frame of analysis. No statistical method was used to predetermine sample size. Imaging of prepupae. Prepupae were initially photographed in original MM media vials used to set up crosses. Images were acquired on a Leica MZ 16F fluorescent stereomicroscope with DC 500 camera. Following low-magnification imaging, the prepupae were then transferred to glass bottom culture dishes (MatTek) containing 50 ml media. A 13-mm, 0.05 mm, polycarbonate membrane filter (Sterlitech) was gently placed on top of the prepupae and used to roll the Image analysis. Microscopy images were opened in Fiji and individual frames were exported as still images or Movies. Movies were subsequently formatted using Apple Final Cut Pro, Adobe Media Encoder or Apple Quicktime software. Imaris Bitplane software was used to perform surface rendering of 4D Lifeact experiments. Adobe Illustrator was used to generate figures from individual still images. 8 NATURE COMMUNICATIONS 6:10098 DOI: /ncomms
9 ARTICLE References 1. Masedunskas, A., Porat-Shliom, N. & Weigert, R. Regulated exocytosis: novel insights from intravital microscopy. Traffic 13, (2012). 2. Nightingale, T. D., Cutler, D. F. & Cramer, L. P. Actin coats and rings promote regulated exocytosis. Trends Cell Biol. 22, (2012). 3. Porat-Shliom, N., Milberg, O., Masedunskas, A. & Weigert, R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell Mol. Life Sci. 70, (2013). 4. Brown, A. C. et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol. 9, e (2011). 5. Giner, D. et al. Real-time dynamics of the F-actin cytoskeleton during secretion from chromaffin cells. J. Cell Sci. 118, (2005). 6. Trifaro, J. M., Gasman, S. & Gutierrez, L. M. Cytoskeletal control of vesicle transport and exocytosis in chromaffin cells. Acta Physiol. (Oxf) 192, (2008). 7. Sokac, A. M., Co, C., Taunton, J. & Bement, W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat. Cell Biol. 5, (2003). 8. Sokac, A. M., Schietroma, C., Gundersen, C. B. & Bement, W. M. Myosin-1c couples assembling actin to membranes to drive compensatory endocytosis. Dev. Cell 11, (2006). 9. Yu, H. Y. & Bement, W. M. Control of local actin assembly by membrane fusion-dependent compartment mixing. Nat. Cell Biol. 9, (2007). 10. Yu, H. Y. & Bement, W. M. Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis. Mol. Biol. Cell 18, (2007). 11. Jerdeva, G. V. et al. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J. Cell Sci. 118, (2005). 12. Miklavc, P. et al. Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. J. Cell Sci. 128, (2015). 13. Miklavc, P. et al. Actin coating and compression of fused secretory vesicles are essential for surfactant secretion--a role for Rho, formins and myosin II. J. Cell Sci. 125, (2012). 14. Nightingale, T. D. et al. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J. Cell Biol. 194, (2011). 15. Larina, O. et al. Dynamic regulation of the large exocytotic fusion pore in pancreatic acinar cells. Mol. Biol. Cell 18, (2007). 16. Masedunskas, A. et al. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc. Natl Acad. Sci. USA 108, (2011). 17. Faix, J. & Grosse, R. Staying in shape with formins. Dev. Cell 10, (2006). 18. Gasman, S., Chasserot-Golaz, S., Hubert, P., Aunis, D. & Bader, M. F. Identification of a potential effector pathway for the trimeric Go protein associated with secretory granules. Go stimulates a granule-bound phosphatidylinositol 4-kinase by activating RhoA in chromaffin cells. J. Biol. Chem. 273, (1998). 19. Goode, B. L. & Eck, M. J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 76, (2007). 20. Goley, E. D. & Welch, M. D. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, (2006). 21. Pollard, T. D. & Cooper, J. A. Actin, a central player in cell shape and movement. Science 326, (2009). 22. Firat-Karalar, E. N. & Welch, M. D. New mechanisms and functions of actin nucleation. Curr. Opin. Cell Biol. 23, 4 13 (2011). 23. Rodriguez-Mesa, E., Abreu-Blanco, M. T., Rosales-Nieves, A. E. & Parkhurst, S. M. Developmental expression of Drosophila Wiskott-Aldrich Syndrome family proteins. Dev. Dyn. 241, (2012). 24. Rotty, J. D., Wu, C. & Bear, J. E. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat. Rev. Mol. Cell Biol. 14, 7 12 (2013). 25. Blanchoin, L. et al. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404, (2000). 26. Mullins, R. D., Heuser, J. A. & Pollard, T. D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl Acad. Sci. USA 95, (1998). 27. Uenishi, E. et al. Actin dynamics regulated by the balance of neuronal Wiskott- Aldrich syndrome protein (N-WASP) and cofilin activities determines the biphasic response of glucose-induced insulin secretion. J. Biol. Chem. 288, (2013). 28. Gorr, S. U., Venkatesh, S. G. & Darling, D. S. Parotid secretory granules: crossroads of secretory pathways and protein storage. J. Dent. Res. 84, (2005). 29. Burgess, J. et al. Type II phosphatidylinositol 4-kinase regulates trafficking of secretory granule proteins in Drosophila. Development 139, (2012). 30. Burgess, J. et al. AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila. Mol. Biol. Cell 22, (2011). 31. Ashburner, M. & Berendes, H. D. Puffing of polytene chromosomes (Academic Press, 1978). 32. Biyasheva, A., Do, T. V., Lu, Y., Vaskova, M. & Andres, A. J. Glue secretion in the Drosophila salivary gland: a model for steroid-regulated exocytosis. Dev. Biol. 231, (2001). 33. Boyd, M. & Ashburner, M. The hormonal control of salivary gland secretion in Drosophila melanogaster: studies in vitro. J. Insect Physiol. 23, (1977). 34. Hatan, M., Shinder, V., Israeli, D., Schnorrer, F. & Volk, T. The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures. J. Cell Biol. 192, (2011). 35. Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, (2008). 36. Pfeiffer, I., Petronis, S., Koper, I., Kasemo, B. & Zach, M. Vesicle adsorption and phospholipid bilayer formation on topographically and chemically nanostructured surfaces. J. Phys. Chem. B 114, (2010). 37. Shewan, A., Eastburn, D. J. & Mostov, K. Phosphoinositides in cell architecture. Cold Spring Harbor Perspect. Biol. 3, a (2011). 38. Bothe, I., Deng, S. & Baylies, M. PI (4,5)P2 regulates myoblast fusion through Arp2/3 regulator localization at the fusion site. Development 141, (2014). 39. Higgs, H. N. & Pollard, T. D. Activation by Cdc42 and PIP(2) of Wiskott- Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol. 150, (2000). 40. Verstreken, P. et al. Tweek, an evolutionarily conserved protein, is required for synaptic vesicle recycling. Neuron 63, (2009). 41. Buszczak, M. et al. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics 175, (2007). 42. Lee, D. M. & Harris, T. J. An Arf-GEF regulates antagonism between endocytosis and the cytoskeleton for Drosophila blastoderm development. Curr. Biol. 23, (2013). 43. Birchenough, G. M., Johansson, M. E., Gustafsson, J. K., Bergstrom, J. H. & Hansson, G. C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 8, (2015). 44. Nilsson, H. E. et al. Intestinal MUC2 mucin supramolecular topology by packing and release resting on D3 domain assembly. J. Mol. Biol. 426, (2014). 45. Gutierrez, L. M. New insights into the role of the cortical cytoskeleton in exocytosis from neuroendocrine cells. Int. Rev. Cell Mol. Biol. 295, (2012). 46. Jang, Y., Soekmadji, C., Mitchell, J. M., Thomas, W. G. & Thorn, P. Real-time measurement of F-actin remodelling during exocytosis using Lifeact-EGFP transgenic animals. PLoS ONE 7, e39815 (2012). 47. Ambort, D. et al. Calcium and ph-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl Acad. Sci. USA 109, (2012). 48. Schutte, A. et al. Microbial-induced meprin beta cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proc. Natl Acad. Sci. USA 111, (2014). 49. Rajan, A., Tien, A. C., Haueter, C. M., Schulze, K. L. & Bellen, H. J. The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors. Nat Cell Biol. 11, (2009). 50. Dietzl, G. et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, (2007). 51. Stevenson, V., Hudson, A., Cooley, L. & Theurkauf, W. E. Arp2/3-dependent pseudocleavage furrow assembly in syncytial Drosophila embryos. Curr. Biol. 12, (2002). Acknowledgements We thank our colleagues for many helpful discussions. We thank Jia Guo for technical assistance. We also thank the Vienna Drosophila RNAi Center, the Bloomington Stock Center and the Developmental Studies Hybridoma Bank for fly stocks and other reagents. This work was supported by the Intramural Research Program of the NIDCR, National Institutes of Health (Z01-DE to K.G.T.H.). Author contributions D.T.T. and K.G.T.H. designed and planned the research with input from R.W. and A. M.; D.T.T. performed the experiments; D.T.T., K.G.T.H., R.W. and A.M. analysed NATURE COMMUNICATIONS 6:10098 DOI: /ncomms
Supplementary Figure 1. Real time in vivo imaging of SG secretion. (a) SGs from Drosophila third instar larvae that express Sgs3-GFP (green) and
 Supplementary Figure 1. Real time in vivo imaging of SG secretion. (a) SGs from Drosophila third instar larvae that express Sgs3-GFP (green) and Lifeact-Ruby (red) were imaged in vivo to visualize secretion
Supplementary Figure 1. Real time in vivo imaging of SG secretion. (a) SGs from Drosophila third instar larvae that express Sgs3-GFP (green) and Lifeact-Ruby (red) were imaged in vivo to visualize secretion
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
Neurite initiation. Neurite formation begins with a bud that sprouts from the cell body. One or several neurites can sprout at a time.
 Neurite initiation. Neuronal maturation initiation f-actin polarization and maturation tubulin stage 1: "spherical" neuron stage 2: neurons extend several neurites stage 3: one neurite accelerates its
Neurite initiation. Neuronal maturation initiation f-actin polarization and maturation tubulin stage 1: "spherical" neuron stage 2: neurons extend several neurites stage 3: one neurite accelerates its
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
Protein Sorting, Intracellular Trafficking, and Vesicular Transport
 Protein Sorting, Intracellular Trafficking, and Vesicular Transport Noemi Polgar, Ph.D. Department of Anatomy, Biochemistry and Physiology Email: polgar@hawaii.edu Phone: 692-1422 Outline Part 1- Trafficking
Protein Sorting, Intracellular Trafficking, and Vesicular Transport Noemi Polgar, Ph.D. Department of Anatomy, Biochemistry and Physiology Email: polgar@hawaii.edu Phone: 692-1422 Outline Part 1- Trafficking
Neurite formation & neuronal polarization
 Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
The neuron as a secretory cell
 The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
Neurite formation & neuronal polarization. The cytoskeletal components of neurons have characteristic distributions and associations
 Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Cell Biology Review. The key components of cells that concern us are as follows: 1. Nucleus
 Cell Biology Review Development involves the collective behavior and activities of cells, working together in a coordinated manner to construct an organism. As such, the regulation of development is intimately
Cell Biology Review Development involves the collective behavior and activities of cells, working together in a coordinated manner to construct an organism. As such, the regulation of development is intimately
FREEMAN MEDIA INTEGRATION GUIDE Chapter 7: Inside the Cell
 FREEMAN MEDIA INTEGRATION GUIDE Chapter 7: Inside the Cell All media is on the Instructors Resource CD/DVD JPEG Resources Figures, Photos, and Tables PowerPoint Resources Chapter Outline with Figures Lecture
FREEMAN MEDIA INTEGRATION GUIDE Chapter 7: Inside the Cell All media is on the Instructors Resource CD/DVD JPEG Resources Figures, Photos, and Tables PowerPoint Resources Chapter Outline with Figures Lecture
Molecular Cell Biology 5068 In Class Exam 1 September 30, Please print your name:
 Molecular Cell Biology 5068 In Class Exam 1 September 30, 2014 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Molecular Cell Biology 5068 In Class Exam 1 September 30, 2014 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
CHAPTER 3. Cell Structure and Genetic Control. Chapter 3 Outline
 CHAPTER 3 Cell Structure and Genetic Control Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell Nucleus and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division
CHAPTER 3 Cell Structure and Genetic Control Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell Nucleus and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division
SUPPLEMENTARY INFORMATION
 GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
!"#$%&'%()*%+*,,%-&,./*%01%02%/*/3452*%3&.26%&4752*,,*1%%
 !"#$%&'%()*%+*,,%-&,./*%01%02%/*/3452*%3&.26%&4752*,,*1%% !"#$%&'(")*++*%,*'-&'./%/,*#01#%-2)#3&)/% 4'(")*++*% % %5"0)%-2)#3&) %%% %67'2#72'*%%%%%%%%%%%%%%%%%%%%%%%4'(")0/./% % 8$+&'&,+"/7 % %,$&7&/9)7$*/0/%%%%%%%%%%
!"#$%&'%()*%+*,,%-&,./*%01%02%/*/3452*%3&.26%&4752*,,*1%% !"#$%&'(")*++*%,*'-&'./%/,*#01#%-2)#3&)/% 4'(")*++*% % %5"0)%-2)#3&) %%% %67'2#72'*%%%%%%%%%%%%%%%%%%%%%%%4'(")0/./% % 8$+&'&,+"/7 % %,$&7&/9)7$*/0/%%%%%%%%%%
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Cell Structure and Cell Cycle
 E X E R C I S E 4 Cell Structure and Cell Cycle Materials model or diagram of a cell compound microscopes and lens paper prepared slides of human skeletal muscle cells, pseudostratified ciliated columnar
E X E R C I S E 4 Cell Structure and Cell Cycle Materials model or diagram of a cell compound microscopes and lens paper prepared slides of human skeletal muscle cells, pseudostratified ciliated columnar
Cell biology: Death drags down the neighbourhood
 Cell biology: Death drags down the neighbourhood The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Vasquez,
Cell biology: Death drags down the neighbourhood The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Vasquez,
Lecture 3 13/11/2018
 Lecture 3 13/11/2018 1 Plasma membrane ALL cells have a cell membrane made of proteins and lipids. protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump Lipid bilayer allows water, carbon
Lecture 3 13/11/2018 1 Plasma membrane ALL cells have a cell membrane made of proteins and lipids. protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump Lipid bilayer allows water, carbon
Chapter 16. Cellular Movement: Motility and Contractility. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 16 Cellular Movement: Motility and Contractility Lectures by Kathleen Fitzpatrick Simon Fraser University Two eukaryotic motility systems 1. Interactions between motor proteins and microtubules
Chapter 16 Cellular Movement: Motility and Contractility Lectures by Kathleen Fitzpatrick Simon Fraser University Two eukaryotic motility systems 1. Interactions between motor proteins and microtubules
Evidence that Cytochalasin B depolymerizes F-actin filaments involved in. Pseudopod formation in Amoeba proteus
 Evidence that Cytochalasin B depolymerizes F-actin filaments involved in Pseudopod formation in Amoeba proteus Ali Hussain Independent Research Project Report Biology 219 Cell Biology, Wheaton College,
Evidence that Cytochalasin B depolymerizes F-actin filaments involved in Pseudopod formation in Amoeba proteus Ali Hussain Independent Research Project Report Biology 219 Cell Biology, Wheaton College,
Shavenbaby Couples Patterning to Epidermal Cell Shape Control. Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S (2006) PLoS Biol 4(9): e290
 Shavenbaby Couples Patterning to Epidermal Cell Shape Control. Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S (2006) PLoS Biol 4(9): e290 Question (from Introduction): How does svb control the
Shavenbaby Couples Patterning to Epidermal Cell Shape Control. Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S (2006) PLoS Biol 4(9): e290 Question (from Introduction): How does svb control the
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo
 Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Role of Mitochondrial Remodeling in Programmed Cell Death in
 Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
Developmental Cell, Vol. 12 Supplementary Data Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster Gaurav Goyal, Brennan Fell, Apurva Sarin, Richard J. Youle, V. Sriram.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
Reception The target cell s detection of a signal coming from outside the cell May Occur by: Direct connect Through signal molecules
 Why Do Cells Communicate? Regulation Cells need to control cellular processes In multicellular organism, cells signaling pathways coordinate the activities within individual cells that support the function
Why Do Cells Communicate? Regulation Cells need to control cellular processes In multicellular organism, cells signaling pathways coordinate the activities within individual cells that support the function
Signal Transduction. Dr. Chaidir, Apt
 Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Signal Transduction Dr. Chaidir, Apt Background Complex unicellular organisms existed on Earth for approximately 2.5 billion years before the first multicellular organisms appeared.this long period for
Guided Reading Activities
 Name Period Chapter 4: A Tour of the Cell Guided Reading Activities Big Idea: Introduction to the Cell Answer the following questions as you read Modules 4.1 4.4: 1. A(n) uses a beam of light to illuminate
Name Period Chapter 4: A Tour of the Cell Guided Reading Activities Big Idea: Introduction to the Cell Answer the following questions as you read Modules 4.1 4.4: 1. A(n) uses a beam of light to illuminate
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
Article. Coordination of Rho Family GTPase Activities to Orchestrate Cytoskeleton Responses during Cell Wound Repair
 Current Biology 24, 144 155, January 20, 2014 ª2014 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2013.11.048 Coordination of Rho Family GTPase Activities to Orchestrate Cytoskeleton
Current Biology 24, 144 155, January 20, 2014 ª2014 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2013.11.048 Coordination of Rho Family GTPase Activities to Orchestrate Cytoskeleton
Lecture 6 - Intracellular compartments and transport I
 01.26.11 Lecture 6 - Intracellular compartments and transport I Intracellular transport and compartments 1. Protein sorting: How proteins get to their appropriate destinations within the cell 2. Vesicular
01.26.11 Lecture 6 - Intracellular compartments and transport I Intracellular transport and compartments 1. Protein sorting: How proteins get to their appropriate destinations within the cell 2. Vesicular
Student Learning Outcomes: Nucleus distinguishes Eukaryotes from Prokaryotes
 9 The Nucleus Student Learning Outcomes: Nucleus distinguishes Eukaryotes from Prokaryotes Explain general structures of Nuclear Envelope, Nuclear Lamina, Nuclear Pore Complex Explain movement of proteins
9 The Nucleus Student Learning Outcomes: Nucleus distinguishes Eukaryotes from Prokaryotes Explain general structures of Nuclear Envelope, Nuclear Lamina, Nuclear Pore Complex Explain movement of proteins
RNA Synthesis and Processing
 RNA Synthesis and Processing Introduction Regulation of gene expression allows cells to adapt to environmental changes and is responsible for the distinct activities of the differentiated cell types that
RNA Synthesis and Processing Introduction Regulation of gene expression allows cells to adapt to environmental changes and is responsible for the distinct activities of the differentiated cell types that
Introduction to cells
 Almen Cellebiologi Introduction to cells 1. Unity and diversity of cells 2. Microscopes and visualization of cells 3. Prokaryotic cells, eubacteria and archaea 4. Eucaryotic cells, nucleus, mitochondria
Almen Cellebiologi Introduction to cells 1. Unity and diversity of cells 2. Microscopes and visualization of cells 3. Prokaryotic cells, eubacteria and archaea 4. Eucaryotic cells, nucleus, mitochondria
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
A. The Cell: The Basic Unit of Life. B. Prokaryotic Cells. D. Organelles that Process Information. E. Organelles that Process Energy
 The Organization of Cells A. The Cell: The Basic Unit of Life Lecture Series 4 The Organization of Cells B. Prokaryotic Cells C. Eukaryotic Cells D. Organelles that Process Information E. Organelles that
The Organization of Cells A. The Cell: The Basic Unit of Life Lecture Series 4 The Organization of Cells B. Prokaryotic Cells C. Eukaryotic Cells D. Organelles that Process Information E. Organelles that
Membrane transport 1. Summary
 Membrane transport 1. Summary A. Simple diffusion 1) Diffusion by electrochemical gradient no energy required 2) No channel or carrier (or transporter protein) is needed B. Passive transport (= Facilitated
Membrane transport 1. Summary A. Simple diffusion 1) Diffusion by electrochemical gradient no energy required 2) No channel or carrier (or transporter protein) is needed B. Passive transport (= Facilitated
Supplementary Methods
 Supplementary Methods Modeling of magnetic field In this study, the magnetic field was generated with N52 grade nickel-plated neodymium block magnets (K&J Magnetics). The residual flux density of the magnets
Supplementary Methods Modeling of magnetic field In this study, the magnetic field was generated with N52 grade nickel-plated neodymium block magnets (K&J Magnetics). The residual flux density of the magnets
Cells. Steven McLoon Department of Neuroscience University of Minnesota
 Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Imaging structural changes of exo- & endocytosis in live cells Imaging Structural Changes Vesicle Fusion and
 Imaging structural changes of exo- & endocytosis in live cells Imaging Structural Changes Vesicle Fusion and Ling-Gang Wu Endocytosis National Institute of in Neurological Live Cells Disorders and Stroke,
Imaging structural changes of exo- & endocytosis in live cells Imaging Structural Changes Vesicle Fusion and Ling-Gang Wu Endocytosis National Institute of in Neurological Live Cells Disorders and Stroke,
Overview of Cells. Prokaryotes vs Eukaryotes The Cell Organelles The Endosymbiotic Theory
 Overview of Cells Prokaryotes vs Eukaryotes The Cell Organelles The Endosymbiotic Theory Prokaryotic Cells Archaea Bacteria Come in many different shapes and sizes.5 µm 2 µm, up to 60 µm long Have large
Overview of Cells Prokaryotes vs Eukaryotes The Cell Organelles The Endosymbiotic Theory Prokaryotic Cells Archaea Bacteria Come in many different shapes and sizes.5 µm 2 µm, up to 60 µm long Have large
Chapter 6: A Tour of the Cell
 AP Biology Reading Guide Fred and Theresa Holtzclaw Chapter 6: A Tour of the Cell Name Period Chapter 6: A Tour of the Cell Concept 6.1 To study cells, biologists use microscopes and the tools of biochemistry
AP Biology Reading Guide Fred and Theresa Holtzclaw Chapter 6: A Tour of the Cell Name Period Chapter 6: A Tour of the Cell Concept 6.1 To study cells, biologists use microscopes and the tools of biochemistry
Class Work 31. Describe the function of the Golgi apparatus? 32. How do proteins travel from the E.R. to the Golgi apparatus? 33. After proteins are m
 Eukaryotes Class Work 1. What does the word eukaryote mean? 2. What is the one major difference between eukaryotes and prokaryotes? 3. List the different kingdoms of the eukaryote domain in the order in
Eukaryotes Class Work 1. What does the word eukaryote mean? 2. What is the one major difference between eukaryotes and prokaryotes? 3. List the different kingdoms of the eukaryote domain in the order in
Supporting Information
 Supporting Information Fleissner et al. 10.1073/pnas.0907039106 Fig. S1. (A) MAK-2-GFP localized to CATs tips is not bound by membrane. his-3::pccg1 mak-2-gfp; mak-2 strain labeled with membrane dye FM4
Supporting Information Fleissner et al. 10.1073/pnas.0907039106 Fig. S1. (A) MAK-2-GFP localized to CATs tips is not bound by membrane. his-3::pccg1 mak-2-gfp; mak-2 strain labeled with membrane dye FM4
Advanced Higher Biology. Unit 1- Cells and Proteins 2c) Membrane Proteins
 Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Biology, 7e (Campbell) Chapter 6: A Tour of the Cell
 Biology, 7e (Campbell) Chapter 6: A Tour of the Cell Chapter Questions 1) What limits the resolving power of a light microscope? A) the type of lens used to magnify the object under study B) the shortest
Biology, 7e (Campbell) Chapter 6: A Tour of the Cell Chapter Questions 1) What limits the resolving power of a light microscope? A) the type of lens used to magnify the object under study B) the shortest
 DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
DOI: 10.1038/ncb2819 Gαi3 / Actin / Acetylated Tubulin Gαi3 / Actin / Acetylated Tubulin a a Gαi3 a Actin Gαi3 WT Gαi3 WT Gαi3 WT b b Gαi3 b Actin Gαi3 KO Gαi3 KO Gαi3 KO # # Figure S1 Loss of protein
7.06 Cell Biology EXAM #3 KEY
 7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016
 Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Molecular Cell Biology 5068 In Class Exam 2 November 8, 2016 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
CELB40060 Membrane Trafficking in Animal Cells. Prof. Jeremy C. Simpson. Lecture 2 COPII and export from the ER
 CELB40060 Membrane Trafficking in Animal Cells Prof. Jeremy C. Simpson Lecture 2 COPII and export from the ER Today s lecture... The COPII coat - localisation and subunits Formation of the COPII coat at
CELB40060 Membrane Trafficking in Animal Cells Prof. Jeremy C. Simpson Lecture 2 COPII and export from the ER Today s lecture... The COPII coat - localisation and subunits Formation of the COPII coat at
Supplementary Information
 Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Identifying cells using Immunofluorscence Rachel Scalzo Bio-300, 001 Professor Bentley 9/24/2014
 Identifying cells using Immunofluorscence Rachel Scalzo Bio-300, 001 Professor Bentley 9/24/2014 2 Abstract In this experiment, we used immunofluorescence to isolate certain organelles and see where they
Identifying cells using Immunofluorscence Rachel Scalzo Bio-300, 001 Professor Bentley 9/24/2014 2 Abstract In this experiment, we used immunofluorescence to isolate certain organelles and see where they
Principles of Experimental Embryology
 Biology 4361 Developmental Biology Principles of Experimental Embryology June 16, 2008 Overview What forces affect embryonic development? The embryonic environment: external and internal How do forces
Biology 4361 Developmental Biology Principles of Experimental Embryology June 16, 2008 Overview What forces affect embryonic development? The embryonic environment: external and internal How do forces
horseradish peroxidase-labeled anti-mouse secondary antibody were procured from
 SUPPORTING INFORMATION Characterization of anti-platelet properties of silver nanoparticles Siddhartha Shrivastava, Tanmay Bera, Sunil K. Singh, Gajendra Singh, P. Ramachandrarao and Debabrata Dash 1.
SUPPORTING INFORMATION Characterization of anti-platelet properties of silver nanoparticles Siddhartha Shrivastava, Tanmay Bera, Sunil K. Singh, Gajendra Singh, P. Ramachandrarao and Debabrata Dash 1.
Chapter 6: A Tour of the Cell
 Chapter 6: A Tour of the Cell 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when Robert Hooke first looked at dead cells from an oak tree.
Chapter 6: A Tour of the Cell 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when Robert Hooke first looked at dead cells from an oak tree.
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Under the Radar Screen: How Bugs Trick Our Immune Defenses
 Under the Radar Screen: How Bugs Trick Our Immune Defenses Session 2: Phagocytosis Marie-Eve Paquet and Gijsbert Grotenbreg Whitehead Institute for Biomedical Research Salmonella Gram negative bacteria
Under the Radar Screen: How Bugs Trick Our Immune Defenses Session 2: Phagocytosis Marie-Eve Paquet and Gijsbert Grotenbreg Whitehead Institute for Biomedical Research Salmonella Gram negative bacteria
T H E J O U R N A L O F C E L L B I O L O G Y
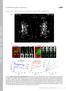 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Eisner et al., http://www.jcb.org/cgi/content/full/jcb.201312066/dc1 Figure S1. Mitochondrial continuity in adult skeletal muscle. (A)
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Eisner et al., http://www.jcb.org/cgi/content/full/jcb.201312066/dc1 Figure S1. Mitochondrial continuity in adult skeletal muscle. (A)
BIOH111. o Cell Biology Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Biology Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook
BIOH111 o Cell Biology Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook
NucView TM 488 Caspase-3 Assay Kit for Live Cells
 NucView TM 488 Caspase-3 Assay Kit for Live Cells Catalog Number: 30029 (100-500 assays) Contact Information Address: Biotium, Inc. 3423 Investment Blvd. Suite 8 Hayward, CA 94545 USA Telephone: (510)
NucView TM 488 Caspase-3 Assay Kit for Live Cells Catalog Number: 30029 (100-500 assays) Contact Information Address: Biotium, Inc. 3423 Investment Blvd. Suite 8 Hayward, CA 94545 USA Telephone: (510)
Exam 1 ID#: October 4, 2007
 Biology 4361 Name: KEY Exam 1 ID#: October 4, 2007 Multiple choice (one point each) (1-25) 1. The process of cells forming tissues and organs is called a. morphogenesis. b. differentiation. c. allometry.
Biology 4361 Name: KEY Exam 1 ID#: October 4, 2007 Multiple choice (one point each) (1-25) 1. The process of cells forming tissues and organs is called a. morphogenesis. b. differentiation. c. allometry.
Life is Cellular Section 7.1
 Life is Cellular Section 7.1 Objectives Understand Cell theory Distinguish between prokaryotes and eukaryotes Understand different types of microscopy, and how they work in more detail What is a Cell?
Life is Cellular Section 7.1 Objectives Understand Cell theory Distinguish between prokaryotes and eukaryotes Understand different types of microscopy, and how they work in more detail What is a Cell?
1. The plasma membrane of eukaryotic cells is supported by a. actin filaments. b. microtubules. c. lamins. d. intermediate filaments.
 ANALYSIS AND MODELING OF CELL MECHANICS Homework #2 (due 1/30/13) This homework involves comprehension of key biomechanical concepts of the cytoskeleton, cell-matrix adhesions, and cellcell adhesions.
ANALYSIS AND MODELING OF CELL MECHANICS Homework #2 (due 1/30/13) This homework involves comprehension of key biomechanical concepts of the cytoskeleton, cell-matrix adhesions, and cellcell adhesions.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature09450 Supplementary Table 1 Summary of kinetic parameters. Kinetic parameters were V = V / 1 K / ATP and obtained using the relationships max ( + m [ ]) V d s /( 1/ k [ ATP] + 1 k ) =,
doi:10.1038/nature09450 Supplementary Table 1 Summary of kinetic parameters. Kinetic parameters were V = V / 1 K / ATP and obtained using the relationships max ( + m [ ]) V d s /( 1/ k [ ATP] + 1 k ) =,
Contains ribosomes attached to the endoplasmic reticulum. Genetic material consists of linear chromosomes. Diameter of the cell is 1 m
 1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
Biology: Life on Earth
 Teresa Audesirk Gerald Audesirk Bruce E. Byers Biology: Life on Earth Eighth Edition Lecture for Chapter 4 Cell Structure and Function Copyright 2008 Pearson Prentice Hall, Inc. Chapter 4 Outline 4.1 What
Teresa Audesirk Gerald Audesirk Bruce E. Byers Biology: Life on Earth Eighth Edition Lecture for Chapter 4 Cell Structure and Function Copyright 2008 Pearson Prentice Hall, Inc. Chapter 4 Outline 4.1 What
Bulk Transport. Active Transport. cell drinking. Highly specific! cell eating
 Bulk Transport cell eating cell drinking Active Transport Highly specific! Bulk transport is the active intracellular membrane transport of large numbers of solute particles or a large volume of solution
Bulk Transport cell eating cell drinking Active Transport Highly specific! Bulk transport is the active intracellular membrane transport of large numbers of solute particles or a large volume of solution
Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Tuesday, December 27, 16
 Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Enduring understanding 3.B: Expression of genetic information involves cellular and molecular
Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Enduring understanding 3.B: Expression of genetic information involves cellular and molecular
Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.
 Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.441/HST522J INTEGRINS I.V. Yannas, Ph.D. and M. Spector, Ph.D. Regulator
Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.441/HST522J INTEGRINS I.V. Yannas, Ph.D. and M. Spector, Ph.D. Regulator
16 The Cell Cycle. Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization
 The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
Chapter 11. Development: Differentiation and Determination
 KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
CELL SIGNALLING and MEMBRANE TRANSPORT. Mark Louie D. Lopez Department of Biology College of Science Polytechnic University of the Philippines
 CELL SIGNALLING and MEMBRANE TRANSPORT Mark Louie D. Lopez Department of Biology College of Science Polytechnic University of the Philippines GENERIC SIGNALLING PATHWAY CELL RESPONSE TO SIGNALS CELL RESPONSE
CELL SIGNALLING and MEMBRANE TRANSPORT Mark Louie D. Lopez Department of Biology College of Science Polytechnic University of the Philippines GENERIC SIGNALLING PATHWAY CELL RESPONSE TO SIGNALS CELL RESPONSE
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
CELL BIOLOGY - CLUTCH CH. 9 - TRANSPORT ACROSS MEMBRANES.
 !! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
!! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
In order to confirm the contribution of diffusion to the FRAP recovery curves of
 Fonseca et al., Supplementary Information FRAP data analysis ) Contribution of Diffusion to the recovery curves In order to confirm the contribution of diffusion to the FRAP recovery curves of PH::GFP
Fonseca et al., Supplementary Information FRAP data analysis ) Contribution of Diffusion to the recovery curves In order to confirm the contribution of diffusion to the FRAP recovery curves of PH::GFP
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
Chapter Life Is Cellular
 Chapter 7 7-1 Life Is Cellular The Discovery of the Cell Anton van Leeuwenhoek used a single-lens microscope to observe tiny little organisms in pond water. The Discovery of the Cell In 1665, Robert Hooke
Chapter 7 7-1 Life Is Cellular The Discovery of the Cell Anton van Leeuwenhoek used a single-lens microscope to observe tiny little organisms in pond water. The Discovery of the Cell In 1665, Robert Hooke
Regulation and signaling. Overview. Control of gene expression. Cells need to regulate the amounts of different proteins they express, depending on
 Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Course Outcomes Guide (COG)
 Course Outcomes Guide (COG) Directions: Please complete this form to document your progress toward improving student learning. For each item, indicate your progress and your anticipated next steps. Thank
Course Outcomes Guide (COG) Directions: Please complete this form to document your progress toward improving student learning. For each item, indicate your progress and your anticipated next steps. Thank
1
 http://photos1.blogger.com/img/13/2627/640/screenhunter_047.jpg 1 The Nose Knows http://graphics.jsonline.com/graphics/owlive/img/mar05/sideways.one0308_big.jpg 2 http://www.stlmoviereviewweekly.com/sitebuilder/images/sideways-253x364.jpg
http://photos1.blogger.com/img/13/2627/640/screenhunter_047.jpg 1 The Nose Knows http://graphics.jsonline.com/graphics/owlive/img/mar05/sideways.one0308_big.jpg 2 http://www.stlmoviereviewweekly.com/sitebuilder/images/sideways-253x364.jpg
Hair Cells: The Sensory Transducers of the Inner Ear
 Chapter 1 Hair Cells: The Sensory Transducers of the Inner Ear Hair cells are specialized cells that transform a mechanical motion into changes in membrane potential. Such changes, whereby one form of
Chapter 1 Hair Cells: The Sensory Transducers of the Inner Ear Hair cells are specialized cells that transform a mechanical motion into changes in membrane potential. Such changes, whereby one form of
Nobel Lecture. The Principle of Membrane Fusion in the Cell. James E. Rothman Yale University. Karolinska Institutet Stockholm December 7, 2013
 Nobel Lecture James E. Rothman Yale University The Principle of Membrane Fusion in the Cell Karolinska Institutet Stockholm December 7, 2013 Stockholm 10:32 am October 7, 2013 Dr. Göran Hansson Manhattan
Nobel Lecture James E. Rothman Yale University The Principle of Membrane Fusion in the Cell Karolinska Institutet Stockholm December 7, 2013 Stockholm 10:32 am October 7, 2013 Dr. Göran Hansson Manhattan
Diffusion and Cell Membranes - I
 Diffusion and Cell Membranes - I Objectives 1. Define the following terms: solute, solvent, concentration gradient, osmotic pressure, and selectively permeable. 2. Define the following processes and identify
Diffusion and Cell Membranes - I Objectives 1. Define the following terms: solute, solvent, concentration gradient, osmotic pressure, and selectively permeable. 2. Define the following processes and identify
Chapter 03. Lecture and Animation Outline
 Chapter 03 Lecture and Animation Outline To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off. Please Note: Once you have
Chapter 03 Lecture and Animation Outline To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off. Please Note: Once you have
Cytokinesis in fission yeast: Modeling the assembly of the contractile ring
 Cytokinesis in fission yeast: Modeling the assembly of the contractile ring Nikola Ojkic, Dimitrios Vavylonis Department of Physics, Lehigh University Damien Laporte, Jian-Qiu Wu Department of Molecular
Cytokinesis in fission yeast: Modeling the assembly of the contractile ring Nikola Ojkic, Dimitrios Vavylonis Department of Physics, Lehigh University Damien Laporte, Jian-Qiu Wu Department of Molecular
Heather Currinn, Benjamin Guscott, Zita Balklava, Alice Rothnie and Thomas Wassmer*
 Online Resources APP controls the formation of PI(3,5)P 2 vesicles through its binding of the PIKfyve complex. Cellular and Molecular Life Sciences Heather Currinn, Benjamin Guscott, Zita Balklava, Alice
Online Resources APP controls the formation of PI(3,5)P 2 vesicles through its binding of the PIKfyve complex. Cellular and Molecular Life Sciences Heather Currinn, Benjamin Guscott, Zita Balklava, Alice
CELL PRACTICE TEST
 Name: Date: 1. As a human red blood cell matures, it loses its nucleus. As a result of this loss, a mature red blood cell lacks the ability to (1) take in material from the blood (2) release hormones to
Name: Date: 1. As a human red blood cell matures, it loses its nucleus. As a result of this loss, a mature red blood cell lacks the ability to (1) take in material from the blood (2) release hormones to
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY METHODS Mef2 primary screen. RNAi hairpins from the VDRC collection were crossed to Mef2-GAL4 at 27 C. After 2 weeks lethality rate and stage was scored, and if possible 20-30 males containing
SUPPLEMENTARY METHODS Mef2 primary screen. RNAi hairpins from the VDRC collection were crossed to Mef2-GAL4 at 27 C. After 2 weeks lethality rate and stage was scored, and if possible 20-30 males containing
Honors Biology Reading Guide Chapter 11
 Honors Biology Reading Guide Chapter 11 v Promoter a specific nucleotide sequence in DNA located near the start of a gene that is the binding site for RNA polymerase and the place where transcription begins
Honors Biology Reading Guide Chapter 11 v Promoter a specific nucleotide sequence in DNA located near the start of a gene that is the binding site for RNA polymerase and the place where transcription begins
A. The Cell: The Basic Unit of Life. B. Prokaryotic Cells. C. Eukaryotic Cells. D. Organelles that Process Information
 The Organization of Cells A. The Cell: The Basic Unit of Life Lecture Series 4 The Organization of Cells B. Prokaryotic Cells C. Eukaryotic Cells D. Organelles that Process Information E. Organelles that
The Organization of Cells A. The Cell: The Basic Unit of Life Lecture Series 4 The Organization of Cells B. Prokaryotic Cells C. Eukaryotic Cells D. Organelles that Process Information E. Organelles that
BIO 311C Spring 2010
 BIO 311C Spring 2010 Prokaryotic cells contain structures that are very similar to structures of the eukaryotic cytoskeleton. Prokaryotic cytoskeletal elements are required for cell division, maintaining
BIO 311C Spring 2010 Prokaryotic cells contain structures that are very similar to structures of the eukaryotic cytoskeleton. Prokaryotic cytoskeletal elements are required for cell division, maintaining
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice Supplementary Figure 2.
 Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
Figure 1. Automated 4D detection and tracking of endosomes and polarity markers.
 A t = 0 z t = 90 B t = 0 z t = 90 C t = 0-90 t = 0-180 t = 0-300 Figure 1. Automated 4D detection and tracking of endosomes and polarity markers. A: Three different z planes of a dividing SOP shown at
A t = 0 z t = 90 B t = 0 z t = 90 C t = 0-90 t = 0-180 t = 0-300 Figure 1. Automated 4D detection and tracking of endosomes and polarity markers. A: Three different z planes of a dividing SOP shown at
The Cell. C h a p t e r. PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas
 C h a p t e r 2 The Cell PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Introduction
C h a p t e r 2 The Cell PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Introduction
Dr. Ketki Assistant Professor Department of Biochemistry Heritage IMS, Varanasi
 TRANSPORT MECHANISMS Dr. Ketki Assistant Professor Department of Biochemistry Heritage IMS, Varanasi Membrane selectivity allows adjustments of cell composition and function If plasma membrane is relatively
TRANSPORT MECHANISMS Dr. Ketki Assistant Professor Department of Biochemistry Heritage IMS, Varanasi Membrane selectivity allows adjustments of cell composition and function If plasma membrane is relatively
16 CONTROL OF GENE EXPRESSION
 16 CONTROL OF GENE EXPRESSION Chapter Outline 16.1 REGULATION OF GENE EXPRESSION IN PROKARYOTES The operon is the unit of transcription in prokaryotes The lac operon for lactose metabolism is transcribed
16 CONTROL OF GENE EXPRESSION Chapter Outline 16.1 REGULATION OF GENE EXPRESSION IN PROKARYOTES The operon is the unit of transcription in prokaryotes The lac operon for lactose metabolism is transcribed
Chapter 4 Active Reading Guide A Tour of the Cell
 Name: AP Biology Mr. Croft Chapter 4 Active Reading Guide A Tour of the Cell Section 1 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when
Name: AP Biology Mr. Croft Chapter 4 Active Reading Guide A Tour of the Cell Section 1 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when
