Functional Connectivity and Selective Odor Responses of Excitatory Local Interneurons in Drosophila Antennal Lobe
|
|
|
- Ira Kelley
- 5 years ago
- Views:
Transcription
1 Article Functional Connectivity and Selective Odor Responses of Excitatory Local Interneurons in Drosophila Antennal Lobe Ju Huang, 1,2 Wei Zhang, 1,2 Wenhui Qiao, 1,2 Aiqun Hu, 1 and Zuoren Wang 1, * 1 Institute of Neuroscience, State Key Laboratory of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai , China 2 Graduate School of Chinese Academy of Sciences, Shanghai , China *Correspondence: zuorenwang@ion.ac.cn DOI /j.neuron SUMMARY Local interneurons in the Drosophila antennal lobe are thought to play important roles in shaping odor responses. However, the physiological properties of excitatory local interneurons (elns) and their connectivity in the antennal lobe remain unclear. We first characterized the firing patterns of krasavietz-gal4-labeled elns (krasavietz elns) in response to depolarizing currents. Paired recordings of krasavietz elns and PNs showed reciprocal excitatory connections mediated by dendrodendritic cholinergic synapses and gap junctions. Reciprocal connections were also found between two krasavietz elns but were rare between krasavietz elns and inhibitory LNs. Analysis of response onset latencies showed that krasavietz elns received monosynaptic inputs from ORNs. Furthermore, each eln responded with distinct patterns to different odors, and each odor elicited distinct responses in different elns, with specific temporal patterns of spiking, indicating that elns serve specific coding functions in addition to global excitation in Drosophila olfactory processing. INTRODUCTION Olfaction is a critical sensation for insects. In Drosophila, odor information received by the olfactory receptor neurons (ORNs) in the antennae and the maxillary palps is relayed to projection neurons (PNs) in the antennal lobe (AL), where the axons of ORNs expressing the same odorant receptors make synapses with the dendrites of corresponding PNs in the glomeruli (Buck and Axel, 1991; de Bruyne et al., 2001; Stocker et al., 1990; Vosshall et al., 2000). The olfactory information is processed in the antennal lobe by local circuits consisting of both PNs and local interneurons (LNs), before being further transmitted to the mushroom bodies and the protocerebral region (lateral horn) of the fly s brain (Ng et al., 2002; Wilson and Laurent, 2005; Wilson et al., 2004). In the Drosophila antennal lobe, PNs relay the olfactory information vertically from ORNs to mushroom bodies and the protocerebrum, whereas inhibitory LNs (ilns) and excitatory LNs (elns) provide lateral connections among different glomeruli that presumably endow PNs with variable spatial and temporal coding capabilities (Asahina et al., 2009; Ng et al., 2002; Olsen et al., 2007; Shang et al., 2007; Wilson and Laurent, 2005; Wilson et al., 2004). Upon odor stimulation, the firing patterns and coding properties of PNs are markedly different from those in ORNs, as a result of both intrinsic properties of PNs and lateral interactions from LNs in antennal lobe (Bhandawat et al., 2007; Silbering et al., 2008; Wilson et al., 2004). How elns contribute to the lateral modulation of PNs and their coding properties remains unclear. The vertebrate olfactory bulb and Drosophila antennal lobe contain analogous structures and may share similar coding strategies in olfactory processing (Wilson, 2008). The powerful fly genetic techniques offer many useful tools for studying the neural circuits in the antennal lobe, including a variety of promoter- or enhancer-trap Gal4 lines that can be used for labeling specific neurons. For example, the GH146-Gal4 line can be used to label two-thirds of PNs, whereas the GH298-Gal4, Np1227-Gal4, and Np2426-Gal4 lines are specific for different subsets of GABAergic ilns (Das et al., 2008; Okada et al., 2009; Stocker et al., 1997). The krasavietz-gal4 and KL107-Gal4 lines label a group of cholinergic elns that coimmunostain with the choline acetyltransferase (ChAT) (Shang et al., 2007). Previous studies have extensively characterized the inhibition of PNs by ilns and shown that they mediate olfactory gain control mainly through presynaptic inhibition of the axon terminals of ORNs, providing uniform and nonspecific global inhibition of various glomerular channels (Ng et al., 2002; Olsen and Wilson, 2008; Root et al., 2007). In addition, antennal lobe ilns are broadly tuned to odors, although with specific odor preferences (Wilson and Laurent, 2005; Wilson et al., 2004). Recent studies demonstrated that the LNs in AL displayed vast variability in their neurotransmitter profiles, connectivity and physiological properties (Chou et al., 2010). Previous work has also identified excitatory lateral excitation of PNs in the antennal lobe and suggested that neurons labeled in the krasavietz-gal4 and KL107-Gal4 lines are responsible (Olsen et al., 2007; Shang et al., 2007). However, in contrast to ilns, the connectivity of these elns with PNs or ORNs and their Neuron 67, , September 23, 2010 ª2010 Elsevier Inc. 1021
2 Figure 1. Identification of Two Subtypes of krasavietz-gal4-labeled Local Interneurons (A and B) Morphological and electrophysiological properties of krasavietz-gal4-labeled local interneurons in the antennal lobe. Left panels: anti-gfp staining (green) shows the expression pattern of krasavietz-gal4-labeled neurons in the antennal lobe of krasavietz-gal4 > UAS-mCD8::GFP fly. Avidin-rhodamine staining of biocytin (red) shows the morphology of a single recorded krasavietz neuron, the cell body of which is indicated by a white arrowhead. The biocytin (red) spread from the recorded type I krasavietz neuron (A), but not from the type II krasavietz neuron (B), to many other antennal lobe neurons. Anti-nc82 staining (blue) shows the structure of the AL. Scale bar, 20 mm. Right panels: a step-depolarizing current under current clamp (top panel) or a voltage ramp from 90 to 10 mv under voltage clamp (bottom panel) was applied to the type I krasavietz neuron (A) and type II krasavietz neuron (B), and their action potential patterns were recorded. The vertical and horizontal scale bars in current clamp are 10 mv and 100 ms, respectively. The vertical and horizontal scale bars in voltage clamp are 100 pa and 50 ms, respectively. (C and D) Quantification of the initial action potential frequency during the first 150 ms following the injection of the step-depolarizing current (C; n = 52 for type I krasavietz neurons, n = 33 for type II krasavietz neurons) and the total number of action currents during the 500 ms voltage ramp (D; n = 52 for type I krasavietz neurons, n = 33 for type II krasavietz neurons). Black arrowheads and red arrowheads marked the data points corresponding to the examples in (A) and (B), respectively. See also Figure S2. functional importance in olfactory processing remain to be elucidated. In the present study, we examined the physiological and synaptic properties of elns labeled in the krasavietz-gal4 by paired whole-cell recording. We also characterized in vivo response profiles of these elns to various odors. These results provide a basis for further understanding the function of elns in olfactory processing in the Drosophila antennal lobe. RESULTS Two Subtypes of Local Interneurons Labeled in krasavietz-gal4 Line In order to study the functional role of antennal lobe elns, we first examined their electrophysiological properties in comparison to those of ilns. We were able to visualize several neurons located on the lateral side of the antennal lobe by the membrane-localized green fluorescent protein (mgfp) signal in krasavietz-gal4 > UAS-mCD8::GFP flies (Figure 1A). Whole-cell recording showed that these mgfp-expressing neurons consisted of two subtypes of interneurons with distinct electrophysiological properties. When a step-depolarizing current was injected into the neuron under current clamp, the type I krasavietz neurons (Figure 1A) exhibited a transient high-frequency burst spiking followed by sparse spiking, whereas the type II krasavietz neurons (Figure 1B) showed a low-frequency adapting spike train. In addition, in response to a voltage ramp from 90 to 10 mv under voltage clamp recording, type I krasavietz neurons showed a train of action currents with a higher frequency than type II krasavietz neurons (Figures 1A and 1B). These two distinct properties were further quantified by measuring the action potential frequency during the first 150 ms following the injection of the step-depolarizing current (Figure 1C) and by the total number of action currents observed during the 500 ms voltage ramp (Figure 1D). Biocytin was loaded into the neuron during the whole-cell recording, and post hoc staining with avidin-rhodamine showed the morphology of the recorded neuron. Both types 1022 Neuron 67, , September 23, 2010 ª2010 Elsevier Inc.
3 of krasavietz neurons arborized widely throughout the antennal lobe (Figures 1A and 1B, and see Figure S2 available online). However, we found that for the great majority of type I krasavietz neurons (23/27), biocytin diffused from the single recorded krasavietz neuron to many other antennal lobe neurons, whereas none of type II krasavietz neurons (0/7) showed detectable spread of biocytin (Figures 1A and 1B). This suggests that type I krasavietz neurons are coupled to other neurons in the antennal lobe via gap junctions (see below). Reciprocal Excitatory Connections between krasavietz elns and PNs Previous studies have shown that krasavietz-gal4-driven mgfpexpressing neurons can be colabeled with either choline acetyltransferase (ChAT) or gamma-aminobutyric acid (GABA), indicating that krasavietz neurons include both excitatory and inhibitory interneurons (Shang et al., 2007). In order to further characterize the nature of these neurons, we performed paired whole-cell recording of krasavietz neuron-pn pairs in dissected fly brains. We identified krasavietz neurons by mgfp fluorescence in krasavietz-gal4 > UAS-mCD8::GFP flies (Figure 2A 1 ), and PNs were randomly chosen in the anterior-dorsal region of the antennal lobe, where PNs are known to be present in a cluster (Jefferis et al., 2001). The identity of PNs was judged by the typical small amplitudes of their action potentials (<10 mv; Figure S1A) and further confirmed by their morphology, as revealed by post hoc biocytin staining (Figure 2A 2 ). An example of a paired recording is shown in Figure 2B. When the type I krasavietz neuron was stimulated with step-depolarizing currents for 100 ms which elicited a defined number (4 6) of spikes in the stimulated neuron (Figure 2B), postsynaptic depolarization responses were induced in the paired PN, indicating that the type I krasavietz neuron was an eln. In 35 pairs (from 16 flies) examined, krasavietz type I neuron could elicit detectable excitatory responses in the PNs (Figure 2C). Thus we conclude that type I krasavietz neurons are elns, referred to hereafter as krasavietz elns. In these paired recordings, when 100-ms step currents were applied to the PN, postsynaptic depolarization responses were also induced in the paired krasavietz eln (Figures 2B and 2C). These results indicate that reciprocal excitatory connections between krasavietz elns and PNs are widespread across the antennal lobe. Furthermore, the relative strengths (for 100 ms stimulation) of these reciprocal connections varied greatly among the pairs recorded (Figure 2C). For some flies (e.g., fly 1 to 13 in Figure 2C), we tested the connectivity of the same krasavietz eln to several (2 to 5) PNs, and found that the responses in different PNs varied markedly. Thus, there are indeed differences in the strength of connections from the same krasavietz eln to different PNs. These results suggest that such eln-pn connectivity may be cell specific. The average amplitude of PN depolarization (for 100 ms eln stimulation) was about 0.9 mv, and that from PN to eln was about 0.6 mv (Figure 2D). Finally, we found that the reciprocal excitation from PNs to krasavietz elns was largely unaffected after the axonal outputs of PNs were eliminated by excising the mushroom bodies and lateral horns (Figure 2E), indicating that excitation of krasavietz elns by PNs was not mediated indirectly by axonal outputs of PNs to higher brain centers, but by dendrodendritic connections within the antennal lobe. Similarly, we examined by paired recording whether type II krasavietz neurons are synaptically connected with PNs. In sharp contrast to our findings on type I krasavietz neurons described above, stimulation of type II krasavietz neurons did not elicit significant inhibitory or excitatory responses in all 15 paired PNs (Figure S3). Because antennal lobe ilns can act through presynaptic inhibition on ORNs without inhibiting PNs directly (Olsen and Wilson, 2008), we conclude that type II krasavietz neurons are probably ilns and are unlikely to be elns. Furthermore, we also performed paired recordings of identified ilns and PNs in order to determine whether lateral inhibition could be recorded by the present method. We found that activation of three types of identified ilns (labeled by the GH298-, Np1227-, and Np2426-Gal4 drivers) caused very small inhibitory responses in the paired PNs (Figures S4A S4C). However, ATP: P2X 2 (Lima and Miesenböck, 2005) mediated activation of iln populations did induce inhibitory responses in PNs (Figures S4D and S4E), indicating that the inhibition of a single iln on PN exists but is too small to be detected by the paired recording method. Although stimulation of type II krasavietz neurons induced no significant responses in PNs, they could be excited by PNs in most cases (Figure S3), similar to what we observed with the other identified ilns (Figure S4). Gap Junctions and Cholinergic Synapses Mediate Reciprocal Excitation We next examined whether the reciprocal excitation between krasavietz elns and PNs was mediated by chemical synapses and the nature of transmitters involved. Because krasavietz elns express ChAT (Shang et al., 2007), and PNs are known to be cholinergic, we tested whether this reciprocal excitation involves cholinergic transmission by examining the effect of the nicotinic acetylcholine receptor (AChR) antagonists mecamylamine (Mec) and a-bungarotoxin (a-btx). We found that bath application of mecamylamine (100 mm) or a-btx (5 mm) did not inhibit or only partially inhibited the excitatory postsynaptic potentials in PNs elicited by krasavietz eln activation, respectively (Figure 3A). In contrast, application of mecamylamine or a-btx largely inhibited the excitatory postsynaptic potentials in krasavietz elns elicited by PN activation (Figure 3B). The residual potentials after pharmacological treatment indicated that the reciprocal excitation must involve other synaptic mechanism besides cholinergic transmission, such as gap junction coupling. When hyperpolarization and subthreshold depolarization were induced by current injections into a krasavietz eln, small but detectable membrane potential changes of the same polarity were observed in the paired PN (Figure 3C). We also observed the converse electrical coupling from a PN to a krasavietz eln (Figure 3C). These results demonstrate the existence of gap junctions between krasavietz elns and PNs. We further measure the coupling coefficient/ratio (post-dv/pre-dv)for both hyperpolarization and depolarization, obtained from 20 trials for each eln-pn pair, and found that the coupling coefficient/ratio (for depolarization) from eln to PN was in general larger than that of the reciprocal PN to eln connection (Figure 3D). These results Neuron 67, , September 23, 2010 ª2010 Elsevier Inc. 1023
4 Figure 2. Reciprocal Excitatory Connections between krasavietz elns and PNs (A) Illustration of the method of paired recording (A1). One electrode recorded a krasavietz neuron that was identified by mgfp fluorescence in krasavietz-gal4 > UAS-mCD8:GFP flies, and another electrode simultaneously recorded a projection neuron (PN) that was located in the anterior dorsal region of the AL. The identity of the recorded PN was confirmed through its typical morphology shown with post-hoc anti-biocytin staining (A2). (B) When a krasavietz eln was activated with a step-depolarizing current injection for 100 ms which elicited a defined number (4 6) of spikes in the stimulated neuron (left, top), postsynaptic depolarization responses were recorded in the paired PN (left, bottom). When a reciprocal protocol was applied to activating the PN (right, top), postsynaptic depolarization responses could also be recorded in the paired krasavietz eln (right, bottom). The average of 10 original recording trials (gray) is shown in blue (left) and black (right). (C) Amplitude of postsynaptic voltage responses induced by 100-ms presynaptic current pulses between 35 krasavietz eln-pn pairs were plotted (n = 35 pairs from 16 flies). In each fly, the connectivity strength of the same krasavietz eln with several different PNs (e.g., 5 PNs in fly1) was recorded subsequently. Reciprocal excitation between each krasavietz eln-pn pairs was presented. Black arrows marked the data points corresponding to the example in (B). (D) Quantification of the postsynaptic response amplitude of the reciprocal excitation between krasavietz elns and PNs (n = 35). (E) Excitation from PNs to krasavietz elns was largely unaffected when PN axons were eliminated by excising the mushroom bodies and lateral horns (yellow circles; n = 15), as compared to that in control flies (gray circles; p = 0.937, t test). See also Figure S3. suggest that electrical coupling contributes more to eln to PN connection than to PN to eln connection. The electrical coupling between krasavietz elns and PNs is consistent with the intercellular spread of biocytin from a single recorded krasavietz eln to other neurons in the AL (Figures 1A and 3J). In order to demonstrate that some of the dye recipient neurons are PNs, we recorded and loaded biocytin into single krasavietz eln in GH146-LexA::GAD/UAS-DsRed ; LexAopmCD2::GFP/krasavietz-Gal4 flies. Post hoc staining showed that several PNs were labeled by both biocytin and GFP staining 1024 Neuron 67, , September 23, 2010 ª2010 Elsevier Inc.
5 (Figure 3J). Thus, PNs are among the recipient neurons of dye spreading from krasavietz elns. To further examine whether both chemical synapse and gap junction coexist between eln and PNs, we analyzed the spiketriggered average (STA) of the postsynaptic membrane potentials (Figures 3E 3I). In trials, a brief stimulation (40 ms for eln and 30 ms for PN) was applied to trigger a single spike in the presynaptic neuron, and the responses in the postsynaptic neuron were recorded and averaged. As shown in Figures 3E 3I, we found that prior to the appearance of the presynaptic spike, the postsynaptic cell already displayed a depolarization in response to the depolarizing membrane potential of the presynaptic neuron. These results were observed for eln-pn connections in both directions, and fully support the existence of gap junction between elns and PNs. Furthermore, our finding that the amplitudes of STA between distinct krasavietz eln-pn pairs (Figure 3K) are different is consistent with the results in Figure 2C. Taken together, our results suggest that the connection from elns to PNs is largely mediated by gap junctions, whereas the PNs to elns connection is predominantly mediated by chemical synapses. Reciprocal Excitatory Connections among krasavietz elns Similarly, we also examined whether there is reciprocal excitatory connection between two krasavietz elns. Paired recording was performed on two krasavietz elns, and 100-ms step depolarizations of one eln induced excitatory responses in the other eln (Figures 4A and 4B), indicating reciprocal excitatory connections. Subthreshold depolarization and hyperpolarization of an eln also induced detectable responses of the same polarity in the paired eln with similar coupling coefficients in both directions, indicating the existence of gap junctions (Figures 4C and 4D). The results of STA analysis between two elns showed that prior to the appearance of the presynaptic spike, the postsynaptic eln already displayed a depolarization in response to the depolarizing membrane potential of the presynaptic eln (Figures 4E and 4F). Dye coupling experiments in krasavietz- Gal4 > UAS-mCD8::GFP flies showing the spread of biocytin from a single recorded eln to another eln which was labeled by both biocytin and GFP staining further confirmed the existence of gap junctions among elns (Figure 4G). Connections between ilns and krasavietz elns We first recorded the spontaneous activity of krasavietz elns and found rhythmic spontaneous IPSPs (or IPSCs) in about 50% krasavietz elns (Figure 5A). The amplitude and frequency of the rhythmic spontaneous IPSPs of krasavietz elns were about 0.8 mv and 10 Hz, respectively (Figure 5C). In addition, these rhythmic inhibitory responses could be largely inhibited by either GABA A R blocker picrotoxin (200 mm) or GABA B R blocker CGP54626 (50 mm) (Figures 5B and 5D), suggesting that krasavietz elns receive inhibitory inputs from GABAergic neurons (most likely ilns) and the involvement of both GABA A Rs and GABA B Rs in the generation of rhythmic inhibition of krasavietz elns. In order to further examine whether krasavietz elns are connected to ilns, paired recordings of iln and eln were made by selecting potential ilns from neurons with action potential amplitude > 30 mv in the dorsal lateral region of AL (Figures S1A and S1B). Out of 10 potential iln - eln pairs, only one pair showed that activation of putative iln induced inhibitory responses in krasavietz eln that were abolished by picrotoxin (Figure 5E). Furthermore, no detectable excitation in ilns was observed by activation of krasavietz elns. Therefore, the connections between krasavietz elns and ilns are rare. Monosynaptic Connections from ORNs to krasavietz elns We next examined whether krasavietz elns receive input from primary ORNs, and found that electric stimulation of the antennal nerve fiber which includes axons of most ORNs induced responses in krasavietz elns (Figure 6A). In order to determine whether these connections are mono- or polysynaptic, we measured the onset latency of krasavietz eln responses elicited by stimulation of the antennal nerve. The whole antennal nerve was stimulated with brief (0.1 ms) pulses of different intensities, and the onset latency was measured from the end of the stimulus artifact to the response onset. As a positive control for monosynaptic connections, we first showed that PNs in the antennal lobe exhibited a short onset latency of about 2 to 3 ms regardless of the different intensities of antennal nerve stimulation (Figure 6B), in agreement with the monosynaptic synapses made by ORNs on PNs (Jefferis et al., 2001; Stocker et al., 1990). We then measured the onset latency of krasavietz elns under similar antennal nerve stimulation with different intensities, and found that the krasavietz elns showed invariant latencies (Figures 6A and 6B). The distribution of the latencies of krasavietz eln responses to the weak antennal nerve stimulation was similar (<3.5 ms) to that of the PN responses (Figure 6C). These results indicate that ORNs make monosynaptic connections with krasavietz elns. Selective Odor-Elicited Responses in krasavietz elns We next examined the responses of krasavietz elns to different odorant stimuli. We applied 11 different odorants and a solvent control (paraffin oil) sequentially to the antennae of immobilized flies, and the responses of krasavietz elns were monitored by in vivo whole-cell recording, with each odorant stimulus applied six times (for 1 s each) at 30 s intervals. As shown in Figure 7A for an example krasavietz eln neuron, we observed strong responses to two (2,3-butanedione, acetone) of the four ketones and only one (ethyl acetate) of the four esters tested. Very little or no response was induced by benzaldehyde and two alcohols. Thus, krasavietz elns exhibited selective odor responses. However, a very different pattern of responses to the same set of odorants in another krasavietz eln from a different fly was observed (Figure 7B), showing moderate responses at the onset of the majority of odorant stimuli, but robust responses to 7 of 11 tested odorants at 0.5 to 1 s after the termination of the odorant stimulus. The responses of these two example neurons show that odorant-selective response patterns in elns may be reflected in either On or Off responses. Neuron 67, , September 23, 2010 ª2010 Elsevier Inc. 1025
6 Figure 3. Gap Junctions and Cholinergic Synapses Mediate Reciprocal Excitation between krasavietz elns and PNs (A) Comparison of the excitatory postsynaptic potentials of PN induced by 100 ms eln activation before (black trace) and after application of AChR blocker mecamylamine (blue trace) or a-btx (green trace). The effect of mecamylamine (blue bar, n = 8, p = 0.834, paired t test) and a-btx (green bar, n = 7, p < 0.01, paired t test) 1026 Neuron 67, , September 23, 2010 ª2010 Elsevier Inc.
7 In total, we examined odorant response patterns in 12 krasavietz elns, of which four example cells with prominent On responses and four with Off responses are shown in Figures 7C and 7D, respectively. We quantified the On and Off responses simply by counting the total number of spikes elicited within 0 to 1.5 s and 1.5 s to 3 s after the onset of the 1 s odorant stimulus, respectively. These were normalized to the maximum response elicited in that krasavietz eln across the 11-odor test set. The results showed that each eln responded with distinct patterns to each of the tested odors, and each odor elicited distinct responses in different elns. Furthermore, distinct patterns of odorant responses were reflected by either On or Off responses in these elns. DISCUSSION Drosophila olfactory information processing involves lateral modulation by local interneurons in the antennal lobe. Previous work characterized the connectivity and synaptic mechanisms of ilns in modulating the coding function (Olsen and Wilson, 2008; Root et al., 2007; Wilson and Laurent, 2005; Wilson et al., 2004). In the present study, we have further investigated the physiological and functional properties of elns in the antennal lobe, using krasavietz-gal4 that has been reported to label elns (Shang et al., 2007). Using paired recording, we found reciprocal connections between PNs and elns and identified their synaptic properties. Reciprocal connections were also found between two elns, but rare between elns and ilns. Analysis of response onset latencies showed that elns make monosynaptic connections with ORNs. We also characterized the in vivo responses of these elns to various odors. These results provide new insights into the function of elns in Drosophila olfactory processing. Excitatory LN-PN Connections Although previous experimental studies and computational modeling of the mammalian olfactory bulb indicated that lateral modulation of PNs by LNs is sparse (Davison and Katz, 2007), recent Drosophila studies suggested that the lateral excitation of PNs in the fly antennal lobes could be dense (Olsen et al., 2007). It is confirmed by our results on the connections between PNs and elns. We also observed distinct connectivity between elns and PNs, with large variation in the strength of excitatory synaptic connections. This distinct excitatory connectivity is consistent with the previous finding that different responses to the activation of a specific type of ORNs were induced in PNs of different glomeruli by lateral excitation (Olsen et al., 2007). We found that the reciprocal excitation from elns to PNs was predominately mediated by gap junctions, whereas that from PNs to elns was mediated by both gap junctions and cholinergic transmission. Direct electrical coupling between elns and PNs and the spread of biocytin from a single recorded eln to PNs and other cells confirmed the presence of gap junctions between these two types of cells. These gap junctions show rectifying properties, with elns to PNs junctions showing significantly higher coupling ratio (Figure 3D). Whereas there is wide spread of biocytin from elns to PNs (Figures 1A and 3J), we observed only a small subset of PNs showing biocytin spread to very few other cells (not the krasavietz elns) in the antennal lobe (data not shown), suggesting more severe rectification of dye spreading than electrical coupling across the gap junctions between PNs to elns. Similar observations of dye coupling rectification have already been reported in other systems, such as in leech and crayfish (Antonsen and Edwards, 2003; Fan et al., 2005; Giaume and Korn, 1984). It might be a feature of rectifying gap junctions. This asymmetry of dye diffusion here between elns and PNs might also be due to the heterotypic composition of innexins that form the junction channels (Dykes et al., 2004). Inhibitory LN-PN Connections GABAergic ilns are thought to play important roles in the antennal lobe. PNs express both GABA A - and GABA B -like receptors, and PNs odor responses are disinhibited by GABA on the EPSPs of PNs was quantified. Amplitude of the EPSPs before drug application was set to 100%. The vertical and horizontal scale bars are 0.5 mv and 100ms, respectively. (B) Comparison of the excitatory postsynaptic potentials of eln induced by 100 ms PN activation before (black trace) and after application of mecamylamine (blue trace) or a-btx (green trace). The effect of mecamylamine (blue bar, n = 6, p < 0.005, paired t test) and a-btx (green bar, n = 6, p < 0.01, paired t test) on the EPSPs of elns was quantified. The vertical and horizontal scale bars are 0.5 mv and 100 ms, respectively. (C) Electrical coupling between krasavietz elns and PNs. Membrane potential changes of the same polarity were induced in a PN (left, bottom) and krasavietz eln (right, bottom) in response to 200-ms step hyperpolarization and subthreshold depolarization of the paired krasavietz eln (left, top) and PN (right, top), respectively. The average of 20 original recording trials (gray) was shown in black. (D) Electrical coupling ratios were larger from krasavietz elns to PNs than those from PNs to krasavietz elns in depolarization condition (top, n = 12, p < 0.005, paired t test,), while they were similar in hyperpolarization condition (bottom, n = 12, p = 0.116, paired t test). (E and F) Single spikes were triggered in the presynaptic krasavietz eln and the responses in the postsynaptic PN was recorded and averaged from 50 trials (E). Spike triggered average (STA) traces were obtained from 9 krasavietz eln to PN pairs (F). Dashed black line and gray line indicate the start point of depolarization and the peak point of triggered spike of presynaptic neuron, respectively. The average traces were shown in red. (G and H) Single spikes were triggered in the presynaptic PN and the response in the postsynaptic krasavietz eln was averaged from 50 trials (G). STA traces (red) were obtained from 7 PN to krasavietz eln pairs (H). (I) Quantification of the percentage of postsynaptic responses before the appearance of action potential in the presynaptic neuron to the peak amplitude. (J) An example of dye coupling between krasavietz eln and PN. Biocytin was injected to a krasavietz eln in the GH146-LexA::GAD/UAS-DsRed ; LexAopmCD2::GFP/krasavietz-Gal4 fly. Post-hoc biocytin staining (middle) showed dye spread from the recorded krasavietz eln (white arrowhead) to several PNs (open white arrowhead) which were colabeled by GFP and biocytin signal (right). The regions within the white frames are windowed out and enlarged (up). Scale bar is 20 mm. (K) Amplitudes of STA between krasavietz elns and PNs were plotted (n = 12 pairs from 10 flies). In the first two flies, STA responses were recorded from the same krasavietz eln paired with two different PNs (N.A., not available). Black arrows marked the data points corresponding to the examples in (E) and (G). See also Figure S4. Neuron 67, , September 23, 2010 ª2010 Elsevier Inc. 1027
8 Figure 4. Reciprocal Excitatory Connections among krasavietz elns (A) When a krasavietz eln was activated with a 100 ms step depolarization (left, top), postsynaptic excitatory responses were recorded in another paired krasavietz eln (left, bottom). The excitation between two krasavietz elns was reciprocal (right). The average of 10 original recording trials (gray) is shown in blue (left) and black (right). (B) Postsynaptic responses of reciprocal excitation from 10 krasavietz eln-eln pairs were plotted. The first pair was the same as that shown in (A). (C) Electrical coupling between two krasavietz elns. Membrane potential changes of the same polarity were induced in one krasavietz eln (bottom) by 200 ms step hyperpolarization and subthreshold depolarization of the paired krasavietz eln (top). The average of 20 original recording trials (gray) was shown in black. (D) Electrical coupling ratio between krasavietz elns were presented in both hyperpolarization condition (black, n = 14) and depolarization condition (red, n = 14) Neuron 67, , September 23, 2010 ª2010 Elsevier Inc.
9 Figure 5. Connections between ilns and krasavietz elns (A and C) Recording spontaneous IPSCs (A, up) and IPSPs (A, bottom) in voltage clamp and current clamp in krasavietz elns, respectively. (C) Quantification of the amplitude of sipsps (left, n = 45) and frequency of sipsps of krasavietz elns (right, n = 45). (B and D) Comparison of the amplitude of sipsps before and after bath application of the GABA A R antagonist picrotoxin and GABA B R antagonist CGP (D) Quantification of the inhibitory effect of picrotoxin (n = 5, p < 0.005, paired t test,) and of CGP54626 (n = 5, p < 0.005, paired t test) on sipsps of krasavietz elns. Amplitude of the sipsps before drug application was set to 100%. (E) Paired recording of a krasavietz eln and a putative iln. Inhibition of krasavietz eln by iln was abolished by bath application of picrotoxin. See also Figure S1. receptor antagonists (Wilson and Laurent, 2005). Olsen et al. (2007) did not observe iln-induced postsynaptic inhibition of PNs. Although activation of a single iln did not induce any obvious responses in PNs by paired recording of iln and PN, our results show that ATP: P2X 2 mediated activation of iln populations did induce inhibitory responses in PNs (Figure S4), indicating that the inhibition of a single iln on PN exists but is too small to be detected. Activation of a single iln may be insufficient to induce substantial membrane hyperpolarization in a PN and the induced response may be highly restricted to the dendrite of the PN. Notably, our results show that PNs send excitatory connections to both elns and ilns (Figures 2, S3, and S4), presumably via dendrodendritic synapses (Figure 2E) in the glomeruli, similar to those found between mitral cells and ilns in the mammalian olfactory bulb (Nowycky et al., 1981). Excitatory LN-Inhibitory LN Connections We found spontaneous inhibitory inputs to elns (Figure 5A), but the connection between ilns and elns are rare (Figure 5E). The difference may be due to the following two reasons. First, the spontaneous IPSPs of krasavietz elns were probably induced by inputs from many ilns but not only a single iln. In addition, the ilns that we recorded were restricted to the dorsolateral region of the AL. It is possible that ilns at other regions of the AL do have connections to krasavietz elns. We found that connections among elns are wide spread, but those between elns and ilns are rare, suggesting they work separately to excite or inhibit the activity of antennal lobe. This is consistent with distinct functions (excitation versus inhibition) of these two groups of interneurons in modulating olfactory processing in the antennal lobe. Difference between krasavietz elns and KL107 elns In this study, we characterized the eln and iln subtypes in both the krasavietz-gal4 and KL107-Gal4 lines and further compared the physiological and morphological properties of krasavietz elns and KL107 elns (Figures 1 and S6). We found that krasavietz elns exhibit dense arborization that covers almost all glomeruli (Figure S2), whereas KL107 elns display relatively sparse arbors that are confined to limited areas in the antennal lobe. The action potential patterns in response to step-depolarizing current are also distinct between these two types of elns, with krasavietz elns exhibiting phasic high-frequency burst (E and F) Single spikes in one krasavietz eln were triggered and the responses in another krasavietz eln were recorded and averaged from 70 trials (E). STA traces were obtained from 10 krasavietz eln-eln pairs (F). Dashed black line and gray line indicate the start point of depolarization and the peak point of triggered spike of presynaptic neuron, respectively. The average traces were shown in red. (G) An example of dye coupling between two krasavietz elns. Biocytin was injected to a single krasavietz eln in the krasavietz-gal4/+;uas-mcd8::gfp/+ fly. Post hoc biocytin staining (middle) showed dye spread from the recorded krasavietz eln (white arrowhead) to another krasavietz eln (open white arrowhead) which was colabeled by GFP and biocytin signal (bottom). The regions within the white frames are windowed out and enlarged. Scale bar is 20 mm. See also Figure S6. Neuron 67, , September 23, 2010 ª2010 Elsevier Inc. 1029
10 Figure 6. Monosynaptic Connections from ORNs to krasavietz elns (A) Representative traces of the depolarizing responses induced in a krasavietz eln by stimulating the ORN nerve with current pulses at three different intensities. The initial responses in each trace are enlarged on the right. The onset latency of the response was measured from the end of the stimulus artifact to the response onset. This electric stimulation induced responses could be abolished by application of a-btx. (B) Average of onset response latency of krasavietz elns and PNs under ORN nerve stimulation with weak, medium, and strong intensities. Both krasavietz elns (n = 16, p = 0.641, ANOVA test) and PNs (n = 15, p = 0.405, ANOVA test) showed invariant latencies. (C) Similar cumulative distribution of onset latencies of krasavietz elns (n = 27) and PNs (n = 25) to weak ORN nerve stimulation (p = 0.235, Kolmogorov-Smirnov test). See also Figure S5. spiking of large amplitude and KL107 elns showing tonic spiking of small amplitude. Paired recording studies further revealed that reciprocal excitatory connections exist widely between krasavietz elns and PNs but are much more restricted between KL107 elns and PNs (Figures 2 and S6), consistent with the neurite arborization patterns of these two types of elns (Figures 1 and S6). These results suggest that the krasavietz- and KL107-Gal4 lines label two distinct subtypes of elns that may serve different functions in olfactory processing within the antennal lobe. Selective Odor Response Patterns and Function of krasavietz elns in Olfactory Processing In the Drosophila antennal lobe, global lateral excitation is responsible for the broader odor tuning of PNs relative to their presynaptic ORNs (Wilson et al., 2004). Excitatory LNs provide the neuronal basis for mediating this lateral excitation (Shang et al., 2007). In this study, we examined the odor response patterns of krasavietz elns in living flies and found that krasavietz elns responded selectively to odor stimuli (Figure 7). There are three aspects of this odor selectivity. First, each krasavietz eln responded with distinct firing patterns to different odors, with strong responses to some odors but weak or no response to others. Second, each odor elicited distinct responses in different krasavietz elns, with strong responses in some krasavietz elns but weak or no responses in others. Third, some krasavietz elns displayed predominantly On responses while others showed Off responses to odor stimuli, with odor specificity reflected in both On and Off responses. Compared with the odor responses of ilns (Wilson and Laurent, 2005; Wilson et al., 2004), the response profiles of krasavietz elns shown here are much more specific in terms of both the intensity of the responses to various odors as well as the temporal patterns of odor-evoked spiking. Our results indicated that krasavietz elns are widely connected to PNs and other elns. The broadly reciprocal connections of elns to PNs and other elns suggest that activation of krasavietz elns might globally increase the excitability of the olfactory circuits in the AL. However, in contrast to the similar responses of ilns to distinct odors, our results showed that the odor responses of krasavietz elns are highly selective. In addition, by comparing the responses of simultaneously recorded PN and krasavietz eln to low-intensity ORN nerve stimulation, we found that the krasavietz eln responses were much higher than those of PNs (Figure S5). Thus, odor-specific responses of a krasavietz eln at low concentrations of odors may help to increase the excitation of its differentially connected PNs. Importantly, due to accessibility of PNs for recording, we only examined connections between krasavietz elns and PNs located in the anterior-dorsal region of the AL. Although the connections of krasavietz elns to the recorded PNs are widespread, we cannot exclude the possibility that krasavietz elns to PNs in other regions exhibit different properties from those described here, including their synaptic mechanisms. In summary, our results on the eln connectivity and odor selectivity in the antennal lobe suggest that elns play an odorspecific role in Drosophila olfactory processing, beyond their global function of lateral excitation. EXPERIMENTAL PROCEDURES Fly Strains Drosophila stocks were raised on standard cornmeal-agar-molasses medium at constant conditions of 25 C, 60% relative humidity. Fly stocks used in this study were kindly provided as follows: krasavietz-gal4, KL107-Gal4 and UAS- P2X 2 by Dr. Gero Miesenböck (University of Oxford, UK); GH146-Gal4 and GH298-Gal4 by Dr. R.F. Stocker (University of Fribourg, Switzerland); Np1227-Gal4 and Np2426-Gal4 by Dr. Kei Ito (University of Tokyo, Japan), GH146-LexA::GAD, LexAOP-mCD2::GFP, UAS-DsRed by Dr. Tzumin Lee (Janelia Farm Research Campus). UAS-mCD8::GFP flies were obtained from the Bloomington Stock Center. Fly Preparation for Electrophysiological Recording For in vitro recording, 1-day-old female flies were anesthetized in a vial by CO 2. Fly brains were dissected in extracellular solution containing (in mm) NaCl 103, 1030 Neuron 67, , September 23, 2010 ª2010 Elsevier Inc.
11 Figure 7. Selective Odor-Elicited Responses in krasavietz elns (A and B) Rasters show odor-induced spiking responses of individual krasavietz eln to 11 different odorant stimuli and a paraffin oil control. Odor pulses were applied for 1 s (gray bar) and repeated six times. For each kind of odor stimulation, six repeated trials of spikes in a total 3 s time window (starting from 500 ms before odor onset) are shown. Corresponding PSTHs of each odor responses were shown on the right of rasters, and the averaged spike numbers in 50 ms per bin were presented in PSTHs. The example krasavietz eln #1 displayed selective On responses to odors (A), whereas the exampled krasavietz eln #5 exhibited selective Off responses (B). (C and D) Response profiles of krasavietz elns with prominent On responses (C) or Off responses (D). We quantified the On responses simply by counting the total number of spikes elicited within 0 to 1.5 s after the onset of the 1 s odor stimuli (C), and Off responses within 1.5 to 3 s after the onset of 1 s odor stimuli (D). These were normalized to the maximum response elicited in that krasavietz eln across the 11 odor test set. The number of spikes for each odor was plotted as a percentage of the maximum (% of max) response for each cell. KCl 3, TES 5, trehalose 10, glucose 10, sucrose 7, NaHCO 3 26, NaH2PO 4 1, CaCl 2 1.5, and MgCl 2 4 and adjusted to ph 7.3 and 280 mosm. The perineural sheath of the brain was gently removed with fine tweezers. The dissected brains were transferred to the recording chamber and fixed with a holder as previously described (Gu and O Dowd, 2006). For in vivo recording of odor responses, 2-day-old female flies were briefly anesthetized by ice then immobilized in a small hole at the bottom of the recording chamber (Wilson et al., 2004). The body of the fly was fixed on the bottom of the recording chamber by melted wax and the proboscis was stuck in front of the head with an insect pin. The cuticle between the compound eyes was removed to reveal the dorsal side and anterior side of the brain. Fat and connective tissues around the brain were removed, and the middle head muscles were cut to prevent movement during recording. The antennae of the fly extended below the bottom of the plastic plane and stayed in the air. The brain tissues were submerged in and continuously perfused with extracellular solution bubbled with 95% O 2 and 5% CO 2. Paired Whole-Cell Patch-Clamp Recording Whole-cell patch recordings were performed as previously described (Wilson and Laurent, 2005). Recording electrodes (10 15 MU) were filled with internal solution containing (in mm) D-gluconic acid potassium salt 160, HEPES 10, Mg-ATP 4, Na 3 GTP 0.5, and EGTA 1 and adjusted to ph 7.3 and 265 mosm. Biocytin (1%, Sigma) was added to the internal solution. Neurons were visualized by using a Nikon FN1 microscope with a 40 3 water-immersion objective. Electrophysiological signals were acquired by an Axon-700B multiclamp amplifier, digitized at 10 khz by a Digidata 1440A D-A converter and Bessel filtered at 2 khz. In eln-pn paired recordings, one krasavietz eln was recorded under the guidance of GFP fluorescence in krasavietz-gal4/+;uasmcd8::gfp/+ fly, and one PN in the anteriordorsal part of the antennal lobe was recorded simultaneously. The identity of PNs was judged by their typical electrophysiological properties and further confirmed by post hoc biocytin staining. One channel was used to depolarize the presynaptic neuron by injecting 100-ms inward current pulses which usually triggered 4 6 spikes, and the other channel recorded the postsynaptic membrane potential changes in the paired neuron (typically 10 trials for each recording). Only one krasavietz eln was recorded in each brain. In some flies, in order to compare the connectivity strength, several different PNs (2 5) in the same brain were paired with the same krasavietz eln subsequently. In eln-eln paired recording, the two krasavietz elns in the same AL were recorded simultaneously under the guidance of GFP fluorescence in krasavietz-gal4/+;uas-mcd8::gfp/+ fly. In eln-iln paired recording, the eln was recorded as described above, while the putative iln, whose identity was judged by the typical action potential pattern of iln, was randomly picked to record in the dorsal lateral region of AL. As for electrical coupling assay, hyperpolarization and subthreshold depolarization were induced by 200-ms current injections into presynaptic neuron, postsynaptic membrane potential changes were recorded in the paired Neuron 67, , September 23, 2010 ª2010 Elsevier Inc. 1031
12 neuron (typically 20 trials for each recording). Electrical coupling ratio was calculated as post-dv/pre-dv. As for spike triggered average (STA) assay, presynaptic neuron was activated by a brief (30 ms) current pulse which triggered only single spike, the postsynaptic membrane potentials were recorded in the postsynaptic neuron ( trials for each recording). STA results were obtained by averaging the postsynaptic responses (by Matlab program) after all pre- and postsynaptic responses were aligned according to the peak point of the presynaptic action potential. Drug Application AChR antagonist a-btx (Sigma) was used at a final concentration of 5 mm and mecamylamine (Sigma) was 100 mm. GABA A R antagonist picrotoxin (Sigma) was 200 mm and GABA B R antagonist CGP54626 (Sigma) was 50 mm. All of the drugs were applied directly into bath solution, and the effect of drugs was observed in 20 min after drug application. In Vivo Odor Response Recording Odors were delivered as described previously (Wilson et al., 2004). A constant stream of air (400 ml/min) from a mineral oil vial was puffed toward the antenna of the living fly throughout the recording. After a synchronizational triggering signal, 10% (40 ml/min) of the airstream was replaced with an odor from the odor vial (at 1:100 dilution in paraffin oil) through a three-way solenoid valve (The Lee Co). The ends of the odor-delivering tubes were positioned about 1 cm in front of the fly. In order to record the krasavietz eln in vivo, some neurons in the dorsolateral side of the antennal lobe were first removed to expose the soma of the deeply located krasavietz elns. The identity of krasavietz elns was confirmed by their GFP fluorescence and their special spike firing patterns. Odor pulses lasted for 1 s and were repeated for six trials at 30 s intervals for each odor. The odors used in this study were 2,3-butanedione, acetone, cyclohexanone, carvone, methyl salicylate, ethyl acetate, isoamyl acetate, pentyl acetate, benzaldehyde, 3-octanol, and methylcyclohexanol. Pure paraffin oil served as the control in each experiment. All odorants were purchased from Sigma. Antennal Nerve Stimulation Antennal nerves were carefully preserved during brain dissection and protected from pulling. The antennal nerve was sucked into a wide-tipped glass electrode that was filled with external solution, and a brief electric pulse ( s) of current was given by using a stimulus isolator (AMPI, Jerusalem, Israel) controlled by a Master 8 stimulator (AMPI). Different intensity of stimulation was applied to antennal nerve and the onset response latency of krasavietz elns or PNs were measured. The weak stimulation was defined as a very low intensity that evoked small EPSCs without action currents. The strong stimulation was defined as the intensity that could elicit several action current in the recording neuron, while medium stimulation was defined as the intensity which was between weak and strong. Onset response latency was measured from the stop point of the stimulation artifact to the beginning point of the response onset. Activation of P2X 2 -Expressing Cells by ATP ATP (1 mm), the ligand for P2X 2 receptors, was puffed onto the neurons which expressed the P2X 2 receptors by an electrically gated valve (Picospritzer III, Parker Hannifin Corp.) under the control of a pulse generator (Master-8 stimulator, AMPI). The tip of the pipette was positioned as close as possible to the stimulated LNs. PN s membrane potential changes were recorded in response to ATP application. Extracellular solution was perfused through the recording chamber with a flow direction that minimized the potential effects of diffusion of ATP to unstimulated neurons. Biocytin Loading and Staining Biocytin (1%) was loaded into the target neuron through the electrode (10 MU) during whole-cell recording. The dye only loaded into one neuronal soma per brain. For about 1 hr following loading, dye was allowed to diffuse within the recording neuron, and then through gap junctions, if there are, to other neurons. After recording, the brain was transferred into 4% paraformaldehyde for 2 hr fixation on ice. The brains were then rinsed in PBS several times and blocked in blocking buffer (0.1M Tris-HCl, 0.1% Triton X-100, 10% goat serum) for 2 hr at room temperature. The brains were incubated with the mouse nc82 antibody (diluted 1:50) and with a rabbit anti-gfp antibody (Molecular Probes; 1:500) overnight at 4 C. After 3 times wash in PBS with 0.1% Triton X-100, the brains were incubated with rhodamine-avidin (Vector Laboratories; 1:200) and Alexa Fluor 488-conjugated goat anti-rabbit antibody and Alexa Fluor 633-conjugated goat anti-mouse antibody (both from Molecular Probes and diluted 1:200) for 2 hr at room temperature. The brains were briefly washed three times, transferred into a glass-bottomed chamber filled with PBS, and immobilized by a holder. A ZEISS LSM 5 Pascal confocal system with a 403 water-immersion objective was used to obtain the confocal optical slices (6 mm each) of the antennal lobes. As for eln-pn dye coupling assay, double labeling transgenic flies GH146- LexA::GAD/UAS-DsRed ; LexAop-mCD2::GFP/krasavietz-Gal4 were produced to label PNs with mcd2::gfp and label krasavietz neurons with DsRed. Only one krasavietz eln in each brain was recorded and loaded with biocytin. Post hoc biocytin staining was performed to show the dye spread from the krasavietz eln to other neurons, including the PNs which were labeled by mcd2::gfp under the driven of GH146-LexA. As for the eln-eln dye coupling assay, krasavietz-gal4/+; UAS-mCD8::GFP/+ flies were used. Similarly, biocytin was loaded into one krasavietz eln to see the dye spread to another krasavietz eln which was labeled by mcd8::gfp under the driven of krasavietz- Gal4. Data Analysis and Statistics Electrophysiological data analysis was carried out with IGOR Pro (Wavemetrics, Inc.) or Clampfit 10.1 (Molecular Devices Co) software. Excitatory postsynaptic responses of eln-pn or eln-eln by paired-patch recordings were averaged from 10 trials. Electrical coupling recordings were averaged from 20 trials and electrical coupling ratio was calculated as post-dv/pre-dv. Single spike triggered postsynaptic responses were averaged from trials. In the odor responses, the action potentials in a 3 s time window (starting from 500 ms before the odor triggering signal) of 6 repeated trials were plotted as rasters (small black bars). Corresponding PSTHs were shown with 50 ms per bin. In the responses to antennal nerve stimulation, the onset response latencies were measured from the stop point of the stimulation artifact to the beginning point of the response onset. Statistics were carried out by using Stata 9.0, and all statistic data are presented as mean ± SEM. SUPPLEMENTAL INFORMATION Supplemental Information includes six figures and can be found with this article online at doi: /j.neuron ACKNOWLEDGMENTS We thank Dr. G. Miesenböck (University of Oxford, UK) for providing Krasavietz-Gal4, KL107-Gal4, and UAS-P2X 2 flies, Dr. R.F. Stocker (University of Fribourg, Switzerland) for GH146-Gal4 and GH298-Gal4 flies, Dr. Kei Ito (University of Tokyo, Japan) for Np1227-Gal4 and Np2426-Gal4 flies, Dr. Tzumin Lee (Janelia Farm Research Campus) for GH146-LexA::GAD, LexAOPmCD2::GFP, UAS-DsRed flies, and Dr. E. Buchner (University of Würzburg, Germany) for providing nc82 antibody. We thank Dr. R.I. Wilson and Dr. O.R. Shawn (Harvard University) for technical guidance. We thank Dr. M.M. Poo for critical reading of and comments on the manuscript. This work was supported by the CB806604, NSF to Z.W. Accepted: July 16, 2010 Published: September 22, 2010 REFERENCES Antonsen, B.L., and Edwards, D.H. (2003). Differential dye coupling reveals lateral giant escape circuit in crayfish. J. Comp. Neurol. 466, Asahina, K., Louis, M., Piccinotti, S., and Vosshall, L.B. (2009). A circuit supporting concentration-invariant odor perception in Drosophila. J. Biol. 8, Neuron 67, , September 23, 2010 ª2010 Elsevier Inc.
Excitatory Interactions between Olfactory Processing Channels in the Drosophila Antennal Lobe
 Article Excitatory Interactions between Olfactory Processing Channels in the Drosophila Antennal Lobe Shawn R. Olsen, 1,2 Vikas Bhandawat, 1,2 and Rachel I. Wilson 1, * 1 Department of Neurobiology, Harvard
Article Excitatory Interactions between Olfactory Processing Channels in the Drosophila Antennal Lobe Shawn R. Olsen, 1,2 Vikas Bhandawat, 1,2 and Rachel I. Wilson 1, * 1 Department of Neurobiology, Harvard
Chapter 9. Nerve Signals and Homeostasis
 Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Chapter 48 Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
Information processing. Divisions of nervous system. Neuron structure and function Synapse. Neurons, synapses, and signaling 11/3/2017
 Neurons, synapses, and signaling Chapter 48 Information processing Divisions of nervous system Central nervous system (CNS) Brain and a nerve cord Integration center Peripheral nervous system (PNS) Nerves
Neurons, synapses, and signaling Chapter 48 Information processing Divisions of nervous system Central nervous system (CNS) Brain and a nerve cord Integration center Peripheral nervous system (PNS) Nerves
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Electrical Coupling between Olfactory Glomeruli
 rticle Emre Yaksi 1 and Rachel I. Wilson 1, 1 epartment of Neurobiology, Harvard Medical School, 22 Longwood venue, oston M, 2115, US orrespondence: rachel_wilson@hms.harvard.edu OI 1.116/j.neuron.21.8.41
rticle Emre Yaksi 1 and Rachel I. Wilson 1, 1 epartment of Neurobiology, Harvard Medical School, 22 Longwood venue, oston M, 2115, US orrespondence: rachel_wilson@hms.harvard.edu OI 1.116/j.neuron.21.8.41
MEMBRANE POTENTIALS AND ACTION POTENTIALS:
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
NN-A29932: Supplementary Data 1 Diversity and Wiring Variability of Olfactory Local Interneurons in the Drosophila Antennal Lobe
 NN-A29932: Supplementary Data 1 Diversity and Wiring Variability of Olfactory Local Interneurons in the Drosophila Antennal Lobe Ya-Hui Chou, Maria L. Spletter, Emre Yaksi, Jonathan C. S. Leong, Rachel
NN-A29932: Supplementary Data 1 Diversity and Wiring Variability of Olfactory Local Interneurons in the Drosophila Antennal Lobe Ya-Hui Chou, Maria L. Spletter, Emre Yaksi, Jonathan C. S. Leong, Rachel
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Neurons, Synapses, and Signaling
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lectures for Biology, Eighth Edition Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp and Janette Lewis Copyright
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lectures for Biology, Eighth Edition Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp and Janette Lewis Copyright
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Neurons, Synapses, and Signaling
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
BIOLOGY. 1. Overview of Neurons 11/3/2014. Neurons, Synapses, and Signaling. Communication in Neurons
 CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
لجنة الطب البشري رؤية تنير دروب تميزكم
 1) Hyperpolarization phase of the action potential: a. is due to the opening of voltage-gated Cl channels. b. is due to prolonged opening of voltage-gated K + channels. c. is due to closure of the Na +
1) Hyperpolarization phase of the action potential: a. is due to the opening of voltage-gated Cl channels. b. is due to prolonged opening of voltage-gated K + channels. c. is due to closure of the Na +
Nervous System Organization
 The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
BIOLOGY 11/10/2016. Neurons, Synapses, and Signaling. Concept 48.1: Neuron organization and structure reflect function in information transfer
 48 Neurons, Synapses, and Signaling CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Concept 48.1: Neuron organization
48 Neurons, Synapses, and Signaling CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Concept 48.1: Neuron organization
Organization of the nervous system. Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2
 Nervous system Organization of the nervous system Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2 Autonomic and somatic efferent pathways Reflex arc - a neural pathway that
Nervous system Organization of the nervous system Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2 Autonomic and somatic efferent pathways Reflex arc - a neural pathway that
37 Neurons, Synapses, and Signaling
 CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: Lines of Communication
CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: Lines of Communication
Membrane Potentials, Action Potentials, and Synaptic Transmission. Membrane Potential
 Cl Cl - - + K + K+ K + K Cl - 2/2/15 Membrane Potentials, Action Potentials, and Synaptic Transmission Core Curriculum II Spring 2015 Membrane Potential Example 1: K +, Cl - equally permeant no charge
Cl Cl - - + K + K+ K + K Cl - 2/2/15 Membrane Potentials, Action Potentials, and Synaptic Transmission Core Curriculum II Spring 2015 Membrane Potential Example 1: K +, Cl - equally permeant no charge
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #4 Wednesday, October 4, 2006 7. CHEMICAL SYNAPSES AND GAP JUNCTIONS We will look at: Chemical synapses in the nervous system Gap junctions in
ECE 795: Quantitative Electrophysiology Notes for Lecture #4 Wednesday, October 4, 2006 7. CHEMICAL SYNAPSES AND GAP JUNCTIONS We will look at: Chemical synapses in the nervous system Gap junctions in
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Consider the following spike trains from two different neurons N1 and N2:
 About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
Dendrites - receives information from other neuron cells - input receivers.
 The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
BIOLOGY. Neurons, Synapses, and Signaling CAMPBELL. Reece Urry Cain Wasserman Minorsky Jackson
 CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 48 Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Lines of Communication The
CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 48 Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Lines of Communication The
80% of all excitatory synapses - at the dendritic spines.
 Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn.
 Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn. The Chemical Senses: Olfaction Mary ET Boyle, Ph.D. Department
Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn. The Chemical Senses: Olfaction Mary ET Boyle, Ph.D. Department
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34
 NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NOTES: CH 48 Neurons, Synapses, and Signaling
 NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
Sensory Encoding of Smell in the Olfactory System of Drosophila
 Sensory Encoding of Smell in the Olfactory System of Drosophila (reviewing Olfactory Information Processing in Drosophila by Masse et al, 2009) Ben Cipollini COGS 160 May 11, 2010 Smell Drives Our Behavior...
Sensory Encoding of Smell in the Olfactory System of Drosophila (reviewing Olfactory Information Processing in Drosophila by Masse et al, 2009) Ben Cipollini COGS 160 May 11, 2010 Smell Drives Our Behavior...
Nerve Signal Conduction. Resting Potential Action Potential Conduction of Action Potentials
 Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
Microsystems for Neuroscience and Medicine. Lecture 9
 1 Microsystems for Neuroscience and Medicine Lecture 9 2 Neural Microsystems Neurons - Structure and behaviour Measuring neural activity Interfacing with neurons Medical applications - DBS, Retinal Implants
1 Microsystems for Neuroscience and Medicine Lecture 9 2 Neural Microsystems Neurons - Structure and behaviour Measuring neural activity Interfacing with neurons Medical applications - DBS, Retinal Implants
The Neuron - F. Fig. 45.3
 excite.org(anism): Electrical Signaling The Neuron - F. Fig. 45.3 Today s lecture we ll use clickers Review today 11:30-1:00 in 2242 HJ Patterson Electrical signals Dendrites: graded post-synaptic potentials
excite.org(anism): Electrical Signaling The Neuron - F. Fig. 45.3 Today s lecture we ll use clickers Review today 11:30-1:00 in 2242 HJ Patterson Electrical signals Dendrites: graded post-synaptic potentials
Propagation& Integration: Passive electrical properties
 Fundamentals of Neuroscience (NSCS 730, Spring 2010) Instructor: Art Riegel; email: Riegel@musc.edu; Room EL 113; time: 9 11 am Office: 416C BSB (792.5444) Propagation& Integration: Passive electrical
Fundamentals of Neuroscience (NSCS 730, Spring 2010) Instructor: Art Riegel; email: Riegel@musc.edu; Room EL 113; time: 9 11 am Office: 416C BSB (792.5444) Propagation& Integration: Passive electrical
Control and Integration. Nervous System Organization: Bilateral Symmetric Animals. Nervous System Organization: Radial Symmetric Animals
 Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Ch. 5. Membrane Potentials and Action Potentials
 Ch. 5. Membrane Potentials and Action Potentials Basic Physics of Membrane Potentials Nerve and muscle cells: Excitable Capable of generating rapidly changing electrochemical impulses at their membranes
Ch. 5. Membrane Potentials and Action Potentials Basic Physics of Membrane Potentials Nerve and muscle cells: Excitable Capable of generating rapidly changing electrochemical impulses at their membranes
Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions:
 Nervous System Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions: Sensory Input: Obtaining stimulation from the environment (light, heat, pressure, vibration, chemical,
Nervous System Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions: Sensory Input: Obtaining stimulation from the environment (light, heat, pressure, vibration, chemical,
doi: /j.neulet
 doi: 10.1016/j.neulet.2012.08.052 *Manuscript Click here to view linked References Title: Whole-cell recording from Kenyon cells in silkmoths Author affiliation: a. Department of Advanced Interdisciplinary
doi: 10.1016/j.neulet.2012.08.052 *Manuscript Click here to view linked References Title: Whole-cell recording from Kenyon cells in silkmoths Author affiliation: a. Department of Advanced Interdisciplinary
Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling
 Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Neurons. The Molecular Basis of their Electrical Excitability
 Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
External Tufted Cells: A Major Excitatory Element That Coordinates Glomerular Activity
 6676 The Journal of Neuroscience, July 28, 2004 24(30):6676 6685 Cellular/Molecular External Tufted Cells: A Major Excitatory Element That Coordinates Glomerular Activity Abdallah Hayar, Sergei Karnup,
6676 The Journal of Neuroscience, July 28, 2004 24(30):6676 6685 Cellular/Molecular External Tufted Cells: A Major Excitatory Element That Coordinates Glomerular Activity Abdallah Hayar, Sergei Karnup,
Overview Organization: Central Nervous System (CNS) Peripheral Nervous System (PNS) innervate Divisions: a. Afferent
 Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Synaptic dynamics. John D. Murray. Synaptic currents. Simple model of the synaptic gating variable. First-order kinetics
 Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Biosciences in the 21st century
 Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Structure and Measurement of the brain lecture notes
 Structure and Measurement of the brain lecture notes Marty Sereno 2009/2010!"#$%&'(&#)*%$#&+,'-&.)"/*"&.*)*-'(0&1223 Neurons and Models Lecture 1 Topics Membrane (Nernst) Potential Action potential/voltage-gated
Structure and Measurement of the brain lecture notes Marty Sereno 2009/2010!"#$%&'(&#)*%$#&+,'-&.)"/*"&.*)*-'(0&1223 Neurons and Models Lecture 1 Topics Membrane (Nernst) Potential Action potential/voltage-gated
Lecture 11 : Simple Neuron Models. Dr Eileen Nugent
 Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
PROPERTY OF ELSEVIER SAMPLE CONTENT - NOT FINAL. The Nervous System and Muscle
 The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
Olfactory Information Processing in Drosophila
 Current Biology 19, R7 R713, August 25, 29 ª29 Elsevier Ltd All rights reserved DOI 1.116/j.cub.29.6.26 Olfactory Information Processing in Drosophila Review Nicolas Y. Masse 1, Glenn C. Turner 2, and
Current Biology 19, R7 R713, August 25, 29 ª29 Elsevier Ltd All rights reserved DOI 1.116/j.cub.29.6.26 Olfactory Information Processing in Drosophila Review Nicolas Y. Masse 1, Glenn C. Turner 2, and
Introduction and the Hodgkin-Huxley Model
 1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
Olfactory Representations by Drosophila Mushroom Body Neurons NY 11724
 Page 1 of 49 Articles in PresS. J Neurophysiol (December 19, 27). doi:1.1152/jn.1283.27 Olfactory Representations by Drosophila Mushroom Body Neurons Glenn C. Turner 1,2, Maxim Bazhenov 3, Gilles Laurent
Page 1 of 49 Articles in PresS. J Neurophysiol (December 19, 27). doi:1.1152/jn.1283.27 Olfactory Representations by Drosophila Mushroom Body Neurons Glenn C. Turner 1,2, Maxim Bazhenov 3, Gilles Laurent
CELL BIOLOGY - CLUTCH CH. 9 - TRANSPORT ACROSS MEMBRANES.
 !! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
!! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
Regulation of interneuron excitability by gap junction coupling with principal cells
 Regulation of interneuron excitability by gap junction coupling with principal cells Pierre F Apostolides 1,2 & Laurence O Trussell 2 Electrical coupling of inhibitory interneurons can synchronize activity
Regulation of interneuron excitability by gap junction coupling with principal cells Pierre F Apostolides 1,2 & Laurence O Trussell 2 Electrical coupling of inhibitory interneurons can synchronize activity
Inhibition of S532C by MTSET at intracellular ph 6.8 indicates accessibility in the closed
 Supplementary Text Inhibition of S532C by MTSET at intracellular ph 6.8 indicates accessibility in the closed state It is difficult to examine accessibility of cysteine-substituted mutants in the fully
Supplementary Text Inhibition of S532C by MTSET at intracellular ph 6.8 indicates accessibility in the closed state It is difficult to examine accessibility of cysteine-substituted mutants in the fully
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES In Physiology Today Ohm s Law I = V/R Ohm s law: the current through a conductor between two points is directly proportional to the voltage across the
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES In Physiology Today Ohm s Law I = V/R Ohm s law: the current through a conductor between two points is directly proportional to the voltage across the
Integration of synaptic inputs in dendritic trees
 Integration of synaptic inputs in dendritic trees Theoretical Neuroscience Fabrizio Gabbiani Division of Neuroscience Baylor College of Medicine One Baylor Plaza Houston, TX 77030 e-mail:gabbiani@bcm.tmc.edu
Integration of synaptic inputs in dendritic trees Theoretical Neuroscience Fabrizio Gabbiani Division of Neuroscience Baylor College of Medicine One Baylor Plaza Houston, TX 77030 e-mail:gabbiani@bcm.tmc.edu
Sensory/ Motor Systems March 10, 2008
 Sensory/ Motor Systems March 10, 2008 Greg Suh greg.suh@med.nyu.edu Title: Chemical Senses- periphery Richard Axel and Linda Buck Win Nobel Prize for Olfactory System Research 2 3 After Leslie Vosshal
Sensory/ Motor Systems March 10, 2008 Greg Suh greg.suh@med.nyu.edu Title: Chemical Senses- periphery Richard Axel and Linda Buck Win Nobel Prize for Olfactory System Research 2 3 After Leslie Vosshal
Neurochemistry 1. Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906
 Neurochemistry 1 Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906 How Many Neurons Do We Have? The human brain contains ~86 billion neurons and
Neurochemistry 1 Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906 How Many Neurons Do We Have? The human brain contains ~86 billion neurons and
SUPPLEMENTARY INFORMATION
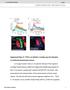 Supplemental Figure S1. WISL is not elicited by courtship song and is dependent on chordotonal mechanosensory neurons. (a) Average locomotor velocity vs. time plots for wild-type CS flies exposed to recordings
Supplemental Figure S1. WISL is not elicited by courtship song and is dependent on chordotonal mechanosensory neurons. (a) Average locomotor velocity vs. time plots for wild-type CS flies exposed to recordings
Synapses. Electrophysiology and Vesicle release
 Synapses Electrophysiology and Vesicle release Major point Cell theory (cells being separated) implies that cells must communicate with each other through extracellular connections most communication is
Synapses Electrophysiology and Vesicle release Major point Cell theory (cells being separated) implies that cells must communicate with each other through extracellular connections most communication is
Vertebrate Physiology 437 EXAM I NAME, Section (circle): am pm 23 September Exam is worth 100 points. You have 75 minutes.
 1 Vertebrate Physiology 437 EXAM I NAME, Section (circle): am pm 23 September 2004. Exam is worth 100 points. You have 75 minutes. True or False (write true or false ; 10 points total; 1 point each) 1.
1 Vertebrate Physiology 437 EXAM I NAME, Section (circle): am pm 23 September 2004. Exam is worth 100 points. You have 75 minutes. True or False (write true or false ; 10 points total; 1 point each) 1.
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
Neurons: Cellular and Network Properties HUMAN PHYSIOLOGY POWERPOINT
 POWERPOINT LECTURE SLIDE PRESENTATION by LYNN CIALDELLA, MA, MBA, The University of Texas at Austin Additional text by J Padilla exclusively for physiology at ECC UNIT 2 8 Neurons: PART A Cellular and
POWERPOINT LECTURE SLIDE PRESENTATION by LYNN CIALDELLA, MA, MBA, The University of Texas at Austin Additional text by J Padilla exclusively for physiology at ECC UNIT 2 8 Neurons: PART A Cellular and
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell
 Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Housekeeping, 26 January 2009
 5 th & 6 th Lectures Mon 26 & Wed 28 Jan 2009 Vertebrate Physiology ECOL 437 (MCB/VetSci 437) Univ. of Arizona, spring 2009 Neurons Chapter 11 Kevin Bonine & Kevin Oh 1. Finish Solutes + Water 2. Neurons
5 th & 6 th Lectures Mon 26 & Wed 28 Jan 2009 Vertebrate Physiology ECOL 437 (MCB/VetSci 437) Univ. of Arizona, spring 2009 Neurons Chapter 11 Kevin Bonine & Kevin Oh 1. Finish Solutes + Water 2. Neurons
Neurons. 5 th & 6 th Lectures Mon 26 & Wed 28 Jan Finish Solutes + Water. 2. Neurons. Chapter 11
 5 th & 6 th Lectures Mon 26 & Wed 28 Jan 2009 Vertebrate Physiology ECOL 437 (MCB/VetSci 437) Univ. of Arizona, spring 2009 Neurons Chapter 11 Kevin Bonine & Kevin Oh 1. Finish Solutes + Water 2. Neurons
5 th & 6 th Lectures Mon 26 & Wed 28 Jan 2009 Vertebrate Physiology ECOL 437 (MCB/VetSci 437) Univ. of Arizona, spring 2009 Neurons Chapter 11 Kevin Bonine & Kevin Oh 1. Finish Solutes + Water 2. Neurons
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten. Lecture 2a. The Neuron - overview of structure. From Anderson (1995)
 Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Biology September 2015 Exam One FORM G KEY
 Biology 251 17 September 2015 Exam One FORM G KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Biology 251 17 September 2015 Exam One FORM G KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Biology September 2015 Exam One FORM W KEY
 Biology 251 17 September 2015 Exam One FORM W KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
Biology 251 17 September 2015 Exam One FORM W KEY PRINT YOUR NAME AND ID NUMBER in the space that is provided on the answer sheet, and then blacken the letter boxes below the corresponding letters of your
2002NSC Human Physiology Semester Summary
 2002NSC Human Physiology Semester Summary Griffith University, Nathan Campus Semester 1, 2014 Topics include: - Diffusion, Membranes & Action Potentials - Fundamentals of the Nervous System - Neuroanatomy
2002NSC Human Physiology Semester Summary Griffith University, Nathan Campus Semester 1, 2014 Topics include: - Diffusion, Membranes & Action Potentials - Fundamentals of the Nervous System - Neuroanatomy
Nervous System AP Biology
 Nervous System 2007-2008 Why do animals need a nervous system? What characteristics do animals need in a nervous system? fast accurate reset quickly Remember Poor think bunny! about the bunny signal direction
Nervous System 2007-2008 Why do animals need a nervous system? What characteristics do animals need in a nervous system? fast accurate reset quickly Remember Poor think bunny! about the bunny signal direction
Ch 33. The nervous system
 Ch 33 The nervous system AP bio schedule Tuesday Wed Thursday Friday Plant test Animal behavior lab Nervous system 25 Review Day (bring computer) 27 Review Day (bring computer) 28 Practice AP bio test
Ch 33 The nervous system AP bio schedule Tuesday Wed Thursday Friday Plant test Animal behavior lab Nervous system 25 Review Day (bring computer) 27 Review Day (bring computer) 28 Practice AP bio test
This script will produce a series of pulses of amplitude 40 na, duration 1ms, recurring every 50 ms.
 9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
Modeling of Action Potential Generation in NG cells
 Modeling of Action Potential Generation in NG108-15 cells NanoScience Technology Center University of Central Florida 144 Research Parkway, Suite 400 Orlando, FL 386 * Corresponding author Email: pmolnar@mail.ucf.edu
Modeling of Action Potential Generation in NG108-15 cells NanoScience Technology Center University of Central Florida 144 Research Parkway, Suite 400 Orlando, FL 386 * Corresponding author Email: pmolnar@mail.ucf.edu
Neuroscience 201A Exam Key, October 7, 2014
 Neuroscience 201A Exam Key, October 7, 2014 Question #1 7.5 pts Consider a spherical neuron with a diameter of 20 µm and a resting potential of -70 mv. If the net negativity on the inside of the cell (all
Neuroscience 201A Exam Key, October 7, 2014 Question #1 7.5 pts Consider a spherical neuron with a diameter of 20 µm and a resting potential of -70 mv. If the net negativity on the inside of the cell (all
Vertebrate Physiology 437 EXAM I 26 September 2002 NAME
 437 EXAM1.DOC Vertebrate Physiology 437 EXAM I 26 September 2002 NAME 0. When you gaze at the stars, why do you have to look slightly away from the really faint ones in order to be able to see them? (1
437 EXAM1.DOC Vertebrate Physiology 437 EXAM I 26 September 2002 NAME 0. When you gaze at the stars, why do you have to look slightly away from the really faint ones in order to be able to see them? (1
Hair Cells: The Sensory Transducers of the Inner Ear
 Chapter 1 Hair Cells: The Sensory Transducers of the Inner Ear Hair cells are specialized cells that transform a mechanical motion into changes in membrane potential. Such changes, whereby one form of
Chapter 1 Hair Cells: The Sensory Transducers of the Inner Ear Hair cells are specialized cells that transform a mechanical motion into changes in membrane potential. Such changes, whereby one form of
Membrane Potentials and Bioelectricity
 Membrane Potentials and Bioelectricity Hugh Purdy Honors University Physics II November 29, 2010 Most, if not all, cells in the human body have a net electric charge to some degree on either side of their
Membrane Potentials and Bioelectricity Hugh Purdy Honors University Physics II November 29, 2010 Most, if not all, cells in the human body have a net electric charge to some degree on either side of their
Nature Methods: doi: /nmeth Supplementary Figure 1. In vitro screening of recombinant R-CaMP2 variants.
 Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
Nervous system. 3 Basic functions of the nervous system !!!! !!! 1-Sensory. 2-Integration. 3-Motor
 Nervous system 3 Basic functions of the nervous system 1-Sensory 2-Integration 3-Motor I. Central Nervous System (CNS) Brain Spinal Cord I. Peripheral Nervous System (PNS) 2) Afferent towards afferent
Nervous system 3 Basic functions of the nervous system 1-Sensory 2-Integration 3-Motor I. Central Nervous System (CNS) Brain Spinal Cord I. Peripheral Nervous System (PNS) 2) Afferent towards afferent
Olfactory Transduction and Taste Processing in Drosophila
 Olfactory Transduction and Taste Processing in Drosophila The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Accessed
Olfactory Transduction and Taste Processing in Drosophila The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Accessed
Introduction to electrophysiology. Dr. Tóth András
 Introduction to electrophysiology Dr. Tóth András Topics Transmembran transport Donnan equilibrium Resting potential Ion channels Local and action potentials Intra- and extracellular propagation of the
Introduction to electrophysiology Dr. Tóth András Topics Transmembran transport Donnan equilibrium Resting potential Ion channels Local and action potentials Intra- and extracellular propagation of the
A. Visceral and somatic divisions. B. Sympathetic and parasympathetic divisions. C. Central and peripheral divisions
 Ch 8: Neurons: Cellular and Network Properties, Part 1 Review of the Nervous System Objectives: Describe the Cells of the NS Explain the creation and propagation of an electrical signal in a nerve cell
Ch 8: Neurons: Cellular and Network Properties, Part 1 Review of the Nervous System Objectives: Describe the Cells of the NS Explain the creation and propagation of an electrical signal in a nerve cell
UNIT I INTRODUCTION TO ARTIFICIAL NEURAL NETWORK IT 0469 NEURAL NETWORKS
 UNIT I INTRODUCTION TO ARTIFICIAL NEURAL NETWORK IT 0469 NEURAL NETWORKS Elementary Neuro Physiology Neuron: A neuron nerve cell is an electricallyexcitable cell that processes and transmits information
UNIT I INTRODUCTION TO ARTIFICIAL NEURAL NETWORK IT 0469 NEURAL NETWORKS Elementary Neuro Physiology Neuron: A neuron nerve cell is an electricallyexcitable cell that processes and transmits information
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Adaptation in the Neural Code of the Retina
 Adaptation in the Neural Code of the Retina Lens Retina Fovea Optic Nerve Optic Nerve Bottleneck Neurons Information Receptors: 108 95% Optic Nerve 106 5% After Polyak 1941 Visual Cortex ~1010 Mean Intensity
Adaptation in the Neural Code of the Retina Lens Retina Fovea Optic Nerve Optic Nerve Bottleneck Neurons Information Receptors: 108 95% Optic Nerve 106 5% After Polyak 1941 Visual Cortex ~1010 Mean Intensity
The sense of smell Outline Main Olfactory System Odor Detection Odor Coding Accessory Olfactory System Pheromone Detection Pheromone Coding
 The sense of smell Outline Main Olfactory System Odor Detection Odor Coding Accessory Olfactory System Pheromone Detection Pheromone Coding 1 Human experiment: How well do we taste without smell? 2 Brief
The sense of smell Outline Main Olfactory System Odor Detection Odor Coding Accessory Olfactory System Pheromone Detection Pheromone Coding 1 Human experiment: How well do we taste without smell? 2 Brief
Bio 449 Fall Exam points total Multiple choice. As with any test, choose the best answer in each case. Each question is 3 points.
 Name: Exam 1 100 points total Multiple choice. As with any test, choose the best answer in each case. Each question is 3 points. 1. The term internal environment, as coined by Clause Bernard, is best defined
Name: Exam 1 100 points total Multiple choice. As with any test, choose the best answer in each case. Each question is 3 points. 1. The term internal environment, as coined by Clause Bernard, is best defined
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
1
 http://photos1.blogger.com/img/13/2627/640/screenhunter_047.jpg 1 The Nose Knows http://graphics.jsonline.com/graphics/owlive/img/mar05/sideways.one0308_big.jpg 2 http://www.stlmoviereviewweekly.com/sitebuilder/images/sideways-253x364.jpg
http://photos1.blogger.com/img/13/2627/640/screenhunter_047.jpg 1 The Nose Knows http://graphics.jsonline.com/graphics/owlive/img/mar05/sideways.one0308_big.jpg 2 http://www.stlmoviereviewweekly.com/sitebuilder/images/sideways-253x364.jpg
Olfactory Bulb External Tufted Cells Are Synchronized by Multiple Intraglomerular Mechanisms
 The Journal of Neuroscience, September 7, 2005 25(36):8197 8208 8197 Cellular/Molecular Olfactory Bulb External Tufted Cells Are Synchronized by Multiple Intraglomerular Mechanisms Abdallah Hayar, 1 Michael
The Journal of Neuroscience, September 7, 2005 25(36):8197 8208 8197 Cellular/Molecular Olfactory Bulb External Tufted Cells Are Synchronized by Multiple Intraglomerular Mechanisms Abdallah Hayar, 1 Michael
SUPPLEMENTARY INFORMATION
 Figure S1 Multiplicative scaling of the granule cell input-output relation is not dependent on input rate. Input-output relations with short-term depression (STD) from Fig. 1d after normalizing by the
Figure S1 Multiplicative scaling of the granule cell input-output relation is not dependent on input rate. Input-output relations with short-term depression (STD) from Fig. 1d after normalizing by the
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
Temporal whitening by power-law adaptation in neocortical neurons
 Temporal whitening by power-law adaptation in neocortical neurons Christian Pozzorini, Richard Naud, Skander Mensi and Wulfram Gerstner School of Computer and Communication Sciences and School of Life
Temporal whitening by power-law adaptation in neocortical neurons Christian Pozzorini, Richard Naud, Skander Mensi and Wulfram Gerstner School of Computer and Communication Sciences and School of Life
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals?
 LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
Lecture 04, 04 Sept 2003 Chapters 4 and 5. Vertebrate Physiology ECOL 437 University of Arizona Fall instr: Kevin Bonine t.a.
 Lecture 04, 04 Sept 2003 Chapters 4 and 5 Vertebrate Physiology ECOL 437 University of Arizona Fall 2003 instr: Kevin Bonine t.a.: Bret Pasch Vertebrate Physiology 437 1. Membranes (CH4) 2. Nervous System
Lecture 04, 04 Sept 2003 Chapters 4 and 5 Vertebrate Physiology ECOL 437 University of Arizona Fall 2003 instr: Kevin Bonine t.a.: Bret Pasch Vertebrate Physiology 437 1. Membranes (CH4) 2. Nervous System
