18. Heat Engine, Entropy and the second law of thermodynamics
|
|
|
- Meagan Hall
- 6 years ago
- Views:
Transcription
1 8. Heat Engne, Entropy and te seond law o terodynas In nature, ost o proesses are rreversble. due to te seond Law o terodynas Heat alwasys lows ro Hot to old. 8-. Heat Engne and te eond Law o erodynas Engne absorbs eat ro te ot reservor. Engne does a ork. Engne releases Heat to te old reservor. tea Engne ( 0) eral eeny e < e e ust u saller tan 00%. (e seond law o terodynas)
2 8-2. Reversble and Irreversble Proesses Free expanson Irreversble : dabat and Isoteral Proess No ork P P nr ( : onstant) P2 2 In te ase tat ork s appled to go bak : U U Pd U + eperature nreases. ery low (Equlbru at all nstant) Reversble Oter tan tat eperature nrease nstantly and release Heat. ( xed)
3 D 8-3. arnot Engne e ost eent engne P P nr P D D U D ad arnot ( ) U nr U bsorb Heat Pd xed P P U : onstant nr xed P P D D P P U U nr U D nr D
4 P- Dagra or arnot yle Eeny e Isoteral e D dabat dabat e Isoteral I 0 K, e. e nr ( ) nr ( D ) nr ( ) ( ) ( D ) ( ) In te adabat proess: γ ons tant γ 5/3 or an deal Gas γ γ γ γ D D γ ( ) γ ( ) D D e <
5 8-4. Heat Pup and Rergerators Heat pup (lower oolant lud) absorbs eat ro te old reservor. ork s gven to te pup. (opress te oolant to beoe a ot, g pressure vapor ) Pup (ot vapor) releases Heat to te Hot reservor. Heat Pup : old r outsde, or r nsde oeent o Perorane o Heat Pup: For arnot eat pup OP OP Rergerator : old r nsde, or r outsde oeent o Perorane o Rergerator: OP For arnot Rergerator OP
6 8-5. e 2nd law o terodynas (alternatve stateent) e st law: Energy onservaton U e 2nd law: eral eeny e < n alternatve stateent o te 2nd law: e Heat always lows ro te Hot objet to te old objet Entropy te 2nd law d d r ange n Entropy : Marosop denton (lausus s denton) r : denotes a reversble proess. Entropy : Degree o Dsorder (Mrosop denton) - n solated syste tends toward Dsorder. e 2nd law: e entropy nreases n all natural proess. d d r (reversble pat) - In te ase o an dabat Proess : 0 0 Isentrop proess
7 arnot Heat Engne, D D 0 dabat Isoteral Isoteral dabat 0 e or arnot engne d d 0 r or any losed reversble yle - Reversble proess ust always be n teral equlbru. Entropy, Pase transton, and Latent Heat Ie ater at 0 º e syste beoes ore dsorder. L L L : Latent eat
8 8-7. Entropy anges n Irreversble Proesses Entropy depends only on te tate o an yste. e entropy ange depends only on te ntal and nal states, not on te pat. 0 n any solated syste. - te 2nd law o terodynas Heat onduton Reservor Reservor, > 0 Free Expanson (Irreversble) 0 and 0 U U, Entropy ; r n te reversble proess.
9 - Equvalent Reversble proess xed Isoteral proess P nr ; onstant dr nr Pd > 0 r Irreversble Heat ranser, , 2 ( ) 22( ) d + 2 d d d For an nntesal ange d d >
10 8-8. Entropy on Mrosop ew Entropy Degree o Dsorder Free Expanson N partles ssue ea oleule oupes soe rosop volue,. Nuber o ases per a oleule : Nuber o ases or N-oleule : N >> N ( )( 2) ( N + ) P ( ) N N Intal state: Fnal state:,, ( ) N ( ) N N k Nk knn nr
11 In te Isoteral Expanson r nr nr Pd d k k k nr Nk k : Entropy s a easureent o rosop dsorder.
11/19/2013. PHY 113 C General Physics I 11 AM 12:15 PM MWF Olin 101
 PHY 113 C General Pyss I 11 AM 12:15 PM MWF Oln 101 Plan or Leture 23: Capter 22: Heat engnes 1. ermodynam yles; work and eat eeny 2. Carnot yle 3. Otto yle; desel yle 4. Bre omments on entropy 11/19/2013
PHY 113 C General Pyss I 11 AM 12:15 PM MWF Oln 101 Plan or Leture 23: Capter 22: Heat engnes 1. ermodynam yles; work and eat eeny 2. Carnot yle 3. Otto yle; desel yle 4. Bre omments on entropy 11/19/2013
Physics 41 Chapter 22 HW Serway 7 th Edition
 yss 41 apter H Serway 7 t Edton oneptual uestons: 1,, 8, 1 roblems: 9, 1, 0,, 7, 9, 48, 54, 55 oneptual uestons: 1,, 8, 1 1 Frst, te effeny of te automoble engne annot exeed te arnot effeny: t s lmted
yss 41 apter H Serway 7 t Edton oneptual uestons: 1,, 8, 1 roblems: 9, 1, 0,, 7, 9, 48, 54, 55 oneptual uestons: 1,, 8, 1 1 Frst, te effeny of te automoble engne annot exeed te arnot effeny: t s lmted
Chapter 18: The Laws of Thermodynamics
 Capter 18: e Laws o ermodynams Answers to Even-Numbered Coneptual uestons. (a) Yes. Heat an low nto te system at te same tme te system expands, as n an sotermal expanson o a gas. (b) Yes. Heat an low out
Capter 18: e Laws o ermodynams Answers to Even-Numbered Coneptual uestons. (a) Yes. Heat an low nto te system at te same tme te system expands, as n an sotermal expanson o a gas. (b) Yes. Heat an low out
Physical Chemistry I for Biochemists. Lecture 18 (2/23/11) Announcement
 Physcal Chestry I or Bochests Che34 Lecture 18 (2/23/11) Yoshtaka Ish Ch5.8-5.11 & HW6 Revew o Ch. 5 or Quz 2 Announceent Quz 2 has a slar orat wth Quz1. e s the sae. ~2 ns. Answer or HW5 wll be uploaded
Physcal Chestry I or Bochests Che34 Lecture 18 (2/23/11) Yoshtaka Ish Ch5.8-5.11 & HW6 Revew o Ch. 5 or Quz 2 Announceent Quz 2 has a slar orat wth Quz1. e s the sae. ~2 ns. Answer or HW5 wll be uploaded
P REVIEW NOTES
 P34 - REIEW NOTES Capter 1 Energy n Termal Pyss termal equlbrum & relaxaton tme temperature & termometry: fxed ponts, absolute temperature sale P = nrt deal gas law: ( ) ( T ) ( / n) C( T ) ( ) + / n vral
P34 - REIEW NOTES Capter 1 Energy n Termal Pyss termal equlbrum & relaxaton tme temperature & termometry: fxed ponts, absolute temperature sale P = nrt deal gas law: ( ) ( T ) ( / n) C( T ) ( ) + / n vral
Lecture 27: Entropy and Information Prof. WAN, Xin
 General Pysis I Leture 27: Entropy and Information Prof. WAN, Xin xinwan@zju.edu.n ttp://zimp.zju.edu.n/~xinwan/ 1st & 2nd Laws of ermodynamis e 1st law speifies tat we annot get more energy out of a yli
General Pysis I Leture 27: Entropy and Information Prof. WAN, Xin xinwan@zju.edu.n ttp://zimp.zju.edu.n/~xinwan/ 1st & 2nd Laws of ermodynamis e 1st law speifies tat we annot get more energy out of a yli
Thermodynamics and Gases
 hermodynamcs and Gases Last tme Knetc heory o Gases or smple (monatomc) gases Atomc nature o matter Demonstrate deal gas law Atomc knetc energy nternal energy Mean ree path and velocty dstrbutons From
hermodynamcs and Gases Last tme Knetc heory o Gases or smple (monatomc) gases Atomc nature o matter Demonstrate deal gas law Atomc knetc energy nternal energy Mean ree path and velocty dstrbutons From
Physics 231 Lecture 35
 ysis 1 Leture 5 Main points of last leture: Heat engines and effiieny: eng e 1 Carnot yle and Carnot engine. eng e 1 is in Kelvin. Refrigerators CO eng Ideal refrigerator CO rev reversible Entropy ΔS Computation
ysis 1 Leture 5 Main points of last leture: Heat engines and effiieny: eng e 1 Carnot yle and Carnot engine. eng e 1 is in Kelvin. Refrigerators CO eng Ideal refrigerator CO rev reversible Entropy ΔS Computation
1. Which two values of temperature are equivalent to the nearest degree when measured on the Kelvin and on the
 . Whih two values of teperature are equivalent to the nearest degree when easured on the Kelvin and on the Celsius sales of teperature? Kelvin sale Celsius sale A. 40 33 B. 273 00 C. 33 40 D. 373 0 2.
. Whih two values of teperature are equivalent to the nearest degree when easured on the Kelvin and on the Celsius sales of teperature? Kelvin sale Celsius sale A. 40 33 B. 273 00 C. 33 40 D. 373 0 2.
Thermodynamics Second Law Entropy
 Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Chapter 6 Second Law of Thermodynamics
 Capter 6 Second Law o Termodynamcs Te rst law o termodynamcs s an energy conservaton statement. It determnes weter or not a process can take place energetcally. It does not tell n wc drecton te process
Capter 6 Second Law o Termodynamcs Te rst law o termodynamcs s an energy conservaton statement. It determnes weter or not a process can take place energetcally. It does not tell n wc drecton te process
PHYSICS 212 MIDTERM II 19 February 2003
 PHYSICS 1 MIDERM II 19 Feruary 003 Exam s losed ook, losed notes. Use only your formula sheet. Wrte all work and answers n exam ooklets. he aks of pages wll not e graded unless you so request on the front
PHYSICS 1 MIDERM II 19 Feruary 003 Exam s losed ook, losed notes. Use only your formula sheet. Wrte all work and answers n exam ooklets. he aks of pages wll not e graded unless you so request on the front
Physics 41 Chapter 22 HW
 Pysis 41 apter 22 H 1. eat ine performs 200 J of work in ea yle and as an effiieny of 30.0%. For ea yle, ow mu energy is (a) taken in and (b) expelled as eat? = = 200 J (1) e = 1 0.300 = = (2) From (2),
Pysis 41 apter 22 H 1. eat ine performs 200 J of work in ea yle and as an effiieny of 30.0%. For ea yle, ow mu energy is (a) taken in and (b) expelled as eat? = = 200 J (1) e = 1 0.300 = = (2) From (2),
ENGINEERING OF NUCLEAR REACTORS. Due November 17, 2006 by 12:00 pm
 .3 ENINEEIN OF NUCEA EACOS ue Noveer 7, 006 y :00 AKE HOME QUIZ (SOUION Prole (60% Hydraul Analyss o te Eereny Core Sray Syste n a BW e nuern o te relevant loatons wtn te syste s sown n Fure elow. 3 e
.3 ENINEEIN OF NUCEA EACOS ue Noveer 7, 006 y :00 AKE HOME QUIZ (SOUION Prole (60% Hydraul Analyss o te Eereny Core Sray Syste n a BW e nuern o te relevant loatons wtn te syste s sown n Fure elow. 3 e
Chapter 5 rd Law of Thermodynamics
 Entropy and the nd and 3 rd Chapter 5 rd Law o hermodynamcs homas Engel, hlp Red Objectves Introduce entropy. Derve the condtons or spontanety. Show how S vares wth the macroscopc varables,, and. Chapter
Entropy and the nd and 3 rd Chapter 5 rd Law o hermodynamcs homas Engel, hlp Red Objectves Introduce entropy. Derve the condtons or spontanety. Show how S vares wth the macroscopc varables,, and. Chapter
Heat Engines, Entropy, and the Second Law of Thermodynamics
 Heat Engnes, Entropy, and te Seond Law o ermodynams HER OULINE.1 Heat Engnes and te Seond Law o ermodynams. Heat umps and Rergerators. Reversble and Irreversble roesses.4 e arnot Engne. Gasolne and esel
Heat Engnes, Entropy, and te Seond Law o ermodynams HER OULINE.1 Heat Engnes and te Seond Law o ermodynams. Heat umps and Rergerators. Reversble and Irreversble roesses.4 e arnot Engne. Gasolne and esel
Thermodynamics. Temperature Scales Fahrenheit: t F. Thermal Expansion and Stress. Temperature and Thermal Equilibrium
 herodynaics Fro the Greek theros eaning heat and dynais eaning power is a branch of physics that studies the effects of changes in teperature, pressure, and volue on physical systes at the acroscopic scale
herodynaics Fro the Greek theros eaning heat and dynais eaning power is a branch of physics that studies the effects of changes in teperature, pressure, and volue on physical systes at the acroscopic scale
Thermodynamics. Temperature Scales Fahrenheit: t F. Thermal Expansion and Strss. Temperature and Thermal Equilibrium
 herodynaics Fro the Greek theros eaning heat and dynais eaning power is a branch of physics that studies the effects of changes in teperature, pressure, and volue on physical systes at the acroscopic scale
herodynaics Fro the Greek theros eaning heat and dynais eaning power is a branch of physics that studies the effects of changes in teperature, pressure, and volue on physical systes at the acroscopic scale
Physics 207 Lecture 23
 ysics 07 Lecture ysics 07, Lecture 8, Dec. Agenda:. Finis, Start. Ideal gas at te molecular level, Internal Energy Molar Specific Heat ( = m c = n ) Ideal Molar Heat apacity (and U int = + W) onstant :
ysics 07 Lecture ysics 07, Lecture 8, Dec. Agenda:. Finis, Start. Ideal gas at te molecular level, Internal Energy Molar Specific Heat ( = m c = n ) Ideal Molar Heat apacity (and U int = + W) onstant :
Lecture 6. Entropy of an Ideal Gas (Ch. 3)
 Lecture 6. Entropy o an Ideal Gas (Ch. oday we wll acheve an portant goal: we ll derve the equaton(s o state or an deal gas ro the prncples o statstcal echancs. We wll ollow the path outlned n the prevous
Lecture 6. Entropy o an Ideal Gas (Ch. oday we wll acheve an portant goal: we ll derve the equaton(s o state or an deal gas ro the prncples o statstcal echancs. We wll ollow the path outlned n the prevous
Maximum work for Carnot-like heat engines with infinite heat source
 Maximum work for arnot-like eat engines wit infinite eat soure Rui Long and Wei Liu* Sool of Energy and Power Engineering, Huazong University of Siene and enology, Wuan 4374, ina orresponding autor: Wei
Maximum work for arnot-like eat engines wit infinite eat soure Rui Long and Wei Liu* Sool of Energy and Power Engineering, Huazong University of Siene and enology, Wuan 4374, ina orresponding autor: Wei
Chapter 3. Problem Solutions
 Capter. Proble Solutions. A poton and a partile ave te sae wavelengt. Can anyting be said about ow teir linear oenta opare? About ow te poton's energy opares wit te partile's total energy? About ow te
Capter. Proble Solutions. A poton and a partile ave te sae wavelengt. Can anyting be said about ow teir linear oenta opare? About ow te poton's energy opares wit te partile's total energy? About ow te
NUCLEAR THERMAL-HYDRAULIC FUNDAMENTALS
 NUCLEAR THERMAL-HYDRAULIC FUNDAMENTALS Dr. J. Micael Doster Departent of Nuclear Engineering Nort Carolina State University Raleig, NC Copyrigted POER CYCLES Te analysis of Terodynaic Cycles is based alost
NUCLEAR THERMAL-HYDRAULIC FUNDAMENTALS Dr. J. Micael Doster Departent of Nuclear Engineering Nort Carolina State University Raleig, NC Copyrigted POER CYCLES Te analysis of Terodynaic Cycles is based alost
General Formulas applicable to ALL processes in an Ideal Gas:
 Calormetrc calculatons: dq mcd or dq ncd ( specc heat) Q ml ( latent heat) General Formulas applcable to ALL processes n an Ideal Gas: P nr du dq dw dw Pd du nc d C R ( monoatomc) C C R P Specc Processes:
Calormetrc calculatons: dq mcd or dq ncd ( specc heat) Q ml ( latent heat) General Formulas applcable to ALL processes n an Ideal Gas: P nr du dq dw dw Pd du nc d C R ( monoatomc) C C R P Specc Processes:
Main components of the above cycle are: 1) Boiler (steam generator) heat exchanger 2) Turbine generates work 3) Condenser heat exchanger 4) Pump
 Introducton to Terodynacs, Lecture -5 Pro. G. Cccarell (0 Applcaton o Control olue Energy Analyss Most terodynac devces consst o a seres o coponents operatng n a cycle, e.g., stea power plant Man coponents
Introducton to Terodynacs, Lecture -5 Pro. G. Cccarell (0 Applcaton o Control olue Energy Analyss Most terodynac devces consst o a seres o coponents operatng n a cycle, e.g., stea power plant Man coponents
Chapter One Mixture of Ideal Gases
 herodynacs II AA Chapter One Mxture of Ideal Gases. Coposton of a Gas Mxture: Mass and Mole Fractons o deterne the propertes of a xture, we need to now the coposton of the xture as well as the propertes
herodynacs II AA Chapter One Mxture of Ideal Gases. Coposton of a Gas Mxture: Mass and Mole Fractons o deterne the propertes of a xture, we need to now the coposton of the xture as well as the propertes
Energy can be interchanged in various ways (ΔU = q + W). But nothing about why unnatural processes do not occur E.g. allowed by the 1 st
 9//00 e Seond law o eodynas Enegy an be nteanged n vaous ways ( + W. But notng about wy unnatual oesses do not ou E.g. allowed by te st law: Metal od @ oo te an suddenly beoe ot on end, as long as otal
9//00 e Seond law o eodynas Enegy an be nteanged n vaous ways ( + W. But notng about wy unnatual oesses do not ou E.g. allowed by te st law: Metal od @ oo te an suddenly beoe ot on end, as long as otal
11. Ideal Gas Mixture
 . Ideal Ga xture. Geeral oderato ad xture of Ideal Gae For a geeral xture of N opoet, ea a pure ubtae [kg ] te a for ea opoet. [kol ] te uber of ole for ea opoet. e al a ( ) [kg ] N e al uber of ole (
. Ideal Ga xture. Geeral oderato ad xture of Ideal Gae For a geeral xture of N opoet, ea a pure ubtae [kg ] te a for ea opoet. [kol ] te uber of ole for ea opoet. e al a ( ) [kg ] N e al uber of ole (
Lecture 27: Entropy and Information Prof. WAN, Xin
 General Pysis I Leture 27: Entropy and Information Prof. WAN, Xin xinwan@zju.edu.n ttp://zimp.zju.edu.n/~xinwan/ Outline Introduing entropy e meaning of entropy Reversibility Disorder Information Seleted
General Pysis I Leture 27: Entropy and Information Prof. WAN, Xin xinwan@zju.edu.n ttp://zimp.zju.edu.n/~xinwan/ Outline Introduing entropy e meaning of entropy Reversibility Disorder Information Seleted
EF 152 Exam #3, Fall, 2012 Page 1 of 6
 EF 5 Exam #3, Fall, 0 Page of 6 Name: Setion: Guidelines: ssume 3 signifiant figures for all given numbers. Sow all of your work no work, no redit Write your final answer in te box provided - inlude units
EF 5 Exam #3, Fall, 0 Page of 6 Name: Setion: Guidelines: ssume 3 signifiant figures for all given numbers. Sow all of your work no work, no redit Write your final answer in te box provided - inlude units
Earlier Lecture. This gas tube is called as Pulse Tube and this phenomenon is called as Pulse Tube action.
 31 1 Earlier Leture In te earlier leture, we ave seen a Pulse Tube (PT) ryoooler in wi te meanial displaer is removed and an osillating gas flow in te tin walled tube produes ooling. Tis gas tube is alled
31 1 Earlier Leture In te earlier leture, we ave seen a Pulse Tube (PT) ryoooler in wi te meanial displaer is removed and an osillating gas flow in te tin walled tube produes ooling. Tis gas tube is alled
Collisions Short, Sharp Shocks
 16-8 Satterng R 1b n, b 19-1 Collson Colatons L1 Collsons 1 R 1 9 d, 15, 11 Derent Reerene Fraes, 111 ranslatonal ngular Moentu Quz 1 R 11a; HW1: r s 13*, 1, 3, 39 Collsons Short, Shar Shoks Sak! F Whh
16-8 Satterng R 1b n, b 19-1 Collson Colatons L1 Collsons 1 R 1 9 d, 15, 11 Derent Reerene Fraes, 111 ranslatonal ngular Moentu Quz 1 R 11a; HW1: r s 13*, 1, 3, 39 Collsons Short, Shar Shoks Sak! F Whh
TEST 5 (phy 240) 2. Show that the volume coefficient of thermal expansion for an ideal gas at constant pressure is temperature dependent and given by
 ES 5 (phy 40). a) Wrte the zeroth law o thermodynamcs. b) What s thermal conductvty? c) Identyng all es, draw schematcally a P dagram o the arnot cycle. d) What s the ecency o an engne and what s the coecent
ES 5 (phy 40). a) Wrte the zeroth law o thermodynamcs. b) What s thermal conductvty? c) Identyng all es, draw schematcally a P dagram o the arnot cycle. d) What s the ecency o an engne and what s the coecent
EF 152 Exam #3, Spring 2016 Page 1 of 6
 EF 5 Exam #3, Spring 06 Page of 6 Name: Setion: Instrutions Do not open te exam until instruted to do so. Do not leave if tere is less tan 5 minutes to go in te exam. Wen time is alled, immediately stop
EF 5 Exam #3, Spring 06 Page of 6 Name: Setion: Instrutions Do not open te exam until instruted to do so. Do not leave if tere is less tan 5 minutes to go in te exam. Wen time is alled, immediately stop
Physical Chemistry I for Biochemists. Chem340. Lecture 16 (2/18/11)
 hyscal Chemstry I or Bochemsts Chem34 Lecture 16 (/18/11) Yoshtaka Ish Ch4.6, Ch5.1-5.5 & HW5 4.6 Derental Scannng Calormetry (Derental hermal Analyss) sample = C p, s d s + dh uson = ( s )Kdt, [1] where
hyscal Chemstry I or Bochemsts Chem34 Lecture 16 (/18/11) Yoshtaka Ish Ch4.6, Ch5.1-5.5 & HW5 4.6 Derental Scannng Calormetry (Derental hermal Analyss) sample = C p, s d s + dh uson = ( s )Kdt, [1] where
Nonequilibrium Thermodynamics of open driven systems
 1 Boynam Otal Imagng Center (BIOPIC) 2 Beng Internatonal Center for Matematal Resear (BICMR) Peng Unversty, Cna Nonequlbrum ermoynams of oen rven systems Hao Ge A sngle boemal reaton yle B + AP 1 C + ADP
1 Boynam Otal Imagng Center (BIOPIC) 2 Beng Internatonal Center for Matematal Resear (BICMR) Peng Unversty, Cna Nonequlbrum ermoynams of oen rven systems Hao Ge A sngle boemal reaton yle B + AP 1 C + ADP
Physics 123. Exam #1. October 11, 2006
 hyscs Exa # October, 006 roble /0 roble /0 roble /0 roble 4 /0 roble 5 /0 roble 6 /0 roble 7 /0 roble 8 /0 roble 9 /0 roble 0 /0 Total /00 Free-Response robles: lease show all work n order to receve partal
hyscs Exa # October, 006 roble /0 roble /0 roble /0 roble 4 /0 roble 5 /0 roble 6 /0 roble 7 /0 roble 8 /0 roble 9 /0 roble 0 /0 Total /00 Free-Response robles: lease show all work n order to receve partal
ESCI 341 Atmospheric Thermodynamics Lesson 11 The Second Law of Thermodynamics
 ESCI 341 Atmosperi ermodynamis Lesson 11 e Seond Law of ermodynamis Referenes: Pysial Cemistry (4 t edition), Levine ermodynamis and an Introdution to ermostatistis, Callen HE SECOND LAW OF HERMODYNAMICS
ESCI 341 Atmosperi ermodynamis Lesson 11 e Seond Law of ermodynamis Referenes: Pysial Cemistry (4 t edition), Levine ermodynamis and an Introdution to ermostatistis, Callen HE SECOND LAW OF HERMODYNAMICS
Physics 2B Chapter 17 Notes - Calorimetry Spring 2018
 Physs 2B Chapter 17 Notes - Calormetry Sprng 2018 hermal Energy and Heat Heat Capaty and Spe Heat Capaty Phase Change and Latent Heat Rules or Calormetry Problems hermal Energy and Heat Calormetry lterally
Physs 2B Chapter 17 Notes - Calormetry Sprng 2018 hermal Energy and Heat Heat Capaty and Spe Heat Capaty Phase Change and Latent Heat Rules or Calormetry Problems hermal Energy and Heat Calormetry lterally
PES 2130 Fall 2014, Spendier Lecture 7/Page 1
 PES 2130 Fall 2014, Spender Lecture 7/Page 1 Lecture today: Chapter 20 (ncluded n exam 1) 1) Entropy 2) Second Law o hermodynamcs 3) Statstcal Vew o Entropy Announcements: Next week Wednesday Exam 1! -
PES 2130 Fall 2014, Spender Lecture 7/Page 1 Lecture today: Chapter 20 (ncluded n exam 1) 1) Entropy 2) Second Law o hermodynamcs 3) Statstcal Vew o Entropy Announcements: Next week Wednesday Exam 1! -
Reversibility. Processes in nature are always irreversible: far from equilibrium
 Reversibility Processes in nature are always irreversible: far from equilibrium Reversible process: idealized process infinitely close to thermodynamic equilibrium (quasi-equilibrium) Necessary conditions
Reversibility Processes in nature are always irreversible: far from equilibrium Reversible process: idealized process infinitely close to thermodynamic equilibrium (quasi-equilibrium) Necessary conditions
Obtaining U and G based on A above arrow line: )
 Suary or ch,,3,4,5,6,7 (Here soe olar propertes wthout underlne) () he three laws o herodynacs - st law: otal energy o syste (SYS) plus surroundng (SUR) s conserved. - nd law: otal change o entropy o the
Suary or ch,,3,4,5,6,7 (Here soe olar propertes wthout underlne) () he three laws o herodynacs - st law: otal energy o syste (SYS) plus surroundng (SUR) s conserved. - nd law: otal change o entropy o the
Outline. Unit Eight Calculations with Entropy. The Second Law. Second Law Notes. Uses of Entropy. Entropy is a Property.
 Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Force = F Piston area = A
 CHAPTER III Ths chapter s an mportant transton between the propertes o pure substances and the most mportant chapter whch s: the rst law o thermodynamcs In ths chapter, we wll ntroduce the notons o heat,
CHAPTER III Ths chapter s an mportant transton between the propertes o pure substances and the most mportant chapter whch s: the rst law o thermodynamcs In ths chapter, we wll ntroduce the notons o heat,
General Physics I. New Lecture 27: Carnot Cycle, The 2nd Law, Entropy and Information. Prof. WAN, Xin
 General Pysics I New Lecture 27: Carnot Cycle, e 2nd Law, Entropy and Information Prof. AN, Xin xinwan@zju.edu.cn ttp://zimp.zju.edu.cn/~xinwan/ Carnot s Engine Efficiency of a Carnot Engine isotermal
General Pysics I New Lecture 27: Carnot Cycle, e 2nd Law, Entropy and Information Prof. AN, Xin xinwan@zju.edu.cn ttp://zimp.zju.edu.cn/~xinwan/ Carnot s Engine Efficiency of a Carnot Engine isotermal
3-1 Introduction: 3-2 Spontaneous (Natural) Process:
 - Introducton: * Reversble & Irreversble processes * Degree of rreversblty * Entropy S a state functon * Reversble heat engne Carnot cycle (Engne) * Crteron for Eulbrum SU,=Smax - Spontaneous (Natural)
- Introducton: * Reversble & Irreversble processes * Degree of rreversblty * Entropy S a state functon * Reversble heat engne Carnot cycle (Engne) * Crteron for Eulbrum SU,=Smax - Spontaneous (Natural)
Introduction to Statistical Methods
 Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Topics Adiabatic Combustion Adiabatic Flame Temperature Example Combustion Efficiency Second and First Law Efficiencies Contrasted Example Problem
 ME 410 Day 12 opis diabati Combustion diabati Flame emperature Example Combustion Eiieny Seond and First Law Eiienies Contrasted Example roblem 1. he Case o diabati Combustion Here is it assumed that there
ME 410 Day 12 opis diabati Combustion diabati Flame emperature Example Combustion Eiieny Seond and First Law Eiienies Contrasted Example roblem 1. he Case o diabati Combustion Here is it assumed that there
Heat exchanger. Heat exchanger
 s are deves n w eat s transferred between tw fluds at dfferent teperatures wtut any xng f fluds. type. Dret eat transfer type 2. Strage type 3. Dret ntat type ttps://www.faebk./0000085304058/vdes/96230780490756/.
s are deves n w eat s transferred between tw fluds at dfferent teperatures wtut any xng f fluds. type. Dret eat transfer type 2. Strage type 3. Dret ntat type ttps://www.faebk./0000085304058/vdes/96230780490756/.
total If no external forces act, the total linear momentum of the system is conserved. This occurs in collisions and explosions.
 Lesson 0: Collsons, Rotatonal netc Energy, Torque, Center o Graty (Sectons 7.8 Last te we used ewton s second law to deelop the pulse-oentu theore. In words, the theore states that the change n lnear oentu
Lesson 0: Collsons, Rotatonal netc Energy, Torque, Center o Graty (Sectons 7.8 Last te we used ewton s second law to deelop the pulse-oentu theore. In words, the theore states that the change n lnear oentu
WYSE Academic Challenge 2004 Sectional Physics Solution Set
 WYSE Acadec Challenge 004 Sectnal Physcs Slutn Set. Answer: e. The axu pssble statc rctn r ths stuatn wuld be: ax µ sn µ sg (0.600)(40.0N) 4.0N. Snce yur pushng rce s less than the axu pssble rctnal rce,
WYSE Acadec Challenge 004 Sectnal Physcs Slutn Set. Answer: e. The axu pssble statc rctn r ths stuatn wuld be: ax µ sn µ sg (0.600)(40.0N) 4.0N. Snce yur pushng rce s less than the axu pssble rctnal rce,
3B SCIENTIFIC PHYSICS
 3B SCIENTIFIC PHYSICS Peltier Heat Pump 0076 Instrution manual 05/7 TL/JS Transport ase Semati view 3 Stirrer unit 4 Connetor for stirrer unit 5 Connetor for power supply 6 Stirring rod old side 7 Peltier
3B SCIENTIFIC PHYSICS Peltier Heat Pump 0076 Instrution manual 05/7 TL/JS Transport ase Semati view 3 Stirrer unit 4 Connetor for stirrer unit 5 Connetor for power supply 6 Stirring rod old side 7 Peltier
Thermodynamics Lecture Series
 Therodynac Lecture Sere Dynac Enery Traner Heat, ork and Ma ppled Scence Educaton Reearch Group (SERG) Faculty o ppled Scence Unvert Teknolo MR Pure utance Properte o Pure Sutance- Revew CHPTER eal: drjjlanta@hotal.co
Therodynac Lecture Sere Dynac Enery Traner Heat, ork and Ma ppled Scence Educaton Reearch Group (SERG) Faculty o ppled Scence Unvert Teknolo MR Pure utance Properte o Pure Sutance- Revew CHPTER eal: drjjlanta@hotal.co
1 Proving the Fundamental Theorem of Statistical Learning
 THEORETICAL MACHINE LEARNING COS 5 LECTURE #7 APRIL 5, 6 LECTURER: ELAD HAZAN NAME: FERMI MA ANDDANIEL SUO oving te Fundaental Teore of Statistical Learning In tis section, we prove te following: Teore.
THEORETICAL MACHINE LEARNING COS 5 LECTURE #7 APRIL 5, 6 LECTURER: ELAD HAZAN NAME: FERMI MA ANDDANIEL SUO oving te Fundaental Teore of Statistical Learning In tis section, we prove te following: Teore.
Chapter 8. Momentum Impulse and Collisions. Analysis of motion: 2 key ideas. Newton s laws of motion. Conservation of Energy
 Chapter 8 Moentu Ipulse and Collsons Analyss o oton: key deas Newton s laws o oton Conseraton o Energy Newton s Laws st Law: An object at rest or traelng n unor oton wll rean at rest or traelng n unor
Chapter 8 Moentu Ipulse and Collsons Analyss o oton: key deas Newton s laws o oton Conseraton o Energy Newton s Laws st Law: An object at rest or traelng n unor oton wll rean at rest or traelng n unor
Quotes. Review - First Law. Review - First Law. Review - First Law. Review - First Law. Thermodynamics Lecture Series
 8//005 herodynaics ecture Series Entropy uantifyg Energy Degradation Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti eknologi MARA eail: drjjlanita@hotail.co http://www3.uit.edu.y/staff/drjj/
8//005 herodynaics ecture Series Entropy uantifyg Energy Degradation Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti eknologi MARA eail: drjjlanita@hotail.co http://www3.uit.edu.y/staff/drjj/
Homework Chapter 21 Solutions!!
 Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
THERMODYNAMICS Lecture 15: Heat exchangers
 HERMODYNAMICS Leture 5: Heat exangers Pierwsza strona Introdution to Heat Exangers Wat Are Heat Exangers? Heat exangers are units designed to transfer eat from a ot flowing stream to a old flowing stream
HERMODYNAMICS Leture 5: Heat exangers Pierwsza strona Introdution to Heat Exangers Wat Are Heat Exangers? Heat exangers are units designed to transfer eat from a ot flowing stream to a old flowing stream
PHYS 1443 Section 002 Lecture #20
 PHYS 1443 Secton 002 Lecture #20 Dr. Jae Condtons for Equlbru & Mechancal Equlbru How to Solve Equlbru Probles? A ew Exaples of Mechancal Equlbru Elastc Propertes of Solds Densty and Specfc Gravty lud
PHYS 1443 Secton 002 Lecture #20 Dr. Jae Condtons for Equlbru & Mechancal Equlbru How to Solve Equlbru Probles? A ew Exaples of Mechancal Equlbru Elastc Propertes of Solds Densty and Specfc Gravty lud
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2013
 Lecture 8/8/3 Unversty o Washngton Departent o Chestry Chestry 45/456 Suer Quarter 3 A. The Gbbs-Duhe Equaton Fro Lecture 7 and ro the dscusson n sectons A and B o ths lecture, t s clear that the actvty
Lecture 8/8/3 Unversty o Washngton Departent o Chestry Chestry 45/456 Suer Quarter 3 A. The Gbbs-Duhe Equaton Fro Lecture 7 and ro the dscusson n sectons A and B o ths lecture, t s clear that the actvty
Recommended Reading. Entropy/Second law Thermodynamics
 Lecture 7. Entropy and the second law of therodynaics. Recoended Reading Entropy/econd law herodynaics http://en wikipedia http://en.wikipedia.org/wiki/entropy http://2ndlaw.oxy.edu/index.htl. his site
Lecture 7. Entropy and the second law of therodynaics. Recoended Reading Entropy/econd law herodynaics http://en wikipedia http://en.wikipedia.org/wiki/entropy http://2ndlaw.oxy.edu/index.htl. his site
THE ESSENCE OF QUANTUM MECHANICS
 THE ESSENCE OF QUANTUM MECHANICS Capter belongs to te "Teory of Spae" written by Dariusz Stanisław Sobolewski. Http: www.tsengines.o ttp: www.teoryofspae.info E-ail: info@tsengines.o All rigts resered.
THE ESSENCE OF QUANTUM MECHANICS Capter belongs to te "Teory of Spae" written by Dariusz Stanisław Sobolewski. Http: www.tsengines.o ttp: www.teoryofspae.info E-ail: info@tsengines.o All rigts resered.
Lecture 09 Systems of Particles and Conservation of Linear Momentum
 Lecture 09 Systes o Partcles and Conseraton o Lnear oentu 9. Lnear oentu and Its Conseraton 9. Isolated Syste lnear oentu: P F dp dt d( dt d dt a solated syste F ext 0 dp dp F, F dt dt dp dp d F F 0, 0
Lecture 09 Systes o Partcles and Conseraton o Lnear oentu 9. Lnear oentu and Its Conseraton 9. Isolated Syste lnear oentu: P F dp dt d( dt d dt a solated syste F ext 0 dp dp F, F dt dt dp dp d F F 0, 0
Physics 3A: Linear Momentum. Physics 3A: Linear Momentum. Physics 3A: Linear Momentum. Physics 3A: Linear Momentum
 Recall that there was ore to oton than just spee A ore coplete escrpton of oton s the concept of lnear oentu: p v (8.) Beng a prouct of a scalar () an a vector (v), oentu s a vector: p v p y v y p z v
Recall that there was ore to oton than just spee A ore coplete escrpton of oton s the concept of lnear oentu: p v (8.) Beng a prouct of a scalar () an a vector (v), oentu s a vector: p v p y v y p z v
Chemical Engineering 160/260 Polymer Science and Engineering. Lecture 10 - Phase Equilibria and Polymer Blends February 7, 2001
 Checal Engneerng 60/60 Polyer Scence and Engneerng Lecture 0 - Phase Equlbra and Polyer Blends February 7, 00 Therodynacs of Polyer Blends: Part Objectves! To develop the classcal Flory-Huggns theory for
Checal Engneerng 60/60 Polyer Scence and Engneerng Lecture 0 - Phase Equlbra and Polyer Blends February 7, 00 Therodynacs of Polyer Blends: Part Objectves! To develop the classcal Flory-Huggns theory for
a) Read over steps (1)- (4) below and sketch the path of the cycle on a P V plot on the graph below. Label all appropriate points.
 Prole 3: Crnot Cyle of n Idel Gs In this prole, the strting pressure P nd volue of n idel gs in stte, re given he rtio R = / > of the volues of the sttes nd is given Finlly onstnt γ = 5/3 is given You
Prole 3: Crnot Cyle of n Idel Gs In this prole, the strting pressure P nd volue of n idel gs in stte, re given he rtio R = / > of the volues of the sttes nd is given Finlly onstnt γ = 5/3 is given You
(T > w) F R = T - w. Going up. T - w = ma
 ANSES Suspended Acceleratng-Objects A resultant orce causes a syste to accelerate. he drecton o the acceleraton s n the drecton o the resultant orce. As llustrated belo, hen suspended objects accelerate,
ANSES Suspended Acceleratng-Objects A resultant orce causes a syste to accelerate. he drecton o the acceleraton s n the drecton o the resultant orce. As llustrated belo, hen suspended objects accelerate,
Primordial Black Holes and the QCD Phase Transition
 Primordial Blak Holes and te QCD Pase ransition odd Springer and Joe Kapusta University of Minnesota arxiv:0706.1111 DSU 07 June 8 t, 007 Outline Formation of PBH s Desription of te QCD pase transition
Primordial Blak Holes and te QCD Pase ransition odd Springer and Joe Kapusta University of Minnesota arxiv:0706.1111 DSU 07 June 8 t, 007 Outline Formation of PBH s Desription of te QCD pase transition
THE SECOND LAW OF THERMODYNAMICS
 HE SECOND LAW OF HERMODYNAMICS 9 EXERCISES Setions 9. and 9.3 e Seond Law of ermodynamis and Its Appliations 3. INERPRE is problem requires us to alulate te effiieny of reversible eat engines tat operate
HE SECOND LAW OF HERMODYNAMICS 9 EXERCISES Setions 9. and 9.3 e Seond Law of ermodynamis and Its Appliations 3. INERPRE is problem requires us to alulate te effiieny of reversible eat engines tat operate
Chem/Biochem 471 Exam 3 12/18/08 Page 1 of 7 Name:
 Che/Bioche 47 Exa /8/08 Pae of 7 Please leave the exa paes stapled toether. The forulas are on a separate sheet. This exa has 5 questions. You ust answer at least 4 of the questions. You ay answer ore
Che/Bioche 47 Exa /8/08 Pae of 7 Please leave the exa paes stapled toether. The forulas are on a separate sheet. This exa has 5 questions. You ust answer at least 4 of the questions. You ay answer ore
Fermi-Dirac statistics
 UCC/Physcs/MK/EM/October 8, 205 Fer-Drac statstcs Fer-Drac dstrbuton Matter partcles that are eleentary ostly have a type of angular oentu called spn. hese partcles are known to have a agnetc oent whch
UCC/Physcs/MK/EM/October 8, 205 Fer-Drac statstcs Fer-Drac dstrbuton Matter partcles that are eleentary ostly have a type of angular oentu called spn. hese partcles are known to have a agnetc oent whch
Problem Free Expansion of Ideal Gas
 Problem 4.3 Free Expanon o Ideal Ga In general: ds ds du P dv P dv NR V dn Snce U o deal ga ndependent on olume (du=), and N = cont n the proce: dv In a ere o nntemal ree expanon, entropy change by: S
Problem 4.3 Free Expanon o Ideal Ga In general: ds ds du P dv P dv NR V dn Snce U o deal ga ndependent on olume (du=), and N = cont n the proce: dv In a ere o nntemal ree expanon, entropy change by: S
Final Exam Solutions, 1998
 58.439 Fnal Exa Solutons, 1998 roble 1 art a: Equlbru eans that the therodynac potental of a consttuent s the sae everywhere n a syste. An exaple s the Nernst potental. If the potental across a ebrane
58.439 Fnal Exa Solutons, 1998 roble 1 art a: Equlbru eans that the therodynac potental of a consttuent s the sae everywhere n a syste. An exaple s the Nernst potental. If the potental across a ebrane
5/24/2007 Collisions ( F.Robilliard) 1
 5/4/007 Collsons ( F.Robllard) 1 Interactons: In our earler studes o orce and work, we saw, that both these quanttes arse n the context o an nteracton between two bodes. We wll now look ore closely at
5/4/007 Collsons ( F.Robllard) 1 Interactons: In our earler studes o orce and work, we saw, that both these quanttes arse n the context o an nteracton between two bodes. We wll now look ore closely at
System in Weibull Distribution
 Internatonal Matheatcal Foru 4 9 no. 9 94-95 Relablty Equvalence Factors of a Seres-Parallel Syste n Webull Dstrbuton M. A. El-Dacese Matheatcs Departent Faculty of Scence Tanta Unversty Tanta Egypt eldacese@yahoo.co
Internatonal Matheatcal Foru 4 9 no. 9 94-95 Relablty Equvalence Factors of a Seres-Parallel Syste n Webull Dstrbuton M. A. El-Dacese Matheatcs Departent Faculty of Scence Tanta Unversty Tanta Egypt eldacese@yahoo.co
x(t) y(t) c c F(t) F(t) EN40: Dynamics and Vibrations Homework 6: Forced Vibrations Due Friday April 5, 2018
 EN40: Dynais and Vibrations Hoewor 6: Fored Vibrations Due Friday April 5, 2018 Shool of Engineering Brown University 1. The vibration isolation syste shown in the figure has =20g, = 19.8 N / = 1.259 Ns
EN40: Dynais and Vibrations Hoewor 6: Fored Vibrations Due Friday April 5, 2018 Shool of Engineering Brown University 1. The vibration isolation syste shown in the figure has =20g, = 19.8 N / = 1.259 Ns
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture : Transition State Teory. tkins & DePaula: 7.6-7.7 University o Wasinton Departent o Ceistry Ceistry 453 Winter Quarter 05. ctivated Kinetics Kinetic rate uations are overned by several principles.
Lecture : Transition State Teory. tkins & DePaula: 7.6-7.7 University o Wasinton Departent o Ceistry Ceistry 453 Winter Quarter 05. ctivated Kinetics Kinetic rate uations are overned by several principles.
2/24/2014. The point mass. Impulse for a single collision The impulse of a force is a vector. The Center of Mass. System of particles
 /4/04 Chapte 7 Lnea oentu Lnea oentu of a Sngle Patcle Lnea oentu: p υ It s a easue of the patcle s oton It s a vecto, sla to the veloct p υ p υ p υ z z p It also depends on the ass of the object, sla
/4/04 Chapte 7 Lnea oentu Lnea oentu of a Sngle Patcle Lnea oentu: p υ It s a easue of the patcle s oton It s a vecto, sla to the veloct p υ p υ p υ z z p It also depends on the ass of the object, sla
Lecture 3 Examples and Problems
 Lecture 3 Examles and Problems Mechancs & thermodynamcs Equartton Frst Law of Thermodynamcs Ideal gases Isothermal and adabatc rocesses Readng: Elements Ch. 1-3 Lecture 3, 1 Wllam Thomson (1824 1907) a.k.a.
Lecture 3 Examles and Problems Mechancs & thermodynamcs Equartton Frst Law of Thermodynamcs Ideal gases Isothermal and adabatc rocesses Readng: Elements Ch. 1-3 Lecture 3, 1 Wllam Thomson (1824 1907) a.k.a.
Answers to Coursebook questions Chapter 4.1
 Carige Physis or the IB Diploa nswers to Courseook questions Chapter 41 1 n osillation is any otion in whih the isplaeent o a partile ro a ixe point keeps hanging iretion an there is a perioiity in the
Carige Physis or the IB Diploa nswers to Courseook questions Chapter 41 1 n osillation is any otion in whih the isplaeent o a partile ro a ixe point keeps hanging iretion an there is a perioiity in the
How does the momentum before an elastic and an inelastic collision compare to the momentum after the collision?
 Experent 9 Conseraton o Lnear Moentu - Collsons In ths experent you wll be ntroduced to the denton o lnear oentu. You wll learn the derence between an elastc and an nelastc collson. You wll explore how
Experent 9 Conseraton o Lnear Moentu - Collsons In ths experent you wll be ntroduced to the denton o lnear oentu. You wll learn the derence between an elastc and an nelastc collson. You wll explore how
AP Physics Thermodynamics Wrap-up
 AP Physics herodynaics Wrap-up Here are your basic equations for therodynaics. here s a bunch of the. 3 his equation converts teperature fro Fahrenheit to Celsius. his is the rate of heat transfer for
AP Physics herodynaics Wrap-up Here are your basic equations for therodynaics. here s a bunch of the. 3 his equation converts teperature fro Fahrenheit to Celsius. his is the rate of heat transfer for
Least Squares Fitting of Data
 Least Squares Fttng of Data Davd Eberly Geoetrc Tools, LLC http://www.geoetrctools.co/ Copyrght c 1998-2014. All Rghts Reserved. Created: July 15, 1999 Last Modfed: February 9, 2008 Contents 1 Lnear Fttng
Least Squares Fttng of Data Davd Eberly Geoetrc Tools, LLC http://www.geoetrctools.co/ Copyrght c 1998-2014. All Rghts Reserved. Created: July 15, 1999 Last Modfed: February 9, 2008 Contents 1 Lnear Fttng
Dissipated energy and Entropy Production for an Unconventional Heat Engine: The Stepwise Circular Cycle
 sspated energy and Entropy roducton for an nconventonal Heat Engne: he Stepwse rcular ycle Francesco d Lberto, Raffaele astore and Fulvo erugg partmento Scenze Fsche - nverstà d apol Federco II - SI-R,
sspated energy and Entropy roducton for an nconventonal Heat Engne: he Stepwse rcular ycle Francesco d Lberto, Raffaele astore and Fulvo erugg partmento Scenze Fsche - nverstà d apol Federco II - SI-R,
Chapter 20 The First Law of Thermodynamics
 Chapter he Frst aw o hermodynamcs. developng the concept o heat. etendng our concept o work to thermal processes 3. ntroducng the rst law o thermodynamcs. Heat and Internal Energy Internal energy: s the
Chapter he Frst aw o hermodynamcs. developng the concept o heat. etendng our concept o work to thermal processes 3. ntroducng the rst law o thermodynamcs. Heat and Internal Energy Internal energy: s the
4.5. QUANTIZED RADIATION FIELD
 4-1 4.5. QUANTIZED RADIATION FIELD Baground Our treatent of the vetor potental has drawn on the onohroat plane-wave soluton to the wave-euaton for A. The uantu treatent of lght as a partle desrbes the
4-1 4.5. QUANTIZED RADIATION FIELD Baground Our treatent of the vetor potental has drawn on the onohroat plane-wave soluton to the wave-euaton for A. The uantu treatent of lght as a partle desrbes the
Irreversible Work of Separation and Heat-Driven Separation
 J. Phys. Che. B 004, 08, 6035-604 6035 Irreversble Wor of Separaton and Heat-Drven Separaton Anatoly M. Tsrln and Vladr Kazaov*, Progra Syste Insttute, Russan Acadey of Scence, set. Botc, PerejaslaVl-Zalesy,
J. Phys. Che. B 004, 08, 6035-604 6035 Irreversble Wor of Separaton and Heat-Drven Separaton Anatoly M. Tsrln and Vladr Kazaov*, Progra Syste Insttute, Russan Acadey of Scence, set. Botc, PerejaslaVl-Zalesy,
Problem Set #6 solution, Chem 340, Fall 2013 Due Friday, Oct 11, 2013 Please show all work for credit
 Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
THERMODYNAMICS of COMBUSTION
 Internal Cobuston Engnes MAK 493E THERMODYNAMICS of COMBUSTION Prof.Dr. Ce Soruşbay Istanbul Techncal Unversty Internal Cobuston Engnes MAK 493E Therodynacs of Cobuston Introducton Proertes of xtures Cobuston
Internal Cobuston Engnes MAK 493E THERMODYNAMICS of COMBUSTION Prof.Dr. Ce Soruşbay Istanbul Techncal Unversty Internal Cobuston Engnes MAK 493E Therodynacs of Cobuston Introducton Proertes of xtures Cobuston
Chapters 18 & 19: Themodynamics review. All macroscopic (i.e., human scale) quantities must ultimately be explained on the microscopic scale.
 Chapters 18 & 19: Themodynamcs revew ll macroscopc (.e., human scale) quanttes must ultmately be explaned on the mcroscopc scale. Chapter 18: Thermodynamcs Thermodynamcs s the study o the thermal energy
Chapters 18 & 19: Themodynamcs revew ll macroscopc (.e., human scale) quanttes must ultmately be explaned on the mcroscopc scale. Chapter 18: Thermodynamcs Thermodynamcs s the study o the thermal energy
ATMO 551a Fall The Carnot Cycle
 What is a arnot ycle and Why do we care The arnot ycle arnot was a French engineer who was trying to understand how to extract usable mechanical work from a heat engine, that is an engine where a gas or
What is a arnot ycle and Why do we care The arnot ycle arnot was a French engineer who was trying to understand how to extract usable mechanical work from a heat engine, that is an engine where a gas or
Chapter 12 Lyes KADEM [Thermodynamics II] 2007
![Chapter 12 Lyes KADEM [Thermodynamics II] 2007 Chapter 12 Lyes KADEM [Thermodynamics II] 2007](/thumbs/79/79128935.jpg) Chapter 2 Lyes KDEM [Therodynacs II] 2007 Gas Mxtures In ths chapter we wll develop ethods for deternng therodynac propertes of a xture n order to apply the frst law to systes nvolvng xtures. Ths wll be
Chapter 2 Lyes KDEM [Therodynacs II] 2007 Gas Mxtures In ths chapter we wll develop ethods for deternng therodynac propertes of a xture n order to apply the frst law to systes nvolvng xtures. Ths wll be
Order (Boltzmann 1875) Information (Shannon 1948) Entropy (Clausius 1855) dq T Cycle. 2 nd LAW. 5. ENTROPY & 2 nd LAW OF THERMODYNAMICS.
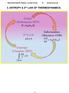 5. ENROPY & nd LAW OF HERMODYNAMIC. Order (Boltzmann 875) ln k B e nd LAW 0 Inormaton (hannon 948) Q bt k B ln e Entropy (Clausus 855) dq Cycle 0 4 5. he Clausus Inequalty. wo emprcal statements o the
5. ENROPY & nd LAW OF HERMODYNAMIC. Order (Boltzmann 875) ln k B e nd LAW 0 Inormaton (hannon 948) Q bt k B ln e Entropy (Clausus 855) dq Cycle 0 4 5. he Clausus Inequalty. wo emprcal statements o the
Classical systems in equilibrium
 35 Classical systes in equilibriu Ideal gas Distinguishable particles Here we assue that every particle can be labeled by an index i... and distinguished fro any other particle by its label if not by any
35 Classical systes in equilibriu Ideal gas Distinguishable particles Here we assue that every particle can be labeled by an index i... and distinguished fro any other particle by its label if not by any
Combined Gas Law (1) Answer Key
 CHAER 4 Cobined Gas Law (1) Answer Key BL 4.1.1A 1. 1 1 1 1 1 1 100.8 ka 4. L 48.15 K 71.15 K10.0 ka.8l. he balloons will decrease in volue. 1 1 1 1 6.0L80% 4.8L 7.8 º C (body teperature) 10.95 K 1 1 1
CHAER 4 Cobined Gas Law (1) Answer Key BL 4.1.1A 1. 1 1 1 1 1 1 100.8 ka 4. L 48.15 K 71.15 K10.0 ka.8l. he balloons will decrease in volue. 1 1 1 1 6.0L80% 4.8L 7.8 º C (body teperature) 10.95 K 1 1 1
On Pfaff s solution of the Pfaff problem
 Zur Pfaff scen Lösung des Pfaff scen Probles Mat. Ann. 7 (880) 53-530. On Pfaff s soluton of te Pfaff proble By A. MAYER n Lepzg Translated by D. H. Delpenc Te way tat Pfaff adopted for te ntegraton of
Zur Pfaff scen Lösung des Pfaff scen Probles Mat. Ann. 7 (880) 53-530. On Pfaff s soluton of te Pfaff proble By A. MAYER n Lepzg Translated by D. H. Delpenc Te way tat Pfaff adopted for te ntegraton of
Variable Range Hopping Conductivity: Case of the non-constant density of states
 Varable Range Hoppng Condutvty: Case of the non-onstant densty of states Saïd Bouthe Dept. de Physue Unverste de Behar 8 Behar Algera e_learnng_@hotal.o The varable range hoppng theory has been wdely used
Varable Range Hoppng Condutvty: Case of the non-onstant densty of states Saïd Bouthe Dept. de Physue Unverste de Behar 8 Behar Algera e_learnng_@hotal.o The varable range hoppng theory has been wdely used
Humidity parameters. Saturation (equilibrium) vapor pressure Condensation balances evaporation
 uidity paraeters Saturation (equilibriu) vapor pressure Condensation balances evaporation Miing ratio & specific huidity Mass ratio of water vapor and air and water content and wet air. Dew point & frost
uidity paraeters Saturation (equilibriu) vapor pressure Condensation balances evaporation Miing ratio & specific huidity Mass ratio of water vapor and air and water content and wet air. Dew point & frost
3 Thermodynamics and Statistical mechanics
 Therodynaics and Statistical echanics. Syste and environent The syste is soe ortion of atter that we searate using real walls or only in our ine, fro the other art of the universe. Everything outside the
Therodynaics and Statistical echanics. Syste and environent The syste is soe ortion of atter that we searate using real walls or only in our ine, fro the other art of the universe. Everything outside the
