Fundamentals of Solid State Ionics I Defect Chemistry
|
|
|
- Melinda Lamb
- 5 years ago
- Views:
Transcription
1 The research leadng to these results has receed fundng from the European Unon's Seenth Framework Programme (FP7/ ) for the Fuel Cells and Hydrogen Jont Technology Intate under grant agreement n [6144. Fundamentals of Sold State Ioncs I Defect Chemstry Tutoral lecture at the Summer School Valenca, September 3-5, 015 Truls Norby Man purposes Introduce defect chemstry to newbes Focus on some mportant prncples and good practces for oldes Department of Chemstry Unersty of slo Centre for Materals Scence and Nanotechnology (SMN) FERMI slo Research Park (Forsknngsparken) truls.norby@kjem.uo.no utlne What are defects and why are they mportant? Random dffuson and onc conductty Defect reactons and equlbrum thermodynamcs Examples nclude M, Zr, BaZr 3 L on battery materals Computatonal defect chemstry Summarsng adce
2 Stochometrc compounds; Pont defects form n pars: Intrnsc pont defect dsorders Schottky defects Caton and anon acances Frenkel defects Caton acances and ntersttals Ant- or anon-frenkel defects Anon acances and ntersttals
3 Stochometrc compounds: Electronc defects: Intrnsc electronc dsorder Domnates n undoped semconductors wth moderate bandgaps Defect electrons n the conducton band and electron holes n the alence band
4 Random dffuson and self dffuson Mass transport n crystallne solds s dren by thermal energy kt Leads to random dffuson If the dffusng speces s a consttuent t s also called self-dffuson Two most mportant mechansms: Vacancy mechansm Intersttal mechansm Defects are needed n both
5 Dffusty s a dffcult entty to understand. Frst warm-up: Dffusty: a matter of geometry and jump rates Consttuent by acancy mechansm rthogonal drectons D Jump rate 1 r, c 6 s c 1 6 s ZX Number of neghbourng stes Lkelyhood of target ste to be acant Jump dstance Rate of suffcently energetc attempts Vacancy D r, s s Z Consttuent by ntersttal mechansm D 1 r, c 6 s c 1 6 s ZX Lkelyhood to be ntersttal Intersttal D r 1, s s Z( 1 X ) s Z ΔGm ω exp exp RT ΔS R m exp ΔH RT m
6 Dffusty next exercse to look at what t s: The Nernst-Ensten relaton lnkng moblty and dffusty Applcaton of a force F ges the randomly dffusng partcles a net drft elocty : B F The proportonalty B s called mechancal moblty («beweglchket») Mechancal moblty B (beweglchket) s the dffusty D oer the thermal energy kt: B D kt D B kt Ths s the Nernst-Ensten relaton
7 Electrcal feld; force, flux densty, and current densty An electrcal feld s the downhll gradent n electrcal potental: E d dx It ges rse to a force on a charged partcle gen as F z ee z e d dx The flux densty j s the olume concentraton c multpled wth the drft elocty : j c c B F c B z ee Current densty by multplcaton wth charge: z ej z ec c B ( z e) E
8 Mobltes and conductty We now defne a charge moblty u u z eb We then obtan for the current densty: Charge moblty u s n physcs often denoted μ. We here use u to aod confuson wth chemcal potental. c B ( z e) E z ec u E We now defne electrcal conductty σ z and obtan ec u Very mportant! Know t! Charge x concentraton x charge moblty z ec u E E Ths s one form of hm s law. Conductty has unts S/cm or S/m.
9 Ionc conductty for acancy mechansm Consttuent by acancy mechansm Vacancy kt ZX s c e z kt D c e z B c e z u ec z c c c c c c c, 6 1,,,,,,, ) ( ) ( ) ( kt Z s c e z kt D c e z B c e z u ec z 6 1,,,,,,, ) ( ) ( ) ( Volume concentraton of acances Charge moblty of acances (~ concentraton ndependent) Volume concentraton of acances Regardless of whether you consder the consttuent or the defect, you need the concentraton of the defect ndrectly or drectly.
10 Before we moe on... Formal oxdaton number nteger charges We know that bonds n onc compounds are not fully onc, n the sense that all alence electrons are not entrely shfted to the anon. But f the bondng s broken - as when somethng, lke a defect, moes the electrons hae to stay or go. Electrons cannot splt n half. And mostly they go wth the anon - the most electronegate atom. That s why the onc model apples n defect chemstry and transport And t s why t s ery useful to know and apply the rules of formal oxdaton numbers, the number of charges an on gets when the alence electrons hae to make the choce z are nteger numbers
11 Defect chemstry Allows us to descrbe processes nolng defects Allows applcaton of statstcal thermodynamcs Equlbrum coeffcents; Enthalpes and entropes Yelds defect structure (concentratons of all defects) under gen condtons The defect concentratons for transport coeffcents (e.g. conductty) Requres nomenclature Requres rules for wrtng proper reactons Addtonal requrements: Electroneutralty, ste balances
12 Kröger-Vnk notaton In modern defect chemstry, we use Kröger-Vnk notaton. It can descrbe any entty n a crystallne structure; defects and perfects. Man symbol A, a subscrpt S, and a superscrpt C: What the entty s, as the man symbol (A) Chemcal symbol or (for acancy) Where the entty s the ste - as subscrpt (S) Chemcal symbol of the normal occupant of the ste or for ntersttal (normally empty) poston A C S Kröger and Vnk used uppercase V for acances and I for ntersttal stes, perhaps because that s natural for nouns n German. Its charge, real or effecte, as superscrpt (C) +, -, or 0 for real charges or., /, or x for effecte poste, negate, or no charge I say: How would you then do defect chemstry for anadum odde VI 3? I clam that lowercase and are much better n all respects, and hereby use and. Basta. The use of effecte charge of a few defects oer the real charge of all the perfects s preferred and one of the key ponts n defect chemstry. We wll learn what t s n the followng sldes
13 Effecte charge The effecte charge s defned as the charge an entty n a ste has relate to (.e. mnus) the charge the same ste would hae had n the deal structure. Example: An oxde on - n an ntersttal ste () Real charge of defect: - - Real charge of ntersttal (empty) ste n deal structure: 0 Effecte charge: = - //
14 Effecte charge more examples Example: An oxde on acancy Real charge of defect (acancy = nothng): 0 Real charge of oxde on - n deal structure: - Effecte charge: 0 - (-) = + Example: A zrconum on acancy, e.g. n Zr Real charge of defect: 0 Real charge of zrconum on Zr 4+ n deal structure: +4 Effecte charge: 0-4 = -4 //// Zr
15 Kröger-Vnk notaton more examples Dopants and mpurtes Y 3+ substtutng Zr 4+ n Zr L + ntersttals / Y Zr L Electronc defects Defect electrons n conducton band Electron holes n alence band / e h
16 We wll now make use of the thermodynamcs of chemcal reactons comprsng defects In order to do that correctly, we need to obey 3 rules for wrtng and balancng defect chemcal reacton equatons: Conseraton of mass - mass balance Conseraton of charge - charge balance Conseraton of ste rato (host structure)
17 We wll now use Schottky defect par as our smple example to learn many thngs: Schottky defects n M We start by wrtng the releant defect formaton reacton: M x M x // M M x M x whch we can smplfy to 0 // M We then wrte ts equlbrum coeffcent: K a a // X X // S M M [ [ // [ M [M Acttes a For pont defects, acttes are expressed n terms of ste fractons X The ste fracton s the concentraton of defects oer the concentraton of stes
18 Schottky defects n M 0 // M K s are often smplfed. There are arous reasons why: Because you sometmes can do t properly; Because the smplfcaton often s a reasonable approxmaton; Because you are perhaps not nterested n the dfference between the exact and smplfed K (ths often means that you dsregard the possblty to assess the entropy change); Because nether the full nor smplfed forms make much sense n terms of entropy, so they are equally useful or accurate (or naccurate), and then we may well choose the smplest. If we express concentratons n molar fractons (mol/mol M), then [M = [ = 1, and we may smplfy to K S [ // [ [ M // [ M [ [M
19 Schottky defects n M 0 // M NTE: At equlbrum, an equlbrum coeffcent expresson s always ald and must be satsfed at all tmes! K S [ [ // M Thus the product of the concentratons of oxygen and metal acances s always constant (at constant T). We may well stress ths by nstead wrtng: [ [ // M K S Whle K S represents nformaton about the system, we hae two unknowns, namely the two defect concentratons, so ths s not enough. We need one more pece of ndependent nput.
20 Schottky defects n M 0 // M The second pece of nput s the electroneutralty expresson. If the two defects of the Schottky par are the domnatng defects, we may wrte [ [ // M or [ [ // M It s now mportant to understand that ths s NT an eternal truth the electroneutralty statement s a choce: We choose to belee or assume that these are the domnatng defects. The next step s to combne the two sets of nformaton; we nsert the electroneutralty nto the equlbrum coeffcent: [ [ [ [ // M // M [ // M K K [ K 1/ S // M S S K 1/ S [ [ // M Vola! We hae now found the expresson for the concentraton of the defects. In ths case, they are only a functon of K S.
21 Schottky defects n M 0 From the general temperature dependency of K, K we obtan [ S // M ΔG exp RT [ 0 S ΔS exp R 0 SS R ΔH exp RT H RT // 1/ M KS exp exp 0 S 0 S 0 S Note: Ths not the Gbbs energy change (whch becomes zero at equlbrum) It s the standard Gbbs energy change. What does standard refer to? The square root and number arse from the reacton contanng defects. ln or log defect concentratons s 1/T (an t Hoff plots): ln[ log[ ln[ log[ 0 SS R H R // M 0 SS Rln10 0 S 1 T 0 H S Rln10 // M 1 T ln10=.303
22 Schottky defects n M 0 // M an t Hoff plot Standard entropy and enthalpy changes can be found from ntercept wth y axs and slope, respectely, after multplcaton wth R and -R. log[ plots can be more ntellgble, but requre the addtonal multplcatons wth ln10 =.303. ΔS S0 /R ln [ -ΔH S0 /R [.. =[ M // The standard enthalpy change can hae any alue: Fndng t s a result! The standard entropy change can be estmated: Fndng t s therefore a control! Dare to try? Get nterested n pre-exponentals and entropes! 1/T Man contrbuton to entropy changes s gas s condensed phases: ~10 J/molK!
23 Recap before we moe on 0 // M The soluton we found assumes that the two Schottky defects are domnatng. The standard entropy and enthalpy changes of the Schottky reacton refer to the reacton when the reactants and products are n the standard state. For defects, the standard state s a ste fracton of unty! Ths s a hypothetcal state, but neertheless the state we hae agreed on as standard. Therefore, the entropy as dered and used here s only ald f the pont defect concentratons are entered (plotted) n unts of ste fracton (whch n M happens to be the same as mole fracton). ther speces gases, electrons, condensed phases should be expressed as acttes, referrng to ther defned standard states, f possble. The model also assumes dealty,.e. that the acttes of defects are proportonal to ther concentratons. It s a dlute soluton case.
24 Now a detour to a more dffcult and perhaps controersal case; electronc defects Intrnsc onsaton of electronc defects For conducton band electrons and alence band holes, the releant reacton s / 0 e h N C The equlbrum coeffcent may be wrtten / [e [h K a / a e h N N C V n N C Here, the acttes of electrons and holes are expressed n terms of the fracton of ther concentraton oer the densty of states of the conducton and alence bands, respectely. The reason s that electrons behae quantum-mechancally and therefore populate dfferent energy states rather than dfferent stes. p N V N V * 8m ekt h 3/ * 8m hkt h 3/ The standard state s accordng to ths: n 0 = N C and p 0 = N V
25 Intrnsc onsaton of electronc defects If we choose to apply the concepts of standard Gbbs energy, entropy, and enthalpy changes as before, we obtan K n N C p N V G exp RT Ths s possble and useful, but not commonly adopted. 0 S exp R In semconductor physcs t s nstead more common to use smply: 0 H exp RT 0 K / [ / e [ h n p N C N V exp E RT g Ths states that the product of n and p s constant at a gen temperature, as expected for the equlbrum coeffcent for the reacton. Howeer, the concept of actty s not appled, as standard states for electronc defects are not commonly defned. For ths reason, we here use a prme on the K / to sgnfy the dfference to a normal K from whch the entropy could hae been dered.
26 Intrnsc onsaton of electronc defects From K n N C p N V exp G RT 0 exp S R 0 exp H RT 0 and K / [ / e [ h n p N C N V exp E RT g we see that the band gap E g s to a frst approxmaton the Gbbs energy change of the ntrnsc onsaton, whch n turn conssts manly of the enthalpy change. We shall not enter nto the fner detals or of the dfferences here, just stress that np = constant at a gen temperature. Always! Physcsts mostly use E g /kt wth E g n ev per electron, whle chemsts often use E g /RT (or ΔG 0 /RT) wth E g n J or kj per mole electrons. Ths s a tral conerson (factor 1 ev = J/mol = kj/mol).
27 Intrnsc onsaton of electronc defects If we choose that electrons and holes domnate the defect structure; n p We nsert nto the equlbrum coeffcent expresson and get n p n n p K K / N C N V E exp RT / 1/ 1/ ( NC NV ) exp g E g RT A logarthmc plot of n or p s 1/T wll thus hae a slope that seems to reflect E g / as the apparent enthalpy. Because of the temperature dependences of the densty of states t should howeer be more approprate to plot nt -3/ or pt -3/ s 1/T to obtan a slope that reflects E g / more correctly.
28 xygen defcent oxdes xygen acances are formed accordng to It s common for most purposes to neglect the dson by N C, to assume [ x = 1 and to remoe p 0 = 1 bar, so that we get ) ( 1 / g e x 1/ 0 C 1/ 0 C 1/ ) ( / N n [ [ [ [ N n [ [ x x g e p p p p a a a a K x Ths bg expresson may seem unnecessary, but s meant to help you understand 1/ / [ C p n K N K Then fnally, a case of nonstochometry, nolng onc and electronc defects: I use agan the prme n K / to sgnfy ths neglectance
29 xygen defcent oxdes We now choose to assume that the oxygen acances and electrons are the two domnatng defects. The electroneutralty then reads [ n We now nsert ths nto the equlbrum coeffcent and get K / 3 1/ 4[ p We fnally sole wth respect to the concentraton of defects: [ ( 1 4 K / ) 1/3 p 1/6 n [ (K / ) 1/3 p 1/6
30 xygen defcent oxdes We splt K / nto a pre-exponental and the enthalpy term: n [ (K / ) 1/3 p 1/6 (K /,0 1/3 exp 3 ) H RT 0 p 1/6 From ths, to a frst approxmaton, a plot of the logarthm of the defect concentratons s 1/T wll ge lnes wth slope of ΔH 0 /3R The number 3 relates to the formaton of 3 defects n the defect reacton x / 1 e ( g)
31 xygen defcent oxdes n [ (K / ) 1/3 p 1/ 6 By takng the logarthm: / 1 log n log log[ 1 3 log( K) 6 log p we see that a plot of logn s logp ges a straght lne wth a slope of -1/6. Ths knd of plot s a Brouwer dagram Note that log[.. s a parallel lne log = 0.30 unts lower.
32 Electroneutralty ne of the key ponts n defect chemstry s the ablty to express electroneutralty n terms of the few defects and ther effecte charges and to skp the real charges of all the normal structural elements poste charges = negate charges can be replaced by poste effecte charges = negate effecte charges poste effecte charges - negate effecte charges = 0
33 Electroneutralty The number of charges s counted oer a olume element, and so we use the concentraton of the defect speces s multpled wth the number of charges z S s z Example: M wth oxygen acances, metal ntersttals, and electrons: [ s zs [ s 0 [M -[e / 0 or [ [M [e / If oxygen acances domnate oer metal ntersttals we can smplfy: [ [e / Note: These are not chemcal reactons, they are mathematcal relatons and must be read as that. For nstance, n the aboe: Are there two acances for each electron or ce ersa?
34 Equlbra and electroneutraltes In defect chemstry, we combne nformaton from equlbrum coeffcents and electroneutralty expressons There s a potental ptfall For defect equlbra, you should use ste fractons n order to get the entropes rght Dfferent defects hae dfferent reference frames (ther host sublattces) For electroneutraltes, you must use olume concentratons, molar fractons, or formula unt fractons All defects must hae the same frame when countng ther charges They can be the same, but are n general not
35 Impurtes Dopng Substtuton
36 We wll only stop at a few mportant ponts for a sngle mportant case - YSZ: Zr -y doped substtutonally wth Y 3 Note: Dopng reactons are almost neer at equlbrum! They are most often fxed or frozen! Y / x 3 YZr 3 What would t take to hae them n equlbrum? Note: Electrons donated from oxygen acancy are accepted by Y dopants; no electronc defects n the bands. Dopant (secondary) phase must be present as source and snk Temperature must be ery hgh
37 Phase dagrams and defect chemstry All sold solutons and ther phase boundares are determned by defect thermodynamcs But suprsngly few studes attempt at takng adantage of ths, e.g. to ratonalse solublty and phase dagram studes
38 xde on conductors xde on conducton of YSZ Zr 0.9 Y The conductty has to a frst approxmaton a smple temperature dependency gen only by the moblty and hence random dffusty of the constant concentraton of oxygen acances. I hae chose to neglect two thngs: * nly a plot of log(σt) would ge a truly straght lne (remember why?) * Defects nteract: xygen acances and acceptor dopants assocate, lowerng the concentraton of free moble acances - or ther moblty f you prefer at lower temperatures.
39 can be hydrated to become proton conductors Y: BaZr 3 : A proton conductng oxde Zr 0.9 Y Ba BaZr 0.9 Y H (g) x H E a,h + 3 E a, From Kreuer,.K-D.
40 Three sldes for the noce on ternary and hgher compounds Ternary and hgher compounds Wth ternary and hgher compounds the ste rato conseraton becomes a lttle more troublesome to handle, that s all. For nstance, consder the peroskte CaT 3. To form Schottky defects n ths we need to form acances on both caton stes, n the proper rato: 0 // Ca //// T 3 And to form e.g. metal defcency we need to do somethng smlar: 3 (g) // Ca //// T 3 x 6h but oxygen defcency or excess would be just as smple as for bnary oxdes, snce the two catons stes are not affected n ths case
41 What f a ternary oxde has a strong preference for one of the caton defects? It can choose to make a selecton of the defects by throwng out one of the components, n order to not brake the ste rato conseraton rule. Example: Schottky defects n AB 3 wth only A and acances: A x A x // A A(g) Example: xdaton of AB 3 by formng metal defcency only on the A ste: x 1 // AA (g) A h A(s) Note: Choce of A(s) (secondary phase) or A(g) (eaporaton) are arbtrarly hosen to llustrate the possbltes
42 Dopng of ternary compounds The same rule apples: Wrte the dopng as you magne the synthess s done: If you are dopng by substtutng one component, you hae to remoe some of the component t s replacng, and thus hang some left of the other component to react wth the dopant. For nstance, to make undoped LaSc 3, you would probably react La 3 and Sc 3 and you could wrte ths as: 1 1 x x x La 3 Sc 3 La La Sc Sc 3 Now, to dope t wth Ca + substtutng La 3+ you would replace some La 3 wth Ca and let that Ca react wth the aalable Sc 3 : Ca 1 / x 5 x 1 Sc 3 Ca La Sc Sc The latter s thus a proper dopng reacton for dopng Ca nto LaSc 3, replacng La 3.
43 Defect chemstry of battery materals?
44 Sold-state L on conductor: L : La /3 T 3 The peroskte has two structurally dfferent A stes; /3 La, and 1/3 empty: La /3 1/3 T 3 Substtute 1 L for 1 La on the La ste, and add L on the empty ste: La /3-x L 3x T 3 or (La /3-x L x )(L x 1/3-x )T 3 [L // La [L Dopng reacton: L // 4 x x T 3 LLa 3 L T T 3
45 LFeP 4 cathode materal Man defect dsorder s L defcency Can be wrtten n seeral ways: Wrtten as an extracton of L : x 1 / 1 L L (g) L h L (s) 4 More releant: Extracton of L(s) to the anode: L x L / L h L(s) Een more releant: Extracton of L + ons to the electrolyte: L x L / L h L e ften donor doped. Total electroneutralty: [D [h [ / L - Normally, neer mx real and effecte charges For battery electrode materals, t may stll be useful: Both types of charges must then be consered separately
46 Computatonal defect chemstry Generate a computatonal cell wth many atoms (ons) and few defects Try to make t charge neutral Establsh boundary condtons by surroundng the cell wth copes of tself Calculate energy mnmum by densty functonal theory (DFT) Defect formaton Gbbs energy; dfference between defecte and perfect lattce; Chemcal potental of gas speces: Defect concentratons: c defect f ΔE N exp- kbt defect Numercally ft to electroneutralty. You enter p s (e.g. p ) and you obtan the Ferm leel μ e You can obtan all defect concentratons s T, p, dopng leel, etc. x 1 (g) e The standard entropy of gases s a frst approxmaton of entropes, that enables you to calculate equlbrum defect concentratons at fnte T, p, etc. We can also calculate lattce and hence defect entropes a further refnement. /
47 Summarsng adce Be honest! Admt and admre your defects! Ramble! That s what your defects do and keep you don! Learn! The nomenclature, the three rules, and wrtng electroneutraltes! Combne! Defect equlbra and the lmtng electroneutralty! Practce! Be brae! Do the statstcal thermodynamcs rght (standard states and ste fractons) and get the pre-exponentals and entropes. Check! Combne DFT and defect chemstry! Become an Almghty Computatonal Defect Chemst! (ACDC) Not a UCDP
between standard Gibbs free energies of formation for products and reactants, ΔG! R = ν i ΔG f,i, we
 hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
or the formation of a Schottky pair in MO, that we here choose to write; x O or the excitation of electronic defects, that we here choose to write;
 3. Defect equlbra 3. Defect equlbra Introducton After hang learned to formulate defect reactons n a compound, we now turn our nterest towards the equlbrum relatons for those reactons. These correlate the
3. Defect equlbra 3. Defect equlbra Introducton After hang learned to formulate defect reactons n a compound, we now turn our nterest towards the equlbrum relatons for those reactons. These correlate the
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
4.2 Chemical Driving Force
 4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
Electrochemical Equilibrium Electromotive Force
 CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
G = G 1 + G 2 + G 3 G 2 +G 3 G1 G2 G3. Network (a) Network (b) Network (c) Network (d)
 Massachusetts Insttute of Technology Department of Electrcal Engneerng and Computer Scence 6.002 í Electronc Crcuts Homework 2 Soluton Handout F98023 Exercse 21: Determne the conductance of each network
Massachusetts Insttute of Technology Department of Electrcal Engneerng and Computer Scence 6.002 í Electronc Crcuts Homework 2 Soluton Handout F98023 Exercse 21: Determne the conductance of each network
The three rules for writing defect reactions
 Introducton As dscussed n the prevous chapter several dfferent types of pont defects may be formed n metal odes and other norganc compounds. In prncple, all types of defects wll be present, but n general,
Introducton As dscussed n the prevous chapter several dfferent types of pont defects may be formed n metal odes and other norganc compounds. In prncple, all types of defects wll be present, but n general,
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
A quote of the week (or camel of the week): There is no expedience to which a man will not go to avoid the labor of thinking. Thomas A.
 A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
Appendix II Summary of Important Equations
 W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
Thermodynamics and Kinetics of Solids 33. III. Statistical Thermodynamics. Â N i = N (5.3) N i. i =0. Â e i = E (5.4) has a maximum.
 hermodynamcs and Knetcs of Solds 33 III. Statstcal hermodynamcs 5. Statstcal reatment of hermodynamcs 5.1. Statstcs and Phenomenologcal hermodynamcs. Calculaton of the energetc state of each atomc or molecular
hermodynamcs and Knetcs of Solds 33 III. Statstcal hermodynamcs 5. Statstcal reatment of hermodynamcs 5.1. Statstcs and Phenomenologcal hermodynamcs. Calculaton of the energetc state of each atomc or molecular
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
CHEMICAL REACTIONS AND DIFFUSION
 CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
5. Diffusion. Introduction. Models of diffusion. Fourier s law of heat flux. 5 Diffusion
 5. Dffuson Introducton Numerous chemcal reactons and mcrostructural changes n solds take place through sold state dffuson. In crystallne solds, the dffuson takes place because of the presence of defects;
5. Dffuson Introducton Numerous chemcal reactons and mcrostructural changes n solds take place through sold state dffuson. In crystallne solds, the dffuson takes place because of the presence of defects;
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
II. Equilibrium Thermodynamics. Lecture 8: The Nernst Equation
 II. Equlbrum Thermodynamcs Lecture 8: The Nernst Equaton Notes by MIT Student, re-wrtten by MZB 2014 February 27, 2014 1 Chemcal Actvty The fundamental thermodynamc quantty controllng transport and reactons
II. Equlbrum Thermodynamcs Lecture 8: The Nernst Equaton Notes by MIT Student, re-wrtten by MZB 2014 February 27, 2014 1 Chemcal Actvty The fundamental thermodynamc quantty controllng transport and reactons
Problem Points Score Total 100
 Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
CinChE Problem-Solving Strategy Chapter 4 Development of a Mathematical Model. formulation. procedure
 nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
3.2 Terminal Characteristics of Junction Diodes (pp )
 /9/008 secton3_termnal_characterstcs_of_juncton_odes.doc /6 3. Termnal Characterstcs of Juncton odes (pp.47-53) A Juncton ode I.E., A real dode! Smlar to an deal dode, ts crcut symbol s: HO: The Juncton
/9/008 secton3_termnal_characterstcs_of_juncton_odes.doc /6 3. Termnal Characterstcs of Juncton odes (pp.47-53) A Juncton ode I.E., A real dode! Smlar to an deal dode, ts crcut symbol s: HO: The Juncton
NAME and Section No.
 Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
Chemstry 391 Fall 2007 Exam I KEY (Monday September 17) 1. (25 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). Defne the terms: open system, closed system and solated system
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
1) Silicon oxide has a typical surface potential in an aqueous medium of ϕ,0
 1) Slcon oxde has a typcal surface potental n an aqueous medum of ϕ, = 7 mv n 5 mm l at ph 9. Whch concentraton of catons do you roughly expect close to the surface? What s the average dstance between
1) Slcon oxde has a typcal surface potental n an aqueous medum of ϕ, = 7 mv n 5 mm l at ph 9. Whch concentraton of catons do you roughly expect close to the surface? What s the average dstance between
Problem Set 9 Solutions
 Desgn and Analyss of Algorthms May 4, 2015 Massachusetts Insttute of Technology 6.046J/18.410J Profs. Erk Demane, Srn Devadas, and Nancy Lynch Problem Set 9 Solutons Problem Set 9 Solutons Ths problem
Desgn and Analyss of Algorthms May 4, 2015 Massachusetts Insttute of Technology 6.046J/18.410J Profs. Erk Demane, Srn Devadas, and Nancy Lynch Problem Set 9 Solutons Problem Set 9 Solutons Ths problem
Multicomponent Flows
 Mole Fracton emperature (K) ransport School of Aerospace Engneerng Equatons for Multcomponent Flows Jerry Setzman.2 25.15 2.1.5 CH4 H2O HCO x 1 emperature Methane Flame.1.2.3 Dstance (cm) 15 1 5 ransport
Mole Fracton emperature (K) ransport School of Aerospace Engneerng Equatons for Multcomponent Flows Jerry Setzman.2 25.15 2.1.5 CH4 H2O HCO x 1 emperature Methane Flame.1.2.3 Dstance (cm) 15 1 5 ransport
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
SUMMARY OF STOICHIOMETRIC RELATIONS AND MEASURE OF REACTIONS' PROGRESS AND COMPOSITION FOR MULTIPLE REACTIONS
 UMMAY OF TOICHIOMETIC ELATION AND MEAUE OF EACTION' POGE AND COMPOITION FO MULTIPLE EACTION UPDATED 0/4/03 - AW APPENDIX A. In case of multple reactons t s mportant to fnd the number of ndependent reactons.
UMMAY OF TOICHIOMETIC ELATION AND MEAUE OF EACTION' POGE AND COMPOITION FO MULTIPLE EACTION UPDATED 0/4/03 - AW APPENDIX A. In case of multple reactons t s mportant to fnd the number of ndependent reactons.
Structure and Drive Paul A. Jensen Copyright July 20, 2003
 Structure and Drve Paul A. Jensen Copyrght July 20, 2003 A system s made up of several operatons wth flow passng between them. The structure of the system descrbes the flow paths from nputs to outputs.
Structure and Drve Paul A. Jensen Copyrght July 20, 2003 A system s made up of several operatons wth flow passng between them. The structure of the system descrbes the flow paths from nputs to outputs.
Poisson brackets and canonical transformations
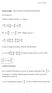 rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
Electrochemistry Thermodynamics
 CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
If two volatile and miscible liquids are combined to form a solution, Raoult s law is not obeyed. Use the experimental data in Table 9.
 9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
Rate of Absorption and Stimulated Emission
 MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
Integrals and Invariants of Euler-Lagrange Equations
 Lecture 16 Integrals and Invarants of Euler-Lagrange Equatons ME 256 at the Indan Insttute of Scence, Bengaluru Varatonal Methods and Structural Optmzaton G. K. Ananthasuresh Professor, Mechancal Engneerng,
Lecture 16 Integrals and Invarants of Euler-Lagrange Equatons ME 256 at the Indan Insttute of Scence, Bengaluru Varatonal Methods and Structural Optmzaton G. K. Ananthasuresh Professor, Mechancal Engneerng,
Be true to your work, your word, and your friend.
 Chemstry 13 NT Be true to your work, your word, and your frend. Henry Davd Thoreau 1 Chem 13 NT Chemcal Equlbrum Module Usng the Equlbrum Constant Interpretng the Equlbrum Constant Predctng the Drecton
Chemstry 13 NT Be true to your work, your word, and your frend. Henry Davd Thoreau 1 Chem 13 NT Chemcal Equlbrum Module Usng the Equlbrum Constant Interpretng the Equlbrum Constant Predctng the Drecton
Estimation of the composition of the liquid and vapor streams exiting a flash unit with a supercritical component
 Department of Energ oltecnco d Mlano Va Lambruschn - 05 MILANO Eercses of Fundamentals of Chemcal rocesses rof. Ganpero Gropp Eercse 8 Estmaton of the composton of the lqud and vapor streams etng a unt
Department of Energ oltecnco d Mlano Va Lambruschn - 05 MILANO Eercses of Fundamentals of Chemcal rocesses rof. Ganpero Gropp Eercse 8 Estmaton of the composton of the lqud and vapor streams etng a unt
Outlet temperature of a WGS reactor (Stage I) for the conversion of CO, applied for the abatement of CO to a fixed value.
 Department of Energy Poltecnco d Mlano Va Lambruschn 56 MILAN Exercses of Fundamentals of Chemcal Processes Prof. Ganpero Gropp Exercse utlet temperature of a WGS reactor (Stage I for the converson of
Department of Energy Poltecnco d Mlano Va Lambruschn 56 MILAN Exercses of Fundamentals of Chemcal Processes Prof. Ganpero Gropp Exercse utlet temperature of a WGS reactor (Stage I for the converson of
Gasometric Determination of NaHCO 3 in a Mixture
 60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
CSci 6974 and ECSE 6966 Math. Tech. for Vision, Graphics and Robotics Lecture 21, April 17, 2006 Estimating A Plane Homography
 CSc 6974 and ECSE 6966 Math. Tech. for Vson, Graphcs and Robotcs Lecture 21, Aprl 17, 2006 Estmatng A Plane Homography Overvew We contnue wth a dscusson of the major ssues, usng estmaton of plane projectve
CSc 6974 and ECSE 6966 Math. Tech. for Vson, Graphcs and Robotcs Lecture 21, Aprl 17, 2006 Estmatng A Plane Homography Overvew We contnue wth a dscusson of the major ssues, usng estmaton of plane projectve
Lecture 10. Reading: Notes and Brennan Chapter 5
 Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Graphical Analysis of a BJT Amplifier
 4/6/2011 A Graphcal Analyss of a BJT Amplfer lecture 1/18 Graphcal Analyss of a BJT Amplfer onsder agan ths smple BJT amplfer: ( t) = + ( t) O O o B + We note that for ths amplfer, the output oltage s
4/6/2011 A Graphcal Analyss of a BJT Amplfer lecture 1/18 Graphcal Analyss of a BJT Amplfer onsder agan ths smple BJT amplfer: ( t) = + ( t) O O o B + We note that for ths amplfer, the output oltage s
Solid state reactions. Often defined as reactions between two solids. Here the definition is extended to any reaction involving a solid:
 Sold state reactons Often defned as reactons between two solds Here the defnton s extended to any reacton nvolvng a sold: Sold/sold Sold/gas (Reacton, decomposton) (Sold/lqud) Intercalaton 1 2 Sold-State
Sold state reactons Often defned as reactons between two solds Here the defnton s extended to any reacton nvolvng a sold: Sold/sold Sold/gas (Reacton, decomposton) (Sold/lqud) Intercalaton 1 2 Sold-State
Difference Equations
 Dfference Equatons c Jan Vrbk 1 Bascs Suppose a sequence of numbers, say a 0,a 1,a,a 3,... s defned by a certan general relatonshp between, say, three consecutve values of the sequence, e.g. a + +3a +1
Dfference Equatons c Jan Vrbk 1 Bascs Suppose a sequence of numbers, say a 0,a 1,a,a 3,... s defned by a certan general relatonshp between, say, three consecutve values of the sequence, e.g. a + +3a +1
PETE 310 Lectures # 24 & 25 Chapter 12 Gas Liquid Equilibrium
 ETE 30 Lectures # 24 & 25 Chapter 2 Gas Lqud Equlbrum Thermal Equlbrum Object A hgh T, Object B low T Intal contact tme Intermedate tme. Later tme Mechancal Equlbrum ressure essels Vale Closed Vale Open
ETE 30 Lectures # 24 & 25 Chapter 2 Gas Lqud Equlbrum Thermal Equlbrum Object A hgh T, Object B low T Intal contact tme Intermedate tme. Later tme Mechancal Equlbrum ressure essels Vale Closed Vale Open
Numerical Heat and Mass Transfer
 Master degree n Mechancal Engneerng Numercal Heat and Mass Transfer 06-Fnte-Dfference Method (One-dmensonal, steady state heat conducton) Fausto Arpno f.arpno@uncas.t Introducton Why we use models and
Master degree n Mechancal Engneerng Numercal Heat and Mass Transfer 06-Fnte-Dfference Method (One-dmensonal, steady state heat conducton) Fausto Arpno f.arpno@uncas.t Introducton Why we use models and
Module 1 : The equation of continuity. Lecture 1: Equation of Continuity
 1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Electrostatic Potential from Transmembrane Currents
 Electrostatc Potental from Transmembrane Currents Let s assume that the current densty j(r, t) s ohmc;.e., lnearly proportonal to the electrc feld E(r, t): j = σ c (r)e (1) wth conductvty σ c = σ c (r).
Electrostatc Potental from Transmembrane Currents Let s assume that the current densty j(r, t) s ohmc;.e., lnearly proportonal to the electrc feld E(r, t): j = σ c (r)e (1) wth conductvty σ c = σ c (r).
Determination of Structure and Formation Conditions of Gas Hydrate by Using TPD Method and Flash Calculations
 nd atonal Iranan Conference on Gas Hydrate (ICGH) Semnan Unersty Determnaton of Structure and Formaton Condtons of Gas Hydrate by Usng TPD Method and Flash Calculatons H. Behat Rad, F. Varamnan* Department
nd atonal Iranan Conference on Gas Hydrate (ICGH) Semnan Unersty Determnaton of Structure and Formaton Condtons of Gas Hydrate by Usng TPD Method and Flash Calculatons H. Behat Rad, F. Varamnan* Department
Chapter 18, Part 1. Fundamentals of Atmospheric Modeling
 Overhead Sldes for Chapter 18, Part 1 of Fundamentals of Atmospherc Modelng by Mark Z. Jacobson Department of Cvl & Envronmental Engneerng Stanford Unversty Stanford, CA 94305-4020 January 30, 2002 Types
Overhead Sldes for Chapter 18, Part 1 of Fundamentals of Atmospherc Modelng by Mark Z. Jacobson Department of Cvl & Envronmental Engneerng Stanford Unversty Stanford, CA 94305-4020 January 30, 2002 Types
THE CURRENT BALANCE Physics 258/259
 DSH 1988, 005 THE CURRENT BALANCE Physcs 58/59 The tme average force between two parallel conductors carryng an alternatng current s measured by balancng ths force aganst the gravtatonal force on a set
DSH 1988, 005 THE CURRENT BALANCE Physcs 58/59 The tme average force between two parallel conductors carryng an alternatng current s measured by balancng ths force aganst the gravtatonal force on a set
Prof. Dr. I. Nasser Phys 630, T Aug-15 One_dimensional_Ising_Model
 EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
2E Pattern Recognition Solutions to Introduction to Pattern Recognition, Chapter 2: Bayesian pattern classification
 E395 - Pattern Recognton Solutons to Introducton to Pattern Recognton, Chapter : Bayesan pattern classfcaton Preface Ths document s a soluton manual for selected exercses from Introducton to Pattern Recognton
E395 - Pattern Recognton Solutons to Introducton to Pattern Recognton, Chapter : Bayesan pattern classfcaton Preface Ths document s a soluton manual for selected exercses from Introducton to Pattern Recognton
V T for n & P = constant
 Pchem 365: hermodynamcs -SUMMARY- Uwe Burghaus, Fargo, 5 9 Mnmum requrements for underneath of your pllow. However, wrte your own summary! You need to know the story behnd the equatons : Pressure : olume
Pchem 365: hermodynamcs -SUMMARY- Uwe Burghaus, Fargo, 5 9 Mnmum requrements for underneath of your pllow. However, wrte your own summary! You need to know the story behnd the equatons : Pressure : olume
Amplification and Relaxation of Electron Spin Polarization in Semiconductor Devices
 Amplfcaton and Relaxaton of Electron Spn Polarzaton n Semconductor Devces Yury V. Pershn and Vladmr Prvman Center for Quantum Devce Technology, Clarkson Unversty, Potsdam, New York 13699-570, USA Spn Relaxaton
Amplfcaton and Relaxaton of Electron Spn Polarzaton n Semconductor Devces Yury V. Pershn and Vladmr Prvman Center for Quantum Devce Technology, Clarkson Unversty, Potsdam, New York 13699-570, USA Spn Relaxaton
THE SUMMATION NOTATION Ʃ
 Sngle Subscrpt otaton THE SUMMATIO OTATIO Ʃ Most of the calculatons we perform n statstcs are repettve operatons on lsts of numbers. For example, we compute the sum of a set of numbers, or the sum of the
Sngle Subscrpt otaton THE SUMMATIO OTATIO Ʃ Most of the calculatons we perform n statstcs are repettve operatons on lsts of numbers. For example, we compute the sum of a set of numbers, or the sum of the
COMPOSITE BEAM WITH WEAK SHEAR CONNECTION SUBJECTED TO THERMAL LOAD
 COMPOSITE BEAM WITH WEAK SHEAR CONNECTION SUBJECTED TO THERMAL LOAD Ákos Jósef Lengyel, István Ecsed Assstant Lecturer, Professor of Mechancs, Insttute of Appled Mechancs, Unversty of Mskolc, Mskolc-Egyetemváros,
COMPOSITE BEAM WITH WEAK SHEAR CONNECTION SUBJECTED TO THERMAL LOAD Ákos Jósef Lengyel, István Ecsed Assstant Lecturer, Professor of Mechancs, Insttute of Appled Mechancs, Unversty of Mskolc, Mskolc-Egyetemváros,
Entropy generation in a chemical reaction
 Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
CHAPTER 14 GENERAL PERTURBATION THEORY
 CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
Linear Momentum. Equation 1
 Lnear Momentum OBJECTIVE Obsere collsons between two carts, testng or the conseraton o momentum. Measure energy changes durng derent types o collsons. Classy collsons as elastc, nelastc, or completely
Lnear Momentum OBJECTIVE Obsere collsons between two carts, testng or the conseraton o momentum. Measure energy changes durng derent types o collsons. Classy collsons as elastc, nelastc, or completely
For now, let us focus on a specific model of neurons. These are simplified from reality but can achieve remarkable results.
 Neural Networks : Dervaton compled by Alvn Wan from Professor Jtendra Malk s lecture Ths type of computaton s called deep learnng and s the most popular method for many problems, such as computer vson
Neural Networks : Dervaton compled by Alvn Wan from Professor Jtendra Malk s lecture Ths type of computaton s called deep learnng and s the most popular method for many problems, such as computer vson
The Order Relation and Trace Inequalities for. Hermitian Operators
 Internatonal Mathematcal Forum, Vol 3, 08, no, 507-57 HIKARI Ltd, wwwm-hkarcom https://doorg/0988/mf088055 The Order Relaton and Trace Inequaltes for Hermtan Operators Y Huang School of Informaton Scence
Internatonal Mathematcal Forum, Vol 3, 08, no, 507-57 HIKARI Ltd, wwwm-hkarcom https://doorg/0988/mf088055 The Order Relaton and Trace Inequaltes for Hermtan Operators Y Huang School of Informaton Scence
Econ107 Applied Econometrics Topic 3: Classical Model (Studenmund, Chapter 4)
 I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
Electrochemistry High Temperature Concepts
 Electrochemstry Hgh Temperature Concepts Ngel Sammes POSTECH South Korea nsammes@postech.ac.kr 2 nd Jont European Summer School on Fuel Cell and Hydrogen Technology OK Let s Get Started Introducton to
Electrochemstry Hgh Temperature Concepts Ngel Sammes POSTECH South Korea nsammes@postech.ac.kr 2 nd Jont European Summer School on Fuel Cell and Hydrogen Technology OK Let s Get Started Introducton to
Lectures - Week 4 Matrix norms, Conditioning, Vector Spaces, Linear Independence, Spanning sets and Basis, Null space and Range of a Matrix
 Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
THE CHINESE REMAINDER THEOREM. We should thank the Chinese for their wonderful remainder theorem. Glenn Stevens
 THE CHINESE REMAINDER THEOREM KEITH CONRAD We should thank the Chnese for ther wonderful remander theorem. Glenn Stevens 1. Introducton The Chnese remander theorem says we can unquely solve any par of
THE CHINESE REMAINDER THEOREM KEITH CONRAD We should thank the Chnese for ther wonderful remander theorem. Glenn Stevens 1. Introducton The Chnese remander theorem says we can unquely solve any par of
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Gouy-Chapman model (1910) The double layer is not as compact as in Helmholtz rigid layer.
 CHE465/865, 6-3, Lecture 1, 7 nd Sep., 6 Gouy-Chapman model (191) The double layer s not as compact as n Helmholtz rgd layer. Consder thermal motons of ons: Tendency to ncrease the entropy and make the
CHE465/865, 6-3, Lecture 1, 7 nd Sep., 6 Gouy-Chapman model (191) The double layer s not as compact as n Helmholtz rgd layer. Consder thermal motons of ons: Tendency to ncrease the entropy and make the
CHAPTER 13. Exercises. E13.1 The emitter current is given by the Shockley equation:
 HPT 3 xercses 3. The emtter current s gen by the Shockley equaton: S exp VT For operaton wth, we hae exp >> S >>, and we can wrte VT S exp VT Solng for, we hae 3. 0 6ln 78.4 mv 0 0.784 5 4.86 V VT ln 4
HPT 3 xercses 3. The emtter current s gen by the Shockley equaton: S exp VT For operaton wth, we hae exp >> S >>, and we can wrte VT S exp VT Solng for, we hae 3. 0 6ln 78.4 mv 0 0.784 5 4.86 V VT ln 4
Chapter 9: Statistical Inference and the Relationship between Two Variables
 Chapter 9: Statstcal Inference and the Relatonshp between Two Varables Key Words The Regresson Model The Sample Regresson Equaton The Pearson Correlaton Coeffcent Learnng Outcomes After studyng ths chapter,
Chapter 9: Statstcal Inference and the Relatonshp between Two Varables Key Words The Regresson Model The Sample Regresson Equaton The Pearson Correlaton Coeffcent Learnng Outcomes After studyng ths chapter,
The Multiple Classical Linear Regression Model (CLRM): Specification and Assumptions. 1. Introduction
 ECONOMICS 5* -- NOTE (Summary) ECON 5* -- NOTE The Multple Classcal Lnear Regresson Model (CLRM): Specfcaton and Assumptons. Introducton CLRM stands for the Classcal Lnear Regresson Model. The CLRM s also
ECONOMICS 5* -- NOTE (Summary) ECON 5* -- NOTE The Multple Classcal Lnear Regresson Model (CLRM): Specfcaton and Assumptons. Introducton CLRM stands for the Classcal Lnear Regresson Model. The CLRM s also
1. Mean-Field Theory. 2. Bjerrum length
 1. Mean-Feld Theory Contnuum models lke the Posson-Nernst-Planck equatons are mean-feld approxmatons whch descrbe how dscrete ons are affected by the mean concentratons c and potental φ. Each on mgrates
1. Mean-Feld Theory Contnuum models lke the Posson-Nernst-Planck equatons are mean-feld approxmatons whch descrbe how dscrete ons are affected by the mean concentratons c and potental φ. Each on mgrates
,..., k N. , k 2. ,..., k i. The derivative with respect to temperature T is calculated by using the chain rule: & ( (5) dj j dt = "J j. k i.
 Suppleentary Materal Dervaton of Eq. 1a. Assue j s a functon of the rate constants for the N coponent reactons: j j (k 1,,..., k,..., k N ( The dervatve wth respect to teperature T s calculated by usng
Suppleentary Materal Dervaton of Eq. 1a. Assue j s a functon of the rate constants for the N coponent reactons: j j (k 1,,..., k,..., k N ( The dervatve wth respect to teperature T s calculated by usng
Department of Statistics University of Toronto STA305H1S / 1004 HS Design and Analysis of Experiments Term Test - Winter Solution
 Department of Statstcs Unversty of Toronto STA35HS / HS Desgn and Analyss of Experments Term Test - Wnter - Soluton February, Last Name: Frst Name: Student Number: Instructons: Tme: hours. Ads: a non-programmable
Department of Statstcs Unversty of Toronto STA35HS / HS Desgn and Analyss of Experments Term Test - Wnter - Soluton February, Last Name: Frst Name: Student Number: Instructons: Tme: hours. Ads: a non-programmable
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
matter consists, measured in coulombs (C) 1 C of charge requires electrons Law of conservation of charge: charge cannot be created or
 Basc Concepts Oerew SI Prefxes Defntons: Current, Voltage, Power, & Energy Passe sgn conenton Crcut elements Ideal s Portland State Unersty ECE 221 Basc Concepts Ver. 1.24 1 Crcut Analyss: Introducton
Basc Concepts Oerew SI Prefxes Defntons: Current, Voltage, Power, & Energy Passe sgn conenton Crcut elements Ideal s Portland State Unersty ECE 221 Basc Concepts Ver. 1.24 1 Crcut Analyss: Introducton
Name ID # For relatively dilute aqueous solutions the molality and molarity are approximately equal.
 Name ID # 1 CHEMISTRY 212, Lect. Sect. 002 Dr. G. L. Roberts Exam #1/Sprng 2000 Thursday, February 24, 2000 CLOSED BOOK EXM No notes or books allowed. Calculators may be used. tomc masses of nterest are
Name ID # 1 CHEMISTRY 212, Lect. Sect. 002 Dr. G. L. Roberts Exam #1/Sprng 2000 Thursday, February 24, 2000 CLOSED BOOK EXM No notes or books allowed. Calculators may be used. tomc masses of nterest are
Assignment 4. Adsorption Isotherms
 Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
= z 20 z n. (k 20) + 4 z k = 4
 Problem Set #7 solutons 7.2.. (a Fnd the coeffcent of z k n (z + z 5 + z 6 + z 7 + 5, k 20. We use the known seres expanson ( n+l ( z l l z n below: (z + z 5 + z 6 + z 7 + 5 (z 5 ( + z + z 2 + z + 5 5
Problem Set #7 solutons 7.2.. (a Fnd the coeffcent of z k n (z + z 5 + z 6 + z 7 + 5, k 20. We use the known seres expanson ( n+l ( z l l z n below: (z + z 5 + z 6 + z 7 + 5 (z 5 ( + z + z 2 + z + 5 5
8.6 The Complex Number System
 8.6 The Complex Number System Earler n the chapter, we mentoned that we cannot have a negatve under a square root, snce the square of any postve or negatve number s always postve. In ths secton we want
8.6 The Complex Number System Earler n the chapter, we mentoned that we cannot have a negatve under a square root, snce the square of any postve or negatve number s always postve. In ths secton we want
Robert Eisberg Second edition CH 09 Multielectron atoms ground states and x-ray excitations
 Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
6.01: Introduction to EECS I Lecture 7 March 15, 2011
 6.0: Introducton to EECS I Lecture 7 March 5, 20 6.0: Introducton to EECS I Crcuts The Crcut Abstracton Crcuts represent systems as connectons of elements through whch currents (through arables) flow and
6.0: Introducton to EECS I Lecture 7 March 5, 20 6.0: Introducton to EECS I Crcuts The Crcut Abstracton Crcuts represent systems as connectons of elements through whch currents (through arables) flow and
Exercises of Fundamentals of Chemical Processes
 Department of Energ Poltecnco d Mlano a Lambruschn 4 2056 MILANO Exercses of undamentals of Chemcal Processes Prof. Ganpero Gropp Exercse 7 ) Estmaton of the composton of the streams at the ext of an sothermal
Department of Energ Poltecnco d Mlano a Lambruschn 4 2056 MILANO Exercses of undamentals of Chemcal Processes Prof. Ganpero Gropp Exercse 7 ) Estmaton of the composton of the streams at the ext of an sothermal
8.592J: Solutions for Assignment 7 Spring 2005
 8.59J: Solutons for Assgnment 7 Sprng 5 Problem 1 (a) A flament of length l can be created by addton of a monomer to one of length l 1 (at rate a) or removal of a monomer from a flament of length l + 1
8.59J: Solutons for Assgnment 7 Sprng 5 Problem 1 (a) A flament of length l can be created by addton of a monomer to one of length l 1 (at rate a) or removal of a monomer from a flament of length l + 1
Energy Storage Elements: Capacitors and Inductors
 CHAPTER 6 Energy Storage Elements: Capactors and Inductors To ths pont n our study of electronc crcuts, tme has not been mportant. The analyss and desgns we hae performed so far hae been statc, and all
CHAPTER 6 Energy Storage Elements: Capactors and Inductors To ths pont n our study of electronc crcuts, tme has not been mportant. The analyss and desgns we hae performed so far hae been statc, and all
CHEM 112 Exam 3 Practice Test Solutions
 CHEM 11 Exam 3 Practce Test Solutons 1A No matter what temperature the reacton takes place, the product of [OH-] x [H+] wll always equal the value of w. Therefore, f you take the square root of the gven
CHEM 11 Exam 3 Practce Test Solutons 1A No matter what temperature the reacton takes place, the product of [OH-] x [H+] wll always equal the value of w. Therefore, f you take the square root of the gven
Lagrange Multipliers. A Somewhat Silly Example. Monday, 25 September 2013
 Lagrange Multplers Monday, 5 September 013 Sometmes t s convenent to use redundant coordnates, and to effect the varaton of the acton consstent wth the constrants va the method of Lagrange undetermned
Lagrange Multplers Monday, 5 September 013 Sometmes t s convenent to use redundant coordnates, and to effect the varaton of the acton consstent wth the constrants va the method of Lagrange undetermned
4.1 The Ideal Diode. Reading Assignment: pp Before we get started with ideal diodes, let s first recall linear device behavior!
 1/25/2012 secton3_1the_ideal_ode 1/2 4.1 The Ideal ode Readng Assgnment: pp.165-172 Before we get started wth deal dodes, let s frst recall lnear dece behaor! HO: LINEAR EVICE BEHAVIOR Now, the deal dode
1/25/2012 secton3_1the_ideal_ode 1/2 4.1 The Ideal ode Readng Assgnment: pp.165-172 Before we get started wth deal dodes, let s frst recall lnear dece behaor! HO: LINEAR EVICE BEHAVIOR Now, the deal dode
Canonical transformations
 Canoncal transformatons November 23, 2014 Recall that we have defned a symplectc transformaton to be any lnear transformaton M A B leavng the symplectc form nvarant, Ω AB M A CM B DΩ CD Coordnate transformatons,
Canoncal transformatons November 23, 2014 Recall that we have defned a symplectc transformaton to be any lnear transformaton M A B leavng the symplectc form nvarant, Ω AB M A CM B DΩ CD Coordnate transformatons,
Name: SID: Discussion Session:
 Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
Name: SID: Dscusson Sesson: Chemcal Engneerng Thermodynamcs 141 -- Fall 007 Thursday, November 15, 007 Mdterm II SOLUTIONS - 70 mnutes 110 Ponts Total Closed Book and Notes (0 ponts) 1. Evaluate whether
10.34 Numerical Methods Applied to Chemical Engineering Fall Homework #3: Systems of Nonlinear Equations and Optimization
 10.34 Numercal Methods Appled to Chemcal Engneerng Fall 2015 Homework #3: Systems of Nonlnear Equatons and Optmzaton Problem 1 (30 ponts). A (homogeneous) azeotrope s a composton of a multcomponent mxture
10.34 Numercal Methods Appled to Chemcal Engneerng Fall 2015 Homework #3: Systems of Nonlnear Equatons and Optmzaton Problem 1 (30 ponts). A (homogeneous) azeotrope s a composton of a multcomponent mxture
KJM5120 and KJM9120 Defects and Reactions
 KJM510 and KJM910 Defects and Reactions Truls Norby Ch. Defect reactions Department of Chemistry University of slo Centre for Materials Science and Nanotechnology (SMN) = v / 1 ) + + e ( g ) FERMI slo
KJM510 and KJM910 Defects and Reactions Truls Norby Ch. Defect reactions Department of Chemistry University of slo Centre for Materials Science and Nanotechnology (SMN) = v / 1 ) + + e ( g ) FERMI slo
Thermodynamics II. Department of Chemical Engineering. Prof. Kim, Jong Hak
 Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
Thermodynamcs II Department of Chemcal Engneerng Prof. Km, Jong Hak Soluton Thermodynamcs : theory Obectve : lay the theoretcal foundaton for applcatons of thermodynamcs to gas mxture and lqud soluton
Generalized Linear Methods
 Generalzed Lnear Methods 1 Introducton In the Ensemble Methods the general dea s that usng a combnaton of several weak learner one could make a better learner. More formally, assume that we have a set
Generalzed Lnear Methods 1 Introducton In the Ensemble Methods the general dea s that usng a combnaton of several weak learner one could make a better learner. More formally, assume that we have a set
