Controlled FTIR measurements of acid gas (NO, mixed NO/SO 2 ) capture in water at high pressure
|
|
|
- Randolph Charles
- 5 years ago
- Views:
Transcription
1 ANLECR&D project Controlled FTIR measurements of acid gas (NO, mixed NO/SO 2 ) capture in water at high pressure Sub contract to Macquarie University Report of ANLECR&D Project on: Products formed from gas impurities in oxyfuel derived CO 2 compression: Emissions on depressurisation, stability, disposal and utilisation potential By Peter F Nelson 1, Rohan Stanger 2 and Terry Wall 2 1 Department of Environmental Sciences, Macquarie University, NSW Australia 2 Chemical Engineering, the University of Newcastle, NSW, Australia March
2 Executive Summary Additional experiments made on the UoN small pressure rig were undertaken with NO x and mixed NO+SO x injection using a combined gas analysis system (FTIR/NO x /N 2 O). This complementary work was made because industrial NO x analysers do not differentiate NO 2 from other forms of NO x (HONO, HNO 3, N 2 O). The intention was to provide tightly controlled gas flows to enable the FTIR identification and quantification of other species formed during the compression process. It was found that the variable amounts of water vapour had an over riding influence on baseline FTIR measurements which required specific deviations from previous methods. Overall, this work has identified N 2 O being formed during the injection of a mixed acid gas stream in similar quantities to those measured by the dedicated N 2 O analyser. It has also confirmed the presence of HONO released during de gassing of the liquid phase from a 25 bar condition. However, the trace levels (~1.3ppm) indicate that the measured NO and NO 2 traces are likely real gas concentrations evolved from desorbing out of the depressurised liquid. This also suggests that the 1 hour residence time at 25 bar is sufficient to oxidise dissolved HONO formed in the NO x absorption process. The FTIR also confirmed the presence of HNO 3 desorbing out the back sections of the reactor at concentrations similar to those measured with the NO x analyser. The presence of HNO 3 represents a potential safety hazard during maintenance of an oxyfuel compression circuit. 2
3 Introduction The Callide Oxyfuel Project CO 2 Processing Unit (COP CPU) was designed to remove SO x at atmospheric pressure using a 2 column caustic scrubbing unit (shown as Scrubber 1 in Figure 1). The removal of NO x occurred at higher pressure during compression and in a high pressure column (Scrubber 2 in Figure 1). However, other gas vendors have proposed alternative circuits involving co removal of SO x and NO x at high pressure as a method of improving overall capture and reducing CPU footprint. Air Products Sour Gas Compression process (Figure 2) involves high pressure co removal of SO x and NO x in a 2 column system operated at 15 and 30 bar, respectively. The application of this process is suited to Australia, as both NO x and SO x are present at reasonable levels in flue gas in Australian coal fired power stations, and their co removal avoids the cost of the addition of separate SO x removal technologies. Capture at higher pressure also provides a method of reducing overall unit size and thus capital requirements. Notionally, the presence of NO 2 also acts as an oxidiser for SO 2, providing greater SO x capture. This mechanism is based on the lead chamber process used in sulfuric acid manufacturing. However, the co capture of SO x and NO x at atmospheric pressure has been linked with the formation of N 2 O a potent greenhouse gas equivalent to 300 the impact of CO 2. The current work extends this finding to high pressures. The UoN has previously reported on the formation of N 2 O during co removal of mixed acid streams on both the small pressure system and the bench scale compression unit [2]. In that report the presence of N 2 O was measured using a dedicated industrial N 2 O analyser. This work reports on additional experiments made on the small pressure system which included the use of an FTIR spectrometer to alternatively verify the presence of N 2 O and estimate its concentration. This combined analytical approach was also extended to NO x only measurements (ie no SO 2 injected) made under controlled conditions to quantify other nitrogen containing species present in various parts of the experimental system. Identification and quantification of nitrogen containing species observed in experiments at the University of Newcastle at high pressure from the compression of oxyfuel gas were described in our previous report [3]. Importantly, the use of an industrial chemiluminescent NO x analyser measures the presence of NO and by difference through a convertor Total NO x. Typically, the difference between NO and Total NO x is attributed to NO 2. However, our research has suggested that this measurement may also include other nitrogen containing species HNO 2, HNO 3 and N 2 O, depending on the experimental conditions. Here we report additional laboratory FTIR measurements to the presence of nitrous oxide (N 2 O) in the products of reactants comprising 1000 ppm NO, 1000 ppm SO 2, at 5 bar, and in the presence of water. In addition further experiments which attempted to identify water soluble de gassing products were also undertaken. 3
4 Figure 1. Air Liquide s CPU design for the Callide Oxyfuel Process with atmospheric scrubbing of SO 2 followed by high pressure removal of NO x. Figure 2. Air Products Sour Gas Compression process with co removal of SO x and NO x at high pressure using two columns at 15 and 30 bar respectively (taken from [4]). The mechanisms of NO x transformations and capture have been studied extensively by the UoN, in part as they relate to mercury capture prior to the brazed aluminium heat exchanger in the COP CPU. Figure 3 shows a summary of these processes presented in an invited webinar by the Global CCS Institute in Importantly, it shows the challenge of obtaining a nitrogen mass balance and the need for different experimental measurements to capture these impacts. 4
5 NO NO 2 Figure 3. High pressure mechanisms of NOx and mercury capture during oxyfuel flue gas compression showing capture in condensates (as stable nitrites) and as acid condensation. Dissolved gases may potentially be an emission when condensates are depressurised, as indicated by the experimental evidence. Taken from [5]. Experimental The small pressurised reactor used at the UoN for COP CPU mechanistic studies has three modes of experimental operation (Figure 4); these being (1) Absorption of NO x into liquid water at high pressure; (2) de gassing of the liquid after the experiment and (3) desorption of condensed acids from the back end of the reactor after the experiment. (1) Absorption by liquid (2) De gassing of liquid (3) Desorption of condensed acid NO x (+SO 2 ) N 2 5% O 2 NO x (from surfaces) H 2 O H 2 O bypassed Air NO x (from liquid) Figure 4. Diagram showing the modes of experimental operation showing (a) absorption of NO x (and/or SO x ) into liquid water at pressures; (b) de gassing of volatile NO x species out of the liquid after absorption and (c) desorption of NO x species from the non wetted surfaces after the reactor (bypassing liquid). 5
6 Mode 1 (absorption) involves the initial contacting of NO x gases with liquid water in which capture occurs through the gas phase oxidation of NO to NO 2 and the subsequent absorption of soluble NO 2 as a mixed acid (HNO 2 /HNO 3 ). Higher pressure has been shown to increase the oxidation of NO and improve overall NO x capture. Previous mixed acid work has shown that the addition of SO 2 injected into the small pressure system results in the formation of N 2 O at 5 and 25 bar to different extents. This work replicates those experiments at 5 bar with FTIR measurements as verification. Mode 2 (de gassing) involved the controlled removal of the liquid after absorption of NO x only (over ~1 hour at 25 bar). The liquid was dropped out in 40 50mL aliquots into a sealed vessel with a constant flow of air at 3LPM. Dissolved gases present in the liquid were then allowed to de gas over 1 hour before the next aliquot was taken. It was hypothesized that the absorption of NO 2 into a mixture of HNO 2 /HNO 3 would result in a build up of HNO 2 dissolved in the liquid phase (HNO 2 being fully ionised). The FTIR measurements taken during this period were averaged over several minutes to obtain sufficient signal. This is important as much of the dissolved gases were observed to be removed over the course of several minutes and hence much of the transient changes are averaged out by the FTIR. Mode 3 (desorption) involved bypassing the liquid section of the reactor and depressurising the transport lines. This allowed for the dissolved gases to be contained in the reactor while the backsections of the system could be flushed. Importantly, this mode of analysis has previously measured a large peak in NO 2 (of the order of several 1000ppm) over the course of several minutes. It was hypothesized that the gas phase formation of HNO 3 in the back sections of the system could occur because of the presence of water vapour derived from the reactor. Once condensed onto the surface, the lack of a liquid water phase could allow the HNO 3 to retain sufficient vapour pressure to be evaporated once depressurised. The FTIR experiments were taken after a 1 hour absorption of NO x only injection at 5 bar. FTIR Operation The FTIR spectra in this series of experiments, performed in November 2014, were collected under the same conditions as previous NO x only experiments. The outlet flow of the high pressure batch reactor at atmospheric pressure (ie after reduction of the reactor pressure) was connected to a Nicolet 6700 FTIR. The FTIR cell was operated in flow through mode (the flow was determined by the conditions of the experiment) in order to minimally perturb the concentrations of reactive nitrogen species measured in the products and to avoid the condensation/ disappearance of these species. Specific to these experiments was the need to provide the FTIR analyser with a sample gas with constant H 2 O vapour. This required a minor change to previously reported work, where the experiment was started by bubbling N 2 only through the pressurised system. Once a H 2 O baseline was established the NO and SO 2 gases were turned on through custom designed Mass Flow Controller (MFC) software. A folded beam gas cell with a total path length of 2.4 meters (Infrared Analysis Inc., Model 2.4 H) was custom fitted to the FTIR. The FTIR was operated using a liquid nitrogen cooled MCT/A detector with the following beam parameters: 128 scans were co added to obtain spectra at 0.25 cm 1 resolution with a scanning range between cm 1. Spectra were recorded using FTIR software (Thermo Scientific Nicolet OmnicTM). 6
7 Peak identity was confirmed and concentrations estimated by comparison with standard spectra provided by a Database of 386 Digitized Quantitative Gas Phase Reference Spectra (Infrared Analysis, Inc QASOFT Package 4.0). Results (Mode 1) Confirmation of nitrous oxide (N 2 O) formation from mixed acid gas (SO2/NO) injection This experiment was performed 18/11/2014 under the following conditions: flow 3 SLPM, 1000 ppm NO, 1000 ppm SO 2, 5 bar, and 150 ml water in the bubble reactor. Figure 5 shows FTIR spectra for the frequency range of cm 1 at three times after the commencement of this experiment, and also for the feed gas. All show the characteristic features for gas phase spectra of N 2 O. The feed gas for this experiment shows small amounts of N 2 O (~10 ppm) probably as an impurity in the NO gas used. Each of the product gas spectra show significantly enhanced concentrations of N 2 O: 200 ppm about 2 3 minutes after the commencement of the experiment, increasing to 240 ppm once steady state conditions had been reached. This matches measurements of N 2 O made in UoN Milestone 4.1 report (with a dedicated N 2 O analyser) which showed a peak N 2 O concentration of 230ppm with the same feed conditions. The observation of N 2 O formation under these conditions is not unexpected given previous research [6] which showed significant amounts of N 2 O formed from coal combustion gases containing similar amounts of NO x and SO 2. As N 2 O has a global warming potential times that of CO 2 for a 100 year timescale, the formation and fate of this species in full scale oxy combustion systems requires investigation and evaluation. 7
8 0.4 Absorbance a b c d Wavenumber (cm -1 ) Figure 5: FTIR spectra of the region of N 2 O absorbance for feed and product gas from 5 bar experiment in University of Newcastle pressure reactor. (a) product gas spectrum 2 3 minutes after commencement of flow; (b) & (c) product gas spectra under steady state conditions. (d) feed gas spectrum with N 2 made prior to acid gas injection into 150 ml water in bubble reactor, showing trace N 2 O impurity. 8
9 (Mode 2) Species observed during de gassing of liquid water after NOx only absorption at 25 bar This experiment was based on replicating previous NO x only measurements made on the small UoN pressure system. Initially, experiments with the FTIR attached were made to confirm the presence of other NO x species (Nelson and Bray, 2015). In replicating the work, a more controlled set of measurements could be made to quantify the concentrations of these other NO x species, specifically HONO and HNO 3. Spectra were collected at a range of times after a 25 bar experiment with NO injected at 1000 ppm, O 2 set to 5% and collection of potential products in liquid water. Specific to this experiment was the addition of liquid water in the degassing vessel, to ensure that the H 2 O vapour was constant. The pressurised liquids were dropped out into a smaller beaker sitting in this water bath. Figure 6 shows the FTIR product gas spectra from this experiment. Trace concentrations of N 2 O of ~1.3ppm are observed but no nitric acid (compare the large amounts of nitric acid observed in Figure 19 of our previous report [3]). The NO and NO 2 measurements taken with the NO x analyser showed that NO 2 reached a peak of 35ppm during these de gassing experiments. This suggests that the high pressure conditions produced a relatively stable liquid. Previous work had confirmed the presence of HONO (ie nitrous, sometimes written as HNO 2 ) during volatile release experiments, leading to the conclusion that it was the main volatile species released during de gassing. However, these controlled experiments have allowed quantification of HONO concentration at trace levels. This suggests that the main volatile species evolved from high pressure liquids are in fact dissolved gases NO and NO 2. 5 Absorbance Wavenumber (cm -1 ) Figure 6: Product gas spectrum in region of HONO and HNO 3 absorbance from collected water experiment; features due to trace HONO (~1.3ppm) observed but no HNO 3. Figure shows the spectra at intervals after the commencement of the desorption phase from the collected water. These spectra are complicated by the very large absorption due to gas phase water but Figure (a) and (b) show evidence of very broad absorption. It is not clear what species might be responsible for these features but water condensing in the FTIR cell is one possibility. 9
10 a 2.0 Absorbance b c Wavenumber (cm -1 ) Figure 7: Spectra collected from collected water during desorption. Sharp lines due to gas phase water. (a) to (c) spectra collected at intervals of ~5 minutes at increasing times after commencement of desorption 10
11 (Mode 3) Species observed during acid desorption test after NO x injection at 5 bar As with Mode 2, these experiments were designed to replicate previous NO x only measurements. The acid desorption at the end of the NO x injection experiments has previously been observed as a NO 2 peak well above the measurement range of the NO x analyser. It was theorised that this material was HNO 3 produced in transport lines downstream of the pressure vessel (in the presence of H 2 O vapour) which was subsequently desorbed into the gas phase. In this experiment, the NO 2 was observed to peak above 500ppm, then drop to 20ppm within 20 minutes. A smaller secondary peak was then observed to occur over ~45 minutes. The purpose of these measurements was to identify that this species is HNO 3 and quantify its concentration. Figure shows spectra obtained during acid desorption. The time elapsed from the beginning of these spectral collections increase in approximately 20 minute intervals from Figure 8(a) to (b) to (c). The fine lines are due to gas phase water and these decrease in intensity with time after the commencement of the desorption. The spectra also include features due to gas phase nitric acid which vary in intensity but which are present at all three times. The identity of the features as due to nitric acid was confirmed by removing the lines due to water; the resulting spectrum is presented as Figure, with peaks at 1710, 1304, and 879 cm 1 confirming the identity of the nitric acid, HNO 3 at a concentration of ~55ppm. The large negative peaks are due to NO and NO 2 in the subtraction spectrum used. TABLE 1. Summary of FTIR experiments and findings FTIR Experiments Inputs Output Comment Mode 1 absorption Present in the reactor (on page 8) exit gas: NO 1000 ppm SO ppm O 2 5% H 2 O added as 150mL liquid (batch) 240 ppm N 2 O (from FTIR) Specific to Air Products Sour Gas Compression process (with presence of SO 2 ) Pressure 5 bar Residual SO 2, NO, NO 2 Mode 2 degassing of liquid after absorption (on page 9 10) NO 1000 ppm SO 2 nil O 2 5% H 2 O added as 150mL liquid (batch) Pressure 25 bar De gassing: Air 3 LPM H 2 O vapour from reactor Present in liquid off gas during degassing: Simulating conditions of the high pressure condensate removal stage at the COP CPU (after cooler), where liquids exit the system Mode 3 desorption of condensed acid (on page 12 13) NO 1000 ppm SO 2 nil O 2 5% H 2 O sat vapour after reactor Pressure 5 bar NO 2 Trace HONO No HNO 3 Present in the desorbing gas stream: Simulating conditions after the lower pressure condensate removal at the COP CPU (intercooler), where water vapour and NOx gases are present Desorption: 5% O 2 in N 2 HNO 3 11
12 a 2.0 Absorbance b c Wavenumber (cm -1 ) Figure 8: FTIR spectra of desorption gas with time from commencement of desorption increasing in approximately 20 minute intervals from (a) to (b) to (c) 12
13 cm cm 1 879cm 1 Absorbance Wavenumber (cm -1 ) 1000 Figure 9: FTIR Difference spectra after subtraction of water lines from acid desorption experiment (from Figure 4b above). Peaks at 1710, 1304, and 879 cm 1 confirm the identity of HNO 3 at a concentration of ~55ppm. 800 Conclusions Additional experiments made on the UoN small pressure rig were undertaken with NO x and mixed NO+SO x injection using a combined gas analysis system (FTIR/NO x /N 2 O) with Table 1 summarising the experiments. It was found that the variable amounts of water vapour had an over riding influence on baseline FTIR measurements which required experimental deviations from previous methods. Overall, this work has identified N 2 O being formed during the injection of a mixed acid gas stream in similar quantities to those measured by the dedicated N 2 O analyser. It has also confirmed the presence of HONO at trace levels when released during de gassing of the liquid phase from the 25 bar condition. This indicates that the measured NO and NO 2 traces are likely real gas concentrations evolved from desorbing out of the depressurised liquid. This also suggests that the 1 hour residence time at 25 bar is sufficient to oxidise dissolved HONO formed in the NOx absorption process. The FTIR also confirmed the presence of HNO 3 desorbing out the back sections of the reactor at concentrations similar to those measured with the NO x analyser. This indicates that for this specific experimental condition the NO 2 trace measured with the NO x analyser is reflective of real HNO 3 concentrations. The presence of high HNO 3 concentration in the gas phase represents a significant safety hazard during maintenance and unplanned shut downs of the high pressure circuit. It also confirms the previous theory on potential avenues for NOx capture in an oxyfuel compression system. 13
14 References 1. Spero, C., Callide Oxyfuel Project Lessons Learned, Report to the Global CCS Institute, oxyfuel project lessons learned, accessed, 14/09/ Stanger, R. and T. Wall, ANLECR&D project Milestone Report 4.1 Products formed from gas impurities in oxyfuel derived CO2 compression: Emissions on depressurisation, stability, disposal and utilisation potential March Nelson, P.F. and P.S. Bray, Formation of nitrogen containing gases under high pressure during compression of OxyFuel gas. ANLECR&D PROJECT : Gas quality impacts, assessment and control in oxy fuel technology for CCS: Part 2. Mercury removal with SO3 in the fabric filter and with NOx as liquids in CO2 compression. 2015, Report of a subcontract from the University of Newcastke to Macquarie University. p Li, H., et al., Gas quality control in oxy pf technology for carbon capture and storage a literature review, Report to Xstrata Coal Low Emissions Research and Development Corporation Wall, T. and R. Stanger, Gas quality impacts, assessment and control in oxy fuel and Gas quality impacts, assessment and control in oxy fuel: part 1, webinar to Global CCS Insitute, 21 July Linak, W.P., et al., Nitrous oxide emissions from fossil fuel combustion. Journal of Geophysical Research: Atmospheres, (D6): p
SO x /NO x co-removal during compression of oxy-fuel flue gas
 ANLECR&D project 6-215-234 SO x /NO x co-removal during compression of oxy-fuel flue gas Milestone 4.1 Report of ANLECR&D Project 6-215-243 on: Products formed from gas impurities in oxyfuel-derived CO
ANLECR&D project 6-215-234 SO x /NO x co-removal during compression of oxy-fuel flue gas Milestone 4.1 Report of ANLECR&D Project 6-215-243 on: Products formed from gas impurities in oxyfuel-derived CO
International Journal of Greenhouse Gas Control
 International Journal of Greenhouse Gas Control 42 (2015) 485 493 Contents lists available at ScienceDirect International Journal of Greenhouse Gas Control j ourna l h o mepage: www.elsevier.com/locate/ijggc
International Journal of Greenhouse Gas Control 42 (2015) 485 493 Contents lists available at ScienceDirect International Journal of Greenhouse Gas Control j ourna l h o mepage: www.elsevier.com/locate/ijggc
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
Reporting of well stirred scrubber results: scrubbing of SO 2 and CO 2 by caustic solutions at atmospheric pressure
 ANLECR&D project Milestone Report for Project 6-0710-0061 on Gas quality impacts, assessment and control in oxy-fuel technology for CCS Reporting of well stirred scrubber results: scrubbing of SO 2 and
ANLECR&D project Milestone Report for Project 6-0710-0061 on Gas quality impacts, assessment and control in oxy-fuel technology for CCS Reporting of well stirred scrubber results: scrubbing of SO 2 and
Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System
 Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System Presented at the BevTech Conference, Albuquerque, NM 2018 by Charles M. Phillips Ph.D., Max Analytical Technologies Mark Taylor, Vice
Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System Presented at the BevTech Conference, Albuquerque, NM 2018 by Charles M. Phillips Ph.D., Max Analytical Technologies Mark Taylor, Vice
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR
 AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Controlling NO x Emission from Post-blast Process Gases
 Controlling NO x Emission from Post-blast Process Gases Farzad Pouralinazar*, Eric M. Kennedy, Bogdan Z. Dlugogorski, John C. Mackie School of Engineering, The University of Newcastle, Callaghan NSW 2308,
Controlling NO x Emission from Post-blast Process Gases Farzad Pouralinazar*, Eric M. Kennedy, Bogdan Z. Dlugogorski, John C. Mackie School of Engineering, The University of Newcastle, Callaghan NSW 2308,
Apportioning of Fuel and Thermal NO x
 DEVELOPMENT OF STABLE NITROGEN ISOTOPE RATIO MEASUREMENTS OBJECTIVES The main aim of the project was to develop a nitrogen-stable isotope measurement technique for NO x and to ascertain whether it can
DEVELOPMENT OF STABLE NITROGEN ISOTOPE RATIO MEASUREMENTS OBJECTIVES The main aim of the project was to develop a nitrogen-stable isotope measurement technique for NO x and to ascertain whether it can
CHLORINE RECOVERY FROM HYDROGEN CHLORIDE
 CHLORINE RECOVERY FROM HYDROGEN CHLORIDE The Project A plant is to be designed for the production of 10,000 metric tons per year of chlorine by the catalytic oxidation of HCl gas. Materials Available 1.
CHLORINE RECOVERY FROM HYDROGEN CHLORIDE The Project A plant is to be designed for the production of 10,000 metric tons per year of chlorine by the catalytic oxidation of HCl gas. Materials Available 1.
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
CoalGen 2009 Dynamic Control of SCR Minimum Operating Temperature
 CoalGen 2009 Dynamic Control of SCR Minimum Operating Temperature Charles A. Lockert, Breen Energy Solution, 104 Broadway Street, Carnegie, PA 15106 Peter C. Hoeflich and Landis S. Smith, Progress Energy
CoalGen 2009 Dynamic Control of SCR Minimum Operating Temperature Charles A. Lockert, Breen Energy Solution, 104 Broadway Street, Carnegie, PA 15106 Peter C. Hoeflich and Landis S. Smith, Progress Energy
Mass Transfer Operations I Prof. Bishnupada Mandal Department of Chemical Engineering Indian Institute of Technology, Guwahati
 Mass Transfer Operations I Prof. Bishnupada Mandal Department of Chemical Engineering Indian Institute of Technology, Guwahati Module - 4 Absorption Lecture - 4 Packed Tower Design Part - 3 Welcome to
Mass Transfer Operations I Prof. Bishnupada Mandal Department of Chemical Engineering Indian Institute of Technology, Guwahati Module - 4 Absorption Lecture - 4 Packed Tower Design Part - 3 Welcome to
The Claviature of Gas Analysis
 The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
EXECUTIVE SUMMARY. especially in last 50 years. Industries, especially power industry, are the large anthropogenic
 EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
DISPLAY YOUR STUDENT ID CARD ON THE TOP OF YOUR DESK NOW UNIVERSITY OF VICTORIA. CHEMISTRY 102 Midterm Test 1 February 1, pm (60 minutes)
 SECTION: (circle one): A01 MR (Dr. Lipson) A02 (Dr. Briggs) A03 MWR (Dr. Brolo) NAME Student No. V0 (Please print clearly.) DISPLAY YOUR STUDENT ID CARD ON THE TOP OF YOUR DESK NOW Version A UNIVERSITY
SECTION: (circle one): A01 MR (Dr. Lipson) A02 (Dr. Briggs) A03 MWR (Dr. Brolo) NAME Student No. V0 (Please print clearly.) DISPLAY YOUR STUDENT ID CARD ON THE TOP OF YOUR DESK NOW Version A UNIVERSITY
Chromatography. Gas Chromatography
 Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Same theme covered in Combined but extra content Extra parts atomic symbols (first 20, Group 1 and Group 7)
 Co-teaching document new ELC Science 5960 and Foundation Level GCSE Combined Science: Trilogy (8464) Chemistry: Component 3 Elements, mixtures and compounds ELC Outcomes Summary of content covered in ELC
Co-teaching document new ELC Science 5960 and Foundation Level GCSE Combined Science: Trilogy (8464) Chemistry: Component 3 Elements, mixtures and compounds ELC Outcomes Summary of content covered in ELC
CHEMICAL ENGINEERING II (MASTERY) Professor K. Li Dr. S. Kalliadasis Professor R. Kandiyoti
 2 ND YEAR COURSE OBJECTIVES CHEMICAL ENGINEERING II (MASTERY) Professor K. Li Dr. S. Kalliadasis Professor R. Kandiyoti ChE.201 The aim of mastery in the 2 nd year is to further develop students ability
2 ND YEAR COURSE OBJECTIVES CHEMICAL ENGINEERING II (MASTERY) Professor K. Li Dr. S. Kalliadasis Professor R. Kandiyoti ChE.201 The aim of mastery in the 2 nd year is to further develop students ability
Energy Procedia
 Energy Procedia 4 (2011) 2847 2854 Energy Procedia 00 (2010) 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 SO x and NO x absorption based removal into acidic
Energy Procedia 4 (2011) 2847 2854 Energy Procedia 00 (2010) 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-10 SO x and NO x absorption based removal into acidic
Topsøe Catalysis Forum 2009
 Mercury Behaviour in Combustion Flue Gases Topsøe Catalysis Forum 9 Munkerupgaard 7 th -8 th of August 9 Dr. Harald Thorwarth Energie braucht Impulse Introduction clean gas Cr Co Ni Cd As Cu Pb Hg Input
Mercury Behaviour in Combustion Flue Gases Topsøe Catalysis Forum 9 Munkerupgaard 7 th -8 th of August 9 Dr. Harald Thorwarth Energie braucht Impulse Introduction clean gas Cr Co Ni Cd As Cu Pb Hg Input
Sampling. Information is helpful in implementing control measures for reducing pollutant concentration to acceptable levels
 Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
á233ñ ELEMENTAL IMPURITIES PROCEDURES
 Second Supplement to USP 38 NF 33 Chemical Tests / á233ñ Elemental Impurities Procedures 1 á233ñ ELEMENTAL IMPURITIES PROCEDURES INTRODUCTION This chapter describes two analytical procedures (Procedures
Second Supplement to USP 38 NF 33 Chemical Tests / á233ñ Elemental Impurities Procedures 1 á233ñ ELEMENTAL IMPURITIES PROCEDURES INTRODUCTION This chapter describes two analytical procedures (Procedures
Supporting Information for. Suppression of OH Generation from the Photo-Fenton Reaction in the Presence of α-pinene Secondary Organic Aerosol Material
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Supporting Information for Suppression of OH Generation from the Photo-Fenton Reaction in the Presence of α-pinene Secondary Organic Aerosol Material Rachel F. Hems *,
1 2 3 4 5 6 7 8 9 10 11 12 13 14 Supporting Information for Suppression of OH Generation from the Photo-Fenton Reaction in the Presence of α-pinene Secondary Organic Aerosol Material Rachel F. Hems *,
3.2 Alkanes. Refining crude oil. N Goalby chemrevise.org 40 C 110 C 180 C. 250 C fuel oil 300 C 340 C. Fractional Distillation: Industrially
 3.2 Alkanes Refining crude oil Fractional Distillation: Industrially Petroleum is a mixture consisting mainly of alkane hydrocarbons Petroleum fraction: mixture of hydrocarbons with a similar chain length
3.2 Alkanes Refining crude oil Fractional Distillation: Industrially Petroleum is a mixture consisting mainly of alkane hydrocarbons Petroleum fraction: mixture of hydrocarbons with a similar chain length
Be prepared to discuss the quantitative comparison method in the oral exam.
 Subject: Ring Experiment III 8 Shell and Tube Heat Exchanger Control The shell and Tube Heat Exchanger has two control valves: one on the process fluid flowing to the tubes and one on the cooling water
Subject: Ring Experiment III 8 Shell and Tube Heat Exchanger Control The shell and Tube Heat Exchanger has two control valves: one on the process fluid flowing to the tubes and one on the cooling water
10/2/2008. hc λ. νλ =c. proportional to frequency. Energy is inversely proportional to wavelength And is directly proportional to wavenumber
 CH217 Fundamentals of Analytical Chemistry Module Leader: Dr. Alison Willows Electromagnetic spectrum Properties of electromagnetic radiation Many properties of electromagnetic radiation can be described
CH217 Fundamentals of Analytical Chemistry Module Leader: Dr. Alison Willows Electromagnetic spectrum Properties of electromagnetic radiation Many properties of electromagnetic radiation can be described
Soil Cation Analysis Using High-Performance Capillary Zone Electrophoresis Last Modified: October 20, 2006
 Soil Cation Analysis Using High-Performance Capillary Zone Electrophoresis Last Modified: October 20, 2006 Introduction: Capillary electrophoresis (CE) is a relatively new, but rapidly growing separation
Soil Cation Analysis Using High-Performance Capillary Zone Electrophoresis Last Modified: October 20, 2006 Introduction: Capillary electrophoresis (CE) is a relatively new, but rapidly growing separation
Mercury Stack Monitor SM-4
 Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Vapourtec UV-150 Photochemical Reactor. Application notes 37-39, 41-44, 47, 48
 Vapourtec UV-150 Photochemical Reactor Application notes 37-39, 41-44, 47, 48 Bringing photochemistry to the bench - Photochemistry has a number of important applications in nature and industry. - Traditional
Vapourtec UV-150 Photochemical Reactor Application notes 37-39, 41-44, 47, 48 Bringing photochemistry to the bench - Photochemistry has a number of important applications in nature and industry. - Traditional
Acid Rain Drops Keep Falling on My Head Investigating Acid Rain
 Investigating Acid Rain Carbonic acid is produced when carbon dioxide gas dissolves in rain droplets of unpolluted air: (1) CO 2(g) + H 2 O (l) H 2 CO 3(aq) CO 2 Nitrous acid and nitric acid result from
Investigating Acid Rain Carbonic acid is produced when carbon dioxide gas dissolves in rain droplets of unpolluted air: (1) CO 2(g) + H 2 O (l) H 2 CO 3(aq) CO 2 Nitrous acid and nitric acid result from
IMPROVED ADIABATIC CALORIMETRY IN THE PHI-TEC APPARATUS USING AUTOMATED ON-LINE HEAT LOSS COMPENSATION
 # 27 IChemE IMPROVED ADIABATIC CALORIMETRY IN THE PHI-TEC APPARATUS USING AUTOMATED ON-LINE HEAT LOSS COMPENSATION B Kubascikova, D.G. Tee and S.P. Waldram HEL Ltd, 5 Moxon Street, Barnet, Hertfordshire,
# 27 IChemE IMPROVED ADIABATIC CALORIMETRY IN THE PHI-TEC APPARATUS USING AUTOMATED ON-LINE HEAT LOSS COMPENSATION B Kubascikova, D.G. Tee and S.P. Waldram HEL Ltd, 5 Moxon Street, Barnet, Hertfordshire,
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
COMBUSTION OF FUEL 12:57:42
 COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
HYPHENATED TECHNOLOGY GUIDE
 HYPHENATED TECHNOLOGY GUIDE TABLE OF CONTENTS TG-IR...3 Hiden Analytical MS Systems for TG-MS...4 TG-MS...5 TG-GC/MS...6 TG-IR-GC/MS...8 HYPHENATED TECHNOLOGY GUIDE Coupling, or hyphenating, two instruments
HYPHENATED TECHNOLOGY GUIDE TABLE OF CONTENTS TG-IR...3 Hiden Analytical MS Systems for TG-MS...4 TG-MS...5 TG-GC/MS...6 TG-IR-GC/MS...8 HYPHENATED TECHNOLOGY GUIDE Coupling, or hyphenating, two instruments
H 2 CO 3 (aq) HNO 2 (aq) + HNO 3 (aq)
 ACID RAIN LAB ENV 1.PALM From Science with Handhelds, Vernier Software & Technology, 2002. INTRODUCTION In this experiment, you will observe the formation of four acids that occur in acid rain: carbonic
ACID RAIN LAB ENV 1.PALM From Science with Handhelds, Vernier Software & Technology, 2002. INTRODUCTION In this experiment, you will observe the formation of four acids that occur in acid rain: carbonic
Monitoring Flammable Vapors and Gases in Industrial Processes
 Flammability Hazards Industrial fires and explosions happen more frequently than most people think. They cause downtime, property damage, injury and sometimes death. These fires and explosions result from
Flammability Hazards Industrial fires and explosions happen more frequently than most people think. They cause downtime, property damage, injury and sometimes death. These fires and explosions result from
Acid Rain. Evaluation copy
 Acid Rain Computer 22 In this experiment, you will observe the formation of four acids that occur in acid rain: carbonic acid, H 2 CO 3 nitrous acid, HNO 2 nitric acid, HNO 3 sulfurous acid, H 2 SO 3 Carbonic
Acid Rain Computer 22 In this experiment, you will observe the formation of four acids that occur in acid rain: carbonic acid, H 2 CO 3 nitrous acid, HNO 2 nitric acid, HNO 3 sulfurous acid, H 2 SO 3 Carbonic
Atomic Absorption Spectrophotometry. Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon
 Atomic Absorption Spectrophotometry Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon Defination In analytical chemistry, Atomic absorption spectroscopy is a
Atomic Absorption Spectrophotometry Presentation by, Mrs. Sangita J. Chandratre Department of Microbiology M. J. college, Jalgaon Defination In analytical chemistry, Atomic absorption spectroscopy is a
Elements and Their Oxides
 Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
PROCEDURES. Pharmacopeial Forum 2 Vol. 36(1) [Jan. Feb. 2009]
![PROCEDURES. Pharmacopeial Forum 2 Vol. 36(1) [Jan. Feb. 2009] PROCEDURES. Pharmacopeial Forum 2 Vol. 36(1) [Jan. Feb. 2009]](/thumbs/87/96349630.jpg) 2 Vol. 36(1) [Jan. Feb. 2009] BRIEFING h233i Elemental Impurities Procedures. This proposed new general test chapter is the second of two being developed to replace the general test chapter Heavy Metals
2 Vol. 36(1) [Jan. Feb. 2009] BRIEFING h233i Elemental Impurities Procedures. This proposed new general test chapter is the second of two being developed to replace the general test chapter Heavy Metals
Fundamentals of Combustion
 Fundamentals of Combustion Lec 3: Chemical Thermodynamics Dr. Zayed Al-Hamamre Content Process Heat Transfer 1-3 Process Heat Transfer 1-4 Process Heat Transfer 1-5 Theoretical and Excess Air Combustion
Fundamentals of Combustion Lec 3: Chemical Thermodynamics Dr. Zayed Al-Hamamre Content Process Heat Transfer 1-3 Process Heat Transfer 1-4 Process Heat Transfer 1-5 Theoretical and Excess Air Combustion
Systems Engineering Spring Group Project #1: Process Flowsheeting Calculations for Acetic Anhydride Plant. Date: 2/25/00 Due: 3/3/00
 10.551 Systems Engineering Spring 2000 Group Project #1: Process Flowsheeting Calculations for Acetic Anhydride Plant Date: 2/25/00 Due: 3/3/00 c Paul I. Barton, 14th February 2000 At our Nowhere City
10.551 Systems Engineering Spring 2000 Group Project #1: Process Flowsheeting Calculations for Acetic Anhydride Plant Date: 2/25/00 Due: 3/3/00 c Paul I. Barton, 14th February 2000 At our Nowhere City
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Estimation of accidental environmental release based on containment measurements
 Estimation of accidental environmental release based on containment measurements Péter Szántó, Sándor Deme, Edit Láng, Istvan Németh, Tamás Pázmándi Hungarian Academy of Sciences Centre for Energy Research,
Estimation of accidental environmental release based on containment measurements Péter Szántó, Sándor Deme, Edit Láng, Istvan Németh, Tamás Pázmándi Hungarian Academy of Sciences Centre for Energy Research,
Pharmacopeial Forum Vol. 36(1) [Jan. Feb. 2010] 1
![Pharmacopeial Forum Vol. 36(1) [Jan. Feb. 2010] 1 Pharmacopeial Forum Vol. 36(1) [Jan. Feb. 2010] 1](/thumbs/74/71142578.jpg) Vol. 36(1) [Jan. Feb. 2010] 1 Page 1 of 23 Time:10:03 Date:10/14/09 Instance: g:/pf/production/final PF36(1)/m5193.xml Template:s:/Pf/Template/PFRedesign/Pf353/PFR-pf-server-2009.3f BRIEFING h233i Elemental
Vol. 36(1) [Jan. Feb. 2010] 1 Page 1 of 23 Time:10:03 Date:10/14/09 Instance: g:/pf/production/final PF36(1)/m5193.xml Template:s:/Pf/Template/PFRedesign/Pf353/PFR-pf-server-2009.3f BRIEFING h233i Elemental
High-Pressure Volumetric Analyzer
 High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES
 JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
Methodology for Analysis of Metallurgical Processes
 Methodology for Analysis of Metallurgical Processes Metallurgical and chemical processes are classified as batch, continuous and semibatch 1. Batch processes The feed is charged into a vessel at the beginning
Methodology for Analysis of Metallurgical Processes Metallurgical and chemical processes are classified as batch, continuous and semibatch 1. Batch processes The feed is charged into a vessel at the beginning
HYPHENATED TECHNOLOGY GUIDE
 HYPHENATED TECHNOLOGY GUIDE TABLE OF CONTENTS TG-IR...3 Hiden Analytical MS Systems for TG-MS...4 TG-MS...5 TG-GC/MS...6 TG-IR-GC/MS...8 HYPHENATED TECHNOLOGY GUIDE Coupling, or hyphenating, two instruments
HYPHENATED TECHNOLOGY GUIDE TABLE OF CONTENTS TG-IR...3 Hiden Analytical MS Systems for TG-MS...4 TG-MS...5 TG-GC/MS...6 TG-IR-GC/MS...8 HYPHENATED TECHNOLOGY GUIDE Coupling, or hyphenating, two instruments
3. Chemical industry. Because of their modular design, the instruments in the TOC-L series can be equipped for any possible measurement
 3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
EXPERIMENTAL AND KINETIC
 EXPERIMENTAL AND KINETIC MODELING STUDY OF THE EFFECT OF SO 2 ON FUEL OXIDATION IN AN O 2 /CO 2 ATMOSPHERE J. Giménez*, M. Martinez, A. Millera, R. Bilbao, M.U. Alzueta I3A - University of Zaragoza - Spain
EXPERIMENTAL AND KINETIC MODELING STUDY OF THE EFFECT OF SO 2 ON FUEL OXIDATION IN AN O 2 /CO 2 ATMOSPHERE J. Giménez*, M. Martinez, A. Millera, R. Bilbao, M.U. Alzueta I3A - University of Zaragoza - Spain
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
It is the purpose of this paper to demonstrate that the detection limit is directly related to the sample size, for both TG and TG/FTIR.
 BIGGER IS BETTER: PUSHING THE LIMIT OF TG AND TG/FTIR Abstract In Thermogravimetry (TG) and TG/FTIR systems, there are many variables that can affect the detection limit of the system. It is demonstrated
BIGGER IS BETTER: PUSHING THE LIMIT OF TG AND TG/FTIR Abstract In Thermogravimetry (TG) and TG/FTIR systems, there are many variables that can affect the detection limit of the system. It is demonstrated
Hydra-C Application Note: 1076
 Hydra-C Application Note: 1076 The Determination of Mercury in Samples by U.S. EPA SOW 846 Method 7473 using the Hydra-C Mercury Analyzer Introduction The accurate determination of mercury in various samples
Hydra-C Application Note: 1076 The Determination of Mercury in Samples by U.S. EPA SOW 846 Method 7473 using the Hydra-C Mercury Analyzer Introduction The accurate determination of mercury in various samples
SECOND ENGINEER REG. III/2 APPLIED HEAT
 SECOND ENGINEER REG. III/2 APPLIED HEAT LIST OF TOPICS A B C D E F G H I J K Pressure, Temperature, Energy Heat Transfer Internal Energy, Thermodynamic systems. First Law of Thermodynamics Gas Laws, Displacement
SECOND ENGINEER REG. III/2 APPLIED HEAT LIST OF TOPICS A B C D E F G H I J K Pressure, Temperature, Energy Heat Transfer Internal Energy, Thermodynamic systems. First Law of Thermodynamics Gas Laws, Displacement
Downloaded from
 Matter in Our Surroundings 1. Which state of matter is characterized by the following properties : (0 A substance with a fixed arrangement of particles. (I'O A substance that has large distances between
Matter in Our Surroundings 1. Which state of matter is characterized by the following properties : (0 A substance with a fixed arrangement of particles. (I'O A substance that has large distances between
Unit C1: Chemistry in our world Page 1 of 5
 Unit C1: Chemistry in our world Page 1 of 5 Lesson Specification learning outcomes Edexcel 360 Science Specification match Edexcel 360 Science GCSE Science Students Book page reference Additional information
Unit C1: Chemistry in our world Page 1 of 5 Lesson Specification learning outcomes Edexcel 360 Science Specification match Edexcel 360 Science GCSE Science Students Book page reference Additional information
(for tutoring, homework help, or help with online classes)
 www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 18.2-30 What is the final stage in municipal water treatment? A. aeration B. settling C. removal of added fluoride
www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 18.2-30 What is the final stage in municipal water treatment? A. aeration B. settling C. removal of added fluoride
Chapter 1. Matter and Measurements. Our main concern is the understanding of the principles that govern chemical reactions.
 Chapter 1 Matter and Measurements Our main concern is the understanding of the principles that govern chemical reactions. 1. Types of matter- pure vs mixture, element vs compound 2. Measurements uncertainties,
Chapter 1 Matter and Measurements Our main concern is the understanding of the principles that govern chemical reactions. 1. Types of matter- pure vs mixture, element vs compound 2. Measurements uncertainties,
Acids and Bases 2 Science Notes JC-Learn. JC-Learn. Science Notes Acids and Bases 2. 1 P a g e
 JC-Learn Science Notes Acids and Bases 2 1 P a g e Acids and Bases 2 The two most common laboratory acids are hydrochloric acid (HCl) and sulfuric acid (H2SO4). The two most common laboratory bases are
JC-Learn Science Notes Acids and Bases 2 1 P a g e Acids and Bases 2 The two most common laboratory acids are hydrochloric acid (HCl) and sulfuric acid (H2SO4). The two most common laboratory bases are
Dynamic simulation and Control of a CO 2 Compression and Purification Unit for Oxy-Coal-Fired Power Plants
 Dynamic simulation and Control of a CO 2 Compression and Purification Unit for Oxy-Coal-Fired Power Plants Authors A. Chansomwong, K.E. Zanganeh, A. Shafeen, P.L. Douglas,E. Croiset, L.A. Ricardez-Sandoval,
Dynamic simulation and Control of a CO 2 Compression and Purification Unit for Oxy-Coal-Fired Power Plants Authors A. Chansomwong, K.E. Zanganeh, A. Shafeen, P.L. Douglas,E. Croiset, L.A. Ricardez-Sandoval,
THE NEW QUANTITATIVE ANALYTICAL METHOD FOR ULTRATRACE SULFUR COMPOUNDS IN NATURAL GAS
 International Gas Union Research Conference 14 THE NEW QUANTITATIVE ANALYTICAL METHOD FOR ULTRATRACE SULFUR COMPOUNDS IN NATURAL GAS Main author Hironori IMANISHI Tokyo Gas Co., Ltd. JAPAN himanishi@tokyo-.co.jp
International Gas Union Research Conference 14 THE NEW QUANTITATIVE ANALYTICAL METHOD FOR ULTRATRACE SULFUR COMPOUNDS IN NATURAL GAS Main author Hironori IMANISHI Tokyo Gas Co., Ltd. JAPAN himanishi@tokyo-.co.jp
Liquid storage: Holds the solvent which is going to act as the mobile phase. Pump: Pushes the solvent through to the column at high pressure.
 High performance liquid chromatography (HPLC) is a much more sensitive and useful technique than paper and thin layer chromatography. The instrument used for HPLC is called a high performance liquid chromatograph.
High performance liquid chromatography (HPLC) is a much more sensitive and useful technique than paper and thin layer chromatography. The instrument used for HPLC is called a high performance liquid chromatograph.
ECH 4224L Unit Operations Lab I Thin Film Evaporator. Introduction. Objective
 Introduction In this experiment, you will use thin-film evaporator (TFE) to separate a mixture of water and ethylene glycol (EG). In a TFE a mixture of two fluids runs down a heated inner wall of a cylindrical
Introduction In this experiment, you will use thin-film evaporator (TFE) to separate a mixture of water and ethylene glycol (EG). In a TFE a mixture of two fluids runs down a heated inner wall of a cylindrical
Final Report. Characterisation of Sample Report. Job No 2016/11/12-34 AS No. 1234A. Client Example Contact Sample. Signed Date 2017.
 Final Report Title Characterisation of Job No 2016/11/12-34 AS No. 1234A Client Contact Sample Author report Signed Date 2017 Easy Reach Report 2017 v2.docx 1 of 33 Contents 1. Study Summary Page 3 2.
Final Report Title Characterisation of Job No 2016/11/12-34 AS No. 1234A Client Contact Sample Author report Signed Date 2017 Easy Reach Report 2017 v2.docx 1 of 33 Contents 1. Study Summary Page 3 2.
Catalysis CAPABILITIES
 Catalysis www.extrel.com CAPABILITIES Contents Extrel instruments have been recognized for their exceptional performance by the world s leading researchers for more than 50 years. Reliability and flexibility
Catalysis www.extrel.com CAPABILITIES Contents Extrel instruments have been recognized for their exceptional performance by the world s leading researchers for more than 50 years. Reliability and flexibility
Ways to Improve the Energy Efficiency of AN Solution Plants
 Ways to Improve the Energy Efficiency of AN Solution Plants by Axel Erben Uhde GmbH Dortmund, Germany Prepared for Presentation at NITROGEN 2009 International Conference Rome, Italy 22-25 February 2009
Ways to Improve the Energy Efficiency of AN Solution Plants by Axel Erben Uhde GmbH Dortmund, Germany Prepared for Presentation at NITROGEN 2009 International Conference Rome, Italy 22-25 February 2009
3. Chemical industry. Because of their modular design, the instruments in the TOC-L series can be equipped for any possible measurement
 3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
Jianfei Peng et al. Correspondence to: Jianfei Peng Min Hu and Renyi Zhang
 Supplement of Atmos. Chem. Phys., 17, 10333 10348, 2017 https://doi.org/10.5194/acp-17-10333-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Supplement of Atmos. Chem. Phys., 17, 10333 10348, 2017 https://doi.org/10.5194/acp-17-10333-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Distillation is a method of separating mixtures based
 Distillation Distillation is a method of separating mixtures based on differences in their volatilities in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process,
Distillation Distillation is a method of separating mixtures based on differences in their volatilities in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process,
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY
 5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY SUB-TOPIC 1.2 METHODS OF PURIFICATION AND ANALYSIS LEARNING
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY SUB-TOPIC 1.2 METHODS OF PURIFICATION AND ANALYSIS LEARNING
Sodium Chloride - Analytical Standard
 Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
ADVANCED DES SIMULATIONS OF OXY-GAS BURNER LOCATED INTO MODEL OF REAL MELTING CHAMBER
 ADVANCED DES SIMULATIONS OF OXY-GAS BURNER LOCATED INTO MODEL OF REAL MELTING CHAMBER Ing. Vojtech Betak Ph.D. Aerospace Research and Test Establishment Department of Engines Prague, Czech Republic Abstract
ADVANCED DES SIMULATIONS OF OXY-GAS BURNER LOCATED INTO MODEL OF REAL MELTING CHAMBER Ing. Vojtech Betak Ph.D. Aerospace Research and Test Establishment Department of Engines Prague, Czech Republic Abstract
Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth
 University of Groningen Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
THE FUTURE OF THE CHEMISTRY: CONTINUOUS FLOW REACTIONS BASEL 2016
 THE FUTURE OF THE CHEMISTRY: CONTINUOUS FLOW REACTIONS BASEL 2016 CHEMICAL PLANT CONTINUOUS FLOW REACTOR The continuous flow reactor is a safe system, running chemical reactions in reduced volume with
THE FUTURE OF THE CHEMISTRY: CONTINUOUS FLOW REACTIONS BASEL 2016 CHEMICAL PLANT CONTINUOUS FLOW REACTOR The continuous flow reactor is a safe system, running chemical reactions in reduced volume with
Module: 5. Lecture: 29
 Module: 5 Lecture: 29 METHYL CHLORIDE and Dichloromethane INTRODUCTION METHYL CHLORIDE Methyl chloride (CH3Cl) which is also known as chloromethane, R-40 or HCC 40, is a chemical compound of the group
Module: 5 Lecture: 29 METHYL CHLORIDE and Dichloromethane INTRODUCTION METHYL CHLORIDE Methyl chloride (CH3Cl) which is also known as chloromethane, R-40 or HCC 40, is a chemical compound of the group
Instrumental Analysis
 Instrumental Analysis Bonds and Infrared Energy Covalent bonds can absorb infrared energy, which causes the bonds to vibrate. Depending on the masses of the bonded atoms, different frequencies of radiation
Instrumental Analysis Bonds and Infrared Energy Covalent bonds can absorb infrared energy, which causes the bonds to vibrate. Depending on the masses of the bonded atoms, different frequencies of radiation
Gaseous CEMS technology
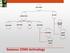 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Chemistry. Exam Choice. Student Number PRELIMINARY COURSE EXAMINATION. Total marks 75. General Instructions
 Student Number Exam Choice 2008 PRELIMINARY COURSE EXAMINATION Chemistry Total marks 75 General Instructions Reading time 5 minutes Working time 2 hours Write using black or blue pen Draw diagrams using
Student Number Exam Choice 2008 PRELIMINARY COURSE EXAMINATION Chemistry Total marks 75 General Instructions Reading time 5 minutes Working time 2 hours Write using black or blue pen Draw diagrams using
Complex Compounds Background of Complex Compound Technology
 Complex Compounds For more than 20 years, Rocky Research has been a pioneer in the field of sorption refrigeration utilizing complex compounds. Our technology earned special recognition from NASA in 1999.
Complex Compounds For more than 20 years, Rocky Research has been a pioneer in the field of sorption refrigeration utilizing complex compounds. Our technology earned special recognition from NASA in 1999.
Chem 434 Instrumental Analysis Test 1
 Chem 434 Instrumental Analysis Test 1 Name: 1. (15 points) In Chapter 5 we discussed four sources of instrumental noise: Thermal Noise, Shot Noise, Flicker Noise, and Environmental noise. Discuss the differences
Chem 434 Instrumental Analysis Test 1 Name: 1. (15 points) In Chapter 5 we discussed four sources of instrumental noise: Thermal Noise, Shot Noise, Flicker Noise, and Environmental noise. Discuss the differences
Importance of in situ Monitoring in MOCVD Process and Future Prospects
 G u e s t F o r u m Guest Forum Series of Lectures by Screening Committees of the Second Masao Horiba Awards Importance of in situ Monitoring in MOCVD Process and Future Prospects Hiroshi Funakubo Tokyo
G u e s t F o r u m Guest Forum Series of Lectures by Screening Committees of the Second Masao Horiba Awards Importance of in situ Monitoring in MOCVD Process and Future Prospects Hiroshi Funakubo Tokyo
ANALYTICAL METHOD DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Air sampling and analysis
 DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
Optimal design of a CO 2 absorption unit and assessment of solvent degradation
 Optimal design of a CO 2 absorption unit and assessment of solvent degradation Mid-term Presentation Grégoire Léonard Table of Content 1. Introduction 2. Objectives 3. Modeling and optimal design 4. Solvent
Optimal design of a CO 2 absorption unit and assessment of solvent degradation Mid-term Presentation Grégoire Léonard Table of Content 1. Introduction 2. Objectives 3. Modeling and optimal design 4. Solvent
2B Air, Oxygen, Carbon Dioxide and Water
 Air, Oxygen, Carbon Dioxide and Water Air, oxygen and carbon dioxide are important chemicals in our everyday lives. Knowledge of their properties helps us to develop an understanding of the role they play.
Air, Oxygen, Carbon Dioxide and Water Air, oxygen and carbon dioxide are important chemicals in our everyday lives. Knowledge of their properties helps us to develop an understanding of the role they play.
The fertiliser industry
 The fertiliser industry The industrial production of fertilisers For more information on this section refer to the Chemical Industries Resource Pack. The industrial production of fertilisers involves several
The fertiliser industry The industrial production of fertilisers For more information on this section refer to the Chemical Industries Resource Pack. The industrial production of fertilisers involves several
Atmospheric Composition הרכב האטמוספירה
 Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
*Correspondence to:
 Supporting Information for Carbonate-promoted hydrogenation of carbon dioxide to multi-carbon carboxylates Aanindeeta Banerjee 1 and Matthew W. Kanan 1 * 1 Department of Chemistry, Stanford University,
Supporting Information for Carbonate-promoted hydrogenation of carbon dioxide to multi-carbon carboxylates Aanindeeta Banerjee 1 and Matthew W. Kanan 1 * 1 Department of Chemistry, Stanford University,
Midterm 2 Scores. Class average: 40/50. # of students. Exam score
 Global Warming Midterm 2 Scores Class average: 40/50 # of students Exam score Learning Objectives (LO) Lecture 19: Global Warming and Energy Use Read: Chapter 14 Homework due Thursday Nov. 5 What we ll
Global Warming Midterm 2 Scores Class average: 40/50 # of students Exam score Learning Objectives (LO) Lecture 19: Global Warming and Energy Use Read: Chapter 14 Homework due Thursday Nov. 5 What we ll
Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation
 Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation Philip Davies TA Instruments UK Thermogravimetric Analysis Change in a samples weight (increase or decrease) as a function
Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation Philip Davies TA Instruments UK Thermogravimetric Analysis Change in a samples weight (increase or decrease) as a function
Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor
 13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC13-3158 Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey
13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC13-3158 Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey
Chapter 1 Introduction and Basic Concepts
 Chapter 1 Introduction and Basic Concepts 1-1 Thermodynamics and Energy Application Areas of Thermodynamics 1-2 Importance of Dimensions and Units Some SI and English Units Dimensional Homogeneity Unity
Chapter 1 Introduction and Basic Concepts 1-1 Thermodynamics and Energy Application Areas of Thermodynamics 1-2 Importance of Dimensions and Units Some SI and English Units Dimensional Homogeneity Unity
In terms of production, nitric acid is the third most widely produced acid across the world.
 In terms of production, nitric acid is the third most widely produced acid across the world. It has a wide range of uses in agriculture, industry and medicine where it is used as a fertiliser and in the
In terms of production, nitric acid is the third most widely produced acid across the world. It has a wide range of uses in agriculture, industry and medicine where it is used as a fertiliser and in the
Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR)
 Technical articles Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR) Tadashi Arii* 1. Introduction Simultaneous
Technical articles Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR) Tadashi Arii* 1. Introduction Simultaneous
Project S66 Benchscale Testing of Gas Absorption in Wet Scrubbers. SEMATECH Technology Transfer A-ENG
 Project S66 Benchscale Testing of Gas Absorption in Wet Scrubbers Technology Transfer 95052814A-ENG and the logo are registered service marks of, Inc. Apple and Macintosh are registered trademarks of Apple
Project S66 Benchscale Testing of Gas Absorption in Wet Scrubbers Technology Transfer 95052814A-ENG and the logo are registered service marks of, Inc. Apple and Macintosh are registered trademarks of Apple
Reference pg and in Textbook
 Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
Glossary of Common Laboratory Terms
 Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
