Optimal design of a CO 2 absorption unit and assessment of solvent degradation
|
|
|
- Evangeline Goodwin
- 5 years ago
- Views:
Transcription
1 Optimal design of a CO 2 absorption unit and assessment of solvent degradation Mid-term Presentation Grégoire Léonard
2 Table of Content 1. Introduction 2. Objectives 3. Modeling and optimal design 4. Solvent degradation 5. Conclusion and perspectives 2
3 1. Introduction
4 1. Introduction CO 2 capture in coal power plants 4
5 1. Introduction Chemical reactions taking place during the CO 2 capture in Monoethanolamine (MEA): 2 H 2 O <--> H 3 O + + OH - C 2 H 7 NO + H 3 O + <--> C 2 H 8 NO + + H 2 O CO H 2 O <--> H 3 O + + HCO - 3 HCO H 2 O <--> H 3 O + + CO 2-3 C 2 H 7 NO + HCO - 3 <--> C 3 H 6 NO H 2 O 5
6 2. Objectives
7 2. Objectives Context: reduction of the CO 2 capture cost for large scale power plants => Energy requirement and degradation induced costs are among the largest operative costs!
8 2. Objectives To establish a link between modeling and degradation 8
9 2. Objectives To establish a link between modeling and degradation The result will be a proposal for optimal operating conditions in the CO 2 capture process taking into account process efficiency and solvent degradation i.e. cost and environmental impacts of PCC 9
10 3. Modeling and optimal design
11 3.1 Objectives Model building Sensitivity study of key parameters Simulation of process improvements Validation based on experimental results Implementation of degradation results Multi-objective optimization 11
12 3.1 Objectives Model building Sensitivity study of key parameters Simulation of process improvements Validation based on experimental results Implementation of degradation results Multi-objective optimization 12
13 3.2 Model Building Two different modeling approaches Model parameters: 2500 NM³ flue gaz, 14vol-% CO 2, 90% Capture rate, MEA 30wt-% 13
14 3.2 Model Building Simulation tool: Aspen Plus V7.2 VENTGAS WASHER W-IN PRODUCT SPLITTER WEX CONDENS FLUE-OFF W-OUT ABSORBER VAP FLUEGAS BLOWER MEA-LEAN COOLER MEA-L-C LIQ LRHEATX FG-P MEA-R-C MEA-R-H PUMP STRIPPER MEA-RICH MEA-L-H 14
15 Regeneration energy (GJ/tonne CO2) Regeneration energy (GJ/tonne CO2) 3.3 Sensitivity study Sensitivity study of process key variables Equilibrium Ratesep Equilibrium Ratesep Stripper Pressure (bar) Solvent flow rate (m³/h) Results Stripper Pressure Solvent concentration Solvent flow rate Base Case Value 1.2 bar 30 wt-% 15 m³/h Optimized Value 2.2 bar 37 wt-% 12.4 m³/h Gain in regeneration energy -16.9% -5.4% -2.8% 15
16 3.4 Model improvements Impact study of process improvements 16
17 Absorber Height (m) Regeneration Energy (GJ/tonne CO2) 3.4 Results summary Impact study of process improvements Intercooler position Regeneration energy (GJ/tonne CO2) Split Flow return stage in the absorber Process modifications Lean vapor compression Absorber intercooling Split-flow configuration Gain in regeneration energy -14% (exergy) -4% -4% 17
18 3.5 Conclusion It is possible to identify optimal operating conditions using modeling Simulation results are less costly than lab experiments and can help to the decision for process improvements Good model knowledge has been gained and can be useful for further simulation However, simulation results still don t take secondary effects into account (degradation, corrosion, )
19 3.6 Perspectives Writing of an article and submission for publication to a journal in the field Energetics and Engineering => planed at the beginning of Validation of the model may occur soon, depending on pilot experimental results => validation based on: - Regeneration energy = f(solvent flow rate) - Absorber and stripper temperature profiles - Solvent lean and rich loading 19
20 3.6 Perspectives Follow-up of a master thesis with subject «Simulation of the dynamic behavior of a pilot plant for CO 2 capture» => First semester 2012 => Objective is to develop a dynamic model using Aspen Dynamics => Eventually modeling of process improvements First tests for the implementation of degradation parameters in Aspen Plus 20
21 4. Solvent degradation
22 4.1 Objectives Design and construction of a degradation test rig Detailed screening of MEA degradation Study of the impact of operating conditions (temperature, gas composition, flow, ) Study of the effect of additives (degradation inhibitors, metal) Test of 1 or 2 other solvents Results implementation in the process model 22
23 4.1 Objectives Design and construction of a degradation test rig Detailed screening of MEA degradation Study of the impact of operating conditions (temperature, gas composition, flow, ) Study of the effect of additives (degradation inhibitors, metal) Test of 1 or 2 other solvents Results implementation in the process model 23
24 4.2 Literature review 3 types of degradation mecanisms: Temperature, O 2, CO 2 24
25 4.2 Literature review 25
26 4.3 Degradation Test Rig 1. Reactor 2. Gas supply 4 3 b 1 3 a 3. Water balance 4. Gas flow Control panel 26
27 4.3 Degradation Test Rig Test rig continuously improved to face experimental problems 27
28 4.4 Analytical Methods Liquid phase: HPLC (High Pressure Liquid Chromatography): MEA quantification GC-FID (Gas Chromatography): identification & quantification of the degradation products IC (Ionic Chromatography): quantification of organic anions Karl-Fischer Titration: water quantification AAS (Atomic Absorption Spectroscopy) and CE (Capillar Electrophoresis): quantification of inorganic ions Gas phase: FTIR (Fourier Transform Infrared Spectroscopy): NH 3 and MEA quantification 28
29 4.4.1 HPLC Quantification of MEA: 3 different columns have been tested C18 Pyramid Nucleosil SA HILIC
30 4.4.1 HPLC With different results C18: no retention Degraded MEA
31 4.4.1 HPLC Nucleosil SA: Better separation but bad peak shape Degraded MEA
32 4.4.1 HPLC Nucleosil SA: quantification of MEA possible: => calibration curve
33 4.4.1 HPLC HILIC: Better peak shape but separation still to be improved Degraded MEA
34 4.4.2 GC-FID GC-FID: - Analytical method has been developed - Identification of degradation products possible
35 4.4.2 GC-FID - Quantification using an internal standard (1% of 2-Methoxyethanol)
36 4.4.3 Organic Ions Analysis Organic ions may lead to heat stable salts (HSS) HSS can not be regenerated and lead to a loss of efficiency Analysis of Formate, Acetate, Glycolate, Oxalate 36
37 4.4.4 Karl Fischer Titration Potentiometric titration of water based on the equation: 2 H 2 O + SO 2 + I 2 SO I + 4 H + => Water quantification in degraded amine samples => In relation with the mass balance
38 4.4.5 Inorganic Ion Analysis Quantification of elementar ions because their presence can be directly related to corrosion Fe, Cr, Ni: components of SS 316 Si: found in many analyses F, Cl: corrosion accelerator
39 4.4.6 FTIR Quantification of NH 3 and MEA in the gas phase at the reactor exhaust Quantification of H 2 O, CO 2, MEA and NH 3
40 4.4.6 FTIR Heating rope from reactor to FTIR => Combined with a pre-heating of the dilution gas => Prevents condensation of the gas sample in the line
41 4.4.6 FTIR Calibration of liquid samples Air outlet to FTIR Air supply Syringe pump Heated plate
42 4.4.6 FTIR Calibration of NH 3 and MEA
43 4.5 Results Summary 43
44 4.5 Results Summary Experimental feed-back of the 1 st test campaign Corrosion Crystal formation Mass balance regulation Temperature regulation Agitation 44
45 MEA concentration (wt-%) HPLC HPLC quantification of MEA in degraded samples Base case 30 Pressure 25 Temperature 20 Batch New base case 15 BC Strong Repetability T and gas flow Experiment number 45
46 4.5.2 GC-FID GC Identification of degradation products Std Exp Acetamide????? HEEDA x x x HEA x OZD x x x x x x x x HEI x x x x x x x x HEIA x x x x x x 46
47 detector response (µv) GC-FID GC Identification of degradation products: Experiment MEA Me OZD HEI Product 2 Product 3 HEIA Product Time (min)
48 detector response (µv) GC-FID GC Identification of degradation products: Experiment MEA Product Me Product OZD HEI HEIA Product Time (min)
49 4.5.2 GC-FID GC Comparison with pilot plant results Experiment Long Term testing at Esbjerg
50 4.5.2 GC-FID GC Comparison with pilot plant results Experiment 10 Long Term Testing at Esbjerg => Experiment 10 has been chosen as the new base case for next test campaign: C - 4 bar - 5%O 2, 15%CO 2, 80%N mln/min gas flow - 2 weeks 50
51 4.5.3 Inorganic Ions Quantification of inorganic ions Fe Cr Ni Si Cl F MEA 30% 0.44 < 0.10 < < Experiment < Experiment Experiment Experiment Experiment 6 14, < Experiment < Experiment < Experiment < Experiment => More ions during Experiments 2, 6 and 7 51
52 4.5.4 Organic Ions Identification of organic ions performed at Laborelec Experiment 5 Experiment 10 52
53 Mass and water balances (%) Karl Fischer Titration Quantification of water in degraded amine sample Good correspondance with mass balance results! Water balance Mass balance Experiment number 53
54 4.6 Conclusion First test campaign has brought practical experience using the degradation test rig that may be very useful for future experiments Analysis methods are numerous and allow for a complete screening of MEA degradation Observed degradation products were expected and may be explained in relation with previous studies Unexpected degradation products are also obtained, but similar to pilot plant results
55 4.6 Conclusion First tests have permitted the definition of a new base case, with results similar to pilot plant s Influence of temperature, gas flow rate, gas composition and pressure may already be observed. Results are in accordance with previous litterature Corrosion follow-up is pursued
56 4.7 Perspectives Development of analytical methods, especially FTIR and GC-FID for quantification of degraded products Next degradation tests must ensure repetability => the new base case experiment will be repeated Second test campaign with MEA to determine more precisely the influence of gas composition and temperature => first experiments will study the influence of O 2 and CO 2 separately 56
57 4.7 Perspectives Bibliography studies about degradation inhibitors and additives that may be tested with MEA Test of alternative solvents furnished by Laborelec Collaboration with the University of Mons in order to determine the influence of degradation on the CO 2 capture process Participation to a conference at the University of Texas in Austin 57
58 5. Conclusion and perspectives
59 5 Conclusions Good knowledge about the CO 2 capture process considers the energetical efficiency but also takes solvent degradation into account! Results prove that the study of MEA degradation under accelerated conditions can be related to pilot scale results In the coming year, the construction of a model including solvent degradation parameters will begin. This model will have to be validated with experimental data in order to perform a multi-objective optimisation of the CO 2 capture process. 59
60 Thanks for your attention! 60
Mass Transfer in a Small Scale Flue Gas Absorber Experimental and Modeling
 Mass Transfer in a Small Scale Flue Gas Absorber Experimental and Modeling P.J.G. Huttenhuis, E.P. van Elk, S. Van Loo, G.F. Versteeg Procede Gas Treating B.V., The Netherlands 11 th MEETING of the INTERNATIONAL
Mass Transfer in a Small Scale Flue Gas Absorber Experimental and Modeling P.J.G. Huttenhuis, E.P. van Elk, S. Van Loo, G.F. Versteeg Procede Gas Treating B.V., The Netherlands 11 th MEETING of the INTERNATIONAL
Py x P P P. Py x P. sat. dq du PdV. abs Q S. An Innovative Approach in the G U TS PV P P G U TS PV T H U PV H U PV. abs. Py x P. sat.
 E a 1 1 sat sat ln Py x P Py x P K H k Ae R E sat a Py x P 1 1 sat ln K1 R Py x P K H k Ae R 1 CO P H 1 1 abs ln K H H 1/ R Q C 1 1 CO P ln S K H K1 R 1 P H abs H P K1 R CP 1 K1 R 1/ R S Q P 1 E a E du
E a 1 1 sat sat ln Py x P Py x P K H k Ae R E sat a Py x P 1 1 sat ln K1 R Py x P K H k Ae R 1 CO P H 1 1 abs ln K H H 1/ R Q C 1 1 CO P ln S K H K1 R 1 P H abs H P K1 R CP 1 K1 R 1/ R S Q P 1 E a E du
CO 2 Capture by Absorption with Potassium Carbonate First Quarterly Report 2007
 CO 2 Capture by Absorption with Potassium Carbonate First Quarterly Report 2007 Quarterly Progress Report Reporting Period Start Date: January 1, 2007 Reporting Period End Date: March 31, 2007 Authors:
CO 2 Capture by Absorption with Potassium Carbonate First Quarterly Report 2007 Quarterly Progress Report Reporting Period Start Date: January 1, 2007 Reporting Period End Date: March 31, 2007 Authors:
Outline DISCLAIMER SOLUTION MONITORING GUIDELINES FOR AMINE SYSTEMS
 SOLUTION MONITORING GUIDELINES FOR AMINE SYSTEMS Amine Best Practices Group (ABPG) Represented by Nate Hatcher 2011 Brimstone-STS Sulphur Recovery Symposium Vail, CO DISCLAIMER The information and data
SOLUTION MONITORING GUIDELINES FOR AMINE SYSTEMS Amine Best Practices Group (ABPG) Represented by Nate Hatcher 2011 Brimstone-STS Sulphur Recovery Symposium Vail, CO DISCLAIMER The information and data
ScienceDirect. Impact of heat stable salts on equilibrium CO 2 absorption
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1781 1794 GHGT-12 Impact of heat stable salts on equilibrium CO 2 absorption Ugochukwu Edwin Aronu*, Kristin Giske Lauritsen,
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1781 1794 GHGT-12 Impact of heat stable salts on equilibrium CO 2 absorption Ugochukwu Edwin Aronu*, Kristin Giske Lauritsen,
Current status of R&D in post combustion CO 2 capture
 Current status of R&D in post combustion CO 2 capture Kaj Thomsen, Ph.D. Center for Energy Resources Engineering, CERE DTU Chemical Engineering Technical University of Denmark Outline Choice of solvent
Current status of R&D in post combustion CO 2 capture Kaj Thomsen, Ph.D. Center for Energy Resources Engineering, CERE DTU Chemical Engineering Technical University of Denmark Outline Choice of solvent
Ionic Liquids for Post Combustion CO 2 -Absorption
 Ionic Liquids for Post Combustion CO 2 -Absorption 12th MEETING of the INTERNATIONAL POST-COMBUSTION CO 2 CAPTURE NETWORK David Wappel 1), Guenter Gronald 2), Roland Kalb 3) and Josef Draxler 1) 1) University
Ionic Liquids for Post Combustion CO 2 -Absorption 12th MEETING of the INTERNATIONAL POST-COMBUSTION CO 2 CAPTURE NETWORK David Wappel 1), Guenter Gronald 2), Roland Kalb 3) and Josef Draxler 1) 1) University
Density modelling NH 3 -CO 2 -H 2 O liquid mixtures. Technology for a better society
 1 Density modelling NH 3 -CO 2 -H 2 O liquid mixtures 2 Liquid density model in Aspen Plus Clarke model (for aqueous electrolyte molar volume) Molar volume for electrolyte solutions (V m l ), applicable
1 Density modelling NH 3 -CO 2 -H 2 O liquid mixtures 2 Liquid density model in Aspen Plus Clarke model (for aqueous electrolyte molar volume) Molar volume for electrolyte solutions (V m l ), applicable
A rational approach to amine mixture formulation for CO 2 capture applications. Trondheim CCS Conference - 6 June 14 16, 2011 Graeme Puxty
 A rational approach to amine mixture formulation for CO 2 capture applications Trondheim CCS Conference - 6 June 14 16, 2011 Graeme Puxty The ideal solvent for CO 2 post-combustion capture: Process challenges
A rational approach to amine mixture formulation for CO 2 capture applications Trondheim CCS Conference - 6 June 14 16, 2011 Graeme Puxty The ideal solvent for CO 2 post-combustion capture: Process challenges
A NEW SOLVENT FOR CO2 CAPTURE R.
 A NEW SOLVENT FOR CO 2 CAPTURE R. Viscardi, G. Vanga and V. Barbarossa vincenzo.barbarossa@enea.it C.R. Casaccia ENEA; via Anguillarese, 301; 00123 S. M. Galeria-Roma Abstract This experimental study describes
A NEW SOLVENT FOR CO 2 CAPTURE R. Viscardi, G. Vanga and V. Barbarossa vincenzo.barbarossa@enea.it C.R. Casaccia ENEA; via Anguillarese, 301; 00123 S. M. Galeria-Roma Abstract This experimental study describes
EXECUTIVE SUMMARY. especially in last 50 years. Industries, especially power industry, are the large anthropogenic
 EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
Available online at ScienceDirect. Energy Procedia 63 (2014 ) GHGT-12
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1854 1862 GHGT-12 Screening tests of new hybrid solvents for the post-combustion CO 2 capture process by chemical absorption
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1854 1862 GHGT-12 Screening tests of new hybrid solvents for the post-combustion CO 2 capture process by chemical absorption
Regeneration Section of CO 2 Capture Plant by MEA Scrubbing with a Rate-Based Model
 A publication of 1849 CHEMICAL ENGINEERING TRANSACTIONS VOL. 3, 013 Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright 013, AIDIC Servizi S.r.l., ISBN 978-88-95608-3-5; ISSN 1974-9791 The Italian
A publication of 1849 CHEMICAL ENGINEERING TRANSACTIONS VOL. 3, 013 Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright 013, AIDIC Servizi S.r.l., ISBN 978-88-95608-3-5; ISSN 1974-9791 The Italian
Available online at ScienceDirect. Energy Procedia 63 (2014 ) GHGT USA
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1487 1496 GHGT-12 CO 2 mass transfer and solubility in aqueous primary and secondary amine Le Li a, Gary Rochelle a, *
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 1487 1496 GHGT-12 CO 2 mass transfer and solubility in aqueous primary and secondary amine Le Li a, Gary Rochelle a, *
The Refined Electrolyte-NRTL Model applied to CO 2 -H 2 O-alkanolamine systems
 1 The Refined Electrolyte-NRTL Model applied to CO 2 -H 2 O-alkanolamine systems - Equilibrium model predictions - Implementation into the CO2SIM simulator., Finn Andrew Tobiesen*, Mehdi Karimi, Xiao Luo,
1 The Refined Electrolyte-NRTL Model applied to CO 2 -H 2 O-alkanolamine systems - Equilibrium model predictions - Implementation into the CO2SIM simulator., Finn Andrew Tobiesen*, Mehdi Karimi, Xiao Luo,
Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth
 University of Groningen Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
Reaction Monitoring in Chemical Engineering with Quantitative Online NMR Spectroscopy
 Reaction Monitoring in Chemical Engineering with Quantitative nline MR Spectroscopy Hans Hasse 1, Sebastian Hoch 1, Erik von Harbou 1, Klaus Albert 2 1 Laboratory of, Technische Universität Kaiserslautern,
Reaction Monitoring in Chemical Engineering with Quantitative nline MR Spectroscopy Hans Hasse 1, Sebastian Hoch 1, Erik von Harbou 1, Klaus Albert 2 1 Laboratory of, Technische Universität Kaiserslautern,
Certificate of Analysis
 Certificate of Analysis Reference Material - Primary Standard Product Name: Metoprolol Tartrate 1.0 mg/ml in Methanol (as free base) Catalogue Number: LGCAMP0027.00-01 Lot Number: 8525 CAS Number: 56392-17-7
Certificate of Analysis Reference Material - Primary Standard Product Name: Metoprolol Tartrate 1.0 mg/ml in Methanol (as free base) Catalogue Number: LGCAMP0027.00-01 Lot Number: 8525 CAS Number: 56392-17-7
CHEMISTRY 31 FINAL EXAM May 15, points total
 CHEMISTRY 31 FINAL EXAM May 15, 2017-150 points total NAME Lab Sect. Debye-Hückel Equation: 2 0.51 z µ logγ = 1+ ( α µ / 305) Where: γ = activity coefficient, μ = ionic strength, α = hydrated ion radius,
CHEMISTRY 31 FINAL EXAM May 15, 2017-150 points total NAME Lab Sect. Debye-Hückel Equation: 2 0.51 z µ logγ = 1+ ( α µ / 305) Where: γ = activity coefficient, μ = ionic strength, α = hydrated ion radius,
Certificate of Analysis
 Certificate of Analysis LGC Quality ISO Guide 34:2009 DAkkS D-RM-14176-01-00 ISO/IEC 17025:2005 DAkkS D-PL-14176-01-00 ISO 9001:2008 DQS 102448 QM08 Primary Reference Standard Tilidine Hydrochloride Hemihydrate
Certificate of Analysis LGC Quality ISO Guide 34:2009 DAkkS D-RM-14176-01-00 ISO/IEC 17025:2005 DAkkS D-PL-14176-01-00 ISO 9001:2008 DQS 102448 QM08 Primary Reference Standard Tilidine Hydrochloride Hemihydrate
Designing Chemical Products. Dr. Kevin G. Joback Molecular Knowledge Systems, Inc.
 Designing Chemical Products Dr. Kevin G. Joback http://www.molecularknowledge.com {1} O C O O O C O CH2 CH2 {1} {1} C C O CH2 CH2 O {1} Group -CH3 -OH >C=O Intercept O SP,p -0.591-0.377 5.548 4.670 4.729
Designing Chemical Products Dr. Kevin G. Joback http://www.molecularknowledge.com {1} O C O O O C O CH2 CH2 {1} {1} C C O CH2 CH2 O {1} Group -CH3 -OH >C=O Intercept O SP,p -0.591-0.377 5.548 4.670 4.729
Introduction to Pharmaceutical Chemical Analysis
 Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
Heat of Absorption of CO 2 in Aqueous Solutions of DEEA, MAPA and their Mixture
 Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 1532 1542 GHGT-11 Heat of Absorption of CO 2 in Aqueous Solutions of DEEA, MAPA and their Mixture Muhammad Waseem Arshad a, Nicolas
Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 1532 1542 GHGT-11 Heat of Absorption of CO 2 in Aqueous Solutions of DEEA, MAPA and their Mixture Muhammad Waseem Arshad a, Nicolas
METHODS FOR THE ANALYSIS OF ORGANIC CHEMISTRY ON TITAN. Thesis by. Robert Hodyss. In Partial Fulfillment of the Requirements.
 METHODS FOR THE ANALYSIS OF ORGANIC CHEMISTRY ON TITAN Thesis by Robert Hodyss In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy CALIFORNIA INSTITUTE OF TECHNOLOGY Pasadena,
METHODS FOR THE ANALYSIS OF ORGANIC CHEMISTRY ON TITAN Thesis by Robert Hodyss In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy CALIFORNIA INSTITUTE OF TECHNOLOGY Pasadena,
Development of reactive chemical absorbents at the CSIRO
 Development of reactive chemical absorbents at the CSIRO HiPerCap Workshop, March 25 2015 Graeme Puxty Research Team Leader CSIRO ENERGY FLAGSHIP CSIRO s chemical absorbent research program Concept Kinetics,
Development of reactive chemical absorbents at the CSIRO HiPerCap Workshop, March 25 2015 Graeme Puxty Research Team Leader CSIRO ENERGY FLAGSHIP CSIRO s chemical absorbent research program Concept Kinetics,
Period of validity: to date of issue:
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-RM-14176-01-00 according to the relevant clauses of ISO Guide 34: and DIN EN ISO/IEC 17025:2005 Period of validity: 11.01.2016
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-RM-14176-01-00 according to the relevant clauses of ISO Guide 34: and DIN EN ISO/IEC 17025:2005 Period of validity: 11.01.2016
Certificate of Analysis
 Certificate of Analysis Reference Material - Primary Standard Product Name: LSD (Lysergic Acid Diethylamide) 1.0 mg/ml in Acetonitrile Catalogue Number: Lot Number: 16905 LGCAMP1346.00-11 O CAS Number:
Certificate of Analysis Reference Material - Primary Standard Product Name: LSD (Lysergic Acid Diethylamide) 1.0 mg/ml in Acetonitrile Catalogue Number: Lot Number: 16905 LGCAMP1346.00-11 O CAS Number:
Certificate of Analysis
 Certificate of Analysis Reference Substance N-Desmethylclobazam (Norclobazam) Catalogue Number: Lot Number: 16595 LGCFOR0910.01 Molecular Formula: C 15 H 11 ClN 2 O 2 Molecular Weight: 286.71 CAS Number:
Certificate of Analysis Reference Substance N-Desmethylclobazam (Norclobazam) Catalogue Number: Lot Number: 16595 LGCFOR0910.01 Molecular Formula: C 15 H 11 ClN 2 O 2 Molecular Weight: 286.71 CAS Number:
Certificate of Analysis
 Certificate of Analysis Reference Substance Dehydronorketamine Hydrochloride Catalogue Number: Lot Number: 14579 LGCFOR0144.07 Molecular Formula: C 12 H 12 ClNO HCl Molecular Weight: 258.14 CAS Number:
Certificate of Analysis Reference Substance Dehydronorketamine Hydrochloride Catalogue Number: Lot Number: 14579 LGCFOR0144.07 Molecular Formula: C 12 H 12 ClNO HCl Molecular Weight: 258.14 CAS Number:
Introduction to biochemical practicals. Vladimíra Kvasnicová
 Introduction to biochemical practicals Vladimíra Kvasnicová arrangement of practicals laboratory safety regulations laboratory equipment dealing with automatic pipette instructions: http://vyuka.lf3.cuni.cz/
Introduction to biochemical practicals Vladimíra Kvasnicová arrangement of practicals laboratory safety regulations laboratory equipment dealing with automatic pipette instructions: http://vyuka.lf3.cuni.cz/
Certificate of Analysis
 Certificate of Analysis Reference Material - Primary Standard Product Name: Oxycodone 1.0 mg/ml in Methanol Catalogue Number: Lot Number: 37583 LGCAMP0672.10-01 CAS Number: 76-42-6 Molecular Formula: C
Certificate of Analysis Reference Material - Primary Standard Product Name: Oxycodone 1.0 mg/ml in Methanol Catalogue Number: Lot Number: 37583 LGCAMP0672.10-01 CAS Number: 76-42-6 Molecular Formula: C
Rigorous Modeling of a CO 2 -MEA Stripping System
 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 57, 2017 Guest Editors: Sauro Pierucci, Jiří Jaromír Klemeš, Laura Piazza, Serafim Bakalis Copyright 2017, AIDIC Servizi S.r.l. ISBN 978-88-95608-48-8;
A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 57, 2017 Guest Editors: Sauro Pierucci, Jiří Jaromír Klemeš, Laura Piazza, Serafim Bakalis Copyright 2017, AIDIC Servizi S.r.l. ISBN 978-88-95608-48-8;
Influence Of The Substrates On Water-based Paints Emissions Fabio Abbà,1*, Mikaela Decio 2, Tiziano Cerulli 2 and Roberto Leoni 2
 Influence Of The Substrates On Water-based Paints Emissions Fabio Abbà,1*, Mikaela Decio 2, Tiziano Cerulli 2 and Roberto Leoni 2 1 Vinavil S.p.A. R & D Laboratory, Via Toce, 7, 28844 Villadossola, Italy
Influence Of The Substrates On Water-based Paints Emissions Fabio Abbà,1*, Mikaela Decio 2, Tiziano Cerulli 2 and Roberto Leoni 2 1 Vinavil S.p.A. R & D Laboratory, Via Toce, 7, 28844 Villadossola, Italy
(RS)-2-(4-isobutyrylphenyl)propanoic acid Ibuprofen Impurity J
 Reference Material Product Information Sheet Epichem's Quality System conforms to ISO9001:2015 as certified by ECAAS Pty Ltd - Certification number 616061. Name BP Name Synonym(s) O (RS)-2-(4-isobutyrylphenyl)propanoic
Reference Material Product Information Sheet Epichem's Quality System conforms to ISO9001:2015 as certified by ECAAS Pty Ltd - Certification number 616061. Name BP Name Synonym(s) O (RS)-2-(4-isobutyrylphenyl)propanoic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
Available online at Energy Procedia 00 (2008) GHGT-9
 Available online at www.sciencedirect.com Energy Procedia (8) Energy Procedia www.elsevier.com/locate/xxx GHGT-9 Quantitative Evaluation of the Aqueous-Ammonia Process for CO Capture Using Fundamental
Available online at www.sciencedirect.com Energy Procedia (8) Energy Procedia www.elsevier.com/locate/xxx GHGT-9 Quantitative Evaluation of the Aqueous-Ammonia Process for CO Capture Using Fundamental
Certificate of Analysis
 Certificate of Analysis LGC Quality ISO Guide 34:2009 DAkkS D-RM-14176-01-00 ISO/IEC 17025:2005 DAkkS D-PL-14176-01-00 ISO 9001:2008 DQS 102448 QM08 Primary Reference Standard Granisetron Hydrochloride
Certificate of Analysis LGC Quality ISO Guide 34:2009 DAkkS D-RM-14176-01-00 ISO/IEC 17025:2005 DAkkS D-PL-14176-01-00 ISO 9001:2008 DQS 102448 QM08 Primary Reference Standard Granisetron Hydrochloride
Annex to the Accreditation Certificate D-RM according to ISO Guide 34:2009 and DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-RM-14176-01-00 according to ISO Guide 34:2009 and DIN EN ISO/IEC 17025:2005 Period of validity: 21.04.2017 to 04.08.2019 date
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-RM-14176-01-00 according to ISO Guide 34:2009 and DIN EN ISO/IEC 17025:2005 Period of validity: 21.04.2017 to 04.08.2019 date
Volatility of MEA and Piperazine
 Volatility of MEA and Piperazine Department of Chemical Engineering The University of Texas at Austin Austin, Texas 78712 USA Marcus Hilliard, John M c Lees, Dr. Gary T. Rochelle June 16, 2006 This presentation
Volatility of MEA and Piperazine Department of Chemical Engineering The University of Texas at Austin Austin, Texas 78712 USA Marcus Hilliard, John M c Lees, Dr. Gary T. Rochelle June 16, 2006 This presentation
Experimental study into carbon dioxide solubility and species distribution in aqueous alkanolamine solutions
 Air Pollution XX 515 Experimental study into carbon dioxide solubility and species distribution in aqueous alkanolamine solutions H. Yamada, T. Higashii, F. A. Chowdhury, K. Goto S. Kazama Research Institute
Air Pollution XX 515 Experimental study into carbon dioxide solubility and species distribution in aqueous alkanolamine solutions H. Yamada, T. Higashii, F. A. Chowdhury, K. Goto S. Kazama Research Institute
Certificate of Analysis
 Certificate of Analysis Reference Substance Benzoic Acid Catalogue Number: Lot Number: 43480 LGCFOR0278.01 Molecular Formula: C 7 H 6 O 2 Molecular Weight: 122.12 CAS Number: [ 65-85-0 ] Long-term Storage:
Certificate of Analysis Reference Substance Benzoic Acid Catalogue Number: Lot Number: 43480 LGCFOR0278.01 Molecular Formula: C 7 H 6 O 2 Molecular Weight: 122.12 CAS Number: [ 65-85-0 ] Long-term Storage:
Pilot / lab scale study of CO 2 separation with ionic liquid blending
 12 June-14 June, 2017 Pilot / lab scale study of CO 2 separation with ionic liquid blending Dawei Shang, Xiangping Zhang, Suojiang Zhang Institute of Process Engineering, Chinese Academy of Sciences 1
12 June-14 June, 2017 Pilot / lab scale study of CO 2 separation with ionic liquid blending Dawei Shang, Xiangping Zhang, Suojiang Zhang Institute of Process Engineering, Chinese Academy of Sciences 1
Summit:Technology and Opportunity
 Study of the gas-liquid CO 2 absorption in aqueous monoethanolamine solutions: development of a new experimental tool C. Wylock, S. Dehaeck, E. Boulay, P. Colinet and B. Haut CO 2 Summit:Technology and
Study of the gas-liquid CO 2 absorption in aqueous monoethanolamine solutions: development of a new experimental tool C. Wylock, S. Dehaeck, E. Boulay, P. Colinet and B. Haut CO 2 Summit:Technology and
ANALYSIS OF CHLORINATED BY-PRODUCTS IN SWIMMING POOL WATER BY MEMBRANE INTRODUCTION MASS SPECTROMETRY- INFLUENCE OF WATER PHYSICOCHEMICAL PARAMETERS
 ANALYSIS OF CHLORINATED BY-PRODUCTS IN SWIMMING POOL WATER BY MEMBRANE INTRODUCTION MASS SPECTROMETRY- INFLUENCE OF WATER PHYSICOCHEMICAL PARAMETERS N. CIMETIERE. N. GARANDEL, P. HUMEAU INTRODUCTION Chlorine
ANALYSIS OF CHLORINATED BY-PRODUCTS IN SWIMMING POOL WATER BY MEMBRANE INTRODUCTION MASS SPECTROMETRY- INFLUENCE OF WATER PHYSICOCHEMICAL PARAMETERS N. CIMETIERE. N. GARANDEL, P. HUMEAU INTRODUCTION Chlorine
Certificate of Analysis
 Certificate of Analysis Reference Standard 4,5α-Epoxy-14-hydroxy-3-methoxymorphinan-6-one Hydrochloride (Noroxycodone Hydrochloride) Catalogue Number: MM0672.03 Molecular Formula: C 17 H 19 NO 4 HCl Molecular
Certificate of Analysis Reference Standard 4,5α-Epoxy-14-hydroxy-3-methoxymorphinan-6-one Hydrochloride (Noroxycodone Hydrochloride) Catalogue Number: MM0672.03 Molecular Formula: C 17 H 19 NO 4 HCl Molecular
Survey Calculations Single Surveys
 Survey Calculations Single Surveys Introduction Survey calculations are single point calculations strung together in series. They are also referred to as multiple point calculations. These calculations
Survey Calculations Single Surveys Introduction Survey calculations are single point calculations strung together in series. They are also referred to as multiple point calculations. These calculations
Analytical Topics to Consider in preparation for the MFAT/GRE
 Analytical Topics to Consider in preparation for the MFAT/GRE 1. Solutions and Measurement: (CHEM 222) Describe the steps in a chemical analysis Know the meaning and use of the following solution concentrations.
Analytical Topics to Consider in preparation for the MFAT/GRE 1. Solutions and Measurement: (CHEM 222) Describe the steps in a chemical analysis Know the meaning and use of the following solution concentrations.
Australian Journal of Basic and Applied Sciences
 ISSN:1991-8178 Australian Journal of Basic and Applied Sciences Journal home page: www.ajbasweb.com Dynamic Modelling of Carbon Dioxide For Different Solvent Faezah Isa, Haslinda Zabiri, Pravin Chandran
ISSN:1991-8178 Australian Journal of Basic and Applied Sciences Journal home page: www.ajbasweb.com Dynamic Modelling of Carbon Dioxide For Different Solvent Faezah Isa, Haslinda Zabiri, Pravin Chandran
STEADY STATE SIMULATION AND EXERGY ANALYSIS OF SUPERCRITICAL COAL-FIRED POWER PLANT (SCPP) WITH CO 2 CAPTURE
 10 th European Conference on Coal Research and its Application () STEADY STATE SIMULATION AND EXERGY ANALYSIS OF SUPERCRITICAL COAL-FIRED POWER PLANT (SCPP) WITH CO 2 CAPTURE Akeem K Olaleye Process and
10 th European Conference on Coal Research and its Application () STEADY STATE SIMULATION AND EXERGY ANALYSIS OF SUPERCRITICAL COAL-FIRED POWER PLANT (SCPP) WITH CO 2 CAPTURE Akeem K Olaleye Process and
Certificate of Analysis
 Certificate of Analysis Reference Substance Atorvastatin Calcium Catalogue Number: Lot Number: 55085 LGCFOR0400.00 Molecular Formula: 2C 33 H 34 FN 2 O 5 Ca Molecular Weight: 1155.34 CAS Number: [ 134523-03-8
Certificate of Analysis Reference Substance Atorvastatin Calcium Catalogue Number: Lot Number: 55085 LGCFOR0400.00 Molecular Formula: 2C 33 H 34 FN 2 O 5 Ca Molecular Weight: 1155.34 CAS Number: [ 134523-03-8
Certificate of Analysis
 Certificate of Analysis LGC Quality ISO Guide 34:2009 DAkkS D-RM-14176-01-00 ISO/IEC 17025:2005 DAkkS D-PL-14176-01-00 ISO 9001:2008 DQS 102448 QM08 Primary Reference Standard Paracetamol H N Catalogue
Certificate of Analysis LGC Quality ISO Guide 34:2009 DAkkS D-RM-14176-01-00 ISO/IEC 17025:2005 DAkkS D-PL-14176-01-00 ISO 9001:2008 DQS 102448 QM08 Primary Reference Standard Paracetamol H N Catalogue
Certificate of Analysis
 Certificate of Analysis Reference Substance Cefalexin Monohydrate Catalogue Number: Lot Number: 38530 LGCFOR0605.00 Molecular Formula: C 16 H 17 N 3 O 4 S H 2 O Molecular Weight: 365.40 CAS Number: [ 23325-78-2
Certificate of Analysis Reference Substance Cefalexin Monohydrate Catalogue Number: Lot Number: 38530 LGCFOR0605.00 Molecular Formula: C 16 H 17 N 3 O 4 S H 2 O Molecular Weight: 365.40 CAS Number: [ 23325-78-2
Prepared for Presentation at the 2004 Annual Meeting, Austin, TX, Nov. 7-12
 Investigation of Methyl Acetate Production by Reactive Extraction Christian Rohde, Rolf Marr Department of Chemical Engineering and Environmental Technology University of Technology Graz Inffeldgasse 25,
Investigation of Methyl Acetate Production by Reactive Extraction Christian Rohde, Rolf Marr Department of Chemical Engineering and Environmental Technology University of Technology Graz Inffeldgasse 25,
Technologies and Approaches of CO 2 Capture
 Southwest Regional Partnership Project Technologies and Approaches of CO 2 Capture Liangxiong Li, Brian McPherson, Robert Lee Petroleum Recovery Research Center New Mexico Institute of Mining and Technology,
Southwest Regional Partnership Project Technologies and Approaches of CO 2 Capture Liangxiong Li, Brian McPherson, Robert Lee Petroleum Recovery Research Center New Mexico Institute of Mining and Technology,
The Role of Modeling in Addressing Scaling with Triazine Based H 2 S Scavengers
 The Role of Modeling in Addressing Scaling with Triazine Based H 2 S Scavengers OLI Simulation Conference 2014 Tuesday 21 st October Dr Neil Goodwin Technical Manager New Developments and Engineering Services
The Role of Modeling in Addressing Scaling with Triazine Based H 2 S Scavengers OLI Simulation Conference 2014 Tuesday 21 st October Dr Neil Goodwin Technical Manager New Developments and Engineering Services
Available online at Energy Procedia 1 (2009) (2008) GHGT-9
 Available online at www.sciencedirect.com Energy Procedia 1 (2009) (2008) 1257 1264 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-9 Solubility of CO 2 in Aqueous
Available online at www.sciencedirect.com Energy Procedia 1 (2009) (2008) 1257 1264 000 000 Energy Procedia www.elsevier.com/locate/procedia www.elsevier.com/locate/xxx GHGT-9 Solubility of CO 2 in Aqueous
Tar analysis by Solid Phase Adsorption (SPA) associated with Thermal Desorption (TD) and Gas Chromatography (GC) analysis
 Tar analysis by Solid Phase Adsorption (SPA) associated with Thermal Desorption (TD) and Gas Chromatography (GC) analysis E. Masson (CRITT Bois, Epinal, France) A. Dufour (CNRS, Nancy, France) International
Tar analysis by Solid Phase Adsorption (SPA) associated with Thermal Desorption (TD) and Gas Chromatography (GC) analysis E. Masson (CRITT Bois, Epinal, France) A. Dufour (CNRS, Nancy, France) International
Chem 321 Name Answer Key D. Miller
 1. For a reversed-phase chromatography experiment, it is noted that the retention time of an analyte decreases as the percent of acetonitrile (CH 3 CN) increases in a CH 3 CN/H 2 O mobile phase. Explain
1. For a reversed-phase chromatography experiment, it is noted that the retention time of an analyte decreases as the percent of acetonitrile (CH 3 CN) increases in a CH 3 CN/H 2 O mobile phase. Explain
Updating 8 m 2MPZ and Independence Models
 Updating 8 m 2MPZ and Independence Models Quarterly Report for January 1 March 31, 2013 by Brent Sherman Supported by the Texas Carbon Management Program and Carbon Capture Simulation Initiative McKetta
Updating 8 m 2MPZ and Independence Models Quarterly Report for January 1 March 31, 2013 by Brent Sherman Supported by the Texas Carbon Management Program and Carbon Capture Simulation Initiative McKetta
Copyright. Akinleye Olaolu Alawode
 Copyright by Akinleye Olaolu Alawode 2005 Oxidative Degradation of Piperazine in the Absorption of Carbon Dioxide by Akinleye Olaolu Alawode, B.S. Thesis Presented to the Faculty of the Graduate School
Copyright by Akinleye Olaolu Alawode 2005 Oxidative Degradation of Piperazine in the Absorption of Carbon Dioxide by Akinleye Olaolu Alawode, B.S. Thesis Presented to the Faculty of the Graduate School
CO 2 Capture by Absorption with Potassium Carbonate
 CO 2 Capture by Absorption with Potassium Carbonate Quarterly Progress Report Reporting Period Start Date: April 1, 2004 Reporting Period End Date: June 30, 2004 Authors: Gary T. Rochelle, Eric Chen, J.
CO 2 Capture by Absorption with Potassium Carbonate Quarterly Progress Report Reporting Period Start Date: April 1, 2004 Reporting Period End Date: June 30, 2004 Authors: Gary T. Rochelle, Eric Chen, J.
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Simulation of CO 2 removal in a split-flow gas sweetening process
 Korean J. Chem. Eng., 28(3), 643-648 (2011) DOI: 10.1007/s11814-010-0446-6 INVITED REVIEW PAPER Simulation of CO 2 removal in a split-flow gas sweetening process Hyung Kun Bae, Sung Young Kim, and Bomsock
Korean J. Chem. Eng., 28(3), 643-648 (2011) DOI: 10.1007/s11814-010-0446-6 INVITED REVIEW PAPER Simulation of CO 2 removal in a split-flow gas sweetening process Hyung Kun Bae, Sung Young Kim, and Bomsock
Luminescence transitions. Fluorescence spectroscopy
 Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Controlled FTIR measurements of acid gas (NO, mixed NO/SO 2 ) capture in water at high pressure
 ANLECR&D project 6 0215 0234 Controlled FTIR measurements of acid gas (NO, mixed NO/SO 2 ) capture in water at high pressure Sub contract to Macquarie University Report of ANLECR&D Project 6 0215 0243
ANLECR&D project 6 0215 0234 Controlled FTIR measurements of acid gas (NO, mixed NO/SO 2 ) capture in water at high pressure Sub contract to Macquarie University Report of ANLECR&D Project 6 0215 0243
Chromatography. Gas Chromatography
 Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Instrumental Analysis
 Chem 454 Name: Instrumental Analysis Exam I February 5, 1999 80 possible points 1] 5 points Which of the following samples would be suitable for analysis by a calibration curve technique using a potentiometric
Chem 454 Name: Instrumental Analysis Exam I February 5, 1999 80 possible points 1] 5 points Which of the following samples would be suitable for analysis by a calibration curve technique using a potentiometric
Analytical Chemistry
 Analytical Chemistry Chromatographic Separations KAM021 2016 Dr. A. Jesorka, 6112, aldo@chalmers.se Introduction to Chromatographic Separations Theory of Separations -Chromatography Terms Summary: Chromatography
Analytical Chemistry Chromatographic Separations KAM021 2016 Dr. A. Jesorka, 6112, aldo@chalmers.se Introduction to Chromatographic Separations Theory of Separations -Chromatography Terms Summary: Chromatography
Systems Engineering Spring Group Project #1: Process Flowsheeting Calculations for Acetic Anhydride Plant. Date: 2/25/00 Due: 3/3/00
 10.551 Systems Engineering Spring 2000 Group Project #1: Process Flowsheeting Calculations for Acetic Anhydride Plant Date: 2/25/00 Due: 3/3/00 c Paul I. Barton, 14th February 2000 At our Nowhere City
10.551 Systems Engineering Spring 2000 Group Project #1: Process Flowsheeting Calculations for Acetic Anhydride Plant Date: 2/25/00 Due: 3/3/00 c Paul I. Barton, 14th February 2000 At our Nowhere City
School of Chemistry UNIVERSITY OF KWAZULU-NATAL, WESTVILLE CAMPUS JUNE 2009 EXAMINATION CHEM340: INSTRUMENTAL ANALYSIS.
 School of Chemistry UNIVERSITY OF KWAZULU-NATAL, WESTVILLE CAMPUS JUNE 2009 EXAMINATION CHEM340: INSTRUMENTAL ANALYSIS DURATION: 3 HOURS TOTAL MARKS: 100 Internal Examiners: Professor A Kindness Dr T Msagati
School of Chemistry UNIVERSITY OF KWAZULU-NATAL, WESTVILLE CAMPUS JUNE 2009 EXAMINATION CHEM340: INSTRUMENTAL ANALYSIS DURATION: 3 HOURS TOTAL MARKS: 100 Internal Examiners: Professor A Kindness Dr T Msagati
Topsøe Catalysis Forum 2009
 Mercury Behaviour in Combustion Flue Gases Topsøe Catalysis Forum 9 Munkerupgaard 7 th -8 th of August 9 Dr. Harald Thorwarth Energie braucht Impulse Introduction clean gas Cr Co Ni Cd As Cu Pb Hg Input
Mercury Behaviour in Combustion Flue Gases Topsøe Catalysis Forum 9 Munkerupgaard 7 th -8 th of August 9 Dr. Harald Thorwarth Energie braucht Impulse Introduction clean gas Cr Co Ni Cd As Cu Pb Hg Input
Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth
 University of Groningen Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Carbon dioxide removal processes by alkanolamines in aqueous organic solvents Hamborg, Espen Steinseth IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
CHEMISTRY 31 FINAL EXAM Dec. 12, points total
 NAME CHEMISTRY 31 FINAL EXAM Dec. 12, 2016-150 points total Equations: Debye-Hückel Equation: 2 0.51 z µ logγ = 1+ ( α µ / 305) Where: γ = activity coefficient, μ = ionic strength, α = hydrated ion radius,
NAME CHEMISTRY 31 FINAL EXAM Dec. 12, 2016-150 points total Equations: Debye-Hückel Equation: 2 0.51 z µ logγ = 1+ ( α µ / 305) Where: γ = activity coefficient, μ = ionic strength, α = hydrated ion radius,
Substance Characterisation for REACH. Dr Emma Miller Senior Chemist
 Substance Characterisation for REACH Dr Emma Miller Senior Chemist Submission of a REACH registration dossier, either as a lead registrant or a joint registrant Submission of an Inquiry to ECHA Submission
Substance Characterisation for REACH Dr Emma Miller Senior Chemist Submission of a REACH registration dossier, either as a lead registrant or a joint registrant Submission of an Inquiry to ECHA Submission
Troubleshooting a Contaminated Refinery Tail Gas Amine Treater ABSTRACT. Introduction
 Troubleshooting a Contaminated Refinery Tail Gas Amine Treater Nathan A. Hatcher & Alfred E. Keller ConocoPhillips Company Ponca City, Oklahoma Ralph H. Weiland Optimized Gas Treating, Inc. Clarita, Oklahoma
Troubleshooting a Contaminated Refinery Tail Gas Amine Treater Nathan A. Hatcher & Alfred E. Keller ConocoPhillips Company Ponca City, Oklahoma Ralph H. Weiland Optimized Gas Treating, Inc. Clarita, Oklahoma
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Simultaneous analysis of the major metal cations and ammonium by CZE
 Simultaneous analysis of the major metal cations and ammonium by CZE This method has been published. You can download a copy of the PDF. Cite this method as: Warren CR, Adams MA (2004) Capillary electrophoresis
Simultaneous analysis of the major metal cations and ammonium by CZE This method has been published. You can download a copy of the PDF. Cite this method as: Warren CR, Adams MA (2004) Capillary electrophoresis
HPLC. GRATE Chromatography Lab Course. Dr. Johannes Ranke. September 2003
 HPLC GRATE Chromatography Lab Course Dr. Johannes Ranke Organisation The groups Start at 9:00 am End at 18:00 pm at the latest Friday, 19th we will finish at 2:00 pm Thursday, 11th: Lecture at 08:15 am
HPLC GRATE Chromatography Lab Course Dr. Johannes Ranke Organisation The groups Start at 9:00 am End at 18:00 pm at the latest Friday, 19th we will finish at 2:00 pm Thursday, 11th: Lecture at 08:15 am
3590/ Laboratory
 3590/3550 - Laboratory Time: Monday to Thursday 2:30 5:30 Location: 318 Parker Bldg. Five two week labs must be completed. Two students per experiment. Individual lab reports, sharing same data. There
3590/3550 - Laboratory Time: Monday to Thursday 2:30 5:30 Location: 318 Parker Bldg. Five two week labs must be completed. Two students per experiment. Individual lab reports, sharing same data. There
CO 2 capture by Adsorption Processes: From Materials to Process Development to Practical Implementation
 CO 2 capture by Adsorption Processes: From Materials to Process Development to Practical Implementation Paul A. Webley Dept. of Chemical Engineering Talloires, July 2010 Outline Adsorption for Post-Combustion
CO 2 capture by Adsorption Processes: From Materials to Process Development to Practical Implementation Paul A. Webley Dept. of Chemical Engineering Talloires, July 2010 Outline Adsorption for Post-Combustion
SIMULATION OF METHYLDIETHANOLAMINE- CARBON DIOXIDE-WATER SYSTEM USING EQUILIBRIUM APPROACH
 SIMULATION OF METHYLDIETHANOLAMINE- CARBON DIOXIDE-WATER SYSTEM USING EQUILIBRIUM APPROACH A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Bachelor of Technology in Chemical
SIMULATION OF METHYLDIETHANOLAMINE- CARBON DIOXIDE-WATER SYSTEM USING EQUILIBRIUM APPROACH A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Bachelor of Technology in Chemical
CHEMISTRY 31 FINAL EXAM - SOLUTIONS Dec. 10, points total
 CHEMISTRY 31 FINAL EXAM - SOLUTIONS Dec. 10, 2012 150 points total A. Multiple Choice and Short answer Section. Multiple Choice Answers in bold 1. A student calculates their unknown soda ash % NaCO 3 as
CHEMISTRY 31 FINAL EXAM - SOLUTIONS Dec. 10, 2012 150 points total A. Multiple Choice and Short answer Section. Multiple Choice Answers in bold 1. A student calculates their unknown soda ash % NaCO 3 as
Simulation of Electrolyte Processes: Status and Challenges
 Simulation of Electrolyte Processes: Status and Challenges Paul M Mathias and Chau-Chyun Chen Aspen Technology, Inc. 12 March 2002 AIChE Spring 2002 Meeting AIChE 2002 Spring Meeting. Summary Opportunities/needs
Simulation of Electrolyte Processes: Status and Challenges Paul M Mathias and Chau-Chyun Chen Aspen Technology, Inc. 12 March 2002 AIChE Spring 2002 Meeting AIChE 2002 Spring Meeting. Summary Opportunities/needs
Why Water Is Your Friend
 Why Water Is Your Friend Water has several properties which make life possible. Transparency Cohesion Capillary action Surface tension: watch this Fluid nature Temperature buffer Solvent ability 2.A.3.a.3.
Why Water Is Your Friend Water has several properties which make life possible. Transparency Cohesion Capillary action Surface tension: watch this Fluid nature Temperature buffer Solvent ability 2.A.3.a.3.
Nexera UC Unified Chromatography
 Nexera UC Unified Chromatography The latest addition to the chromatography toolbox Dr. Gesa J. Schad Shimadzu Europa GmbH A brief history of SFC ϒ Late 1800 s: it was found that heavy, non-volatile organic
Nexera UC Unified Chromatography The latest addition to the chromatography toolbox Dr. Gesa J. Schad Shimadzu Europa GmbH A brief history of SFC ϒ Late 1800 s: it was found that heavy, non-volatile organic
April 23, 2015 Lab 1 - Gravimetric Analysis of a Metal Carbonate
 Lab 1 - Gravimetric Analysis of a Metal Carbonate Purpose - to determine the identity of the cation in X 2 CO 3 by gravimetric analysis Lab Highlights Include: SLOW gravity filtration Primary Experimental
Lab 1 - Gravimetric Analysis of a Metal Carbonate Purpose - to determine the identity of the cation in X 2 CO 3 by gravimetric analysis Lab Highlights Include: SLOW gravity filtration Primary Experimental
Figure 1. Structures for Vitamin B2 and Vitamin B1.
 CH 461 & CH 461H F 18 Name Experiment 2C Integrated Laboratory Experiment DETERMINATION OF RIBOFLAVIN: A COMPARISON OF TECHNIQUES PART C. HIGH PERFORMANCE LIQUID CHROMATOGRAPHY The purpose of this experiment
CH 461 & CH 461H F 18 Name Experiment 2C Integrated Laboratory Experiment DETERMINATION OF RIBOFLAVIN: A COMPARISON OF TECHNIQUES PART C. HIGH PERFORMANCE LIQUID CHROMATOGRAPHY The purpose of this experiment
Development of Safe and Scalable Continuous-Flow Methods for. Palladium-Catalyzed Aerobic Oxidation Reactions
 Supplementary Data for Development of Safe and Scalable Continuous-Flow Methods for Palladium-Catalyzed Aerobic Oxidation Reactions Xuan Ye, a Martin D. Johnson, *b Tianning Diao, a Matthew H. Yates *b
Supplementary Data for Development of Safe and Scalable Continuous-Flow Methods for Palladium-Catalyzed Aerobic Oxidation Reactions Xuan Ye, a Martin D. Johnson, *b Tianning Diao, a Matthew H. Yates *b
Methods of pollution control and waste management - laboratory. Adsorptive removal of volatile organic compounds from gases streams
 Methods of pollution control and waste management - laboratory Adsorptive removal of volatile organic compounds from gases streams Manual for experiment 17 dr Hanna Wilczura-Wachnik and dr inż. Jadwiga
Methods of pollution control and waste management - laboratory Adsorptive removal of volatile organic compounds from gases streams Manual for experiment 17 dr Hanna Wilczura-Wachnik and dr inż. Jadwiga
Gaseous CEMS technology
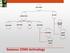 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
CHEMICALLY COMPLEXING IONIC LIQUIDS FOR POST-COMBUSTION CO 2 CAPTURE
 CHEMICALLY COMPLEXING IONIC LIQUIDS FOR POST-COMBUSTION CO 2 CAPTURE Burcu E. Gurkan, Juan C. de la Fuente, Elaine M. Mindrup, Lindsay E. Ficke, Brett F. Goodrich, Erica A. Price, William F. Schneider*,
CHEMICALLY COMPLEXING IONIC LIQUIDS FOR POST-COMBUSTION CO 2 CAPTURE Burcu E. Gurkan, Juan C. de la Fuente, Elaine M. Mindrup, Lindsay E. Ficke, Brett F. Goodrich, Erica A. Price, William F. Schneider*,
*Correspondence to:
 Supporting Information for Carbonate-promoted hydrogenation of carbon dioxide to multi-carbon carboxylates Aanindeeta Banerjee 1 and Matthew W. Kanan 1 * 1 Department of Chemistry, Stanford University,
Supporting Information for Carbonate-promoted hydrogenation of carbon dioxide to multi-carbon carboxylates Aanindeeta Banerjee 1 and Matthew W. Kanan 1 * 1 Department of Chemistry, Stanford University,
Environmental Engineering and Reactor Technology
 Environmental Engineering and Reactor Technology Teaching and research principles of separation, reactor technology and process design The largest research group in the department about 40 PhD-students
Environmental Engineering and Reactor Technology Teaching and research principles of separation, reactor technology and process design The largest research group in the department about 40 PhD-students
CO 2 Capture by Aqueous Absorption
 CO 2 Capture by Aqueous Absorption Summary of First Quarterly Progress Reports 2012 by Gary T. Rochelle Supported by the Luminant Carbon Management Program and the Industrial Associates Program for CO
CO 2 Capture by Aqueous Absorption Summary of First Quarterly Progress Reports 2012 by Gary T. Rochelle Supported by the Luminant Carbon Management Program and the Industrial Associates Program for CO
Multiple Choice (60%)
 Chemistry Test 4-3 (May) Please use the answer sheet. Thank you! Multiple Choice (60%) 1) Please consider a 5.00-mL sample of lead with a density of 11.4 g/ml and a 5.00-mL sample of gold with a density
Chemistry Test 4-3 (May) Please use the answer sheet. Thank you! Multiple Choice (60%) 1) Please consider a 5.00-mL sample of lead with a density of 11.4 g/ml and a 5.00-mL sample of gold with a density
Compounds in Aqueous Solution
 1 Compounds in Aqueous Solution Many reactions involve ionic compounds, especially reactions in water KMnO 4 in water K + (aq) ) + MnO 4- (aq) 2 CCR, page 149 3 How do we know ions are present in aqueous
1 Compounds in Aqueous Solution Many reactions involve ionic compounds, especially reactions in water KMnO 4 in water K + (aq) ) + MnO 4- (aq) 2 CCR, page 149 3 How do we know ions are present in aqueous
Supporting Information to Accompany:
 SI-1 Supporting Information to Accompany: Mixer based on Magnetically Driven Agitation in a Tube (MDAT) Affords ast og-resistant Mixing to acilitate low Chemistry. Sarah J. Dolman, Jason L. Nyrop and Jeffrey
SI-1 Supporting Information to Accompany: Mixer based on Magnetically Driven Agitation in a Tube (MDAT) Affords ast og-resistant Mixing to acilitate low Chemistry. Sarah J. Dolman, Jason L. Nyrop and Jeffrey
This is a repository copy of Optimised PEI impregnation of activated carbons - Enhancement of CO2 capture under post-combustion conditions.
 This is a repository copy of Optimised PEI impregnation of activated carbons - Enhancement of CO2 capture under post-combustion conditions. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/89498/
This is a repository copy of Optimised PEI impregnation of activated carbons - Enhancement of CO2 capture under post-combustion conditions. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/89498/
Introduction to Chromatographic Separations
 Introduction to Chromatographic Separations Analysis of complex samples usually involves previous separation prior to compound determination. Two main separation methods instrumentation are available:
Introduction to Chromatographic Separations Analysis of complex samples usually involves previous separation prior to compound determination. Two main separation methods instrumentation are available:
Gas Chromatography (GC)! Environmental Organic Chemistry CEE-PUBH Analysis Topic 5
 Gas Chromatography (GC)! Environmental Organic Chemistry CEE-PUBH 5730-6730 Analysis Topic 5 Chromatography! Group of separation techniques based on partitioning (mobile phase/stationary phase). Two immiscible
Gas Chromatography (GC)! Environmental Organic Chemistry CEE-PUBH 5730-6730 Analysis Topic 5 Chromatography! Group of separation techniques based on partitioning (mobile phase/stationary phase). Two immiscible
