Introduction to electron microscopes: electron optics, interactions and signals
|
|
|
- Charles Glenn
- 5 years ago
- Views:
Transcription
1 Introduction to electron microscopes: electron optics, interactions and signals J.L. Lábár 1 Research Institute for Technical Physics and Materials Science, H-1121, Budapest, Konkoly- Thege u , Hungary Introduction Present paper serves as a general introduction to the topics of the 15 tutorials following it. By sketching the general layout of electron microscopes, first we shall discuss what are the similarities and differences between the individual types of electron microscopes, namely the scanning electron microscope (SEM), the transmission electron microscope (TEM) and the analytical electron microscope (AEM). We shall see what tells apart a microprobe from a scanning electron microscope. Next, we schematically follow the scatterings of a bombarding electron within the sample and classify the elastic and different inelastic scattering processes. Both the primary scattering event and the ensuing secondary physical processes produce a multitude of signal used both in different modes of imaging and in spectroscopic and diffraction analysis. The main structure of that broad topic and the relation between the parts are delineated here. The details will be given in the ensuing lectures. What makes an "electron microscope"? This workshop is about the examination of different materials with electrons as we do it in an electron microscope or a microprobe. Examination includes both imaging aspects and analytical aspects. Since electrons interact strongly with any form of material and we want to restrict the interaction to the sample (in contrast to its surrounding), the samples are examined in vacuum. The schematic diagrams of the general principle is sketched in Fig. 1. The electron source emits electrons that are accelerated. That monoenergetic primary electron beam is formed and shaped by the condenser lenses and by the (upper part of the) objective lens. This lens-system facilitates changing the current in the electron beam that bombards our sample and controls the beam convergence (from parallel broad beam illumination in a TEM to finely focused convergent beam in an SEM, EPMA or AEM). The interaction of the electrons with the matter in our sample produces different signals (electrons, photons from the infrared through visible till the X-ray range, etc.) These signals can be used for imaging different sample characteristics, like surface morphology or distribution of electrically active crystal defects or local composition, etc. Beside the two-dimensional (2D) distributions (images), 1D distributions (line-scans) and 0D characteristics (point measurements) can also be obtained. The measured characteristics can be presented qualitatively and interpreted intuitively (e.g. looking at an image and recognizing a broken metallization in an integrated circuit (IC)) or can be presented quantitatively (e.g. by determining the local chemical composition). If the sample is semi-infinite (as it is in an SEM), the detectors can only be located on the same halfspace as the electron source. Images are generated electronically using the scanning principle in these machines and imaging in the (electron)optical sense is not present. In case of thin, transparent samples, signals can be detected from both sides. In 1 labar@mfa.kfki.hu Lábár: Introduction 1 / 14
2 this latter case, further lenses may be present at the other side of the sample (as in a TEM). These lenses can be used to form a true electronoptical image or may not be present (as in a special form of the AEM: the dedicated scanning transmission electron microscope, the DSTEM). First, we are going to give a brief overview of the general components of such an instrument. Then we classify the main mechanisms of electron-matter interaction in order to understand what kinds of signals are generated and how can we detect them. Finally, we summarize how these hardware-components and signals appear in the different instruments and what are they used for. Figure 1. Main components of an "abstract electron beam instrument" that includes different electron microscopes and the microprobe as special cases. Vacuum system The electron column (comprising the electron gun and the lenses) and the specimen chamber (also including the specimen holder / specimen stage) form the body of the microscope. The evacuated space between the gun and the specimen is ensured by different vacuum pumps. Starting from atmospheric pressure, rough vacuum (~10-1 Pa) is reached with fore-vacuum pumps, namely rotary pumps or membrane pumps. These same pump-types serve for the backing of the high-vacuum (HV) pumps that further reduce the pressure into the Pa in the specimen chamber and into ultra high vacuum (UHV: Pa) in the gun chamber (in case of a field emission gun). Diffusion pumps or turbomolecular pumps can reach the normal HV (or UHV) region that can also be maintained with the help of an iongetter pump. Lábár: Introduction 2 / 14
3 Figure 2/a: Operation of a rotary (mechanical) pump. The vanes protruding from the eccentric rotor are always in touch with the pump chamber. Gas molecules are compressed in the outlet side, while a vacuum is created in the inlet side during rotation. Backstreaming of the lubricating oil into the evacuated microscope column is generally prevented by a cooled oil trap above the next pump stage. Figure 2/b: Structure of a diffusion pump. Boiling oil produces vapor jets that drag gas molecules with them. These molecules are freed as the oil condenses on the cooled pump wall and are extracted by the backing mechanical pump. Diffusion pumps, producing a vacuum pressure down to 10-9 Pa can transport several hundreds of liters or air per second. Figure 2/c: A Ti iongetter pump emits electrons from its cathode. The electrons ionize gas atoms while spiraling in the magnetic field. The ionized gas atoms are accelerated by the electric field and sputter Ti atoms from the anode during their impact. The condensing Ti atoms trap gas atoms on every surface. The pump can be switched on at a vacuum <10 3 Pa. Pirani and Penning vacuum gauges (manometers) can monitor the pressure down to 1 Pa and 10-3 Pa, respectively, while hot cathode ionization vacuum meters can be used in the Pa range. Pirani gauges measure the heat conduction in a thin metallic wire (Pt or W). Reproduced from [5] Lábár: Introduction 3 / 14
4 Monotonic change of heat conduction with pressure is calibrated with more expensive manometers. Spontaneous ionization of gas molecules is used in a Penning gauge. An external magnetic field is applied to spiral the charged particles between the cold cathode and the anode, enhancing further ionizations. The large number of electrons emitted from a hot cathode produces enough ionizations without a magnetic field in the ionization gauges. Current is a calibrated function of the gas pressure in both types. The current in an iongetter pump is also a monotonic function of the (low) gas pressure, so the pump is its own vacuum gauge ( Pa). A pressure of 10-1 Pa would be enough to prevent our primary electrons from colliding on the gas atoms. The better vacuum is needed to ensure as clean a sample surface, as it is possible. Electron guns Electron guns produce the monoenergetic (monochromatic) primary electrons used to illuminate (bombard) the sample. Their function includes both emission and acceleration of the electrons. The electrons sources (acting as cathodes in the gun) can be classified on the basis of the physical principle behind their electron emission as either thermionic or field emission (FE) sources. The type of electron source is fixed at manufacture time and thermionic guns can not be interchanged with FE guns later. The current density obtained from a thermionic source is described by the Richardson formula J Φ 2 k T = A T e (1) where both A and the "work function", Φ are material-constants, T is the temperature (in Kelvin) and k is Boltzmann's constant. J can be increased either by increasing T or by reducing Φ. Only a few refractory (high melting temperature) materials (like W) can withstand the required high temperature to obtain reasonable current density and increase of the temperature beyond saturation reduces cathode lifetime with minor gain in current. Materials with lower work function (like LaB 6 or CeB 6 ) can be operated at lower temperatures and still produce higher brightness (current density/solid angle) and live longer (See Table 1). Reactivity of these materials requires a vacuum, better by 2 orders of magnitude than a W cathode. Brightness limits microscope (and microprobe) performance when the largest signal is needed in the smallest possible spot. Brightness of thermionic sources is linearly increasing with primary beam energy (accelerating voltage). The higher voltage is the better from that point of view. A field emission gun (FEG) emits electrons through the tunneling effect. The high electric field (that increases with the curvature) around a sharp tip of a W needle cathode sucks the electrons out of the cathode by tunneling them below the potential barrier (in contrast to elevating them above it). The cathode is at room temperature and must be extremely clean. That is why FEGs operate at Pa. Even though, regular cleaning ("flushing") of the cathode is needed. The gun contains two anodes. The first one provides the "extraction field" and the second accelerates the electrons to the required energy. Due to this construction the brightness does not depends significantly on the accelerating voltage. Although the brightness is the highest for FEGs, the maximum emitted current (needed for low magnification operation) is the lowest for them and thermionic cathodes surpass FEGs in that respect. Long term stability is also poorer with FEGs (<5%/hour with feedback regulating electronics). Lábár: Introduction 4 / 14
5 Thermally assisted FEGs or Schottky emitters eliminate contamination of the cathode (and the need for flushing) by the elevated temperature at a price of a slightly increased energy spread. Except of a few applications, they provide a good compromise. Units W LaB 6 FE Φ ev Operating temperature K Brightness A/m 2 /sr * Energy spread ev Vacuum Pa Lifetime h >1000 Table 1. Comparison of electron sources at 100 kv. Electromagnetic lenses Electrons (being charged particles) can be deflected (focused) with the help of an electromagnetic field. The effect of an (electro)magnetic lens on the moving electron is twofold. On the one hand, deflection towards the optical axis is identical to the focusing effect of a convex (magnifying) lens in light optics. On the other hand, an additional rotation around the optical axis occurs in the case of the electrons. The two effects can be treated separately. Ray diagrams, based on optics can be easily drawn to understand most of the effects in the electron microscope. The additional rotation of the electron beam can be taken into account separately. Both analytical applications and imaging based on the scanning principle need a finely focused beam with beam size and beam current as the two most important parameters. An ideal (aberration free) illumination system (condenser lenses + the (upper) objective lens) produces a demagnified real image of the electron source in the focused beam at the surface of the sample where every point in the electron source is imaged into an ideal point on the sample. As the magnification of the illumination system (do NOT confuse with the magnification of the imaging system!) is decreased, the (Gaussian) probe size is decreased proportionally. Probe current is reduced simultaneously, since the virtual solid angle subtended by the source (looked from the sample) is reduced. That is why brightness is the relevant parameter determining the achievable current density at the sample. The action of the lens is realized through the magnetic field generated by the current flowing through a coil. That field is concentrated in a gap by a polepiece. Perfectness of the field is determined by the quality of the polepiece. The effect of a real lens deviates from the ideal one due to the same types of aberrations that we know from light optics. In contrast to light optics, the quality of the best electromagnetic lenses is poor. Deviation from rotational symmetry is called astigmatism and can be corrected for by exciting an additional quadrupol lens asymmetrically. Chromatic aberration means that the focal length depends on the energy of the electrons (wavelength, i.e. color in optics). Since the primary electrons are monochromatic (<2 ev for LaB 6 and <0.6 ev for FEG), chromatic aberration is not a limiting factor in reducing the probe size of the primary electrons (which is of importance in our analytical applications), at least at higher energies. The probe-size limiting aberration is spherical aberration, i.e. the imperfection of the lens that nominal focal length (Gaussian focus) is only valid for the rays parallel to the optical axis. The larger an electron (or light) ray Lábár: Introduction 5 / 14
6 deviates from being parallel to the optical axis, the shorter the focal length is. The effect is a blurred disk at the focal plane, as shown in Fig. 3. Figure 3: Spherical aberration blurs the image or an ideal point into a finite disk. The resulting probe size can be reduced in either of two ways. Either the focus is shifted from the Gaussian focus to the "disk of minimum confusion", or the size of the probe-forming aperture is reduced, excluding the rays passing far from the optical axis. The first solution can not reach the minimum possible probe size, but the beam current is not compromised. The second solution produces minimum probe size at a price paid in beam current. Remember: ultimate probe size (for a given probe current) also depends on the selection of the probe-forming aperture! Real beam size is a superposition of the Gaussian probe size and the broadening caused by the spherical aberration. Since a given signal is needed for detection with a given precision, the best resolution of our image (or analysis) will be a function of gun brightness, lens aberration and selection of the operating parameters (beam forming aperture and correct alignment). Sample stages (eucentricity) The function of specimen stages / specimen holders is that a preselected location of the larger sample should be positioned suitably for imaging and analysis. Selection of the area requires lateral movement in two independent directions (called "x" and "y" directions). Tilting (in at least one or preferably two directions) is needed for orienting the preselected sample feature into the direction optimal for either imaging or analysis. For optimal examination, the preselected sample point should be on the optical axis at the object distance, determined by the objective lens. (The object distance is strictly determined for a TEM and can be varied for an SEM with variable working distance.) Lateral movement in the plane of the holder is trivial, so let's examine tilting. Lábár: Introduction 6 / 14
7 It is desirable that tilting do not induce shift in the image (the examined object should not move out of the field of view when we want to view it from a different orientation). Separation of tilt from lateral shift is achieved by eucentric stages (holders). In a eucentric holder, any preselected point of the sample can be made coincide with the tilting axis (which crosses the optical axis in a well-aligned microscope). Points on the tilting axis remain stationary (but viewed from different angles) while tilting. Points at a distance "r" from the tilting axis move along an arc with radius "r" while tilting. Fine shift possibility perpendicular to the directions of lateral shifts (called "z"-direction) ensures that the preselected point can be placed on the tilting axis. In double-tilting holders, it is only the main tilt that is eucentric (in order to keep both manufacturing and operation simple). The secondary tilt usually introduces lateral shift. Samples and sampled volume Most of our microscopes and microprobes operate in a vacuum and bombard the sample with energetic electrons. Consequently, solid samples can be examined which are not altered by either the vacuum or by the electron bombardment. Electrical conductivity is also needed in order to prevent artifacts and instabilities caused by charging. (Exception is the low pressure (environmental) SEM, which can examine insulating, wet and even living objects.) Many of the charging artifacts can be suppressed by evaporating a thin conductive layer on the sample. Au (or Pd/Au) is preferred from imaging in the SEM and carbon is preferred for TEM or analysis. SEM imaging does not need further sample preparation. Quantitative microprobe analysis relies on correction calculations, which assume defined sample geometry. Consequently, a polished, smooth and flat sample surface, positioned perpendicular to the electron beam is assumed and that requires sample preparation. Maximum sample size is determined by the sample stage (holder). For a TEM, thickness is additionally restricted to ensure electron transparency. High-resolution imaging and analysis in a TEM further restricts thickness down to a few nanometers. Sample preparation (thinning) is more complicated correspondingly. Last but not least, the sample should be representative of the property or process we want to examine. The rules of correct sampling can not be overemphasized. The volume of signal generation and the penetrating power of the selected signal together determine the volume we sample. For signals that cross the material without significant loss (like hard X-rays), the sampled volume coincide with the excited volume (varying from the µm 3 range for bulk samples EPMA down to nm 3 range for thin samples in an AEM). For strongly absorbed signals (like seconder electrons or Auger electrons) only a depth of a few atomic layers is sampled in an area determined by the probe size, irrespective of the excited depth. The physical basis of both imaging and analysis: electron scattering Whenever the energy balance of the collision of two particles (bodies) can be described with kinetic energies only, we speak of elastic scattering. When either other forms of energy or the appearance of new particles must be taken into account for energy conservation, the scattering is inelastic. Conservation of momentum applies to both forms of scattering. Elastic scattering It is known from classical physics that the partitioning of the kinetic energy between two colliding bodies (with masses m1 and m2) only depends on the ratio of their masses: m1/m2. Since the mass of the electron is negligible as compared to the mass of any finite sample (or Lábár: Introduction 7 / 14
8 even with a single atomic nucleus), the kinetic energy taken by the sample can be neglected. Energy conservation during an elastic scattering means conservation of the kinetic energy for the primary electron (i.e. the conservation of its velocity). Elastic scattering means a change in the direction of the electron's speed and it is mainly caused by the collision with atomic nuclei. General theory of microanalysis is based on the assumption that the sample is homogeneous and isotropic, so crystalline effects are not taken into account. For such materials (like an amorphous sample), calculation of elastic scattering can be restricted to the calculation of scattering angle. Elastic scattering is characterized with relatively large average scattering angles (in contrast to inelastic scattering), resulting that a considerable fraction of the primary electrons may "turn back" and leave the bulk sample, producing the useful backscattered electron signal. The isotropic approximation is used in Monte Carlo (MC) calculations which calculate the measured signal intensity by following the individual scattering events of the primary electron (and of the secondaries) and summing up the probabilities of signal generation during all of these scattering events. Effects due to crystallinity are out of the scope of such calculations and of all the correction procedures used to convert measured X-ray signals into chemical compositions. Consequently, the presence of crystalline material is a source of systematic error for X-ray microanalysis. This error is rare and small for EPMA of bulk materials, but it can be significant during EDS analysis of thin samples. Crystalline effects are also needed to explain all diffraction patterns, including electron backscattering (EBSD) patterns in an SEM and to simulate the contrast in a (HR)TEM. Kinematic (single scattering) theory corresponds to the Bragg-equation of diffraction. The Kikuchi lines in a selected area electron diffraction (SAED) pattern in a TEM or the (pseudo)kikuchi-lines in an EBSD pattern manifest the traces of the atomic planes in Braggcondition (for the given orientation of the given structure). Interpretation of diffraction patterns is based on the calculation of electron states in a periodic crystal that are described by Bloch-waves, which are the solutions of a Schrödinger-like equation. Dynamic (multiple elastic scattering) effects are also included in the Bloch description. Deviation from the kinematic theory is mainly restricted to the crossing-points of such lines. Elastic scattering permutates the electron between Bloch-states corresponding to the same total energy. Interference between such coherent Bloch-waves produces the atomic resolution phase contrast image in the conventional HRTEM. Correct interpretation of such images can only be done on the basis of extended image simulations. Inelastic and quasielastic scattering "Loss" of kinetic energy (of the primary electron) is mainly caused by the interaction with the electrons of the sample. The simplest inelastic scattering process is single electron excitation or ionization. In that case a bound electron of the sample is kicked off its bound state and receives kinetic energy. It is called a secondary electron (in contrast to the primary electron). The most probable value of the received kinetic energy is a few ev and it is below 50 ev for most of the cases. Strictly speaking of quantum mechanics, the primary and secondary electrons can not be distinguished. However, we shall call primary electron the one with the large part of the kinetic energy and secondary electron the one with the small kinetic energy. If the secondary is generated close to the sample surface, it can leave the sample with high probability and can be collected as a signal electron. If it is generated deep in the volume, it is absorbed with high probability and can not escape. The kinetic energy lost by the primary is a sum of the kinetic energy and the original binding energy of the secondary. The binding energies are quantized and characteristic of the atoms (for inner shells), so the minimum energy-loss for each such event-type represents a characteristic loss (onset of an edge-type Lábár: Introduction 8 / 14
9 loss) and serves as a basis for electron energy loss spectroscopy (EELS) in the TEM. Since the binding energies of inner shells are large, these infrequent inelastic collisions cause large individual losses (up to several tens of kev). One of the two competing processes that brings the atom back to its ground state after inner shell ionization is X-ray emission used in EPMA and AEM. The frequent event of single electron excitation in an outer shell is characterized by a binding energy in the 1 ev - multiples of 10 ev range and by the production of secondary electrons. The production of electron-hole pairs in a semiconductor also belongs to single electron excitations. The second type of inelastic scattering is the interaction with electrons of the conduction (valence) band. These electrons respond with a collective oscillation (correlated motion) to the excitation by the primary electron. The quantized pseudo-particle describing this collective oscillation is the so-called plasmon. Its energy (lost by the primary) is in the ev range. It is a frequent scattering event resulting in a strong peak in the EELS spectrum. The same process is responsible for the multiple scattering that smears out the characteristic EELS edges when the sample thickness increases. The third type of inelastic scattering is frequently called quasielastic, because (although strictly speaking it is inelastic) the loss in the kinetic energy of the primary is so low that we can not separate it from elastic scattering with the usual electron energy loss spectrometers. These losses of the order of 10-2 ev are caused by excitation of lattice vibrations, the so-called phonons (dissipating as heat). Since the nuclei are well localized, the scattering in reciprocal space extends to large angles, resulting in diffuse scattering between the Bragg peaks. The high angle annular dark field (HAADF) electron detector unique of the best AEM, the socalled dedicated scanning transmission electron microscope (DSTEM) uses this thermal diffuse scattered signal to produce atomic resolution incoherent images that can be interpreted intuitively. Thermal diffuse scattering is the most probable event at room temperature. The resulting effect of the multitude of inelastic scattering events is that the primary electron is slowed down in a bulk sample. Most of the inelastic scattering events cause a small angle scattering (as compared to elastic scattering). That is why MC calculations frequently approximate the net effect of many inelastic scatterings with the so-called continuous slowing down approximation which is nothing else than calculating the most probable energy loss between two consecutive elastic scatterings from an analytical formula (in contrast to calculating many inelastic events individually). Sample thickness, single, multiple and plural scattering; Monte Carlo simulations The larger the sample thickness, the larger the probability that the primary electron is scattered more than once within the sample. It is always the case for bulk samples in the SEM. If a few scattering happens we call it multiple scattering. Reduction of the elastic peak and appearance of peaks at multiples of plasmon energy in the EELS spectrum are characteristic of multiple scattering for thin samples. EELS edges are rounded, elemental analysis is difficult in this range. The name of a large number of consecutive scatterings of a primary electron is plural scattering. EELS analysis is impossible in this thickness range. Sophisticated MC calculations follow all the elastic scattering events and the inner shell ionizations (with large individual energy losses) individually. They take the effect of plural inelastic scattering events into account by the continuous slowing down approximation. The Monte Carlo name originates from probability. (Reasoning that generating random numbers is the same as gambling in a Casino in Monte Carlo.) The most probable values of scattering angle, traveled distance and lost energy are determined by random numbers. The probabilities Lábár: Introduction 9 / 14
10 are scaled to give back physically meaningful values for the sum of a large number of scattering events. The main advantage is that arbitrary geometrical boundary conditions can be taken into account during the calculations. Generated signals and signal detectors As we mentioned in connection with inelastic scattering, a large number of secondary electrons (SE) with low energy are generated in the sample and the SEs generated close to the surface escape and can be detected. Due to their low energy, SEs can be efficiently collected by an attractive electric field (of 10 kev) into the scintillator of an Everhardt-Thornley detector. The attractive field collects the SEs from practically every direction, ensuring that the collection solid angle approximates to a hemisphere. The scintillator converts the electron signal into light, which is detected by a photomultiplier. The analog output of the multiplier can modulate the intensity of a TV display (old terminology is cathode ray tube, CRT) when the image is formed on the basis of the scanning principle. SE images are sensitive to surface morphology. Edges and sharp tips show up bright, since an inner point is close to the surface in multiple directions in these regions, resulting in enhanced SE emission. Emission of these low energy SEs is sensitive to even small changes of local surface potential. The resulting potential contrast (voltage contrast) can be used to map the local electric potential (e.g. over the surface of an operating integrated circuit). As we saw, many primary electron turn back from a bulk sample due to the large angle of elastic scattering and due to the large number of scattering events. (It does not make too much difference if some of them were inelastic. It only reducing the energy if the backscattered electron, resulting in a broader spectrum of BE energies.) These energetic backscattered electrons (BE) carry twofold information. On the one hand, elastic scattering (and hence the fraction of electrons that backscatter) depends on the average atomic number in a monotonic manner, serving as a basis for atomic number contrast. On the other hand, backscattering coefficient also depends on the elevation of the emitting surface, resulting in a topographic contrast component. The two signal components can be separated with a pair of symmetrically positioned BE detectors. In most of the cases a large area, reverse-biased p-n junction serves as a BE detector. The energetic BEs generate a wealth of electron-hole pairs in the detector collected at the opposite electrodes by the bias field. The current of the detector is proportional to the number of BEs. The detector is more efficient at higher accelerating voltages (more energetic BEs). Directional dependence of the BEs from a single crystal (due to Bragg reflection) can be mapped with a 2D charged couple device (CCD) detector. The small modulation superposed over a large isotropic background is separated in EBSD. Computer processing identifies the Kikuchi-lines in the weak contrast and compares to patterns simulated for the given (known) crystal structure. Orientation of individual grains is determined and processing of thousands of grains yields an orientation map. Pole figures for textures and misorientation maps can also be deduced. If the sample is connected to Earth-potential, the absorbed current through its connection gives a mixed contrast of both atomic number and topographic contrast. Although no special detector is needed, separation of the contrast components is impossible, limiting its usage. Transmitted electrons (in case of a thin sample in the TEM) carry mass thickness (mainly for amorphous samples) and diffraction contrast information (for crystalline samples). The bright field (BF) and the dark field (DF) detectors in a STEM use these contrast mechanisms. Lábár: Introduction 10 / 14
11 Internal electric fields in the sample can be imaged in a (semiconducting) sample if an external short-circuit (with an included current meter) is attached to it. When the primary beam excites a small volume far from the internal field region, the generated electron-hole pairs are recombined within their diffusion range and no signal is generated in the external short circuit. However, the electron-hole pairs are separated by the internal field when they are generated at the field region (within the diffusion range of the charge carriers) and a compensation current flows in the external circuit. This signal is called electron beam induced current (EBIC). Typically p-n junctions, Schottky-barriers and electrically active dislocations in semiconductors and grain boundaries in several ceramics are examined by EBIC. Cathodoluminescence (CL) gives information complementary to EBIC: the distribution of recombination centers for the charge carriers. Recombination of the electron-hole pairs emits a photon in the infrared (IR), or in the visible range. The signal of a photon detector shows the regions of recombination centers bright in the CL image while the beam is scanned. Electron energy analyzers are applied for two different purposes. On the one hand, the EELS detector in a TEM measures the energy distribution of electrons having passed the thin sample. Beside additional information, the characteristic edges of inner shell ionizations provide the chemical composition of the sample. On the other hand, the Auger electron spectrometer (AES) measures the kinetic energy of electrons leaving the surface of a bulk sample. The Auger-process is the alternative of X-ray emission after inner shell ionization and identifies the atoms by the characteristic energies of the emitted electron, similarly to the characteristic X-rays (see Fig. 4). The main signal for this community is X-rays, generated in the secondary process (after the primary process, ionization). The inner hole is filled from an outer shell and the difference in the energies of the two shells is carried away by an X-ray photon (see Fig. 4). The energies of a given X-ray line are a monotonic function of the atomic number, as described by Moseley's law. This monotonic dependence facilitates qualitative analysis. By measuring the energies of the characteristic X-ray lines (from Moseley's law) we obtain a list of the elements present. The two usual types of X-ray detectors are treated in two different lectures. Quantitative analysis requires the measurement of the number of photons for one selected X-ray line per every element present in the sample. (In X-ray analysis we superficially call the number of photons "intensity", although strictly speaking, intensity in physics has a different dimension.) The correction procedure, needed to convert the measured X-ray intensities into chemical compositions is elaborated in a different talk. The effect of crystal structure; ALCHEMI The systematic error in the chemical composition (measured from X-ray intensities) caused by dynamic electron diffraction in a single crystal is turned into a source of additional information by the method atomic locations by channeling enhanced microanalysis (ALCHEMI). Crystallographic location of minor components in a thin crystalline sample can also be determined via this method (beside composition) in a TEM. Lábár: Introduction 11 / 14
12 Figure 4. There are two alternative ways of deexitation from the highly excited state of an inner shell being ionized. These are the emission of either an X-ray photon or an Auger electron. X-ray emission is a single-electron process that shifts the inner "hole" (i.e. the electron state that is empty due to ionization) to an outer shell. The difference between the energies of the two shells is transferred to the X-ray photon. Since the energies of the shells are well defined in an atom (being quantized), the energy of the photon is characteristic to the emitting atom. Several outer shells are available for a given inner shell as possible routes of deexitation by photon emission. The appearance of a series of X-ray lines produced in response to the ionization of a single inner shell is the manifestation of the existence of the alternative routes. If the ionized shell is the innermost (most bound) K- shell with principal quantum number equal to 1, the series of lines is called the K-series. If the shell with principal quantum number 2 (L-shell) is ionized, the series is the L-series, etc. The shells with principal quantum number >1 (L, M, etc.) are separated into subshells with slightly different energies (as compared to the energy difference between the main shells). The alternative Auger-electron emission is a two-electron process. While the inner hole is shifted to one of the outer shells, the energy difference is given to another outer-shell electron (in contrast to a photon). This energy exceeds the binding energy of this other electron and it is emitted from the atom (leaving it in a doubly ionized state). Since the kinetic energy of the emitted electron is a difference between 3 binding energies, it is also characteristic of the atom, making Auger spectroscopy an alternative analytical technique. The probability of X- ray emission is called the fluorescence yield (ω), while the probability of Auger emission is 1- ω, the two processes being the only alternatives for deexitation. Since ω gives the total probability of photon emission (irrespective of which line of the series it belongs to), we need a further atomic constant, the weight of line to obtain the probability of the emission of a selected X-ray line. A special type of Auger transition is when the two outer subshells involved in the transition belong to the same shell. In that case we speak of a Coster-Kronig transition, characterized with Coster-Kronig transition rates, f ij for transitions between the i th and j th subshell of the same shall. The importance of Koster-Cronig transitions in X-ray analysis is that they redistribute the (primary) holes between the different inner subshells prior to the X-ray emission. Consequently they change the intensities of the individual X-ray lines from the values pertinent to the primary ionization by our bombarding electrons. Scanning electron microscopes (SEM) All SEMs use the scanning principle for imaging (hence the name). The focused electron beam is scanned over the surface of the bulk sample synchronized with the scanning of another electron beam in a TV tube. Any selected signal from the sample (varying as a Lábár: Introduction 12 / 14
13 function of beam position) can modulate the intensity of the TV tube. Magnification is determined by the ratio of the lengths of the two respective scanned lines. Typical accelerating voltage varies from 30 kev down to 200 ev. Resolution is the best at the higher voltages (~1-2 nm for FEG at 30 kev). The main advantages of an SEM is good spatial resolution (intermediate between optical microscopy and TEM), large depth of focus (giving a clear image simultaneously from hills and valleys of a rough surface) and moderate (or no) sample preparation. If an X-ray detector is attached (either EDS or WDS), the analytical capacity becomes close to a microprobe. Working distance is variable in many SEMs; low for high-resolution imaging and large for EDS/WDS. Conventional SEMs operate in HV. Low vacuum (low pressure / environmental) SEMs do not evacuate the vacuum chamber to that level. Even critical water pressure can be maintained to examine a water droplet or a living creature. Insulating samples can also be examined, because the gas atoms carry away the excess charge and prevent the sample from charging. Transmission electron microscopes (TEM) TEMs are operated at kev to image thin samples. Elastic scattering is used in the formation of true electronoptical images by electrons transmitted through the sample. Diffraction contrast dominates low and medium magnification images. High-resolution images are based on phase contrast. Assessment of crystal defects is a main application of conventional TEM. The sample is illuminated by a broad (nearly) parallel beam and the image is formed by the imaging lenses at the other side of the sample. Magnification has nothing to do with the size of the illuminated area, it is determined by the imaging lenses. The image is formed on an electroluminescent screen (or film or detector) in a fixed position in both modes of operation. In imaging mode, the image plane of the objective lens is projected to the screen. In diffraction mode, the focal plane of the same objective lens (containing a diffraction pattern) is projected to the same screen. Focused (convergent), fine electron probe is used for analysis in an AEM. The possibility to form an intense fine beam and the presence of an EDS or an EELS detector makes an AEM from a TEM. The microprobe (EPMA) A microprobe is similar to an SEM that is equipped with (an EDS and) several WDS detectors for X-ray analysis. Quantitative point analysis is the main feature with possibilities of elemental mapping and line scan. The point to analyze is typically selected from the compositional BE image of the polished sample. The three main characteristics that tell apart a microprobe from an SEM is stability, maximum beam current and the number of spectrometers. SEMs are optimized for SE resolution, while microprobes are optimized for long-term stability and higher currents. Quantification of the results is based on the fact that the measured X-ray intensities (generally) do not depend on the chemical bonding of the given element. In that sense EPMA / EDS / WDS gives "elemental" concentrations (in contrast to chemical composition in the chemical sense that includes the chemical form the element is in). In the exceptional cases (of mainly light elements), a chemical shift and some distortion of the measured peak can be detected, as will be elaborated in a separate lecture. Lábár: Introduction 13 / 14
14 Conclusions The introduction tried to delineate the structure of the "forest". The individual "trees" will be examined in detail during the workshop. Although most of the topics were touched superficially, I hope this skimming helps the audience to place the "trees" of the coming lectures into their correct positions and to see the "forest" in its full beauty. Further reading 1. J.I. Goldstein, D.E. Newbury, P. Echlin, D.C. Joy, C.E. Fiori and E. Lifschin, Scanning electron microscopy, Plenum Press, D.E. Newbury, D.C. Joy, P. Echlin, C.E. Fiori and J.I. Goldstein, Advanced scanning electron microscopy, Plenum Press, R.F. Egerton, Electron energy-loss spectroscopy, Plenum Press, S.J.B. Reed, Electron microprobe analysis, Cambridge University Press, D.B. Williams and C.B. Carter, Transmission electron microscopy, Plenum Press, F. Salvat and X. Llovet, Interaction of electrons and photons with matter, in Proc. EMAS'98, p L. Reimer, Electron-specimen interactions in X-ray microanalysis, Proc. EMAS'2000, Acknowledgement Financial support of the Hungarian Research Fund (OTKA ) is acknowledged. Lábár: Introduction 14 / 14
Transmission Electron Microscopy
 L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
Basic structure of SEM
 Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction
Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction
Part II: Thin Film Characterization
 Part II: Thin Film Characterization General details of thin film characterization instruments 1. Introduction to Thin Film Characterization Techniques 2. Structural characterization: SEM, TEM, AFM, STM
Part II: Thin Film Characterization General details of thin film characterization instruments 1. Introduction to Thin Film Characterization Techniques 2. Structural characterization: SEM, TEM, AFM, STM
Everhart-Thornley detector
 SEI Detector Everhart-Thornley detector Microscope chamber wall Faraday cage Scintillator Electrons in Light pipe Photomultiplier Electrical signal out Screen Quartz window +200 V +10 kv Always contains
SEI Detector Everhart-Thornley detector Microscope chamber wall Faraday cage Scintillator Electrons in Light pipe Photomultiplier Electrical signal out Screen Quartz window +200 V +10 kv Always contains
Weak-Beam Dark-Field Technique
 Basic Idea recall bright-field contrast of dislocations: specimen close to Bragg condition, s î 0 Weak-Beam Dark-Field Technique near the dislocation core, some planes curved to s = 0 ) strong Bragg reflection
Basic Idea recall bright-field contrast of dislocations: specimen close to Bragg condition, s î 0 Weak-Beam Dark-Field Technique near the dislocation core, some planes curved to s = 0 ) strong Bragg reflection
Praktikum zur. Materialanalytik
 Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
Chapter 9. Electron mean free path Microscopy principles of SEM, TEM, LEEM
 Chapter 9 Electron mean free path Microscopy principles of SEM, TEM, LEEM 9.1 Electron Mean Free Path 9. Scanning Electron Microscopy (SEM) -SEM design; Secondary electron imaging; Backscattered electron
Chapter 9 Electron mean free path Microscopy principles of SEM, TEM, LEEM 9.1 Electron Mean Free Path 9. Scanning Electron Microscopy (SEM) -SEM design; Secondary electron imaging; Backscattered electron
Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist
 12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
Gaetano L Episcopo. Scanning Electron Microscopy Focus Ion Beam and. Pulsed Plasma Deposition
 Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist
 12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
Electron Microscopy I
 Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
object objective lens eyepiece lens
 Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
High-Resolution. Transmission. Electron Microscopy
 Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
M2 TP. Low-Energy Electron Diffraction (LEED)
 M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
Modern Optical Spectroscopy
 Modern Optical Spectroscopy X-Ray Microanalysis Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Backscattered
Modern Optical Spectroscopy X-Ray Microanalysis Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Backscattered
MSE 321 Structural Characterization
 Optical Microscope Plan Lenses In an "ideal" single-element lens system all planar wave fronts are focused to a point at distance f from the lens; therefore: Image near the optical axis will be in perfect
Optical Microscope Plan Lenses In an "ideal" single-element lens system all planar wave fronts are focused to a point at distance f from the lens; therefore: Image near the optical axis will be in perfect
4. Inelastic Scattering
 1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Electron beam scanning
 Electron beam scanning The Electron beam scanning operates through an electro-optical system which has the task of deflecting the beam Synchronously with cathode ray tube which create the image, beam moves
Electron beam scanning The Electron beam scanning operates through an electro-optical system which has the task of deflecting the beam Synchronously with cathode ray tube which create the image, beam moves
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN
 KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN 20970725 HACETTEPE UNIVERSITY DEPARTMENT OF CHEMICAL ENGINEERING, SPRING 2011,APRIL,ANKARA CONTENTS
KMÜ 396 MATERIALS SCIENCE AND TECH. I PRESENTATION ELECTRON ENERGY LOSS SPECTROSCOPY (EELS) TUĞÇE SEZGİN 20970725 HACETTEPE UNIVERSITY DEPARTMENT OF CHEMICAL ENGINEERING, SPRING 2011,APRIL,ANKARA CONTENTS
AP5301/ Name the major parts of an optical microscope and state their functions.
 Review Problems on Optical Microscopy AP5301/8301-2015 1. Name the major parts of an optical microscope and state their functions. 2. Compare the focal lengths of two glass converging lenses, one with
Review Problems on Optical Microscopy AP5301/8301-2015 1. Name the major parts of an optical microscope and state their functions. 2. Compare the focal lengths of two glass converging lenses, one with
Scanning Electron Microscopy
 Scanning Electron Microscopy Field emitting tip Grid 2kV 100kV Anode ZEISS SUPRA Variable Pressure FESEM Dr Heath Bagshaw CMA bagshawh@tcd.ie Why use an SEM? Fig 1. Examples of features resolvable using
Scanning Electron Microscopy Field emitting tip Grid 2kV 100kV Anode ZEISS SUPRA Variable Pressure FESEM Dr Heath Bagshaw CMA bagshawh@tcd.ie Why use an SEM? Fig 1. Examples of features resolvable using
Chemical Analysis in TEM: XEDS, EELS and EFTEM. HRTEM PhD course Lecture 5
 Chemical Analysis in TEM: XEDS, EELS and EFTEM HRTEM PhD course Lecture 5 1 Part IV Subject Chapter Prio x-ray spectrometry 32 1 Spectra and mapping 33 2 Qualitative XEDS 34 1 Quantitative XEDS 35.1-35.4
Chemical Analysis in TEM: XEDS, EELS and EFTEM HRTEM PhD course Lecture 5 1 Part IV Subject Chapter Prio x-ray spectrometry 32 1 Spectra and mapping 33 2 Qualitative XEDS 34 1 Quantitative XEDS 35.1-35.4
Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn?
 Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn? EPMA - what is it? Precise and accurate quantitative chemical analyses of micron-size
Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn? EPMA - what is it? Precise and accurate quantitative chemical analyses of micron-size
Practical course in scanning electron microscopy
 Practical course in scanning electron microscopy Fortgeschrittenen Praktikum an der Technischen Universität München Wintersemester 2017/2018 Table of contents 1. Introduction 3 2. Formation of an electron
Practical course in scanning electron microscopy Fortgeschrittenen Praktikum an der Technischen Universität München Wintersemester 2017/2018 Table of contents 1. Introduction 3 2. Formation of an electron
Imaging Methods: Scanning Force Microscopy (SFM / AFM)
 Imaging Methods: Scanning Force Microscopy (SFM / AFM) The atomic force microscope (AFM) probes the surface of a sample with a sharp tip, a couple of microns long and often less than 100 Å in diameter.
Imaging Methods: Scanning Force Microscopy (SFM / AFM) The atomic force microscope (AFM) probes the surface of a sample with a sharp tip, a couple of microns long and often less than 100 Å in diameter.
Interactions with Matter
 Manetic Lenses Manetic fields can displace electrons Manetic field can be produced by passin an electrical current throuh coils of wire Manetic field strenth can be increased by usin a soft ferromanetic
Manetic Lenses Manetic fields can displace electrons Manetic field can be produced by passin an electrical current throuh coils of wire Manetic field strenth can be increased by usin a soft ferromanetic
SEM Doctoral Course MS-636. April 11-13, 2016
 Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Electron Sources, Optics and Detectors SEM Doctoral Course MS-636 April 11-13, 2016 Summary Electron propagation is only possible through
Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Electron Sources, Optics and Detectors SEM Doctoral Course MS-636 April 11-13, 2016 Summary Electron propagation is only possible through
Why microscopy?
 Electron Microscopy Why microscopy? http://www.cellsalive.com/howbig.htm 2 Microscopes are used as magnifying tools (although not exclusively as will see later on). The resolution of the human eye is limited
Electron Microscopy Why microscopy? http://www.cellsalive.com/howbig.htm 2 Microscopes are used as magnifying tools (although not exclusively as will see later on). The resolution of the human eye is limited
QUANTUM PHYSICS. Limitation: This law holds well only for the short wavelength and not for the longer wavelength. Raleigh Jean s Law:
 Black body: A perfect black body is one which absorbs all the radiation of heat falling on it and emits all the radiation when heated in an isothermal enclosure. The heat radiation emitted by the black
Black body: A perfect black body is one which absorbs all the radiation of heat falling on it and emits all the radiation when heated in an isothermal enclosure. The heat radiation emitted by the black
CHARACTERIZATION of NANOMATERIALS KHP
 CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
h p λ = mν Back to de Broglie and the electron as a wave you will learn more about this Equation in CHEM* 2060
 Back to de Broglie and the electron as a wave λ = mν h = h p you will learn more about this Equation in CHEM* 2060 We will soon see that the energies (speed for now if you like) of the electrons in the
Back to de Broglie and the electron as a wave λ = mν h = h p you will learn more about this Equation in CHEM* 2060 We will soon see that the energies (speed for now if you like) of the electrons in the
Analytical Methods for Materials
 Analytical Methods for Materials Lesson 21 Electron Microscopy and X-ray Spectroscopy Suggested Reading Leng, Chapter 3, pp. 83-126; Chapter 4, pp. 127-160; Chapter 6, pp. 191-219 P.J. Goodhew, J. Humphreys
Analytical Methods for Materials Lesson 21 Electron Microscopy and X-ray Spectroscopy Suggested Reading Leng, Chapter 3, pp. 83-126; Chapter 4, pp. 127-160; Chapter 6, pp. 191-219 P.J. Goodhew, J. Humphreys
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
CHEM 681 Seminar Mingqi Zhao April 20, 1998 Room 2104, 4:00 p.m. High Resolution Transmission Electron Microscopy: theories and applications
 CHEM 681 Seminar Mingqi Zhao April 20, 1998 Room 2104, 4:00 p.m. High Resolution Transmission Electron Microscopy: theories and applications In materials science, people are always interested in viewing
CHEM 681 Seminar Mingqi Zhao April 20, 1998 Room 2104, 4:00 p.m. High Resolution Transmission Electron Microscopy: theories and applications In materials science, people are always interested in viewing
Practical 1P4 Energy Levels and Band Gaps
 Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
Practical 1P4 Energy Levels and Band Gaps
 Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
Practical 1P4 Energy Levels and Band Gaps What you should learn from this practical Science This practical illustrates some of the points from the lecture course on Elementary Quantum Mechanics and Bonding
April 10th-12th, 2017
 Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Introduction: Basics of Transmission Electron Microscopy (TEM) TEM Doctoral Course MS-637 April 10th-12th, 2017 Outline 1. What is microcopy?
Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Introduction: Basics of Transmission Electron Microscopy (TEM) TEM Doctoral Course MS-637 April 10th-12th, 2017 Outline 1. What is microcopy?
Invited Lecture. "Different Aspects of Electron Microscopy. Sardar Vallabhbhai National Institute of Technology, Surat. Deepak Rajput & S.K.
 Invited Lecture on "Different Aspects of Electron Microscopy at Sardar Vallabhbhai National Institute of Technology, Surat Deepak Rajput & S.K. Tiwary R&D and Product Development Essar Steel Limited Abstract
Invited Lecture on "Different Aspects of Electron Microscopy at Sardar Vallabhbhai National Institute of Technology, Surat Deepak Rajput & S.K. Tiwary R&D and Product Development Essar Steel Limited Abstract
Chapter Six: X-Rays. 6.1 Discovery of X-rays
 Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Electron-Matter Interactions
 Electron-Matter Interactions examples of typical EM studies properties of electrons elastic electron-matter interactions scattering processes; coherent and incoherent image formation; chemical contrast;
Electron-Matter Interactions examples of typical EM studies properties of electrons elastic electron-matter interactions scattering processes; coherent and incoherent image formation; chemical contrast;
Advanced Lab Course. X-Ray Photoelectron Spectroscopy 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT Qualitative analysis Chemical Shifts 7
 Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Part I Basics and Methods
 j1 Part I Basics and Methods In-situ Electron Microscopy: Applications in Physics, Chemistry and Materials Science, First Edition. Edited by Gerhard Dehm, James M. Howe, and Josef Zweck. Ó 2012 Wiley-VCH
j1 Part I Basics and Methods In-situ Electron Microscopy: Applications in Physics, Chemistry and Materials Science, First Edition. Edited by Gerhard Dehm, James M. Howe, and Josef Zweck. Ó 2012 Wiley-VCH
Electron Microprobe Analysis and Scanning Electron Microscopy
 Electron Microprobe Analysis and Scanning Electron Microscopy Electron microprobe analysis (EMPA) Analytical technique in which a beam of electrons is focused on a sample surface, producing X-rays from
Electron Microprobe Analysis and Scanning Electron Microscopy Electron microprobe analysis (EMPA) Analytical technique in which a beam of electrons is focused on a sample surface, producing X-rays from
HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS. SCSAM Short Course Amir Avishai
 HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS SCSAM Short Course Amir Avishai RESEARCH QUESTIONS Sea Shell Cast Iron EDS+SE Fe Cr C Objective Ability to ask the
HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS SCSAM Short Course Amir Avishai RESEARCH QUESTIONS Sea Shell Cast Iron EDS+SE Fe Cr C Objective Ability to ask the
Elastic and Inelastic Scattering in Electron Diffraction and Imaging
 Elastic and Inelastic Scattering in Electron Diffraction and Imaging Contents Introduction Symbols and definitions Part A Diffraction and imaging of elastically scattered electrons Chapter 1. Basic kinematical
Elastic and Inelastic Scattering in Electron Diffraction and Imaging Contents Introduction Symbols and definitions Part A Diffraction and imaging of elastically scattered electrons Chapter 1. Basic kinematical
Ma5: Auger- and Electron Energy Loss Spectroscopy
 Ma5: Auger- and Electron Energy Loss Spectroscopy 1 Introduction Electron spectroscopies, namely Auger electron- and electron energy loss spectroscopy are utilized to determine the KLL spectrum and the
Ma5: Auger- and Electron Energy Loss Spectroscopy 1 Introduction Electron spectroscopies, namely Auger electron- and electron energy loss spectroscopy are utilized to determine the KLL spectrum and the
Application Note GA-301E. MBMS for Preformed Ions. Extrel CMS, 575 Epsilon Drive, Pittsburgh, PA I. SAMPLING A CHEMICAL SOUP
 Application Note MBMS for Preformed Ions, 575 Epsilon Drive, Pittsburgh, PA 15238 (Poster Presented at 45th ASMS Conference on Mass Spectrometry, June 1-5, 1997) In order to accurately characterize a plasma
Application Note MBMS for Preformed Ions, 575 Epsilon Drive, Pittsburgh, PA 15238 (Poster Presented at 45th ASMS Conference on Mass Spectrometry, June 1-5, 1997) In order to accurately characterize a plasma
The illumination source: the electron beam
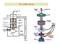 The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
Lecture 5. X-ray Photoemission Spectroscopy (XPS)
 Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
6. Analytical Electron Microscopy
 Physical Principles of Electron Microscopy 6. Analytical Electron Microscopy Ray Egerton University of Alberta and National Institute of Nanotechnology Edmonton, Canada www.tem-eels.ca regerton@ualberta.ca
Physical Principles of Electron Microscopy 6. Analytical Electron Microscopy Ray Egerton University of Alberta and National Institute of Nanotechnology Edmonton, Canada www.tem-eels.ca regerton@ualberta.ca
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu
 Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
Chapter 4 Scintillation Detectors
 Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Angular Correlation Experiments
 Angular Correlation Experiments John M. LoSecco April 2, 2007 Angular Correlation Experiments J. LoSecco Notre Dame du Lac Nuclear Spin In atoms one can use the Zeeman Effect to determine the spin state.
Angular Correlation Experiments John M. LoSecco April 2, 2007 Angular Correlation Experiments J. LoSecco Notre Dame du Lac Nuclear Spin In atoms one can use the Zeeman Effect to determine the spin state.
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
Surface Sensitivity & Surface Specificity
 Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
CHEM-E5225 :Electron Microscopy Imaging
 CHEM-E5225 :Electron Microscopy Imaging 2016.10 Yanling Ge Outline Planar Defects Image strain field WBDF microscopy HRTEM information theory Discuss of question homework? Planar Defects - Internal Interface
CHEM-E5225 :Electron Microscopy Imaging 2016.10 Yanling Ge Outline Planar Defects Image strain field WBDF microscopy HRTEM information theory Discuss of question homework? Planar Defects - Internal Interface
Massachusetts Institute of Technology. Dr. Nilanjan Chatterjee
 Massachusetts Institute of Technology Dr. Nilanjan Chatterjee Electron Probe Micro-Analysis (EPMA) Imaging and micrometer-scale chemical compositional analysis of solids Signals produced in The Electron
Massachusetts Institute of Technology Dr. Nilanjan Chatterjee Electron Probe Micro-Analysis (EPMA) Imaging and micrometer-scale chemical compositional analysis of solids Signals produced in The Electron
ELECTROMAGNETIC WAVES
 VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES SPECTRA PRODUCED BY DISCHARGE TUBES CATHODE RAYS (electron beams) Streams of electrons (negatively charged particles) observed in vacuum
VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES SPECTRA PRODUCED BY DISCHARGE TUBES CATHODE RAYS (electron beams) Streams of electrons (negatively charged particles) observed in vacuum
CBE Science of Engineering Materials. Scanning Electron Microscopy (SEM)
 CBE 30361 Science of Engineering Materials Scanning Electron Microscopy (SEM) Scale of Structure Organization Units: micrometer = 10-6 m = 1µm nanometer= 10-9 m = 1nm Angstrom = 10-10 m = 1Å A hair is
CBE 30361 Science of Engineering Materials Scanning Electron Microscopy (SEM) Scale of Structure Organization Units: micrometer = 10-6 m = 1µm nanometer= 10-9 m = 1nm Angstrom = 10-10 m = 1Å A hair is
Lecture 20 Optical Characterization 2
 Lecture 20 Optical Characterization 2 Schroder: Chapters 2, 7, 10 1/68 Announcements Homework 5/6: Is online now. Due Wednesday May 30th at 10:00am. I will return it the following Wednesday (6 th June).
Lecture 20 Optical Characterization 2 Schroder: Chapters 2, 7, 10 1/68 Announcements Homework 5/6: Is online now. Due Wednesday May 30th at 10:00am. I will return it the following Wednesday (6 th June).
3.1 Introduction to Semiconductors. Y. Baghzouz ECE Department UNLV
 3.1 Introduction to Semiconductors Y. Baghzouz ECE Department UNLV Introduction In this lecture, we will cover the basic aspects of semiconductor materials, and the physical mechanisms which are at the
3.1 Introduction to Semiconductors Y. Baghzouz ECE Department UNLV Introduction In this lecture, we will cover the basic aspects of semiconductor materials, and the physical mechanisms which are at the
Secondary Ion Mass Spectrometry (SIMS)
 CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
Ecole Franco-Roumaine : Magnétisme des systèmes nanoscopiques et structures hybrides - Brasov, Modern Analytical Microscopic Tools
 1. Introduction Solid Surfaces Analysis Group, Institute of Physics, Chemnitz University of Technology, Germany 2. Limitations of Conventional Optical Microscopy 3. Electron Microscopies Transmission Electron
1. Introduction Solid Surfaces Analysis Group, Institute of Physics, Chemnitz University of Technology, Germany 2. Limitations of Conventional Optical Microscopy 3. Electron Microscopies Transmission Electron
EDS User School. Principles of Electron Beam Microanalysis
 EDS User School Principles of Electron Beam Microanalysis Outline 1.) Beam-specimen interactions 2.) EDS spectra: Origin of Bremsstrahlung and characteristic peaks 3.) Moseley s law 4.) Characteristic
EDS User School Principles of Electron Beam Microanalysis Outline 1.) Beam-specimen interactions 2.) EDS spectra: Origin of Bremsstrahlung and characteristic peaks 3.) Moseley s law 4.) Characteristic
Structure analysis: Electron diffraction LEED TEM RHEED
 Structure analysis: Electron diffraction LEED: Low Energy Electron Diffraction SPA-LEED: Spot Profile Analysis Low Energy Electron diffraction RHEED: Reflection High Energy Electron Diffraction TEM: Transmission
Structure analysis: Electron diffraction LEED: Low Energy Electron Diffraction SPA-LEED: Spot Profile Analysis Low Energy Electron diffraction RHEED: Reflection High Energy Electron Diffraction TEM: Transmission
MEMS Metrology. Prof. Tianhong Cui ME 8254
 MEMS Metrology Prof. Tianhong Cui ME 8254 What is metrology? Metrology It is the science of weights and measures Refers primarily to the measurements of length, weight, time, etc. Mensuration- A branch
MEMS Metrology Prof. Tianhong Cui ME 8254 What is metrology? Metrology It is the science of weights and measures Refers primarily to the measurements of length, weight, time, etc. Mensuration- A branch
DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD
 Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
5.8 Auger Electron Spectroscopy (AES)
 5.8 Auger Electron Spectroscopy (AES) 5.8.1 The Auger Process X-ray and high energy electron bombardment of atom can create core hole Core hole will eventually decay via either (i) photon emission (x-ray
5.8 Auger Electron Spectroscopy (AES) 5.8.1 The Auger Process X-ray and high energy electron bombardment of atom can create core hole Core hole will eventually decay via either (i) photon emission (x-ray
Generation of X-Rays in the SEM specimen
 Generation of X-Rays in the SEM specimen The electron beam generates X-ray photons in the beam-specimen interaction volume beneath the specimen surface. Some X-ray photons emerging from the specimen have
Generation of X-Rays in the SEM specimen The electron beam generates X-ray photons in the beam-specimen interaction volume beneath the specimen surface. Some X-ray photons emerging from the specimen have
1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Physics (A-level)
 1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Physics (A-level) Electromagnetic induction (Chapter 23): For a straight wire, the induced current or e.m.f. depends on: The magnitude of the magnetic
1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Physics (A-level) Electromagnetic induction (Chapter 23): For a straight wire, the induced current or e.m.f. depends on: The magnitude of the magnetic
Physical Principles of Electron Microscopy. 2. Electron Optics
 Physical Principles of Electron Microscopy 2. Electron Optics Ray Egerton University of Alberta and National Institute of Nanotechnology Edmonton, Canada www.tem-eels.ca regerton@ualberta.ca Properties
Physical Principles of Electron Microscopy 2. Electron Optics Ray Egerton University of Alberta and National Institute of Nanotechnology Edmonton, Canada www.tem-eels.ca regerton@ualberta.ca Properties
SOLID STATE PHYSICS PHY F341. Dr. Manjuladevi.V Associate Professor Department of Physics BITS Pilani
 SOLID STATE PHYSICS PHY F341 Dr. Manjuladevi.V Associate Professor Department of Physics BITS Pilani 333031 manjula@bits-pilani.ac.in Characterization techniques SEM AFM STM BAM Outline What can we use
SOLID STATE PHYSICS PHY F341 Dr. Manjuladevi.V Associate Professor Department of Physics BITS Pilani 333031 manjula@bits-pilani.ac.in Characterization techniques SEM AFM STM BAM Outline What can we use
Vacuum Pumps. Two general classes exist: Gas transfer physical removal of matter. Mechanical, diffusion, turbomolecular
 Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Solid Surfaces, Interfaces and Thin Films
 Hans Lüth Solid Surfaces, Interfaces and Thin Films Fifth Edition With 427 Figures.2e Springer Contents 1 Surface and Interface Physics: Its Definition and Importance... 1 Panel I: Ultrahigh Vacuum (UHV)
Hans Lüth Solid Surfaces, Interfaces and Thin Films Fifth Edition With 427 Figures.2e Springer Contents 1 Surface and Interface Physics: Its Definition and Importance... 1 Panel I: Ultrahigh Vacuum (UHV)
SEM stands for Scanning Electron Microscopy. The earliest known work describing
 1. HISTORY ABOUT SEM SEM stands for Scanning Electron Microscopy. The earliest known work describing the concept of a Scanning Electron Microscope was by M. Knoll (1935) who, along with other pioneers
1. HISTORY ABOUT SEM SEM stands for Scanning Electron Microscopy. The earliest known work describing the concept of a Scanning Electron Microscope was by M. Knoll (1935) who, along with other pioneers
ET3034TUx Utilization of band gap energy
 ET3034TUx - 3.3.1 - Utilization of band gap energy In the last two weeks we have discussed the working principle of a solar cell and the external parameters that define the performance of a solar cell.
ET3034TUx - 3.3.1 - Utilization of band gap energy In the last two weeks we have discussed the working principle of a solar cell and the external parameters that define the performance of a solar cell.
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons
 Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Overview of scattering, diffraction & imaging in the TEM
 Overview of scattering, diffraction & imaging in the TEM Eric A. Stach Purdue University Scattering Electrons, photons, neutrons Radiation Elastic Mean Free Path (Å)( Absorption Length (Å)( Minimum Probe
Overview of scattering, diffraction & imaging in the TEM Eric A. Stach Purdue University Scattering Electrons, photons, neutrons Radiation Elastic Mean Free Path (Å)( Absorption Length (Å)( Minimum Probe
Nova 600 NanoLab Dual beam Focused Ion Beam IITKanpur
 Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 Q1. (a) The diagram below shows a narrow beam of electrons produced by attracting electrons emitted from a filament wire to a metal plate which has a small hole in it. (i) Why
PhysicsAndMathsTutor.com 1 Q1. (a) The diagram below shows a narrow beam of electrons produced by attracting electrons emitted from a filament wire to a metal plate which has a small hole in it. (i) Why
Chemistry Instrumental Analysis Lecture 19 Chapter 12. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Structure of Surfaces
 Structure of Surfaces C Stepped surface Interference of two waves Bragg s law Path difference = AB+BC =2dsin ( =glancing angle) If, n =2dsin, constructive interference Ex) in a cubic lattice of unit cell
Structure of Surfaces C Stepped surface Interference of two waves Bragg s law Path difference = AB+BC =2dsin ( =glancing angle) If, n =2dsin, constructive interference Ex) in a cubic lattice of unit cell
Name Final Exam May 1, 2017
 Name Final Exam May 1, 217 This test consists of five parts. Please note that in parts II through V, you can skip one question of those offered. Some possibly useful formulas appear below. Constants, etc.
Name Final Exam May 1, 217 This test consists of five parts. Please note that in parts II through V, you can skip one question of those offered. Some possibly useful formulas appear below. Constants, etc.
Electron Rutherford Backscattering, a versatile tool for the study of thin films
 Electron Rutherford Backscattering, a versatile tool for the study of thin films Maarten Vos Research School of Physics and Engineering Australian National University Canberra Australia Acknowledgements:
Electron Rutherford Backscattering, a versatile tool for the study of thin films Maarten Vos Research School of Physics and Engineering Australian National University Canberra Australia Acknowledgements:
Chapter 10: Wave Properties of Particles
 Chapter 10: Wave Properties of Particles Particles such as electrons may demonstrate wave properties under certain conditions. The electron microscope uses these properties to produce magnified images
Chapter 10: Wave Properties of Particles Particles such as electrons may demonstrate wave properties under certain conditions. The electron microscope uses these properties to produce magnified images
Techniques EDX, EELS et HAADF en TEM: possibilités d analyse et applications
 Techniques EDX, EELS et HAADF en TEM: possibilités d analyse et applications Thomas Neisius Université Paul Cézanne Plan Imaging modes HAADF Example: supported Pt nanoparticles Electron sample interaction
Techniques EDX, EELS et HAADF en TEM: possibilités d analyse et applications Thomas Neisius Université Paul Cézanne Plan Imaging modes HAADF Example: supported Pt nanoparticles Electron sample interaction
Table of Content. Mechanical Removing Techniques. Ultrasonic Machining (USM) Sputtering and Focused Ion Beam Milling (FIB)
 Table of Content Mechanical Removing Techniques Ultrasonic Machining (USM) Sputtering and Focused Ion Beam Milling (FIB) Ultrasonic Machining In ultrasonic machining (USM), also called ultrasonic grinding,
Table of Content Mechanical Removing Techniques Ultrasonic Machining (USM) Sputtering and Focused Ion Beam Milling (FIB) Ultrasonic Machining In ultrasonic machining (USM), also called ultrasonic grinding,
PHI 5000 Versaprobe-II Focus X-ray Photo-electron Spectroscopy
 PHI 5000 Versaprobe-II Focus X-ray Photo-electron Spectroscopy The very basic theory of XPS XPS theroy Surface Analysis Ultra High Vacuum (UHV) XPS Theory XPS = X-ray Photo-electron Spectroscopy X-ray
PHI 5000 Versaprobe-II Focus X-ray Photo-electron Spectroscopy The very basic theory of XPS XPS theroy Surface Analysis Ultra High Vacuum (UHV) XPS Theory XPS = X-ray Photo-electron Spectroscopy X-ray
CHEM-E5225 :Electron Microscopy X-Ray Spectrometry
 CHEM-E5225 :Electron Microscopy X-Ray Spectrometry 2016.11 Yanling Ge Outline X-ray Spectrometry X-ray Spectra and Images Qualitative and Quantitative X-ray Analysis and Imaging Discussion of homework
CHEM-E5225 :Electron Microscopy X-Ray Spectrometry 2016.11 Yanling Ge Outline X-ray Spectrometry X-ray Spectra and Images Qualitative and Quantitative X-ray Analysis and Imaging Discussion of homework
Methods of surface analysis
 Methods of surface analysis Nanomaterials characterisation I RNDr. Věra Vodičková, PhD. Surface of solid matter: last monoatomic layer + absorbed monolayer physical properties are effected (crystal lattice
Methods of surface analysis Nanomaterials characterisation I RNDr. Věra Vodičková, PhD. Surface of solid matter: last monoatomic layer + absorbed monolayer physical properties are effected (crystal lattice
CHAPTER 3 The Experimental Basis of Quantum Theory
 CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
PHYS-E0541:Special Course in Physics Gas phase synthesis of carbon nanotubes for thin film application. Electron Microscopy. for
 PHYS-E0541:Special Course in Physics Gas phase synthesis of carbon nanotubes for thin film application Electron Microscopy for Introduction to Electron Microscopy Carbon Nanomaterials (nanotubes) Dr. Hua
PHYS-E0541:Special Course in Physics Gas phase synthesis of carbon nanotubes for thin film application Electron Microscopy for Introduction to Electron Microscopy Carbon Nanomaterials (nanotubes) Dr. Hua
tip conducting surface
 PhysicsAndMathsTutor.com 1 1. The diagram shows the tip of a scanning tunnelling microscope (STM) above a conducting surface. The tip is at a potential of 1.0 V relative to the surface. If the tip is sufficiently
PhysicsAndMathsTutor.com 1 1. The diagram shows the tip of a scanning tunnelling microscope (STM) above a conducting surface. The tip is at a potential of 1.0 V relative to the surface. If the tip is sufficiently
Chapter 2 Instrumentation for Analytical Electron Microscopy Lecture 5. Chapter 2 CHEM 793, 2011 Fall 1
 Chater Instrumentation for Analytical Electron Microscoy Lecture 5 Chater CHEM 793, 011 Fall 1 Outline Electron Sources (Electron Guns) Thermionic: LaB 6 or W Field emission gun: cold or Schottky Lenses
Chater Instrumentation for Analytical Electron Microscoy Lecture 5 Chater CHEM 793, 011 Fall 1 Outline Electron Sources (Electron Guns) Thermionic: LaB 6 or W Field emission gun: cold or Schottky Lenses
Nano-Microscopy. Lecture 2. Scanning and Transmission Electron Microscopies: Principles. Pavel Zinin HIGP, University of Hawaii, Honolulu, USA
 GG 711: Advanced Techniques in Geophysics and Materials Science Nano-Microscopy. Lecture 2 Scanning and Transmission Electron Microscopies: Principles Pavel Zinin HIGP, University of Hawaii, Honolulu,
GG 711: Advanced Techniques in Geophysics and Materials Science Nano-Microscopy. Lecture 2 Scanning and Transmission Electron Microscopies: Principles Pavel Zinin HIGP, University of Hawaii, Honolulu,
EE 527 MICROFABRICATION. Lecture 5 Tai-Chang Chen University of Washington
 EE 527 MICROFABRICATION Lecture 5 Tai-Chang Chen University of Washington MICROSCOPY AND VISUALIZATION Electron microscope, transmission electron microscope Resolution: atomic imaging Use: lattice spacing.
EE 527 MICROFABRICATION Lecture 5 Tai-Chang Chen University of Washington MICROSCOPY AND VISUALIZATION Electron microscope, transmission electron microscope Resolution: atomic imaging Use: lattice spacing.
