Structural and dynamical properties of liquid and solid phases of ionic liquids confined inside nanoporous materials
|
|
|
- Silvester Potter
- 5 years ago
- Views:
Transcription
1 Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2012 Structural and dynamical properties of liquid and solid phases of ionic liquids confined inside nanoporous materials Ramesh Singh Louisiana State University and Agricultural and Mechanical College, Follow this and additional works at: Part of the Chemical Engineering Commons Recommended Citation Singh, Ramesh, "Structural and dynamical properties of liquid and solid phases of ionic liquids confined inside nanoporous materials" (2012). LSU Doctoral Dissertations This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please
2 STRUCTURAL AND DYNAMICAL PROPERTIES OF LIQUID AND SOLID PHASES OF IONIC LIQUIDS CONFINED INSIDE NANOPOROUS MATERIALS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor in Philosophy In The Gordon A. and Mary Cain Department of Chemical Engineering By Ramesh Singh B.Tech., Indian Institute of Technology, Roorkee, 2001 M.S.,SUNY-ESF, Syracuse, 2007 M.S., Louisiana State University, 2010 May 2012
3 ACKNOWLEDGEMENTS First and foremost I would like to express my deepest gratitude to my advisor Dr. Francisco R. Hung, for his invaluable guidance, consistent encouragement, and support. I have been fortunate to gain the benefit of his expertise in the field of molecular modeling and simulation. He has always been a patient listener to my research ideas and constantly gave me positive feedback in order to implement them. His delightful nature taught me that serious research work can be done in a light and charming environment. I sincerely thank dean s representative Dr. Jeffrey C. Clayton and my thesis committee members Dr. John C. Flake, Dr. Krishnaswamy Nandakumar, and Dr. Dorel Moldovan for their invaluable suggestions on my research work. My special thanks to Dr. Erik Santiso who patiently guided me in the studies of solid phases of ionic liquids from time to time. I am also very thankful to Dr. Joshua D Monk, my fellow group members and my friends for their support and for making my life easy. I would also like to thank all those in the Cain Department of Chemical Engineering at LSU. I gratefully acknowledge funding from the American Chemical Society Petroleum Research Foundation (ACS-PRF), the LONI Institute and the Cain Department of Chemical Engineering, LSU. I also thank the Cain Chemical Engineering Department at LSU for giving me opportunity to pursue my Ph.D degree here. I also thank all the professors, staff members and other graduate students of the department. I am indebted to my parents, brother Suresh Kumar Singh, and sister Lakshmi Chaudhary for their ever-present love and support. Last but not the least, I would thank my loving, and patient wife Geeta and son Nilesh who faithfully supported me during my Ph.D. ii
4 TABLE OF CONTENTS ACKNOWLEDGEMENTS... ii ABSTRACT... v CHAPTER 1 INTRODUCTION Background and Motivation Outline of Dissertation... 3 CHAPTER 2 A COMPUTATIONAL STUDY OF THE BEHAVIOR OF IONIC LIQUID [BMIM + ][PF6 - ] CONFINED INSIDE MULTI-WALLED CARBON NANOTUBES Introduction Computational Details Results and Discussion Structural Properties Dynamical Properties Concluding Remarks CHAPTER 3 HETEROGENEITY IN THE DYNAMICS OF THE IONIC LIQUID [BMIM + ][PF 6 - ] CONFINED IN A SLIT NANOPORE Introduction Computational Details Results and Discussion Mean Squared Displacement (MSD) and Self-diffusion Coefficients Self-part of the Van Hove Correlation Function Incoherent Intermediate Scattering Function Relaxation Times Radial Distribution Functions Concluding Remarks CHAPTER 4 MOLECULAR DYNAMIC SIMULATIONS OF IL [EMIM + ][TFMSI - ] CONFINED INSIDE RUTILE (110) NANOPORE: EFFECT OF PORE LOADING ON STRUCTURAL AND DYNAMICAL PROPERTIES Introduction Computational Details Results and Discussion Structural Properties Dynamical Properties Concluding Remarks CHAPTER 5 EFFECT OF ACETONITRILE SOLVENT ON THE STRUCTURAL AND DYNAMICAL PROPERTIES OF IL [EMIM + ][TFMSI - ] CONFINED INSIDE SLIT-LIKE GRAPHITIC PORE: A MOLECULAR DYNAMIC SIMULATION STUDY Introduction Computational Details Results and Discussion iii
5 5.3.1 Density Profiles Radial Distribution Function Preferential Orientation Mean Square Displacement Concluding Remarks CHAPTER 6 CONCLUSION AND FUTURE WORK Conclusion Proposed Work Defining Order Parameters to Characterize Local and Global Degree of Crystallinity Study of the Effect of Charged Electrodes on the Properties of the Confined ILs REFERENCES APPENDIX A: PERMISSION LETTERS A.1 Permission for Chapter A.2 Permission for Chapter APPENDIX B: SUPPORTING INFORMATION (CHAPTER 3) B.1 Representative Gromacs Input File with MD Parameters (.mdp file) B.2 Relaxation Times VITA iv
6 ABSTRACT The purpose of this research is to investigate the physical properties of ionic liquids (ILs) confined inside nanopores of different materials and morphologies. We are interested to study the effect of pore material, morphology and addition of organic solvents on the properties of confined ILs. Understanding the behavior of ILs inside nanopores is relevant to potential applications of these systems in electrochemical double layer capacitors (EDLCs) and dye sensitized solar cells (DSSCs). Such a fundamental understanding is also crucial to optimize the synthesis of hard-templated 1D nanostructures (nanorods, nanotubes, nanowires) based on organic salts, which may be imparted properties (e.g., magnetic, optical) that are desirable for different applications (magnetic hyperthermia cancer treatment, medical imaging, sensors). In this work we have used molecular dynamics (MD) simulations to investigate systems of representative ILs, [BMIM + ][PF - 6 ] and [EMIM + ][TFMSI - ], inside several model materials (e.g., slit-shaped graphitic and titania pores, carbon nanotubes). Formation of different layers of ions was observed for the confined ILs irrespective of variations in pore size, shape, material and amount of solvent. In all cases, change in pore loading leads to lower densities of ions in the center of the pore. The cations close to the pore walls tend to align with their imidazolium rings parallel to the pore surface in the case of carbon materials, and multiple preferential orientations are observed in the case of titania pores. For all porous materials studied, the dynamics of the ions depend strongly on their location with respect to the surface; bulk-like dynamics are generally observed for the ions in the center regions of the pore, with the dynamics becoming slower as the ions get closer to the pore surfaces. Addition of acetonitrile solvent also shows similar layering behavior for ILs and solvent near the pore wall with less variation in the center of the pore. Preferential orientations of the ions remain unaffected and solvent molecules tend to v
7 align flat near the pore surface. The dynamics of the ions and molecules increases linearly with increase in IL molar concentration both near the wall and in the center of the pore. vi
8 CHAPTER 1 INTRODUCTION 1.1 Background and Motivation Ionic liquids (ILs) are salts composed of an organic cation and an organic or inorganic anion with melting point below 100 C. Their unique properties such as great thermal, chemical, and electrochemical stability and their nonvolatile, nonexplosive and nonflammable nature have gained them immense attention in recent years [1-10]. Because of their numerous applications and unique properties, there is a rapidly growing scientific and commercial interest in ILs. It has been observed that properties of ILs changes drastically under confinement. It has been reported that the melting points and the thermal decomposition temperature of several ILs increase by more than 200 C when confined inside multi-walled carbon nanotubes (MWCNTs) [2, 3]. Experimental results [11] suggest that organic cations are strongly adsorbed on surfaces with negative charges (e.g., mica, silica), and interact strongly with graphitic surfaces; surface roughness is also observed to affect the structure of ILs at the interface. Understanding the behavior of ILs inside nanopores is relevant to potential applications of these systems in electrochemical double layer capacitors (EDLCs) [12-19]. These devices have specific power higher than batteries and fuel cells but lack in specific energy. The conventional electrolytes that are used in these devices are only stable up to 2.5 volts hence limiting the performance in terms of both specific power and specific energy. ILs with their unique properties, especially higher electrochemical window (up to 6.0 volts) have the potential to replace the conventional electrolytes that are used in these devices. ILs also have potential to replace the conventional electrolytes in dye sensitized solar cells (DSSCs), a class of solar cell based on a semiconductor formed between a photo-sensitized anode and an electrolyte. The major problems with the conventional electrolytes in DSSCs are 1
9 that they catch fire, are explosive and evaporate with time [20-22]. This not only undermines the performance of the device, but also causes environmental hazards. Current research suggests that ILs with their unique properties have potential to tackle these problems and improve the performance of DSSCs [22-25]. Understanding the behavior of ILs confined inside nanopores is also relevant to the development of nanoparticles based on organic salts. Recently, Warner et al. synthesized nonmagnetic [26, 27], magnetic [27] and optically-active (fluorescent) [28] nanoparticles based on ILs that are in solid phase at room temperature. They called these nanoparticles GUMBOS (Group of Uniform Materials Based on Organic Salts). These nanomaterials have potential uses in biomedical applications ranging from magnetic hyperthermia cancer treatment to medical imaging [29-37]. As further tunability of the magnetic and optical properties can be achieved by introducing shape anisotropy [29-37], the Warner group also synthesized 1D nanomaterials (e.g., nanorods, nanotubes and nanowires) based on organic salts, by introducing these compounds inside hard templates with cylindrical nanopores, e.g., multi-walled carbon nanotubes (MWCNTs), and anodic aluminum oxide membranes (AAO). Nanomaterials based on ILs hold enormous promise, due to their highly tunable properties [5] and the fact that they can be prepared from FDA-approved compounds [38-41], and thus could be free of the toxic effects that other nanomaterials currently considered for biomedical applications may have [42, 43]. The properties of a nanoconfined fluid phase can be different from those of bulk phase [44]. It is very complicated to gain a molecular-level picture of the behavior of ILs inside nanopores from experiments alone. Characterization techniques usually give an incomplete picture of the pore morphology and surface chemistry of the porous material. Moreover, in experiments it is difficult to determine the individual effects of each variable (e.g., pore size, 2
10 shape, interconnectivity) on the system s properties. Molecular simulations, on the other hand, allow us to work with a model porous material that is completely characterized (the positions and chemical identities of all the atoms are known a priori). In consequence, molecular simulations are well suited to perform a systematic study of the relevant physical variables and determine their individual effects on the system. A reliable computational model can assist in the interpretation of experimental results and provide accurate predictions of the behavior of the system, eliminating the need for costly trial-and-error experiments. Furthermore, molecular simulations of confined ILs will be fundamental to develop theories for the electric double layer for ILs, which are lacking [45] and will be useful to obtain rapid predictions for these systems. 1.2 Outline of Dissertation As stated before, the main objective of this work is to use molecular simulation techniques to study the structural, dynamical and thermodynamic properties of ILs confined in nanopores of different geometries and materials. Chapter 2 is published work (Singh, R., J. Monk, and F.R. Hung, A Computational Study of the Behavior of the Ionic Liquid [BMIM + ][PF - 6 ] Confined Inside Multiwalled Carbon Nanotubes, The Journal of Physical Chemistry C, 2010, 114(36): p ) in which we have performed molecular dynamics (MD) simulations to study the structural and dynamical properties of a typical (IL) [BMIM + ][PF - 6 ] confined inside multi-walled carbon nanotubes with inner diameters ranging between 2.0 and 3.7 nm. Chapter 3 is published work (Singh, R., J. Monk, and F.R. Hung, Heterogeneity in the Dynamics of the Ionic Liquid [BMIM + ][PF - 6 ] Confined in a Slit Nanopore, The Journal of Physical Chemistry C, 2011, 115(33): p ) in which we have performed MD simulations to study the dynamics of the IL [BMIM + ][PF - 6 ] confined in a slit nanopore of pore 3
11 size 5.4 nm. The main focus of this work was to study the effect of temperature on the dynamics the ions and different time regimes that are present in the MSD plots. Chapter 4 includes our MD simulation study of the IL [EMIM + ][TFMSI - ] confined inside rutile (110) pore of pore size 5.2 nm. We studied the effect of pore loading on the structural and dynamical properties of the ILs, and contrast those against our results obtained for the same IL inside a slit graphitic nanopore of the same size. Chapter 5 includes our MD simulation study of mixtures of acetonitrile and varying concentrations of the IL [EMIM + ][TFMSI - ] confined inside slit-like graphitic pore of pore size 5.2 nm. We studied the effect of organic solvent on the structural and dynamical properties of the IL at various IL molar concentrations. Chapter 6 has the summary of the main finding of this work. Ideas for future research in this area are also discussed. 4
12 CHAPTER 2 A COMPUTATIONAL STUDY OF THE BEHAVIOR OF IONIC LIQUID [BMIM + ][PF6 - ] CONFINED INSIDE MULTI-WALLED CARBON NANOTUBES 1 Contents of this chapter have already been published (Singh, R., J. Monk, and F.R. Hung, A Computational Study of the Behavior of the Ionic Liquid [BMIM + ][PF - 6 ] Confined Inside Multiwalled Carbon Nanotubes. The Journal of Physical Chemistry C, 2010, 114(36): p ). In this chapter, we report molecular dynamics (MD) results of the structural and dynamical properties of the IL [BMIM + ][PF - 6 ] confined inside multi-walled carbon nanotubes with inner diameters ranging between 2.0 and 3.7 nm. The main objective of this study was to understand the effect of pore size and pore filling on the structural and dynamical properties of the confined IL. The rest of this chapter is structured as follows. Section 2.1 is the introduction. Section 2.2 contains a description of our computational models and methods. In Section 2.3 we present results and discussion of the structural and dynamic properties of the confined ILs, and in Section 2.4 we summarize our main findings Introduction Ionic liquids (ILs) have attracted extensive attention in recent years for applications ranging from green solvents for chemical synthesis, catalysis and separations, to electrolytes for electrochemistry and photovoltaics, among others [1-10]. When confined inside nm-sized pores, ILs exhibit physical properties that are different from those observed in bulk systems. For example, the melting points and the thermal decomposition temperature of several ILs have been reported to increase by more than 200 C when confined inside multi-walled carbon nanotubes (MWCNTs) [2, 3]. In contrast, when confined inside nanoporous silica [46], ILs can exhibit 1 Reprinted with permission from Singh, R., J. Monk, and F.R. Hung, The Journal of Physical Chemistry C, (36).Copyright (2010) American Chemical Society. 5
13 similar mobility as the bulk phases, and their melting points decrease significantly [1, 47]. This melting point depression is enhanced by decreasing the pore size. Experimental results [11] also suggest that organic cations are strongly adsorbed on surfaces with negative charges (e.g., mica, silica), and interact strongly with graphitic surfaces; surface roughness is also observed to affect the structure of ILs at the interface. All these observations agree with prior studies considering simpler molecules adsorbed inside nm-sized pores [48-50]. Understanding the behavior of ILs inside nanopores is relevant to potential applications of these systems in electrochemical double layer capacitors (EDLCs) [12-19]. These devices have a structure similar to a battery, consisting of two carbon-based nanoporous electrodes, an electrolyte and an ion-permeable separator. EDLCs store charge in the interface between a nanoporous electrode and an electrolyte; during the charging process, a difference of potential induces charges in the negative and positive electrodes, attracting the cations and the anions in the electrolyte. EDLCs can store more energy than traditional capacitors, since the nanoporous electrodes exhibit a large surface area, and the thickness of the electric double layer has nanoscale dimensions. EDLCs cannot store as much energy as fuel cells and batteries, but exhibit fast charge/discharge times and can provide bursts of energy very quickly. As a result, EDLCs can complement the capabilities of internal combustion engines, fuel cells and batteries in hybrid vehicles, industrial equipment and electronic devices. Understanding the behavior of ILs confined inside nanopores is also relevant to the development of nanoparticles based on organic salts. Recently, Warner et al. synthesized non-magnetic [26, 27], magnetic [27] and opticallyactive (fluorescent) [28] nanoparticles based on organic salts that are in solid phase at room temperature. These nanomaterials have potential uses in biomedical applications ranging from magnetic hyperthermia cancer treatment to medical imaging [29-37]. As further tunability of the 6
14 magnetic and optical properties can be achieved by introducing shape anisotropy [29-37], the Warner group also synthesized 1D nanomaterials (e.g., nanorods, nanotubes and nanowires) based on organic salts, by introducing these compounds inside hard templates with cylindrical nanopores, e.g., multi-walled carbon nanotubes (MWCNTs), and anodic aluminum oxide membranes (AAO). Nanomaterials based on organic salts hold enormous promise, due to their highly tunable properties[5] and the fact that they can be prepared from FDA-approved compounds,[38-41] and thus could be free of the toxic effects that other nanomaterials currently considered for biomedical applications may have.[42, 43] Optimization of the applications mentioned above therefore requires a fundamental understanding of the behavior of ILs when confined inside nm-sized pores. In this study, we have used molecular simulation to investigate systems of ILs inside multi-walled carbon nanotubes (MWCNTs). There has been several simulation studies of ILs adsorbed on surfaces such as rutile and graphite [51-55]. A few simulation studies have been published on ILs confined inside nanopores with slit-like [52, 56-61], and cylindrical geometry [62-64]. Only three of these previously mentioned reports considered the formation of solid phases inside nanopores [59, 60, 63]. In these prior studies, the authors considered very narrow pores (pore widths or diameters < 1.4 nm) and very small system sizes (in some cases, only four ion pairs were inside the nanopores). Although understanding the solidification of organic salts inside nanopores is relevant to optimize the synthesis of 1D-nanostructures based on organic salts [26, 28], as a first step we have concentrated on studying liquid phases of organic salts confined inside MWCNTs. The main objective of this study is to understand the effect of pore size and pore filling on the structural and dynamical properties of the confined IL 1-butyl-3- methylimidazolium hexafluorophosphate, [BMIM + ][PF - 6 ]. Recent studies of ILs confined inside 7
15 carbon nanotubes [62-64] have either considered other ILs, or have not studied their dynamical properties Computational Details The software Nanotube Modeler (JCrystalSoft, 2009) was used to generate the xyz coordinates of the carbon atoms in the multi-walled carbon nanotubes (MWCNTs). The MWCNTs were kept Figure 2.1 Schematic representation of the IL [BMIM + ][PF - 6 ]. in fixed positions and were filled with varying amounts of the IL 1-butyl-3-methylimidazolium hexafluorophosphate, [BMIM + ][PF - 6 ]. A scheme of the IL is presented in Figure 2.1. Constant volume and temperature (NVT) molecular dynamics simulations have been performed using the GROMACS MD package [65]. The carbon atoms in the MWCNTs were modeled as LJ spheres with σ c = nm and ε c /k = 28.0 K. The IL was modeled using the all-atom force field developed by Lopes et al. [66-68], which is based on the optimized potential for liquids simulation/all atom (OPLS-AA) of Jorgensen et al.[69] Prior to studying confined systems, we carried out preliminary MD simulations of bulk [BMIM + ][PF - 6 ] in the NVT and NPT ensembles. From these simulations we obtained a value of 1.35 g/cm 3 for the bulk liquid density, and 42.2 kcal/mol for the heat of vaporization at 25 C (this last quantity was determined following the 8
16 procedure detailed by Morrow and Maginn).[70] These simulated values are comparable to results from previous simulations, [70] and to reported experimental values (1.36 g/cm 3 ; and 37.0 kcal/mol and 45.8 kcal/mol). [71, 72] For our simulations with confined ILs, we considered four different multi-walled carbon nanotubes (MWCNTs) having a length of L = 5.0 nm and internal pore diameters D equal to 2.0, 2.5, 3.0 and 3.7 nm. These pore diameters D are measured as the center-to-center distance between diametrically-opposed carbon atoms in the inner wall of the MWCNTs. In our first set of simulations, we placed a total of 33, 60, 96 and 147 ion pairs initially arranged in an arbitrary lattice inside the MWCNTs mentioned above. These numbers of ion pairs were chosen for the confined [BMIM + ][PF - 6 ] to have a density of 1.35 g/cm 3. We also performed additional simulations at different degrees of pore loadings, where we reduced the number of ion pairs inside the MWCNTs to 80% and 65% of the bulk density. These densities for the confined IL were calculated using a volume Vmin D c L 4 2. Our results for the radial density profiles (Figure 3) indicate that the density of the confined ions reach a value of zero at r D 2 ~ c. Such an observation leads us to believe that the volume we have used in our calculations give an accurate estimate of the density of the confined IL in our systems. We also ran several simulations considering longer MWCNTs (L = 10 nm), obtaining results similar to those obtained with L = 5.0 nm. In all our simulations, the Lennard-Jones interactions were cut off at 1.2 nm, and the long range coulomb interactions were handled by the particle-mesh Ewald (PME) method[73] with a cutoff of 1.0 nm and a grid spacing of 0.1 nm. Periodic boundary conditions were applied in the x, y, and z directions. The ILs/nanotube system was kept in vacuum in the center of the 9
17 orthorhombic box in such a way that one side of the box was equal to the length of the nanotube (5.0 nm), and the length of the other two sides of the box (12.0 nm) was long enough to avoid any artificial effect caused by the periodic boundary conditions. From preliminary simulations it was determined that a 5.0 nm axial length was long enough to remove any finite-size effects due to periodic boundaries in the system. The improved velocity-rescaling algorithm recently proposed by Parrinello et al.[74, 75] was used to mimic weak coupling at 300 K with a coupling constant of 0.1 ps. D =2.0 nm D =2.5 nm D =3.0 nm D =3.7 nm Figure 2.2 Representative simulation snapshots of [BMIM + ][PF 6 - ] confined inside MWCNTs of different diameters. 10
18 We first minimized the energy of our initial configurations using the steepest descent method. Afterwards, we ran MD simulations using a time step of 0.5 fs. All of the systems were run for 2 ns at 600 K, and then annealed from 600 to 300 K in six stages: 200 ps at 550 K, 300 ps at 500 K, 300 ps at 450 K, 200 ps at 400 K, 500 ps at 350 K and 6.5 ns at 300 K. Afterwards, we took the final configuration of our systems and repeated the above steps two additional times. We subjected our systems to a total of three cycles of simulated annealing in an attempt to overcome the difficulties posed by the slow dynamics of ILs, which are likely to be exacerbated when these compounds are confined inside nanopores. Our final run at 300 K was further extended to up to 40 ns, and our measured properties were averaged over the final ~30 ns of simulation time. Representative simulation snapshots for our systems are depicted in Figure Results and Discussion Structural Properties In Figure 2.3 we present the radial density profiles of the ions (in kg/m 3 ) confined inside MWCNTs of different diameters, when the confined IL has a density similar to that of a bulk IL (ρ bulk ). The density profiles of the ions are calculated from the individual atomic positions. The mass density profiles of the confined IL are found to be oscillatory with a local maximum in the density close to the pore walls, due to the stacking of the ions near the wall surface. Layering behavior is observed for both the cations and anions and all pore diameters considered. The oscillation patterns observed in the radial density profiles depend strongly on the diameter of the MWCNTs. The number of layers of IL increases from two to four as the diameter of the nanotubes increases from 2.0 nm to 3.7 nm. Another interesting observation regards the positions and the widths of the peaks of the radial density profiles for the ions. The peaks for both the cations and anions are observed in similar positions, and the peak width is about nm, 11
19 Mass density (Kg/m 3 ) Electron density (e/nm 3 ) Mass density (Kg/m 3 ) Electron density (e/nm 3 ) Mass density (Kg/m 3 ) Electron density (e/nm 3 ) Mass density (Kg/m 3 ) Electron density (e/nm 3 ) showing that the cations and anions stack together inside the MWCNTs. The results presented in Figure 2.3 are in good agreement with previous results obtained for a different IL inside single D = 2.0 nm Cation Anion Radial distance (nm) D = 2.5 nm Cation Anion Radial distance (nm) D = 3.0 nm Cation Anion Radial distance (nm) D = 3.7 nm Cation Anion Radial distance (nm) D = 2.0 nm Cation Anion Radial distance (nm) D = 2.5 nm Cation Anion Radial distance (nm) D = 3.0 nm Cation Anion Radial distance (nm) D = 3.7 nm Cation Anion Radial distance (nm) Figure 2.3 Mass density profiles (left) and electron density profiles (right) of the confined ions along the radial direction. In all cases, the confined IL has a density similar to ρ bulk. 12
20 Mass density (Kg/m 3 ) Mass density (Kg/m 3 ) Mass density (Kg/m 3 ) Mass density (Kg/m 3 ) and double-walled carbon nanotubes [62]. These observations are also similar to what was obtained for the density profiles of ILs inside pores of slit-like geometry [56]. We also present in Figure 2.3 the electron density profiles for the cations and anions in the radial direction, which were calculated by the procedure described by Bhargava and Balasubramanian.[76] X-ray reflectivity experiments have been used in the past to measure the electron density profiles as a function of depth at the [BMIM + ][PF - 6 ]-vapor interface [77]. The electron and the mass density profiles in the radial direction are very similar. Both profiles show oscillatory patterns, with the positions of the peaks for the cations and anions completely in phase (i.e., the peaks for the cations and anions in both electron and mass density profiles are observed at similar radial positions). These observations are in contrast to what was found in simulation studies for the ρ ~ 0.45ρ Bulk Cation Anion ρ ~ 0.65ρ Bulk Cation Anion Radial distance (nm) Radial distance (nm) ρ ~ 0.80ρ Bulk Cation Anion Radial distance (nm) ρ ~ ρ Bulk Cation Anion Radial distance (nm) Figure 2.4 Mass density profiles along the radial direction of the cations and anions confined with a MWCNT of D = 3.7 nm, for different pore loadings (expressed as percentages of ρ bulk ). 13
21 Mass density (Kg/m 3 ) Mass density (Kg/m 3 ) Mass density (Kg/m 3 ) Mass density (Kg/m 3 ) Mass density (Kg/m 3 ) Mass density (Kg/m 3 ) [BMIM + ][PF - 6 ]-vapor interface,[76] where the positions of all of the ion peaks were out of phase with the exception of the one that was exactly at the vapor-il planar interface. These differences in behaviors can be attributed to the important differences in geometry (cylindrical vs. planar) and the confinement effects due to the MWCNT walls D =2.0 nm Axial distance (nm) 1500 D =2.0 nm Axial distance (nm) 1500 D =2.0 nm Axial distance (nm) 1500 D =3.7 nm Axial distance (nm) 1500 D =3.7 nm Axial distance (nm) 1500 D =3.7 nm Axial distance (nm) Figure 2.5 Mass density profiles along the axial direction of the ions confined inside MWCNTs of D = 2.0 nm (left) and D = 3.7 nm (right) and different pore loadings: ρ ~ ρ bulk (top), ρ ~ 0.8 ρ bulk (center) and ρ ~ 0.65 ρ bulk (bottom). Continuum and dashed lines represent the densities of cations and anions. 14
22 Orientation (Cos (θ)) In Figure 2.4 we present mass density profiles in the radial direction for both cations and anions confined inside a MWCNT with D = 3.7 nm at different pore loadings. Our results show that reductions in the pore loading lead to a decrease in the mass density near the center of the nanotube, and to an increase in the local density near the surface of the nanotube. We also studied the axial density profiles of the confined cations and anions at different pore loadings; results for the case of D = 2.0 and 3.7 nm are shown in Figure 2.5. When the confined IL has a density similar to ρ bulk, the axial density profiles are relatively uniform for both pore sizes, but as the pore loading decreases, we observe local variations in the axial density profiles, which exhibit regions of high and low densities (Figure 2.5). The variations in the axial density are particularly pronounced for the confined cations nm nanotube 3.0 nm nanotube 3.7 nm nanotube Radial distance (nm) Figure 2.6 Orientation distribution profile (continuum lines) of the imidazolium ring of the cation as a function of radial position for different pore sizes and ρ ~ 0.8 ρ bulk. Cos(θ) is defined as the angle between the radial vector and the vector perpendicular to the imidazolium ring. The radial position of the inner surfaces of the carbon atoms at the pore walls are represented as dotted lines. 15
23 In order to understand the structure of the ILs near the nanotube surface, we determined the orientation of the cations as measured by cos(θ), where θ is the angle between the radial vector and the vector normal to the imidazolium ring. In Figure 2.6 we present results for the average value of cos(θ) as a function of the radial position for MWCNTs of different diameters when the confined IL has a density similar to 80% of ρ bulk. Our results show that cos(θ) has a relatively large value near the surfaces of the MWCNTs, indicating that the imidazolium ring of the cations tend to align parallel to the surface when close to the walls. Another factor that affects the ordering of the cations is the available surface area, which increases as the nanotube diameter becomes larger. For the MWCNTs with D = 3.0 and 3.7 nm, the average values of cos(θ) drop as we approach the center of the nanotube (Figure 2.6), suggesting a loss in orientational order with increasing distance from the pore walls. These results are consistent with similar findings obtained for ILs confined inside slit-like pores [56], as well as inside single- and double-walled nanotubes [62] Dynamical Properties In Figure 2.7 we present results for the mean square displacements in the axial direction of [BMIM + ][PF - 6 ] confined inside MWCNTs of D = 3.7 nm and 3.0 nm at 300 K and different pore loadings (expressed as a percentage of the bulk density of the IL at the same temperature). These MSD results suggest that the cations move faster than the anions, in analogy to previous studies for bulk systems [70, 78-83]. At any given value of t, the MSD for the bulk cations and anions are larger than those observed for the confined ions, indicating that the dynamics for bulk systems are faster than for confined systems. Our results also suggest that, for a given pore size, the dynamics are faster when the confined IL has a density lower than the bulk density. Systems at lower pore loadings have larger MSDs and reach the diffusive regime (MSD ~ t) faster; in 16
24 MSD, Axial Direction (nm 2 ) MSD,Axial Direction (nm 2 ) 1 D = 3.0 nm 0.1 Fickian Time ( ns ) 1 D = 3.7 nm 0.1 Fickian Time ( ns ) Figure 2.7 Mean square displacement in the axial direction for the ions confined inside MWCNTs with D = 3.0 nm (top) and D = 3.7 nm (bottom), and different pore loadings: ρ ~ ρ bulk (red), ρ ~ 0.8 ρ bulk (green) and ρ ~ 0.65 ρ bulk (blue). Black lines represent the bulk behavior. Continuum and dashed lines represent the MSDs of cations and anions. The continuous purple line indicates the behavior expected for the Fickian regime (MSD t). contrast, systems at the largest pore loading have barely reached the Fickian regime close to the end of our simulations. Also, for all pore loadings considered, it is apparent that there is a long 17
25 time interval in which the MSD increases slowly with t, which is a signature of the so-called cage effect where ions are temporarily trapped by their surrounding neighbors. The cage effect has been observed in bulk ILs [78, 84], as well as in supercooled LJ liquids confined in nanopores [85]. The time intervals for this cage regime are larger for confined systems, as compared to those observed for bulk systems. Our results suggest that for a given pore loading, there is a non-monotonic dependence of MSD with pore size. Our results suggest that, for pore loadings equivalent to 100% and 80% of the bulk density, the axial MSD decreases with increasing degree of confinement. However, for a pore loading equivalent to 65% of the bulk density, the axial MSD observed for D=3.0 nm is larger than that observed for D=3.7 nm. Furthermore, our results suggest that for a given pore size, there is a non-monotonic dependence of MSD with pore loading. For a MWCNT with D = 3.0 nm, the axial MSD increases with decreasing pore filling, but for the MWCNs with D = 3.7 nm, the smaller MSD is observed when the density of the confined IL is similar to the bulk density, and the largest MSD is observed at ~80% of the bulk density. Intermediate values of the axial MSD are observed when the confined IL has a density similar to 65% of the bulk density. Furthermore, for this latter pore size, when the IL has a density similar to 80% of ρ bulk it reaches the diffusive regime faster than when it has a density similar to 65% of ρ bulk. The axial MSDs for an IL confined inside a MWCNT with D = 3.0 nm (data not shown) have a behavior similar to that we have described for a MWCNT with D = 3.7 nm. These non-monotonic behaviors of the axial MSD for varying pore sizes and fixed pore loadings, and for fixed pore sizes and varying pore loadings, may be due to strong local fluctuations in the axial density profile of the confined IL, as the pore filling decreases (Figure 2.5). We intend to study this behavior in more detail in an upcoming manuscript. 18
26 C(t)/C(0) MSD, Axial Direction (nm 2 ) C(t)/C(0) MSD, Axial Direction (nm 2 ) 1.2 D =3.0 nm (ρ ~0.80 ρ Bulk ) Time ( ns ) 1.2 D =3.0 nm (ρ ~ ρ Bulk ) Time ( ns ) Cr Cr (NN) Cr (NH) Cv Cr Cr (NN) Cr (NH) Cv 0.03 D =3.0 nm (ρ ~0.80 ρ Bulk ) Time ( ns ) D =3.0 nm (ρ ~ ρ Bulk ) Time ( ns ) Cation Anion Cation Anion Figure 2.8 Single-particle time correlation functions for the cations (left panel) and mean square displacement in the axial direction for cations and anions (right panel). The IL is confined inside a MWCNT with D = 3.0 nm and two pore loadings are considered: ρ ~ 0.8ρ bulk (top panel) and ρ ~ ρ bulk (bottom panel). The correlation functions depicted are the following: Cv = velocity of the center of mass of the cation (Cv); Cr = reorientation of the cation around an axis perpendicular to the imidazolium ring; Cr (NN) = reorientation of the cation around an axis in the same plane as the imidazolium ring, but perpendicular to the vector joining the N atoms (see Figure 1); and Cr (NH) = similar to Cr (NN), but now the vector is in the direction of the C-H bond of the CR carbon (Figure 2.1). Motivated by the simulation results presented by Urahata and Ribeiro [79] for bulk ILs, in Figure 2.8 we present several single-particle time correlation functions of the cations, as well 19
27 as the axial MSD of the cations and anions confined within a MWCNT of D = 3.0 nm and two different pore loadings. These results indicate that for both pore fillings, the velocity of the center of mass of the cations decorrelate very quickly, as this correlation function reaches a value close to zero in less than 1 ns of simulation time. In contrast, the reorientational motion of the ring of the cations around different rotation axes is orders of magnitude slower. In particular, when the confined IL has a density similar to that of a bulk IL, ~30 ns of simulation time are not enough for these rotational motions to completely decorrelate. Nevertheless, reducing the density of the confined fluid to about 80% of ρ bulk allow the reorientational motion of the imidazolium ring of the cations to decorrelate after ~30 ns. Even though the translational and the angular velocities of the cations are not directly compared here, the single-particle time correlation functions presented serve as a suitable measure of different timescales involved in the translational and rotational motion of the cations for different pore loadings. Previous simulations for bulk ILs also pointed out large differences in characteristic timescales for the translational and rotational motions of the cations of ILs [78, 79]. In Figure 2.9 we present data for the axial MSD of the cations and anions according to their radial position within a MWCNT of D = 3.0 nm and a density similar to that of a bulk system. There are slight differences in the MSDs for the different layers of ions. The cations in the inner layer (center of the pore) move faster than those in the middle layer, which in turn have faster dynamics than the cations close to the walls of the nanotube (outer layer). These results are in agreement with those observed for supercooled LJ fluids in confinement [85]. Similarly, the anions close to the walls have slower dynamics as compared to those in the inner and middle layers. 20
28 MSD,Axial Direction (nm 2 ) D =3.0 nm Time ( ns ) Cation (0 < r < 0.5) Cation (0.5 < r <1.0) Cation (1.0 < r <1.5) Anion (0 < r < 0.5) Anion (0.5 < r < 1.0) Anion (1.0 < r < 1.5) Figure 2.9 Mean square displacement in the axial direction for the cations and anions in different layers according to their radial position (Figures 2 and 3). The ions are confined inside a MWCNT of D = 3.0 nm and have ρ ~ ρ bulk. Thick, continuous lines represent the cations, and thin, dashed lines represent the anions. All r distances are in nm. Nevertheless, the axial MSD of the anions in the inner and middle layers are similar in magnitude. The cations in the inner and outer layers have larger MSDs than the anions in those layers; in contrast, the MSDs of cations and anions in the middle layers are comparable in magnitude. Similar results are observed in our simulations for MWCNTs of different sizes when the density of the confined IL is similar to that of a bulk IL (data not shown). These results suggest that the local dynamics of the confined ions are heterogeneous and depend strongly on their radial position. 2.4 Concluding Remarks We have studied the structural and dynamical properties of the IL [BMIM + ][PF 6 - ] confined inside MWCNTs of different diameters. Our results indicate that the diameter of the MWCNT and the pore loading have a profound influence on the structural and dynamical 21
29 properties of the confined IL. The mass density profiles of the ions in the radial direction show layering behavior, with the peaks of the cations and anions taking place at similar values of r. Increasing the pore diameter results in an increase in the number of layers from two (D = 2.0 nm) to four (D = 3.7 nm). Reductions in the pore loading lead to regions of low density in the center of the pore. The density profiles in the axial direction also exhibit important variations, with regions of high and low densities as the pore loading decreases. Our simulations also indicate that cations close to the pore surface tend to align with their imidazolium ring parallel to the surfaces. Our MSD results in the axial direction indicate that the confined ions show dynamics that are slower than those observed in bulk systems. The confined cations move faster than the anions, in analogy to bulk systems; however, the confined ions spend a significantly larger amount of time in the cage regime than bulk systems, before finally reaching the Fickian (diffusive) regime. In analogy to bulk systems, there are large differences in the characteristic timescales for the translational and rotational motion of the confined cations. The former decorrelate very quickly (much less than 1 ns of simulation time), but the rotational motion of the cation is orders of magnitude slower. The rotation of the imidazolium ring of the cation decorrelates faster when the pore loading is reduced. Our results also suggest that the cations in the center of the pore tend to move faster than those close to the pore walls, in analogy to the behavior observed for simple fluids in confinement. A similar behavior is observed for the distinct layers of anions, although the differences in dynamics are not as marked as those observed between layers of cations. Finally, our results suggest that for some pore sizes, the axial MSD increases with decreasing pore filling; however, for some pore sizes, the axial MSD seems to have a non-monotonic dependence with pore loading. Likewise, there is a non-monotonic 22
30 dependence of MSD with pore size and fixed pore loadings. These non-monotonic behaviors are possibly due to the presence of local variations in the axial density profile of the IL as the pore loading decreases. We intend to study this behavior in more detail in an upcoming manuscript. Similarly, in upcoming studies we will consider confinement of ILs in pores of different geometries, as well as studying the behavior of solid phases of organic salts inside nanopores. 23
31 CHAPTER 3 HETEROGENEITY IN THE DYNAMICS OF THE IONIC LIQUID [BMIM + ][PF 6 - ] CONFINED IN A SLIT NANOPORE 2 Contents of this chapter have already been published (Singh, R., J. Monk, and F.R. Hung, Heterogeneity in the Dynamics of the Ionic Liquid [BMIM + ][PF - 6 ] Confined in a Slit Nanopore, The Journal of Physical Chemistry C, 2011, 115(33): p ). In this chapter, we report molecular dynamics (MD) results of the structural and dynamical properties of the IL [BMIM + ][PF - 6 ] when confined inside an uncharged slit-like graphitic pore of width H = 5.4 nm, in the temperature range K. The main objective of this work was to study in detail the dynamical properties of the same IL, [BMIM + ][PF6 - ], when confined inside a much simpler pore geometry, namely an uncharged slit-like graphitic pore. The rest of this chapter is structured as follows. Section 3.1 is the introduction. Section 3.2 contains a description of our computational models and methods. In Section 3.3 we present results and discussion of the structural and dynamic properties of the confined ILs, and in Section 3.4 we summarize our main findings. 3.1 Introduction Ionic liquids (ILs) are organic salts with a melting temperature below the boiling point of water.[86] The properties of ILs are highly tunable; as many as 10 9 to ILs could be formed by varying the cations or the anions.[86] ILs have applications as green solvents for chemical synthesis, catalysis and separations, and as electrolytes for electrochemistry and photovoltaics, among others.[6, 7, 86] A fundamental understanding of the properties of ILs inside nanopores is relevant for applications of ILs as electrolytes for energy storage in electric 2 Reprinted with permission from Singh, R., J. Monk, and F.R. Hung, The Journal of Physical Chemistry C, (33).Copyright (2011) American Chemical Society. 24
32 double-layer capacitors (EDLCs),[12, 13, 87-93] and in dye-sensitized solar cells (DSSCs) for conversion of solar energy.[93-95] Furthermore, ILs confined inside sol-gels (ionogels) have potential applications as electrolyte membranes and in optics, catalysis and biocatalysis, drug delivery and sensing and biosensing.[10, 96-98] In addition, very recently nanoparticles (NPs) based on frozen ILs with interesting magnetic[26, 27] and optically-active (fluorescent)[28, ] properties were synthesized. As further tunability of the magnetic and optical properties can be achieved by introducing shape anisotropy,[29-37, 102] the Warner group has also synthesized 1D-nanomaterials (e.g., nanorods, nanotubes and nanowires) based on organic salts,[101] by introducing these compounds inside hard templates with cylindrical nanopores, e.g., anodized alumina membranes. Nanomaterials based on organic salts hold enormous promise, because they share the inherent functionality and highly tunable properties of ILs,[7, 86] and can be prepared via simple procedures.[26-28, ] Furthermore, nanomaterials based on organic salts can be prepared from FDA-approved compounds,[38-41] and thus could be free of the toxicity effects that other nanomaterials may have.[ ] Possible applications of these organic salts-based nanomaterials are envisioned in fields as diverse as optoelectronics, photovoltaics, separations, analytical and biomedical. The macroscopic performance of IL-based EDLCs and DSSCs, as well as the macroscopic properties of ionogels and IL-based 1D-nanomaterials, are determined by the structural and dynamical properties of the IL confined inside the nanopores. Nevertheless, a fundamental understanding of the structural and dynamical properties of ILs inside nanopores is still lacking. Such knowledge is crucial for the rational design of EDLCs, DSSCs, ionogels and IL-based nanomaterials with optimal properties. Molecular simulations, in close interplay with 25
33 experiments, are well positioned to provide such fundamental understanding and advance those technologies well beyond their current state of the art. Several studies have reported simulations of ILs and molten salts adsorbed on surfaces such as rutile, graphite, quartz and sapphire.[51-55, ] A few simulation reports have been published on ILs confined inside nanopores with slit-like,[52, 56-58, 61, 113, ] cylindrical[62-64, 113, 122] and complex pore geometries.[123] In a recent paper,[122] we have studied the structural and dynamical properties of the IL [BMIM + ][PF - 6 ] confined inside multiwalled carbon nanotubes of diameters ranging from 1.5 to 4.7 nm. In this study, we found that the dynamics of the confined cations and anions are heterogeneous and depend strongly on their distance to the pore walls; for example, the cations and anions in the center of the pore had faster dynamics than those close to the pore walls. However, in that study we used the force field proposed by Lopes et al.,[66-68] which has important limitations in reproducing experimental values of the transport properties of bulk ILs. For example, the diffusion coefficients of bulk [BMIM + ][PF - 6 ], as predicted by the force field of Lopes et al., are on average about 6 times smaller than reported experimental values in the temperature range between 300 K and 450 K.[124, 125] Although the absolute values of the dynamics of the confined IL as estimated in our previous study[122] are likely to be off, we expect that the trends observed for the dynamics of the confined IL as a function of pore size and pore loading, as well as the heterogeneities observed in the dynamics of the confined ions as a function of their distance to the pore walls, are still going to be consistent with the trends observed in a similar real system. The aim of our present work is to study in detail the dynamical properties of the same IL, [BMIM + ][PF - 6 ], when confined inside a much simpler pore geometry, namely an uncharged slitlike graphitic pore. We have modeled the IL using the non-polarizable force field developed by 26
34 Bhargava and Balasubramanian,[124] which can give accurate predictions of the structure and dynamics of bulk [BMIM + ][PF - 6 ]. Furthermore, this force field was used recently to study heterogeneity in the dynamics of [BMIM + ][PF - 6 ] in the bulk.[87] In addition to mean squared displacements (MSDs), we also report results for the radial distribution function, self-part of the time-dependent van Hove correlation function G s (r,t), and the incoherent intermediate scattering function F s (q,t). These properties have been used to detect and characterize dynamical heterogeneities in previous studies of bulk ILs (see ref. [78] for a recent review) and supercooled Lennard-Jones (LJ) liquids in a slit pore.[85] Heterogeneities in the dynamics are explained in terms of structural properties, namely local density profiles and radial distribution functions. Previous simulation studies of ILs and molten salts confined in slit pores [52, 56-58, 61, 113, ] have not placed a special focus on dynamical heterogeneities. 3.2 Computational Details Constant volume and temperature (NVT) molecular dynamics simulations have been performed using the GROMACS MD package.[65] We considered a slit-like graphitic pore of size H = 5.4 nm, where the two pore walls are formed by three graphene layers of area (5.70 nm 5.61 nm) fixed in space (Figure 3.1). The carbon atoms in the graphite sheets were modeled as LJ spheres with σ c = nm and ε c /k = 28.0 K. Regarding the force field for the IL 1-butyl-3-methylimidazolium hexafluorophosphate, [BMIM + ][PF - 6 ], in our previous study[122] we used the model proposed by Lopes et al. [66-68] to study the structural and the dynamical properties of the IL inside carbon nanotubes. However, this force field has important limitations in reproducing experimental values of the transport properties of bulk ILs; for example, the diffusion coefficients of the cation and anion of [BMIM + ][PF - 6 ] as predicted by the force field of Lopes et al. are on average about 6 times 27
35 smaller than reported experimental values in the temperature range between 300 K and 450 K.[124, 125] In the present work, the IL was modeled using the non-polarizable force field developed by Bhargava and Balasubramanian.[124] In this model, charges on the cations and anions are chosen to be 0.8e and -0.8e respectively (see refs. [124] and [78] for a discussion of the reasons behind this choice of net charges for the ions). Bhargava and Balasubramanian have reported simulated density values within 1.4% of the experimental values, and diffusion coefficients that are within 20% of experimental values in the temperature range between 300 K and 500 K. Prior to studying confined system, we carried out preliminary MD simulations of bulk [BMIM + ][PF - 6 ] in the NVT and NPT ensembles. From these simulations we obtained a value of 1.38 g/cm 3 for the bulk liquid density at 25 C; diffusion coefficients for the cations and anions were found to be m 2 s -1 and m 2 s -1 respectively. Our simulated values are comparable to results from previous simulations, and to reported experimental values.[87, 124] In our simulations, the slit pore was filled with a varying number of ion pairs so that our systems had a density similar to the bulk density at the temperatures of interest. Therefore, the number of ion pairs we used were 486, 470 and 455 at 300 K, 350 K and 400 K, so that the confined IL had a density of 1.37 g/cm 3, 1.33 g/cm 3 and 1.28 g/cm 3 at these temperatures. The temperature range considered in our simulations ( K) is well above the experimental glass transition temperature of K reported for [BMIM + ][PF - 6 ].[126] The density for the V xy z confined IL was calculated using a volume min c. Our results for the mass density profile (Figure 1) indicate that the density of the confined ions reach a value of zero at z ~ c 2. We have also run simulations at different temperatures keeping constant the number of ion pairs, 28
36 and the results were very similar to those obtained when our confined IL had a density similar to the bulk value at the temperatures of interest. In all our simulations, the Lennard-Jones interactions were cut off at 1.2 nm, and the long range coulomb interactions were handled by the particle-mesh Ewald (PME) method [73] with a cutoff of 1.0 nm and a grid spacing of 0.1 nm. Periodic boundary conditions were applied in the x, y, and z directions. The ILs/graphite sheet system was kept in vacuum in the center of the orthorhombic box in such a way that two sides of the box were equal to the sides of the graphite sheets (5.70 nm 5.61 nm), and the length of the other one side of the box (10.0 nm) was long enough to avoid any artificial effect caused by the periodic boundary conditions. The improved velocity-rescaling algorithm recently proposed by Parrinello et al.[74, 75] was used to mimic weak coupling at different temperatures with a coupling constant of 0.1 ps. We first minimized the energy of our initial configuration using the steepest descent method. Afterwards, we ran MD simulation using a time step of 0.5 fs. The systems were melted at 600 K for 2 ns, and then annealed from 600 to 300 K in stages: 200 ps at 550 K, 300 ps at 500 K, 300 ps at 450 K, 200 ps at 400 K, and 500 ps at 350 K. Afterwards, our systems were equilibrated and the properties of interest were sampled for 6.5 ns at 300 K. In order to make sure that our systems were properly equilibrated, apart from monitoring standard variables (e.g., energy), we also examined the mean squared displacement (MSD) and confirmed that the confined IL has reached the diffusive regime (Figures 3.3 and 3.4). In addition, and as we did in our previous study,[122] we also monitored several single-particle time correlation functions for the cations (namely, the correlation functions for the velocity of the center of mass of the cation, and the reorientation of the cation around different rotation axes). These results (not shown for 29
37 brevity) indicate that our simulation times are long enough to decorrelate the reorientational motion of the ring of the cations around different rotation axes. After finishing this first production run, we took the final configuration of our system and repeated the above steps (melting, annealing, equilibration and averaging) at least one additional time. Our reported results were averaged over at least two independent realizations of the same system. Such a procedure was adopted in an attempt to overcome the difficulties posed by the slow dynamics of ILs, which are likely to be exacerbated when these compounds are confined inside a nm-sized slit pore. A similar procedure was used to carry out simulations of the confined IL at 350 and 400 K; likewise, we also performed simulations of the bulk IL at 300, 350 and 400 K using a similar procedure. A representative simulation snapshot of the IL confined at 300 K is depicted in Figure Results and Discussion Prior to the study of the dynamics of the confined system, we measured the mass density profile of the ions in the IL for different temperatures ranging from 300 to 400 K (Figure 3.1). Layering behavior was observed at all temperatures, with two density peaks close to each of the graphite sheets and a region in the center of the pore where the IL has a density similar to the bulk density (Figure 3.1). It is also observed that the positions of the peaks do not change appreciably with variations in the temperature; however, the heights of the peaks decrease as the temperature increases. According to our previous simulation study of ILs confined in cylindrical nanopores,[122] the dynamical properties of the ions will depend strongly on their position from the pore walls. As a result, and based on the density profiles shown in Figure 3.1, we split the ions into three different layers/regions according to their distance from the pore walls: the first layers (closest to the pore walls), the second layers and the center regions. We monitored the 30
38 Mass Density (Kg/m 3 ) coordinates of the ions as a function of time during the sampling period from the simulation trajectories, and we did not observe significant exchange of ions within the different layers/regions. The fact that ions remain in their layers/regions during the sampling period allowed us to accurately sample and report the properties of each layer/region of the confined IL, as indicated below K 350 K 400 K Z (nm) Figure 3.1 Left panel: Representative simulation snapshot of the IL [BMIM + ][PF 6 - ] confined inside a slit-like graphitic pore of size H = 5.4 nm at 300 K. Cations and anions are depicted in purple and green. The density of the confined IL is similar to that of the bulk IL at the same temperature. Right panel: Mass density profile of the IL along the z direction at different temperatures. 31
39 MSD (nm 2 ) MSD (nm 2 ) 10 First layers 10 Center region Time (ps) Time (ps) Figure 3.2 Parallel (x- and y-) and z-component of the mean square displacements of the ions in the first layers (left panel) and in the center region of the pore (right panel) at 400 and 300 K. Solid and dashed lines represent cations and anions. Red and purple lines depict the parallel and z-components of the MSD at 400 K; black and green lines represent the parallel and z- components of the MSD at 300 K Mean Squared Displacement (MSD) and Self-diffusion Coefficients In Figure 3.2, we show a semi-log plot of the MSDs of the ions in the first layers and in the center region for 300 K and 400 K. Depicted in this figure are the parallel and the z- component of the MSD of both cations and anions. Results for the second layers are in-between those for the first layers and the center region, and thus are not shown. As expected, the dynamics of the ions increase with increase in temperature. It is also observed that cations move faster than anions independent of their distance from the pore walls. The dynamics of the ions in the z-direction are slower as compared to those in the parallel direction due to the presence of the 32
40 MSD (nm 2 ) MSD (nm 2 ) Cation, first layers Cation, second layers Cation, center region Diffusive regime Cation,bulk Sub-Diffusive regime Ballistic regime Time (ps) Anion, first layers Anion, second layers Anion, center region Diffusive regime 0.01 Anion, bulk Sub-Diffusive regime Ballistic regime Time (ps) Figure 3.3 Parallel component of the mean squared displacement for the cations (top panel) and anions (bottom panel) at 300 K, as a function of their distance from the pore walls. The continuous blue lines depict the slopes expected for the ballistic and diffusive regimes. 33
41 walls and confinement effects. As expected, the dynamics of the confined IL become faster with an increase in the temperature. In Figure 3.3 we show a log-log plot of the parallel component of the MSD of the confined cations and anions in the different layers/regions at 300 K, and compare against the simulated MSD for the bulk IL at the same temperature. The dynamics of the confined ions are heterogeneous: the slowest dynamics are observed for the ions in the first layers (closest to the pore walls), and the dynamics of the ions in the center of the pore are very similar to those observed for the ions in the bulk IL. These results agree with our previous findings for an IL confined in a cylindrical pore,[122] as well as with previous results for a supercooled LJ liquid confined in a slit pore with rough walls.[85] All MSDs exhibit the three dynamic regimes (ballistic, sub-diffusive and diffusive or Fickian).[85, 127] The ballistic regime occurs in the first few picoseconds when the molecules have not interacted with other neighbors, and thus the dynamics are faster than in the other regimes, MSD t 2.[85, 127] After the ballistic regime, a plateau is observed and the system is in the sub-diffusive regime. In this regime the dynamics slow down due to the ions becoming temporarily trapped by a cage formed by their neighbors. The time window in which this plateau is observed is slightly larger for the ions close to the pore walls, and becomes shorter for the ions in the center of the pore and for the ions in the bulk IL (Figure 3.3). Eventually, particles escape the cage formed by their neighbors, the MSDs become proportional to t and the dynamics reach the diffusive regime. An interesting observation is that the bulk and the ions in the center of the pore reach the diffusive regime faster than the ions closer to the pore walls; the ions in the first layers (closest to the pore walls) spend the longest time in the sub-diffusive regime. As temperature increases, the plateau corresponding to the sub-diffusive regime becomes weaker and the ions tend to reach the diffusive regime faster, as can be observed in Figure 3.4. In this figure we depict the variations in the overall MSD for 34
42 MSD (nm 2 ) MSD (nm 2 ) K 350K 300K Cation, center region Diffusive regime 0.01 Sub-Diffusive regime Ballistic regime Time (ps) K 350K 300K Anion, center region Diffusive regime 0.01 Sub-Diffusive regime Ballistic regime Time (ps) Figure 3.4 Overall mean square displacement of the cations (top panel) and anions (bottom panel) in the center region of the pore at 300 K, 350 K and 400 K. The continuous blue lines depict the slopes expected for the ballistic and diffusive regimes. 35
43 the ions in the center region of the pore as the temperature changes. Similar trends with temperature are observed for the ions in the first and second layers (results not shown). The self-diffusion coefficients of the ions are obtained from the MSDs in the diffusive regime by using the appropriate Einstein relation.[128, 129] The obtained values of the diffusion coefficient of the confined ions in the parallel direction are compared with the bulk values at 300 K, 350 K and 400 K and are reported in Table 3.1. Only for comparison purposes, we also show in this table the self-diffusion coefficients in the parallel direction that would be obtained for the ions in the different layers/regions of the pore. Results from this table indicate that at all temperatures, the self-diffusivities of the confined ions in the parallel direction are always smaller than the bulk values (or at most equal, within the reported uncertainties). In addition, and consistent with our MSD results (Figures ), the ions close to the graphite have the slowest dynamics and the ions in the center of the pore have dynamics similar to those in the bulk IL. Table 3.1 Diffusion coefficient of the confined ions in the parallel direction at different temperatures. Bulk results obtained from simulation and experiment (parentheses) are taken from Bhargava and Balasubramanian.[124] Cation, Ds (m 2 s -1 ) T [K] first layers second layers center region overall Bulk (+/-0.8) 6.4 (+/-0.1) 7.2 (+/-0.5) 6.1 (+/-0.8) 6.7 (8.0) (+/-1.2) 23.3 (+/-0.7) 48.6 (+/-1.2) 36.1 (+/-0.8) 77.6 (64.2) (+/-18.9) (+/-2.1) (+/-2.6) (+/-3.4) (209.2) Anion, Ds (m 2 s -1 ) T [K] first layers second layers center region overall Bulk (+/-0.8) 3.6 (+/-0.2) 4.7 (+/-0.7) 4.0 (+/-0.6) 4.7 (5.9) (+/-2.7) 13.0 (+/-1.8) 32.5 (+/-0.4) 23.8 (+/-3.3) 52.7 (51.4) (+/-1.7) 76.3 (+/-10.5) (+/-3.1) 94.4 (+/-1.5) (178.6) 36
44 Self-part of the Van Hove Correlation Function The results presented in the previous section indicate that the dynamics of the confined ions are heterogeneous and depend on their distance to the pore walls. Further insights of these dynamical heterogeneities can be obtained by studying the time-dependence of the self part of the van Hove correlation function of the ions.[130, 131] G s (r,t) represents the probability of finding a particle at time t a distance r away from the place it was at t = 0. Figure 3.5 represents the G s (r,t) of the center of mass of the confined cations and anions in the different layers/regions of the pore as a function of r at 300 K and three different characteristic times. These results are compared against the G s (r,t) of the bulk ions at the same temperature. At time t = 0.10 ps, the ions in all regions of the pore and in the bulk system are in the ballistic regime (Figure 3.3). Therefore, G s (r,t) decays to zero rapidly at a very short distance, and G s (r,t) is very similar for all confined ions independently of their distance from the pore walls. G s (r,t) for the bulk ions is also similar to those observed for the confined ions in all layers/regions; slightly larger differences are seen for the cations than for the anions, but this fact is consistent with the larger differences observed between the MSDs of the cations in bulk and in confinement, as compared to the bulk and confined anions (Figure 3.3). For the intermediate time t =10 ps, when the bulk and confined systems are in the subdiffusive regime (Figure 3.3), we observe different behaviors for G s (r,t) for the confined ions depending on their distance from the graphitic walls. At this time, G s (r,t) for the anions in the center of the pore have its maximum at r ~ 0.06 nm. In contrast, the anions in the first layers (closest to the pore walls) have a G s (r,t) with a maximum at r ~ nm, which highlights again that the dynamics of the confined anions depend on their distance to the pore walls. The short distances at which the maxima are observed in G s (r,t), when coupled with the results for the 37
45 G s (r,t) G s (r,t) G s (r,t) G s (r,t) G s (r,t) G s (r,t) t=0.1ps,t= 300 K Cation, bulk Cation, first layers Cation, second layers Cation, center region r (nm) t=0.1ps,t= 300 K Anion, bulk Anion, first layers Anion, second layers Anion, center region r (nm) t=10 ps, T= 300 K t=10 ps, T= 300 K r (nm) r (nm) 10 8 t=2500 ps,t= 300 K 10 8 t=2500 ps,t= 300 K r (nm) r (nm) Figure 3.5 Time dependence of the self part of the van Hove correlation function G s (r,t) for the confined cations (left) and anions (right) in the different layers/regions of the pore at 300 K. Results of G s (r,t) for the bulk cations and anions at the same temperature are also presented. 38
46 MSDs (Figure 3.4) suggests that at t = 10 ps the anions have not yet escaped the initial cage formed by their neighbors at t = 0. Another observation is that G s (r,t) for the anions in the second layers has properties in between those observed for G s (r,t) for the anions in the center of the pore and the first layers. A surprising observation is that, at this time, G s (r,t) for the anions in the second layers is very similar to the G s (r,t) of the bulk anions. The qualitative trends described for the anions is also observed in the G s (r,t) of the cations, with differences observed in the specific values of G s (r,t) and the positions of the peaks (since the cations move faster than the anions). At t = 2500 ps, G s (r,t) for the bulk ions becomes similar to G s (r,t) for the ions in the center of the pore. However, G s (r,t) for the ions in the layers near the pore walls are significantly different from those of the ions in the center of the pore or the bulk system, highlighting the fact that the ions closer to the pore walls exhibit dynamics that are much slower than those of the ions in the center of the pore or in a bulk system at the same temperature Incoherent Intermediate Scattering Function The space Fourier transform of G s (r,t), which is better known as the incoherent or selfintermediate scattering function, F s (q,t) can be measured experimentally, for example using inelastic neutron scattering. In Figure 3.6 we show F s (q,t) for the center of the mass of the ions as a function of time, for the bulk system at 300 K and for the confined ions in the different layers/regions of the pore, and at different wave vectors (in the case of the confined system, the wave vectors were parallel to the graphitic walls). F s (q,t) for the bulk system agrees with simulated values reported previously,[87] and shows the features typically observed in supercooled liquids and other ILs.[78, 84, 85, 87, ] First, at very short timescales, F s (q,t) F q, t 1 t 2 can be described by s s 2, where Ω s is the microscopic frequency 39
47 (a) First layers (b) Second layers (c) Center region (d) Bulk Figure 3.6 Incoherent intermediate scattering function F s (q,t) of the center of the mass of the ions with time (log scale) at 300 K in the different layers/regions of the pore. The scattering functions are calculated at the wave vectors q = 5.0, 10.0, 15.0, 20.0, 25.0 and 30.0 nm -1 (shown from top to bottom). (a) First layers (b) second layers (c) center of the pore and (d) bulk. Cations and anions are represented by solid and dashed lines. k T 1 2 defined as s q B.[135] This corresponds to the ballistic regime, when ions are free to move. Following the ballistic motion of the ions at short time, F s (q,t) decays to a plateau, 40
48 which corresponds to the cage effect described before in which ions are temporarily trapped in a cage formed by their neighbors. The time window in which F s (q,t) is in this plateau represents the β-relaxation regime, in which the ions diffuse only inside the cage formed by their neighbors. Afterwards, at longer timescales, F s (q,t) falls below the plateau, which corresponds to a second relaxation process (α-relaxation) in which the ions escape the cage and reach the diffusive regime. From Figure 3.6, it is observed that, independently of wave vector, the F s (q,t) for the cations decays to zero faster than that of the anions in the bulk system and for all layers/regions of the pore. Such a result indicates that cations relax faster than the anions. In addition, the plateau corresponding to the β-relaxation regime is more noticeable for certain values of the wave vector (Figure 6). Furthermore, results for F s (q,t) for the confined ions in the center of the pore are similar to those observed for the bulk IL. All these observations are consistent with the MSD results reported in Figure 3.3. Figure 3.7 shows F s (q,t) as a function of time at 350 K for the confined ions in the different layers/regions of the pore. The scattering functions are calculated at the wave vector q = 15.0 nm -1 (maximum of the structure factor for the bulk IL). For this wave vector, the correlation function decays to zero within the length of the MD trajectory (Figure 3.6), which suggests that the run is long enough to equilibrate the system.[136] From Figure 3.7, the ions in the center regions of the pore have a F s (q,t) that decays faster as compared to the layers of ions closer to the pore walls, which is consistent to the results presented in Figures 3.3 and 3.5. Furthermore, the results for the center region and for the bulk system are very similar. In Figure 3.8 we show the time dependence of F s (q,t) of the confined ions in the second layers at different temperatures (results for the other layers/regions exhibit similar trends and are not shown for brevity). It is observed that as temperature decreases, the length of the plateau becomes longer; afterwards, 41
49 F s (q,t) decays very fast with increase in the temperature, which indicates that the confined ions reach the diffusive regime faster as temperature increases, in agreement with the results presented in Figures 3.2 and 3.4. Figure 3.7 Comparison of the incoherent intermediate scattering function F s (q,t) of the confined cation (left panel) and anion (right panel) with time (log scale) at 350 K for the different layers/regions of the pore. The scattering functions are calculated at the wave vector q = 15.0 nm -1 (maximum of the structure factor). Results of F s (q,t) for the bulk cations and anions at the same temperature are also presented. 42
50 Figure 3.8 Incoherent intermediate scattering function F s (q,t) of the center of the mass of cation (left panel) and anion (right panel) with time (log scale) for the second layers at different temperatures. The scattering functions are calculated at the wave vector q =15.0 nm Relaxation Times To quantify the times associated with the β- and α-relaxation processes, we fitted F s (q,t) to a modified Kohlrausch-Williams-Watts (KWW) function at the wave vector q =15 nm -1, following the methodology used by Sarangi et al.,[87] who modeled bulk [BMIM + ][PF - 6 ] using the same force field used by us. In particular we used the following equation:[87, 134] F s t t exp q, t Aexp 1 A (3.1) Where A and (1 A) represent the amplitudes of the β- and α-relaxation processes, and β is the stretching parameter of the α-relaxation. Following Sarangi et al.,[87] the time parameter associated with the α-relaxation process, <τ α >, was obtained using the following relation: 43
51 <τ α > or τ β (ps) β 1 (3.2) In Figure B-1 (Appendix B) we show the original F s (q,t) data and the fitted equation (3.1) for the cations in the center regions of the pore at 350 K. The fitted parameters, standard errors, R 2 values and other relevant statistical parameters are also presented in this figure. In the Cation First layers Second layers Center region Anion First layers Second layers Center region z (nm) Figure 3.9 Time associated with α and β relaxation along the z direction at 350 K. The values are calculated at the wave vector q = 15.0 nm -1 (maximum of the structure factor). The top and bottom curves denote values for <τ α > and τ β respectively. Inset: variation of the KWW stretching parameter β of the α-relaxation regime. Dotted lines are drawn as a guide to the eye. 44
52 case we obtained an excellent value for R 2 = Similar fit qualities were obtained for all of our results, which are summarized in Figures All numerical fits were performed using the OriginPro 8.5 software. In Figure 3.9 we present the time parameters associated with the α- and β-relaxation processes, <τ α > and τ β. These time parameters are determined for the confined cations and anions in the different layers/regions of the pore at 350 K. The time parameters <τ α > and τ β for the confined ions exhibit a decreasing trend as we move from a position close to the pore walls toward the center of the pore. This trend is consistent with results observed for a supercooled Lennard-Jones fluid confined in a slit pore.[85] The fitted time parameters <τ α > and τ β for the confined cations and anions are very similar in magnitude to those reported by Sarangi et al.[87] in their simulation study for bulk [BMIM + ][PF - 6 ]. The relaxation times for the confined anions are slightly larger than those of the cations in all regions of the pore, which is consistent with the results reported in Figs In contrast, Sarangi et al.[87] report τ β values that are slightly larger for the cation, and <τ α > values that are larger for the anion. These results suggest that in the bulk IL, at short timescales the anion relaxes slightly faster than the cation, but the trend reverses at longer timescales. In the inset of Figure 3.9 we show the variation of β, the stretching parameter of the α-relaxation process for the confined ions in the different layers/regions of the pore. In analogy to <τ α > and τ β, the stretching parameter β decreases as we move towards the center of the pore. In Figure 3.10 we show the effect of temperature on the relaxation of the confined cations and anions in the second layers. Results for the other layers/regions of the pore follow similar trends and are not reported here for brevity. As expected, increases in the temperature causes a reduction in both the α- and β-relaxation times <τ α > and τ β. The inset in the figure 3.10 shows the variation of the KWW stretching parameter β of the relaxation regime for the ions. It is observed 45
53 <τ α > or τ β (ps) β that the value of β increase with increase in temperature. These findings are consistent with what has been reported by Sarangi et al. for the bulk IL.[87] In Figure 3.11 we show the dependence of <τ α > and τ β with the wave vectors, for the confined ions in the center of the pore. It is observed 10, , Cation Anion Temperature (K) Figure 3.10 Time associated with α and β relaxation for the second layers at different temperature along the z direction. The values are calculated at the wave vector q = 15.0 nm -1. At every point, higher and lower values are <τ α > and τ β respectively. Inset: variation of the KWW stretching parameter β of the α relaxation regime. Dotted lines are drawn as a guide to the eye. 46
54 <τ α > or τ β (ps) β 1, Cation Anion q (nm -1 ) Figure 3.11 Time associated with α and β relaxation for the center region along the z direction at 300 K. The values are calculated at the wave vectors q = 10.0, 15.0, 20.0, 25.0 and 30.0 nm -1. At every point, higher and lower values are <τ α > and τ β respectively. Inset: variation of the KWW stretching parameter β of the α relaxation regime. Dotted lines are drawn as a guide to the eye. that both relaxation times decrease with an increase in the wave vectors. τ β shows a much weaker dependence than <τ α > similar to what has been found in the bulk.[87] The inset of Figure 3.10 shows the variation of the KWW stretching parameter β of the α-relaxation regime for the confined ions in the center of the pore. β decreases with an increase in the wave vectors, in analogy to what was found for the bulk IL. 47
55 Radial Distribution Functions Insights on the structure of the confined IL can be obtained from analysis of relevant radial distribution functions g(r). In Figure 3.12 we have depicted the g(r) for cation-cation, anion-anion and cation-anion for the IL in the different layers/regions of the slit pore, and for the bulk IL at 300 K. To obtain these g(r) we used the N atom bonded to the butyl group in the cation, and the P atom in the anion. All radial distribution functions reported in Figure 3.12 were calculated in 3D. Strictly speaking, the first and second layers of IL inside the pore are not 2D systems, because the density profiles of Figure 3.1 show that these layers have non-negligible thickness (rather, they are quasi-2d systems). The IL in the center regions of the pore is a 3D system; however, it exhibits a slab geometry (i.e., its size in the z-direction is smaller than its size in the x- and y-directions). Because we are carrying out a conventional 3D g(r) calculation for the IL in these quasi-2d and slab systems, we observe some distortions in the height of the peaks, and the g(r) of the confined IL do not go to unity at large distances (Figure 3.12). These distortions are examples of excluded volume effects.[ ] These excluded volume effects affect mainly the height of the peaks in g(r) and the value of g(r) at large distances [for example, compare Figures 5-7 in ref. [137], which show as-measured g(r) for water inside Vycor glass nanopores, against Figures 3-5 in ref. [138], which show the same g(r) corrected for excluded volume effects]. Excluded volume effects do not change the location of the peaks of g(r); furthermore, these effects do not produce additional, artificial peaks or features to arise in g(r). In Figure 3.12, we are mostly interested in comparing the position of the peaks/features of g(r) between the bulk ions and the confined ions in the different layers/regions, rather than in comparing the height of the peaks/features. 48
56 g (r) g (r) g (r) K Cat-Cat (first layers) Cat-Cat (second layers) Cat-Cat (center region) Cat-Cat (bulk) Distance (nm) K An-An (first layers) An-An (second layers) 6 An-An (center region) 4 An-An (bulk) Distance (nm) K Cat-An (first layers) Cat-An (second layers) Cat-An (center region) Cat-An (bulk) Distance (nm) Figure 3.12 Radial distribution function g(r) of cation-cation (top panel), anion-anion (center panel) and cation-anion (bottom panel) for different layers/regions at T=300 K. Results of the bulk ILs at the same temperature are also presented. 49
57 From Figure 3.12, it is apparent that the cation-cation, anion-anion and cation-anion g(r) for the confined ions in the center of the pore and for the bulk ions are very similar, suggesting that the ions in the center of the pore have a structure that closely resembles that of the bulk IL. In contrast, the g(r) for the confined ions in the first layers exhibit marked differences with respect to their counterparts for the bulk IL. In particular, more correlation at longer distances is observed for the confined ions in the first layers as compared to the ions in the bulk IL; also, the first peak in the cation-cation and anion-anion g(r) are observed at somewhat longer values of r as compared to those in the bulk IL. The g(r) for the confined ions in the second layers exhibit features that are in between those observed in the g(r) for the bulk ions and for the ions in the first layer. The results shown in Figure 3.12 suggest that the confined ions in the first and second layers and in the center of the pore have marked structural differences. Such structural heterogeneity is likely linked to the dynamical heterogeneity described by the results shown before, in analogy to what was observed for bulk ILs.[84, 87] 3.4. Concluding Remarks We have studied the dynamics of the IL [BMIM + ][PF - 6 ] when confined inside an uncharged slit-like graphitic nanopore of width H = 5.4 nm, in the temperature range K. We have modeled the IL using the non-polarizable force field of Bhargava and Balasubramanian,[124] which has been shown to give accurate predictions for the structural and dynamical properties of bulk [BMIM + ][PF - 6 ]. The same force field was used recently to study heterogeneity in the dynamics of [BMIM + ][PF - 6 ] in the bulk.[87] For the particular pore size considered by us, the local density profile (Figure 3.1) for the confined cations and anions show three distinct regions: a first layer closest to the pore walls, a second layer and a center region. Our results for the MSDs, self-diffusivities, self-part of the van Hove correlation functions, and 50
58 incoherent intermediate scattering functions for the confined ions in the different regions of the pore indicate that the dynamics of the confined IL are highly heterogeneous, and depend strongly on the distance of the ions with the pore walls. In particular, the ions in the center regions of the pore have dynamics and relaxation times that are very similar to those observed in a bulk IL at the same temperature. In contrast, the dynamics slow down appreciably as the ions get closer to the pore walls, and their relaxation times increase markedly. Ions closer to the pore walls spend a significantly larger amount of time inside a cage formed by their neighbors ( cage regime ), as compared to their counterparts in the center of the pore, before finally reaching the diffusive (Fickian) regime. The diffusivities in the parallel (x, y) direction for the confined ions in the different layers/regions of the pore are found to be always smaller (or at most equal, within the numerical uncertainties) than the diffusivities of the bulk ions at the same temperature. The overall diffusivities in the parallel (x, y) direction for the confined ions are found to be always smaller than the bulk diffusivities. Inspection of the radial distribution functions g(r) suggests that these dynamical heterogeneities are linked to structural features. Although the g(r) of the ions in the center of the pore are similar to those of the bulk IL, marked differences were observed between these g(r) and those of the confined ions in the first and second layers. We also studied the effect of temperature on the dynamics, observing that in analogy to bulk systems, an increase in temperature cause the confined IL to have faster dynamics and smaller relaxation times. It is interesting to compare the simulation results presented in this paper with those obtained for the same IL (with a different force field, however) confined inside a cylindrical multi-walled carbon nanotube (MWCNT).[122] The pore diameters considered in that study (up to D = 3.7 nm) were significant smaller than the pore size considered here (H = 5.4 nm). 51
59 Significant layering was observed for the IL inside the two different pore geometries. Likewise, in both pore geometries, in general the cations exhibited faster dynamics than anions, and the dynamics of the ions decrease monotonically as we go from the center of the pore to the IL layer near the pore walls. However, evidence of complex dynamics was observed for the IL inside a cylindrical pore. MSD results observed for the IL inside a MWCNT of D = 3.0 nm (for which three layers of ions are observed) indicate that in the second layer, the MSD of the cations and anions are similar in magnitude; furthermore, the anions in this second layer have dynamics that are similar to those observed for the anions in the center of the pore (see Figure 9 in ref. [122]). Evidence of similar complex dynamics was not observed for the same IL inside a slit pore in this study. It is also interesting to note that the dynamic properties of the confined IL in the center region of the pore (which is only ~1.25 nm away from the walls) are very similar to bulk-like. Similar results have been observed experimentally in other glass-forming systems inside nanopores or near surfaces, including polymer thin films[143] and organic glass-formers.[49, 144] 52
60 CHAPTER 4 MOLECULAR DYNAMIC SIMULATIONS OF IL [EMIM + ][TFMSI - ] CONFINED INSIDE RUTILE (110) NANOPORE: EFFECT OF PORE LOADING ON STRUCTURAL AND DYNAMICAL PROPERTIES In this chapter, the structural and dynamical properties of the IL [EMIM + ][TFMSI - ] confined inside a rutile (110) pore are investigated by means of classical molecular dynamics simulations. The simulations are carried out at different pore loading ranging from 0.1ρ bulk to ρ bulk with the pore size (H) of 5.2 nm at 333 K. Various equilibrium and time dependent properties such as density profiles, radial density profiles (RDF), orientation, auto-correlation functions and mean square displacements (MSDs) are calculated and the effect of the confinement on the above properties are studied. We contrast these results against those obtained by us for the same IL confined inside a slit graphitic nanopore of the same width. The rest of this chapter is structured as follows. Section 4.1 is the introduction. Section 4.2 contains a description of our computational models and methods. In Section 4.3 we present results and discussion of the structural and dynamic properties of the confined ILs, and in Section 4.4 we summarize our main findings. 4.1 Introduction Ionic liquids (ILs) are molten salts that are usually composed of bulky organic cations and smaller organic or inorganic anions. Due to their bulky structure and loose packing IL remain liquid at or near room temperature. ILs are also considered green chemicals for properties such as low vapor pressure (non-volatile), nonflammability, low viscosities, high conductivity, excellent thermal and chemical stabilities. These unique properties make them potential candidates for the replacement of the conventional chemicals in diverse fields such as green solvents for the synthesis [6, 145, 146], in CO 2 sequestration [ ], in extraction [156, 157] and as electrolytes in electrochemical double layer capacitors (EDLCs) [ ] and dye 53
61 sensitized solar cells (DSSCs) [20, 22-25, ]. The latter is a class of solar cell based on a semiconductor formed between a photo-sensitized anode and an electrolyte. A schematic diagram of a DSSC is given in Figure 4.1. The major problems with the conventional electrolytes in DSSCs are that they catch fire, are explosive and evaporate with time [20-22]. This not only undermines the performance of the device, but also causes environmental hazards. Current research suggests that ILs with their unique properties have potential to tackle these problems and improve the performance of DSSCs [22-25]. It is possible to synthesize ILs with the desired properties by selecting the right combination of cation and anion while keeping in mind that the properties of ILs strongly depend on the structure of the ions. Previous experimental [ ] and simulation studies [52, 56-64, 113, , ] have helped in understanding these complex ions under confinements; however the effect of the structure and dynamics of ILs on the performance of the device is not well understood yet. Electrodes are the other main components of the DSSCs; the characteristics of the electrodes determine the overall performance and life of these devices. Some of the semiconductor materials that have potential as an anode are TiO 2, ZnO and CuO [20-22, ]. Among these TiO 2 is one of the most extensively studied materials over the last few decades due to its unique chemical, electrical, optical, and photochemical properties [184, ]. The ability to harness the solar energy can be further improved by the choice of electrode material and IL electrolyte. Many IL/solid interfaces have been studied [175, 176, 178, 180, 181, 183, ]; however it is not possible to test all possibilities experimentally [ , , 205, 206]. Computer simulations enable us to have atomic-level understanding at the IL/solid interface. A few simulation studies have been published on ILs near TiO 2 surface [180, 208]. In these prior studies only structural properties and preferential orientation of the ILs 54
62 [BMIM + ][PF - 6 ] and [BMIM + ][NO - 3 ] have been discussed. The main objective of this work is to study in detail the structural as well as dynamical properties of the IL [EMIM + ][TFMSI - ] near TiO 2 electrode. In the present study we considered the interaction between the IL [EMIM + ][TFMSI - ] and the rutile (110) surface at different pore loadings. In this work molecular dynamics simulations are performed with the goal of predicting and understanding the behavior of the IL [EMIM + ][TFMSI - ] as an electrolyte near TiO 2 electrodes. Achieving such an objective is crucial in developing new electrolytes by choosing the most appropriate cation-anion combination for applications in DSSCs and other devices such as fuel cells and EDLCs. The rest of this paper is structured as follows. Section 4.2 contains a description of our computational models and methods. In section 4.3, we present results and a discussion of the structural and dynamic properties of the confined ILs, and in section 4.4, we summarize our main findings. 4.2 Computational Details The ionic liquid [EMIM + ][TFMSI - ] (Figure 4.2) was confined inside two parallel (110) rutile TiO 2 walls with a pore size of H = 5.2 nm and simulations were performed at a temperature of 333 K to characterize its structural and dynamical properties. Various pore loadings ranging from 0.1ρ bulk to ρ bulk are considered by changing the IL pairs inside the fixed pore volume. The numbers of IL pairs at 0.1ρ bulk, 0.2ρ bulk, 0.4ρ bulk, 0.6ρ bulk, 0.8ρ bulk, and ρ bulk loadings are 36, 73, 145, 218, 290, and 363 respectively. Classical molecular dynamic simulations at constant volume and temperature (NVT) ensemble were performed using the GROMACS MD package [65]. A temperature higher than the melting point of [EMIM + ][TFMSI - ], T m = K, was chosen to ensure significant ionic displacement. The two pore walls are formed by repeating the rutile unit cell along the [100], [010], and [001] and then cutting the box 55
63 Electric bulb e e e e Solar energy e e e Electrically conductive FTO glass electrode TiO2 nanowires loaded with dye Ionic liquid electrolyte Counter Pt electrode Figure 4.1 Schematic diagram of a dye sensitized solar cell (DSSC). H1 H1 HA H1 HA NA CR NA CE C1 C2 HA CW CW HA HA HE HE HE FJ FJ CJ OJ FJ SJ OJ NJ FJ SJ OJ OJ FJ CJ FJ [EMIM + ] [TFMSI - ] 1-ethyl-3-methylimidazolium Bis-(trifluoromethanesulfonyl)imide Figure 4.2 A schematic representation of the IL [EMIM + ][TFMSI - ]. The labels are atom notations used throughout in this work. 56
64 Oxygen atoms Titanium atoms Figure 4.3 Representative snapshot of the rutile (110) surfaces. Oxygen and titanium atoms are colored red and white respectively. A vector perpendicular to the surface is shown. along the (110) plane to form a slab (Figure 4.3) of the dimensions (5.60 nm 5.10 nm). The oxygen and titanium atoms in the rutile surface were modeled as LJ spheres with σ o = 0.303, σ Ti = 0.392, nm, ε o = 0.12 kcal/mol, and ε Ti = kcal/mole [209, 210]. The partial charges on oxygen and titanium atoms are e and 2.196e respectively[211]. The force field parameters for the cations [EMIM + ] and anions [TFMSI - ] were taken from Maginn et al. [212]. Partial charges were located on cations and anions according to the values reported by Maginn et al. Force field parameters for [EMIM + ][TFMSI - ] from Maginn et al. adequately reproduced the experimental density, heat of vaporization and diffusion coefficient of the bulk system. The density for the confined IL was calculated using a volume V min xy z c. Our results for the number density profile (Figure 4.5) indicate that the density of the confined ions reach a value of zero at z 2. ~ c 57
65 (a) (b) (c) Z X (d) (e) (f) Figure 4.4 Representative simulation snapshots of the IL [EMIM + ][TFSI - ] confined inside a rutile (110) surfaces (H = 5.2 nm) at 333 K and different pore loadings: (a) ρ = 0.1ρ bulk, (b) ρ = 0.2 ρ bulk, (c) ρ = 0.4 ρ bulk, (d) ρ = 0.6 ρ bulk, (e) ρ = 0.8 ρ bulk and (f) ρ = ρ bulk. Cations and anions are depicted in purple and green. In all our simulations, the Lennard-Jones interactions were cut off at 1.2 nm, and the long range coulomb interactions were handled by the particle-mesh Ewald (PME) method[73] with a cutoff of 1.0 nm and a grid spacing of 0.1 nm. Periodic boundary conditions were applied in all directions. The ILs/rutile system was kept in vacuum in the center of the orthorhombic box in such a way that two sides of the box were equal to the sides of the graphite sheets (5.60 nm 5.10 nm), and the length of the other one side of the box (12.0 nm) was long enough to avoid any artificial effect caused by the periodic boundary conditions. The improved velocity-rescaling algorithm recently proposed by Parrinello et al.[74, 75] was used to mimic weak coupling at different temperatures with a coupling constant of 0.1 ps. 58
66 We first minimized the energy of our initial configuration using the steepest descent method. Afterwards, we ran MD simulation using a time step of 1.0 fs. The systems were melted at 600 K for 1 ns, and then annealed from 600 to 333 K in stages: 400 ps at 550 K, 400 ps at 500 K, 200 ps at 450 K, 200 ps at 400 K, 200 ps at 350 K, and 200 ps at 333 K. Afterwards, our systems were equilibrated and the properties of interest were sampled for 20.0 ns at 333 K. Afterwards, we took the final configuration of our system and repeated the above steps (melting, annealing, equilibration and averaging) at least one additional time. Our reported results are averaged over at least two independent realizations of the same system. Such a procedure is adopted in an attempt to overcome the difficulties posed by the slow dynamics of ILs, which are likely to be exacerbated when these compounds are confined inside a nm-sized slit pore. Representative simulation snapshots of the ILs confined inside a rutile(110) pore at 333 K and different loadings are depicted in Figure Results and Discussion Structural Properties We studied the mass and number density profiles of the ions along the pore length. The density profiles of the ions are calculated by computing the average in different bins inside the pore. Figure 4.5 presents the number density profiles of the ions (ions/nm 3 ) confined inside the rutile (110) pore, when the confined IL has a density ranging from 0.1 ρ bulk to bulk density (ρ bulk ) at 333 K. It is found that both cations and anions have the highest density near the surface with layering behavior evident throughout the system. The oscillations are observed to reduce in amplitude away from the surface and are minimal at the center of the pore. It can be summarized that the increased density profiles near the surface are due to a strong interaction between the ions and the titanium and oxygen atoms that form the surface shown in Figure 4.5. At lower pore 59
67 loadings, the intensity of the fluctuations decrease monotonically in the center of the pore; the density of the ions in the first layer is minimally affected by pore loadings above 20%. At a density equal to 0.1 ρ bulk, the height of the peak drops down due to less number of ions in the pore which results in the IL s inability to cover the entire surface. It is also observed that anions have higher mass densities than cations in every pore loading study. The mass density profiles of the ions are also examined (not reported for brevity). Similar profile characteristics are observed with the exception that the anions have a higher mass density peaks than the anions. This is explained by the higher molecular weight of the anions. Both mass density and number density profiles show that the local maxima (first peak) for both the ions were present at the same position from the wall. This indicates that both ions are stacking at the same distance from the wall, forming a layer of average thickness nm. The results presented in Figure 4.5 are in good agreement with previous results obtained for different ILs inside the slit like pores of different materials such as graphite, silica, mica, quartz, sapphire and rutile [52, 56-58, 61-64, 113, ]. To further understand the structural differences of the ions in different regions and at different pore loading, we studied the radial distribution function g(r) of the ions inside the pore. The RDFs of the ions are calculated by taking the CR atom of the cation and the NJ atom of the anion. Figure 4.6 represents the site-site intermolecular RDF for cation-cation, anion-anion and anion-cation confined inside a rutile (110) pore of 5.2 nm at 333 K. The results for the first layers are shown in the left panel and right panel shows the results of the center regions at different pore loadings. We observed drastic change in the liquid structure of IL in the first layers at different pore loadings indicating structural heterogeneity inside the pore. Highly ordered structure is observed at lower pore loadings as there are very few ions in the center of the pore. 60
68 Number Density (ions/nm 3 ) Number Density (ions/nm 3 ) Cation Anion Distance (nm) Distance (nm) Figure 4.5 Number density profiles of the confined ions along the z-direction at different pore loading. The confined IL has a density similar to bulk density (1.49 g/cm 3 ). The vertical dotted lines indicate how the confined ions were divided into different layers/regions for further study. Different color lines are used for different loadings: gray (ρ = 0.1ρ bulk ), orange (ρ = 0.2 ρ bulk ), green (ρ = 0.4 ρ bulk ), purple (ρ = 0.6 ρ bulk ), red (ρ = 0.8 ρ bulk ) and blue (ρ = ρ bulk ). In the center of the pore we observed increase in the height of the peaks with decrease in pore loading but the position of peaks remains same at different pore loadings. This indicates that the pore loading does not affect the overall 3D structure of IL in the center of the pore. The results are similar to our previous studies of ILs confined inside the graphitic slit like pore. The RDF results in the center of the pore at different loading are similar and indicate homogeneous structure in the center of the pore at various pore loadings. No such similarities are observed in the first layers with more ordered structure at lower loadings. 61
69 g(r) g(r) g(r) g(r) g(r) g(r) 10 8 First layers (a) Distance(nm) (b) Center region Distance(nm) 10.0 ρ ~ ρ bulk 8.0 ρ ~ 0.80 ρ bulk 6.0 ρ ~ 0.60 ρ bulk 4.0 ρ ~ 0.40 ρ bulk ρ ~ 0.20 ρ bulk 2.0 ρ ~ 0.10 ρ bulk Distance(nm) (c) Distance(nm) Distance(nm) Distance(nm) Figure 4.6 Radial distribution functions g(r) of (a) cation cation, (b) cation anion, and (c) anion anion for the different regions of [EMIM + ][TFMSI - ] confined inside a slit pore of H = 5.2 nm and different pore loadings at 333 K. Left panel shows the results for the first layers and right panel for the center region. 62
70 Cation Anion Figure 4.7 Left panel shows the center of mass of cations and anions in the first layer. Different colors are used to show different orientation. In case of cations orange dots indicates [EMIM + ] laying parallel (θ <25 0 ) to surface and blue indicates [EMIM + ] tilted (θ >25 0 ) to the surface. In case of anions green dots indicates ions laying parallel to surface (θ <25 0 ) and purple indicates ions tilted to surface (θ >25 0 ). In the right panel, two vectors are shown that are used in orientation calculations. Right panel shows multiple preferential orientations of the cations (top) and anions (bottom) at the rutile (110) surface at 333 K. The orientation of the cations near the rutile surfaces was determined by calculating cos(θ), where θ is the angle between the vector normal to the slab surface and the vector normal to the imidazolium ring. Similarly, the orientations of the anions are determined by calculating the cos(θ), where θ is the angle between the vector normal to the slab surface and the vector formed by joining the atoms CJ and CJ of the ion (see Figure 4.2 for the nomenclature). Figure 4.7 (left panel) shows the center of mass of the ions in the first layer; two different color dots are used to distinguish the ions that are laying parallel to the surface (θ <25 0 ) we use orange for cations and green from anions. Any ion that is not parallel, thus is tilted or perpendicular to the surface (θ>25 0 ) are identified by blue for cations and purple for anions. It is interesting to note 63
71 that, although most cations orient tilted or perpendicular to the surface, a significant fraction of them arrange parallel to the surface. This heterogeneous layering behavior is not common in imidazolium based cations and these results present new layering arrangements not observed in previously reported results where ions are definitively flat or perpendicular near carbon and rutile surfaces [122, 123, 180, 208]. Since ions can have multiple orientations, it would be interesting to know the most preferred orientation of ions. Figure 4.7 (right panel) shows the arrangement of the ions near the surface. In case of the cations, the most common orientation results is the interaction of the rutile oxygen atoms and both hydrogen atoms connected to CW atoms (Figure 4.2). The second most common orientation is found when the hydrogen atom connected to CR atom is attracted to the rutile oxygen (Figure 4.2). This is due to the partial positive charge on the ring hydrogen atoms and partially negatively charged oxygen atoms. The most preferential arrangements of the anions have a strong interaction between the anions OJ and the titanium in the rutile (110) surface and are also shown in the right panel of figure 4.7. No preferential orientation of the cations and anions are observed in the center region of the pore (results not shown) Dynamical Properties Although extensive research has been done on confined fluids, there are only few studies where dynamics of the ions has been studied in detail. From our previous studies where we mainly focused on carbon based pores of different morphologies [122, 123, 183, 213], we found that dynamics of the ions inside the pore are very complex and heterogeneous. It is found that the ions near the surface have the slowest dynamics and the behavior changes non-monotonically with pore loadings. The ions in the center of the pores behave very similar to the bulk if the pore size is larger than 5.0 nm [183]. It was also observed that lower loadings slow down the 64
72 C(t)/C(0) C(t)/C(0) C(t)/C(0) C(t)/C(0) Cation (First layers) Anion (First layers) Time (ns) Time(ns) Cation (Center region) Anion (Center region) Time (ns) Time (ns) Figure 4.8 Single-particle time correlation functions for the cations (left panel) and anions (right panel) in different layers/regions. In case of cations, reorientation around an axis perpendicular to the imidazolium ring. In case of anion, reorientation around a vector formed by joining the atoms CJ and CJ of the ion (see Figure 4.2). Different color lines are used for different loadings: gray (ρ = 0.1ρ bulk ), orange (ρ = 0.2 ρ bulk ), green (ρ = 0.4 ρ bulk ), purple (ρ = 0.6 ρ bulk ), red (ρ = 0.8 ρ bulk ) and blue (ρ = ρ bulk ). dynamics due to the formation of the void spaces inside the pores. In the present work, we want to extend our previous efforts to understand the dynamics inside rutile (110) pore. The study of the ions inside rutile pore is more relevant for DCSCs application where the internal resistance and charge screening entirely depends on the dynamic of the ions. The dynamical behavior of [EMIM + ][TFMSI - ] was first observed by plotting single-particle time auto correlation functions for the reorientation of the ions as presented by Urahata and Ribeiro [214]. Figure 4.8 shows the single-particle time correlation functions of the cations (left panel) and anion (right panel) at different loadings in different layers/regions at 333 K. In case of cations, reorientation motion is 65
73 calculated around an axis perpendicular to the imidazolium ring. The results indicate that for cations at ρ~ ρ bulk, the reorientation in the center region decorrelate very quickly. The correlation function reaches a value close to zero in less than 1.0 ns of simulation time. In contrast, cations near the surface hardly decorrelate after 20 ns due to their strong interaction to the surface. The reorientation motion of the anions is calculated around a vector formed by joining the atoms CJ and CJ of the ion (see Figure 4.2); similar results are obtained at ρ~ ρ bulk. Correlation functions for the center ions reach a value close to zero in less than 1 ns of simulation time. Reduction in pore density causes slower dynamics for both cations and anions in the center of the pore and ions take longer time to decorrelate with the longest time at 0.1ρ bulk since the ratio of surface ions to center ions increases with decreasing loading. In case of the first layers, there is a nonmonotonic decorrelation for both ions at different pore loading. The results are consistent with our previous studies of ILs inside graphitic slit-like pores. Mean square displacements (MSDs) of the ions in the direction perpendicular to the confinement (x- and y- direction) are calculated. The dynamics of the ions in different layers/regions are compared in the above mentioned direction. Figure 4.9 shows the dynamics of the cations (left panel) parallel to the surface (x- and y- direction) in the center of the pore at three different pore loadings. It is found that lowering the loading slows down the mobility if the ions. It is due to the formation of the void spaces inside the pores. Figure 4.9 (right panel) shows the dynamics of the anions in the center of the pore at different loading and similar behavior is observed. Dynamics of cations and anions are faster along the x and y direction as compared to the z direction (results are not shown for brevity). This indicates that the confinement hinders the motion of the IL in the confined direction. These findings are in analogy to our previous results of IL under 66
74 MSD (nm 2 ) MSD (nm 2 ) MSD (nm 2 ) MSD (nm 2 ) 0.08 Cation (First layers) 0.08 Anion (First layers) Time (ns) 5.0 Cation (Center region) Time (ns) 5.0 Anion (Center region) Time (ns) Time (ns) Figure 4.9 Mean square displacements (MSDs) of the ions in the first layers and center region of the pore. Left panel shows the parallel (x- and y-) component of cation MSDs and right panel shows the parallel (x- and y-) component of anion MSDs. Different color lines are used for different loadings: gray (ρ = 0.1ρ bulk ), orange (ρ = 0.2 ρ bulk ), green (ρ = 0.4 ρ bulk ), purple (ρ = 0.6 ρ bulk ), red (ρ = 0.8 ρ bulk ) and blue (ρ = ρ bulk ). confinement. [122, 183] The results also indicate that MSD values haves a strong correlation to the distance of ions from the surface. To visually represent the slower dynamics near the surface, the displacement in nm between the initial and final timesteps are represented (Figure 4.10) for three pore loading systems, 0.4 ρ bulk, 0.6 ρ bulk, and 1.0 ρ bulk. It can be observed that the mobility of the cations and anions in the first layers are slower and the dynamics are very complex as compared to the central region and are not shown for brevity. 67
75 (a) (b) (c) Figure 4.10 (Color) Displacement of the each atom was calculated between T = 0 ns and at 20 ns for the systems with a) 0.4 ρ bulk b) 0.6 ρ bulk and c) 1.0 ρ bulk. The legend on the right indicates the coloring scheme where blue has minimal displacement and red has the maximum displacement. Titanium is white and oxygen is red in rutile (110) structure Concluding Remarks Structural and dynamical properties of IL [EMIM + ][TFMSI - ] inside a titania slit nanopore is studied. It is found that both cations and anions form local density maxima near the pore surface with slight oscillation in the center of the pore, consistent with our previous studies of ILs inside slit-like graphitic pores, nanotubes and CMK-3. We observed drastic change in the liquid structure of IL in the first layers at different pore loadings; however the overall 3D structure of IL in the center of the pore remains unchanged. It is interesting to see the orientation of the ions, especially near the surface; our results indicate that cations have multiple orientation preferences near the surface with most of the cations tilted. Cations are oriented in such a way that the hydrogen atoms of the imidazolium ring attracted to the partially negatively charged surface oxygen atoms. Similar results are observed for anions with oxygen atoms of the ions attracted to the titanium atom of the rutile surface. Self autocorrelation results indicate that at a density equal to bulk density, ions in the center of the pore decorrelate faster than the ions on the 68
76 surface. Reduction in the pore loading results in slower decorrelation. The ions in the first layers are strongly attached to the surface and have very poor decorrealtion at all different loadings. We studied the dynamics of the ions by measuring the mean square displacements of the ions. We find that the ions in the center of the pore have the fastest dynamics at a density equal to bulk density. At lower pore loading, dynamics slows down linearly due to the formation of the void space in the center. The dynamics in the first layers is the slowest, complex and non-monotonic with pore loadings. The dynamical behavior of ILs inside rutile pores is similar to our previous studies of ILs confined inside carbon nanopores at different pore loadings. However the mobility of the ions is much slower in rutile pores indicating stronger interaction of ions for the rutile surface. We have gained atomic level insight on the rutile (110) pore. However there are several aspects of ILs and pore materials that need further attention. Pore surface plays an important role in determining the behavior of the ILs, it is essential to consider other surfaces of the same polymorph of titania. It is also important to understand the effect of the charged surface on the behavior of ILs, especially on the dynamics of the ions to enhance the performance of DSSCs. 69
77 CHAPTER 5 EFFECT OF ACETONITRILE SOLVENT ON THE STRUCTURAL AND DYNAMICAL PROPERTIES OF IL [EMIM + ][TFMSI - ] CONFINED INSIDE SLIT-LIKE GRAPHITIC PORE: A MOLECULAR DYNAMIC SIMULATION STUDY In this chapter we studied the effect of acetonitrile (ACN) solvent on the properties of the IL [EMIM + ][TFMSI - ] inside an uncharged slit-like graphitic pore. The effect of different concentrations of IL (1.0 M, 2.0M, and 3.0M) on the structural and dynamical properties of the system is considered. The rest of this chapter is structured as follows. Sections 5.1 and 5.2 contain an introduction and a description of our computational models and methods. In Section 5.3 we present results and discussion of the dynamic properties of the confined ILs, and in Section 5.4 we summarize our main findings. 5.1 Introduction Electrochemical double layer capacitors (EDLCs) have attracted extensive attention due to very fast charging and discharging ability, which results in high specific power [12, ]. However, these devices have low specific energies as compared to batteries. Carbon-based materials have been extensively used as electrode materials due to their low cost and properties such as high electrical conductivity, large surface area, stiffness and chemical and thermal inertness [ , ]. The conventional electrolytes used in these devices are either aqueous solution of sulfuric acid and potassium hydroxide or organic electrolytes based on acetonitrile (ACN) and propylene carbonate (PC) solvent[226, 238]. There are also some studies on using polymer gel electrolytes for EDLCs [239]. It is found that aqueous electrolytes have the problem of decomposition at higher voltage whereas organic electrolytes suffer due to low ion conductivity limiting the performance of the device. Ionic liquids (ILs), which are organic salts with melting points below C, have the potential to replace conventional electrolytes, due to unique properties such as very low vapor pressure (non-volatile), excellent thermal and chemical 70
78 stability, non-toxicity, non-flammability and wide electrochemical window. When compared to conventional electrolytes that are only stable up to 2.5 volts, ILs are stable up to 6 volts and have the potential to improve the efficiency of EDLCs in term of both specific power and specific energy. Some of these simulations [61, 64, 113, , 121, ] suggest that the electrode capacitance attains maxima at certain specific conditions of pore size, pore geometry, surface charge density and electrode potential. These simulation and theoretical studies also supported and provided rational explanations to the experimental findings that the specific (or area-normalized) capacitance of nanopores filled with organic electrolytes or ILs increased anomalously as the pore size decreased[ ] (however, a recent experimental study[251] reports a relatively constant specific capacitance for the electrolyte (C 2 H 5 ) 4 NBF 4 /acetonitrile inside carbons with pore sizes ranging between 0.7 and 15 nm). Furthermore, very recent simulation and theoretical studies[ ] suggest that the specific capacitance has a decaying oscillatory behavior as the pore size increases. Both experimental and computational studies have been done in order to understand the microscopic and macroscopic behavior of ILs near electrodes of different materials (e.g. silica, quartz, rutile, and graphite) and morphologies (e.g. slit-like pore, nanotube, and CMK-3) [51-58, 60-64, , 122, 183, 213, ]. It has been observed that nm-size confinements have profound effect on both structural and dynamical properties. Previous computational studies on ILs inside nanopores suggest a layering behavior of ILs near the wall due to strong interaction forces exerted by the wall atoms. The dynamics of the confined cations and anions are heterogeneous and depend strongly on distance from the pore walls; for example, cations and anions in the center of the pore have faster dynamics than those close to the pore walls. 71
79 The purpose of using organic solvents in EDLCs is to reduce viscosity of ILs in order to improve the mobility of ions inside the pores. It is important to understand the effect of solvent and variation in IL concentration on the molecular level properties of EDLCs. Previous experimental study has shown that acetonitrile can reduce the viscosity of ILs and improve the performance of EDLCs [256]. Although many computational studies have considered pure ILs near electrodes of different materials [51-58, 60-64, , 122, 183, 213, 252, 253], very limited attention has been given on the effect of organic solvents on the behavior of ILs [179, ]. Some of the computational studies that have been done in this area show a change in the capacitance with changing the ion size and concentration. It is also observed that addition of solvent affects the ion distribution and orientation inside the pore. However, a fundamental understanding of how the structure and dynamics of the ions inside the pore change with variations in IL concentrations is still missing. Following our previous work on pure IL inside nanopores of different pore size, pore morphology and pore material, we extend our efforts at understanding the effect of organic solvent on the properties of confined ILs. In the present work, we intend to study the effect of varying concentrations of acetonitrile (ACN) solvent on the properties of the IL [EMIM + ][TFMSI - ] inside an uncharged slit-like graphitic pore. IL at different ionic concentration (1.0M, 2.0M, and 3.0M) is considered and both structural and dynamical properties are measured. We aim at understanding the atomic distribution of the ions and ACN molecules inside the slit-like pores. We are also interested in understanding the effect of the IL concentrations on the mass and number density profiles. Orientation of the ions plays a crucial role in determining the electrical double layer at the electrode; preferential orientation of the ions will be calculated. Dynamics of the ions will be analyzed by calculating the mean square displacement (MSD) of the ions at different concentrations. 72
80 The rest of this paper is structured as follows. Section 5.2 contains a description of our computational models and methods. In Section 5.3 we present results and discussion of the dynamical properties of the confined ILs, and in Section 5.4 we summarize our main findings. H1 H1 HA H1 HA NA CR NA CE C1 HA CW CW HA C2 HA HE HE HE FJ FJ CJ OJ FJ SJ OJ NJ FJ SJ OJ OJ FJ CJ FJ HT HT HT CT CB NB [EMIM + ] [TFMSI - ] (ACN) 1-ethyl-3-methylimidazolium Bis-(trifluoromethanesulfonyl)imide Acetonitrile (a) (b) (c) Figure 5.1 A schematic representation of the IL [EMIM + ][TFMSI - ] (panel a and b)and acetonitrile (ACN) molecule (panel c). The labels are atom notations used throughout in this work. Vectors shown are used to define the orientation of the ions/molecule. 5.2 Computational Details GROMACS molecular dynamics simulation package was used to study the effect of ACN solvent on the properties of the IL [EMIM + ][TFMSI - ] inside graphitic slit-like pore of pore size H = 5.2 nm. A schematic representation of the IL [EMIM + ][TFMSI - ] and ACN molecule are shown in Figure 5.1. The ensemble of choice was NVT due to the fixed dimensions (5.70 nm 5.61 nm 5.2 nm) of the pore. The pore walls were modeled by taking three carbon sheets and fixing them in space keeping the pore size H =5.2 nm. The carbon atoms in the graphite sheets were modeled as LJ spheres with σ c = nm and ε c /k = 28.0 K. Three different IL molar concentrations 1.0 M, 2.0M and 3.0M including pure ACN were considered in this study. The number of IL pairs and ACN molecules in different cases are reported in table
81 Table 5.1 Detail description of the IL ion pairs and ACN molecules in different cases. System [EMIM + ][TFMSI - ] pairs ACN molecules 1.0 M IL M IL M IL Pure ACN H= 5.2 nm Z Y Figure 5.2 A snapshot of 3.0 M IL [EMIM + ][TFMSI - ]electrolyte in ACN solvent confined inside a slit-like graphitic pore of size H = 5.2 nm at 333 K. Cations, anions, ACN molecules are colored in green, purple, and red respectively. The non-polarizable force field force field parameters for IL [EMIM + ][TFMSI - ] used in this work are taken from Maginn et al. [212]. The force field parameters for ACN molecules are taken form Nikitin et al. [261]. A representative simulation snapshot of the slit-like graphitic pore containing 3.0 M IL at 333 K is depicted in Figure
82 Most of the simulation procedure used in this work is similar to our previous work and a detailed description could be found in recent publications [122, 123, 183, 213]. In all our simulations, the Lennard-Jones interactions were cut off at 1.2 nm, and the long range coulomb interactions were handled by the particle-mesh Ewald (PME) method with a cutoff of 1.0 nm and a grid spacing of 0.1 nm. Periodic boundary conditions were applied in the x, y, and z directions. The ILs/ACN/graphite sheet system was kept in vacuum in the center of the orthorhombic box in such a way that two sides of the box were equal to the sides of the graphite sheets (5.70 nm 5.61 nm), and the length of the other one side of the box (12.0 nm) was long enough to avoid any artificial effect caused by the periodic boundary conditions. The improved velocity-rescaling algorithm recently proposed by Parrinello et al. was used to mimic weak coupling at different temperatures with a coupling constant of 0.1 ps. 5.3 Results and Discussion Density Profiles Density of the ions near the electrode surface affects the electrical double layer formation. In case of pure IL near an uncharged surface, higher density is reported close to wall than in the center of the pore [56, 80, 183, 213, 252, 253]. Figure 5.3 shows the mass density profiles along the length of the pore (Z- direction, Figure 5.2) of ions and ACN molecules at three different IL molar concentrations (1.0M, 2.0M and 3.0M) including pure ACN molecules. From the density profile, the confined ions were divided into two different layers/regions: the first layers (closest to the pore walls), the center region. In case of pure ACN molecules (case (a), Figure 5.3), a strong layering behavior is observed throughout the pore length with highest density near the pore wall caused by strong surface forces; density decreases linearly as we move toward the center of the pore. The density in the first layer is roughly five times higher 75
83 Mass Density (Kg/m 3 ) Mass Density (Kg/m 3 ) Mass Density (Kg/m 3 ) Mass Density (Kg/m 3 ) 5000 (a) 2500 (b) Distance (nm) 2000 (c) Distance (nm) 2000 (d) Distance (nm) Distance (nm) Figure 5.3 Mass density profiles of the IL [EMIM + ][TFMSI - ] in acetonitrile (ACN) solvent at different molar concentrations along the length of the pore: (a) Pure ACN, (b) 1M [EMIM + ][TFMSI - ], (c) 2M IL [EMIM + ][TFMSI - ], and (d) 3M IL [EMIM + ][TFMSI - ]. Cations, anions and ACN molecules are shown in green, purple and red color respectively. Dotted horizontal line in case (a), represent bulk ACN density (780 Kg/m 3 ) and dotted vertical lines distinguish the first layers. than the bulk density. It is observed that the higher density in the first few layers induces a void space in the center of the pore; the density in the center is lower than the bulk density (780 Kg/m 3 ). The thicknesses of the layers are approximately nm and are consistent inside the pore. At different IL molar concentration (case (b), (c) and (d), Figure 5.3), significantly different density profiles for ILs and ACN molecules are observed. Addition of ions in the 76
84 system starts replacing ACN molecules that are present in the first layers. At 1.0M concentration, ACN molecules dominate first layers; the density in the first layer is two times higher than the bulk density. Anions have second highest density (850 Kg/m 3 ) followed by cations (410 Kg/m 3 ). In the center, densities are lower for the ions/molecules than in bulk. At 2.0 and 3.0 M, the density of the ions in the first layer is higher than ACN molecules with cations and anion have the maximum densities of 900 and 1400 Kg/m 3 (2.0M) and 1000 and 1500 Kg/m 3 (3.0M) respectively. The density of the ACN molecules in the first layers decreases from 1000 Kg/m 3 to 500 Kg/m 3 after changing the concentration form 2.0M to 3.0M. Density results in the center of the pore at 2.0M and 3.0M are fluctuating, similar to 1.0M and depend on the size and shape of the void formed. We also studied number density profiles at various IL molar concentrations (Results are not show for brevity). Observations from the number density profiles are similar to mass density results and are consistent with our previous studies of confined ILs [213] Radial Distribution Function To understand the local structure of IL confined inside slit pore at different molar concentration, the radial distribution function (RDF) was computed. Figure 5.4 represents the site-site intermolecular RDF of anion anion, cation cation, cation anion, anion ACN, and cation ACN for the first layers and the center region at 333 K. CR carbon atom of [EMIM + ], NJ nitrogen atom for [TFMSI - ] and CB atom for ACN were chosen for plotting RDF. Result indicates that structural heterogeneity is observed in the RDF of ions at different molar concentrations and this heterogeneity in the structure is more significant at the surface as compared to the center region. We observed loss of the first coordinations shell of anion-anion and formation of first coordination shell of anion-acn with increase in concentration in the first layers. In the first layers the RDF of cation-cation shows upto three coordination shells at 1 M 77
85 g (r) g (r) g (r) g (r) g (r) g (r) g (r) g (r) g (r) g (r) 6.0 (a) First layers 3.0 Center region Distance (nm) 6.0 (b) Distance (nm) Distance (nm) 12.0 (c) Distance (nm) Distance (nm) 8.0 (d) Distance (nm) 8.0 (e) Distance (nm) Distance (nm) Distance (nm) Distance (nm) Figure 5.4 Radial distribution functions g(r) of the (a) anion anion, (b) cation cation, (c) cation anion, (d) anion CAN, and (e) cation ACN for the first layers/center region of [EMIM + ][TFMSI ] confined inside a slit pore of H = 5.2 nm at different concentrations. Red, green and purple colors are used to show the results of 1.0M, 2.0M, and 3.0M respectively. 78
86 3.5 nm 2.5 nm 1.2 nm Top view Side view Figure 5.5 Snapshots of the top and side views of the ions and ACN molecules in the center of the pore at different IL molar concentrations. Side views show the width of the void formed at different concentrations. Top panel shows 1.0M [EMIM + ][TFMSI - ], center panel 2.0M [EMIM + ][TFMSI - ], and bottom panel 3.0M [EMIM + ][TFMSI - ]. Cations, anions and ACN molecules are shown in green, purple and red color respectively. 79
87 concentration however at 2 and 3 M concentration only one broader and weaker peak is observed suggesting weak structuring of cation-cation at 2 and 3 M concentrations. The results show that the local structure of anion-anion and cation-cation at 1M concentrations is significantly different from 2M and 3M concentrations at the surface indicating that presence of high number of ACN molecules at the surface affect the interaction among cations and anions. Unlike our previous findings RDF of cation-cation, cation-anion and anion-anions shows some structural heterogeneity in the center [183, 213]. This could be due to formation of void at the center by ACN molecules where IL is trapped. As seen from Figure 5.5 that width of void formed in 1M concentration is more as compared to 2 and 3 M, hence more ions close to the surface gets affected by the void in 1 M concentration Preferential Orientation Preferential orientations of the ions/molecules inside the pore are studied by calculating the average P 1 (Cos θ), where θ is the angle between a vector normal to the pore surface and a vector defining the orientation of the ions/molecules. The pore is sliced into bins and averaged P 1 (Cos θ) is calculated for 20 ns long trajectories (frames are saved after every 50 ps). In case of cations, we defined a vector that is perpendicular to the imidazolium ring as shown in panel (a) of figure 5.1. Panel (b) of figure 5.1 shows the orientation vector for anions; the vector is defined by joining the CJ- CJ atoms of the anions. Similarly CT and NB atoms are used to define the orientation vector for the ACN molecules (panel (c), figure 5.1). Figure 5.6 shows the average P 1 (Cos θ) for the ions/molecules at 1.0M and 3.0M IL concentrations. Results at 2.0M are similar and are not reported. In all different cases, the ions/ molecules that are in the center of the pores, have no preferential orientation; the average P 1 (Cos θ) value is in between 0.45 to The average P 1 (Cos θ) value for cations that are close to the surface, is close to 1 and for anions and 80
88 P 1 (Cos θ) P 1 (Cos θ) M IL [EMIM + ][TFMSI - ] M IL [EMIM + ][TFMSI - ] First layer Center First layer Figure 5.6 Orientation of the ions and ACN molecules at 1.0M and 3.0M [EMIM + ][TFMSI - ] concentrations. Average P 1 (Cosθ) are calculated using a vector perpendicular to the pore surface and orientation vectors as shown in figure 5.1. Average P 1 (Cosθ) for cations, anions and ACN molecules are shown in green, purple and red color respectively. ACN molecules is close to 0, showing that ions/molecules tend to align themselves parallel to the surface. The results can be further confirmed by looking at the snapshots of the first layer ions/molecules shown in Figure 5.7. These finding are consistent with other reported and our previous studies of the confined ILs inside the graphitic slit pore [183, 213, 252]. 81
89 MSD (nm 2 ) MSD (nm 2 ) (a) (b) (c) Figure 5.7 Snapshots of the ions and ACN molecules in the first layers at different IL molar concentrations: (a) 1.0M [EMIM + ][TFMSI - ], (b) 2.0M [EMIM + ][TFMSI - ], and (c) 3.0M [EMIM + ][TFMSI - ]. 0.3 First layers 0.9 Center region Time (ns) Time (ns) Figure 5.8 Lateral (x and y direction) mean square displacement of the ions and ACN molecules in the first layer (left panel) and in the center region (right panel) of the pore at various concentration. Solid lines, dashed lines and dotted lines are used to represent 3.0M, 2.0M and 1.0M [EMIM + ][TFMSI - ] concentrations respectively. Results for cations, anions and ACN molecules are shown in green, purple and red color respectively. 82
90 5.3.4 Mean Square Displacement Dynamics of the electrolyte play a very crucial role in determining the performance of electrochemical devices; faster dynamics results in less internal resistance and improved specific power. Mean square displacements (MSDs) of the ions and ACN molecules are calculated in the plane parallel to the wall surface at various IL concentrations. Figure 5.8 shows the MSDs of the ions and ACN at 1.0 M, 2.0 and 3.0M IL concentrations. Left panel shows the behavior of the ions/molecules in the first layers and right panel in the center of the pore. It is interesting to find that dynamics of the ions/molecules decreases linearly with decrease in the molar concentration form 3.0M to 1.0M in the center of the pore. The result contradicts the previous studies of ILs solvent mixtures in bulk [262]. The dynamics of the ACN molecules is found to be between the ions, consistent at different concentrations. The slower dynamics is the result of the clustering of the ions/molecules and large size void formation in the center of the pore. Among the ions/molecules, cations have the fastest dynamics and anion slowest. Figure 5.5 shows the top and side view of ions/molecules in the center of the pores. It is found that at 1.0M concentration, there is a large void formation caused by local clustering of the ions and molecules. The thickness of the void is approximately 3.5 nm. As we increase the IL concentration, the size of the void decreases results in improved dynamics. From our previous studies, it has been observed that void formations are the results are local clustering and dynamics slow down drastically once voids are formed. It is also interesting to see that ions prefer to cluster themselves at the liquid/air interface. At 3.0M, void diminishes results in improved dynamics. The dynamics in the first layer is much slower than in the center of the pore but follows the same trends as in the center of the pore. 83
91 5.4 Concluding Remarks We performed MD simulations to study the effect of acetonitrile solvent on the properties of the IL [EMIM + ][TFMSI ] confined inside uncharged slit-like graphitic nanopores of pore width 5.2 nm. The structural and dynamical properties of the confined IL were studied at three different IL molar concentrations including a case when pure ACN was confined inside the nanopore. Mass density results indicate a very strong layering pattern throughout the pore width in case when pure ACN solvent is confined. The density of the solvent decreases linearly as we move toward the center of the pore. Addition of IL in the solvent continued showing similar behavior for ILs and solvent near the pore wall with less variation in the center of the pore. Variation in the density depends strongly on the concentration of the confined IL. In all cases some very low density region (cavity) formation is observed due to the clustering of the ions/molecules near the pore wall. Variations in the concentration do not cause significant changes in the overall (local) 3-d liquid structure of the confined IL and solvent in the center of the pore. Significant change is observed in the structure of the first layers showing structural heterogeneity under confinement at different concentrations. These finding are consistent with our previous studies of ILs in slit-like pores [183, 213]. Structural heterogeneity could be further confirmed by looking at the preferential orientation of the ions/molecules at different concentrations. Irrespective of the concentration, the ions/ molecules in the first layer like to align themselves parallel to the wall surface whereas no preferential orientation is observed in the center of the pore. The dynamics of the IL/ACN at various concentrations is studied from the mean square displacement (MSD) results. It is interesting to find that dynamics of the ions/molecules decreases linearly with decrease in the molar concentration form 3.0M to 1.0M. It is due to clustering of the ions/molecules and large size void formation in the center of the pore 84
92 in the center of the pore. At higher concentration, clustering diminishes results in improved dynamics. Among the ions/molecules, cations have the fastest dynamics and anion slowest. The dynamics of the ACN molecules is found to be between the ions, consistent at different concentrations. Surprisingly the results obtained in this chapter indicate that addition of solvent deteriorate the dynamics of the ions inside the nanopores. A factor that needs to be considered in future simulation studies in this area is to increase the solvent concentration to avoid formation of voids at different IL molar concentration. These studies are currently in progress and will be described in detail in an upcoming paper. 85
93 CHAPTER 6 CONCLUSION AND FUTURE WORK 6.1 Conclusion In this work we have used molecular dynamics (MD) simulation to investigate systems of representative ILs, [BMIM + ][PF - 6 ] and [EMIM + ][TFMSI - ], inside several model materials (e.g., slit-shaped graphitic and titania pores, carbon nanotubes) having pores of different sizes and morphologies. Our goal was to understand how several properties of the ILs (e.g., local density profiles, radial distribution functions, orientation of the ions, diffusivities and mean squared displacements) depend on variables such as pore size and shape, porous material and the amount of IL inside the pore. We also studied the effect of mixing varying amounts of the IL [EMIM + ][TFMSI - ] with an organic solvent, acetonitrile (ACN), on the properties of the confined ILs inside a slit-like graphitic pore. In general, we observed that the ILs exhibit similar layering effects near the surface of the nanoporous irrespective of the pore size, shape and morphology. Variation in the pore loading (pure ILs) and IL molar concentration (IL-ACN mixtures) had a similar effect on the structural properties, with the IL having similar oscillations in the density profiles with lower densities in the center. The densities close to the wall surface depend on the pore loading and IL molar concentration. Orientation distribution profiles indicate that the imidazolium ring of the cations tend to align parallel to the surface when close to the walls in case of slit-shaped graphitic pores, and carbon nanotubes. In case of titania pores, cations tend to have multiple preferential orientations near the surface. Varying molar concentration of IL has no effect on the preferential orientation of the ions near the wall surface. MSD results show that the dynamics of the ions depend strongly on their location with respect to the surface, with slower dynamics generally observed near the pore surfaces in all studied model materials. 86
94 It is interesting to compare the simulation results of ILs inside a slit-like graphitic pore [183] with those obtained for the same IL (with a different force field, however) confined inside a cylindrical multi-walled carbon nanotube (MWCNT) [122]. Significant layering was observed for the IL inside the two different pore geometries. Likewise, in both pore geometries, in general the cations exhibited faster dynamics than anions, and the dynamics of the ions decrease monotonically as we go from the center of the pore to the IL layer near the pore walls. However, evidence of complex dynamics was observed for the IL inside a cylindrical pore. MSD results observed for the IL inside a MWCNT of D = 3.0 nm (for which three layers of ions are observed) indicate that in the second layer, the MSDs of the cations and anions are similar in magnitude; furthermore, the anions in this second layer have dynamics that are similar to those observed for the anions in the center of the pore (compare Figures 2.9 and 3.3). Evidence of similar complex dynamics was not observed for the same IL inside a slit pore. It is also interesting to note that the dynamical properties of the confined IL in the center region of the pore are very similar to bulklike. Similar results have been observed experimentally in other glass-forming systems inside nanopores or near surfaces, including polymer thin films[143] and organic glass-formers.[49, 144] When comparing the properties of ILs confined inside slit-like graphitic and rutile pore, significant difference in both structural and dynamical properties was observed. Multiple preferential orientations for the ions were observed near the rutile wall. However, for slit-like graphitic pores ions prefer to align flat at the pore wall. No preferential orientation was observed in the center region for both pore materials. We also observed that 3-d structure of ions in the center region remains unchanged. The dynamical behavior of ILs inside rutile pores is similar to our previous studies of ILs confined inside carbon nanopores at different pore loadings. However 87
95 the mobility of the ions is much slower in rutile pores indicating stronger interaction of ions for the rutile surface. Addition of solvent in IL does not affect the preferential orientation of the ions near the wall and in the center of the pore when compared with neat IL; most of the ions tend to align flat near the surface. We observed that solvent molecules also lie flat at the surface. Unexpectedly the dynamics of confined IL slows down with addition of solvent. This may be due to the clustering of the ions/molecules and large size void formation in the center of the pore. 6.2 Proposed Work Defining Order Parameters to Characterize Local and Global Degree of Crystallinity A proposed research in this area could be studying the solid phases of ILs in bulk and under confinement. A fundamental understanding of the solidification of organic salts inside cylindrical nanopores is essential to optimize the properties of the resulting 1D-nanoGUMBOS. One important aspect of the proposed simulation studies is establishing a suitable set of collective variables (order parameters) that can accurately characterize the nanocrystalline structure of the ILs in bulk and under confinement. These order parameters should allow us to distinguish between different crystal polymorphs and liquid/amorphous phases, as well as permit the detection of local order in nm-sized regions. A method to calculate the order parameters have been recently proposed by Santiso and Trout,[263, 264] which are based on the generalized pair distribution function of molecular fluids. This method has been tested successfully to study the nucleation of α-glycine crystals in solution, the crystallization of benzene from the melt, and the polymorph transformation of terephthalic acid in bulk systems.[263, 264] This methodology could be extended to study the solidification of organic salts in bulk and inside nanopores. 88
96 Figure 6.1 Snapshot from a preliminary MD simulation of bulk [DMIM + ][Cl - ]. Cations are color-coded by their locally-averaged bond orientation order parameter [eqn. (7)]. Red = liquidlike, white/blue = crystal-like. The calculations of local, locally- and globally-averaged order parameters is already implemented in C++ libraries for NAMD [265] and available under the GNU Lesser General Public License [266]. These local parameters usually take larger values for crystal-like configurations and small values for liquid-like/amorphous configurations. One then could calculate local and global averages of these concentration parameters and use those as a measure of the local and global degree of crystallinity in our simulated systems. In Fig. 6.1 we show a snapshot from a preliminary MD simulation of bulk [DMIM + ][Cl - ] exhibiting distinct liquid-like (disordered) and crystal-like regions. The cations are color-coded according to their locallyaveraged bond orientation order parameter. These preliminary results strongly suggest that the proposed order parameters can indeed be used to distinguish between crystalline and amorphous nm-sized regions in systems of ILs. 89
97 6.2.2 Study of the Effect of Charged Electrodes on the Properties of the Confined ILs Another proposed research in this area could be the study of confined ILs in which pore surfaces are positively/negatively charged. It will be interesting to study the effect of charge on the structural and dynamical properties of the confined ILs inside nanopores of different pore material and morphology. The study could be further extended to the case where solvents are used along with ILs inside confinement. This will give a more realistic picture of the relations between electrode/electrolyte surface and its ability to store charge at the interface. A more complex model of electroactive potential can also be studied [240, 267]. By fundamentally understanding how the electrical, structural and dynamical properties of ILs are affected by the properties of nanoporous materials (i.e pore size and shape, surface density of electrical charge, presence of chemical functional groups at the pore surfaces, and various degree of heterogeneities in these properties), a solid platform will be in place to rationally design EDLCs, DSSCs, ionogels and IL-based electrochemical systems with optimal properties. A very simple approach can be used to study how the properties of the ILs are affected by different values of surface charge density (σ) in the nanopores. A charged nanoporous material can be modeled by uniformly distributing a constant charge q= ±ne among the wall atoms (where e is the charge of one electron; n=0 for an uncharged porous material).[57, 61, 62, 64, 268]. A system with an uncharged porous material will contain an equal number of cations and anions. In contrast, a system where the porous material has a charge, q= ±ne, n/2 randomly selected cations (anions) can be replaced by anions (cations) for the case of positively (negatively) charged nanopores, to ensure that the whole system is electrically neutral. The total charge q will be kept fixed throughout the simulations. Replacing cations for anions and vice 90
98 versa will lead to variations in the density of the IL, and therefore different system sizes will be evaluated in an attempt to minimize the effects of these variations. A number of dynamical properties such as MSD, self part of van Hove correlation function, intermediate scattering function can be determined in the proposed simulations for charged systems in order to understand the dynamics of ILs inside nanopores at the molecular level. 91
99 REFERENCES 1. Bideau, J.L., et al., Effect of confinement on ionic liquids dynamics in monolithic silica ionogels: 1H NMR study. Physical Chemistry Chemical Physics, (40): p Chen, S., et al., Morphology and Melting Behavior of Ionic Liquids inside Single-Walled Carbon Nanotubes. Journal of the American Chemical Society, (41): p Chen, S., et al., Transition of Ionic Liquid [bmim][pf6] from Liquid to High-Melting- Point Crystal When Confined in Multiwalled Carbon Nanotubes. Journal of the American Chemical Society, (9): p Wang, P., et al., A Binary Ionic Liquid Electrolyte to Achieve >7% Power Conversion Efficiencies in Dye-Sensitized Solar Cells. Chemistry of Materials, (14): p Wasserscheid, P. and T. Welton, eds. Ionic Liquids in Synthesis 2nd ed, 2008, Wiley- VCH: Weinheim. 6. Welton, T., Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chemical Reviews, (8): p Plechkova, N.V. and K.R. Seddon, Applications of ionic liquids in the chemical industry. Chemical Society Reviews, (1): p Xu, W. and C.A. Angell, Solvent-Free Electrolytes with Aqueous Solution-Like Conductivities. Science, (5644): p Noda, A., et al., Bronsted Acid∠Base Ionic Liquids as Proton-Conducting Nonaqueous Electrolytes. The Journal of Physical Chemistry B, (17): p Néouze, M.-A., et al., Ionogels, New Materials Arising from the Confinement of Ionic Liquids within Silica-Derived Networks. Chemistry of Materials, (17): p
100 11. Hayes, R., G.G. Warr, and R. Atkin, At the interface: solvation and designing ionic liquids. Physical Chemistry Chemical Physics, (8): p Pandolfo, A.G. and A.F. Hollenkamp, Carbon properties and their role in supercapacitors. Journal of Power Sources, (1): p Frackowiak, E., Carbon materials for supercapacitor application. Physical Chemistry Chemical Physics, (15): p Arbizzani, C., et al., Safe, high-energy supercapacitors based on solvent-free ionic liquid electrolytes. Journal of Power Sources, (2): p Lazzari, M., F. Soavi, and M. Mastragostino, High voltage, asymmetric EDLCs based on xerogel carbon and hydrophobic IL electrolytes. Journal of Power Sources, (1): p Arbizzani, C., et al., Electrode materials for ionic liquid-based supercapacitors. Journal of Power Sources, (2): p Mastragostino, M. and F. Soavi, Strategies for high-performance supercapacitors for HEV. Journal of Power Sources, (1): p Balducci, A., et al., High temperature carbon-carbon supercapacitor using ionic liquid as electrolyte. Journal of Power Sources, (2): p Galinski, M., A. Lewandowski, and I. Stepniak, Ionic liquids as electrolytes. Electrochimica Acta, (26): p Hagfeldt, A., et al., Dye-Sensitized Solar Cells. Chemical Reviews, (11): p Sambandam, A., Recent improvements and arising challenges in dye-sensitized solar cells. Solar Energy Materials and Solar Cells, (9): p Wei, D., Dye Sensitized Solar Cells. International Journal of Molecular Sciences, (3): p
101 23. Kuang, D., et al., Organic Dye-Sensitized Ionic Liquid Based Solar Cells: Remarkable Enhancement in Performance through Molecular Design of Indoline Sensitizers. Angewandte Chemie International Edition, (10): p Gorlov, M. and L. Kloo, Ionic liquid electrolytes for dye-sensitized solar cells. Dalton Transactions, 2008(20): p Cao, Y., et al., Dye-Sensitized Solar Cells with Solvent-Free Ionic Liquid Electrolytes. The Journal of Physical Chemistry C, (35): p Tesfai, A.E.-Z., B.; Bwambok, D. K.; Baker, G. A.; Fakayode, S. O.; Lowry, M.; Warner, I. M., Controllable Formation of Ionic Liquid Micro- and Nanoparticles via a Melt- Emulsion-Quench Approach. Nano Lett., : p Tesfai, A., et al., Magnetic and Nonmagnetic Nanoparticles from a Group of Uniform Materials Based on Organic Salts. ACS Nano, (10): p Bwambok, D.K., et al., Near-Infrared Fluorescent NanoGUMBOS for Biomedical Imaging. ACS Nano, (12): p Vereda, F., J.d. Vicente, and R. Hidalgo-Álvarez, Physical Properties of Elongated Magnetic Particles: Magnetization and Friction Coefficient Anisotropies13. Chemphyschem, (8): p Sun, L., et al., Tuning the properties of magnetic nanowires. IBM J. Res. Dev., (1): p Murphy, C.J., et al., Gold Nanoparticles in Biology: Beyond Toxicity to Cellular Imaging. Accounts of Chemical Research, (12): p Mornet, S., et al., Magnetic nanoparticle design for medical applications. Progress in Solid State Chemistry, (2-4): p Huang, X., et al., Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. Journal of the American Chemical Society, (6): p
102 34. Gupta, A.K. and M. Gupta, Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials, (18): p Day, E.S., J.G. Morton, and J.L. West, Nanoparticles for Thermal Cancer Therapy. Journal of Biomechanical Engineering, (7): p Boisselier, E. and D. Astruc, Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chemical Society Reviews, (6): p Fortin, J.-P., et al., Size-Sorted Anionic Iron Oxide Nanomagnets as Colloidal Mediators for Magnetic Hyperthermia. Journal of the American Chemical Society, (9): p Ohno, H. and K. Fukumoto, Amino Acid Ionic Liquids. Accounts of Chemical Research, (11): p Li, M.D.R., S. L.; Bwambok, D. K.; El-Zahab, B.; DiTusa, J. F.; Warner, I. M., Magnetic chiral ionic liquids derived from amino acids. Chem. Commun., 2009: p Hough-Troutman, W.L.S., M.; Griffin, S.; Reichert, W. M.; Mirska, I.; Jodynis-Liebert, J.; Adamska, T.; Nawrot, J.; Stasiewicz, M.; Rogers,R.D.; Pernak, J, Ionic Liquids with Dual Biological Function: Sweet and Anti-Microbial, Hydrophobic Quaternary Ammonium-Based Salts. New J. Chem., : p Hough, W.L.S., M.; Rodriguez, H.; Swatloski, R. P.; Spear, S. K.; Daly, D. T.; Pernak, J.; Grisel, J. E.; Carliss, R. D.; Soutullo, M. D.; Davis, J. J. H.; Rogers, R. D, The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem., : p Derfus, A.M.C., W. C. W.; Bhatia, S. N., Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett., : p Porter, A.E.G., M.; Muller, K.; Skepper, J. N.; Midgley, P. A.; Welland, M., Direct Imaging of Single-Walled Carbon nanotubes in Cells. Nat. Nanotechnol., : p
103 44. Lev, D.G. and et al., Phase separation in confined systems. Reports on Progress in Physics, (12): p Kornyshev, A.A., Double-Layer in Ionic Liquids: Paradigm Change? The Journal of Physical Chemistry B, (20): p Goebel, R., et al., Surprisingly high, bulk liquid-like mobility of silica-confined ionic liquids. Physical Chemistry Chemical Physics, (19): p Kanakubo, M., et al., Melting point depression of ionic liquids confined in nanospaces. Chemical Communications, 2006(17): p Alba-Simionesco, C., et al., Effects of confinement on freezing and melting. Journal of Physics-Condensed Matter, (6): p. R15-R Alcoutlabi, M. and G.B. McKenna, Effects of confinement on material behaviour at the nanometre size scale. Journal of Physics-Condensed Matter, (15): p. R461-R Gelb, L.D., et al., Phase separation in confined systems. Reports on Progress in Physics, (12): p Liu, L., et al., Well-ordered structure at ionic liquid/rutile (110) interface. Journal of Physical Chemistry C, (33): p Lynden-Bell, R.M., et al., Simulations of ionic liquids, solutions, and surfaces. Accounts of Chemical Research, (11): p Sha, M.L., et al., Ordering layers of bmim PF6 ionic liquid on graphite surfaces: Molecular dynamics simulation. Journal of Chemical Physics, (13): p Kislenko, S.A., I.S. Samoylov, and R.H. Amirov, Molecular dynamics simulation of the electrochemical interface between a graphite surface and the ionic liquid BMIM PF6. Physical Chemistry Chemical Physics, (27): p Wang, S., et al., Molecular Dynamic Simulations of Ionic Liquids at Graphite Surface. Journal of Physical Chemistry C, (2): p
104 56. Pinilla, C., et al., Structure and Dynamics of a Confined Ionic Liquid. Topics of Relevance to Dye-Sensitized Solar Cells. The Journal of Physical Chemistry B, (38): p Pinilla, C., et al., Polarization relaxation in an ionic liquid confined between electrified walls. Journal of Physical Chemistry B, (18): p Reed, S.K., P.A. Madden, and A. Papadopoulos, Electrochemical charge transfer at a metallic electrode: A simulation study. Journal of Chemical Physics, (12): p Sha, M., et al., Liquid-to-Solid Phase Transition of a 1,3-Dimethylimidazolium Chloride Ionic Liquid Monolayer Confined between Graphite Walls. The Journal of Physical Chemistry C, (47): p Sha, M., et al., Drastic Phase Transition in Ionic Liquid [Dmim][Cl] Confined Between Graphite Walls: New Phase Formation. The Journal of Physical Chemistry C, (11): p Feng, G., J.S. Zhang, and R. Qiao, Microstructure and Capacitance of the Electrical Double Layers at the Interface of Ionic Liquids and Planar Electrodes. Journal of Physical Chemistry C, (11): p Shim, Y. and H.J. Kim, Solvation of Carbon Nanotubes in a Room-Temperature Ionic Liquid. Acs Nano, (7): p Dong, K., et al., Structural Evidence for the Ordered Crystallites of Ionic Liquid in Confined Carbon Nanotubes. The Journal of Physical Chemistry C, (23): p Shim, Y. and H.J. Kim, Nanoporous Carbon Supercapacitors in an Ionic Liquid: A Computer Simulation Study. Acs Nano, (4): p Hess, B., et al., GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. Journal of Chemical Theory and Computation, (3): p
105 66. Canongia Lopes, J.N., A.A.H. Padua, and K. Shimizu, Molecular Force Field for Ionic Liquids IV: Trialkylimidazolium and Alkoxycarbonyl-Imidazolium Cations; Alkylsulfonate and Alkylsulfate Anions. The Journal of Physical Chemistry B, (16): p Canongia Lopes, J.N. and A.l.A.H. PÃ dua, Molecular Force Field for Ionic Liquids III:â Imidazolium, Pyridinium, and Phosphonium Cations; Chloride, Bromide, and Dicyanamide Anions. The Journal of Physical Chemistry B, (39): p Canongia Lopes, J.N., J. Deschamps, and A.A.H. Padua, Modeling Ionic Liquids Using a Systematic All-Atom Force Field. The Journal of Physical Chemistry B, (6): p Jorgensen, W.L. and J. Tirado-Rives, The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. Journal of the American Chemical Society, (6): p Morrow, T.I. and E.J. Maginn, Molecular dynamics study of the ionic liquid 1-n-butyl-3- methylimidazolium hexafluorophosphate. Journal of Physical Chemistry B, (49): p Zaitsau, D.H., et al., Experimental vapor pressures of 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imides and a correlation scheme for estimation of vaporization enthalpies of ionic liquids. Journal of Physical Chemistry A, (22): p Swiderski, K., et al., Estimates of internal energies of vaporisation of some room temperature ionic liquids. Chemical Communications, 2004(19): p Darden, T., D. York, and L. Pedersen, PARTICLE MESH EWALD - AN N.LOG(N) METHOD FOR EWALD SUMS IN LARGE SYSTEMS. Journal of Chemical Physics, (12): p Bussi, G., D. Donadio, and M. Parrinello, Canonical sampling through velocity rescaling. Journal of Chemical Physics, : p
106 75. Bussi, G., T. Zykova-Timan, and M. Parrinello, Isothermal-isobaric molecular dynamics using stochastic velocity rescaling. Journal of Chemical Physics, : p Bhargava, B.L. and S. Balasubramanian, Layering at an ionic liquid-vapor interface: A molecular dynamics simulation study of bmim PF6. Journal of the American Chemical Society, (31): p Sloutskin, E., et al., Surface layering in ionic liquids: An X-ray reflectivity study (vol 127, pg 7796, 2005). Journal of the American Chemical Society, (51): p Maginn, E.J., Molecular simulation of ionic liquids: current status and future opportunities. Journal of Physics-Condensed Matter, (37): p Urahata, S.M. and M.C.C. Ribeiro, Single particle dynamics in ionic liquids of 1-alkyl-3- methylimidazolium cations. Journal of Chemical Physics, (2): p Del Popolo, M.G. and G.A. Voth, On the Structure and Dynamics of Ionic Liquids. The Journal of Physical Chemistry B, (5): p Margulis, C.J., H.A. Stern, and B.J. Berne, Computer Simulation of a â œgreen Chemistryâ Room-Temperature Ionic Solvent. The Journal of Physical Chemistry B, (46): p de Andrade, J., E.S. Boes, and H. Stassen, Computational Study of Room Temperature Molten Salts Composed by 1-Alkyl-3-methylimidazolium CationsForce-Field Proposal and Validation. The Journal of Physical Chemistry B, (51): p Tsuzuki, S., et al., Molecular Dynamics Simulations of Ionic Liquids: Cation and Anion Dependence of Self-Diffusion Coefficients of Ions. Journal of Physical Chemistry B, (31): p Hu, Z.H. and C.J. Margulis, Heterogeneity in a room-temperature ionic liquid: Persistent local environments and the red-edge effect. Proceedings of the National Academy of Sciences of the United States of America, (4): p Scheidler, P., W. Kob, and K. Binder, The relaxation dynamics of a supercooled liquid confined by rough walls. Journal of Physical Chemistry B, (21): p
107 86. Wasserscheid, P. and T. Welton, eds. Ionic Liquids in Synthesis 2nd ed. 2008, Wiley- VCH: Weinheim. 87. Sarangi, S.S., et al., Correlation between Dynamic Heterogeneity and Local Structure in a Room-Temperature Ionic Liquid: A Molecular Dynamics Study of bmim PF6. Chemphyschem, (9): p Liu, C., et al., Advanced Materials for Energy Storage. Advanced Materials, (8): p. E Zhang, L.L. and X.S. Zhao, Carbon-based materials as supercapacitor electrodes. Chemical Society Reviews, (9): p Simon, P. and Y. Gogotsi, Materials for electrochemical capacitors. Nature Materials, (11): p Arico, A.S., et al., Nanostructured materials for advanced energy conversion and storage devices. Nature Materials, (5): p Hall, P.J., et al., Energy storage in electrochemical capacitors: designing functional materials to improve performance. Energy & Environmental Science, (9): p Armand, M., et al., Ionic-liquid materials for the electrochemical challenges of the future. Nature Materials, (8): p Gratzel, M., Recent Advances in Sensitized Mesoscopic Solar Cells. Accounts of Chemical Research, (11): p Kamat, P.V., Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. Journal of Physical Chemistry C, (7): p Vioux, A., et al., Use of ionic liquids in sol-gel; ionogels and applications. Comptes Rendus Chimie, (1-2): p Lunstroot, K., et al., Lanthanide-doped luminescent ionogels. Dalton Transactions, 2009(2): p
108 98. Lunstroot, K., et al., Luminescent ionogels based on europium-doped ionic liquids confined within silica-derived networks. Chemistry of Materials, (24): p Das, S., et al., Nontemplated Approach to Tuning the Spectral Properties of Cyanine- Based Fluorescent NanoGUMBOS. Langmuir, (15): p Dumke, J.C., et al., Lanthanide-Based Luminescent NanoGUMBOS. Langmuir, (19): p de Rooy, S.L., et al., Fluorescent One-Dimensional Nanostructures from a Group of Uniform Materials Based on Organic Salts. Chemical Communications, 2011: p. submitted Hergt, R., et al., Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy. Journal of Physics-Condensed Matter, (38): p. S2919-S Derfus, A.M., W.C.W. Chan, and S.N. Bhatia, Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett., : p Porter, A.E., et al., Direct Imaging of Single-Walled Carbon nanotubes in Cells. Nat. Nanotechnol., : p Poland, C.A., et al., Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nature Nanotechnology, (7): p Lam, C.W., et al., A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Critical Reviews in Toxicology, (3): p Jeng, H.A. and J. Swanson, Toxicity of metal oxide nanoparticles in mammalian cells. Journal of Environmental Science and Health Part a-toxic/hazardous Substances & Environmental Engineering, (12): p Connor, E.E., et al., Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small, (3): p
109 109. Mezger, M., et al., Layering of BMIM (+)-based ionic liquids at a charged sapphire interface. Journal of Chemical Physics, (9): p Dou, Q.A., et al., Mass Distribution and Diffusion of 1-Butyl-3-methylimidazolium Y Ionic Liquids Adsorbed on the Graphite Surface at K. Chemphyschem, (11): p Sha, M.L., et al., Double-Layer Formation of Bmim PF6 Ionic Liquid Triggered by Surface Negative Charge. Langmuir, (15): p Sieffert, N. and G. Wipff, Ordering of Imidazolium-Based Ionic Liquids at the alpha- Quartz(001) Surface: A Molecular Dynamics Study. Journal of Physical Chemistry C, (49): p Vatamanu, J., O. Borodin, and G.D. Smith, Molecular dynamics simulations of atomically flat and nanoporous electrodes with a molten salt electrolyte. Physical Chemistry Chemical Physics, (1): p Dou, Q., et al., Molecular dynamics simulation of the interfacial structure of C(n)mim PF6 adsorbed on a graphite surface: effects of temperature and alkyl chain length. Journal of Physics-Condensed Matter, (17): p Sha, M.L., et al., Liquid-to-Solid Phase Transition of a 1,3-Dimethylimidazolium Chloride Ionic Liquid Monolayer Confined between Graphite Walls. Journal of Physical Chemistry C, (47): p Sha, M.L., et al., Drastic Phase Transition in Ionic Liquid [Dmim][CI] Confined Between Graphite Walls: New Phase Formation. Journal of Physical Chemistry C, (11): p Tazi, S., et al., Potential-Induced Ordering Transition of the Adsorbed Layer at the Ionic Liquid/Electrified Metal Interface. Journal of Physical Chemistry B, (25): p Fedorov, M.V. and A.A. Kornyshev, Ionic liquid near a charged wall: Structure and capacitance of electrical double layer. Journal of Physical Chemistry B, (38): p
110 119. Reed, S.K., O.J. Lanning, and P.A. Madden, Electrochemical interface between an ionic liquid and a model metallic electrode. Journal of Chemical Physics, (8): p Vatamanu, J., O. Borodin, and G.D. Smith, Molecular Insights into the Potential and Temperature Dependences of the Differential Capacitance of a Room-Temperature Ionic Liquid at Graphite Electrodes. Journal of the American Chemical Society, (42): p Vatamanu, J., O. Borodin, and G.D. Smith, Molecular Simulations of the Electric Double Layer Structure, Differential Capacitance, and Charging Kinetics for N-Methyl-Npropylpyrrolidinium Bis(fluorosulfonyl)imide at Graphite Electrodes. Journal of Physical Chemistry B, (12): p Singh, R., J. Monk, and F.R. Hung, A computational study of the behavior of the ionic liquid [BMIM+][PF6-] confined inside multi-walled carbon nanotubes. Journal of Physical Chemistry C, : p Monk, J., R. Singh, and F.R. Hung, Effects of Pore Size and Pore Loading on the Properties of Ionic Liquids Confined Inside Nanoporous CMK-3 Carbon Materials. Journal of Physical Chemistry C, (7): p Bhargava, B.L. and S. Balasubramanian, Refined potential model for atomistic simulations of ionic liquid [bmim][pf[sub 6]]. The Journal of Chemical Physics, (11): p Borodin, O., Polarizable Force Field Development and Molecular Dynamics Simulations of Ionic Liquids. Journal of Physical Chemistry B, (33): p Tokuda, H., et al., How ionic are room-temperature ionic liquids? An indicator of the physicochemical properties. Journal of Physical Chemistry B, (39): p Dubbeldam, D. and R.Q. Snurr, Recent developments in the molecular modeling of diffusion in nanoporous materials. Molecular Simulation, (4-5): p Allen, M.P. and D.J. Tildesley, Computer Simulation of Liquids. 1987, Oxford: Clarendon Press. 103
111 129. Frenkel, D. and B. Smit, Understanding Molecular Simulation: From Algorithms to Applications. 2nd ed. 2002, San Diego: Academic Press Hansen, J.-P. and I.R. McDonald, Theory of simple liquids. 1986, London: Academic Press Barrat, J.-L. and J.-P. Hansen, Basic concepts for simple and complex liquids. 2003, Cambridge: Cambridge University Press Jiang, W., et al., Molecular dynamics simulation of the energetic room-temperature ionic liquid, 1-hydroxyethyl-4-amino-1,2,4-triazolium nitrate (HEATN). Journal of Physical Chemistry B, (10): p Habasaki, J. and K.L. Ngai, Heterogeneous dynamics of ionic liquids from molecular dynamics simulations. Journal of Chemical Physics, (19): p Triolo, A., et al., Quasielastic neutron scattering characterization of the relaxation processes in a room temperature ionic liquid. Journal of Chemical Physics, (16): p Varnik, F., J. Baschnagel, and K. Binder, Static and dynamic properties of supercooled thin polymer films. The European Physical Journal E: Soft Matter and Biological Physics, (2): p Kob, W. and H.C. Andersen, Scaling Behavior in the beta -Relaxation Regime of a Supercooled Lennard-Jones Mixture. Physical Review Letters, (10): p Bruni, F., M.A. Ricci, and A.K. Soper, Water confined in Vycor glass. I. A neutron diffraction study. Journal of Chemical Physics, (4): p Soper, A.K., F. Bruni, and M.A. Ricci, Water confined in Vycor glass. II. Excluded volume effects on the radial distribution functions. Journal of Chemical Physics, (4): p Morineau, D. and C. Alba-Simionesco, Liquids in confined geometry: How to connect changes in the structure factor to modifications of local order. Journal of Chemical Physics, (20): p
112 140. Soper, A.K., The excluded volume effect in confined fluids and liquid mixtures. Journal of Physics-Condensed Matter, (11): p Gallo, P., M.A. Ricci, and M. Rovere, Layer analysis of the structure of water confined in vycor glass. Journal of Chemical Physics, (1): p Hung, F.R., et al., Molecular modeling of freezing of simple fluids confined within carbon nanotubes. Journal of Chemical Physics, (14): p Keddie, J.L., R.A.L. Jones, and R.A. Cory, INTERFACE AND SURFACE EFFECTS ON THE GLASS-TRANSITION TEMPERATURE IN THIN POLYMER-FILMS. Faraday Discussions, : p Arndt, M., et al., Dielectric investigations of the dynamic glass transition in nanopores. Physical Review E, (5): p Rogers, R.D. and K.R. Seddon, Ionic Liquids--Solvents of the Future? Science, (5646): p Reichardt, C. and T. Welton, Front Matter, in Solvents and Solvent Effects in Organic Chemistry. 2010, Wiley-VCH Verlag GmbH & Co. KGaA. p. I-XXVI Aparicio, S., et al., Study on Hydroxylammonium-Based Ionic Liquids. II. Computational Analysis of CO2 Absorption. The Journal of Physical Chemistry B. 115(43): p Chen, Y., et al., Ionic Liquid/Metal Organic Framework Composite for CO2 Capture: A Computational Investigation. The Journal of Physical Chemistry C, (44): p Zhang, X., et al., Microscopic Structure, Interaction, and Properties of a Guanidinium- Based Ionic Liquid and Its Mixture with CO2. Industrial & Engineering Chemistry Research, (13): p Wu, H., et al., Structure and Dynamics of Neat and CO2-Reacted Ionic Liquid Tetrabutylphosphonium 2-Cyanopyrrolide. Industrial & Engineering Chemistry Research, (15): p
113 151. Perez-Blanco, M.E. and E.J. Maginn, Molecular Dynamics Simulations of Carbon Dioxide and Water at an Ionic Liquid Interface. The Journal of Physical Chemistry B, (35): p Perez-Blanco, M.E. and E.J. Maginn, Molecular Dynamics Simulations of CO2 at an Ionic Liquid Interface: Adsorption, Ordering, and Interfacial Crossing. The Journal of Physical Chemistry B, (36): p Maginn, E.J., Molecular simulation of ionic liquids: current status and future opportunities. Journal of Physics: Condensed Matter, (37): p Bates, E.D., et al., CO2 Capture by a Task-Specific Ionic Liquid. Journal of the American Chemical Society, (6): p Gurkan, B., et al., Molecular Design of High Capacity, Low Viscosity, Chemically Tunable Ionic Liquids for CO2 Capture. The Journal of Physical Chemistry Letters, (24): p Wasserscheid, P. and A. Jess, Deep desulfurization of oil refinery streams by extraction with ionic liquids. Green Chemistry, (7): p Poole, C.F. and S.K. Poole, Extraction of organic compounds with room temperature ionic liquids. Journal of Chromatography A, (16): p Sato, T., G. Masuda, and K. Takagi, Electrochemical properties of novel ionic liquids for electric double layer capacitor applications. Electrochimica Acta, (21): p Ue, M., et al., Application of Low-Viscosity Ionic Liquid to the Electrolyte of Double- Layer Capacitors. Journal of The Electrochemical Society, (4): p. A499-A Ue, M., et al., Ionic Liquids with Low Melting Points and Their Application to Double- Layer Capacitor Electrolytes. Electrochemical and Solid-State Letters, (6): p. A119-A Orita, A., K. Kamijima, and M. Yoshida, Allyl-functionalized ionic liquids as electrolytes for electric double-layer capacitors. Journal of Power Sources, (21): p
114 162. Matsumoto, K. and R. Hagiwara, Electrochemical Properties of the Ionic Liquid 1-Ethyl- 3-methylimidazolium Difluorophosphate as an Electrolyte for Electric Double-Layer Capacitors. Journal of The Electrochemical Society, (5): p. A578-A Balducci, A., et al., Ionic liquids for hybrid supercapacitors. Electrochemistry Communications, (6): p Frackowiak, E., G. Lota, and J. Pernak, Room-temperature phosphonium ionic liquids for supercapacitor application. Applied Physics Letters, (16): p Mysyk, R., et al., Pseudo-capacitance of nanoporous carbons in pyrrolidinium-based protic ionic liquids. Electrochemistry Communications, (3): p Wang, P., et al., A New Ionic Liquid Electrolyte Enhances the Conversion Efficiency of Dye-Sensitized Solar Cells. The Journal of Physical Chemistry B, (48): p Kawano, R., et al., High performance dye-sensitized solar cells using ionic liquids as their electrolytes. Journal of Photochemistry and Photobiology A: Chemistry, (1-3): p Ito, S., et al., Bifacial dye-sensitized solar cells based on an ionic liquid electrolyte. Nat Photon, (11): p Fabregat-Santiago, F., et al., Correlation between Photovoltaic Performance and Impedance Spectroscopy of Dye-Sensitized Solar Cells Based on Ionic Liquids. The Journal of Physical Chemistry C, (17): p Kuang, D., et al., Stable Mesoscopic Dye-Sensitized Solar Cells Based on Tetracyanoborate Ionic Liquid Electrolyte. Journal of the American Chemical Society, (24): p Wang, P., et al., Gelation of Ionic Liquid-Based Electrolytes with Silica Nanoparticles for Quasi-Solid-State Dye-Sensitized Solar Cells. Journal of the American Chemical Society, (5): p Jeon, J., et al., The Role of Confined Water in Ionic Liquid Electrolytes for Dye-Sensitized Solar Cells. The Journal of Physical Chemistry Letters, (4): p
115 173. Hočevar, M., et al., Dye-Sensitized Solar, Cells Sol-Gel Processing for Conventional and Alternative Energy. 2012, Springer US. p Machesky, M., et al., Comparison of Cation Adsorption by Isostructural Rutile and Cassiterite. Langmuir, (8): p Cremer, T., et al., Liquid/Solid Interface of Ultrathin Ionic Liquid Films: [C1C1Im][Tf2N] and [C8C1Im][Tf2N] on Au(111). Langmuir, (7): p Alam, M.T., et al., Differential Capacitance at Au(111) in 1-Alkyl-3-methylimidazolium Tetrafluoroborate Based Room-Temperature Ionic Liquids. The Journal of Physical Chemistry C, (40): p Wu, C., et al., Modeling the Interaction between Integrin-Binding Peptide (RGD) and Rutile Surface: The Effect of Na+ on Peptide Adsorption. The Journal of Physical Chemistry C, (45): p Sieffert, N. and G. Wipff, Ordering of Imidazolium-Based Ionic Liquids at the α- Quartz(001) Surface: A Molecular Dynamics Study. The Journal of Physical Chemistry C, (49): p Shim, Y., Y. Jung, and H.J. Kim, Graphene-Based Supercapacitors: A Computer Simulation Study. The Journal of Physical Chemistry C, (47): p Liu, L., et al., Well-Ordered Structure at Ionic Liquid/Rutile (110) Interface. The Journal of Physical Chemistry C, (33): p Klaver, T.P.C., et al., DFT Study of 1,3-Dimethylimidazolium Tetrafluoroborate on Al and Cu(111) Surfaces. The Journal of Physical Chemistry C, (30): p Feng, G., D.-e. Jiang, and P.T. Cummings, Curvature Effect on the Capacitance of Electric Double Layers at Ionic Liquid/Onion-Like Carbon Interfaces. Journal of Chemical Theory and Computation, (3): p
116 183. Singh, R., J. Monk, and F.R. Hung, Heterogeneity in the Dynamics of the Ionic Liquid [BMIM+][PF6-] Confined in a Slit Nanopore. The Journal of Physical Chemistry C, (33): p Liu, B. and E.S. Aydil, Growth of Oriented Single-Crystalline Rutile TiO2 Nanorods on Transparent Conducting Substrates for Dye-Sensitized Solar Cells. Journal of the American Chemical Society, (11): p Martinson, A.B.F., et al., ZnO Nanotube Based Dye-Sensitized Solar Cells. Nano Letters, (8): p Anandan, S., X. Wen, and S. Yang, Room temperature growth of CuO nanorod arrays on copper and their application as a cathode in dye-sensitized solar cells. Materials Chemistry and Physics, (1): p Chen, D., et al., Mesoporous Anatase TiO2 Beads with High Surface Areas and Controllable Pore Sizes: A Superior Candidate for High-Performance Dye-Sensitized Solar Cells. Advanced Materials, (21): p Kang, T.-S., et al., Fabrication of Highly-Ordered TiO2 Nanotube Arrays and Their Use in Dye-Sensitized Solar Cells. Nano Letters, (2): p Shao, F., et al., Template-Free Synthesis of Hierarchical TiO2 Structures and Their Application in Dye-Sensitized Solar Cells. ACS Applied Materials & Interfaces, (6): p Zhou, Z.-j., et al., Effect of Highly Ordered Single-Crystalline TiO2 Nanowire Length on the Photovoltaic Performance of Dye-Sensitized Solar Cells. ACS Applied Materials & Interfaces, (11): p Ghadiri, E., et al., Enhanced Electron Collection Efficiency in Dye-Sensitized Solar Cells Based on Nanostructured TiO2 Hollow Fibers. Nano Letters, (5): p Kim, Y.J., et al., Formation of Highly Efficient Dye-Sensitized Solar Cells by Hierarchical Pore Generation with Nanoporous TiO2 Spheres. Advanced Materials, (36): p
117 193. Roy, P., et al., Improved efficiency of TiO2 nanotubes in dye sensitized solar cells by decoration with TiO2 nanoparticles. Electrochemistry Communications, (5): p Zhu, K., et al., Enhanced Charge-Collection Efficiencies and Light Scattering in Dye- Sensitized Solar Cells Using Oriented TiO2 Nanotubes Arrays. Nano Letters, (1): p Zhang, J., X. Tang, and D. Li, One-Step Formation of Crystalline TiO2 Nanotubular Arrays with Intrinsic p-n Junctions. The Journal of Physical Chemistry C, (44): p Ghatee, M.H. and F. Moosavi, Physisorption of Hydrophobic and Hydrophilic 1-Alkyl-3- methylimidazolium Ionic Liquids on the Graphenes. The Journal of Physical Chemistry C, (13): p Rivera-Rubero, S. and S. Baldelli, Surface Spectroscopy of Room-temperature Ionic Liquids on a Platinum Electrode: A Sum Frequency Generation Study. The Journal of Physical Chemistry B, (39): p Atkin, R. and G.G. Warr, Self-Assembly of a Nonionic Surfactant at the Graphite/Ionic Liquid Interface. Journal of the American Chemical Society, (34): p Liu, Y., et al., Coexistence of Liquid and Solid Phases of Bmim-PF6 Ionic Liquid on Mica Surfaces at Room Temperature. Journal of the American Chemical Society, (23): p Köhler, R., et al., Liquid- or Solid-Like Behavior of [omim][bf4] at a Solid Interface? The Journal of Physical Chemistry Letters, (13): p Rollins, J.B., B.D. Fitchett, and J.C. Conboy, Structure and Orientation of the Imidazolium Cation at the Room-Temperature Ionic Liquid/SiO2 Interface Measured by Sum-Frequency Vibrational Spectroscopy The Journal of Physical Chemistry B, (18): p Romero, C. and S. Baldelli, Sum Frequency Generation Study of the Room-Temperature Ionic Liquids/Quartz Interface. The Journal of Physical Chemistry B, (12): p
118 203. Hayes, R., et al., Double Layer Structure of Ionic Liquids at the Au(111) Electrode Interface: An Atomic Force Microscopy Investigation. The Journal of Physical Chemistry C, (14): p Sha, M., et al., Double-Layer Formation of [Bmim][PF6] Ionic Liquid Triggered by Surface Negative Charge. Langmuir, (15): p Lauw, Y., et al., Electrical Double-Layer Capacitance in Room Temperature Ionic Liquids: Ion-Size and Specific Adsorption Effects. The Journal of Physical Chemistry B, (34): p Drüschler, M., et al., Hysteresis Effects in the Potential-Dependent Double Layer Capacitance of Room Temperature Ionic Liquids at a Polycrystalline Platinum Interface. The Journal of Physical Chemistry C, (8): p Vatamanu, J., O. Borodin, and G.D. Smith, Molecular Simulations of the Electric Double Layer Structure, Differential Capacitance, and Charging Kinetics for N-Methyl-Npropylpyrrolidinium Bis(fluorosulfonyl)imide at Graphite Electrodes. The Journal of Physical Chemistry B, (12): p Wang, S., et al., A molecular dynamics simulation of the structure of ionic liquid (BMIM+/PF 6 )/rutile (110) interface. Science in China Series B: Chemistry, (9): p Utesch, T., G. Daminelli, and M.A. Mroginski, Molecular Dynamics Simulations of the Adsorption of Bone Morphogenetic Protein-2 on Surfaces with Medical Relevance. Langmuir, (21): p Borodin, O., et al., Molecular Dynamics Study of the Influence of Solid Interfaces on Poly(ethylene oxide) Structure and Dynamics. Macromolecules, (20): p Bandura, A.V. and J.D. Kubicki, Derivation of Force Field Parameters for TiO2∠H2O Systems from ab Initio Calculations. The Journal of Physical Chemistry B, (40): p Kelkar, M.S. and E.J. Maginn, Effect of Temperature and Water Content on the Shear Viscosity of the Ionic Liquid 1-Ethyl-3-methylimidazolium 111
119 Bis(trifluoromethanesulfonyl)imide As Studied by Atomistic Simulations. The Journal of Physical Chemistry B, (18): p Rajput, N.N., et al., On the Influence of Pore Size and Pore Loading on Structural and Dynamical Heterogeneities of an Ionic Liquid Confined in a Slit Nanopore. The Journal of Physical Chemistry C, (8): p Urahata, S.M. and M.C.C. Ribeiro, Collective excitations in an ionic liquid. The Journal of Chemical Physics, (7): p Yang, J., et al., Carbon Electrode Material with High Densities of Energy and Power. Acta Physico-Chimica Sinica, (1): p Abruna, H.D., Y. Kiya, and J.C. Henderson, Batteries and electrochemical capacitors. Physics Today, (12): p Meyer, J.C., et al., The structure of suspended graphene sheets. Nature, (7131): p Vivekchand, S., et al., Graphene-based electrochemical supercapacitors. Journal of Chemical Sciences, (1): p Wang, H., et al., Real-Time NMR Studies of Electrochemical Double-Layer Capacitors. Journal of the American Chemical Society, (48): p Li, Q., et al., Synthesis of mesoporous carbon spheres with a hierarchical pore structure for the electrochemical double-layer capacitor. Carbon, (4): p Du, X., et al., Graphene nanosheets as electrode material for electric double-layer capacitors. Electrochimica Acta, (16): p Huang, C.-W., et al., Mesoporous carbon spheres grafted with carbon nanofibers for high-rate electric double layer capacitors. Carbon, (3): p Stoner, B.R., et al., Graphenated carbon nanotubes for enhanced electrochemical double layer capacitor performance. Applied Physics Letters, (18): p
120 224. Kotz, R. and M. Carlen, Principles and applications of electrochemical capacitors. Electrochimica Acta, (15-16): p Balducci, A., et al., High temperature carbon-carbon supercapacitor using ionic liquid as electrolyte. Journal of Power Sources, (2): p Endo, M., et al., Capacitance and Pore-Size Distribution in Aqueous and Nonaqueous Electrolytes Using Various Activated Carbon Electrodes. Journal of The Electrochemical Society, (8): p. A910-A Weng, T.-C. and H. Teng, Characterization of High Porosity Carbon Electrodes Derived from Mesophase Pitch for Electric Double-Layer Capacitors. Journal of The Electrochemical Society, (4): p. A368-A Aurbach, D., et al., Cation Trapping in Highly Porous Carbon Electrodes for EDLC Cells. Journal of The Electrochemical Society, (10): p. A745-A Ye, J.-S., et al., Electrochemical oxidation of multi-walled carbon nanotubes and its application to electrochemical double layer capacitors. Electrochemistry Communications, (3): p Li, C., et al., Oxidation behavior of CNTs and the electric double layer capacitor made of the CNT electrodes. Science in China Series E: Technological Sciences, (4): p Stoller, M.D., et al., Graphene-Based Ultracapacitors. Nano Letters, (10): p Xu, B., et al., Room temperature molten salt as electrolyte for carbon nanotube-based electric double layer capacitors. Journal of Power Sources, (1): p Yang, H., et al., Improvement of Commercial Activated Carbon and Its Application in Electric Double Layer Capacitors. Electrochemical and Solid-State Letters, (6): p. A141-A Prabaharan, S.R.S., R. Vimala, and Z. Zainal, Nanostructured mesoporous carbon as electrodes for supercapacitors. Journal of Power Sources, (1): p
121 235. Ma, R., et al., Processing and Performance of Electric Double-Layer Capacitors with Block-Type Carbon Nanotube Electrodes. Bulletin of the Chemical Society of Japan, (11): p Li, C., et al., Oxidation of multiwalled carbon nanotubes by air: benefits for electric double layer capacitors. Powder Technology, (2-3): p Yoon, B.-J., et al., Electrical properties of electrical double layer capacitors with integrated carbon nanotube electrodes. Chemical Physics Letters, (1-3): p Janes, A. and E. Lust, Organic carbonate-organic ester-based non-aqueous electrolytes for electrical double layer capacitors. Electrochemistry Communications, (5): p Liu, X. and T. Osaka, Properties of Electric Double-Layer Capacitors with Various Polymer Gel Electrolytes. Journal of The Electrochemical Society, (9): p Wu, P., et al., Complex Capacitance Scaling in Ionic Liquids-Filled Nanopores. Acs Nano, (11): p Feng, G. and P.T. Cummings, Supercapacitor Capacitance Exhibits Oscillatory Behavior as a Function of Nanopore Size. The Journal of Physical Chemistry Letters, 2011: p Jiang, D.-e., Z. Jin, and J. Wu, Oscillation of Capacitance inside Nanopores. Nano Letters, : p Kondrat, S., et al., A superionic state in nano-porous double-layer capacitors: insights from Monte Carlo simulations. Physical Chemistry Chemical Physics, (23): p Kondrat, S. and A. Kornyshev, Superionic state in double-layer capacitors with nanoporous electrodes. Journal of Physics-Condensed Matter, (2): p Feng, G., et al., Ion Distribution in Electrified Micropores and Its Role in the Anomalous Enhancement of Capacitance. ACS Nano, (4): p
122 246. Chmiola, J., et al., Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science, (5794): p Chmiola, J., et al., Monolithic Carbide-Derived Carbon Films for Micro- Supercapacitors. Science, (5977): p Raymundo-Pinero, E., et al., Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon, (12): p Largeot, C., et al., Relation between the ion size and pore size for an electric double-layer capacitor. Journal of the American Chemical Society, (9): p Lin, R., et al., Solvent effect on the ion adsorption from ionic liquid electrolyte into subnanometer carbon pores. Electrochimica Acta, (27): p Centeno, T.A., O. Sereda, and F. Stoeckli, Capacitance in carbon pores of 0.7 to 15 nm: a regular pattern. Physical Chemistry Chemical Physics, (27): p Singh, R., J. Monk, and F.R. Hung, A Computational Study of the Behavior of the Ionic Liquid [BMIM+][PF6-] Confined Inside Multiwalled Carbon Nanotubes. The Journal of Physical Chemistry C, (36): p Gao, J., W.D. Luedtke, and U. Landman, Layering Transitions and Dynamics of Confined Liquid Films. Physical Review Letters, (4): p Largeot, C., et al., Microporous Carbon-Based Electrical Double Layer Capacitor Operating at High Temperature in Ionic Liquid Electrolyte. Electrochemical and Solid- State Letters, (12): p. A174-A Lin, R., et al., Capacitive Energy Storage from -50 to 100 C Using an Ionic Liquid Electrolyte. The Journal of Physical Chemistry Letters, (19): p Frackowiak, E., G. Lota, and J. Pernak, Room-temperature phosphonium ionic liquids for supercapacitor application. Applied Physics Letters, (16): p
123 257. Frolov, A.I., et al., Molecular-scale insights into the mechanisms of ionic liquids interactions with carbon nanotubes. Faraday Discussions. 154(0) Shim, Y., H.J. Kim, and Y. Jung, Graphene-based supercapacitors in the parallel-plate electrode configuration: Ionic liquids versus organic electrolytes. Faraday Discussions. 154(0) Liu, W., et al., Electrochemical behavior of graphene nanosheets in alkylimidazolium tetrafluoroborate ionic liquid electrolytes: influences of organic solvents and the alkyl chains. Journal of Materials Chemistry, (35) Bester-Rogac, M., et al., Association of ionic liquids in solution: a combined dielectric and conductivity study of [bmim][cl] in water and in acetonitrile. Physical Chemistry Chemical Physics, (39): p Nikitin, A.M. and A.P. Lyubartsev, New six-site acetonitrile model for simulations of liquid acetonitrile and its aqueous mixtures. Journal of computational chemistry, (12): p Wu, X., et al., Molecular dynamics simulation of room-temperature ionic liquid mixture of [bmim][bf4] and acetonitrile by a refined force field. Physical Chemistry Chemical Physics, (14): p Santiso, E.E. and B.L. Trout, A general set of order parameters for molecular crystals. The Journal of Chemical Physics, (6): p Santiso, E.E., Personal communication, Phillips, J.C., et al., Scalable molecular dynamics with NAMD. Journal of computational chemistry, (16): p < (last accessed on Mar. 02, 2010) Feng, G., et al., Atomistic Insight on the Charging Energetics in Subnanometer Pore Supercapacitors. The Journal of Physical Chemistry C, (41): p
124 268. Pinilla, C., et al., Structure and Dynamics of a Confined Ionic Liquid. Topics of Relevance to Dye-Sensitized Solar Cells. The Journal of Physical Chemistry B, (38): p
125 APPENDIX A: PERMISSION LETTERS A.1 Permission for Chapter 2 118
Molecular Simula-on studies of Organic salts inside carbon nanopores Harsha Dissanayake Dr. Francisco R. Hung Dr. Joshua D.
 Molecular Simula-on studies of Organic salts inside carbon nanopores Harsha Dissanayake Dr. Francisco R. Hung Dr. Joshua D. Monk Ramesh Singh Cain Department of Chemical Engineering, Louisiana State University,
Molecular Simula-on studies of Organic salts inside carbon nanopores Harsha Dissanayake Dr. Francisco R. Hung Dr. Joshua D. Monk Ramesh Singh Cain Department of Chemical Engineering, Louisiana State University,
Materials and Structural Design for Advanced Energy Storage Devices
 Materials and Structural Design for Advanced Energy Storage Devices Imran Shakir Sustainable Energy Technologies Center (SET) King Saud University Saudi Arabia Specific Power (W/kg) Introduction and Motivation
Materials and Structural Design for Advanced Energy Storage Devices Imran Shakir Sustainable Energy Technologies Center (SET) King Saud University Saudi Arabia Specific Power (W/kg) Introduction and Motivation
INNOVATIVE MATERIALS FOR RESEARCH AND INDUSTRY. Elena-Oana CROITORU 1
 INNOVATIVE MATERIALS FOR RESEARCH AND INDUSTRY Elena-Oana CROITORU 1 ABSTRACT Research, development and implementation of products and innovative technologies that aim to reduce or eliminate the use and
INNOVATIVE MATERIALS FOR RESEARCH AND INDUSTRY Elena-Oana CROITORU 1 ABSTRACT Research, development and implementation of products and innovative technologies that aim to reduce or eliminate the use and
The micro-properties of [hmpy+] [Tf 2 N-] Ionic liquid: a simulation. study. 1. Introduction
![The micro-properties of [hmpy+] [Tf 2 N-] Ionic liquid: a simulation. study. 1. Introduction The micro-properties of [hmpy+] [Tf 2 N-] Ionic liquid: a simulation. study. 1. Introduction](/thumbs/72/66941683.jpg) ISBN 978-1-84626-081-0 Proceedings of the 2010 International Conference on Application of Mathematics and Physics Volume 1: Advances on Space Weather, Meteorology and Applied Physics Nanjing, P. R. China,
ISBN 978-1-84626-081-0 Proceedings of the 2010 International Conference on Application of Mathematics and Physics Volume 1: Advances on Space Weather, Meteorology and Applied Physics Nanjing, P. R. China,
Molecular Dynamics Simulation of the Structure, Dynamics and Crystallization of Ionic Liquids under Confinement and Low Temperature
 Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2015 Molecular Dynamics Simulation of the Structure, Dynamics and Crystallization of Ionic Liquids under Confinement
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2015 Molecular Dynamics Simulation of the Structure, Dynamics and Crystallization of Ionic Liquids under Confinement
Electrophoretic Deposition. - process in which particles, suspended in a liquid medium, migrate in an electric field and deposit on an electrode
 Electrophoretic Deposition - process in which particles, suspended in a liquid medium, migrate in an electric field and deposit on an electrode no redox differs from electrolytic in several ways deposit
Electrophoretic Deposition - process in which particles, suspended in a liquid medium, migrate in an electric field and deposit on an electrode no redox differs from electrolytic in several ways deposit
Electrolyte Concentration Dependence of Ion Transport through Nanochannels
 Electrolyte Concentration Dependence of Ion Transport through Nanochannels Murat Bakirci mbaki001@odu.edu Yunus Erkaya yerka001@odu.edu ABSTRACT The magnitude of current through a conical nanochannel filled
Electrolyte Concentration Dependence of Ion Transport through Nanochannels Murat Bakirci mbaki001@odu.edu Yunus Erkaya yerka001@odu.edu ABSTRACT The magnitude of current through a conical nanochannel filled
Chapter 6 Magnetic nanoparticles
 Chapter 6 Magnetic nanoparticles Magnetic nanoparticles (MNPs) are a class of nanoparticle which can be manipulated using magnetic field gradients. Such particles commonly consist of magnetic elements
Chapter 6 Magnetic nanoparticles Magnetic nanoparticles (MNPs) are a class of nanoparticle which can be manipulated using magnetic field gradients. Such particles commonly consist of magnetic elements
Ion-Gated Gas Separation through Porous Graphene
 Online Supporting Information for: Ion-Gated Gas Separation through Porous Graphene Ziqi Tian, Shannon M. Mahurin, Sheng Dai,*,, and De-en Jiang *, Department of Chemistry, University of California, Riverside,
Online Supporting Information for: Ion-Gated Gas Separation through Porous Graphene Ziqi Tian, Shannon M. Mahurin, Sheng Dai,*,, and De-en Jiang *, Department of Chemistry, University of California, Riverside,
Chapter - 8. Summary and Conclusion
 Chapter - 8 Summary and Conclusion The present research explains the synthesis process of two transition metal oxide semiconductors SnO 2 and V 2 O 5 thin films with different morphologies and studies
Chapter - 8 Summary and Conclusion The present research explains the synthesis process of two transition metal oxide semiconductors SnO 2 and V 2 O 5 thin films with different morphologies and studies
Chapter 5. Effects of Photonic Crystal Band Gap on Rotation and Deformation of Hollow Te Rods in Triangular Lattice
 Chapter 5 Effects of Photonic Crystal Band Gap on Rotation and Deformation of Hollow Te Rods in Triangular Lattice In chapter 3 and 4, we have demonstrated that the deformed rods, rotational rods and perturbation
Chapter 5 Effects of Photonic Crystal Band Gap on Rotation and Deformation of Hollow Te Rods in Triangular Lattice In chapter 3 and 4, we have demonstrated that the deformed rods, rotational rods and perturbation
CHAPTER 4. SYNTHESIS, CHARACTERIZATION OF TiO 2 NANOTUBES AND THEIR APPLICATION IN DYE SENSITIZED SOLAR CELL
 93 CHAPTER 4 SYNTHESIS, CHARACTERIZATION OF TiO 2 NANOTUBES AND THEIR APPLICATION IN DYE SENSITIZED SOLAR CELL 4.1 INTRODUCTION TiO 2 -derived nanotubes are expected to be applicable for several applications,
93 CHAPTER 4 SYNTHESIS, CHARACTERIZATION OF TiO 2 NANOTUBES AND THEIR APPLICATION IN DYE SENSITIZED SOLAR CELL 4.1 INTRODUCTION TiO 2 -derived nanotubes are expected to be applicable for several applications,
Seminars in Nanosystems - I
 Seminars in Nanosystems - I Winter Semester 2011/2012 Dr. Emanuela Margapoti Emanuela.Margapoti@wsi.tum.de Dr. Gregor Koblmüller Gregor.Koblmueller@wsi.tum.de Seminar Room at ZNN 1 floor Topics of the
Seminars in Nanosystems - I Winter Semester 2011/2012 Dr. Emanuela Margapoti Emanuela.Margapoti@wsi.tum.de Dr. Gregor Koblmüller Gregor.Koblmueller@wsi.tum.de Seminar Room at ZNN 1 floor Topics of the
Research to Improve Photovoltaic (PV) Cell Efficiency by Hybrid Combination of PV and Thermoelectric Cell Elements.
 UNIVERSITY OF CENTRAL FLORIDA Research to Improve Photovoltaic (PV) Cell Efficiency by Hybrid Combination of PV and Thermoelectric Cell Elements. Page 129 PI: Nicoleta Sorloaica-Hickman, Robert Reedy Students:
UNIVERSITY OF CENTRAL FLORIDA Research to Improve Photovoltaic (PV) Cell Efficiency by Hybrid Combination of PV and Thermoelectric Cell Elements. Page 129 PI: Nicoleta Sorloaica-Hickman, Robert Reedy Students:
electrodeposition is a special case of electrolysis where the result is deposition of solid material on an electrode surface.
 Electrochemical Methods Electrochemical Deposition is known as electrodeposition - see CHEM* 1050 - electrolysis electrodeposition is a special case of electrolysis where the result is deposition of solid
Electrochemical Methods Electrochemical Deposition is known as electrodeposition - see CHEM* 1050 - electrolysis electrodeposition is a special case of electrolysis where the result is deposition of solid
Boyan Iliev, Marcin Smiglak, Anna Świerczyńska, Thomas Schubert. IOLITEC Ionic Liquids Technologies GmbH Salzstraße 184 D Heilbronn
 ILSEPT, Sitges, Spain, September 6th Boyan Iliev, Marcin Smiglak, Anna Świerczyńska, Thomas Schubert IOLITEC Ionic Liquids Technologies GmbH Salzstraße 184 D-74076 Heilbronn Outline 1 Introduction 2 Solubility
ILSEPT, Sitges, Spain, September 6th Boyan Iliev, Marcin Smiglak, Anna Świerczyńska, Thomas Schubert IOLITEC Ionic Liquids Technologies GmbH Salzstraße 184 D-74076 Heilbronn Outline 1 Introduction 2 Solubility
Optimization of MnO2 Electrodeposits using Graphenated Carbon Nanotube Electrodes for Supercapacitors
 Optimization of MnO2 Electrodeposits using Graphenated Carbon Nanotube Electrodes for Supercapacitors Waleed Nusrat, 100425398 PHY 3090U Material Science Thursday April 9 th 2015 Researchers optimize the
Optimization of MnO2 Electrodeposits using Graphenated Carbon Nanotube Electrodes for Supercapacitors Waleed Nusrat, 100425398 PHY 3090U Material Science Thursday April 9 th 2015 Researchers optimize the
The first three categories are considered a bottom-up approach while lithography is a topdown
 Nanowires and Nanorods One-dimensional structures have been called in different ways: nanowires, nanorod, fibers of fibrils, whiskers, etc. The common characteristic of these structures is that all they
Nanowires and Nanorods One-dimensional structures have been called in different ways: nanowires, nanorod, fibers of fibrils, whiskers, etc. The common characteristic of these structures is that all they
Optimization of new Chinese Remainder theorems using special moduli sets
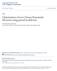 Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2010 Optimization of new Chinese Remainder theorems using special moduli sets Narendran Narayanaswamy Louisiana State
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2010 Optimization of new Chinese Remainder theorems using special moduli sets Narendran Narayanaswamy Louisiana State
The Effect of Viscosity and Ion Size Copley, Hubbard, Maisano on the Transduction of Ionic. Polymer Metal Composite
 The Effect of Viscosity and Ion Size Copley, Hubbard, Maisano on the Transduction of Ionic Keywords ionic polymer metal Polymer Metal Composite composite (IPMC), actuators, ionic liquids, Actuators viscosity
The Effect of Viscosity and Ion Size Copley, Hubbard, Maisano on the Transduction of Ionic Keywords ionic polymer metal Polymer Metal Composite composite (IPMC), actuators, ionic liquids, Actuators viscosity
Adsorption Processes. Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad
 Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Physics of disordered materials. Gunnar A. Niklasson Solid State Physics Department of Engineering Sciences Uppsala University
 Physics of disordered materials Gunnar A. Niklasson Solid State Physics Department of Engineering Sciences Uppsala University Course plan Familiarity with the basic description of disordered structures
Physics of disordered materials Gunnar A. Niklasson Solid State Physics Department of Engineering Sciences Uppsala University Course plan Familiarity with the basic description of disordered structures
Lecture 3 Charged interfaces
 Lecture 3 Charged interfaces rigin of Surface Charge Immersion of some materials in an electrolyte solution. Two mechanisms can operate. (1) Dissociation of surface sites. H H H H H M M M +H () Adsorption
Lecture 3 Charged interfaces rigin of Surface Charge Immersion of some materials in an electrolyte solution. Two mechanisms can operate. (1) Dissociation of surface sites. H H H H H M M M +H () Adsorption
In today s lecture, we will cover:
 In today s lecture, we will cover: Metal and Metal oxide Nanoparticles Semiconductor Nanocrystals Carbon Nanotubes 1 Week 2: Nanoparticles Goals for this section Develop an understanding of the physical
In today s lecture, we will cover: Metal and Metal oxide Nanoparticles Semiconductor Nanocrystals Carbon Nanotubes 1 Week 2: Nanoparticles Goals for this section Develop an understanding of the physical
INVESTIGATION OF THE ABSORPTION OF CO 2 IN IONIC LIQUID. Kalyan Dhar 1 * and Syed Fahim 1
 Bangladesh J. Sci. Res. 29(1): 41-46, 2016 (June) INVESTIGATION OF THE ABSORPTION OF CO 2 IN IONIC LIQUID Kalyan Dhar 1 * and Syed Fahim 1 Dept. di Chimica Materiali e Ingegneria chimica G. Natta, Politecnico
Bangladesh J. Sci. Res. 29(1): 41-46, 2016 (June) INVESTIGATION OF THE ABSORPTION OF CO 2 IN IONIC LIQUID Kalyan Dhar 1 * and Syed Fahim 1 Dept. di Chimica Materiali e Ingegneria chimica G. Natta, Politecnico
What Does an Ionic Liquid Surface Really Look Like? Unprecedented Details from Molecular Simulations
 Page 1 of 7 PCCP Dynamic Article Links Cite this: DOI: 10.1039/c0xx00000x www.rsc.org/xxxxxx COMMUNICATION What Does an Ionic Liquid Surface Really Look Like? Unprecedented Details from Molecular Simulations
Page 1 of 7 PCCP Dynamic Article Links Cite this: DOI: 10.1039/c0xx00000x www.rsc.org/xxxxxx COMMUNICATION What Does an Ionic Liquid Surface Really Look Like? Unprecedented Details from Molecular Simulations
An Introduction to Ionic Liquids. Michael Freemantle. RSC Publishing
 An to Ionic Liquids Michael Freemantle RSC Publishing Chapter 1 1 1.1 Definition of Ionic Liquids 1 1.2 Synonyms 1 1.3 Attraction of Ionic Liquids 2 1.4 Cations and Anions 3 1.5 Shorthand Notation for
An to Ionic Liquids Michael Freemantle RSC Publishing Chapter 1 1 1.1 Definition of Ionic Liquids 1 1.2 Synonyms 1 1.3 Attraction of Ionic Liquids 2 1.4 Cations and Anions 3 1.5 Shorthand Notation for
Energetic Ionic Liquids
 Energetic Ionic Liquids The AMPAC-GaTech Partnership fosters research aimed at opening and developing new avenues and compounds that fit the American Pacific Corporation (AMPAC) s potential market. Under
Energetic Ionic Liquids The AMPAC-GaTech Partnership fosters research aimed at opening and developing new avenues and compounds that fit the American Pacific Corporation (AMPAC) s potential market. Under
NANOMEDICINE. WILEY A John Wiley and Sons, Ltd., Publication DESIGN AND APPLICATIONS OF MAGNETIC NANOMATERIALS, NANOSENSORS AND NANOSYSTEMS
 NANOMEDICINE DESIGN AND APPLICATIONS OF MAGNETIC NANOMATERIALS, NANOSENSORS AND NANOSYSTEMS Vijay K. Varadan Linfeng Chen Jining Xie WILEY A John Wiley and Sons, Ltd., Publication Preface About the Authors
NANOMEDICINE DESIGN AND APPLICATIONS OF MAGNETIC NANOMATERIALS, NANOSENSORS AND NANOSYSTEMS Vijay K. Varadan Linfeng Chen Jining Xie WILEY A John Wiley and Sons, Ltd., Publication Preface About the Authors
The goal of this project is to enhance the power density and lowtemperature efficiency of solid oxide fuel cells (SOFC) manufactured by atomic layer
 Stanford University Michael Shandalov1, Shriram Ramanathan2, Changhyun Ko2 and Paul McIntyre1 1Department of Materials Science and Engineering, Stanford University 2Division of Engineering and Applied
Stanford University Michael Shandalov1, Shriram Ramanathan2, Changhyun Ko2 and Paul McIntyre1 1Department of Materials Science and Engineering, Stanford University 2Division of Engineering and Applied
Hands-on : Model Potential Molecular Dynamics
 Hands-on : Model Potential Molecular Dynamics OUTLINE 0. DL_POLY code introduction 0.a Input files 1. THF solvent molecule 1.a Geometry optimization 1.b NVE/NVT dynamics 2. Liquid THF 2.a Equilibration
Hands-on : Model Potential Molecular Dynamics OUTLINE 0. DL_POLY code introduction 0.a Input files 1. THF solvent molecule 1.a Geometry optimization 1.b NVE/NVT dynamics 2. Liquid THF 2.a Equilibration
Diffusion of Water and Diatomic Oxygen in Poly(3-hexylthiophene) Melt: A Molecular Dynamics Simulation Study
 Diffusion of Water and Diatomic Oxygen in Poly(3-hexylthiophene) Melt: A Molecular Dynamics Simulation Study Julia Deitz, Yeneneh Yimer, and Mesfin Tsige Department of Polymer Science University of Akron
Diffusion of Water and Diatomic Oxygen in Poly(3-hexylthiophene) Melt: A Molecular Dynamics Simulation Study Julia Deitz, Yeneneh Yimer, and Mesfin Tsige Department of Polymer Science University of Akron
Structure. relevant. Example. What atomic. How can the. Research
 Structure property studies; intermetallics and functional oxides In this project you will combine methods like X ray or neutron diffraction that enables determination of the atomic arrangement of crystalline
Structure property studies; intermetallics and functional oxides In this project you will combine methods like X ray or neutron diffraction that enables determination of the atomic arrangement of crystalline
Demystifying Transmission Lines: What are They? Why are They Useful?
 Demystifying Transmission Lines: What are They? Why are They Useful? Purpose of This Note This application note discusses theory and practice of transmission lines. It outlines the necessity of transmission
Demystifying Transmission Lines: What are They? Why are They Useful? Purpose of This Note This application note discusses theory and practice of transmission lines. It outlines the necessity of transmission
quantum dots, metallic nanoparticles, and lanthanide ions doped upconversion
 Chapter 1 Introduction 1.1 Background Nanostructured materials have significantly different characteristics from their bulk counterparts. 1 Inorganic nanoparticles such as semiconductor quantum dots, metallic
Chapter 1 Introduction 1.1 Background Nanostructured materials have significantly different characteristics from their bulk counterparts. 1 Inorganic nanoparticles such as semiconductor quantum dots, metallic
Chapter 2 Mesoporous Silica Mcm 41 Si Mcm 41
 We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer, you have convenient answers with chapter 2 mesoporous
We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer, you have convenient answers with chapter 2 mesoporous
High-energy Hydrogen III Teacher Page
 High-energy Hydrogen III Teacher Page Student Objective The student: will be able to explain how hydrogen can be extracted from water will be able to design and conduct an experiment demonstrating how
High-energy Hydrogen III Teacher Page Student Objective The student: will be able to explain how hydrogen can be extracted from water will be able to design and conduct an experiment demonstrating how
Communicating Research to the General Public
 Communicating Research to the General Public At the March 5, 2010 UW-Madison Chemistry Department Colloquium, the director of the Wisconsin Initiative for Science Literacy (WISL) encouraged all Ph.D. chemistry
Communicating Research to the General Public At the March 5, 2010 UW-Madison Chemistry Department Colloquium, the director of the Wisconsin Initiative for Science Literacy (WISL) encouraged all Ph.D. chemistry
London Dispersion Forces (LDFs) Intermolecular Forces Attractions BETWEEN molecules. London Dispersion Forces (LDFs) London Dispersion Forces (LDFs)
 LIQUIDS / SOLIDS / IMFs Intermolecular Forces (IMFs) Attractions BETWEEN molecules NOT within molecules NOT true bonds weaker attractions Represented by dashed lines Physical properties (melting points,
LIQUIDS / SOLIDS / IMFs Intermolecular Forces (IMFs) Attractions BETWEEN molecules NOT within molecules NOT true bonds weaker attractions Represented by dashed lines Physical properties (melting points,
II.1.4 Nanoengineering of Hybrid Carbon Nanotube-Metal Nanocluster Composite Materials for Hydrogen Storage
 II.1.4 Nanoengineering of Hybrid Carbon Nanotube-Metal Nanocluster Composite Materials for Hydrogen Storage Investigators Kyeongjae (KJ) Cho, Assistant Professor of Mechanical Engineering; Bruce Clemens,
II.1.4 Nanoengineering of Hybrid Carbon Nanotube-Metal Nanocluster Composite Materials for Hydrogen Storage Investigators Kyeongjae (KJ) Cho, Assistant Professor of Mechanical Engineering; Bruce Clemens,
Experimental designs for multiple responses with different models
 Graduate Theses and Dissertations Graduate College 2015 Experimental designs for multiple responses with different models Wilmina Mary Marget Iowa State University Follow this and additional works at:
Graduate Theses and Dissertations Graduate College 2015 Experimental designs for multiple responses with different models Wilmina Mary Marget Iowa State University Follow this and additional works at:
Synthesis of LiFePO 4 Nanostructures for Lithium-Ion Batteries by Electrochemical Deposition
 Synthesis of LiFePO 4 Nanostructures for Lithium-Ion Batteries by Electrochemical Deposition Erika Aragon and Alfredo A. Martinez-Morales Southern California Research Initiative for Solar Energy College
Synthesis of LiFePO 4 Nanostructures for Lithium-Ion Batteries by Electrochemical Deposition Erika Aragon and Alfredo A. Martinez-Morales Southern California Research Initiative for Solar Energy College
Cation Redistribution Upon Water Adsorption in Titanosilicate ETS-10
 Cation Redistribution Upon Water Adsorption in Titanosilicate ETS-10 Anjaiah Nalaparaju, George X. S. Zhao and Jianwen Jiang* Department of Chemical and Bimolecular Engineering National University of Singapore,
Cation Redistribution Upon Water Adsorption in Titanosilicate ETS-10 Anjaiah Nalaparaju, George X. S. Zhao and Jianwen Jiang* Department of Chemical and Bimolecular Engineering National University of Singapore,
Chapter 12 - Modern Materials
 Chapter 12 - Modern Materials 12.1 Semiconductors Inorganic compounds that semiconduct tend to have chemical formulas related to Si and Ge valence electron count of four. Semiconductor conductivity can
Chapter 12 - Modern Materials 12.1 Semiconductors Inorganic compounds that semiconduct tend to have chemical formulas related to Si and Ge valence electron count of four. Semiconductor conductivity can
CITY UNIVERSITY OF HONG KONG. Theoretical Study of Electronic and Electrical Properties of Silicon Nanowires
 CITY UNIVERSITY OF HONG KONG Ë Theoretical Study of Electronic and Electrical Properties of Silicon Nanowires u Ä öä ªqk u{ Submitted to Department of Physics and Materials Science gkö y in Partial Fulfillment
CITY UNIVERSITY OF HONG KONG Ë Theoretical Study of Electronic and Electrical Properties of Silicon Nanowires u Ä öä ªqk u{ Submitted to Department of Physics and Materials Science gkö y in Partial Fulfillment
Chapter 11. Intermolecular Forces and Liquids & Solids
 Chapter 11 Intermolecular Forces and Liquids & Solids The Kinetic Molecular Theory of Liquids & Solids Gases vs. Liquids & Solids difference is distance between molecules Liquids Molecules close together;
Chapter 11 Intermolecular Forces and Liquids & Solids The Kinetic Molecular Theory of Liquids & Solids Gases vs. Liquids & Solids difference is distance between molecules Liquids Molecules close together;
AP* States of Matter & Intermolecular Forces Free Response KEY page 1
 AP* States of Matter & Intermolecular Forces Free Response KEY page 1 1991 a) three points MgCl 2 is ionic and SiCl 4 is covalent. The elctrostatic, interionic forces in MgCl 2 are much stronger than the
AP* States of Matter & Intermolecular Forces Free Response KEY page 1 1991 a) three points MgCl 2 is ionic and SiCl 4 is covalent. The elctrostatic, interionic forces in MgCl 2 are much stronger than the
RESEARCH HIGHLIGHTS. Computationally-guided Design of Polymer Electrolytes
 RESEARCH HIGHLIGHTS From the Resnick Sustainability Institute Graduate Research Fellows at the California Institute of Technology Computationally-guided Design of Polymer Electrolytes Global Significance
RESEARCH HIGHLIGHTS From the Resnick Sustainability Institute Graduate Research Fellows at the California Institute of Technology Computationally-guided Design of Polymer Electrolytes Global Significance
Analysis of the simulation
 Analysis of the simulation Marcus Elstner and Tomáš Kubař January 7, 2014 Thermodynamic properties time averages of thermodynamic quantites correspond to ensemble averages (ergodic theorem) some quantities
Analysis of the simulation Marcus Elstner and Tomáš Kubař January 7, 2014 Thermodynamic properties time averages of thermodynamic quantites correspond to ensemble averages (ergodic theorem) some quantities
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary References
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary References Supplementary Figure 1. SEM images of perovskite single-crystal patterned thin film with
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary References Supplementary Figure 1. SEM images of perovskite single-crystal patterned thin film with
Properties of Compounds
 Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Enhanced Power Systems Through Nanotechnology
 Enhanced Power Systems Through Nanotechnology Applied Power Electronics Conference and Exposition Fort Worth, Texas March 19, 2014 Dale Teeters Chemistry and Biochemistry The University of Tulsa The Movie,
Enhanced Power Systems Through Nanotechnology Applied Power Electronics Conference and Exposition Fort Worth, Texas March 19, 2014 Dale Teeters Chemistry and Biochemistry The University of Tulsa The Movie,
Free volume and Phase Transitions of 1-Butyl-3-Methylimidazolium Based Ionic Liquids: Positron Lifetime
 Free volume and Phase Transitions of 1-Butyl-3-Methylimidazolium Based Ionic Liquids: Positron Lifetime Positron Annihilation Laboratory Yu, Yang Oct. 12th. 211 Outline 2 Introduction to free volume Positron
Free volume and Phase Transitions of 1-Butyl-3-Methylimidazolium Based Ionic Liquids: Positron Lifetime Positron Annihilation Laboratory Yu, Yang Oct. 12th. 211 Outline 2 Introduction to free volume Positron
Amino Acids and Proteins at ZnO-water Interfaces in Molecular Dynamics Simulations: Electronic Supplementary Information
 Amino Acids and Proteins at ZnO-water Interfaces in Molecular Dynamics Simulations: Electronic Supplementary Information Grzegorz Nawrocki and Marek Cieplak Institute of Physics, Polish Academy of Sciences,
Amino Acids and Proteins at ZnO-water Interfaces in Molecular Dynamics Simulations: Electronic Supplementary Information Grzegorz Nawrocki and Marek Cieplak Institute of Physics, Polish Academy of Sciences,
FACULTY OF ENGINEERING ALEXANDRIA UNVERSITY. Solid State lab. Instructors Dr. M. Ismail El-Banna Dr. Mohamed A. El-Shimy TA Noha Hanafy
 FACULTY OF ENGINEERING ALEXANDRIA UNVERSITY Solid State lab Instructors Dr. M. Ismail El-Banna Dr. Mohamed A. El-Shimy TA Noha Hanafy 2017-2018 first term A. Experiments 1- Relationship between the intensity
FACULTY OF ENGINEERING ALEXANDRIA UNVERSITY Solid State lab Instructors Dr. M. Ismail El-Banna Dr. Mohamed A. El-Shimy TA Noha Hanafy 2017-2018 first term A. Experiments 1- Relationship between the intensity
Computer simulation methods (2) Dr. Vania Calandrini
 Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
MATHEMATICAL MODELING OF DISBONDED COATING AND CATHODIC DELAMINATION SYSTEMS KERRY N. ALLAHAR
 MATHEMATICAL MODELING OF DISBONDED COATING AND CATHODIC DELAMINATION SYSTEMS By KERRY N. ALLAHAR A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE
MATHEMATICAL MODELING OF DISBONDED COATING AND CATHODIC DELAMINATION SYSTEMS By KERRY N. ALLAHAR A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE
Contents. Foreword by Darrell H. Reneker
 Table of Foreword by Darrell H. Reneker Preface page xi xiii 1 Introduction 1 1.1 How big is a nanometer? 1 1.2 What is nanotechnology? 1 1.3 Historical development of nanotechnology 2 1.4 Classification
Table of Foreword by Darrell H. Reneker Preface page xi xiii 1 Introduction 1 1.1 How big is a nanometer? 1 1.2 What is nanotechnology? 1 1.3 Historical development of nanotechnology 2 1.4 Classification
Solids. properties & structure
 Solids properties & structure Determining Crystal Structure crystalline solids have a very regular geometric arrangement of their particles the arrangement of the particles and distances between them is
Solids properties & structure Determining Crystal Structure crystalline solids have a very regular geometric arrangement of their particles the arrangement of the particles and distances between them is
Supplementary Information Supplementary Figures
 Supplementary Information Supplementary Figures Supplementary Figure 1 SEM images of the morphologies of Li metal after plating on Cu (1st cycle) from different electrolytes. The current density was 0.5
Supplementary Information Supplementary Figures Supplementary Figure 1 SEM images of the morphologies of Li metal after plating on Cu (1st cycle) from different electrolytes. The current density was 0.5
Chem 1075 Chapter 13 Liquids and Solids Lecture Outline
 Chem 1075 Chapter 13 Liquids and Solids Lecture Outline Slide 2-3 Properties of Liquids Unlike gases, liquids respond dramatically to temperature and pressure changes. We can study the liquid state and
Chem 1075 Chapter 13 Liquids and Solids Lecture Outline Slide 2-3 Properties of Liquids Unlike gases, liquids respond dramatically to temperature and pressure changes. We can study the liquid state and
Small-scale demo, large-scale promise of novel bromine battery 27 June 2014, by Nancy W. Stauffer
 Small-scale demo, large-scale promise of novel bromine battery 27 June 2014, by Nancy W. Stauffer Figure 1 The availability of low-cost, high-capacity energy storage technology could profoundly change
Small-scale demo, large-scale promise of novel bromine battery 27 June 2014, by Nancy W. Stauffer Figure 1 The availability of low-cost, high-capacity energy storage technology could profoundly change
A MOLECULAR DYNAMICS SIMULATION OF A BUBBLE NUCLEATION ON SOLID SURFACE
 A MOLECULAR DYNAMICS SIMULATION OF A BUBBLE NUCLEATION ON SOLID SURFACE Shigeo Maruyama and Tatsuto Kimura Department of Mechanical Engineering The University of Tokyo 7-- Hongo, Bunkyo-ku, Tokyo -866,
A MOLECULAR DYNAMICS SIMULATION OF A BUBBLE NUCLEATION ON SOLID SURFACE Shigeo Maruyama and Tatsuto Kimura Department of Mechanical Engineering The University of Tokyo 7-- Hongo, Bunkyo-ku, Tokyo -866,
Supporting Information for
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Supporting Information for Controllable Growth of High-Quality Metal Oxide/Conducting
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Supporting Information for Controllable Growth of High-Quality Metal Oxide/Conducting
WATER PERMEATION THROUGH GRAPHENE NANOSLIT BY MOLECULAR DYNAMICS SIMULATION
 WATER PERMEATION THROUGH GRAPHENE NANOSLIT BY MOLECULAR DYNAMICS SIMULATION Taro Yamada 1 and Ryosuke Matsuzaki 2 1 Department of Mechanical Engineering, Tokyo University of Science, 2641 Yamazaki, Noda,
WATER PERMEATION THROUGH GRAPHENE NANOSLIT BY MOLECULAR DYNAMICS SIMULATION Taro Yamada 1 and Ryosuke Matsuzaki 2 1 Department of Mechanical Engineering, Tokyo University of Science, 2641 Yamazaki, Noda,
Intermolecular Forces and Liquids and Solids. Chapter 11. Copyright The McGraw Hill Companies, Inc. Permission required for
 Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw Hill Companies, Inc. Permission required for 1 A phase is a homogeneous part of the system in contact with other parts of the
Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw Hill Companies, Inc. Permission required for 1 A phase is a homogeneous part of the system in contact with other parts of the
Effect of Metal Concentration on Shape and Composition Changes in Gold-Silver Bimetallic Systems Md. Jahangir Alam
 Noto-are 15542466: Chemical technology. 2013-07-15. Effect of Metal Concentration on Shape and Composition Changes in Gold-Silver Bimetallic Systems Md. Jahangir Alam Department of Agronomy and Agricultural
Noto-are 15542466: Chemical technology. 2013-07-15. Effect of Metal Concentration on Shape and Composition Changes in Gold-Silver Bimetallic Systems Md. Jahangir Alam Department of Agronomy and Agricultural
SEPARATION BY BARRIER
 SEPARATION BY BARRIER SEPARATION BY BARRIER Phase 1 Feed Barrier Phase 2 Separation by barrier uses a barrier which restricts and/or enhances the movement of certain chemical species with respect to other
SEPARATION BY BARRIER SEPARATION BY BARRIER Phase 1 Feed Barrier Phase 2 Separation by barrier uses a barrier which restricts and/or enhances the movement of certain chemical species with respect to other
Molecular modelling of ionic liquids in the ordered mesoporous carbon CMK-5
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln NASA Publications National Aeronautics and Space Administration 2015 Molecular modelling of ionic liquids in the ordered
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln NASA Publications National Aeronautics and Space Administration 2015 Molecular modelling of ionic liquids in the ordered
Hydrogen adsorption by graphite intercalation compounds
 62 Chapter 4 Hydrogen adsorption by graphite intercalation compounds 4.1 Introduction Understanding the thermodynamics of H 2 adsorption in chemically modified carbons remains an important area of fundamental
62 Chapter 4 Hydrogen adsorption by graphite intercalation compounds 4.1 Introduction Understanding the thermodynamics of H 2 adsorption in chemically modified carbons remains an important area of fundamental
Numerical Modeling of the Bistability of Electrolyte Transport in Conical Nanopores
 Numerical Modeling of the Bistability of Electrolyte Transport in Conical Nanopores Long Luo, Robert P. Johnson, Henry S. White * Department of Chemistry, University of Utah, Salt Lake City, UT 84112,
Numerical Modeling of the Bistability of Electrolyte Transport in Conical Nanopores Long Luo, Robert P. Johnson, Henry S. White * Department of Chemistry, University of Utah, Salt Lake City, UT 84112,
Nanowires and nanorods
 Nanowires and nanorods One-dimensional structures have been called in different ways: nanowires, nanorod, fibers of fibrils, whiskers, etc. These structures have a nanometer size in one of the dimensions,
Nanowires and nanorods One-dimensional structures have been called in different ways: nanowires, nanorod, fibers of fibrils, whiskers, etc. These structures have a nanometer size in one of the dimensions,
Shear Properties and Wrinkling Behaviors of Finite Sized Graphene
 Shear Properties and Wrinkling Behaviors of Finite Sized Graphene Kyoungmin Min, Namjung Kim and Ravi Bhadauria May 10, 2010 Abstract In this project, we investigate the shear properties of finite sized
Shear Properties and Wrinkling Behaviors of Finite Sized Graphene Kyoungmin Min, Namjung Kim and Ravi Bhadauria May 10, 2010 Abstract In this project, we investigate the shear properties of finite sized
Fabrication Methods: Chapter 4. Often two methods are typical. Top Down Bottom up. Begins with atoms or molecules. Begins with bulk materials
 Fabrication Methods: Chapter 4 Often two methods are typical Top Down Bottom up Begins with bulk materials Begins with atoms or molecules Reduced in size to nano By thermal, physical Chemical, electrochemical
Fabrication Methods: Chapter 4 Often two methods are typical Top Down Bottom up Begins with bulk materials Begins with atoms or molecules Reduced in size to nano By thermal, physical Chemical, electrochemical
Chapter 10: Liquids, Solids, and Phase Changes
 Chapter 10: Liquids, Solids, and Phase Changes In-chapter exercises: 10.1 10.6, 10.11; End-of-chapter Problems: 10.26, 10.31, 10.32, 10.33, 10.34, 10.35, 10.36, 10.39, 10.40, 10.42, 10.44, 10.45, 10.66,
Chapter 10: Liquids, Solids, and Phase Changes In-chapter exercises: 10.1 10.6, 10.11; End-of-chapter Problems: 10.26, 10.31, 10.32, 10.33, 10.34, 10.35, 10.36, 10.39, 10.40, 10.42, 10.44, 10.45, 10.66,
Synthetic Neural Interface via electrically controlled ion pump
 Synthetic Neural Interface via electrically controlled ion pump Presented By: Jason Farkas Electrical Engineering Ventura Community College Mentor: Samuel Beach Faculty Advisor: Luke Theogarajan The Big
Synthetic Neural Interface via electrically controlled ion pump Presented By: Jason Farkas Electrical Engineering Ventura Community College Mentor: Samuel Beach Faculty Advisor: Luke Theogarajan The Big
Cover Page. The handle holds various files of this Leiden University dissertation
 Cover Page The handle http://hdl.handle.net/1887/29891 holds various files of this Leiden University dissertation Author: Roobol, Sander Bas Title: The structure of a working catalyst : from flat surfaces
Cover Page The handle http://hdl.handle.net/1887/29891 holds various files of this Leiden University dissertation Author: Roobol, Sander Bas Title: The structure of a working catalyst : from flat surfaces
Chapter 10. Lesson Starter. Why did you not smell the odor of the vapor immediately? Explain this event in terms of the motion of molecules.
 Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
for sodium ion (Na + )
 3.4 Unit 2 Chemistry 2 Throughout this unit candidates will be expected to write word equations for reactions specified. Higher tier candidates will also be expected to write and balance symbol equations
3.4 Unit 2 Chemistry 2 Throughout this unit candidates will be expected to write word equations for reactions specified. Higher tier candidates will also be expected to write and balance symbol equations
Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia
 University of Groningen Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia IMPORTANT NOTE: You are advised to consult the publisher's version
University of Groningen Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia IMPORTANT NOTE: You are advised to consult the publisher's version
Chapter 6 Thesis Highlights and Scope for Future
 Chapter 6 Thesis Highlights and Scope for Future Summary: Chapter 6 Developing intelligent or smart nanomaterials with multiple functions and tailoring their properties at the molecular level is one of
Chapter 6 Thesis Highlights and Scope for Future Summary: Chapter 6 Developing intelligent or smart nanomaterials with multiple functions and tailoring their properties at the molecular level is one of
Stage 1. Chemistry. Written by. Mr Ian Kershaw. BSc Dip Ed
 Stage 1 Written by Chemistry Mr Ian Kershaw BSc Dip Ed The Author Ian Kershaw B.Sc., Dip.Ed. Ian has taught senior Chemistry since 1976. He was a member of the SSABSA Subject Advisory Committee for some
Stage 1 Written by Chemistry Mr Ian Kershaw BSc Dip Ed The Author Ian Kershaw B.Sc., Dip.Ed. Ian has taught senior Chemistry since 1976. He was a member of the SSABSA Subject Advisory Committee for some
Nanostrukturphysik (Nanostructure Physics)
 Nanostrukturphysik (Nanostructure Physics) Prof. Yong Lei & Dr. Yang Xu Fachgebiet 3D-Nanostrukturierung, Institut für Physik Contact: yong.lei@tu-ilmenau.de; yang.xu@tu-ilmenau.de Office: Unterpoerlitzer
Nanostrukturphysik (Nanostructure Physics) Prof. Yong Lei & Dr. Yang Xu Fachgebiet 3D-Nanostrukturierung, Institut für Physik Contact: yong.lei@tu-ilmenau.de; yang.xu@tu-ilmenau.de Office: Unterpoerlitzer
Heterogeneous nucleation from a supercooled ionic liquid on a carbon surface
 Heterogeneous nucleation from a supercooled ionic liquid on a carbon surface Xiaoxia He, 1,* Yan Shen, 1,*, Francisco R. Hung 1,2,, and Erik E. Santiso 3, 1 Cain Department of Chemical Engineering, Louisiana
Heterogeneous nucleation from a supercooled ionic liquid on a carbon surface Xiaoxia He, 1,* Yan Shen, 1,*, Francisco R. Hung 1,2,, and Erik E. Santiso 3, 1 Cain Department of Chemical Engineering, Louisiana
Matter mass space atoms solid, a liquid, a gas, or plasm elements compounds mixtures atoms Compounds chemically combined Mixtures not chemically
 SOL PS.2 THE NATURE OF MATTER Matter is anything that has mass and occupies space. All matter is made up of small particles called atoms. Matter can exist as a solid, a liquid, a gas, or plasma. Matter
SOL PS.2 THE NATURE OF MATTER Matter is anything that has mass and occupies space. All matter is made up of small particles called atoms. Matter can exist as a solid, a liquid, a gas, or plasma. Matter
Intermolecular Forces and Liquids and Solids
 PowerPoint Lecture Presentation by J. David Robertson University of Missouri Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
PowerPoint Lecture Presentation by J. David Robertson University of Missouri Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Multiscale modeling and simulation of shock wave propagation
 University of Iowa Iowa Research Online Theses and Dissertations Spring 2013 Multiscale modeling and simulation of shock wave propagation Cory Nelsen University of Iowa Copyright 2013 Cory Nelsen This
University of Iowa Iowa Research Online Theses and Dissertations Spring 2013 Multiscale modeling and simulation of shock wave propagation Cory Nelsen University of Iowa Copyright 2013 Cory Nelsen This
arxiv: v1 [physics.chem-ph] 8 Mar 2010
![arxiv: v1 [physics.chem-ph] 8 Mar 2010 arxiv: v1 [physics.chem-ph] 8 Mar 2010](/thumbs/88/116239288.jpg) arxiv:1003.1678v1 [physics.chem-ph] 8 Mar 2010 A new battery-charging method suggested by molecular dynamics simulations Ibrahim Abou Hamad 1,, M. A. Novotny 1,2, D. Wipf 3, and P. A. Rikvold 4 1 HPC 2,
arxiv:1003.1678v1 [physics.chem-ph] 8 Mar 2010 A new battery-charging method suggested by molecular dynamics simulations Ibrahim Abou Hamad 1,, M. A. Novotny 1,2, D. Wipf 3, and P. A. Rikvold 4 1 HPC 2,
RW Session ID = MSTCHEM1 Intermolecular Forces
 RW Session ID = MSTCHEM1 Intermolecular Forces Sections 9.4, 11.3-11.4 Intermolecular Forces Attractive forces between molecules due to charges, partial charges, and temporary charges Higher charge, stronger
RW Session ID = MSTCHEM1 Intermolecular Forces Sections 9.4, 11.3-11.4 Intermolecular Forces Attractive forces between molecules due to charges, partial charges, and temporary charges Higher charge, stronger
Sunyia Hussain 06/15/2012 ChE210D final project. Hydration Dynamics at a Hydrophobic Surface. Abstract:
 Hydration Dynamics at a Hydrophobic Surface Sunyia Hussain 6/15/212 ChE21D final project Abstract: Water is the universal solvent of life, crucial to the function of all biomolecules. Proteins, membranes,
Hydration Dynamics at a Hydrophobic Surface Sunyia Hussain 6/15/212 ChE21D final project Abstract: Water is the universal solvent of life, crucial to the function of all biomolecules. Proteins, membranes,
m WILEY- ADSORBENTS: FUNDAMENTALS AND APPLICATIONS Ralph T. Yang Dwight F. Benton Professor of Chemical Engineering University of Michigan
 ADSORBENTS: FUNDAMENTALS AND APPLICATIONS Ralph T. Yang Dwight F. Benton Professor of Chemical Engineering University of Michigan m WILEY- INTERSCIENCE A JOHN WILEY & SONS, INC., PUBLICATION Preface xi
ADSORBENTS: FUNDAMENTALS AND APPLICATIONS Ralph T. Yang Dwight F. Benton Professor of Chemical Engineering University of Michigan m WILEY- INTERSCIENCE A JOHN WILEY & SONS, INC., PUBLICATION Preface xi
D DAVID PUBLISHING. Study the Synthesis Parameter of Tin Oxide Nanostructure. 1. Introduction. 2. Experiment
 Journal of Materials Science and Engineering B 5 (9-10) (2015) 353-360 doi: 10.17265/2161-6221/2015.9-10.003 D DAVID PUBLISHING Study the Synthesis Parameter of Tin Oxide Nanostructure Gyanendra Prakash
Journal of Materials Science and Engineering B 5 (9-10) (2015) 353-360 doi: 10.17265/2161-6221/2015.9-10.003 D DAVID PUBLISHING Study the Synthesis Parameter of Tin Oxide Nanostructure Gyanendra Prakash
In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium salt to produce potassium
 Q1. This question is about potassium. (a) Humphrey Davy was a professor of chemistry. In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium
Q1. This question is about potassium. (a) Humphrey Davy was a professor of chemistry. In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium
Structural order in glassy water
 Structural order in glassy water Nicolas Giovambattista, 1 Pablo G. Debenedetti, 1 Francesco Sciortino, 2 and H. Eugene Stanley 3 1 Department of Chemical Engineering, Princeton University, Princeton,
Structural order in glassy water Nicolas Giovambattista, 1 Pablo G. Debenedetti, 1 Francesco Sciortino, 2 and H. Eugene Stanley 3 1 Department of Chemical Engineering, Princeton University, Princeton,
Figure 2: Simulation results of concentration by line scan along the x-axis at different partitioning coefficients after 0.48 ms.
 Executive Summary One of the critical hurdles of the success of many clean energy technologies is energy storage. For this, new and advanced computational tools to predict battery properties are very important.
Executive Summary One of the critical hurdles of the success of many clean energy technologies is energy storage. For this, new and advanced computational tools to predict battery properties are very important.
PEROVSKITE STRUCTURES AND ELECRICAL COMPONENTS
 PEROVSKITE STRUCTURES AND ELECRICAL COMPONENTS Subject Area(s) Physical Science, Chemistry Associated Unit Energy, energy resources, physical and chemical structures Lesson Title Using perovskite structures
PEROVSKITE STRUCTURES AND ELECRICAL COMPONENTS Subject Area(s) Physical Science, Chemistry Associated Unit Energy, energy resources, physical and chemical structures Lesson Title Using perovskite structures
CHAPTER 4 CHEMICAL MODIFICATION OF ACTIVATED CARBON CLOTH FOR POTENTIAL USE AS ELECTRODES IN CAPACITIVE DEIONIZATION PROCESS
 CHAPTER 4 CHEMICAL MODIFICATION OF ACTIVATED CARBON CLOTH FOR POTENTIAL USE AS ELECTRODES IN CAPACITIVE DEIONIZATION PROCESS 4.1 INTRODUCTION Capacitive deionization (CDI) is one of the promising energy
CHAPTER 4 CHEMICAL MODIFICATION OF ACTIVATED CARBON CLOTH FOR POTENTIAL USE AS ELECTRODES IN CAPACITIVE DEIONIZATION PROCESS 4.1 INTRODUCTION Capacitive deionization (CDI) is one of the promising energy
Nanoparticles, nanorods, nanowires
 Nanoparticles, nanorods, nanowires Nanoparticles, nanocrystals, nanospheres, quantum dots, etc. Drugs, proteins, etc. Nanorods, nanowires. Optical and electronic properties. Organization using biomolecules.
Nanoparticles, nanorods, nanowires Nanoparticles, nanocrystals, nanospheres, quantum dots, etc. Drugs, proteins, etc. Nanorods, nanowires. Optical and electronic properties. Organization using biomolecules.
Nanotechnology Fabrication Methods.
 Nanotechnology Fabrication Methods. 10 / 05 / 2016 1 Summary: 1.Introduction to Nanotechnology:...3 2.Nanotechnology Fabrication Methods:...5 2.1.Top-down Methods:...7 2.2.Bottom-up Methods:...16 3.Conclusions:...19
Nanotechnology Fabrication Methods. 10 / 05 / 2016 1 Summary: 1.Introduction to Nanotechnology:...3 2.Nanotechnology Fabrication Methods:...5 2.1.Top-down Methods:...7 2.2.Bottom-up Methods:...16 3.Conclusions:...19
Thomas Roussel, Roland J.-M. Pellenq, Christophe Bichara. CRMC-N CNRS, Campus de Luminy, Marseille, cedex 09, France. Abstract.
 A GRAND CANONICAL MONTE-CARLO STUDY OF H ADSORPTION IN PRISTINE AND Li-DOPED CARBON REPLICAS OF FAUJASITE ZEOLITE Thomas Roussel, Roland J.-M. Pellenq, Christophe Bichara CRMC-N CNRS, Campus de Luminy,
A GRAND CANONICAL MONTE-CARLO STUDY OF H ADSORPTION IN PRISTINE AND Li-DOPED CARBON REPLICAS OF FAUJASITE ZEOLITE Thomas Roussel, Roland J.-M. Pellenq, Christophe Bichara CRMC-N CNRS, Campus de Luminy,
