COLUMN SELECTION OVERVIEW
|
|
|
- Antony Carson
- 5 years ago
- Views:
Transcription
1 COLUMN SELECTION OVERVIEW 30
2 COLUMN SELECTION OVERVIEW Making the right column choice At Canadian Life Science we understand that the column is an important piece of the puzzle and we therefore place paramount importance on helping you choose the right column for your application. Since we opened in 1997, we have greatly broadened our expertise and today we have the complete spectrum of ingredients for your critical analysis. In following the history of irregular, irreproducible 10µm and larger particle sized silica in the late 60ʼs to modern highly reproducible silica manufacturing with a % purity, we can better understand silica behaviour and applicability. Most applications can, by choosing the right column and conditions, be solved. increased number of applications in our catalogue. We demonstrate this by an Often you have challenging compounds which create a need for different, alternative chemistries. As the experts, we can provide you with an excellent choice of product ranging from TiO 2, fluorinated phases, mixed-mode, HILIC, newly developed columns for high ph as well as traditional phase columns. For your chiral applications we provide the widest range of columns available in the Canadian market. Our chiral columns cover 98% of the available phases. We provide polymeric bonded phases for wider ph range and size exclusion chromatography. Our column selection overview gives you an excellent broad range of reproducible, quality, robust phases with pages indicating which column is applicable for your analysis based on a variety of criteria including molecular weight, polarity and hydrophobicity. Discover the benefits of dealing with the column experts - contact us today to help solve your difficult separation! View our catalogue online at To search products or order online, click on the Webstore link found on our website. Not registered? Please call us toll free at to find out how! Technical Desk - tede@lifescience.ca Sales - sales@lifescience.ca General information - info@lifescience.ca 31
3 COLUMN SELECTION OVERVIEW Base Material SILICA is the most popular base material. It has a high physical strength and a surface which is easily chemically modified to give phases suitable for use in a broad range of HPLC modes. However silicas dissolve in water at ph 6.5 whilst bonded silicas are usually unstable at ph 2.5. Newer bonded silicas may have an extended ph range of POLYMERIC materials have minimal ph restrictions but are less physically stable and exhibit lower separation efficiencies than silica for small molecules. For large molecules such as proteins or synthetic polymers their performance is comparable to that of silica based materials. TITANIA (TiO 2 ) is stable over a wide ph range and at elevated temperatures. In contrast to silica, the surface of titania is alkaline, which can be beneficial in the analysis of phosphopeptides and basic drugs, but separation efficiencies are generally lower. ALUMINA has greater ph stability than silica but cannot be easily chemically modified. Separation Mode See next page for Selection Guide. For some molecules, more than one technique may be appropriate Selection of the appropriate chromatographic separation mode is guided by the soluteʼs molecular size and polarity. SeQuant from EMD Chemicals Inc. Contact us for application and column selection advice. Toll-free:
4 COLUMN SELECTION OVERVIEW HPLC COLUMN SELECTION TREE Sample MW Sample Solubility Separation Mode Our Recommended Column Page Hexane- Soluble Normal Phase Adsorption ACE Silica Inertsil SIL-100Å Nucleodur SiOH MW<5000 Estimate MW of Sample Organic- Soluble Aqueous- Soluble Methanol Methanol/H 2 O Soluble THF-Soluble Non Ionic Ionic Normal Phase Bonded Reversed Phase Bonded Gel Permeation Chromatography (GPC) Reversed Phase Reversed Phase Ion Pairing/Suppression Ion-Exchange ACE CN Inertsil CN-3, DIOL Inertsil NH 2 Nucleodur NH 2,CN Jordi Organic GPC Shodex Organic GPC ACE C8, C18, C18-AR HALO C18 Inertsil ODS-4, ODS-3 Inertsil C8-4, ODS-SP, ODS-EP Nucleodur Isis,Gravity,Pyramid Nucleodur Sphinx RP, C18ec Nucleodur C8 Gravity, C8e ACE C8, C18 HALO C18 Inertsil C8, C8-3 Inertsil ODS-3, ODS-4 Nucleodur C18 Gravity, C8 Gravity Inertsil AX, CX Jordi SAX ACE C8, C18, C18-AR Epic C18, SD, Aquasep HALO C Inertsil ODS-4, ODS-3 Inertsil C8-4, C8-3, ODS-SP Inertsil ODS-EP, ODS-P 156 Nucleodur Isis,Gravity,Pyramid 188 Nucleodur Sphinx RP, C18ec Nucleodur C8 Gravity, C8e Enq. Peptides Proteins Reversed Phase ACE C8, C18, Ph Inertsil ODS-4, PEPTIDE C18 Nucleodur HILIC ZIC-HILIC , MW>5000 Organic- Soluble Aqueous- Soluble Gel Permeation Chromatography (GPC) Gel Filtration Aqueous GFC/SEC Ion-Exchange Reversed Phase Hydrophobic Interaction (HIC)º Affinity/Bioaffinity Unknown MW Range Known MW Range ph ph>7.5 Cation- Exchange Anion- Exchange ph ph>7.5 Inertsil WP300 Diol Jordi Organic GPC Shodex Organic GPC Inertsil Diol Jordi Organic GPC Shodex Organic GPC Inertsil WP300 Diol Jordi Aqueous GPC Shodex OH pak Jordi Aqueous GPC Shodex IEC CM-825 Nucleosil DEAE ACE 300A C4, C8, C18, Ph Hamilton PRP Inertsil WP300-C18, WP300-C8 Inertsil WP300-C4 Macrosep Nucleosil DEAE Hamilton PRP-3 PolySULFOETHYL A ZIC-HILIC Inertsil HILIC Nucleodur HILIC Shodex AF pak Enq. 168 Enq. Enq Enq. Enq
5 COLUMN SELECTION BY CHROMATOGRAPHIC MODE REVERSED-PHASE (Narrow Pore) > Most popular Narrow pore silica or other materials Eluent: Methanol-water Acetonitrile-water Chemistry: C18, C8, C6, Phenyl, C4, C3, C2, C1, CN Polymer, Carbon Ace (p65) ES Industries (p100) Halo (p106) Hamilton (p116) Hypersil (p120) Inertsil (p133) Jordi (p167) LiChrosorb (p184) LiChrospher (p185) NUCLEODUR (p187) Nucleosil (p193) Partisil (p203) Partisphere (p204) Ultrasphere (p235) MIXED-MODE (Narrow Pore) Most universal Narrow pore silica Eluent: Acetonitrile-water buffer Chemistry: C12-COO, C12-+NH, Phenyl-COO Inertsil (p133) Primesep (p211) Shodex (p224) < ION-EXCHANGE (Narrow Pore) Narrow pore silica or Polymer Strong cation - SCX Strong anion - SAX Charge mainly ph independent Low capacity for ion chromatography Hamilton (p116) Inertsil AX and CX (p150) Jordi (p167) Nucleosil (p193) Partisil (p203) Partisphere (p204) NORMAL-PHASE Classical mode Organic eluent Polar stationary phase: Silica Alumina - more retentive Diol - less polar Amino - most polar NO 2 - charge transfer Ace (p65) Hypersil (p120) Inertsil (p133) Jordi (p167) LiChrosorb (p184) LiChrospher (p185) NUCLEODUR (p187) Nucleosil (p193) Partisil (p203) REVERSED-PHASE (Wide Pore) Wide pore silica Eluent: Acetonitrile-water (TFA) Chemistry: C18, C8, C4, Phenyl, CN Ace (p65) Inertsil (p133) Jordi (p167) NUCLEODUR (p187) Nucleosil (p192) 34
6 COLUMN SELECTION BY CHROMATOGRAPHIC MODE ION-EXCHANGE (Wide Pore) Wide pore silica or polymer Strong and weak Anion and cation Jordi (p167) PolyLC (p205) Shodex (p224) GEL FILTRATION Wide pore silica or polymer Pore size - MW correlation Inertsil (p133), Jordi (p167) Shodex (p224) HYDROPHILIC INTERACTION (HILIC) Silica based phases Pore size - 60Å Å Solutes elute in order of increasing hydrophilicity (polarity), opposite of RP ES Industries (p100), Inertsil (p133), Halo (p106), NUCLEODUR (p187), PolyLC (p205) Primesep (p211), SeQuant (p222) HYDROPHOBIC INTERACTION (HIC) Wide pore silica Chemistry: C3, C2, C1 Eluent : Aqueous buffer. Inorganic salt gradient elutes solute PolyLC (p205) Shodex (p224) GEL PERMEATION Styrene-divinylbenzene matrix Wide-ranging pore size Jordi (p167) Shodex (p224) Contact us for application and column selection advice. Toll-free:
7 SPECIFICATIONS OF C18-BONDED RP MATERIALS The efficiency of a column is determined not only by the quality of packing material utilized, but also by its quality of manufacture. The smaller the particle size and the tighter its distribution, the greater the column performance. Typical performances for C18 bonded phases are: 3µm > 120,000 plates/metre 5µm > 80,000 plates/metre 10µm > 40,000 plates/metre Spherical packing materials generally give columns a higher performance than those packed with irregular material due to improved particle uniformity. Table 1 lists physical characteristics and column efficiencies of a range of C18 bonded silicas. Table 1 Octadecylsilyl-bonded silicas Phase Particle Size (µm) Pore Size (Å) * Surface Area (m 2 /g) Carbon Load Endcapping Page Ace C18 1 3, 5, Fully 66 Ace C18-AR 1 3, 5, Fully 68 Ace C18-HL 1 3, 5, 10, Fully 71 BetaBasic C18 1 3, Fully 125 BETASIL C18 3, 5, Fully 124 Capcell Pak AG C Fully Call Capcell Pak MG C18 3, Fully Call Cosmosil 5C18-AR-II Fully Call Cosmosil 5C18-MS-II Fully Call Develosil ODS-UG 1 3, Fully Call Develosil ODS-MG Fully Call Develosil ODS-HG 1 3, Fully Call Develosil ODS-SR Fully Call ES Industries Epic C18 1 3, Fully 100 ES Industries Epic C18 SD 1 3, Fully Call ES Industries ProTec C18 1 3, Uncapped Call ES Industries BAS-C Uncapped Call ES Industries HC-C Uncapped Call ES Industries C Uncapped Call ES Industries C18-BD Uncapped Call ES Industries C18-AI Uncapped Call Exsil ODS 3, 5, Fully Call Exsil ODS1 3, Partially Call Exsil ODSB 3, Fully Call HALO C Proprietary Fully 106 HALO Peptides ES-C Proprietary Fully 108 Hichrom C , Fully Call Hichrom RPB 1, 2 3.5, 5, Fully 118 Hypersil ODS 3, 5, Fully 122 Hypersil ODS-2 3, Fully 122 Hypersil BDS C18 3, Fully 123 Hypersil GOLD 1 1.9, 3.5, 8, Fully 120 Inertsil ODS Fully Call Inertsil ODS Fully 151 Inertsil ODS-3 1 2, 3, Fully 142 Inertsil ODS-4 1 2, 3, Fully 134 InertSustain C18 1 3, Fully 140 Inertsil ODS-P Uncapped High purity silica 2 Mixed alkyl mode C18/C8 *For 300Å C18 phases, see page 58 36
8 SPECIFICATIONS OF C18-BONDED RP MATERIALS Table 1 Octadecylsilyl-bonded silicas (continued) Phase Particle Size (µm) Pore Size (Å) Surface Area (m 2 /g) Carbon Load Endcapping Page Inertsil Peptide C Proprietary Fully 150 Inertsil ODS-Sprint 1 3, Fully 148 Kromasil C18 3.5, 5, Fully 183 LiChrosorb RP-18 5, Uncapped 184 LiChrospher RP-18 5, Uncapped 185 LiChrospher RP-18e 5, Fully 185 Nucleodur C18 ec 1 3, Fully 187 Nucleodur C18 Gravity 1 1.8, 3, Fully 187 Nucleodur C18 Isis 1 1.8, 3, Fully 187 Nucleodur C18 Pyramid 1 1.8, 3, Fully 187 Nucleodur C18 Sphinx RP 1 1.8, 3, Fully 188 Nucleosil C18 3, 5, 7, Fully 193 3, 5, 7, Fully 193 Nucleosil C18AB Fully Call Nucleosil C18HD 3, Fully Call Partisil ODS Uncapped 203 Partisil ODS Partially 203 Partisil ODS3 5, Fully 203 PartiSphere C Fully High purity silica 3 Other sizes available Common Methods Used for Retaining Highly Polar Compounds Silica based C18 columns with highly aqueous mobile phase Short retention time Limited selectivity Poor reproducibility AQ type phases with polar endcapping Improvement of Kʼ over traditional C18 Good retention and resolution Eliminates need for ion-pair additives Polar embedded phases If compound is not retained on C18 it is unlikely to be retained If compound is retained on C18 chemistry, may offer additional selectivity Significant phase bleed with MS detection Ion-pairing retention Complex mobile phase Requires dedicated column Not compatible with MS, ELSD Not compatible with gradient elution 37
9 SPECIFICATIONS OF C1- TO C6- BONDED RP MATERIALS Octyl-bonded phases are the most common medium polarity alternative to C18-bonded phases (see page 39). Very short chain alkyl-bonded phases are less stable. The shorter the alkyl chain the greater the vulnerability of the material to aqueous dissolution or loss of bonded phase. A number of such materials have been withdrawn in recent times. For wide pore silicas the C4-chemistry retains high popularity. Table 1 lists the physical characteristics and efficiencies of a range of C1- to C8-bonded narrow pore silicas. Table 1 Short chain alkyl-bonded silicas. Phase Particle Size (µm) Pore Size (Å) * Surface Area (m 2 /g) Carbon Load Endcapping Page C1-Bonded Betasil C Fully 124 ES Industries TMS (C1) Call Excil C1 3, Call Hypersil SAS 3, 5, C2-Bonded ES Industries Chromegabond C Uncapped Call Nucleosil C Uncapped 193 C4-Bonded Ace C4 1 3, Fully 66 BetaBasic 4 3, Fully 125 ES Industries Chromegabond C Uncapped Call Epic C4-SD 3, 5, Fully Call Hypersil Butyl (C4) 3, 5, Inertsil C Fully 151 Nucleosil C Fully 193 C6-Bonded Betasil C6 3, Fully 124 ES Industries Chromegabond C Uncapped Call ES Industries Chromegabond MC-CC Fully Call 1 New generation phases 2 Other sizes available *For 300Å C4 phases, see page 58 Existing Column Stationary Phases Technology A significant number of LC applications can be covered with few types of stationary phases. Reverse phase and ion-exchange mechanism are two dominant processes utilized for separation of small molecules. Reverse phase columns Ion-exchange columns Normal phase columns 38
10 SPECIFICATIONS OF C8-BONDED RP MATERIALS Table 1 Short chain alkyl-bonded silicas. Phase Particle Size (µm) Pore Size (Å) * Surface Area (m 2 /g) Carbon Load Endcapping Page Ace C8 1 3, Fully 66 BetaBasic 8 3, Fully 125 BETASIL C8 3, 5, Fully 124 ES Industries Epic C8 1 3, ES Industries AquaSep 1 3, Call ES Industries ProTec C8 1 3, Call ES Industries BAS C Uncapped Call ES Industries Chromegabond C Uncapped Call ES Industries MC Fully Call ES Industries C8-BD Uncapped Call Exsil C8 3, Fully Call HALO C Proprietary Fully 106 Hichrom C , Fully Call Hypersil MOS (C8) 3.5, Uncapped 122 Hypersil BDS C8 3, Fully 123 Hypersil GOLD C8 1.9, 3, 5, 8, Fully 120 Inertsil C Fully 151 Inertsil C , 3, Fully 142 Inertsil C , 3, Fully 137 LiChrosorb RP-8 5, Uncapped 184 LiChrospher RP Uncapped 185 LiChrospher RP-8e Fully 185 Nucleodur C8 Gravity 1 1.8, 3, Fully 187 Nucleodur C8 ec 1 3, Fully 187 5, 7, Nucleosil C8 Uncapped 3, 5, 7, Nucleosil C8HD 3, Fully 193 Partisil C8 5, Fully 203 PartiSphere C Fully New generation phases *For 300Å C8 phases, see page 58 39
11 CHARACTERIZATION OF REVERSED-PHASE MATERIALS The performance of reversed-phase materials depends on many parameters. Two key properties, hydrophobicity and polarity, are of practical importance and dominate their selection. Hydrophobicity The strength of hydrophobic interaction can be measured by the retention of neutral (non-polar) molecules. The percentage of carbon in the material is a simplistic but useful guide to the retention characteristics of the column. In Figure 1 this loose correlation is demonstrated by the increase in retention observed when alkyl chain length (ie. carbon load) is increased. This results in an increase in hydrophobicity of the stationary phase. Figures 2 and 3 compare the retention obtained for a selection of non-polar solutes with a range of commercially available C8 and C18 columns. Figure 1. Increase in retention with alkyl chain length C4 Bonded Silica (8% C) 6 1. Uracil 2. Benzamide 3. Methyl benzoate 4. Toluene 5. Propyl benzene 6. Butyl benzene C8 Bonded Silica (12% C) C18 Bonded Silica 5 6 (19% C) minutes Column: 250mm x 4.6 mm Eluent: CH 3 CN - H 2 O (70:30) Flow rate: 2ml/min. Figure 2. C8 Hydrophobicity comparison Separation of dimethyl phthalate, toluene and biphenyl on C8 bonded phases using a methanol-water (90:10) eluent 40
12 CHARACTERIZATION OF REVERSED-PHASE MATERIALS (CONT D) Figure 3. C18 Hydrophobicity comparison Separation of dimethyl phthalate, toluene, biphenyl and phenanthrene on C18 bonded phases using a methanol-water (90:10) eluent 1. LiChrospher RP-18e 2. Inertsil ODS-3 3. LiChrospher RP Ace C18-HL 5. YMC J sphere ODS-H80 6. Nucleosil 100 C18 7. Kromasil C18 8. Develosil ODS-MG 9. Partisil ODS2 10. LiChrosorb RP Symmetry C Symmetry Shield C Inertsil ODS 14. Develosil ODS-UG 15. Purospher RP-18e 16. Prodigy ODS YMC ProC Zorbax ODS 19. Nucleosil 100 C18HD 20. Inertsil ODS2 21. YMC ODS-A 22. YMC ODS-AM 23. YMC ODS-AQ 24. Prodigy ODS TSK ODS-80T M 26. Nucleosil 120 C Ace C ProTec C Develosil ODS 30. Genesis C Luna C18(2) 32. Nucleosil 100 C18AB 33. Hichrom C Waters Spherisorb ODS2 35. Zorbax Extend-C Develosil ODS-HG 37. Zorbax Rx-C Resolve C Zorbax XDB-C Hypersil BDS-C Ultrasphere ODS 42. Hypersil HyPURITY C TSK ODS-120T 44. Partisil ODS3 45. Hichrom RPB 46. Waters Spherisorb ODS1 47. µ Bondapak C Exsil 100 ODS 49. Capcell Pak AG 120 C YMC J sphere ODS M Capcell Pak SG 120 C Capcell Pak UG 120 C Zorbax SB-C XTerra MS C Hypersil ODS 56. Nova-Pak C SynChropak CR Exsil 100 ODS1 59. Ace AQ 60. Ace C Vydac SelectaPore 90M 62. Vydac SelectaPore 300P 63. Vydac 218TP 64. Partisil ODS 65. Vydac SelectaPore 300M A more comprehensive characterization and comparison of all leading C18 reversed-phase materials has been done by NIST. Please visit and look at our technical pages to retrieve this report. 41
13 CHARACTERIZATION OF REVERSED-PHASE MATERIALS (CONT D) Polarity (Silanol Activity) The second key property of reversed-phase materials is their silanol activity, often discussed in terms of polarity. This can be measured on a relative basis by comparing the retention of a polar solute (involving hydrophobic and ionic interactions) to that of a neutral solute (involving hydrophobic interaction only). High purity base deactivated phases In recent years a number of new alkyl bonded silicas have been introduced. The cumulative metal ion impurity level within these base silicas has in some cases been reduced to <10ppm. As a result the number of isolated silanol groups and hence the polarity of the silica surface is also reduced. When coupled with the use of more effective and reproducible bonding processes, a new generation of reversed-phase materials is produced, which gives significantly improved chromatography for the more basic polar solute molecules. Use of bonded alkyl groups containing hydrophilic substituents (i.e. polar embedded) can either enhance the above effect and/or offer alternative selectivity. Figure 4 demonstrates the reduced polarity of high purity base deactivated materials compared to lower purity products. Toluene is used as a hydrophobic reference. High purity (low polarity) materials generally give better peak shape with strongly basic compounds. However, low purity (high polarity) materials may offer a unique selectivity. Figure 4. Reduction in phase polarity as purity is increased 3 Today 5 6 High Purity C18 1 (low polarity) Uracil 2. Phenylpropanolamine 3. Nortriptyline 4. Toluene 5. Imipramine 6. Amitriptyline 1 4 Mid 80ʼs Intermediate Purity C18 3 (moderate polarity) minutes Column: 25mm0 x 4.6 mm, 5µm Eluent: CH 3 CN - 10mM Na 3 PO 4, ph ʼs Low Purity C18 (high polarity) 5 6 Flow rate: 1.5 ml/min Temperature: 40 C Optimizing selectivity Figure 5. Variation of phase polarity with change in hydrophobicity Figure 5 illustrates the change in polarity and hydrophobicity for C18, C8 and C4 materials. As discussed previously, a decrease in hydrophobicity on reducing alkyl chain length is observed (see p.43). Greater ligand density and hence lower polarity is also seen as the length of the alkyl chain is reduced from C18 to C4. Polarity C8 C18 Such variations offer the possibility of reduced analysis time and improvements in peak shape, but no major change in selectivity. Changing the chemistry of the bonded phase (e.g. from C18 to cyano or phenyl) is a more powerful tool in altering the selectivity. C4 Hydrophobicity 42
14 CHARACTERIZATION OF REVERSED-PHASE MATERIALS (CONT D) Traditional C18 (ODS) phases are hydrophobic and have a high polarity due to the lower purity silicas on which they are based. Use of the new high purity silicas reduces the resultant phasesʼ silanol activity and improves reproducibility. Employing a polar embedded functionality may also result in a reduced polarity material. Shorter alkyl chain phases are found at the lower hydrophobicity area of the graph. Alternative bonded phases (including phenyl, cyano and fluorinated phases) based on high purity silicas are best considered to effect changes in polarity. For any separation it is possible to select the most suitable phase from Figure 6. For basic solutes which will interact strongly with surface silanols, lower polarity phases are recommended. If a column of significantly different polar selectivity is required, select phases from a different section of the graph. If only differences in hydrophobicity are desired, simply select phases that are well separated on the hydrophobicity axis. Figure 6. Hydrophobicity and polarity comparison Low Moderate High Ace CN (p65) HALO HILIC (p107) Inertsil CN-3 (p142) Inertsil Ph-3 (p142) Inertsil Diol (p142) Inertsil HILIC (p142) NUCLEODUR HILIC (p190) Ace Phenyl (p65) FluoroSep-RP Octyl (p103) HALO Peptide ES-C18 (p108) HALO PFP (p110) HALO Phenyl Hexyl (p107) Inertsil Phenyl-3 (p142) Primesep A,B,B2,C,100, (p211) FluoroSep-RP Phenyl (FSP) (Call) Hypersil ODS (p122) Inertsil ODS-P (p144) LiChrospher RP-18 (p185) Nucleosil 100 C18 (p193) Nucleosil 120 C18 (p193) High POLARITY Inertsil Ph (p151) AquaSep (Call) Inertsil ODS-EP (p145) LiChrospher RP-select B (p185) NUCLEODUR Sphinx RP (p188) Ace C18-AR (p68) HALO RP-Amide (p107) Hypersil BDS C18 (p123) Inertsil ODS 80Å (Call) Inertsil ODS-2 (p151) NUCLEODUR C18 ISIS (p187) NUCLEODUR C18 Pyramid (p187) Moderate Ace C4 (p65) Inertsil C4 (p151) Ace C8 (p65) Epic C8 (p100) HALO C8 (p106) Inertsil C8-3 (p142) Inertsil C8-4 (p137) Inertsil ODS-SP (p147) NUCLEODUR Gravity C8 (p187) NUCLEODUR C8ec (p187) Ace C18 (p65) Epic C18 (p100) HALO C18 (p106) Hichrom RPB (p118) Inertsil ODS-80Å (Call) Inertsil ODS-4 (p134) Inertsil ODS-3 (p142) Inertsil ODS-3V (p144) NUCLEODUR C18ec (p187) NUCLEODUR Gravity C18 (p187) Low HYDROPHOBICITY 1 Primesep is a special phase with embedded ionizable groups which mask silanol interaction but produce additional ionic or polar interaction with different analytes (p211) 43
15 CHIRAL PHASES In many biological processes the activity of one member of an enantiomeric pair can be contrasted with the inactivity or even harmful activity of the other. The successful development of chiral stationary phases (CSP) for HPLC now allows us to monitor the optical purity of a bulk drug and its presence in formulations or biological fluids. Further applications can be found within the agrochemical, environmental and related industries. The main types of CSPs are discussed. Chiral Technologies has introduced LC-MS columns for Chiral applications. 1. Immobilized Polysaccharide CSPs Coated polysaccharide CSPs are limited in the solvents that may be used in the mobile phase and as sample diluents. Immobilized CSPs allow the use of a more robust and expanded range of solvents and bring new selectivity and higher sample solubility relative to conventionally coated CSPs. Based on high-efficiency spherical silica, Immobilized Polysaccharide CSPs utilize a chiral polymer or unique chiral selector based on the 3,5-dichlorophenylcarbamate of cellulose. 2. Protein Bound Proteins are high molecular weight polymers containing chiral sub-units. When bound to silica they act as very effective CSPs. The binding or complexation of small enantiomeric molecules is often stereospecific, especially for serum proteins such as α 1 -acid glycoprotein (AGP) or human serum albumin (HSA). The additional stability of the Ultron ES-OVM and ES-Pepsin columns allow them to be used with high organic content eluents. Immobilized enzymes can similarly be used. Protein immobilized CSPs are typically used in buffered aqueous eluents compatible with many biological samples. They offer good selectivity. Enantiomer retention and stereoselectivity can often be significantly altered by changes in eluent ph or modifier concentration. They are primarily analytical columns, however can also be used for semi-preparative separations. 3. Cellulose and Amylose Bound Cellulose and amylose are linear polymers of optically active glucose units with molecular weights of 250,000 to 1,000,000. Crosslinked derivatives of these materials coated onto silica give unique chiral selectivity. Their chiral recognition properties depend on the steric fit of guest enantiomers into the materialʼs cavities. Choice of eluent is the key factor affecting chiral recognition. 4. Brush-Type Although brush-type (Pirkle) chiral selectors are relatively simple molecules, their well defined structure contains three types of functional groups capable of participating in charge transfer (π-π bonding), hydrogen bonding ( dipole stacking interactions) and steric effects. The monolayer of chiral selector covalently bound to the silica surface usually gives a column of relatively high capacity and efficiency but often with limited chiral discrimination ability. Since the synthesis of the popular D-3,5-dinitrobenzoylphenylglycine phase, significant numbers of these multiple interaction CSPs have been synthesized. Polyaromatic hydrocarbon derivative CSPs are the most recent additions to the range. All brush-type phases are typically used with normal-phase eluents. 5. Ligand Exchange Ligand exchange chiral phases are characterized by the attachment of a chiral chelating ligand to the stationary support. In the presence of an appropriate transition metal cation such as copper (ll), a molecular complex is formed with the chiral stationary phase ligand and the analyte. Compounds that are suitable for chiral ligand exchange are α-amino acids, hydroxy acids and small peptides. 6. Crown Ether Chiral recognition with crown ether phases is achieved when a complex is formed between the crown ether and an ammonium ion from the analyte. These phases are used for solutes with a primary amino group at or near its chiral centre, such as amino acids and amino alcohols. 7. Cyclodextrin Inclusion Cyclodextrins are a class of oligosaccharides containing six to twelve optically active glucose units. They are covalently bound to silica to form the corresponding CSP. The physical shape of these molecules is that of a truncated cone, the internal diameter of which is proportional to the number of glucose units. The interior of the cavity is relatively hydrophobic. Secondary hydroxyl groups at the entrance to the cavity contribute to the separation process. The relative stability of the inclusion complexes formed by the enantiomers of the guest molecule at the edge of the cyclodextrin cavity dictates the degree of separation. β-cyclodextrin and its derivatives are the most commonly used CSPs of this type. Cyclodextrin CSPs are used in reversed-phase and are suitable for preparative separations. 44
16 CHIRAL PHASES (CONT D) Phase Manufacturer Chiral Type Chiral Selector Particle Size (µm) Features Page D-Phenylglycine CHIRA-chrom-1 L-Phenylglycine High efficiency and Hichrom Brush 5 capacity. L-Leucine Low cost 119 CHIRA-chrom-2 Dinitrophenyltartramide ChiraDex ß-Cyclodextrin Forms inclusion Merck Cyclodextrin 5 ChiraDex GAMMA γ-cyclodextrin complexes Call CHIRAL-AGP Protein α 1 -acid glycoprotein 86 Widely used. CHIRAL-CBH ChromTech Enzyme Cellobiohydrolase 5 86 ph variation useful tool CHIRAL-HSA Protein Human Serum Albumin 86 CHIRALCEL OA, OB, OC, Cellulose Cellulose derivative Unique separation OD, OF, OG, OJ, OK, OZ 5, 10, 20 applications. 90 CHIRALPAK AD, AS, AY, AZ Amylose Amylose derivative Most versatile 90 CHIRALPAK IA Amylose Immobilized Amylose derivative 5, CHIRALPAK IB Cellulose Immobilized Cellulose derivative 5 Broad application 85 Daicel CHIRALPAK IC Cellulose Immobilized Cellulose derivative 5, 20 range 85 CHIRALPAK ID Amylose Immobilized Amylose derivative 5 85 CHIRALPAK QD-AX Quinidine derivative 91 Anion Exchange 5 Useful for chiral acids CHIRALPAK QN-AX Quinidine derivative 91 CROWNPAK Crown Ether 18-crown-6 type crown ether 5 Amino acids, primary amines 92 ChiroSil RStech Crown Ether (18-crown-6)-tetracarboxylic acid 5, 10 Amino acids, primary amines 221 Nucleodex ß-OH ß-Cyclodextrin Nucleodex α-pm Reversed-phase Cyclodextrin Permethylated α-, ß- and γ-cyclodextrins 5 Nucleodex ß-PM Macherey- applications respectively 194 Nucleodex γ-pm Nagel Nucleosil Chiral-1 Ligand exchange L-Hydroxyproline-Cu 2+ complex 5 α Amino acids typical example Resolvosil BSA-7PX Protein Bovine Serum Albumin 7 Early generation DACH-DNB π electron acceptor/donor. ULMO 218 Widely used. Whelk-01/Whelk-02 3,5-Dinitrobenzoyl α-burke 2 Regis Brush derivatives 5 ß-GEM 1 Leucine Phenylglycine π electron acceptor 217 Pirkle-1J ß-lactase ORpak CDA-453 HQ α Cyclodextrin 6 Call ORpak CDB-453 HQ β Cyclodextrin 6 Suitable for optical and Call Cyclodextrin structural isomers that have ORpak CDC-453 HQ γ Cyclodextrin 6 similar structure Call ORpak CDBS-453 Shodex β Cyclodextrin 3 Call ORpak CRX-853 Ligand Exchange L-Amino Acid 6 Amino acids and acid hydroxyl groups Call AFpak ABA-894 Protein Bovine Serum Albumin 6 Amino acids and carboxylic acids 228 Ultron ES-OVM Shinwa Chem. Ovomucoid 5 Call Protein Stable phase Ultron ES-Pepsin Ind. Pepsin 5 Call 45
17 FUSED-CORE PARTICLE TECHNOLOGY Fused-Core particles are designed for high speed, high resolution liquid chromatography. They are unique particles consisting of a 0.5µm porous silica halo fused to a 1.7µm solid silica core (see Figures 1 and 2). The high density and extremely narrow size distribution of these Fused-Core particles facilitate the packing of columns with unexpectedly high efficiencies - efficiencies more in line with what one would expect from columns packed with sub-2µm particles. UHPLC columns packed with Fused-Core particles do, however, generate the back pressure expected from columns packed with 2.7µm size particles. This pressure is low enough to permit these UHPLC columns to be used effectively with conventional HPLC equipment. In addition, the narrow size distribution of the Fused-Core particles permits the use of 2µm porosity inlet frits on columns. This is the same inlet frit porosity typically found on columns packed with 5µm particles. The result is columns capable of delivering the speed and resolution of other UHPLC columns, but with the ease of use and durability of columns packed with 5µm particles. Figure 1. Fused-Core particle The science behind Fused-Core particle technology The well known van Deemter equation identifies the three main sources of band broadening. H = A + B/u + Cu The value of the A term, eddy diffusion, reflects the multiple flow paths through a column. Packing particle size, particle size distribution and the uniformity of the packed bed all determine the value of A. Because of the high density and extremely narrow size distribution of Fused-Core particles, columns can be packed with well ordered beds that have A term values significantly smaller than what is typically seen with columns packed with totally porous particles. This is one of the reasons that columns packed with Fused-Core particles deliver column plate numbers that are much higher than what would normally be expected from their particle size. The C term of the van Deemter equation, the coefficient of mass transfer, reflects the time it takes for analyte to diffuse in and out of the stationary phase. The C term is directly related to mobile phase velocity because higher velocity interferes with the equilibrium between the analyte, mobile phase, and stationary phase. The longer the path an analyte has to travel within the pores of the stationary phase support particles, the more detrimental will be the effect of mobile phase velocity on column efficiency. The path a solute has to travel within the pores of a stationary phase can be reduced by using smaller size particles and this is typically the strategy that is used by column manufacturers when making columns for fast HPLC. Smaller particles have shorter path lengths and, therefore, are less affected by increases in mobile phase velocity. Fused-Core particles, by virtue of their 0.5 micron porous shell, have reduced the diffusional mass transfer path by one third compared to 3 micron particles. The result is a column that can achieve faster separations and higher sample throughput (see Figure 3). Figure 2. Scanning electron microscope (SEM) photograph of HALO particles Figure 3. Effect of particle size and type This SEM photograph of HALO particles illustrates two important attributes of this unique column packing. First, the incredibly narrow particle size distribution is apparent. Second, this SEM photo shows some of the HALO particles sliced in half so that the solid core and the porous outer layer, the halo of the particle is evident 46
18 Guide to Ultra-Fast HPLC Quick Tips for Converting Conventional Reversed-Phase HPLC Separations to Ultra-Fast Separations There has been enormous interest recently in so-called ultrafast HPLC columns that can reduce run times by 70% or more. These ultra-fast columns are typically packed with particles considerably smaller than what is packed in conventional columns, giving them the advantage of equivalent separating power in much shorter length columns as well as the advantage that they maintain their separating power at higher mobile phase flow rates. The ability to use shorter columns and higher flow rates offers an opportunity to reduce analysis time and increase sample throughput significantly by substituting an ultra-fast column for a conventional column in an established method. This guide is intended to assist you in selecting an ultra-fast column and modifying conditions for a faster run time. In addition, this guide will help you estimate how the new ultra-fast conditions will affect run time, resolution, and back pressure. First, one qualification: An important chromatographic parameter that seems to be ignored in most discussions of ultra-fast HPLC is selectivity. Although selectivity is beyond the scope of this guide, you should be aware that converting from a conventional column to an ultra-fast column will sometimes be accompanied by a change in selectivity for one or more peak pairs in your chromatogram. This may be true if you continue to use the same bonded phase chemistry, such as C18, and even true if the ultrafast column you choose is the same brand as the conventional column that it is replacing. However, in most cases the change in selectivity will be minor enough that the estimation models used in this guide will still be useful. Suggested steps for converting a conventional reversed-phase separation to an ultra-fast separation 1. Select the shortest ultra-fast column that can provide resolution equivalent to or better than the conventional column (see Figure 1). Figure 1: Resolving power as a function of particle size and column length Instructions: This chart plots resolving power (the ability of a column to separate components in a mixture) versus column length for 7 different columns. Three of the columns are packed with conventional particles (5.0μm, 3.5μm, and 3.0μm) and four are packed with ultrafast particles (2.2μm, 2.7μm Fused-Core, 1.8μm, and 1.7μm). As column length increases, so does Resolving Power, but run time also increases. Notice that the ultra-fast columns provide greater Resolving Power in much shorter column lengths compared to the conventional columns. When converting a conventional separation to an ultra-fast separation, choose the shortest ultra-fast column that provides Resolving Power equal to or better than the conventional column it is replacing. This will allow you to minimize run time and maintain acceptable resolution. 2. Estimate the back pressure for the selected ultra-fast column (see Figure 2). If the pressure exceeds the maximum acceptable pressure for your system, select an alternate column with lower back pressure, most likely one packed with larger particles. You could elect to operate at a lower flow rate to keep the pressure acceptable, but this would also increase the run time, negating the purpose of converting to an ultra-fast column. Figure 2: Relative back pressure versus particle size Instructions: For the ultra-fast column configuration selected in Step 1 (length, particle size), estimate the expected back pressure on this column by multiplying the pressure observed on the conventional column by the ratio of the Relative Pressure of the ultra-fast column to the conventional column and then by the ratio of the column lengths. Note: This calculation assumes that the mobile phase velocity is the same for both the conventional column and the ultra-fast column. Example: A 100mm ultra-fast column packed with 2.7μm Fused-Core particles meets the criteria of providing equal or better Resolving Power compared to a 250mm column packed with conventional 5μm particles. This ultra-fast column is an appropriate choice for replacing the 250mm length conventional column in an ultra-fast method. Example: A 100mm ultra-fast column packed with 1.8μm particles will generate approximately 3 times the back pressure of a 250mm conventional column packed with 5μm particles. 47
19 Guide to Ultra-Fast HPLC 3. Confirm that the selectivity and resolution of the ultra-fast column is adequate. Since the selectivity of the ultra-fast column may be different from the selectivity of the conventional column (this may be true even if both the conventional column and the ultra-fast column are the same brand), run your separation with the ultra-fast column and calculate resolution. If the resolution does not meet the minimum required resolution, you may have to choose a longer column, or possibly even a different brand of ultra-fast column (with a different selectivity) to achieve acceptable resolution. If the resolution exceeds the required resolution, you may be able to use an even shorter ultrafast column, or at least operate at a higher mobile phase flow rate to reduce the run time even further. 4. Once a column has been selected that provides acceptable resolution and pressure, increase the flow rate to minimize the run time while maintaining acceptable resolution and pressure. Considerable time savings and greater sample throughput can be achieved by operating at higher flow rates with an ultra-fast column, as long as you donʼt exceed your systemʼs maximum back pressure (see Figure 3). Figure 3: Resolution versus mobile phase flow rate Instructions: If the resolution on the selected ultra-fast column exceeds the minimum required resolution for the separation and does not exceed the pressure limit, you will be able to reduce analysis time further by increasing the flow rate. Since the optimum flow velocity (for maximum resolution) of an ultra-fast column is 3 to 4 times faster than for a conventional column, you may actually be able to both reduce the run time and increase resolution by operating at a higher flow rate. This chart estimates change in resolution with changes in mobile phase velocity. Not only do ultra-fast columns have their optimum efficiency at higher mobile phase velocities, they also sacrifice less of their efficiency as mobile phase velocity is increased beyond their optimum. 5. If the separation uses gradient elution, you will need to adjust the gradient time (tg) to the volume of the ultra-fast column and for any changes in flow rate (see Figure 4). Figure 4: Adjusting gradient time (tg) for changes in column volume and flow rate See Table on page 50 for estimates of column volumes for most commercially available column dimensions. Important note: the system dwell volume (gradient mixing volume) can have a significant effect on the chromatography when using gradients because it adds an isocratic hold to the beginning of the gradient. The time of this hold is equal to the dwell volume divided by the flow rate. When the flow rate is changed, this isocratic hold will also change. This change in gradient hold will generally have more of an effect on early eluting peaks, but it will also affect all peaks in the chromatogram to some extent. To minimize the effect on your separation, keep the dwell volume as small as possible by using micro gradient mixers and keeping the tubing volume in the system to a minimum. Example: A conventional method uses a column 4.6mm x 150mm (1.57 ml), a flow rate of 1.0 ml/min, and a gradient of 15% B to 35% B in 20.0 minutes. The gradient time for an ultra-fast method that uses a column 4.6mm x 50mm (2.7μm Fused-Core, 0.42ml) and a flow rate of 2.0ml/min is: 6. Adjust the injection volume to the ultra-fast columnʼs volume (see Figure 5). Figure 5: Adjust sample injection volume for changes in column dimension Example: An ultra-fast column packed with 2.7μm Fused-Core particles can be operated at a relatively fast mobile phase velocity of 0.7 cm/sec and still retain over 96% of its resolving power. A conventional column packed with 5μm particles run at the same flow velocity would retain only about 82% of its resolving power. Example: A conventional method uses a sample injection volume of 20μl on a column 4.6mm x 150mm. The sample volume that should be injected on to a 4.6mm x 50mm ultra-fast column (2.7μm Fused- Core) is: 48
20 Conventional HPLC Separation Conditions COLUMN: 4.6mm x 250mm, 5μm FLOW RATE: 1.5 ml/min MOBILE PHASE: Isocratic RUN TIME: 10 minutes PRESSURE: 1,580psi, 109bar Maximum acceptable pressure = 4,000psi, 275bar RESOLUTION: 3.0 SAMPLE INJECTION VOLUME: 20μl Converting to Ultra-Fast Separation Conditions 1. Select the shortest ultra-fast column that provides resolution equivalent to or better than the conventional column (See Relative Resolution chart in Figure 1 pg. 47) A column 4.6mm x 100mm packed with 1.7μm particles is selected for further investigation. 2. Estimate back pressure (See relative pressure table in Figure 2 pg. 47) Since this ultra-fast column exceeds our maximum acceptable back pressure (4,000psi), a different ultrafast column is selected for investigation. The alternative ultra-fast column selected is a 4.6mm x 100mm packed with 2.7μm Fused-Core particles (HALO HPLC column). The back pressure on this column is: Guide to Ultra-Fast HPLC An Example of Converting a Conventional Separation to an Ultra-Fast Separation 5. Adjust the gradient time This is an isocratic separation, so no adjustment to gradient time is required. 6. Adjust the injection volume (See Figure 4 pg. 48 for calculations and table with estimated column volumes). Ultra-Fast Conditions COLUMN: 4.6mm x 100mm, 2.7μm Fused-Core (HALO ) FLOW RATE: 2.5 ml/min RUN TIME: 0.84 ml 1.5 ml/min 10 min x x = 1.9 min* 2.62 ml 2.5 ml/min RESOLUTION: 3.1 PRESSURE: 3,582psi SAMPLE INJECTION VOLUME: 6.4μl *Run time for the ultra-fast separation can be estimated by multiplying the run time on the conventional column by the ratio of the volumes of ultra-fast column to the conventional column and then by the inverse ratio of the flow rates on the two columns (See page 50) Conventional Separation Column: 250mm x 4.6mm, 5µm. C18 Flow Rate: 1.5mL/min Resolution: 3,0 Pressure: 1,580psi Run Time: 10 minutes Sample Injection Volume: 20µl This ultra-fast column provides both acceptable resolution and acceptable back pressure for our method. 3. Confirm that the selectivity and resolution of the ultrafast column is adequate For simplicity, we will assume that the selectivity of this ultra-fast column is almost identical to the selectivity of the conventional column and, therefore, the resolution is adequate. 4. Optimize flow rate to minimize run time (See Figure 3 pg. 48 to estimate changes in resolution with changes in flow rate.) We can further reduce run time by operating the ultrafast column at a higher flow rate. We just have to make sure we stay within the requirements of minimum resolution and maximum pressure. The ultra-fast column we selected has low enough back pressure that we can operate at a flow rate of 2.5 ml/min and still stay within our defined limits of pressure and resolution. Ultra-Fast Separation Column: 100mm x 4.6mm, 2.7µm Fused-Core. C18 Halo Flow Rate: 2.5mL/min Resolution: 3,1 Pressure: 3,582psi Run Time: 1.9 minutes Sample Injection Volume: 6.4µl 49
21 Guide to Ultra-Fast HPLC Reference Tables and Equations for Quick Estimates Table 1: Estimated volume, Vm, for a variety of available column dimension Note: Column volumes listed here are estimates only. However, most commercial columns can be expected to have volumes within about 5% of what is reported here. Columns packed with Fused-Core particles are an exception and, therefore, are listed separately. Table 2: Column plate number, N, for columns packed with different size/type particles Note: Estimates in this table are for near ideal conditions. Column plate number is dependent on many factors including the solute, mobile phase viscosity and flow velocity and it is not unusual under real-world conditions for column plate numbers to be over 20% lower than what is reported here. Estimating changes in run time with changes in column volume and flow rate RT = Run Time V = Column Volume F = Flow rate (If column ID changes, mobile phase velocity should be used instead of flow rate.) Estimating changes in resolution with changes in column plate number Rs = Resolution N = Column plate number 50
22 HIGH AQUEOUS REVERSED-PHASE MATERIALS Introduction When separating very polar, water-soluble compounds, eluents containing less than 5% organic modifier are commonly used to achieve sufficient retention. However, operation under such highly aqueous conditions can lead to poor chromatographic reproducibility. The alkyl bonded phase of conventional C8 and C18 columns undergoes ʻphase collapseʼ or ʻmattingʼ under these conditions. The long alkyl chains fold over on themselves, excluding water and reducing the accessible bonded phase hence decreasing retention times. This phenomenon may occur either very quickly or more gradually. High Aqueous Phases The problem of phase collapse has been addressed by either embedding a polar group in the alkyl phase, or by using hydrophilic (polar) endcapping reagents (see Figure 1). Both these approaches, or the use of a C30 phase, have the effect of keeping the alkyl phase extended in the eluent, even when using 100% aqueous eluent. Both polar and non-polar analytes are able to interact with the stationary phase. In addition to their role in stabilizing the alkyl chains, the polar groups can also interact with polar functions on the sample molecules, introducing an alternative selectivity compared with conventional C8/C18 columns. Good retention and resolution for polar compounds It is claimed by the manufacturers of these phases that the alkyl chains do not show any signs of folding-over and phase collapse in highly aqueous eluents, even after several days or weeks. This results in reproducible retention times and improved peak shapes for acidic, basic and zwitterionic analytes. Figure 1. Polar embedded (A) and hydrophilic endcapped (B) phases (A) (B) Alternative selectivity Conventional C18 phases depend predominantly on hydrophobic interactions between analyte and stationary phase to provide separation. In addition, ʻAQʼ type phases also show hydrophilic (polar) interactions via H-bonding and dipole-dipole forces. This can influence retention time and improve selectivity for polar analytes. Eliminate need for ion-pair additives Many separations of very polar analytes are performed using ion-pair chromatography in order to provide adequate retention. The use of an ʻAQʼ phase generally enables reproducible results to be obtained using conventional aqueous/organic eluents. Typical applications Typical applications of these ʻAQʼ type phases include carboxylic acids, water soluble vitamins, catecholamines, nucleic acid bases and various polar pharmaceuticals. High Aqueous Phases Phase Manufacturer Particle Size (µm) Pore Size (Å) * Properties Page Ace AQ ACT 3, 5, C18 phase with integral polar functionality 66 AquaSep ES Industries 3, Embedded ether group Call Chromegabond ODS-PI ES Industries 3, Ureide embedded polar group Call Epic Polar ES Industries 3, 5, Embedded ether group Call HALO RP-Amide AMT Wettable polar amide group 107 Inertsil ODS-4 2, 3, C18 phase with high resistance to dewetting 134 Inertsil ODS-EP GL Sciences C18 phase with polar embedded group 145 Inertsil ODS-Sprint 3, C18 phase with high resistance to dewetting 147 NUCLEODUR C18 Pyramid 3, C18 phase with hydrophilic endcapping 187 NUCLEODUR PolarTec Macherey-Nagel 1.8, 3, C18 phase with embedded polar group NUCLEOSIL Nautilus 3, Call ProTec-RP ES Industries 3, C8, C18 or Phenyl phase with embedded amide group Call *For 300Å AQS phases, see page 58 51
23 HYDROPHILIC INTERACTION CHROMATOGRAPHY Introduction Hydrophilic Interaction Chromatography (HILIC) is a variant of normal-phase chromatography which is performed using polar stationary phases with partially aqueous eluents. Solutes elute in order of increasing hydrophilicity (polarity), the opposite of reversed-phase. Mode of operation Retention in HILIC is proportional to the amount of organic solvent in the eluent. Typical HILIC eluents contain 65-80% acetonitrile, methanol or propanol. The low proportion of water in the eluent generates a stagnant aqueous layer on the surface of the stationary phase. This enables solutes to partition between the eluent and aqueous layers (Figure 1). In addition, weak electrostatic interactions between solute and stationary phase contribute to overall selectivity. Gradient elution may be performed either with a decreasing organic or increasing salt gradient. Salt is not required for uncharged solutes such as carbohydrates, but 10mM salt is necessary with charged solutes such as peptides. Ammonium formate and acetate are suitable volatile buffers for LC-MS. Other salts with good solubility in HILIC eluents include potassium methylphosphonate, triethylamine phosphate and sodium perchlorate. Applications HILIC phases are particularly useful for compounds that are weakly retained or eluted in the void volume of reversedphase columns. Typical application areas include carbohydrates, oligonucleotides, peptides and proteins, amino acids, natural products and phosphorylated compounds. HILIC phases do not react with reduceable sugars, making them a good substitute for amino columns in analyzing carbohydrates. Figures 2 and 3 illustrate the separation selectivity of HILIC compared to reversed-phase. Figure 1. Hypothetical partition mechanism of Hydrophilic Interaction Chromatography (HILIC) Mobile Phase (mostly organic) Solute Solute Mobile Phase (stagnant; mostly aqueous) H-Y-D-R-O-P-H-I-L-I-C SILICA C-O-A-T-I-N-G Figure 2. HILIC vs. RP: Inverse selectivity HILIC PolyLC Arabidopsis thaliana. leaf extract Figure 3. HILIC vs. RP: Inverse selectivity Eluent: A: 60/40 acetonitrile/ 10mM ammonium acetate, ph 7 B: 5/95 acetonitrile/ 10mM ammonium acetate, ph 7 Flow Rate: 1.0 ml/min Injection: 20 µl Detection: UV, 214nm Sample: about 0.25 mg/ml of each peptide in eluent Column A ZIC -HILIC, 150mm x 4.6mm PEEK, 5µm 200Å RP C18 Hypersil Column B Kromasil C18, 150mm x 4.6mm SS, 5µm 185Å Peak 3 not displayed: FGGF (>20 min. retention) HILIC Phases Phase Manufacturer Functional Group Particle Size (µm) Pore Size (Å) Page HALO HILIC AMT Inertsil HILIC GL Sciences Propyl alcohol 3, NUCLEODUR HILIC Macherey-Nagel Ammonium-sulphonic acid 3, PolyGLYCOPLEX - 5, PolyHYDROXYETHYL A PolyLC Hydroxyethylaspartamide 3, PolySULFOETHYL A Sulfoethylaspartamide 3, ZIC-HILIC SeQuant (E.M.D.) Sulfobetaine 3.5, 5, ZIC-pHILIC SeQuant (E.M.D.) Sulfobetaine 3,
24 ION-EXCHANGE PHASES Introduction Ion-exchange phases separate solutes on the basis of ionic charge. Retention in ion-exchange chromatography is determined by the ph of the eluent, the nature and ionic strength of the buffer and temperature. Column efficiencies are lower than in reversed-phase HPLC. Eluents are normally aqueous but can contain some organic component. Base material Both silica-based and polymer-based ion-exchangers are available. For the former, ionic species are attached to the silica surface, whereas for the latter the ion-exchange groups are distributed throughout the matrix. Silica based materials maintain a mechanical strength and higher efficiency advantage whereas the polymer based materials have greater ph stability. Classification Ion-Exchange Ion-Exchange Phases Application Ion-exchange is used for the analysis of small ions but its key application area is in the separation of biomolecules such as proteins and nucleic acids. Weak ion-exchangers are used for the analysis of inorganic ions, a technique more specifically termed ion-chromatography. Ion-Exchange capacity The exchange capacity of an ion-exchanger is an important measure of its retentivity (typically measured in milliequivalents per gram material). For any one column the packing density of the phase must also be taken into account. Wide pore materials will typically have lower ion-exchange capacities. Type Strength Nomenclature Typical Functionality ph Ionization Range Anion Cation Weak WAX Amine Ionized at specific ph Strong SAX Quaternary Ammonium Ionized over complete ph range Weak SCX Sulphonic Acid Strong WCX Carboxylic Acid Ionized at specific ph Phase Manufacturer Base Material Classification Particle Size (µm) Pore Size (Å) Applications and Features Page Asahipak ES-502N WAX Asahipak ES-502C WCX 9 2,000 Proteins, peptides, oligonucleotides, catecholamines 229 IEC QA SAX 12 5,000 IEC DEAE 8 5,000 WAX IEC DEAE3N Polymer Shodex IEC SP ,000 SCX IEC SP-420N Proteins, peptides, DNA, RNA 225 IEC CM WCX 8 5,000 AXpak WA WAX 10 2,000 Nucleotides Call CXpak P S-DVB SCX 6 Amino acids Call Hamilton PRP-X100 WAX 5, 10, Anions, inorganic and organic Hamilton PRP-X110 WAX Lower level anions Hamilton PRP-X500 SAX, WAX 7 Superficially porous Large proteins and labeled DNA Hamilton PRP-X600 SAX, WAX 7 Superficially porous Labeled and unlabeled DNA Hamilton RCX-10 AX Carbohydrate oligomers up to DP8 Hamilton RCX-30 AX Complex carbohydrates Hamilton PRP-X200 WCX Inorganic and organic cations Hamilton PRP-X400 Hamilton Polymer CX 7, Glyphosate, sugar alcohols 116 Hamilton PRP-X800 CX 7 - Mono and divalent cations/ Transition metals Hamilton HC-40 CX Gel-type Sugar oligomers up to DP8 Hamilton HC-75 H Organic acids and sugars Hamilton HC-75 C CX 9 Gel-type Mono and disaccharides Hamilton HC-75 Pb Sugar alcohols Inertsil AX, CX GL Sciences Silica SAX, SCX Small molecule analysis 150 Jordi SAX 1 Jordi Quat DVB SAX Inorganic ions NUCLEOGEL Anion l Polymer SAX Inorganic anions NUCLEOGEL SAX Polymer SAX 8, 10 1,000, 4,000 Biological macromolecules NUCLEOGEL SCX Polymer SAX 8, 10 1,000, 4,000 Biological macromolecules Call NUCLEOSIL Anion Silica SAX Macherey-Nagel NUCLEOSIL Anion ll Silica SAX Inorganic anions NUCLEOSIL PEI Polymer coated silica WAX Proteins and peptides NUCLEOSIL SA SCX 5, Silica Inorganic and organic small molecules NUCLEOSIL SB SAX 5, Partisil SAX, SCX Whatman Silica SAX, SCX Small molecule analysis Partisphere SAX, SCX PolyCAT A WCX 300, 1000, 1500 Aspartic acid functionality PolySULFOETHYL A PolyLC Silica SCX 3, 5, , 1000 Sulfoethylaspartamide 205 PolyWAX WAX 100, , 5000 Proteins with isoelectricº point <6.0 1 WCX and WAX available upon request 53
25 PHENYL-BONDED PHASES Phenyl-bonded silica columns offer an alternative reversedphase selectivity. They show similar retention characteristics to C8-bonded phases but exhibit a different selectivity for aromatic compounds. Nitrophenyl- and pentafluorophenyl-bonded phases offer further selectivity alternatives, especially for fluorine-containing molecules. Pentafluorophenyl phases enhance interaction with aromatics, halogens, conjugated systems, and epimers. Pentafluoropheny phases can often separate impurities that co-elute on an ODS column. Traditional phenyl phases tend to be less stable than the corresponding C8 or C18 reversed-phases. The larger steric size of the phenyl group reduces surface coverage leaving a greater number of exposed silanol sites. More recently introduced phenyl phases show greater stability. The use of a purer silica base, more effective and reproducible bonding procedures and the availability of a sterically protected phenylsilane all contribute to greater phase robustness. Phenyl-Bonded Phases Phase ACE-C18 AR 1 Manufacturer Particle Size (µm) Pore Size (Å) Surface Area Page 3, 5, ACE-C18 PFP 1 ACT 3, 5, Ace Phenyl 1 3, 5, ES Industries FluoroSep Pentafluorophenyl 3, Call ES Industries ProTec Phenyl Call ES Industries BAS Phenyl ES Industries 3, Call ES Industries Diisopropyl/Phenyl 3, Call Fluophase PFP Call HALO PFP 1 AMT Hypersil GOLD Phenyl 1 1.9, 3, Hypersil Phenyl 3, 5, Thermo Scientific Hypersil Phenyl-2 3, 5, Hypersil BDS Phenyl 3, Inertsil Phenyl GL Sciences Inertsil Phenyl-3 1 2, 3, Nucleodur PFP 1 1.8, 3, Macherey-Nagel Nucleosil Phenyl 5, 7 100, , Primesep P Sielc 5, New generation phases 54
26 POLAR BONDED PHASES Introduction Polar bonded silicas offer an alternative selectivity to alkyl bonded materials (see p.30-39). In general they have a lower hydrophobicity but higher polarity. Cyano-, amino- and diol-bonded phases can be used in both normal- and reversedphase modes. In normal-phase they equilibrate more rapidly with the eluent than silica itself and are not deactivated by traces of water. Availability Cyano-, amino- and diol-bonded phases are available from a number of manufacturers. The cyano phase is commonly used in reversed-phase. Wide pore silicas are now available for the analysis of hydrophobic proteins. Due to its rapid equilibration it is very suitable for normal-phase gradient elution. In addition to normal-phase separations, amino-bonded material can be used in reversed-phase mode for the separation of polar compounds such as carbohydrates or as a weak anion-exchanger. The diol phase can be used in reversed-phase, normal-phase and HILIC modes. Differing pore size materials are used in sizeexclusion separations. Polar Bonded Phases Phase Manufacturer Particle Size (µm) Pore Size (Å) Surface Area Page Ace CN 1 ACT 3, 5, Chromegabond BAS-CN Chromegabond CN-BD Chromegabond CN ES Industries 3, 5, 10 Chromegabond CN/HS Chromegabond D (Diol) 475, , 100 Chromegabond A (Amine) 310 Epic Polar 1 3, Call Hypersil APS-2 3, 5, Hypersil BDS CPS 3, Hypersil CPS Thermo Scientific 3, 5, Hypersil CPS-2 5, Hypersil GOLD CN 1 1.9, 3, Inertsil CN-3 1 3, 5, Inertsil NH 2 GL Sciences Inertsil Diol LiChrosorb CN LiChrosorb NH 2 LiChrosorb OH LiChrospher CN LiChrospher NH 2 LiChrospher OH Nucleodur CN 1 / CN-RP 1 Merck 5, Call , Nucleodur NH 2 1 / NH 2 -RP Nucleodur PolarTec 1 1.8, 3, Nucleosil CN Macherey-Nagel 5, 7, , , 200 Nucleosil NH 2 3, 5, 7 100, , 200 Nucleosil NO Nucleosil OH 5, New generation phases 55
27 SILICA PHASES Silica introduction Despite its porosity, spherical porous HPLC silica exhibits a high mechanical strength compared with other materials. Additionally, it is readily chemically modified. A wide range of porous silicas are available for normal-phase HPLC, characterized by surface area, pore size and particle size measurements. Particle size For analytical work, as the quality and reproducibility of porous silica improves, the use of 1.8, 3 or 3.5 particle size materials increases. 10µm particles are less commonly used but remain a key particle size for preparative applications. For economic reasons irregular silicas still share some of this latter market. Purity and quality The physical characteristics of the newer silica particles have been improved in several ways. Surface activity A lower level of the unwanted, free and isolated silanol groups is observed. The lower metal ion contaminant level partly contributes to this drop in surface activity. Basic compounds interact less strongly with the silica surface resulting in improved chromatography. Physical properties Improved control of physical properties such as surface area, pore volume, mean pore diameter and particle size have given the new silicas better lot-to-lot reproducibility. Purity The level of metal ion impurities have in some cases been reduced to cumulative figures < 10ppm. Undesirable chelation of metal ion and solute has been minimized. Inertsil pure silica gel Silica Phases Phase Manufacturer Particle Size (µm) Pore Size (Å) Surface Area Page ACE ACT 3, 5, Hypersil Thermo Scientific 3, 5, Inertsil GL Sciences 1 3, LiChrosorb Merck 5, 10 60, , LiChrospher Merck 5 60, , NUCLEODUR Macherey-Nagel 1 3, NUCLEOSIL Macherey-Nagel 3, 5, , , Partisil Whatman 5, Preparative grades available 56
28 SIZE EXCLUSION CHROMATOGRAPHY PHASES Introduction SEC columns separate components according to their molecular size in solution, larger molecules eluting first. Separation is achieved by the differential exclusion or inclusion of components within the packing material particles. In addition to the separation of discrete components, the technique is used for characterizing the molecular weight distribution of polymers. Base material Modes of operation Gel permeation chromatography (GPC) refers to the SEC separation of organic soluble polymers using an organic solvent as the eluent. Gel filtration chromatography (GFC) refers to the separation of water soluble polymers in aqueous capacity compared with adsorptive HPLC techniques. Applications Silica based SEC materials generally exhibit higher resolving power than polymer based materials. However, polymer based materials show greater stability for use with high ph eluents. Polymeric packing materials are generally available in larger particle sizes, which may be more practical for large-scale preparative separations. Size Exclusion Chromatography Phases Phase/Series Manufacturer Base Material/ Bonding Mode SEC analyses do not normally result in the denaturation of samples, making the technique a suitable choice for biological samples where activity must be retained. A wide range of bio-molecules and organic polymers are separated by SEC. For samples of wide molecular weight distribution, it can be useful to use a mixed pore size phase or to couple columns of one or more pore sizes in series. Pore Sizes (Å) Typical Applications Gel DVB DVB GPC 100, 500, 10 3, 10 4, 10 5 Organic Solvent GPC 168 Hydroxylated DVB DVB (OH) GPC 100, 500, 10 3, 10 4, 10 5 H 2 O/Organic Solvent GPC Call Sulfonated DVB DVB (SO 3 ) GPC 100, 500, 10 3, , 10 Aqueous GPC of neutral or Neg. charged polymers 168 Polar Pac WAX DVB Jordi DVB (WAX) GPC 100, 500, 10 3, , 10 Aqueous GPC of neutral or Pos. charged polymers Call Fl.A.S.H Fluorinated DVB DVB (F) GPC 100, 500, 10 3, 10 4, 10 5 High Speed GPC 170 Glucose GBR DVB DVB (Glucose) GPC 100, 500, 10 3, , 10 Aqueous GPC of noncharged polymers 172 Silica with hydroxyethylaspartamide coating 1000, , 100, 200, 300, 500, PolyHYDROXYETHYL A PolyLC GPC Peptides, proteins, carbohydrates, small molecules 205 KF-800 Max. 50, 150, 300, 500, 1500, 5000, 10,000, 20,000 Call LF Max. 3,000 Call KF-600 Max. 50, 150, 300, 500, 1500, 5000, 10,000, 20,000 General synthetic polymer Call Porous SDVB GPC Max. 50, 150, 300, 500, KF-400HQ 1500, 5000, 10,000 Call K-800 Max. 50, 150, 300, 500, 1500, 5000, 10,000, 20,000 Call Max. 50, 150, 300, 500, KD , 5000, 10,000, 20,000 Polar polymer (polymide, polyvinylpyrrolidone etc.) Call Max. 100, 200, 800, 2,000, SB-800 HQ PHM gel GPC/GFC 7,000, 15,000, 30,000 Polar polymer/biological macromolecule/water-soluble polymer 224 SB400 PHM gel GPC/GFC Max. 40, 200, 800, 2,000 Polar polymer/biological macromolecule/water-soluble polymer 224 HT-800 Shodex GPC Max. 500, 1,500, 5,000, 10,000, 20,000 Analysis at High Temperature Call UT-800 GPC Max. 300, 10,000, 20,000 Analysis at High Temperature Call AT-806MS Porous SDVB GPC Max. 10,000 Analysis at High Temperature Call Max. 500, 1,500, 5,000, HFIP-800 GPC 10,000, 20,000 Engineering resin analysis at room temperature Call HFIP-600 GPC Max. 500, 1,500, 5,000, 10,000, 20,000 Page Engineering resin analysis at room temperature Call Proteins, glycoproteins and peptides 224 KW-800 GFC Max. 400, 1,000, 1,500 Porous silica gel KW400 GFC Max. 400, 800, 1,500, 2,000 Biological macromolecule 224 Max, 20, 60,100, 200, KS-800 Sulfonated PS Gel GFC Mono, di, tri, oligo and polysaccharides, starches and celluloses Call 500,1000 GS-HQ GFC Max. 150, 400, 2,000, 7,000 Oligosaccharide, polysaccharide Call PVA GF-HQ GPC/GFC Max. 400, 2,000, 10,000 Aqueous and organic Call 57
29 WIDE PORE (300Å) REVERSED-PHASE MATERIALS Introduction In order for a sample molecule to freely access the interior of the pores of the packing material, its diameter must be smaller than the average pore diameter. For high molecular weight solutes, the use of lower pore size materials of Å may result in frictional drag within the pore leading to restricted diffusion and reduced column efficiency. The use of larger pore silica-based bonded phases leads to improvements in resolution, capacity and recovery of proteins and other biomolecules, due to a reduction in size-exclusion mechanism and enhanced molecular diffusion rates. A pore size of 300Å has become the accepted standard for wide pore silicas, and has been found to be suitable for a broad range of molecular weight proteins, peptides and oligonucleotides. In general, peptides exceeding about 50 amino acids and oligonucleotides greater than 25 residues are preferentially analyzed on 300Å materials. Separations of very large biomolecules (MW >100,000 Da) may require larger pore size packings (500 to 4000Å). Bonded phases Alkyl bonded silica phases are the most commonly used materials for the reversed-phase separation of biomolecules. The shorter C4 matrices are generally recommended for large hydrophobic peptides and most proteins. Peptide maps, natural and synthetic peptides and small hydrophilic proteins are best chromatographed on C8 columns. C18 columns are often chosen for the analysis of small peptides. Other bonded wide pore phases, including cyano, diol and phenyl, are available in some brands. The table below summarizes a range of wide pore alkylbonded reversed-phase silica materials. Column dimensions Wide pore silica phases are available in a range of column dimensions from rapid analysis to preparative and process scale. Increased column capacity favours these wide pore materials for preparative separations of samples with molecular weight >5000 Da. 300Å Reversed-Phase Bonded Silica Phases Phase Manufacturer Particle Size (µm) Surface Area (m 2 /g) CarbonLoad (%) 2.6 Ace C ACT 3, 5, Ace C Ace C ACE CN Inertsil WP300-C4 1 3 Inertsil WP300-C8 1 4 Inertsil WP300-C18 1 GL Sciences Inertsil WP300-Diol 1 9 Inertsil WP300-SIL 1 0 MacroSep C4 1.8 MacroSep C8 3.7 MacroSep C18 7 ES Industries 3, MacroSep AQS - MacroSep CN - MacroSep HPR - Nucleosil 300 C4 5, 7 2 Nucleosil 300 C8 Macherey-Nagel Nucleosil 300 C Page Call Call 1 New generation phases 58
30 COLUMN SELECTION BY TRADENAME A distributor of world class LC columns, Canadian Life Science has gained a unique knowledge of the worldʼs packing materials. We distribute 70% of the worldʼs trademarked columns. We appreciate that the chromatographer can choose from hundreds of different packing materials. The following pages give some guidance, but please consult us for your specific needs. In recent times we have supplied most of the popular columns listed in the table. There are many other LC columns not listed, on which we can offer guidance. For more information, please call or us. We are here to assist you! Material Tradename Manufacturer Description Page Ace ACT Base deactivated spherical silica 65, 236 Adsorbosphere Alltech Spherical silica - Apex Jones Chromatography Spherical silica - Aquapore Perkin Elmer Wide-pore spherical silica Call AquaSep ES Industries C18 spherical silica for high water content eluents Call Asahipak Shodex Polymer based gel 225, 229 BetaBasic Thermo Scientific Base deactivated spherical silica 125 Betasil Thermo Scientific Base deactivated spherical silica 124 BioBasic Thermo Scientific Base deactivated wide pore spherical silica Call Bioptic GL Sciences Glycoprotein bonded silica Call µbondapak Waters Irregular silica Call Brownlee Perkin Elmer Range of silica based materials Call Capcell Pak Shiseido Polymer coated silica Call CHIRA-chrom Hichrom Chiral Pirkle silica 119 ChiraDex Merck Chiral cyclodextrin-bonded silica Call CHIRAL-AGP, CBH, HSA Daicel Chiral protein-bound silica 86 CHIRALCEL Daicel Chiral cellulose-bonded silica 90 CHIRALPAK Daicel Chiral cellulose-bonded silica 85, 90 ChiroSil RStech Chiral Covalently bonded silica 221 Chromegabond ES Industries Silica and alumina 102 Chromolith Merck Monolithic silica Call Cosmosil Nacalai Tesque Spherical silica Call Cyclobond Astec Chiral cyclodextrin-bonded silica Call DACH-DNB Regis Pirkle-type chiral silica 217 Daisogel Daiso Base deactivated spherical silica 245 Develosil Nomura Base deactivated spherical silica Call Dynamax Varian Spherical silica with dynamic axial compression hardware Call Eurospher Knauer Base deactivated spherical silica Call EPIC ES Industries Base deactivated spherical silica 100 Exsil Grace Davison Spherical silica Call Fluophase Thermo Scientific Fluorinated silica Call FluoroSep-RP ES Industries Fluoroacyl bound silica 103 Gammabond Alumina ES Industries Spherical alumina containing polymer coating Call Genesis Grace Davison Base deactivated spherical silica - HALO Advanced Materials Technology Solid silica core with porous outer shell 106 Hichrom Hichrom Base deactivated spherical silica 118 Hypercarb Thermo Scientific Graphitic carbon Call Hypersil Thermo Scientific Spherical silica 122 Hypersil BDS Thermo Scientific Base deactivated spherical silica 123 Hypersil GOLD Thermo Scientific Base deactivated spherical silica 120 HyPURITY Thermo Scientific Base deactivated spherical silica Call Inertsil ODS-4, ODS-3, ODS Inertsil 80Å GL Sciences Base deactivated spherical silica Call Inertsil Sustain 140 Jordi-Gel Jordi & Associates Polymerized divinylbenzene 167 Kromasil Eka Chemicals Base deactivated spherical silica 183 L-Column CERI Base deactivated spherical silica Call LiChrosorb Merck Irregular silica 184,186 LiChrospher Merck Spherical silica 185,186 1 New generation phases 59
31 COLUMN SELECTION BY TRADENAME (CONT D) Material Tradename Manufacturer Description Page MetaSil Varian/Agilent Spherical silica Call MacroSep ES Industries Wide pore spherical silica Call MCI GEL Mitsubishi Chemicals Polymeric phases Call Microsorb Varian Spherical silica - Mightysil Kanto Kagaku Spherical silica Call Nova-Pak Waters Spherical silica - NUCLEODUR Macherey-Nagel Base deactivated spherical silica 187 NUCLEOGEN Macherey-Nagel DEAE anion exchange silica Call NUCLEOSIL Macherey-Nagel Spherical silica 193 OBELISC SIELC RP/ion-exchange mixed mode silica 213 Partisil Whatman Irregular silica 203 PartiSphere Whatman Spherical silica 204 PLgel Polymer Labs GPC polystyrene-divinylbenzene gel Call PLRP-S Polymer Labs Reversed-phase polystyrene-divinylbenzene gel Call PolarTec Macherey-Nagel Base deactivated spherical silica 192 PolyCAT A PolyLC Weak cation exchange silica POLYGOSIL Macherey-Nagel Irregular silica 205 PolyHYDROXYETHYL PolyLC Hydrophilic interaction silica µporasil Waters Irregular silica - Poroshell Agilent Technologies Superficially porous material Call Primesep SIELC RP/ion-exchange mixed mode silica 211 ProC18 YMC Base deactivated silica - ProntoSIL Bischoff Base deactivated spherical silica Call ProTec RP ES Industries Base deactivated silica Call PRP-1 Hamilton Reversed-phase polystyrene-divinylbenzene gel PRP-X100 Hamilton Anion exchange polystyrene-divinylbenzene gel 116 Purospher Merck Base deactivated silica 186 Regis Regis Technologies Spherical silica 217 Resolve Waters Spherical silica - RESOLVOSIL Macherey-Nagel Chiral protein-bound silica 194 Shodex Showa Denko Spherical silica and polymer 224 Spherisorb Waters Spherical silica and alumina Call Sugar-Pak Waters Call Sulphonated styrene-divinylbenzene gel Sunfira Waters Call Supelcosil ABZ + Supelco Base deactivated spherical silica - Supelcosil-LC Supelco Spherical silica - SynChropak Eprogen Spherical silica - Symmetry Waters Spherical silica - Titansphere GL Sciences Titania based phase 248 TSK Tosoh Bioscience Spherical silica and polymer - ULMO Regis Technologies Pirkle-type chiral silica 217 Ultrasphere Beckman Spherical silica 235 Ultron ES-OVM Shinwa Chemical Industries Chiral protein-bound silica Call VHP Grace Vydac Wide pore ion exchange - Vydac Grace Vydac Wide pore spheroidal silica - Wakosil Wako Chemicals Base deactivated spherical silica Call Whelk-01/Whelk-02 Regis Pirkle-type chiral silica 217 YMC YMC Irregular and spherical silica - ZIC-HILIC SeQuant Zwitterionic spherical silica 222 Zorbax Agilent Technologies Spherical silica - Zorbax Rx Agilent Technologies Base deactivated silica - Zorbax Bioseries Agilent Technologies Wide pore spherical silica - Zorbax Bonus-RP Agilent Technologies Polar-linked alkyl bonded silica - Zorbax Eclipse XDB Agilent Technologies Spherical silica (extra dense bonding) - Zorbax Extend-C18 Agilent Technologies Bidentate bonded silica - Zorbax SB Agilent Technologies Stablebond spherical silica - 60
32 COLUMN SELECTION BY USP SPECIFICATIONS The following list of USP (United States Pharmacopoeia) column specifications (USP 24) includes a selection of recommended columns within each category. L1 Widely available (p.36-37) L2 L3 L4 L5 L6 L7 L8 L9 L10 L11 L12 L13 L14 L15 L16 L17 L18 L19 L20 L21 L22 L23 Octadecylsilane chemically bonded to porous silica or ceramic particles, 3 to 10µm in diameter. For most cases there are several columns available within a given category, but in a few indicated instances a packing very closely fitting the specification has been included. Octadecylsilane chemically bonded to silica gel of a controlled surface porosity that has been bonded to a solid spherical core, 30 to 50µm in diameter. Pellicular ODS (Whatman) Porous silica particles, 3 to 10µm in diameter. Widely available (p.56) Silica gel of controlled surface porosity bonded to a solid spherical core, 30 to 50µm in diameter. Pellicular silica (Whatman) Alumina of controlled surface porosity bonded to a solid spherical core, 30 to 50µm in diameter. Please enquire Strong cation exchange packing - sulphonated fluorocarbon polymer coated on a solid spherical core, 30 to 50µm in diameter Please enquire Octylsilane chemically bonded to totally porous silica particles, 3 to 10µm in diameter. Widely available (p.39) An essentially monomolecular layer of aminopropylsilane chemically bonded to a totally porous silica gel support, 10µm in diameter Widely available (p.55) Totally porous silica gel, with a chemically bonded strongly acidic cation-exchange coating, 3 to 10µm in diameter. Inertsil CX (p.53) Partisil 10 SCX (p.203) Nitrile groups chemically bonded to porous silica particles, 3 to 10µm in diameter. Widely available (p.55) Phenyl groups chemically bonded to porous silica particles, 5 to 10µm in diameter. Widely available (p.54) Strong anion-exchange packing made by chemically bonding a quaternary amine to a solid silica spherical core, 30 to 50µm in diameter. Fractogel EMD TMAE(S) (Merck) Trimethylsilane chemically bonded to porous silica particles, 3 to 10µm in diameter. Shodex Silica 5TMS (Call) Silica gel, 10µm in diameter, with a chemically bonded strongly basic quaternary ammonium anion exchanger coating. Nucleosil SB (Call) Partisil SAX (p.203) Hexylsilane chemically bonded to totally porous silica particles, 3 to 10µm in diameter. Chromegabond C6 (Call) Dimethylsilane chemically bonded to totally porous silica particles, 5 to 10µm in diameter Chromegabond C2 (Call) Nucleosil C2 (p.193) Strong cation exchange resin consisting of sulphonated cross-linked styrene-divinylbenzene copolymer in the hydrogen form, 7 to 11µm in diameter Shodex RSpak KC-811 (p.227) Shodex SUGAR SH1011 (p.229) Shodex SUGAR SH1821 (p.229) Amino and cyano groups chemically bonded to porous silica particles, 3 to 10µm in diameter Partisil PAC (Call) Strong cation exchange resin consisting of sulphonated cross-linked styrene-divinylbenzene copolymer in the calcium form, about 9µm in diameter Shodex SUGAR SC1011 (p.227) Shodex SUGAR SH1211 (p.227) Shodex SUGAR SH1821 (p.227) Dihydroxypropane groups chemicallybonded to porous silica particles, 3 to 10µm in diameter Inertsil Diol (p.150) Shodex PROTEIN KW-800 series (p.224) Shodex PROTEIN KW400 series (p.224) A rigid, spherical styrene-divinylbenzene copolymer, 5 to 10µm in diameter Shodex RSpak DS-613, DS-413, RP18-413, RP (p.227) Shodex GPC KF,K,KD,GPC LF (Call) A cation exchange resin made of porous polystyrene gel with sulphonic acid groups, about 10µm in size. Shodex SUGAR SH1011, SP0810, SC1011, KS-800 RSpak DC-613, KC-811 (p.229) An ion exchange resin made of porous polymethacrylate or polyacrylate gel with quaternary ammonium groups, about 10µm in size Shodex IEC QA-825 (Call) 61
33 COLUMN SELECTION BY USP SPECIFICATIONS L24 L25 L26 L27 L28 L29 L30 L31 A semi-rigid hydrophilic gel consisting of vinyl polymers with numerous hydroxyl groups on the matrix surface, 32 to 63µm in diameter. Toyopearl HW 40F 1 Packing having the capacity to separate compounds with a molecular weight range from 100 to 5000 daltons (as determined by polyethylene oxide), applied to neutral, anionic and cationic water-soluble polymers. A polymethacrylate resin base, cross-linked with polyhydroxylated ether (surface contained some residual carboxyl functional groups) was found suitable. Shodex OHpak SB-802 HQ (p.224) Shodex SB402.5 (p.224) Butylsilane chemically bonded to totally porous silica particles, 5 to 10µm in diameter. Widely available (p.38) Porous silica particles, 30 to 50µm in diameter. Daisogel (p.245) A multifunctional support which consists of a high purity 100Å spherical silica substrate that has been bonded with anionic (amine) functionality in addition to a conventional reversed-phase C8 functionality. ProTec C8 (Call) Gamma alumina, reversed-phase, low carbon percentage by weight, alumina-based polybutadiene spherical particles, 5µm in diameter with a pore diameter of 80Å. GammaBond RP1 (Call) Ethyl silane chemically bonded to totally porous silica particles, 3 to 10µm in diameter As for L16 1 A strong anion exchange resin - quaternary amine bonded on latex particles attached to a core of 8.5µm macroporous particles having a pore size of 2000Å and consisting of ethylvinylbenzene cross-linked with 55% divinylbenzene. L32 A chiral ligand-exchange packing - L-proline copper complex covalently bonded to irregularly shaped silica particles, 5 to 10µm in diameter. Nucleosil Chiral-1 (p.194) L33 Packing having the capacity to separate proteins by molecular size over a range of 4,000 to 400,000 daltons. It is spherical, silica-based and processed to provide ph stability. Shodex PROTEIN KW-800 series, KW-400 series (p.224) L34 Strong cation exchange resin consisting of sulphonated cross-linked styrene-divinylbenzene copolymer in the lead form, about 9µm in diameter Hamilton HC-75 Pb 2+ (p.116) 7µm Shodex SUGAR SP0810 (Call) L35 A zirconium-stabilised spherical silica packing with a hydrophilic (diol-type) molecular monolayer bonded phase having a pore size of 150Å. L36 L37 L38 L39 L40 L41 L42 L43 L44 A 3,5-dinitrobenzoyl derivative of L-phenylglycine covalently bonded to 5µm aminopropyl silica. Hichrom CHIRA-chrom-1 (p.119) Nucleosil CHIRAL-3 (p.194) Polymethacrylate gel packing having the capacity to separate proteins by molecular size over a range of 2,000 to 40,000 daltons. Shodex OHpak SB-803HQ (p.224) Shodex SB-403 (p.224) A methacrylate-based size-exclusion packing for water-soluble samples. Shodex OHpak SB-800HQ series (p.224) Shodex SB400 series (p.224) A hydrophilic polyhydroxymethacrylate gel of totally porous spherical resin. Shodex OHpak SB-800HQ series (p.224) Shodex SB400 series (p.224) Shodex ODP2 HP (p.226) Shodex RSpak DM-614 (p.227) Cellulose tris-3,5-dimethylphenylcarbamate coated porous silica particles, 5 to 20µm in diameter CHIRALCEL OD (p.90) Immobilized α 1 -acid glycoprotein on spherical silica particles, 5µm in diameter CHIRAL-AGP (p.88) Octylsilane and octadecylsilane groups chemically bonded to porous silica particles, 5µm in diameter Hichrom RPB (p.118) Pentafluorophenyl groups chemically bonded to silica particles, 5 to 10µm in diameter ACE PFP (p.70) HALO PFP (p.110) A multifunctional support, which consists of a high purity, 60Å, spherical silica substrate that has been bonded with a cationic exchanger, sulphonic acid functionality in addition to a conventional reversed-phase C8 functionality Chromegabond RP-SCX (Call) 1 Column represents the closest match to USP specifications 62
34 COLUMN SELECTION BY USP SPECIFICATIONS L45 L46 L47 L48 L49 L50 Beta cyclodextrin bonded to porous silica particles, 5 to 10µm in diameter ChiraDex Shodex ORpak CDBS-453 (call) Polystyrene/divinylbenzene substrate agglomerated with quaternary amine functionalized latex beads, 10µm in diameter CarboPac PA1 (Dionex) CarboPac PA100 (Dionex) High capacity anion exchange microporous substrate, 8µm in diameter, dimension 250mm x 4.0mm CarboPac MA1 (Dionex) Sulphonated, cross linked polystyrene with an outer layer of sub-micron porous, anion exchange microbeads, 15µm in diameter Ion Pac AS7 A reversed-phase packing made by coating a thin layer of polybutadiene on to spherical porous zirconia particles, 3 to 10µm in diameter Multifunction resin with reversed-phase retention and strong anion-exchange functionalities. The resin consists of ethylvinylbenzene, 55% cross-linked with divinylbenzene copolymer, 3 to 15µm in diameter, and a surface area of not less than 350m 2 /g. Substrate is coated with quartenary ammonium functionalized latex particles consisting of styrene crosslinked with divinylbenzene. L51 L52 L53 Amylose tris-3,5-dimethylphenylcarbamate-coated, porous, spherical silica particles, 5 to 10µm diameter. Chiralpak AD (Call) A strong cation exchange resin made of porous silica with sulfopropyl groups, 5 to 10µm in diameter. BioBasic SCX (Call) Weak cation-exchange resin consisting of ethylvinylbenzene, 55% cross-linked with divinylbenzene copolymer, 3 to 15µm in diameter. Substrate is surface grafted with carboxylic acid and/or phosphoric acid functionalized monomers. Capacity not less than 500 µeq/column. L54 A size exclusion medium made of covalent bonding of dextran to highly cross-linked porous agarose beads, about 13µm in diameter. Superdex peptide HR 10/30 (Call) L55 A strong cation exchange resin made of porous silica coated with polybutadiene-maleic acid copolymer, about 5µm in diameter. Universal Cation Universal Cation HR L56 Isopropyl silane chemically bonded to totally porous silica particles, 3 to 10µm in diameter. L57 L58 L59 L60 L61 L62 L63 L64 A chiral-recognition protein, ovomucoid, chemically bonded to silica particles, about 5µm in diameter, with a pore size of 120 angstroms Ultron ES-OVM (Call) Strong cation-exchange resin consisting of sulfonated cross-linked styrene-divinylbenzene copolymer in the sodium form, about 7 to 11µm diameter. Shodex CXpak P-421S (Call) Shodex RSpak DC-613pak P-421S (p.227) Shodex SUGAR KS-800 series (p.224) Packing having the capacity to separate proteins by molecular weight over the range of 10 to 7000kDa. It is spherical (5-10µm), silica-based and processed to provide hydrophilic characteristics and ph stability. Shodex PROTEIN KW-800 series, KW-400 series (p.224) Spherical porous silica gel, 10µm or less in diameter, the surface of which has been covalently modified with alkyl amide groups and endcapped. HALO RP-Amide (p.107) A hydroxide-selective, strong anion-exchange resin consisting of a highly cross-linked core of 13µm microporous particles having a pore size less that 10 angstrom and consisting of ethylvinylbenzene cross-linked with 55% divinylbenzene with a latex coating composed of 85nm diameter microbeads bonded with alkanol quaternary ammonium ions (6%). C30 silane bonded phase on a fully porous spherical silica, 3 to 15µm in diameter. Chromegabond C30 (p.102) Glycopeptide teicoplanin linked through multiple covalent bonds to a 100Å spherical silica Shodex SUGAR KS-800 series (p.224) Strongly basic anion exchange resin consisting of 8% crosslinked styrene divinylbenzene copolymer with a quartenary ammonium group in the chloride form, 45 to 180µm in diameter AG 1-X8 63
35 COLUMN SELECTION BY USP SPECIFICATIONS L65 L66 L67 L68 L69 L70 L71 L72 L73 L74 Strongly acidic cation exchange resin consisting of 8% sulfonated crosslinked styrene divinylbenzene copolymer with a sulfonic acid group in the hydrogen form, 63 to 250µm in diameter. AG 50W-X2 A crown ether coated on a 5µm particle size silica gel substrate. The active site is (S)-18-crown-6-ether Crownpak CR(+) (p.92) Porous vinyl alcohol copolymer with a C18 alkyl group attached to the hydroxyl group of the polymer, 2 to 10µm in diameter Shodex Asahipak ODP-40 (p.226) Shodex Asahipak ODP-50 (p.226) Spherical, porous silica, 10µm or less in diameter, the surface of which has been covalently modified with alkyl amide groups and not endcapped Ethylvinylbenzene/divinylbenzene substrate agglomerated with quaternary amine funtionalized 130nm latex beads, about 6.5µm in diameter CarboPac PA20 (Call) Cellulose tris(phenyl carbamate) coated on 5µm silica CHIRALCEL OC-H (p.90) A rigid, spherical polymetacrylate, 4 to 6µm in diameter Shodex RSpak DE-613 (p.227) Shodex RSpak DE-413 (Call) Shodex RSpak DE-213 (p.call) (R)-phenylglycine and 3,5-dinitroanaline urea linkage covalently bonded to silica A rigid, spherical polydivinylbenzene particle, 5 to 10µm in diameter Jorid-Gel DVB (p.174) A strong anion-exchange resin consisting of a highly cross-linked core of 7µm macroporous particles having a 100Å average pore size and consisting of ethylvinylbenzene cross-linked with 55% divinylbenzene and an anion-exchange layer grafted to the surface which is functionalized with alkyl quartenary ammonium ions Shodex SI-904E (p.231) We supply over 70% of the worldʼs leading brands of HPLC columns. Contact us for the appropriate column to suit your USP method. 64
Comparison Guide to C18 Reversed Phase HPLC Columns
 Comparison Guide to C18 Reversed Phase HPLC Columns Comparison Data on Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
Comparison Guide to C18 Reversed Phase HPLC Columns Comparison Data on Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
Product Bulletin. ACE LC/MS and Rapid Analysis HPLC Columns. HPLC Columns
 Product Bulletin HPLC Columns ACE LC/MS and Rapid Analysis HPLC Columns 0 mm, 30 mm, 35 mm and 50 mm column lengths.0,., 3.0, 4.0 and 4.6 mm column diameters Configured for High Sample Throughput Specially
Product Bulletin HPLC Columns ACE LC/MS and Rapid Analysis HPLC Columns 0 mm, 30 mm, 35 mm and 50 mm column lengths.0,., 3.0, 4.0 and 4.6 mm column diameters Configured for High Sample Throughput Specially
COMPARISON GUIDE. to C18 Reversed Phase HPLC Columns. Comparison Data on 60 Commonly Used C18 Phases. Stationary Phase Specifications
 COMPARISON GUIDE to C18 Reversed Phase HPLC Columns Comparison Data on 60 Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
COMPARISON GUIDE to C18 Reversed Phase HPLC Columns Comparison Data on 60 Commonly Used C18 Phases Stationary Phase Specifications Phases Compared According to Relative Hydrophobicity Phases Compared According
Packings for HPLC. Packings for HPLC
 Summary of packings for HPLC In analytical HPLC, packings with particle sizes of 3 to 10 µm are preferred. For preparative separation tasks, also particles with diameters larger than 10 µm are applied.
Summary of packings for HPLC In analytical HPLC, packings with particle sizes of 3 to 10 µm are preferred. For preparative separation tasks, also particles with diameters larger than 10 µm are applied.
LC and LC/MS Column Selection Flow Chart
 LC and LC/MS Column Selection Flow Chart To use the column selection diagram below, simply follow the path for your analyte and mobile phase. At the far right, follow your final column selection to the
LC and LC/MS Column Selection Flow Chart To use the column selection diagram below, simply follow the path for your analyte and mobile phase. At the far right, follow your final column selection to the
Optimisil HPLC & UPLC Column catalogue
 Optimisil HPLC & UPLC Column catalogue Analytical Hub Plot No: 37, P & T Colony, Vikrampuri, Secunderabad 500009 Tel: 040-64621313, Mobile: +91 7799931313 Website: www.analyticalhub.com email: contact@analyticalhub.com
Optimisil HPLC & UPLC Column catalogue Analytical Hub Plot No: 37, P & T Colony, Vikrampuri, Secunderabad 500009 Tel: 040-64621313, Mobile: +91 7799931313 Website: www.analyticalhub.com email: contact@analyticalhub.com
penta-hilic UHPLC COLUMNS
 penta-hilic UHPLC COLUMNS penta-hilic Highly retentive, proprietary penta-hydroxy-ligand Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
penta-hilic UHPLC COLUMNS penta-hilic Highly retentive, proprietary penta-hydroxy-ligand Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
HICHROM. Chromatography Columns and Supplies. LC COLUMNS Zorbax. Catalogue 9. Hichrom Limited
 HICHROM Chromatography Columns and Supplies LC COLUMNS Zorbax Catalogue 9 1 The Markham Centre, Station Road Theale, Reading, Berks, RG7 4PE, UK Tel: +44 (0)118 930 3660 Fax: +44 (0)118 932 3484 Email:
HICHROM Chromatography Columns and Supplies LC COLUMNS Zorbax Catalogue 9 1 The Markham Centre, Station Road Theale, Reading, Berks, RG7 4PE, UK Tel: +44 (0)118 930 3660 Fax: +44 (0)118 932 3484 Email:
Phenyl-Hexyl. UHPLC Columns. Alternate, complementary selectivity to C18 and C8 bonded phases
 Phenyl-Hexyl UHPLC Columns Alternate, complementary selectivity to C8 and C8 bonded phases Particularly recommended for compounds containing aromatic groups Excellent bonded phase stability for durable,
Phenyl-Hexyl UHPLC Columns Alternate, complementary selectivity to C8 and C8 bonded phases Particularly recommended for compounds containing aromatic groups Excellent bonded phase stability for durable,
P R O D U C T B U L L E T I N. Fused-Core particle technology for hyper-fast and super-rugged HPLC columns
 P R O D U C T B U L L E T I N Fused-Core particle technology for hyper-fast and super-rugged HPLC columns HALO column packings are not made the typical way. Instead, the particles packed into HALO columns
P R O D U C T B U L L E T I N Fused-Core particle technology for hyper-fast and super-rugged HPLC columns HALO column packings are not made the typical way. Instead, the particles packed into HALO columns
LC Technical Information
 LC Technical Information Method Transfer to Accucore.6 μm Columns Containing solid core particles, which are engineered to a diameter of.6μm and a very narrow particle size distribution; Accucore HPLC
LC Technical Information Method Transfer to Accucore.6 μm Columns Containing solid core particles, which are engineered to a diameter of.6μm and a very narrow particle size distribution; Accucore HPLC
New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns
 New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns have: the highest plate number versus any other 5-micron
New 5-micron HALO-5 columns based on Fused- Core particle technology boost the performance of HPLC. Compared to other HPLC columns, HALO-5 columns have: the highest plate number versus any other 5-micron
NUCLEODUR (Macherey-Nagel)
 NUCLEDUR (Macherey-Nagel) Ultrapure silica Excellent mechanical and chemical stability Low metal content Enhanced ph stability Manufactured by Macherey-Nagel GmbH & Co. KG, Düren, Germany, NUCLEDUR is
NUCLEDUR (Macherey-Nagel) Ultrapure silica Excellent mechanical and chemical stability Low metal content Enhanced ph stability Manufactured by Macherey-Nagel GmbH & Co. KG, Düren, Germany, NUCLEDUR is
ChromegaChiral TM CSP Media and Columns
 ChromegaChiral TM CSP Media and Columns p h a r m a c e u t i c a l e n v i r o n m e n t a l c h e m i c a l b i o c h e m i c a l s e p a r a t i o n & p u r i f i c a t i o n ES Industries 701 S. Route
ChromegaChiral TM CSP Media and Columns p h a r m a c e u t i c a l e n v i r o n m e n t a l c h e m i c a l b i o c h e m i c a l s e p a r a t i o n & p u r i f i c a t i o n ES Industries 701 S. Route
Chapter content. Reference
 Chapter 7 HPLC Instrumental Analysis Rezaul Karim Environmental Science and Technology Jessore University of Science and Technology Chapter content Liquid Chromatography (LC); Scope; Principles Instrumentation;
Chapter 7 HPLC Instrumental Analysis Rezaul Karim Environmental Science and Technology Jessore University of Science and Technology Chapter content Liquid Chromatography (LC); Scope; Principles Instrumentation;
Bringing uhplc Performance to the Separation of Peptides
 P e p t i d e E S - C 1 8 U H P L C C o l u m n s Bringing uhplc Performance to the Separation of Peptides Compatible with UHPLC and conventional HPLC equipment. Sumpfstr. 3, CH-6300 Zug; Fax: 041 748
P e p t i d e E S - C 1 8 U H P L C C o l u m n s Bringing uhplc Performance to the Separation of Peptides Compatible with UHPLC and conventional HPLC equipment. Sumpfstr. 3, CH-6300 Zug; Fax: 041 748
ChromegaChiral TM CSP Media and Columns
 ChromegaChiral TM CSP Media and Columns p h a r m a c e u t i c a l e n v i r o n m e n t a l c h e m i c a l b i o c h e m i c a l s e p a r a t i o n & p u r i f i c a t i o n ES Industries 701 S. Route
ChromegaChiral TM CSP Media and Columns p h a r m a c e u t i c a l e n v i r o n m e n t a l c h e m i c a l b i o c h e m i c a l s e p a r a t i o n & p u r i f i c a t i o n ES Industries 701 S. Route
penta-hilic UHPLC COLUMNS
 penta-hilic UHPLC COLUMNS Highly retentive, proprietary penta-hydroxy-ligand penta-hilic Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
penta-hilic UHPLC COLUMNS Highly retentive, proprietary penta-hydroxy-ligand penta-hilic Excellent peak shape for polar compounds with a variety of functional groups: acids, bases, zwitterions strong and
ACE Ultra Inert Base Deactivated HPLC Columns
 ACE Ultra Inert Base Deactivated HPLC Columns Contents Page ACE HPLC Columns...- Independent Comparison of HPLC Columns #... Independent Comparison of HPLC Columns #... ACE 00Å HPLC Columns Specifications...-
ACE Ultra Inert Base Deactivated HPLC Columns Contents Page ACE HPLC Columns...- Independent Comparison of HPLC Columns #... Independent Comparison of HPLC Columns #... ACE 00Å HPLC Columns Specifications...-
ProntoSIL C18-EPS Reversed-Phase HPLC Columns
 ProntoSIL C8-EPS Reversed-Phase HPLC Columns Provides excellent separation of polar compounds Better peak shape for acids and bases Stabilized bonded phase for rugged, robust HPLC methods More retentive
ProntoSIL C8-EPS Reversed-Phase HPLC Columns Provides excellent separation of polar compounds Better peak shape for acids and bases Stabilized bonded phase for rugged, robust HPLC methods More retentive
Too Polar for Reversed Phase What Do You Do?
 Too Polar for Reversed Phase What Do You Do? June 20, 2013 Mark Powell Columns and Consumables Technical Support Page 1 C8 or C18 Doesn t Always Do the Job Typical reversed phase conditions involve water/buffer
Too Polar for Reversed Phase What Do You Do? June 20, 2013 Mark Powell Columns and Consumables Technical Support Page 1 C8 or C18 Doesn t Always Do the Job Typical reversed phase conditions involve water/buffer
HICHROM. Chromatography Columns and Supplies. LC COLUMNS SIELC Primesep. Catalogue 9. Hichrom Limited
 HICHROM Chromatography Columns and Supplies LC COLUMNS SIELC Primesep Catalogue 9 The Markham Centre, Station Road Theale, Reading, Berks, RG7 4PE, UK Tel: +44 (0)8 90 660 Fax: +44 (0)8 9 484 Email: sales@hichrom.co.uk
HICHROM Chromatography Columns and Supplies LC COLUMNS SIELC Primesep Catalogue 9 The Markham Centre, Station Road Theale, Reading, Berks, RG7 4PE, UK Tel: +44 (0)8 90 660 Fax: +44 (0)8 9 484 Email: sales@hichrom.co.uk
Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS. Table of Contents
 No. 089 SEPARATION REPORT Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS Table of Contents 1. Introduction 1 2. Column Specification 1 3. Features of Packing Materials
No. 089 SEPARATION REPORT Packed Column for Ultra-Fast Reversed-Phase Liquid Chromatography, TSKgel Super-ODS Table of Contents 1. Introduction 1 2. Column Specification 1 3. Features of Packing Materials
zorbax Packed by Phenomenex Material Characteristics High efficiency Guaranteed quality Phenomenex columns packed with Zorbax
 Packed by Phenomenex High efficiency Guaranteed quality Phenomenex columns packed with 7 μm materials offer the advantages of lower cost as well as lower operating backpressures, which result in long column
Packed by Phenomenex High efficiency Guaranteed quality Phenomenex columns packed with 7 μm materials offer the advantages of lower cost as well as lower operating backpressures, which result in long column
Orosil HPLC Columns. OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph.
 Orosil OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph. Excellent organic base selectivity with very low asymmetry values Compatible
Orosil OroSil HPLC columns are designed for the separation of polar, semi-polar, and nonpolar compounds at low to medium ph. Excellent organic base selectivity with very low asymmetry values Compatible
State-of-the-art C18 HPLC Columns
 An HPLC GL Sciences Newest and Most Advanced ODS Phase-New For 00 State-of-the-art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with 00%
An HPLC GL Sciences Newest and Most Advanced ODS Phase-New For 00 State-of-the-art C HPLC s Improved Peak Shapes and Heights Enhancing Sensitivity High Resolution Fast Equilibration Compatible with 00%
Transferring Yesterdays Methods to Tomorrows Technology: The Evolution from HPLC to UPLC TM
 Transferring Yesterdays Methods to Tomorrows Technology: The Evolution from HPLC to UPLC TM Eric S. Grumbach Thomas E. Wheat Chuck Phoebe Joe Arsenault Jeffrey R. Mazzeo Diane M. Diehl UPLC TECHNOLOGY
Transferring Yesterdays Methods to Tomorrows Technology: The Evolution from HPLC to UPLC TM Eric S. Grumbach Thomas E. Wheat Chuck Phoebe Joe Arsenault Jeffrey R. Mazzeo Diane M. Diehl UPLC TECHNOLOGY
Analysis - HPLC A.136. Primesep 5 µm columns B.136
 Primesep 5 µm columns Primesep columns feature double functionality of the bonding i.e : alkyl chain with anionic or cationic group, chelating group. This feature creates unique selectivities when using
Primesep 5 µm columns Primesep columns feature double functionality of the bonding i.e : alkyl chain with anionic or cationic group, chelating group. This feature creates unique selectivities when using
InertSustainSwift C8
 Physical Properties Silica Particle Size Surface Area Pore Size Pore Volume Bonded Phase End-capping Carbon Loading ph Range USP Code :ES (Evolved Surface) Silica Gel :.9 μm, μm, μm :00 m /g :00 Å (0 nm)
Physical Properties Silica Particle Size Surface Area Pore Size Pore Volume Bonded Phase End-capping Carbon Loading ph Range USP Code :ES (Evolved Surface) Silica Gel :.9 μm, μm, μm :00 m /g :00 Å (0 nm)
Hypersil BDS Columns TG 01-05
 TG 0-0 Hypersil BDS Columns Introduction Hypersil BDS columns have gained a reputation over the years as one of the most robust, reproducible and reliable HPLC column brands available. This Technical Guide
TG 0-0 Hypersil BDS Columns Introduction Hypersil BDS columns have gained a reputation over the years as one of the most robust, reproducible and reliable HPLC column brands available. This Technical Guide
Optical Isomer Separation Columns and Packing Materials
 02 Optical Isomer Separation s and Packing Materials CHIRAL ART----------------------------------- 26~29 YMC CHIRAL NEA (R), (S)-----------------------30 YMC CHIRAL CD BR------------------------------31
02 Optical Isomer Separation s and Packing Materials CHIRAL ART----------------------------------- 26~29 YMC CHIRAL NEA (R), (S)-----------------------30 YMC CHIRAL CD BR------------------------------31
Capital HPLC Columns and HPLC Cartridges. Optimal HPLC Range
 Capital HPLC Columns and HPLC Cartridges Specialty Columns HPLC Columns & HPLC Cartridges Download Capital Catalog Adsorbosphere Apex Chromegabond Diasogel Exsil Hypersil Inertsil ODS-2 Partisil Rosil
Capital HPLC Columns and HPLC Cartridges Specialty Columns HPLC Columns & HPLC Cartridges Download Capital Catalog Adsorbosphere Apex Chromegabond Diasogel Exsil Hypersil Inertsil ODS-2 Partisil Rosil
Ch.28 HPLC. Basic types of Liquid Chromatography Partition (LLC) Adsorption (LSC) Ion Exchange (IC) Size Exclusion (SEC or Gel Chromatography)
 Ch.28 HPLC 28.1 Basic types of Liquid Chromatography Partition (LLC) Adsorption (LSC) Ion Exchange (IC) Size Exclusion (SEC or Gel Chromatography) High Performance (Pressure) LC Glass column st.steel (high
Ch.28 HPLC 28.1 Basic types of Liquid Chromatography Partition (LLC) Adsorption (LSC) Ion Exchange (IC) Size Exclusion (SEC or Gel Chromatography) High Performance (Pressure) LC Glass column st.steel (high
( )( ) Selectivity Choices in Reversed-Phase Fast LC. Introduction. R s = 1 a 1 k 4 a 1 + k
 Selectivity Choices in Reversed-Phase Fast LC Luisa Pereira, Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20543 Key Words Accucore, Hypersil GOLD, Syncronis, Column Chemistry,
Selectivity Choices in Reversed-Phase Fast LC Luisa Pereira, Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20543 Key Words Accucore, Hypersil GOLD, Syncronis, Column Chemistry,
HICHROM. Chromatography Columns and Supplies. LC COLUMNS HALO and HALO-5. Catalogue 9. Hichrom Limited
 HICHROM Chromatography Columns and Supplies LC COLUMNS HALO and HALO- Catalogue 9 The Markham Centre, Station Road Theale, Reading, Berks, RG PE, UK Tel: + (0)8 90 0 Fax: + (0)8 9 8 Email: sales@hichrom.co.uk
HICHROM Chromatography Columns and Supplies LC COLUMNS HALO and HALO- Catalogue 9 The Markham Centre, Station Road Theale, Reading, Berks, RG PE, UK Tel: + (0)8 90 0 Fax: + (0)8 9 8 Email: sales@hichrom.co.uk
ACE SuperC18. ACE UHPLC and HPLC Columns. Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability
 ACE SuperC8 TM Ultra-Inert UHPLC and HPLC s with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS compatible buffers between ph.. High efficiency µm, µm, µm and
ACE SuperC8 TM Ultra-Inert UHPLC and HPLC s with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS compatible buffers between ph.. High efficiency µm, µm, µm and
Fast Separation of Vastly Different Compounds by Isocratic HPLC
 Fast Separation of Vastly Different Compounds by Isocratic HPLC Aimee N. Heyrman and Yury Zelechonok Resolution Systems, Inc. 590 E. 32nd St. Holland. MI, 49423 SIELC Technologies, 15 E. Palatine Rd, Suite
Fast Separation of Vastly Different Compounds by Isocratic HPLC Aimee N. Heyrman and Yury Zelechonok Resolution Systems, Inc. 590 E. 32nd St. Holland. MI, 49423 SIELC Technologies, 15 E. Palatine Rd, Suite
Chemistry Instrumental Analysis Lecture 28. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
Chemistry 4631 Instrumental Analysis Lecture 28 High Performance Liquid Chromatography () Instrumentation Normal Phase Chromatography Normal Phase - a polar stationary phase with a less polar mobile phase.
Shodex TM ODP2 HP series columns
 HPLC Columns Shodex TM ODP2 HP series columns Better retention of highly polar substances Technical notebook No. 6 Contents 1. Introduction 1-1. Specifications 1-2. Eluent Compatibility of ODP2 HP Series
HPLC Columns Shodex TM ODP2 HP series columns Better retention of highly polar substances Technical notebook No. 6 Contents 1. Introduction 1-1. Specifications 1-2. Eluent Compatibility of ODP2 HP Series
Advantages of polymerbased. an alternative for ODS
 Advantages of polymerbased HPLC columns an alternative for ODS Yukiko Higai Shodex, 1 Content (1) Comparison between Silica and Polymer-based RP columns (2) Characteristics of Polymer RP columns - an example:
Advantages of polymerbased HPLC columns an alternative for ODS Yukiko Higai Shodex, 1 Content (1) Comparison between Silica and Polymer-based RP columns (2) Characteristics of Polymer RP columns - an example:
HPLC Background Chem 250 F 2008 Page 1 of 24
 HPLC Background Chem 250 F 2008 Page 1 of 24 Outline: General and descriptive aspects of chromatographic retention and separation: phenomenological k, efficiency, selectivity. Quantitative description
HPLC Background Chem 250 F 2008 Page 1 of 24 Outline: General and descriptive aspects of chromatographic retention and separation: phenomenological k, efficiency, selectivity. Quantitative description
LIQUID CHROMATOGRAPHY
 LIQUID CHROMATOGRAPHY RECENT TECHNIQUES HPLC High Performance Liquid Chromatography RRLC Rapid Resolution Liquid Chromatography UPLC Ultra Performance Liquid Chromatography UHPLC Ultra High Pressure Liquid
LIQUID CHROMATOGRAPHY RECENT TECHNIQUES HPLC High Performance Liquid Chromatography RRLC Rapid Resolution Liquid Chromatography UPLC Ultra Performance Liquid Chromatography UHPLC Ultra High Pressure Liquid
Overview Applications of NUCLEODUR HPLC Phases
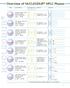 verview Applications of HPLC Phases Phase Specification Characteristics* Stability Structure A B C octadecyl phase, high density coating multi-endcapping 8% C USP L ph stability -, suitable for LC/MS (Si-
verview Applications of HPLC Phases Phase Specification Characteristics* Stability Structure A B C octadecyl phase, high density coating multi-endcapping 8% C USP L ph stability -, suitable for LC/MS (Si-
Method Development Kits
 Method Development Kits sales representative for ordering information or contact...77 Method Development Kits First column choice for Pharma Development USP L C Method Development Kits Maximize retention
Method Development Kits sales representative for ordering information or contact...77 Method Development Kits First column choice for Pharma Development USP L C Method Development Kits Maximize retention
Inertsil Technical Library
 HPLC COLUMN INERTSIL TECHNICAL LIBRARY H P L C C o l u m n Inertsil Technical Library Inertsil ODS-4, C8-4 Comparison of Performance List of Compared Columns Experimental Explanation, Analytical Conditions
HPLC COLUMN INERTSIL TECHNICAL LIBRARY H P L C C o l u m n Inertsil Technical Library Inertsil ODS-4, C8-4 Comparison of Performance List of Compared Columns Experimental Explanation, Analytical Conditions
Open Column Chromatography, GC, TLC, and HPLC
 Open Column Chromatography, GC, TLC, and HPLC Murphy, B. (2017). Introduction to Chromatography: Lecture 1. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy, Chicago. USES OF CHROMATOGRAPHY
Open Column Chromatography, GC, TLC, and HPLC Murphy, B. (2017). Introduction to Chromatography: Lecture 1. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy, Chicago. USES OF CHROMATOGRAPHY
Comparison of different aqueous mobile phase HPLC techniques
 June 009 ewsletter Pharmaceutical Analysis: What is Your Problem? SIELC Technologies, Inc., Prospect Heights, IL 0070 Pharmaceutical analysis involves liquid chromatography of various compounds, from active
June 009 ewsletter Pharmaceutical Analysis: What is Your Problem? SIELC Technologies, Inc., Prospect Heights, IL 0070 Pharmaceutical analysis involves liquid chromatography of various compounds, from active
Sample Preparation TLC Plates
 TLC PLATES TLC Plates Economical separation method High sample throughput Pilot procedure for HPLC and flash chromatography Versatile range of ready-to-use layers Thin layer chromatography (TLC) is a simple,
TLC PLATES TLC Plates Economical separation method High sample throughput Pilot procedure for HPLC and flash chromatography Versatile range of ready-to-use layers Thin layer chromatography (TLC) is a simple,
Inertsil ODS-EP Technical Information
 Technical Information Advantages of The selectivity is completely different from those of conventional columns such as ODS column due to its specific polar group in the stationary phase. Durability can
Technical Information Advantages of The selectivity is completely different from those of conventional columns such as ODS column due to its specific polar group in the stationary phase. Durability can
HPLC Column Selection: Solve the Separation Mystery. The world leader in serving science
 HPLC Column Selection: Solve the Separation Mystery The world leader in serving science verview Introduction Historical verview in Stationary Phases Basics in Column Selection Resolution, Efficiency and
HPLC Column Selection: Solve the Separation Mystery The world leader in serving science verview Introduction Historical verview in Stationary Phases Basics in Column Selection Resolution, Efficiency and
Successfully Scaling and Transferring HPLC and UPLC Methods
 Successfully Scaling and Transferring HPLC and UPLC Methods Esa Lehtorinne Esa_Lehtorinne@waters.com Tel: +358-9-5659 6288 Fax: +358-9-5659 6282 Waters Finland Kutomotie 16 00380 Helsinki 2013 Waters Corporation
Successfully Scaling and Transferring HPLC and UPLC Methods Esa Lehtorinne Esa_Lehtorinne@waters.com Tel: +358-9-5659 6288 Fax: +358-9-5659 6282 Waters Finland Kutomotie 16 00380 Helsinki 2013 Waters Corporation
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Guide to Choosing and Using Polymer Reversed Phase Columns!
 Guide to Choosing and Using Polymer Reversed Phase Columns! Choosing the best RP Column to use: Silica is normally used as the packing material for HPLC columns for a number of reasons. It is very strong,
Guide to Choosing and Using Polymer Reversed Phase Columns! Choosing the best RP Column to use: Silica is normally used as the packing material for HPLC columns for a number of reasons. It is very strong,
Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity
 Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20544 Key Words Hydrophilic, HILIC,
Hydrophilic Interaction Liquid Chromatography: Some Aspects of Solvent and Column Selectivity Monica Dolci, Thermo Fisher Scientific, Runcorn, Cheshire, UK Technical Note 20544 Key Words Hydrophilic, HILIC,
Kromasil. for your analytical HPLC. Kromasil. The way to peak performance in liquid chromatography
 Kromasil for your analytical HPLC Kromasil The way to peak performance in liquid chromatography Kromasil is known, worldwide, for its high performance and excellent total economy in preparative and industrial
Kromasil for your analytical HPLC Kromasil The way to peak performance in liquid chromatography Kromasil is known, worldwide, for its high performance and excellent total economy in preparative and industrial
Cation Exchange HPLC Columns
 Cation Exchange HPLC Columns Hamilton offers seven polymeric packing materials for cation exchange separations. Type Recommended Application(s) PRP-X00 PRP-X00 PRP-X800 HC-0 HC-7 Ca + HC-7 H + HC-7 Pb
Cation Exchange HPLC Columns Hamilton offers seven polymeric packing materials for cation exchange separations. Type Recommended Application(s) PRP-X00 PRP-X00 PRP-X800 HC-0 HC-7 Ca + HC-7 H + HC-7 Pb
SGE is excited to launch a new HPLC product line under the ProteCol brand.
 The Role of Pore Size in Reversed Phase HPLC SGE is excited to launch a new HPLC product line under the ProteCol brand. Fundamental to the new ProteCol line of columns is the continued focus on inert column
The Role of Pore Size in Reversed Phase HPLC SGE is excited to launch a new HPLC product line under the ProteCol brand. Fundamental to the new ProteCol line of columns is the continued focus on inert column
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 25: CHROMATOGRAPHIC METHODS AND CAPILLARY ELECTROPHORESIS
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 25: CHROMATOGRAPHIC METHODS AND CAPILLARY ELECTROPHORESIS CHAPTER 25: Opener Aa CHAPTER 25: Opener Ab CHAPTER 25: Opener B 25-1 Ion-Exchange
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 25: CHROMATOGRAPHIC METHODS AND CAPILLARY ELECTROPHORESIS CHAPTER 25: Opener Aa CHAPTER 25: Opener Ab CHAPTER 25: Opener B 25-1 Ion-Exchange
HICHROM. Chromatography Columns and Supplies. LC COLUMNS SeQuant ZIC-HILIC. Catalogue 9. Hichrom Limited
 HICHROM Chromatography Columns and Supplies LC COLUMNS SeQuant ZIC-HILIC Catalogue 9 The Markham Centre, Station Road Theale, Reading, Berks, RG7 PE, UK Tel: + (0)8 90 660 Fax: + (0)8 9 8 Email: sales@hichrom.co.uk
HICHROM Chromatography Columns and Supplies LC COLUMNS SeQuant ZIC-HILIC Catalogue 9 The Markham Centre, Station Road Theale, Reading, Berks, RG7 PE, UK Tel: + (0)8 90 660 Fax: + (0)8 9 8 Email: sales@hichrom.co.uk
There s Great Chemistry Between Us.
 There s Great Chemistry Between Us. Introduction HPLC Columns Theory, Technology and Practice Uwe D. Neue, Waters Corporation, Milford, MA published by John Wiley & Sons High performance liquid chromatography
There s Great Chemistry Between Us. Introduction HPLC Columns Theory, Technology and Practice Uwe D. Neue, Waters Corporation, Milford, MA published by John Wiley & Sons High performance liquid chromatography
Thermo Scientific Accucore XL HPLC Columns. Technical Manual
 Thermo Scientific Accucore XL HPLC Columns Technical Manual Thermo Scientific Accucore XL HPLC Columns Based on Core Enhanced Technology using µm solid core particles, Accucore XL HPLC columns allow users
Thermo Scientific Accucore XL HPLC Columns Technical Manual Thermo Scientific Accucore XL HPLC Columns Based on Core Enhanced Technology using µm solid core particles, Accucore XL HPLC columns allow users
Chromatographic Separation
 What is? is the ability to separate molecules using partitioning characteristics of molecule to remain in a stationary phase versus a mobile phase. Once a molecule is separated from the mixture, it can
What is? is the ability to separate molecules using partitioning characteristics of molecule to remain in a stationary phase versus a mobile phase. Once a molecule is separated from the mixture, it can
Chiral Flash Columns
 6 -I -I SFC Chiral Columns Chiral Flash/MPLC Columns -I -I Immobilized Crown ether HPLC columns for separation in acidic mobile phase SFC Chiral Columns Chiral Flash Columns CHIRAL FLASH / MPLC Columns
6 -I -I SFC Chiral Columns Chiral Flash/MPLC Columns -I -I Immobilized Crown ether HPLC columns for separation in acidic mobile phase SFC Chiral Columns Chiral Flash Columns CHIRAL FLASH / MPLC Columns
Part 2. Overview of HPLC Media
 Part 1. General Chromatographic Theory Part 2. Overview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use 1 HPLC Particle Technology Core-Shell Particle Fully
Part 1. General Chromatographic Theory Part 2. Overview of HPLC Media Part 3. The Role of the Mobile Phase in Selectivity Part 4. Column Care and Use 1 HPLC Particle Technology Core-Shell Particle Fully
QUALITY HPLC COLUMNS PARTICLE PORE PAGE SIZE SIZE. C18 Bidentate C18 Extremely long column life and rugged 4um 100Å 3
 Cogent HPLC Columns Manufactured by Table of Contents QUALITY HPLC COLUMNS PARTICLE PORE PAGE SIZE SIZE C18 Bidentate C18 Extremely long column life and rugged 4um 100Å 3 C8 Bidentate C8 Extremely long
Cogent HPLC Columns Manufactured by Table of Contents QUALITY HPLC COLUMNS PARTICLE PORE PAGE SIZE SIZE C18 Bidentate C18 Extremely long column life and rugged 4um 100Å 3 C8 Bidentate C8 Extremely long
InertSustainSwift C8
 HPLC, LC/MS Columns InertSustainSwift TM C8 New! InertSustainSwift C8 is an octyl group (C8) bonded column offering the same extreme inertness to any type of compounds just like InertSustainSwift C8, which
HPLC, LC/MS Columns InertSustainSwift TM C8 New! InertSustainSwift C8 is an octyl group (C8) bonded column offering the same extreme inertness to any type of compounds just like InertSustainSwift C8, which
C18 Column. Care & Use Sheet
 C18 Column Care & Use Sheet HALO Description HALO C18 is a high-speed, high-performance liquid chromatography column based on a new Fused-CoreTM particle design. The Fused-Core particle provides a thin
C18 Column Care & Use Sheet HALO Description HALO C18 is a high-speed, high-performance liquid chromatography column based on a new Fused-CoreTM particle design. The Fused-Core particle provides a thin
HPLC Column Material - Bulk Ware
 HPLC Column Material - Bulk Ware Constantly growing demands on separation efficiency and availability of solid phase material for packing HPLC columns and column packing stations have motivated us to provide
HPLC Column Material - Bulk Ware Constantly growing demands on separation efficiency and availability of solid phase material for packing HPLC columns and column packing stations have motivated us to provide
Liquid Chromatography
 Liquid Chromatography 1. Introduction and Column Packing Material 2. Retention Mechanisms in Liquid Chromatography 3. Method Development 4. Column Preparation 5. General Instrumental aspects 6. Detectors
Liquid Chromatography 1. Introduction and Column Packing Material 2. Retention Mechanisms in Liquid Chromatography 3. Method Development 4. Column Preparation 5. General Instrumental aspects 6. Detectors
Introduction to Chromatography
 Introduction to Chromatography Dr. Sana Mustafa Assistant Professor Department of Chemistry, Federal Urdu University of Arts, Science & Technology, Karachi. What is Chromatography? Derived from the Greek
Introduction to Chromatography Dr. Sana Mustafa Assistant Professor Department of Chemistry, Federal Urdu University of Arts, Science & Technology, Karachi. What is Chromatography? Derived from the Greek
for Acclaim Mixed-Mode HILIC-1 Column
 for Acclaim Mixed-Mode HILIC-1 Column Product Manual for ACCLAIM Mixed-Mode HILIC-1 Page 1 of 17 Product Manual for ACCLAIM Mixed-Mode HILIC-1 Column 5µm, 4.6 x 250mm, P/N 066844 5µm, 4.6 x 150mm, P/N
for Acclaim Mixed-Mode HILIC-1 Column Product Manual for ACCLAIM Mixed-Mode HILIC-1 Page 1 of 17 Product Manual for ACCLAIM Mixed-Mode HILIC-1 Column 5µm, 4.6 x 250mm, P/N 066844 5µm, 4.6 x 150mm, P/N
New Product Update. HPLC Columns
 New Product Update HPLC Columns ZORBAX MicroBore HPLC Columns ZORBAX Extend-C18 Columns ZORBAX Bonus-RP Columns Inertsil ODS-2 Cartridge Columns ZORBAX Carbohydrate Analysis Columns ZORBAX Eclipse dsdna
New Product Update HPLC Columns ZORBAX MicroBore HPLC Columns ZORBAX Extend-C18 Columns ZORBAX Bonus-RP Columns Inertsil ODS-2 Cartridge Columns ZORBAX Carbohydrate Analysis Columns ZORBAX Eclipse dsdna
ACE Excel SuperC18. ACE UHPLC and HPLC Columns. Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability
 ACE Excel SuperC8 TM Ultra-Inert UHPLC and HPLC s with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS-compatible buffers between ph.. High efficiency µm, µm,
ACE Excel SuperC8 TM Ultra-Inert UHPLC and HPLC s with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS-compatible buffers between ph.. High efficiency µm, µm,
Protein separation and characterization
 Address:800 S Wineville Avenue, Ontario, CA 91761,USA Website:www.aladdin-e.com Email USA: tech@aladdin-e.com Email EU: eutech@aladdin-e.com Email Asia Pacific: cntech@aladdin-e.com Protein separation
Address:800 S Wineville Avenue, Ontario, CA 91761,USA Website:www.aladdin-e.com Email USA: tech@aladdin-e.com Email EU: eutech@aladdin-e.com Email Asia Pacific: cntech@aladdin-e.com Protein separation
Uptisphere CS Evolution Core Shell columns for fast & highly efficient identification & quantification of small molecules
 Uptisphere CS Evolution Core Shell columns for fast & highly efficient identification & quantification of small molecules www.interchim.com www.blog.interchim.com www.forum.interchim.com www.facebook.com/interchim
Uptisphere CS Evolution Core Shell columns for fast & highly efficient identification & quantification of small molecules www.interchim.com www.blog.interchim.com www.forum.interchim.com www.facebook.com/interchim
Liquid Chromatography
 Liquid Chromatography 1. Introduction and Column Packing Material 2. Retention Mechanisms in Liquid Chromatography 3. Method Development 4. Column Preparation 5. General Instrumental aspects 6. Detectors
Liquid Chromatography 1. Introduction and Column Packing Material 2. Retention Mechanisms in Liquid Chromatography 3. Method Development 4. Column Preparation 5. General Instrumental aspects 6. Detectors
8. Methods in Developing Mobile Phase Condition for C18 Column
 I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
I. HPLC Columns Technical Information 8. Methods in Developing Mobile Phase Condition for C18 Column Introduction In reversed phase HPLC, octadecyl group bonded silica columns (C18, ODS) are the most widely
 CAPCELL PAK C18 IF2 Type http://hplc.shiseido.co.jp/e/column/html/if2_index.htm Page 1 of 2 List of Sales Representatives Contact Shiseido Technical Materials Catalog List HPLC Columns HPLC Instruments
CAPCELL PAK C18 IF2 Type http://hplc.shiseido.co.jp/e/column/html/if2_index.htm Page 1 of 2 List of Sales Representatives Contact Shiseido Technical Materials Catalog List HPLC Columns HPLC Instruments
columns Acclaim Mixed-Mode WCX-1 for Separating Basic Molecules
 columns Acclaim Mixed-Mode WCX- for Separating Basic Molecules The Acclaim Mixed-Mode WCX- is a novel, high-efficiency, silica-based column specially designed for separating various basic analytes. This
columns Acclaim Mixed-Mode WCX- for Separating Basic Molecules The Acclaim Mixed-Mode WCX- is a novel, high-efficiency, silica-based column specially designed for separating various basic analytes. This
ACE SuperC18. ACE UHPLC and HPLC Columns. Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability
 ACE SuperC8 TM Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS compatible buffers between ph.. High efficiency µm, µm,
ACE SuperC8 TM Ultra-Inert UHPLC and HPLC Columns with Extended ph Stability Exploit selectivity at low, intermediate and high ph Use with LC/MS compatible buffers between ph.. High efficiency µm, µm,
Handling Products. Sumitomo Chemical Co., Ltd. SUMICHIRAL ~192
 15 Sumitomo Chemical Co., Ltd. SUMICHIRAL ------------------------------ 188~192 YMC_GC_Vol12_15_CS4.indd 187 15/09/24 9:49 15 Chiral columns for enantiomer separation by HPLC [SUMICHIRAL OA] *SUMICHIRAL
15 Sumitomo Chemical Co., Ltd. SUMICHIRAL ------------------------------ 188~192 YMC_GC_Vol12_15_CS4.indd 187 15/09/24 9:49 15 Chiral columns for enantiomer separation by HPLC [SUMICHIRAL OA] *SUMICHIRAL
ChromegaChiral TM Columns. The right choice for chiral purification
 ChromegaChiral TM Columns The right choice for chiral purification Chiral Chromatography The Background... 01 Chiral Stationary Phases (CSP) and Chirality Comparison of Chiral and Achiral Molecules. (a)
ChromegaChiral TM Columns The right choice for chiral purification Chiral Chromatography The Background... 01 Chiral Stationary Phases (CSP) and Chirality Comparison of Chiral and Achiral Molecules. (a)
Agilent s New Weak Anion Exchange (WAX) Solid Phase Extraction Cartridges: SampliQ WAX
 Agilent s New Weak Anion Exchange (WAX) Solid Phase Extraction Cartridges: SampliQ WAX Technical Note Agilent s SampliQ WAX provides Applications for strongly acidic, acidic and neutral compounds Excellent
Agilent s New Weak Anion Exchange (WAX) Solid Phase Extraction Cartridges: SampliQ WAX Technical Note Agilent s SampliQ WAX provides Applications for strongly acidic, acidic and neutral compounds Excellent
Choosing Columns and Conditions for the Best Peak Shape
 Choosing Columns and Conditions for the Best Peak Shape What is Good Peak Shape and Why is it Important? Good peak shape can be defined as a symmetrical or gaussian peak and poor peak shape can include
Choosing Columns and Conditions for the Best Peak Shape What is Good Peak Shape and Why is it Important? Good peak shape can be defined as a symmetrical or gaussian peak and poor peak shape can include
Chromatography. Intro basic terminology types Partition and Adsorption C Ion-Exchange C Gel Filtration (aka Exclusion or Molecular Sieve) C Affinity C
 Chromatography Intro basic terminology types Partition and Adsorption C Ion-Exchange C Gel Filtration (aka Exclusion or Molecular Sieve) C Affinity C Extremely varied and widely used methodology for separation
Chromatography Intro basic terminology types Partition and Adsorption C Ion-Exchange C Gel Filtration (aka Exclusion or Molecular Sieve) C Affinity C Extremely varied and widely used methodology for separation
Chiral Columns for enantiomer separation by HPLC
 Chiral Columns for enantiomer separation by HPLC SUMICHIRAL OA columns are high-performance chiral columns for enantiomer separation by HPLC. On SUMICHIRAL OA columns direct separation of various enantiomers
Chiral Columns for enantiomer separation by HPLC SUMICHIRAL OA columns are high-performance chiral columns for enantiomer separation by HPLC. On SUMICHIRAL OA columns direct separation of various enantiomers
Quick guide to selecting columns and standards for Gel Permeation Chromatography and Size Exclusion Chromatography SELECTION GUIDE
 Quick guide to selecting columns and standards for Gel Permeation Chromatography and Size Exclusion Chromatography SELECTION GUIDE Introduction Gel permeation chromatography (GPC) and size exclusion chromatography
Quick guide to selecting columns and standards for Gel Permeation Chromatography and Size Exclusion Chromatography SELECTION GUIDE Introduction Gel permeation chromatography (GPC) and size exclusion chromatography
The Secrets of Rapid HPLC Method Development. Choosing Columns for Rapid Method Development and Short Analysis Times
 The Secrets of Rapid HPLC Method Development Choosing Columns for Rapid Method Development and Short Analysis Times Rapid Analysis Is More Than Run Time It is developing a method to meet a goal and developing
The Secrets of Rapid HPLC Method Development Choosing Columns for Rapid Method Development and Short Analysis Times Rapid Analysis Is More Than Run Time It is developing a method to meet a goal and developing
LC Columns with Liquid Separation Cell Technology
 Obelisc LC Columns with Liquid Separation Cell Technology Obelisc HPLC Columns - Liquid Separation Cell Technology Introduction Obelisc HPLC columns are the latest, innovative columns from SIELC Technologies,
Obelisc LC Columns with Liquid Separation Cell Technology Obelisc HPLC Columns - Liquid Separation Cell Technology Introduction Obelisc HPLC columns are the latest, innovative columns from SIELC Technologies,
Analysis of Metals, Halides, and Inorganic Ions Using Hydrophilic Interaction Chromatography
 Application Note Inorganic Ions, Water Testing, Minerals, Metals, Basic Chemicals Analysis of Metals, Halides, and Inorganic Ions Using Hydrophilic Interaction Chromatography Authors Anne Mack, Adam Bivens
Application Note Inorganic Ions, Water Testing, Minerals, Metals, Basic Chemicals Analysis of Metals, Halides, and Inorganic Ions Using Hydrophilic Interaction Chromatography Authors Anne Mack, Adam Bivens
How proteins separate on reverse-phase HPLC
 1 Reverse Phase How proteins separate on reverse-phase HPLC RP chromatography separates proteins through the interaction of the hydrophobic foot of the protein with a nonpolar surface of the particle RP
1 Reverse Phase How proteins separate on reverse-phase HPLC RP chromatography separates proteins through the interaction of the hydrophobic foot of the protein with a nonpolar surface of the particle RP
Gel Permeation Chromatography Basics and Beyond eseminar March 13, Jean Lane Technical and Applications Support LSCA, Columns and Supplies
 Gel Permeation Chromatography Basics and Beyond eseminar March 13, 2013 Jean Lane Technical and Applications Support LSCA, Columns and Supplies 1 Content Overview of GPC/SEC What is it? Why do we use it?
Gel Permeation Chromatography Basics and Beyond eseminar March 13, 2013 Jean Lane Technical and Applications Support LSCA, Columns and Supplies 1 Content Overview of GPC/SEC What is it? Why do we use it?
Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS
 Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS 1. List two advantages of temperature programming in GC. a) Allows separation of solutes with widely varying retention factors in a reasonable
Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS 1. List two advantages of temperature programming in GC. a) Allows separation of solutes with widely varying retention factors in a reasonable
Chiral separations efficient, fast and productive
 Chiral separations efficient, fast and productive By Per Jageland*, Mattias Bengtsson and Kristina Hallman AkzoNobel Eka Chemicals AB, Separation Products SE 445 80 Bohus Sweden Introduction Chromatographic
Chiral separations efficient, fast and productive By Per Jageland*, Mattias Bengtsson and Kristina Hallman AkzoNobel Eka Chemicals AB, Separation Products SE 445 80 Bohus Sweden Introduction Chromatographic
Instrumental Chemical Analysis
 L2 Page1 Instrumental Chemical Analysis Chromatography (General aspects of chromatography) Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester,
L2 Page1 Instrumental Chemical Analysis Chromatography (General aspects of chromatography) Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester,
William E. Barber, Ph.D. Applications Chemist October
 William E. Barber, Ph.D. Applications Chemist ctober 0 00 Secrets of Good Peak Shape in HPLC Time: :00 p.m. CET Telephone Number: + 44 0 76 088 Chairperson: John Vis The Secrets of Good Peak Shape in HPLC
William E. Barber, Ph.D. Applications Chemist ctober 0 00 Secrets of Good Peak Shape in HPLC Time: :00 p.m. CET Telephone Number: + 44 0 76 088 Chairperson: John Vis The Secrets of Good Peak Shape in HPLC
GPC-/SEC-Columns. MZ-Gel SD. MZ-Gel Super FG SEC with fluorinated Eluents
 GPC-/SEC-Columns & 2 accessories2 0 18 direct from Producer packed in new generation column hardware! MZ-Gel SD MZ-Gel SD LS MZ-Super FG Development Manufacturing Distribution Consulting HPLC-Columns New
GPC-/SEC-Columns & 2 accessories2 0 18 direct from Producer packed in new generation column hardware! MZ-Gel SD MZ-Gel SD LS MZ-Super FG Development Manufacturing Distribution Consulting HPLC-Columns New
Changing the way you think about HPLC
 3521 Silverside Road Suite 1-K Quillen Building Wilmington, DE 19810 USA info@advanced-materials-tech.com Changing the way you think about HPLC Changing the way you think about HPLC New 5-micron HALO-5
3521 Silverside Road Suite 1-K Quillen Building Wilmington, DE 19810 USA info@advanced-materials-tech.com Changing the way you think about HPLC Changing the way you think about HPLC New 5-micron HALO-5
High Performance Liquid Chromatography
 STANDARDBASE techniques: High Performance Liquid Chromatography Drenthe College, The Netherlands 1. Introduction HPLC. High Performance Liquid Chromatography High Performance Liquid Chromatography (HPLC)
STANDARDBASE techniques: High Performance Liquid Chromatography Drenthe College, The Netherlands 1. Introduction HPLC. High Performance Liquid Chromatography High Performance Liquid Chromatography (HPLC)
