A B C D E F Q1
|
|
|
- Jordan Darcy Pierce
- 5 years ago
- Views:
Transcription
1 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) 1) Li 2) Q1 3) H + A B C G - None of these products are a major product of the reaction that is shown. D E F
2 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) 1) Li 2) Q1 3) H + A B C G - None of these products are a major product of the reaction that is shown. D E F
3 Chem 234, Fall 2016 Exam 3 F r e q u e n c y Count 181 Average 84.0% Max 100.0% Min 16.4% 0 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Exam Score
4 Exam by Day Exam Average # Test Takers 92.00% 90.00% 88.00% 86.00% 84.00% 82.00% 80.00% 78.00% 90.50% 89.50% 82.10% % 0
5 Exam 4 (Cumulative Exam) Time: Thursday, December 8: 2:00 4:00PM OR Saturday, December 10: 10:00 am Noon OR Saturday, December 10: 1:00 4:00PM Location Soc/Anthro Testing Center Chapters will be covered in this order: Chapter 18, 19, 20 Practice Exams are Posted Ex4-90A Practice Final Exam Ex4-90B Practice Final Exam Deadline for alternate arrangements is Monday, 12/5/2016 at 4:30 PM (i.e., close of business) An oral make-up exam will be required for making up the exam for all students not taking the exam on the above dates or having already made prior arrangements
6 Assignment Due Date Ex4-01-B B Claisen Condensation Friday, November 11, 2016 Ex4-02-B C Claisen Condensation Saturday, November 12, 2016 Ex4-03-B B A-B Unsaturated Rxns Sunday, November 13, 2016 Ex4-04-B C A-B Unsaturated Rxns Monday, November 14, 2016 Ex4-05-B A Carb Classification Tuesday, November 15, 2016 Ex4-06-B Hemiacetal Formation Wednesday, November 16, 2016 Ex4-07-B Carbohydrate Reactions Thursday, November 17, 2016 Ex4-08-B Kiliani-Fischer Synthesis Friday, November 18, 2016 Ex4-09-B Important Carbohydrates Monday, November 28, 2016 Ex4-10-B Carbs in Blood Types Monday, November 28, 2016 Thanksgiving Break Ex4-11-B Amino Acid Nomenclature Tuesday, November 29, 2016 Ex4-12-B B Amino Acid Naming Wednesday, November 30, 2016 Ex4-13-B Amino Acid Acid Base Thursday, December 1, 2016 Ex4-14-B Edmann Degradation Friday, December 2, 2016 Ex4-15-B Merrified Peptide Synthesis Saturday, December 3, 2016 Ex4-16-B Synthesis in Peptides Sunday, December 4, 2016
7 Veterans
8 No Class Next Friday!!!
9 Carbohydrates Simple Elemental Formula C x (H 2 O) x D-glyceraldehyde C 3 H 6 O 3 OR C 3 (H 2 O) 3 L-glyceraldehyde Enantiomers Stereoisomers which are not superimposable, but are mirror-image related
10 Aldoses vs Ketoses Aldoses Contain an aldehyde Ketoses Contain a ketone
11 D-Saccharides vs L-Saccharides Arrange so that most oxidized end of the molecule is at the top D-Saccharides Bottom-most OH to right L-Saccharides Bottom-most OH to left
12 Classify the following carbohydrate as D- or L- and as an aldose or a ketose. A. D-aldose B. D-ketose C. L-aldose D. L-ketose Q2
13 Classify the following carbohydrate as D- or L- and as an aldose or a ketose. A. D-aldose B. D-ketose C. L-aldose D. L-ketose Q2
14 How many stereoisomers are there of an aldopentose? (Hint: Remember that the number of stereoisomers is 2 n, where n is the number of chiral centers). Give your answer as an integer number Q3
15 How many stereoisomers are there of an aldopentose? (Hint: Remember that the number of stereoisomers is 2 n, where n is the number of chiral centers). Give your answer as an integer number. 3 chiral centers Q3 Number of stereoisomers = 2 3 Number of stereoisomers = 8
16 Hemiacetal Formation or 6-membered ring formation are good H + CH3OH
17 Hemiacetal Formation 4 H CH3OH 4-membered ring, too high in energy and hemiacetal does not form. Normal acetal formation occurs H + CH3OH membered ring is good for intramolecular hemiacetal formation.
18 Glucose Hemiacetal Formation From a Fischer Projection Perspective H + H2O
Give the major organic product(s) of the following reaction.
 Give the major organic product(s) of the following reaction. 1) NaNO2, HCl 0 o C 2) CuCN 2016-09-23 Q1 A D B C E F There is no reaction or the correct product is not listed here. 5 of 7 Give the major
Give the major organic product(s) of the following reaction. 1) NaNO2, HCl 0 o C 2) CuCN 2016-09-23 Q1 A D B C E F There is no reaction or the correct product is not listed here. 5 of 7 Give the major
A B C D Q1
 Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx
Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx
Give the major organic product(s) of the following reaction.
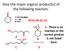 Give the major organic product(s) of the following reaction. 1) CH3CH2MgBr 2) H3O 2016-09-26 Q1 + E. There is no reaction or the correct product A B is not listed here. C D Explanation CH3CH2MgBr 1) CH3CH2MgBr
Give the major organic product(s) of the following reaction. 1) CH3CH2MgBr 2) H3O 2016-09-26 Q1 + E. There is no reaction or the correct product A B is not listed here. C D Explanation CH3CH2MgBr 1) CH3CH2MgBr
Give a common name for the following compound.
 Give a common name for the following compound. A. Benzyl Phenoate B. Phenyl Phenoate C. Benzyl Benzoate D. Phenyl Benzoate E. Phenyl Phenylacetate F. Phenyl Phenylethanoate G. Phenyl Benzylacetate 2016-10-07
Give a common name for the following compound. A. Benzyl Phenoate B. Phenyl Phenoate C. Benzyl Benzoate D. Phenyl Benzoate E. Phenyl Phenylacetate F. Phenyl Phenylethanoate G. Phenyl Benzylacetate 2016-10-07
Give the major product(s) of the following reaction.
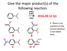 Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
H3O+ heat I Q1
 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) H3O + heat A B C D E F G
Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) H3O + heat A B C D E F G
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
11/14/2016. NaBH 4. Superimposable on mirror image. (achiral) D-ribose. NaBH 4 NaBH 4. Not superimposable on mirror image.
 There are multiple ways to relate the structures of ketoses to aldoses. Reduction of a ketose with NaB 4 forms a new stereocenter and thus forms a mixture of two alditols. An aldopentose forms a chiral
There are multiple ways to relate the structures of ketoses to aldoses. Reduction of a ketose with NaB 4 forms a new stereocenter and thus forms a mixture of two alditols. An aldopentose forms a chiral
A B C D E F G Q1. heat
 Give the final product of the following reaction. Give your answer as a text answer. If more than one species is correct, put your answers in alphabetical order. HNO3 H2SO4 A B C D E F G 2016-09-09 Q1
Give the final product of the following reaction. Give your answer as a text answer. If more than one species is correct, put your answers in alphabetical order. HNO3 H2SO4 A B C D E F G 2016-09-09 Q1
Q1. Intensity of M+1 peak x Intensity of M peak 2.57% x 46.60% Number of carbon atoms = Number of carbon atoms =
 The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 46.60% and an M+1 peak of 2.57%. How many carbon atoms are in the compound? (Fill in an integer number) Number
The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 46.60% and an M+1 peak of 2.57%. How many carbon atoms are in the compound? (Fill in an integer number) Number
BIO 311C Spring 2010
 BIO 311C Spring 2010 Grades for Exam 1 will be available on BlackBoard by the end of today. Your graded exam will be returned to you during your discussion period on Friday or Monday. The mean grade for
BIO 311C Spring 2010 Grades for Exam 1 will be available on BlackBoard by the end of today. Your graded exam will be returned to you during your discussion period on Friday or Monday. The mean grade for
Chem 109 C Fall 2015 Armen Zakarian Office: Chemistry Bldn 2217
 Chem 109 C Fall 2015 Armen Zakarian ffice: Chemistry Bldn 2217 http://web.chem.ucsb.edu/~zakariangroup/courses.html syllabus! ffice ours Mon, Wed - 9:00-9:50 am (or email)! Chemistry building, room 2217!
Chem 109 C Fall 2015 Armen Zakarian ffice: Chemistry Bldn 2217 http://web.chem.ucsb.edu/~zakariangroup/courses.html syllabus! ffice ours Mon, Wed - 9:00-9:50 am (or email)! Chemistry building, room 2217!
Importance of Carbohydrates
 Chapter 25 Importance of Carbohydrates Distributed widely in nature Key intermediates of metabolism (sugars) Structural components of plants (cellulose) Central to materials of industrial products: paper,
Chapter 25 Importance of Carbohydrates Distributed widely in nature Key intermediates of metabolism (sugars) Structural components of plants (cellulose) Central to materials of industrial products: paper,
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Tentative Daily Schedule CHEM 125 Fall 2012
 Tentative Daily Schedule CHEM 125 Fall 2012 Atomic Structure and Periodic Trends Thursday, Aug 30 th, Day 2 Resources: o Text and Connect Passcode (available at bookstore): Chemical Structure and Properties,
Tentative Daily Schedule CHEM 125 Fall 2012 Atomic Structure and Periodic Trends Thursday, Aug 30 th, Day 2 Resources: o Text and Connect Passcode (available at bookstore): Chemical Structure and Properties,
Organic Chemistry. Carbohydrates
 Organic Chemistry by Nurlin Abu Samah, Dr. Md. Shaheen & Dr. Nadeem Akhtar Faculty of Industrial Sciences & Technology nurlin@ump.edu.my Chapter Description Aims The students should understand the fundamental
Organic Chemistry by Nurlin Abu Samah, Dr. Md. Shaheen & Dr. Nadeem Akhtar Faculty of Industrial Sciences & Technology nurlin@ump.edu.my Chapter Description Aims The students should understand the fundamental
ORGANIC - CLUTCH CH CARBOHYDRATES.
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
!! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
ORGANIC - BRUICE 8E CH THE ORGANIC CHEMISTRY OF CARBOHYDRATES
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
!! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
Loudon Chapter 24 Review: Carbohydrates CHEM 3331, Jacquie Richardson, Fall Page 1
 CEM 3331, Jacquie Richardson, Fall 2010 - Page 1 This chapter is about carbohydrates molecules with the general formula of C n ( 2 ) n, or in other words C n 2n n. This is a very common formula for sugars
CEM 3331, Jacquie Richardson, Fall 2010 - Page 1 This chapter is about carbohydrates molecules with the general formula of C n ( 2 ) n, or in other words C n 2n n. This is a very common formula for sugars
Experiment 8 Optical Isomers. In this experiment you will be given the opportunity to see the 3-dimensional aspects of
 Experiment 8 Optical Isomers In this experiment you will be given the opportunity to see the 3-dimensional aspects of stereochemistry and optical isomers. Previously in class you were exposed to the concept
Experiment 8 Optical Isomers In this experiment you will be given the opportunity to see the 3-dimensional aspects of stereochemistry and optical isomers. Previously in class you were exposed to the concept
Tentative Chem 125 Schedule
 Tentative Chem 125 Schedule Introduction Monday, Aug 26 th As we have a shortened class schedule on the first day, we are move to ASC 202 & 204 to where you will take the Colorado Survey, log into Connect,
Tentative Chem 125 Schedule Introduction Monday, Aug 26 th As we have a shortened class schedule on the first day, we are move to ASC 202 & 204 to where you will take the Colorado Survey, log into Connect,
Carbohydrates - Introduction
 arbohydrates - Introduction arbohydrates - General Description A. Polyhydroxy Aldehydes or Ketones ARBN AIN B. Serve a variety of functions ARBN AIN ARBN AIN 1. Energy storage (Glucose, Glycogen, Starch)
arbohydrates - Introduction arbohydrates - General Description A. Polyhydroxy Aldehydes or Ketones ARBN AIN B. Serve a variety of functions ARBN AIN ARBN AIN 1. Energy storage (Glucose, Glycogen, Starch)
Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017)
 Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017) Class: Organic Chemistry II Instructor: Dr. David J. Leaver Room: WSB 307 Office: WSB 318 Time: MWF 9:00-9:50am Office
Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017) Class: Organic Chemistry II Instructor: Dr. David J. Leaver Room: WSB 307 Office: WSB 318 Time: MWF 9:00-9:50am Office
ORGANIC - EGE 5E EXTRA: CARBOHYDRATES.
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
!! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
FARMINGDALE STATE COLLEGE DEPARTMENT OF CHEMISTRY. CONTACT HOURS: Lecture: 3 Laboratory: 4
 FARMINGDALE STATE COLLEGE DEPARTMENT OF CHEMISTRY COURSE OUTLINE: COURSE TITLE: Prepared by: Dr. M. DeCastro September 2011 Organic Chemistry II COURSE NUMBER: CHM 271 CREDITS: 5 CONTACT HOURS: Lecture:
FARMINGDALE STATE COLLEGE DEPARTMENT OF CHEMISTRY COURSE OUTLINE: COURSE TITLE: Prepared by: Dr. M. DeCastro September 2011 Organic Chemistry II COURSE NUMBER: CHM 271 CREDITS: 5 CONTACT HOURS: Lecture:
ORGANIC - BROWN 8E CH CARBOHYDRATES.
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
!! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
CHEMISTRY 150. April 2012
 CHEMISTRY 150 Dr. B. MacLean April 2012 NAME: (please print) ID #: This is a three hour exam. SUGGESTION: Read over the entire exam before beginning, and begin by doing those questions which you find easiest.
CHEMISTRY 150 Dr. B. MacLean April 2012 NAME: (please print) ID #: This is a three hour exam. SUGGESTION: Read over the entire exam before beginning, and begin by doing those questions which you find easiest.
Exam 3 Review (4/12/2011) Lecture note excerpt covering lectures (Exam 3 topics: Chapters 8, 12, 14 & 15)
 Exam 3 Review (4/12/2011) Lecture note excerpt covering lectures 17-23 (Exam 3 topics: Chapters 8, 12, 14 & 15) Enzyme Kinetics, Inhibition, and Regulation Chapter 12 Enzyme Kinetics When the concentration
Exam 3 Review (4/12/2011) Lecture note excerpt covering lectures 17-23 (Exam 3 topics: Chapters 8, 12, 14 & 15) Enzyme Kinetics, Inhibition, and Regulation Chapter 12 Enzyme Kinetics When the concentration
CH 331 Syllabus Fall 2012
 Instructor Information: Dr. Daniel J. T. Myles Office: Gilbert Hall 145 Phone: 541-737-6756 E-mail: daniel.myles@oregonstate.edu All course information, updates, and announcements are posted via Blackboard
Instructor Information: Dr. Daniel J. T. Myles Office: Gilbert Hall 145 Phone: 541-737-6756 E-mail: daniel.myles@oregonstate.edu All course information, updates, and announcements are posted via Blackboard
KJM 3200 Required Reading (Pensum), Fall 2016
 KJM 3200 Required Reading (Pensum), Fall 2016 John McMurry: Organic Chemistry 8 nd ed. or Paula Y. Bruice, Organic Chemistry 7 nd ed. as specified below, as specified below. Lise-Lotte Gundersen KJM 3200.
KJM 3200 Required Reading (Pensum), Fall 2016 John McMurry: Organic Chemistry 8 nd ed. or Paula Y. Bruice, Organic Chemistry 7 nd ed. as specified below, as specified below. Lise-Lotte Gundersen KJM 3200.
LECTURE 5: TYPES OF CARBOHYRATE LECTURE OUTCOMES
 LECTURE 5: TYPES OF CARBOHYRATE LECTURE OUTCOMES After completing this lecture and mastering the lecture materials, students are expected to be able 1. 2. 3. 4. 5. to explain the origin of plants carbohydrates
LECTURE 5: TYPES OF CARBOHYRATE LECTURE OUTCOMES After completing this lecture and mastering the lecture materials, students are expected to be able 1. 2. 3. 4. 5. to explain the origin of plants carbohydrates
Outline. Organic Compounds. Overview: Carbon: The Backbone of Life. I. Organic compounds II. Bonding with Carbon III. Isomers IV.
 Chapter 4 Carbon and the Molecular Diversity of Life Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups Organic Compounds What is organic We think of organic produce
Chapter 4 Carbon and the Molecular Diversity of Life Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups Organic Compounds What is organic We think of organic produce
02/07/2017. Isomerism. Structural isomerism. 1. Structural isomerism different linkages of atoms. Same molecular formula Different structural formulae
 hain isomerism Position isomerism Metamerism Tautomerism Functional group isomerism Geometrical isomerism Optical isomerism 02/07/2017 Isomerism The presence of two or more compounds which has the same
hain isomerism Position isomerism Metamerism Tautomerism Functional group isomerism Geometrical isomerism Optical isomerism 02/07/2017 Isomerism The presence of two or more compounds which has the same
Announcements Monday, November 13
 Announcements Monday, November 13 The third midterm is on this Friday, November 17. The exam covers 3.1, 3.2, 5.1, 5.2, 5.3, and 5.5. About half the problems will be conceptual, and the other half computational.
Announcements Monday, November 13 The third midterm is on this Friday, November 17. The exam covers 3.1, 3.2, 5.1, 5.2, 5.3, and 5.5. About half the problems will be conceptual, and the other half computational.
1. Make two superimposable models of bromochloroiodomethane. Position your models on your desk to prove that they are superimposable.
 HM 204 Organic hemistry Introduction to Stereochemistry Recall that two models are identical if they can be superimposed without breaking bonds. Recall that conformations (conformers) are structures that
HM 204 Organic hemistry Introduction to Stereochemistry Recall that two models are identical if they can be superimposed without breaking bonds. Recall that conformations (conformers) are structures that
CHEM 203. Final Exam December 18, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Marking Scheme For The Exam QUESTION #
 Chemistry 2420 S10 Spring 2005 Term Exam #1 110 Minutes ame: Student umber Marking Scheme For The Exam QUESTI # 1 2 3 4 5 6 7 8 6 12 14 14 13 39 12 9 TTAL 119 % Question 1. (6 Marks) Under mildly acidic
Chemistry 2420 S10 Spring 2005 Term Exam #1 110 Minutes ame: Student umber Marking Scheme For The Exam QUESTI # 1 2 3 4 5 6 7 8 6 12 14 14 13 39 12 9 TTAL 119 % Question 1. (6 Marks) Under mildly acidic
Experiment: Carbohydrates and Optical Activity
 Experiment: Carbohydrates and Optical Activity This lab will explore properties of sugars, one type of carbohydrate. In this lab you will build molecular models of three types of sugars and explore the
Experiment: Carbohydrates and Optical Activity This lab will explore properties of sugars, one type of carbohydrate. In this lab you will build molecular models of three types of sugars and explore the
A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6
 GEORGIA INSTITUTE OF TECHNOLOGY School of Chemistry and Biochemistry Spring 2004 A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6 Instructor: Marcus Weck Office: Boggs 3-85 Phone:
GEORGIA INSTITUTE OF TECHNOLOGY School of Chemistry and Biochemistry Spring 2004 A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6 Instructor: Marcus Weck Office: Boggs 3-85 Phone:
Chapter 24 Carbohydrates
 hapter arbohydrates Review of oncepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of hapter. Each of the sentences below appears verbatim in the
hapter arbohydrates Review of oncepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of hapter. Each of the sentences below appears verbatim in the
DAILY QUESTIONS 28 TH JUNE 18 REASONING - CALENDAR
 DAILY QUESTIONS 28 TH JUNE 18 REASONING - CALENDAR LEAP AND NON-LEAP YEAR *A non-leap year has 365 days whereas a leap year has 366 days. (as February has 29 days). *Every year which is divisible by 4
DAILY QUESTIONS 28 TH JUNE 18 REASONING - CALENDAR LEAP AND NON-LEAP YEAR *A non-leap year has 365 days whereas a leap year has 366 days. (as February has 29 days). *Every year which is divisible by 4
Chapter 25 Organic and Biological Chemistry
 Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Topics in General Chemistry Chemistry 103 Fall 2017
 Topics in General Chemistry Chemistry 103 Fall 2017 Instructor: Professor Oertel, N280 Science Center, 775-8989, catherine.oertel@oberlin.edu Class meeting: MWF 11-11:50 am, Science Center A255 Laboratory
Topics in General Chemistry Chemistry 103 Fall 2017 Instructor: Professor Oertel, N280 Science Center, 775-8989, catherine.oertel@oberlin.edu Class meeting: MWF 11-11:50 am, Science Center A255 Laboratory
Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop
 Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop What are isomers? Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types
Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop What are isomers? Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types
Chemistry Organic Chemistry II, Spring 2018
 Chemistry 2320 Organic Chemistry II, Spring 2018 Instructor: Dr. Tom Chang Office: Widtsoe 337 Phone: 797-3545 Email: tom.chang@usu.edu Meeting Time/Place: MWF 10:30-11:20 am, Eccles Business Building
Chemistry 2320 Organic Chemistry II, Spring 2018 Instructor: Dr. Tom Chang Office: Widtsoe 337 Phone: 797-3545 Email: tom.chang@usu.edu Meeting Time/Place: MWF 10:30-11:20 am, Eccles Business Building
ASTR 101L: Motion of the Sun Take Home Lab
 Name: CWID: Section: Introduction Objectives This lab is designed to help you understand the Sun s apparent motion in the sky over the course of the year. In Section 2 you are asked to answer some questions
Name: CWID: Section: Introduction Objectives This lab is designed to help you understand the Sun s apparent motion in the sky over the course of the year. In Section 2 you are asked to answer some questions
CHEM 2322: Organic Chemistry II Fall 2011
 CHEM 2322: Organic Chemistry II Fall 2011 Instructor: Dr. Jimmy R. Rogers Office Number: Baker Chemistry Research Building Room 104 Office Telephone Number: 817-272-5442 Email and Website: jimrogers@uta.edu
CHEM 2322: Organic Chemistry II Fall 2011 Instructor: Dr. Jimmy R. Rogers Office Number: Baker Chemistry Research Building Room 104 Office Telephone Number: 817-272-5442 Email and Website: jimrogers@uta.edu
4 Carbon and the Molecular Diversity of Life
 4 Carbon and the Molecular Diversity of Life CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups
4 Carbon and the Molecular Diversity of Life CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups
Announcements Monday, November 13
 Announcements Monday, November 13 The third midterm is on this Friday, November 17 The exam covers 31, 32, 51, 52, 53, and 55 About half the problems will be conceptual, and the other half computational
Announcements Monday, November 13 The third midterm is on this Friday, November 17 The exam covers 31, 32, 51, 52, 53, and 55 About half the problems will be conceptual, and the other half computational
The lecture schedule is only a rough guide and will be likely changed as needed.
 CEHM 239 ORGANIC CHEMISTRY II Spring 2010 Instructor: Professor Hyun-Soon Chong Chemistry Division, BCPS Dept, IIT, LS 398, Chong@iit.edu, 312-567-3235 Course Hours: TR 1:50pm-3:05pm in LS 111 Office Hours:
CEHM 239 ORGANIC CHEMISTRY II Spring 2010 Instructor: Professor Hyun-Soon Chong Chemistry Division, BCPS Dept, IIT, LS 398, Chong@iit.edu, 312-567-3235 Course Hours: TR 1:50pm-3:05pm in LS 111 Office Hours:
EASTERN ARIZONA COLLEGE Fundamental Organic Chemistry
 EASTERN ARIZONA COLLEGE Fundamental Organic Chemistry Course Design 2013-2014 Course Information Division Science Course Number CHM 230 (SUN# CHM 2230) Title Fundamental Organic Chemistry Credits 4 Developed
EASTERN ARIZONA COLLEGE Fundamental Organic Chemistry Course Design 2013-2014 Course Information Division Science Course Number CHM 230 (SUN# CHM 2230) Title Fundamental Organic Chemistry Credits 4 Developed
Henderson - Hasselbalch equation
 Structure & Properties of Water Bent geometry, O- bond length of 0.958Å Can form ydrogen bonds Comprehensive Exam eview 12/03/2009 enderson - asselbalch equation From [ ][ ] K = [ ] The 6 step approach
Structure & Properties of Water Bent geometry, O- bond length of 0.958Å Can form ydrogen bonds Comprehensive Exam eview 12/03/2009 enderson - asselbalch equation From [ ][ ] K = [ ] The 6 step approach
JANUARY Single Ad Business Card Size: 3.5 x 2. Single Ad Business Card Size: 3.5 x 2. Double Ad Ad Size: 3.5 x 4.125
 JANUARY 2019 Sunday MONday TUESday WEDNESday THURSday FRIday SATURday 1 2 3 4 5 New Year s Day New Moon 6 7 8 9 10 11 12 13 14 15 16 17 18 19 First Quarter 20 21 22 23 24 25 26 Martin Luther King, Jr.
JANUARY 2019 Sunday MONday TUESday WEDNESday THURSday FRIday SATURday 1 2 3 4 5 New Year s Day New Moon 6 7 8 9 10 11 12 13 14 15 16 17 18 19 First Quarter 20 21 22 23 24 25 26 Martin Luther King, Jr.
CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide
 CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide page 1 CHEM 243 Exam II is scheduled for Monday, November 5 in DMF 477 & 481 (lab rooms). You must take the exam during your normal
CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide page 1 CHEM 243 Exam II is scheduled for Monday, November 5 in DMF 477 & 481 (lab rooms). You must take the exam during your normal
MODULE CONTENT YEAR TERM CREDITS TYPE. I - Fundamentals Chemistry BASIC. Postal address, telephone n o, address
 SUBJECT GUIDE Academic year 2015-2016 ORGANIC CHEMISTRY MODULE CONTENT YEAR TERM CREDITS TYPE I - Fundamentals Chemistry 1 1 6 BASIC LECTURER(S) Prof. Mónica Díaz Gavilán (MDG) Prof. Francisco Franco Montalbán
SUBJECT GUIDE Academic year 2015-2016 ORGANIC CHEMISTRY MODULE CONTENT YEAR TERM CREDITS TYPE I - Fundamentals Chemistry 1 1 6 BASIC LECTURER(S) Prof. Mónica Díaz Gavilán (MDG) Prof. Francisco Franco Montalbán
CHM 151: GENERAL CHEMISTRY I Department of Chemistry College of Arts and Sciences Northern Arizona University
 CHM 151: GENERAL CHEMISTRY I Department of Chemistry College of Arts and Sciences Northern Arizona University Instructor: Dr. Brandon Cruickshank Office: Chem: Rm 121, and 125 Phone: 523-9602 Web site:
CHM 151: GENERAL CHEMISTRY I Department of Chemistry College of Arts and Sciences Northern Arizona University Instructor: Dr. Brandon Cruickshank Office: Chem: Rm 121, and 125 Phone: 523-9602 Web site:
4 Carbon and the Molecular Diversity of Life
 4 Carbon and the Molecular Diversity of Life CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Vitalism vs Mechanism
4 Carbon and the Molecular Diversity of Life CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Vitalism vs Mechanism
Final Exam Version 1. Chemistry 140C. Fall Good Luck! Dec 5, :30 am 2:30 pm This exam accounts for 50% of the final grade.
 Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
Chapter 1 0+7= 1+6= 2+5= 3+4= 4+3= 5+2= 6+1= 7+0= How would you write five plus two equals seven?
 Chapter 1 0+7= 1+6= 2+5= 3+4= 4+3= 5+2= 6+1= 7+0= If 3 cats plus 4 cats is 7 cats, what does 4 olives plus 3 olives equal? olives How would you write five plus two equals seven? Chapter 2 Tom has 4 apples
Chapter 1 0+7= 1+6= 2+5= 3+4= 4+3= 5+2= 6+1= 7+0= If 3 cats plus 4 cats is 7 cats, what does 4 olives plus 3 olives equal? olives How would you write five plus two equals seven? Chapter 2 Tom has 4 apples
Randa AbiRafi Jaber Office: Chem Bldg. Rm 212 Office Hrs: Tue, Wed 11:00 Thu: 2:00 3:00 pm
 Faraj Hasanayn Office Chem Bldg. Rm 522 Office Hrs: Mon: 10:30 11:30 am Tuesday: 1:30 2:30 pm, and by appointment Email: fh19@aub.edu.lb, Phone Ext: 3994 Randa AbiRafi Jaber Office: Chem Bldg. Rm 212 Office
Faraj Hasanayn Office Chem Bldg. Rm 522 Office Hrs: Mon: 10:30 11:30 am Tuesday: 1:30 2:30 pm, and by appointment Email: fh19@aub.edu.lb, Phone Ext: 3994 Randa AbiRafi Jaber Office: Chem Bldg. Rm 212 Office
Chemical Reactions - Oxidation. Reactions Involving Aldehydes and Ketones. Learning Check. Learning Check. Chemical Reactions - Addition of Hydrogen
 Reactions Involving Aldehydes and Ketones Chemical Reactions - Oxidation When aldehydes are prepared by oxidizing primary alcohols with KMnO 4 or K 2 Cr 2 O 7, the reaction may continue and produce carboxylic
Reactions Involving Aldehydes and Ketones Chemical Reactions - Oxidation When aldehydes are prepared by oxidizing primary alcohols with KMnO 4 or K 2 Cr 2 O 7, the reaction may continue and produce carboxylic
CHE 322 Summer 2012 Final Exam Form Choose the lowest unoccupied molecular orbital (LUMO) of the pentadienyl cation.
 Multiple Choice Questions: 100 points 1. Choose the lowest unoccupied molecular orbital (LUMO) of the pentadienyl cation. 2. Select the possible reactants of the following reaction. A i B ii C iii D i
Multiple Choice Questions: 100 points 1. Choose the lowest unoccupied molecular orbital (LUMO) of the pentadienyl cation. 2. Select the possible reactants of the following reaction. A i B ii C iii D i
Course Outline. TERM EFFECTIVE: Fall 2016 CURRICULUM APPROVAL DATE: 03/14/2016
 5055 Santa Teresa Blvd Gilroy, CA 95023 Course Outline COURSE: CHEM 12B DIVISION: 10 ALSO LISTED AS: TERM EFFECTIVE: Fall 2016 CURRICULUM APPROVAL DATE: 03/14/2016 SHORT TITLE: ORGANIC CHEMISTRY LONG TITLE:
5055 Santa Teresa Blvd Gilroy, CA 95023 Course Outline COURSE: CHEM 12B DIVISION: 10 ALSO LISTED AS: TERM EFFECTIVE: Fall 2016 CURRICULUM APPROVAL DATE: 03/14/2016 SHORT TITLE: ORGANIC CHEMISTRY LONG TITLE:
The Final Learning Experience
 Chemistry 212 and 300 rganic Chemistry II Winter Semester 2000 Dr. Rainer Glaser Examination #5 Carbon-Carbon Bond Forming Reactions, Sugars & Nucleic Acids The Final Learning Experience Friday, May 5,
Chemistry 212 and 300 rganic Chemistry II Winter Semester 2000 Dr. Rainer Glaser Examination #5 Carbon-Carbon Bond Forming Reactions, Sugars & Nucleic Acids The Final Learning Experience Friday, May 5,
PSI Chemistry. 3) How many electron pairs does carbon share in order to complete its valence shell? A) 1 B) 2 C) 3 D) 4 E) 8
 Organic Chemistry HW PSI Chemistry Name I - Organic Introduction 1) Organic chemistry is a science based on the study of A) functional groups. B) vital forces interacting with matter. C) carbon compounds.
Organic Chemistry HW PSI Chemistry Name I - Organic Introduction 1) Organic chemistry is a science based on the study of A) functional groups. B) vital forces interacting with matter. C) carbon compounds.
CHEM 203. Final Exam December 18, 2013 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
Chem 221b. Problem Set 9, Chapter 23. Carbohydrates. Due: Monday, April 18, Last year's PS9 was so good, here it is again.
 Chem 221b Problem Set 9, Chapter 23 Carbohydrates Due: Monday, April 18, 2005 Last year's PS9 was so good, here it is again. The graphic on the left was conceived by M. A. Rosanoff in 1906 as a means of
Chem 221b Problem Set 9, Chapter 23 Carbohydrates Due: Monday, April 18, 2005 Last year's PS9 was so good, here it is again. The graphic on the left was conceived by M. A. Rosanoff in 1906 as a means of
Principles of Inorganic, Organic and Biological Chemistry Chemistry 11 Course Syllabus
 Principles of Inorganic, rganic and Biological Chemistry Chemistry 11 Course Syllabus Instructor: Ms. Julie Lowe Phone: 661-395-4310 ffice: SE-5B Email: JLowe@bakersfieldcollege.edu Include a subject of
Principles of Inorganic, rganic and Biological Chemistry Chemistry 11 Course Syllabus Instructor: Ms. Julie Lowe Phone: 661-395-4310 ffice: SE-5B Email: JLowe@bakersfieldcollege.edu Include a subject of
Research Project Summaries and Homework (all equally graded): 60% Final: 30% Attendance/Participation: 10%
 GEOL 520 001 Adv Topic:Igm/Metam Petrology Syllabus Professor Dr. Justin Filiberto Office Hours: Open Door Park 203 Course Time: M W F 9 9:50 Parkinson 101 Email: Filiberto@siu.edu Phone: 618 453 4849
GEOL 520 001 Adv Topic:Igm/Metam Petrology Syllabus Professor Dr. Justin Filiberto Office Hours: Open Door Park 203 Course Time: M W F 9 9:50 Parkinson 101 Email: Filiberto@siu.edu Phone: 618 453 4849
A is one of the categories into which qualitative data can be classified.
 Chapter 2 Methods for Describing Sets of Data 2.1 Describing qualitative data Recall qualitative data: non-numerical or categorical data Basic definitions: A is one of the categories into which qualitative
Chapter 2 Methods for Describing Sets of Data 2.1 Describing qualitative data Recall qualitative data: non-numerical or categorical data Basic definitions: A is one of the categories into which qualitative
CHE Organic Chemistry Exam 4, May 4, 2004
 CE 232 - rganic Chemistry Exam 4, May 4, 2004 ame KEY Student ID o. Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second:
CE 232 - rganic Chemistry Exam 4, May 4, 2004 ame KEY Student ID o. Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second:
GOVERNMENT COLLEGE (AUTONOMOUS), RAJAMAHENDRAVARAM DEPARTMENT OF CHEMISTRY CBCS Syllabus for B.Sc. III Year Effective from onwards
 GOVERNMENT COLLEGE (AUTONOMOUS), RAJAMAHENDRAVARAM DEPARTMENT OF CHEMISTRY CBCS Syllabus for B.Sc. III Year Effective from 2018 2019 onwards Paper VI Semester V Applied Physical Chemistry and Organic Chemistry
GOVERNMENT COLLEGE (AUTONOMOUS), RAJAMAHENDRAVARAM DEPARTMENT OF CHEMISTRY CBCS Syllabus for B.Sc. III Year Effective from 2018 2019 onwards Paper VI Semester V Applied Physical Chemistry and Organic Chemistry
CHE 325 ORGANIC CHEMISTRY II Spring 2017
 Instructor: CHE 325 ORGANIC CHEMISTRY II Spring 2017 Professor James Kallmerten 4-014A Center for Science and Technology Phone: 3-2854 Email: jkallmer@syr.edu Office Hours: Monday 11 am -1 pm, Wednesday
Instructor: CHE 325 ORGANIC CHEMISTRY II Spring 2017 Professor James Kallmerten 4-014A Center for Science and Technology Phone: 3-2854 Email: jkallmer@syr.edu Office Hours: Monday 11 am -1 pm, Wednesday
Carbonyl Group in Aldehydes and Ketones
 Lecture 4: Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Carbonyl Group in Aldehydes and Ketones A carbonyl group (C=) In an aldehyde is attached to at least one atom. In a ketone
Lecture 4: Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Carbonyl Group in Aldehydes and Ketones A carbonyl group (C=) In an aldehyde is attached to at least one atom. In a ketone
UCSC, Binder. CHEM 8B Organic Chemistry II FINAL EXAM (300 points)
 UCSC, Binder Name CEM 8B rganic Chemistry II FINAL EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry conventions to answer the questions in the proper manner.
UCSC, Binder Name CEM 8B rganic Chemistry II FINAL EXAM (300 points) In each of the following problems, use your knowledge of organic chemistry conventions to answer the questions in the proper manner.
Loudon Chapter 24 Review: Carbohydrates Jacquie Richardson, CU Boulder Last updated 4/26/2018
 This chapter is about carbohydrates molecules with the general formula of C n( 2O) n, or in other words C n 2nO n. This is a very common formula for sugars and many other natural products. The structure
This chapter is about carbohydrates molecules with the general formula of C n( 2O) n, or in other words C n 2nO n. This is a very common formula for sugars and many other natural products. The structure
As you enter... (Write down the learning objective, the question and your answer.) What do you think it means for a reaction to occur spontaneously?
 Monday, March 23rd Learning objective 5.13: The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively)
Monday, March 23rd Learning objective 5.13: The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively)
CHEMISTRY 101 DETAILED WEEKLY TEXTBOOK HOMEWORK & READING SCHEDULE *
 CHEMISTRY 101 COURSE POLICIES 15 CHEMISTRY 101 DETAILED WEEKLY TEXTBOOK HOMEWORK & READING SCHEDULE * * Refer to textbook homework assignment and pre-lecture assignment for corresponding chapters to read.
CHEMISTRY 101 COURSE POLICIES 15 CHEMISTRY 101 DETAILED WEEKLY TEXTBOOK HOMEWORK & READING SCHEDULE * * Refer to textbook homework assignment and pre-lecture assignment for corresponding chapters to read.
Schedule and Reading Assignments 8.01
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Physics Physics 8.01 Fall 2013 Website: http://web.mit.edu/8.01t/www/ Schedule and Reading Assignments 8.01 Reading Sources: Classical Mechanics: MIT
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Physics Physics 8.01 Fall 2013 Website: http://web.mit.edu/8.01t/www/ Schedule and Reading Assignments 8.01 Reading Sources: Classical Mechanics: MIT
1. This diagram illustrates a sound wave. The peak of the wave is its maximum on the y-axis. The trough of the wave is its minimum on the y-axis.
 M.7.NS.1 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. This diagram illustrates a sound wave. The peak of the wave is its maximum on the y-axis. The
M.7.NS.1 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. This diagram illustrates a sound wave. The peak of the wave is its maximum on the y-axis. The
Course information. Required Text
 Course information EHS 101: Fundamentals of Chemistry for Environmental Health UCLA School of Public Health https://ccle.ucla.edu/course/view/16f-envhlt101-1 Syllabus Fall 2016 Thursdays, 1-2:50 pm, in
Course information EHS 101: Fundamentals of Chemistry for Environmental Health UCLA School of Public Health https://ccle.ucla.edu/course/view/16f-envhlt101-1 Syllabus Fall 2016 Thursdays, 1-2:50 pm, in
Objective: SWBAT identify a compound as either ionic, covalent or an acid after reviewing terminology at 85% accuracy.
 Mrs. Chausse s Chemistry Gifted Lesson Plan: Unit 11 Chemical Reactions January 4 18, 2018 Wednesday, January 3, 2018 Naming and writing formulas Objective: SWBAT identify a compound as either ionic, covalent
Mrs. Chausse s Chemistry Gifted Lesson Plan: Unit 11 Chemical Reactions January 4 18, 2018 Wednesday, January 3, 2018 Naming and writing formulas Objective: SWBAT identify a compound as either ionic, covalent
Chemistry 39. Final Exam
 Chemistry 39 Final Exam Tuesday, May 7, 2005 ame (printed) 11:10 am S.S. # Signature Please show your picture I.D. to a proctor when you hand in your exam. TTAL 1 There are 25 questions on this exam. Check
Chemistry 39 Final Exam Tuesday, May 7, 2005 ame (printed) 11:10 am S.S. # Signature Please show your picture I.D. to a proctor when you hand in your exam. TTAL 1 There are 25 questions on this exam. Check
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chemistry 1406 Chemistry for the Health Professions Spring Dr. Janet L. Maxwell CAV 229B,
 Chemistry 1406 Chemistry for the Health Professions Spring 2019 Dr. Janet L. Maxwell CAV 229B, 325-486-6624 janet.maxwell@angelo.edu Office Hours: MWF 11:00 am noon Tuesday 2:00 3:00, Thursday 11 am -noon
Chemistry 1406 Chemistry for the Health Professions Spring 2019 Dr. Janet L. Maxwell CAV 229B, 325-486-6624 janet.maxwell@angelo.edu Office Hours: MWF 11:00 am noon Tuesday 2:00 3:00, Thursday 11 am -noon
1. Organic Molecules. Elements in Biological Molecules 2/13/2016. Chapter 4: Carbon and the Molecular Diversity of Life
 Chapter 4: Carbon and the Molecular Diversity of Life 1. Organic Molecules 2. Chemical Groups 1. Organic Molecules Chapter Reading pp. 57-62 Elements in Biological Molecules Biological macromolecules are
Chapter 4: Carbon and the Molecular Diversity of Life 1. Organic Molecules 2. Chemical Groups 1. Organic Molecules Chapter Reading pp. 57-62 Elements in Biological Molecules Biological macromolecules are
Chapter 4: Carbon and the Molecular Diversity of Life. 1. Organic Molecules 2. Chemical Groups
 Chapter 4: Carbon and the Molecular Diversity of Life 1. Organic Molecules 2. Chemical Groups 1. Organic Molecules Chapter Reading pp. 57-62 Elements in Biological Molecules Biological macromolecules are
Chapter 4: Carbon and the Molecular Diversity of Life 1. Organic Molecules 2. Chemical Groups 1. Organic Molecules Chapter Reading pp. 57-62 Elements in Biological Molecules Biological macromolecules are
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
The Basics of General, Organic, and Biological Chemistry
 The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
Chem 204. Mid-Term Exam I. July 21, There are 3 sections to this exam: Answer ALL questions
 Chem 204 Mid-Term Exam I July 21, 2009 Name: Answer Key Student ID: There are 3 sections to this exam: Answer ALL questions Section I: Multiple-Choice 20 questions, 2 pts each Section II: Fill-in-the-Blank
Chem 204 Mid-Term Exam I July 21, 2009 Name: Answer Key Student ID: There are 3 sections to this exam: Answer ALL questions Section I: Multiple-Choice 20 questions, 2 pts each Section II: Fill-in-the-Blank
TEACHING ASSISTANT POSITIONS (CUPE 3902, Unit 1) Emergency Posting Winter Department of Geography, University of Toronto Mississauga
 TEACHING ASSISTANT POSITIONS (CUPE 3902, Unit 1) Emergency Posting Winter 2019 Department of Geography, University of Toronto Mississauga Posted on: November 30, 2018 Applications due: December 7, 2018
TEACHING ASSISTANT POSITIONS (CUPE 3902, Unit 1) Emergency Posting Winter 2019 Department of Geography, University of Toronto Mississauga Posted on: November 30, 2018 Applications due: December 7, 2018
Lecture 22 Organic Chemistry 1
 CEM 232 rganic Chemistry I at Chicago Lecture 22 rganic Chemistry 1 Professor Duncan Wardrop April 1, 2010 1 Self Test Question Which starting material could not be used to prepare the tribromide below?
CEM 232 rganic Chemistry I at Chicago Lecture 22 rganic Chemistry 1 Professor Duncan Wardrop April 1, 2010 1 Self Test Question Which starting material could not be used to prepare the tribromide below?
ORGANIC - BROWN 8E CH.3 - STEREOISOMERISM AND CHIRALITY.
 !! www.clutchprep.com CONCEPT: TYPES OF ISOMERS Isomers are used to describe relationships between similar molecules. We can order these relationships in order of increasing similarity Page 2 CONCEPT:
!! www.clutchprep.com CONCEPT: TYPES OF ISOMERS Isomers are used to describe relationships between similar molecules. We can order these relationships in order of increasing similarity Page 2 CONCEPT:
Carbon and the Molecular Diversity of Life
 Chapter 4 Carbon and the Molecular Diversity of Life Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 4: 1. Organic chemistry is the study of carbon compounds 2. Carbon atoms
Chapter 4 Carbon and the Molecular Diversity of Life Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 4: 1. Organic chemistry is the study of carbon compounds 2. Carbon atoms
Alabama. Department of. Postsecondary Education
 Date Adopted: July 1, 1998 Date Reviewed: December 1, 1999 Date Revised: 1999, 2007, 2011, 2013 Alabama Department of Postsecondary Education Representing Alabama s Public Two-Year College System Jefferson
Date Adopted: July 1, 1998 Date Reviewed: December 1, 1999 Date Revised: 1999, 2007, 2011, 2013 Alabama Department of Postsecondary Education Representing Alabama s Public Two-Year College System Jefferson
Chapter 10 Study Guide The Mole Section 10 1
 Chapter 10 Study Guide The Mole Section 10 1 Measuring Matter The electron configuration of hydrogen is like that of Group 1 metals. 4. Study Guide - Chapter 10 The Mole Section 10.1 Measuring Matter 1.
Chapter 10 Study Guide The Mole Section 10 1 Measuring Matter The electron configuration of hydrogen is like that of Group 1 metals. 4. Study Guide - Chapter 10 The Mole Section 10.1 Measuring Matter 1.
Chapter 16 Aldehydes and Ketones Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 16 Aldehydes and Ketones Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 16 Aldehydes and Ketones Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Syllabus: CHEM 4610/5560 Advanced Inorganic Chemistry I Fall Semester credit hours; lecture only
 Syllabus: CHEM 4610/5560 Advanced Inorganic Chemistry I Fall Semester 2014 3 credit hours; lecture only Instructor: Dr. LeGrande M. Slaughter Chemistry Building Rm. 307E Office phone: 565-4350; legrande.slaughter@unt.edu
Syllabus: CHEM 4610/5560 Advanced Inorganic Chemistry I Fall Semester 2014 3 credit hours; lecture only Instructor: Dr. LeGrande M. Slaughter Chemistry Building Rm. 307E Office phone: 565-4350; legrande.slaughter@unt.edu
Chemistry 401: Modern Inorganic Chemistry (3 credits) Fall 2017
 Chemistry 401: Modern Inorganic Chemistry (3 credits) Fall 2017 Monday, Wednesday, Friday 9:10-10:00 am in Troy G5 Syllabus Instructor: Professor Qiang Zhang Office: Troy 220 Phone: 509-335-1269 Email:
Chemistry 401: Modern Inorganic Chemistry (3 credits) Fall 2017 Monday, Wednesday, Friday 9:10-10:00 am in Troy G5 Syllabus Instructor: Professor Qiang Zhang Office: Troy 220 Phone: 509-335-1269 Email:
