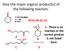
Give a common name for the following compound.
|
|
|
- Basil Shaw
- 5 years ago
- Views:
Transcription
1 Give a common name for the following compound. A. Benzyl Phenoate B. Phenyl Phenoate C. Benzyl Benzoate D. Phenyl Benzoate E. Phenyl Phenylacetate F. Phenyl Phenylethanoate G. Phenyl Benzylacetate Q1
2 Give a common name for the following compound. A. Benzyl Phenoate B. Phenyl Phenoate C. Benzyl Benzoate D. Phenyl Benzoate E. Phenyl Phenylacetate F. Phenyl Phenylethanoate G. Phenyl Benzylacetate Q1
3 Exam 2 Time: Tuesday, October 18: 7:00 9:00PM OR Wednesday, October 19: 7:00 9:00PM OR Thursday, October 20: 7:00 10:00PM Location Soc/Anthro Testing Center Chapters will be covered in this order: Chapter 19, 12 Practice Exams are Posted Ex Practice Exam 2A Ex Practice Exam 2B Deadline for alternate arrangements is Monday, 10/17/2016 at 4:30 PM (i.e., close of business) An oral make-up exam will be required for making up the exam for all students not taking the exam on the above dates or having already made prior arrangements
4 Order of Coverage (Exam 2) Homework Assignment Due Date 13 Ex2-07-B A Carbox Acid Rxns Thursday, October 6, Ex2-07-B B Carbox Acid Rxns Friday, October 7, Ex2-08-B A Naming Carbox Acid Derivatives 16 Ex2-08-B B Naming Carbox Acid Derivatives Saturday, October 8, 2016 Sunday, October 9, Ex2-09-B A Rxns Acid Chlorides Monday, October 10, Ex2-09-B B Rxns Acid Chlorides Tuesday, October 11, Ex2-10-B A Rxns Esters Wednesday, October 12, Ex2-10-B B Rxns Esters Thursday, October 13, Ex2-11-B A Rxns Amides Friday, October 14, Ex2-11-B B Rxns Amides Saturday, October 15, Ex2-12-B A Step Growth Polymers Sunday, October 16, 2016 Exam 2 October 18, 19, 20
5 Reactions of Acid Chlorides Most reactive carboxylic acid derivative Addition of Nucleophiles! LiAlH 4 (Similar to esters) RMgBr (Similar to esters) R 2 CuLi (Stops at the ketone) Conversion into other carboxylic acids under all conditions Base-catalyzed Acid-catalyzed
6 Acid Chlorides and LiAlH 4 1) LiAlH 4 2) H 3 O + H +
7 2 nd Example: Acid Chlorides and LiAlH 4 1) LiAlH 4 2) H 3 O + H +
8 Acid Chlorides and Special LiAlH 4 Reagent 1) LiAlH 4 2) H 3 O + Steric hindrance of the t-butyl groups and diminished reactivity of the Al-H stop the reaction at the aldehyde stage. X LiAlH(OC(CH 3 ) 3 stops at the aldehyde and does not react further!
9 Acid Chlorides and RMgBr H +
10 Acid Chlorides and R 2 CuLi 1) (CH 3 ) 2 CuLi 2) H 3 O + X 2 nd addition does not occur, since the cuprate is not a hard enough base.
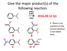
11 Give the major organic product(s) of the following reaction. 1) LiAlH 4 2) H 3 O Q2 A B C D E F G. There is no reaction under these conditions or the correct product is not listed here.
12 Give the major organic product(s) of the following reaction. 1) LiAlH 4 2) H 3 O Q2 A B C D E F G. There is no reaction under these conditions or the correct product is not listed here.
13 Give the major organic product(s) of the following reaction. Ph 2 CuLi Ether, -78 o C Q3 A B C D E F. There is no reaction under these conditions or the correct product is not listed here.
14 Give the major organic product(s) of the following reaction. Ph 2 CuLi Ether, -78 o C Q3 A B C D E F. There is no reaction under these conditions or the correct product is not listed here.
15 Base-Catalyzed Reactions of Carboxylic Acid Derivatives X Y - Y X - X - Y
16 Acid-Catalyzed Reactions of Carboxylic Acid Derivatives H + X HY X HY + X -H + Y -H + -HX Y X + H +H + Y X
17 Carboxylic Acid Derivatives are a Mixture of a Carboxylic Acid and another Heteroatom. Ethyl Benzoate Aspartame
18 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx ab) Q4 A B C D E - None of these products are a major product of the reaction that is shown.
19 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx ab) Q4 A B C D E - None of these products are a major product of the reaction that is shown.
20 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx ab) Q5 NaOH A B E - None of these products are a major product of the reaction that is shown. C D
21 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx ab) Q5 NaOH A B E - None of these products are a major product of the reaction that is shown. C D
Give the major organic product(s) of the following reaction.
 Give the major organic product(s) of the following reaction. 1) CH3CH2MgBr 2) H3O 2016-09-26 Q1 + E. There is no reaction or the correct product A B is not listed here. C D Explanation CH3CH2MgBr 1) CH3CH2MgBr
Give the major organic product(s) of the following reaction. 1) CH3CH2MgBr 2) H3O 2016-09-26 Q1 + E. There is no reaction or the correct product A B is not listed here. C D Explanation CH3CH2MgBr 1) CH3CH2MgBr
Give the major organic product(s) of the following reaction.
 Give the major organic product(s) of the following reaction. 1) NaNO2, HCl 0 o C 2) CuCN 2016-09-23 Q1 A D B C E F There is no reaction or the correct product is not listed here. 5 of 7 Give the major
Give the major organic product(s) of the following reaction. 1) NaNO2, HCl 0 o C 2) CuCN 2016-09-23 Q1 A D B C E F There is no reaction or the correct product is not listed here. 5 of 7 Give the major
H3O+ heat I Q1
 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) H3O + heat A B C D E F G
Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) H3O + heat A B C D E F G
A B C D Q1
 Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx
Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx
Give the major product(s) of the following reaction.
 Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
A B C D E F Q1
 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) 1) Li 2) 2016-11-11 Q1 3)
Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) 1) Li 2) 2016-11-11 Q1 3)
A B C D E F G Q1. heat
 Give the final product of the following reaction. Give your answer as a text answer. If more than one species is correct, put your answers in alphabetical order. HNO3 H2SO4 A B C D E F G 2016-09-09 Q1
Give the final product of the following reaction. Give your answer as a text answer. If more than one species is correct, put your answers in alphabetical order. HNO3 H2SO4 A B C D E F G 2016-09-09 Q1
Q1. Intensity of M+1 peak x Intensity of M peak 2.57% x 46.60% Number of carbon atoms = Number of carbon atoms =
 The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 46.60% and an M+1 peak of 2.57%. How many carbon atoms are in the compound? (Fill in an integer number) Number
The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 46.60% and an M+1 peak of 2.57%. How many carbon atoms are in the compound? (Fill in an integer number) Number
Chapter 19 Substitutions at the Carbonyl Group
 Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
CHEM Chapter 21. Carboxylic Acid Derivatives_Nucleophilic Acyl Substitution Reactions (homework) W
 Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry II Examination #3 - March 31, 2003
 INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Exam 3 Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3331-100 Spring 2008 Exam 3 Professor R. oenigman igh = 102 Low = 15 Average = 68 Signature ame (printed) Last four digits of your student ID number Recitation
I pledge to uphold the CU onor Code: CEM 3331-100 Spring 2008 Exam 3 Professor R. oenigman igh = 102 Low = 15 Average = 68 Signature ame (printed) Last four digits of your student ID number Recitation
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
LECTURE #22 Thurs., Nov.15, 2007
 Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
CHEM*2700 ORGANIC CHEMISTRY I (Winter Semester 2007) Information Sheet and Course Outline-Revised
 CHEM*2700 ORGANIC CHEMISTRY I (Winter Semester 2007) Information Sheet and Course Outline-Revised Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHEM*2700 ORGANIC CHEMISTRY I (Winter Semester 2007) Information Sheet and Course Outline-Revised Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
Ch 17 Alcohols and Phenols
 Ch 17 Alcohols and Phenols Alcohols are compounds with a hydroxyl group (OH) attached to an sp 3 C. Phenols are compounds with a hydroxyl group (OH) attached to an aromatic sp 2 C. Classification of Alcohols
Ch 17 Alcohols and Phenols Alcohols are compounds with a hydroxyl group (OH) attached to an sp 3 C. Phenols are compounds with a hydroxyl group (OH) attached to an aromatic sp 2 C. Classification of Alcohols
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution Reactions. McMurray Text Chapter 21
 Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Learning Guide for Chapter 15 - Alcohols (II)
 Learning Guide for Chapter 15 - Alcohols (II) I. Introduction to alcohol reactivity II. Reactions of alcohols with acids III. Reactions of alcohols with electrophiles alogenated phosphorus and sulfur compounds
Learning Guide for Chapter 15 - Alcohols (II) I. Introduction to alcohol reactivity II. Reactions of alcohols with acids III. Reactions of alcohols with electrophiles alogenated phosphorus and sulfur compounds
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2009) Information Sheet and Course Outline
 CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2009) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2009) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHE 325 ORGANIC CHEMISTRY II Spring 2017
 Instructor: CHE 325 ORGANIC CHEMISTRY II Spring 2017 Professor James Kallmerten 4-014A Center for Science and Technology Phone: 3-2854 Email: jkallmer@syr.edu Office Hours: Monday 11 am -1 pm, Wednesday
Instructor: CHE 325 ORGANIC CHEMISTRY II Spring 2017 Professor James Kallmerten 4-014A Center for Science and Technology Phone: 3-2854 Email: jkallmer@syr.edu Office Hours: Monday 11 am -1 pm, Wednesday
FIRST EXAMINATION. Name: CHM 332
 ame: CM 332 FIRST EXAMIATI All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is,
ame: CM 332 FIRST EXAMIATI All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is,
CHEM Fall Exam 2 Professor R. Hoenigman. Signature. Name (printed) Last four digits of your student ID number.
 Average = 68 I pledge to uphold the CU on Code: CEM 3311-200 Fall 2006 Exam 2 Profess R. oenigman igh = 98 Low = 18 Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation
Average = 68 I pledge to uphold the CU on Code: CEM 3311-200 Fall 2006 Exam 2 Profess R. oenigman igh = 98 Low = 18 Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation
I pledge on my honor that I have neither given nor received unauthorized aid on this examination
 Chemistry 220b, Section 1 Exam 2 (100 pts) Thursday, ebruary 26, 2015 Chapters 13, 15-19 ame Write and sign the VU onor Pledge: I pledge on my honor that I have neither given nor received unauthorized
Chemistry 220b, Section 1 Exam 2 (100 pts) Thursday, ebruary 26, 2015 Chapters 13, 15-19 ame Write and sign the VU onor Pledge: I pledge on my honor that I have neither given nor received unauthorized
Chemistry Organic Chemistry II, Spring 2018
 Chemistry 2320 Organic Chemistry II, Spring 2018 Instructor: Dr. Tom Chang Office: Widtsoe 337 Phone: 797-3545 Email: tom.chang@usu.edu Meeting Time/Place: MWF 10:30-11:20 am, Eccles Business Building
Chemistry 2320 Organic Chemistry II, Spring 2018 Instructor: Dr. Tom Chang Office: Widtsoe 337 Phone: 797-3545 Email: tom.chang@usu.edu Meeting Time/Place: MWF 10:30-11:20 am, Eccles Business Building
ORGANIC - BROWN 8E CH CARBOXYLIC ACIDS.
 RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS!! www.clutchprep.com RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS CNCEPT: CARBXYLIC ACID NMENCLATURE IUPAC: Replace alkane -e with Substituents are located using
RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS!! www.clutchprep.com RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS CNCEPT: CARBXYLIC ACID NMENCLATURE IUPAC: Replace alkane -e with Substituents are located using
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
ALDEH. I. Multiple Choice Questions (Type-I)
 Unit 12 ALDEHYDE ALDEH YDES, KETONE KET ONES AND CARBOX C ARBOXYLIC ACIDS I. Multiple Choice Questions (Type-I) 1. Addition of water to alkynes occurs in acidic medium and in the presence of Hg 2+ ions
Unit 12 ALDEHYDE ALDEH YDES, KETONE KET ONES AND CARBOX C ARBOXYLIC ACIDS I. Multiple Choice Questions (Type-I) 1. Addition of water to alkynes occurs in acidic medium and in the presence of Hg 2+ ions
Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
 Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017)
 Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017) Class: Organic Chemistry II Instructor: Dr. David J. Leaver Room: WSB 307 Office: WSB 318 Time: MWF 9:00-9:50am Office
Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017) Class: Organic Chemistry II Instructor: Dr. David J. Leaver Room: WSB 307 Office: WSB 318 Time: MWF 9:00-9:50am Office
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION
 CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
ORGANIC - CLUTCH CH ALCOHOLS AND CARBONYL COMPOUNDS.
 !! www.clutchprep.com CONCEPT: INTRO TO REDOX Oxidation reactions involve an increase in the content of a molecule Reduction reactions involve an increase in the content of a molecule EXAMPLE: Label the
!! www.clutchprep.com CONCEPT: INTRO TO REDOX Oxidation reactions involve an increase in the content of a molecule Reduction reactions involve an increase in the content of a molecule EXAMPLE: Label the
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chapter 16. Ethers, Epoxides, and Sulfides. Class Notes. 2. Epoxides: cyclic ethers, 3-membered rings. 1. Symmetrical and unsymmetrical (mixed) ethers
 Chapter 16 Ethers, Epoxides, and Sulfides Chapter 16 suggested problems: 21, 25, 26, 31, 32, 33 Class Notes I. Nomenclature of ethers, epoxides, and sulfides A. General B. Ethers 1. Ethers: C-O-C 2. Epoxides:
Chapter 16 Ethers, Epoxides, and Sulfides Chapter 16 suggested problems: 21, 25, 26, 31, 32, 33 Class Notes I. Nomenclature of ethers, epoxides, and sulfides A. General B. Ethers 1. Ethers: C-O-C 2. Epoxides:
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2010) Information Sheet and Course Outline
 CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2010) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2010) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
Organic Chemistry 1 CHM 2210 Exam 4 (December 10, 2001)
 Exam 4 (December 10, 2001) Name (print): Signature: Student ID Number: There are 12 multiple choice problems (4 points each) on this exam. Record the answers to the multiple choice questions on THIS PAGE.
Exam 4 (December 10, 2001) Name (print): Signature: Student ID Number: There are 12 multiple choice problems (4 points each) on this exam. Record the answers to the multiple choice questions on THIS PAGE.
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Name: Student Number: University of Manitoba - Department of Chemistry CHEM Introductory Organic Chemistry II - Term Test 1
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 11, 2016; 7-9 PM This is a 2-hour test, marked out
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 11, 2016; 7-9 PM This is a 2-hour test, marked out
CHEMISTRY MIDTERM # 1 answer key February 10, 2005
 CEMSTRY 314-0 MDTERM # 1 answer key February 10, 005 Statistics: Average: 77 pts (77%); ighest: 99 pts (99%); Lowest: 40 pts (40%) Number of students performing at or above average: 5 (54%) 1. (8 pts)
CEMSTRY 314-0 MDTERM # 1 answer key February 10, 005 Statistics: Average: 77 pts (77%); ighest: 99 pts (99%); Lowest: 40 pts (40%) Number of students performing at or above average: 5 (54%) 1. (8 pts)
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CARBOXYLIC ACIDS AND THEIR DERIVATIVES. 3. Predict the relative acidity and basicity of compounds and ions. Important criteria include:
 CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
Conjugate Addition Reactions 2:02 PM
 1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
c. Oxidizing agent shown here oxidizes 2º alcohols to ketones and 1º alcohols to carboxylic acids. 3º alcohols DO NOT REACT.
 Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
An alcohol is a compound obtained by substituting a hydoxyl group ( OH) for an H atom on a carbon atom of a hydrocarbon group.
 Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Chapter 12 Alcohols from Carbonyl Compounds: Oxidation-Reduction and Organometallic Compounds
 Chapter 12 Alcohols from Carbonyl Compounds: Oxidation-Reduction and Organometallic Compounds Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols
Chapter 12 Alcohols from Carbonyl Compounds: Oxidation-Reduction and Organometallic Compounds Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols
CH101 - Chemistry. Instructors: Dr. David Cheung, Prof. Paul Murphy, Dr. Luca Ronconi (Coordinator)
 CH101 - Chemistry Instructors: Dr. David Cheung, Prof. Paul Murphy, Dr. Luca Ronconi (Coordinator) Module Overview and General Aims This Module lays a broad foundation in chemistry for students who have
CH101 - Chemistry Instructors: Dr. David Cheung, Prof. Paul Murphy, Dr. Luca Ronconi (Coordinator) Module Overview and General Aims This Module lays a broad foundation in chemistry for students who have
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch16_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which type of compound does not contain a carbonyl group? ketone B) aldehyde C) amine D)
Ch16_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which type of compound does not contain a carbonyl group? ketone B) aldehyde C) amine D)
A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6
 GEORGIA INSTITUTE OF TECHNOLOGY School of Chemistry and Biochemistry Spring 2004 A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6 Instructor: Marcus Weck Office: Boggs 3-85 Phone:
GEORGIA INSTITUTE OF TECHNOLOGY School of Chemistry and Biochemistry Spring 2004 A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6 Instructor: Marcus Weck Office: Boggs 3-85 Phone:
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
1) Which type of compound does not contain a carbonyl group? A) ketone B) aldehyde C) amine D) ester E) carboxylic acid
 1) Which type of compound does not contain a carbonyl group? ketone aldehyde amine ester carboxylic acid 2) Which functional group contains a carbonyl group and a hydroxyl group bonded to the same carbon
1) Which type of compound does not contain a carbonyl group? ketone aldehyde amine ester carboxylic acid 2) Which functional group contains a carbonyl group and a hydroxyl group bonded to the same carbon
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
ASTR 101L: Motion of the Sun Take Home Lab
 Name: CWID: Section: Introduction Objectives This lab is designed to help you understand the Sun s apparent motion in the sky over the course of the year. In Section 2 you are asked to answer some questions
Name: CWID: Section: Introduction Objectives This lab is designed to help you understand the Sun s apparent motion in the sky over the course of the year. In Section 2 you are asked to answer some questions
Alcohol Synthesis. Dr. Sapna Gupta
 Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
Class XII - Chemistry Aldehydes, Ketones and Carboxylic Acid Chapter-wise Problems
 Class XII - Chemistry Aldehydes, Ketones and Carboxylic Acid Chapter-wise Problems I. Multiple Choice Questions (Type-I) 1. Addition of water to alkynes occurs in acidic medium and in the presence of Hg
Class XII - Chemistry Aldehydes, Ketones and Carboxylic Acid Chapter-wise Problems I. Multiple Choice Questions (Type-I) 1. Addition of water to alkynes occurs in acidic medium and in the presence of Hg
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Week 8/Tu: Lecture Unit 19
 Week 8/Tu: Lecture Unit 19 Unit 18: Periodicity -- electron configurations -- periodicity of elements -- radii, ionization, affinity -- electronegativity Unit 19: Chemical Bonding -- Basis of bonding --
Week 8/Tu: Lecture Unit 19 Unit 18: Periodicity -- electron configurations -- periodicity of elements -- radii, ionization, affinity -- electronegativity Unit 19: Chemical Bonding -- Basis of bonding --
A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D. III > IV > I > II
 Practice Final Exam, Chemistry 2220, Organic Chemistry II 1. Rank the designated protons by 1 H NMR chemical shift ( ), highest first. A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D.
Practice Final Exam, Chemistry 2220, Organic Chemistry II 1. Rank the designated protons by 1 H NMR chemical shift ( ), highest first. A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D.
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
AMSC/MATH 673, CLASSICAL METHODS IN PDE, FALL Required text: Evans, Partial Differential Equations second edition
 AMSC/MATH 673, CLASSICAL METHODS IN PDE, FALL 2018. MWF 2:00pm - 2:50pm MTH 0407 Instructor: M. Machedon Office: MTH 3311 e-mail: mxm@math.umd.edu Required text: Evans, Partial Differential Equations second
AMSC/MATH 673, CLASSICAL METHODS IN PDE, FALL 2018. MWF 2:00pm - 2:50pm MTH 0407 Instructor: M. Machedon Office: MTH 3311 e-mail: mxm@math.umd.edu Required text: Evans, Partial Differential Equations second
CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide
 CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide page 1 CHEM 243 Exam II is scheduled for Monday, November 5 in DMF 477 & 481 (lab rooms). You must take the exam during your normal
CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide page 1 CHEM 243 Exam II is scheduled for Monday, November 5 in DMF 477 & 481 (lab rooms). You must take the exam during your normal
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Answer Key Question 1.
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Answer Key Question 1.
Organic Chemistry. Introduction to Organic Molecules and Functional Groups
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
Grant MacEwan University Fall 2010 CHEM 362 (270) Advanced Organic Chemistry. Organic Chemistry by Clayden, Greeves, Warren and Wothers
 1 Instructor: Office: Grant MacEwan University Fall 2010 CHEM 362 (270) Advanced Organic Chemistry Dr. Manzar Saberi 5-172 K, City Center Campus Phone: 497-4634 Lectures: Tuesday, Thursday, 12:30-2:00
1 Instructor: Office: Grant MacEwan University Fall 2010 CHEM 362 (270) Advanced Organic Chemistry Dr. Manzar Saberi 5-172 K, City Center Campus Phone: 497-4634 Lectures: Tuesday, Thursday, 12:30-2:00
Reading: Finish reading Ch. 9 if you haven't already HW - finish smartwork above and begin working on competition problems from the Ch. 9 Handout.
 Ch. 8 Smartwork available - due Tomorrow @ 10 AM - don't forget to check for carbocation rearrangements! Ch. 9 Smartwork available - due Saturday (12/9) @ 11:59 PM Final Exam (Monday 12/11 @ 8 AM here)
Ch. 8 Smartwork available - due Tomorrow @ 10 AM - don't forget to check for carbocation rearrangements! Ch. 9 Smartwork available - due Saturday (12/9) @ 11:59 PM Final Exam (Monday 12/11 @ 8 AM here)
UNIVERSITY OF MANITOBA DEPARTMENT OF CHEMISTRY
 PAGE 1 of 7 UNIVERSITY F MANITBA DEPARTMENT F CEMISTRY 2.339 STRUCTURAL TRANSFRMATINS IN RGANIC CEMISTRY FINAL EAMINATIN Dr. Phil ultin Thursday December 14, 2000. NAME: ANSWERS STUDENT NUMBER: 1) (15
PAGE 1 of 7 UNIVERSITY F MANITBA DEPARTMENT F CEMISTRY 2.339 STRUCTURAL TRANSFRMATINS IN RGANIC CEMISTRY FINAL EAMINATIN Dr. Phil ultin Thursday December 14, 2000. NAME: ANSWERS STUDENT NUMBER: 1) (15
A. B. C. D. 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal?
 Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
3) Between aldehyde and ketones which one is confirmed using Tollen s reagent.
 UNIT ALDEHYDES KETONES AND CARBOXYLIC ACIDS ) What are aldehydes? Aldehydes are the organic compounds containing carbonyl group,linked with one hydrogen and one alkyl /aryl group. ) What are carboxylic
UNIT ALDEHYDES KETONES AND CARBOXYLIC ACIDS ) What are aldehydes? Aldehydes are the organic compounds containing carbonyl group,linked with one hydrogen and one alkyl /aryl group. ) What are carboxylic
Level 3 Chemistry, 2013
 91391 913910 3SUPERVISOR S Level 3 Chemistry, 2013 91391 Demonstrate understanding of the properties of organic compounds 2.00 pm Tuesday 19 November 2013 Credits: Five Achievement Achievement with Merit
91391 913910 3SUPERVISOR S Level 3 Chemistry, 2013 91391 Demonstrate understanding of the properties of organic compounds 2.00 pm Tuesday 19 November 2013 Credits: Five Achievement Achievement with Merit
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Organic Chemistry II KEY March 27, 2013
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
Chapter 17: Alcohols and Phenols. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Q. No. 4 Chlorination of toluene in the presence of light and heat followed by treatment with aqueous NaOH gives o cresol p cresol 2, 4 dihydroxy tolu
 Q. No. 1 Glycerine has One primary and two secondary OH groups One secondary and two primary OH groups Three primary OH groups Three secondary OH groups Q. No. 2 The structural formula of cyclohexanol
Q. No. 1 Glycerine has One primary and two secondary OH groups One secondary and two primary OH groups Three primary OH groups Three secondary OH groups Q. No. 2 The structural formula of cyclohexanol
Allyl radicals are especially stable due to resonance ( and double bond switch places):
 Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
Organic Chemistry II KEY March 27, Which of the following reaction intermediates will form the fastest in the reaction below?
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
Chapter 3. Organic Compounds: Alkanes and Their Stereochemistry
 Chapter 3. Organic Compounds: Alkanes and Their Stereochemistry Functional Group: Be able to identify and name any of the functional groups listed on Table 3.1, pages 76-77. Summary of important functional
Chapter 3. Organic Compounds: Alkanes and Their Stereochemistry Functional Group: Be able to identify and name any of the functional groups listed on Table 3.1, pages 76-77. Summary of important functional
Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton)
 314 Arrow Pushing practice/eauchamp 1 Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton) ucleophile = nucleus/positive loving = any general electron
314 Arrow Pushing practice/eauchamp 1 Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton) ucleophile = nucleus/positive loving = any general electron
Organic Chemistry II KEY March 25, a) I only b) II only c) II & III d) III & IV e) I, II, III & IV
 rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
1 a) Name the following substances with the used common names or according to IUPAC (4p): b) Draw detailed structures of the substances below.
 EGLIS VERSI Exam rganic Chemistry 2 (KD1100) Wednesday May 21, 2008, 08.00-13.00 Allowed answering aid: molecular models Periodic system and tables of bond energies, pk a -values and MR-shifts are attached
EGLIS VERSI Exam rganic Chemistry 2 (KD1100) Wednesday May 21, 2008, 08.00-13.00 Allowed answering aid: molecular models Periodic system and tables of bond energies, pk a -values and MR-shifts are attached
DO NOT WRITE YOUR NAME UNTIL TOLD TO START! CHEM 8B Organic Chemistry II EXAM 2, Summer 2018 (300 points)
 UCSC, Binder Exam 2, SS 18 Full First and Last Name Underline the first initial of your last name D NT WRITE YUR NAME UNTIL TLD T START! CHEM 8B rganic Chemistry II EXAM 2, Summer 2018 (300 points) 1 (50)
UCSC, Binder Exam 2, SS 18 Full First and Last Name Underline the first initial of your last name D NT WRITE YUR NAME UNTIL TLD T START! CHEM 8B rganic Chemistry II EXAM 2, Summer 2018 (300 points) 1 (50)
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
CH 320/328 N Summer II 2018
 CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
Chem 315/316 Reactions. Bromo organic compounds C1 & C2 carbon skeletons C3 carbon skeletons C4 carbon skeletons. Alcohols. Chem 316 / Beauchamp 1
 hem 316 / Beauchamp 1 hem 315/316 eactions Name available sources of carbon 4 3 NaN methyl 3 2 ethyl 3 2 2 n-propyl 3 3 isopropyl 2 3 2 3 3 2 2 3 3 3 n-butyl sec butyl t-butyl phenyl available until topic
hem 316 / Beauchamp 1 hem 315/316 eactions Name available sources of carbon 4 3 NaN methyl 3 2 ethyl 3 2 2 n-propyl 3 3 isopropyl 2 3 2 3 3 2 2 3 3 3 n-butyl sec butyl t-butyl phenyl available until topic
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Full First and Last Name DO NOT WRITE YOUR NAME UNTIL TOLD TO START! CHEM 8B Organic Chemistry II EXAM 2, Winter 2017 (200 points)
 UCSC, Binder Exam 2, W17 Full First and Last Name D NT WRITE YUR NAME UNTIL TLD T START! CHEM 8B rganic Chemistry II EXAM 2, Winter 2017 (200 points) In each of the following problems, use your knowledge
UCSC, Binder Exam 2, W17 Full First and Last Name D NT WRITE YUR NAME UNTIL TLD T START! CHEM 8B rganic Chemistry II EXAM 2, Winter 2017 (200 points) In each of the following problems, use your knowledge
Chemistry 262: Organic Chemistry II Winter 2018
 Chemistry 262: Organic Chemistry II Winter 2018 The second of a three quarter sequence in organic chemistry for university transfer, intended primarily for science majors, and those fulfilling requirements
Chemistry 262: Organic Chemistry II Winter 2018 The second of a three quarter sequence in organic chemistry for university transfer, intended primarily for science majors, and those fulfilling requirements
CHAPTER 20: MORE ABOUT OXIDATION REDUCTION REACTIONS Oxidation Reduction Reactions of Organic Compounds: An Overview
 CHAPTER 20: MORE ABOUT OXIDATION REDUCTION REACTIONS In an oxidation-reduction reaction (redox reaction), one species loses electrons and one gains electrons. The species that loses electrons is oxidized,
CHAPTER 20: MORE ABOUT OXIDATION REDUCTION REACTIONS In an oxidation-reduction reaction (redox reaction), one species loses electrons and one gains electrons. The species that loses electrons is oxidized,
CHAPTER 22 HW: CO 2 H DERIVATIVES
 APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
