CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
|
|
|
- Jeremy Atkinson
- 5 years ago
- Views:
Transcription
1 CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting materials with aldehydes; equilibrium favors the products with ketones. Equilibrium favors the products with aldehydes; equilibrium favors the starting materials with ketones. Equilibrium favors the starting materials with both aldehydes and ketones. Equilibrium favors the products with both aldehydes and ketones. 2. In a Michael reaction, where does the nucleophile attack the a,b-unsaturated carbonyl component? Carbonyl carbon a-carbon Carbonyl carbon and b-carbon b-carbon 3. What is the product of the following Claisen reaction? IV I III
2 4. Would this crossed Aldol reaction work well? Why or why not? Yes, the diketone is significantly more acidic, so this enolate can be formed selectively. No, the diketone is significantly more acidic, so this enolate cannot be formed selectively. Yes, the aldehyde is significantly more acidic, so this enolate can be formed selectively. No, the aldehyde is significantly more acidic, so this enolate cannot be formed selectively. 5. What is an intramolecular Claisen reaction called? Robinson reaction Dieckmann reaction Hoffman reaction Michael reaction 6. What reaction type is a Claisen reaction? Electrophilic substitution Nucleophilic addition Electrophilic addition Nucleophilic substitution 7. What product (including stereochemistry) is formed in the following intermolecular reaction? IV I III
3 8. Which of the following compounds can undergo an Aldol with itself? I III Both I and II 9. There are several variations of the Aldol reaction. Which of the following types of reactants leads to only one possible product with the Aldol condensation reaction? Any aldehyde and ketone mixed together can react to form a single condensation product. Two different ketones with a-hydrogens are able to form a single aldol condensation product. Any pair of aldehyde or ketone reactants where one of the reactants has no a-hydrogens will lead to a single aldol product. Two different aldehydes with a-hydrogens are able to form a single aldol condensation product. 10. What is the name given to an Aldol reaction between two different carbonyl compounds? Multiple Aldol reaction Crossed Aldol reaction Differential Aldol reaction Versatile Aldol reaction 11. What is the general name for the class of products formed in an Aldol condensation reaction? a,g-unsaturated carbonyl compound a,b-unsaturated carbonyl compound b,g-unsaturated carbonyl compound b-hydroxy carbonyl compound
4 12. Complete this statement: A major difference between the Aldol condensation and the Claisen condensation reactions is that the Aldol reaction is base catalyzed while the Claisen reaction requires a full equivalent of base. the Aldol reaction is acid catalyzed while the Claisen reaction is base-catalyzed. the Aldol reaction involves substitution while the Claisen reaction involves addition. the Aldol reaction is base catalyzed while the Claisen reaction is acid-catalyzed. 13. The b-hydroxy carbonyl product of an Aldol reaction is oftentimes not the final isolated product; what is the explanation for this result? Hydroxide is eliminated via an enolate intermediate. The hydroxy group is oxidized to a carbonyl. The hydroxy group reacts with the carbonyl to form a ketal. It undergoes elimination, since water is a good leaving group. 14. In what situation can the yield of a single crossed Aldol product be increased? The nucleophilic carbonyl component is relatively hindered and is used in limited amount. The electrophilic carbonyl carbon component is relatively hindered and is used in limited amount. The electrophilic carbonyl component is relatively unhindered and is used in excess. The nucleophilic carbonyl component is relatively unhindered and is used in excess. 15. The following reaction is an example of what type of reaction? Claisen condensation Dieckmann condensation Mixed Aldol reaction Robinson annulation
5 16. What is the Aldol addition product formed from the reaction of acetone, (CH 3 ) 2 CO,withitself? III I IV 17. Complete this statement: A major difference between the Aldol condensation and the Claisen condensation reactions is that the Aldol reaction is base catalyzed while the Claisen reaction requires a full equivalent of base. the Aldol reaction is acid catalyzed while the Claisen reaction is base-catalyzed. the Aldol reaction involves substitution while the Claisen reaction involves addition. the Aldol reaction is base catalyzed while the Claisen reaction is acid-catalyzed. 18. What is the name given to the Claisen reaction between two different esters? Versatile Claisen reaction Crossed Claisen reaction Differential Claisen reaction Multiple Claisen reaction
6 19. Which is the unsaturated carbonyl compound formed in the dehydration of the following b-hydroxy carbonyl compound? I and II I None of the choices 20. What is the Aldol addition product formed from reaction of the following compound with itself? III I IV
7 21. What cyclic product is formed in the intramolecular Aldol condensation when the following compound is treated with aqueous NaOH? I I IV II 22. What are the two starting materials for a Robinson annulation? an a,b-unsaturated carbonyl compound and an enolate a 1,3-Dicarbonyl compound and an enolate a 1,5-Dicarbonyl compound and an enolate a b-ketoester and an enolate 23. Which of the following carbonyl compounds does not undergo Aldol addition reactions when treated with aqueous sodium hydroxide? III IV I II
8 24. What is the general name of the product of a crossed Claisen reaction between a ketone and an ester? a,b-dicarbonyl compound b-dicarbonyl compound g-dicarbonyl compound b-keto ester 25. What is the general name for the reaction product formed in an aldol addition reaction? b-hydroxy carbonyl compound g-hydroxy carbonyl compound a,b-hydroxy carbonyl compound a-hydroxy carbonyl compound
ORGANIC - CLUTCH CH CONDENSATION CHEMISTRY.
 !! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
!! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Summary of π Bond Chemistry
 Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Conjugate Addition Reactions 2:02 PM
 1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
Chapter 23. Alpha Substitution of Carbonyl Compounds.
 1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
What is in Common for the Following Reactions, and How Do They Work?
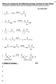 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Reversible Additions to carbonyls: Weak Nucleophiles Relative Reactivity of carbonyls: Hydration of Ketones and Aldehydes
 Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
18: Reactions of Enolate Ions and Enols
 18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
20.10 Conjugate Additions
 894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
State University of New York at Stony Brook Department of Chemistry. CHE 322, Organic Chemistry II Exam II March 17, 2004 Form 1
 State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
Another Equilibrium: Reaction At The α-position
 Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
ENOLATES IN ORGANIC SYNTHESIS
 ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
CHAPTER 24 HW: CARBONYL CONDENSATIONS
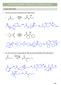 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
Chapter 21 Ester Enolates
 hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry II Examination #3 - March 31, 2003
 INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Important Concepts. Problems. Chapter Problems. Cuprate additions followed by enolate alkylations. 15. Michael Addition (Section 18-11)
 Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Luckily this intermediate has three saturated carbons between the carbonyls, which again points to a Michael reaction:
 Retrosynthesis Practice Problems Answer Key October 1, 2013 1. Draw a retrosynthesis for how to make the compound shown below from starting materials with eight or fewer carbon atoms. The first step is
Retrosynthesis Practice Problems Answer Key October 1, 2013 1. Draw a retrosynthesis for how to make the compound shown below from starting materials with eight or fewer carbon atoms. The first step is
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
(5) a. b. (6) N H CH 3 N H O H O (7) CH 3 O (8) OCH 3 2. explanation:
 ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
O O O CH 2 O 7. 2 = C=O hydration H B. 6 = reverse aldol H O. 9b = acetal formation add alcohol (step 2)
 1 equences For Practice 1. 1 2 3 7 2 6 5 4 8 9 Possible Key 3 = AD + oxidation 1 2 3 4 5 3 2 1 AD + 7 1 = AD + oxidation 7 = aldol AD 2 = = hydration 2 6 6 = aldol AD + AD 5 5 = β-keto decarboxylation
1 equences For Practice 1. 1 2 3 7 2 6 5 4 8 9 Possible Key 3 = AD + oxidation 1 2 3 4 5 3 2 1 AD + 7 1 = AD + oxidation 7 = aldol AD 2 = = hydration 2 6 6 = aldol AD + AD 5 5 = β-keto decarboxylation
Carbonyl Condensation Reactions
 24 arbonyl ondensation eactions 24.1 The aldol reaction 24.2 rossed aldol reactions 24.3 Directed aldol reactions 24.4 Intramolecular aldol reactions 24.5 The laisen reaction 24.6 The crossed laisen and
24 arbonyl ondensation eactions 24.1 The aldol reaction 24.2 rossed aldol reactions 24.3 Directed aldol reactions 24.4 Intramolecular aldol reactions 24.5 The laisen reaction 24.6 The crossed laisen and
22.4 ALDOL ADDITION AND ALDOL CONDENSATION
 .4 ALDL ADDITIN AND ALDL CNDENATIN 06 H C $ C L C L CH ) H C H C $ CA CL CH ) H C not an important structure (.8) In addition, the carbocation is destabilized by its unfavorable electrostatic interaction
.4 ALDL ADDITIN AND ALDL CNDENATIN 06 H C $ C L C L CH ) H C H C $ CA CL CH ) H C not an important structure (.8) In addition, the carbocation is destabilized by its unfavorable electrostatic interaction
Chem 312 SS05 Page 1 of 6 Kantorowski. 17 August 2005 Name:
 Chem 32 SS05 Page of 6 Kantorowski Exam III Key 7 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
Chem 32 SS05 Page of 6 Kantorowski Exam III Key 7 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
12BL Experiment 8: Green Chem: Solvent-Free Aldol Condensation-Dehydration
 12BL Experiment 8: Green Chem: Solvent-Free Aldol Condensation-Dehydration Safety: Proper lab goggles/glasses must be worn (even over prescription glasses). As always, ask where organic waste containers
12BL Experiment 8: Green Chem: Solvent-Free Aldol Condensation-Dehydration Safety: Proper lab goggles/glasses must be worn (even over prescription glasses). As always, ask where organic waste containers
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
Expt 9: The Aldol Condensation
 Expt 9: The Aldol Condensation INTRDUCTIN Reactions that form carbon-carbon bonds are particularly important in organic chemistry as they allow the synthesis of more complex structures from simpler molecules.
Expt 9: The Aldol Condensation INTRDUCTIN Reactions that form carbon-carbon bonds are particularly important in organic chemistry as they allow the synthesis of more complex structures from simpler molecules.
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
Experiment 2 Solvent-free Aldol Condensation between 3,4-dimethoxybenzaldehyde and 1-indanone
 Experiment 2 Solvent-free Aldol Condensation between 3,4-dimethoxybenzaldehyde and 1-indanone Chemical Concepts Carbonyl chemistry, base catalyzed aldol reaction, melting point, recrystallization Green
Experiment 2 Solvent-free Aldol Condensation between 3,4-dimethoxybenzaldehyde and 1-indanone Chemical Concepts Carbonyl chemistry, base catalyzed aldol reaction, melting point, recrystallization Green
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Aldehydes and Ketones 2. Based on Organic Chemistry, J. G. Smith 3rde.
 Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
How to make pyridines: the Hantzsch pyridine synthesis
 ow to make pyridines: the antzsch pyridine synthesis 1191 Zinc in acetic acid (Chapter 24) reduces the oxime to the amine and we can start the synthesis by doing the conjugate addition and then reducing
ow to make pyridines: the antzsch pyridine synthesis 1191 Zinc in acetic acid (Chapter 24) reduces the oxime to the amine and we can start the synthesis by doing the conjugate addition and then reducing
Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione
 Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione The purpose of this experiment was to synthesize 5-isopropyl-1,3-cyclohexanedione from commercially available compounds. To do this, acetone and
Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione The purpose of this experiment was to synthesize 5-isopropyl-1,3-cyclohexanedione from commercially available compounds. To do this, acetone and
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
Answers To Chapter 7 Problems.
 Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Exam III 17 August 2005 Name:
 Chem 312 SS05 Page 1 of 6 Kantorowski Exam III 17 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
Chem 312 SS05 Page 1 of 6 Kantorowski Exam III 17 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Final Exam Version 1. Chemistry 140C. Fall Good Luck! Dec 5, :30 am 2:30 pm This exam accounts for 50% of the final grade.
 Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
Also note here that the product is always a six membered ring with a double bond in it.
 Diels Alder Class 1 March 3, 2011 I want to talk in some detail over the next few classes about Diels Alder reactions. I am sure that most of you have heard of Diels Alder reactions before, but we will
Diels Alder Class 1 March 3, 2011 I want to talk in some detail over the next few classes about Diels Alder reactions. I am sure that most of you have heard of Diels Alder reactions before, but we will
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
O Enolate. = Electrophile/ Lewis acid. = Electrophile/ Lewis acid O B. Enolate. = Electrophile/ Lewis acid. Enolate. O O Enolate
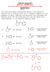 CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
Answers to HT2, Chemistry A
 Answers to T2, hemistry 302-302A - 2004 1. There are many ways to do these problems - here is just one. For all three parts we can use diphenyl ketone (benzophenone) so let s make that first: 1. 2 Eq.
Answers to T2, hemistry 302-302A - 2004 1. There are many ways to do these problems - here is just one. For all three parts we can use diphenyl ketone (benzophenone) so let s make that first: 1. 2 Eq.
HO \ /CH3. b. (2 pts) Which compound(s) (A-C) would be completely deprotonated by NaOH? (pka of water is 16
 All\ 1 rn 3l ~ ~')'\ CHEM 243 Exam #2A Spring 216 1. (6 pts) Identify the compounds below as an: acetal, hemi-acetal, ketal, hemi-ketal, enol, imine, enamine or hydrate. HO o'c(ch 3 Q OCH 3 HO \ /CH3 c
All\ 1 rn 3l ~ ~')'\ CHEM 243 Exam #2A Spring 216 1. (6 pts) Identify the compounds below as an: acetal, hemi-acetal, ketal, hemi-ketal, enol, imine, enamine or hydrate. HO o'c(ch 3 Q OCH 3 HO \ /CH3 c
Chapter 12: Carbonyl Compounds II
 Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Aldehydes, Ketones and Carboxylic acids
 Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
Aldehydes and Ketones
 9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
CuI CuI eage lic R tal ome rgan gbr ommon
 Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Lecture 19. Carboxylic Acids O C OH O R C O - + H + O - Chemistry 328N
 Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr.
 April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr. Rainer Glaser Announced Reading: Prins Cyclization Reactions 1 Question 1. Aldol-Prins
April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr. Rainer Glaser Announced Reading: Prins Cyclization Reactions 1 Question 1. Aldol-Prins
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
DEPARTMENT: Chemistry
 CODE CHEM 204 TITLE: Organic Chemistry II INSTITUTE: STEM DEPARTMENT: Chemistry COURSE DESCRIPTION: A continuation of CHEM-203, students will extend their studies into topics including aromatic hydrocarbons,
CODE CHEM 204 TITLE: Organic Chemistry II INSTITUTE: STEM DEPARTMENT: Chemistry COURSE DESCRIPTION: A continuation of CHEM-203, students will extend their studies into topics including aromatic hydrocarbons,
12BL Experiment 8: Green Chem: Solvent-Free Aldol Condensation-Dehydration
 12BL Experiment 8 Green Chem Solvent-Free Aldol Condensation-Dehydration Safety Proper lab goggles/glasses must be worn (even over prescription glasses). As always, ask where organic waste containers are
12BL Experiment 8 Green Chem Solvent-Free Aldol Condensation-Dehydration Safety Proper lab goggles/glasses must be worn (even over prescription glasses). As always, ask where organic waste containers are
Chapter 22 The Chemistry of Enolate Ions, Enols, and
 Organic Chemistry, 5th ed. Marc Loudon Chapter 22 The Chemistry of Enolate Ions, Enols, and α,β Unsaturated Carbonyl Compounds Eric J. Kantorowski California Polytechnic State University San Luis Obispo,
Organic Chemistry, 5th ed. Marc Loudon Chapter 22 The Chemistry of Enolate Ions, Enols, and α,β Unsaturated Carbonyl Compounds Eric J. Kantorowski California Polytechnic State University San Luis Obispo,
Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction.
 Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction. References: Brown & Foote, Chapters 16, 19, 23 INTRODUCTION: This experiment continues the saga of carbon-carbon
Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction. References: Brown & Foote, Chapters 16, 19, 23 INTRODUCTION: This experiment continues the saga of carbon-carbon
A. Loupy, B.Tchoubar. Salt Effects in Organic and Organometallic Chemistry
 A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
