CHEM 234: Organic Chemistry II Reaction Sheets
|
|
|
- Kristin Barnett
- 6 years ago
- Views:
Transcription
1 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles: acyl exchange u: u u =, l For =, reactions are generally reversible with u = and can occur under acidic or basic conditions. f u =, reaction is generally irreversible due to deprotonation of the product acid. ucleophilic addition at carbonyl groups: xygen and nitrogen nucleophiles, = or 2, = or 2, = or
2 Enol and enolate formation Base acid or base = or 2, 2 ' 2 General reactions of enolates
3 eductions of aldehydes and ketones. ab 4 M or LiAl 4 acidic workup M ab 4 acidic workup or LiAl 4 Li 3 LiAl 4 Al 3 3 LiAl 3 (Me) LiAl 4 Barbier reaction (1899) 1. 3, Mg 2. Acid workup Grignard reaction (1900) 1. 3 Mg 3 Mg 2. Acid workup Grignard reagent 1. Mg Mg 2. Acid workup
4 Grignard additions and reductions of esters. Mg 3 Mg 3 3 MeMg Mg Mg Mg 3 workup 3 3 Synthesis and reactions of epoxides: Me Me, Me Me Me Mea Me Me Mg will give similar opening ( replaces Me)
5 Formation of alkyl halides from alcohols 3 Alcohols - dry or l 1 Alcohols - P 3 or Sl 2 P 3 Me 3 Si Me PPh 3 Me 3 Si Me xidation of alcohols 2 r 4 acetone 2 r 4 acetone P 2 l hemiacetal (unstable) 3 acetal (stable) 3 2 hemiaminal An imine
6 2 The Wittig reaction An enamine PPh 3 PPh3 PPh 3 n-buli PPh 3 Li PPh 3 unstablized ylides give cis 3 Ph 3 P Me Me stabilized ylides give trans orner-wadsworth-emmons reaction: A Wittig variant base Et P Me Et To generate enolates, use strong base. Et P Et Me Me 3 a a 3 3 Li a 3
7 3 The aloform eaction ad D 2 2 D D 3 3 a a 2 2 The Aldol eaction a a 2 rossed aldol reactions. Practical when one component contains no!-hydrogens Ketones: equilibrium unfavourable a a Dehydration is also very common in acid catalyzed aldol reactions. 2 l 2
8 ntramolecular aldol: cyclizations to form rings. a or l a or l The use of strong base is advantageous for directed aldol reactions Li Li i Pr 2 Li Ph mild acid workup Strong base is also useful for generating enolates of esters and amides. i Pr 2 Li Li 78 pka ~ i Pr 2 Li Li 78 pka ~ 27-28
9 Additional uses of lithium enolates: alkylation Li i Pr 2 Li % Michael additions work well when only one component can enolize and one component can undergo addition 45% Good reaction: 2 Li t-bu i Et t-bu Pr 2 Li t-bu 78 Li t-bu i Et t-bu Pr 2 Li t-bu 78 Poor reaction: Many possible combinations t-bu t-bu Et Et t-bu t-bu Poor reaction: Many possible combinations aet obinson Annulation aet aet 65% yield Sir obert obinson obel 1947 Most classic obinson annulations use!-diketones as they direct the reaction better. Et a 2 Ph 43% yield
10 a 2 Ph 43% yield Ph Ac, aac benzene reflux 2, reflux Synthesis of esters from alcohols and carboxylic acids: esterification Saponification: irreversible hydrolysis of an ester 3 3 a 3 a 3 Sl 2 l Pl 3 and Pl 5 accomplish the same reaction Acid chlorides and anhydrides react with alcohols to form esters. base l base
11 Amines react easily with acid chlorides and anhydrides to form amides. atalysts are not generally needed. l Et 3 2 Et 3 Acid blocking groups: esters 3 3 3, Me a l 3 3 Amine blocking groups: carbamates Most common example: the tert-butyloxycarbonyl group (Boc) 3 3 a, dioxane/ 2 F 3 2 3
12 Dipeptide synthesis: 3 l 3 3 D, Et 3 (Bt) 3 3 Although acid chloride or acid anhydrides might be used in theory, in practice they lead to some racemization of the acid component. We thus use D or other carbodimides, sometimes in combination with Bt or other nucleophiles. 1. a 2. F Dicyclohexylcarbodiimide (D) and -hydroxybenzotriazole (Bt) in amide formation. 2 ' (D) (Bt) Acid hydrolysis of amides is very slow. ommon conditions 6 M l ' 3 ' slow ' " " ydrolysis of nitriles produces 1 amides or carboxylic acids. 3 2
13 itriles are more conveniently prepared by nucleophilic reactions: alide displacement S 2 a onjugate addition a Formation of cyanohydrins a Formation of an!-aminonitrile ' ' 2 ydrolysis of nitriles produces 1 amides or carboxylic acids. 3 2
14 laisen ondensation Esters are enolized by weaker bases. f we have a methyl ester, we use sodium methoxide so that transesterifcation is redundant. Mea a Mea acid workup a Mea a Mea acid workup a Mea a rossed laisen condensations are sometimes difficult to carry out. As with aldol, they work out well if one component has no!-hydrogens. Mea o thermodynamic sink Mea a Mea a acid workup ntramolecular laisen condensation: Dieckmann condensation Me Me Mea Me a Me Me Mea Me a acid workup
15 omparison of simple ketone alkylation vs. acetoacetic ester method aet K a heat LDA LDA Malonic esters have similar chemistry to acetoacetates Et Et aet Et Ph Et K Et Ph Et Et Et a heat Ph Ph 2 Ph
16 Synthesis of amines Simple S 2 displacement of alkyl halides Synthesis of amines Azide is a good replacement for ammonia in a simple S2. a 3 LiAl 4 or Ph 3 P, 2 2 Gabriel synthesis: Phthalimide as an ammonia equivalent. K K 2 2 2
17 eductive amination Et 2 LiAl 4 ab 3 Ac eductions of nitriles and amides LiAl 4 or B 3 LiAl 4 or B 3 Primary amines from amides via offman and urtuis rearrangement a a 3 l heat, 2 2
18 Diazotization 2 a 2 2 S 4 S 4 arenediazonium ion Arenediazonium ions are more stable (up to 5 ) and are synthetically useful. Sandmeyer reaction Y u = l,, Also u 2 to get = 2 a 2 2 S 4 S 4 P Alkyl diazonium ions mainly useful in amino acids. 2 Azo compounds by diazo coupling. a 2, 2 S 4 a Me 2 Me 2 a 2 l 2 l aac ofmann elimination gives different regioselectivity compared to normal eliminations. 3 Ag 2 2 Me 3 ame heat
19 The Mannich reaction is a good way to install methylene groups adjacent to ketones. Me; a n some cases, aryl halides can undergo displacement with nucleophiles. l a 350 l 2 a l 2 a l 2 a
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
Carbonyl Chemistry. aldehydes ketones. carboxylic acid and derivatives. Wednesday, April 29, 2009
 Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Lecture Notes Chemistry Mukund P. Sibi Lecture 36 Synthesis of Amines
 Lecture otes hemistry 42-2008 Mukund P. Sibi Synthesis of Amines Amines can be prepared from a variety of starting materials. All of these methods involve functional group transformations. The main methods
Lecture otes hemistry 42-2008 Mukund P. Sibi Synthesis of Amines Amines can be prepared from a variety of starting materials. All of these methods involve functional group transformations. The main methods
Chapter 22: Amines. Organic derivatives of ammonia, NH 3. Nitrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic
 hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
CH 3 CHCH 3 CH 3 CHCH 3 Isopropyl cation. Oxomium ion intermediate. intermediate (an electrophile)
 Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Amines Amines (e.g. RNH 2 ) are organic derivatives of ammonia, NH 3 similar to ammonia
 148Exam 2 notes rganic hemistry (e.g. 2 ) are organic derivatives of ammonia, 3 similar to ammonia omenclature: suffix 2 prefix 2 ( 3 ) 2 Types: Primary (1 o ), secondary (2 o ), tertiary (3 o ) or quaternary
148Exam 2 notes rganic hemistry (e.g. 2 ) are organic derivatives of ammonia, 3 similar to ammonia omenclature: suffix 2 prefix 2 ( 3 ) 2 Types: Primary (1 o ), secondary (2 o ), tertiary (3 o ) or quaternary
Appendix A. Common Abbreviations, Arrows, and Symbols. Abbreviations
 smi97462_apps.qxd 12/6/04 1:09 PM Page A-1 Appendix A ommon Abbreviations, Arrows, and Symbols Abbreviations Ac acetyl, 3 BBN 9-borabicyclo[3.3.1]nonane BINAP 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl
smi97462_apps.qxd 12/6/04 1:09 PM Page A-1 Appendix A ommon Abbreviations, Arrows, and Symbols Abbreviations Ac acetyl, 3 BBN 9-borabicyclo[3.3.1]nonane BINAP 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl
Loudon Chapter 19 Review: Aldehydes and Ketones CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Synthetic possibilities Chem 315 Beauchamp 1
 Synthetic possibilities hem Beauchamp Propose reasonable syntheses f the following target molecules (TM-#). You can use the given starting materials and any typical ganic reagents studied in our course
Synthetic possibilities hem Beauchamp Propose reasonable syntheses f the following target molecules (TM-#). You can use the given starting materials and any typical ganic reagents studied in our course
Loudon Chapter 20 & 21 Review: Carboxylic Acids & Derivatives CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Chapter 12: Carbonyl Compounds II
 Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 18: Carbonyl Compounds II
 Chapter 18: Carbonyl Compounds II Learning bjectives: 1. ecognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 18: Carbonyl Compounds II Learning bjectives: 1. ecognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Loudon Chapter 23 Review: Amines CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 23 eview: Amines CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions
Loudon Chapter 23 eview: Amines CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis]
![Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis]](/thumbs/86/93838793.jpg) Lecture 13A 05/11/12 Amines [Sn2; ofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Curtius and ofmann rearrangements Both of these, in principle,
Lecture 13A 05/11/12 Amines [Sn2; ofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Curtius and ofmann rearrangements Both of these, in principle,
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Chem 345 Reaction List: Chem 343 Reactions: Page 1 (You do not need to know the mechanism for the reactions in the boxes).
 Chem 345 eaction List: Chem 343 eactions: Page 1 (You do not need to know the mechanism for the reactions in the boxes). uc - optically active LG 2 optically active uc Alpha carbon (carbon attached to
Chem 345 eaction List: Chem 343 eactions: Page 1 (You do not need to know the mechanism for the reactions in the boxes). uc - optically active LG 2 optically active uc Alpha carbon (carbon attached to
Aldehydes and Ketones
 Aldehydes and Ketones Preparation of Aldehydes xidation of Primary Alcohols --- 2 P 1o alcohol ydroboration of a Terminal Alkyne, followed by Tautomerization --- 1. B 3, TF 2. 2 2, K 2 terminal alkyne
Aldehydes and Ketones Preparation of Aldehydes xidation of Primary Alcohols --- 2 P 1o alcohol ydroboration of a Terminal Alkyne, followed by Tautomerization --- 1. B 3, TF 2. 2 2, K 2 terminal alkyne
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
Summary of π Bond Chemistry
 Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
NH 2 O O O O OCH (6 pts) Provide an acceptable name for each of the following compounds: NH 2 COOH HOOC COOH. piperidine
 CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Synthesis of Amines Amine Alkylation by SN2 reaction II. Reductive Amination
 Synthesis of Amines I. Amine Alkylation by SN2 reaction Amines can be alkylated in SN2 fashion by alkyl halides; primary halides are best for this purpose. This is not a practical reaction for formation
Synthesis of Amines I. Amine Alkylation by SN2 reaction Amines can be alkylated in SN2 fashion by alkyl halides; primary halides are best for this purpose. This is not a practical reaction for formation
Cape Cod Community College
 Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chem 263 Nov 28, Reactions of Carboxylic Acids and Derivatives: Strong Nucleophiles
 Chem 263 ov 28, 2013 eactions of Carboxylic Acids and Derivatives: Strong ucleophiles The strong nucleophiles (u: - ) that we have learned in this course are either hydride anion ( - ) or alkyl anion (
Chem 263 ov 28, 2013 eactions of Carboxylic Acids and Derivatives: Strong ucleophiles The strong nucleophiles (u: - ) that we have learned in this course are either hydride anion ( - ) or alkyl anion (
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Topic 4 Aldehydes and Ketones
 4-1 Topic 4 Aldehydes and Ketones 16.1 4-2 Aldehydes and Ketones ' aldehyde ketone The polarized oxygen-carbon -bond renders aldehydes and ketones electrophilic: ' The electrophilicity of the oxygen-carbon
4-1 Topic 4 Aldehydes and Ketones 16.1 4-2 Aldehydes and Ketones ' aldehyde ketone The polarized oxygen-carbon -bond renders aldehydes and ketones electrophilic: ' The electrophilicity of the oxygen-carbon
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
CHEMISTRY MIDTERM # 4 ANSWER KEY April 16, 2002
 CEMISTY 314-01 MIDTEM # 4 ASWE KEY April 16, 00 1. (6 pts) Mark as true (T) or false (F) the following statements. Do not explain! (F) Amides are less reactive than acid chlorides but more reactive than
CEMISTY 314-01 MIDTEM # 4 ASWE KEY April 16, 00 1. (6 pts) Mark as true (T) or false (F) the following statements. Do not explain! (F) Amides are less reactive than acid chlorides but more reactive than
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
O O O CH 2 O 7. 2 = C=O hydration H B. 6 = reverse aldol H O. 9b = acetal formation add alcohol (step 2)
 1 equences For Practice 1. 1 2 3 7 2 6 5 4 8 9 Possible Key 3 = AD + oxidation 1 2 3 4 5 3 2 1 AD + 7 1 = AD + oxidation 7 = aldol AD 2 = = hydration 2 6 6 = aldol AD + AD 5 5 = β-keto decarboxylation
1 equences For Practice 1. 1 2 3 7 2 6 5 4 8 9 Possible Key 3 = AD + oxidation 1 2 3 4 5 3 2 1 AD + 7 1 = AD + oxidation 7 = aldol AD 2 = = hydration 2 6 6 = aldol AD + AD 5 5 = β-keto decarboxylation
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chemistry Final Examinations Summer 2006 answers
 Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Carbonyl Condensation Reactions
 24 arbonyl ondensation eactions 24.1 The aldol reaction 24.2 rossed aldol reactions 24.3 Directed aldol reactions 24.4 Intramolecular aldol reactions 24.5 The laisen reaction 24.6 The crossed laisen and
24 arbonyl ondensation eactions 24.1 The aldol reaction 24.2 rossed aldol reactions 24.3 Directed aldol reactions 24.4 Intramolecular aldol reactions 24.5 The laisen reaction 24.6 The crossed laisen and
Chapter 17: Carbonyl Compounds II
 Chapter 17: Carbonyl Compounds II Learning bjectives: 1. ecognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 17: Carbonyl Compounds II Learning bjectives: 1. ecognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid R C N + H 2 O + H O R
 arboxylic Acid Derivatives Addition/Elimination Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid X = l, Br addition/elimination X acid
arboxylic Acid Derivatives Addition/Elimination Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid X = l, Br addition/elimination X acid
Chapter 11 Reaction of Alcohols
 Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
The Organic Acids. Carboxylic Acids * *
 arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
ROADMAP FOR REACTIONS Chapter 6
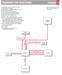 RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
CHAPTER 22: Amines R 2 H N N N R 1. cadeverine N CH 3. nicotine
 Based on notes by JZ updated 4/2018 Amines are Biologically Important CAPTE 22: Amines Amino acids are the basis of all peptides and proteins. These are the tissue building blocks and nature's catalysts
Based on notes by JZ updated 4/2018 Amines are Biologically Important CAPTE 22: Amines Amino acids are the basis of all peptides and proteins. These are the tissue building blocks and nature's catalysts
Structure and Reactivity: Prerequired Knowledge
 Structure and eactivity: Prerequired Knowledge!!! The concepts presented in this summary are required for lecture and examination!!! 1. Important Principles in rganic Chemistry In general, structures which
Structure and eactivity: Prerequired Knowledge!!! The concepts presented in this summary are required for lecture and examination!!! 1. Important Principles in rganic Chemistry In general, structures which
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
22.7 Reactions of Amines: A Review and a Preview
 22.7 Reactions of Amines: A Review and a Preview Preparation of Amines Two questions to answer: 1) How is the C N C N bond to be formed? 2) How do we obtain the correct oxidation state of nitrogen (and
22.7 Reactions of Amines: A Review and a Preview Preparation of Amines Two questions to answer: 1) How is the C N C N bond to be formed? 2) How do we obtain the correct oxidation state of nitrogen (and
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Carbonyls (Ch ketones and aldehydes and carboxylic acids derivatives)
 arbonyls (h 16-19 ketones and aldehydes and carboxylic acids derivatives) +δ -δ ' - sp 2 - trigonal planar (120 0 ) - strongly polarized double bond eactivity? addition nucleophilic 1 Nucleophilic Addition
arbonyls (h 16-19 ketones and aldehydes and carboxylic acids derivatives) +δ -δ ' - sp 2 - trigonal planar (120 0 ) - strongly polarized double bond eactivity? addition nucleophilic 1 Nucleophilic Addition
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution Reactions. McMurray Text Chapter 21
 Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton)
 314 Arrow Pushing practice/eauchamp 1 Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton) ucleophile = nucleus/positive loving = any general electron
314 Arrow Pushing practice/eauchamp 1 Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton) ucleophile = nucleus/positive loving = any general electron
R N R N R N. primary secondary tertiary
 Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
896 Chapter 21 Amines H H N R R R N R H R N R H O H R 3 N CH 2 NH 2 NHCH 3
 896 Chapter 21 Amines 21.20 Summary Section 21.1 Section 21.2 Section 21.3 Section 21.4 Alkylamines are compounds of the type shown, where R, R, and R are alkyl groups. ne or more of these groups is an
896 Chapter 21 Amines 21.20 Summary Section 21.1 Section 21.2 Section 21.3 Section 21.4 Alkylamines are compounds of the type shown, where R, R, and R are alkyl groups. ne or more of these groups is an
Chapter 22 Amines. Nomenclature Amines are classified according to the degree of substitution at nitrogen.
 CH. 22 Chapter 22 Amines Amines are very important in biological chemistry. Most of the bases in biological acid-base reactions are amines. They are also very important nucleophiles in biochemical reactions.
CH. 22 Chapter 22 Amines Amines are very important in biological chemistry. Most of the bases in biological acid-base reactions are amines. They are also very important nucleophiles in biochemical reactions.
TOPIC 3 - ALDEHYDES AND KETONES (Chapters 12 & 16)
 TPIC 3 - ALDEYDES AND KETNES (Chapters 12 & 16) Lecture 15 Web12 12.1 Introduction 16.1 Introduction 16.2 Nomenclature of Aldehydes and Ketones 16.3 ysical Properties 12.2 xidation Reduction Reactions
TPIC 3 - ALDEYDES AND KETNES (Chapters 12 & 16) Lecture 15 Web12 12.1 Introduction 16.1 Introduction 16.2 Nomenclature of Aldehydes and Ketones 16.3 ysical Properties 12.2 xidation Reduction Reactions
Aldehydes and Ketones Reactions. Dr. Sapna Gupta
 Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
THE CHEMISTRY OF THE CARBONYL GROUP
 TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
Chemistry of Carbonyl Compounds
 Chemistry of Carbonyl Compounds ucleophilic addition (1,2-add) / substitution Conjugate addition (1,4 add) obinson annulation (McM 23.12) Alkylation of enolate anions namines as enolate equivs. (McM 23.11)
Chemistry of Carbonyl Compounds ucleophilic addition (1,2-add) / substitution Conjugate addition (1,4 add) obinson annulation (McM 23.12) Alkylation of enolate anions namines as enolate equivs. (McM 23.11)
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Chapter 20: Aldehydes and Ketones
 hem A225 Notes Page 67 I. Introduction hapter 20: Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group (=) with no other heteroatoms attached. An aldehyde has at least one hydrogen attached;
hem A225 Notes Page 67 I. Introduction hapter 20: Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group (=) with no other heteroatoms attached. An aldehyde has at least one hydrogen attached;
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
S N 2 and E2 Mechanisms (strong base/nucleophile competition reacting at a carbon or reacting at a proton)
 Electrophile (Lewis Acid) = electron loving = any general electron pair acceptor (can also be an acidic proton = onsted acid) ucleophile (Lewis Base) = nucleus/positive loving = any general electron pair
Electrophile (Lewis Acid) = electron loving = any general electron pair acceptor (can also be an acidic proton = onsted acid) ucleophile (Lewis Base) = nucleus/positive loving = any general electron pair
CHEMISTRY MIDTERM # 2 November 02, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
Chapter 14 Aldehydes and Ketones: Addition Reactions at Electrophilic Carbons Overview of Chapter Structures of aldehydes and ketones
 hem 215 F12 otes Dr. Masato Koreeda - Page 1 of 11. Date: September 19, 2012 hapter 14 Aldehydes and Ketones: Addition eactions at Electrophilic arbons verview of hapter 14 1. Structures of aldehydes and
hem 215 F12 otes Dr. Masato Koreeda - Page 1 of 11. Date: September 19, 2012 hapter 14 Aldehydes and Ketones: Addition eactions at Electrophilic arbons verview of hapter 14 1. Structures of aldehydes and
Chapter 20: Aldehydes and Ketones
 hapter 20: Aldehydes and Ketones [hapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ketone ' aldehyde 2. eview of the Synthesis of Aldehydes and Ketones Br Br f
hapter 20: Aldehydes and Ketones [hapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ketone ' aldehyde 2. eview of the Synthesis of Aldehydes and Ketones Br Br f
Chapter 19 Substitutions at the Carbonyl Group
 Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
