22.4 ALDOL ADDITION AND ALDOL CONDENSATION
|
|
|
- Merry Daniels
- 6 years ago
- Views:
Transcription
1 .4 ALDL ADDITIN AND ALDL CNDENATIN 06 H C $ C L C L CH ) H C H C $ CA CL CH ) H C not an important structure (.8) In addition, the carbocation is destabilized by its unfavorable electrostatic interaction with the bond dipole of the carbonyl group that is, with the partial positive charge on the carbonyl carbon atom. (CH ) C d C d+ CH destabilizing electrostatic interaction PRBLEM.6 What product is formed when (a) phenylacetic acid is treated first with Br and one equivalent of PBr, then with a large excess of ethanol? (b) propionic acid is treated first with Br and one equivalent of PBr, then with a large excess of ammonia?.7 Give the structure of the product expected in each of the following reactions. (a) CH CH CCH Br + -bromo--butanone N pyridine (b) BrCH C L Ph + CH Na CL a-bromoacetophenone sodium acetate.8 Give a curved-arrow mechanism for the reaction in Eq..4. Your mechanism should show why two equivalents of NaH must be used..4 ALDL ADDITIN AND ALDL CNDENATIN A. Base-Catalyzed Aldol Reactions In aqueous base, acetaldehyde undergoes a reaction called the aldol addition. H C L CH acetaldehyde NaH H H H C L CH L CH L CH -hydroxybutanal (aldol) (50 yield) (.9)
2 064 CHAPTER THE CHEMITRY F ENLATE IN, ENL, AND a,b-unaturated CARBNYL CMPUND The term aldol is both a traditional name for -hydroxybutanal and a generic name for b- hydroxy aldehydes. An aldol addition is a reaction of two aldehyde molecules to form a b-hydroxy aldehyde. The aldol addition is a very important and general reaction of aldehydes and ketones that have a-hydrogens. Notice that this reaction provides another method of forming carbon carbon bonds. The base-catalyzed aldol addition involves an enolate ion as an intermediate. In this reaction, an enolate ion, formed by the reaction of acetaldehyde with aqueous NaH, adds to a second molecule of acetaldehyde. H H L CH L CH H C L CH + H enolate ion (.40a) H C L CH H C L CH enolate ion H C L CH L CH L CH H L H H H C L CH L CH L CH + H (.40b) The aldol addition is another nucleophilic addition to a carbonyl group. In this reaction, the nucleophile is an enolate ion. The reaction may look more complicated than some additions because of the number of carbon atoms in the product. However, it is not conceptually different from other nucleophilic additions, such as cyanohydrin formation. Cyanohydrin formation: Aldol addition: nucleophile + aldehyde CN H C L CH A H C L CH A H C L CH A protonation CN H C LCH L H C L CH A H C LCH L H L CN H L H addition product CN H C L CH L H + CN H C L CH A H C LCH LH + H (.4) PRBLEM.9 Use the reaction mechanism to deduce the product of the aldol addition reaction of (a) phenylacetaldehyde; (b) propionaldehyde. The aldol addition is reversible. Like many other carbonyl addition reactions (ec. 9.7B), the equilibrium for the aldol addition is more favorable for aldehydes than for ketones.
3 .4 ALDL ADDITIN AND ALDL CNDENATIN 065 H Ba(H) H C LCLCH H CLCLCH LC LCH acetone (equilibrium CH lies to the left) 4-hydroxy-4-methyl--pentanone (diacetone alcohol) (.4) In this aldol addition reaction of acetone, the equilibrium favors the ketone reactant rather than the addition product, diacetone alcohol. This product can be isolated in good yield only if an apparatus is used that allows the product to be removed from the base catalyst as it is formed. Under more severe conditions (higher base concentration, or heat, or both), the product of aldol addition undergoes a dehydration reaction. CH CH CH CH A butanal M NaH 80 C H CH CH CH CH L CHCH A CH CH -ethyl--hydroxyhexanal (the aldol addition product) CH CH CH CH A CCH A CH CH -ethyl--hexenal (86 yield) + H (.4) The sequence of reactions consisting of the aldol addition followed by dehydration, as in Eq..4, is called the aldol condensation. (A condensation is a reaction in which two molecules combine to form a larger molecule with the elimination of a small molecule, in many cases water.) The term aldol condensation has been used historically to refer to the aldol addition reaction as well as to the addition and dehydration reactions together. To eliminate ambiguity, aldol condensation is used in this text only for the addition-dehydration sequence. The term aldol reactions is used to refer generically to both addition and condensation reactions. The dehydration part of the aldol condensation is a b-elimination reaction catalyzed by base, and it occurs in two distinct steps through an enolate-ion intermediate. H L C L C LC L H aldol addition product carbanion intermediate a,b-unsaturated carbonyl compound H (.44) This is not a concerted b-elimination. In this respect, it differs from the E reaction. (ee Problem 9.6, p. 40.) H C L LC L C LC L $ H + CA C + ) H $ )
4 066 CHAPTER THE CHEMITRY F ENLATE IN, ENL, AND a,b-unaturated CARBNYL CMPUND A base-catalyzed dehydration reaction of simple alcohols is unknown; ordinary alcohols do not dehydrate in base. However, b-hydroxy aldehydes and b-hydroxy ketones do for two reasons. First, their a-hydrogens are relatively acidic. Recall that base-promoted b-eliminations are particularly rapid when acidic hydrogens are involved (ecs. 7.B and 9.5B). econd, the product is conjugated and therefore is particularly stable. To the extent that the transition state of the dehydration reaction resembles the a,b-unsaturated ketone, it too is stabilized by conjugation, and the elimination reaction is accelerated (Hammond s postulate). The product of the aldol condensation is an a,b-unsaturated carbonyl compound. The aldol condensation is an important method for the preparation of certain a,b-unsaturated carbonyl compounds. Whether the aldol addition product or the condensation product is formed depends on reaction conditions, which must be worked out on a case-by-case basis. You can assume for purposes of problem-solving, unless stated otherwise, that either the addition product or the condensation product can be prepared. Musical History of the Aldol Condensation Discovery of the aldol condensation is usually attributed solely to Charles Adolphe Wurtz, a French chemist who trained Friedel and Crafts. However, the reaction was first investigated during the period by Aleksandr Borodin (8 887), a Russian chemist who was also a self-taught and proficient musician and composer. (Borodin s musical themes were used as the basis of songs in the musical Kismet.) Borodin found it difficult to compete with Wurtz s large, modern, well-funded laboratory. Borodin also lamented that his professional duties so burdened him with examinations and commissions that he could only compose when he was at home ill. Knowing this, his musical friends used to greet him, Aleksandr, I hope you are ill today! B. Acid-Catalyzed Aldol Condensation Aldol condensations are also catalyzed by acid. H C LC LCH acetone acid H C $ C A CH LC LCH + H ) H C (.45) Acid-catalyzed aldol condensations, as in this example, generally give a,b-unsaturated carbonyl compounds as products; addition products cannot be isolated. In acid-catalyzed aldol condensations, the conjugate acid of the aldehyde or ketone is a key reactive intermediate. mesityl oxide (79 yield) H L H H H C LC LCH H C LC LCH + H (.46a) This protonated ketone plays two roles. First, it serves as a source of the enol, as shown in Eq..7b on p econd, the protonated ketone is the electrophilic species in the reaction. It reacts as an electrophile with the p electrons of the enol to give an a-hydroxy carbocation, which is also the conjugate acid of the addition product:
5 .4 ALDL ADDITIN AND ALDL CNDENATIN 067 a second molecule of the protonated ketone H H C LC LCH H H one molecule of the protonated ketone H CH H CH L H $ A C H C C L CH H L C L CH L C L CH enol CH CH an a-hydroxy carbocation the carbonyl carbon accepts (a protonated ketone; ec. 9.6) p electrons from the enol CH H L C L CH L C L CH + H CH H (.46b) As the second part of Eq..46b shows, the a-hydroxy carbocation loses a proton to give the b-hydroxy ketone product. Under the acidic conditions, this material spontaneously undergoes acid-catalyzed dehydration to give an a,b-unsaturated carbonyl compound: TUDY GUIDE LINK. Dehydration of b-hydroxy Carbonyl Compounds CH H C H H L C LCH LC LCH $ CH LC LCH H ) C A + CH H C (.46c) This dehydration drives the aldol condensation to completion. (Recall that without this dehydration, the aldol condensation of ketones is unfavorable; Eq..4). Let s contrast the species involved in the acid- and base-catalyzed aldol reactions. An enol, not an enolate ion, is the nucleophilic species in an acid-catalyzed aldol condensation. Enolate ions are too basic to exist in acidic solution. Although an enol is much less nucleophilic than an enolate ion, it reacts at a useful rate because the protonated carbonyl compound (an a-hydroxy carbocation) with which it reacts is a potent electrophile. In a base-catalyzed aldol reaction, an enolate ion is the nucleophile. A protonated carbonyl compound is not an intermediate because it is too acidic to exist in basic solution. The electrophile that reacts with the enolate ion is a neutral carbonyl compound. To summarize: Reaction Nucleophile Electrophile Base-catalyzed aldol reaction enolate ion neutral carbonyl compound Acid-catalyzed aldol condensation enol protonated carbonyl compound C. pecial Types of Aldol Reactions Crossed Aldol Reactions The preceding discussion considered only aldol reactions between two molecules of the same aldehyde or ketone. When two different carbonyl compounds are used, the reaction is called a crossed aldol reaction. In many cases, the result of a crossed aldol reaction is a difficult-to-separate mixture, as tudy Problem. illustrates.
6 068 CHAPTER THE CHEMITRY F ENLATE IN, ENL, AND a,b-unaturated CARBNYL CMPUND tudy Problem. Give the structures of the aldol addition products expected from the base-catalyzed reaction of acetaldehyde and propionaldehyde. olution uch a reaction involves four different species: acetaldehyde (A) and its enolate ion (A9), as well as propionaldehyde (P) and its enolate ion (P9): H C LC LH H C LCLH CH CH LCLH CH CH LC LH A A Four possible addition products can arise from the reaction of each enolate ion with each aldehyde: P P H CH CHCH CH A A + A H CH CH CHCH CH A P + A H CH CHCHCH A CH H CH CH CHCHCH A CH A + P P + P (Be sure you see how each product is formed; write a mechanism for each, if necessary.) Notice also a further complication: diastereomers are possible for the last two products because each has two asymmetric carbons. Crossed aldol reactions that provide complex mixtures, such as the one in tudy Problem., are not very useful because the product of interest is not formed in very high yield, and because isolation of one product from a complex mixture is in most cases extremely tedious. Although conditions that favor one product or another in crossed aldol reactions have been worked out in specific cases, as a practical matter, under the usual conditions (aqueous or alcoholic acid or base), useful crossed aldol reactions are limited to situations in which a ketone with a-hydrogens is condensed with an aldehyde that has no a-hydrogens. An important example of this type is the Claisen chmidt condensation. In a Claisen chmidt condensation, a ketone with a-hydrogens acetone in the following example is condensed with an aromatic aldehyde that has no a-hydrogens benzaldehyde in this case. H L CH aqueous NaH $ PhCH A + H C LC LCH C A C + H ) $ benzaldehyde acetone Ph H (excess) ) C benzalacetone (4-phenyl--buten--one) (65 78 yield) (.47) Notice that the addition product cannot be isolated in this reaction; the highly conjugated condensation product is formed as its most stable stereoisomer the trans isomer in Eq..47. In view of the complex mixture obtained in the example used in tudy Problem., it is reasonable to ask why only one product is obtained from the crossed aldol condensation in Eq..47. The analysis of this case highlights several important principles of carbonyl compound
7 .4 ALDL ADDITIN AND ALDL CNDENATIN 069 TUDY GUIDE LINK.4 Understanding Condensation Reactions Further Exploration. Directed Aldol Addition Reactions reactivity. First, because the aldehyde in the Claisen chmidt reaction has no a-hydrogens, it cannot act as the enolate component of the aldol condensation; consequently, two of the four possible crossed aldol products cannot form. The other possible side reaction is the aldol addition reaction of the ketone with itself, as in Eq..4; why doesn t this reaction occur? The enolate ion from acetone can react either with another molecule of acetone or with benzaldehyde. Recall that addition to a ketone occurs more slowly than addition to an aldehyde (ec. 9.7C). Furthermore, even if addition to acetone does occur, the aldol addition reaction of two ketones is reversible (Eq..4) and addition to an aldehyde has a more favorable equilibrium constant than addition to a ketone (ec. 9.7B). Thus, in Eq..47, both the rate and equilibrium for addition to benzaldehyde are more favorable than they are for addition to a second molecule of acetone. Thus, the product shown in Eq..47 is the only one formed. The Claisen chmidt condensation, like other aldol condensations, can also be catalyzed by acid. Ph LCH A + H C LC LPh H 4 CH C H H L Ph $ ) C C A C ) $ Ph H (95 yield) (.48) A great deal of research has been devoted to finding solutions to the crossed aldol problem, particularly in the reactions of aldehydes with the enolate ions derived from ketones. ome of this work is described in Further Exploration.. Intramolecular Aldol Condensation When a molecule contains more than one aldehyde or ketone group, an intramolecular reaction (a reaction within the same molecule) is possible. In such a case, the aldol condensation results in formation of a ring. Intramolecular aldol condensations are particularly favorable when five- and six-membered rings can be formed because of the proximity effect (ec..7). H C L L H CH CH CCH CH CH (acid catalyst) + H # C CH # A L (.49) PRBLEM.0 Predict the product(s) in each of the following aldol condensations. (a) ) NaH LCH A + H C LC LCH ) H (equal molar amounts) NaH (b) acetophenone hexanal (c) + CH LC LCH CH CH CH KH
8 070 CHAPTER THE CHEMITRY F ENLATE IN, ENL, AND a,b-unaturated CARBNYL CMPUND. A reverse aldol addition is an important step in the glycolytic pathway, the process by which hexoses (six-carbon sugars) are metabolized as energy sources. The following reaction is catalyzed by the enzyme aldolase. H H PCH C H H H H fructose,6-diphosphate CH P HCH C aldolase (an enzyme) CH P + PCH CH dihydroxyacetone phosphate H H glyceraldehyde--phosphate Give a curved-arrow mechanism for this reaction, using B as a base catalyst (which is part of the enzyme) and BH as its conjugate acid. D. ynthesis with the Aldol Condensation The aldol condensation can be applied to the synthesis of a wide variety of a,b-unsaturated aldehydes and ketones, and it is also another method for the formation of carbon carbon bonds. (ee the complete list in Appendix VI.) If you want to prepare a particular a,bunsaturated aldehyde or ketone by the aldol condensation, you must ask two questions: () What starting materials are required in the aldol condensation? () With these starting materials, is the aldol condensation of these compounds a feasible one? The starting materials for an aldol condensation can be determined by mentally splitting the a,b-unsaturated carbonyl compound at the double bond: this portion is derived from the carbonyl compound that reacts with the enolate ion or enol R C L R $ ) C AA C ) $ R R this portion is derived from the enolate ion or enol implies R $ C LC LR ) A + H C R R (.50) That is, work backward from the desired synthetic objective by replacing the double bond on the carbonyl side by two hydrogens and on the other side by a carbonyl oxygen (A) to obtain the structures of the starting materials in the aldol condensation. Knowing the potential starting materials for an aldol condensation is not enough; you must also know whether the condensation is one that works, or whether instead it is one that is likely
9 .4 ALDL ADDITIN AND ALDL CNDENATIN 07 to give troublesome mixtures. (Review tudy Problem 4.9 on p. 5). In other words, you can t make every conceivable a,b-unsaturated aldehyde or ketone by the aldol condensation only certain ones. This point is illustrated in tudy Problem.. tudy Problem. Determine whether the following a,b-unsaturated ketone can be prepared by an aldol condensation. H C $ C CH C CH CH ) A L L H C olution Following the procedure in Eq..50, analyze the desired product as follows: H C $ C CH C CH CH ) A L L H C H C $ C ) A + H C LC LCH CH H C -butanone acetone required starting materials The desired product requires a crossed condensation between two similar ketones: acetone and -butanone. The question, then, is whether the desired product is the only one that could form, or whether other competing aldol reactions would occur. First, either acetone and -butanone could serve as either the enolate component or the carbonyl component of the aldol addition, and there is no reason to presume that the desired reaction will be the only one observed. (This situation is analogous to the one presented in tudy Problem..) To complicate matters even more, -butanone has two nonequivalent a-carbons at which enolate ions (or enols) could form. This opens yet other possibilities for aldol reactions and thus for complex product mixtures. Hence, the reaction of acetone and -butanone would not be a useful for preparing the desired ketone because a large number of constitutionally isomeric products would be expected. PRBLEM. ome of the following molecules can be synthesized in good yield using an aldol condensation. Identify these and give the structures of the required starting materials. thers cannot be synthesized in good yield by an aldol condensation. Identify these, and explain why the required aldol condensation would not be likely to succeed. (a) CH L LCH A C LC LCH CH CH (b) L CH A C LC LCH CH CH CH
10 07 CHAPTER THE CHEMITRY F ENLATE IN, ENL, AND a,b-unaturated CARBNYL CMPUND (c) CH A (d) (e) CH Ph CH Ph Ph Ph (f) Ph L CH ACH LC LCH ACH L Ph (g) (CH ) C ACH L CH A (h). Analyze the aldol condensation in Eq..49 on p. 069 using the method given in Eq..50. how that four possible aldol condensation products might in principle result from the starting material. Explain why the observed product is the most reasonable one..5 CNDENATIN REACTIN INVLVING ETER ENLATE IN With this section, we begin the use of more compact abbreviations for several commonly occurring organic groups. These abbreviations, shown in Table., not only save space but also make the structures of large molecules less cluttered and easier to read. Just as PhL is used to symbolize the phenyl ring, MeL can be used for methyl, EtL for ethyl, PrL for propyl, and so on. Thus, ethyl acetate is abbreviated EtAc; sodium ethoxide (Na C H 5 ) is simply written as NaEt; and methanol is abbreviated as MeH. PRBLEM.4 Write the structure that corresponds to each of the following abbreviations. (ee Table..) (a) Et CLH (b) i-prlph (c) t-buac (d) PrLH (e) Ac (f) AcLPh A. Claisen Condensation The base-catalyzed aldol reactions discussed in the previous section involve enolate ions derived from aldehydes and ketones. This section discusses condensation reactions that involve the enolate ions of esters. Ethyl acetate undergoes a condensation reaction in the presence of one equivalent of sodium ethoxide in ethanol to give ethyl -oxobutanoate, which is known commonly as ethyl acetoacetate. H C LC LEt ethyl acetate NaEt ( equiv.) EtH H H CLCLCH LC LEt + EtH ethyl acetoacetate (75 76 yield) (.5)
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
CHAPTER 23 HW: ENOLS + ENOLATES
 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CHAPTER 24 HW: CARBONYL CONDENSATIONS
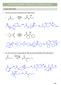 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
ORGANIC - CLUTCH CH CONDENSATION CHEMISTRY.
 !! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
!! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Reactions of Chapter 10 Worksheet and Key
 1) Alcohol Fermentation Reactions of Chapter 10 Worksheet and Key Alcohol fermentation is a series of chemical reaction that convert sugar molecules, such a glucose, into ethanol and C 2. The overall reaction
1) Alcohol Fermentation Reactions of Chapter 10 Worksheet and Key Alcohol fermentation is a series of chemical reaction that convert sugar molecules, such a glucose, into ethanol and C 2. The overall reaction
Nomenclature of Aldehydes and Ketones
 Aldehydes and Ketones Nomenclature of Aldehydes and Ketones Common aldehydes H H Methanl (formaldehyde) H3C H CH3CH2 ethanl (acetaldehyde) H propanal (propionaldehyde) CH3CH2CH2 butanal (n-butyraldehyde)
Aldehydes and Ketones Nomenclature of Aldehydes and Ketones Common aldehydes H H Methanl (formaldehyde) H3C H CH3CH2 ethanl (acetaldehyde) H propanal (propionaldehyde) CH3CH2CH2 butanal (n-butyraldehyde)
Aldehydes and Ketones
 9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
ALDEHYDES AND KETONES
 11 R R R ALDEYDES AND KETNES APTER SUMMARY 111 Structure of Aldehydes and Ketones Aldehydes and ketones both have a carbonyl group (carbonoxygen double bond); aldehydes have at least one carbon bonded
11 R R R ALDEYDES AND KETNES APTER SUMMARY 111 Structure of Aldehydes and Ketones Aldehydes and ketones both have a carbonyl group (carbonoxygen double bond); aldehydes have at least one carbon bonded
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
State University of New York at Stony Brook Department of Chemistry. CHE 322, Organic Chemistry II Exam II March 17, 2004 Form 1
 State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
What is in Common for the Following Reactions, and How Do They Work?
 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
CH 320/328 N Summer II 2018
 CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Chapter 23 Aldehydes and Ketones
 Chapter 23 Aldehydes and Ketones Ketones are common solvents for quickdrying paints. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan
Chapter 23 Aldehydes and Ketones Ketones are common solvents for quickdrying paints. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
20.10 Conjugate Additions
 894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
2/26/18. Practice Questions. Practice Questions B F. How many steps are there in this reaction?
 Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry II Examination #3 - March 31, 2003
 INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
Chapter 21 Ester Enolates
 hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Carbonyl Compounds. Introduction
 Carbonyl Compounds Introduction 1 Introduction Two broad classes of compounds contain the carbonyl group: [1] Compounds that have only carbon and hydrogen atoms bonded to the carbonyl [2] Compounds that
Carbonyl Compounds Introduction 1 Introduction Two broad classes of compounds contain the carbonyl group: [1] Compounds that have only carbon and hydrogen atoms bonded to the carbonyl [2] Compounds that
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Reversible Additions to carbonyls: Weak Nucleophiles Relative Reactivity of carbonyls: Hydration of Ketones and Aldehydes
 Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
p Bonds as Electrophiles
 Chapter 7 p Bonds as Electrophiles REACTIONS OF CARBONYLS AND RELATED FUNCTIONAL GROUPS Copyright 2018 by Nelson Education Limited 1 7.2.1 Orbital structure of the carbonyl group Because oxygen is more
Chapter 7 p Bonds as Electrophiles REACTIONS OF CARBONYLS AND RELATED FUNCTIONAL GROUPS Copyright 2018 by Nelson Education Limited 1 7.2.1 Orbital structure of the carbonyl group Because oxygen is more
Química Orgânica I. Ciências Farmacêuticas Bioquímica Química AFB QO I 2007/08 1
 Química rgânica I Ciências Farmacêuticas Bioquímica Química AFB Q I 2007/08 1 alcohols Adaptado de rganic Chemistry, 6th Edition; Wade rganic Chemistry, 6 th Edition; McMurry AFB Q I 2007/08 2 Typical
Química rgânica I Ciências Farmacêuticas Bioquímica Química AFB Q I 2007/08 1 alcohols Adaptado de rganic Chemistry, 6th Edition; Wade rganic Chemistry, 6 th Edition; McMurry AFB Q I 2007/08 2 Typical
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
CHAPTER 24 Organic Chemistry
 CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Aldol Condensation Notes
 Reminder: These notes are meant to supplement, not replace, the laboratory manual. Aldol Condensation Notes History and Application Condensation reactions are molecular transformations that join together
Reminder: These notes are meant to supplement, not replace, the laboratory manual. Aldol Condensation Notes History and Application Condensation reactions are molecular transformations that join together
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
CHEM 345 Problem Set 07 Key
 CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Theophylline (TH), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma.
 1 EXAM SCIETIIC CULTURE CEMISTRY PRBLEM 1: Theophylline (T), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma. 3 C 7 1 3 9 1.1 Structural study and acid-base
1 EXAM SCIETIIC CULTURE CEMISTRY PRBLEM 1: Theophylline (T), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma. 3 C 7 1 3 9 1.1 Structural study and acid-base
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
BRCC CHM102 Chapter 17 Notes Class Notes Page 1 of 8
 BR HM102 hapter 17 Notes lass Notes Page 1 of 8 hapter 17 Aldehydes and Ketones arbonyl group - found in fats, carbohydrates, proteins, nucleic s, and other important biological compounds. * Aldehydes
BR HM102 hapter 17 Notes lass Notes Page 1 of 8 hapter 17 Aldehydes and Ketones arbonyl group - found in fats, carbohydrates, proteins, nucleic s, and other important biological compounds. * Aldehydes
12BL Experiment 8: Green Chem: Solvent-Free Aldol Condensation-Dehydration
 12BL Experiment 8 Green Chem Solvent-Free Aldol Condensation-Dehydration Safety Proper lab goggles/glasses must be worn (even over prescription glasses). As always, ask where organic waste containers are
12BL Experiment 8 Green Chem Solvent-Free Aldol Condensation-Dehydration Safety Proper lab goggles/glasses must be worn (even over prescription glasses). As always, ask where organic waste containers are
Answers to HT2, Chemistry A
 Answers to T2, hemistry 302-302A - 2004 1. There are many ways to do these problems - here is just one. For all three parts we can use diphenyl ketone (benzophenone) so let s make that first: 1. 2 Eq.
Answers to T2, hemistry 302-302A - 2004 1. There are many ways to do these problems - here is just one. For all three parts we can use diphenyl ketone (benzophenone) so let s make that first: 1. 2 Eq.
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Alcohols, Ethers and Epoxides. Chapter Organic Chemistry, 8th Edition John McMurry
 Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction.
 Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction. References: Brown & Foote, Chapters 16, 19, 23 INTRODUCTION: This experiment continues the saga of carbon-carbon
Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction. References: Brown & Foote, Chapters 16, 19, 23 INTRODUCTION: This experiment continues the saga of carbon-carbon
12BL Experiment 8: Green Chem: Solvent-Free Aldol Condensation-Dehydration
 12BL Experiment 8: Green Chem: Solvent-Free Aldol Condensation-Dehydration Safety: Proper lab goggles/glasses must be worn (even over prescription glasses). As always, ask where organic waste containers
12BL Experiment 8: Green Chem: Solvent-Free Aldol Condensation-Dehydration Safety: Proper lab goggles/glasses must be worn (even over prescription glasses). As always, ask where organic waste containers
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Expt 9: The Aldol Condensation
 Expt 9: The Aldol Condensation INTRDUCTIN Reactions that form carbon-carbon bonds are particularly important in organic chemistry as they allow the synthesis of more complex structures from simpler molecules.
Expt 9: The Aldol Condensation INTRDUCTIN Reactions that form carbon-carbon bonds are particularly important in organic chemistry as they allow the synthesis of more complex structures from simpler molecules.
The Chemistry of Ethers, Epoxides, Glycols, and Sulfides
 The Chemistry of Ethers, Epoxides, Glycols, and Sulfides The chemistry of ethers is closely intertwined with the chemistry of alkyl halides, alcohols, and alkenes. Ethers, however, are considerably less
The Chemistry of Ethers, Epoxides, Glycols, and Sulfides The chemistry of ethers is closely intertwined with the chemistry of alkyl halides, alcohols, and alkenes. Ethers, however, are considerably less
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
INSTRUCTIONS FOR. LITERATURE PROBLEM REPORT CHEM 30121, Fall 2018
 NSTRUCTNS FR LTERATURE PRBLEM REPRT CEM 30121, Fall 2018 Provide a reaction scheme for a fluorescent dye that features at least one reaction that you have performed and/or was covered during the lectures
NSTRUCTNS FR LTERATURE PRBLEM REPRT CEM 30121, Fall 2018 Provide a reaction scheme for a fluorescent dye that features at least one reaction that you have performed and/or was covered during the lectures
Polar Reactions. Indian Institute of Technology Madras CH 3 OH 2 -H 2 O CH 2. HCl. H 3 C CH 3 neopentyl alcohol CH 3 CH 3. 1,2-shift -H + H 3 C
 Polar Reactions Important points to be discussed in this module: 1. Chemistry of Carbocations with respect to: a. 1,2-shifts of carbocations b. Pinacol-pinacolone rearrangement c. Semipinacolone rearrangement
Polar Reactions Important points to be discussed in this module: 1. Chemistry of Carbocations with respect to: a. 1,2-shifts of carbocations b. Pinacol-pinacolone rearrangement c. Semipinacolone rearrangement
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch16_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which type of compound does not contain a carbonyl group? ketone B) aldehyde C) amine D)
Ch16_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which type of compound does not contain a carbonyl group? ketone B) aldehyde C) amine D)
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Allylic and Benzylic Reactivity
 17 17 Allylic and Benzylic Reactivity An allylic group is a group on a carbon adjacent to a double bond. A benzylic group is a group on a carbon adjacent to a benzene ring or substituted benzene ring.
17 17 Allylic and Benzylic Reactivity An allylic group is a group on a carbon adjacent to a double bond. A benzylic group is a group on a carbon adjacent to a benzene ring or substituted benzene ring.
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
2/28/2011. Chapter 6 An Overview of Organic Reactions. Organic Chemical Reactions. 6.1 Kinds of Organic Reactions
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 6 An Overview of Organic Reactions CHP 6 Problems: 6.1-13, 17-36. Richard Morrison University of Georgia, Athens Organic Chemical Reactions
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 6 An Overview of Organic Reactions CHP 6 Problems: 6.1-13, 17-36. Richard Morrison University of Georgia, Athens Organic Chemical Reactions
Answers To Chapter 7 Problems.
 Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Answer Key Question 1.
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Answer Key Question 1.
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
18: Reactions of Enolate Ions and Enols
 18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
