A B C D Q1
|
|
|
- Dayna Lyons
- 6 years ago
- Views:
Transcription
1 Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx ab) Q1 A B C D
2 Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx ab) Q1 A B C D Answer = CAD OR CADAC
3 Exam 3 Time: Tuesday, November 8: 7:00 9:00PM OR Wednesday, November 9: 7:00 9:00PM OR Thursday, November 10: 7:00 10:00PM Location Soc/Anthro Testing Center Chapters will be covered in this order: Chapter 17, 18 Practice Exams are Posted Ex3A Practice Exam 3A Ex3B Practice Exam 3B Deadline for alternate arrangements is Monday, 11/7/2016 at 4:30 PM (i.e., close of business) An oral make-up exam will be required for making up the exam for all students not taking the exam on the above dates or having already made prior arrangements
4 Exam 3 Lecture Planning Ex3-01-B A Ketone Aldehyde Naming Friday, October 22 Ex3-01-B B Aldehyde Ketone Naming Saturday, October 22 Ex3-02-B A Ald Ket Rxns O-Nucl Saturday, October 23 Ex3-02-B B Ald Ket O-Nucleophiles Sunday, October 24 Ex3-02-B C Ald Ket Rxns O-Nucl Monday, October 25 Ex3-03-B A Ald Ket with N-Nucl Tuesday, October 26 Ex3-03-B B Ald Ket with N-Nucl Wednesday, October 27 Ex3-03-B C Ald Ket with N-Nucl Thursday, October 28 Ex3-04-B A Ald Ket with C-Nucl Friday, October 29 Ex3-04-B B Ald Ket with C-Nucl Saturday, October 29 Ex3-04-B C Ald Ket with C-Nucl Sunday, October 30 Ex3-05-B Tautomers Sunday, October 30 Ex3-06-B B Alpha-Bromination Monday, October 31 Ex3-06-B C Alpha-Bromination Tuesday, November 1 Ex3-07-B B Alkylation Alpha-C=O Wednesday, November 2 Ex3-07-B C Alkylation Alpha-C=O Thursday, November 3 Ex3-08-B B Malonic Ester Synthesis Friday, November 4 Ex3-08-B C Malonic Ester Synthesis Saturday, November 5 Ex3-09-B Fatty Acids Sunday, November 6 Exam 3 November 8, 9, 10
5 Aldehydes and Ketones with C-Nucleophiles Grignard Reactions HCN additions and their implications Carboxylic Acid Formation Amine Formation Wittig Reaction
6 Aldehydes and Ketones with Grignard Reagents 1) 2) H + 2) H3O + We have already spent lots of time on the Grignard reaction. By class affirmation, we are not reviewing anymore examples here. As drawn in WE_LEARN
7 Aldehyde and Ketone Reactions with HCN CN HCN - CN 1) NaCN 2) H + 2) H + - CN HOCΞN Cyanohydrin
8 Implications of HCN Addition: Chain Elongation 1) NaCN 2) H + H2 Pt 1) NaCN 2) H + H3O + heat
9 Nitrile Hydrolysis H3O + + H2O heat - H + + H2O + H + - H + + H +
10 Give the major organic product(s) of the following reaction Q2 1) 2) H + A B C D E F - None of these products are a major product of the reaction that is shown.
11 Give the major organic product(s) of the following reaction Q2 1) 2) H + A B C D E F - None of these products are a major product of the reaction that is shown.
12 Give the major organic product(s) of the following reaction. 1) Q3 2) H + A B C D E F F - None of these products are a major product of the reaction that is shown.
13 Give the major organic product(s) of the following reaction. 1) Q3 2) H + A B C D E F F - None of these products are a major product of the reaction that is shown.
14 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx ab) 1) HCN 2) H2, Pt Q6 A B C D E F - None of these products are a major product of the reaction that is shown.
15 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx ab) 1) HCN 2) H2, Pt Q6 A B C D E F - None of these products are a major product of the reaction that is shown.
16 Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx ab) H3O + heat Q7 A B C D E F G H I
17 Correct Answer H3O + heat Q7 correct answer H C D E F B G I A Correct answer: HCDEFBGAI
18 Reacts this way! strong base Wittig Reaction Pronounce Vittig (i.e., the German pronounciation) More nicely represented this way! End here! Start here!
19 Simplified View of the Wittig Reaction Trade Ends of the Double Bonds! Cis preferred, when there is a choice Inorganic Product! Ignore!
Give the major organic product(s) of the following reaction.
 Give the major organic product(s) of the following reaction. 1) NaNO2, HCl 0 o C 2) CuCN 2016-09-23 Q1 A D B C E F There is no reaction or the correct product is not listed here. 5 of 7 Give the major
Give the major organic product(s) of the following reaction. 1) NaNO2, HCl 0 o C 2) CuCN 2016-09-23 Q1 A D B C E F There is no reaction or the correct product is not listed here. 5 of 7 Give the major
Give the major organic product(s) of the following reaction.
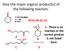 Give the major organic product(s) of the following reaction. 1) CH3CH2MgBr 2) H3O 2016-09-26 Q1 + E. There is no reaction or the correct product A B is not listed here. C D Explanation CH3CH2MgBr 1) CH3CH2MgBr
Give the major organic product(s) of the following reaction. 1) CH3CH2MgBr 2) H3O 2016-09-26 Q1 + E. There is no reaction or the correct product A B is not listed here. C D Explanation CH3CH2MgBr 1) CH3CH2MgBr
Give a common name for the following compound.
 Give a common name for the following compound. A. Benzyl Phenoate B. Phenyl Phenoate C. Benzyl Benzoate D. Phenyl Benzoate E. Phenyl Phenylacetate F. Phenyl Phenylethanoate G. Phenyl Benzylacetate 2016-10-07
Give a common name for the following compound. A. Benzyl Phenoate B. Phenyl Phenoate C. Benzyl Benzoate D. Phenyl Benzoate E. Phenyl Phenylacetate F. Phenyl Phenylethanoate G. Phenyl Benzylacetate 2016-10-07
Give the major product(s) of the following reaction.
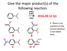 Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
H3O+ heat I Q1
 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) H3O + heat A B C D E F G
Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) H3O + heat A B C D E F G
A B C D E F Q1
 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) 1) Li 2) 2016-11-11 Q1 3)
Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) 1) Li 2) 2016-11-11 Q1 3)
A B C D E F G Q1. heat
 Give the final product of the following reaction. Give your answer as a text answer. If more than one species is correct, put your answers in alphabetical order. HNO3 H2SO4 A B C D E F G 2016-09-09 Q1
Give the final product of the following reaction. Give your answer as a text answer. If more than one species is correct, put your answers in alphabetical order. HNO3 H2SO4 A B C D E F G 2016-09-09 Q1
Q1. Intensity of M+1 peak x Intensity of M peak 2.57% x 46.60% Number of carbon atoms = Number of carbon atoms =
 The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 46.60% and an M+1 peak of 2.57%. How many carbon atoms are in the compound? (Fill in an integer number) Number
The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 46.60% and an M+1 peak of 2.57%. How many carbon atoms are in the compound? (Fill in an integer number) Number
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHE 325 ORGANIC CHEMISTRY II Spring 2017
 Instructor: CHE 325 ORGANIC CHEMISTRY II Spring 2017 Professor James Kallmerten 4-014A Center for Science and Technology Phone: 3-2854 Email: jkallmer@syr.edu Office Hours: Monday 11 am -1 pm, Wednesday
Instructor: CHE 325 ORGANIC CHEMISTRY II Spring 2017 Professor James Kallmerten 4-014A Center for Science and Technology Phone: 3-2854 Email: jkallmer@syr.edu Office Hours: Monday 11 am -1 pm, Wednesday
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Chemistry 3720, Spring 2004 Exam 3 Name:
 Chemistry 3720, Spring 2004 Exam 3 Name: This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data
Chemistry 3720, Spring 2004 Exam 3 Name: This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
CHEM 114 Principles of Chemistry (CRN points)
 SCHOOL OF CHEMICAL AND PHYSICAL SCIENCES Te Wânanga Matû CHEM 114 Principles of Chemistry (CRN 17148-15 points) Course Outline trimester 1, 2012 Course coordinator/lecturers Prof James Johnston (Coordinator)
SCHOOL OF CHEMICAL AND PHYSICAL SCIENCES Te Wânanga Matû CHEM 114 Principles of Chemistry (CRN 17148-15 points) Course Outline trimester 1, 2012 Course coordinator/lecturers Prof James Johnston (Coordinator)
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
Chapter 18: Ketones and Aldehydes. I. Introduction
 1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
LECTURE #22 Thurs., Nov.15, 2007
 Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See
 Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Chapter 20: Aldehydes and Ketones
 Chapter 20: Aldehydes and Ketones [Chapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ' ketone aldehyde f both aldehydes and ketones, the parent chain is the longest
Chapter 20: Aldehydes and Ketones [Chapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ' ketone aldehyde f both aldehydes and ketones, the parent chain is the longest
CHEM*2700 ORGANIC CHEMISTRY I (Winter Semester 2007) Information Sheet and Course Outline-Revised
 CHEM*2700 ORGANIC CHEMISTRY I (Winter Semester 2007) Information Sheet and Course Outline-Revised Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHEM*2700 ORGANIC CHEMISTRY I (Winter Semester 2007) Information Sheet and Course Outline-Revised Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2009) Information Sheet and Course Outline
 CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2009) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2009) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
Exam 3 Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3331-100 Spring 2008 Exam 3 Professor R. oenigman igh = 102 Low = 15 Average = 68 Signature ame (printed) Last four digits of your student ID number Recitation
I pledge to uphold the CU onor Code: CEM 3331-100 Spring 2008 Exam 3 Professor R. oenigman igh = 102 Low = 15 Average = 68 Signature ame (printed) Last four digits of your student ID number Recitation
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Lecture Notes Chemistry Mukund P. Sibi Lecture 36 Synthesis of Amines
 Lecture otes hemistry 42-2008 Mukund P. Sibi Synthesis of Amines Amines can be prepared from a variety of starting materials. All of these methods involve functional group transformations. The main methods
Lecture otes hemistry 42-2008 Mukund P. Sibi Synthesis of Amines Amines can be prepared from a variety of starting materials. All of these methods involve functional group transformations. The main methods
Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017)
 Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017) Class: Organic Chemistry II Instructor: Dr. David J. Leaver Room: WSB 307 Office: WSB 318 Time: MWF 9:00-9:50am Office
Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017) Class: Organic Chemistry II Instructor: Dr. David J. Leaver Room: WSB 307 Office: WSB 318 Time: MWF 9:00-9:50am Office
Ketones and Aldehydes Reading Study Problems Key Concepts and Skills Lecture Topics: Structure of Ketones and Aldehydes Structure:
 Ketones and Aldehydes Reading: Wade chapter 18, sections 18-1- 18-21 Study Problems: 18-43, 18-44,18-50, 18-51, 18-52, 18-59, 18-60, 18-62, 18-64, 18-72. Key Concepts and Skills: Interpret the IR, NMR,
Ketones and Aldehydes Reading: Wade chapter 18, sections 18-1- 18-21 Study Problems: 18-43, 18-44,18-50, 18-51, 18-52, 18-59, 18-60, 18-62, 18-64, 18-72. Key Concepts and Skills: Interpret the IR, NMR,
ORGANIC REACTIONS Chem223 (Winter 2019)
 ORGANIC REACTIONS Chem223 (Winter 2019) Lectures: Mondays 10:30-11:30 am Wednesdays 9:30-10:30 am Fridays 8:30-9:30 am Location: Stirling B (lectures), Che118 (labs) Course instructor: Dr Anne Petitjean
ORGANIC REACTIONS Chem223 (Winter 2019) Lectures: Mondays 10:30-11:30 am Wednesdays 9:30-10:30 am Fridays 8:30-9:30 am Location: Stirling B (lectures), Che118 (labs) Course instructor: Dr Anne Petitjean
ORGANIC REACTIONS Chem223 (Winter 2018) Chernoff Hall, room 215
 ORGANIC REACTIONS Chem223 (Winter 2018) Lectures: Mondays 9:30-10:30 am Wednesdays 8:30-9:30 am Thursdays 10:30-11:30 am Location: Stirling C (lectures), Che118 (labs) Voluntary tutorials: Wednesdays 10:30-11:30
ORGANIC REACTIONS Chem223 (Winter 2018) Lectures: Mondays 9:30-10:30 am Wednesdays 8:30-9:30 am Thursdays 10:30-11:30 am Location: Stirling C (lectures), Che118 (labs) Voluntary tutorials: Wednesdays 10:30-11:30
CHAPTER 23 HW: ENOLS + ENOLATES
 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Chemistry 234 Practice Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
Chapter 20: Carboxylic Acids
 1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
FIRST EXAMINATION. Name: CHM 332
 ame: CM 332 FIRST EXAMIATI All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is,
ame: CM 332 FIRST EXAMIATI All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is,
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
The problem is that your product still has a-protons, and can keep on forming enolates to get more methyl groups added:
 Lecture 13 Notes November 7, 2012 We are going to start with some examples of proline-catalyzed reactions. In each case, you can draw a mechanism that would involve proline as a chiral catalyst, and one
Lecture 13 Notes November 7, 2012 We are going to start with some examples of proline-catalyzed reactions. In each case, you can draw a mechanism that would involve proline as a chiral catalyst, and one
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
TOPIC 3 - ALDEHYDES AND KETONES (Chapters 12 & 16)
 TPIC 3 - ALDEYDES AND KETNES (Chapters 12 & 16) Lecture 15 Web12 12.1 Introduction 16.1 Introduction 16.2 Nomenclature of Aldehydes and Ketones 16.3 ysical Properties 12.2 xidation Reduction Reactions
TPIC 3 - ALDEYDES AND KETNES (Chapters 12 & 16) Lecture 15 Web12 12.1 Introduction 16.1 Introduction 16.2 Nomenclature of Aldehydes and Ketones 16.3 ysical Properties 12.2 xidation Reduction Reactions
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2010) Information Sheet and Course Outline
 CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2010) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2010) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
Columbia University C99ORG22ak.DOC Chem S3444Q Summer 99 Professor Irving J. Borowitz Exam No. 2 Answer Key July 28, 1999
 olumbia University 99RG22ak. hem SQ Summer 99 Professor Irving J. Borowitz Exam No. 2 Answer Key July 28, 1999 Name: Grade: Please use a non-red pen. Answer questions in the provided space. If you write
olumbia University 99RG22ak. hem SQ Summer 99 Professor Irving J. Borowitz Exam No. 2 Answer Key July 28, 1999 Name: Grade: Please use a non-red pen. Answer questions in the provided space. If you write
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry
 Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry Examination #4 Aldehydes & Ketones, Carboxylic Acids & Carboxylic Acid Derivatives, Lipids & Detergents, and Amines. Handout: Tuesday,
Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry Examination #4 Aldehydes & Ketones, Carboxylic Acids & Carboxylic Acid Derivatives, Lipids & Detergents, and Amines. Handout: Tuesday,
Score: Homework Problem Set 6 Iverson CH320N Due Friday, March 10. NAME (Print): Chemistry 320N Dr. Brent Iverson 6th Homework March 3, 2017
 omework Problem Set 6 Iverson 320 Due Friday, March 10 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 6th omework March 3, 2017 Please print the first three letters of your last name in the three
omework Problem Set 6 Iverson 320 Due Friday, March 10 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 6th omework March 3, 2017 Please print the first three letters of your last name in the three
22.7 Reactions of Amines: A Review and a Preview
 22.7 Reactions of Amines: A Review and a Preview Preparation of Amines Two questions to answer: 1) How is the C N C N bond to be formed? 2) How do we obtain the correct oxidation state of nitrogen (and
22.7 Reactions of Amines: A Review and a Preview Preparation of Amines Two questions to answer: 1) How is the C N C N bond to be formed? 2) How do we obtain the correct oxidation state of nitrogen (and
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Aldehydes and Ketones: Nucleophilic Addition Reactions
 Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Chemistry 3720 Old Exams. Practice Exams & Keys
 Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Option G: Further organic chemistry (15/22 hours)
 Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Chem Final Examination August 7, 2004
 Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
Organic Chemistry, Third Edition. Janice Gorzynski Smith University of Hawai i. Chapter 21. Aldehydes and Ketones Nucleophilic Addition
 Organic Chemistry, Third Edition Janice Gorzynski Smith University of Hawai i Chapter 21 Aldehydes and Ketones Nucleophilic Addition Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Organic Chemistry, Third Edition Janice Gorzynski Smith University of Hawai i Chapter 21 Aldehydes and Ketones Nucleophilic Addition Prepared by Rabi Ann Musah State University of New York at Albany Copyright
A. B. C. D. 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal?
 Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
Chemistry 330 Fall 2015 Organic Chemistry I
 Chemistry 330 Fall 2015 Organic Chemistry I Instructor: John G. Kodet Contact Information: Office: Faraday Hall 335 Email: jkodet@niu.edu Office Hours: MW 2:00-3:00 pm, and by appointment Lecture: MWF
Chemistry 330 Fall 2015 Organic Chemistry I Instructor: John G. Kodet Contact Information: Office: Faraday Hall 335 Email: jkodet@niu.edu Office Hours: MW 2:00-3:00 pm, and by appointment Lecture: MWF
Grant MacEwan University Fall 2010 CHEM 362 (270) Advanced Organic Chemistry. Organic Chemistry by Clayden, Greeves, Warren and Wothers
 1 Instructor: Office: Grant MacEwan University Fall 2010 CHEM 362 (270) Advanced Organic Chemistry Dr. Manzar Saberi 5-172 K, City Center Campus Phone: 497-4634 Lectures: Tuesday, Thursday, 12:30-2:00
1 Instructor: Office: Grant MacEwan University Fall 2010 CHEM 362 (270) Advanced Organic Chemistry Dr. Manzar Saberi 5-172 K, City Center Campus Phone: 497-4634 Lectures: Tuesday, Thursday, 12:30-2:00
Chemistry Organic Chemistry II, Spring 2018
 Chemistry 2320 Organic Chemistry II, Spring 2018 Instructor: Dr. Tom Chang Office: Widtsoe 337 Phone: 797-3545 Email: tom.chang@usu.edu Meeting Time/Place: MWF 10:30-11:20 am, Eccles Business Building
Chemistry 2320 Organic Chemistry II, Spring 2018 Instructor: Dr. Tom Chang Office: Widtsoe 337 Phone: 797-3545 Email: tom.chang@usu.edu Meeting Time/Place: MWF 10:30-11:20 am, Eccles Business Building
1- Reaction at the carbonyl carbon (Nucleophilic addition reactions).
 Reactions of aldehydes and Ketones Aldehydes and Ketones undergo many reactions to give a wide variety of useful derivatives. There are two general kinds of reactions that aldehydes and ketones undergo:
Reactions of aldehydes and Ketones Aldehydes and Ketones undergo many reactions to give a wide variety of useful derivatives. There are two general kinds of reactions that aldehydes and ketones undergo:
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
Announcements Monday, November 13
 Announcements Monday, November 13 The third midterm is on this Friday, November 17. The exam covers 3.1, 3.2, 5.1, 5.2, 5.3, and 5.5. About half the problems will be conceptual, and the other half computational.
Announcements Monday, November 13 The third midterm is on this Friday, November 17. The exam covers 3.1, 3.2, 5.1, 5.2, 5.3, and 5.5. About half the problems will be conceptual, and the other half computational.
CHEMISTRY 341. Final Exam Tuesday, December 16, Problem 1 15 pts Problem 9 8 pts. Problem 2 5 pts Problem pts
 CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide
 CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide page 1 CHEM 243 Exam II is scheduled for Monday, November 5 in DMF 477 & 481 (lab rooms). You must take the exam during your normal
CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide page 1 CHEM 243 Exam II is scheduled for Monday, November 5 in DMF 477 & 481 (lab rooms). You must take the exam during your normal
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
Organic Chemistry 1 CHM 2210 Exam 4 (December 10, 2001)
 Exam 4 (December 10, 2001) Name (print): Signature: Student ID Number: There are 12 multiple choice problems (4 points each) on this exam. Record the answers to the multiple choice questions on THIS PAGE.
Exam 4 (December 10, 2001) Name (print): Signature: Student ID Number: There are 12 multiple choice problems (4 points each) on this exam. Record the answers to the multiple choice questions on THIS PAGE.
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Course Syllabus. Department: Science & Technology. Date: April I. Course Prefix and Number: CHM 212. Course Name: Organic Chemistry II
 Department: Science & Technology Date: April 2012 I. Course Prefix and Number: CHM 212 Course Name: Organic Chemistry II Course Syllabus Credit Hours and Contact Hours: 5 credit hours and 7 (3:3:1) contact
Department: Science & Technology Date: April 2012 I. Course Prefix and Number: CHM 212 Course Name: Organic Chemistry II Course Syllabus Credit Hours and Contact Hours: 5 credit hours and 7 (3:3:1) contact
UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 READ ALL THE INSTRUCTIONS CAREFULLY
 WEDNESDAY MARCH 9th, 2016 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
WEDNESDAY MARCH 9th, 2016 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
Chapter 19 Substitutions at the Carbonyl Group
 Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Ch 20 Carboxylic Acids and Nitriles
 Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6
 GEORGIA INSTITUTE OF TECHNOLOGY School of Chemistry and Biochemistry Spring 2004 A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6 Instructor: Marcus Weck Office: Boggs 3-85 Phone:
GEORGIA INSTITUTE OF TECHNOLOGY School of Chemistry and Biochemistry Spring 2004 A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6 Instructor: Marcus Weck Office: Boggs 3-85 Phone:
Chapter 20: Aldehydes and Ketones
 hapter 20: Aldehydes and Ketones [hapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ketone ' aldehyde 2. eview of the Synthesis of Aldehydes and Ketones Br Br f
hapter 20: Aldehydes and Ketones [hapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ketone ' aldehyde 2. eview of the Synthesis of Aldehydes and Ketones Br Br f
Mechanisms. . CCl2 F + Cl.
 Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
12.1 The Nature of Organic molecules
 12.1 The Nature of Organic molecules Organic chemistry: : The chemistry of carbon compounds. Carbon is tetravalent; it always form four bonds. Prentice Hall 2003 Chapter One 2 Organic molecules have covalent
12.1 The Nature of Organic molecules Organic chemistry: : The chemistry of carbon compounds. Carbon is tetravalent; it always form four bonds. Prentice Hall 2003 Chapter One 2 Organic molecules have covalent
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
Products from reactions of carbon nucleophiles and carbon electrophiles used in the 14 C Game and our course:
 synthesis strategies, hem / / Beauchamp roducts from reactions of carbon nucleophiles and carbon electrophiles used in the Game and our course: arbon electrophiles methyl primary organolithium reagents
synthesis strategies, hem / / Beauchamp roducts from reactions of carbon nucleophiles and carbon electrophiles used in the Game and our course: arbon electrophiles methyl primary organolithium reagents
Announcements Monday, September 18
 Announcements Monday, September 18 WeBWorK 1.4, 1.5 are due on Wednesday at 11:59pm. The first midterm is on this Friday, September 22. Midterms happen during recitation. The exam covers through 1.5. About
Announcements Monday, September 18 WeBWorK 1.4, 1.5 are due on Wednesday at 11:59pm. The first midterm is on this Friday, September 22. Midterms happen during recitation. The exam covers through 1.5. About
Important Note: We will NOT accept papers written in pencil back for re-marking after they have been returned to you. Please do not ask!
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 2 Thursday, March 15, 2012; 7-9 PM This is a 2-hour test, marked out of
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 2 Thursday, March 15, 2012; 7-9 PM This is a 2-hour test, marked out of
Introduction to Organic Chemistry
 Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
Lecture 23 March 12, 2018 Chap 7.3
 Statics - TAM 210 & TAM 211 Lecture 23 March 12, 2018 Chap 7.3 Announcements Upcoming deadlines: Monday (3/12) Mastering Engineering Tutorial 9 Tuesday (3/13) PL HW 8 Quiz 5 (3/14-16) Sign up at CBTF Up
Statics - TAM 210 & TAM 211 Lecture 23 March 12, 2018 Chap 7.3 Announcements Upcoming deadlines: Monday (3/12) Mastering Engineering Tutorial 9 Tuesday (3/13) PL HW 8 Quiz 5 (3/14-16) Sign up at CBTF Up
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
CHEM 203. Midterm Exam 1 October 31, 2008 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CHEM Fall Exam 2 Professor R. Hoenigman. Signature. Name (printed) Last four digits of your student ID number.
 Average = 68 I pledge to uphold the CU on Code: CEM 3311-200 Fall 2006 Exam 2 Profess R. oenigman igh = 98 Low = 18 Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation
Average = 68 I pledge to uphold the CU on Code: CEM 3311-200 Fall 2006 Exam 2 Profess R. oenigman igh = 98 Low = 18 Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation
