H3O+ heat I Q1
|
|
|
- Barrie Jefferson
- 6 years ago
- Views:
Transcription
1 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) H3O + heat A B C D E F G H I - None of these products are a major product of the reaction that is shown Q1
2 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) H3O + heat A B C D E F G H I - None of these products are a major product of the reaction that is shown Q1
3 Exam 2 Time: Tuesday, October 18: 7:00 9:00PM OR Wednesday, October 19: 7:00 9:00PM OR Thursday, October 20: 7:00 10:00PM Location Soc/Anthro Testing Center Chapters will be covered in this order: Chapter 19, 12 Practice Exams are Posted Ex Practice Exam 2A Ex Practice Exam 2B Deadline for alternate arrangements is Monday, 10/17/2016 at 4:30 PM (i.e., close of business) An oral make-up exam will be required for making up the exam for all students not taking the exam on the above dates or having already made prior arrangements
4 Order of Coverage (Exam 2) Homework Assignment Due Date 13 Ex2-07-B A Carbox Acid Rxns Thursday, October 6, Ex2-07-B B Carbox Acid Rxns Friday, October 7, Ex2-08-B A Naming Carbox Acid Derivatives 16 Ex2-08-B B Naming Carbox Acid Derivatives Saturday, October 8, 2016 Sunday, October 9, Ex2-09-B A Rxns Acid Chlorides Monday, October 10, Ex2-09-B B Rxns Acid Chlorides Tuesday, October 11, Ex2-10-B A Rxns Esters Wednesday, October 12, Ex2-10-B B Rxns Esters Thursday, October 13, Ex2-11-B A Rxns Amides Friday, October 14, Ex2-11-B B Rxns Amides Saturday, October 15, Ex2-12-B A Step Growth Polymers Sunday, October 16, 2016 Exam 2 October 18, 19, 20
5 Mistake #1 in the Last Lecture Lecture H2O HO - No Reaction WE_LEARN H2O HO - Esters to Carboxylic Acids Either answer will be counted as corrected on the exam (although you may have to appeal to get the credit.
6 Mistake #2 in the Last Lecture Lecture CH3OH CH3O - CH3OH CH3O -, heat WE_LEARN CH3OH CH3O - CH3OH CH3O -, heat Trans-Esterification Either answer will be counted as corrected on the exam (although you may have to appeal to get the credit.
7 Carboxylic Acid Derivatives Acid Halide Anhydride Carboxylic Acid Ester Amide Most Reactive Least Reactive Most Stable Least Stable
8 Amides to Acid Chlorides No direct route! Must convert to a carboxylic acid, then to the acid chloride H3O + heat 1) HO -, heat 2) H +
9 H2O H +, heat H2O H + H2O No Reaction No Reaction Amides to Carboxylic Acids H2O HO - No Reaction H2O HO -, heat
10 CH3OH H +, heat Amides to Esters CH3OH H + No Reaction CH3OH No Reaction CH3OH CH3O - No Reaction CH3OH CH3O -, heat
11 Amide Reduction by LiAlH 4 Acidic Work-up 1) LiAlH 4 2) H 3 O + H + Basic Work-up NaOH
12 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) NaOH heat A B Q2 E - None of these products are a major product of the reaction that is shown. C D
13 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) NaOH heat A B Q2 E - None of these products are a major product of the reaction that is shown. C D
14 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) PCl 3 heat A B C Q3 D E F G - None of these products are a major product of the reaction that is shown.
15 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) PCl 3 heat A B C Q3 D E F G - None of these products are a major product of the reaction that is shown.
16 Towards Step-Growth Polymers 2 ends to react Only 1 end to react Only 1 end to react No ends left to react with
17 Step-Growth Polymers
18 Step-Growth Polymers
19 Step-Growth Polymers All of these are representations of the same polymer!
20 Aliphatic Homopolymer Polyesters PGA Polyglycolide PLA PCL Polylactic acid Polycaprolactone Ester Linkages Lead to Biodegradability PHA Polyhydroxyalkanoate PHB Polyhydroxybutyrate
21 Aliphatic Copolymer Polyesters PEA Polyethylene adipate PBS Polybutylene succinate PHBV Poly(3-hydroxybutyrate-co-3- hydroxyvalerate)
22 PET Semiaromatic Copolymer Polyesters Polyethylene terephthalate PBT Polybutylene terephthalate PTT PEN Polytrimethylene terephthalate Polyethylene naphthalate
23 Polyamides Nylon 6,6 Kevlar
24 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) E - None of these products are a major product of the reaction that is shown. A n B n Q4 C n D
25 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) E - None of these products are a major product of the reaction that is shown. A n B n Q4 C n D
Give the major organic product(s) of the following reaction.
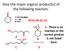 Give the major organic product(s) of the following reaction. 1) CH3CH2MgBr 2) H3O 2016-09-26 Q1 + E. There is no reaction or the correct product A B is not listed here. C D Explanation CH3CH2MgBr 1) CH3CH2MgBr
Give the major organic product(s) of the following reaction. 1) CH3CH2MgBr 2) H3O 2016-09-26 Q1 + E. There is no reaction or the correct product A B is not listed here. C D Explanation CH3CH2MgBr 1) CH3CH2MgBr
Give a common name for the following compound.
 Give a common name for the following compound. A. Benzyl Phenoate B. Phenyl Phenoate C. Benzyl Benzoate D. Phenyl Benzoate E. Phenyl Phenylacetate F. Phenyl Phenylethanoate G. Phenyl Benzylacetate 2016-10-07
Give a common name for the following compound. A. Benzyl Phenoate B. Phenyl Phenoate C. Benzyl Benzoate D. Phenyl Benzoate E. Phenyl Phenylacetate F. Phenyl Phenylethanoate G. Phenyl Benzylacetate 2016-10-07
Give the major organic product(s) of the following reaction.
 Give the major organic product(s) of the following reaction. 1) NaNO2, HCl 0 o C 2) CuCN 2016-09-23 Q1 A D B C E F There is no reaction or the correct product is not listed here. 5 of 7 Give the major
Give the major organic product(s) of the following reaction. 1) NaNO2, HCl 0 o C 2) CuCN 2016-09-23 Q1 A D B C E F There is no reaction or the correct product is not listed here. 5 of 7 Give the major
A B C D Q1
 Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx
Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx
Give the major product(s) of the following reaction.
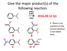 Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
A B C D E F Q1
 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) 1) Li 2) 2016-11-11 Q1 3)
Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) 1) Li 2) 2016-11-11 Q1 3)
A B C D E F G Q1. heat
 Give the final product of the following reaction. Give your answer as a text answer. If more than one species is correct, put your answers in alphabetical order. HNO3 H2SO4 A B C D E F G 2016-09-09 Q1
Give the final product of the following reaction. Give your answer as a text answer. If more than one species is correct, put your answers in alphabetical order. HNO3 H2SO4 A B C D E F G 2016-09-09 Q1
Q1. Intensity of M+1 peak x Intensity of M peak 2.57% x 46.60% Number of carbon atoms = Number of carbon atoms =
 The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 46.60% and an M+1 peak of 2.57%. How many carbon atoms are in the compound? (Fill in an integer number) Number
The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 46.60% and an M+1 peak of 2.57%. How many carbon atoms are in the compound? (Fill in an integer number) Number
A polymer is a very large molecule that is built from monomers. A monomer is one of the repeating units that make up a polymer.
 1.8 Polymers The General Structure of Polymers A polymer is a very large molecule that is built from monomers. A monomer is one of the repeating units that make up a polymer. Many biological molecules,
1.8 Polymers The General Structure of Polymers A polymer is a very large molecule that is built from monomers. A monomer is one of the repeating units that make up a polymer. Many biological molecules,
Lecture 4 Chapter 13 - Polymers. Functional Groups Condensation Rxns Free Radical Rxns
 Lecture 4 Chapter 13 - Polymers Functional Groups Condensation Rxns Free Radical Rxns Chemistry the whole year on one page Last semester Basic atomic theory Stoichiometry, balancing reactions Thermodynamics
Lecture 4 Chapter 13 - Polymers Functional Groups Condensation Rxns Free Radical Rxns Chemistry the whole year on one page Last semester Basic atomic theory Stoichiometry, balancing reactions Thermodynamics
21.1 Introduction Carboxylic Acids
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. Klein, Organic Chemistry 1e 21-1 The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. Klein, Organic Chemistry 1e 21-1 The US produces over 2.5 million tons of acetic acid per year, which
Polymers are high molecular mass macromolecules composed of repeating structural
 Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
ASTR 101L: Motion of the Sun Take Home Lab
 Name: CWID: Section: Introduction Objectives This lab is designed to help you understand the Sun s apparent motion in the sky over the course of the year. In Section 2 you are asked to answer some questions
Name: CWID: Section: Introduction Objectives This lab is designed to help you understand the Sun s apparent motion in the sky over the course of the year. In Section 2 you are asked to answer some questions
POLYMERISATION. A process in which small molecules called monomers join together into large molecules consisting of repeating units.
 Polymers 1 PLYMERISATI General A process in which small molecules called monomers join together into large molecules consisting of repeating units. There are two basic types ADDITI & DESATI ADDITI PLYMERS
Polymers 1 PLYMERISATI General A process in which small molecules called monomers join together into large molecules consisting of repeating units. There are two basic types ADDITI & DESATI ADDITI PLYMERS
Week 8/Tu: Lecture Unit 19
 Week 8/Tu: Lecture Unit 19 Unit 18: Periodicity -- electron configurations -- periodicity of elements -- radii, ionization, affinity -- electronegativity Unit 19: Chemical Bonding -- Basis of bonding --
Week 8/Tu: Lecture Unit 19 Unit 18: Periodicity -- electron configurations -- periodicity of elements -- radii, ionization, affinity -- electronegativity Unit 19: Chemical Bonding -- Basis of bonding --
Design at the Molecular Level. All rules of thumb are half-truths some are useful. Marty Mulvihill, Class 13, Monday March 7 th.
 Design at the Molecular Level All rules of thumb are half-truths some are useful. Marty Mulvihill, Class 13, Monday March 7 th Definitions 1. Degradation: Breakdown of chemicals, through physical, chemical
Design at the Molecular Level All rules of thumb are half-truths some are useful. Marty Mulvihill, Class 13, Monday March 7 th Definitions 1. Degradation: Breakdown of chemicals, through physical, chemical
Downloaded from Polymer. (one mark questions Q1 to Q20)
 Polymer (one mark questions Q1 to Q20) Q1.Give the name and structure of reagent used for initiating a free radical chain reaction. Ans: Name- benzoylperoxide,c 6H 5-CO-O-O-CO-H 5C 6 Q2.Classify them as
Polymer (one mark questions Q1 to Q20) Q1.Give the name and structure of reagent used for initiating a free radical chain reaction. Ans: Name- benzoylperoxide,c 6H 5-CO-O-O-CO-H 5C 6 Q2.Classify them as
The functionality of a monomer is the number of binding sites that is/are present in that monomer.
 Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
Question 15.1: Explain the terms polymer and monomer. Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (10
Isomerism and Carbonyl Compounds
 Isomerism and Carbonyl Compounds 18 Section B Answer all questions in the spaces provided. 7 Esters have many important commercial uses such as solvents and artificial flavourings in foods. Esters can
Isomerism and Carbonyl Compounds 18 Section B Answer all questions in the spaces provided. 7 Esters have many important commercial uses such as solvents and artificial flavourings in foods. Esters can
Note: Brief explanation should be no more than 2 sentences.
 Her \Hmher UNIVERSITY OF TORONTO FACULTY OF APPLIED SCIENCE AND ENGINEERING FINAL EXAMINATION, April 26, 2017 DURATION: 2 and /2 hrs MSE245 - HiS - Second Year - MSE Organic Material Chemistry & Processing
Her \Hmher UNIVERSITY OF TORONTO FACULTY OF APPLIED SCIENCE AND ENGINEERING FINAL EXAMINATION, April 26, 2017 DURATION: 2 and /2 hrs MSE245 - HiS - Second Year - MSE Organic Material Chemistry & Processing
Due by 3:00 PM Tuesday, June 26 NO LATE PAPERS ACCEPTED!
 HEM 100 Name Exam 3 Summer 2011 Due by 3:00 PM Tuesday, June 26 N LATE PAPERS AEPTED! Follow instructions on the attached exam. learly mark your answers. YU MUST SHW YUR WRK T REEIVE REDIT. Instructions
HEM 100 Name Exam 3 Summer 2011 Due by 3:00 PM Tuesday, June 26 N LATE PAPERS AEPTED! Follow instructions on the attached exam. learly mark your answers. YU MUST SHW YUR WRK T REEIVE REDIT. Instructions
DAILY QUESTIONS 28 TH JUNE 18 REASONING - CALENDAR
 DAILY QUESTIONS 28 TH JUNE 18 REASONING - CALENDAR LEAP AND NON-LEAP YEAR *A non-leap year has 365 days whereas a leap year has 366 days. (as February has 29 days). *Every year which is divisible by 4
DAILY QUESTIONS 28 TH JUNE 18 REASONING - CALENDAR LEAP AND NON-LEAP YEAR *A non-leap year has 365 days whereas a leap year has 366 days. (as February has 29 days). *Every year which is divisible by 4
AMSC/MATH 673, CLASSICAL METHODS IN PDE, FALL Required text: Evans, Partial Differential Equations second edition
 AMSC/MATH 673, CLASSICAL METHODS IN PDE, FALL 2018. MWF 2:00pm - 2:50pm MTH 0407 Instructor: M. Machedon Office: MTH 3311 e-mail: mxm@math.umd.edu Required text: Evans, Partial Differential Equations second
AMSC/MATH 673, CLASSICAL METHODS IN PDE, FALL 2018. MWF 2:00pm - 2:50pm MTH 0407 Instructor: M. Machedon Office: MTH 3311 e-mail: mxm@math.umd.edu Required text: Evans, Partial Differential Equations second
POLYMERISATION. A process in which small molecules called monomers join together into large molecules consisting of repeating units.
 Polymers 2814 1 PLYMERISATIN General A process in which small molecules called monomers join together into large molecules consisting of repeating units. There are two basic types ADDITIN & NDENSATIN ADDITIN
Polymers 2814 1 PLYMERISATIN General A process in which small molecules called monomers join together into large molecules consisting of repeating units. There are two basic types ADDITIN & NDENSATIN ADDITIN
Due by 3:00 PM Tuesday, June 26 NO LATE PAPERS ACCEPTED!
 HEM 100 Name Exam 3 Summer 2011 Due by 3:00 PM Tuesday, June 26 N LATE PAPERS AEPTED! Follow instructions on the attached exam. learly mark your answers. YU MUST SHW YUR WRK T REEIVE REDIT. Instructions
HEM 100 Name Exam 3 Summer 2011 Due by 3:00 PM Tuesday, June 26 N LATE PAPERS AEPTED! Follow instructions on the attached exam. learly mark your answers. YU MUST SHW YUR WRK T REEIVE REDIT. Instructions
Chapter : 15. POLYMERS. Level-1:Questions
 1) What are polymers? Chapter : 15. POLYMERS Level-1:Questions A: These are referred to as Macromolecules which are formed by joining of repeating structural units on a large scale. 2) Give two examples
1) What are polymers? Chapter : 15. POLYMERS Level-1:Questions A: These are referred to as Macromolecules which are formed by joining of repeating structural units on a large scale. 2) Give two examples
Level 3 Chemistry Demonstrate understanding of the properties of organic compounds
 1 ANSWERS Level 3 Chemistry 91391 Demonstrate understanding of the properties of organic compounds Credits: Five Achievement Achievement with Merit Achievement with Excellence Demonstrate understanding
1 ANSWERS Level 3 Chemistry 91391 Demonstrate understanding of the properties of organic compounds Credits: Five Achievement Achievement with Merit Achievement with Excellence Demonstrate understanding
LECTURE #22 Thurs., Nov.15, 2007
 Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Te Marau: Mātai Matū (Chemistry) Te Taumata: 2 Kaiako: Matua Matt Cassidy
 Te Marau: Mātai Matū (Chemistry) Te Taumata: 2 Kaiako: Matua Matt Cassidy Curriculum level: 7 Curriculum document: Medium of instruction: NZC English Tauira need to have gained 12 credits from T1 Pūtaiao
Te Marau: Mātai Matū (Chemistry) Te Taumata: 2 Kaiako: Matua Matt Cassidy Curriculum level: 7 Curriculum document: Medium of instruction: NZC English Tauira need to have gained 12 credits from T1 Pūtaiao
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #4 Enols and Aldol Reaction, Carboxylic Acids and Carboxylic Acid Derivatives. Thursday, November 14, 2013,
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #4 Enols and Aldol Reaction, Carboxylic Acids and Carboxylic Acid Derivatives. Thursday, November 14, 2013,
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2009) Information Sheet and Course Outline
 CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2009) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2009) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
ABBREVIATIONS AND SYMBOLS
 ABBREVIATIONS AND SYMBOLS AA ACA or P 1 6-ACA Actis 12-ADA 11-AUA BHET BPA-PC CAPEX CD CHDM CL COOH [COOH] CSD CSTR CVD DEG DHI DLC acetaldehyde aminocaproic acid 6-amino-n-caproic acid amorphous carbon
ABBREVIATIONS AND SYMBOLS AA ACA or P 1 6-ACA Actis 12-ADA 11-AUA BHET BPA-PC CAPEX CD CHDM CL COOH [COOH] CSD CSTR CVD DEG DHI DLC acetaldehyde aminocaproic acid 6-amino-n-caproic acid amorphous carbon
Plastics are synthetic substances that can be moulded (often under heat and pressure) and retain the shape they are moulded into.
 5.7: Polymers Plastics are synthetic substances that can be moulded (often under heat and pressure) and retain the shape they are moulded into. Polymers are large molecules that are made by linking together
5.7: Polymers Plastics are synthetic substances that can be moulded (often under heat and pressure) and retain the shape they are moulded into. Polymers are large molecules that are made by linking together
CHEM Chapter 21. Carboxylic Acid Derivatives_Nucleophilic Acyl Substitution Reactions (homework) W
 Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Lecture 23 March 12, 2018 Chap 7.3
 Statics - TAM 210 & TAM 211 Lecture 23 March 12, 2018 Chap 7.3 Announcements Upcoming deadlines: Monday (3/12) Mastering Engineering Tutorial 9 Tuesday (3/13) PL HW 8 Quiz 5 (3/14-16) Sign up at CBTF Up
Statics - TAM 210 & TAM 211 Lecture 23 March 12, 2018 Chap 7.3 Announcements Upcoming deadlines: Monday (3/12) Mastering Engineering Tutorial 9 Tuesday (3/13) PL HW 8 Quiz 5 (3/14-16) Sign up at CBTF Up
Chapter 1 0+7= 1+6= 2+5= 3+4= 4+3= 5+2= 6+1= 7+0= How would you write five plus two equals seven?
 Chapter 1 0+7= 1+6= 2+5= 3+4= 4+3= 5+2= 6+1= 7+0= If 3 cats plus 4 cats is 7 cats, what does 4 olives plus 3 olives equal? olives How would you write five plus two equals seven? Chapter 2 Tom has 4 apples
Chapter 1 0+7= 1+6= 2+5= 3+4= 4+3= 5+2= 6+1= 7+0= If 3 cats plus 4 cats is 7 cats, what does 4 olives plus 3 olives equal? olives How would you write five plus two equals seven? Chapter 2 Tom has 4 apples
Determination of the degree of randomness in condensation copolymers containing both symmetrical and unsymmetrical monomer units: a theoretical study
 e-polymers 2003, no. 030. http://www.e-polymers.org ISSN 1618-7229 Determination of the degree of randomness in condensation copolymers containing both symmetrical and unsymmetrical monomer units: a theoretical
e-polymers 2003, no. 030. http://www.e-polymers.org ISSN 1618-7229 Determination of the degree of randomness in condensation copolymers containing both symmetrical and unsymmetrical monomer units: a theoretical
Announcements Monday, September 18
 Announcements Monday, September 18 WeBWorK 1.4, 1.5 are due on Wednesday at 11:59pm. The first midterm is on this Friday, September 22. Midterms happen during recitation. The exam covers through 1.5. About
Announcements Monday, September 18 WeBWorK 1.4, 1.5 are due on Wednesday at 11:59pm. The first midterm is on this Friday, September 22. Midterms happen during recitation. The exam covers through 1.5. About
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2010) Information Sheet and Course Outline
 CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2010) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHEM*2700 ORGANIC CHEMISTRY I (Spring/Summer Semester 2010) Information Sheet and Course Outline Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
As you enter... (Write down the learning objective, the question and your answer.) What do you think it means for a reaction to occur spontaneously?
 Monday, March 23rd Learning objective 5.13: The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively)
Monday, March 23rd Learning objective 5.13: The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively)
CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide
 CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide page 1 CHEM 243 Exam II is scheduled for Monday, November 5 in DMF 477 & 481 (lab rooms). You must take the exam during your normal
CHEM 243 ORGANIC CHEMISTRY I Fall 2018 Exam II Information and Study Guide page 1 CHEM 243 Exam II is scheduled for Monday, November 5 in DMF 477 & 481 (lab rooms). You must take the exam during your normal
CHEM*2700 ORGANIC CHEMISTRY I (Winter Semester 2007) Information Sheet and Course Outline-Revised
 CHEM*2700 ORGANIC CHEMISTRY I (Winter Semester 2007) Information Sheet and Course Outline-Revised Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
CHEM*2700 ORGANIC CHEMISTRY I (Winter Semester 2007) Information Sheet and Course Outline-Revised Instructor: Professor William Tam Office: MacN 332 Phone: 824-4120 (Ext.52268) E-mail: wtam@uoguelph.ca
JBA 2018 Chemistry Exam 2. Name: Score: /100 = /80
 JBA 2018 hemistry Exam 2 ame: Score: /100 = /80 Multiple choice questions are worth two points each. 1. onstitutional isomers are compounds that have a. the same chemical formulas and molecular structures
JBA 2018 hemistry Exam 2 ame: Score: /100 = /80 Multiple choice questions are worth two points each. 1. onstitutional isomers are compounds that have a. the same chemical formulas and molecular structures
Exam 3 Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3331-100 Spring 2008 Exam 3 Professor R. oenigman igh = 102 Low = 15 Average = 68 Signature ame (printed) Last four digits of your student ID number Recitation
I pledge to uphold the CU onor Code: CEM 3331-100 Spring 2008 Exam 3 Professor R. oenigman igh = 102 Low = 15 Average = 68 Signature ame (printed) Last four digits of your student ID number Recitation
Instructions: Cut individually and fold/glue. Can be either laminated or folded around cardboard.
 NCEA Chemistry 3.5 Flash Cards Instructions: Cut individually and fold/glue. Can be either laminated or folded around cardboard. Ideas for Use: 1. Group reactants (or products) into functional groups 2.
NCEA Chemistry 3.5 Flash Cards Instructions: Cut individually and fold/glue. Can be either laminated or folded around cardboard. Ideas for Use: 1. Group reactants (or products) into functional groups 2.
Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017)
 Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017) Class: Organic Chemistry II Instructor: Dr. David J. Leaver Room: WSB 307 Office: WSB 318 Time: MWF 9:00-9:50am Office
Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017) Class: Organic Chemistry II Instructor: Dr. David J. Leaver Room: WSB 307 Office: WSB 318 Time: MWF 9:00-9:50am Office
CYCLOALKANES, POLYMERS, ALCOHOLS AND ETHERS Home Assignment
 CYCLOALKANES, POLYMERS, ALCOHOLS AND ETHERS Home Assignment 1. The tendency of cylopropane (I), Cyclobutane (II), cyclopentane (III) to form addition compounds is in the order : a) I > II > III b) I =
CYCLOALKANES, POLYMERS, ALCOHOLS AND ETHERS Home Assignment 1. The tendency of cylopropane (I), Cyclobutane (II), cyclopentane (III) to form addition compounds is in the order : a) I > II > III b) I =
I pledge on my honor that I have neither given nor received unauthorized aid on this examination
 Chemistry 220b, Section 1 Exam 2 (100 pts) Thursday, ebruary 26, 2015 Chapters 13, 15-19 ame Write and sign the VU onor Pledge: I pledge on my honor that I have neither given nor received unauthorized
Chemistry 220b, Section 1 Exam 2 (100 pts) Thursday, ebruary 26, 2015 Chapters 13, 15-19 ame Write and sign the VU onor Pledge: I pledge on my honor that I have neither given nor received unauthorized
Notes Friday, September 09, 2016
 Notes Friday, September 09, 2016 Day 1 - Polynomials Page 1 Notes Friday, September 09, 2016 Day 1 - Polynomials Page 2 Notes Friday, September 09, 2016 Day 1 - Polynomials Page 3 Notes Friday, September
Notes Friday, September 09, 2016 Day 1 - Polynomials Page 1 Notes Friday, September 09, 2016 Day 1 - Polynomials Page 2 Notes Friday, September 09, 2016 Day 1 - Polynomials Page 3 Notes Friday, September
POLYMERS Mega molecules Out of Small Ones
 Back Recommended grades level(s) 11-12 Time Duration: - 50 minutes POLYMERS Mega molecules Out of Small Ones Objective(s): The learner will produce a linear and a cross-linked polyester. The learner will
Back Recommended grades level(s) 11-12 Time Duration: - 50 minutes POLYMERS Mega molecules Out of Small Ones Objective(s): The learner will produce a linear and a cross-linked polyester. The learner will
 Top concepts Chapter : Polymers 1. Polymers are high molecular mass substance consisting of large number of repeating structural units. As polymers are single, giant molecules i.e. big size molecules,
Top concepts Chapter : Polymers 1. Polymers are high molecular mass substance consisting of large number of repeating structural units. As polymers are single, giant molecules i.e. big size molecules,
Reminders : Sign up to make up Labs (Tues/Thurs from 3 4 pm)
 Monday, 5.11.15 Learning Target : I can identify nuclear reactions based on the characteristics of their chemical equations. Homework: Bingo packet 2 due Thursday What is a fission reaction? What is a
Monday, 5.11.15 Learning Target : I can identify nuclear reactions based on the characteristics of their chemical equations. Homework: Bingo packet 2 due Thursday What is a fission reaction? What is a
Name: Score: /100. Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each
 ame: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. When l is added to pure water, l molecules lose protons, while water molecules gain protons.
ame: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. When l is added to pure water, l molecules lose protons, while water molecules gain protons.
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution Reactions. McMurray Text Chapter 21
 Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
2A - Amines. 2 H atoms replaced: 2 attached C's to N. 3 H atom replaced: 3 attached C's to N Ammonia, NH3 Primary amine Secondary amine Tertiary amine
 2A - Amines Something fishy about amines: Have an NH 2, amine group. Amines are derivatives of ammonia: 3 H atoms 1 H atom replaced: 1 attached C to N 2 H atoms replaced: 2 attached C's to N 3 H atom replaced:
2A - Amines Something fishy about amines: Have an NH 2, amine group. Amines are derivatives of ammonia: 3 H atoms 1 H atom replaced: 1 attached C to N 2 H atoms replaced: 2 attached C's to N 3 H atom replaced:
Name Date Class. aryl halides substitution reaction
 23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
Lecture 25 POLYMERS. April 19, Chemistry 328N
 Lecture 25 POLYMERS Wallace Carothers April 19, 2016 Paul Flory Wallace Hume Carothers 1896-1937 Carothers at Dupont 1.Commercializion of Nylon https://www.chemheritage.org/ Nylon was first used for fishing
Lecture 25 POLYMERS Wallace Carothers April 19, 2016 Paul Flory Wallace Hume Carothers 1896-1937 Carothers at Dupont 1.Commercializion of Nylon https://www.chemheritage.org/ Nylon was first used for fishing
HIGHER 1 Polymers. Polymers are giant molecules made by linking together smaller molecules called monomers.
 IGER 1 Polymers PLYMERS Polymers are giant molecules made by linking together smaller molecules called monomers. The reaction is called polymerisation. The linking can be done in two ways: by addition
IGER 1 Polymers PLYMERS Polymers are giant molecules made by linking together smaller molecules called monomers. The reaction is called polymerisation. The linking can be done in two ways: by addition
CHE 325 ORGANIC CHEMISTRY II Spring 2017
 Instructor: CHE 325 ORGANIC CHEMISTRY II Spring 2017 Professor James Kallmerten 4-014A Center for Science and Technology Phone: 3-2854 Email: jkallmer@syr.edu Office Hours: Monday 11 am -1 pm, Wednesday
Instructor: CHE 325 ORGANIC CHEMISTRY II Spring 2017 Professor James Kallmerten 4-014A Center for Science and Technology Phone: 3-2854 Email: jkallmer@syr.edu Office Hours: Monday 11 am -1 pm, Wednesday
, :...21
 , 20.,,,,, 2017 ......3 1...6 1.1 :,,.6 1.1.1..6 1.1.2 :,,, 11 1.2.19 1.2.1,..19 1.2.2 :....21 2 26 2.1. 26 2.2. 28.39 40 ,..,,. ё (,,..), :, [1], [2],.. [3] ( ) (, ) ( ),,,, [4]. : -3-
, 20.,,,,, 2017 ......3 1...6 1.1 :,,.6 1.1.1..6 1.1.2 :,,, 11 1.2.19 1.2.1,..19 1.2.2 :....21 2 26 2.1. 26 2.2. 28.39 40 ,..,,. ё (,,..), :, [1], [2],.. [3] ( ) (, ) ( ),,,, [4]. : -3-
Chapter 19 Substitutions at the Carbonyl Group
 Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Welcome in the Master programme
 Welcome in the Master programme Prof. Axel Görlitz, HHU Düsseldorf 11.10.2013 Master Programme in Physics Physics Focus (Schwerpunkt) Plasma Physics, Quantum Optics and Quantum Information, Solid State
Welcome in the Master programme Prof. Axel Görlitz, HHU Düsseldorf 11.10.2013 Master Programme in Physics Physics Focus (Schwerpunkt) Plasma Physics, Quantum Optics and Quantum Information, Solid State
Tentative Daily Schedule CHEM 125 Fall 2012
 Tentative Daily Schedule CHEM 125 Fall 2012 Atomic Structure and Periodic Trends Thursday, Aug 30 th, Day 2 Resources: o Text and Connect Passcode (available at bookstore): Chemical Structure and Properties,
Tentative Daily Schedule CHEM 125 Fall 2012 Atomic Structure and Periodic Trends Thursday, Aug 30 th, Day 2 Resources: o Text and Connect Passcode (available at bookstore): Chemical Structure and Properties,
CH101 - Chemistry. Instructors: Dr. David Cheung, Prof. Paul Murphy, Dr. Luca Ronconi (Coordinator)
 CH101 - Chemistry Instructors: Dr. David Cheung, Prof. Paul Murphy, Dr. Luca Ronconi (Coordinator) Module Overview and General Aims This Module lays a broad foundation in chemistry for students who have
CH101 - Chemistry Instructors: Dr. David Cheung, Prof. Paul Murphy, Dr. Luca Ronconi (Coordinator) Module Overview and General Aims This Module lays a broad foundation in chemistry for students who have
CHM 151: GENERAL CHEMISTRY I Department of Chemistry College of Arts and Sciences Northern Arizona University
 CHM 151: GENERAL CHEMISTRY I Department of Chemistry College of Arts and Sciences Northern Arizona University Instructor: Dr. Brandon Cruickshank Office: Chem: Rm 121, and 125 Phone: 523-9602 Web site:
CHM 151: GENERAL CHEMISTRY I Department of Chemistry College of Arts and Sciences Northern Arizona University Instructor: Dr. Brandon Cruickshank Office: Chem: Rm 121, and 125 Phone: 523-9602 Web site:
Welcome to Math 102 Section 102
 Welcome to Math 102 Section 102 Mingfeng Qiu Sep. 5, 2018 Math 102: Announcements Instructor: Mingfeng Qiu Email: mqiu@math.ubc.ca Course webpage: https://canvas.ubc.ca Check the calendar!!! Sectional
Welcome to Math 102 Section 102 Mingfeng Qiu Sep. 5, 2018 Math 102: Announcements Instructor: Mingfeng Qiu Email: mqiu@math.ubc.ca Course webpage: https://canvas.ubc.ca Check the calendar!!! Sectional
Announcements Monday, November 13
 Announcements Monday, November 13 The third midterm is on this Friday, November 17. The exam covers 3.1, 3.2, 5.1, 5.2, 5.3, and 5.5. About half the problems will be conceptual, and the other half computational.
Announcements Monday, November 13 The third midterm is on this Friday, November 17. The exam covers 3.1, 3.2, 5.1, 5.2, 5.3, and 5.5. About half the problems will be conceptual, and the other half computational.
3.1 Poly(ethylene terephthalate) (PET)
 3 Thermo-Oxidative Degradation 3.1 Poly(ethylene terephthalate) (PET) The mechanism developed originally by Bolland and Gee [1] to explain the thermal oxidation of rubbers and polyolefins has been successfully
3 Thermo-Oxidative Degradation 3.1 Poly(ethylene terephthalate) (PET) The mechanism developed originally by Bolland and Gee [1] to explain the thermal oxidation of rubbers and polyolefins has been successfully
CHEMISTRY 1A Fall 2010 Final Exam Key
 CHEMISTRY 1A Fall 2010 Final Exam Key YOU MIGHT FIND THE FOLLOWING USEFUL; 0.008314 kj H E ( n)rt R = K mol 0.00418 kj q C cal m w T g C H rxn = H f (products) H f (reactants) Electronegativities H 2.2
CHEMISTRY 1A Fall 2010 Final Exam Key YOU MIGHT FIND THE FOLLOWING USEFUL; 0.008314 kj H E ( n)rt R = K mol 0.00418 kj q C cal m w T g C H rxn = H f (products) H f (reactants) Electronegativities H 2.2
1. This diagram illustrates a sound wave. The peak of the wave is its maximum on the y-axis. The trough of the wave is its minimum on the y-axis.
 M.7.NS.1 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. This diagram illustrates a sound wave. The peak of the wave is its maximum on the y-axis. The
M.7.NS.1 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. This diagram illustrates a sound wave. The peak of the wave is its maximum on the y-axis. The
Chemistry 250: Reactivity 1
 Chemistry 250: Reactivity 1 Spring 2013 Tentative Even Day Class Schedule Resources Workbook: Principles of Reactivity 1 Available in the CSB Bookstore Text: Choose one or more of the following options:
Chemistry 250: Reactivity 1 Spring 2013 Tentative Even Day Class Schedule Resources Workbook: Principles of Reactivity 1 Available in the CSB Bookstore Text: Choose one or more of the following options:
CALCULUS 2 FIVES SHEET F=Fun at home I=Incunabula V=Variety E=Endeavors S=Specimens Thursday, 12/3
 Thursday, 12/3 F #70 (yes, it is strange that this 5 sheet is #70) As shown in the figure above, water is draining from a conical tank with height 12 feet and diameter 8 feet into a cylindrical tank that
Thursday, 12/3 F #70 (yes, it is strange that this 5 sheet is #70) As shown in the figure above, water is draining from a conical tank with height 12 feet and diameter 8 feet into a cylindrical tank that
Chemistry Syllabus Fall Term 2017
 Chemistry 9 - Syllabus Fall Term 17 Date Lecture Number - General Subject Chapter W 8/30 F 9/1 1 - Introduction and orgo I review X - Review, friendly diagnostic exam M 9/4 2 - Orgo I review, exam highlights
Chemistry 9 - Syllabus Fall Term 17 Date Lecture Number - General Subject Chapter W 8/30 F 9/1 1 - Introduction and orgo I review X - Review, friendly diagnostic exam M 9/4 2 - Orgo I review, exam highlights
Structure and Properties of Polymers Prepared by Polymerization of 2,2-Dimethyl-1,3-Propandiol and - Caprolactone Monomer
 78 ITB J. Sci. Vol. 41 A, No. 2, 2009, 78-87 Structure and Properties of Polymers Prepared by Polymerization of 2,2-Dimethyl-1,3-Propandiol and - Caprolactone Monomer I Made Arcana*, M. Hasan, Shinta Dewi
78 ITB J. Sci. Vol. 41 A, No. 2, 2009, 78-87 Structure and Properties of Polymers Prepared by Polymerization of 2,2-Dimethyl-1,3-Propandiol and - Caprolactone Monomer I Made Arcana*, M. Hasan, Shinta Dewi
Topic, Assignments, Agenda, Board Configurations
 Lesson Plans (Advanced and Regular Physical Science) UNIT 23: The Periodic Table (6 days) Date Range: 4/13/15-4/17/15 (chapter 9) Topic, Assignments, Agenda, Board Configurations Standard/ Homeroom: Announcements
Lesson Plans (Advanced and Regular Physical Science) UNIT 23: The Periodic Table (6 days) Date Range: 4/13/15-4/17/15 (chapter 9) Topic, Assignments, Agenda, Board Configurations Standard/ Homeroom: Announcements
Chapter 22 Hydrocarbon Compounds
 Chapter 22 Hydrocarbon Compounds 1 ORGANIC COMPOUNDS Organic compounds are carbon compounds and there are over a million. The simplest organic compounds are hydrocarbons and they are composed of hydrogen
Chapter 22 Hydrocarbon Compounds 1 ORGANIC COMPOUNDS Organic compounds are carbon compounds and there are over a million. The simplest organic compounds are hydrocarbons and they are composed of hydrogen
Differential Equations MTH 205
 A Course Title & Number MTH 205 B Pre/Co requisite(s) MTH 104 C Number of credits 3 0 3 D Faculty Name Ayman Badawi E Term/ Year Fall 2014 F Sections CRN Days Time Location UTR 11:00 11:50 Office Hours:
A Course Title & Number MTH 205 B Pre/Co requisite(s) MTH 104 C Number of credits 3 0 3 D Faculty Name Ayman Badawi E Term/ Year Fall 2014 F Sections CRN Days Time Location UTR 11:00 11:50 Office Hours:
CHEMISTRY 850 Exam I Saturday, October 20, 2012 NAME (Print)
 CEMISTRY 850 Exam I Saturday, ctober 20, 2012 AME (Print) Question Max Points Score 1 12 2 8 3 12 4 10 5 14 6 10 7 12 8 24 9 16 10 8 11 8 12 14 13 16 14 36 BUS 8 Exam Total 1 1) Draw the detailed arrow
CEMISTRY 850 Exam I Saturday, ctober 20, 2012 AME (Print) Question Max Points Score 1 12 2 8 3 12 4 10 5 14 6 10 7 12 8 24 9 16 10 8 11 8 12 14 13 16 14 36 BUS 8 Exam Total 1 1) Draw the detailed arrow
Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
 Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Chemistry (A-level)
 1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Electrophoresis (Chapter 27): Chemistry (A-level) Electrophoresis: the separation of charged particles by their different rates of movement in
1 P a g e h t t p s : / / w w w. c i e n o t e s. c o m / Electrophoresis (Chapter 27): Chemistry (A-level) Electrophoresis: the separation of charged particles by their different rates of movement in
AS 203 Principles of Astronomy 2 Introduction to Stellar and Galactic Astronomy Syllabus Spring 2012
 AS 203 Principles of Astronomy 2 Introduction to Stellar and Galactic Astronomy Syllabus Spring 2012 Instructor Prof. Elizabeth Blanton Room: CAS 519 Email: eblanton@bu.edu Phone: 617-353-2633 Office hours:
AS 203 Principles of Astronomy 2 Introduction to Stellar and Galactic Astronomy Syllabus Spring 2012 Instructor Prof. Elizabeth Blanton Room: CAS 519 Email: eblanton@bu.edu Phone: 617-353-2633 Office hours:
School of Mathematics and Science MATH 163 College Algebra Section: EFA
 CCBC Essex School of Mathematics and Science MATH 163 College Algebra Section: EFA CLASSROOM LOCATION: L311 SEMESTER: Spring 2012 CLASS MEETING DAYS: MWF CLASS MEETING TIMES:12:20-1:15 INSTRUCTOR: D. TRUSZKOWSKI
CCBC Essex School of Mathematics and Science MATH 163 College Algebra Section: EFA CLASSROOM LOCATION: L311 SEMESTER: Spring 2012 CLASS MEETING DAYS: MWF CLASS MEETING TIMES:12:20-1:15 INSTRUCTOR: D. TRUSZKOWSKI
1.2 Inductive Reasoning
 1.2 Inductive Reasoning Goal Use inductive reasoning to make conjectures. Key Words conjecture inductive reasoning counterexample Scientists and mathematicians look for patterns and try to draw conclusions
1.2 Inductive Reasoning Goal Use inductive reasoning to make conjectures. Key Words conjecture inductive reasoning counterexample Scientists and mathematicians look for patterns and try to draw conclusions
Red Sox - Yankees. Baseball can not get more exciting than these games. Physics 121, April 17, Kinetic theory of gases.
 Red Sox - Yankees. Baseball can not get more exciting than these games. Physics 121, April 17, 2008. Kinetic theory of gases. http://eml.ou.edu/physics/module/thermal/ketcher/idg4.avi Physics 121. April
Red Sox - Yankees. Baseball can not get more exciting than these games. Physics 121, April 17, 2008. Kinetic theory of gases. http://eml.ou.edu/physics/module/thermal/ketcher/idg4.avi Physics 121. April
A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6
 GEORGIA INSTITUTE OF TECHNOLOGY School of Chemistry and Biochemistry Spring 2004 A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6 Instructor: Marcus Weck Office: Boggs 3-85 Phone:
GEORGIA INSTITUTE OF TECHNOLOGY School of Chemistry and Biochemistry Spring 2004 A SURVEY OF ORGANIC CHEMISTRY CHEMISTRY 1315 TuTr 9:35-10:55 am, Boggs B6 Instructor: Marcus Weck Office: Boggs 3-85 Phone:
Lecture 27 More Polymers
 Lecture 27 More Polymers Step Chain April 25, 2018 Where: MEZ 1.306!! Final Exam When: Friday, May 11 th, 2:00 5:00 PM Do: Study lecture notes, homework, reading Practice: Hydrolysis, signatures and synthesis.
Lecture 27 More Polymers Step Chain April 25, 2018 Where: MEZ 1.306!! Final Exam When: Friday, May 11 th, 2:00 5:00 PM Do: Study lecture notes, homework, reading Practice: Hydrolysis, signatures and synthesis.
H 5. CH(OH)COOH, is used in some skin creams and can be converted into a condensation polymer., is used to make some artificial fingernails.
 1 Mandelic acid (2-phenyl-2-hydroxyethanoic acid), 6 5 (), is used in some skin creams and can be converted into a condensation polymer. The addition polymer of ethyl methacrylate (ethyl 2-methyl-2-propenoate),
1 Mandelic acid (2-phenyl-2-hydroxyethanoic acid), 6 5 (), is used in some skin creams and can be converted into a condensation polymer. The addition polymer of ethyl methacrylate (ethyl 2-methyl-2-propenoate),
Summer Program Schedule
 TIME MONDAY 23 DEC TUESDAY 24 DEC WEDNESDAY 25 DEC THURSDAY 26 DEC FRIDAY 27 DEC SATURDAY 28 DEC SUNDAY 29 DEC TIME * BREAKFAST THE RHYTHM DIVINE Geoff Wood Cathy Van Extel hosts our comprehensive coverage
TIME MONDAY 23 DEC TUESDAY 24 DEC WEDNESDAY 25 DEC THURSDAY 26 DEC FRIDAY 27 DEC SATURDAY 28 DEC SUNDAY 29 DEC TIME * BREAKFAST THE RHYTHM DIVINE Geoff Wood Cathy Van Extel hosts our comprehensive coverage
Lecture 22: Harmonic Waves. Physics 2210 Fall Semester 2014
 Lecture 22: Harmonic Waves Physics 2210 Fall Semester 2014 Announcements Unit 21 Simple and Physical Pendula (Nov 24th ) HW Due 11/25 th as usual No new material Wednesday November 26th. In-class discussion
Lecture 22: Harmonic Waves Physics 2210 Fall Semester 2014 Announcements Unit 21 Simple and Physical Pendula (Nov 24th ) HW Due 11/25 th as usual No new material Wednesday November 26th. In-class discussion
MATH 341, Section 001 FALL 2014 Introduction to the Language and Practice of Mathematics
 MATH 341, Section 001 FALL 2014 Introduction to the Language and Practice of Mathematics Class Meetings: MW 9:30-10:45 am in EMS E424A, September 3 to December 10 [Thanksgiving break November 26 30; final
MATH 341, Section 001 FALL 2014 Introduction to the Language and Practice of Mathematics Class Meetings: MW 9:30-10:45 am in EMS E424A, September 3 to December 10 [Thanksgiving break November 26 30; final
Name Date Class FUNCTIONAL GROUPS. SECTION 23.1 INTRODUCTION TO FUNCTIONAL GROUPS (pages )
 Name Date lass 23 FUNTINAL GRUPS SETIN 23.1 INTRDUTIN T FUNTINAL GRUPS (pages 725 729 This section defines a functional group and gives several examples. It also describes halocarbons and the substitution
Name Date lass 23 FUNTINAL GRUPS SETIN 23.1 INTRDUTIN T FUNTINAL GRUPS (pages 725 729 This section defines a functional group and gives several examples. It also describes halocarbons and the substitution
CHEM Fall Exam 2 Professor R. Hoenigman. Signature. Name (printed) Last four digits of your student ID number.
 Average = 68 I pledge to uphold the CU on Code: CEM 3311-200 Fall 2006 Exam 2 Profess R. oenigman igh = 98 Low = 18 Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation
Average = 68 I pledge to uphold the CU on Code: CEM 3311-200 Fall 2006 Exam 2 Profess R. oenigman igh = 98 Low = 18 Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation
Topics in General Chemistry Chemistry 103 Fall 2017
 Topics in General Chemistry Chemistry 103 Fall 2017 Instructor: Professor Oertel, N280 Science Center, 775-8989, catherine.oertel@oberlin.edu Class meeting: MWF 11-11:50 am, Science Center A255 Laboratory
Topics in General Chemistry Chemistry 103 Fall 2017 Instructor: Professor Oertel, N280 Science Center, 775-8989, catherine.oertel@oberlin.edu Class meeting: MWF 11-11:50 am, Science Center A255 Laboratory
Year 13 Chemistry (NCEA) Student Information
 Year 13 Chemistry (NCEA) Student Information Science Department Onslow College 2015 Introduction Year 13 Chemistry is a full year course, of five topics, that works towards gaining Level Three credits
Year 13 Chemistry (NCEA) Student Information Science Department Onslow College 2015 Introduction Year 13 Chemistry is a full year course, of five topics, that works towards gaining Level Three credits
AS The Astronomical Universe. Prof. Merav Opher - Fall 2013
 SYLLABUS AS 102 - The Astronomical Universe Prof. Merav Opher - Fall 2013 Course Catalog Summary: The birth and death of stars; red giants, white dwarfs, black holes; our galaxy, the Milky Way, and other
SYLLABUS AS 102 - The Astronomical Universe Prof. Merav Opher - Fall 2013 Course Catalog Summary: The birth and death of stars; red giants, white dwarfs, black holes; our galaxy, the Milky Way, and other
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Polymer chemistry Prof. Dibakar Dhara Department of Chemistry Indian Institute of Technology, Kharagpur. Lecture - 4 Step-growth Polymerization
 Polymer chemistry Prof. Dibakar Dhara Department of Chemistry Indian Institute of Technology, Kharagpur Lecture - 4 Step-growth Polymerization (Refer Slide Time: 00:27) In the last lecture, we were discussing
Polymer chemistry Prof. Dibakar Dhara Department of Chemistry Indian Institute of Technology, Kharagpur Lecture - 4 Step-growth Polymerization (Refer Slide Time: 00:27) In the last lecture, we were discussing
