BCM Protein crystallography - II Isomorphous Replacement Anomalous Scattering and Molecular Replacement Model Building and Refinement
|
|
|
- Dora Hunter
- 5 years ago
- Views:
Transcription
1 BCM Protein crystallography - II Isomorphous Replacement Anomalous Scattering and Molecular Replacement Model Building and Refinement
2 Changing practice in de novo structure determination Hendrickson W. Quarterly Reviews of Biophysics 47, 1 (2014), pp BCM
3 MAD Phasing Collection of anomalous scattering data at specific wavelengths where heavy atoms scatter strongly. Multiwavelength Anomalous Diffraction Advantage Perfect isomorphism Single crystal Requires suitable K- or L-edge absorption Element may be natural (e.g. Zn 2+, Fe 3+ ) Data treatment similar to SIR Fewer errors in phases (one crystal) but anomalous signals are small and require accurate intensity measurements High quality experimental ρ(x,y,z) maps Most synchrotrons set up to conduct MAD/SAD SAD can be done on home sources BCM
4 X-ray scattering X-ray scattering by atoms involves the following process:- 1. The electromagnetic field of the incident X-ray beam exerts a force on the electrons within the atoms. 2. To a first approximation the electrons can be regarded as free electrons which oscillate at the same frequency as the incident X-rays. 3. These electrons then hence emit X-rays of the same wavelength as the incident X-rays. 4. The phase of the scattered X-rays differs by 180 from that of the incident X-rays. This is called the free-electron approximation to X-ray scattering. BCM
5 Anomalous X-ray scattering The free electron approximation breaks down for "high" Z atoms. 1. The inner electrons are more tightly attached to the nucleus than the outer electrons, and at certain X-ray wavelengths these electrons may be ejected from the inner shell (say K) into the continuous energy region. This happens if the incident wavelength is close to what is termed the absorption edge. 2. The electron then re-emits an X-ray photon as it falls back into a lower energy shell (say L). 3. The emitted photon does not necessarily differ in phase by 180 from the incident photon. The existence of anomalous dispersion always implies that the total scattering radiation diminishes, because a fraction of the scattered radiation is absorbed to produce the electronic transition. BCM
6 Anomalous scattering Experimentally, there is a sharp discontinuity in the dependence of the absorption coefficient on energy (wavelength) at the energy corresponding to the energy required to eject an inner-shell electron. The discontinuity is known as an absorption edge. For Cu, the K-absorption edge is at 1.380Å. Compared to K α at Å Near absorption edge, electrons in an atom can no longer be considered as free electrons. The consequence is anomalous scattering. Heavy atoms (Hg, Pt, Se) are common anomalous scatterers because their absorption edges fall within usable X-ray energy ranges. K absorption edge The atomic absorption coefficient for Cu The energy of an photon is E=hc/λ λ BCM
7 When the incident photon has relatively low energy When the incident photon has high enough energy The photon is scattered, as it has insufficient energy to excite any of the available electronic transitions. The scattering effect of the atom may be adequately described by using the normal atomic scattering coefficient f 0 only. Some photons are scattered. Some photons are absorbed and re-emitted at lower energy (fluorescence). The scattered photon gains an imaginary component to its phase (f" scattering coefficient becomes non-zero); i.e. it is retarded compared to a normally scattered photon. BCM
8 Anomalous scattering factor When these electron transitions occur, the corresponding atomic scattering factor (f) behaves as a complex number, appearing with two correction terms, a real number (f ') and an imaginary one (f ''). In order to interpret how this correction factors modify the scattering factor, one has to take into account that the real component (f ') is 180 degrees out of phase with the normally scattered radiation, and that the imaginary component (f '') is 90 degrees out of phase with the normally scattered radiation. Free electron scattering Negative for heavy atoms! -Δf Bound electron scattering Therefore, the existence of anomalous dispersion always implies that the total scattering radiation diminishes because a fraction of the scattered radiation is absorbed to produce the electronic transition. BCM
9 Physical interpretation of the real and imaginary correction factors of f f = f o + Δf + iδf real component, Δf A small component of the scattered radiation is 180 out of phase with the normally scattered radiation given by f o. Always diminishes f o. Absorption of X-rays imaginary component, Δf A small component of the scattered radiation is 90 out of phase with the normally scattered radiation given by f o. This phase shift is always oriented counterclockwise relative to the phase of the free electron scatter f o in the Argand diagram. BCM
10 Anomalous scattering Anomalous scattering factor for different atom types Regular scattering factor decreases as resolution increases But anomalous component is rather independent of resolution because it is mainly caused by inner electrons. The anomalous component is a function of both Z and wavelength, and tables of Δf and f exist for each wavelength and Z. BCM
11 Elements with absorption edges accessible for anomalous scattering in ~ 5-22 kev range 5-22 kev energy range most home sources and synchrotron beamlines X-ray Absorption BCM
12 Measuring f to determine f X-ray Anomalous scattering factor corrections are influenced by the chemical environment of the absorbing atom. Changes in scattering factors are pronounced near the absorption edge. Detailed spectral features appear. Scattering corrections cannot be assumed to be the same as free-atom anomalous scattering factors. Such corrections are accurate only at energies distant from the absorption edge. Cryostat Fluorescence detector Fluorescence Incident beam Crystal The anomalous scattering f is experimentally accessible: proportional to the atomic absorption coefficient, which is proportional to the fluorescence, which is turn allows f to be calculated (from the Kramers-Kronig transformation). BCM
13 Tunable synchrotron radiation maximizes effect of anomalous scattering f f Fluorescence scan of Se-element in a selenomethionine protein sample. Experimental values scattering factors f' and f'' as a function of x-ray energy obtained from the fluorescence scan using the program CHOOCH BCM
14 X-ray Absorption Edges (theory vs reality) The exact edge position depends on the oxidation state of the atom as the inner shell electron can be more tightly bound when valence outer shell electrons are removed. Finally the X-ray wavelength bandpass needs to be considered because inherent effects can be masked if this is broader than the linewidths of the features naturally present. Theory isn't good enough - measure it yourself The actual absorption edge is shifted relative to the idealized edge for an isolated Cu atom. The largest part of this shift is due to the oxidation state of the Cu atom in the protein. The local chemical environment introduces "ripples" (EXAFS) into the scattering spectrum. The maximum achievable f" is actually larger than the edge jump from theory. (The effect isn't very large in this particular example, but sometimes it is substantial). The maximum achievable f', however, is smaller than the theoretical value. This is largely limited by the energy bandwidth of the x-ray source. BCM
15 Anomalous Scattering Thus, the total structure factor F PH contains: the normal scattering of all atoms F P +F H 0, the dispersive component of the anomalously scattering atoms ΔF H and the anomalous component of the anomalously scattering atoms ΔF H. Note: Symbols F H and F A will be used interchangeably to describe the anomalous scatterers with λ F A h = 0 F A h + λ F A h +i λ ΔF " A h, where the contribution of anomalous scatterers to λ F A h is broken down into their λ independent component, 0 F A h, and λ dependent components - λ F A h being dispersive - Δf (λ), and λ ΔF " A h being anomalous - Δf (λ). BCM
16 Anomalous Scattering breaks Friedel's Law F ph+ F ph- α hkl α -h-k-l Friedel s law does not hold under conditions of anomalous diffraction. The correction to the scattering factor, ΔF H, is always positive in imaginary plane. If all atoms scatter anomalously, the amplitude stays the same, but the phase is shifted. When there are mixed non-anomalous and anomalous scatterers (this shifts the amplitude and phase of F hkl vs F -h-k-l ) with accompanying breakdown. Equivalent representations BCM
17 Bijvoet Pairs are Related by Space Group Symmetry Bijvoet pairs are similar to Friedel mates, except they are equivalent by space group symmetry. For example, in the case of a 2-fold along b, F + hkl = F hkl = F -hk-l and F - hkl = F -h-k-l = F h-kl Bijvoet pair equivalence may break down with anomalous diffraction. F hkl F -hk-l and F -h-k-l F h-kl The Bijvoet differences is defined as: Δ F hkl = F + hkl - F - hkl The difference can be used to locate heavy atoms that cause anomalous scattering in a Bijvoet Difference Patterson map with coefficients (Δ F hkl 2 ). Like the anomalous difference Fourier, the Bijvoet differences can be used for phasing as long as the phase is adjusted by 90. BCM
18 SIRAS - Single Isomorphous Replacement with Anomalous Scattering Remember: F ph = F p + F H F p = - F H + F ph Harker Construction BCM
19 Centric and acentric reflections Space group symmetry sometimes constrains phases of certain reflections to have only a limited, finite number of phase possibilities. For example, in orthorhombic space groups, any reflection of the type (hk0), (h0l) and (0kl) may only have a phase of 0 or 180. The axial planes in reciprocal space are thus referred to as centric zones, and the reflections within these planes are referred to as centric reflections. Reflections with unrestricted phases are referred to as acentric reflections. The probability distributions associated with these two classes of reflections are different, and in any phasing program, statistics are often reported separately for the two classes of reflections. It is in general "easier" to determine the phase angle of centric reflections. BCM
20 MAD Phasing Data Sets to Collect X-ray diffraction data are usually collected at 3 or more wavelengths to provide observations to solve simultaneous equations: The edge or inflection (λ 1 ) f where the real component of the scattering correction is maximum. The peak or white line (λ 2 ) f where the imaginary component of the correction is maximum. The remote (λ 3 ), where f and f are small. This can be located on either side of the absorption edge. A 2 nd remote (λ 4 ) may be chosen at low energy. Wavelengths are chosen to maximize the differences in intensity between Bijvoet pairs (or Friedel mates). Fluorescence Scan Fit to Kramers-Kronig Relation λ 4 f f λ 1 λ 2 λ 3 BCM
21 The MAD Method BCM
22 Goal of MAD Phasing Experiment Locate the anomalously diffracting atoms F A in the unit cell, F A is redefined as 0 F A h + λ F A h Note: the sum of 0 F A h and λ F A h does not change the phase angle of F A This allows calculation of the corresponding phase φ A. The MAD Equations then estimate Δφ and F T (where Δφ is the difference in phase angle between anomalous and normal scattering atoms components. In the simplest case, the phase of F T is Δφ + φ A. The Fourier transform with amplitudes F T and Δφ + φ A phase will give an electron density map of all atoms in the structure. The wavelength dependent scattering for each atom type is: f = fo + f (λ) + i f (λ) BCM
23 Recapitulation Δφ = φ T - φ A Only F λ is measured F λ is the total amplitude of the unknown structure whose phase is needed for the electron density. It is a wavelength dependent. F T is the normal scattering from all atoms. F A is the scattering due to the partial structure of the anomalously diffracting atoms. Δφ is the phase angle difference between normal (φ T )and anomalously diffracting atoms (φ A ). If we can locate the anomalous scattering atoms in the unit cell, we know their phase angle, φ A. We can then generate an estimate for Δφ and F T. BCM
24 MAD Phasing Scattering Correction Karle (1980) and Hendrickson (1985) formulated algebraic expressions that separate the non-anomalous (λ independent) from the anomalous scattering (λ dependent) atoms of F hkl. The form of the corrected scattering factor is: f = f 0 + f (λ) + if (λ) The contribution of the non-anomalously scattering atoms and those of the anomalous scatterers can be depicted in separate groups Then the structure factor for a given F hkl at wavelength λ can be recast as follows: Δφ λ F (hkl) 2 = 0 F T (hkl) 2 + a(λ) 0 F A (hkl) 2 + b(λ) 0 F T (hkl) 0 F A (hkl) cos[ 0 φ T (hkl) 0 φ A (hkl) ] + c(λ) 0 F T (hkl) 0 F A (hkl) sin[ 0 φ T (hkl) 0 φ A (hkl) ] a(λ) = (f λ 2 + f λ 2 )/(f 02 ); b(λ) = 2(f λ / f 0 ); c(λ) = 2(f λ /f 0 ) Jerome Karle Wayne Hendrickson W.A. Hendrickson, J.L. Smith & S. Sheriff (1985). "Direct Phase Determination Based on Anomalous Scattering" Methods Enzymol. 115, BCM
25 Solving the MAD Equations λ F (h) 2 = F T 2 + a(λ) F A 2 + b(λ) F T F A cos[δφ] + c(λ) F T F A sin[δφ] Δφ = φ T - φ A Each measurements of λ F (h) at some wavelength gives us one instance of the equation above, and the separate instances may be treated as a system of simultaneous equations from which we want to obtain the quantities F A, F T, and Δφ. One equation for λ F (h) and one for λ F (-h). Therefore at least two wavelengths are needed to solve the equations. Rewriting F A h as λ F A h = 0 F A h + λ F A h +i λ F A " h, where as before the contribution of anomalous scatterers to λ F A h is broken down into their λ independent component, 0 F A h, and λ dependent components - λ F A h and λ ΔF A " h. Then defining F ano = λ F h λ F h, and using λ F A h as defined above, we have F ano 2 λ F " A h sin F ano is thus uniquely determined by the properties of anomalously scattering atoms BCM
26 Locating the Anomalous Scattering Sites In practice, we obtain estimates for F T, F A and Δφ from programs such as Phenix or CCP4 suite of programs. The goal is to find the protein phase, φ T. Recall that φ T = Δφ + φ A, so if one knew φ A the problem would be solved. Like MIR methods, the anomalous scatter location is found by solving a difference Patterson map (n atom structure, where n = number of anomalous scatterers). Calculate an anomalous difference Patterson with coefficients F2 ano = [ λ F h λ F h ] 2 using Bijvoet or Friedel pairs Since [ λ F h λ F h ] 2 4 [ λ F A " h ] 2 sin 2, F ano will be maximal if the phase angle φ A is perpendicular to φ T and zero if both vectors are collinear, which is the opposite to the MIR case The anomalous difference Patterson will have peaks of anomalous scatterers with heights proportional to 2 [ λ F A " h ] 2 as <sin 2 > = ½ Then to maximize signal-to-noise, choose the peak wavelength. The anomalous scattering can be used to "generate" multiple derivatives when the incident wavelength λ is changed (MAD). BCM
27 Bijvoet Difference Patterson Map to Locate Anomalous Atoms Anomalous differences F2 ano can be used to locate the positions of the anomalously scattering atoms Harker section calculated using the anomalous signal from a single Cu atom in a 96 residue metalloprotein CBP [Guss, et al. 1989]; This map is rather noisy!! Why? At the maximum f, there are only 4.2 e - Instead, use estimate of F A from the MAD equations. u = 1/2 Harker Section F2 ano coefficients from λ peak λ = Å, f = 4.17e The Patterson map with coefficients F ano 2 requires only a home source of fixed wavelength, accurate intensity measurements, and good software to solve the Patterson map! BCM
28 Use of F A 2 Patterson Map Using the solution for F A greatly improves the signal-to-noise since it was derived from all data sets. Now you have φ A and the estimate for Δφ, it is possible to solve for φ T = Δφ + φ A. Δφ = φ T - φ A Cu metalloprotein CBP u = 1/2 Harker Section F A 2 coefficients derived from four wavelengths BCM
29 Signal-to-Noise of a MAD Experiment Theoretical calculations will indicate whether there is a sufficient signal. < F λmin F λmax > < F T > N 2 f λmin f λmax < F T > < F + F > < F T > N 2 f" λpeak < F T > N = # anomalous scatterers < F T > = scattering power of macromolecule λmin and λmax measured from experiment but estimated for feasibility < F PH F P > < F P > N 2 f 0 < F P > f 0 is the uncorrected scattering factor for the heavy atom The anomalous diffraction signal does not diminish as a function of resolution. However signal to noise, I/σ(I), of data decreases with increased S = 2 sinθ/λ Generally, the signal to detect must be greater then the noise level, i.e. I > σ(i). This is indicated by the I/σ(I) value plotted versus resolution. BCM
30 Use of Direct Methods or Automation Often the use of Se-Met methods causes the introduction of multiple anomalously diffracting sites. In general, there will be N 2 total vectors in the Patterson, where N is the number of atoms There will be N self-vectors on the origin. This leaves (N 2 - N) non-self vectors to sort out on the Patterson. For Se atoms, there are still 90 to 380 vectors, which can make the Harker Sections complicated (also see cross-vectors). In general, one needs about 1 Se per amino acids for phasing. For these reasons, crystallographers often turn to automated methods to solve difference Patterson maps. These include Direct Methods (Shake-n-Bake, ShelXd). Also see, Patterson methods like SOLVE/RESOLVE (heavy atom search and superposition HASSP). BCM
31 Finding the Heavy Atom Substructure Thus finding the heavy atom substructure in MAD uses exactly the same methods as in MIR, except we use primarily the anomalous (f'') signal rather than the isomorphous (F PH -F P ) signal between two datasets. The simplest and tedious way is to calculate an anomalous difference Patterson, find the peaks on the Harker sections and calculate the relationship between the Harker peaks and the real space locations of the heavy atoms (Se, in this case). This is in fact how most of the MIR structures were done before the days of more reliable programs that automate this task. More recently, the programs SOLVE and SHELX have supplied us with powerful tools that can quite predictably locate heavy atoms from anomalous or isomorphous data. Direct Methods program is routinely used to solve small molecule crystal structures (at very high resolutions) and has been adapted so that it can solve heavy atom substructures at much more modest resolutions (the number of reflections per atom are not dissimilar in these two situations). Direct Methods is based on a simple set of probabilistic relationships where: φ -H +φ -K +φ H-K ~ 0 for a set of three strong reflections. BCM
32 Increasing S/n with Redundancy 3.2Å Bijvoet difference Patterson maps calculated at 90 intervals of crystal rotation for ALR (augmenter of liver regeneration) crystals and the corresponding 3.2Å Fcalc Patterson map calculated from the cadmium positions. All maps are contoured starting at 1σ (map) with a 1σ step between contours. The marked improvement in the Patterson map is due to the increase in Bijvoet redundancy from 0.8 (90 sweep) to 9.0 (720 sweep). Patterson coefficients based on cadmium (Δf" = 4.7) anomalous signal for intensity data measured with a Cu rotating anode BCM
33 Se-Met: selenomethionine Se-Met MAD Phasing : Most popular method currently in use for proteins. Methionine is a relatively rare amino acid: 2.4% (vs. average of 5%) For Se-Met phasing, require 1 Met per ~100 amino acids. This really depends on the quality of data (signal-to-noise). Se can be introduced by growth of Met auxotrophic bacteria in the presence of Se-Met Conditions that inhibit Met synthesis can be used, and the media supplemented with Se-Met (nonauxotrophic strains). Other added ions or endogenous metals can be used too. Use of the method is relatively restricted to E. coli. Grayhack has developed a strain of Se-Met tolerant yeast. Air oxidation of protein must be vigorously avoided (DTT) Se=O can abolish the MAD signal. Methionine Selenomethionine Ref: Van Duyne et al. & Clardy (1993) J. Mol. Biol. 229, ; Doublie (1997) Methods in Enzymol. 276, BCM
34 Se-Met MAD Phasing Substitution in E. coli can reach 100%. The replacement of Met with Se-Met has advantages in that the side chain often packs in a hydrophobic environment and is well-ordered. However, the N-terminal (AUG) start signal is often disordered. The natural frequency of Met in a protein ~ 1 in 75 amino acids. This represents about 2.5% of F P signal, which is detectable with a good crystal. It has also been possible to introduce Met sites for MAD phasing by site- directed mutagenesis. Se-Met is often isomorphous with Met and has good anomalous signal (as much as 18 e - ) Se-Met can help determine the sequence, or to find NCS. Use of 5-Br-U is better for DNA than RNA. Recent studies showed that 5-Br-U RNA can change the global fold Anomalous data are collected from 1 crystal at Se K-edge ( kev). MAD data are collected at Edge, Inflection, and remote wavelengths BCM
35 Sulfur S-SAS: experimental realities The ultimate goal of the SAS method is the use of S-SAS to phase protein data since most proteins contain sulfur as cysteines and methionines. However sulfur has a very weak anomalous scattering signal with f = 0.56 e - for Cu X-rays. The use of soft X-rays such as Cr K ( = Å) doubles the sulfur signal ( f = 1.14 e - ). S-SAS method requires careful data collection and large data redundancies in order to maximize S/n and crystals that diffract to high resolution. A high symmetry space group (more symmetry equivalents) increases the chance of success. There are increasing number of S-SAS structures in the Protein Data Bank. BCM
36 Pushing the limits of sulfur SAD phasing Single-wavelength anomalous dispersion of S atoms (S-SAD) is an elegant phasing method to determine crystal structures that does not require heavy-atom incorporation or selenomethionine derivatization. Nevertheless, this technique has been limited by the paucity of the signal at the usual X-ray wavelengths, requiring very accurate measurement of the anomalous differences. [ F(+) - F(-) ] /σ Successful structure solution of the N-terminal domain of the ectodomain of HCV E1 from crystals that diffracted very weakly. By combining data from 32 crystals, it was possible to solve the sulfur substructure and calculate initial maps at 7 Å resolution, and after density modification and phase extension using a higher resolution native data set to 3.5 Å resolution, model building was achievable. d /sig(d ) as a function of resolution. The graph shows the signal to noise from the anomalous differences. In the red part of the graph the anomalous signal is considered to be nonexistent. El Omari K, Iourin O, Kadlec J, Fearn R, Hall DR, Harlos K, Grimes JM, Stuart DI. Pushing the limits of sulfur SAD phasing: de novo structure solution of the N-terminal domain of the ectodomain of HCV E1. Acta Crystallogr D Biol Crystallogr Aug;70(Pt 8): BCM
37 Improvement of SAS electron-density maps The blue meshes show the electron density contoured at 1σ. (a) Electron-density maps at 7 Å resolution after density modification by phenix.autosol using a solvent content of 75%. (b) Electron-density maps at 3.5 Å resolution after density modification by phenix.autobuild using sixfold NCS. (c) Final 2 F o F c electron-density maps at 3.5 Å resolution after refinement with autobuster. (d) Structure of HCV ne1 fitted into the electrondensity maps described in (c). The six monomers composing the asymmetric unit are colored differently. BCM
38 Resolve the SIR or SAS phase ambiguity (Handedness) by Solvent Flattening The ISAS process is carried twice, once with heavy atom site(s) at refined locations (+++), and once in their inverted locations (---). Data FOM 1 Handedness FOM 2 R-Factor Corr. coeff RHE 0.54 Correct Incorrect NP With I Correct Incorrect NP With I & S Correct Incorrect : Figure of merit before solvent flattening 2 : Figure of merit after one filter and four cycles of solvent flattening 3 : Four Iodine were used for phasing 4 : Four Iodine and 56 Sulfur atoms were used for phasing Heavy Atom Handedness and Protein Structure Determination using Single-wavelength Anomalous Scattering Data, ACA Annual Meeting, Montreal, July 25, BCM
39 Calculation of phases R-factors The isomorphous and anomalous lack of closures (ε iso and ε ano ) are minimized for each derivative using least-squares or maximum likelihood methods During the refinement, the contribution that each data set is making to the phasing may be judged by inspecting the values of several R-factors which are a measure of the average Bijvoet or dispersive anomalous differences to their respective lack of closures. For isomorphous (dispersive) differences in acentric reflections, a Cullis type R-factor may be quoted where R acentric C iso = ε iso and for centric reflections we may use Δ iso R centric C iso = F ph ± F p F calc F ph ± F p For anomalous differences, we equivalently define R C ano = All Cullis R-factor values should be less than unity. ε ano Δ Bijvoet BCM
40 Signal Strength and Data Quality Typical values SIR: R = 100 Σ hkl F PH - F P / Σ hkl F P 15-30% SAD: R anom = 200 Σ hkl I + - I - / Σ hkl I + + I - 5% S-SAD: R anom =.. 1% Requires high quality photon counters (Pilatus) BCM
41 Signal Strength and Data Quality R merge = Σ hkl Σ i I i,hkl - <I hkl > / Σ hkl Σ i I i,hkl R r.i.m. = Σ hkl (N/(N-1)) 1/2 Σ i I i,hkl - <I hkl > / Σ hkl Σ i I i,hkl = R meas R p.i.m. = Σ hkl (1/(N-1)) 1/2 Σ i I i,hkl - <I hkl > / Σ hkl Σ i I i,hkl R anom = Σ hkl I hkl - I -h-k-l / (1/2 Σ hkl I hkl + I -h-k-l ) BCM
42 Molecular Replacement BCM
43 Molecular Replacement Molecular replacement has proven effective for solving macromolecular crystal structures based upon the knowledge of homologous structures. The method is straightforward and reduces the time and effort required for structure determination because there is no need to prepare heavy atom derivatives and collect their data. However S-SAS phasing is obviating the need to prepare derivatives and can be used, if only, at a last resort to solve the crystal structure Model building is also simplified, since little or no chain tracing is required. BCM
44 Molecular Replacement: Practical Considerations The 3-dimensional structure of the search model must be very close (< 2Å rmsd) to that of the unknown structure for the technique to work efficiently. Sequence homology between the model and unknown protein is helpful but not strictly required. Success has been observed using search models having as low as 17% sequence similarity. Several computer programs such as AmoRe, X-PLOR/CNS, PHASER are available for MR calculations. BCM
45 How Molecular Replacment works Use a model of the protein to estimate phases Must be a structural homologue (RMSD < 2Å) Two-step process: rotation and translation Find orientation of model (red black) Find location of oriented model (black blue) px.cryst.bbk.ac.uk/03/sample/molrep.htm BCM
46 Using a protein model to estimate phases: the rotation function We need to determine the orientation of the model in the crystal unit cell We use a Patterson search approach in (,, ), which are Euler angles associated with the rotational space BCM
47 Euler angles for rotation function The coordinate system is rotated by: an angle α around the original z axis; then by an angle β around the new y axis; and then by an angle γ around the final z axis. xyz convention BCM
48 Using a protein model to estimate phases: translation function We need to determine the oriented model s location in crystal unit cell We do this with an R-factor search, where R = h F h obs k F h (calc) F h (obs) h BCM
49 Translation functions Oriented model is stepped through the crystal unit cell using small increments in x, y, and z (e.g. x x+ step) The point where R is lowest represents the correct location There exists an alternative method that uses maximum likelihood to find the translation peak; this notion is embodied in the software package PHASER by Randy Read BCM
50 Molecular Replacement Translation search Exp. Patterson Map Oriented Model Patterson TF(xyz) Rotation Function RF(αβγ) Model Patterson Map Exp. Patterson BCM
51 Acknowledgements John Rose, ACA Summer School 2006, Reorganized by Andy Howard, Spring 2008 Mike Lawrence, Walter & Eliza Hall Institute of Medical Research, Melbourne, Australia Joseph Wedekind, University of Rochester Medical Center, Rochester, NY Manfred S. Weiss, Helmholtz-Zentrum Berlin, Macromolecular Crystallography (HZB-MX), D Berlin, Germany Yizhi Jane Tao, Rice University, Houston, Texas BCM
52 BCM
SHELXC/D/E. Andrea Thorn
 SHELXC/D/E Andrea Thorn What is experimental phasing? Experimental phasing is what you do if MR doesn t work. What is experimental phasing? Experimental phasing methods depend on intensity differences.
SHELXC/D/E Andrea Thorn What is experimental phasing? Experimental phasing is what you do if MR doesn t work. What is experimental phasing? Experimental phasing methods depend on intensity differences.
Scattering by two Electrons
 Scattering by two Electrons p = -r k in k in p r e 2 q k in /λ θ θ k out /λ S q = r k out p + q = r (k out - k in ) e 1 Phase difference of wave 2 with respect to wave 1: 2π λ (k out - k in ) r= 2π S r
Scattering by two Electrons p = -r k in k in p r e 2 q k in /λ θ θ k out /λ S q = r k out p + q = r (k out - k in ) e 1 Phase difference of wave 2 with respect to wave 1: 2π λ (k out - k in ) r= 2π S r
Anomalous dispersion
 Selenomethionine MAD Selenomethionine is the amino acid methionine with the Sulfur replaced by a Selenium. Selenium is a heavy atom that also has the propery of "anomalous scatter" at some wavelengths,
Selenomethionine MAD Selenomethionine is the amino acid methionine with the Sulfur replaced by a Selenium. Selenium is a heavy atom that also has the propery of "anomalous scatter" at some wavelengths,
Experimental phasing, Pattersons and SHELX Andrea Thorn
 Experimental phasing, Pattersons and SHELX Andrea Thorn What is experimental phasing? Experimental phasing is what you do if MR doesn t work. What is experimental phasing? Experimental phasing methods
Experimental phasing, Pattersons and SHELX Andrea Thorn What is experimental phasing? Experimental phasing is what you do if MR doesn t work. What is experimental phasing? Experimental phasing methods
What is the Phase Problem? Overview of the Phase Problem. Phases. 201 Phases. Diffraction vector for a Bragg spot. In General for Any Atom (x, y, z)
 Protein Overview of the Phase Problem Crystal Data Phases Structure John Rose ACA Summer School 2006 Reorganized by Andy Howard,, Spring 2008 Remember We can measure reflection intensities We can calculate
Protein Overview of the Phase Problem Crystal Data Phases Structure John Rose ACA Summer School 2006 Reorganized by Andy Howard,, Spring 2008 Remember We can measure reflection intensities We can calculate
Biology III: Crystallographic phases
 Haupt/Masterstudiengang Physik Methoden moderner Röntgenphysik II: Streuung und Abbildung SS 2013 Biology III: Crystallographic phases Thomas R. Schneider, EMBL Hamburg 25/6/2013 thomas.schneider@embl-hamburg.de
Haupt/Masterstudiengang Physik Methoden moderner Röntgenphysik II: Streuung und Abbildung SS 2013 Biology III: Crystallographic phases Thomas R. Schneider, EMBL Hamburg 25/6/2013 thomas.schneider@embl-hamburg.de
CCP4 Diamond 2014 SHELXC/D/E. Andrea Thorn
 CCP4 Diamond 2014 SHELXC/D/E Andrea Thorn SHELXC/D/E workflow SHELXC: α calculation, file preparation SHELXD: Marker atom search = substructure search SHELXE: density modification Maps and coordinate files
CCP4 Diamond 2014 SHELXC/D/E Andrea Thorn SHELXC/D/E workflow SHELXC: α calculation, file preparation SHELXD: Marker atom search = substructure search SHELXE: density modification Maps and coordinate files
Protein crystallography. Garry Taylor
 Protein crystallography Garry Taylor X-ray Crystallography - the Basics Grow crystals Collect X-ray data Determine phases Calculate ρ-map Interpret map Refine coordinates Do the biology. Nitrogen at -180
Protein crystallography Garry Taylor X-ray Crystallography - the Basics Grow crystals Collect X-ray data Determine phases Calculate ρ-map Interpret map Refine coordinates Do the biology. Nitrogen at -180
Protein Crystallography
 Protein Crystallography Part II Tim Grüne Dept. of Structural Chemistry Prof. G. Sheldrick University of Göttingen http://shelx.uni-ac.gwdg.de tg@shelx.uni-ac.gwdg.de Overview The Reciprocal Lattice The
Protein Crystallography Part II Tim Grüne Dept. of Structural Chemistry Prof. G. Sheldrick University of Göttingen http://shelx.uni-ac.gwdg.de tg@shelx.uni-ac.gwdg.de Overview The Reciprocal Lattice The
X-ray Crystallography. Kalyan Das
 X-ray Crystallography Kalyan Das Electromagnetic Spectrum NMR 10 um - 10 mm 700 to 10 4 nm 400 to 700 nm 10 to 400 nm 10-1 to 10 nm 10-4 to 10-1 nm X-ray radiation was discovered by Roentgen in 1895. X-rays
X-ray Crystallography Kalyan Das Electromagnetic Spectrum NMR 10 um - 10 mm 700 to 10 4 nm 400 to 700 nm 10 to 400 nm 10-1 to 10 nm 10-4 to 10-1 nm X-ray radiation was discovered by Roentgen in 1895. X-rays
Determination of the Substructure
 Monday, June 15 th, 2009 Determination of the Substructure EMBO / MAX-INF2 Practical Course http://shelx.uni-ac.gwdg.de Overview Substructure Definition and Motivation Extracting Substructure Data from
Monday, June 15 th, 2009 Determination of the Substructure EMBO / MAX-INF2 Practical Course http://shelx.uni-ac.gwdg.de Overview Substructure Definition and Motivation Extracting Substructure Data from
Crystal lattice Real Space. Reflections Reciprocal Space. I. Solving Phases II. Model Building for CHEM 645. Purified Protein. Build model.
 I. Solving Phases II. Model Building for CHEM 645 Purified Protein Solve Phase Build model and refine Crystal lattice Real Space Reflections Reciprocal Space ρ (x, y, z) pronounced rho F hkl 2 I F (h,
I. Solving Phases II. Model Building for CHEM 645 Purified Protein Solve Phase Build model and refine Crystal lattice Real Space Reflections Reciprocal Space ρ (x, y, z) pronounced rho F hkl 2 I F (h,
Direct Method. Very few protein diffraction data meet the 2nd condition
 Direct Method Two conditions: -atoms in the structure are equal-weighted -resolution of data are higher than the distance between the atoms in the structure Very few protein diffraction data meet the 2nd
Direct Method Two conditions: -atoms in the structure are equal-weighted -resolution of data are higher than the distance between the atoms in the structure Very few protein diffraction data meet the 2nd
Patterson Methods
 59-553 Patterson Methods 113 In 1935, Patterson showed that the unknown phase information in the equation for electron density: ρ(xyz) = 1/V h k l F(hkl) exp[iα(hkl)] exp[-2πi(h x + k y + l z)] can be
59-553 Patterson Methods 113 In 1935, Patterson showed that the unknown phase information in the equation for electron density: ρ(xyz) = 1/V h k l F(hkl) exp[iα(hkl)] exp[-2πi(h x + k y + l z)] can be
Crystals, X-rays and Proteins
 Crystals, X-rays and Proteins Comprehensive Protein Crystallography Dennis Sherwood MA (Hons), MPhil, PhD Jon Cooper BA (Hons), PhD OXFORD UNIVERSITY PRESS Contents List of symbols xiv PART I FUNDAMENTALS
Crystals, X-rays and Proteins Comprehensive Protein Crystallography Dennis Sherwood MA (Hons), MPhil, PhD Jon Cooper BA (Hons), PhD OXFORD UNIVERSITY PRESS Contents List of symbols xiv PART I FUNDAMENTALS
Likelihood and SAD phasing in Phaser. R J Read, Department of Haematology Cambridge Institute for Medical Research
 Likelihood and SAD phasing in Phaser R J Read, Department of Haematology Cambridge Institute for Medical Research Concept of likelihood Likelihood with dice 4 6 8 10 Roll a seven. Which die?? p(4)=p(6)=0
Likelihood and SAD phasing in Phaser R J Read, Department of Haematology Cambridge Institute for Medical Research Concept of likelihood Likelihood with dice 4 6 8 10 Roll a seven. Which die?? p(4)=p(6)=0
Direct Methods and Many Site Se-Met MAD Problems using BnP. W. Furey
 Direct Methods and Many Site Se-Met MAD Problems using BnP W. Furey Classical Direct Methods Main method for small molecule structure determination Highly automated (almost totally black box ) Solves structures
Direct Methods and Many Site Se-Met MAD Problems using BnP W. Furey Classical Direct Methods Main method for small molecule structure determination Highly automated (almost totally black box ) Solves structures
Overview - Macromolecular Crystallography
 Overview - Macromolecular Crystallography 1. Overexpression and crystallization 2. Crystal characterization and data collection 3. The diffraction experiment 4. Phase problem 1. MIR (Multiple Isomorphous
Overview - Macromolecular Crystallography 1. Overexpression and crystallization 2. Crystal characterization and data collection 3. The diffraction experiment 4. Phase problem 1. MIR (Multiple Isomorphous
Experimental phasing in Crank2
 Experimental phasing in Crank2 Pavol Skubak and Navraj Pannu Biophysical Structural Chemistry, Leiden University, The Netherlands http://www.bfsc.leidenuniv.nl/software/crank/ X-ray structure solution
Experimental phasing in Crank2 Pavol Skubak and Navraj Pannu Biophysical Structural Chemistry, Leiden University, The Netherlands http://www.bfsc.leidenuniv.nl/software/crank/ X-ray structure solution
Phase problem: Determining an initial phase angle α hkl for each recorded reflection. 1 ρ(x,y,z) = F hkl cos 2π (hx+ky+ lz - α hkl ) V h k l
 Phase problem: Determining an initial phase angle α hkl for each recorded reflection 1 ρ(x,y,z) = F hkl cos 2π (hx+ky+ lz - α hkl ) V h k l Methods: Heavy atom methods (isomorphous replacement Hg, Pt)
Phase problem: Determining an initial phase angle α hkl for each recorded reflection 1 ρ(x,y,z) = F hkl cos 2π (hx+ky+ lz - α hkl ) V h k l Methods: Heavy atom methods (isomorphous replacement Hg, Pt)
Macromolecular Phasing with shelxc/d/e
 Sunday, June 13 th, 2010 CCP4 Workshop APS Chicago, June 2010 http://shelx.uni-ac.gwdg.de Overview Substructure Definition and Motivation Extracting Substructure Data from measured Data Substructure Solution
Sunday, June 13 th, 2010 CCP4 Workshop APS Chicago, June 2010 http://shelx.uni-ac.gwdg.de Overview Substructure Definition and Motivation Extracting Substructure Data from measured Data Substructure Solution
X-ray Crystallography
 2009/11/25 [ 1 ] X-ray Crystallography Andrew Torda, wintersemester 2009 / 2010 X-ray numerically most important more than 4/5 structures Goal a set of x, y, z coordinates different properties to NMR History
2009/11/25 [ 1 ] X-ray Crystallography Andrew Torda, wintersemester 2009 / 2010 X-ray numerically most important more than 4/5 structures Goal a set of x, y, z coordinates different properties to NMR History
Structure solution from weak anomalous data
 Structure solution from weak anomalous data Phenix Workshop SBGrid-NE-CAT Computing School Harvard Medical School, Boston June 7, 2014 Gábor Bunkóczi, Airlie McCoy, Randy Read (Cambridge University) Nat
Structure solution from weak anomalous data Phenix Workshop SBGrid-NE-CAT Computing School Harvard Medical School, Boston June 7, 2014 Gábor Bunkóczi, Airlie McCoy, Randy Read (Cambridge University) Nat
PAN-modular Structure of Parasite Sarcocystis muris Microneme Protein SML-2 at 1.95 Å Resolution and the Complex with 1-Thio-β-D-Galactose
 Supplementary Material to the paper: PAN-modular Structure of Parasite Sarcocystis muris Microneme Protein SML-2 at 1.95 Å Resolution and the Complex with 1-Thio-β-D-Galactose Jürgen J. Müller, a Manfred
Supplementary Material to the paper: PAN-modular Structure of Parasite Sarcocystis muris Microneme Protein SML-2 at 1.95 Å Resolution and the Complex with 1-Thio-β-D-Galactose Jürgen J. Müller, a Manfred
PSD '17 -- Xray Lecture 5, 6. Patterson Space, Molecular Replacement and Heavy Atom Isomorphous Replacement
 PSD '17 -- Xray Lecture 5, 6 Patterson Space, Molecular Replacement and Heavy Atom Isomorphous Replacement The Phase Problem We can t measure the phases! X-ray detectors (film, photomultiplier tubes, CCDs,
PSD '17 -- Xray Lecture 5, 6 Patterson Space, Molecular Replacement and Heavy Atom Isomorphous Replacement The Phase Problem We can t measure the phases! X-ray detectors (film, photomultiplier tubes, CCDs,
research papers 1. Introduction Thomas C. Terwilliger a * and Joel Berendzen b
 Acta Crystallographica Section D Biological Crystallography ISSN 0907-4449 Discrimination of solvent from protein regions in native Fouriers as a means of evaluating heavy-atom solutions in the MIR and
Acta Crystallographica Section D Biological Crystallography ISSN 0907-4449 Discrimination of solvent from protein regions in native Fouriers as a means of evaluating heavy-atom solutions in the MIR and
Web-based Auto-Rickshaw for validation of the X-ray experiment at the synchrotron beamline
 Web-based Auto-Rickshaw for validation of the X-ray experiment at the synchrotron beamline Auto-Rickshaw http://www.embl-hamburg.de/auto-rickshaw A platform for automated crystal structure determination
Web-based Auto-Rickshaw for validation of the X-ray experiment at the synchrotron beamline Auto-Rickshaw http://www.embl-hamburg.de/auto-rickshaw A platform for automated crystal structure determination
Molecular Biology Course 2006 Protein Crystallography Part I
 Molecular Biology Course 2006 Protein Crystallography Part I Tim Grüne University of Göttingen Dept. of Structural Chemistry November 2006 http://shelx.uni-ac.gwdg.de tg@shelx.uni-ac.gwdg.de Overview Overview
Molecular Biology Course 2006 Protein Crystallography Part I Tim Grüne University of Göttingen Dept. of Structural Chemistry November 2006 http://shelx.uni-ac.gwdg.de tg@shelx.uni-ac.gwdg.de Overview Overview
SOLVE and RESOLVE: automated structure solution, density modification and model building
 Journal of Synchrotron Radiation ISSN 0909-0495 SOLVE and RESOLVE: automated structure solution, density modification and model building Thomas Terwilliger Copyright International Union of Crystallography
Journal of Synchrotron Radiation ISSN 0909-0495 SOLVE and RESOLVE: automated structure solution, density modification and model building Thomas Terwilliger Copyright International Union of Crystallography
Practical aspects of SAD/MAD. Judit É Debreczeni
 Practical aspects of SAD/MAD Judit É Debreczeni anomalous scattering Hg sinθ/λ CuKα 0 Å - 0.4 Å - 0.6 Å - Å.5Å 0.83Å f 80 53 4 f total (θ, λ) f(θ) + f (λ) + if (λ) f f -5 8-5 8-5 8 f total increasing with
Practical aspects of SAD/MAD Judit É Debreczeni anomalous scattering Hg sinθ/λ CuKα 0 Å - 0.4 Å - 0.6 Å - Å.5Å 0.83Å f 80 53 4 f total (θ, λ) f(θ) + f (λ) + if (λ) f f -5 8-5 8-5 8 f total increasing with
Phaser: Experimental phasing
 Phaser: Experimental phasing Using SAD data in Phaser R J Read, Department of Haematology Cambridge Institute for Medical Research Diffraction with anomalous scatterers SAD: single-wavelength anomalous
Phaser: Experimental phasing Using SAD data in Phaser R J Read, Department of Haematology Cambridge Institute for Medical Research Diffraction with anomalous scatterers SAD: single-wavelength anomalous
Practical applications of synchrotron radiation in the determination of bio-macromolecule three-dimensional structures. M. Nardini and M.
 Practical applications of synchrotron radiation in the determination of bio-macromolecule three-dimensional structures M. Nardini and M. Bolognesi Department of Biomolecular Sciences and Biotechnology,
Practical applications of synchrotron radiation in the determination of bio-macromolecule three-dimensional structures M. Nardini and M. Bolognesi Department of Biomolecular Sciences and Biotechnology,
Experimental Phasing with SHELX C/D/E
 WIR SCHAFFEN WISSEN HEUTE FÜR MORGEN Dr. Tim Grüne :: Paul Scherrer Institut :: tim.gruene@psi.ch Experimental Phasing with SHELX C/D/E CCP4 / APS School Chicago 2017 22 nd June 2017 1 - The Phase Problem
WIR SCHAFFEN WISSEN HEUTE FÜR MORGEN Dr. Tim Grüne :: Paul Scherrer Institut :: tim.gruene@psi.ch Experimental Phasing with SHELX C/D/E CCP4 / APS School Chicago 2017 22 nd June 2017 1 - The Phase Problem
Structure factors again
 Structure factors again Remember 1D, structure factor for order h F h = F h exp[iα h ] = I 01 ρ(x)exp[2πihx]dx Where x is fractional position along unit cell distance (repeating distance, origin arbitrary)
Structure factors again Remember 1D, structure factor for order h F h = F h exp[iα h ] = I 01 ρ(x)exp[2πihx]dx Where x is fractional position along unit cell distance (repeating distance, origin arbitrary)
Determining Protein Structure BIBC 100
 Determining Protein Structure BIBC 100 Determining Protein Structure X-Ray Diffraction Interactions of x-rays with electrons in molecules in a crystal NMR- Nuclear Magnetic Resonance Interactions of magnetic
Determining Protein Structure BIBC 100 Determining Protein Structure X-Ray Diffraction Interactions of x-rays with electrons in molecules in a crystal NMR- Nuclear Magnetic Resonance Interactions of magnetic
EXAFS. Extended X-ray Absorption Fine Structure
 AOFSRR Cheiron School 2010, SPring-8 EXAFS Oct. 14th, 2010 Extended X-ray Absorption Fine Structure Iwao Watanabe Ritsumeikan University EXAFS Theory Quantum Mechanics Models Approximations Experiment
AOFSRR Cheiron School 2010, SPring-8 EXAFS Oct. 14th, 2010 Extended X-ray Absorption Fine Structure Iwao Watanabe Ritsumeikan University EXAFS Theory Quantum Mechanics Models Approximations Experiment
Resolution: maximum limit of diffraction (asymmetric)
 Resolution: maximum limit of diffraction (asymmetric) crystal Y X-ray source 2θ X direct beam tan 2θ = Y X d = resolution 2d sinθ = λ detector 1 Unit Cell: two vectors in plane of image c* Observe: b*
Resolution: maximum limit of diffraction (asymmetric) crystal Y X-ray source 2θ X direct beam tan 2θ = Y X d = resolution 2d sinθ = λ detector 1 Unit Cell: two vectors in plane of image c* Observe: b*
Diffraction Geometry
 Diffraction Geometry Diffraction from a crystal - Laue equations Reciprocal lattice Ewald construction Data collection strategy Phil Evans LMB May 2013 MRC Laboratory of Molecular Biology Cambridge UK
Diffraction Geometry Diffraction from a crystal - Laue equations Reciprocal lattice Ewald construction Data collection strategy Phil Evans LMB May 2013 MRC Laboratory of Molecular Biology Cambridge UK
An Introduction to Diffraction and Scattering. School of Chemistry The University of Sydney
 An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
Macromolecular X-ray Crystallography
 Protein Structural Models for CHEM 641 Fall 07 Brian Bahnson Department of Chemistry & Biochemistry University of Delaware Macromolecular X-ray Crystallography Purified Protein X-ray Diffraction Data collection
Protein Structural Models for CHEM 641 Fall 07 Brian Bahnson Department of Chemistry & Biochemistry University of Delaware Macromolecular X-ray Crystallography Purified Protein X-ray Diffraction Data collection
X-ray Diffraction. Diffraction. X-ray Generation. X-ray Generation. X-ray Generation. X-ray Spectrum from Tube
 X-ray Diffraction Mineral identification Mode analysis Structure Studies X-ray Generation X-ray tube (sealed) Pure metal target (Cu) Electrons remover inner-shell electrons from target. Other electrons
X-ray Diffraction Mineral identification Mode analysis Structure Studies X-ray Generation X-ray tube (sealed) Pure metal target (Cu) Electrons remover inner-shell electrons from target. Other electrons
SUPPLEMENTARY INFORMATION
 Table of Contents Page Supplementary Table 1. Diffraction data collection statistics 2 Supplementary Table 2. Crystallographic refinement statistics 3 Supplementary Fig. 1. casic1mfc packing in the R3
Table of Contents Page Supplementary Table 1. Diffraction data collection statistics 2 Supplementary Table 2. Crystallographic refinement statistics 3 Supplementary Fig. 1. casic1mfc packing in the R3
Protein Crystallography. Mitchell Guss University of Sydney Australia
 Protein Crystallography Mitchell Guss University of Sydney Australia Outline of the talk Recap some basic crystallography and history Highlight the special requirements for protein (macromolecular) structure
Protein Crystallography Mitchell Guss University of Sydney Australia Outline of the talk Recap some basic crystallography and history Highlight the special requirements for protein (macromolecular) structure
ANODE: ANOmalous and heavy atom DEnsity
 Bruker Webinar ANODE: ANOmalous and heavy atom DEnsity Andrea Thorn 03 December, 2013 Michael Ruf Welcome Dr. Michael Ruf Product Manager, SC-XRD Bruker AXS Inc. Madison, WI, USA Andrea Thorn Crystallographic
Bruker Webinar ANODE: ANOmalous and heavy atom DEnsity Andrea Thorn 03 December, 2013 Michael Ruf Welcome Dr. Michael Ruf Product Manager, SC-XRD Bruker AXS Inc. Madison, WI, USA Andrea Thorn Crystallographic
X-Ray Damage to Biological Crystalline Samples
 X-Ray Damage to Biological Crystalline Samples Gerd Rosenbaum Structural Biology Center, ANL and Dept. of Biochemistry, UGA ACA Summer School IIT, 19 July 2007 A U.S. Department of Energy laboratory managed
X-Ray Damage to Biological Crystalline Samples Gerd Rosenbaum Structural Biology Center, ANL and Dept. of Biochemistry, UGA ACA Summer School IIT, 19 July 2007 A U.S. Department of Energy laboratory managed
ANODE: ANOmalous and heavy atom DEnsity
 Bruker User s Meeting 2012 ANODE: ANOmalous and heavy atom DEnsity Andrea Thorn September 26 th, 2012 Typical SHELXC/D/E workflow The anomalous or heavy atom signal is used to find the substructure of
Bruker User s Meeting 2012 ANODE: ANOmalous and heavy atom DEnsity Andrea Thorn September 26 th, 2012 Typical SHELXC/D/E workflow The anomalous or heavy atom signal is used to find the substructure of
Roger Johnson Structure and Dynamics: X-ray Diffraction Lecture 6
 6.1. Summary In this Lecture we cover the theory of x-ray diffraction, which gives direct information about the atomic structure of crystals. In these experiments, the wavelength of the incident beam must
6.1. Summary In this Lecture we cover the theory of x-ray diffraction, which gives direct information about the atomic structure of crystals. In these experiments, the wavelength of the incident beam must
Part II. Fundamentals of X-ray Absorption Fine Structure: data analysis
 Part II Fundamentals of X-ray Absorption Fine Structure: data analysis Sakura Pascarelli European Synchrotron Radiation Facility, Grenoble, France Page 1 S. Pascarelli HERCULES 2016 Data Analysis: EXAFS
Part II Fundamentals of X-ray Absorption Fine Structure: data analysis Sakura Pascarelli European Synchrotron Radiation Facility, Grenoble, France Page 1 S. Pascarelli HERCULES 2016 Data Analysis: EXAFS
ACORN - a flexible and efficient ab initio procedure to solve a protein structure when atomic resolution data is available
 ACORN - a flexible and efficient ab initio procedure to solve a protein structure when atomic resolution data is available Yao Jia-xing Department of Chemistry, University of York, Heslington, York, YO10
ACORN - a flexible and efficient ab initio procedure to solve a protein structure when atomic resolution data is available Yao Jia-xing Department of Chemistry, University of York, Heslington, York, YO10
Linking data and model quality in macromolecular crystallography. Kay Diederichs
 Linking data and model quality in macromolecular crystallography Kay Diederichs Crystallography is highly successful Can we do better? Error in experimental data Error = random + systematic Multiplicity
Linking data and model quality in macromolecular crystallography Kay Diederichs Crystallography is highly successful Can we do better? Error in experimental data Error = random + systematic Multiplicity
The Crystallographic Process
 Experimental Phasing Macromolecular Crystallography School Madrid, May 2017 Paul Adams Lawrence Berkeley Laboratory and Department of Bioengineering UC Berkeley The Crystallographic Process Crystallization
Experimental Phasing Macromolecular Crystallography School Madrid, May 2017 Paul Adams Lawrence Berkeley Laboratory and Department of Bioengineering UC Berkeley The Crystallographic Process Crystallization
Data Collection. Overview. Methods. Counter Methods. Crystal Quality with -Scans
 Data Collection Overview with a unit cell, possible space group and computer reference frame (orientation matrix); the location of diffracted x-rays can be calculated (h k l) and intercepted by something
Data Collection Overview with a unit cell, possible space group and computer reference frame (orientation matrix); the location of diffracted x-rays can be calculated (h k l) and intercepted by something
Supplementary Material for. Herapathite
 Supplementary Material for Herapathite Bart Kahr, John Freudenthal, Shane Phillips, Werner Kaminsky Department of Chemistry, Box 351700, University of Washington, Seattle WA 98195-1700 Crystal Structure
Supplementary Material for Herapathite Bart Kahr, John Freudenthal, Shane Phillips, Werner Kaminsky Department of Chemistry, Box 351700, University of Washington, Seattle WA 98195-1700 Crystal Structure
Molecular Replacement (Alexei Vagin s lecture)
 Molecular Replacement (Alexei Vagin s lecture) Contents What is Molecular Replacement Functions in Molecular Replacement Weighting scheme Information from data and model Some special techniques of Molecular
Molecular Replacement (Alexei Vagin s lecture) Contents What is Molecular Replacement Functions in Molecular Replacement Weighting scheme Information from data and model Some special techniques of Molecular
Fan, Hai-fu Institute of Physics, Chinese Academy of Sciences, Beijing , China
 Direct Methods in Crystallography Fan, Hai-fu Institute of Physics, Chinese Academy of Sciences, Beijing 100080, China An important branch of crystallography is the X-ray diffraction analysis of crystal
Direct Methods in Crystallography Fan, Hai-fu Institute of Physics, Chinese Academy of Sciences, Beijing 100080, China An important branch of crystallography is the X-ray diffraction analysis of crystal
Changing and challenging times for service crystallography. Electronic Supplementary Information
 Changing and challenging times for service crystallography Simon J Coles,* a and Philip A Gale* a Electronic Supplementary Information Instrument descriptions and experimental protocols The following firstly
Changing and challenging times for service crystallography Simon J Coles,* a and Philip A Gale* a Electronic Supplementary Information Instrument descriptions and experimental protocols The following firstly
BC530 Class notes on X-ray Crystallography
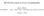 BC530 Class notes on X-ray Crystallography web material: Ethan A Merritt http://skuld.bmsc.washington.edu/~merritt/bc530/ October 11, 2016 Growing Crystals It should be self-evident that in order to do
BC530 Class notes on X-ray Crystallography web material: Ethan A Merritt http://skuld.bmsc.washington.edu/~merritt/bc530/ October 11, 2016 Growing Crystals It should be self-evident that in order to do
11/6/2013. Refinement. Fourier Methods. Fourier Methods. Difference Map. Difference Map Find H s. Difference Map No C 1
 Refinement Fourier Methods find heavy atom or some F s phases using direct methods locate new atoms, improve phases continue until all atoms found in more or less correct position starting point of refinement
Refinement Fourier Methods find heavy atom or some F s phases using direct methods locate new atoms, improve phases continue until all atoms found in more or less correct position starting point of refinement
Experimental Phasing of SFX Data. Thomas Barends MPI for Medical Research, Heidelberg. Conclusions
 Experimental Phasing of SFX Data Thomas Barends MP for Medical Researc Heidelberg Conclusions X-ray Free-Electron Lasers are pushing back the boundaries of possibility in biological crystallography: -Data
Experimental Phasing of SFX Data Thomas Barends MP for Medical Researc Heidelberg Conclusions X-ray Free-Electron Lasers are pushing back the boundaries of possibility in biological crystallography: -Data
Generalized Method of Determining Heavy-Atom Positions Using the Difference Patterson Function
 Acta Cryst. (1987). A43, 1- Generalized Method of Determining Heavy-Atom Positions Using the Difference Patterson Function B THOMAS C. TERWILLIGER* AND SUNG-Hou KIM Department of Chemistry, University
Acta Cryst. (1987). A43, 1- Generalized Method of Determining Heavy-Atom Positions Using the Difference Patterson Function B THOMAS C. TERWILLIGER* AND SUNG-Hou KIM Department of Chemistry, University
Protein Structure Determination. Part 1 -- X-ray Crystallography
 Protein Structure Determination Part 1 -- X-ray Crystallography Topics covering in this 1/2 course Crystal growth Diffraction theory Symmetry Solving phases using heavy atoms Solving phases using a model
Protein Structure Determination Part 1 -- X-ray Crystallography Topics covering in this 1/2 course Crystal growth Diffraction theory Symmetry Solving phases using heavy atoms Solving phases using a model
Two Lectures in X-ray Crystallography
 Biochemistry 503 Michael Wiener (mwiener@virginia.edu, 3-2731, Snyder 360) Two Lectures in X-ray Crystallography Outline 1. Justification & introductory remarks 2. Experimental setup 3. Protein crystals
Biochemistry 503 Michael Wiener (mwiener@virginia.edu, 3-2731, Snyder 360) Two Lectures in X-ray Crystallography Outline 1. Justification & introductory remarks 2. Experimental setup 3. Protein crystals
The SHELX approach to the experimental phasing of macromolecules. George M. Sheldrick, Göttingen University
 The SHELX approach to the experimental phasing of macromolecules IUCr 2011 Madrid George M. Sheldrick, Göttingen University http://shelx.uni-ac.gwdg.de/shelx/ Experimental phasing of macromolecules Except
The SHELX approach to the experimental phasing of macromolecules IUCr 2011 Madrid George M. Sheldrick, Göttingen University http://shelx.uni-ac.gwdg.de/shelx/ Experimental phasing of macromolecules Except
Charles Ballard (original GáborBunkóczi) CCP4 Workshop 7 December 2011
 Experimental phasing Charles Ballard (original GáborBunkóczi) CCP4 Workshop 7 December 2011 Anomalous diffraction F P protein F A anomalous substructure ano F A " -FA" A F F -* F A F P Phasing Substructure
Experimental phasing Charles Ballard (original GáborBunkóczi) CCP4 Workshop 7 December 2011 Anomalous diffraction F P protein F A anomalous substructure ano F A " -FA" A F F -* F A F P Phasing Substructure
Basic Crystallography Part 1. Theory and Practice of X-ray Crystal Structure Determination
 Basic Crystallography Part 1 Theory and Practice of X-ray Crystal Structure Determination We have a crystal How do we get there? we want a structure! The Unit Cell Concept Ralph Krätzner Unit Cell Description
Basic Crystallography Part 1 Theory and Practice of X-ray Crystal Structure Determination We have a crystal How do we get there? we want a structure! The Unit Cell Concept Ralph Krätzner Unit Cell Description
Molecular replacement. New structures from old
 Molecular replacement New structures from old The Phase Problem phase amplitude Phasing by molecular replacement Phases can be calculated from atomic model Rotate and translate related structure Models
Molecular replacement New structures from old The Phase Problem phase amplitude Phasing by molecular replacement Phases can be calculated from atomic model Rotate and translate related structure Models
PX-CBMSO Course (2) of Symmetry
 PX-CBMSO Course (2) The mathematical description of Symmetry y PX-CBMSO-June 2011 Cele Abad-Zapatero University of Illinois at Chicago Center for Pharmaceutical Biotechnology. Lecture no. 2 This material
PX-CBMSO Course (2) The mathematical description of Symmetry y PX-CBMSO-June 2011 Cele Abad-Zapatero University of Illinois at Chicago Center for Pharmaceutical Biotechnology. Lecture no. 2 This material
Scattering Lecture. February 24, 2014
 Scattering Lecture February 24, 2014 Structure Determination by Scattering Waves of radiation scattered by different objects interfere to give rise to an observable pattern! The wavelength needs to close
Scattering Lecture February 24, 2014 Structure Determination by Scattering Waves of radiation scattered by different objects interfere to give rise to an observable pattern! The wavelength needs to close
Table 1. Crystallographic data collection, phasing and refinement statistics. Native Hg soaked Mn soaked 1 Mn soaked 2
 Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
Table 1. Crystallographic data collection, phasing and refinement statistics Native Hg soaked Mn soaked 1 Mn soaked 2 Data collection Space group P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 P2 1 2 1 2 1 Cell
S-SAD and Fe-SAD Phasing using X8 PROTEUM
 S-SAD and Fe-SAD Phasing using X8 PROTEUM Kristina Djinovic Carugo Dept. for Structural and Computational Biology Max F. Perutz Labs Univ. Vienna, Austria Outline Fe-SAD on chlorite dismutase from Candidatus
S-SAD and Fe-SAD Phasing using X8 PROTEUM Kristina Djinovic Carugo Dept. for Structural and Computational Biology Max F. Perutz Labs Univ. Vienna, Austria Outline Fe-SAD on chlorite dismutase from Candidatus
Fast, Intuitive Structure Determination IV: Space Group Determination and Structure Solution
 Fast, Intuitive Structure Determination IV: Space Group Determination and Structure Solution November 25, 2013 Welcome I I Dr. Michael Ruf Product Manager Crystallography Bruker AXS Inc. Madison, WI, USA
Fast, Intuitive Structure Determination IV: Space Group Determination and Structure Solution November 25, 2013 Welcome I I Dr. Michael Ruf Product Manager Crystallography Bruker AXS Inc. Madison, WI, USA
X-ray Spectroscopy. Danny Bennett and Maeve Madigan. October 12, 2015
 X-ray Spectroscopy Danny Bennett and Maeve Madigan October 12, 2015 Abstract Various X-ray spectra were obtained, and their properties were investigated. The characteristic peaks were identified for a
X-ray Spectroscopy Danny Bennett and Maeve Madigan October 12, 2015 Abstract Various X-ray spectra were obtained, and their properties were investigated. The characteristic peaks were identified for a
Experimental phasing in Crank2
 Experimental phasing in Crank2 Pavol Skubak and Navraj Pannu Biophysical Structural Chemistry, Leiden University, The Netherlands http://www.bfsc.leidenuniv.nl/software/crank/ Crank2 for experimental phasing
Experimental phasing in Crank2 Pavol Skubak and Navraj Pannu Biophysical Structural Chemistry, Leiden University, The Netherlands http://www.bfsc.leidenuniv.nl/software/crank/ Crank2 for experimental phasing
SI Text S1 Solution Scattering Data Collection and Analysis. SI references
 SI Text S1 Solution Scattering Data Collection and Analysis. The X-ray photon energy was set to 8 kev. The PILATUS hybrid pixel array detector (RIGAKU) was positioned at a distance of 606 mm from the sample.
SI Text S1 Solution Scattering Data Collection and Analysis. The X-ray photon energy was set to 8 kev. The PILATUS hybrid pixel array detector (RIGAKU) was positioned at a distance of 606 mm from the sample.
Circular Dichroism & Optical Rotatory Dispersion. Proteins (KCsa) Polysaccharides (agarose) DNA CHEM 305. Many biomolecules are α-helical!
 Circular Dichroism & Optical Rotatory Dispersion Polysaccharides (agarose) DNA Proteins (KCsa) Many biomolecules are α-helical! How can we measure the amount and changes in amount of helical structure
Circular Dichroism & Optical Rotatory Dispersion Polysaccharides (agarose) DNA Proteins (KCsa) Many biomolecules are α-helical! How can we measure the amount and changes in amount of helical structure
Theory of X-ray diffraction
 Theory of X-ray diffraction A users perspective Disclaimer: I am not a physicist but there will be equations! Phil Evans Diamond December 2016 MRC Laboratory of Molecular Biology Cambridge UK Acknowledgements:
Theory of X-ray diffraction A users perspective Disclaimer: I am not a physicist but there will be equations! Phil Evans Diamond December 2016 MRC Laboratory of Molecular Biology Cambridge UK Acknowledgements:
The Phase Problem of X-ray Crystallography
 163 The Phase Problem of X-ray Crystallography H.A. Hauptman Hauptman-Woodward Medical Research Institute, Inc. 73 High Street Buffalo, NY, USA hauptman@hwi.buffalo.edu ABSTRACT. The intensities of a sufficient
163 The Phase Problem of X-ray Crystallography H.A. Hauptman Hauptman-Woodward Medical Research Institute, Inc. 73 High Street Buffalo, NY, USA hauptman@hwi.buffalo.edu ABSTRACT. The intensities of a sufficient
Twinning. Andrea Thorn
 Twinning Andrea Thorn OVERVIEW Introduction: Definitions, origins of twinning Merohedral twins: Recognition, statistical analysis: H plot, Yeates Padilla plot Example Refinement and R values Reticular
Twinning Andrea Thorn OVERVIEW Introduction: Definitions, origins of twinning Merohedral twins: Recognition, statistical analysis: H plot, Yeates Padilla plot Example Refinement and R values Reticular
Supplementary materials. Crystal structure of the carboxyltransferase domain. of acetyl coenzyme A carboxylase. Department of Biological Sciences
 Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Molecular Biology Course 2006 Protein Crystallography Part II
 Molecular Biology Course 2006 Protein Crystallography Part II Tim Grüne University of Göttingen Dept. of Structural Chemistry December 2006 http://shelx.uni-ac.gwdg.de tg@shelx.uni-ac.gwdg.de Overview
Molecular Biology Course 2006 Protein Crystallography Part II Tim Grüne University of Göttingen Dept. of Structural Chemistry December 2006 http://shelx.uni-ac.gwdg.de tg@shelx.uni-ac.gwdg.de Overview
Pseudo translation and Twinning
 Pseudo translation and Twinning Crystal peculiarities Pseudo translation Twin Order-disorder Pseudo Translation Pseudo translation b Real space a Reciprocal space Distance between spots: 1/a, 1/b Crystallographic
Pseudo translation and Twinning Crystal peculiarities Pseudo translation Twin Order-disorder Pseudo Translation Pseudo translation b Real space a Reciprocal space Distance between spots: 1/a, 1/b Crystallographic
Part 1: What is XAFS? What does it tell us? The EXAFS equation. Part 2: Basic steps in the analysis Quick overview of typical analysis
 Introduction to XAFS Part 1: What is XAFS? What does it tell us? The EXAFS equation Part 2: Basic steps in the analysis Quick overview of typical analysis Tomorrow Measurement methods and examples The
Introduction to XAFS Part 1: What is XAFS? What does it tell us? The EXAFS equation Part 2: Basic steps in the analysis Quick overview of typical analysis Tomorrow Measurement methods and examples The
CS273: Algorithms for Structure Handout # 13 and Motion in Biology Stanford University Tuesday, 11 May 2003
 CS273: Algorithms for Structure Handout # 13 and Motion in Biology Stanford University Tuesday, 11 May 2003 Lecture #13: 11 May 2004 Topics: Protein Structure Determination Scribe: Minli Zhu We acknowledge
CS273: Algorithms for Structure Handout # 13 and Motion in Biology Stanford University Tuesday, 11 May 2003 Lecture #13: 11 May 2004 Topics: Protein Structure Determination Scribe: Minli Zhu We acknowledge
Supplemental Data. Structure of the Rb C-Terminal Domain. Bound to E2F1-DP1: A Mechanism. for Phosphorylation-Induced E2F Release
 Supplemental Data Structure of the Rb C-Terminal Domain Bound to E2F1-DP1: A Mechanism for Phosphorylation-Induced E2F Release Seth M. Rubin, Anne-Laure Gall, Ning Zheng, and Nikola P. Pavletich Section
Supplemental Data Structure of the Rb C-Terminal Domain Bound to E2F1-DP1: A Mechanism for Phosphorylation-Induced E2F Release Seth M. Rubin, Anne-Laure Gall, Ning Zheng, and Nikola P. Pavletich Section
X-ray Data Collection. Bio5325 Spring 2006
 X-ray Data Collection Bio535 Spring 006 Obtaining I hkl and α (Ihkl) from Frame Images Braggs Law -predicts conditions for in-phase scattering by equivalent atoms lying in planes that transect a crystal.
X-ray Data Collection Bio535 Spring 006 Obtaining I hkl and α (Ihkl) from Frame Images Braggs Law -predicts conditions for in-phase scattering by equivalent atoms lying in planes that transect a crystal.
The Reciprocal Lattice
 59-553 The Reciprocal Lattice 61 Because of the reciprocal nature of d spacings and θ from Bragg s Law, the pattern of the diffraction we observe can be related to the crystal lattice by a mathematical
59-553 The Reciprocal Lattice 61 Because of the reciprocal nature of d spacings and θ from Bragg s Law, the pattern of the diffraction we observe can be related to the crystal lattice by a mathematical
Biological Small Angle X-ray Scattering (SAXS) Dec 2, 2013
 Biological Small Angle X-ray Scattering (SAXS) Dec 2, 2013 Structural Biology Shape Dynamic Light Scattering Electron Microscopy Small Angle X-ray Scattering Cryo-Electron Microscopy Wide Angle X- ray
Biological Small Angle X-ray Scattering (SAXS) Dec 2, 2013 Structural Biology Shape Dynamic Light Scattering Electron Microscopy Small Angle X-ray Scattering Cryo-Electron Microscopy Wide Angle X- ray
Protein Structure Determination 9/25/2007
 One-dimensional NMR spectra Ethanol Cellulase (36 a.a.) Branden & Tooze, Fig. 18.16 1D and 2D NMR spectra of inhibitor K (57 a.a.) K. Wuthrich, NMR of Proteins and Nucleic Acids. (Wiley, 1986.) p. 54-55.
One-dimensional NMR spectra Ethanol Cellulase (36 a.a.) Branden & Tooze, Fig. 18.16 1D and 2D NMR spectra of inhibitor K (57 a.a.) K. Wuthrich, NMR of Proteins and Nucleic Acids. (Wiley, 1986.) p. 54-55.
Electronic Supplementary Information (ESI) for Chem. Commun. Unveiling the three- dimensional structure of the green pigment of nitrite- cured meat
 Electronic Supplementary Information (ESI) for Chem. Commun. Unveiling the three- dimensional structure of the green pigment of nitrite- cured meat Jun Yi* and George B. Richter- Addo* Department of Chemistry
Electronic Supplementary Information (ESI) for Chem. Commun. Unveiling the three- dimensional structure of the green pigment of nitrite- cured meat Jun Yi* and George B. Richter- Addo* Department of Chemistry
An Introduction to XAFS
 An Introduction to XAFS Matthew Newville Center for Advanced Radiation Sources The University of Chicago 21-July-2018 Slides for this talk: https://tinyurl.com/larch2018 https://millenia.cars.aps.anl.gov/gsecars/data/larch/2018workshop
An Introduction to XAFS Matthew Newville Center for Advanced Radiation Sources The University of Chicago 21-July-2018 Slides for this talk: https://tinyurl.com/larch2018 https://millenia.cars.aps.anl.gov/gsecars/data/larch/2018workshop
Materials 286C/UCSB: Class VI Structure factors (continued), the phase problem, Patterson techniques and direct methods
 Materials 286C/UCSB: Class VI Structure factors (continued), the phase problem, Patterson techniques and direct methods Ram Seshadri (seshadri@mrl.ucsb.edu) Structure factors The structure factor for a
Materials 286C/UCSB: Class VI Structure factors (continued), the phase problem, Patterson techniques and direct methods Ram Seshadri (seshadri@mrl.ucsb.edu) Structure factors The structure factor for a
Handout 13 Interpreting your results. What to make of your atomic coordinates, bond distances and angles
 Handout 13 Interpreting your results What to make of your atomic coordinates, bond distances and angles 1 What to make of the outcome of your refinement There are several ways of judging whether the outcome
Handout 13 Interpreting your results What to make of your atomic coordinates, bond distances and angles 1 What to make of the outcome of your refinement There are several ways of judging whether the outcome
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/22715 holds various files of this Leiden University dissertation. Author: Waterreus, Willem-Jan Title: Software developments in automated structure solution
Cover Page The handle http://hdl.handle.net/1887/22715 holds various files of this Leiden University dissertation. Author: Waterreus, Willem-Jan Title: Software developments in automated structure solution
X-ray Fluorescence Imaging Following Synchrotron Beam Excitation
 Conference on Applied Digital Imaging Techniques for Understanding the Palimpsest X-ray Fluorescence Imaging Following Synchrotron Beam Excitation Uwe Bergmann Stanford Synchrotron Radiation Laboratory
Conference on Applied Digital Imaging Techniques for Understanding the Palimpsest X-ray Fluorescence Imaging Following Synchrotron Beam Excitation Uwe Bergmann Stanford Synchrotron Radiation Laboratory
research papers Reduction of density-modification bias by b correction 1. Introduction Pavol Skubák* and Navraj S. Pannu
 Acta Crystallographica Section D Biological Crystallography ISSN 0907-4449 Reduction of density-modification bias by b correction Pavol Skubák* and Navraj S. Pannu Biophysical Structural Chemistry, Leiden
Acta Crystallographica Section D Biological Crystallography ISSN 0907-4449 Reduction of density-modification bias by b correction Pavol Skubák* and Navraj S. Pannu Biophysical Structural Chemistry, Leiden
Non-merohedral Twinning in Protein Crystallography
 Bruker Users Meeting 2010 Karlsruhe, 22 nd September 2010 Non-merohedral Twinning in Protein Crystallography Regine Herbst-Irmer rherbst@shelx.uni-ac.gwdg.de http://shelx.uni-ac.gwdg.de/~rherbst/twin.html
Bruker Users Meeting 2010 Karlsruhe, 22 nd September 2010 Non-merohedral Twinning in Protein Crystallography Regine Herbst-Irmer rherbst@shelx.uni-ac.gwdg.de http://shelx.uni-ac.gwdg.de/~rherbst/twin.html
Resolution and data formats. Andrea Thorn
 Resolution and data formats Andrea Thorn RESOLUTION Motivation Courtesy of M. Sawaya Map resolution http://www.bmsc.washington.edu/people/verlinde/experiment.html Data quality indicators Resolution accounts
Resolution and data formats Andrea Thorn RESOLUTION Motivation Courtesy of M. Sawaya Map resolution http://www.bmsc.washington.edu/people/verlinde/experiment.html Data quality indicators Resolution accounts
Reflection = EM strikes a boundary between two media differing in η and bounces back
 Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
General theory of diffraction
 General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
