EE 232 Lightwave Devices. Photodiodes
|
|
|
- Randell Jonas Harrell
- 5 years ago
- Views:
Transcription
1 EE 3 Lgwav Dvcs Lcur 8: oocoucors a p-- ooos Rag: Cuag, Cap. 4 Isrucor: Mg C. Wu Uvrsy of Calfora, Brkly Elcrcal Egrg a Compur Sccs Dp. EE3 Lcur 8-8. Uvrsy of Calfora oocoucors ω + - x Ara w L Euval Crcu ω Dark curr: ( p) J σ E μ + p μ E Lg llumao gra lcro-ol pars, crasg coucvy: δ δ G Say sa: / --> δ G ( p) Δ J δ μ + μ E oocoucor rurs bo coacs cs o b Omc EE3 Lcur 8-8. Uvrsy of Calfora
2 oocarrr Grao Ra ω Lg Isy α x + - x Ara w L op ( R ) x ( α ) op op G η : poocarrr grao ra [ ] 3 ω lw cm s η η ( R)( α ) R : rflcvy of poocoucor surfac α: absorpo coffc : absorpo lg α : fraco of lg rmas afr absorpo lg EE3 Lcur Uvrsy of Calfora oocoucv Ga ( )( μ μp) Δ I lwδ J lw G + E op Δ I lw η ( μ + μp) E ω lw op Δ I η ( μ E ) η v ω ω : ras m v I Δ η op ω op oocurr oocoucv Ga EE3 Lcur Uvrsy of Calfora
3 Aalogy o Curr Ga Bpolar Trassor Curr ga bpolar rassor: E B C I C β I B T curr ga ca also b xprss as rb β :rasm m rb : carrr rcombao lfm bas EE3 Lcur Uvrsy of Calfora N op N η ω lw Small sgal rspos: N N + N jω N jωn η ω lw N η ω ( lw ) jω+ / ( ) I J lw N v lw Frucy of oocoucors Rspos (B) ooc coucv /, /, / / / I η ω jω + ( DC Quaum Effccy ) ( oocoucv Ga ) ( Normalz Frucy Rspos) EE3 Lcur Uvrsy of Calfora Frucy (Hz)
4 Rvrs-bas p-- juco Mos of volag rop across -rgo, ma absorpo rgo Hg fl sparas poogra lcro a ol -- ooo ν N Larg bagap marals ar us for a N f possbl Ec Ev Fas rspos ν Low os No ga (uaum ffccy 4 < %) F x EE3 Lcur Uvrsy of Calfora I-V Curv kt B Dark curr: I I ( ) V I oocurr: I η p op ω Quaum ffccy: η η ( R)( α ) V kt B Toal curr: I I ( ) + I p V I p I p Loa L for Basg D EE3 Lcur Uvrsy of Calfora
5 Absorpo Coffc Lg sy cays xpoally smcoucor: I( x) I αx Drc bagap smcoucor as a sarp absorpo g S absorbs poos w v > E g. V, bu absorpo coffc s small Suffc for CCD A gr rgy (~ 3 V), absorpo coffc of S bcoms larg aga, u o rc bagap raso o gr CB EE3 Lcur Uvrsy of Calfora Two Typs of p-- ooos L Surfac-Illuma p-- η η ( R)( α ) η : ral uaum ffccy R : rflcvy : absorpo layr ckss Wavgu p-- Γ L η η ( R)( α ) η : ral uaum ffccy R : rflcvy Γ : cofm facor L : lg of wavgu D EE3 Lcur 8-8. Uvrsy of Calfora
6 Ramo s Torm A T curr caus xral crcu by a movg carg movg a a volocy v ( ) a paralll pla w a sparao of a a volag bas of V s v() () roof: Work o o carg: W Forc Dsplacm V Ex x Work prov by powr supply: W () V V V () x x v () () EE3 Lcur 8-8. Uvrsy of Calfora Rspos of O oogra Elcro-Hol ar v () () + () / () N v / () Tm EE3 Lcur 8- x x x Tm v v Hol Trajcory Elcro Trajcory 8. Uvrsy of Calfora Toal carg gra: Q () + () v x v x + v v O absorb poo o carg c
7 Tras Tm () () + () N () () May lcro-ol pars gra v v Tm Elcro curr s w las lcro gra ar -s racs N-lcro: v Hol curr s w las ol gra ar N-s racs -lcro: v Hol s usually slowr A cosrvav sma of ras m: v EE3 Lcur Uvrsy of Calfora Toal Rspos Tm of p-- () m: ε A R ( A : ara of p--) () Tras m: v Toal rspos m: f 3B + π Absorpo layr ckss for opmum frucy rspos: Rε A + + v Rε A v v f 3B f3 B,max π 4π Rε A Opmum baw occurs w Rε A v opmum Rε Av EE3 Lcur Uvrsy of Calfora
8 Mor Rgorous Aalyss of p-- Rspos Tm Small-sgal aalyss: assum pu lg s moula a frucy ω, poocurr s proporoal o ( ( ) ) log H f ω Tras s Tm () + log H( f jω ω ) T frs rm s sgl-pol rspos from, wl sco rm s pas lay u o Toal ras m rspos. ( ) ( ( ) ) log H3 f f f EE3 Lcur Uvrsy of Calfora f Comparso of Numrc Exampls Exampl: 4.4 ps ps f 3B 4.6 GHz π + ω s () H( ω) + j ω ω Solvg H( ω ), f3b 9.7 GHz T scrpacy s smallr w omas, a largr w ras m omas. (Tras m rspos as a sarp rop-off). EE3 Lcur Uvrsy of Calfora ( ( ) ) log H f Tras Tm log H f ( ( ) ) Toal ( ( ) ) log H3 f f f f
9 Baw-Effccy rouc () For surfac-lluma p-- (assum AR coag: R%), xrm of absorbg layr a ras-m-oma rspos: α η η ( ) η ( ( α )) ηα v f3b π v ηα v Baw-ffccy pr ouc: f3b η ( ηα ) π π () O or a, ffccy of wavgu p-- s ΓαL η η ( ) ηγαl -lm baw: f3b π RεLw ηγα Baw-ffccy prouc: f3b η ( ηγ αl) π R ε L w π R ε w I gral, r s a baw-ffccy ra-off EE3 Lcur Uvrsy of Calfora
EE"232"Lightwave"Devices Lecture"16:"p7i7n"Photodiodes"and" Photoconductors"
 EE"232"Lgwav"Dvcs Lcur"16:"p77n"Pooos"an" Pooconucors" Rang:"Cuang,"Cap."15"(2 n E) Insrucor:"Mng"C."Wu Unvrsy"of"Calforna,"Brkly Elcrcal"Engnrng"an"Compur"Scncs"Dp. EE232$Lcur$16-1 Rvrs"bas%p""n%juncon
EE"232"Lgwav"Dvcs Lcur"16:"p77n"Pooos"an" Pooconucors" Rang:"Cuang,"Cap."15"(2 n E) Insrucor:"Mng"C."Wu Unvrsy"of"Calforna,"Brkly Elcrcal"Engnrng"an"Compur"Scncs"Dp. EE232$Lcur$16-1 Rvrs"bas%p""n%juncon
Lecture 1: Photoconductors and p-i-n Photodiodes
 Lcur 1: Poocoucors a p-i- Pooios Isrucor: Mig C. Wu Uivrsiy of Califoria, Brkly Elcrical Egirig a Compur Scics Dp. 1 Prof. Mig Wu Poocors Covrs lig o lcric sigals Mai yps of poocors Poocoucors P-i- pooios
Lcur 1: Poocoucors a p-i- Pooios Isrucor: Mig C. Wu Uivrsiy of Califoria, Brkly Elcrical Egirig a Compur Scics Dp. 1 Prof. Mig Wu Poocors Covrs lig o lcric sigals Mai yps of poocors Poocoucors P-i- pooios
Chapter 5 Transient Analysis
 hpr 5 rs Alyss Jsug Jg ompl rspos rs rspos y-s rspos m os rs orr co orr Dffrl Equo. rs Alyss h ffrc of lyss of crcus wh rgy sorg lms (ucors or cpcors) & m-ryg sgls wh rss crcus s h h quos rsulg from r
hpr 5 rs Alyss Jsug Jg ompl rspos rs rspos y-s rspos m os rs orr co orr Dffrl Equo. rs Alyss h ffrc of lyss of crcus wh rgy sorg lms (ucors or cpcors) & m-ryg sgls wh rss crcus s h h quos rsulg from r
Lecture 12: Introduction to nonlinear optics II.
 Lcur : Iroduco o olar opcs II r Kužl ropagao of srog opc sgals propr olar ffcs Scod ordr ffcs! Thr-wav mxg has machg codo! Scod harmoc grao! Sum frqucy grao! aramrc grao Thrd ordr ffcs! Four-wav mxg! Opcal
Lcur : Iroduco o olar opcs II r Kužl ropagao of srog opc sgals propr olar ffcs Scod ordr ffcs! Thr-wav mxg has machg codo! Scod harmoc grao! Sum frqucy grao! aramrc grao Thrd ordr ffcs! Four-wav mxg! Opcal
Physics 160 Lecture 3. R. Johnson April 6, 2015
 Physics 6 Lcur 3 R. Johnson April 6, 5 RC Circui (Low-Pass Filr This is h sam RC circui w lookd a arlir h im doma, bu hr w ar rsd h frquncy rspons. So w pu a s wav sad of a sp funcion. whr R C RC Complx
Physics 6 Lcur 3 R. Johnson April 6, 5 RC Circui (Low-Pass Filr This is h sam RC circui w lookd a arlir h im doma, bu hr w ar rsd h frquncy rspons. So w pu a s wav sad of a sp funcion. whr R C RC Complx
A L A BA M A L A W R E V IE W
 A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
Multi-fluid magnetohydrodynamics in the solar atmosphere
 Mul-flud magohydrodyams h solar amoshr Tmuraz Zaqarashvl თეიმურაზ ზაქარაშვილი Sa Rsarh Isu of Ausra Aadmy of Ss Graz Ausra ISSI-orksho Parally ozd lasmas asrohyss 6 Jauary- Fbruary 04 ISSI-orksho Parally
Mul-flud magohydrodyams h solar amoshr Tmuraz Zaqarashvl თეიმურაზ ზაქარაშვილი Sa Rsarh Isu of Ausra Aadmy of Ss Graz Ausra ISSI-orksho Parally ozd lasmas asrohyss 6 Jauary- Fbruary 04 ISSI-orksho Parally
P a g e 5 1 of R e p o r t P B 4 / 0 9
 P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
OH BOY! Story. N a r r a t iv e a n d o bj e c t s th ea t e r Fo r a l l a g e s, fr o m th e a ge of 9
 OH BOY! O h Boy!, was or igin a lly cr eat ed in F r en ch an d was a m a jor s u cc ess on t h e Fr en ch st a ge f or young au di enc es. It h a s b een s een by ap pr ox i ma t ely 175,000 sp ect at
OH BOY! O h Boy!, was or igin a lly cr eat ed in F r en ch an d was a m a jor s u cc ess on t h e Fr en ch st a ge f or young au di enc es. It h a s b een s een by ap pr ox i ma t ely 175,000 sp ect at
02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr
 Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Chap 2: Reliability and Availability Models
 Chap : lably ad valably Modls lably = prob{s s fully fucog [,]} Suppos from [,] m prod, w masur ou of N compos, of whch N : # of compos oprag corrcly a m N f : # of compos whch hav fald a m rlably of h
Chap : lably ad valably Modls lably = prob{s s fully fucog [,]} Suppos from [,] m prod, w masur ou of N compos, of whch N : # of compos oprag corrcly a m N f : # of compos whch hav fald a m rlably of h
By Joonghoe Dho. The irradiance at P is given by
 CH. 9 c CH. 9 c By Joogo Do 9 Gal Coao 9. Gal Coao L co wo po ouc, S & S, mg moocomac wav o am qucy. L paao a b muc ga a. Loca am qucy. L paao a b muc ga a. Loca po obvao P a oug away om ouc o a a P wavo
CH. 9 c CH. 9 c By Joogo Do 9 Gal Coao 9. Gal Coao L co wo po ouc, S & S, mg moocomac wav o am qucy. L paao a b muc ga a. Loca am qucy. L paao a b muc ga a. Loca po obvao P a oug away om ouc o a a P wavo
5 questions, 3 points each, 15 points total possible. 26 Fe Cu Ni Co Pd Ag Ru 101.
 Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
The Periodic Table. Periodic Properties. Can you explain this graph? Valence Electrons. Valence Electrons. Paramagnetism
 Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
P a g e 3 6 of R e p o r t P B 4 / 0 9
 P a g e 3 6 of R e p o r t P B 4 / 0 9 p r o t e c t h um a n h e a l t h a n d p r o p e r t y fr om t h e d a n g e rs i n h e r e n t i n m i n i n g o p e r a t i o n s s u c h a s a q u a r r y. J
P a g e 3 6 of R e p o r t P B 4 / 0 9 p r o t e c t h um a n h e a l t h a n d p r o p e r t y fr om t h e d a n g e rs i n h e r e n t i n m i n i n g o p e r a t i o n s s u c h a s a q u a r r y. J
as nonrigid Carnot groups
 Th Th Th V 5 5 34 356 V V crcc 5 c5 5 Hdr 5 34 356 Vr 34 dh 356 crcc-c 5 Hdr c Vr d Cr r c d Cr r c r c c r c 5 B Hdr Wrhr B Wrhr Vr Ccd b G cr Ccd b cr Abrc G c cr W d rdc r c d Cr hch Abrc r W d Cr rdc
Th Th Th V 5 5 34 356 V V crcc 5 c5 5 Hdr 5 34 356 Vr 34 dh 356 crcc-c 5 Hdr c Vr d Cr r c d Cr r c r c c r c 5 B Hdr Wrhr B Wrhr Vr Ccd b G cr Ccd b cr Abrc G c cr W d rdc r c d Cr hch Abrc r W d Cr rdc
Last 4 Digits of USC ID:
 Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
C Line Stations. What will the C Line look like in your neighborhood? Larger station at high-ridership stops. Upgrades to custom shelter
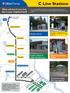 C L Sas Wa w C L k k yur gbrd? Bass Lak d Brky Cr Tras Cr Xrxs 56 T C L w ffr smar srvc ad sa faurs A L Sg Avu. Ts mags sw smar sas wa s pad C L. 57 Av Brky Buvard 49 Av 100 Mdum sa Smar gbrd sa Largr
C L Sas Wa w C L k k yur gbrd? Bass Lak d Brky Cr Tras Cr Xrxs 56 T C L w ffr smar srvc ad sa faurs A L Sg Avu. Ts mags sw smar sas wa s pad C L. 57 Av Brky Buvard 49 Av 100 Mdum sa Smar gbrd sa Largr
1 of 5 14/10/ :21
 X-ray absorption s, characteristic X-ray lines... 4.2.1 Home About Table of Contents Advanced Search Copyright Feedback Privacy You are here: Chapter: 4 Atomic and nuclear physics Section: 4.2 Absorption
X-ray absorption s, characteristic X-ray lines... 4.2.1 Home About Table of Contents Advanced Search Copyright Feedback Privacy You are here: Chapter: 4 Atomic and nuclear physics Section: 4.2 Absorption
Lecture 14. P-N Junction Diodes: Part 3 Quantitative Analysis (Math, math and more math) Reading: Pierret 6.1
 Lctur 4 - ucto ods art 3 Quattatv alyss Math, math ad mor math Radg rrt 6. Gorga Tch ECE 3040 - r. la oolttl Quattatv - od Soluto ssumtos stady stat codtos o- dgrat dog 3 o- dmsoal aalyss 4 low- lvl jcto
Lctur 4 - ucto ods art 3 Quattatv alyss Math, math ad mor math Radg rrt 6. Gorga Tch ECE 3040 - r. la oolttl Quattatv - od Soluto ssumtos stady stat codtos o- dgrat dog 3 o- dmsoal aalyss 4 low- lvl jcto
PERIODIC TABLE OF THE ELEMENTS
 Useful Constants and equations: K = o C + 273 Avogadro's number = 6.022 x 10 23 d = density = mass/volume R H = 2.178 x 10-18 J c = E = h = hc/ h = 6.626 x 10-34 J s c = 2.998 x 10 8 m/s E n = -R H Z 2
Useful Constants and equations: K = o C + 273 Avogadro's number = 6.022 x 10 23 d = density = mass/volume R H = 2.178 x 10-18 J c = E = h = hc/ h = 6.626 x 10-34 J s c = 2.998 x 10 8 m/s E n = -R H Z 2
Guide to the Extended Step-Pyramid Periodic Table
 Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Engineering Circuit Analysis 8th Edition Chapter Nine Exercise Solutions
 Engnrng rcu naly 8h Eon hapr Nn Exrc Soluon. = KΩ, = µf, an uch ha h crcu rpon oramp. a For Sourc-fr paralll crcu: For oramp or b H 9V, V / hoo = H.7.8 ra / 5..7..9 9V 9..9..9 5.75,.5 5.75.5..9 . = nh,
Engnrng rcu naly 8h Eon hapr Nn Exrc Soluon. = KΩ, = µf, an uch ha h crcu rpon oramp. a For Sourc-fr paralll crcu: For oramp or b H 9V, V / hoo = H.7.8 ra / 5..7..9 9V 9..9..9 5.75,.5 5.75.5..9 . = nh,
Advanced Placement. Chemistry. Integrated Rates
 Advanced Placement Chemistry Integrated Rates 204 47.90 9.22 78.49 (26) 50.94 92.9 80.95 (262) 52.00 93.94 83.85 (263) 54.938 (98) 86.2 (262) 55.85 0. 90.2 (265) 58.93 02.9 92.2 (266) H Li Na K Rb Cs Fr
Advanced Placement Chemistry Integrated Rates 204 47.90 9.22 78.49 (26) 50.94 92.9 80.95 (262) 52.00 93.94 83.85 (263) 54.938 (98) 86.2 (262) 55.85 0. 90.2 (265) 58.93 02.9 92.2 (266) H Li Na K Rb Cs Fr
Parts Manual. EPIC II Critical Care Bed REF 2031
 EPIC II Critical Care Bed REF 2031 Parts Manual For parts or technical assistance call: USA: 1-800-327-0770 2013/05 B.0 2031-109-006 REV B www.stryker.com Table of Contents English Product Labels... 4
EPIC II Critical Care Bed REF 2031 Parts Manual For parts or technical assistance call: USA: 1-800-327-0770 2013/05 B.0 2031-109-006 REV B www.stryker.com Table of Contents English Product Labels... 4
FILTER BANK MULTICARRIER WITH LAPPED TRANSFORMS
 FILTER BANK ULTICARRIER WITH LAPPED TRANSFORS aurc Bllagr, CNA Davd attra, aro Tada, Uv.Napol arch 5 Obctvs A multcarrr approach to mprov o OFD for futur wrlss systms - asychroous mult-usr accss - spctral
FILTER BANK ULTICARRIER WITH LAPPED TRANSFORS aurc Bllagr, CNA Davd attra, aro Tada, Uv.Napol arch 5 Obctvs A multcarrr approach to mprov o OFD for futur wrlss systms - asychroous mult-usr accss - spctral
B. X : in phase; Y: out of phase C. X : out of phase; Y: in phase D. X : out of phase; Y: out of phase
 2015 April 24 Exam 3 Physics 106 Circle the letter of the single best answer. Each question is worth 1 point Physical Constants: proton charge = e = 1.60 10 19 C proton mass = m p = 1.67 10 27 kg electron
2015 April 24 Exam 3 Physics 106 Circle the letter of the single best answer. Each question is worth 1 point Physical Constants: proton charge = e = 1.60 10 19 C proton mass = m p = 1.67 10 27 kg electron
single-layer transition metal dichalcogenides MC2
 single-layer transition metal dichalcogenides MC2 Period 1 1 H 18 He 2 Group 1 2 Li Be Group 13 14 15 16 17 18 B C N O F Ne 3 4 Na K Mg Ca Group 3 4 5 6 7 8 9 10 11 12 Sc Ti V Cr Mn Fe Co Ni Cu Zn Al Ga
single-layer transition metal dichalcogenides MC2 Period 1 1 H 18 He 2 Group 1 2 Li Be Group 13 14 15 16 17 18 B C N O F Ne 3 4 Na K Mg Ca Group 3 4 5 6 7 8 9 10 11 12 Sc Ti V Cr Mn Fe Co Ni Cu Zn Al Ga
State Observer Design
 Sa Obsrvr Dsgn A. Khak Sdgh Conrol Sysms Group Faculy of Elcrcal and Compur Engnrng K. N. Toos Unvrsy of Tchnology Fbruary 2009 1 Problm Formulaon A ky assumpon n gnvalu assgnmn and sablzng sysms usng
Sa Obsrvr Dsgn A. Khak Sdgh Conrol Sysms Group Faculy of Elcrcal and Compur Engnrng K. N. Toos Unvrsy of Tchnology Fbruary 2009 1 Problm Formulaon A ky assumpon n gnvalu assgnmn and sablzng sysms usng
High Accuracy EUV Reflectometry and Scattering at the Advanced Light Source
 High Accuracy EUV Reflectometry and Scattering at the Advanced Light Source Eric Gullikson Lawrence Berkeley National Laboratory 1 Reflectometry and Scattering Beamline (ALS 6.3.2) Commissioned Fall 1994
High Accuracy EUV Reflectometry and Scattering at the Advanced Light Source Eric Gullikson Lawrence Berkeley National Laboratory 1 Reflectometry and Scattering Beamline (ALS 6.3.2) Commissioned Fall 1994
Nucleus. Electron Cloud
 Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
Chemistry 431 Practice Final Exam Fall Hours
 Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
Metallurgical Chemistry. An Audio Course for Students
 Laval University From the SelectedWorks of Fathi Habashi February, 1987 Metallurgical Chemistry. An Audio Course for Students Fathi Habashi Available at: https://works.bepress.com/fathi_habashi/27/ METALLURGICAL
Laval University From the SelectedWorks of Fathi Habashi February, 1987 Metallurgical Chemistry. An Audio Course for Students Fathi Habashi Available at: https://works.bepress.com/fathi_habashi/27/ METALLURGICAL
Speed of light c = m/s. x n e a x d x = 1. 2 n+1 a n π a. He Li Ne Na Ar K Ni 58.
 Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
The Periodic Table of Elements
 The Periodic Table of Elements 8 Uuo Uus Uuh (9) Uup (88) Uuq (89) Uut (8) Uub (8) Rg () 0 Ds (9) 09 Mt (8) 08 Hs (9) 0 h () 0 Sg () 0 Db () 0 Rf () 0 Lr () 88 Ra () 8 Fr () 8 Rn () 8 At (0) 8 Po (09)
The Periodic Table of Elements 8 Uuo Uus Uuh (9) Uup (88) Uuq (89) Uut (8) Uub (8) Rg () 0 Ds (9) 09 Mt (8) 08 Hs (9) 0 h () 0 Sg () 0 Db () 0 Rf () 0 Lr () 88 Ra () 8 Fr () 8 Rn () 8 At (0) 8 Po (09)
H STO RY OF TH E SA NT
 O RY OF E N G L R R VER ritten for the entennial of th e Foundin g of t lair oun t y on ay 8 82 Y EEL N E JEN K RP O N! R ENJ F ] jun E 3 1 92! Ph in t ed b y h e t l a i r R ep u b l i c a n O 4 1922
O RY OF E N G L R R VER ritten for the entennial of th e Foundin g of t lair oun t y on ay 8 82 Y EEL N E JEN K RP O N! R ENJ F ] jun E 3 1 92! Ph in t ed b y h e t l a i r R ep u b l i c a n O 4 1922
Almost unbiased exponential estimator for the finite population mean
 Almos ubasd poal smaor for f populao ma Rajs Sg, Pakaj aua, ad rmala Saa, Scool of Sascs, DAVV, Idor (M.P., Ida (rsgsa@aoo.com Flor Smaradac ar of Dparm of Mamacs, Uvrs of Mco, Gallup, USA (smarad@um.du
Almos ubasd poal smaor for f populao ma Rajs Sg, Pakaj aua, ad rmala Saa, Scool of Sascs, DAVV, Idor (M.P., Ida (rsgsa@aoo.com Flor Smaradac ar of Dparm of Mamacs, Uvrs of Mco, Gallup, USA (smarad@um.du
MANY ELECTRON ATOMS Chapter 15
 MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
ESE319 Introduction to Microelectronics. Feedback Basics
 Feedback Basics Feedback concept Feedback in emitter follower Stability One-pole feedback and root locus Frequency dependent feedback and root locus Gain and phase margins Conditions for closed loop stability
Feedback Basics Feedback concept Feedback in emitter follower Stability One-pole feedback and root locus Frequency dependent feedback and root locus Gain and phase margins Conditions for closed loop stability
Circle the letters only. NO ANSWERS in the Columns!
 Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
The real E-k diagram of Si is more complicated (indirect semiconductor). The bottom of E C and top of E V appear for different values of k.
 Modr Smcoductor Dvcs for Itgratd rcuts haptr. lctros ad Hols Smcoductors or a bad ctrd at k=0, th -k rlatoshp ar th mmum s usually parabolc: m = k * m* d / dk d / dk gatv gatv ffctv mass Wdr small d /
Modr Smcoductor Dvcs for Itgratd rcuts haptr. lctros ad Hols Smcoductors or a bad ctrd at k=0, th -k rlatoshp ar th mmum s usually parabolc: m = k * m* d / dk d / dk gatv gatv ffctv mass Wdr small d /
8. Relax and do well.
 CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
Chapter 3: Stoichiometry
 Chapter 3: Stoichiometry Chem 6A Michael J. Sailor, UC San Diego 1 Announcements: Thursday (Sep 29) quiz: Bring student ID or we cannot accept your quiz! No notes, no calculators Covers chapters 1 and
Chapter 3: Stoichiometry Chem 6A Michael J. Sailor, UC San Diego 1 Announcements: Thursday (Sep 29) quiz: Bring student ID or we cannot accept your quiz! No notes, no calculators Covers chapters 1 and
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Conventional Hot-Wire Anemometer
 Convnonal Ho-Wr Anmomr cro Ho Wr Avanag much mallr prob z mm o µm br paal roluon array o h nor hghr rquncy rpon lowr co prormanc/co abrcaon roc I µm lghly op p layr 8µm havly boron op ch op layr abrcaon
Convnonal Ho-Wr Anmomr cro Ho Wr Avanag much mallr prob z mm o µm br paal roluon array o h nor hghr rquncy rpon lowr co prormanc/co abrcaon roc I µm lghly op p layr 8µm havly boron op ch op layr abrcaon
Chapter 8. Second-Harmonic Generation and Parametric Oscillation
 Chapr 8 Sco-Haroc Grao a ararc Oscao 8 Irouco Sco-Haroc grao : ararc Oscao : Rfrc : RW Boy Noar Opcs Chapr Th oar Opca Suscpby Noar Opcs ab Hayag Uv Th Noar Opca Suscpby Gra for of uc poarao : whr : ar
Chapr 8 Sco-Haroc Grao a ararc Oscao 8 Irouco Sco-Haroc grao : ararc Oscao : Rfrc : RW Boy Noar Opcs Chapr Th oar Opca Suscpby Noar Opcs ab Hayag Uv Th Noar Opca Suscpby Gra for of uc poarao : whr : ar
(C) Pavel Sedach and Prep101 1
 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach
(C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach
PART 1 Introduction to Theory of Solids
 Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:1 Trim:165 240MM TS: Integra, India PART 1 Introduction to Theory of Solids Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:2
Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:1 Trim:165 240MM TS: Integra, India PART 1 Introduction to Theory of Solids Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:2
[ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34.
![[ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34. [ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34.](/thumbs/74/69911105.jpg) .. - 2-2009 [661.87.+661.88]:543.4(075.8) 35.20:34.2373-60..,..,..,..,.. -60 : /... ;. 2-. : -, 2008. 134. ISBN 5-98298-299-7 -., -,,. - «,, -, -», - 550800,, 240600 «-», -. [661.87.+661.88]:543.4(075.8)
.. - 2-2009 [661.87.+661.88]:543.4(075.8) 35.20:34.2373-60..,..,..,..,.. -60 : /... ;. 2-. : -, 2008. 134. ISBN 5-98298-299-7 -., -,,. - «,, -, -», - 550800,, 240600 «-», -. [661.87.+661.88]:543.4(075.8)
CLASS TEST GRADE 11. PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials 1
 CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You
CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Periodicity & Many-Electron Atoms
 Chap. 8 ELECTRON CONFIGURAT N & CEMICAL PERIODICITY 8.1-8.2 Periodicity & Many-Electron Atoms Understand the correlation of electron configuration and the periodic character of atomic properties such as
Chap. 8 ELECTRON CONFIGURAT N & CEMICAL PERIODICITY 8.1-8.2 Periodicity & Many-Electron Atoms Understand the correlation of electron configuration and the periodic character of atomic properties such as
Chapter 12 The Atom & Periodic Table- part 2
 Chapter 12 The Atom & Periodic Table- part 2 Electrons found outside the nucleus; negatively charged Protons found in the nucleus; positive charge equal in magnitude to the electron s negative charge Neutrons
Chapter 12 The Atom & Periodic Table- part 2 Electrons found outside the nucleus; negatively charged Protons found in the nucleus; positive charge equal in magnitude to the electron s negative charge Neutrons
Atoms and the Periodic Table
 Atoms and the Periodic Table Parts of the Atom Proton Found in the nucleus Number of protons defines the element Charge +1, mass 1 Parts of the Atom Neutron Found in the nucleus Stabilizes the nucleus
Atoms and the Periodic Table Parts of the Atom Proton Found in the nucleus Number of protons defines the element Charge +1, mass 1 Parts of the Atom Neutron Found in the nucleus Stabilizes the nucleus
BROOKLYN COLLEGE Department of Chemistry. Chemistry 1 Second Lecture Exam Nov. 27, Name Page 1 of 5
 BROOKLYN COLLEGE Department of Chemistry Chemistry 1 Second Lecture Exam Nov. 27, 2002 Name Page 1 of 5 Circle the name of your lab instructor Kobrak, Zhou, Girotto, Hussey, Du Before you begin the exam,
BROOKLYN COLLEGE Department of Chemistry Chemistry 1 Second Lecture Exam Nov. 27, 2002 Name Page 1 of 5 Circle the name of your lab instructor Kobrak, Zhou, Girotto, Hussey, Du Before you begin the exam,
c. What is the average rate of change of f on the interval [, ]? Answer: d. What is a local minimum value of f? Answer: 5 e. On what interval(s) is f
![c. What is the average rate of change of f on the interval [, ]? Answer: d. What is a local minimum value of f? Answer: 5 e. On what interval(s) is f c. What is the average rate of change of f on the interval [, ]? Answer: d. What is a local minimum value of f? Answer: 5 e. On what interval(s) is f](/thumbs/87/95703678.jpg) Essential Skills Chapter f ( x + h) f ( x ). Simplifying the difference quotient Section. h f ( x + h) f ( x ) Example: For f ( x) = 4x 4 x, find and simplify completely. h Answer: 4 8x 4 h. Finding the
Essential Skills Chapter f ( x + h) f ( x ). Simplifying the difference quotient Section. h f ( x + h) f ( x ) Example: For f ( x) = 4x 4 x, find and simplify completely. h Answer: 4 8x 4 h. Finding the
Radiometric Dating (tap anywhere)
 Radiometric Dating (tap anywhere) Protons Neutrons Electrons Elements on the periodic table are STABLE Elements can have radioactive versions of itself called ISOTOPES!! Page 1 in your ESRT has your list!
Radiometric Dating (tap anywhere) Protons Neutrons Electrons Elements on the periodic table are STABLE Elements can have radioactive versions of itself called ISOTOPES!! Page 1 in your ESRT has your list!
! 94
 ! 94 4 : - : : / : : : : ( :) : : : - : / : / : : - 4 : -4 : : : : : -5 () ( ) : -6 : - - : : : () : : : :4 : -7. : : -8. (. : ( : -9 : ( ( ( (5 (4 4 : -0! : ( : ( :. : (. (. (. (4. ( ( ( : ( 4 : - : :
! 94 4 : - : : / : : : : ( :) : : : - : / : / : : - 4 : -4 : : : : : -5 () ( ) : -6 : - - : : : () : : : :4 : -7. : : -8. (. : ( : -9 : ( ( ( (5 (4 4 : -0! : ( : ( :. : (. (. (. (4. ( ( ( : ( 4 : - : :
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
". :'=: "t',.4 :; :::-':7'- --,r. "c:"" --; : I :. \ 1 :;,'I ~,:-._._'.:.:1... ~~ \..,i ... ~.. ~--~ ( L ;...3L-. ' f.':... I. -.1;':'.
 = 47 \ \ L 3L f \ / \ L \ \ j \ \ 6! \ j \ / w j / \ \ 4 / N L5 Dm94 O6zq 9 qmn j!!! j 3DLLE N f 3LLE Of ADL!N RALROAD ORAL OR AL AOAON N 5 5 D D 9 94 4 E ROL 2LL RLLAY RL AY 3 ER OLLL 832 876 8 76 L A
= 47 \ \ L 3L f \ / \ L \ \ j \ \ 6! \ j \ / w j / \ \ 4 / N L5 Dm94 O6zq 9 qmn j!!! j 3DLLE N f 3LLE Of ADL!N RALROAD ORAL OR AL AOAON N 5 5 D D 9 94 4 E ROL 2LL RLLAY RL AY 3 ER OLLL 832 876 8 76 L A
Chemistry 1 First Lecture Exam Fall Abbasi Khajo Levine Mathias Mathias/Ortiz Metlitsky Rahi Sanchez-Delgado Vasserman
 Chemistry 1 First Lecture Exam Fall 2011 Page 1 of 9 NAME Circle the name of your recitation/lab instructor(s) Abbasi Khajo Levine Mathias Mathias/Ortiz Metlitsky Rahi Sanchez-Delgado Vasserman Before
Chemistry 1 First Lecture Exam Fall 2011 Page 1 of 9 NAME Circle the name of your recitation/lab instructor(s) Abbasi Khajo Levine Mathias Mathias/Ortiz Metlitsky Rahi Sanchez-Delgado Vasserman Before
CHEM 10113, Quiz 5 October 26, 2011
 CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
CHEM 107 (Spring-2005) Exam 3 (100 pts)
 CHEM 107 (Spring-2005) Exam 3 (100 pts) Name: ------------------------------------------------------------------------, Clid # ------------------------------ LAST NAME, First (Circle the alphabet segment
CHEM 107 (Spring-2005) Exam 3 (100 pts) Name: ------------------------------------------------------------------------, Clid # ------------------------------ LAST NAME, First (Circle the alphabet segment
M11/4/CHEMI/SPM/ENG/TZ2/XX CHEMISTRY STANDARD LEVEL PAPER 1. Monday 9 May 2011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M11/4/CHEMI/SPM/ENG/TZ/XX 116116 CHEMISTRY STANDARD LEVEL PAPER 1 Monday 9 May 011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M11/4/CHEMI/SPM/ENG/TZ/XX 116116 CHEMISTRY STANDARD LEVEL PAPER 1 Monday 9 May 011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
Fourier Series: main points
 BIOEN 3 Lcur 6 Fourir rasforms Novmbr 9, Fourir Sris: mai pois Ifii sum of sis, cosis, or boh + a a cos( + b si( All frqucis ar igr mulipls of a fudamal frqucy, o F.S. ca rprs ay priodic fucio ha w ca
BIOEN 3 Lcur 6 Fourir rasforms Novmbr 9, Fourir Sris: mai pois Ifii sum of sis, cosis, or boh + a a cos( + b si( All frqucis ar igr mulipls of a fudamal frqucy, o F.S. ca rprs ay priodic fucio ha w ca
Solutions and Ions. Pure Substances
 Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Guest Lectures for Dr. MacFarlane s EE3350
 Guest Lectures for Dr. MacFarlane s EE3350 Michael Plante Sat., -08-008 Write name in corner.. Problem Statement Amplifier Z S Z O V S Z I Z L Transducer, Antenna, etc. Coarse Tuning (optional) Amplifier
Guest Lectures for Dr. MacFarlane s EE3350 Michael Plante Sat., -08-008 Write name in corner.. Problem Statement Amplifier Z S Z O V S Z I Z L Transducer, Antenna, etc. Coarse Tuning (optional) Amplifier
(please print) (1) (18) H IIA IIIA IVA VA VIA VIIA He (2) (13) (14) (15) (16) (17)
 CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
T h e C S E T I P r o j e c t
 T h e P r o j e c t T H E P R O J E C T T A B L E O F C O N T E N T S A r t i c l e P a g e C o m p r e h e n s i v e A s s es s m e n t o f t h e U F O / E T I P h e n o m e n o n M a y 1 9 9 1 1 E T
T h e P r o j e c t T H E P R O J E C T T A B L E O F C O N T E N T S A r t i c l e P a g e C o m p r e h e n s i v e A s s es s m e n t o f t h e U F O / E T I P h e n o m e n o n M a y 1 9 9 1 1 E T
M10/4/CHEMI/SPM/ENG/TZ2/XX+ CHEMISTRY. Wednesday 12 May 2010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
Using the Periodic Table
 MATH SKILLS TRANSPARENCY WORKSHEET Using the Periodic Table 6 Use with Chapter 6, Section 6.2 1. Identify the number of valence electrons in each of the following elements. a. Ne e. O b. K f. Cl c. B g.
MATH SKILLS TRANSPARENCY WORKSHEET Using the Periodic Table 6 Use with Chapter 6, Section 6.2 1. Identify the number of valence electrons in each of the following elements. a. Ne e. O b. K f. Cl c. B g.
8. Relax and do well.
 CHEM 1215 Exam III John III. Gelder November 11, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1215 Exam III John III. Gelder November 11, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 172 EXAMINATION 1. January 15, 2009
 CHEM 17 EXAMINATION 1 January 15, 009 Dr. Kimberly M. Broekemeier NAME: Circle lecture time: 9:00 11:00 Constants: c = 3.00 X 10 8 m/s h = 6.63 X 10-34 J x s J = kg x m /s Rydberg Constant = 1.096776 x
CHEM 17 EXAMINATION 1 January 15, 009 Dr. Kimberly M. Broekemeier NAME: Circle lecture time: 9:00 11:00 Constants: c = 3.00 X 10 8 m/s h = 6.63 X 10-34 J x s J = kg x m /s Rydberg Constant = 1.096776 x
INSTRUCTIONS: Exam III. November 10, 1999 Lab Section
 CHEM 1215 Exam III John III. Gelder November 10, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1215 Exam III John III. Gelder November 10, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CATAVASII LA NAȘTEREA DOMNULUI DUMNEZEU ȘI MÂNTUITORULUI NOSTRU, IISUS HRISTOS. CÂNTAREA I-A. Ήχος Πα. to os se e e na aș te e e slă ă ă vi i i i i
 CATAVASII LA NAȘTEREA DOMNULUI DUMNEZEU ȘI MÂNTUITORULUI NOSTRU, IISUS HRISTOS. CÂNTAREA I-A Ήχος α H ris to os s n ș t slă ă ă vi i i i i ți'l Hris to o os di in c ru u uri, în tâm pi i n ți i'l Hris
CATAVASII LA NAȘTEREA DOMNULUI DUMNEZEU ȘI MÂNTUITORULUI NOSTRU, IISUS HRISTOS. CÂNTAREA I-A Ήχος α H ris to os s n ș t slă ă ă vi i i i i ți'l Hris to o os di in c ru u uri, în tâm pi i n ți i'l Hris
The far field calculation: Approximate and exact solutions. Persa Kyritsi November 10th, 2005 B2-109
 Th fa fl calculao: Appoa a ac oluo Pa K Novb 0h 005 B-09 Oul Novb 0h 005 Pa K Iouco Appoa oluo flco fo h gou ac oluo Cocluo Pla wav fo Ic fl: pla wav k ( ) jk H ( ) λ λ ( ) Polaao fo η 0 0 Hooal polaao
Th fa fl calculao: Appoa a ac oluo Pa K Novb 0h 005 B-09 Oul Novb 0h 005 Pa K Iouco Appoa oluo flco fo h gou ac oluo Cocluo Pla wav fo Ic fl: pla wav k ( ) jk H ( ) λ λ ( ) Polaao fo η 0 0 Hooal polaao
Faculty of Natural and Agricultural Sciences Chemistry Department. Semester Test 1 MEMO. Analytical Chemistry CMY 283
 Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 MEMO Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 90
Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 MEMO Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 90
Atomic Emission Spectra. and. Flame Tests. Burlingame High School Chemistry
 Atomic Structure Atomic Emission Spectra and Flame Tests Flame Tests Sodium potassium lithium When electrons are excited they bump up to a higher energy level. As they bounce back down they release energy
Atomic Structure Atomic Emission Spectra and Flame Tests Flame Tests Sodium potassium lithium When electrons are excited they bump up to a higher energy level. As they bounce back down they release energy
Quick Review. ESE319 Introduction to Microelectronics. and Q1 = Q2, what is the value of V O-dm. If R C1 = R C2. s.t. R C1. Let Q1 = Q2 and R C1
 Quick Review If R C1 = R C2 and Q1 = Q2, what is the value of V O-dm? Let Q1 = Q2 and R C1 R C2 s.t. R C1 > R C2, express R C1 & R C2 in terms R C and ΔR C. If V O-dm is the differential output offset
Quick Review If R C1 = R C2 and Q1 = Q2, what is the value of V O-dm? Let Q1 = Q2 and R C1 R C2 s.t. R C1 > R C2, express R C1 & R C2 in terms R C and ΔR C. If V O-dm is the differential output offset
EE415/515 Fundamentals of Semiconductor Devices Fall 2012
 3 EE4555 Fudmls of Smicoducor vics Fll cur 8: PN ucio iod hr 8 Forwrd & rvrs bis Moriy crrir diffusio Brrir lowrd blcd by iffusio rducd iffusio icrsd mioriy crrir drif rif hcd 3 EE 4555. E. Morris 3 3
3 EE4555 Fudmls of Smicoducor vics Fll cur 8: PN ucio iod hr 8 Forwrd & rvrs bis Moriy crrir diffusio Brrir lowrd blcd by iffusio rducd iffusio icrsd mioriy crrir drif rif hcd 3 EE 4555. E. Morris 3 3
Modified from: Larry Scheffler Lincoln High School IB Chemistry 1-2.1
 Modified from: Larry Scheffler Lincoln High School IB Chemistry 1-2.1 The development of the periodic table brought a system of order to what was otherwise an collection of thousands of pieces of information.
Modified from: Larry Scheffler Lincoln High School IB Chemistry 1-2.1 The development of the periodic table brought a system of order to what was otherwise an collection of thousands of pieces of information.
Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
1- I. M. ALGHROUZ: A New Approach To Fractional Derivatives, J. AOU, V. 10, (2007), pp
 Jourl o Al-Qus Op Uvrsy or Rsrch Sus - No.4 - Ocobr 8 Rrcs: - I. M. ALGHROUZ: A Nw Approch To Frcol Drvvs, J. AOU, V., 7, pp. 4-47 - K.S. Mllr: Drvvs o or orr: Mh M., V 68, 995 pp. 83-9. 3- I. PODLUBNY:
Jourl o Al-Qus Op Uvrsy or Rsrch Sus - No.4 - Ocobr 8 Rrcs: - I. M. ALGHROUZ: A Nw Approch To Frcol Drvvs, J. AOU, V., 7, pp. 4-47 - K.S. Mllr: Drvvs o or orr: Mh M., V 68, 995 pp. 83-9. 3- I. PODLUBNY:
Earth Materials I Crystal Structures
 Earth Materials I Crystal Structures Isotopes same atomic number, different numbers of neutrons, different atomic mass. Ta ble 1-1. Su mmar y of quantu m num bers Name Symbol Values Principal n 1, 2,
Earth Materials I Crystal Structures Isotopes same atomic number, different numbers of neutrons, different atomic mass. Ta ble 1-1. Su mmar y of quantu m num bers Name Symbol Values Principal n 1, 2,
CHM 101 PRACTICE TEST 1 Page 1 of 4
 CHM 101 PRACTICE TEST 1 Page 1 of 4 Please show calculations (stuffed equations) on all mathematical problems!! On the actual test, "naked answers, with no work shown, will receive no credit even if correct.
CHM 101 PRACTICE TEST 1 Page 1 of 4 Please show calculations (stuffed equations) on all mathematical problems!! On the actual test, "naked answers, with no work shown, will receive no credit even if correct.
pn Junction Under Reverse-Bias Conditions 3.3 Physical Operation of Diodes
 3.3 Physcal Orato of os Jucto Ur vrs-bas Cotos rft Currt S : ato to th ffuso Currt comot u to majorty carrr ffuso, caus by thrmally grat morty carrrs, thr ar two currt comots lctros mov by rft from to
3.3 Physcal Orato of os Jucto Ur vrs-bas Cotos rft Currt S : ato to th ffuso Currt comot u to majorty carrr ffuso, caus by thrmally grat morty carrrs, thr ar two currt comots lctros mov by rft from to
30 Zn(s) 45 Rh. Pd(s) Ag(s) Cd(s) In(s) Sn(s) white. 77 Ir. Pt(s) Au. Hg(l) Tl. 109 Mt. 111 Uuu. 112 Uub. 110 Uun. 65 Tb. 62 Sm. 64 Gd. 63 Eu.
 Enthalpy changes: experimentally it is much easier to measure heat flow at const pressure - this is enthalpy q p = )H : also nearly all chemical reactions are done at constant pressure. Enthalpy (heat)
Enthalpy changes: experimentally it is much easier to measure heat flow at const pressure - this is enthalpy q p = )H : also nearly all chemical reactions are done at constant pressure. Enthalpy (heat)
signal amplification; design of digital logic; memory circuits
 hatr Th lctroc dvc that s caabl of currt ad voltag amlfcato, or ga, cojucto wth othr crcut lmts, s th trasstor, whch s a thr-trmal dvc. Th dvlomt of th slco trasstor by Bard, Bratta, ad chockly at Bll
hatr Th lctroc dvc that s caabl of currt ad voltag amlfcato, or ga, cojucto wth othr crcut lmts, s th trasstor, whch s a thr-trmal dvc. Th dvlomt of th slco trasstor by Bard, Bratta, ad chockly at Bll
Chapter 4 Transients. Chapter 4 Transients
 Chapter 4 Transients Chapter 4 Transients 1. Solve first-order RC or RL circuits. 2. Understand the concepts of transient response and steady-state response. 1 3. Relate the transient response of first-order
Chapter 4 Transients Chapter 4 Transients 1. Solve first-order RC or RL circuits. 2. Understand the concepts of transient response and steady-state response. 1 3. Relate the transient response of first-order
Faculty of Natural and Agricultural Sciences Chemistry Department. Semester Test 1. Analytical Chemistry CMY 283. Time: 120 min Marks: 100 Pages: 6
 Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 120 min
Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 120 min
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start. Page # Points possible Points awarded
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
CHEM 171 EXAMINATION 1. October 9, Dr. Kimberly M. Broekemeier. NAME: Key
 CHEM 171 EXAMINATION 1 October 9, 008 Dr. Kimberly M. Broekemeier NAME: Key I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gase s 1 H 1.008 Li.941 11 Na.98 19 K 9.10 7
CHEM 171 EXAMINATION 1 October 9, 008 Dr. Kimberly M. Broekemeier NAME: Key I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gase s 1 H 1.008 Li.941 11 Na.98 19 K 9.10 7
Secondary Support Pack. be introduced to some of the different elements within the periodic table;
 Secondary Support Pack INTRODUCTION The periodic table of the elements is central to chemistry as we know it today and the study of it is a key part of every student s chemical education. By playing the
Secondary Support Pack INTRODUCTION The periodic table of the elements is central to chemistry as we know it today and the study of it is a key part of every student s chemical education. By playing the
Made the FIRST periodic table
 Made the FIRST periodic table 1869 Mendeleev organized the periodic table based on the similar properties and relativities of certain elements Later, Henri Moseley organized the elements by increasing
Made the FIRST periodic table 1869 Mendeleev organized the periodic table based on the similar properties and relativities of certain elements Later, Henri Moseley organized the elements by increasing
ε = R d ρ v ρ d ρ m CIVE322 BASIC HYDROLOGY I O = ds dt e s ( 1 T )] (T ) = 611exp[ L R v = = P + R 1 ΔS s + R g R 2 T s I E s
![ε = R d ρ v ρ d ρ m CIVE322 BASIC HYDROLOGY I O = ds dt e s ( 1 T )] (T ) = 611exp[ L R v = = P + R 1 ΔS s + R g R 2 T s I E s ε = R d ρ v ρ d ρ m CIVE322 BASIC HYDROLOGY I O = ds dt e s ( 1 T )] (T ) = 611exp[ L R v = = P + R 1 ΔS s + R g R 2 T s I E s](/thumbs/79/80168614.jpg) CVE3 BSC HYDROOGY Hydrlgc Scc ad Egrg Cvl ad Evrmal Egrg Dparm Fr Clls, CO 853-37 Fall (97 49-76 Hydrlgc Budg Equas O ds d S ds d O d S ΔS s P + R R + R g E s s Saura vapr prssur s ( 6xp[ ( 73.5 ] ε.6
CVE3 BSC HYDROOGY Hydrlgc Scc ad Egrg Cvl ad Evrmal Egrg Dparm Fr Clls, CO 853-37 Fall (97 49-76 Hydrlgc Budg Equas O ds d S ds d O d S ΔS s P + R R + R g E s s Saura vapr prssur s ( 6xp[ ( 73.5 ] ε.6
EE 529 Remote Sensing Techniques. Review
 59 Rmo Snsing Tchniqus Rviw Oulin Annna array Annna paramrs RCS Polariaion Signals CFT DFT Array Annna Shor Dipol l λ r, R[ r ω ] r H φ ηk Ilsin 4πr η µ - Prmiiviy ε - Prmabiliy
59 Rmo Snsing Tchniqus Rviw Oulin Annna array Annna paramrs RCS Polariaion Signals CFT DFT Array Annna Shor Dipol l λ r, R[ r ω ] r H φ ηk Ilsin 4πr η µ - Prmiiviy ε - Prmabiliy
Chemistry 185 Exam #2 - A November 5, Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
