CHAPTER VII. Thermometry
|
|
|
- Aubrey Goodman
- 6 years ago
- Views:
Transcription
1 CHAPTER VII Thermometry 1. Introduction Temperature is a measure of the degree of warmth or cold in a material. We can perceive through touch that one bath of water is warmer than another. To make such a concept precise we need to define a quantity called temperature in such a way that the higher the value of the temperature of a body, the hotter it is. We also have to devise practical and convenient methods of measuring temperature. The definition and methods must be such as to lead to the same value of the temperature of a body measured by different observers, using different methods. A thermometer is a device to measure temperature. It uses a property of a material that changes as it is heated or cooled. This property can be the volume of the material, or the pressure of a gas at constant volume or some electrical property such as the resistance. We have to choose two different baths and arbitrarily set a value for the temperature T 1 for the cold bath and a value T 2, higher than T 1, for the temperature of the warmer bath. We bring the thermometric device in contact with the bath, wait till thermal equilibrium is achieved, and measure the value P of the property. Let P(T 1 ) be the property value when the material is kept in the cold standard bath and P(T 2 ) be its property value when the material is kept in the warm standard bath. If we now have a bath, the temperature T of which is to be measured, the thermometer is brought to thermal equilibrium with this bath and the property value P is measured. The temperature T of this bath is then defined by the relation T = T 1 +{[P P(T 1 )]/ [ P(T 2 ) P(T 1 )]} (T 2 T 1 ) (VII.1.1) The temperature of a bath defined in this way will depend both on the property that is measured and the material of the thermometer. For example, if we take the property to be the resistance of a metallic wire which forms the thermometer, there is no justifiable reason to expect that the way the resistance changes with temperature will be the same for wires of different material. This implies that temperature defined in this fashion will not be absolute in the sense that measurements made with different material and using different properties will generally give different values for the temperature of the same bath. This will create chaos. To define an absolute temperature, we have to look for a device and a property such that the variation of the property with temperature will be independent of the working substance used. The device may only be a theoretical one that will not be possible to be realized in practice. Still, such an absolute temperature scale will be useful since it 93
2 provides a benchmark against which the actual performance of practical thermometers can be tested. 2. Absolute temperature The Carnot engine is a heat engine in which the working substance undergoes a cycle of changes in its thermodynamic state. In each cycle it absorbs a quantity, Q 1, of heat from a warm reservoir and delivers a smaller quantity, Q 2, of heat to a cold reservoir. The difference W = (Q 1 Q 2 ) is available as work. The important feature of the Carnot cycle is that its efficiency, η, defined by the relation η = W/Q 1 = (1 Q 2 / Q 1 ) (VII.2..1) is independent of the working substance in the heat engine or the nature of the property used in converting heat to work in the heat engine. This is a consequence of the second law of thermodynamics. So the efficiency of a Carnot engine can be used to define an absolute temperature scale. If we take the temperature of the standard cold reservoir as T 2, and the warm reservoir has a temperature T, we may define the efficiency by η = (1 T 2 /T) (VII.2.2) The coldest reservoir that one can use must have a temperature T 2 = 0 since the efficiency of any engine cannot be greater than 1. This is called the Absolute Zero of Temperature and all bodies must have a positive temperature on this scale. A scale of temperature defined thus is called the absolute or Kelvin scale Ideal Gas Scale While it is nice to be able to define such an absolute scale, is there any practical way of realizing the Kelvin scale? Here the ideal gas laws come to our help. Boyle and Charles performed experiments on the pressure, P, of a given volume, v, of a gas in different warm baths. They found that if the gas is at low pressure, the product of the pressure and volume, Pv, remains constant in a given bath. This quantity increases as the bath becomes warmer. One can therefore take a mole of a gas at low pressure and determine the product PV (here V is the volume of 1 mole of the gas) in different baths. One can define a practical temperature scale using this product PV. If we take the temperature of a bath of melting ice as 0 and temperature of water boiling under atmospheric pressure as 100, 1 we may define a gas scale of temperature by T = {[PV(T) PV(0) ] / [PV(100) PV(0)]} 100 (VII.2.3) This relation implies that on this scale PV = C + RT (VII.2.4) 94
3 The above constant, C, can be written as RT 0. In such a case, PV = R (T+T 0 ) (VII..2.5) Experiments show that (a) in the limit of low pressure all gases show the same behaviour and (b) the constants R and T 0 have the same values for all gases. The constant R has a value Joules/K and T 0 has a value We may now define a new scale of temperature, called the Ideal Gas Temperature Scale, in which the ice point temperature T 2 ig = and the temperature of boiling point water under atmospheric pressure T 1 ig = This is also called the Celsius Scale. We may use any gas as our thermometric substance and measure the pressure at a constant volume of the gas. The temperature of a bath deduced from such a measurement, in the limit of pressure of the gas tending to zero, will be the same. Also it will be independent of the gas. The relation PV = RT ig (VII.2.6) is called the Charles law. At high pressures, the density of the gas becomes high and interatomic interactions play a role in causing deviations from Charles law Relation of ideal gas scale to the absolute scale How does the ideal gas scale of temperature relate to the absolute temperature scale based on Carnot cycle? If we use an ideal gas as the working substance in the Carnot engine and calculate from Charles law the heat, Q 1, absorbed at the warm temperature T ig and the heat, Q 2, rejected at the cold temperature T 2 ig we find And hence, Q 2ig /Q 1ig = T 2ig /T ig η = (1 T 2ig /T ig ) (VII.2.7) (VII.2.8) Comparing the equations (VII.2.2) and (VII.2.8), we find that the ideal gas temperature T ig should be proportional to the absolute temperature T K. It is convenient to choose the constant of proportionality to be unity. Hence we see that the ideal gas temperature scale coincides with the absolute temperature scale. So the absolute temperature scale is realizable in practice using a gas thermometer filled at very low pressure (i. e. in the limit P tending to zero). The ideal gas thermometer is called a primary thermometer. In actual practice a gas thermometer can be filled only at a finite pressure and there will be small deviations from Charles law. But one can apply corrections for temperatures measured by the gas thermometer and obtain the temperature on the absolute scale. These corrections will depend on the nature of the gas used. 95
4 In practice one cannot use the gas thermometer in experiments. One will have to use secondary thermometers that are small in size and weight and that are more convenient for measurement and control. There are a variety of such thermometers available. These are calibrated at a few fixed points using certain interpolation formulae as agreed upon by International convention. We shall describe the International Temperature Scale currently in use. 3. International temperature scale (ITS90) The ITS90 was adopted by the International Commission on Weights and Measures in This scale supercedes the practical temperature scale of 1968 and the provisional 0.5 to 30 K Temperature Scale of The temperature measured on the ITS90 scale is written as T 90. The ITS 90 is defined in different temperature ranges as follows: Between 0.65 to 5 K, T 90 is defined in terms of vapour pressure of 3 He and 4 He. The temperature T 90 is represented in terms of the vapour pressure by the following equation: 9 T 90 = A 0 + Σ A i { [ln(p) B]/C} i (VII.3.1) i = 1 The vapour pressure P is in Pascals. Table VII.1 gives the constants for 3 He and 4 He and the temperature range in which the constants are valid is indicated. 96
5 Between 4.2 K to triple point of Ne, a 4 He gas thermometer calibrated at three fixed points (at the triple point of Ne, triple point of e-hydrogen, and one other temperature between 3 and 5 K as determined by vapour pressure thermometer) is used. The equation to be used to obtain T 90 is T 90 = a + bp +cp 2 (VII.3.2) Here P is the pressure of the Helium gas. The constants a, b and c are determined from the calibration at the above three temperatures. Between the triple point of e- hydrogen and melting point of silver, a platinum resistance thermometer calibrated at specified sets of defined fixed points using specific interpolations defines T 90. We define W(T 90 ) by the ratio R(T 90 )/R(273.16) where R(T) is the resistance of the Pt thermometer at temperature T. The thermometer should be made of pure strain-free Platinum wire satisfying at least one of the following two conditions: W (MP of Ga) (VII.3.3) and W (TP of Hg) (VII.3.4) If it is to be used till the freezing point of Ag it must also satisfy the condition W (FP of Ag) (VII.3.5) From to K the temperature can be calculated from W (T) by the relation 15 T 90 = B 0 + Σ i B i [{W 1/6 (T) 0.65)/0.35] i i=1 (VII.3.6) The constants B i are listed in Table VII.2. Table VII.3 gives the temperature of a few fixed points. 97
6 4. Secondary thermometers A secondary thermometer is a device of low thermal capacity having a property, which changes with temperature. It should have a well-characterized and reproducible response to variations in temperature. It will be useful if the response can be fitted to a welldefined equation containing a few parameters that can be obtained by calibrating the thermometer at a few fixed points. The sensitivity of the thermometer (i.e. the change in signal for one degree change in temperature) should be high. At the same time the thermometer should respond quickly to changes in temperature. This is achieved by making the thermal capacity of the thermometer small and having a good thermal contact with the object, the temperature of which is to be measured. For cryogenic thermometry it is necessary to have long leads connecting the sensor at low temperature to electrical leads at room temperature. One must take adequate precautions to see that the heat leaking through the leads is small. Otherwise the temperature indicated by the thermometer will be higher than the actual temperature of the object. One should use as thin and as long leads as possible to increase the thermal resistance of the leads. It will be good to anchor the leads to a point that is at the temperature of the object at low temperature. Then all the heat from room temperature will go to the cold object and ultimately to the refrigerating medium used to cool the object. Heat leak to the thermometer will become small. This is indicated in Figure VII.1. The thermometer leads from the feed through (FT) at the room temperature flange is wrapped round the specimen chamber and fixed to it with a thin layer of GE varnish before being connected to the thermometer. Such anchoring is necessary in all thermometric measurements at low temperature. 98
7 5. Resistance thermometers One of the most widely used techniques for measurement of temperature is resistance thermometry. In this technique the resistance of a material is measured and the temperature is deduced from the value of resistance. One can have materials with a positive temperature coefficient (PTC) of resistance. In these materials the resistance increases with increase in temperature. This is usually the behaviour of metals and metallic alloys. The semi-conducting materials, on the other hand, have a negative temperature coefficient (NTC) of resistance. In these materials, the resistance increases (often by a few orders of magnitude) as the sensor is cooled. In the case of PTC sensors one uses a constant current source to drive a constant current through the material. The voltage across the sensor is measured with a digital voltmeter. As the temperature falls the power dissipated in the sensor will decrease. With NTC materials, it is usual to use a constant voltage across the resistor and measure the current. The power dissipated is inversely proportional to the resistance. So, with this method of measurement, the power dissipated will decrease in NTC sensors as the sensor cools. 99
8 5. 1. Self Heating Why are we worried about the power dissipated in the sensor? This power will have to be conducted away to the cold object, the temperature of which is being measured. If the thermal resistance of the contact between the thermometer and the object is ρ contact and P is the power dissipated in the sensor during measurement, there will be a temperature difference T between the thermometer and the object given by T = ρ contact P (VII.5.1) If the object temperature is T, the temperature recorded by the thermometer will be T+ T. This is called the self-heating effect. The contact resistance increases as the temperature falls and varies as T 3 below 1 K. It is therefore necessary to keep P small enough so that T is a small fraction of T. The lower the temperature the smaller should be the value of P. That is the reason for using a constant current mode of measurement with PTC sensors and a constant voltage mode of measurement with NTC sensors. The power dissipated depends on the square of the current while the measuring signal will be proportional to the current. It is therefore possible to choose a current value that will give a large enough signal to measure the temperature accurately and that, at the same time, will keep the power dissipated small enough to make the error T smaller than a pre-assigned value Two Wire and Four-Wire Measurement: 100
9 Figures VII..2(a) and 2(b) show the connections for a two-wire and a four-wire measurement of the resistance of the sensor. In the two-wire measurement the DVM measures the voltage across the resistance thermometer and the current leads. So the resistance measured includes the resistance of the current leads, which will vary with temperature in a manner different from the behaviour of the resistance thermometer. Unless the resistance of the current leads is negligibly small compared to the resistance of the thermometer, the measured temperature will be in error. In the four-wire measurement the current and voltage leads are connected to the terminals of RT. So the DVM measures only the voltage across RT and the resistance of the current leads does not enter into the measurement AC Measurement: One can also measure the resistance of RT by AC methods using either a bridge circuit or a lock in amplifier. This involves expensive instrumentation and is justified only if one requires a very high sensitivity in measurement Platinum resistance thermometer (PRT) The platinum resistance thermometer is widely used in the temperature range 20K to room temperature and above. The thermometer is made of a thin, high purity wire of platinum mounted strain-free and encapsulated in a ceramic tube or a glass tube. It comes with two leads. The PRT has a diameter of about 2 mm and a length varying between 5 to 20 mm. For four-wire measurement the voltage and current leads will have to be soldered to the two leads of the PRT. The resistance thermometer usually has a nominal resistance of 100 Ohms at K. For such a thermometer the sensitivity is roughly about 0.4 Ohm/K from 40 K to 500 K. Below 30 K the sensitivity falls off steeply. The temperature variation of resistance is shown in Figure VII
10 If the thermometer is calibrated, its accuracy in measuring temperature is around 5 to 10 mk and reproducibility is also of the same magnitude. The response time of the thermometer depends on (a) its mass and (b) the temperature. The response time is a few seconds at low temperature and increases as the temperature increases. The excitation current is usually 1 ma, which will give a self-heating power of 100 microwatts at 273 K. The Platinum thermometer can be used above 50 K in magnetic fields as the magnetoresistance of platinum causes only an error of a few per cent in the measurement of temperature Rhodium-Iron resistance thermometer The temperature variation of resistance of Rhodium-iron alloy depends on its composition. It is possible to choose a composition for which the resistance increases monotonically in the range 1.4 to 300 K as the temperature increases. Thermometers are made either with a thin film of Rh-Fe alloy or with a thin wire. Because of the small thermal capacity in the former case, the response time is much shorter (of the order of a few milliseconds at 4.2 K to about twenty milliseconds at 300 K) than the response time (a few seconds) with the wire wound thermometer. The sensitivity is high from K. Below 100 K the sensitivity drops till 30 K. The sensitivity rises again to a high value below 10 K. This thermometer is unsuitable for measurement in magnetic fields below 77 K NTC Resistance Thermometers Doped Germanium resistance thermometer 102
11 Germanium (Ge) is a semi-conductor and appropriate doping can alter its electrical conductivity. The resistance increases sharply as the temperature is reduced. Various models are available with different suppliers, for use in the temperature range 0.05 K to 30 K. The rapid increase in sensitivity below 4.2 K makes it well suited for sub-milli Kelvin control of temperature. A typical resistance temperature curve for a GRT is shown in Figure VII.4. It is seen that above 30 K the sensitivity of the thermometer becomes poor. It has to be used with a constant voltage excitation. At 0.5 K the excitation voltage will be a few tens of microvolts and this is increased to 10 mv at 100 K. This excitation voltage is chosen so that the self-heating power at 4.2 K is about 0.1 µw and at 0.5 K is about 0.1 pico- Watt. Typical thermal response time is a few hundred milliseconds at 4.2 K and a few seconds at 77K Cernox Thermometers These are ceramic oxides with a negative temperature coefficient of resistance. They are useful in the temperature range 1.4 to 300 K. These thermometers are characterised by a very low magneto-resistance and so are useful to measure temperature in the presence of high magnetic fields. These are used with constant voltage excitation of a few millivolts such that the self-heating power is about 10 µwatt at 300 K and 0.1 µw at 4.2 K. The thermal response time is a few milliseconds in the range 0.3 to 4.2 K. Figure VII.5 shows the resistance temperature graph of commercial Cernox thermometers. 103
12 Other NTC resistance thermometers Carbon glass sensors, which consist of carbon fibres trapped in a glass matrix, can be used from 1.4 to 300 K. They have a negative temperature coefficient of resistance. Below 10 K they have a high sensitivity. However above 100 K their sensitivity falls below 1Ohm/K. Above 100 K they can only provide rough temperature measurement to an accuracy of 0.1 K. They have a low magneto-resistance, this being negative below 20K. They can be used in magnetic fields as high as 19T and the correction for the temperature amounts only to a few per cent. Ruthenium oxide sensors are thick film thermometers. They are useful in the range 50 mk to 30 K. The magnetic field dependence of resistance is small. Allen-Bradley carbon resistors have been used for thermometry. They are inexpensive. But they need frequent calibration. 6. Diode thermometers At a constant current, the voltage across a forward biased p-n junction diode changes with temperature. This property is used in diode thermometry. The advantage of diode thermometers is their sensitivity (change in forward bias voltage for unit change in temperature) is large and nearly constant from 300 K down to about 25 K in the case of Si diodes and 40 K in the case of GaAs diodes. Below 25 K in Si diodes and 40 K in GaAs diodes, the forward voltage increases rapidly as the temperature decreases. Figure VII.6 Forward voltage of a Si diode and a Ga-As diode as a function of temperature at constant current. The forward voltage as a function of temperature at a constant current is shown in Figure VII.6 for both Si and GaAs diodes. Si diodes are relatively inexpensive. Though they are less sensitive than GaAs diodes they are more stable. Above 1 K one can achieve an accuracy of about ± 25 mk in temperature measurement. The excitation current is 10 µa. Problems can arise if there is an AC component of current from the DC 104
13 source. One should take precautions like proper electrical shielding and grounding to get precise measurements. The GaAs diodes can be used in moderate magnetic fields. 7. Thermocouples Thermocouples have been used often in the past for the measurement of cryogenic temperatures. They are inexpensive. When two dissimilar metals A and B are joined together and the two junctions are maintained at different temperatures T 1 and T 2 an electromotive force is generated which can be measured on a digital voltmeter. The typical measurement arrangement is shown in Figure VII.7. Figure VII.7: A and B are wires of two dissimilar metals joined together to form two junctions maintained at temperatures T 1 and T 2. The open ends of A are connected to the terminals of a DVM. If T 1 is different from T 2 the DVM indicates an emf in millivolts. This is called the thermo-emf. The thermo-emf is a function of temperature difference (T 1 T 2 ) provided the junctions of leads A and A to the terminals of the DVM are at the same temperature. If T 2 is a temperature of a reference bath such as melting ice then one can measure the unknown temperature T 1. Usually the reference bath is dispensed with. The open ends of A and B are connected to the DVM. But at the junction to the DVM a resistance thermometer measures the temperature and produces an appropriate voltage by a bridge circuit. Figure VII. 8: Thermo-emf of standard thermocouples with the reference junction at K 105
14 There are three standard thermocouples used in the range of cryogenic temperature. These are type E (chromel-constantan), type K (chromel-alumel) and type T (copperconstantan). In Figure VII.8 the thermoelectric emf of these standard junctions are plotted in millivolts against temperature T 1, taking the reference temperature to be that of melting ice ( K). The slope of the curve gives the sensitivity. We see that the curves flatten out at the low temperature end. The sensitivity falls at low temperature and so these thermocouples are not usable below 30 K. For example the thermoelectric power at 20 K for E, K and T type thermocouples are 8.5, 4.1, and 4.5 µv/k. Since the wires find themselves in a region where temperature gradient exists, it is essential that the wires A and B must be homogeneous. Otherwise the temperature gradient along the length of the thermocouple wires will produce an emf of its own. The Gold- (0.7 at %) Fe Chromel thermocouple is suited for temperatures below 30 K as its thermo-electric power remains high at 15 µv/k above 10 K. One can measure the temperature to an accuracy of about 1 K. Relative changes in temperature can however be measured more accurately. The advantage of thermocouple thermometry is the low response time of the thermometer. 8. Capacitance thermometers The dielectric constant of strontium titanate shows an appreciable variation with temperature. A capacitance of a few nano-farads made with this material as the dielectric medium can be used as a thermometer below 100 K. The capacitance will have to be measured by an AC bridge with about a few volts of excitation. The disadvantage of these thermometers is that the calibration changes after thermal cycling. They have to be re-calibrated frequently. The advantage of the capacitance thermometer is that it is totally insensitive to magnetic fields. The capacitance thermometer is useful for controlling temperature in the presence of magnetic fields. Their thermal response time is high, of the order of minutes. 9. Magnetic susceptibility thermometer Temperatures of a few tens of a millikelvin can be obtained either by adiabatic demagnetization of a paramagnetic salt or with the help of the dilution refrigerator.. For the measurement of such low temperatures the magnetic susceptibility thermometer can be used. The paramagnetic susceptibility of a dilute system of of magnetic atoms varies with temperature as χ = C / T (VII..9.1) 106
15 This is known as the Curie law. In a solid, two effects cause a change in the behaviour of the susceptibility represented by Curie s law. One is the exchange interaction between neighbouring magnetic atoms, which cause the magnetic moments of the atoms to become ordered below a certain temperature leading to ferro-, antiferro- or ferrimagnetism. This interaction drops off rapidly as the distance between the neighbouring magnetic atoms increases. Secondly the degenerate levels of the ground state of the free atom are split by crystal field effect. The paramagnetic salt should be in good thermal contact with the object the temperature of which is to be measured. The magnetic susceptibility is measured by using a Hartshorne bridge or a ratio transformer system. Cerium magnesium nitrate is the thermometric substance commonly used below 1 K since its magnetic ordering temperature is 4 mk. The other material is Pd containing a few parts per million of iron atoms. The spin freezing temperature of this material is 0.1 mk and it has a giant atomic magnetic moment of 10 Bohr magnetons. 10. Vapour pressure thermometry The pressure exerted by the saturated vapour over the liquid surface is a definite function of temperature and hence can be used to measure the temperature of the liquid. In fact, in the International Scale of Temperature (ITS90), the vapour pressure of 3 He and 4 He are used for defining the temperature scale between 0.65K and 5K. With a good pressure measuring arrangement, the vapour pressure thermometer is an excellent secondary standard, since the temperature response depends on the physical property of a pure element. The vapour pressure is expressed by semi-empirical formulae with fit coefficients. The vapour pressure equations for many common cryogenic fluids are given in Table VII.4 and the constants in Table VII
16 The different fixed points of common vapour pressure thermometers are given in Table VII.6. The vapour pressure thermometer is useful for temperature measurement in the range from the triple point to the critical point of the liquid. One of the important advantages of vapour pressure thermometer is the extreme sensitivity in the range over which they can be used. The disadvantage is that it is useful only over a limited temperature range. The thermometers are most accurate in the range of the normal boiling point of the liquid chosen. The constants for the vapour pressure equations are given in Tables VII.5 and VII
17 The apparatus for vapour pressure determination is quite simple. There are many variations of vapour pressure thermometers, but one described by Scott is shown in Figure VII.9. The copper bulb having the liquid is connected to an accurate mercury manometer through a thin walled vacuum jacketed stainless steel tube and a coiled copper tubing. It should be obvious that the tubing between the bulb and manometer should nowhere be colder than the bulb since this can cause refluxing and the pressure readings will be low. Also, the gas purity is quite important. With Hydrogen, the ortho / para ratio is an important parameter due to the strong dependence of the vapour pressure on this composition. In the case of helium, hydrogen is the possible source of impurity, since all the other impurities will be solidified. As mentioned earlier, the vapour pressure thermometry is quite sensitive. It also has good response and is not affected by magnetic fields and does not need calibration. 11. Conclusion There are other thermometric techniques like noise thermometry and nuclear orientation thermometry which are outside the purview of the book. Since temperature measurement is an important component of any low temperature measurement one must pay special attention to thermometry. 109
18 REFERENCES 1. Thomas M. Flynn, Cryogenic Engineering, Mercel Dekker Inc., N.Y Randall F Barron, Cryogenic Systems, 2 nd edition, Oxford University Press, New York, Scott, R. B., Cryogenic Engineering, D. Van Nostrand Co., Inc., Princeton, New Jersy, McClintock, M., Cryogenics, Reinhold Publishing Corp., New York, Guy K. White, Experimental Techniques in low temperature Physics, 3 rd edition, Oxford University Press, New York, A. C. Rose-Innes, Low Temperature Technique, The English Universities Press Ltd., London, S I Data sheets. 8. Lab Facility, U. K. 9. Lakeshore Cryotronics. 110
Temperature Scales. Temperature, and Temperature Dependent on Physical Properties. Temperature. Temperature Scale
 Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Lecture 11 Temperature Sensing. ECE 5900/6900 Fundamentals of Sensor Design
 EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
Temperature. 3
 Temperature In 1848, Sir William Thomson (Lord Kelvin) stated the zero principle of dynamics. This principle enabled him to define thermodynamic temperature and to establish an objective method of measuring
Temperature In 1848, Sir William Thomson (Lord Kelvin) stated the zero principle of dynamics. This principle enabled him to define thermodynamic temperature and to establish an objective method of measuring
Temperature measurement
 emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
4. Thermometry. Temperature and Heat Flow Temperature Scales Thermometers
 4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
Section 7. Temperature Measurement
 Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Using a Mercury itc with thermocouples
 Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
THERMOCOUPLE CHARACTERISTICS TRAINER
 THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
Sensors and Actuators Sensors Physics
 Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
THERMOMETRY above 1 Kelvin
 THERMOMETRY above 1 Kelvin Florence LEVY-BERTRAND from the Highly correlated systems team www.neel.cnrs.fr 20/09/2011 cryocourse 1 /28 THERMOMETRY above 1 K Introduction Thermometer types Measurement techniques
THERMOMETRY above 1 Kelvin Florence LEVY-BERTRAND from the Highly correlated systems team www.neel.cnrs.fr 20/09/2011 cryocourse 1 /28 THERMOMETRY above 1 K Introduction Thermometer types Measurement techniques
Sensing, Computing, Actuating
 Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect
 Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form
Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form
19. Thermometry in Magnetic Fields
 160 19. Thermometry in Magnetic Fields Most thermometers change their calibration in the presence of a magnetic field. In this chapter we discuss by how much traceability to the original calibration can
160 19. Thermometry in Magnetic Fields Most thermometers change their calibration in the presence of a magnetic field. In this chapter we discuss by how much traceability to the original calibration can
Lecture 36: Temperatue Measurements
 Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
Temperature Measurement
 MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
EXPERIMENT VARIATION OF THERMO-EMF WITH TEMPERATURE. Structure. 7.1 Introduction Objectives
 EXPERIMENT 7 VARIATION OF THERMO-EMF WITH TEMPERATURE Thermo-EMF Structure 7.1 Introduction Objectives 7.2 Working Principle of a Potentiometer 7.3 Measurement of Thermo-EMF and its Variation with Temperature
EXPERIMENT 7 VARIATION OF THERMO-EMF WITH TEMPERATURE Thermo-EMF Structure 7.1 Introduction Objectives 7.2 Working Principle of a Potentiometer 7.3 Measurement of Thermo-EMF and its Variation with Temperature
Thermodynamics INTRODUCTION AND BASIC CONCEPTS. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics INTRODUCTION AND BASIC CONCEPTS Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. THERMODYNAMICS AND ENERGY Thermodynamics: The science of energy.
Thermodynamics INTRODUCTION AND BASIC CONCEPTS Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. THERMODYNAMICS AND ENERGY Thermodynamics: The science of energy.
15. Compare the result with the value you have taken above Compare the calculated pressure value with the actual pressure value that you have
 105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
Chapter 17 Temperature and heat
 Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
I. Introduction and Objectives
 Calibration and Measurement of Temperatures using K, T-Type Thermocouples, and a Thermistor Ben Sandoval 1 8/28/2013 Five K and five T type thermocouples were calibrated using ice water, a hot bath (boiling
Calibration and Measurement of Temperatures using K, T-Type Thermocouples, and a Thermistor Ben Sandoval 1 8/28/2013 Five K and five T type thermocouples were calibrated using ice water, a hot bath (boiling
SENSORS and TRANSDUCERS
 SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
Thermometry at Low and Ultra-low Temperatures
 Thermometry at Low and Ultra-low Temperatures Temperature is a thermodynamic property of state It can be defined by a reversible cycle, like a carnot cycle but this is not very practical General Considerations
Thermometry at Low and Ultra-low Temperatures Temperature is a thermodynamic property of state It can be defined by a reversible cycle, like a carnot cycle but this is not very practical General Considerations
This book is under copyright to A-level Physics Tutor. However, it may be distributed freely provided it is not sold for profit.
 2 This book is under copyright to A-level Physics Tutor. However, it may be distributed freely provided it is not sold for profit. CONTENTS thermometry what is temperature?, fixed points, Kelvin (Absolute),
2 This book is under copyright to A-level Physics Tutor. However, it may be distributed freely provided it is not sold for profit. CONTENTS thermometry what is temperature?, fixed points, Kelvin (Absolute),
Dr.Salwa Alsaleh fac.ksu.edu.sa/salwams
 Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams What is Temperature? It is the measurement of the AVERAGE kinetic energy of the particles of matter. Temperature We associate the concept of temperature
Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams What is Temperature? It is the measurement of the AVERAGE kinetic energy of the particles of matter. Temperature We associate the concept of temperature
Temperature Measurements
 Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
MEASURING INSTRUMENTS
 Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Temperature Measurement
 Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
ECNG3032 Control and Instrumentation I
 sensor ECNG3032 Control and Instrumentation I Lecture 1 Temperature Sensors Sensors The sensor is the first element in the measurement system. Measurand Transducer Principle Excitation Signal Interface
sensor ECNG3032 Control and Instrumentation I Lecture 1 Temperature Sensors Sensors The sensor is the first element in the measurement system. Measurand Transducer Principle Excitation Signal Interface
Chapter 1 INTRODUCTION AND BASIC CONCEPTS
 Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu University of Gaziantep
Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu University of Gaziantep
Properties of Gases. The perfect gas. States of gases Gas laws Kinetic model of gases (Ch th ed, th ed.) Real gases
 Properties of Gases Chapter 1 of Physical Chemistry - 6th Edition P.W. Atkins. Chapter 1 and a little bit of Chapter 24 of 7th Edition. Chapter 1 and a little bit of Chapter 21 of 8th edition. The perfect
Properties of Gases Chapter 1 of Physical Chemistry - 6th Edition P.W. Atkins. Chapter 1 and a little bit of Chapter 24 of 7th Edition. Chapter 1 and a little bit of Chapter 21 of 8th edition. The perfect
Measurements of ultralow temperatures
 Measurements of ultralow temperatures Anssi Salmela 1 Outline Motivation Thermometry below 1K Methods below 1K (Adiabatic melting experiment) 2 Motivation Why tedious refrigeration is worthwhile? Reduced
Measurements of ultralow temperatures Anssi Salmela 1 Outline Motivation Thermometry below 1K Methods below 1K (Adiabatic melting experiment) 2 Motivation Why tedious refrigeration is worthwhile? Reduced
1 Written and composed by: Prof. Muhammad Ali Malik (M. Phil. Physics), Govt. Degree College, Naushera
 CURRENT ELECTRICITY Q # 1. What do you know about electric current? Ans. Electric Current The amount of electric charge that flows through a cross section of a conductor per unit time is known as electric
CURRENT ELECTRICITY Q # 1. What do you know about electric current? Ans. Electric Current The amount of electric charge that flows through a cross section of a conductor per unit time is known as electric
Cryogenic Instrumentation I Thermometry OUTLINE Thermometry Pt (pure metal) Temperature Ranges of Thermometer Application Typical Resistive Thermal
 Cryogenic Instrumentation I 1. Thermometry 2. anges of Application 3. Constant Volume 4. Thermocouples 5. Time esponse Data 6. 4 Terminal esistance Measurement OUTLINE 8. Pt (pure metal) 9. Typical esistive
Cryogenic Instrumentation I 1. Thermometry 2. anges of Application 3. Constant Volume 4. Thermocouples 5. Time esponse Data 6. 4 Terminal esistance Measurement OUTLINE 8. Pt (pure metal) 9. Typical esistive
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test
 ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
ASEN 2002 Experimental Laboratory 1: Temperature Measurement and an Blow Dryer Test Assigned 6 September 2000 Individual Lab Reports due 3 October 2000 OBJECTIVES Learn the basic concepts and definitions
I m. R s. Digital. R x. OhmmetersxSeries Shunt Digital. R m
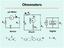 µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
The International Temperature Scale of 1990
 The International Temperature Scale of 199 metrologia Springer-Verlag 199 This copy incorporates textual corrections detailed in Metrologia 27, 17 (199) The International Temperature Scale of 199 (ITS-9)
The International Temperature Scale of 199 metrologia Springer-Verlag 199 This copy incorporates textual corrections detailed in Metrologia 27, 17 (199) The International Temperature Scale of 199 (ITS-9)
AE 3051, Lab #16. Investigation of the Ideal Gas State Equation. By: George P. Burdell. Group E3
 AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
I. Yang, C. H. Song, Y.-G. Kim & K. S. Gam
 Cryostat for Fixed-Point Calibration of Capsule-Type SPRTs I. Yang, C. H. Song, Y.-G. Kim & K. S. Gam International Journal of Thermophysics Journal of Thermophysical Properties and Thermophysics and Its
Cryostat for Fixed-Point Calibration of Capsule-Type SPRTs I. Yang, C. H. Song, Y.-G. Kim & K. S. Gam International Journal of Thermophysics Journal of Thermophysical Properties and Thermophysics and Its
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Cryogenic Engineering
 2017 Fall Semester Cryogenic Engineering 2017 Fall Semester Kim, Min Soo Chapter 6. Measurement Systems for Low Temperatures 6.1 Theoretical plate calculations for columns Temperature measurement from
2017 Fall Semester Cryogenic Engineering 2017 Fall Semester Kim, Min Soo Chapter 6. Measurement Systems for Low Temperatures 6.1 Theoretical plate calculations for columns Temperature measurement from
Measurement of Temperature in the Plastics Industry
 Sawi Mess- und Regeltechnik AG CH 8405 Winterthur-Gotzenwil, Switzerland Telephone +41 52 320 50 50, Fax +41 52 320 50 55 www.sawi.ch Measurement of Temperature in the Plastics Industry Johannes Wild,
Sawi Mess- und Regeltechnik AG CH 8405 Winterthur-Gotzenwil, Switzerland Telephone +41 52 320 50 50, Fax +41 52 320 50 55 www.sawi.ch Measurement of Temperature in the Plastics Industry Johannes Wild,
National 5 Physics. Electricity and Energy. Notes
 National 5 Physics Electricity and Energy Notes Name. 1 P a g e Key Area Notes, Examples and Questions Page 3 Conservation of energy Page 10 Electrical charge carriers and electric fields and potential
National 5 Physics Electricity and Energy Notes Name. 1 P a g e Key Area Notes, Examples and Questions Page 3 Conservation of energy Page 10 Electrical charge carriers and electric fields and potential
5. Temperature and Heat
 Leaving Cert Physics Long Questions 2017-2002 5. Temperature and Heat Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Contents Temperature:
Leaving Cert Physics Long Questions 2017-2002 5. Temperature and Heat Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Contents Temperature:
Resistivity and Temperature Coefficients (at 20 C)
 Homework # 4 Resistivity and Temperature Coefficients (at 0 C) Substance Resistivity, Temperature ( m) Coefficient, (C ) - Conductors Silver.59 x 0-0.006 Copper.6 x 0-0.006 Aluminum.65 x 0-0.0049 Tungsten
Homework # 4 Resistivity and Temperature Coefficients (at 0 C) Substance Resistivity, Temperature ( m) Coefficient, (C ) - Conductors Silver.59 x 0-0.006 Copper.6 x 0-0.006 Aluminum.65 x 0-0.0049 Tungsten
Thermometry. History. History 1/21/18. The art or science of temperature observation
 Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and
Thermometry The art or science of temperature observation History No one person credited with the invention of the thermometer; developed over time Avicenna used this principal to show that hotness and
THERMODYNAMICS SSC-JE STAFF SELECTION COMMISSION MECHANICAL ENGINEERING STUDY MATERIAL THERMODYNAMICS THERMODYNAMICS THERMODYNAMICS
 1 SSC-JE STAFF SELECTION COMMISSION MECHANICAL ENGINEERING STUDY MATERIAL 2 Syllabus: Thermal Engineering (Thermodynamics) Properties of Pure Substances : p-v & P-T diagrams of pure substance like H 2
1 SSC-JE STAFF SELECTION COMMISSION MECHANICAL ENGINEERING STUDY MATERIAL 2 Syllabus: Thermal Engineering (Thermodynamics) Properties of Pure Substances : p-v & P-T diagrams of pure substance like H 2
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
Earlier Lecture. In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide.
 41 1 Earlier Lecture In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide. Silicon diodes have negligible i 2 R losses. Cernox RTDs offer high response
41 1 Earlier Lecture In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide. Silicon diodes have negligible i 2 R losses. Cernox RTDs offer high response
Part 2. Sensor and Transducer Instrument Selection Criteria (3 Hour)
 Part 2 Sensor and Transducer Instrument Selection Criteria (3 Hour) At the end of this chapter, you should be able to: Describe the definition of sensor and transducer Determine the specification of control
Part 2 Sensor and Transducer Instrument Selection Criteria (3 Hour) At the end of this chapter, you should be able to: Describe the definition of sensor and transducer Determine the specification of control
HEAT AND TEMPERATURE Vikasana-Bridge Course 2012
 HEAT AND TEMPERATURE TOPICS Introduction Effects of heat Specific heat Basics of thermodynamics Introduction Heat may be defined as energy in transit from a high temperature region to a lower temperature
HEAT AND TEMPERATURE TOPICS Introduction Effects of heat Specific heat Basics of thermodynamics Introduction Heat may be defined as energy in transit from a high temperature region to a lower temperature
THERMODYNAMICS THEORY & OBJECTIVE CORPORATE OFFICE
 THEORY & OBJECTIVE THERMODYNAMICS State Engineering Services Examinations. ublic Sector Examinations. JEn (SSC, DMRC & State Level). Other Technical Competitive Exams. CORORATE OFFICE 100-102, Ram Nagar,
THEORY & OBJECTIVE THERMODYNAMICS State Engineering Services Examinations. ublic Sector Examinations. JEn (SSC, DMRC & State Level). Other Technical Competitive Exams. CORORATE OFFICE 100-102, Ram Nagar,
Magnetic systems for refrigeration & thermometry
 Magnetic systems for refrigeration & thermometry * Paramagnetic salts * * Hyperfine enhanced systems * * Nuclear spins * Magnetic moments can be aligned by external magnetic field Thermal disorder counteracts
Magnetic systems for refrigeration & thermometry * Paramagnetic salts * * Hyperfine enhanced systems * * Nuclear spins * Magnetic moments can be aligned by external magnetic field Thermal disorder counteracts
Energy. E d. Energy Power = time. E t P = E t = P
 Energy Forms of energy Energy can never be created or destroyed. It can only be transformed from one type to another (or other types). here are many different forms of energy: Kinetic (movement) Energy
Energy Forms of energy Energy can never be created or destroyed. It can only be transformed from one type to another (or other types). here are many different forms of energy: Kinetic (movement) Energy
Thermoelectric effect
 Thermoelectric effect See Mizutani the temperature gradient can also induce an electrical current. linearized Boltzmann transport equation in combination with the relaxation time approximation. Relaxation
Thermoelectric effect See Mizutani the temperature gradient can also induce an electrical current. linearized Boltzmann transport equation in combination with the relaxation time approximation. Relaxation
Chapter 18. Temperature, Heat, and the First Law of Thermodynamics Temperature
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18.2 Temperature 18.3: The Zeroth aw of Thermodynamics If bodies A and B are each in thermal equilibrium with a third body T, then A and
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18.2 Temperature 18.3: The Zeroth aw of Thermodynamics If bodies A and B are each in thermal equilibrium with a third body T, then A and
3 Electric current, resistance, energy and power
 3 3.1 Introduction Having looked at static charges, we will now look at moving charges in the form of electric current. We will examine how current passes through conductors and the nature of resistance
3 3.1 Introduction Having looked at static charges, we will now look at moving charges in the form of electric current. We will examine how current passes through conductors and the nature of resistance
Part I. Temperature Measurements in the Range from 0.1 K to 300 K
 Part I Temperature Measurements in the Range from 0.1 K to 300 K Introduction Part I describes modem methods for measuring temperatures lower than O C based on the use of substances that are gaseous at
Part I Temperature Measurements in the Range from 0.1 K to 300 K Introduction Part I describes modem methods for measuring temperatures lower than O C based on the use of substances that are gaseous at
UNIT II CURRENT ELECTRICITY
 UNIT II CUENT ELECTICITY Weightage : 07 Marks Electric current; flow of electric charges in a metllic conductor, drift velocity, mobility and their relation with electric current. Ohm s law electrical
UNIT II CUENT ELECTICITY Weightage : 07 Marks Electric current; flow of electric charges in a metllic conductor, drift velocity, mobility and their relation with electric current. Ohm s law electrical
Unit 11: Temperature and heat
 Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
PHYS102 Previous Exam Problems. Temperature, Heat & The First Law of Thermodynamics
 PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical Simulation and Measurement
 Publication Reference EA-10/11 EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical PURPOSE This document has been produced by EA as a means of giving advice for calibrating
Publication Reference EA-10/11 EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical PURPOSE This document has been produced by EA as a means of giving advice for calibrating
A thermodynamic system is taken from an initial state X along the path XYZX as shown in the PV-diagram.
 AP Physics Multiple Choice Practice Thermodynamics 1. The maximum efficiency of a heat engine that operates between temperatures of 1500 K in the firing chamber and 600 K in the exhaust chamber is most
AP Physics Multiple Choice Practice Thermodynamics 1. The maximum efficiency of a heat engine that operates between temperatures of 1500 K in the firing chamber and 600 K in the exhaust chamber is most
CHAPTER - 12 THERMODYNAMICS
 CHAPER - HERMODYNAMICS ONE MARK QUESIONS. What is hermodynamics?. Mention the Macroscopic variables to specify the thermodynamics. 3. How does thermodynamics differ from Mechanics? 4. What is thermodynamic
CHAPER - HERMODYNAMICS ONE MARK QUESIONS. What is hermodynamics?. Mention the Macroscopic variables to specify the thermodynamics. 3. How does thermodynamics differ from Mechanics? 4. What is thermodynamic
Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power).
 Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power). Thermodynamics: The science of energy. Conservation of energy principle: During an interaction, energy
Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power). Thermodynamics: The science of energy. Conservation of energy principle: During an interaction, energy
Good practice guide containing experimental results and recommendations for the selection, preparation and calibration of the temperature sensors
 Good practice guide containing experimental results and recommendations for the selection, preparation and calibration of the temperature sensors 1. Scope... 2 2. Introduction... 2 3. Selection of thermocouples
Good practice guide containing experimental results and recommendations for the selection, preparation and calibration of the temperature sensors 1. Scope... 2 2. Introduction... 2 3. Selection of thermocouples
NEEL Phase Change in Chromium At the Néel Temperature
 University of Toronto ADVANCED PHYSICS LABOATOY NEEL Phase Change in Chromium At the Néel Temperature evisions: January 2018: January/August 2016: October 2005: Original: Young-June Kim David Bailey
University of Toronto ADVANCED PHYSICS LABOATOY NEEL Phase Change in Chromium At the Néel Temperature evisions: January 2018: January/August 2016: October 2005: Original: Young-June Kim David Bailey
The Underground Experimental Investigation of Thermocouples
 The Underground Experimental Investigation of Thermocouples April 14, 21 Abstract This experiment investigates a K-type thermocouple in order to demonstrate the Seebeck and Peltier coefficients. Temperature
The Underground Experimental Investigation of Thermocouples April 14, 21 Abstract This experiment investigates a K-type thermocouple in order to demonstrate the Seebeck and Peltier coefficients. Temperature
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Temperature Measurement
 Temperature Measurement Concepts Concept from Claudius Galenus 8 Mixtures of Ice/boiling water Latin temperatura (blending/mixing) At left Galileo s Florentine Thermograph Temperature Measurement Florentine
Temperature Measurement Concepts Concept from Claudius Galenus 8 Mixtures of Ice/boiling water Latin temperatura (blending/mixing) At left Galileo s Florentine Thermograph Temperature Measurement Florentine
International Temperature Scale of 1990 From Wikipedia, the free encyclopedia
 International Temperature Scale of 1990 From Wikipedia, the free encyclopedia The International Temperature Scale of 1990 (ITS-90) published by the Consultative Committee for Thermometry (CCT) of the International
International Temperature Scale of 1990 From Wikipedia, the free encyclopedia The International Temperature Scale of 1990 (ITS-90) published by the Consultative Committee for Thermometry (CCT) of the International
(Refer Slide Time 3:35)
 Mechanical Measurements and Metrology Prof. S. P. Venkateshan Department of Mechanical Engineering Indian Institute of Technology, Madras Module 2 Lecture - 9 Temperature Measurement This will be lecture
Mechanical Measurements and Metrology Prof. S. P. Venkateshan Department of Mechanical Engineering Indian Institute of Technology, Madras Module 2 Lecture - 9 Temperature Measurement This will be lecture
Estimate, for this water, the specific heat capacity, specific heat capacity =... J kg 1 K 1. the specific latent heat of vaporisation.
 1 A kettle is rated as 2.3 kw. A mass of 750 g of water at 20 C is poured into the kettle. When the kettle is switched on, it takes 2.0 minutes for the water to start boiling. In a further 7.0 minutes,
1 A kettle is rated as 2.3 kw. A mass of 750 g of water at 20 C is poured into the kettle. When the kettle is switched on, it takes 2.0 minutes for the water to start boiling. In a further 7.0 minutes,
Electro - Principles I
 Electro - Principles I Page 10-1 Atomic Theory It is necessary to know what goes on at the atomic level of a semiconductor so the characteristics of the semiconductor can be understood. In many cases a
Electro - Principles I Page 10-1 Atomic Theory It is necessary to know what goes on at the atomic level of a semiconductor so the characteristics of the semiconductor can be understood. In many cases a
39th International Physics Olympiad - Hanoi - Vietnam Experimental Problem
 DIFFERENTIAL THERMOMETRIC METHOD In this problem, we use the differential thermometric method to fulfill the two following tasks: 1. Finding the temperature of solidification of a crystalline solid substance.
DIFFERENTIAL THERMOMETRIC METHOD In this problem, we use the differential thermometric method to fulfill the two following tasks: 1. Finding the temperature of solidification of a crystalline solid substance.
Technische Universität Dresden Lehrstuhl für Kälte- und Kryotechnik Dresden, 01062, Germany
 CONSTRUCTION OF A PARA-ORTHO HYDROGEN TEST CRYOSTAT J. Essler, Ch. Haberstroh Technische Universität Dresden Lehrstuhl für Kälte- und Kryotechnik Dresden, 01062, Germany ABSTRACT In a prospective hydrogen
CONSTRUCTION OF A PARA-ORTHO HYDROGEN TEST CRYOSTAT J. Essler, Ch. Haberstroh Technische Universität Dresden Lehrstuhl für Kälte- und Kryotechnik Dresden, 01062, Germany ABSTRACT In a prospective hydrogen
Chapter 3: Electric Current And Direct-Current Circuits
 Chapter 3: Electric Current And Direct-Current Circuits 3.1 Electric Conduction 3.1.1 Describe the microscopic model of current Mechanism of Electric Conduction in Metals Before applying electric field
Chapter 3: Electric Current And Direct-Current Circuits 3.1 Electric Conduction 3.1.1 Describe the microscopic model of current Mechanism of Electric Conduction in Metals Before applying electric field
What is Temperature?
 What is Temperature? Observation: When objects are placed near each other, they may change, even if no work is done. (Example: when you put water from the hot tap next to water from the cold tap, they
What is Temperature? Observation: When objects are placed near each other, they may change, even if no work is done. (Example: when you put water from the hot tap next to water from the cold tap, they
Chapter 10. Thermal Physics. Thermodynamic Quantities: Volume V and Mass Density ρ Pressure P Temperature T: Zeroth Law of Thermodynamics
 Chapter 10 Thermal Physics Thermodynamic Quantities: Volume V and Mass Density ρ Pressure P Temperature T: Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion of Solids and Liquids Ideal
Chapter 10 Thermal Physics Thermodynamic Quantities: Volume V and Mass Density ρ Pressure P Temperature T: Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion of Solids and Liquids Ideal
Heat and Temperature
 Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
SENSORS AND TRANSDUCERS
 Electrical Measurements International Program Department of Electrical Engineering UNIVERSITAS INDONESIA ANDRITTO ABDUL GHAFFAR ANDHIKA ADIEL INSANI Lecturer : Ir. Chairul Hudaya, ST, M.Eng., Ph.D., IPM
Electrical Measurements International Program Department of Electrical Engineering UNIVERSITAS INDONESIA ANDRITTO ABDUL GHAFFAR ANDHIKA ADIEL INSANI Lecturer : Ir. Chairul Hudaya, ST, M.Eng., Ph.D., IPM
Temperature. Sensors. Measuring technique. Eugene V. Colla. 10/25/2017 Physics 403 1
 Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
Gas Thermometer and Absolute Zero
 Chapter 1 Gas Thermometer and Absolute Zero Name: Lab Partner: Section: 1.1 Purpose Construct a temperature scale and determine absolute zero temperature (the temperature at which molecular motion ceases).
Chapter 1 Gas Thermometer and Absolute Zero Name: Lab Partner: Section: 1.1 Purpose Construct a temperature scale and determine absolute zero temperature (the temperature at which molecular motion ceases).
Total radiation measurements of thermodynamic temperature
 Total radiation measurements of thermodynamic temperature metrologia T. J. Quinn and J. E. Martin Abstract. The principles of total radiation thermometry as a method of primary thermometry are presented.
Total radiation measurements of thermodynamic temperature metrologia T. J. Quinn and J. E. Martin Abstract. The principles of total radiation thermometry as a method of primary thermometry are presented.
He II Heat transfer through a Corrugated Tube - Test Report
 He II Heat transfer through a Corrugated Tube - Test Report Authors: Ch. Darve, Y. Huang, T. Nicol, T. Peterson Keywords: LHC inner triplet, heat exchanger, He II heat transfer, Kapitza resistance. Abstract
He II Heat transfer through a Corrugated Tube - Test Report Authors: Ch. Darve, Y. Huang, T. Nicol, T. Peterson Keywords: LHC inner triplet, heat exchanger, He II heat transfer, Kapitza resistance. Abstract
PhysicsAndMathsTutor.com
 Electricity May 02 1. The graphs show the variation with potential difference V of the current I for three circuit elements. PhysicsAndMathsTutor.com When the four lamps are connected as shown in diagram
Electricity May 02 1. The graphs show the variation with potential difference V of the current I for three circuit elements. PhysicsAndMathsTutor.com When the four lamps are connected as shown in diagram
Experiment Aim: Students will describe the magnitude of resistance and define the EMF (electromotive force) of a cell.
 Experiment I: Electromotive force and internal resistance Experiment Aim: Students will describe the magnitude of resistance and define the EMF (electromotive force) of a cell. Experimental tools and materials:
Experiment I: Electromotive force and internal resistance Experiment Aim: Students will describe the magnitude of resistance and define the EMF (electromotive force) of a cell. Experimental tools and materials:
TOPIC 25 RESISTANCE COVERING: R = VII = 3/0.25 = 12 Q
 TOPIC 25 RESISTANCE COVERING: simple measurement of resistance; resistance and resistivity; I-V characteristics; resistors in series and in parallel; e.m.f.; internal resistance; power. In the previous
TOPIC 25 RESISTANCE COVERING: simple measurement of resistance; resistance and resistivity; I-V characteristics; resistors in series and in parallel; e.m.f.; internal resistance; power. In the previous
INTRODUCTION AND BASIC CONCEPTS. Chapter 1. Mehmet Kanoglu. Thermodynamics: An Engineering Approach, 6 th Edition. Yunus A. Cengel, Michael A.
 Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu Copyright The McGraw-Hill Companies, Inc.
Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu Copyright The McGraw-Hill Companies, Inc.
1. Thermal energy is transferred through the glass windows of a house mainly by. D. radiation and convection. (1)
 1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation. C. conduction and convection. D. radiation and convection. 2. The specific latent heat of vaporization
1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation. C. conduction and convection. D. radiation and convection. 2. The specific latent heat of vaporization
Thermocouple Calibrations and Heat Transfer Coefficients
 Laboratory Experiment 5: Thermocouple Calibrations and Heat Transfer Coefficients Presented to the University of California, San Diego Department of Mechanical and Aerospace Engineering MAE 170 Prepared
Laboratory Experiment 5: Thermocouple Calibrations and Heat Transfer Coefficients Presented to the University of California, San Diego Department of Mechanical and Aerospace Engineering MAE 170 Prepared
CHAPTER V. Thermal properties of materials at low temperatures
 CHAPTER V Thermal properties of materials at low temperatures 1. Introduction The mechanical properties of materials were discussed in Chapter IV. In this chapter we shall discuss the thermal properties
CHAPTER V Thermal properties of materials at low temperatures 1. Introduction The mechanical properties of materials were discussed in Chapter IV. In this chapter we shall discuss the thermal properties
1. Mark the correct statement(s)
 1. Mark the correct statement(s) Figure to the right shows a mass measurement scale using a spring. 1.1 The span of the scale is a) 16 kg b) 21 kg c) 11 kg d) 5-16 kg 1.2 The range of the scale is a) 16
1. Mark the correct statement(s) Figure to the right shows a mass measurement scale using a spring. 1.1 The span of the scale is a) 16 kg b) 21 kg c) 11 kg d) 5-16 kg 1.2 The range of the scale is a) 16
ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A
 ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A 1. What is meant by thermodynamics system? (A/M 2006) Thermodynamics system is defined as any space or matter or group of matter
ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A 1. What is meant by thermodynamics system? (A/M 2006) Thermodynamics system is defined as any space or matter or group of matter
Lecture 1 INTRODUCTION AND BASIC CONCEPTS
 Lecture 1 INTRODUCTION AND BASIC CONCEPTS Objectives Identify the unique vocabulary associated with thermodynamics through the precise definition of basic concepts to form a sound foundation for the development
Lecture 1 INTRODUCTION AND BASIC CONCEPTS Objectives Identify the unique vocabulary associated with thermodynamics through the precise definition of basic concepts to form a sound foundation for the development
Mechanical Measurements. Module 2:
 Mechanical Measurements Module : 1. Thermometry Formally we start the study of Mechanical Measurements now! Module will consider the measurement of field quantities like temperature, pressure and fluid
Mechanical Measurements Module : 1. Thermometry Formally we start the study of Mechanical Measurements now! Module will consider the measurement of field quantities like temperature, pressure and fluid
Electric Currents. Resistors (Chapters 27-28)
 Electric Currents. Resistors (Chapters 27-28) Electric current I Resistance R and resistors Relation between current and resistance: Ohm s Law Resistivity ρ Energy dissipated by current. Electric power
Electric Currents. Resistors (Chapters 27-28) Electric current I Resistance R and resistors Relation between current and resistance: Ohm s Law Resistivity ρ Energy dissipated by current. Electric power
Chapter 17. Temperature. Dr. Armen Kocharian
 Chapter 17 Temperature Dr. Armen Kocharian Temperature We associate the concept of temperature with how hot or cold an objects feels Our senses provide us with a qualitative indication of temperature Our
Chapter 17 Temperature Dr. Armen Kocharian Temperature We associate the concept of temperature with how hot or cold an objects feels Our senses provide us with a qualitative indication of temperature Our
fehmibardak.cbu.tr Temporary Office 348, Mühendislik Fakültesi B Blok
 fehmibardak.cbu.tr Temporary Office 348, Mühendislik Fakültesi B Blok 1 Course Progress Introductory level Electrostatic, Coulomb s Law Electric Field, Gauss Law Magnetic field, Maxwell s Equations Current,
fehmibardak.cbu.tr Temporary Office 348, Mühendislik Fakültesi B Blok 1 Course Progress Introductory level Electrostatic, Coulomb s Law Electric Field, Gauss Law Magnetic field, Maxwell s Equations Current,
I. MEASUREMENT OF TEMPERATURE
 I. MEASUREMENT OF TEMPERATURE Most frequent measurement and control Direct contact: thermometer, Indirect contact: pyrometer (detect generated heat or sensing optical properties) 1. Definition of temperature
I. MEASUREMENT OF TEMPERATURE Most frequent measurement and control Direct contact: thermometer, Indirect contact: pyrometer (detect generated heat or sensing optical properties) 1. Definition of temperature
Lecture 2: Zero law of thermodynamics
 Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
