ija 3 m Interakcije spojina makromolekula ska ke acevt Farm doc.dr. Marko Anderluh 11 oktober. 2011
|
|
|
- Ashley Perry
- 6 years ago
- Views:
Transcription
1 acevts ska kem mija 3 Farm Interakcije spojina makromolekula doc.dr. Marko Anderluh 11. oktober 2011
2 Interakcije spojina-makromolekula Vezavne regije Spojina Vezavne skupine Medmolekulske vezi Vezavno mesto Vezavno mesto Spojina Spojina Tarčna makromolekula Tarčna makromolekula Nevezana učinkovina Vezana učinkovina
3 Interakcije spojina-makromolekula Osnova farmakološkega učinka! Sproščanje energije vezavna entalpija + entropija G G = H-T SH T S Sprememba konformacije liganda (učinkovine) i za prileganje vezavnemu mestu Posledice? Induced fit sprememba konformacije vezavnega mesta makromolekule, celotna konformacijska sprememba Zasedanje aktivnega mesta encima
4 Interakcije spojina-makromolekula Termodinamika vezave U(činkovina) R(eceptor) Posledica: K d =10 nm, G =?
5 Interakcije spojina-makromolekula H -T S Kovalentne vezi Hidrofobne interakcije Ionske vezi Izgube rotacijskih, translacijskih prostostnih stopenj Dipolne interakcije interakcije kation interakcije H-vezi VdW interakcije
6 Interakcije spojina-makromolekula Ločimo po: energiji interakcije: močne, šibke interakcije po vrsti interakcije: kovalentene, ionske, H-vezi, hidrofobne itd. po trajanju interakcije: trajne in reverzibilne po specifičnosti
7 Interakcije spojina-makromolekula Moč interakcij Vrsta interakcije Energija Primer Kovalentna a vez cca 420 kj/mol A -B (cca 100 Kcal/mol) Ionska vez cca 20 kj/mol A B dipolarne vezi Vodikova vez cca 4,2 kj/mol Hidrofobni efekt /interakcija/ Van der Waals 0,1 0,2 kj/mol A kj/mol
8 Kovalentne vezi Močna vez ( G = 400 kj/mol) Ireverzibilna Neselektivna Tipična za: alkilirajoče citostatitke (iperiti)! toksičnost alkilantov kancerogenost metabolitov in ksenobiotikov
9 Energije nekaterih kovalentnih vezi v kj/mol C-H C-F C-N C-O C-C N-N N-H C-Cl C=N C=O C=C N=N O-H C-Br C N C CC S-H 347 C-I 213
10 Obstrukcija metabolizma O S O H 2 N O S O N N CF 3 CF 3 N N F SC H 3 C CELEKOKSIB t 1/2 = 211h t 1/2 = 8-12h SI (COX-2/COX-1) = >1000 SI (COX-2/COX-1) = 375
11 Ionske interakcije Najmočnejše medmolekulske vezi (20-40 kj/mol) Sferično simetrična usmerjenost ni pomembna! Moč vezi pada premosorazmerno z razdaljo: E~q i q j / r ij q ij i,j...naboja r i,j...razdalja med nabojema...diel. konstanta (voda=80, vakuum=1) Močnejše interakcije v vakuumu/hidrofobnem okolju Bistvene na začetku interakcij učinkovina-makromolekula Drug O O H 3 N Target Drug NH 3 O Target O
12 Ionske interakcije Skupine, ki omogočajo ionske interakcije ij (kisline, baze) Kationske skupine R + Anionske skupine R -
13 Dipol Dipol je posledica različne elektronske gostote v molekuli Parcialni dipoli v molekuli se seštevajo kot vektorji - + Parcialni dipoli v molekuli se Višja gostota Nižja gostota Cl Simbolni zapis dipola
14 Dipol Primer polarnosti: 1,2; 1,3 in 1,4 diklorbenzen Cl Cl Cl Cl Cl Cl Cl
15 Dipolne interakcije Šibke interakcije Vektorski ki seštevek dipolov Moč odvisna od razdalje in usmeritve Obratnosorazmerne z razdaljo: Ion dipol (E r-2 ) Dipol dipol (E r-3) Ion induciran dipol (E r-4) Dipol induciran dipol (E r-5) Induciran dipol induciran dipol (E r-6)
16 Ion-dipol interakcije Med nabojem ene molekule in dipolom druge molekule Pomembna usmerjenost dipola! Moč pada s kvadratom razdalje: R E~z cos /Dr 2 z...naboj...dipolni l i moment r...razdalja med nabojem in dipolom D...diel. konstanta C O R O C O R C R O H 3 N Vezavno mesto Vezavno mesto
17 Primer interakcije ion-dipol Na + (H 2 O) približno 6 Cl - (H 2 O) približno 6
18 Dipol-dipol interakcije Med dvema dipoloma Dipola se pri vezavi orientirata v nasprotni smeri Orientacija molekule je ključnega pomena! Moč pada s 3 potenco razdalje: E~A 3 /Dr...dipolni moment r...razdalja razdalja med nabojem in dipolom D...diel. konstanta
19 Dipol-dipol interakcije O Dipolni moment R C R lokaloziran dipolni moment R C O R Vezavno mesto Vezavno mesto
20 Interakcije dipol/ion induciran dipol T.i. Induktivne interakcije - naboj ene molekule inducira dipol na drugi E~ z 2 /D 2 r 4 z...naboj...polarizabilnost r...razdalja med nabojem in dipolom D...diel. Konstanta
21 Vodikove vezi (H-vezi) Med z elektroni revnim vodikom (donor vodikove vezi) in z elektroni bogatim heteroatomom (akceptor vodikove vezi) Močno variirajo po moči: nevtralne 2-6 kj/mol, ionske do 20 kj/mol (povprečje 4,2 kj/mol) Interakcije temeljijo na prekrivanju orbital delni značaj kovalentne vezi! Optimalna interakcija: X-H vez kaže direktno na prost elektronski par Y, tako da je kot med X-H...Y 180 X H Y X H Y Hybridised orbital 1s orbital Donor Hybridised orbital Akceptor
22 2 - + Vodikova vez Kovalentna vez Vodikova vez: Privlak med razgaljenim protonom in elektronskim parom KISIKA Vodikova vez: -kratkosežna -šibka -aditivna -prostorsko usmerjena Življenska doba H-vezi je 1-20 ps
23 Vodikove vezi (H-vezi) Najmočnejši akceptor H-vezi?
24 Vpliv števila H vezi na vrelišče spojin Amini i z bruto formulo: C 3 H 9 N.... H 3 C N CH 3 CH 3 CH 2 N CH 3 CH 3 3 H.. CH 3 CH 2 CH 2 N Vrelišče C 37 0 C 49 0 C H H Št. medsebojnih H vezi 0 1 2
25 Voda kot univerzalno topilo Elektrostatske vezi med vodnim dipolom in drugimi i dipoli. Tvorba ionske vezi z ioni. Tvorba vodikovih vezi s topljencem. Tvorba vodikovih vezi med vodnimi molekulami
26 Benzen in voda
27 KAJ JE VODA?
28 Lastnosti posamezne molekule vode Elektronska gostota molekule vode Prosta elektronska k para na kisiku
29 Molekula vode je dipol Elektronska gostota molekule vode Prosta elektronska k para na kisiku
30 DVE MOLEKULI VODE H vez
31 Primer povezanosti ene molekule vode s štirimi sosednjimi molekulami preko vodikovih vezi. Vodikova vez
32 H vez Energija: cca 23 kj/mol (1/5 kov. vezi) Usmerjena vez, ima 10% karakterja kovalentne vezi. Aditivna vez povezana z dipolnim i momentom vodne molekule. Večinski vzrok za nenavadne lastnosti vode.
33 Velika površinska napetost vode Tvorba vodikovih vezi na medfazni površini i je vzrok za tvorbo kože na površini.
34 Struktura ledu je posledica vodikovih vezi med vodnimi molekulami Led: 4 H-vezi med 1 molekulo vode in okolico Tekoča voda: povprečno 3,4 H vezi med molekule vode in okolico
35 Primerjava vrelišč hidridov ogljikove gj in kisikove vertikale* Voda je izjema Vrelišče Molska masa
36 Fazni prehodi, energija in struktura vode Voda E(H-vezi) = 18,9 kj/mol E(O-H) = 462 kj/mol Izparilne toplote: q(voda) = 40,82 kj/mol q(etanol) = 39,39 kj/mol q(benzen) = 30,78 kj/mol Energije pri faznih prehodih: q(izparilna: voda para) = 40,82 kj/mol q(talilna: a: led voda) = 6,04 kj/mol C (toplotna kapaciteta) = 0,07 kj/mol st Izračun za prehod: led v paro 6, x 0, ,82 = 53,86 kj/mol 53,86 : 18,9 = 2,88 ali cca 3 H vezi
37 Primer strukture tekoče vode Vodne molekule interagirajo med seboj: -z vodikovimi vezmi -z ionskimi vezmi, -z dipolarnimi interakcijami
38 Delne strukture tekoče vode Pentamer Biciklooktamer Triciklodekamer
39 Klaster tipa ikozahed aheder er Majhni klastri pri nižji temperaturi tvorijo večje klastre.
40 Idealiziran ikozahedralni klaster (H 2 O) 280 Struktura nizke gostote Kondenzirana struktura
41 = ps Klaster iz molekul vode T = 0 0 C V klastru je: molekul vode. T = C V klastru je: molekul vode.
42 Voda: Ravnotežna dinamična stru truktura Ekspandirana struktura Nižja gostota Počasnejša difuzija Višja viskoznost Zgoščena struktura Višja gostota Večji medsebojni vplivi Bolj reaktivna oblika
43 Vplivi ionov na klastre Ekspandrana struktura Zgoščena struktura Nižja gostota Višja gostota Počasnejša difuzija Večji medsebojni vpliv Višja viskoznost Bolj reaktivna oblika Kaotropni ioni Kozmotropni ioni Cs + > Rb + > K + kationi Ca 2+ > Li + > Na + ClO 4- >H 4- >Cl - 2 PO anioni SO 2-4, HPO 2-4 Leu, Ileu, Lys, Arg Am. kisline Asp, Glu Šibko hidratirani i ionii z Močnoč hidratirani i ionii z difuzno gostoto naboja. visoko gostoto naboja. Stabilizian an centralni n dodekahedron
44 Desolvatacijske izgube H Interakcije v vodnem mediju! Polarne regije proteina in učinkovine so solvatirane! Desolvatacija je nujna in prinaša E izgube Izguba zaradi desolvatacije<entalpija vezave O H O H H H O O O C H C R R H R R O H O H O O H C R R H O Vezavno mesto Vezavno mesto Vezavno mesto Desolvatacija - izguba Vezava porast E!
45 Vodikove vezi (H-vezi)
46 Kation- interakcije Med kationom in kvadrupolnim momentom elektronskega oblaka aromatov po jakosti primerljive H-vezi Aromat kvadrupol?
47 Kation- interakcije Jakost nekaterih kation- interakcij
48 Kation- interakcije Jakost nekaterih kation- interakcij
49 - interakcije Interakcije med aromatskimi obroči Neke vrste vodikove vezi med protoni enega aromata in - elektronskim oblakom drugega Oblika? DNA?! Interkalatorji
50 Van der Waalsove interakcije = Londonove disperzijske sile En trenuten dipol inducira nastanek drugega dipola, privlak je posledica interakcije trenutnih dipolov. Polarizabilnost je zmožnost deformacije elektronskega oblaka molekule Disperzijske interakcijske sile naraščajo z molsko maso. Disperzijske sile so univerzalne, odvisne so od oblike molekule, so sorazmerne kontaktni površini
51 Van der Waalsove interakcije
52 Van der Waalsove interakcije Šibke interakcije (2-4 kjmol-1) Med hidrofobnimi regijami učinkovine in makromolekule med nepolarnimi molekulami! Začasne regije z visokimi/nizkimi elektronskimi gostotami + - Hidrofobne regije Začasni dipol van der Waalsova interakcija + - Učinkovina - + Vezavno mesto
53 Van der Waalsove interakcije Moč vezi pada izjemno hitro z razdaljo - Lennard-Jonesov potencial E ~ E odboj - E privlak Pada z r 12 Pada z r 6 Učinkovina v bližini vezavnega mesta! Celoten doprinos k vezavi učinkovine ponavadi razmeroma velik
54 Plinska faza Tekoča faza Šibke privlačne sile med molekulami
55 Vpliv na vrelišče
56 Hidrofobne interakcije Posredne interakcije, prisotne samo v vodi. Posledica vodikovih vezi med vodnimi molekulami in entropijskih efektov Sorazmerne velikosti nepolarne površine Kratkosežne in usmerjevalne
57 Hidrofobne interakcije Hidrofobne regije makromolekule in tarče niso solvatirane Molekule vode interagirajo med seboj in tvorijo organizirane plasti okoli teh regij-negativna entropija! Interakcije med hidrofobnimi regijami učinkovine in makromolekule sprostijo organizirano vodo Rezultat je povečanje entropije pj in porast celokupne vezavne E E~ 0,1-0,2 kj/molå 2
58 Hidrofobne interakcije Entropija povzroči nastanek komleksnejših struktur v vodi Urejanje vodnih molekul ob nepolarni površini pomeni zmanjpševanje entropije micel
59 Definicije Hidrofobni efekt: nizka topnost ogljikovodikov in drugih nepolarnih molekul l v vodi in njihova povečana tendenca po združevanju. Hidrofobne interakcije: so posredne in vodijo do združevanja nepolarnih molekul v vodi.
60
61 Opis vezave učinkovina-receptor Sterična komplementarnost (+/-) Desolvatacija (-) Izguba translacijskih in rotacijskih p.s.(-) H vezave, H H-vezi med mol. vode (+) Inducirano prileganje Bioaktivna konformacija?!
62 Dikofenak / COX-2
63
64
65 Nekaj vprašanj 2Fbin%2F %2F
66 Literatura predavanj Farmacevts ska kem mija 3 G. L. Patrick: An introduction to medicinal chemistry, Oxford University press, 3nd ed., 2005: 2 poglavje
ija 3 m Kislost-bazi - čnost Hammettove konstante ska ke acevt Farm Izr. prof. dr Izr. prof. dr. Marko Anderluh. Marko Anderluh 23 oktober.
 acevts ska kem mija 3 Farm Kislost-bazičnost Hammettove konstante Izr. prof. dr. Marko Anderluh 23. oktober 2012 Vpliv kislinsko bazičnih lastnosti Vezava na tarčno mesto farmakodinamsko delovanje Topnost/sproščanje
acevts ska kem mija 3 Farm Kislost-bazičnost Hammettove konstante Izr. prof. dr. Marko Anderluh 23. oktober 2012 Vpliv kislinsko bazičnih lastnosti Vezava na tarčno mesto farmakodinamsko delovanje Topnost/sproščanje
METODE ZA PREDVIDEVANJE (NAPOVEDOVANJE) VODOTOPNOSTI (topnosti spojin v vodi)
 METODE ZA PREDVIDEVANJE (NAPOVEDOVANJE) VODOTOPNOSTI (topnosti spojin v vodi) Delitev metod (metode temeljijo na): 1. Prispevki posameznih skupin v molekuli k aktivnostnemu koeficientu spojine v vodi.
METODE ZA PREDVIDEVANJE (NAPOVEDOVANJE) VODOTOPNOSTI (topnosti spojin v vodi) Delitev metod (metode temeljijo na): 1. Prispevki posameznih skupin v molekuli k aktivnostnemu koeficientu spojine v vodi.
ENAČBA STANJA VODE IN VODNE PARE
 ENAČBA STANJA VODE IN VODNE PARE SEMINARSKA NALOGA PRI PREDMETU JEDRSKA TEHNIKA IN ENERGETIKA TAMARA STOJANOV MENTOR: IZRED. PROF. DR. IZTOK TISELJ NOVEMBER 2011 Enačba stanja idealni plin: pv = RT p tlak,
ENAČBA STANJA VODE IN VODNE PARE SEMINARSKA NALOGA PRI PREDMETU JEDRSKA TEHNIKA IN ENERGETIKA TAMARA STOJANOV MENTOR: IZRED. PROF. DR. IZTOK TISELJ NOVEMBER 2011 Enačba stanja idealni plin: pv = RT p tlak,
A L A BA M A L A W R E V IE W
 A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
Transport snovi preko celičnih membran. Lodish et al. 4. izdaja, 15. poglavje (str )
 Transport snovi preko celičnih membran Lodish et al. 4. izdaja, 15. poglavje (str. 578 615) Relativna propustnost fosfolipidnega dvosloja za različne molekule Načini transporta snovi preko celičnih membran
Transport snovi preko celičnih membran Lodish et al. 4. izdaja, 15. poglavje (str. 578 615) Relativna propustnost fosfolipidnega dvosloja za različne molekule Načini transporta snovi preko celičnih membran
CHEM 172 EXAMINATION 1. January 15, 2009
 CHEM 17 EXAMINATION 1 January 15, 009 Dr. Kimberly M. Broekemeier NAME: Circle lecture time: 9:00 11:00 Constants: c = 3.00 X 10 8 m/s h = 6.63 X 10-34 J x s J = kg x m /s Rydberg Constant = 1.096776 x
CHEM 17 EXAMINATION 1 January 15, 009 Dr. Kimberly M. Broekemeier NAME: Circle lecture time: 9:00 11:00 Constants: c = 3.00 X 10 8 m/s h = 6.63 X 10-34 J x s J = kg x m /s Rydberg Constant = 1.096776 x
Solutions and Non-Covalent Binding Forces
 Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
3. Which of the following elements is primarily responsible for the photochemical smog? Chemistry 12, Exam III, Form A, April 4, 2001
 Chemistry 12, Exam III, Form A, April 4, 2001 In all questions involving gases, assume that the ideal-gas laws hold, unless the question specifically refers to the non-ideal behavior. 1. It takes 21.3
Chemistry 12, Exam III, Form A, April 4, 2001 In all questions involving gases, assume that the ideal-gas laws hold, unless the question specifically refers to the non-ideal behavior. 1. It takes 21.3
Atomic and Molecular Dimensions
 1 Atomic and Molecular Dimensions Equilibrium Interatomic Distances When two atoms approach each other, their positively charged nuclei and negatively charged electronic clouds interact. The total interaction
1 Atomic and Molecular Dimensions Equilibrium Interatomic Distances When two atoms approach each other, their positively charged nuclei and negatively charged electronic clouds interact. The total interaction
Funkcije proteinov. Oporna funkcija (strukturni proteini, npr keratini, kolagen...) Obramba pred tujki/invazivnimi organizmi (Imunoglobulini)
 Funkcije proteinov Oporna funkcija (strukturni proteini, npr keratini, kolagen...) Transport/skladiščenje določenih molekul (ligandov, npr. Hb, Mb) Uravnavanje procesov (DNA-vezavni proteini) Obramba pred
Funkcije proteinov Oporna funkcija (strukturni proteini, npr keratini, kolagen...) Transport/skladiščenje določenih molekul (ligandov, npr. Hb, Mb) Uravnavanje procesov (DNA-vezavni proteini) Obramba pred
TOPLJENEC ASOCIIRA LE V VODNI FAZI
 TOPLJENEC ASOCIIRA LE V VODNI FAZI V primeru asociacij molekul topljenca v vodni ali organski fazi eksperimentalno določeni navidezni porazdelitveni koeficient (P n ) v odvisnosti od koncentracije ni konstanten.
TOPLJENEC ASOCIIRA LE V VODNI FAZI V primeru asociacij molekul topljenca v vodni ali organski fazi eksperimentalno določeni navidezni porazdelitveni koeficient (P n ) v odvisnosti od koncentracije ni konstanten.
Bonding/Lewis Dots Lecture Page 1 of 12 Date. Bonding. What is Coulomb's Law? Energy Profile: Covalent Bonds. Electronegativity and Linus Pauling
 Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
3rd Advanced in silico Drug Design KFC/ADD Molecular mechanics intro Karel Berka, Ph.D. Martin Lepšík, Ph.D. Pavel Polishchuk, Ph.D.
 3rd Advanced in silico Drug Design KFC/ADD Molecular mechanics intro Karel Berka, Ph.D. Martin Lepšík, Ph.D. Pavel Polishchuk, Ph.D. Thierry Langer, Ph.D. Jana Vrbková, Ph.D. UP Olomouc, 23.1.-26.1. 2018
3rd Advanced in silico Drug Design KFC/ADD Molecular mechanics intro Karel Berka, Ph.D. Martin Lepšík, Ph.D. Pavel Polishchuk, Ph.D. Thierry Langer, Ph.D. Jana Vrbková, Ph.D. UP Olomouc, 23.1.-26.1. 2018
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
We study bonding since it plays a central role in the understanding of chemical reactions and understanding the chemical & physical properties.
 AP Chapter 8 Notes Bonding We study bonding since it plays a central role in the understanding of chemical reactions and understanding the chemical & physical properties. Chemical Bond: holding atoms together
AP Chapter 8 Notes Bonding We study bonding since it plays a central role in the understanding of chemical reactions and understanding the chemical & physical properties. Chemical Bond: holding atoms together
Sinteza homologov paracetamola
 Katedra za farmacevtsko kemijo Sinteza homologov paracetamola Vaje iz Farmacevtske kemije 3 1 Sinteza N-(4-hidroksifenil)dekanamida Vaje iz Farmacevtske kemije 3 2 Vprašanja: 1. Zakaj uporabimo zmes voda/dioksan?
Katedra za farmacevtsko kemijo Sinteza homologov paracetamola Vaje iz Farmacevtske kemije 3 1 Sinteza N-(4-hidroksifenil)dekanamida Vaje iz Farmacevtske kemije 3 2 Vprašanja: 1. Zakaj uporabimo zmes voda/dioksan?
Acid-Base Character of Salt Solutions. Cations. Cations are potentially acidic, but some have no effect on ph.
 Acid-Base Character of Salt Solutions The ph of a salt solution will depend on the acidbase nature of both the cation and anion. Cations Cations are potentially acidic, but some have no effect on ph. M(H
Acid-Base Character of Salt Solutions The ph of a salt solution will depend on the acidbase nature of both the cation and anion. Cations Cations are potentially acidic, but some have no effect on ph. M(H
Properties of substances are largely dependent on the bonds holding the material together.
 Basics of Chemical Bonding AP Chemistry Lecture Outline Properties of substances are largely dependent on the bonds holding the material together. Basics of Bonding A chemical bond occurs when atoms or
Basics of Chemical Bonding AP Chemistry Lecture Outline Properties of substances are largely dependent on the bonds holding the material together. Basics of Bonding A chemical bond occurs when atoms or
NAME: SECOND EXAMINATION
 1 Chemistry 64 Winter 1994 NAME: SECOND EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
1 Chemistry 64 Winter 1994 NAME: SECOND EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
All chemical bonding is based on the following relationships of electrostatics: 2. Each period on the periodic table
 UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
 lectures accompanying the book: Solid State Physics: An Introduction, by Philip ofmann (2nd edition 2015, ISBN-10: 3527412824, ISBN-13: 978-3527412822, Wiley-VC Berlin. www.philiphofmann.net 1 Bonds between
lectures accompanying the book: Solid State Physics: An Introduction, by Philip ofmann (2nd edition 2015, ISBN-10: 3527412824, ISBN-13: 978-3527412822, Wiley-VC Berlin. www.philiphofmann.net 1 Bonds between
CHEM 10113, Quiz 5 October 26, 2011
 CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
Example 9.1 Using Lewis Symbols to Predict the Chemical Formula of an Ionic Compound
 Example 9.1 Using Lewis Symbols to Predict the Chemical Formula of an Ionic Compound For Practice 9.1 Use Lewis symbols to predict the formula for the compound that forms between magnesium and nitrogen.
Example 9.1 Using Lewis Symbols to Predict the Chemical Formula of an Ionic Compound For Practice 9.1 Use Lewis symbols to predict the formula for the compound that forms between magnesium and nitrogen.
Speed of light c = m/s. x n e a x d x = 1. 2 n+1 a n π a. He Li Ne Na Ar K Ni 58.
 Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
2A skupina zemeljskoalkalijske kovine
 1. NALOGA: V ČEM SE RAZLIKUJETA BeO IN MgO? 1. NALOGA: ODGOVOR Elementi 2. periode (od Li do F) se po fizikalnih in kemijskih lastnostih (diagonalne lastnosti) znatno razlikujejo od elementov, ki so v
1. NALOGA: V ČEM SE RAZLIKUJETA BeO IN MgO? 1. NALOGA: ODGOVOR Elementi 2. periode (od Li do F) se po fizikalnih in kemijskih lastnostih (diagonalne lastnosti) znatno razlikujejo od elementov, ki so v
30 Zn(s) 45 Rh. Pd(s) Ag(s) Cd(s) In(s) Sn(s) white. 77 Ir. Pt(s) Au. Hg(l) Tl. 109 Mt. 111 Uuu. 112 Uub. 110 Uun. 65 Tb. 62 Sm. 64 Gd. 63 Eu.
 Enthalpy changes: experimentally it is much easier to measure heat flow at const pressure - this is enthalpy q p = )H : also nearly all chemical reactions are done at constant pressure. Enthalpy (heat)
Enthalpy changes: experimentally it is much easier to measure heat flow at const pressure - this is enthalpy q p = )H : also nearly all chemical reactions are done at constant pressure. Enthalpy (heat)
PROTEAZE PROTEAZE TRIOZA FOSFAT-IZOMERAZA LAKTAT DEHIDROGENAZA DNA-METIL TRANSFERAZA. tripsinogen. enteropeptidaza autokataliza.
 PRTEAZE TRIZA FSFAT-IZMERAZA LAKTAT DEIDRGEAZA DA-METIL TRASFERAZA PRTEAZE SERISKE PRTEAZE KIMTRIPSI tripsinogen enteropeptidaza autokataliza tripsin aktivacija kimotripsinogena aktivacija proelastaze
PRTEAZE TRIZA FSFAT-IZMERAZA LAKTAT DEIDRGEAZA DA-METIL TRASFERAZA PRTEAZE SERISKE PRTEAZE KIMTRIPSI tripsinogen enteropeptidaza autokataliza tripsin aktivacija kimotripsinogena aktivacija proelastaze
(b) electrovalent and covalent (c) electrovalent and co-ordinate (d) covalent and co-ordinate 10. Which pair is different from others (a) Li Mg (b)
 1. Following triads have approximately equal size (a) Na+, Mg 2+, Al 3+ (iso-electronic) (b) F, Ne, O 2 (iso-electronic) (c) Fe, Co, Ni (d) Mn+, Fe 2+, Cr (iso-electronic) 2. Which of the following halides
1. Following triads have approximately equal size (a) Na+, Mg 2+, Al 3+ (iso-electronic) (b) F, Ne, O 2 (iso-electronic) (c) Fe, Co, Ni (d) Mn+, Fe 2+, Cr (iso-electronic) 2. Which of the following halides
Formulas and Constants (you may remove this page)
 Formulas and Constants (you may remove this page) NA = 6.0x0 3 mol - h = 6.66x0-34 J s c =.99x0 m s - e =.60x0-9 C me = 9.09x0-3 kg Å = x0-0 m = 00 pm atm = 760 torr R = 0.006 L atm K - mol - =.34 J K
Formulas and Constants (you may remove this page) NA = 6.0x0 3 mol - h = 6.66x0-34 J s c =.99x0 m s - e =.60x0-9 C me = 9.09x0-3 kg Å = x0-0 m = 00 pm atm = 760 torr R = 0.006 L atm K - mol - =.34 J K
11/9/15. Intermolecular hydrogen bond: Hydrogen bond: Intramolecular hydrogen bond: Induced dipole moment, polarisability
 Induced dipole moment, polarisability in electric field: Van der Waals forces Intermolecular forces other than covalent bonds or other than electrostatic interactions of ions induced d. moment µ * = α
Induced dipole moment, polarisability in electric field: Van der Waals forces Intermolecular forces other than covalent bonds or other than electrostatic interactions of ions induced d. moment µ * = α
Chemistry Standard level Paper 1
 Chemistry Standard level Paper 1 Thursday 12 May 2016 (morning) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all the questions. For each question,
Chemistry Standard level Paper 1 Thursday 12 May 2016 (morning) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all the questions. For each question,
 Bond characteristics: Bond length, Bond Enthalphy, Bond angle, Bond order 1. Bond Angles i. It is the angle between any two bonded atoms at central atom in a molecule. ii. Bond angles of molecule may (or)
Bond characteristics: Bond length, Bond Enthalphy, Bond angle, Bond order 1. Bond Angles i. It is the angle between any two bonded atoms at central atom in a molecule. ii. Bond angles of molecule may (or)
If anything confuses you or is not clear, raise your hand and ask!
 CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
Lecture 14. Professor Hicks Inorganic Chemistry (CHE151) Shapes of Molecules. The shape of most molecules is not flat as they are drawn on paper
 Lecture 14 Professor Hicks Inorganic Chemistry (CHE151) Shapes of Molecules The shape of most molecules is not flat as they are drawn on paper The three dimensional arrangement of atoms is called the Geometry
Lecture 14 Professor Hicks Inorganic Chemistry (CHE151) Shapes of Molecules The shape of most molecules is not flat as they are drawn on paper The three dimensional arrangement of atoms is called the Geometry
Chapter 8. forces of attraction which hold atoms or ions together. 3 fundamental types of bonding. Ionic - metals & nonmetals
 Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds forces of attraction which hold atoms or ions together 3 fundamental types of bonding Ionic - metals & nonmetals Covalent - nonmetals (semimetals)
Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds forces of attraction which hold atoms or ions together 3 fundamental types of bonding Ionic - metals & nonmetals Covalent - nonmetals (semimetals)
D. Bonchev* D epartment of Physicai Chemistry, Higher Schooi of Chemicai Technoiogy, 8015 Burgas, Buigaria. Received May 12, 1976
 CROATCA CHEMCA ACTA CCACAA 49 (1) 83-88 (1977) CCA-978 CND/2 Study of C2H2 + H2 System D. Bonchev* YU SSN 11-1643 516.11 Note D epartment of Physicai Chemistry, Higher Schooi of Chemicai Technoiogy, 815
CROATCA CHEMCA ACTA CCACAA 49 (1) 83-88 (1977) CCA-978 CND/2 Study of C2H2 + H2 System D. Bonchev* YU SSN 11-1643 516.11 Note D epartment of Physicai Chemistry, Higher Schooi of Chemicai Technoiogy, 815
TERMODINAMIKA, BIOENERGETIKA
 TERMODINAMIKA, BIOENERGETIKA Osnovni termodinamski koncepti Fizikalni pomen termodinamskih količin ph in standardni pogoji Sklopljeni procesi Energijsko bogate biomolekule Osnovni termodinamski koncepti
TERMODINAMIKA, BIOENERGETIKA Osnovni termodinamski koncepti Fizikalni pomen termodinamskih količin ph in standardni pogoji Sklopljeni procesi Energijsko bogate biomolekule Osnovni termodinamski koncepti
M10/4/CHEMI/SPM/ENG/TZ2/XX+ CHEMISTRY. Wednesday 12 May 2010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
Atomic Structure. Atomic weight = m protons + m neutrons Atomic number (Z) = # of protons Isotope corresponds to # of neutrons
 Atomic Structure Neutrons: neutral Protons: positive charge (1.6x10 19 C, 1.67x10 27 kg) Electrons: negative charge (1.6x10 19 C, 9.11x10 31 kg) Atomic weight = m protons + m neutrons Atomic number (Z)
Atomic Structure Neutrons: neutral Protons: positive charge (1.6x10 19 C, 1.67x10 27 kg) Electrons: negative charge (1.6x10 19 C, 9.11x10 31 kg) Atomic weight = m protons + m neutrons Atomic number (Z)
Lecture C2 Microscopic to Macroscopic, Part 2: Intermolecular Interactions. Let's get together.
 Lecture C2 Microscopic to Macroscopic, Part 2: Intermolecular Interactions Let's get together. Most gases are NOT ideal except at very low pressures: Z=1 for ideal gases Intermolecular interactions come
Lecture C2 Microscopic to Macroscopic, Part 2: Intermolecular Interactions Let's get together. Most gases are NOT ideal except at very low pressures: Z=1 for ideal gases Intermolecular interactions come
CHAPTER 8 BONDING: GENERAL CONCEPTS
 Advanced Chemistry Name Hour Advanced Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 8 BONDING: GENERAL CONCEPTS Day Plans for the day Assignment(s)
Advanced Chemistry Name Hour Advanced Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 8 BONDING: GENERAL CONCEPTS Day Plans for the day Assignment(s)
Chapter 8 Test Study Guide AP Chemistry 6 points DUE AT TEST (Wed., 12/13/17) Date:
 Chapter 8 Test Study Guide Name: AP Chemistry 6 points DUE AT TEST (Wed., 12/13/17) Date: Topics to be covered on the December 13, 2017 test: bond bond energy ionic bond covalent bond polar covalent bond
Chapter 8 Test Study Guide Name: AP Chemistry 6 points DUE AT TEST (Wed., 12/13/17) Date: Topics to be covered on the December 13, 2017 test: bond bond energy ionic bond covalent bond polar covalent bond
Organska kemija I. Struktura in reaktivnost organskih spojin
 Organska kemija I Struktura in reaktivnost organskih spojin Prezentacija študentom 2. letnika kemije, zimski semester 2015-2016 I. Uvod: kaj je organska kemija? II. Vezi v organskih spojinah III. Struktura
Organska kemija I Struktura in reaktivnost organskih spojin Prezentacija študentom 2. letnika kemije, zimski semester 2015-2016 I. Uvod: kaj je organska kemija? II. Vezi v organskih spojinah III. Struktura
CHM2045 S13: Exam # MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 CHM2045 S13: Exam #2 2013.03.01 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) How would replacing one of benzene's C atoms and the H atom attached
CHM2045 S13: Exam #2 2013.03.01 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) How would replacing one of benzene's C atoms and the H atom attached
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit. Lecture 2 (9/11/17)
 16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
Circle the letters only. NO ANSWERS in the Columns! (3 points each)
 Chemistry 1304.001 Name (please print) Exam 4 (100 points) April 12, 2017 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 1304.001 Name (please print) Exam 4 (100 points) April 12, 2017 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 105: General Chemistry I Dr. Gutow and Dr. Matsuno Spring 2004 Page 1
 Page 1 1) Name You are to keep this copy of the test. Your name is in case you leave it behind. 2) Use only a #2 pencil on the answer sheet. 3) Before starting the exam fill in your student ID# (not your
Page 1 1) Name You are to keep this copy of the test. Your name is in case you leave it behind. 2) Use only a #2 pencil on the answer sheet. 3) Before starting the exam fill in your student ID# (not your
SEMINAR IZ MOLEKULSKEGA MODELIRANJA PRI FARMACEVTSKI KEMIJI III
 SEMIAR IZ MOLEKULSKEGA MODELIRAJA PRI FARMACEVTSKI KEMIJI III 2. DEL PREDSTAVITEV PROGRAMOV ZA DELO A SEMIARJU doc. dr. Andrej Perdih e-mail: andrej.perdih@ki.si LITERATURA 1. TEMELJA: POGLAVJE O OSOVAH
SEMIAR IZ MOLEKULSKEGA MODELIRAJA PRI FARMACEVTSKI KEMIJI III 2. DEL PREDSTAVITEV PROGRAMOV ZA DELO A SEMIARJU doc. dr. Andrej Perdih e-mail: andrej.perdih@ki.si LITERATURA 1. TEMELJA: POGLAVJE O OSOVAH
Virtual screening for drug discovery. Markus Lill Purdue University
 Virtual screening for drug discovery Markus Lill Purdue University mlill@purdue.edu Lecture material http://people.pharmacy.purdue.edu/~mlill/teaching/eidelberg/ I.1 Drug discovery Cl N Disease I.1 Drug
Virtual screening for drug discovery Markus Lill Purdue University mlill@purdue.edu Lecture material http://people.pharmacy.purdue.edu/~mlill/teaching/eidelberg/ I.1 Drug discovery Cl N Disease I.1 Drug
CHEMISTRY The Molecular Nature of Matter and Change
 CHEMISTRY The Molecular Nature of Matter and Change Third Edition Chapter 12 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 11 INTERMOLECULAR FORCES
CHEMISTRY The Molecular Nature of Matter and Change Third Edition Chapter 12 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 11 INTERMOLECULAR FORCES
Last 4 Digits of USC ID:
 Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
1. Following Dalton s Atomic Theory, 2. In 1869 Russian chemist published a method. of organizing the elements. Mendeleev showed that
 20 CHEMISTRY 11 D. Organizing the Elements The Periodic Table 1. Following Dalton s Atomic Theory, By 1817, chemists had discovered 52 elements and by 1863 that number had risen to 62. 2. In 1869 Russian
20 CHEMISTRY 11 D. Organizing the Elements The Periodic Table 1. Following Dalton s Atomic Theory, By 1817, chemists had discovered 52 elements and by 1863 that number had risen to 62. 2. In 1869 Russian
More Chemical Bonding
 More Chemical Bonding Reading: Ch 10: section 1-8 Ch 9: section 4, 6, 10 Homework: Chapter 10:.31, 33, 35*, 39*, 43, 47, 49* Chapter 9: 43, 45, 55*, 57, 75*, 77, 79 * = important homework question Molecular
More Chemical Bonding Reading: Ch 10: section 1-8 Ch 9: section 4, 6, 10 Homework: Chapter 10:.31, 33, 35*, 39*, 43, 47, 49* Chapter 9: 43, 45, 55*, 57, 75*, 77, 79 * = important homework question Molecular
Aqueous solutions. Solubility of different compounds in water
 Aqueous solutions Solubility of different compounds in water The dissolution of molecules into water (in any solvent actually) causes a volume change of the solution; the size of this volume change is
Aqueous solutions Solubility of different compounds in water The dissolution of molecules into water (in any solvent actually) causes a volume change of the solution; the size of this volume change is
1. How many grams of Cr can be produced by the reaction of 44.1 g of Cr 2 O 3 with 35.0 g of Al according to the following chemical reaction?
 Final Exam Revision 1. How many grams of Cr can be produced by the reaction of 44.1 g of Cr 2 O 3 with 35.0 g of Al according to the following chemical reaction? 2Al + Cr 2 O 3 Al 2 O 3 + 2Cr Ans: 30.2
Final Exam Revision 1. How many grams of Cr can be produced by the reaction of 44.1 g of Cr 2 O 3 with 35.0 g of Al according to the following chemical reaction? 2Al + Cr 2 O 3 Al 2 O 3 + 2Cr Ans: 30.2
Revision of Important Concepts. 1. Types of Bonding
 Revision of Important Concepts 1. Types of Bonding Electronegativity (EN) often molecular often ionic compounds Bonding in chemical substances Bond energy: Is the energy that is released when a bond is
Revision of Important Concepts 1. Types of Bonding Electronegativity (EN) often molecular often ionic compounds Bonding in chemical substances Bond energy: Is the energy that is released when a bond is
NAME: FIRST EXAMINATION
 1 Chemistry 64 Winter 1994 NAME: FIRST EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
1 Chemistry 64 Winter 1994 NAME: FIRST EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
1) V diagramu sta prikazana plazemska koncentracijska profila po večkratnem intravenskem odmerjanju učinkovine v dveh različnih primerih (1 in 2).
 NALOGE ) V diagramu sta prikazana plazemska koncentracijska profila po večkratnem intravenskem odmerjanju učinkovine v dveh različnih primerih ( in ). 0.8 0.6 0.4 0. 0.0 0.08 0.06 0.04 0.0 0.00 0 0 0 30
NALOGE ) V diagramu sta prikazana plazemska koncentracijska profila po večkratnem intravenskem odmerjanju učinkovine v dveh različnih primerih ( in ). 0.8 0.6 0.4 0. 0.0 0.08 0.06 0.04 0.0 0.00 0 0 0 30
9701 CHEMISTRY. Mark schemes should be read in conjunction with the question paper and the Principal Examiner Report for Teachers.
 CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International Advanced Level MARK SCHEME for the May/June 2015 series 9701 CHEMISTRY 9701/42 Paper 4 (Structured Questions), maximum raw mark 100 This mark
CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International Advanced Level MARK SCHEME for the May/June 2015 series 9701 CHEMISTRY 9701/42 Paper 4 (Structured Questions), maximum raw mark 100 This mark
CAPVT IIII TOPLJIVOST
 CAPVT IIII TOPLJIVOST O entalpiji O vodi WATER from a Chemical Point of View or On Eccentricities and Peculiarities of a Very Strange Liquid Vladimir Stilinović Department of Chemistry, Faculty of Science,
CAPVT IIII TOPLJIVOST O entalpiji O vodi WATER from a Chemical Point of View or On Eccentricities and Peculiarities of a Very Strange Liquid Vladimir Stilinović Department of Chemistry, Faculty of Science,
Relative Permittivity in Stern and Diffuse Layers
 241 Scientific paper Relative Permittivity in Stern and Diffuse Layers Ekaterina Gongadze 1,2 and Ale{ Igli~ 1,2, * 1 Laboratory of Biophysics, Faculty of Electrical Engineering, University of Ljubljana,
241 Scientific paper Relative Permittivity in Stern and Diffuse Layers Ekaterina Gongadze 1,2 and Ale{ Igli~ 1,2, * 1 Laboratory of Biophysics, Faculty of Electrical Engineering, University of Ljubljana,
AP CHEMISTRY CHAPTERS 5 & 6 Problem Set #4. (Questions 1-13) Choose the letter that best answers the question or completes the statement.
 NAME: AP CHEMISTRY CHAPTERS 5 & 6 Problem Set #4 (Questions 1-13) Choose the letter that best answers the question or completes the statement. (Questions 1-2) Consider atoms of the following elements.
NAME: AP CHEMISTRY CHAPTERS 5 & 6 Problem Set #4 (Questions 1-13) Choose the letter that best answers the question or completes the statement. (Questions 1-2) Consider atoms of the following elements.
Chapter 12 INTERMOLECULAR FORCES. Covalent Radius and van der Waals Radius. Intraand. Intermolecular Forces. ½ the distance of non-bonded
 Chapter 2 INTERMOLECULAR FORCES Intraand Intermolecular Forces Covalent Radius and van der Waals Radius ½ the distance of bonded ½ the distance of non-bonded Dipole Dipole Interactions Covalent and van
Chapter 2 INTERMOLECULAR FORCES Intraand Intermolecular Forces Covalent Radius and van der Waals Radius ½ the distance of bonded ½ the distance of non-bonded Dipole Dipole Interactions Covalent and van
Red veze za benzen. Slika 1.
 Red veze za benzen Benzen C 6 H 6 je aromatično ciklično jedinjenje. Njegove dve rezonantne forme (ili Kekuléove structure), prema teoriji valentne veze (VB) prikazuju se uobičajeno kao na slici 1 a),
Red veze za benzen Benzen C 6 H 6 je aromatično ciklično jedinjenje. Njegove dve rezonantne forme (ili Kekuléove structure), prema teoriji valentne veze (VB) prikazuju se uobičajeno kao na slici 1 a),
02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr
 Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Chapter 8 The Concept of the Chemical Bond
 Chapter 8 The Concept of the Chemical Bond Three basic types of bonds: Ionic - Electrostatic attraction between ions (NaCl) Metallic - Metal atoms bonded to each other Covalent - Sharing of electrons Ionic
Chapter 8 The Concept of the Chemical Bond Three basic types of bonds: Ionic - Electrostatic attraction between ions (NaCl) Metallic - Metal atoms bonded to each other Covalent - Sharing of electrons Ionic
Chapter 2 - Water 9/8/2014. Water exists as a H-bonded network with an average of 4 H-bonds per molecule in ice and 3.4 in liquid. 104.
 Chapter 2 - Water Water exists as a -bonded network with an average of 4 -bonds per molecule in ice and 3.4 in liquid. 104.5 o -bond: An electrostatic attraction between polarized molecules containing
Chapter 2 - Water Water exists as a -bonded network with an average of 4 -bonds per molecule in ice and 3.4 in liquid. 104.5 o -bond: An electrostatic attraction between polarized molecules containing
MANY ELECTRON ATOMS Chapter 15
 MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
Atomic Structure & Interatomic Bonding
 Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
5 questions, 3 points each, 15 points total possible. 26 Fe Cu Ni Co Pd Ag Ru 101.
 Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
Chem 401 Unit 2 Exam Spr 2018 (Acids/ Bases/ General Equilibria /Acid-Base Equilibria)
 Name: Date: Exam #: _ Chem 401 Unit 2 Exam Spr 2018 (Acids/ Bases/ General Equilibria /Acid-Base Equilibria) Multiple Choice Identify the letter of the choice that best completes the statement or answers
Name: Date: Exam #: _ Chem 401 Unit 2 Exam Spr 2018 (Acids/ Bases/ General Equilibria /Acid-Base Equilibria) Multiple Choice Identify the letter of the choice that best completes the statement or answers
Bonding Test pg 1 of 4 Name: Pd. Date:
 Bonding Test pg 1 of 4 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) How many electrons are shared in a single covalent bond? 1. A) 2 B) 3 C)
Bonding Test pg 1 of 4 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) How many electrons are shared in a single covalent bond? 1. A) 2 B) 3 C)
The Periodic Table. Periodic Properties. Can you explain this graph? Valence Electrons. Valence Electrons. Paramagnetism
 Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Atomic Structure and Bonding. Chapter 1 Organic Chemistry, 8 th Edition John McMurry
 Atomic Structure and Bonding Chapter 1 Organic Chemistry, 8 th Edition John McMurry 1 Common Elements Groups First row Second row In most organic molecules carbon is combined with relatively few elements
Atomic Structure and Bonding Chapter 1 Organic Chemistry, 8 th Edition John McMurry 1 Common Elements Groups First row Second row In most organic molecules carbon is combined with relatively few elements
T h e C S E T I P r o j e c t
 T h e P r o j e c t T H E P R O J E C T T A B L E O F C O N T E N T S A r t i c l e P a g e C o m p r e h e n s i v e A s s es s m e n t o f t h e U F O / E T I P h e n o m e n o n M a y 1 9 9 1 1 E T
T h e P r o j e c t T H E P R O J E C T T A B L E O F C O N T E N T S A r t i c l e P a g e C o m p r e h e n s i v e A s s es s m e n t o f t h e U F O / E T I P h e n o m e n o n M a y 1 9 9 1 1 E T
8. Relax and do well.
 CHEM 1314 Name Exam IV TA Name John IV. Gelder December 14, 1992 Lab Section INSTRUCTIONS: 1. This examination consists of a total of 9 different pages. The last three pages includes a periodic table and
CHEM 1314 Name Exam IV TA Name John IV. Gelder December 14, 1992 Lab Section INSTRUCTIONS: 1. This examination consists of a total of 9 different pages. The last three pages includes a periodic table and
8. Relax and do well.
 CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
Questions 1 13 cover material from Exam 1
 Questions 1 13 cover material from Exam 1 1. Which intermolecular forces are present in H Te(l)? A. dispersion only C. dispersion, dipole-dipole, and hydrogen bonding B. dispersion and dipole-dipole D.
Questions 1 13 cover material from Exam 1 1. Which intermolecular forces are present in H Te(l)? A. dispersion only C. dispersion, dipole-dipole, and hydrogen bonding B. dispersion and dipole-dipole D.
FINAL EXAM April 26, 2004
 CM 1045 (11:15 am Lecture) Dr. Light FINAL EXAM April 26, 2004 Name (please print) Check your recitation section: Sec. 21 5:30-6:20 pm (Popovic) Sec. 24 3:30-4:20 pm (Giunta) Sec. 22 6:30-7:20 pm (Popovic)
CM 1045 (11:15 am Lecture) Dr. Light FINAL EXAM April 26, 2004 Name (please print) Check your recitation section: Sec. 21 5:30-6:20 pm (Popovic) Sec. 24 3:30-4:20 pm (Giunta) Sec. 22 6:30-7:20 pm (Popovic)
4 th Advanced in silico Drug Design KFC/ADD Molecular Modelling Intro. Karel Berka, Ph.D.
 4 th Advanced in silico Drug Design KFC/ADD Molecular Modelling Intro Karel Berka, Ph.D. UP Olomouc, 21.1.-25.1. 2019 Motto A theory is something nobody believes, except the person who made it An experiment
4 th Advanced in silico Drug Design KFC/ADD Molecular Modelling Intro Karel Berka, Ph.D. UP Olomouc, 21.1.-25.1. 2019 Motto A theory is something nobody believes, except the person who made it An experiment
Stopnja protolize(disociacije) - merilo za jakost elektrolita. = c d /c
 Stopnja protolize(disociacije) - merilo za jakost elektrolita = N/N 0 = n/n 0 = c d /c = stopnja protolize (disociacije) N = število disociiranih molekul (HCl) oz. formulskih enot (NaCl) N 0 = število
Stopnja protolize(disociacije) - merilo za jakost elektrolita = N/N 0 = n/n 0 = c d /c = stopnja protolize (disociacije) N = število disociiranih molekul (HCl) oz. formulskih enot (NaCl) N 0 = število
APPLICATION OF THOMAS-FERMI MODEL TO FULLERENE MOLECULE AND NANOTUBE UDC 547. Yuri Kornyushin
 FACTA UNIVERSITATIS Series: Physics, Chemistry and Technology Vol. 5, N o 1, 2007, pp. 11-18 DOI: 10.2298/FUPCT0701011K APPLICATION OF THOMAS-FERMI MODEL TO FULLERENE MOLECULE AND NANOTUBE UDC 547 Yuri
FACTA UNIVERSITATIS Series: Physics, Chemistry and Technology Vol. 5, N o 1, 2007, pp. 11-18 DOI: 10.2298/FUPCT0701011K APPLICATION OF THOMAS-FERMI MODEL TO FULLERENE MOLECULE AND NANOTUBE UDC 547 Yuri
Marks for each question are as indicated in [] brackets.
![Marks for each question are as indicated in [] brackets. Marks for each question are as indicated in [] brackets.](/thumbs/77/75878580.jpg) Name Student Number CHEMISTRY 140 FINAL EXAM December 10, 2002 Numerical answers must be given with appropriate units and significant figures. Please place all answers in the space provided for the question.
Name Student Number CHEMISTRY 140 FINAL EXAM December 10, 2002 Numerical answers must be given with appropriate units and significant figures. Please place all answers in the space provided for the question.
AP CHEMISTRY: BONDING THEORIES REVIEW KEY p. 1
 AP CHEMISTRY: BONDING THEORIES REVIEW KEY p. 1 1) a) O-H PC b) Cs-Cl I c) H-Cl PC d) Br-Br NPC 2) differences in electronegativity determines amount of ity O3 0, P8 0, NO.5, CO2 1.0, CH4.4, H2S.4 answer
AP CHEMISTRY: BONDING THEORIES REVIEW KEY p. 1 1) a) O-H PC b) Cs-Cl I c) H-Cl PC d) Br-Br NPC 2) differences in electronegativity determines amount of ity O3 0, P8 0, NO.5, CO2 1.0, CH4.4, H2S.4 answer
Chpt 8 Chemical Bonding Forces holding atoms together = Chemical Bonds
 Chpt 8 Chemical Bonding Forces holding atoms together = Chemical Bonds Kinds of chemical bonds: 1. Ionic 2. Covalent 3. Metallic Useful guideline: Octet rule Atoms tend to gain, lose, or share e - to achieve
Chpt 8 Chemical Bonding Forces holding atoms together = Chemical Bonds Kinds of chemical bonds: 1. Ionic 2. Covalent 3. Metallic Useful guideline: Octet rule Atoms tend to gain, lose, or share e - to achieve
TOPILO IZSTOPNI VENTIL CILINDER KOLONA DUŠILEC
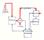 TOPILO VSTOPNI VENTIL CILINDER BAT IZSTOPNI VENTIL KOLONA DUŠILEC HPLC: 1. PORAZDELITVENA ( liquid-liquid ) 2. IONSKA 3. IZKLJUČITVENA oziroma GELSKA 4. ADSORPCIJSKA oziroma LIQUID-SOLID majhni delci (
TOPILO VSTOPNI VENTIL CILINDER BAT IZSTOPNI VENTIL KOLONA DUŠILEC HPLC: 1. PORAZDELITVENA ( liquid-liquid ) 2. IONSKA 3. IZKLJUČITVENA oziroma GELSKA 4. ADSORPCIJSKA oziroma LIQUID-SOLID majhni delci (
CHM1045 Exam 3 Chapters 5, 8, & 9
 1. Which of the following conditions will never result in a decrease in the internal energy of a system? CHM1045 Exam 3 Chapters 5, 8, & 9 a. System loses heat and does work on the surroundings. b. System
1. Which of the following conditions will never result in a decrease in the internal energy of a system? CHM1045 Exam 3 Chapters 5, 8, & 9 a. System loses heat and does work on the surroundings. b. System
CHEM Come to the PASS workshop with your mock exam complete. During the workshop you can work with other students to review your work.
 It is most beneficial to you to write this mock midterm UNDER EXAM CONDITIONS. This means: Complete the midterm in 1.5 hours. Work on your own. Keep your notes and textbook closed. Attempt every question.
It is most beneficial to you to write this mock midterm UNDER EXAM CONDITIONS. This means: Complete the midterm in 1.5 hours. Work on your own. Keep your notes and textbook closed. Attempt every question.
Consider a 1.0 L solution of 0.10 M acetic acid. Acetic acid is a weak acid only a small percent of the weak acid is ionized
 Chemistry 12 Acid- Base Equilibrium V Name: Date: Block: 1. Buffers 2. Hydrolysis Buffers An acid- base buffer is a solution that resists changes in ph following the addition of relatively small amounts
Chemistry 12 Acid- Base Equilibrium V Name: Date: Block: 1. Buffers 2. Hydrolysis Buffers An acid- base buffer is a solution that resists changes in ph following the addition of relatively small amounts
Downloaded from
 I.I.T.Foundation - XI Chemistry MCQ #4 Time: 45 min Student's Name: Roll No.: Full Marks: 90 Chemical Bonding I. MCQ - Choose Appropriate Alternative 1. The energy required to break a chemical bond to
I.I.T.Foundation - XI Chemistry MCQ #4 Time: 45 min Student's Name: Roll No.: Full Marks: 90 Chemical Bonding I. MCQ - Choose Appropriate Alternative 1. The energy required to break a chemical bond to
M09/4/CHEMI/HPM/ENG/TZ2/XX+ CHEMISTRY. Monday 18 May 2009 (afternoon) 1 hour INSTRUCTIONS TO CANDIDATES
 M09/4/CEMI/PM/ENG/TZ/XX+ 096113 CEMISTRY igher level Paper 1 Monday 18 May 009 (afternoon) 1 hour INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer all the
M09/4/CEMI/PM/ENG/TZ/XX+ 096113 CEMISTRY igher level Paper 1 Monday 18 May 009 (afternoon) 1 hour INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer all the
CHAPTER 12 CHEMICAL BONDING
 Chemistry Name Hour Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 12 CHEMICAL BONDING Day Plans for the day Assignment(s) for the day 1 Begin Chapter
Chemistry Name Hour Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 12 CHEMICAL BONDING Day Plans for the day Assignment(s) for the day 1 Begin Chapter
Supplemental Activities. Module: States of Matter. Section: Intermolecular Forces - Key
 Supplemental Activities Module: States of Matter Section: Intermolecular Forces - Key Electrostatic Forces ACTIVITY 1 The purpose of this activity is to practice recognizing the nature of the forces important
Supplemental Activities Module: States of Matter Section: Intermolecular Forces - Key Electrostatic Forces ACTIVITY 1 The purpose of this activity is to practice recognizing the nature of the forces important
Chapter 10. Dipole Moments. Intermolecular Forces (IMF) Polar Bonds and Polar Molecules. Polar or Nonpolar Molecules?
 Polar Bonds and Polar Molecules Chapter 10 Liquids, Solids, and Phase Changes Draw Lewis Structures for CCl 4 and CH 3 Cl. What s the same? What s different? 1 Polar Covalent Bonds and Dipole Moments Bonds
Polar Bonds and Polar Molecules Chapter 10 Liquids, Solids, and Phase Changes Draw Lewis Structures for CCl 4 and CH 3 Cl. What s the same? What s different? 1 Polar Covalent Bonds and Dipole Moments Bonds
CHEM- 457: Inorganic Chemistry
 CHEM- 457: Inorganic Chemistry Midterm II April 17 th, 2014 NAME This exam is comprised of five questions and is 13 pages in length. Please be sure that you have a complete exam and place your name on
CHEM- 457: Inorganic Chemistry Midterm II April 17 th, 2014 NAME This exam is comprised of five questions and is 13 pages in length. Please be sure that you have a complete exam and place your name on
materials and their properties
 materials and their properties macroscopic properties phase state strength / stiffness electrical conductivity chemical properties color / transparence spectroscopical properties surface properties density
materials and their properties macroscopic properties phase state strength / stiffness electrical conductivity chemical properties color / transparence spectroscopical properties surface properties density
Crystal Field Theory
 Crystal Field Theory It is not a bonding theory Method of explaining some physical properties that occur in transition metal complexes. Involves a simple electrostatic argument which can yield reasonable
Crystal Field Theory It is not a bonding theory Method of explaining some physical properties that occur in transition metal complexes. Involves a simple electrostatic argument which can yield reasonable
Circle the letters only. NO ANSWERS in the Columns!
 Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
2 (27) 3 (26) 4 (21) 5 (18) 6 (8) Total (200) Periodic Table
 Chem 3311 Sammakia Fall 2009 Midterm 1 Student ID page points: 2 (27) 3 (26) 4 (21) 5 (18) 6 (8) Total (200) Periodic Table e Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn
Chem 3311 Sammakia Fall 2009 Midterm 1 Student ID page points: 2 (27) 3 (26) 4 (21) 5 (18) 6 (8) Total (200) Periodic Table e Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn
Patrick: An Introduction to Medicinal Chemistry 5e Chapter 01
 Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
